Abstract
Introduction:
Whether existing serological assays are sufficiently robust to measure the lower antibody levels expected following single-dose HPV vaccination is unknown.
Methods:
We evaluated seven assays measuring HPV-16/18 immunological responses overall and by number of doses in 530 serum samples from participants receiving varying doses of Cervarix or Gardasil up to 36-months post-vaccination. Serum was evaluated by simplex (HPV-16 ELISA, HPV-18 ELISA), multiplex (LIA-4, VLP-MIA, M9ELISA, GST-L1), and high-throughput pseudovirion-based neutralization assays (HT-PBNA), and results were compared to the gold standard HPV-16/18 secreted alkaline phosphatase neutralization assay (SEAP-NA). Reproducibility was assessed by the coefficient of variation (CV) and intraclass correlation coefficient (ICC). Percent agreement, Pearson correlation and weighted-kappa were used to assess validity. Determinants of seronegativity were evaluated by chi-squared test.
Results:
HPV-16: Seropositivity range was 97.1–99.5% for single dose and 98.8–99.8% overall. CV range was 4.0–18.0% for single dose and 2.9–19.5% overall. ICC range was 0.77–0.99 for single dose and 0.74–0.99 overall. Correlation with SEAP-NA range was 0.43–0.85 for single dose and 0.51–0.90 overall. Weighted-kappa range was 0.34–0.82 for single dose and 0.45–0.84 overall.
HPV-18: Seropositivity range was 63.9–94.7% for single dose and 86.2–97.9% overall. CV range was 8.1–18.2% for single dose and 4.6–18.6% overall. ICC range was 0.75–0.99 for single dose and 0.83–0.99 overall. Correlation with SEAP-NA range was 0.31–0.99 for single dose and 0.27–0.96 overall. Weighted-kappa range was 0.35–0.83 for single dose and 0.45–0.84 overall.
HPV-16 seronegativity was <5% for all assays. HPV-18 seronegativity range was 5.5%−17.3%. For LIA-4 and GST-L1 where the proportion of seronegativity was >10%, the strongest correlates of seronegativity were receiving a single vaccine dose and receiving Gardasil.
Conclusions:
These results support the utility of existing serological assays to monitor antibody responses following single-dose HPV vaccination.
Keywords: human papillomavirus, vaccine, antibody response, immunogenicity
INTRODUCTION
Multi-dose regimens of human papillomavirus (HPV) virus-like particle (VLP)-based vaccines have been shown to be highly efficacious at preventing infection with targeted oncogenic HPVs and their associated lesions, leading to worldwide vaccination efforts in order to reduce cervical cancer burden in future generations [1]. Several vaccines are currently recommended for use by the World Health Organization (WHO), including the 4-valent Gardasil (HPV-6/11/16/18), the 9-valent Gardasil-9 (HPV-6/11/16/18/31/33/45/52/58), and the bivalent Cervarix (HPV-16/18) [2]. These vaccines provide near complete protection against targeted HPV types, and, in the case of the bivalent vaccine, additional partial protection against non-targeted HPV types [3–5]. While highly effective, multi-dose vaccination programs are costly and difficult to implement, particularly in poorer regions of the world where the bulk of disease burden lies [6].
There is accumulating evidence that a single dose of the bivalent or 4-valent HPV vaccine provides high level of protection over at least 4 years despite lower levels of antibodies generated with a single dose [7–9]. This has led to formal randomized trials to demonstrate efficacy and durability of protection with single-dose virus-like particle (VLP)-based vaccines [10, 11]. As recommended by the WHO, virological outcomes are the primary outcomes being evaluated in these formal trials [12]. In addition, serological immune response measures are important secondary outcomes in these trials to enable a better understanding of minimum antibody levels required for protection. It is likely that these serological measures of vaccine response will become increasingly important for studies aimed at bridging results from formal efficacy trials to other populations and other VLP-based vaccines [13].
The primary assays used to monitor antibody responses to vaccination in the initial multi-dose HPV vaccine trials for licensure were the antigen-binding enzyme-linked immunosorbent assay (ELISA) and the competitive Luminex immunoassay (cLIA) targeting individual vaccine HPV types [14]. Both of these assays have been shown to be highly reproducible and to correlate closely with neutralization potential (as measured by the gold standard SEAP/PBNA assays) in multi-dose recipients [15, 16].
While stable, antibody levels generated following a single HPV vaccine dose are considerably lower than those generated following multi-dose vaccination [8, 9, 17]. It is therefore important to evaluate the performance of immunoassays to measure lower level responses expected following single-dose vaccination, to ensure that they are reproducible and correlate well with more direct measures of neutralization potential. A few studies have explored the ability of current assays to measure responses following a single vaccine dose, but these studies have generally included small numbers of single-dose recipients [15].
Ideal assays to monitor immune responses in ongoing or future single-dose HPV vaccine trials would measure antibody response to all HPV types included in the vaccines being evaluated (up to 9 HPV types in the case of Gardasil-9). At a minimum, the assays should be able to reproducibly and validly measure responses to the two HPV types (HPV-16/18) that cause upwards of 70% of all cervical cancers worldwide. In this study, we aim to evaluate the reproducibility of the simplex ELISA and newer multiplex/high-throughput assays by number of doses received, and to determine whether they are valid proxies for neutralization (as measured by SEAP-NA, the gold standard) even at low antibody levels. We focus on HPV-16/18 for this initial evaluation, since they are the primary HPV types included in all HPV vaccines on the market.
MATERIALS AND METHODS
Study participants and sample selection
Samples for this study were selected from amongst participants in two large-scale HPV vaccine trials described in detail previously, the NCI-sponsored HPV-16/18 Costa Rica Vaccine Trial (CVT; (Clinicaltrials.gov ID: NCT00128661) and the IARC-sponsored HPV-6/11/16/18 Vaccine Trial in India (INDIA; NCT00923702). These studies were selected because they 1) included participants vaccinated with varying number of doses of the HPV vaccine, 2) followed vaccinated individuals prospectively through the antibody plateau phase, and 3) represent the AS04-adjuvanted bivalent and the alum-adjuvanted quadrivalent vaccines.
We selected samples from individuals who received varying number of vaccine doses and from timepoints representing both peak and plateau antibody titers (Table 1). Sample size was driven by specimen availability and an attempt to balance the number of samples from Cervarix and Gardasil recipients from varying timepoints. The intent of this sampling strategy was to obtain samples from a broad representation of vaccine-induced antibody levels. Samples from individuals who received 2 doses six months apart were grouped with those who received 3 doses over six months given published evidence of comparable antibody levels among these two groups [18]. Henceforth, we refer to samples from individuals who received 1 dose as the “single dose group,” those from individuals who received 2 doses over one or two months as the “reduced dose group,” and those from individuals who received either 2 or 3 doses over six months as the “full dose group.”
Table 1.
Number of Participants Selected by Study and Dosing Group*
| Costa Rica HPV-16/18 Vaccine Trial (CVT) | India HPV-6/11/16/18 Trial (INDIA) | |||||
|---|---|---|---|---|---|---|
| Dosing Group** | Study Entry | Month 12/18 Timepoint -N (Median Months of Follow-Up) | Month 24/36 Timepoint -N (Median Months of Follow-Up) | Study Entry | Month 12/18 Timepoint -N (Median Months of Follow-Up) | Month 24/36 Timepoint -N (Median Months of Follow-Up) |
| Single Dose Group | 0 | 30 (14.0) | 60 (35.0) | 0 | 40 (21.0) | 40 (27.0) |
| Reduced Dose Group | 0 | 30 (16.0) | 60 (35.0) | 0 | 40 (21.5) | 40 (28.0) |
| Full Dose Group | 0 | 30 (12.0) | 60 (33.0) | 0 | 40 (20.0) | 40 (34.0) |
| Pre-vaccination Group | 10 | 0 | 0 | 10 | 0 | 0 |
Due to sample volume limitations, not all samples selected were tested by all assays. Proportion tested for each assay are listed in the text and summarized further in Supplementary Table S1.
Single dose group = samples from individuals who received 1-dose; Reduced dose group = samples from individuals who received 2 doses over one or two months; Full dose group = samples from individuals who received either 2 or 3 doses over six months.
For CVT, samples were selected from amongst participants who were seronegative (by HPV-16/18 ELISA) at enrollment, HPV-16/18 DNA-negative during the 6-month vaccination phase, and for whom specimens were available at relevant timepoints described in Table 1. For INDIA, samples were selected from amongst participants for whom specimens were available at relevant timepoints described in Table 1. While virological/serological testing was not performed pre-vaccination for INDIA trial participants, participants are assumed to be HPV-naïve given their age (10–18) and pre-marital status. In addition, we selected a total of 20 pre-vaccination samples among DNA- and seronegative women in CVT and in INDIA to confirm assay specificity. Samples selected for study at different timepoints were not necessarily from the same participants (i.e. this study was not designed to evaluate paired samples across time from the same participants).
In total, 530 samples were selected for testing (Table 1). From these samples, multiple blinded aliquots were generated to enable testing in duplicate by the gold standard HPV-16 and HPV-18 SEAP-NA assays and the 7 simplex and multiplex antibody binding and high-throughput neutralization assays described below. Some samples were insufficient to generate the needed aliquots for duplicate testing by all of the assays. For these samples, an algorithm was designed to balance the use of duplicates across assays. Ultimately, duplicate samples were prepared for testing for the following percentage of study specimens: 61.7% for SEAP-NA, 62.5% for ELISA, 86.0% for LIA-4, 59.8% for HT-PBNA/GST-L1/VLP-MIA, and 68.5% for M9E (see Supplementary Table S1 for detailed breakdown of sample use).
