Abstract
Introduction
Sleep disorders ultimately result in sleep deficiency and poor-quality adversely impacts the immune system, glucose metabolism, body weight control, cardiovascular and cerebrovascular function, cognitive function, psychological stability, work productivity, quality of life, and social safety. Sleep disorders are very common among the elderly and are often comorbid with other diseases such as dementia, and further accelerating the underlying neurodegenerative processes. Initial studies have not clearly revealed the relationship between sleep disorders and dementia. Nonetheless, recent findings have suggested that insomnia and obstructive sleep apnea (OSA) are closely associated with dementia and perhaps they could be good predictors of occurrence of dementia and optimal treatments for sleep deficiencies may prevent or delay the onset dementia.
Methods
Here, we conducted a systematic review based on the criteria of predictive, preventive, and personalized medicine on the association of dementia in elderlies with sleep disorder, namely insomnia and OSA. We included 7432 studies and analyzed a total of 14 publications after applying appropriate exclusion criteria.
Results
We found that OSA patients had a large tendency to develop and/or experience accelerations of both Alzheimer’s disease (AD) and also vascular dementia, whereas insomnia patients only develop and/or experience accelerations of AD. This may be reflected in the fact that AD and vascular dementia have similar and at the same time also different mechanisms of action. Several studies have also revealed that treating sleep disorders in elderly patients prevented or delayed the onset of dementia, mitigating the progression of symptoms in patients who already manifested dementic symptoms and even reversing neurodegeneration in particular brain areas.
Discussion
Currently, the general medical consensus has poorly addressed the role of sleep disorders in exacerbating the risk of dementia. Critically, studies such as the present one emphasizes that the treatment of sleep disorders could be one the preventive measures to evade or to improve dementia symptoms. Additionally, elderly individuals often manifest different sleep deficiency symptoms than younger ones. Given this, an improved age-specific categorization and evaluation methods for sleep deficiency need to be implemented in diagnosing dementia in order to enable personalized assessments and treatments. Collectively, these findings may also assist to improve efforts in predictively detecting and eventually treating dementia.
Keywords: Insomnia, Obstructive sleep apnea, Dementia, Alzheimer’s disease, CPAP, Cognitive function, Predictive preventive personalized medicine
Introduction
Alterations in sleep structure, such as a shorter sleep duration, increased arousal events, and sleep fragmentation, decreased deep non-rapid eye movement (NREM) sleep which is also known as slow wave sleep (SWS); decreased rapid eye movement (REM) sleep are very often observed in elderly subjects. These age-related sleep changes may contribute to neurological changes in some subcortical brain regions, such as the brainstem ascending arousal system, thalamus, and hypothalamus, together with select cortical regions [1]. Any type of sleep disturbance is found to be highly associated with objective and subjective cognitive decline even in healthy elderlies [2]. Cognitive decline left untreated leads to mild cognitive impairment (MCI) and eventually to dementia and often manifest in elderlies. Degeneration of sleep-specific brain areas, such as the suprachiasmatic nucleus, in Alzheimer’s disease (AD) and other dementias often occurs with the presence of sleep disturbances [3] and altered sleep-wake patterns. Interactions between dementia and sleep disorders may also be bidirectional. Studies in both animal models and in humans have suggested that sleep deficiencies lead to amyloid-β (Aβ) deposits in the brain [4, 5], a key component of AD pathology. Whereas, the accumulation of Aβ in brain regions critical for sleep process is a typical pathological characteristic of AD [6]. Impairment of sleep structure is prominently exaggerated in MCI and AD patients compared to normal cognitively functioning elderlies [7–9].
Dementia in elderlies are often associated with the dynamic changes of a number of biomarkers which begins in midlife and goes through a transition over a period of 10 to 25 years prior to the manifestations of severe symptoms [10]. Sleep deficiencies may also contribute to neurodegeneration-induced neuroinflammation [11]. Furthermore, sleep disruptions can increase synaptic and neuronal activity, which in turn dysregulates brain Aβ production in both mice and humans [12].
Among the main mechanism contributing to the Aβ deposits accumulation is the lack of NREM SWS due to high occurrence of sleep fragmentations and shorter total sleep duration (J. Kent Werner et al. 2017). Glymphatic clearance of Aβ basically occurs during NREM SWS [4].
The aim of the present review was to survey literatures to determine the predictive role of the most common sleep disturbances, which are insomnia and obstructive sleep apnea (OSA), in the incidence of dementia.
In the future, preventive measurements should be used to evade or to improve dementia symptoms and additionally, elderly individuals who are undergoing different sleep disorders need personalized age-specific categorization and evaluation methods for its personalized assessments and treatments for dementia symptoms [13–16].
Terminology: sleep disorder
Depending on the field of studies, scientific societies, journals, and other aspects, there are a quite number of terminologies describing the abnormality of an optimal sleep such as sleep disorder, sleep disturbance, sleep deprivation, sleep deficiency, sleep fragmentation, dysgraphia, and many others. Ultimately however, the main concern of any type of sleep abnormalities addresses its impact on the normal or healthy structure of the sleep itself. Hence, in this review, we use the term “sleep disorder” to indicate the state of pathological defective sleep process.
Sleep disorder may include the condition where subjects spending shorter duration of sleep compared to the optimum duration needed. One of the widely known conditions of this is insomnia. Insomnia symptom profiles can be categorized as sleep onset insomnia, sleep maintenance insomnia, combination of both, and neither criterion [17]. Another is a low sleep efficiency where subjects have relatively sufficient duration of sleep, nevertheless having fragmented sleep or imbalance of sleep stages, which ultimately leading to a sleep deficiency state. Some subjects may have subjective excessive daytime sleepiness or other apparent symptoms related to sleep disorder, whereas others may not. This low efficiency sleep may be caused by several know sleep disorders such as OSA, restless leg syndrome, etc.
Method
Data sources
This systematic review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [18]. We executed a systematic search from several main databases including MEDLINE/PubMed, Scopus, and Cochrane library to sort out studies and researches reporting on the association between sleep deficiency, namely insomnia and OSA with dementia. The search keywords used are as follows.
