Abstract
Systolic/diastolic blood pressure of 130–139/80–89 mm Hg has been defined as stage I hypertension by the 2017 American College of Cardiology/American Heart Association (ACC/AHA) hypertension guidelines. Drug treatment is recommended for stage I hypertensive patients aged ≥65 years without cardiovascular disease (CVD) in the 2017 ACC/AHA guidelines, but not in the 2018 Chinese guidelines. However, the cost-effectiveness of drug treatment among this subgroup of Chinese patients is unclear. This study developed a microsimulation model to compare costs and effectiveness of drug treatment and non-drug treatment for the subgroup of stage I hypertensive patients over a lifetime horizon from a government affordability perspective. Event rates of mortality and cardiovascular complications were estimated from three cohorts in Chinese population. Costs and health utilities were obtained from national statistics report and published literature. The model predicted that drug treatment generated quality-adjusted life year (QALY) of 13.52 and associated with expected costs of $6825 in comparison with 13.81 and $7328 produced by non-drug treatment over a lifetime horizon among stage I hypertensive patients aged ≥65 years without CVD. At a willingness-to-pay threshold of $8836/QALY (the gross domestic product per capita in 2017), drug treatment only had a 1.8% probability of being cost-effective compared with non-drug treatment after 10000 probabilistic simulations. Sensitivity analysis of treatment costs, benefits expected from treatment, health utilities, and discount rates did not change the results. Our results suggested that drug treatment was not cost-effective compared to non-drug treatment for stage I hypertensive patients aged ≥65 years without CVD in China.
Keywords: ACC/AHA guidelines, Chinese, cost-effectiveness, drug, stage I hypertension
Graphical Abstract
INTRODUCTION
Stage I hypertension was defined as a systolic blood pressure (SBP) of 130 to 139 mm Hg or diastolic blood pressure (DBP) of 80 to 89 mm Hg in the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guidelines,1 which would markedly increase the prevalence of hypertension. Data from the US and China revealed that the number of people labeled as having hypertension would increase by 26.8% and 45.1% if the new guidelines were adopted, respectively.2 Other studies showed that the prevalence of hypertension was doubled in Mexico,3 Canada,4 Nepal,5 and India6 by the new guidelines.
However, it should be noted that the 2017 ACC/AHA guidelines did not recommend drug treatment for all stage I hypertensive patients but a subgroup of patients who had pre-existing arteriosclerotic cardiovascular disease (ASCVD), with high CVD risk, or aged ≥65 years. Conversely, the 2018 European Society of Cardiology/European Society of Hypertension (ESC/ESH) guidelines7 and the 2018 Chinese guidelines8 maintained the diagnostic threshold of hypertension at 140/90 mm Hg, and recommended drug treatment for ASCVD patients or high risk patients with BPs in the range of 130–139/80–89 mm Hg. Therefore, discrepancy existed for newly defined stage I hypertensive patients aged ≥65 years without ASCVD, where drug treatment was recommended in the 2017 ACC/AHA guidelines but not in the 2018 ESC/ESH guidelines and the 2018 Chinese guidelines.
Recently, several studies have examined the impact of the 2017 ACC/AHA guidelines on CVD events and all-cause mortality in the Chinese adults. Qi et al9 found that stage I hypertension was associated with a 78% increased risk of CVD in the age group of 34–59 years using data from the Chinese Multi-provincial Cohort. Results from the Singapore Chinese Health Study,10 as well as our previous analysis in the Shanghai Women’s Health Study (SWHS), the Shanghai Men’s Health Study (SMHS), and the Dongfeng-Tongji (DFTJ) cohort,11 consistently showed that stage I hypertension was associated with an increased risk of CVD mortality only in those <65 years and/or free of CVD, but not those older than 65 years old. Studies in other populations have reported inconsistent findings.12–15
Adopting a new diagnostic criteria for hypertension would certainly have a big impact on the health care system. A recent study reported that an estimated additional 42.7 billion US dollars of the direct medical cost would be required if the 2017 ACC/AHA guidelines were adopted in China to reach the current treatment rate of hypertension (43.4%).16 Therefore, it is critical to assess the costs and benefits of drug treatment among newly defined stage I hypertensive patients. To our knowledge, no such cost-effectiveness analysis has been conducted in Chinese patients. Thus, we developed economic models to investigate the cost-effectiveness of drug treatment versus non-drug treatment among stage I hypertensive patients aged ≥65 years without CVD using data from three large prospective cohort studies, including the SWHS, the SMHS, and the DFTJ cohort. The study aimed to provide evidence for policy makers and clinicians when weighing the pros and cons about the implementation of the 2017 ACC/AHA guidelines in Chinese population.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
This study conformed to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guidelines and checklist items.17 We develop a Markov microsimulation model based on previously published models.18,19 It estimates the incremental cost-effectiveness ratio (ICER), with the outcomes expressed as quality-adjusted life years (QALYs), between drug treatment and non-drug treatment for stage I hypertensive patients aged ≥65 years without CVD from a government affordability perspective. The ICER less than the gross domestic product (GDP) per capita was considered highly cost-effective. This value was $8836 in China in 2017 (US$1.00=6.75RMB).20 The model development and analyses were performed using TreeAge Pro Suite 2019 (TreeAge Software, Inc, Williamstown, Mass).
Time horizon
The starting age was 65 years for the patients. To quantify lifetime benefits of drug treatment, we simulated all adults were followed until death or age 100 years. Hence, the lifetime horizon was set as 35 years. The average life expectancy is approximately 77 years in Chinese,20 so we also simulated a 12-year time horizon as well as 25-year time horizon.