Laboratory testing
The following assays were performed for this study: secreted alkaline phosphatase pseudovirion-based neutralization assay (SEAP-NA) for HPV-16 and HPV-18, enzyme-linked immunosorbent assay (ELISA) for HPV-16 and HPV-18, 4-plex Luminex immunoassay (LIA-4) for HPV-16/18/6/11 combined, M9-ELISA assay (M9E) for HPV-16/18/6/11/31/33/45/52/58 combined, glutathione S-transferase L1 assay (GST-L1) for HPV-16/18/6/11/31/33/45/52/58 combined, VLP multiplex immune assay (VLP-MIA) for HPV-16/18 combined, and high-throughput pseudovirion-based neutralization assay (HT-PBNA) for HPV-16/18/6/31/33/45/52/58. Three laboratories, each specialized in its own tests, contributed to our study: The SEAP-NA, ELISA, and LIA-4 assays were performed at the Frederick National Laboratory for Cancer Research (Maryland, U.S.A.); the VLP-MIA, GST-L1, and HT-PBNA assays were performed at the German Cancer Research Center (DKFZ; Heidelberg, Germany); the M9E assay was performed at the Centers for Disease Control and Prevention (Georgia, U.S.A.).
The ELISA, LIA-4, M9E, GST-L1, and VLP-MIA assays are polyclonal binding assays. The SEAP-NA and HT-PBNA assays are neutralization assays. The SEAP-NA was considered the gold standard to which other assays were compared. Details of the testing methods used for each of the assays listed above are provided in the Supplementary Materials.
In addition to HPV-16 and HPV-18, some of the assays utilized in this study were designed to measure antibodies against other HPV types. However, for purposes of this analysis (given that serum was collected from individuals vaccinated against HPV-16/18 in CVT and HPV-6/11/16/18 in INDIA), we evaluated results for HPV-16 and HPV-18 testing only.
Statistical analysis
We evaluated the accuracy and reproducibility for each of the seven assays and for each of the two HPV types (HPV-16 and HPV-18); therefore, we considered 14 = 7 × 2 distinct measurements. Furthermore, for each measurement, we performed analyses using all samples and separately using only the single-dose, reduced-dose, or full-dose samples. We first measured the percent of samples that were positive and, using only the positive samples, estimated the geometric mean titer (GMT), the coefficient of variation (CV), and the intraclass correlation coefficients (ICC), with the estimates and confidence intervals for the latter two calculated using statistics from the SAS procedure PROC MIXED. We then categorized both the measurement and SEAP-NA levels into quartiles and quantified their concordance by the proportion of samples classified into the same category for each assay and by the weighted kappa statistic. The p-value for asymmetry was calculated by the McNemar-Bowker Test of Symmetry. Moreover, we estimated the Pearson correlation coefficient between the measurement and SEAP-NA assay. Exact agreement with SEAP-NA was evaluated at the continuous and categorial levels since both approaches to reporting antibody responses are used in the field. Finally, for measurements with >10% seronegativity, we compared seronegativity and sample characteristics using a chi-squared test.
RESULTS
Assay reproducibility and specificity
We first evaluated assay reproducibility for HPV-16 and HPV-18 by comparing results from blinded duplicate testing performed for each of the assays evaluated (ELISA-16, ELISA-18, LIA-4, VLP-MIA, M9E, GST-L1, and HT-PBNA) (Supplementary Figure S1). We examined reproducibility overall and separately by dose group (single-, reduced-, and full-dose groups) (Table 2). Results from sensitivity analyses that evaluated reproducibility further stratified by study and age at entry (Supplementary Table S2) are consistent with our findings in the main analysis. We also evaluated the specificity of the assays under evaluation among samples from 20 individuals seronegative for HPV-16 and HPV-18 by ELISA pre-vaccination (Supplementary Table S3).
Table 2.
Assay Reproducibility for HPV-16/18 by Dose Groups.
| Assay | HPV Type | N Samples (N Results) | Seropositive (%) | GMT* | %CV | %CV 95% CI | ICC | ICC 95% CI |
|---|---|---|---|---|---|---|---|---|
| HPV-16 ASSAYS | ||||||||
| SAMPLES FROM SINGLE DOSE RECIPIENTS | ||||||||
| ELISA | 16 | 105 (210) | 97.1 | 19.2 | 7.1 | (6.1–8.3) | 0.95 | (0.93–0.97) |
| LIA-4 | 16 | 137 (274) | 99.3 | 15.7 | 5.4 | (4.7–6.2) | 0.98 | (0.97–0.99) |
| VLP-MIA | 16 | 83 (166) | 99.4 | 7.4 | 18.0 | (15.2–21.3) | 0.77 | (0.68–0.85) |
| M9E | 16 | 118 (236) | 98.3 | 12.1 | 4.7 | (4.0–5.5) | 0.99 | (0.99–0.99) |
| GST-L1 | 16 | 102 (204) | 99.5 | 760.2 | 4.0 | (3.5–4.6) | 0.90 | (0.86–0.93) |
| HT-PBNA | 16 | 104 (208) | 98.6 | 25.9 | 11.1 | (9.6–13.0) | 0.87 | (0.82–0.91) |
| SAMPLES FROM REDUCED DOSE RECIPIENTS | ||||||||
| ELISA | 16 | 107 (214) | 100.0 | 92.0 | 4.0 | (3.5–4.6) | 0.96 | (0.95–0.98) |
| LIA-4 | 16 | 148 (296) | 100.0 | 72.4 | 3.6 | (3.2–4.0) | 0.98 | (0.97–0.98) |
| VLP-MIA | 16 | 27 (54) | 100.0 | 15.8 | 20.6 | (15.5–27.2) | 0.44 | (0.18–0.73) |
| M9E | 16 | 112 (224) | 100.0 | 81.9 | 2.4 | (2.1–2.8) | 0.99 | (0.98–0.99) |
| GST-L1 | 16 | 97 (194) | 100.0 | 2135.6 | 3.8 | (3.3–4.4) | 0.82 | (0.74–0.87) |
| HT-PBNA | 16 | 101 (202) | 100.0 | 161.2 | 4.8 | (4.1–5.5) | 0.95 | (0.93–0.97) |
| SAMPLES FROM FULL DOSE RECIPIENTS | ||||||||
| ELISA | 16 | 108 (216) | 99.1 | 222.4 | 3.6 | (3.1–4.1) | 0.97 | (0.96–0.98) |
| LIA-4 | 16 | 144 (288) | 99.0 | 184.1 | 7.5 | (6.7–8.5) | 0.84 | (0.79–0.88) |
| VLP-MIA | 16 | 9 (18) | 100.0 | 29.1 | 20.8 | (12.8–33.7) | 0.37 | (0.05–0.87) |
| M9E | 16 | 120 (240) | 100.0 | 209.3 | 2.4 | (2.1–2.7) | 0.99 | (0.98–0.99) |
| GST-L1 | 16 | 98 (196) | 100.0 | 3134.0 | 3.0 | (2.6–3.5) | 0.77 | (0.67–0.84) |
| HT-PBNA | 16 | 99 (198) | 100.0 | 498.7 | 3.7 | (3.2–4.3) | 0.96 | (0.94–0.97) |
| ALL SAMPLES REGARDLESS OF NUMBER OF DOSES | ||||||||
| ELISA | 16 | 320 (640) | 98.8 | 74.8 | 4.5 | (4.2–4.9) | 0.98 | (0.98–0.98) |
| LIA-4 | 16 | 429 (858) | 99.4 | 60.8 | 6.3 | (5.8–6.8) | 0.96 | (0.95–0.97) |
| VLP-MIA | 16 | 119 (238) | 99.6 | 9.8 | 19.5 | (17.0–22.5) | 0.74 | (0.65–0.82) |
| M9E | 16 | 350 (700) | 99.4 | 59.9 | 2.9 | (2.7–3.1) | 0.99 | (0.99–1.00) |
| GST-L1 | 16 | 297 (594) | 99.8 | 1702.2 | 3.6 | (3.3–3.9) | 0.92 | (0.90–0.93) |
| HT-PBNA | 16 | 304 (608) | 99.5 | 125.5 | 5.9 | (5.4–6.5) | 0.96 | (0.96–0.97) |
| HPV-18 ASSAYS | ||||||||
| SAMPLES FROM SINGLE DOSE RECIPIENTS | ||||||||
| ELISA | 18 | 105 (210) | 94.3 | 10.3 | 11.3 | (9.7–13.2) | 0.91 | (0.87–0.93) |
| LIA-4 | 18 | 137 (274) | 63.9 | 6.7 | 18.2 | (14.8–22.3) | 0.96 | (0.95–0.97) |
| VLP-MIA | 18 | 96 (192) | 92.7 | 5.9 | 16.5 | (14.0–19.6) | 0.88 | (0.83–0.92) |
| M9E | 18 | 118 (236) | 87.3 | 6.0 | 8.1 | (6.7–9.7) | 0.99 | (0.98–0.99) |
| GST-L1 | 18 | 102 (204) | 73.0 | 308.2 | 11.6 | (10.0–13.3) | 0.75 | (0.66–0.83) |
| HT-PBNA | 18 | 104 (208) | 94.7 | 8.6 | 10.8 | (9.1–12.8) | 0.95 | (0.92–0.96) |
| SAMPLES FROM REDUCED DOSE RECIPIENTS | ||||||||
| ELISA | 18 | 107 (214) | 99.5 | 35.6 | 6.0 | (5.2–6.9) | 0.95 | (0.93–0.97) |
| LIA-4 | 18 | 148 (296) | 95.3 | 15.8 | 6.3 | (5.5–7.2) | 0.98 | (0.98–0.99) |
| VLP-MIA | 18 | 78 (156) | 100.0 | 19.7 | 17.2 | (14.5–20.5) | 0.74 | (0.63–0.83) |
| M9E | 18 | 112 (224) | 98.2 | 33.1 | 4.3 | (3.7–5.0) | 0.99 | (0.98–0.99) |
| GST-L1 | 18 | 97 (194) | 96.4 | 446.7 | 5.9 | (5.1–6.8) | 0.82 | (0.75–0.88) |
| HT-PBNA | 18 | 101 (202) | 100.0 | 43.2 | 5.1 | (4.4–6.0) | 0.98 | (0.96–0.98) |
| SAMPLES FROM FULL DOSE RECIPIENTS | ||||||||
| ELISA | 18 | 108 (216) | 99.1 | 85.6 | 5.2 | (4.5–6.0) | 0.96 | (0.94–0.97) |
| LIA-4 | 18 | 144 (288) | 98.3 | 37.1 | 7.6 | (6.7–8.6) | 0.95 | (0.93–0.96) |
| VLP-MIA | 18 | 52 (104) | 99.0 | 29.9 | 19.6 | (15.9–24.0) | 0.62 | (0.45–0.77) |
| M9E | 18 | 120 (240) | 99.2 | 74.8 | 3.6 | (3.1–4.1) | 0.99 | (0.98–0.99) |
| GST-L1 | 18 | 98 (196) | 96.4 | 721.8 | 6.3 | (5.4–7.2) | 0.82 | (0.75–0.88) |
| HT-PBNA | 18 | 100 (200) | 99.0 | 115.9 | 3.8 | (3.3–4.5) | 0.98 | (0.98–0.99) |
| ALL SAMPLES REGARDLESS OF NUMBER OF DOSES | ||||||||
| ELISA | 18 | 320 (640) | 97.7 | 32.5 | 6.8 | (6.3–7.5) | 0.97 | (0.96–0.97) |
| LIA-4 | 18 | 429 (858) | 86.2 | 17.9 | 9.0 | (8.2–9.8) | 0.98 | (0.97–0.98) |
| VLP-MIA | 18 | 226 (452) | 96.7 | 13.3 | 18.6 | (16.7–20.7) | 0.85 | (0.80–0.88) |
| M9E | 18 | 350 (700) | 94.9 | 26.1 | 4.6 | (4.2–5.1) | 0.99 | (0.99–0.99) |
| GST-L1 | 18 | 297 (594) | 88.4 | 477.9 | 8.0 | (7.3–8.7) | 0.83 | (0.79–0.86) |
| HT-PBNA | 18 | 305 (610) | 97.9 | 35.2 | 5.8 | (5.2–6.3) | 0.98 | (0.98–0.99) |
GMT is calculated among positive samples. Titers are presented in IU/mL for ELISA, LIA-4, VLP-MIA, M9E, and HT-PBNA. For GST-L1 Titers are presented as Median Fluorescence Intensity.
