Abstract
Memory is a dynamic process that is continuously regulated by both synaptic and intrinsic neural mechanisms. While numerous studies have shown that synaptic plasticity is important in various types and phases of learning and memory, neuronal intrinsic excitability has received relatively less attention, especially regarding the dynamic nature of memory. In this review, we present evidence demonstrating the importance of intrinsic excitability in memory allocation, consolidation, and updating. We also consider the intricate interaction between intrinsic excitability and synaptic plasticity in shaping memory, supporting both memory stability and flexibility.
Keywords: intrinsic excitability, synaptic plasticity, memory allocation, memory consolidation, memory updating, temporal memory-linking
Introduction
The question of how memories are formed and stored has intrigued and inspired neuroscientists for more than a century. Diverse theories have been proposed to describe the fundamental mechanisms underlying learning and memory at the molecular, cellular, and network levels. Following the Hebbian hypothesis (Hebb, 1949), numerous studies over several decades of research have shown that memory storage involves the induction of synaptic plasticity, defined as an activity-dependent modification of the strength of synaptic connections (Levy and Steward, 1979; Bliss and Collingridge, 1993, 2013; Citri and Malenka, 2008; Mayford et al., 2012; Takeuchi et al., 2014). Immediately after learning, newly formed memories are stabilized through the induction of synaptic plasticity, which typically takes the form of long-term potentiation or depression (LTP or LTD) (Bliss and Lømo, 1973; Ito and Kano, 1982). The mechanisms that govern synaptic plasticity have been the subject of extensive investigation. Here, we will focus on another critical, yet much less understood mechanism underlying learning and memory: the modulation of neuronal intrinsic excitability (Disterhoft et al., 1986; Coulter et al., 1989; Thompson et al., 1996; Daoudal and Debanne, 2003; Zhang and Linden, 2003; Disterhoft and Oh, 2006a, 2007; Kuo et al., 2008; Oh et al., 2010, 2016; Sehgal et al., 2013; Oh and Disterhoft, 2015). Neuronal intrinsic excitability is a neuron’s tendency to generate action potentials (APs) upon synaptic integration and is dictated primarily by the distribution and function of voltage-gated ion channels (see Box 1).
Box 1: Experimental quantification of intrinsic excitability.
Experience-dependent alterations in neuronal intrinsic excitability are primarily measured using in vitro whole-cell patch-clamp recordings in brain slices, where changes are reflected in several electrophysiological parameters outlined in Table 1. Overall, excitability is defined as the neuron’s capability to generate APs in response to stimuli (such as somatic current injections or synaptic stimulation). While a simple measurement of the resting membrane potential could reflect excitability, even neurons with the same resting potential could have different excitability. Therefore, studies usually measure other key parameters that may affect AP generation while holding the membrane potential at a certain level (such as −60 mV or −70 mV).
One important parameter to determine intrinsic excitability is the afterhyperpolarization (AHP), which is a hyperpolarized phase after a single or a train of APs where the membrane potential is below the neuron’s resting membrane potential. There are three types of AHPs (Table 1; Fig. 1): the fast AHP (fAHP) that occurs after each AP and lasts 2–5 ms, and the medium (mAHP) and slow (sAHP) AHP, which are evoked after a burst of APs and last 50–300 ms and 1–2 seconds, respectively. Since postburst AHP is generated independent of synaptic transmission, it is considered to be an intrinsic neuronal property (Coulter et al., 1989; Power et al., 2002). Both mAHP and sAHP are mediated largely by calcium-dependent outward K+ currents. The channels that underlie mAHP are generally considered to be a sub-family of Ca2+-dependent K+ channels called apamin-sensitive SK channels (Stocker et al., 1999; Bond et al., 2004; Stocker, 2004), while those that underlie sAHP are not clearly known (Sah and Faber, 2002; Disterhoft and Oh, 2006a; Oh et al., 2010). A reduction in the size of the postburst AHP is considered to indicate an increase in intrinsic excitability, and vice versa. Interestingly, changes in mAHP and sAHP have been observed after learning and in age-related cognitive deficits (Disterhoft and Oh, 2006b, 2006a, 2007; Ohno et al., 2006; Oh et al., 2016).
In addition to postburst AHP, spike frequency accommodation (referred to as accommodation from here forward) is an additional phenotype of AP reflecting intrinsic excitability (Table 1). Accommodation is defined as a reduction in AP firing frequency in response to a sustained depolarization step or a train of depolarizing stimuli. It results from the adaptation of various ionic currents that affect the generation of APs, including the AHP current mediated by Ca2+-dependent K+ channels (Madison and Nicoll, 1984), and is usually considered to be an intrinsic property of a neuron. A reduction in accommodation (i.e. more APs induced by a sustained stimulus) reflects an increase in excitability, and vice versa. Researchers have also used other parameters associated with APs as indicators of neuronal intrinsic excitability (see Table 1). Alterations in these properties reflect changes in the overall expression, distribution, and function of voltage-gated ion channels and are used to quantify changes in intrinsic excitability (Aizenman and Linden, 2000; Armano et al., 2000; Egorov et al., 2002; Crestani et al., 2018).
Specifically, we will highlight several ways in which intrinsic excitability plays a fundamental role in memory formation and memory updating. First, intrinsic excitability regulates the allocation of memory to a specific ensemble of neurons. Next, learning-induced increases in intrinsic excitability likely promote memory consolidation by facilitating the induction of long-term synaptic plasticity. Finally, intrinsic excitability supports the dynamic processes of memory updating, contributing to memory flexibility. In this review, we will examine the current literature supporting a role for neuronal intrinsic excitability across these different phases in the evolution of memories and propose future studies that can advance our understanding of the role of intrinsic excitability in the dynamic processes underlying learning and memory.
Intrinsic excitability and memory allocation
In the early twentieth century, Richard Semon introduced the term “engram” to describe the physical manifestation of memory, defined as “the enduring though primarily latent modification in the irritable substance produced by a stimulus” (Semon, 1921). The biological basis for the engram was elusive, however, as Karl Lashley was unable to find a specific engram within the cortex (Lashley, 1933, 1935). In recent years, new technologies for identifying and controlling cellular activity have enabled us to deepen our understanding of the possible physical “trace” of memory (Josselyn, 2010; Sakaguchi and Hayashi, 2012; Josselyn et al., 2015, 2017; Tonegawa et al., 2015b, 2015a; Eichenbaum, 2016; Poo et al., 2016). Several studies have shown that memories are initially encoded in a sparse population of neurons, or a neural “ensemble” (Guzowski et al., 1999; Reijmers et al., 2007; Han et al., 2009; Zhou et al., 2009; Liu et al., 2012; Josselyn et al., 2015; Tonegawa et al., 2015a, 2015b). Artificially reactivating the neural ensemble originally activated during memory encoding leads to memory retrieval (Garner et al., 2012; Liu et al., 2012; Ramirez et al., 2013; Cowansage et al., 2014; Yiu et al., 2014; Ryan et al., 2015; Rogerson et al., 2016; Frankland et al., 2019). Furthermore, memory allocation, the process of recruiting neurons to form an ensemble representation of the memory, is not random. Rather, neurons with elevated excitability have a higher probability of being recruited into a memory ensemble (Silva et al., 2009; Lisman et al., 2018). Here, we review the literature highlighting the role of intrinsic excitability in regulating memory allocation.
Initial insights into how neuronal excitability modulated memory allocation were derived from a series of studies on the cAMP responsive element-binding protein (CREB), a molecule that plays a key role in the induction of long-term potentiation (LTP) and the consolidation of long-term memory (Yin and Tully, 1996; Alberini, 1999, 2009) (see Box 2). Recent studies found that CREB function regulates memory allocation. Overexpressing wild-type CREB in the amygdala significantly increased the probability of a neuron to be activated during memory encoding and recruited into the memory ensemble, and the expression of dominant negative CREB decreased this probability (Han et al., 2007). CREB’s regulatory role in memory allocation has since been repeatedly found in the amygdala (Han et al., 2009; Zhou et al., 2009; Yiu et al., 2014; Rogerson et al., 2016), the insular cortex (Sano et al., 2014), and the hippocampus (Park et al., 2016). It was postulated that CREB increased memory allocation through increasing neuronal excitability. Neurons with enhanced CREB gene expression exhibited increased excitability while those with suppressed CREB expression had decreased excitability (Dong et al., 2006; Viosca et al., 2009; Zhou et al., 2009).
Box 2: Molecular factors regulating intrinsic excitability.
In addition to direct modulation by the expression, distribution, and function of various ion channels, the intrinsic excitability is also dependent on protein synthesis and second messenger systems (Daoudal and Debanne, 2003; Saar and Barkai, 2003; Zhang and Linden, 2003; Cohen-Matsliah et al., 2010), many of which are shared with the induction and maintenance of long-term potentiation (LTP), which is a major form of long-term synaptic plasticity believed to be essential for memory consolidation (Abel and Lattal, 2001; Kandel, 2001; Dudai, 2004; Citri and Malenka, 2008; Kandel et al., 2014; Dudai et al., 2015).
One important molecule indicated in both synaptic plasticity and intrinsic excitability is the cAMP responsive element-binding protein (CREB), a key regulator in the cAMP-protein kinase A (PKA) signaling pathway. CREB is activated via posttranslational modifications such as phosphorylation, and its activation is synergistically triggered by an increase in intracellular Ca2+ and an increase in cAMP (Lonze and Ginty, 2002). CREB has important functions in the nervous system, including learning and memory, which has been demonstrated in various brain regions across different behavior tasks (Yin and Tully, 1996; Bernabeu et al., 1997; Impey et al., 1998; Alberini, 1999, 2009; Taubenfeld et al., 1999; Stanciu et al., 2001). As a well-studied form of long-term synaptic plasticity supporting memory consolidation, the induction of LTP is regulated by CREB. The CRE-mediated gene expression was markedly increased after the generation of long-lasting LTP (Impey et al., 1996), while CREB knockout mice showed memory deficits and impaired LTP (Bourtchuladze et al., 1994). The essential role of CREB activation in LTP induction is now confirmed in a number of studies (Bito et al., 1996; Deisseroth et al., 1996; Segal and Murphy, 1998; Alberini, 1999, 2009; Kandel, 2001; Barco et al., 2002; Josselyn and Nguyen, 2005; Kandel et al., 2014). Besides supporting synaptic plasticity, CREB also regulates neuronal intrinsic excitability. Virally overexpressing CREB increased neuronal intrinsic excitability in various brain regions, including the hippocampus, amygdala, locus coeruleus, and nucleus accumbens neurons (Dong et al., 2006; Han et al., 2007; Viosca et al., 2009; Zhou et al., 2009; Yiu et al., 2014; Yu et al., 2017), possibly by decreasing voltage-gated K+ currents (Dong et al., 2006) and then reducing the size of postburst AHP (Gu et al., 2005; Oh et al., 2010). Therefore, both synaptic and intrinsic plasticity (LTP and increased excitability, respectively) are induced after CREB activation. These mechanisms may then work synergistically in promoting memory consolidation.
A number of other molecular factors can also alter the expression of ion channels on the membrane, reduce the AHP, and increase intrinsic excitability. Protein kinase A (PKA), protein kinase C (PKC), and calmodulin-dependent protein kinase II (CaMKII), which are major protein kinases within the cAMP-dependent pathway and are involved in different phases of LTP induction (Huang et al., 1996; Abel et al., 1997; Impey et al., 1998, 1999; Abel and Lattal, 2001; Kandel, 2001), have been found to be involved in the reduction of AHP (Melyan et al., 2002; Seroussi et al., 2002; Ohno et al., 2006; Armentia et al., 2007; Grabauskas et al., 2007; Oh et al., 2009; Cohen-Matsliah et al., 2010). The activation of muscarinic receptors and metabotropic glutamate receptors has been shown to reduce AHP via PKC and CaMKII (Malenka et al., 1986; Pineda et al., 1995; Pedarzani and Storm, 1996; Grabauskas et al., 2007), while the activation of monoamine receptors reduced AHP via PKA (Pedarzani and Storm, 1993; Lancaster et al., 2006; Grabauskas et al., 2007; Oh et al., 2009).