Model structure
We simulated two Markov models: one that adopted the 2017 ACC/AHA guidelines and the other one adopted the 2018 Chinese guidelines (Figure 1 and Figure S1). We modelled the following distinct health states: (1) stage I hypertension; (2) stage II hypertension, which was defined as SBP/DBP ≥140/90 mm Hg or having been clinically diagnosed with hypertension or having received any antihypertensive medications; (3) stroke; (4) coronary artery disease (CAD); (5) post-stroke; (6) post-CAD; (7) death. Patients with stage I hypertension can maintain the disease status, progress to stage II hypertension, suffer a stroke/CAD, or die directly. Stroke/CAD patients may move to a chronic health state (post-stroke/post-CAD), suffer a recurrent stroke/CAD, be complicated by CAD/stroke, or die directly. The model cycle length was 1 year.
Figure 1.
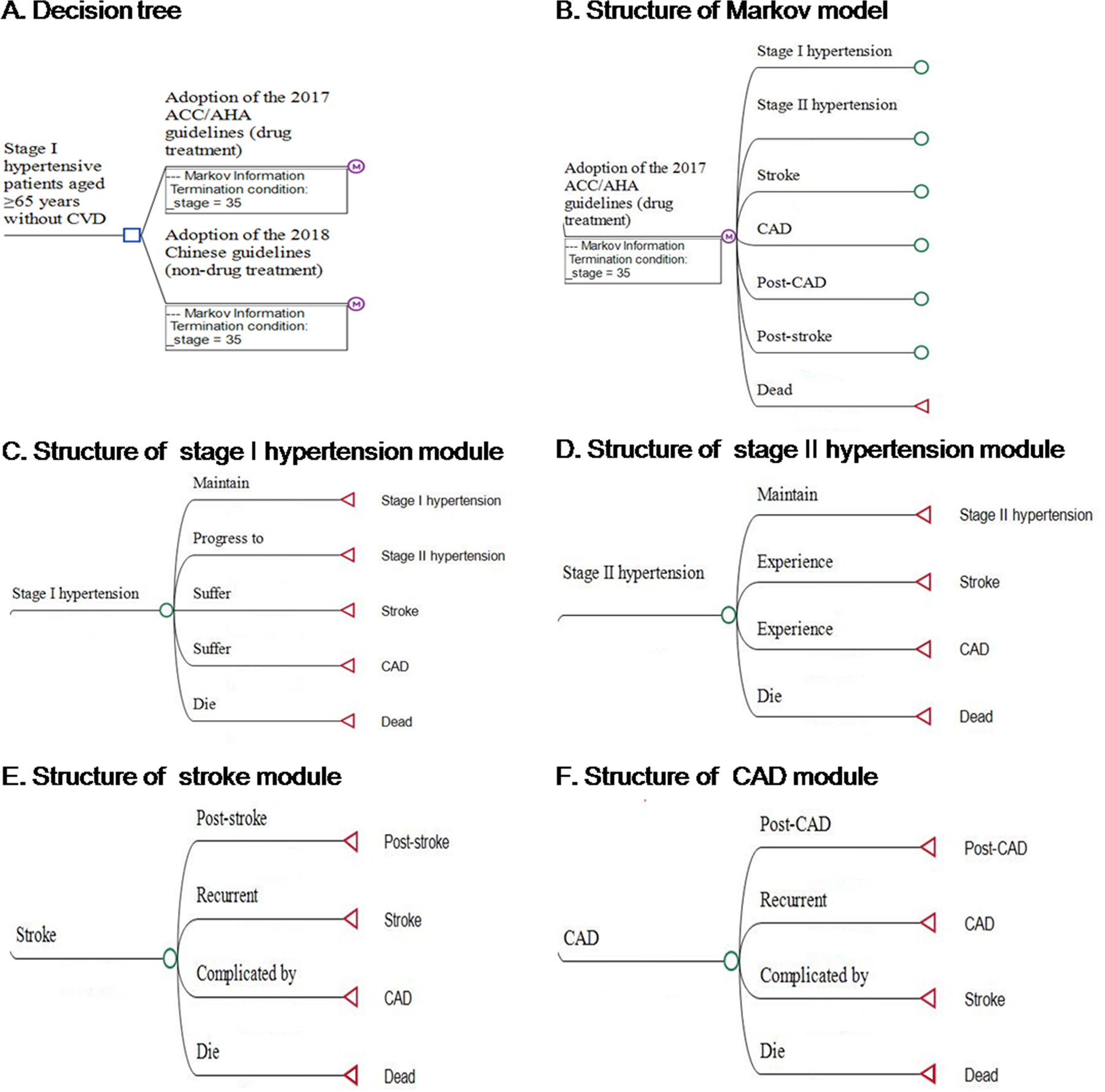
Structure of the hypertension simulation model. A, Decision tree. B. Structure of Markov model. C, Structure of stage I hypertension module. D, Structure of stage II hypertension module. E, Structure of stroke module. F, Structure of CAD module. ACC/AHA indicates American College of Cardiology/American Heart Association; CAD, coronary artery disease; and CVD, cardiovascular disease.
Model population
All-cause mortality and event rates for stroke and CAD were derived from the three Chinese cohorts (the SWHS, SMHS, and DFTJ cohort).21–23 Briefly, the SWHS recruited 74940 women aged 40–70 years from 1996 to 2000 (while the BPs were measured since 2000–2002 follow-up cycle), and the SMHS recruited 61478 men aged 40–74 years from 2002 to 2006, from eight urban neighborhood communities in Shanghai, China. For the SWHS/SMHS, participants were followed with a combination of annual linkage to Shanghai Vital Statistics Registries and home visits every 2–4 years. The DFTJ cohort recruited 27009 retired employees in Dongfeng Motor Corporation (DMC) in 2008 and was followed up for every 5 years. In the DFTJ cohort, health service use, disease incidence and mortality for participants were tracked with the DMC medical insurance system and electronic medical records in the DMC hospitals. After excluding 8305 participants with missing information of baseline BP, 715 participants with missing or invalid data on censor date, and 23483 participants with pre-existing CVD or cancer, a total of 130924 participants were included in the current analysis. A flow chart of sample selection is shown in the Figure S2, and baseline characteristics of study participants as well as comparisons of those with and without baseline BP information are shown in the Tables S1–S2.