Abbreviations: number (N); enzyme-linked immunosorbent assay (ELISA); 4-plex Luminex immunoassay (LIA-4); VLP multiplex immune assay (VLP-MIA); M9-ELISA assay (M9E); glutathione S-transferase L1 assay (GST-L1); high-throughput pseudovirion-based neutralization assay (HT-PBNA); coefficient of variation (CV); intraclass correlation coefficient (ICC); and confidence intervals (CI).
For HPV-16, seropositivity ranged from 97.1 to 99.5% for single dose, 100.0% for reduced dose, 99.0 to 100% for full dose, and 98.8 to 99.8% overall (Table 2). Among seropositives, GMTs (expressed in IU/mL except for GST-L1) ranged from 7.4 to 25.9 for single dose, 15.8 to 161.2 for reduced dose, 29.1 to 498.7 for full dose, and 9.8 to 125.5 overall. GMTs for GST-L1 (expressed in MFI) was 760.2 for single dose, 2135.6 for reduced dose, 3134.0 for full dose, and 1702.2 overall. The range for assay reproducibility (CV) was 4.0–18.0% for single dose, 2.4–20.6% for reduced dose, 2.4–20.8% for full dose, and 2.9–19.5% overall. CV was below 15% for all assays except for the VLP-MIA (CV = 18.0% for single dose, 20.6% for reduced dose, 20.8% for full dose, and 19.5% overall). ICCs were above 0.80 for all assays except for the VLP-MIA (ICC = 0.77 for single dose, 0.44 for reduced dose, 0.37 for full dose, and 0.74 overall) and GST-L1 (ICC = 0.77 for full dose). CV for the SEAP-NA, considered the gold standard in this analysis, was 7.6% (95% CI = 6.6–8.8) for single dose, 5.2% (95% CI = 4.5–5.9) for reduced dose, 4.7% (95% CI = 4.1–5.4) for full dose, and 5.5% (95% CI = 5.1–6.0) overall. Seropositivity among pre-vaccination controls was under 10% for all assays except for the GST-L1 assay (seropositivity = 16.1%; GMT among positives = 150 MFI), providing evidence of the specificity of these assays (Supplementary Table S3).
For HPV-18, seropositivity ranged from 63.9 to 94.7% for single dose, 95.3 to 100.0% for reduced dose, 96.4 to 99.2% for full dose, and from 86.2 to 97.9% overall (Table 2). Among seropositives, GMTs (expressed in IU/mL except for GST-L1) ranged from 5.9 to 10.3 for single dose, 15.8 to 43.2 for reduced dose, 29.9 to 115.9 for full dose, and 13.3 to 35.2 overall. GMTs for GST-L1 (expressed in MFI) was 308.2 for single dose, 446.7 for reduced dose, 721.8 for full dose, and 477.9 overall. The range for assay reproducibility (CV) was 8.1–18.2% for single dose, 4.3–17.2% for reduced dose, 3.6–19.6% for full dose, and 4.6–18.6% overall. CV was below 15% for all assays except for the LIA-4 (CV = 18.2% for single dose) and VLP-MIA (CV = 16.5% for single dose, 17.2% for reduced dose, 19.6% for full dose, and 18.6% overall). ICCs were above 0.80 for all assays regardless of dose group with a few exceptions: GST-L1 (ICC = 0.75 for single dose), VLP-MIA (ICC = 0.74 for reduced dose and 0.62 for full dose). CV for the SEAP-NA was 8.0% (95% CI = 6.9–9.4) for single dose, 6.3% (95% CI = 5.4–7.2) for reduced dose, 5.3% (95% CI = 4.6–6.2) for full dose, and 6.3% (95% CI = 5.8–6.9) overall. Seropositivity among pre-vaccination controls was under 10% for all assays except for the ELISA (seropositivity = 19.4%; GMT among positives = 5.8 IU/mL) and the GST-L1 assay (seropositivity = 16.1%; GMT among positives = 244 MFI), providing evidence of the specificity of these assays (Supplementary Table S3).
Validity of assays as markers of neutralization potential
We examined agreement of each of the assays against SEAP-NA to determine whether these assays correlated well with HPV-16 and HPV-18 neutralization potential (Figure 1). We examined agreement with SEAP-NA overall and separately by dose group. Quartile cuts (as defined in Materials and Methods) were used to calculate exact agreement and kappa levels. Results from the analysis by dose groups and overall are summarized in Table 3. Results from sensitivity analyses that evaluated validity further stratified by study and age at entry (Supplementary Table S4) are consistent with our findings in the main analysis. Results from analyses that evaluated exact agreement using dichotomous cuts (Supplementary Table S5) are also consistent with our main findings.
Figure 1. Comparison between SEAP-NA and HPV-16/18 a) ELISA, b) LIA-4, c) VLP-MIA, d) M9E, e) GST-L1, and f) HT-PBNA.