A critical study tested the direct link between intrinsic excitability and memory allocation by manipulating two AHP-related K+ channels to directly increase and decrease excitability (Yiu et al., 2014). Memory allocation was biased toward neurons with increased intrinsic excitability and away from neurons with decreased intrinsic excitability. Another study used a step function opsin to induce a prolonged depolarization in a subset of amygdala neurons, mimicking a more depolarized resting membrane potential of the hyperexcitable neurons (Rogerson et al., 2016). Fear memory ensembles were biased into the opsin-expressing, depolarized neurons. Beyond experimental findings, theoretical modeling has also supported the potential for excitability to dictate memory allocation. Biophysical modeling of 1000 lateral amygdala cells predicted that principal neurons with higher intrinsic excitability prior to network training on an auditory fear conditioning task were more likely to become “plastic” cells after training (increased response to the conditioned stimulus) compared to cells with lower excitability (Kim et al., 2013). The results of these various studies point to a direct role for neuronal excitability in guiding memory allocation.
Studies describing how place cells emerge have provided converging evidence for the role of intrinsic excitability in memory allocation. A landmark study found that in the hippocampus a specific subset of cells, now known as place cells, fired when the animal was in a particular location (O’Keefe and Dostrovsky, 1971), contrary to “silent cells”, which rarely fired during awake, active behavior (Thompson and Best, 1989). With the advantage of intracellular recording techniques in freely-behaving animals (Lee et al., 2006, 2009), more recent studies started to answer the question of how excitability plays a role in shaping place or silent cells. Place cells exhibited increased excitability compared to silent cells, with lower spiking thresholds and higher burst rates, even before an animal was first introduced to an environment (Epsztein et al., 2011). Furthermore, altering the excitability of CA1 pyramidal cells induced place field formation. Increasing the excitability of previously silent CA1 pyramidal neurons by uniformly depolarizing the somatic membrane potential during spatial exploration led to the emergence of spatially-tuned place fields (Lee et al., 2012), and current injections at specific spatial locations appeared to drive the formation of spatial fields (Bittner et al., 2015; Diamantaki et al., 2018). In vivo intracellular recordings have also shown that exploration of novel environments caused increases in measures of intrinsic excitability, such as reduced action potential (AP) threshold and increased subthreshold membrane voltage “hills” that underlie AP bursts and place fields (Cohen et al., 2017). Together, these findings suggest that the formation of place fields not only requires that cells receive specific spatial input, but that those cells must also have increased intrinsic excitability relative to other cells.
In conclusion, converging evidence has demonstrated that memory allocation is not a random process, but is regulated, at least in part, by neuronal intrinsic excitability. Neurons with higher excitability are preferentially recruited into an ensemble, and direct manipulations of excitability are sufficient to drive the ensemble formation (Lee et al., 2012; Yiu et al., 2014; Bittner et al., 2015; Rogerson et al., 2016; Diamantaki et al., 2018).
Intrinsic excitability and memory consolidation
After memory allocation, a series of processes follows to stabilize the newly acquired information and transform it into long-term memory. These processes are commonly referred to as memory consolidation, which occurs at the synaptic and systems levels on different time scales (Dudai, 1996, 2004; McGaugh, 2000; Kandel et al., 2014; Dudai et al., 2015). Synaptic consolidation is assumed to last minutes to hours after encoding within the local circuit and synapses. Systems consolidation, on the other hand, is considered to take days to months or even longer and involves the distribution of the memory representations across different brain regions. The two levels of consolidation are closely related, since systems consolidation is assumed to involve waves of synaptic consolidation in interacting brain areas (Dudai, 2012). The mechanisms of memory consolidation are generally thought to rely on synaptic plasticity—the strengthening or weakening of synaptic connections—through activation of signaling cascades leading to modulations in gene expression, post-translational modifications, and protein synthesis (Abel and Lattal, 2001; Kandel, 2001; Dudai, 2004; Alberini, 2008; Kandel et al., 2014; Dudai et al., 2015). Synaptic plasticity has been seen in different forms, with the most extensively studied have been long-term potentiation (LTP) and long-term depression (LTD) in the hippocampus (Citri and Malenka, 2008).
While the synaptic mechanisms of memory consolidation have been the subject of considerable investigation, the role of intrinsic excitability in memory consolidation has received much less attention. There is increasing evidence, nonetheless, that intrinsic excitability may have a significant role in memory consolidation. First, learning induces a transient increase in neuronal intrinsic excitability, with a time window coinciding with that of memory consolidation, suggesting a potential role for intrinsic excitability during consolidation. Second, post-learning excitability increase is required for successful learning and may also serve as a general mechanism promoting learning and memory. Last, intrinsic excitability may promote memory consolidation by interacting with LTP, a major form of synaptic plasticity supporting memory consolidation.
After learning, neuronal intrinsic excitability transiently increases, persisting from hours to days. This phenomenon was initially discovered in rabbits after a trace eyeblink conditioning task (Moyer et al., 1996, 2000; Thompson et al., 1996). Once rabbits successfully learned the task, postburst afterhyperpolarization (AHP) (see Box 1 and Fig. 1) was reduced in hippocampal pyramidal neurons in CA1 (Moyer et al., 1996, 2000) and CA3 (Thompson et al., 1996), indicating increased excitability. Enhanced excitability was observed as early as 1 hour after learning, maximizing at 1 day, and returning to baseline within 7 days (Moyer et al., 1996; Thompson et al., 1996). Enhanced excitability has been observed in rodent hippocampal CA1 after learning various tasks, including trace eyeblink conditioning (Kuo et al., 2008; Matthews et al., 2009; Oh et al., 2009), trace and contextual fear conditioning (Kaczorowski and Disterhoft, 2009; Mckay et al., 2009; Song et al., 2012), Morris water maze (Oh et al., 2003), and an olfactory discrimination task (Zelcer et al., 2006). An important feature of this increase in learning-induced excitability is its transient nature. With induction as early as an hour after learning (perhaps sooner, although this has not been measured), the enhancement of intrinsic excitability temporally overlaps with the critical period of synaptic consolidation (Dudai, 2004). Further, this increase in excitability persists for days after learning (Moyer et al., 1996; Thompson et al., 1996), which may facilitate systems consolidation by supporting synaptic plasticity between interacting brain regions.
Figure 1. Three types of afterhyperpolarization (AHP).
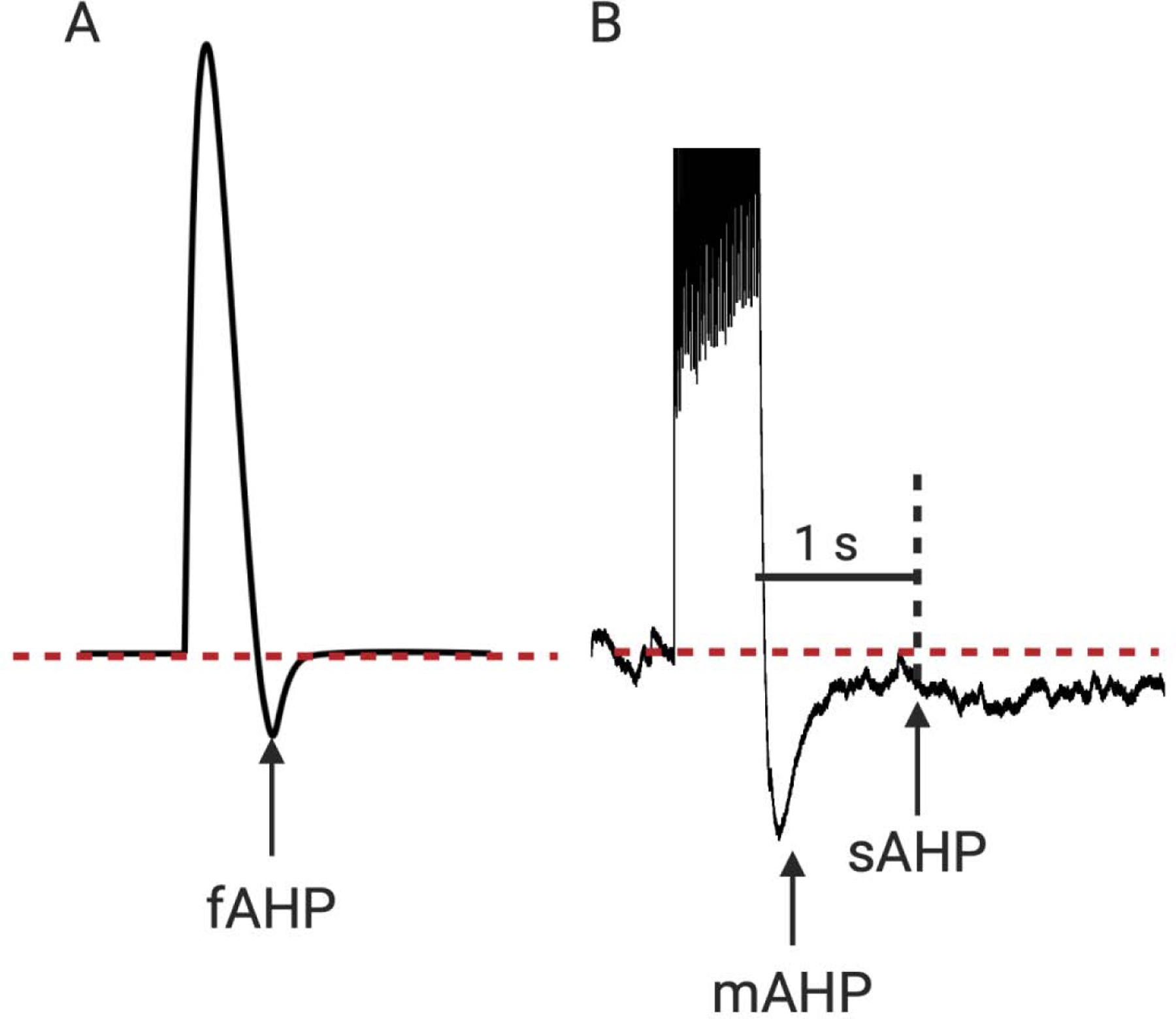
(A) Fast AHP (fAHP) after a single action potential, measured as the negative peak relative to the holding potential after a single action potential (AP). (B) Medium and slow AHP (mAHP and sAHP, respectively) after a burst of APs, measured as the negative peak relative to the holding potential between 50–300 ms (mAHP) or the remaining hyperpolarization at 1 s (sAHP) after the AP train.
Since neuronal hyperexcitability and consolidation processes occur within the same time window after learning, excitability may play a role during the first hours to days to stabilize the memory. Indeed, a number of behavioral observations support this hypothesis. First, the transient increase in excitability correlates with successful learning. Neuronal intrinsic excitability in animals that failed to learn the trace eyeblink conditioning task (non-learners) was comparable to that of control animals and lower than that of successful learners (Moyer et al., 1996, 2000; Thompson et al., 1996; Oh et al., 2003; Kaczorowski and Disterhoft, 2009; Matthews et al., 2009; Song et al., 2012). Further studies of excitability changes with aging supported learning-induced excitability as important for memory consolidation. While middle-aged and aged animals had either similar or lower baseline excitability compared to young animals, it was post-learning excitability that predicted whether aged animals were able to consolidate memories properly (Moyer et al., 2000; Tombaugh et al., 2005; Kaczorowski and Disterhoft, 2009; Matthews et al., 2009). Animals that recalled the memory had higher learning-induced excitability, regardless of whether the subjects were young or aged. Second, excitability increases in the hippocampus lasted for a shorter period of time than the retention of the memory. While excitability generally returned to baseline within 7 days, the retention of the memory for the trace eyeblink conditioning task lasted for months (Moyer et al., 1996; Thompson et al., 1996). Therefore, it would appear that enhanced excitability is not required to retrieve the memory but is instead involved in the initial stabilizing processes after encoding. It is also possible that hyperexcitability in CA1 facilitates not only synaptic consolidation within the hippocampus, but also systems consolidation in associated cortical areas, rendering the memory hippocampus-independent at a later time point (Kim et al., 1995). Whether waves of excitability alterations persist in interacting brain regions after learning, ranging from hours to days and months, remains unclear and will be an exciting area to explore. Taken together, these studies demonstrate that excitability increases after learning may be critical in memory consolidation.
