Abstract
Introduction and Hypothesis
The current study aims to characterize the association between pelvic floor disorder symptoms and bone strength reflecting a potential connective tissue pathophysiology in postmenopausal women.
Methods
A cross-sectional study was conducted in postmenopausal women undergoing osteoporosis evaluation from 2007 to 2010. Urinary incontinence (UI) was defined as urinary leakage ≥2–3 times/week. UI types were defined using the 3 Incontinence Questionnaire. Fecal incontinence was defined as stool leakage ≥1/month, and pelvic organ prolapse as a positive response to “Do you have a bulge or something falling out that you can see or feel in your vaginal area?” Bone quality and quantity were assessed by trabecular bone score (TBS) and bone mineral density, respectively; i) bone strength was defined by combined quality/quantity index, low strength being equivalent to moderate-severe fracture risk ii) low quality as TBS≤1.31; iii) low quantity by T-score <−1 or on osteoporosis medication.
Results
Of 681 subjects, 262 had low bone strength whereas 419 were normal using the combined quality/quantity bone assessment. Characteristics were similar except for age (low bone strength: 69.0±8.2 vs. normal: 65.0±7.1, p<0.01) and smoking (8.8% vs. 3.3%, p<0.01). Low bone strength was associated with any UI (aOR:1.48, 1.05–2.10), stress (aOR:1.53, 1.06–2.21), and mixed (aOR:1.45, 1.02–2.05). Women with low bone quality had increased odds of UI (any, urgency, mixed) whereas none of the pelvic floor disorder symptoms were associated with low bone quantity.
Conclusions
Low bone strength defined by a combined quantity/quality index as well as low bone quality alone were associated with increased risk of UI.
Keywords: Bone mineral density, Incontinence, Osteoporosis, Pelvic floor disorders, Urinary incontinence, Trabecular bone score
Brief summary
Low bone strength defined as a combination of quality and quantity bone assessment is associated with increased risk of urinary incontinence in postmenopausal women.
Introduction
Pelvic floor disorders including urinary incontinence (UI), fecal incontinence (FI), and pelvic organ prolapse (POP) are highly prevalent and debilitating conditions greatly impacting quality of life (QOL).[1–3] Nearly one in four adult women in the United States (US) report at least one pelvic floor disorder symptoms,[4] and prevalence significantly increases with age. With a rapidly growing population of older adults, socioeconomic burdens of pelvic floor disorders will become even more substantial.
Osteoporosis is another major public health concern with significant morbidity and mortality affecting more than half of US adults older than 50 years.[5] Osteoporosis, characterized by low bone mineral density (BMD) reflecting bone quantity, as well as poor microarchitecture (bone quality) results in compromised bone strength and an increased bone fracture risk.[6, 7]
Pelvic floor support consists of pelvic floor muscles and fibromuscular connective tissue (most abundantly, collagen) which are attached to the bony pelvis.[8] Therefore, abnormal collagen metabolism can lead to poor pelvic floor support and function resulting in pelvic floor disorders.[9–13] Bone strength also depends on the quantity and quality of connective tissue influenced by extracellular matrix (predominantly collagen)[7] similar to pelvic floor support. Thus, there may be a pathophysiologic association between pelvic floor disorders and bone strength as a global alteration of extracellular matrix metabolism. Existing data suggesting the potential association between skeletal integrity (osteoporosis) and pelvic floor disorders are limited and solely based on BMD, which only measures bone quantity.[14–16] As both bone quantity and quality determine the overall bone strength, our primary aim was to characterize the association between pelvic floor disorder symptoms and bone strength defined by a combination of quantity and quality bone assessment in postmenopausal women undergoing an osteoporosis/bone density evaluation. We further examined the impact of bone quantity and quality on pelvic floor disorder symptoms separately. Our hypothesis was that there would be an association between overall bone strength and pelvic floor disorder symptoms in postmenopausal women.