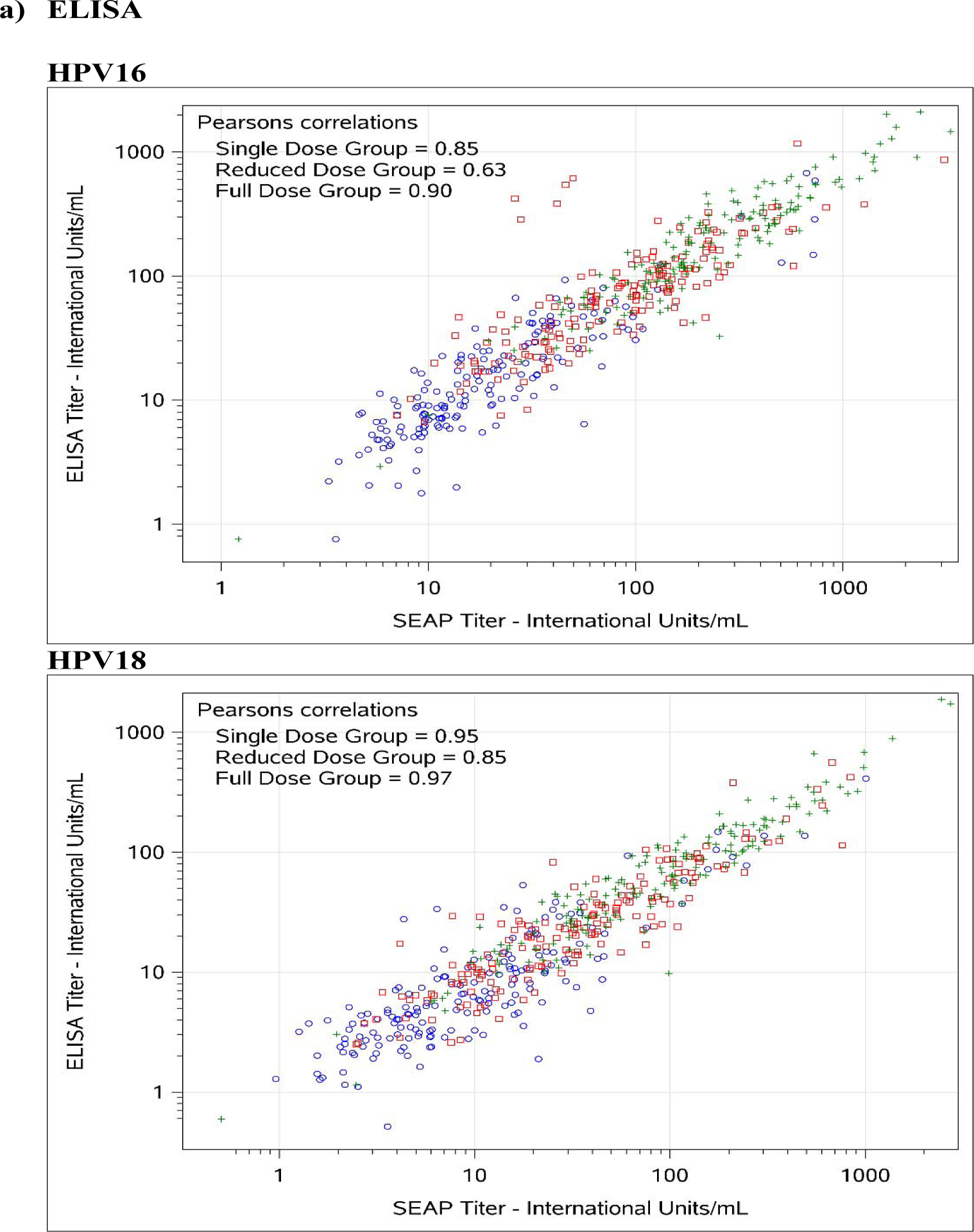
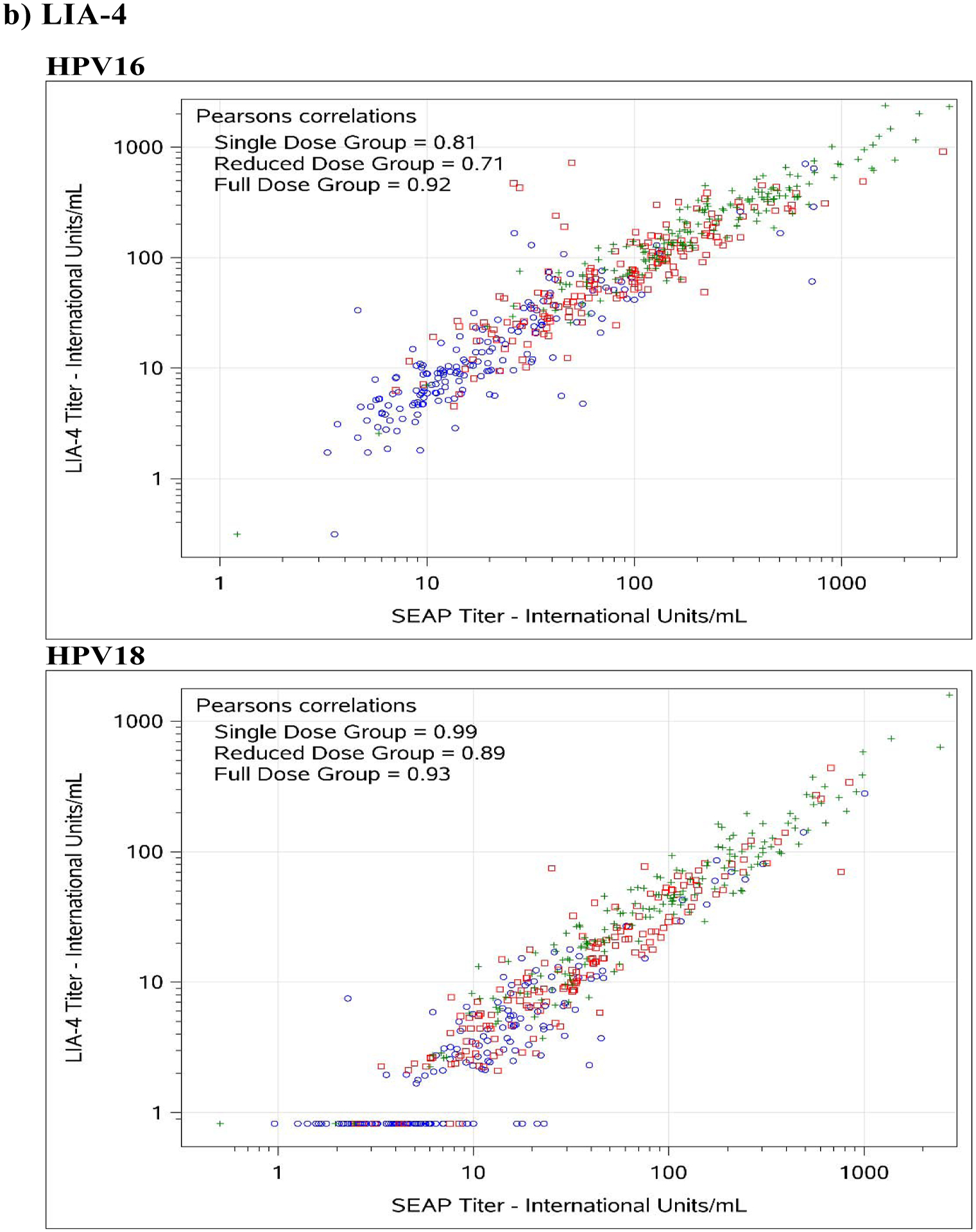
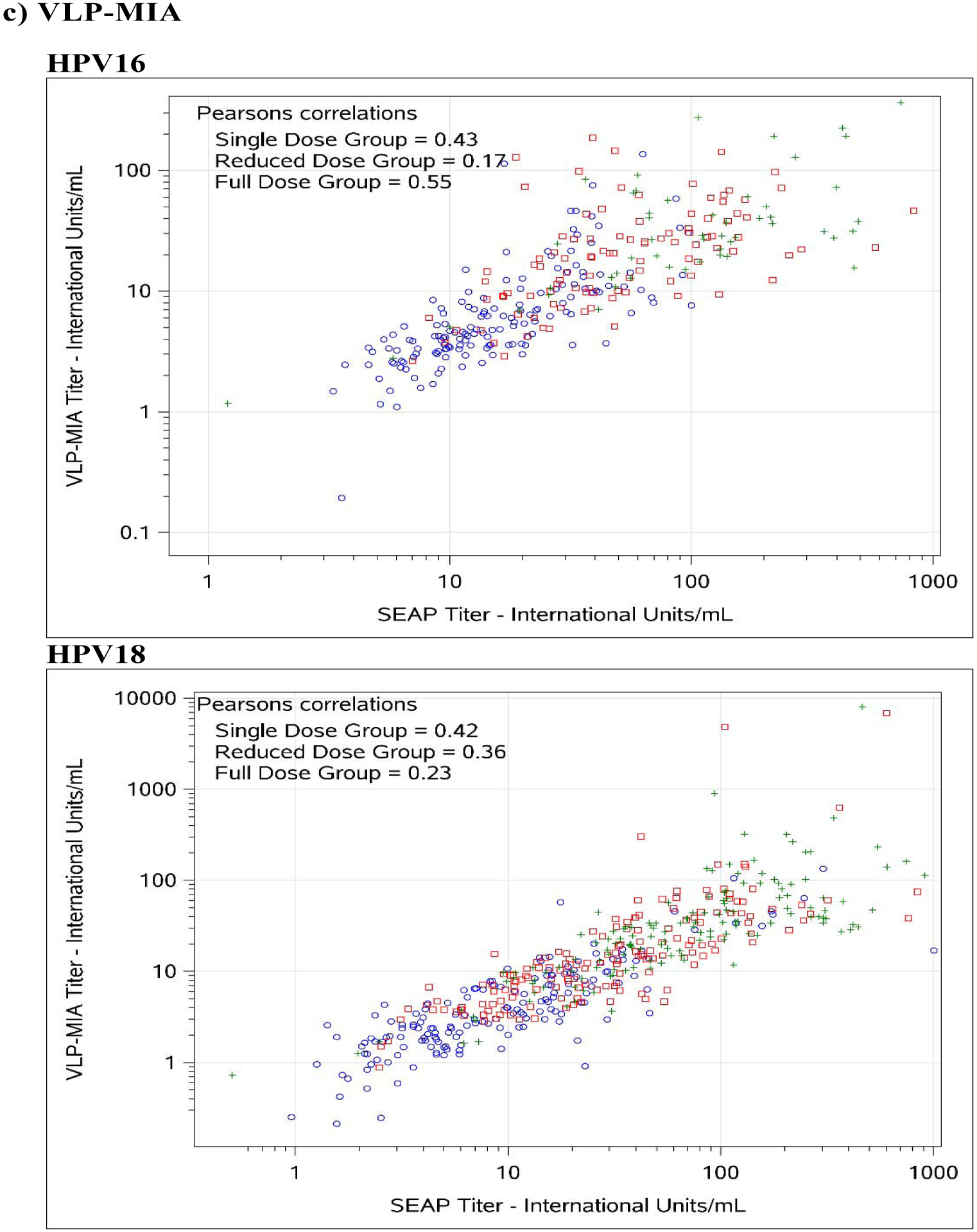
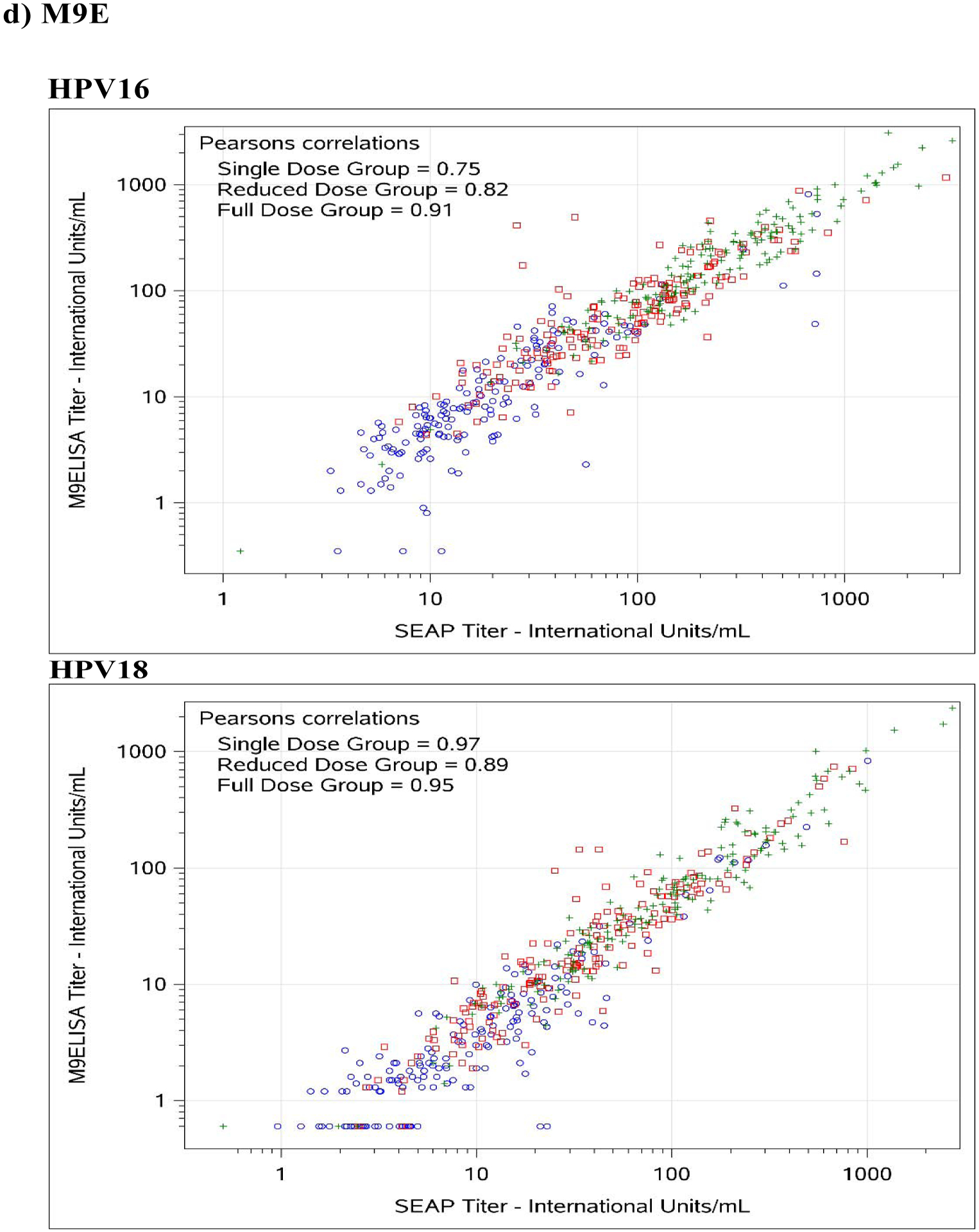
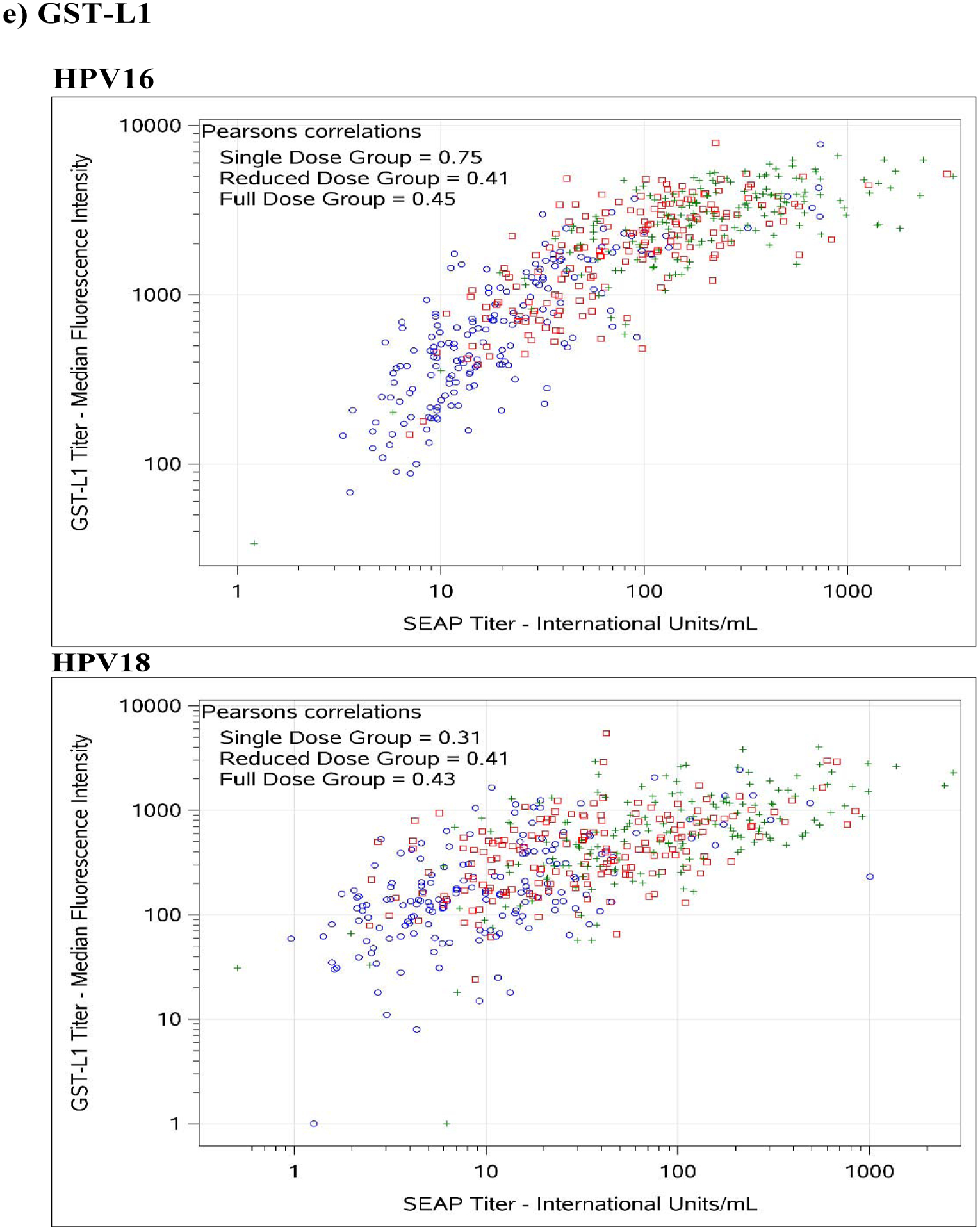