Although a number of studies have shown that the level of intrinsic excitability correlates closely with the quality of learning and memory, there has so far been a dearth of research investigating how intrinsic excitability directly affects consolidation.
Several studies do, nonetheless, describe how excitability regulates LTP. As discussed, LTP is a major form of synaptic plasticity contributing to memory consolidation (Abel and Lattal, 2001; Kandel, 2001; Dudai, 2004; Citri and Malenka, 2008; Kandel et al., 2014; Dudai et al., 2015). These studies suggest a mutual relationship between intrinsic excitability and the induction of LTP, which could provide a mechanistic link between learning-induced excitability increases and memory consolidation. Intrinsic excitability is determined by the distribution and function of voltage-gated ion channels (see Box 1). A sub-class of Ca2+-activated K+ channels, known as the apamin-sensitive SK channels, are a major determinant of intrinsic excitability through regulation of the postburst AHP (Stocker et al., 1999; Sah and Faber, 2002; Bond et al., 2004; Stocker, 2004; Disterhoft and Oh, 2006a; Oh et al., 2010). Inhibiting SK channels reduced the size of postburst AHP (Stocker et al., 1999; Kramár et al., 2004), leading to a higher intrinsic excitability, while activation of SK channels led to an enlarged AHP and reduced excitability (Pedarzani et al., 2001; McKay et al., 2012). Besides mediating intrinsic excitability, SK channels also modulated LTP induction. These channels appear to be highly enriched in the postsynaptic density of dendritic spines in pyramidal neurons (Faber et al., 2005; Ngo-Anh et al., 2005; Bloodgood and Sabatini, 2007; Faber, 2010; Mulholland et al., 2011), where they form a negative feedback loop that limits NMDA receptor activation in response to dendritic Ca2+ influx. Further, activating SK channels in the hippocampus impaired associative learning (McKay et al., 2012), while blocking SK channels in the hippocampus (Behnisch and Reymann, 1998; Norris et al., 1998; Stackman et al., 2002; Kramár et al., 2004), amygdala (Faber et al., 2005, 2008), and cortex (Faber, 2010; Bock and Stuart, 2016; Bock et al., 2019) facilitated LTP induction, increased postsynaptic neuronal excitability, and enhanced spatial and non-spatial memories (Stackman et al., 2002). Therefore, the inhibition of SK channels could conceivably serve as a common mechanism both enhancing intrinsic excitability and facilitating LTP induction after learning. Learning-induced alterations in ion channels directly alter intrinsic excitability and can act as an additional mechanism (besides the classical LTP induction cascade) to further promote the strengthening of synapses. Moreover, maintaining enhanced excitability for a period of time after learning requires protein synthesis and the activation of intracellular signaling pathways, many of which also contribute to the induction and maintenance of LTP (see Box 2). Taken together, these studies suggest a close relationship between the induction and maintenance of intrinsic and synaptic plasticity.
In summary, a transient increase in intrinsic excitability is correlated with successful learning and may serve as a neuronal mechanism to promote memory consolidation. Moreover, the time course of learning-induced excitability increases occurs within the same time as synaptic and early systems consolidation. Given the highly interconnected relationship between intrinsic excitability and LTP, a well-established proxy of memory consolidation, it is very likely that intrinsic excitability contributes to consolidation processes. Within the hippocampal circuits, a transient increase in excitability may facilitate LTP induction and the initial synaptic consolidation. Brain wide, waves of excitability alterations in interacting brain regions may enhance the strengthening of cross-regional synaptic connections, thus contributing to systems consolidation on a longer time scale as well.
Intrinsic excitability and memory updating
In the last few decades, there have been significant advances in our understanding of the mechanisms underlying the formation of single memories (Bliss and Collingridge, 1993; McGaugh, 2000; Kandel, 2001; Kandel et al., 2014). Memory processing, however, involves the integration of multiple memories across time, with single memories affecting how others are processed and stored over time (Schlichting et al., 2015, 2017; Cai et al., 2016; Rashid et al., 2016; Morton et al., 2017; Richards and Frankland, 2017; Yokose et al., 2017). The brain’s ability to organize and integrate different experiences so that it can efficiently ‘file’ and ‘cross reference’ information is critical for daily life. Memories need to maintain stability and fidelity, yet new information must be flexibly integrated into past memories to inform future decision making (Schacter et al., 1998; Routtenberg and Rekart, 2005; Kroes and Fernández, 2012; Nadel et al., 2012; Rule et al., 2019). The dynamic nature of memory makes it possible to integrate new information during memory updating while reducing the influence of outdated knowledge, which is crucial for memory-guided decision making (Richards and Frankland, 2017). Although different forms of memory integration and updating has been observed in animal and human studies, the exact mechanisms driving these phenomena remain largely unclear. Here, we review the literature on neuronal intrinsic excitability regulating the dynamic memory updating processes. Specifically, we will focus on temporal memory-linking, memory integration during retrieval, and rule and schema learning.
Temporal memory-linking
We have reviewed several studies showing that the initial allocation of memory, the process of recruiting neurons to form an ensemble representation of a memory, is not random. Neurons with higher excitability have an increased probability of being recruited into the memory ensemble than neighboring neurons (Han et al., 2007, 2009; Zhou et al., 2009; Sano et al., 2014; Yiu et al., 2014; Park et al., 2016; Rogerson et al., 2016). An important evidence for the memory allocation hypothesis is that learning-induced excitability increases may be a mechanism to temporally link memories encoded close in time (i.e., temporal memory-linking). It has been shown repeatedly in different behavior tasks that after learning, neuronal intrinsic excitability increases transiently within hours and returns to baseline within days (Moyer et al., 1996; Thompson et al., 1996; Zelcer et al., 2006). A contextual memory is encoded into a sparse neural ensemble in the hippocampus (Guzowski et al., 1999; Garner et al., 2012; Liu et al., 2012; Deng et al., 2013; Mckenzie et al., 2014). When a second new environment is introduced a few hours later and the excitability of the ensemble neurons from the first memory is still high, the allocation of the second memory should be biased into many of the same neurons as the first one (Zhou et al., 2009; Dragoi and Tonegawa, 2011; Epsztein et al., 2011; Lee et al., 2012; Yiu et al., 2014). Indeed, with in vivo calcium imaging, a study showed that the ensemble overlap between two memories encoded 5 hours apart was significantly higher than that between two memories encoded 7 days apart (Cai et al., 2016). Sharing a neural ensemble between two memories had important behavioral consequences; recall of one memory triggered recall of the temporally linked memory (encoded 5 hours apart) (Cai et al., 2016). Similar findings have been observed in the lateral amygdala (LA), where two cued fear conditioning sessions administered 6 hours apart were more likely to be encoded by an overlapping population of neurons than those encoded a day apart (Rashid et al., 2016). These memories were also behaviorally linked so that extinguishing one memory also extinguished the other when learned hours apart.
In addition to endogenously changing excitability through learning, other studies artificially manipulated neuronal excitability to examine how excitability may regulate temporal memory-linking. Increasing excitability by virally overexpressing CREB in a sparse population of neurons biased the CREB positive neurons to increase their probability of allocating two memories encoded 24 hours apart, artificially linking two memories typically encoded separately (Rashid et al., 2016). Studies have also used neural activity as a proxy for intrinsic excitability and investigated its potential role in temporal memory-linking. Optogenetic activation of a sparse neural ensemble in the lateral amygdala during the encoding of two temporally separate memories was sufficient to drive the linking of the two memories (Rashid et al., 2016). Separate studies with middle-aged mice with decreased hippocampal CA1 excitability (Murphy et al., 2006; Disterhoft and Oh, 2007; Kaczorowski and Disterhoft, 2009; Oh et al., 2010) showed decreased ensemble overlap for temporally close memories leading to a deficit in temporal memory-linking (Cai et al., 2016). Chemogenetically boosting CA1 neural activity restored normal temporal memory-linking in middle-aged mice by driving temporally close memories into a shared ensemble (Cai et al., 2016). These studies suggest that a group of highly active neurons is sufficient to drive the linking processes. While all of these manipulations affect neuronal excitability or activity, off target effects may contribute to linking. Although CREB increases excitability, it also triggers a molecular cascade of events that could mediate linking. Both optogenetic and chemogenetic manipulations serve only as a proxy for intrinsic excitability. Therefore, future studies directly manipulating excitability-related voltage-gated ion channels are critical to dissect how intrinsic excitability links and separates memories across time.
As we have discussed, an important finding in temporal memory-linking studies is that when memories were encoded close in time and shared an overlapping neuronal ensemble, the first memory enhanced the strength of the second (Cai et al., 2016; Rashid et al., 2016). This enhancement effect was also observed in studies artificially driving excitability during encoding (Yiu et al., 2014; Yu et al., 2017). A possible explanation for this enhancing effect is that the state of transient hyperexcitability of the first memory ensemble drives allocation of the second memory into a shared ensemble, and, subsequently, increased excitability of the shared neurons further facilitates consolidation of the second memory. The phenomenon of temporal memory-linking serves as a demonstration that memories are not formed in isolation, but rather that the formation of a memory may be influenced by prior experiences, possibly through the modulation of intrinsic excitability.
Memory integration during retrieval
The studies reviewed thus far demonstrate that intrinsic excitability appears to play a key role in guiding memory allocation and facilitating memory consolidation, leading to the temporal linking of memories. Intrinsic excitability is not only transiently increased after the initial encoding of a memory, but also after memory retrieval providing a mechanism by which memories can be updated through the integration of new information. A recent study investigated changes in the intrinsic excitability of dentate gyrus granule cells after mice recalled a contextual fear memory (Pignatelli et al., 2019). On Day 1, mice were exposed to a contextual fear conditioning task. On Day 2, mice were re-exposed to the conditioned context to elicit memory retrieval. Researchers prepared acute brain slices from mice that underwent retrieval and observed that eYFP-tagged cells (ensemble cells) exhibited enhanced excitability compared to neighboring non-tagged cells (non-ensemble cells), demonstrated by increased input resistance and decreased rheobase. Interestingly, optogenetic activation of the ensemble cells while animals were in a novel context did not alter their intrinsic excitability, suggesting that the observed heightened excitability was specific to retrieval-induced re-activation but not to artificial activation. Heightened excitability lasted approximately 1 hour during which the animals’ ability to discriminate between the conditioned context and a similar unconditioned context (i.e. pattern separation) was enhanced. Also, the retrieval of a neutral contextual memory enabled the animals to more rapidly associate the neutral context with a foot shock during an immediate shock protocol. To demonstrate that these enhancements were caused by elevated excitability, researchers then overexpressed endogenous Kir2.1, which suppresses intrinsic excitability. Suppressing the excitability of ensemble cells abolished the enhancement in pattern separation and memory integration after retrieval (Pignatelli et al., 2019). Another study also showed the effect of memory retrieval on encoding of a subsequent memory, without, however, measuring excitability. Recalling a fear memory 6 hours but not 1 day before encoding a new memory enhanced the new memory (Rashid et al., 2016). Together, these studies show that recalling a memory can both alter the quality of a memory and facilitate the integration of new information with the recalled memory. Furthermore, similar to learning-induced excitability increases, retrieval-induced excitability increases may also enhance the encoding and consolidation of a memory formed following the recall.