“sleep OR insomnia OR snoring OR sleep disordered breathing OR sleep apnea OR obstructive sleep apnea OR sleep disturbances OR sleep disorder OR sleep problems OR sleep quality OR sleepiness OR somnolence” AND “dementia OR Alzheimer’s disease OR vascular dementia”.
The search keywords were combined into pairs, e.g., sleep apnea AND dementia. The search was limited to English language and publications up until April, 2020. In addition, we also included articles from the reference lists and other related articles that matched our inclusion criteria. Almost all articles are acquired in the form of PDF or Word files.
Type of studies
The inclusion criteria applied for sorting were (1) high associations between sleep disorder and dementia, (2) dementia diagnosed accordingly to international standard, (3) availability of medical records, (4) longitudinal study, and (5) sleep disorder detected by self-reported symptoms, subjective evaluation questionnaires, objective measurements of sleep variables, or sleep related clinical findings. The exclusion criteria were (1) case reports, conference abstracts, reviews, or commentaries; (2) cross-sectional study; and (3) main concern of the study is not dementia, AD, or vascular dementia. In order to avoid overlaps of study subjects, if any same target population were applied in plural publications, we adopted only the publication that used the highest number of subjects. The recruiting process of relevant publication is shown in detail in a flowchart in Fig. 1.
Fig. 1.
Flowchart PRISMA of Systematic Review in OSA, insomnia, and dementia
Depending on each study and its diagnostic formats, the diagnostic criteria of dementia was based on the National Institute of Neurological Disorders and Stroke, Alzheimer’s Disease and Related Disorder Association, International Statistical Classification of Diseases and Related Health Problems, or Diagnostic and Statistical Manual of Mental Disorders (5th edition). The diagnostic criteria of insomnia and OSA were based on International Classification of Sleep Disorder (3rd edition).
Type of study subjects
We included participants who were diagnosed with dementia, AD, cognitive impairment, or vascular dementia and also have a history of or ongoing OSA or insomnia. Limitations on clinical factors and other demographic variables such as age, sex, race, BMI, and others were not taken into account.
Results
Out of 7432 studies included and analyzed for this review, 14 studies were included in this systematic review. Three studies [19–21] found that OSA and dementia are associated but there were no studies to report the opposite (Table 1). There were six [22–27] studies reporting that insomnia and dementia are associated and four [28–31] reporting the opposite. One study [32] assessed the association of insomnia and OSA to dementia and found a high hazard ratio for both sleep disorder.
Table 1.
Longitudinal studies of insomnia and OSA as a risk of dementia
| Author | Year | Total sample | Age at baseline (year) | Follow-up duration (year) | Sleep variables | Type of sleep disturbance | Diagnosis of dementia | Main findings |
|---|---|---|---|---|---|---|---|---|
| Morgan et al. [29] | 1994 | 84 | > 65 | 4 | Self-reported insomnia | Insomnia | DSM-IIIR | Insomnia was not associated with the risk of dementia |
| Forley et al. [30] | 2001 | 2356 | 76.6 ± 3.9 | 3 | Self-reported insomnia | Insomnia | DSM-IIIR | Insomnia was not associated with the risk of dementia |
| Elwood et al. [31] | 2011 | 1986 | 55 69 | 10 | Self-reported insomnia | Insomnia | NINCDS criteria | Insomnia was not associated with incident vascular dementia |
| Osorio et al. [22] | 2011 | 346 | 75.9 ± 6.5 | 7.7 | Self-reported insomnia | Insomnia | DSM-IV and NINCDS-ADRDA | Insomnia was significantly associated with AD (OR = 2.39) |
| Yaffe et al. [19] | 2011 | 298 | 82.3 ± 3.2 | 4.7 | Objective OSA | OSA | DSM-IV | OSA was associated with risk of dementia (adjusted RR = 1.86) |
| Chen et al. [23] | 2012 | 34,158 | > 50 | 3 | ICD-9 diagnostic insomnia | Insomnia | ICD-9 | Insomnia was significantly associated with risk of dementia (HR = 2.34) |
| Jaussent et al. [31] | 2012 | 4894 | > 65 | 8 | Self-reported insomnia | Insomnia | DSM-IV | Insomnia complaints was notassociated with risk of dementia |
| Chang et al. [20] | 2013 | 8484 | > 40 | 5 | ICD-9 diagnostic sleep apnea | OSA | ICD-9 | OSA was associated with risk of dementia (Adjusted RR = 1.70) |
| Benedict et al. [24] | 2015 | 1574 | 49.6 ± 0.6 | 40 | Self-reported insomnia | Insomnia | DSM-IV and NINCDS-ADRDA | Sleep disturbances were associated with risk of dementia (HR = 1.33) |
| Tsapanou et al. [25] | 2015 | 1041 | 79.3 ± 6.4 | 3 | Self-reported insomnia | Insomnia | DSM-IIIR and NINCDS-ADRDA | Insomnia was not associated with risk of dementia |
| Yaffe et al. [32] | 2015 | 179,738 | > 55 | 14.3 | ICD-9 diagnostic insomnia and sleep apnea | Insomnia and OSA | ICD-9 | Insomnia was associated with risk of dementia (HR = 1.29). OSA was associated with risk of dementia (adjusted RR = 1.23) |
| Hung et al. [26] | 2018 | 51,734 | > 20 | 7 | ICD-9 diagnostic insomnia | Insomnia | ICD-9 | Primary insomnia had a higher risk of developing dementia than those without primary insomnia (adjusted RR = 2.14) |
| Sindi et al. [27] | 2018 | 1446 | > 50 | 3–10 | Self-reported insomnia | Insomnia | ICD-9 | Midlife insomnia and late-life terminal insomnia or long sleep duration were associated with a higher late-life dementia risk |
| Bubu et al. [21] | 2019 | 1639 | > 55 | 2.52 ± 0.51 | Self-reported OSA | OSA | DSM-IV and NINCDS-ADRDA | OSA is related to longitudinal increases in amyloid and tau burden in cognitively normal and mild cognitive impairment (MCI) OSA patients |
EDS excessive daytime sleepiness; DSM-IIIR diagnostic and statistical manual of mental disorders, 3rd edition revised; DSM-IV diagnostic and statistical manual of mental disorders, 4th edition; NINCDS-ADRDA National Institute of Neurological and Communicative Diseases and Stroke and Alzheimer’s Disease and Related Disorders Association; ICD-9 International statistical classification of disease, 9th revision; OSA obstructive sleep apnea; AD Alzheimer’s disease; OR odds ratio; RR risk ratio; HR hazard ratio
Discussion
OSA and dementia
Recent meta-analysis studies revealed that middle-aged adults with OSA also have multiple deterioration in cognitive domains. The main cognitive declines are seen in selective attention (concentration), sustained attention (vigilance), episodic and/or working memory, and executive and/or motor function. Despite all these, the domains that govern psychomotor, linguistic, and visuospatial functionality were minimally impacted [33]. Middle-aged adults (40 year old and above) with OSA were 26% more likely to develop significant cognitive decline or if remained untreated progress into dementia in 3 to 15 years [34]. Untreated OSA can worsen the vulnerability of the brain due to the gradual deterioration of its structure and functionality [35]. Consequently, in the occurrence of neurodegenerative events, the chronic apneic brain tends to be vulnerable to its clinical manifestations of dementia [36].