Materials and Methods
A cross-sectional study was conducted using an existing database containing postmenopausal women presenting for osteoporosis evaluation from 2007 to 2010 in the bone densitometry services/osteoporosis clinic at a university hospital. Institutional Review Board (IRB) approval was obtained. This database including clinical and pelvic floor symptom data, as well as bone densitometry images was previously established to characterize pelvic floor disorder symptoms in postmenopausal women.[16] Clinical characteristics obtained from the database include age, race, body mass index, number of vaginal deliveries, pulmonary disease (asthma or chronic obstructive pulmonary disease), prior history of pelvic floor surgery, bilateral salpingo-oophorectomy prior to menopause, menopausal status, hormonal replacement therapy, use of the Food and Drug Administration (FDA) approved BMD medications, chronic use of corticosteroids, daily alcohol use and smoking status. The presence of UI (any UI) was defined as the leakage of urine ≥ 2–3 times per week in the past 4 weeks using the four-item International Consultation on Incontinence Questionnaire-Short Form (ICIQ-SF).[17] As many women report infrequent leakage that is not bothersome, a common definition of UI in epidemiologic studies is at least once weekly leakage which is considered at least moderate UI based on validated measures. [3, 4] Of the available choices in the ICIQ-SF, ≥ 2 leakage episodes per week was felt to be most comparable with other existing studies. Among subjects with UI, the types of UI were determined using the 3 Incontinence Questions (3IQ).[18] Stress urinary incontinence (SUI) was the leakage when “performing some physical activity such as coughing, sneezing, lifting, or exercise”, whereas urgency urinary incontinence (UUI) was leakage when having “the urge or the feeling that you needed to empty your bladder, but you could not get to the toilet fast enough”. Women having urinary leakage “about equally with activity and urge” were classified as having mixed urinary incontinence (MUI). The presence of FI was defined as liquid or solid stool leakage at least once per month using the Fecal Incontinence Severity Index (FISI),[19] and POP as a positive response to “Do you have a bulge or something falling out that you can see or feel in your vaginal area?” from the Pelvic Floor Distress Inventory-20 (PFDI-20).[20] Having a bulge symptom has been shown to correlate with the presence of a vaginal bulge on examination, thus a commonly used definition in epidemiologic studies.[3, 4, 21]
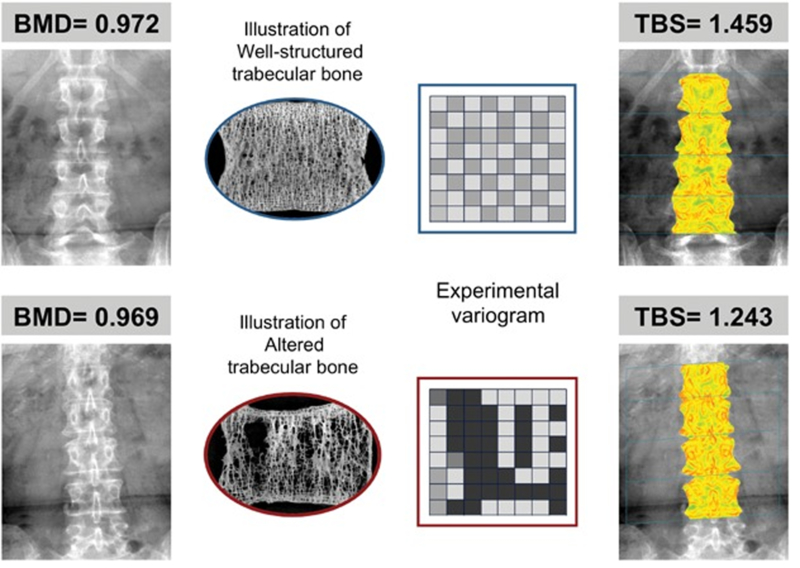
For bone quality assessment, a trabecular bone score (TBS) was obtained using TBS iNsight (Medimaps, Newton, MA) as a surrogate for bone microarchitecture. The TBS is a validated measure to assess bone microarchitecture for predicting the risk of fragility fractures.[6, 22] Low bone quality was defined as TBS ≤ 1.31 corresponding to at least intermediate to high risk of fracture.[6] For bone quantity assessment, BMD measurements were obtained via dual-energy X-ray absorptiometry (DXA), Hologic W (Hologic Inc, Bedford, MA). The recent technology has allowed simultaneous measurements of both TBS and BMD in women undergoing DXA scanning without additional scan time or radiation exposure (Figure 1). Using the unique feature of the iNsight software, TBS was applied retrospectively to existing DXA exams (without additional imaging) and compared to BMD in the subjects identified from the existing database which contained pelvic floor symptoms as well as demographic data at the time of their DXA/osteoporosis evaluation from 2007 to 2010.