The incident events were coded according to the International Classification of Diseases, 9th reversion (ICD-9) for the SWHS/SMHS and the International Classification of Diseases, 10th reversion (ICD-10) for the DFTJ cohort. Stroke was defined by ICD-9 codes 430–438 or ICD-10 I60-I69. CAD included myocardial infarction (ICD-9 410, 412 or ICD-10 I21, I22), angina and other coronary heart disease (ICD-9 411, 413 and 414, or ICD-10 I20, I23-I25).
We supposed that the BP of stage I hypertensive patients in the treatment group would reduce to 120–130/<80 mm Hg as recommended by the guidelines, while the BP of those in control group would remain at 130–140/80–89 mm Hg. In each cohort, person years for each participant were counted from baseline survey until date of loss-to-follow-up, date of death or December 31, 2014 (the SWHS/SMHS) and December 31, 2016 (the DFTJ cohort), whichever came first. The overall event rates were calculated by meta-analyses using random-effects model in the three cohorts (Figures S3–S5). The recurrence rates of stroke and CAD, as well as relative risk (RR) reductions on recurrence events standardized for 10 mm Hg reduction in SBP were derived from published literature.24–29 Event rates are summarized in the Table 1 and Table S3.
Table 1.
Selected Input Value for the Cost-Effectiveness Model
| Data input | Stage I hypertensive patients aged ≥65 years without CVD | Stage II hypertensive patients aged ≥65 years without CVD | |
|---|---|---|---|
| Non-drug treatment | Drug treatment | ||
| First cardiovascular disease event or death among participants with stage I hypertension or stage II hypertension (rate/person-year) | |||
| Stroke incidence* | 0.013 | 0.012 | 0.020 |
| CAD incidence* | 0.016 | 0.014 | 0.023 |
| All-cause mortality* | 0.016 | 0.018 | 0.020 |
| Second cardiovascular disease event or death among participants with stage I hypertension (rate/person-year) | |||
| CAD incidence after stroke* | 0.030 | 0.021 | - |
| One-year recurrent stroke24 | 0.170 | 0.112 | - |
| Long-term recurrent Stroke25 | 0.069 | 0.045 | - |
| All-cause mortality after stroke* | 0.041 | 0.047 | - |
| Stroke incidence after CAD* | 0.024 | 0.012 | - |
| One-year recurrent CAD26 | 0.090 | 0.068 | - |
| Long-term recurrent CAD27 | 0.030 | 0.023 | - |
| All-cause mortality after CAD* | 0.029 | 0.037 | - |
| Frequency of progression to stage II hypertension* | 0.150 | 0.148 | - |
| Effect of drug treatment | |||
| RR reduction of recurrent stroke28 | 0.37 (0.20–0.50)† | ||
| RR reduction of recurrent CAD29 | 0.32 (0.20–0.42)† | ||
| Cost ($)‡ | |||
| Hypertension screening or monitoring visit costs30 | 2.44 (2.15–2.55)† | ||
| Average antihypertensive drug costs of 1.0 standard dose per year31 | 88.92 (51.24–266.76)† | ||
| Annual cost for health management of hypertension32 | 18.12 | ||
| Annual cost for stroke20,34,35 | 3017.58 (954.07–8977.11)† for the first year, 1416.73 (299.24–4250.19)† for the subsequent years | ||
| Annual cost for CAD20,36 | 4374.54 (1285.49–12916.95)† for the first year, 397.69 (130.47–1174.26)† for the subsequent years | ||
| Quality of life weights (health utilities) | |||
| Hypertension37–39 | 0.90 (0.79–0.95)† | ||
| Stroke18,38,39 | 0.63 (0.26–0.89)†; post 0.65 (0.46–0.82)† | ||
| CAD39,40 | 0.76 (0.50–0.89)†; post 0.88 (0.67–0.94)† | ||
| Death | 0 | ||
| Discount rate | 3% (0%−5%)† | ||
CVD indicates cardiovascular disease; CAD, coronary artery disease; and RR, relative risk.
The values inside the brackets represent ranges for sensitivity analyses.
The costs were inflated to the 2017 price level using the average rate of inflation in China from 2010 to 2017 and converted to US dollars (US$1.00=6.75RMB).
means data is not required.
Costs
Costs data were obtained from the China Health Statistics Yearbook report and published literature. The average total cost included direct/indirect medical costs. For costs of stage I hypertension, it included screening and/or monitoring visit costs,30 antihypertensive drug costs,31 and health management costs.32 We calculated average antihypertensive drug costs of 1.0 standard dose per year, using the annual cost of 62 antihypertensive medications and the prescription frequency for each medication.31 For each standard-dose medication, the reduction in BP was calculated on the basis of the pretreatment BP. As observed in a meta-analysis of 354 trials,33 the average reduction was 9 mm Hg SBP at 1.0 standard dose drug treatment when the pretreatment SBP was in the range of 130–139 mm Hg. To achieve a 10-mm Hg reduction in SBP, we assumed a 10% increase in the antihypertensive drug costs. For costs of stroke/CAD, it included the costs of hospitalized patients with stroke/CAD and average annual costs for post-stroke/post-CAD management. Annual hospitalized costs for stroke and CAD were extracted from China Health Statistics Yearbook 2017.20 Annual costs for post-stroke/post-CAD were estimated based on data from published literature.34–36 The costs were inflated to the 2017 price level using the average rate of inflation in China and converted to US dollars. Cost details are summarized in the Table 1 and Table S3. All costs were discounted at 3% annually.