Since OSA and dementia often merge, several large-scale studies suggest that OSA influences the progression of dementia and may even be used as predictor for dementia at an earlier age. A longitudinal study by Sharafkhaneh et al. [37] of more than 4 million patients (average participant age: 57.6 years) examined by the Veterans Health Administration revealed that the risk for dementia was 1.18 times higher among those with an OSA diagnosis than among healthy control subjects. Furthermore, a 4.7-year follow-up study of 298 nondemented subjects aged 65 years and above by Yaffe et al. [19] revealed a 1.8 times higher risk for MCI or dementia in patients with OSA. Similarly, a 5-year prospective study by Chang et al. [20] including 1414 patients with OSA revealed a 1.7 times higher risk of dementia in patients with OSA than in healthy control subjects and 3.2 times higher risk in female subjects aged above 70 years (Table 1).
In addition to dementia, OSA may also influence the progression of cognitive disorders. A study of sleep quality in patients with dementia conducted by Aoki et al. [38] reported that patients with dementia and moderate or severe OSA had significantly worsen dementia severity than those with a lesser OSA severity. This finding was even more pronounced in patients younger than 80 years. These results suggest that OSA not only influences the progression of dementia but also adversely affects the progression of cognitive dysfunction. In all the studies stated above however, the severity of OSA and its impact on the severity of dementia was not studied in detail.
Mechanisms underlying OSA’s role in the progression of dementia
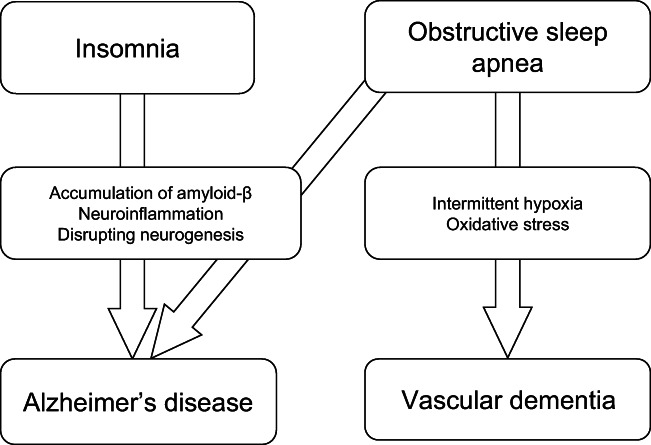
OSA may contribute to dementia via several mechanisms (Fig. 2). Deposition of Aβ, the fundamental protein implicated in dementia, may be one of the most critical factors mediating these mechanisms.
Fig. 2.
Pathological pathway of dementia caused by OSA and insomnia
A further potential mechanism involves blood vessel dysfunction. The intermittent hypoxemia caused by OSA results in cerebral blood vessel and nerve system degeneration via multiple pathways. Two of these are microvasculature degeneration [39] (caused by vascular endothelial dysfunction, chemical reactions, and blood pressure elevations) and degeneration of the white matter of the cerebrum and the hippocampus due to chronic cerebral hypoperfusion. Buratti et al. [40] evaluated vascular degeneration using carotid artery ultrasonography in AD patients. These authors revealed deteriorated cerebrovascular reactivity against the intima complex thickness and altered carbon dioxide loads in patients with OSA-related complications. They also reported aggravated vascular degeneration with OSA in a small subset of AD patients.
Dysregulated oxygen saturation has been implicated as a potential bridging mechanism between neurodegeneration and vascular degeneration or dysfunction. Using polysomnography (PSG) features to detect postmortem brain pathology, Gelber et al. [41] found that in 167 Japanese American subjects who were an average of 84 years old, sleep duration with a peripheral capillary oxygen saturation (SpO2) < 95% was associated with an increased number of microinfarcts (adjusted odds ratio = 3.88). Specifically, the authors found that individuals with more than 71.6% of their sleep achieving less than 95% SpO2 experienced 3.88 times greater levels of microinfarcts than those who experienced less than 13.1% of their sleep with less than 95% SpO2. Several additional studies have also evaluated cerebral morphological changes using MRI and found hippocampal, frontal lobe, and gray matter degeneration in OSA patients [42–44]. Collectively, these results demonstrate that brain health is negatively affected by OSA.
In addition to intermittent hypoxia, less deep sleep due to sleep fragmentation and increased numbers of arousal events also occur in OSA, posing a potentially significant risk to cognitive function. In one study [45], neurophysiological testing and fMRI were combined to determine that OSA led to declines in working memory speed, which were further attributed to decreased activity in the dorsolateral prefrontal cortex. Pointing to the supremacy of sleep disturbances and fragmentation, this relationship was found to be more highly associated with sleep fragmentation than nocturnal hypoxia.