Figure 1:
Comparison of the traditional bone mineral density (BMD) examining bone mass versus trabecular bone score (TBS) evaluating bone microarchitecture.
Permission obtained from John Wiley and Sons©. The original figure published in Journal of Bone and Mineral Research. Silva BC, Leslie WD, Resch H, et al. Trabecular Bone Score: A Noninvasive Analytical Method Based Upon the DXA Image. J Bone Miner Res. 2014. 29: 518–530.
Bone strength was assessed using an existing fracture risk classification system with a combined BMD and TBS index, and low bone strength was defined as having a major osteoporotic fracture risk of greater than 7–10/1000 fractures per year. This is consistent with the current literature of a moderate to high fracture risk in postmenopausal women.[22] Low bone quantity was defined using the traditional World Health Organization criteria, a T-score < −1 (osteopenia or osteoporosis) or currently on FDA approved BMD medication.[23]
Patient characteristics and pelvic floor disorder symptoms were compared between the study groups. Three parallel comparisons were conducted where each definition of compromised skeletal integrity (combined low bone strength, low bone quality, low bone quantity) was compared to the normal group. Student’s t-test was used for continuous variables, whereas Chi-square test, Fisher’s exact test, or Cochran–Mantel–Haenszel test were used for categorical variables as appropriate. Multivariable logistic regression was performed to further assess the association between bone strength and pelvic floor disorder symptoms, adjusting for pertinent covariates potentially influencing the difference observed between groups based on clinical risk factors and bivariate analyses with p < 0.10. Statistical analysis was conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC). Statistical significance was indicated at a 0.05 level.
Results
Of 1665 postmenopausal women undergoing osteoporosis evaluation in the existing database, TBS compatible DXA images were available in 681(40.9%) subjects; 262/681 (38.5%) were classified as low bone strength, and 419/681 (61.5%) as normal using the combined (TBS and BMD) bone strength assessment. Demographic characteristics were similar with the exceptions of older age (low bone strength: 69.0 ± 8.2 years vs. normal: 65.0 ± 7.1 years, p<0.01) and more smokers (low bone strength: 8.8% vs. normal: 3.3%, p<0.01) in the low bone strength group (Table 1). On bivariate analyses, women meeting the low bone strength criteria had a higher rate of any UI (49.4% vs. 41.4%, p=0.042) and SUI (37.5% vs. 30.1%. p=0.047, Table 2). Using the combined bone assessment, multivariable regression revealed women with low bone strength (TBS and BMD) had a significantly increased odds of having any UI (adjusted-OR: 1.48, 95%CI: 1.05–2.10), SUI (aOR: 1.53, 95%CI: 1.06–2.21), and MUI (aOR: 1.45, 95%CI: 1.02–2.05, Table 3) compared to the “normal” bone strength cohort after adjusting for age, obesity, race, the use of oral hormone therapy, smoking, and vaginal delivery.