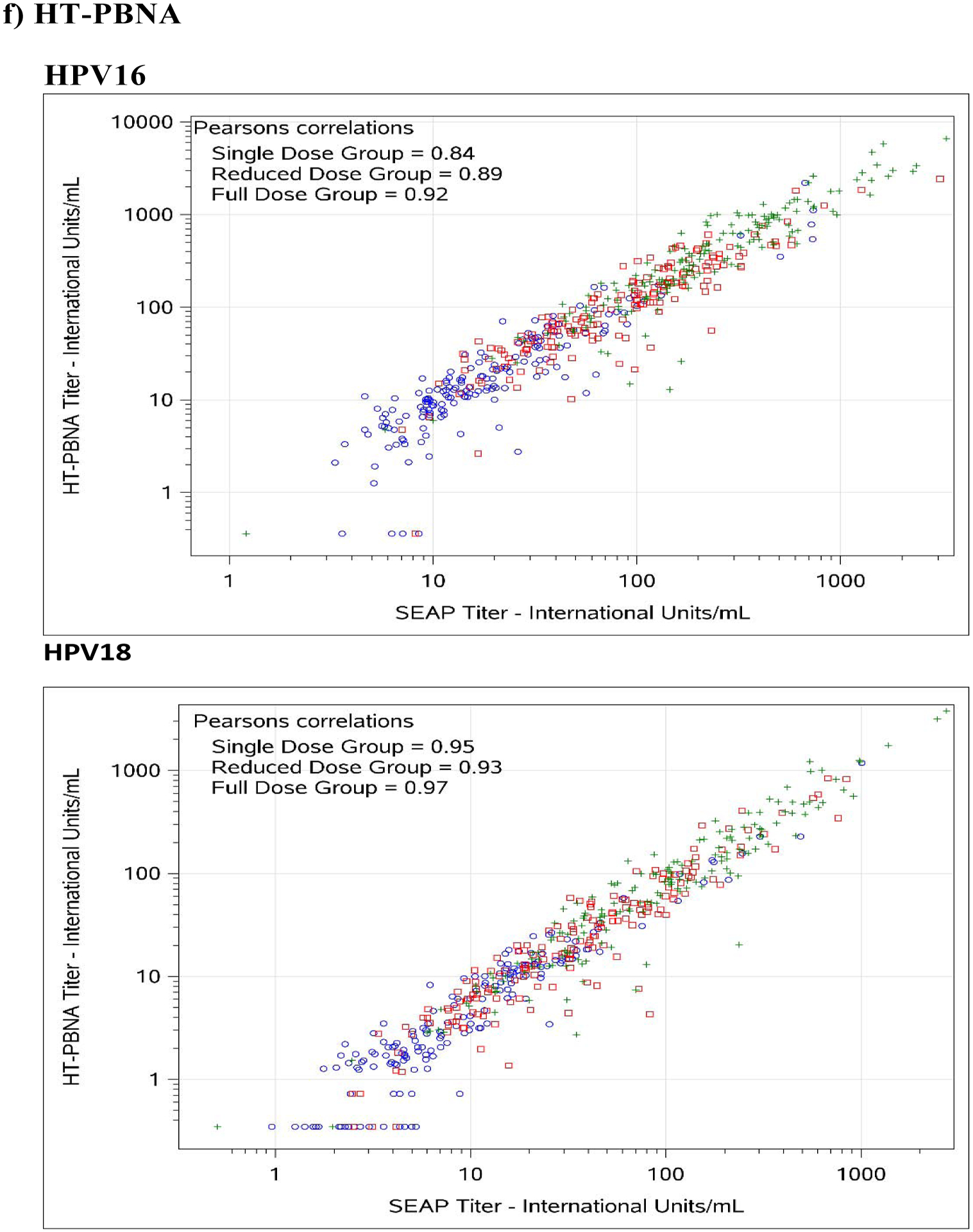
Blue circles represent single dose group; red squares represent reduced dose group; green crosses represent full dose group.