In addition to the findings discussed above, excessive daytime sleepiness (EDS), a typical symptom of OSA, is also considered to be a risk factor for vascular dementia. This was clarified by Elwood et al. [30] in a 10-year-long prospective cohort study of 1225 male subjects wherein those with EDS were found to have 4.44 times higher risk for developing vascular dementia.
As stated above, even though the mechanisms underlying dementia are mainly attributed to vascular lesions or neural degeneration mediated by intermittent hypoxia, these may also be intricately related to a number of other factors, such as alterations in sleep structure and EDS.
Effects of treatments for OSA as the preventive measures of dementia
The most typical and widely applied treatments for OSA are nasal continuous positive airway pressure (CPAP) devices, oral appliances, and upper airway surgeries. Among these, CPAP may be the most common and most effective treatment for moderate to severe OSA in elderly patients. The greatest advantage of this particular treatment method is that an adequate treatment efficacy is attainable even in severe OSA cases by simply applying positive air pressure to prevent airway collapse. It is widely acknowledged that this does not only improve subjective and objective sleepiness but also neurocognitive functionality as well.
Many studies have shown treatment for OSA potentially has positive effects as for preventive treatments for cognitive decline, impairment, or dementia. Canessa et al. [43] investigated the correlations between cerebral MRI findings and cognitive functioning in 17 severe OSA patients and 15 normal, age-matched control subjects (average age: 44). The authors found that cognitive function declined in patients with severe OSA. In these patients, declines were also correlated with reductions in hippocampal, cerebral, left posterior parietal cortex, and right superior frontal gyrus gray matter density. Nevertheless, after 3 months of CPAP treatment, significant improvements in these patients’ cognitive functioning and gray matter density were observed.
A recent large-scale study [46] conducted by the Alzheimer’s Disease Neuroimaging Initiative in America found that OSA may influence the progression of MCI and AD. The authors also reported a high likelihood that CPAP contributes to mitigating the progression of cognitive dysfunction. In this study, 2470 subjects between the ages of 55 and 90 years were divided into three groups: MCI, AD, and normal. The onset age of MCI and AD, the presence of OSA symptoms, and CPAP treatment history for each group were reported. Patients who were diagnosed with MCI or AD and who exhibited OSA symptoms were found to first manifest MCI symptoms at an average age of 77 years, whereas patients without OSA symptoms manifested MCI symptoms at 90, on average. Meanwhile, the symptomatic manifestation of AD in patients with OSA symptoms started at an average age of 83 years, while in those without OSA symptoms, it manifested at 88. Additionally, patients who underwent CPAP treatment began to exhibit MCI and AD symptoms 10 years and 5 years, respectively, after those who did not undergo CPAP treatment.
In a randomized study of 33 OSA patients (average age: 71), a 3-month CPAP treatment improved short-term memory, working memory, selective attention, executive functionality, and fight middle frontal gyrus functional connectivity [47]. In another preliminary study, CPAP treatment on nondemented cohort of elderly subjects were found to delay MCI onset age by approximately 10 years (72 vs. 82 years old). Furthermore, the MCI onset age of OSA group was near to non-CPAP group, indicating that treatment of OSA can mitigate neurodegenerative processes [46]. In a case study on OSA patients with subjective cognitive impairment showed that CPAP treatment for 1 year normalized the CSF Aβ42 and t-tau/Aβ42 ratios and also the cognitive complains [48]. This may suggest that OSA has a potential as a reversible risk factor for dementia. On the contrary, however, in another randomized trial, 1 year of CPAP treatment showed no significant effect on cognitive function in subjects aged 65 and older, despite having significant improvement in sleepiness [49]. The absence of improvement in cognitive functionality in this study could be caused by a ceiling effect, where the subjects showed normal cognitive functions at baseline. This, which in turn can be viewed as a predictive and personalization for CPAP administration, where elderly subjects with manifestation of cognitive functionality impairment at baseline need treatment for OSA more than those with normal performance at baseline.
Given that symptoms such as daytime sleepiness and ailments like cardiovascular disease in elderly OSA patients are both pathologically and clinically distinct from those in a younger population, some may great the prospect of treating elderly OSA patients with some skepticism. Nevertheless, this review indicates that the treatments for OSA that are also found to be most promising from the perspective of cognitive health and cerebral pathology, such as CPAP, should be employed more broadly.
CPAP, however, is not well tolerated by all patients. Here, a personalized treatment for OSA must be considered. Some CPAP treatment may end in failure with no significant improvements despite the high compliance and in some cases, patients may refuse to continue using. In such cases, other alternative treatments should be considered.
The first alternative is oral appliances only if the CPAP treatment remains unsuccessful even after maximum effort. Criteria for patients who meet the best compliance for oral appliances were extensively reviewed [50]. Some oral appliances function to advance the mandible forward during sleep to maintain upper airway dilation, others hold tongue forward avoiding it to collapse onto the upper airway. This review overall concluded that in mild to severe OSA, only 52% of cases were able to control airway maintenance. Patients with severe OSA and very high BMI showed more resistance to the treatment since more fat and soft tissue in the nasal pharyngeal were not affected by these types of oral appliances. As for the overall evaluation, oral appliances showed less effectiveness than CPAP, which was greater than 80% in maintaining airway. One other aspect to consider in oral appliances are the side effects upon adherence such as mandibular joint pain, tooth pain, dry mouth, and tongue pain. All these ultimately lead to an adverse effect of sleep disorder itself where the patients experience more sleep fragmentations due to the frequent arousals caused by the side effects stated above.
Another alternative treatment is surgical interventions to widen the upper airway for patients with very low compliance or improvements after CPAP and oral appliances. One of the most performed surgical procedures for OSA is uvulopalatopharyngoplasty (UPPP), which was overall less successful as OSA treatment compared to CPAP and oral appliances. Only 33% of patients experienced the same improvement of CPAP and oral appliance treatment [51]. This surgical method is more likely to achieve successful improvements in OSA patients who did not show significant improvement with CPAP and oral appliances and with mild OSA and normal BMI. Obese patients with higher BMI have excessive adipose tissue in the lateral parapharyngeal area, the tongue, and the neck. This surgical method has its limitation where it cannot directly address the fat stored at these sites.