Table 1:
Clinical and Demographic Characteristics for Postmenopausal Women with Low versus Normal Bone Strength
| Characteristic | Low Bone Strength (n=262) | Normal (n=419) | p-value |
|---|---|---|---|
| Age | 69.0 ± 8.2 | 65.0 ± 7.1 | <0.001* |
| Race: Caucasian | 200 (76.3) | 344 (82.1) | 0.068† |
| Obese | 57 (21.8) | 104 (24.8) | 0.360† |
| Pulmonary Disease (Asthma, COPD) | 37 (14.1) | 50 (11.9) | 0.405† |
| Chronic Steroid use | 17 (6.5) | 19 (4.5) | 0.268† |
| Hormonal Replacement Therapy | 8 (3.1) | 24 (5.7) | 0.109† |
| Current Smoker | 23 (8.8) | 14 (3.3) | <0.001† |
| Drinker (daily) | 1 (0.4) | 4 (1.0) | 0.654‡ |
| Vaginal Delivery | 0.251§ | ||
| 0 | 44 (18.7) | 77 (19.7) | |
| 1 | 35 (14.9) | 75 (19.2) | |
| 2+ | 156 (66.4) | 238 (61.0) | |
| BSO prior to menopause | 70 (26.7) | 115 (27.5) | 0.835† |
| Prior pelvic floor surgery | 39 (14.9) | 72 (17.2) | 0.430† |
Student’s t-test
Chi-square test of association
Fisher’s exact test
Cochran–Mantel–Haenszel test
Values reported as n (%) or mean ± standard deviation
COPD: Chronic obstructive pulmonary disease
BSO: Bilateral salpingo-oophorectomy
Table 2:
Pelvic Floor Disorders in Postmenopausal Women with Low versus Normal Bone Strength
| Combined TBS & BMD | TBS only (Bone Quality) | BMD only (Bone Quantity) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Low Bone Strength | Normal | p-value | Low TBS | Normal | p-value | Low BMD | Normal | p-value | |
| n=262 | n=419 | n=222 | n=459 | n=300 | n=381 | ||||
| Any UI | 127 (49.4) | 171 (41.4) | 0.042 | 115 (52.5) | 183 (40.6) | 0.004 | 133 (44.8) | 165 (44.2) | 0.888 |
| SUI | 97 (37.5) | 125 (30.1) | 0.047 | 87 (39.2) | 135 (29.8) | 0.015 | 102 (34.3) | 120 (31.8) | 0.476 |
| UUI | 103 (39.8) | 139 (33.3) | 0.090 | 97 (44.1) | 145 (31.8) | 0.002 | 107 (35.8) | 135 (35.8) | 0.995 |
| MUI | 77 (29.5) | 97 (23.2) | 0.068 | 72 (32.4) | 102 (22.3) | 0.005 | 80 (26.8) | 94 (24.7) | 0.550 |
| FI | 80 (34.0) | 126 (32.1) | 0.624 | 69 (34.9) | 137 (31.9) | 0.470 | 91 (34.1) | 115 (31.9) | 0.573 |
| POP | 28 (11.3) | 45 (11.3) | 0.973 | 29 (13.8) | 44 (10.1) | 0.159 | 31 (11.0) | 42 (11.5) | 0.816 |
All values reported as n (%)
TBS: Trabecular bone score
BMD: Bone mineral density
UI: Urinary incontinence
SUI: Stress urinary incontinence
UUI: Urgency urinary incontinence
MUI: Mixed urinary incontinence
FI: Fecal incontinence
POP: Pelvic organ prolapse
Table 3:
Association between Pelvic Floor Disorders and Bone Strength in Postmenopausal Women
| Combined TBS & BMD* | TBS only† | BMD only‡ | ||||
|---|---|---|---|---|---|---|
| aOR | 95% CI | aOR | 95% CI | aOR | 95% CI | |
| Any UI | 1.48 | 1.05–2.10 | 1.57 | 1.08–2.28 | 1.07 | 0.76–1.50 |
| SUI | 1.53 | 1.06–2.21 | 1.46 | 0.99–2.16 | 1.16 | 0.81–1.66 |
| UUI | 1.33 | 0.93–1.90 | 1.54 | 1.05–2.26 | 1.00 | 0.70–1.43 |
| MUI | 1.45 | 1.02–2.05 | 1.55 | 1.06–2.25 | 1.05 | 0.74–1.48 |
| FI | 1.13 | 0.78–1.65 | 1.10 | 0.73–1.65 | 1.11 | 0.76–1.61 |
| POP | 0.95 | 0.55–1.65 | 1.16 | 0.65–2.04 | 1.04 | 0.60–1.78 |
Bolded values indicate statistical significance.