When two memories are encoded close in time, they can be behaviorally linked (Cai et al., 2016; Rashid et al., 2016). Similarly, memories that were initially encoded as separate memories can also be linked during memory retrieval. When mice were trained independently on two different conditioning tasks with distinct unconditioned stimuli (US 1: LiCl injection; US 2: foot shock) and distinct conditioned stimuli (CS1: saccharin solution; CS2: tone), repeated co-retrieval with the presence of the conditioned stimuli caused the two conditioned responses to be co-represented by a shared neural ensemble which also linked them behaviorally: the saccharin solution (CS1) triggered freezing (response to CS2) (Yokose et al., 2017). We see here that memories can be linked not only during initial memory encoding (Cai et al., 2016; Rashid et al., 2016) but also during retrieval. Transiently increased excitability may increase the overlap between two originally independent ensembles during repeated co-reactivation.
In conclusion, studies suggest that recall of an existing memory transiently triggers an increase in the excitability of ensemble neurons, which then opens up a temporal window both for memory enhancement and integration of new information into the existing memory. Memory integration during retrieval is a further demonstration of the dynamic nature of memory, and retrieval-induced intrinsic excitability increases may play an important role in mediating this process.
Rule and schema learning
We have thus far described how memory encoding and retrieval influence the formation of a temporally close memory and the integration of new associations into an existing memory. We can think of this process as the use of past information or experience to guide the formation of new memories and the update of old memories, which is critical for survival. There are, at the same time, other types of learning that appear to associate past and new information. In “rule learning” and “schema learning”, two separately discovered but closely related behavioral phenomena in rats, a rule or schema is created through repeated training. When similar information is subsequently encountered, the brain can utilize the established rule or schema to achieve more efficient learning. Here, we review studies of rule and schema learning and consider how neuronal intrinsic excitability in interacting brain regions may play a role in establishing and maintaining a rule or schema after a sufficient amount of training.
Rule learning describes the phenomenon of an abrupt increase in the learning rate of new cue-reward associations after animals acquire the first one or two associations in the same training paradigm. The behavioral evidence for rule learning came from a series of studies on an odor discrimination task during which water-deprived rats learned to distinguish between pairs of odors in order to obtain a water reward (Staubli et al., 1987; Saar et al., 1998, 1999, 2001, 2002; Saar and Barkai, 2003; Quinlan et al., 2004; Zelcer et al., 2006; Chandra and Barkai, 2018). In general, rats were presented a multi-arm radial maze in which odors were released into two arms, one with a positive odor and a water reward, the other with a negative odor and no reward. Rats needed to learn that water reward was associated with the positive odor. Twenty trials were conducted per session per day, and the criterion for completion of learning on an odor pair was at least 80% positive-cue choices in the last 10 trials of a training session. Researchers found that learning occurred in two distinct phases: a first phase of 7–8 sessions for the animals to learn to discriminate the first pair of odors and get the water reward, and a second phase during which animals could learn to associate a new odor with water reward within one session after being well trained on the first odor pair (Saar et al., 1998, 1999; Saar and Barkai, 2003; Chandra and Barkai, 2018). It was proposed that the animals learned the general rule during the first phase (“rule learning”), which enhanced their capability for learning new cue-reward associations. Notably, the enhancement in learning capability was significant and abrupt immediately after the rule learning phase.
Evidence for how excitability may modulate rule learning comes from studies of the piriform cortex. It has been shown that in the piriform cortex, which receives direct input from the olfactory bulb (Haberly, 1990; Schoenbaum and Eichenbaum, 1995), neuronal intrinsic excitability is strongly correlated with rule learning. Electrophysiology studies revealed that neuronal intrinsic excitability of piriform cortex pyramidal neurons was increased after rule learning, demonstrated by a reduction in both postburst AHP and spike accommodation (Saar et al., 1998; Saar and Barkai, 2003, 2009). This state of hyperexcitability was sustained as long as training continued, and lasted another 1–3 days after training was stopped (Saar et al., 1998). During this time of enhanced excitability, there was enhancement in the learning rate in discriminating new odor pairs. If the training was suspended for 4 or more days (when excitability had returned to baseline levels), there was no benefit from rule learning and animals behaved as if they had not been trained (Saar et al., 1998). This finding suggests that excitability increases in the piriform cortex may be important in maintaining the rule and essential for enhancing the animals’ ability to learn new associations in the same training paradigm.
A similar yet more complex behavior phenomenon was observed in rats, defined as schema learning (Tse et al., 2007). Briefly, rats were trained to dig for flavored food pellets hidden in sand wells placed at specific locations in a familiar arena. Before the start of each trial, rats were cued with a flavor to orient to and dig into a specific sand well for pellets of the same flavor. The rats’ performance was evaluated by the number of incorrect digs before going to the correct well during training sessions, and by the time spent digging at the correct well during probe tests (rats cued but no reward in the sand well). Performance was enhanced incrementally during training across the first 13 sessions. Remarkably, only a single training was required for rats to remember a new paired-associate (new food flavor and new sand well location) after they mastered the task. Moreover, a hippocampal lesion only 24 hours after learning the new pair associations did not disrupt the newly formed memory. This was a surprising finding as this type of paired-associate learning is likely to be initially mediated in the hippocampus (Bunsey and Elchenbaum, 1996; Wirth et al., 2003; Kesner et al., 2005) and later consolidated in the neocortex (Sakai and Miyashita, 1991; Miyashita, 2004), and systems consolidation generally requires more than 48 hours to be completed (Zola-Morgan and Squire, 1990; Kim and Fanselow, 1992; Anagnostaras et al., 1999; Bayley et al., 2005). Together, these results indicate that prior learning of an associative schema may facilitate faster encoding and systems consolidation when learning new associations (Tse et al., 2007).
No studies have directly characterized intrinsic excitability profiles during schema learning and how excitability may facilitate systems consolidation and faster learning. However, excitability changes in hippocampal CA1 during rule learning may have implications for the potential role of excitability in schema learning. The excitability of hippocampal CA1 pyramidal neurons remained unchanged until it increased 1 day before rats acquired the rule, and returned to baseline only 1 day after rule learning, regardless of whether training continued (Zelcer et al., 2006). Compared to learning-induced hyperexcitability in the piriform cortex, which was sustained as long as training continued and could last another 1–3 days if training was stopped (Saar et al., 1998; Saar and Barkai, 2003, 2009), the hippocampus and piriform cortex may have different roles in rule learning. Hippocampal CA1 excitability may drive the local circuit processes essential for learning the initial pairs of associations while sending signals to interacting neocortical regions to enhance excitability and facilitate the establishment of a rule or schema representation in the cortex. Subsequently, the excitability of cortical neurons may increase and further help to stabilize ensembles in cortical structures.
It remains largely unknown whether and how excitability fluctuations in different brain regions mediate rule or schema learning. We speculate that similar to learning-induced and retrieval-induced hyperexcitability promoting memory strength and integration, rule learning through repeated trainings may lead to the formation of a stable ensemble representation with sustained higher excitability that lasts several days. During this temporal window, additional learning may be facilitated by recruiting overlapping neurons from the hyperexcitable ensemble with already established synaptic connectivity. Further, enhanced excitability may promote information flow between interacting brain regions and facilitate systems consolidation and the establishment of schema representations in the neocortex.
Conclusion and future directions
In this review, we have summarized studies that examine how neuronal intrinsic excitability may regulate three fundamental phases of memory: allocation, consolidation, and updating (Fig. 2). First, neurons with higher intrinsic excitability are more likely to be recruited into a neural ensemble during learning. Second, learning induces an increase in the excitability in the ensemble neurons, and this higher excitability might work synergistically with synaptic plasticity to facilitate memory consolidation. Third, intrinsic excitability may also contribute to multiple memory updating processes, such as temporal memory-linking, memory integration during retrieval, and rule and schema learning.
Figure 2. The contribution of intrinsic excitability in memory allocation, consolidation, and updating.
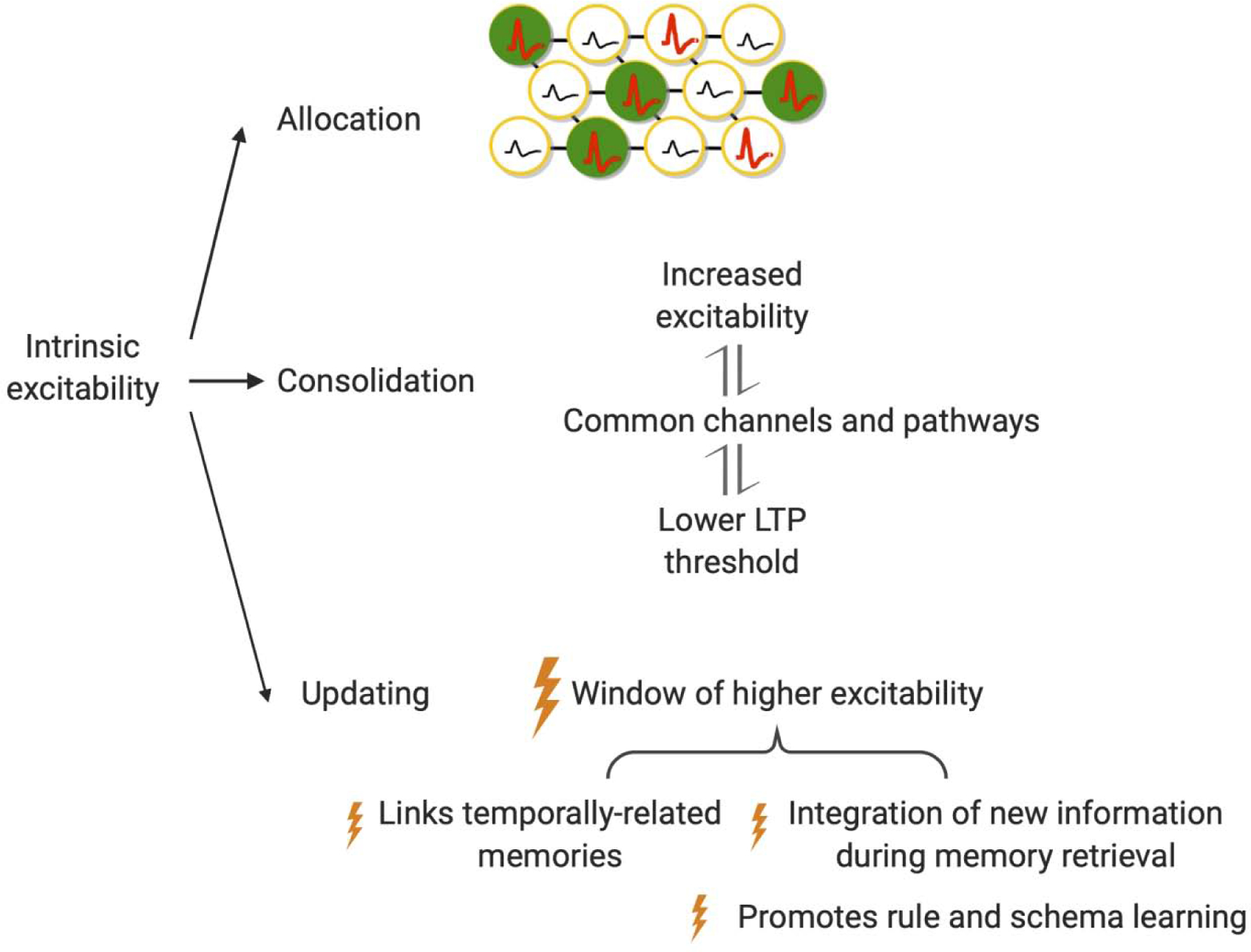
Allocation: neurons with a higher excitability at the time of learning have a higher probability to be recruited into a memory ensemble. Consolidation: increases in neuronal intrinsic excitability lower the threshold for LTP induction, through common underlying voltage-gated ion channels (such as SK channels) and signaling pathways. Updating: excitability is important for memory integration during updating after allocation and consolidation. Here, we have highlighted the role of heightened excitability in three processes: temporal memory-linking, memory integration during retrieval, and rule and schema learning.