To prevent dementia, it is clear that early stage interventions are critical, regardless of OSA onset age. Future randomized clinical trials with larger sample size and longer duration of therapy is necessary and may think about targeting with a high-risk group (e.g., people with MCI).
Insomnia and dementia
Insomnia is a type of sleep disorder characterized by difficulty falling and/or staying asleep. Specifically, individuals with insomnia experience one or more of the following symptoms: difficulty falling asleep, frequent waking during the night with trouble going back to sleep, waking early in the morning, and fatigue upon awakening. Insomnia is one of the most common sleep complaints among elderly adults, with a prevalence of nearly 50% in those aged 65 years and older [52]. In recent years, a number of studies have suggested a high rate of insomnia and dementia comorbidity. Given this, insomnia has attracted attention as possible dementia risk factor or initial symptom. For instance, in one 3-year follow-up longitudinal study of 1041 non-dementia individuals aged over 65 years, those who developed dementia (75 subjects) reported experiencing daytime vigilance difficulties, while those unaffected by dementia (966 subjects) did not [25]. Additionally, subjects who developed dementia also reported inadequate nocturnal sleep. These results suggest a correlation between daytime somnolence and the development of dementia. Daytime somnolence and nocturnal sleep deprivation should further be considered to be risk factors for dementia and could be used as predictor for it.
Epidemiological studies have been mixed, with some showing links between insomnia and dementia and others showing none. In an Italian epidemiological survey of 750 elderly individuals aged over 65 years (664 normal subjects and 86 dementia subjects), 85% were found to have insomnia [53]. Critically, this study found no significant differences in sleep onset latency, nocturnal awakening, OSA, or restless leg syndrome rates between normal patients and those with dementia. It did report, however, that dementia patients slept significantly more during the day than did controls. Conversely, another study of 3857 nondemented individuals aged over 65 years reported a hazard ratio of 1.58 for dementia-specific mortality among those who averaged more than 9 h of daily sleep relative to those who averaged less than 9 h [54].
In particular, the association between insomnia and dementia risk has also been examined. In one such systematic review and meta-analysis, de Almondes et al. [55] identified five population-based prospective cohort studies [22–24, 29, 31] that examined this association more closely. This analysis revealed that insomnia was associated with a significant risk for all-cause dementia (risk ratio = 1.53). However, another systematic review and meta-analysis by Shi et al. [56], which examined four additional prospective studies [25, 28, 30, 32], found that insomnia was not a risk factor for all-cause dementia. Interestingly, Shi et al. also reported that insomnia had differential effects of sleep on dementia subtypes. Specifically, insomnia increased the risk for developing AD but had no effect on vascular dementia. Discrepancies in meta-analysis results may be attributable to differences in the designs of the studies and variability in participants’ subtypes of dementia (Table 1).
Mechanisms underlying insomnia role in the progression of dementia
Insomnia may contribute to dementia via several mechanisms (Fig. 2). Aβ, the fundamental protein implicated in dementia, may be one of the most critical factors mediating these mechanisms. Studies in both animal models and in humans have suggested that sleep deficiencies lead to Aβ deposits in the brain [4, 5], a key component of AD pathology. Sleep deficiencies may also contribute to neurodegeneration-induced neuroinflammation [11]. Furthermore, sleep disruptions can increase synaptic and neuronal activity, which in turn dysregulates brain Aβ production in both mice and humans [12]. As insomnia patients tend to have insufficient duration of NREM sleep, glymphatic clearance of Aβ during NREM may be slowed down.
Effects of treatments for insomnia as the preventive measures of dementia
As described above, sleep disturbances are commonly found in patients with cognitive impairments and vice-versa. Studies done to determine the effectiveness of interventions for insomnia may secondarily facilitate the prevention of dementia in high-risk populations. Thus, it is critical to evaluate whether these insomnia interventions also mitigate cognitive decline.
One common intervention used for the treatment of insomnia is melatonin supplementation, which may also have indirect effects on cognition. Serum melatonin decreases with aging and more prominently low in insomnia patients. However, the direct effects of melatonin onto sleep structure of insomnia patients and its potential onto cognitive decline remain controversial. For example, in one 4-week trial, melatonin administration of 1 mg nightly improved verbal memory and slight improvements in other cognitive function tests [57]. In another 6-month trial, administration of 2 mg of prolonged release melatonin showed positive effects on cognitive functioning and sleep maintenance in AD patients, particularly in those with insomnia comorbidity [58]. Melatonin has been demonstrated to prevent Aβ25–35-induced circadian alterations [59], Aβ-induced oxidative stress [60], and lipid peroxidation [61].
On the other hand, for instance, Xu et al. [62] summarized seven randomized controlled trials of melatonin’s effects on cognition and concluded that melatonin did in fact increase sleep duration and sleep efficiency in patients with dementia but did not offer any significant benefits for cognitive functioning.
Melatonin replacement for insomnia patients must be carefully administered due to its duration of effect in the body. Oral administration melatonin is available mainly in two types. One is immediate release and the another is prolonged release [60, 63–65]. Depending on the manifestation of insomnia symptoms on individuals, physicians need to assess the appropriate type for each patient. Some insomnia patients have high SOL but low WASO; on the other hand, others may have the opposite of this, and some have both these complexes. Another variable of melatonin administration to take into account is the dose dependency [66]. Each insomnia patient requires different dose for an optimum efficacy of sleep depending on age, BMI, sex, comorbidities, and working hours [67–69]. Additionally, sleep hygiene education is also very important to achieve a constant sleep circadian rhythm. The melatonin administration timing needs to be precisely followed since the duration required for it to initiate sleep may differ among patients. Furthermore, light exposure at night time would also interfere with the melatonin function. All these details for treatment must be carefully planned in order for an effective and reliable long-term personalized medication. A mismatch of melatonin type, dose, timing, and interactions with other drugs may cause adverse effects such as somnolence, EDS, fatigue, and other conditions.