Models adjusted for covariates potentially influencing the outcomes based on clinical risk factors and bivariate analyses
Adjusted for age (centered), obesity, race, hormone therapy, smoking, and vaginal delivery
Adjusted for age (centered), obesity, race, hormone therapy, smoking, vaginal delivery, pulmonary disease, and steroid use
Adjusted for age (centered), obesity, race, hormone therapy, and vaginal delivery
TBS: Trabecular bone score
BMD: Bone mineral density
UI: Urinary incontinence
SUI: Stress urinary incontinence
UUI: Urgency urinary incontinence
MUI: Mixed urinary incontinence
FI: Fecal incontinence
POP: Pelvic organ prolapse
Using the TBS only criteria, women with low bone quality had a higher rate of any UI, SUI, UUI, and MUI (all p<0.05, Table 2). Low bone quality was not associated with FI or POP symptoms. In the multivariable regression analysis, women with low bone quality was independently associated with an increased odds of having any UI (aOR: 1.57, 95%CI: 1.08–2.28), UUI (aOR: 1.54, 95%CI: 1.05–2.26), and MUI (aOR: 1.55, 95%CI: 1.06–2.25) adjusting for age, obesity, race, hormone therapy, smoking, vaginal delivery, pulmonary disease, and steroid use (Table 3), but not FI or POP. Using the bone quantity (BMD) only criteria, no difference in UI, FI, or POP was observed between groups (all p>0.05, Table 2) or on multivariable analyses (Table 3). Furthermore, upon expanding the definition of prolapse to either currently having a vaginal bulge symptom or previous history of POP surgery, no association was noted between POP and low bone strength (data not shown) using any of the skeletal integrity criteria.
Discussion
The current study demonstrated that low bone strength defined by the combination assessment (TBS and BMD) as well as low bone quality (reflecting poor microarchitecture, TBS) are associated with an increased risk of having UI but not FI or POP in postmenopausal women undergoing osteoporosis evaluation. However, osteoporosis using the traditional definition (low bone quantity, BMD) was not associated with any of the pelvic floor disorder symptoms.
Existing data based on observational studies regarding the association between pelvic floor disorders and low bone strength are inconsistent. An association has been reported between moderate to severe POP and an increased risk of fragility fractures.[14, 15, 24] However, no relationship was observed between POP symptoms and low bone mass or osteoporosis assessed by BMD via DXA scan.[16] The current literature regarding UI is also conflicting. While some studies showed no association between UI and osteoporosis, population-based studies have reported higher prevalence of UI among older women with osteoporosis.[16, 25, 26] A retrospective study also demonstrated a higher odds of having large volume urine leakage (compared to small leakage) in postmenopausal women with lower BMD (more severe osteoporosis).[16]
The inconsistent findings of the association between pelvic floor disorders and bone strength are possibly due to the limitations of the previous studies. The bone assessment used to define skeletal fragility in existing data are all based on BMD, which only measures bone quantity even though both low bone quantity and quality together adversely impact bone strength.[6] It has been shown that up to 50% of fragility fractures occur in those who are not classified as “osteoporotic” using the traditional BMD criteria.[27] In order to accurately evaluate bone strength, micro-architecture and bone quality are equally critical. To overcome the limitation of previous studies, skeletal integrity was fully evaluated in the present study by utilizing a combination of bone quality (TBS) and quantity (BMD) bone indices. Furthermore, to account for the impact of BMD medications on DXA scores, “low bone mass” was defined using BMD and/or the use of medications. Evaluating the association between pelvic floor disorder symptoms and low bone mass solely based on BMD scores (without including medication in the analysis), similar results were observed (data not shown).
Of the pelvic floor disorder symptoms, UI is the most prevalent condition, affecting at least one in three community dwelling older women in the US.[3, 4, 28] UI is considered a top health priority as it has been shown to be associated with increased hospitalization and long-term care admissions independent of multiple co-morbid conditions in the older population.[29, 30] “Geriatric Syndrome,” referring to multifactorial and highly prevalent conditions in the older population, includes incontinence, frailty, and falls, which are associated with significant morbidity and continue to be a major public health concern. Although epidemiologic studies have identified risk factors for UI, the underlying pathophysiology involved in the development of this condition is not fully understood. Given the potential similarity in the mechanisms of skeletal integrity and continence provided by intact pelvic floor support, a pathophysiologic link may exist between UI and bone strength.