A particularly intriguing characteristic of learning-induced increase in excitability is its transient, or dynamic, nature. The time course of excitability after learning can vary across distinct behavioral tasks, different brain regions and cell types, and the diverse experimental methods used to measure it. In a trace eye-blink conditioning task, the highest excitability in rabbit hippocampal CA1 and CA3 pyramidal neurons occurred at 24 hours and persisted for up to 5 days, as shown by a reduction in the size of postburst AHP in slice preparations, while the reduction in spike accommodation (also indicating higher excitability) lasted as long as 3 days after conditioning (Moyer et al., 1996; Thompson et al., 1996; Disterhoft and Oh, 2007). In rodent studies using a contextual fear conditioning paradigm, hippocampal CA1 neurons had higher excitability in brain slices prepared as long as 2 days after the last training session (Mckay et al., 2009; Crestani et al., 2018), while a recall of fear memory induced hyperexcitability in dentate granule cells persisted for only 1 hour (Pignatelli et al., 2019). In vivo electrophysiology recordings and population activity analyses have revealed that different hippocampal subregions varied in terms of how quickly neural ensemble activity turned over across time (Mankin et al., 2012, 2015; Rangel et al., 2014). Studies have shown that CA1 and CA2 population activity patterns changed dramatically with increasing temporal distances of encoding, while CA3 representations stayed relatively stable across time (Mankin et al., 2012, 2015). Interestingly, the dentate gyrus might recruit populations of hyperexcitable new-born granule cells to organize memories across weeks (Aimone et al., 2006, 2009; Rangel et al., 2014). Nevertheless, we still lack a comprehensive analysis of the time course of excitability change in brain regions involved in learning a particular task (e.g. the amygdala-hippocampus-prefrontal cortex circuit in a contextual fear conditioning paradigm), which will be critical to further understand the dynamic integration and updating of memories, such as temporal memory-linking, and how the brain organizes and stores memories over time. A caveat in systematically studying excitability alteration patterns is that the tools used to measure excitability may influence the results. Therefore, it is important to consider the methods used to examine intrinsic excitability when interpreting results.
Besides serving as a regulator for local ensembles in various brain regions, intrinsic excitability potentially also serves as a link between ensembles across brain regions. Intracellular and extracellular oscillations may bind information processing across brain regions by synchronizing windows of memory allocation and plasticity. For instance, short bursts of excitability, such as those driven by intracellular theta oscillations, may mediate learning in a similar way to the longer-term fluctuations in excitability discussed above. Indeed, the coherence between local oscillations in interactive brain regions enhanced the information flow between them (Von Stein and Sarnthein, 2000; Fries, 2005; Akam and Kullmann, 2010, 2014; Bosman et al., 2012) and this process might be mediated by fluctuations in excitability. Interestingly, in CA1 place cells, intracellular theta increased in frequency and power as the animal entered the cell’s place field (Harvey et al., 2009), which might amplify the effect of oscillations on memory processes. In addition, cells with a higher excitability had stronger intracellular theta both inside and outside of their place fields (Lee et al., 2012) indicating that hyperexcitable cells might be more sensitive to global oscillations, further enhancing their impact on learning. Finally, beyond place cells, the hyperexcitable cells recruited into ensembles across brain regions may engage coherent oscillations from interacting regions and facilitate the synchronization of dispersed ensembles into systems.
Additional insights into excitability and oscillations come from theoretical studies. Computational models have suggested a relationship between intrinsic excitability and gamma oscillations (40–100 Hz), where gamma-paced excitation and inhibition selected the most highly excited cells in a given network to fire during gamma cycles (De Almeida et al., 2009a, 2009b). An explicit parameter in this computational model was the AHP, which varied across brain regions and even across cells within a region. The magnitude of this AHP could influence the oscillatory pattern of spiking of a single cell, and the computational implications of this activity might be different patterns of excitation to downstream regions, leading to activation of different memory ensembles. Thus, the heterogeneous intrinsic excitability of neuronal populations may dictate the identity of neurons active in an ensemble during oscillatory network states.
As we have reiterated throughout this review, there is much research yet to be done to achieve a deeper, more comprehensive understanding of intrinsic excitability across the distinct phases of memory. Currently existing experimental tools have separately provided us with an understanding of the dynamics of single-neuron excitability (whole-cell patch clamp), synaptic integration within a neuron (dendritic patch clamp, dendritic calcium imaging, neurotransmitter release imaging), and population-level activity (IEG expression, TetTag, neuronal calcium imaging, in vivo electrophysiology recordings). Although these techniques can directly or indirectly measure excitability, they all have limitations. Patch-clamp recording can provide direct and accurate quantification of intrinsic excitability but is relatively low throughput. Techniques based on IEG expression, such as TetTag and catFISH, enable visualization of populations of cells, but are done in vitro and can only report cell activation that may or may not be a direct result of changes in excitability. Calcium imaging can indirectly indicate excitability in a large group of neurons but lacks temporal resolution. The advent of large-scale recording techniques that allow simultaneous recording of neuronal and dendritic excitability in a population of neurons in vivo would allow scientists to broadly probe intrinsic excitability changes and structural reorganization dynamically, and provide us with better insight into the neural mechanisms underlying the dynamic processes of memory formation and integration.
While no tool exists that covers the entire breadth of these investigations, promising new technologies have already been developed to advance research in intrinsic excitability. Genetically encoded voltage indicator (GEVI) imaging offers the unique ability to optically record changes in voltage within individual neurons across populations, with a time-scale significantly more sensitive than the current standard of optical imaging (e.g. calcium-based imaging) (Cao et al., 2013; St-Pierre et al., 2014; Yang et al., 2016; Chamberland et al., 2017; Adam et al., 2019; Piatkevich et al., 2019; Villette et al., 2019). Moreover, GEVIs have the ability to capture both subthreshold and suprathreshold activities and have fluorescence modulation across the physiological range of membrane potentials, including hyperpolarization. GEVIs are ideal candidates to monitor changes in intrinsic excitability parameters, such as postburst AHP and single AP properties, in a large population of neurons in awake behaving animals (Adam et al., 2019; Villette et al., 2019), which is difficult to achieve with traditional electrophysiological methods. The recent development of new generations of GEVIs and corresponding ultra-fast two-photon imaging methods have made it possible to further examine alterations in the excitability of subcellular compartments in vivo such as dendritic backpropagating APs (Adam et al., 2019; Villette et al., 2019). Combined with synaptic level tools, including the tagging and manipulation of highly active individual synapses during specified time windows (Hayashi-Takagi et al., 2015; Gobbo et al., 2017), these novel technologies will enable investigation of how synaptic and intrinsic plasticity integrate and interact at a population level and how they correlate with animal behavior.
Finally, while numerous models and theories have been proposed to describe the formation and transformation of memory in animal research, it will be important to test and validate these ideas in the human brain. Recent studies using functional magnetic resonance imaging (fMRI) have provided evidence that memory dynamics observed in rodent studies across time also existed in the human brain (Schlichting and Frankland, 2017). Hippocampal activation patterns evoked by specific encoding events have been shown to be more similar for those encoded close in time than those encoded at distant times in human subjects (Schapiro et al., 2012), and neural pattern similarity predicted a person’s later subjective judgment of the temporal proximity of memories (Ezzyat and Davachi, 2014). Moreover, temporal proximity of memory encoding could both increase the efficiency and accuracy of inferential judgements and enhance the integration of memories in the human brain (Zeithamova and Preston, 2017). Another recent study in humans demonstrated that temporally close memories (encoded 3 hours but not 7 days apart) were linked using a fear conditioning paradigm (Yetton et al., 2019) comparable to the behavioral paradigm used in rodent studies (Cai et al., 2016; Rashid et al., 2016). Another form of memory integration observed in animals, memory schemas (a process of incorporating new information into pre-existing knowledge) (Tse et al., 2007), has also been observed in humans through longitudinal studies using fMRI to track the brain regions and activity involved in their development (Sommer, 2017).
While these studies provide evidence of similar processes in humans to those observed in animals, recording methods with higher spatial and temporal resolution are needed to address a number of important questions, such as how single neuron activity and excitability may be involved in memory formation and updating. The invasive nature of currently available techniques has restricted the recording of single neuron activity in awake behaving humans to a very limited number of circumstances, such as in drug-resistant epileptic patients or with the implantation of a deep brain stimulation device for the treatment of psychiatric or movement disorders (Rutishauser, 2019). Novel minimally invasive techniques that will enable investigations at a higher resolution are needed to achieve a fuller understanding of the dynamic processes of memory in the human brain.
Intrinsic excitability is more than simply a byproduct of neural activity. Intrinsic excitability involves plasticity from alterations in gene expression, as well as changes in the distribution and function of somatic and dendritic ion channels and may consequently exert lasting effects on memory from the initial encoding to consolidation. Moreover, its transient nature makes intrinsic excitability an ideal cellular property governing the dynamic process of integration during memory updating. Intrinsic excitability promotes both the stability and flexibility of memories, helping to shape, update, and organize memories accumulated across a lifetime.
Table 1.
Experimental quantification of neuronal intrinsic excitability.
| Parameters | Regulators | Measurement | Relationship to excitability |
|---|---|---|---|
| fAHP | BK channel; A-type K+ channel ↑ Channel activity ↑ fAHP size |
Negative peak after a single AP relative to the holding potential (Fig. 1A) |
↑ fAHP size ↑ Excitability |
| mAHP | SK channel ↓ SK channel activity ↓ mAHP size |
Negative peak after a train of APs relative to the holding potential (Fig. 1B) |
↓ mAHP size ↑ Excitability |
| sAHP | To be identified | Remaining hyperpolarization relative to the holding potential 1 s after the AP train (Fig. 1B) |
↓ sAHP size ↑ Excitability |
| Spike frequency accommodation | M channel; fast Na+ channel; regulated by postburst AHP ↓ AHP size ↓ Accommodation |
Number of APs elicited during a sustained stimulation |
↓ Accommodation ↑ Excitability |
| AP half-width AP frequency |
Regulated by fAHP ↑ fAHP ↓ AP half-width ↑ AP frequency |
Half-width: the width of an AP at its half-maximal value Frequency: number of APs per second |
↓ AP half-width ↑ AP frequency ↑ Excitability |
| AP threshold Rheobase | Related to the density of Na+ channels, Na+ channel inactivation and K+ channels |
Threshold: the membrane potential at which the rising speed of an AP peaks or exceeds a certain value during depolarization Rheobase: the minimal current amplitude of an infinite duration to elicit a single AP |
↓ AP threshold ↓ Rheobase ↑ Excitability |
| Input resistance | Determined by the total number and conductance of open channels | Calculated from the I-V curve derived from a series of somatic current injections (R = V/I) |
↑ Input resistance ↑ Excitability |
Highlights.
Neuronal intrinsic excitability contributes to different phases of a memory’s evolution.
Intrinsic excitability promotes both the stability and flexibility of memories.
Intrinsic excitability regulates the allocation of memory to specific neural ensembles.
Learning-induced increases in intrinsic excitability may promote memory consolidation.
Alterations in intrinsic excitability support the dynamic process of memory updating.