Apart from melatonin, some additional preventive or therapeutic measures have also been investigated in the context of cognitive dysfunction or dementia and insomnia. For instance, exercise has been shown to improve sleep quality among the elderly and potentially improve cognitive functioning in subjects with or without cognitive impairments [70]. Between sleep treatments and cognitive outcomes, bright light therapy may also be a promising, low-risk treatment for sleep problems. In a recent meta-analysis by Van Maanen et al. [71], the authors concluded that while most effect sizes were only small or medium, light therapy was effective for sleep problems in general, and particularly for circadian outcomes and insomnia symptoms.
Another alternative for non-pharmacological and non-invasive method is cognitive behavioral therapy for chronic insomnia (CBT-i). This therapy successfully improves long-term sleep quality and cognitive decline in insomnia patients [72]. CBT-I intervention may be a strong candidate of treatment as insomnia it is markedly present in aging, MCI, and AD where it in turn increases the risk factor for developing AD.
Conclusions and expert recommendations
It will be of grave importance for physicians, researchers, caregivers, and patients themselves to correctly comprehend about each individual’s tendencies of sleep disorder in order to take correct preventive measures. The present review briefly described a pathogenic risk for dementia that is associated with sleep disorders. Particularly, insomnia may increase the risk for AD onset, and OSA is a risk factor for the development of both AD and vascular dementia (Fig. 2). The predictive role of the most common sleep disorders which are OSA and insomnia could be also used as a predictive standard for dementia. A general clinical consensus indicates that dementia prevention in high-risk patients involves both early stage diagnosis and other necessary patient-personalized interventions. Careful evaluation of sleep structure in these patients should also be highly considered.
Middle-aged subjects having insomnia or OSA may develop or accelerate benign pre-existing dementia in a course of approximately 15 years. Pre-clinical changes in the brain need to be assessed and personalized treatment for each patient is crucial in order to prevent or delay the onset of severe dementia.
Furthermore, to block the aggregation of cerebral Aβ and facilitate its excretion, discrete and early stage interventions for insomnia and also OSA need to be implemented. Superficial improvements in patient education around sleep hygiene and lifestyle may not be sufficient. It is likely that broader benefits can be achieved with these essential and potentially significant clinical treatments of insomnia and OSA in dementia patients and those at greatest risk.
In any event where patients make a visit to medical centers about sleep problems or cognitive decline, the physicians handling sleep problems should not only perform sleep tests but also work on a collaborative test with neurology departments to perform cognitive tests as well. The same approach should be made by neurologists regarding sleep tests.
In the case of middle-aged patients with mild symptoms, prevention measures for sleep disorder or cognitive functions must be prioritized and a long-term treatment should be advised. These patients need to be given necessary education on sleep and cognitive as well. Some patients may also be given lifestyle guidance in order to avoid further progression of the symptoms.
In the case of patients with symptoms which have progressed, a personalized treatment should be implemented carefully. As the cognitive functionality declines, it will be even challenging for the patients themselves to maintain a healthy lifestyle. Here, not only treatments but also educations for patient’s caretakers need to be thoroughly given.
Lastly, the authors would also like to strongly emphasize the crucial necessity of large scaled studies on the various types of sleep disorders for the ascertainments of the demographics of the general population in order to achieve better prediction and prevention measures on any kind of sleep disorders itself long before developing any kind of neurodegenerative disorder such as dementia.
Limitation
In this systematic review, we focused mainly on the association of dementia to insomnia and OSA; however, we did not include other sleep disorders such as sleep wake rhythm changes and hypersomnia, or other pathologies such as chronic fatigue syndrome that may cause or exacerbate dementia.
Funding information
This work was supported by a Grant-in-Aid for Scientific Research (C) (no. 1 6 K 1 1 2 5 1) from the Japanese Society for the Promotion of Science.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mander BA, Winer JR, Walker MP. Sleep and human aging HHS public access. Neuron. 2017;94:19–36. doi: 10.1016/j.neuron.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsapanou A, Vlachos GS, Cosentino S, Gu Y, Manly JJ, Brickman AM, et al. Sleep and subjective cognitive decline in cognitively healthy elderly: results from two cohorts. J Sleep Res. 2019;28:1–18. doi: 10.1111/jsr.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- 4.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s disease? Trends Neurosci. 2016;39:552–566. doi: 10.1016/j.tins.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prinz PN, Peskind ER, Vitaliano PP, Raskind MA, Eisdorfer C, Zemcuznikov HN, et al. Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J Am Geriatr Soc. 1982;30:86–92. doi: 10.1111/j.1532-5415.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 8.Hita-Yanez E, Atienza M, Gil-Neciga E, Cantero JL. Disturbed sleep patterns in elders with mild cognitive impairment: the role of memory decline and ApoE ε4 genotype. Curr Alzheimer Res. 2012;9:290–7. [DOI] [PubMed]
- 9.Westerberg CE, Mander BA, Florczak SM, Weintraub S, Mesulam MM, Zee PC, et al. Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. J Int Neuropsychol Soc. 2012;18:490–500. [DOI] [PMC free article] [PubMed]