The current study suggests that there may be a differential impact of bone strength on pelvic floor disorder symptoms. Pelvic floor muscle training has been shown to be an effective treatment for UI in women. More evidence exists on pelvic floor function and muscle training on urinary symptoms, compared to FI or POP. [31, 32] In addition, there is growing evidence supporting the concept of a “bone-muscle unit” where bone and muscle are recognized as interacting tissues via cross-communication.[33] Thus, the impact of bone-muscle interaction on pelvic floor function and continence mechanism may differ depending on bone strength and is possibly affected by the bony properties determined by extracellular matrix remodeling. The potential association between low bone quality (TBS) and urgency UI may be explained by this bone-muscle interaction influenced by the bony properties, as bladder function and detrusor contraction has been shown to be influenced by pelvic floor muscle activities.[34–36]
Both osteoporosis and pelvic floor disorders are highly prevalent conditions among older women. In addition to the negative impact of aging on skeletal health, the abrupt decline in ovarian function causes an accelerated rate of bone loss during the menopausal transition, contributing to a higher rate of osteoporosis in postmenopausal women. Therefore, menopausal status is one of the criteria for routine osteoporosis screening and fracture risk assessment. The current study aimed to assess the potential association linking the two conditions (bone strength and pelvic floor disorder symptoms) using an existing database containing postmenopausal women presenting for osteoporosis evaluation and where pelvic floor disorder symptoms are ascertained. Menopause is thought to be a risk factor for the development of pelvic floor disorders as pelvic organs and their surrounding muscular/connective tissue are estrogen responsive. However, the exact role of estrogen deficiency on pelvic floor function is somewhat controversial.[37] Given that all subjects are postmenopausal women, the specific impact of estrogen deficiency on pelvic floor function and bone strength could not be determined in the current study.
Limitations of the study include the retrospective methodology, thus causality cannot be inferred. Furthermore, selection bias is possible as the study population was composed of postmenopausal women presenting for bone evaluation at a tertiary referral center which has a subspecialty clinic for osteoporosis and also pelvic floor disorders, possibly inflating the prevalence of these conditions compared to the general population. Additionally, not all subjects in the existing database (41%) had TBS compatible DXA images. Another limitation is that pelvic floor disorders were assessed by subjective measures only. However, the definitions of pelvic floor disorder symptoms used in this study are comparable to existing population-based studies.[3, 4, 21].
Strengths include the robust sample size of nearly 700 postmenopausal women with detailed and robust pelvic floor symptom characterization obtained using validated pelvic floor disorder assessment measures. All bone evaluations were performed at the International Society of Clinical Densitometry (ISCD) accredited DXA facility strictly following the standard operating procedures by the ISCD guidelines. This is particularly important for study reliability and reproducibility as a high degree of variability has been reported in DXA results due to inconsistent operational and technological quality.[38, 39] The novelty of the current study includes that a validated bone quality measure (TBS) was used in addition to the traditional bone quantity assessment (BMD) to enhance the accuracy of bone strength evaluation and perhaps its sensitivity as related to pathophysiologic mechanism. However, the fracture risk stratification model using the combination of TBS and BMD to determine clinical significance requires further investigation.
Low bone strength defined by the combined TBS/BMD measures as well as poor microarchitecture (low TBS) alone were associated with an increased risk of UI but not FI or POP. Given the tremendous burden and negative impact of UI and low bone strength on QOL, data linking these two common conditions in older women would help develop and implement effective preventative strategies and treatment algorithm targeting this high-risk population. Future studies are warranted to further investigate such associations and the possible underlying pathophysiology.
Acknowledgements
The authors would like to thank Elena G Gibson, MD and Rohith S Vadlamudi, MD for their assistance with the data acquisition and manuscript preparation.
Grant Support: Partially supported by the NIH/NICHD Women’s Reproductive Health Research Career Development Program (K12 HD001258). For the remaining authors, none were declared.
Footnotes
Conflict of interest: None
Disclosures
HE Richter: Research Grants: Renovia, Allergan.
For the remaining authors, none were declared.