Table 3.
Comparison of Results (in Quartiles) to the SEAP Pseudovirion Neutralization Assay for HPV-16/18 (Gold Standard) by Dose Groups.
| Assay | HPV Type | Total N | % Exact Agreement (95% CI) | Pearson correlation (95% CI) | Weighted Kappa (95% CI) | Symmetry p-value |
|---|---|---|---|---|---|---|
| HPV-16 ASSAYS | ||||||
| SAMPLES FROM SINGLE DOSE RECIPIENTS | ||||||
| ELISA | 16 | 170 | 78.8% (72.2%−84.5%) | 0.85 (0.81–0.89) | 0.69 (0.60–0.79) | 0.78 |
| LIA-4 | 16 | 170 | 80.6% (74.1%−86.0%) | 0.81 (0.75–0.86) | 0.71 (0.63–0.80) | 0.87 |
| VLP-MIA | 16 | 153 | 53.6% (45.7%−61.4%) | 0.43 (0.29–0.55) | 0.34 (0.26–0.42) | <0.001 |
| M9E | 16 | 167 | 81.4% (75.0%−86.8%) | 0.75 (0.67–0.81) | 0.72 (0.64–0.81) | 0.82 |
| GST-L1 | 16 | 170 | 77.6% (70.9%−83.4%) | 0.75 (0.67–0.81) | 0.68 (0.59–0.78) | 0.64 |
| HT-PBNA | 16 | 170 | 87.1% (81.4%−91.5%) | 0.84 (0.79–0.88) | 0.82 (0.75–0.90) | 1.00 |
| SAMPLES FROM REDUCED DOSE RECIPIENTS | ||||||
| ELISA | 16 | 170 | 68.2% (61.0%−74.9%) | 0.63 (0.53–0.71) | 0.65 (0.56–0.73) | 0.55 |
| LIA-4 | 16 | 170 | 66.5% (59.1%−73.3%) | 0.71 (0.63–0.78) | 0.63 (0.54–0.71) | 0.15 |
| VLP-MIA | 16 | 102 | 29.4% (21.2%−38.8%) | 0.17 (−0.03–0.35) | 0.23 (0.13–0.33) | <0.001 |
| M9E | 16 | 167 | 70.7% (63.4%−77.2%) | 0.82 (0.77–0.87) | 0.68 (0.60–0.76) | 0.54 |
| GST-L1 | 16 | 170 | 49.4% (41.9%−56.9%) | 0.41 (0.28–0.53) | 0.47 (0.38–0.56) | 0.60 |
| HT-PBNA | 16 | 170 | 74.1% (67.1%−80.3%) | 0.89 (0.85–0.91) | 0.73 (0.66–0.80) | 0.72 |
| SAMPLES FROM FULL DOSE RECIPIENTS | ||||||
| ELISA | 16 | 170 | 80.0% (73.5%−85.5%) | 0.90 (0.87–0.93) | 0.74 (0.66–0.82) | 0.74 |
| LIA-4 | 16 | 170 | 85.9% (80.0%−90.5%) | 0.92 (0.89–0.94) | 0.82 (0.75–0.89) | 0.38 |
| VLP-MIA | 16 | 52 | 50.0% (36.6%−63.4%) | 0.55 (0.33–0.72) | 0.41 (0.24–0.58) | 0.0010 |
| M9E | 16 | 168 | 86.3% (80.5%−90.9%) | 0.91 (0.88–0.93) | 0.83 (0.76–0.89) | 0.56 |
| GST-L1 | 16 | 169 | 58.0% (50.4%−65.3%) | 0.45 (0.33–0.57) | 0.48 (0.37–0.58) | 0.50 |
| HT-PBNA | 16 | 170 | 82.9% (76.7%−88.0%) | 0.92 (0.89–0.94) | 0.77 (0.70–0.85) | 0.46 |
| ALL SAMPLES REGARDLESS OF NUMBER OF DOSES | ||||||
| ELISA | 16 | 510 | 75.7% (71.8%−79.3%) | 0.87 (0.84–0.89) | 0.79 (0.76–0.83) | 0.95 |
| LIA-4 | 16 | 510 | 77.6% (73.9%−81.1%) | 0.89 (0.87–0.90) | 0.81 (0.78–0.84) | 0.95 |
| VLP-MIA | 16 | 307 | 45.0% (39.4%−50.6%) | 0.51 (0.42–0.59) | 0.45 (0.40–0.51) | <0.001 |
| M9E | 16 | 502 | 79.5% (75.8%−82.8%) | 0.89 (0.87–0.91) | 0.83 (0.80–0.86) | 1.00 |
| GST-L1 | 16 | 509 | 61.7% (57.4%−65.8%) | 0.55 (0.48–0.60) | 0.67 (0.63–0.71) | 0.57 |
| HT-PBNA | 16 | 510 | 81.4% (77.8%−84.6%) | 0.90 (0.88–0.92) | 0.84 (0.81–0.87) | 0.54 |
| HPV-18 ASSAYS | ||||||
| SAMPLES FROM SINGLE DOSE RECIPIENTS | ||||||
| ELISA | 18 | 170 | 69.4% (62.2%−76.0%) | 0.95 (0.93–0.96) | 0.63 (0.53–0.72) | 0.92 |
| LIA-4 | 18 | 170 | 78.2% (71.6%−84.0%) | 0.99 (0.98–0.99) | 0.74 (0.66–0.82) | 0.71 |
| VLP-MIA | 18 | 166 | 71.1% (63.8%−77.6%) | 0.42 (0.29–0.54) | 0.64 (0.55–0.74) | 0.51 |
| M9E | 18 | 167 | 80.8% (74.3%−86.3%) | 0.97 (0.96–0.98) | 0.78 (0.70–0.85) | 0.54 |
| GST-L1 | 18 | 170 | 50.6% (43.1%−58.1%) | 0.31 (0.16–0.44) | 0.35 (0.24–0.47) | 0.18 |
| HT-PBNA | 18 | 170 | 84.7% (78.7%−89.5%) | 0.95 (0.94–0.97) | 0.83 (0.76–0.89) | 0.88 |
| SAMPLES FROM REDUCED DOSE RECIPIENTS | ||||||
| ELISA | 18 | 170 | 70.6% (63.4%−77.1%) | 0.85 (0.80–0.89) | 0.72 (0.65–0.79) | 0.76 |
| LIA-4 | 18 | 170 | 76.5% (69.7%−82.4%) | 0.89 (0.85–0.92) | 0.78 (0.72–0.85) | 0.80 |
| VLP-MIA | 18 | 160 | 59.4% (51.6%−66.8%) | 0.36 (0.22–0.49) | 0.62 (0.54–0.70) | 0.026 |
| M9E | 18 | 167 | 74.3% (67.2%−80.5%) | 0.89 (0.86–0.92) | 0.76 (0.70–0.83) | 0.84 |
| GST-L1 | 18 | 170 | 36.5% (29.5%−43.9%) | 0.41 (0.27–0.53) | 0.26 (0.15–0.37) | 0.85 |
| HT-PBNA | 18 | 170 | 76.5% (69.7%−82.4%) | 0.93 (0.91–0.95) | 0.78 (0.71–0.84) | 0.68 |
| SAMPLES FROM FULL DOSE RECIPIENTS | ||||||
| ELISA | 18 | 170 | 82.9% (76.7%−88.0%) | 0.97 (0.95–0.97) | 0.81 (0.74–0.88) | 0.80 |
| LIA-4 | 18 | 170 | 82.4% (76.1%−87.5%) | 0.93 (0.91–0.95) | 0.80 (0.74–0.87) | 0.64 |
| VLP-MIA | 18 | 145 | 74.5% (66.9%−81.1%) | 0.23 (0.07–0.38) | 0.72 (0.63–0.81) | 0.30 |
| M9E | 18 | 168 | 82.7% (76.5%−87.9%) | 0.95 (0.94–0.97) | 0.81 (0.75–0.88) | 0.73 |
| GST-L1 | 18 | 169 | 52.1% (44.5%−59.5%) | 0.43 (0.30–0.55) | 0.41 (0.30–0.52) | 0.016 |
| HT-PBNA | 18 | 170 | 81.8% (75.4%−87.0%) | 0.97 (0.96–0.98) | 0.80 (0.73–0.87) | 0.48 |
| ALL SAMPLES REGARDLESS OF NUMBER OF DOSES | ||||||
| ELISA | 18 | 510 | 74.3% (70.4%−78.0%) | 0.95 (0.94–0.96) | 0.78 (0.75–0.82) | 0.98 |
| LIA-4 | 18 | 510 | 79.0% (75.3%−82.4%) | 0.93 (0.92–0.94) | 0.83 (0.80–0.86) | 0.92 |
| VLP-MIA | 18 | 471 | 68.2% (63.8%−72.2%) | 0.27 (0.19–0.35) | 0.73 (0.69–0.77) | 0.0072 |
| M9E | 18 | 502 | 79.3% (75.6%−82.7%) | 0.95 (0.94–0.96) | 0.83 (0.80–0.86) | 0.97 |
| GST-L1 | 18 | 509 | 46.4% (42.1%−50.7%) | 0.46 (0.38–0.52) | 0.45 (0.40–0.51) | 0.33 |
| HT-PBNA | 18 | 510 | 81.0% (77.4%−84.2%) | 0.96 (0.96–0.97) | 0.84 (0.81–0.87) | 0.70 |
Abbreviations: number (N); secreted alkaline phosphatase pseudovirion-based neutralization assay (SEAP-NA); enzyme-linked immunosorbent assay (ELISA); 4-plex Luminex immunoassay (LIA-4); VLP multiplex immune assay (VLP-MIA); M9-ELISA assay (M9E); glutathione S-transferase L1 assay (GST-L1); high-throughput pseudovirion-based neutralization assay (HT-PBNA); and confidence intervals (CI).