- 10.Jack CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010:119–28. [DOI] [PMC free article] [PubMed]
- 11.Zhu B, Dong Y, Xu Z, Gompf HS, Ward SAP, Xue Z, et al. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis. 2012;48:348–55. [DOI] [PMC free article] [PubMed]
- 12.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 13.Golubnitschaja O, Costigliola V, EPMA. General Report & recommendations in predictive, preventive and personalised medicine 2012: White Paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2012;3:14. [DOI] [PMC free article] [PubMed]
- 14.Golubnitschaja O, Costigliola V. EPMA summit 2014 under the auspices of the presidency of Italy in the EU: professional statements. EPMA J. 2015;6:4. doi: 10.1186/s13167-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, et al. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7:23. doi: 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.EPMA World Congress Traditional forum in predictive, preventive and personalised medicine for multi-professional consideration and consolidation. EPMA J. 2017;8:1–54. doi: 10.1007/s13167-017-0081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pillai V, Roth T, Drake CL. The nature of stable insomnia phenotypes. Sleep. 2015;38:127–138. doi: 10.5665/sleep.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang W-P, Liu M-E, Chang W-C, Yang AC, Ku Y-C, Pai J-T, Huang HL, Tsai SJ. Sleep apnea and the risk of dementia: a population-based 5-year follow-up study in Taiwan. PLoS One. 2013;8:e78655. doi: 10.1371/journal.pone.0078655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bubu OM, Andrade AG, Umasabor-Bubu OQ, Hogan MM, Turner AD, de Leon MJ, et al. Obstructive sleep apnea, cognition and Alzheimer’s disease: a systematic review integrating three decades of multidisciplinary research. Sleep Med Rev. 2020;50:101250. [DOI] [PMC free article] [PubMed]
- 22.Osorio RS, Pirraglia E, Agüera-Ortiz LF, During EH, Sacks H, Ayappa I, Walsleben J, Mooney A, Hussain A, Glodzik L, Frangione B, Martínez-Martín P, de Leon MJ. Greater risk of Alzheimer’s disease in older adults with insomnia. J Am Geriatr Soc. 2011;59:559–562. doi: 10.1111/j.1532-5415.2010.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen P-L, Lee W-J, Sun W-Z, Oyang Y-J, Fuh J-L. Risk of dementia in patients with insomnia and long-term use of hypnotics: a population-based retrospective cohort study. PLoS One. 2012;7:e49113. doi: 10.1371/journal.pone.0049113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benedict C, Byberg L, Cedernaes J, Hogenkamp PS, Giedratis V, Kilander L, Lind L, Lannfelt L, Schiöth HB. Self-reported sleep disturbance is associated with Alzheimer’s disease risk in men. Alzheimers Dement. 2015;11:1090–1097. doi: 10.1016/j.jalz.2014.08.104. [DOI] [PubMed] [Google Scholar]
- 25.Tsapanou A, Gu Y, Manly J, Schupf N, Tang M-X, Zimmerman M, Scarmeas N, Stern Y. Daytime sleepiness and sleep inadequacy as risk factors for dementia. Dement Geriatr Cogn Dis Extra. 2015;5:286–295. doi: 10.1159/000431311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung C-M, Li Y-C, Chen H-J, Lu K, Liang C-L, Liliang P-C, et al. Risk of dementia in patients with primary insomnia: a nationwide population-based case-control study. BMC Psychiatry. 2018;18:38. [DOI] [PMC free article] [PubMed]
- 27.Sindi S, Kåreholt I, Johansson L, Skoog J, Sjöberg L, Wang HX, Johansson B, Fratiglioni L, Soininen H, Solomon A, Skoog I, Kivipelto M. Sleep disturbances and dementia risk: a multicenter study. Alzheimers Dement. 2018;14:1235–1242. doi: 10.1016/j.jalz.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Morgan K, Lilley JM. Risk factors among incident cases of dementia in a representative british sample. Int J Geriatr Psychiatry. 1994;9:11–15. [Google Scholar]
- 29.Foley D, Monjan A, Masaki K, Ross W, Havlik R, White L, Launer L. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J Am Geriatr Soc. 2001;49:1628–1632. doi: 10.1046/j.1532-5415.2001.t01-1-49271.x. [DOI] [PubMed] [Google Scholar]
- 30.Elwood PC, Bayer AJ, Fish M, Pickering J, Mitchell C, Gallacher JEJ. Sleep disturbance and daytime sleepiness predict vascular dementia. J Epidemiol Community Health. 2011;65:820–824. doi: 10.1136/jech.2009.100503. [DOI] [PubMed] [Google Scholar]
- 31.Jaussent I, Bouyer J, Ancelin M-L, Berr C, Foubert-Samier A, Ritchie K, Ohayon MM, Besset A, Dauvilliers Y. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep. 2012;35:1201–1207. doi: 10.5665/sleep.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaffe K, Nettiksimmons J, Yesavage J, Byers A. Sleep quality and risk of dementia among older male veterans. Am J Geriatr Psychiatry. 2015;23:651–654. doi: 10.1016/j.jagp.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 34.Leng Y, McEvoy CT, Allen IE, Yaffe K. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: a systematic review and meta-analysis. JAMA. 2017;74:1237–45. [DOI] [PMC free article] [PubMed]
- 35.Rosenzweig I, Glasser M, Polsek D, Leschziner GD, Williams SCR, Morrell MJ. Sleep apnoea and the brain: a complex relationship. Lancet Respir Med. 2015:404–14. [DOI] [PubMed]
- 36.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 37.Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28:1405–1411. doi: 10.1093/sleep/28.11.1405. [DOI] [PubMed] [Google Scholar]
- 38.Aoki K, Matsuo M, Takahashi M, Murakami J, Aoki Y, Aoki N, Mizumoto H, Namikawa A, Hara H, Miyagawa M, Kadotani H, Yamada N. Association of sleep-disordered breathing with decreased cognitive function among patients with dementia. J Sleep Res. 2014;23:517–523. doi: 10.1111/jsr.12167. [DOI] [PubMed] [Google Scholar]
- 39.Zimmerman ME, Aloia MS. Sleep-disordered breathing and cognition in older adults. Curr Neurol Neurosci Rep. 2012;12:537–546. doi: 10.1007/s11910-012-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buratti L, Viticchi G, Falsetti L, Cagnetti C, Luzzi S, Bartolini M, Provinciali L, Silvestrini M. Vascular impairment in Alzheimer’s disease: the role of obstructive sleep apnea. J Alzheimers Dis. 2014;38:445–453. doi: 10.3233/JAD-131046. [DOI] [PubMed] [Google Scholar]