QALY and health utilities
We derived utility values of hypertension, stroke, CAD, post-stroke, and post-CAD from published literature based on the European Quality of Life-5 Dimensions Questionnaire (EQ-5D) and calculated the corresponding mean values by meta-analyses using random-effects model (Table 1 and Table S3).18,37–40 QALY was calculated by multiplying the time duration in a certain health state by the utility value associated with that state. The QALYs after 1 year was discounted at an annual rate of 3%.
Main analysis
The estimated values of model parameters combined with the assumptions of costs and effectiveness made above were used to calculate the ICER between drug treatment and non-drug treatment over the 12-year, 25-year, and lifetime horizon.
Sensitivity analyses
The impact of uncertainty around the model’s parameters on the cost-effectiveness results was assessed using both one-way and probabilistic sensitivity analysis (PSA). First, we conducted one-way sensitivity analyses, in which treatment effect, cost, and utility parameters changed over defined ranges or 95% confidence interval (CI), as described in the Table 1 and Table S3. Second, to assess how sensitive the results were to variations in simultaneous changes of several variables, we conducted a PSA with a set of 10000 produced results characterized the probability distributions of outcomes resulting from the uncertainty around the input parameters. We assumed beta distributions for clinical events rates, transition probabilities, and health utilities; a gamma distribution for costs; and triangular distributions were used where appropriate (Table S4). Third, the cost-effectiveness acceptability curve was constructed to assess the probability of cost-effectiveness at a willingness-to-pay (WTP) thresholds of 1 to 3 times per capita GDP.
Results
Main analysis
The model predicted that drug treatment generated QALY of 13.52 and associated with expected costs of $6825 in comparison with 13.81 and $7328 produced by non-drug treatment over a lifetime horizon. These resulted in an ICER of $1720/QALY gained for non-drug treatment over a lifetime horizon (Table 2). Shortening the time horizon to 25 years and 12 years resulted in lower ICERs for non-drug treatment.
Table 2.
Results for non-drug versus drug treatment among stage I hypertensive patients aged ≥65 years without CVD
| Outcome | 12-year time horizon | 25-year time horizon | lifetime horizon | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-drug treatment | Drug treatment | Changes | Non-drug treatment | Drug treatment | Changes | Non-drug treatment | Drug treatment | Changes | |
| Undiscounted QALYs | 9.37 | 9.30 | 0.07 | 16.55 | 16.25 | 0.30 | 20.43 | 19.88 | 0.55 |
| Undiscounted costs ($) | 3360.32 | 3502.14 | −141.82 | 8665.20 | 8133.20 | 532.00 | 12294.12 | 11096.85 | 1197.27 |
| Costs by hypertension | 513.11 | 975.72 | −462.61 | 971.47 | 1472.71 | −501.24 | 1147.38 | 1652.21 | −504.83 |
| Costs by stroke | 1529.35 | 1365.25 | 164.10 | 4503.27 | 3890.81 | 612.46 | 6675.41 | 5615.58 | 1059.83 |
| Costs by CAD | 1317.86 | 1161.17 | 156.69 | 3190.46 | 2769.68 | 420.78 | 4471.33 | 3829.06 | 642.27 |
| Undiscounted ICER | Cost-saving | $1779.46/QALY | $2162.42/QALY | ||||||
| Discounted QALYs | 7.97 | 7.91 | 0.06 | 12.20 | 12.01 | 0.19 | 13.81 | 13.52 | 0.29 |
| Discounted cost ($) | 2729.79 | 2887.47 | −157.68 | 5820.53 | 5592.71 | 227.82 | 7327.85 | 6825.45 | 502.40 |
| Costs by hypertension | 420.88 | 837.87 | −416.99 | 693.87 | 1135.05 | −441.18 | 767.76 | 1210.49 | −442.73 |
| Costs by stroke | 1228.64 | 1097.80 | 130.84 | 2954.80 | 2567.00 | 387.80 | 3856.30 | 3283.97 | 572.33 |
| Costs by CAD | 1080.27 | 951.80 | 128.47 | 2171.86 | 1890.66 | 281.20 | 2703.79 | 2330.99 | 372.80 |
| Discount ICER | Cost-saving | $1216.58/QALY | $1720.29/QALY | ||||||
CVD indicates cardiovascular disease; CAD, coronary artery disease; ICER, incremental cost-effectiveness ratio; and QALY, quality-adjusted life-year.
US$1.00=6.75RMB; The GDP per capita of China in 2017 was reported to be $8836 from the World Bank report.
Sensitivity analyses
The results of one-way sensitivity analyses are shown in the Figure S6 and Table S5. Sensitivity analysis of treatment costs, benefits expected from treatment, health utilities, and discount rates did not change the ranking of the ICERs. The input parameters that had significant impacts on the ICERs were the CAD incidence and all-cause mortality rate among stage I hypertensive patients in non-drug treatment group. When modeling the CAD incidence and all-cause mortality rate as the upper limit of 95%CI (4.3% and 2.1%) in non-drug treatment group, the ICER would be $13169/QALY and $14299/QALY (>$8836/QALY), respectively. However, when modeling the same CAD incidence or all-cause mortality rate among stage I hypertensive patients in drug and non-drug treatment group, the ICER would be $1454/QALY or $2598/QALY (<$8836/QALY), respectively. Only when the two groups had the same all-cause mortality rates of stage I hypertension, stroke, and CAD, drug treatment was cost-effective as it generated higher QALY gains and lower costs (Table S6).
The cost-effectiveness acceptability curve and cost-effectiveness scatter plots of non-drug treatment versus drug treatment is presented in the Figure 2. At a WTP threshold of $8836/QALY, drug treatment had a 1.8% probability of being cost-effective over the lifetime horizon. At a WTP threshold of $26508/QALY, drug treatment had a 0.0% probability of being cost-effective.
Figure 2.