Contributor Information
Isuzu Meyer, Division of Urogynecology and Pelvic Reconstructive Surgery, Department of Obstetrics and Gynecology, University of Alabama at Birmingham, Alabama
Sarah L Morgan, Division of Clinical Immunology and Rheumatology, UAB Osteoporosis Prevention and Treatment Clinic, University of Alabama at Birmingham, Alabama
Alayne D Markland, Birmingham/Atlanta Geriatrics, Research, Education, and Clinical Center (GRECC) at the Birmingham VA Medical Center, Departments of Medicine, Division of Gerontology, Geriatrics and Palliative Care, University of Alabama at Birmingham, Alabama
Jeff M Szychowski, Department of Biostatistics, University of Alabama at Birmingham, Alabama
Holly E Richter, Division of Urogynecology and Pelvic Reconstructive Surgery, Department of Obstetrics and Gynecology, University of Alabama at Birmingham, Alabama
REFERENCES
- 1.Markland AD, et al. , Incidence and risk factors for fecal incontinence in black and white older adults: a population-based study. J Am Geriatr Soc, 2010. 58(7): p. 1341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markland AD, et al. , Prevalence and trends of urinary incontinence in adults in the United States, 2001 to 2008. J Urol, 2011. 186(2): p. 589–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nygaard I, et al. , Prevalence of symptomatic pelvic floor disorders in US women. JAMA, 2008. 300(11): p. 1311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu JM, et al. , Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet Gynecol, 2014. 123(1): p. 141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright NC, et al. , The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res, 2014. 29(11): p. 2520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hans D, Stenova E, and Lamy O, The Trabecular Bone Score (TBS) Complements DXA and the FRAX as a Fracture Risk Assessment Tool in Routine Clinical Practice. Curr Osteoporos Rep, 2017. 15(6): p. 521–531. [DOI] [PubMed] [Google Scholar]
- 7.Kanis JA, Assessment of osteoporosis at the primary health-care level. Technical report. World Health Organization Collaborating Centre for Metabolic Bone Diseases. UK: University of Sheffield, 2007. [Google Scholar]
- 8.Ashton-Miller JA and DeLancey JO, Functional anatomy of the female pelvic floor. Ann N Y Acad Sci, 2007. 1101: p. 266–96. [DOI] [PubMed] [Google Scholar]
- 9.Edwall L, Carlstrom K, and Fianu Jonasson A, Markers of collagen synthesis and degradation in urogenital tissue and serum from women with and without uterovaginal prolapse. Mol Hum Reprod, 2008. 14(3): p. 193–7. [DOI] [PubMed] [Google Scholar]
- 10.Chen B and Yeh J, Alterations in connective tissue metabolism in stress incontinence and prolapse. J Urol, 2011. 186(5): p. 1768–72. [DOI] [PubMed] [Google Scholar]
- 11.Campeau L, et al. , Pelvic floor disorders: linking genetic risk factors to biochemical changes. BJU Int, 2011. 108(8): p. 1240–7. [DOI] [PubMed] [Google Scholar]
- 12.Lim VF, et al. , Recent studies of genetic dysfunction in pelvic organ prolapse: the role of collagen defects. Aust N Z J Obstet Gynaecol, 2014. 54(3): p. 198–205. [DOI] [PubMed] [Google Scholar]
- 13.Kerkhof MH, Hendriks L, and Brolmann HA, Changes in connective tissue in patients with pelvic organ prolapse--a review of the current literature. Int Urogynecol J Pelvic Floor Dysfunct, 2009. 20(4): p. 461–74. [DOI] [PubMed] [Google Scholar]
- 14.Pal L, et al. , Association of pelvic organ prolapse and fractures in postmenopausal women: analysis of baseline data from the Women’s Health Initiative Estrogen Plus Progestin trial. Menopause, 2008. 15(1): p. 59–66. [DOI] [PubMed] [Google Scholar]
- 15.MacLennan AH, et al. , The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. BJOG, 2000. 107(12): p. 1460–70. [DOI] [PubMed] [Google Scholar]
- 16.Richter HE, et al. , Pelvic floor symptoms and bone mineral density in women undergoing osteoporosis evaluation. Int Urogynecol J, 2013. 24(10): p. 1663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avery K, et al. , ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn, 2004. 23(4): p. 322–30. [DOI] [PubMed] [Google Scholar]