Acknowledgements
This work was supported by the NIH Director’s New Innovator Award, One Mind Rising Star Award, McKnight Memory and Cognitive Disorders Award, Klingenstein-Simons Fellowship, Brain Research Foundation Award, NARSAD Young Investigator Award, Botanical Center Pilot Award, and the Friedman Scholar Award to DJC, and the CURE Taking Flight Award and American Epilepsy Society Junior investigator Award to TS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel T, Lattal KM (2001) Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol 11:180–187. [DOI] [PubMed] [Google Scholar]
- Abel T, Nguyen PV., Barad M, Deuel TAS, Kandel ER, Bourtchouladze R (1997) Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88:615–626. [DOI] [PubMed] [Google Scholar]
- Adam Y et al. (2019) Voltage imaging and optogenetics reveal behaviour-dependent changes in hippocampal dynamics. Nature 569:413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH (2006) Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci 9:723–727. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH (2009) Computational Influence of Adult Neurogenesis on Memory Encoding. Neuron 61:187–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenman CD, Linden DJ (2000) Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat Neurosci 3:109–111. [DOI] [PubMed] [Google Scholar]
- Akam T, Kullmann DM (2010) Oscillations and filtering networks support flexible routing of information. Neuron 67:308–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akam T, Kullmann DM (2014) Oscillatory multiplexing of population codes for selective communication in the mammalian brain. Nat Rev Neurosci 15:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM (1999) Genes to remember. [DOI] [PubMed]
- Alberini CM (2008) The role of protein synthesis during the labile phases of memory: revisiting the skepticism. Neurobiol Learn Mem 89:234–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM (2009) Transcription factors in long-term memory and synaptic plasticity. Physiol Rev 89:121–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Fanselow MS (1999) Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: Within-subjects examination. J Neurosci 19:1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armano S, Rossi P, Taglietti V, D’Angelo E (2000) Long-term potentiation of intrinsic excitability at the mossy fibergranule cell synapse of rat cerebellum. J Neurosci 20:5208–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentia ML de, Jancic D, Olivares R, Alarcon JM, Kandel ER, Barco A (2007) cAMP Response Element-Binding Protein-Mediated Gene Expression Increases the Intrinsic Excitability of CA1 Pyramidal Neurons. J Neurosci 27:13909–13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco A, Alarcon JM, Kandel ER (2002) Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell 108:689–703. [DOI] [PubMed] [Google Scholar]
- Bayley PJ, Gold JJ, Hopkins RO, Squire LR (2005) The neuroanatomy of remote memory. Neuron 46:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnisch T, Reymann KG (1998) Inhibition of apamin-sensitive calcium dependent potassium channels facilitate the induction of long-term potentiation in the CA1 region of rat hippocampus in vitro. Neurosci Lett 253:91–94. [DOI] [PubMed] [Google Scholar]
- Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, Izquierdo I, Medina JH (1997) Involvement of hippocampal cAMPcAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. [DOI] [PMC free article] [PubMed]
- Bito H, Deisseroth K, Tsien RW (1996) CREB phosphorylation and dephosphorylation: A Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell 87:1203–1214. [DOI] [PubMed] [Google Scholar]
- Bittner KC, Grienberger C, Vaidya SP, Milstein AD, Macklin JJ, Suh J, Tonegawa S, Magee JC (2015) Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nat Neurosci 18:1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–39. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL (2013) Expression of NMDA receptor-dependent LTP in the hippocampus: bridging the divide. Mol Brain 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Lømo T (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232:331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood BL, Sabatini BL (2007) Nonlinear Regulation of Unitary Synaptic Signals by CaV2.3 Voltage-Sensitive Calcium Channels Located in Dendritic Spines. Neuron 53:249–260. [DOI] [PubMed] [Google Scholar]
- Bock T, Honnuraiah S, Stuart GJ (2019) Paradoxical excitatory impact of SK channels on dendritic excitability. J Neurosci 39:0105–0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock T, Stuart GJ (2016) Impact of calcium-activated potassium channels on NMDA spikes in cortical layer 5 pyramidal neurons. J Neurophysiol 115:1740–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, Adelman JP (2004) Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci 24:5301–5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman CA, Schoffelen JM, Brunet N, Oostenveld R, Bastos AM, Womelsdorf T, Rubehn B, Stieglitz T, De Weerd P, Fries P (2012) Attentional Stimulus Selection through Selective Synchronization between Monkey Visual Areas. Neuron 75:875–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ (1994) Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 79:59–68. [DOI] [PubMed] [Google Scholar]
- Bunsey M, Elchenbaum H (1996) Conservation of hippocampal memory function in rats and humans. Nature 379:255–257. [DOI] [PubMed] [Google Scholar]
- Cai DJ et al. (2016) A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534:115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Platisa J, Pieribone VA, Raccuglia D, Kunst M, Nitabach MN (2013) Genetically targeted optical electrophysiology in intact neural circuits. Cell 154:904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S, Yang HH, Pan MM, Evans SW, Guan S, Chavarha M, Yang Y, Salesse C, Wu H, Wu JC, Clandinin TR, Toth K, Lin MZ, St-Pierre F (2017) Fast two-photon imaging of subcellular voltage dynamics in neuronal tissue with genetically encoded indicators. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra N, Barkai E (2018) A non-synaptic mechanism of complex learning: Modulation of intrinsic neuronal excitability. Neurobiol Learn Mem 154:30–36. [DOI] [PubMed] [Google Scholar]
- Citri A, Malenka RC (2008) Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology 33:18–41. [DOI] [PubMed] [Google Scholar]
- Cohen-Matsliah SI, Motanis H, Rosenblum K, Barkai E (2010) A novel role for protein synthesis in long-term neuronal plasticity: maintaining reduced postburst afterhyperpolarization. J Neurosci 30:4338–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Bolstad M, Lee AK (2017) Experience-dependent shaping of hippocampal CA1 intracellular activity in novel and familiar environments. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DA, Lo Turco JJ, Kubota M, Disterhoft JF, Moore JW, Alkon DL (1989) Classical conditioning reduces amplitude and duration of calcium-dependent afterhyperpolarization in rabbit hippocampal pyramidal cells. J Neurophysiol 61. [DOI] [PubMed] [Google Scholar]
- Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M (2014) Direct Reactivation of a Coherent Neocortical Memory of Context. Neuron 84:432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani AP, Krueger JN, Barragan EV., Nakazawa Y, Nemes SE, Quillfeldt JA, Gray JA, Wiltgen BJ (2018) Metaplasticity contributes to memory formation in the hippocampus. Neuropsychopharmacology:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoudal G, Debanne D (2003) Long-term plasticity of intrinsic excitability: learning rules and mechanisms. Learn Mem 10:456–465. [DOI] [PubMed] [Google Scholar]
- De Almeida L, Idiart M, Lisman JE (2009a) A second function of gamma frequency oscillations: An E%-max winner-take-all mechanism selects which cells fire. J Neurosci 29:7497–7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Almeida L, Idiart M, Lisman JE (2009b) The input-output transformation of the hippocampal granule cells: From grid cells to place fields. J Neurosci 29:7504–7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Bito H, Tsien RW (1996) Signaling from synapse to nucleus: Postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron 16:89–101. [DOI] [PubMed] [Google Scholar]
- Deng W, Mayford M, Gage FH (2013) Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. Elife 2:e00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantaki M, Coletta S, Nasr K, Zeraati R, Laturnus S, Berens P, Preston-Ferrer P, Burgalossi A (2018) Manipulating Hippocampal Place Cell Activity by Single-Cell Stimulation in Freely Moving Mice. Cell Rep 23:32–38. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Coulter DA, Alkon DL (1986) Conditioning-specific membrane changes of rabbit hippocampal neurons measured in vitro. Proc Natl Acad Sci U S A 83:2733–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disterhoft JF, Oh MM (2006a) Learning, aging and intrinsic neuronal plasticity. Trends Neurosci 29:587–599. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Oh MM (2006b) Pharmacological and molecular enhancement of learning in aging and Alzheimer’s disease. J Physiol Paris 99:180–192. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Oh MM (2007) Alterations in intrinsic neuronal excitability during normal aging. Aging Cell 6:327–336. [DOI] [PubMed] [Google Scholar]
- Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ, Malenka RC (2006) CREB modulates excitability of nucleus accumbens neurons. Nat Neurosci 9:475–477. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Tonegawa S (2011) Preplay of future place cell sequences by hippocampal cellular assemblies. Nature 469:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y (1996) Consolidation: Fragility on the road to the engram. Neuron 17:367–370. [DOI] [PubMed] [Google Scholar]
- Dudai Y (2004) The Neurobiology of Consolidations, Or, How Stable is the Engram? Annu Rev Psychol 55:51–86. [DOI] [PubMed] [Google Scholar]
- Dudai Y (2012) The Restless Engram: Consolidations Never End. Annu Rev Neurosci 35:227–247. [DOI] [PubMed] [Google Scholar]
- Dudai Y, Karni A, Born J (2015) The Consolidation and Transformation of Memory. Neuron 88:20–32. [DOI] [PubMed] [Google Scholar]
- Egorov AV., Hamam BN, Fransén E, Hasselmo ME, Alonso AA (2002) Graded persistent activity in entorhinal cortex neurons. Nature 420:173–178. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H (2016) Still searching for the engram. Learn Behav 25:1032–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epsztein J, Brecht M, Lee AK (2011) Intracellular Determinants of Hippocampal CA1 Place and Silent Cell Activity in a Novel Environment. Neuron 70:109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzyat Y, Davachi L (2014) Similarity breeds proximity: Pattern similarity within and across contexts is related to later mnemonic judgments of temporal proximity. Neuron 81:1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ESL (2010) Functional interplay between NMDA receptors, SK channels and voltage-gated Ca2+ channels regulates synaptic excitability in the medial prefrontal cortex. J Physiol 588:1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ESL, Delaney AJ, Power JM, Sedlak PL, Crane JW, Sah P (2008) Modulation of SK channel trafficking by beta adrenoceptors enhances excitatory synaptic transmission and plasticity in the amygdala. J Neurosci 28:10803–10813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ESL, Delaney AJ, Sah P (2005) SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci 8:635–641. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Josselyn SA, Köhler S (2019) The neurobiological foundation of memory retrieval. Nat Neurosci 22:1576–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P (2005) A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn Sci 9:474–480. [DOI] [PubMed] [Google Scholar]
- Garner AR, Rowland DC, Hwang SY, Baumgaertel K, Roth BL, Kentros C, Mayford M (2012) Generation of a synthetic memory trace. Science (80-) 335:1513–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbo F, Marchetti L, Jacob A, Pinto B, Binini N, Pecoraro Bisogni F, Alia C, Luin S, Caleo M, Fellin T, Cancedda L, Cattaneo A (2017) Activity-dependent expression of Channelrhodopsin at neuronal synapses. Nat Commun 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabauskas G, Lancaster B, O’Connor V, Wheal HV. (2007) Protein kinase signalling requirements for metabotropic action of kainate receptors in rat CA1 pyramidal neurones. J Physiol 579:363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Hu H, Storm JF (2005) Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J Physiol 566:689–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF (1999) Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci 2:1120–124. [DOI] [PubMed] [Google Scholar]