For HPV-16, exact agreement with SEAP-NA was above 75% for all assays except for VLP-MIA (53.6% single dose; 29.4% reduced dose; 50.0% full dose; 45.0% overall), ELISA (68.2% reduced dose), LIA-4 (66.5% reduced dose), M9E (70.7% reduced dose), GST-L1 (49.4% reduced dose; 58.0% full dose; 61.7% overall), and HT-PBNA (74.1% reduced dose) (Table 3). Correlation with SEAP-NA ranged from 0.43 to 0.85 for single dose, 0.17 to 0.89 for reduced dose, 0.45 to 0.92 for full dose, and 0.51 to 0.90 overall. Correlation was above 0.75 for all assays except for VLP-MIA (0.43 single dose; 0.17 reduced dose; 0.55 full dose; 0.51 overall), ELISA (0.63 reduced dose), LIA-4 (0.71 reduced dose), and GST-L1 (0.41 reduced dose; 0.45 full dose; 0.55 overall). Weighted kappa estimates are also summarized in Table 3 and suggest similar patterns as those observed for exact agreement and correlations described above. There was no evidence for asymmetry among discordants, except for VLP-MIA (p-value < 0.001 for single dose, reduced dose, and overall; < 0.01 for full dose).
For HPV-18, exact agreement with SEAP-NA was above 75% for all assays except for ELISA (69.4% single dose; 70.6% reduced dose; 74.3% overall), VLP-MIA (71.1% single dose; 59.4% reduced dose; 74.5% full dose; 68.2% overall), GST-L1 (50.6% single dose; 36.5% reduced dose; 52.1% full dose; 46.4% overall), and M9E (74.3% reduced dose) (Table 3). Correlation with SEAP-NA ranged from 0.31 to 0.99 for single dose, 0.36 to 0.93 for reduced dose, 0.23 to 0.97 for full dose, and 0.27 to 0.96 overall. Correlation was above 0.75 for all assays except for VLP-MIA (0.42 single dose; 0.36 reduced dose; 0.23 full dose; 0.27 overall) and GST-L1 (0.31 single dose; 0.41 reduced dose; 0.43 full dose; 0.46 overall). Weighted kappa estimates are also summarized in Table 3 and suggest similar patterns as those observed for exact agreement and correlations described above. There was no evidence for asymmetry among discordants, except for VLP-MIA (p-value = 0.026 for reduced dose; 0.0072 overall) and GST-L1 (p-value = 0.016 for full dose).
Proportion and determinants of seronegativity
We examined what proportion of post-vaccination samples were seronegative by each of the assays of interest. Seronegativity for HPV-16 antibodies was lower than 5% for all assays (range: 0.33–2.2%). Seronegativity for HPV-18 antibodies ranged from 5.5% (VLP-MIA) to 17.3% (LIA-4). For the two assays with greater than 10% seronegativity for HPV-18, LIA-4 and GST-L1, we evaluated determinants of seronegativity (Table 4). The strongest determinant of seronegativity for both assays was the number of doses received, with 44.1% and 28.8% of single-dose recipients testing HPV-18 negative by LIA-4 and GST-L1, respectively, compared to 1.8% and 6.5% of full-dose recipients (p-values <0.001). In addition, for the LIA-4 assay, we noted that seronegativity was significantly associated with participation in the INDIA trial (30.4% in INDIA vs. 5.6% in CVT; p-value < 0.001) and with younger age at vaccination (p-value < 0.001). The association with age at vaccination was likely explained by the fact that participants from the INDIA trial were younger (9–18 years) than those from CVT (18–25 years). For the GST-L1 assay, seronegativity was significantly associated with time since first vaccine dose (p-value = 0.034).
Table 4.
Correlates of Seronegativity for Assays with >10% Seronegativity Overall.
| HPV-18 LIA-4 (N=510) | HPV-18 GST-L1 (N=509) | |||
|---|---|---|---|---|
| % | N | % | N | |
| Overall | 17.3 | 88 | 13.6 | 69 |
| Covariate: | ||||
| Study | ||||
| CVT | 5.6 | 15 | 11.1 | 30 |
| INDIA | 30.4 | 73 | 16.3 | 39 |
| * p-value: <0.001 | * p-value: 0.087 | |||
| Dosage Schedule | ||||
| Single Dose Group | 44.1 | 75 | 28.8 | 49 |
| Reduced Dose Group | 5.9 | 10 | 5.3 | 9 |
| Full Dose Group | 1.8 | 3 | 6.5 | 11 |
| * p-value: <0.001 | * p-value: <0.001 | |||
| Age at Vaccination (years) | ||||
| 9–12 | 28.6 | 30 | 13.3 | 14 |
| 13–16 | 30.2 | 32 | 14.2 | 15 |
| 17–21 | 8.4 | 17 | 14.9 | 30 |
| 22–25 | 9.3 | 9 | 10.3 | 10 |
| * p-value: <0.001 | * p-value: 0.75 | |||
| Time Since First Vaccine Dose (months) | ||||
| <20 | 12.6 | 13 | 8.7 | 9 |
| 20–24 | 19.7 | 36 | 10.9 | 20 |
| 25+ | 17.4 | 39 | 17.9 | 40 |
| * p-value: 0.32 | * p-value: 0.034 | |||
P-value calculated from chi-squared test.
Abbreviations: number (N); 4-plex Luminex immunoassay (LIA-4); and glutathione S-transferase L1 assay (GST-L1).
DISCUSSION
As evidence for efficacy of single-dose HPV vaccination mounts and clinical trials formally quantifying such efficacy advance, defining assays that can be used to monitor lower level antibody responses induced by a single dose of the HPV vaccine is important. Such assays will become increasingly valuable to understand the protective antibody levels observed among recipients of reduced number of vaccine doses and to bridge findings from the large, ongoing efficacy trials across populations and new VLP-based vaccines as they become available.
In this study, we evaluated the reproducibility of various assays designed to monitor antibody response to HPV vaccination and examined to what extent they correlate with direct measures of neutralization potential currently considered as gold standards to monitor immune response to vaccination. Importantly, we expressed assay results in terms of IU/mL for all assays (except the GST-L1 assay) because the use of international standards is important in assay standardization and comparisons. In total, we evaluated two simplex antibody-binding assays (ELISA-16 and ELISA-18), four multiplex binding assays (LIA, VLP-MIA, M9E, and GST-L1), and one high-throughput multiplex neutralization assay (HT-PBNA). Both the M9E and HT-PBNA assays performed exceedingly well with respect to both reproducibility and agreement with simplex neutralization assays for HPV-16/18. Furthermore, our results demonstrated high reproducibility for most assays, even in the context of modest antibody levels generated after single-dose vaccination. In addition, with a few exceptions discussed below, we have demonstrated that most assays correlate well with the simplex SEAP-NA assays that measure direct neutralization potential, the primary effector mechanism of protection afforded by the HPV vaccines, even in the context of modest antibody levels generated after single-dose vaccination.
While results overall were reassuring, we did note a few exceptions. Despite high reproducibility, the GST-L1 assay tended to have reduced agreement with the SEAP-NA for both HPV-16 and HPV-18 (Table 3). This is not unexpected, given that the antigen constructs used in this assay are GST-L1 fusion proteins rather than HPV VLPs, and thus do not reflect the HPV capsid in its true 3-dimentional conformation. As such, it is likely that some conformation-dependent neutralizing epitopes are not or are poorly detected using this assay.
The two bead-based multiplex binding assays evaluated also deserve some discussion. The VLP-MIA assay had the lowest reproducibility of the assays evaluated, with CVs of 18.0% and 16.5% for HPV-16 and HPV-18, respectively, among single-dose recipients (Table 2). This appears to be partly due to the inability of the assay, in its current configuration, to quantify antibody levels on the higher end of the distribution, as indicated by the considerable proportion of samples that had to be excluded from the analysis due to levels that were above the assay’s upper limit of quantification and increasing variability observed with increasing levels of antibody (i.e. among 2- and 3-dose recipients). Future efforts to optimize the VLP-MIA could benefit from an increase of the dynamic range of the assay, with emphasis on the upper limit of quantification of the assay.
The other bead-based multiplex assay evaluated, the LIA assay, performed well with respect to both reproducibility and correlation to neutralization for HPV-16 but demonstrated reduced reproducibility for HPV-18 among single-dose recipients (CV = 18.2%) (Table 2). Furthermore, up to 44% of samples from 1-dose recipients tested seronegative for HPV-18 antibodies by this assay (Table 4), suggesting the need for further assay development to define cutoff thresholds that maximize sensitivity while retaining specificity.