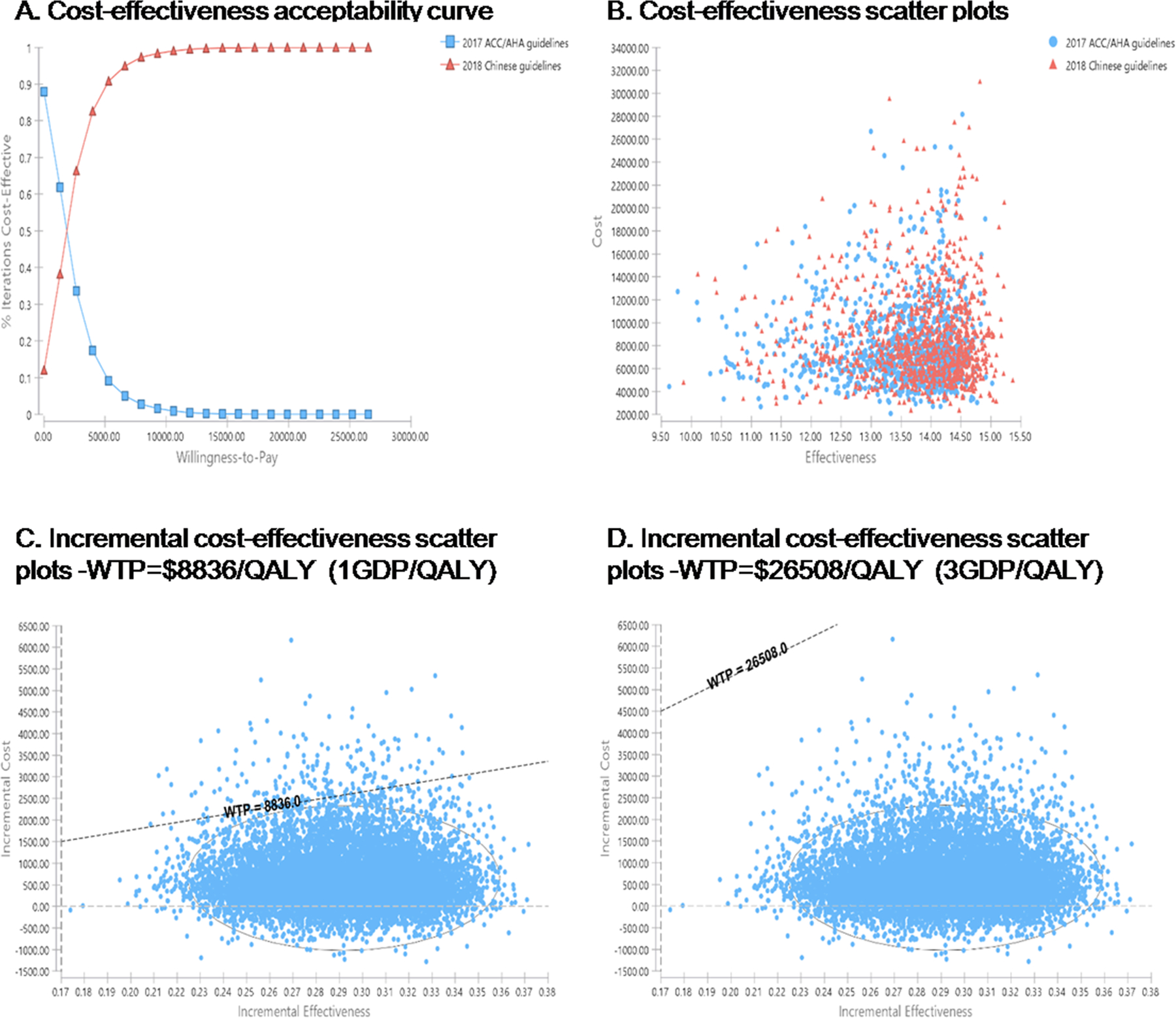
Cost-effectiveness acceptability curve and cost-effectiveness scatter plots of the 2018 Chinese guidelines versus 2017 ACC/AHA guidelines for stage I hypertensive patients. A, Cost-effectiveness acceptability curve. B, Cost-effectiveness scatter plots. C, Incremental cost-effectiveness scatter plots-WTP=$8836/QALY (1GDP/QALY). D, Incremental cost-effectiveness scatter plots-WTP=$26508/QALY (3GDP/QALY). ACC/AHA indicates American College of Cardiology/American Heart Association; QALY, quality-adjusted life year; GDP, gross domestic product; and WTP, willingness-to-pay.
Discussion
To our knowledge, this is the first study in the Chinese population investigating the cost-effectiveness of drug treatment versus non-drug treatment in the newly defined stage I hypertensive patients using local incidence events and cost data. The model-based economic analysis indicated that drug treatment was not cost-effective for stage I hypertensive patients aged ≥65 years without CVD.
After publication of the 2017 ACC/AHA guidelines, there are different views on whether it should be adopted in China and other countries. From a public health perspective, there exists an interest in managing a larger number of individuals to prevent the deleterious effects of hypertension. However, there remains a concern that lower thresholds for diagnosing hypertension and treatment goals might result in adverse events and higher healthcare costs, particularly in China with a large population and a high prevalence of hypertension. In our analysis, the use of 2017 ACC/AHA guidelines would labeled 30277 adults (27.07%) and 4085 adults (21.41%) as having stage I hypertension in the SWHS/SMHS and DFTJ cohort, respectively. Among newly defined stage I hypertensive cases, 22.41% were aged ≥65 years without CVD. However, it should be noted that these patients only had a slightly higher stroke and CAD incidence, but not all-cause mortality and stroke/CAD mortality compared with those with BPs of 120–130/<80 mm Hg (Table 1). This was also the main reason that drug treatment for stage I hypertension would result in fewer QALYs than non-drug treatment. In addition, drug treatment for stage I hypertension entailed more frequent office visits, additional health management, increased antihypertensive medication use, but less costs for stroke and CAD than non-drug treatment, resulting in less total costs. Furthermore, a major concern in the management of hypertension in China is the extremely low control rate of hypertension (5.7%) even with the therapeutic target of 140/90 mm Hg.41 Given the high prevalence of hypertension and low control rate of BP, nationally integrated strategies are urgently warranted to improve the awareness and control of hypertension among patients with BP above 140/90 mm Hg.