- Haberly LB (1990) Comparative Aspects of Olfactory Cortex. In, pp 137–166. Springer, Boston, MA. [Google Scholar]
- Han J-H, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA (2007) Neuronal competition and selection during memory formation. Science (80-) 316:457–460. [DOI] [PubMed] [Google Scholar]
- Han J-H, Kushner SA, Yiu AP, Hsiang H-L (Liz), Buch T, Waisman A, Bontempi B, Neve RL, Frankland PW, Josselyn SA (2009) Selective Erasure of a Fear Memory. Science (80-) 323:1492–1496. [DOI] [PubMed] [Google Scholar]
- Harvey CD, Collman F, Dombeck DA, Tank DW (2009) Intracellular dynamics of hippocampal place cells during virtual navigation. 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Yagishita S, Nakamura M, Shirai F, Wu YI, Loshbaugh AL, Kuhlman B, Hahn KM, Kasai H (2015) Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 525:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO (1949) The organization of behavior, a neuropsychological theory. New York: Wiley. [Google Scholar]
- Huang Y-Y, Nguyen PV, Abel T, Kandel ER (1996) Long-lasting Forms of Synaptic Potentiation in the Mammalian Hippocampus. Learn Mem 3:74–85. [DOI] [PubMed] [Google Scholar]
- Impey S, Mark M, Villacres EC, Poser S, Chavkin C, Storm DR (1996) Induction of CRE-Mediated Gene Expression by Stimuli That Generate Long-Lasting LTP in Area CA1 of the Hippocampus. Neuron 16:973–982. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Storm DR (1999) Making New Connections: Role of ERK/MAP Kinase Signaling in Neuronal Plasticity. Neuron 23:11–14. [DOI] [PubMed] [Google Scholar]
- Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR (1998) Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat Neurosci 1:595–601. [DOI] [PubMed] [Google Scholar]
- Ito M, Kano M (1982) Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett 33:253–258. [DOI] [PubMed] [Google Scholar]
- Josselyn SA (2010) Continuing the search for the engram: examining the mechanism of fear memories. J Psychiatry Neurosci 35:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Köhler S, Frankland PW (2015) Finding the engram. Nat Rev Neurosci 16:521–534. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Köhler S, Frankland PW (2017) Heroes of the Engram. J Neurosci 37:4647–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Nguyen PV. (2005) CREB, synapses and memory disorders: Past progress and future challenges. Curr Drug Targets CNS Neurol Disord 4:481–497. [DOI] [PubMed] [Google Scholar]
- Kaczorowski CC, Disterhoft JF (2009) Memory deficits are associated with impaired ability to modulate neuronal excitability in middle-aged mice. Learn Mem 16:362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER (2001) The molecular biology of memory storage: a dialogue between genes and synapses. Science (80-) 294:1030–1038. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Dudai Y, Mayford MR (2014) The molecular and systems biology of memory. Cell 157:163–186. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR, Gilbert PE (2005) The role of CA1 in the acquisition of an object-trace-odor paired associate task. Behav Neurosci 119:781–786. [DOI] [PubMed] [Google Scholar]
- Kim D, Pare D, Nair SS (2013) Assignment of Model Amygdala Neurons to the Fear Memory Trace Depends on Competitive Synaptic Interactions. J Neurosci 33:14354–14358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF (1995) Hippocampectomy Impairs the Memory of Recently, but Not Remotely, Acquired Trace Eyeblink Conditioned Responses. Behav Neurosci 109:195–203. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS (1992) Modality-specific retrograde amnesia of fear. Science 256:675–677. [DOI] [PubMed] [Google Scholar]
- Kramár EA, Lin B, Lin CY, Arai AC, Gall CM, Lynch G (2004) A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor. J Neurosci 24:5151–5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroes MCW, Fernández G (2012) Dynamic neural systems enable adaptive, flexible memories. Neurosci Biobehav Rev 36:1646–1666. [DOI] [PubMed] [Google Scholar]
- Kuo AG, Lee G, McKay BM, Disterhoft JF (2008) Enhanced neuronal excitability in rat CA1 pyramidal neurons following trace eyeblink conditioning acquisition is not due to alterations in IM. Neurobiol Learn Mem 89:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Hu H, Gibb B, Storm JF (2006) Kinetics of ion channel modulation by cAMP in rat hippocampal neurones. J Physiol 576:403–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashley KS (1933) Integrative functions of the cerebral cortex. Physiol Rev 13:1–42. [Google Scholar]
- Lashley KS (1935) Studies of cerebral function in learning: XI. The behavior of the rat in latch-box situations. Comp Psychol Monogr 11:5–42. [Google Scholar]
- Lee AK, Epsztein J, Brecht M (2009) Head-anchored whole-cell recordings in freely moving rats. Nat Protoc 4:385–392. [DOI] [PubMed] [Google Scholar]
- Lee AK, Manns ID, Sakmann B, Brecht M (2006) Whole-Cell Recordings in Freely Moving Rats. Neuron 51:399–407. [DOI] [PubMed] [Google Scholar]
- Lee D, Lin B-J, Lee AK (2012) Hippocampal Place Fields Emerge upon Single-Cell Manipulation of Excitability During Behavior. Science (80-) 337:849–853. [DOI] [PubMed] [Google Scholar]
- Levy WB, Steward O (1979) Synapses as associative memory elements in the hippocampal formation. Brain Res 175:233–245. [DOI] [PubMed] [Google Scholar]
- Lisman J, Cooper K, Sehgal M, Silva AJ (2018) Memory formation depends on both synapse-specific modifications of synaptic strength and cell-specific increases in excitability. Nat Neurosci 21:309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S (2012) Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484:381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35:605–623. [DOI] [PubMed] [Google Scholar]
- Madison DV., Nicoll RA (1984) Control of the repetitive discharge of rat CA1 pyramidal neurones in vitro. J Physiol 354:319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Madison DV, Andrade R, Nicoll RA (1986) Phorbol esters mimic some cholinergic actions in hippocampal pyramidal neurons. J Neurosci 6:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin EA, Diehl GW, Leutgeb S, Leutgeb JK, Mankin EA, Diehl GW, Sparks FT, Leutgeb S, Leutgeb JK (2015) Hippocampal CA2 Activity Patterns Change over Time to a Larger Extent than between Spatial Article Hippocampal CA2 Activity Patterns Change over Time to a Larger Extent than between Spatial Contexts. Neuron 85:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin EA, Sparks FT, Slayyeh B, Robert J, Leutgeb S, Leutgeb JK (2012) Neuronal code for extended time in the hippocampus. Proc Natl Acad Sci 109:19462–19467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews EA, Linardakis JM, Disterhoft JF (2009) The Fast and Slow Afterhyperpolarizations Are Differentially Modulated in Hippocampal Neurons by Aging and Learning. J Neurosci 29:4750–4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Siegelbaum SA, Kandel ER (2012) Synapses and memory storage. Cold Spring Harb Perspect Biol 4:a005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL (2000) Memory--a century of consolidation. Science (80-) 287:248–251. [DOI] [PubMed] [Google Scholar]
- Mckay BM, Matthews EA, Oliveira FA, Disterhoft JF (2009) Intrinsic Neuronal Excitability Is Reversibly Altered by a Single Experience in Fear Conditioning. J Neurophysiol 102:2763–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BM, Oh MM, Galvez R, Burgdorf J, Kroes RA, Weiss C, Adelman JP, Moskal JR, Disterhoft JF (2012) Increasing SK2 channel activity impairs associative learning. J Neurophysiol 108:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckenzie S, Frank AJ, Kinsky NR, Porter B, Rivie PD (2014) Hippocampal Representation of Related and Opposing Memories Develop within Distinct, Hierarchically Organized Neural Schemas. Neuron 83:202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melyan Z, Wheal HV., Lancaster B (2002) Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron 34:107–114. [DOI] [PubMed] [Google Scholar]
- Miyashita Y (2004) Cognitive memory: cellular and network machineries and their top-down control. Science 306:435–440. [DOI] [PubMed] [Google Scholar]
- Morton NW, Sherrill KR, Preston AR (2017) Memory integration constructs maps of space, time, and concepts. Curr Opin Behav Sci 17:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Power JM, Thompson LT, Disterhoft JF (2000) Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. J Neurosci 20:5476–5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Thompson LT, Disterhoft JF (1996) Trace Eyeblink Conditioning Increases CA1 Excitability in a Transient and Learning-Specific Manner. J Neurosci 16:5536–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Becker HC, Woodward JJ, Chandler LJ (2011) Small conductance calcium-activated potassium type 2 channels regulate alcohol-associated plasticity of glutamatergic synapses. Biol Psychiatry 69:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GG, Rahnama NP, Silva AJ (2006) Investigation of Age-Related Cognitive Decline Using Mice as a Model System: Behavioral Correlates. Am J Geriatr Psychiatry 14:1004–1011. [DOI] [PubMed] [Google Scholar]
- Nadel L, Hupbach A, Gomez R, Newman-Smith K (2012) Memory formation, consolidation and transformation. Neurosci Biobehav Rev 36:1640–1645. [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP (2005) SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci 8:642–649. [DOI] [PubMed] [Google Scholar]
- Norris CM, Halpain S, Foster TC (1998) Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca2+channels. J Neurosci 18:3171–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J (1971) The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 34:171–175. [DOI] [PubMed] [Google Scholar]
- Oh MM, Disterhoft JF (2015) Increased excitability of both principal neurons and interneurons during associative learning. Neuroscientist 21:372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MM, Kuo AG, Wu WW, Sametsky EA, Disterhoft JF (2003) Watermaze learning enhances excitability of CA1 pyramidal neurons. J Neurophysiol 90:2171–2179. [DOI] [PubMed] [Google Scholar]
- Oh MM, McKay BM, Power JM, Disterhoft JF (2009) Learning-related postburst afterhyperpolarization reduction in CA1 pyramidal neurons is mediated by protein kinase A. Proc Natl Acad Sci 106:1620–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MM, Oliveira FA, Disterhoft JF (2010) Learning and aging related changes in intrinsic neuronal excitability. Front Aging Neurosci 2:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MM, Simkin D, Disterhoft JF (2016) Intrinsic Hippocampal Excitability Changes of Opposite Signs and Different Origins in CA1 and CA3 Pyramidal Neurons Underlie Aging-Related Cognitive Deficits. Front Syst Neurosci 10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Sametsky EA, Silva AJ, Disterhoft JF (2006) Differential effects of αCaMKII mutation on hippocampal learning and changes in intrinsic neuronal excitability. Eur J Neurosci 23:2235–2240. [DOI] [PubMed] [Google Scholar]
- Park S, Kramer EE, Mercaldo V, Rashid AJ, Insel N, Frankland PW, Josselyn SA (2016) Neuronal Allocation to a Hippocampal Engram. Neuropsychopharmacology 41:2987–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedarzani P, Mosbacher J, Rivard A, Cingolani LA, Oliver D, Stocker M, Adelman JP, Fakler B (2001) Control of Electrical Activity in Central Neurons by Modulating the Gating of Small Conductance Ca2+-activated K+ Channels. J Biol Chem 276:9762–9769. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Storm JF (1993) Pka mediates the effects of monoamine transmitters on the K+ current underlying the slow spike frequency adaptation in hippocampal neurons. Neuron 11:1023–1035. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Storm JF (1996) Evidence that Ca/calmodulin-dependent protein kinase mediates the modulation of the Ca2+-dependent K+ current, IAHP, by acetylcholine, but not by glutamate, in hippocampal neurons. Pflugers Arch 431:723–728. [PubMed] [Google Scholar]
- Piatkevich KD, Murdock MH, Subach FV. (2019) Advances in engineering and application of optogenetic indicators for neuroscience. Appl Sci 9. [Google Scholar]
- Pignatelli M, Ryan TJ, Roy DS, Lovett C, Smith LM, Muralidhar S, Tonegawa S (2019) Engram Cell Excitability State Determines the Efficacy of Memory Retrieval. Neuron 101:274–284.e5. [DOI] [PubMed] [Google Scholar]