All assays examined had low negativity for HPV-16 detection (< 2.5% seronegativity). For HPV-18, we noted two assays for which the proportion of seronegativity was above 10% (LIA-4 and GST-L1). For these two assays, the main determinants of seronegativity were having received a single dose and participation in the INDIA trial (i.e. Gardasil recipients). These findings are consistent with our understanding that antibody levels are lower among individuals who receive a single dose of vaccine compared to multiple doses and with our knowledge that Gardasil is less immunogenic than Cervarix [8, 9, 19].
The main limitation of our study is the inability to evaluate assay performance to detect antibodies generated in response to vaccination with HPV types other than HPV-16/18 since no serum was available in this study from individuals vaccinated with the nonavalent vaccine. Moreover, it would be more informative to have included a larger (we included only 20 samples among presumed seronegatives pre-vaccination) set of known seronegative individuals (e.g. young children) to allow for more careful evaluation of assay specificity at alternative assay cutoffs. Nonetheless, results from our study are useful to identify the subset of assays for further optimization and to identify specific areas requiring improvement for individual assays.
Strengths of our study include the large number of post-vaccination samples tested (representing the broad range of antibody levels expected following vaccination with 1, 2, or 3 doses of either the bivalent or quadrivalent HPV vaccine currently on the market) and the evaluation of assay performance not only at peak antibody levels observed shortly after administering all requisite doses but also in the out years once plateau responses have been reached.
In summary, our study has shown high reproducibility and correlation with neutralization potential for several existent serological assays designed to measure immune response to HPV vaccination, even in the context of lower antibody responses observed after single-dose vaccination. Our findings also identified specific areas where further assay optimization could be considered. In particular, future attention is needed to define ideal assay cutoffs that optimize assay sensitivity while avoiding false positive results. Ultimately, these assays will be important to monitor immunological responses and to determine the minimum antibody levels required for protection from single-dose HPV vaccination.
The Costa Rica Vaccine Trial Study Group authors:
Bernal Cortés, Paula González, Rolando Herrero, Silvia E. Jiménez, Carolina Porras, Ana Cecilia Rodríguez (Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica); Allan Hildesheim, Aimée R. Kreimer, Douglas R. Lowy, Mark Schiffman, John T. Schiller, Mark Sherman (United States National Cancer Institute, Bethesda, MD, USA); Ligia A. Pinto, Troy J. Kemp (Leidos Biomedical Research, Inc., Frederick National Laboratory for Cancer Research, Frederick, MD, USA); Mary K. Sidawy, (Georgetown University, Washington, DC, USA); Wim Quint, Leen-Jan van Doorn, Linda Struijk (DDL Diagnostic Laboratory, Netherlands); Joel M. Palefsky, Teresa M. Darragh (University of California, San Francisco, CA, USA); Mark H. Stoler (University of Virginia, Charlottesville, VA, USA)
Supplementary Material
Acknowledgments
We extend a special thanks to the women of Guanacaste and Puntarenas, Costa Rica, who gave of themselves in participating in this effort. In Costa Rica, we acknowledge the tremendous effort and dedication of the staff involved in this project; we would like to specifically acknowledge the meaningful contributions by Carlos Avila, Loretto Carvajal, Rebeca Ocampo, Cristian Montero, Diego Guillen, Jorge Morales and Mario Alfaro. In the United States, we extend our appreciation to the team from Information Management Services (IMS) responsible for the development and maintenance of the data system used in the trial and who serve as the data management center for this effort, especially Jean Cyr, Julie Buckland, John Schussler, and Brian Befano. We thank Dr. Diane Solomon (CVT: medical monitor & QC pathologist) for her invaluable contributions during the randomized blinded phase of the trial and the design of the LTFU and Nora Macklin (CVT) and Kate Torres (LTFU) for the expertise in coordinating the study. We thank the members of the Data and Safety Monitoring Board charged with protecting the safety and interest of participants during the randomized, blinded phase of our study (Steve Self, Chair, Adriana Benavides, Luis Diego Calzada, Ruth Karron, Ritu Nayar, and Nancy Roach) and members of the external Scientific HPV Working Group who have contributed to the success of our efforts over the years (Elizabeth Fontham and Henriette Raventós, Co-Chairs, Joanna Cain, Diane Davey, Gypsyamber D’Souza, Anne Gershon, Elizabeth Holly, Silvia Lara, Wasima Rida, Richard Roden, Maria del Rocío Sáenz Madrigal, and Margaret Stanley).
Conflict of Interest and Role of the Funding Source.
The Costa Rica HPV Vaccine Trial is a long-standing collaboration between investigators in Costa Rica and the NCI. The trial is sponsored and funded by the NCI (contract N01-CP-11005), with funding support from the National Institutes of Health Office of Research on Women’s Health. GlaxoSmithKline Biologicals (GSK) provided vaccine and support for aspects of the trial associated with regulatory submission needs of the company under a Clinical Trials Agreement (FDA BB-IND 7920) during the four-year, randomized blinded phase of our study. John T. Schiller and Douglas R. Lowy report that they are named inventors on US Government-owned HPV vaccine patents that are licensed to GlaxoSmithKline and Merck and for which the National Cancer Institute receives licensing fees. They are entitled to limited royalties as specified by federal law. The other authors declare that they have no conflicts of interest. The NCI and Costa Rica investigators are responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript. Registered with Clinicaltrials.gov NCT00128661
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLAIMER
Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer / World Health Organization.
The findings and conclusions of this report are those of the authors and do not necessary represent the official position of the Centers for Disease Control and Prevention.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
REFERENCES
- [1].Schiller J, Lowy D. Explanations for the high potency of HPV prophylactic vaccines. Vaccine. 2018;36:4768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Harper DM, DeMars LR. HPV vaccines - A review of the first decade. Gynecol Oncol. 2017;146:196–204. [DOI] [PubMed] [Google Scholar]
- [3].World Health Organization. Electronic address swi Human papillomavirus vaccines: WHO position paper, May 2017-Recommendations. 2017:241–68. [Google Scholar]
- [4].Hildesheim A, Wacholder S, Catteau G, Struyf F, Dubin G, Herrero R, et al. Efficacy of the HPV-16/18 vaccine: final according to protocol results from the blinded phase of the randomized Costa Rica HPV-16/18 vaccine trial. Vaccine. 2014;32:5087–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Malagon T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:781–9. [DOI] [PubMed] [Google Scholar]
- [6].de Sanjose S, Serrano B, Castellsague X, Brotons M, Munoz J, Bruni L, et al. Human papillomavirus (HPV) and related cancers in the Global Alliance for Vaccines and Immunization (GAVI) countries. A WHO/ICO HPV Information Centre Report. Vaccine. 2012;30 Suppl 4:D1–83, vi. [DOI] [PubMed] [Google Scholar]
- [7].Kreimer AR, Struyf F, Del Rosario-Raymundo MR, Hildesheim A, Skinner SR, Wacholder S, et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA Trials. Lancet Oncol. 2015;16:775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Safaeian M, Porras C, Pan Y, Kreimer A, Schiller JT, Gonzalez P, et al. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila). 2013;6:1242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sankaranarayanan R, Joshi S, Muwonge R, Esmy PO, Basu P, Prabhu P, et al. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine. 2018;36:4783–91. [DOI] [PubMed] [Google Scholar]
- [10].Sampson JN, Hildesheim A, Herrero R, Gonzalez P, Kreimer AR, Gail MH. Design and statistical considerations for studies evaluating the efficacy of a single dose of the human papillomavirus (HPV) vaccine. Contemp Clin Trials. 2018;68:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kreimer AR, Herrero R, Sampson JN, Porras C, Lowy DR, Schiller JT, et al. Evidence for single-dose protection by the bivalent HPV vaccine-Review of the Costa Rica HPV vaccine trial and future research studies. Vaccine. 2018;36:4774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].WHO International Agency for Research on Cancer. Primary End-points for Prophylactic HPV Vaccine Trials. Lyon (FR) 2014. [PubMed] [Google Scholar]
- [13].Lowy DR, Herrero R, Hildesheim A, Participants in the INCIwoPEfPHPVVT. Primary endpoints for future prophylactic human papillomavirus vaccine trials: towards infection and immunobridging. Lancet Oncol. 2015;16:e226–33. [DOI] [PubMed] [Google Scholar]
- [14].Pinto LA, Dillner J, Beddows S, Unger ER. Immunogenicity of HPV prophylactic vaccines: Serology assays and their use in HPV vaccine evaluation and development. Vaccine. 2018;36:4792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Robbins HA, Kemp TJ, Porras C, Rodriguez AC, Schiffman M, Wacholder S, et al. Comparison of antibody responses to human papillomavirus vaccination as measured by three assays. Front Oncol. 2014;3:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brown D, Muller M, Sehr P, Pawlita M, Seitz H, Rubio I, et al. Concordance assessment between a multiplexed competitive Luminex immunoassay, a multiplexed IgG Luminex immunoassay, and a pseudovirion-based neutralization assay for detection of human papillomaviruse types 16 and 18. Vaccine. 2014;32:5880–7. [DOI] [PubMed] [Google Scholar]
- [17].Safaeian M, Sampson JN, Pan Y, Porras C, Kemp TJ, Herrero R, et al. Durability of Protection Afforded by Fewer Doses of the HPV16/18 Vaccine: The CVT Trial. Journal of the National Cancer Institute. 2018;110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309:1793–802. [DOI] [PubMed] [Google Scholar]
- [19].Einstein MH, Takacs P, Chatterjee A, Sperling RS, Chakhtoura N, Blatter MM, et al. Comparison of long-term immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18–45 years: end-of-study analysis of a Phase III randomized trial. Hum Vaccin Immunother. 2014;10:3435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