Several studies have been conducted to explore the cost-effectiveness of hypertension treatment among Chinese adults. Gu et al19 found that giving antihypertensive treatment to all stage II and stage I hypertensive patients was projected to be cost-effective compared with giving antihypertensive treatment to stage II and CVD patients alone, with an ICER of $13000/QALY over a lifetime. However, stage I hypertension was defined differently between that study and ours (SBP/DBP of 140–159/90–99 mm Hg vs. SBP/DBP of 130–139/80–89 mm Hg). Another study found that intensive hypertension control (target, SBP <133 mm Hg) would be more cost-effective than standard hypertension control (target, SBP <140 mm Hg), with an ICER of $1167/QALY over 10 years.42 Similar results have been found in an American study, which indicated that intensive hypertension control (target, SBP <120 mm Hg) was cost-effective compared with standard hypertension control (target, SBP <140 mm Hg), with an ICER of $28000/QALY over a lifetime.18
Inconsistent with our results, Chen et al34 found that drug treatment was cost-effective for prehypertension patients (130–139/85–89 mm Hg) compared with placebo treatment, with an ICER of $12994/QALY over a lifetime. The explanation for the inconsistence may be that the transition probability, treatment effect, and cost variables varied across studies. Specially, the incidence of CVD and hypertension in that study was calculated by prediction models using data from a cross-sectional study in Nanjing. In addition, due to lacking of relevant data on CVD events and mortality, they hypothesized no effect on CVD risk by drug treatment. However, our data showed that stage I hypertension had a slightly higher CVD risk compared with BP 120–130/<80 mm Hg and the model was very sensitive to the incidence and mortality of CVD. In addition, we evaluated the average antihypertensive drug costs of 1.0 standard dose per year from a nationwide cross-sectional survey,31 and added costs of hypertension screening and health management in the analyses.30,32
The study strength lies in the use of three well-established cohorts to derive relevant parameters. Several limitations need to be acknowledged. First, our model only captured a limited number of health states, which might have underestimated the benefits or harms of drug treatment for those patients. Second, as there were no reports discussing the medication compliance of standard dose drug treatment among hypertensive patients in Chinese population, its potential impact on our result may deserve more attentions. Third, the recurrence rates and RR reductions were derived from published literature, in which some participants were aged <65 years and the length of follow-up varied across studies. As expected, the RR reductions were greater in participants aged <65 compared to those aged ≥65 years,28 resulting in the overestimate of QALYs and thus underestimate of ICERs. In addition, some evidence showed that the reduction in stroke/CAD events standardized for a reduction of 10 mm Hg in SBP after only one year of follow-up was similar to the results from the long-term trial or cohort studies.43 Therefore, the difference between the follow-up time and lifetime horizon might have marginal impact on the results. Fourth, the key inputs of the model were derived from three cohorts in China and there was inconsistency in the event rates among different cohorts. Differences in the study populations, such as age, sex, and geographic regions may be one of the major reasons for the inconsistency. Furthermore, considering the treatment costs and event transition probabilities varied by different countries and populations, our results may not be directly extrapolated to other populations. More studies are still needed to confirm our findings and reach consensus.
Perspectives
In China, drug treatment for stage I hypertensive patients aged ≥65 years without CVD based on the 2017 ACC/AHA guidelines may not be cost-effective over a lifetime horizon compared with non-drug treatment from a government affordability perspective. More studies are still needed to validate our results and provide more evidence for the decision making of implementation of the new ACC/AHA guidelines in Chinese population.
Supplementary Material
Novelty and Significance.
What’s is New?
Stage I hypertensive patients aged ≥65 years were considered as the high risk category and drug treatment was recommended in the 2017 ACC/AHA guidelines, but not in the 2018 ESC/ESH guidelines and the 2018 Chinese guidelines.
This is the first study to compare the cost-effectiveness of implementing the 2017 ACC/AHA hypertension guidelines (drug treatment) for stage I hypertensive patients aged ≥65 years without CVD with implementing the 2018 Chinese guidelines (non-drug treatment).
What is relevant?
Adopting a new diagnostic criteria for hypertension would certainly have a big impact on the health care system, particularly in China with a large population.
Recent evidence suggests that higher BP levels are associated with increased CVD risk only in young adults.
Summary:
Drug treatment for stage I hypertensive patients aged ≥65 years without CVD was reported as not a cost-effective strategies than non-drug treatment.
Policy makers and clinicians should be cautious to implement the 2017 ACC/AHA guidelines among different population.
Acknowledgements
We thank all the participants and research staff who took part in the Shanghai Women’s and Men’s Health Studies and the Dongfeng-Tongji cohort for their contributions.
Sources of Funding
A Pan was supported by the National Key Research and Development Program of China (2017YFC0907504). The DFTJ cohort was supported by the National Key Research and Development Program of China (2016YFC0900800, 2016YFC0900801, 2017YFC0907500, and 2017YFC0907501), and the Natural National Scientific Foundation of China (91643202, 81230069, and 81390542). The SWHS/SMHS cohorts were supported by grants from US National Institutes of Health (UM1 CA182910, R01 CA082729, and UM1 CA173640). D Yu was supported by Vanderbilt University Medical Center Faculty Research Scholars Program. The funding agencies were not involved in the study design, data collection and analysis, or preparation of the manuscript.