- Pineda JC, Bargas J, Flores-Hernández J, Galarraga E (1995) Muscarinic receptors modulate the afterhyperpolarizing potential in neostriatal neurons. Eur J Pharmacol 281:271–277. [DOI] [PubMed] [Google Scholar]
- Poo M ming, Pignatelli M, Ryan TJ, Tonegawa S, Bonhoeffer T, Martin KC, Rudenko A, Tsai LH, Tsien RW, Fishell G, Mullins C, Gonçalves JT, Shtrahman M, Johnston ST, Gage FH, Dan Y, Long J, Buzsáki G, Stevens C (2016) What is memory? The present state of the engram. BMC Biol 14:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JM, Wu WW, Sametsky E, Oh MM, Disterhoft JF (2002) Age-related enhancement of the slow outward calcium-activated potassium current in hippocampal CA1 pyramidal neurons in vitro. J Neurosci 22:7234–7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Lebel D, Brosh I, Barkai E (2004) A Molecular Mechanism for Stabilization of Learning-Induced Synaptic Modifications. Neuron 41:185–192. [DOI] [PubMed] [Google Scholar]
- Ramirez S, Liu X, Lin P-A, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S (2013) Creating a False Memory in the Hippocampus. Science (80-) 341:387–391. [DOI] [PubMed] [Google Scholar]
- Rangel LM, Alexander AS, Aimone JB, Wiles J, Gage FH, Chiba AA, Quinn LK (2014) Temporally selective contextual encoding in the dentate gyrus of the hippocampus. Nat Commun 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid AJ, Yan C, Mercaldo V, Hsiang H-L, Park S, Cole CJ, De Cristofaro A, Yu J, Ramakrishnan C, Lee SY, Deisseroth K, Frankland PW, Josselyn SA (2016) Competition between engrams influences fear memory formation and recall. Science (80-) 353:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmers LG, Perkins BL, Matsuo N, Mayford M (2007) Localization of a stable neural correlate of associative memory. Science (80-) 317:1230–1233. [DOI] [PubMed] [Google Scholar]
- Richards BA, Frankland PW (2017) The Persistence and Transience of Memory. Neuron 94:1071–1084. [DOI] [PubMed] [Google Scholar]
- Rogerson T, Jayaprakash B, Cai DJ, Sano Y, Lee YS, Zhou Y, Bekal P, Deisseroth K, Silva AJ (2016) Molecular and cellular mechanisms for trapping and activating emotional memories. PLoS One 11:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routtenberg A, Rekart JL (2005) Post-translational protein modification as the substrate for long-lasting memory. Trends Neurosci 28:12–19. [DOI] [PubMed] [Google Scholar]
- Rule ME, O’Leary T, Harvey CD (2019) Causes and consequences of representational drift. Curr Opin Neurobiol 58:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U (2019) Testing Models of Human Declarative Memory at the Single-Neuron Level. Trends Cogn Sci:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TJ, Roy DS, Pignatelli M, Arons A, Tonegawa S (2015) Engram cells retain memory under retrograde amnesia. Science (80-) 348:1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar D, Barkai E (2003) Long-term modifications in intrinsic neuronal properties and rule learning in rats. Eur J Neurosci 17:2727–2734. [DOI] [PubMed] [Google Scholar]
- Saar D, Barkai E (2009) Long-lasting maintenance of learning-induced enhanced neuronal excitability: Mechanisms and functional significance. Mol Neurobiol 39:171–177. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E (1998) Reduced after-hyperpolarization in rat piriform cortex pyramidal neurons is associated with increased learning capability during operant conditioning. Eur J Neurosci 10:1518–1523. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E (1999) Reduced Synaptic Facilitation between Pyramidal Neurons in the Piriform Cortex After Odor Learning. J Neurosci 19:8616–8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E (2001) Long-Lasting Cholinergic Modulation Underlies Rule Learning in Rats. J Neurosci 21:1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E (2002) Learning-Induced Enhancement of Postsynaptic Potentials in Pyramidal Neurons. J Neurophysiol 87:2358–2363. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ESL (2002) Channels underlying neuronal calcium-activated potassium currents. [DOI] [PubMed]
- Sakaguchi M, Hayashi Y (2012) Catching the engram: Strategies to examine the memory trace. Mol Brain 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Miyashita Y (1991) Neural organization for the long-term memory of paired associates. Nature 354:152–155. [DOI] [PubMed] [Google Scholar]
- Sano Y, Shobe JL, Zhou M, Huang S, Shuman T, Cai DJ, Golshani P, Kamata M, Silva AJ (2014) CREB regulates memory allocation in the insular cortex. Curr Biol 24:2833–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Norman KA, Koutstaal W (1998) The cognitive neuroscience of constructive memory. Annu Rev Psychol 49:289–318. [DOI] [PubMed] [Google Scholar]
- Schapiro AC, Kustner LV., Turk-Browne NB (2012) Shaping of object representations in the human medial temporal lobe based on temporal regularities. Curr Biol 22:1622–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, Frankland PW (2017) Memory allocation and integration in rodents and humans. Curr Opin Behav Sci 17:90–98. [Google Scholar]
- Schlichting ML, Guarino KF, Schapiro AC, Turk-Browne NB, Preston AR (2017) Hippocampal structure predicts statistical learning and associative inference abilities during development. J Cogn Neurosci 29:37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, Mumford JA, Preston AR (2015) Learning-related representational changes reveal dissociable integration and separation signatures in the hippocampus and prefrontal cortex. Nat Commun 6:8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Eichenbaum H (1995) Information coding in the rodent prefrontal cortex. II. Ensemble activity in orbitofrontal cortex. J Neurophysiol 74:751–762. [DOI] [PubMed] [Google Scholar]
- Segal M, Murphy DD (1998) CREB activation mediates plasticity in cultured hippocampal neurons In: Neural Plasticity, pp 1–7. Hindawi Publishing Corporation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal M, Song C, Ehlers VL, Moyer JR (2013) Learning to learn - Intrinsic plasticity as a metaplasticity mechanism for memory formation. Neurobiol Learn Mem 105:186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semon R (1921) The mneme. London: Allen, Unwin. [Google Scholar]
- Seroussi Y, Brosh I, Barkai E (2002) Learning-induced reduction in post-burst after-hyperpolarization (AHP) is mediated by activation of PKC. Eur J Neurosci 16:965–969. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Zhou Y, Rogerson T, Shobe J, Balaji J (2009) Molecular and Cellular Approaches to Memory Allocation in Neural Circuits. Science (80-) 326:391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer T (2017) The Emergence of Knowledge and How it Supports the Memory for Novel Related Information. Cereb Cortex 27:1906–1921. [DOI] [PubMed] [Google Scholar]
- Song C, Detert JA, Sehgal M, Moyer JR (2012) Trace fear conditioning enhances synaptic and intrinsic plasticity in rat hippocampus. J Neurophysiol 107:3397–3408. [DOI] [PubMed] [Google Scholar]
- St-Pierre F, Marshall JD, Yang Y, Gong Y, Schnitzer MJ, Lin MZ (2014) High-fidelity optical reporting of neuronal electrical activity with an ultrafast fluorescent voltage sensor. Nat Neurosci 17:884–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T (2002) Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci 22:10163–10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanciu M, Radulovic J, Spiess J (2001) Phosphorylated cAMP response element binding protein in the mouse brain after fear conditioning: Relationship to Fos production. Mol Brain Res 94:15–24. [DOI] [PubMed] [Google Scholar]
- Staubli U, Fraser D, Faraday R, Lynch G (1987) Olfaction and the "data" memory system in rats. Behav Neurosci 101:757–765. [DOI] [PubMed] [Google Scholar]
- Stocker M (2004) Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci 5:758–770. [DOI] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P (1999) An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A 96:4662–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Duszkiewicz AJ, Morris RGM (2014) The synaptic plasticity and memory hypothesis: encoding, storage and persistence. Philos Trans R Soc B Biol Sci 369:20130288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenfeld SM, Wiig KA, Bear MF, Alberini CM (1999) A molecular correlate of memory and amnesia in the hippocampus. Nat Neurosci 2:309–310. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Best PJ (1989) Place cells and silent cells in the hippocampus of freely-behaving rats. J Neurosci 9:2382–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LT, Moyer JR, Disterhoft JF (1996) Transient changes in excitability of rabbit CA3 neurons with a time course appropriate to support memory consolidation. J Neurophysiol 76:1836–1849. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Rose GM (2005) The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged fisher 344 rats. J Neurosci 25:2609–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S, Liu X, Ramirez S, Redondo R (2015a) Memory Engram Cells Have Come of Age. Neuron 87:918–931. [DOI] [PubMed] [Google Scholar]
- Tonegawa S, Pignatelli M, Roy DS, Ryan TJ (2015b) Memory engram storage and retrieval. Curr Opin Neurobiol 35:101–109. [DOI] [PubMed] [Google Scholar]
- Tse D, Langston RRF, Kakeyama M, Bethus I, Spooner P a, Wood ER, Witter MP, Morris RGM (2007) Schemas and memory consolidation. Science (80-) 316:76–82. [DOI] [PubMed] [Google Scholar]
- Villette V, Chavarha M, Dimov IK, Ding J, Lin MZ (2019) Ultrafast Two-Photon Imaging of a High-Gain Voltage Indicator in Awake Behaving Mice Graphical Abstract Resource Ultrafast Two-Photon Imaging of a High-Gain Voltage Indicator in Awake Behaving Mice.:1590–1608. [Google Scholar]
- Viosca J, Lopez de Armentia M, Jancic D, Barco A (2009) Enhanced CREB-dependent gene expression increases the excitability of neurons in the basal amygdala and primes the consolidation of contextual and cued fear memory. Learn Mem 16:193–197. [DOI] [PubMed] [Google Scholar]
- Von Stein A, Sarnthein J (2000) Different frequencies for different scales of cortical integration: From local gamma to long range alpha/theta synchronization. Int J Psychophysiol 38:301–313. [DOI] [PubMed] [Google Scholar]
- Wirth S, Yanike M, Frank LM, Smith AC, Brown EN, Suzuki WA (2003) Single neurons in the monkey hippocampus and learning of new associations. Science (80-) 300:1578–1581. [DOI] [PubMed] [Google Scholar]
- Xu Y, Padiath QS, Shapiro RE, Jones CR, Wu SC, Saigoh N, Saigoh K, Ptácek LJ, Fu Y-H (2005) Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature 434:640–644. [DOI] [PubMed] [Google Scholar]
- Yang HHH, St-Pierre F, Sun X, Ding X, Lin MZZ, Clandinin TRR (2016) Subcellular Imaging of Voltage and Calcium Signals Reveals Neural Processing In Vivo. Cell 166:245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetton BD, Cai DJ, Spoormaker VI, Silva AJ, Mednick SC (2019) Human Memories Can Be Linked by Temporal Proximity. Front Hum Neurosci 13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JCP, Tully T (1996) CREB and the formation of long-term memory. Curr Opin Neurobiol 6:264–268. [DOI] [PubMed] [Google Scholar]
- Yiu AP, Mercaldo V, Yan C, Richards B, Rashid AJ, Hsiang H-LL, Pressey J, Mahadevan V, Tran MM, Kushner SA, Woodin MA, Frankland PW, Josselyn SA (2014) Neurons Are Recruited to a Memory Trace Based on Relative Neuronal Excitability Immediately before Training. Neuron 83:722–735. [DOI] [PubMed] [Google Scholar]
- Yokose J, Okubo-Suzuki R, Nomoto M, Ohkawa N, Nishizono H, Suzuki A, Matsuo M, Tsujimura S, Takahashi Y, Nagase M, Watabe AM, Sasahara M, Kato F, Inokuchi K (2017) Overlapping memory trace indispensable for linking, but not recalling, individual memories. Science (80-) 355:398–403. [DOI] [PubMed] [Google Scholar]
- Yu XW, Curlik DM, Oh MM, Yin JCP, Disterhoft JF (2017) CREB overexpression in dorsal CA1 ameliorates long-term memory deficits in aged rats. Elife 6:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Preston AR (2017) Temporal proximity promotes integration of overlapping events. J Cogn Neurosci 29:1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer I, Cohen H, Richter-Levin G, Lebiosn T, Grossberger T, Barkai E (2006) A cellular correlate of learning-induced metaplasticity in the hippocampus. Cereb Cortex 16:460–468. [DOI] [PubMed] [Google Scholar]
- Zhang W, Linden DJ (2003) The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci 4:885–900. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ (2009) CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci 12:1438–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire L (1990) The primate hippocampal formation: evidence for a time-limited role in memory storage. Science (80-) 250:288–290. [DOI] [PubMed] [Google Scholar]