- 18.Brown JS, et al. , The sensitivity and specificity of a simple test to distinguish between urge and stress urinary incontinence. Ann Intern Med, 2006. 144(10): p. 715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rockwood TH, et al. , Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum, 1999. 42(12): p. 1525–32. [DOI] [PubMed] [Google Scholar]
- 20.Barber MD, Walters MD, and Bump RC, Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol, 2005. 193(1): p. 103–13. [DOI] [PubMed] [Google Scholar]
- 21.Barber MD, Neubauer NL, and Klein-Olarte V, Can we screen for pelvic organ prolapse without a physical examination in epidemiologic studies? Am J Obstet Gynecol, 2006. 195(4): p. 942–8. [DOI] [PubMed] [Google Scholar]
- 22.Hans D, et al. , Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom, 2011. 14(3): p. 302–12. [DOI] [PubMed] [Google Scholar]
- 23.Kanis JA, et al. , The diagnosis of osteoporosis. J Bone Miner Res, 1994. 9(8): p. 1137–41. [DOI] [PubMed] [Google Scholar]
- 24.Pal L, et al. , Increased incident hip fractures in postmenopausal women with moderate to severe pelvic organ prolapse. Menopause, 2011. 18(9): p. 967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sran MM, Prevalence of urinary incontinence in women with osteoporosis. J Obstet Gynaecol Can, 2009. 31(5): p. 434–9. [DOI] [PubMed] [Google Scholar]
- 26.Johnson TM 2nd, et al. , Self-care practices used by older men and women to manage urinary incontinence: results from the national follow-up survey on self-care and aging. J Am Geriatr Soc, 2000. 48(8): p. 894–902. [DOI] [PubMed] [Google Scholar]
- 27.Siris ES, et al. , Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med, 2004. 164(10): p. 1108–12. [DOI] [PubMed] [Google Scholar]
- 28.Wu JM, et al. , Forecasting the prevalence of pelvic floor disorders in U.S. Women: 2010 to 2050. Obstet Gynecol, 2009. 114(6): p. 1278–83. [DOI] [PubMed] [Google Scholar]
- 29.Thom DH, Haan MN, and Van Den Eeden SK, Medically recognized urinary incontinence and risks of hospitalization, nursing home admission and mortality. Age Ageing, 1997. 26(5): p. 367–74. [DOI] [PubMed] [Google Scholar]
- 30.Morrison A and Levy R, Fraction of nursing home admissions attributable to urinary incontinence. Value Health, 2006. 9(4): p. 272–4. [DOI] [PubMed] [Google Scholar]
- 31.Dumoulin C, Cacciari LP, and Hay-Smith EJC, Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev, 2018. 10: p. CD005654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Gong Y, and Wang B, The efficacy of pelvic floor muscle training for pelvic organ prolapse: a systematic review and meta-analysis. Int Urogynecol J, 2016. 27(7): p. 981–92. [DOI] [PubMed] [Google Scholar]
- 33.Isaacson J and Brotto M, Physiology of Mechanotransduction: How Do Muscle and Bone “Talk” to One Another? Clin Rev Bone Miner Metab, 2014. 12(2): p. 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shafik A, A study of the continence mechanism of the external urethral sphincter with identification of the voluntary urinary inhibition reflex. J Urol, 1999. 162(6): p. 1967–71. [DOI] [PubMed] [Google Scholar]
- 35.Shafik A and Shafik IA, Overactive bladder inhibition in response to pelvic floor muscle exercises. World J Urol, 2003. 20(6): p. 374–7. [DOI] [PubMed] [Google Scholar]
- 36.Griffiths D, et al. , Brain Mechanisms Underlying Urge Incontinence and its Response to Pelvic Floor Muscle Training. J Urol, 2015. 194(3): p. 708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bodner-Adler B, et al. , Effectiveness of hormones in postmenopausal pelvic floor dysfunction-International Urogynecology Association research and development-committee opinion. Int Urogynecol J, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morgan SL and Prater GL, Quality in dual-energy X-ray absorptiometry scans. Bone, 2017. 104: p. 13–28. [DOI] [PubMed] [Google Scholar]
- 39.Lewiecki EM and Lane NE, Common mistakes in the clinical use of bone mineral density testing. Nat Clin Pract Rheumatol, 2008. 4(12): p. 667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]