Footnotes
Disclosures
None.
References
- 1.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 2.Khera R, Lu Y, Lu J, Saxena A, Nasir K, Jiang L, Krumholz HM. Impact of 2017 ACC/AHA guidelines on prevalence of hypertension and eligibility for antihypertensive treatment in United States and China: nationally representative cross sectional study. BMJ. 2018;362:k2357. doi: 10.1136/bmj.k2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez-Rueda AJ, Olivas-Martinez A, Vega-Vega O, Fonseca-Correa JI, Correa-Rotter R. New 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. Hypertension. 2019;73:142–147. doi: 10.1161/HYPERTENSIONAHA.118.11827. [DOI] [PubMed] [Google Scholar]
- 4.Garies S, Hao S, McBrien K, Williamson T, Peng M, Khan NA, Padwal RS, Quan H, Leung AA, for Hypertension Canada’s Research and Evaluation Committee. Prevalence of hypertension, treatment, and blood pressure targets in Canada associated with the 2017 American College of Cardiology and American Heart Association Blood Pressure Guidelines. JAMA network open. 2019;2:e190406. doi: 10.1001/jamanetworkopen.2019.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kibria GMA, Swasey K, Kc A, Mirbolouk M, Sakib MN, Sharmeen A, Chadni MJ, Stafford KA. Estimated change in prevalence of hypertension in Nepal following application of the 2017 ACC/AHA Guideline. JAMA network open. 2018;1:e180606. doi: 10.1001/jamanetworkopen.2018.0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkateshmurthy NS, Geldsetzer P, Jaacks LM, Prabhakaran D. Implications of the new American College of Cardiology Guidelines for hypertension prevalence in India. JAMA Intern Med. 2018;178:1416–1418. doi: 10.1001/jamainternmed.2018.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 8.Liu LS. 2018 Chinese guidelines for the management of hypertension. Chin J Cardiovasc Med. 2019;24:24–55. [Google Scholar]
- 9.Qi Y, Han X, Zhao D, Wang W, Wang M, Sun J, Liu J, Li Y, Gao S, Hao YC, Deng QJ, Liu J. Long-term cardiovascular risk associated with stage 1 hypertension defined by the 2017 ACC/AHA Hypertension Guideline. J Am Coll Cardiol. 2018;72:1201–1210. doi: 10.1016/j.jacc.2018.06.056. [DOI] [PubMed] [Google Scholar]
- 10.Talaei M, Hosseini N, Koh AS, Yuan JM, Koh WP. Association of “elevated blood pressure” and “stage 1 Hypertension” with cardiovascular mortality among an Asian population. J Am Heart Assoc. 2018;7. doi: 10.1161/JAHA.118.008911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu N, Yang JJ, Meng RW, Pan XF, Zhang XM, He MA, Li HL, Gao YT, Xiang YB, Shu XO, Zheng W, Wu TC, Yu DX, Pan A. Associations of blood pressure categories defined by 2017 ACC/AHA guidelines with mortality in China: pooled results from three prospective cohorts. Eur J Prev Cardiol. 2020; 27:345–354. doi: 10.1177/2047487319862066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bundy JD, Mills KT, Chen J, Li C, Greenland P, He J. Estimating the association of the 2017 and 2014 hypertension guidelines with cardiovascular events and deaths in US adults: an analysis of national data. JAMA cardio. 2018;3:572–581. doi: 10.1001/jamacardio.2018.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yano Y, Reis JP, Colangelo LA, Shimbo D, Viera AJ, Allen NB, Gidding SS, Bress AP, Greenland P, Muntner P, Lloyd-Jones DM. Association of blood pressure classification in young adults using the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline with cardiovascular events later in life. JAMA. 2018;320:1774–1782. doi: 10.1001/jama.2018.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Son JS, Choi S, Kim K, Kim SM, Choi D, Lee G, Jeong SM, Park SY, Kim YY, Yun JM, Park SM. Association of blood pressure classification in Korean young adults according to the 2017 American College of Cardiology/American Heart Association Guidelines with subsequent cardiovascular disease events. JAMA. 2018;320:1783–1792. doi: 10.1001/jama.2018.16501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atasoy S, Johar H, Peters A, Ladwig KH. Association of hypertension cut-off values with 10-year cardiovascular mortality and clinical consequences: a real-world perspective from the prospective MONICA/KORA study. Eur Heart J. 2019;40:732–738. doi: 10.1093/eurheartj/ehy694. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Hao G, Wang X, Chen Z, Zhang L, Zhang Z, Hu H, Weintraub WS, Gao R; China hypertension survey investigators. Clinical outcomes and economic impact of the 2017 ACC/AHA guidelines on hypertension in China. J Clin Hypertens. 2019;21:1212–1220. doi: 10.1111/jch.13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E, CHEERS Task Force. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement. Value Health. 2013;16:e1–e5. doi: 10.1016/j.jval.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Bress AP, Bellows BK, King JB, et al. Cost-effectiveness of intensive versus standard blood-pressure control. New Engl J Med. 2017;377:745–755. doi: 10.1056/NEJMsa1616035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu DF, He J, Coxson PG, Rasmussen PW, Huang C, Thanataveerat A, Tzong KY, Xiong JY, Wang M, Zhao D, Goldman L, Moran AE. The cost-effectiveness of low-cost essential antihypertensive medicines for hypertension control in China: a modelling study. PLoS Med. 2015;12:e1001860. doi: 10.1371/journal.pmed.1001860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Ministry of Health of China. China’s health and family planning statistical yearbook 2017. Beijing: China Union Medical University; 2017. [Google Scholar]
- 21.Shu XO, Li H, Yang G, Gao J, Cai H, Takata Y, Zheng W, Xiang YB. Cohort Profile: The Shanghai Men’s Health Study. Int J Epidemiol. 2015;44:810–818. doi: 10.1093/ije/dyv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng W, Chow WH, Yang G, Jin F, Rothman N, Blair A, Li HL, Wen WQ, Ji BT, Li Q, Shu XO, Gao YT. The Shanghai Women’s Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162:1123–1131. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 23.Wang F, Zhu J, Yao P, et al. Cohort Profile: the Dongfeng-Tongji cohort study of retired workers. Int J Epidemiol. 2013;42:731–740. doi: 10.1093/ije/dys053. [DOI] [PubMed] [Google Scholar]
- 24.Wang YL, Xu J, Zhao XQ, Wang D, Wang CX, Liu LP, Wang AX, Meng X, Li H, Wang YJ. Association of hypertension with stroke recurrence depends on ischemic stroke subtype. Stroke. 2013;44:1232–1237. doi: 10.1161/STROKEAHA.111.000302. [DOI] [PubMed] [Google Scholar]
- 25.Dong W, Pan XF, Yu C, Lv J, Guo Y, Bian Z, Yang L, Chen YP, Wu TC, Chen ZM, Pan A, Li LM, China Kadoorie Biobank Collaborative Group. Self-rated health status and risk of incident stroke in 0.5 million Chinese adults: the China Kadoorie Biobank Study. J Stroke. 2018;20:247–257. doi: 10.5853/jos.2017.01732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shang P, Liu GG, Zheng X, Ho PM, Hu S, Li J, Jiang ZH, Li X, Bai XK, Gao Y, Xing C, Wang Y, Normand SL, Krumholz HM. Association between medication adherence and 1-year major cardiovascular adverse events after acute myocardial infarction in China. J Am Heart Asso. 2019;8:e011793. doi: 10.1161/JAHA.118.011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye P, Lu ZL, Du BM, Chen Z, Wu YF, Yu XH, Zhao YC, CCSPS Investigators. Effect of xuezhikang on cardiovascular events and mortality in elderly patients with a history of myocardial infarction: a subgroup analysis of elderly subjects from the China Coronary Secondary Prevention Study. J Am Geriatr Soc. 2007;55:1015–1022. doi: 10.1111/j.1532-5415.2007.01230.x. [DOI] [PubMed] [Google Scholar]
- 28.Arima H, Anderson C, Omae T, Liu L, Tzourio C, Woodward M, Macmahon S, Neal B, Rodgers A, Chalmers J; PROGRESS Collaborative Group. Perindopril-based blood pressure lowering reduces major vascular events in Asian and Western participants with cerebrovascular disease: the PROGRESS trial. J Hypertens; 2010;28:395–400. doi: 10.1097/HJH.0b013e328333b009. [DOI] [PubMed] [Google Scholar]
- 29.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet; 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 30.The World Health Organization. Choosing Interventions that are Cost-Effective (WHO-CHOICE): World Health Organization. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su M, Zhang Q, Bai X, et al. Availability, cost, and prescription patterns of antihypertensive medications in primary health care in China: a nationwide cross-sectional survey. Lancet. 2017;390:2559–2568. doi: 10.1016/S0140-6736(17)32476-5. [DOI] [PubMed] [Google Scholar]
- 32.Department of Primary Health of National Health Commission. National basic public health service project. http://www.nbphsp.org.cn/jbgw/jswd/20171017/1004.html (20 August 2019, data last accessed).
- 33.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003, 326:1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen T, Yu D, Cornelius V, Qin R, Cai Y, Jiang Z, Zhao ZZ. Potential health impact and cost-effectiveness of drug therapy for prehypertension. Int J Cardiol. 2017;240:403–408. doi: 10.1016/j.ijcard.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Wu X, Zou S, Zhu B, Shi J. The hospital costs of stroke patients in Chinese island populations: an 11-year tendency analysis. J Stroke Cerebrovasc Dis. 2015;24:988–992. doi: 10.1016/j.jstrokecerebrovasdis.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 36.Le C, Fang Y, Linxiong W, Shulan Z, Golden AR. Economic burden and cost determinants of coronary heart disease in rural southwest China: a multilevel analysis. Public health. 2015;129:68–73. doi: 10.1016/j.puhe.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Zhou Z, Gao J, Wang D, Zhang Q, Zhou Z, Su M, Li D. Health-related quality of life and its influencing factors for patients with hypertension: evidence from the urban and rural areas of Shaanxi Province, China. BMC Health Serv Res. 2016;16:277. doi: 10.1186/s12913-016-1536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan CW, Cong XL, Zhou HJ, Wang XZ, Sun HP, Xu Y, Wang P. Evaluating health-related quality of life impact of chronic conditions among older adults from a rural town in Suzhou, China. Arch gerontol Geriat. 2018;76:6–11. doi: 10.1016/j.archger.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Geisler BP, Egan BM, Cohen JT, Garner AM, Akehurst RL, Esler MD, Pietzsch JB. Cost-effectiveness and clinical effectiveness of catheter-based renal denervation for resistant hypertension. J Am Coll Cardiol. 2012;60:1271–1277. doi: 10.1016/j.jacc.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Wu YQ, Tang X, Li N, He L, Cao Y, Chen DF, Hu YH. Profile and correlates of health related quality of life in Chinese patients with coronary heart disease. Chinese medical journal. 2015;128:1853–1861. doi: 10.4103/0366-6999.160486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu J, Lu Y, Wang X, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from 1.7 million adults in a population-based screening study (China PEACE Million Persons Project). Lancet. 2017;390:2549–2558. doi: 10.1016/S0140-6736(17)32478-9. [DOI] [PubMed] [Google Scholar]
- 42.Xie X, He T, Kang J, Siscovick DS, Li Y, Pagan JA. Cost-effectiveness analysis of intensive hypertension control in China. Prev Med. 2018;111:110–114. doi: 10.1016/j.ypmed.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 43.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