- 41.Gelber RP, Redline S, Ross GW, Petrovitch H, Sonnen JA, Zarow C, Uyehara-Lock JH, Masaki KH, Launer LJ, White LR. Associations of brain lesions at autopsy with polysomnography features before death. Neurology. 2015;84:296–303. doi: 10.1212/WNL.0000000000001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim H, Yun C-H, Thomas RJ, Lee SH, Seo HS, Cho ER, Lee SK, Yoon DW, Suh S, Shin C. Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle-aged and older general population. Sleep. 2013;36:709–715B. doi: 10.5665/sleep.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canessa N, Castronovo V, Cappa SF, Aloia MS, Marelli S, Falini A, Alemanno F, Ferini-Strambi L. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011;183:1419–1426. doi: 10.1164/rccm.201005-0693OC. [DOI] [PubMed] [Google Scholar]
- 44.Macey PM. Is brain injury in obstructive sleep apnea reversible? Sleep. 2012;35:9–10. doi: 10.5665/sleep.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas RJ, Rosen BR, Stern CE, Weiss JW, Kwong KK. Functional imaging of working memory in obstructive sleep-disordered breathing. J Appl Physiol. 2005;98:2226–2234. doi: 10.1152/japplphysiol.01225.2004. [DOI] [PubMed] [Google Scholar]
- 46.Osorio RS, Gumb T, Pirraglia E, Lu S, Lim J, Wohlleber ME, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84:1964–1971. doi: 10.1212/WNL.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dalmases M, Solé-Padullés C, Torres M, Embid C, Nuñez MD, Martínez-Garcia MÁ, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA a randomized pilot study. Chest. 2015;148:1214–23. [DOI] [PubMed]
- 48.Liguori C, Mercuri NB, Nuccetelli M, Izzi F, Cordella A, Bernardini S, et al. Obstructive sleep apnea may induce orexinergic system and cerebral β-amyloid metabolism dysregulation: is it a further proof for Alzheimer’s disease risk? Sleep Med. 2019;56:171–6. [DOI] [PubMed]
- 49.McMillan A, Bratton DJ, Faria R, Laskawiec-Szkonter M, Griffin S, Davies RJ, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2:804–12. [DOI] [PubMed]
- 50.Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29:244–262. doi: 10.1093/sleep/29.2.244. [DOI] [PubMed] [Google Scholar]
- 51.Caples SM, Rowley JA, Prinsell JR, Pallanch JF, Elamin MB, Katz SG, Harwick JD. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396–1407. doi: 10.1093/sleep/33.10.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolkove N, Elkholy O, Baltzan M, Palayew M. Sleep and aging: 1. Sleep disorders commonly found in older people. CMAJ. 2007;176:1299–1304. doi: 10.1503/cmaj.060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merlino G, Piani A, Gigli GL, Cancelli I, Rinaldi A, Baroselli A, et al. Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: a population-based study. Sleep Med. 2010;11:372–7. [DOI] [PubMed]
- 54.Benito-León J, Louis ED, Villarejo-Galende A, Romero JP, Bermejo-Pareja F. Long sleep duration in elders without dementia increases risk of dementia mortality (NEDICES) Neurology. 2014;83:1530–1537. doi: 10.1212/WNL.0000000000000915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Almondes KM, Costa MV, Malloy-Diniz LF, Diniz BS. Insomnia and risk of dementia in older adults: systematic review and meta-analysis. J Psychiatr Res. 2016;77:109–115. doi: 10.1016/j.jpsychires.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 56.Shi L, Chen SJ, Ma MY, Bao YP, Han Y, Wang YM, et al. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev. 2018;40:4–16. [DOI] [PubMed]
- 57.Peck JS. Cognitive effects of exogenous melatonin administration in elderly persons: a pilot study. Am J Geriatr Psychiatry. 2004;12:432–6. [DOI] [PubMed]
- 58.Wade AG, Farmer M, Harari G, Fund N, Laudon M, Nir T, et al. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: a 6-month, randomized, placebo-controlled, multicenter trial. Clin Interv Aging. 2014;9:947–61. [DOI] [PMC free article] [PubMed]
- 59.Cheng X, Van Breemen RB. Mass spectrometry-based screening for inhibitors of β-amyloid protein aggregation. Anal Chem. 2005;77:7012–7015. doi: 10.1021/ac050556a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Srinivasan V, Zakaria R, Othman Z, Brzezinski A. Melatonin, insomnia and the use of melatonergic drugs. J Endocrinol Reprod. 2012;16:15–24. [Google Scholar]
- 61.Daniels WMU, Rensburg SJ, Zyl JM, Taljaard JJF. Melatonin prevents β‐amyloid‐induced lipid peroxidation. J Pineal Res. 1998;24:78–82. [DOI] [PubMed]
- 62.Xu J, Wang L-L, Dammer EB, Li C-B, Xu G, Chen S-D, Wang G. Melatonin for sleep disorders and cognition in dementia. Am J Alzheimer’s Dis Other Dementiasr. 2015;30:439–447. doi: 10.1177/1533317514568005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zisapel N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br J Pharmacol. 2018;175:3190–3199. doi: 10.1111/bph.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wade A, Zisapel N, Lemoine P. Prolonged-release melatonin for the treatment of insomnia: targeting quality of sleep and morning alertness. Aging Health. 2008;4:11–21. [Google Scholar]
- 65.Suzuki K, Miyamoto M, Hirata K. Sleep disorders in the elderly: diagnosis and management. J Gen Fam Med. 2017;18:61–71. doi: 10.1002/jgf2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vela-Bueno A, Olavarrieta-Bernardino S, Fernández-Mendoza J, Aguirre-Berrocal A. Melatonin, Sleep, and Sleep Disorders. Sleep Med Clin. 2007;2:303–312. [Google Scholar]
- 67.Lemoine P, Nir T, Laudon M, Zisapel N. Prolonged-release melatonin improves sleep quality and morning alertness in insomnia patients aged 55 years and older and has no withdrawal effects. J Sleep Res. 2007;16:372–380. doi: 10.1111/j.1365-2869.2007.00613.x. [DOI] [PubMed] [Google Scholar]
- 68.Zhdanova IV, Wurtman RJ, Regan MM, Taylor JA, Shi JP, Leclair OU. Melatonin treatment for age-related insomnia. J Clin Endocrinol Metab. 2001;86:4727–4730. doi: 10.1210/jcem.86.10.7901. [DOI] [PubMed] [Google Scholar]
- 69.McGrane IR, Corman SL. Role in therapy of melatonin for the treatment of insomnia in children and adults. Ment Health Clin. 2014;4:52–58. [Google Scholar]
- 70.Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86:876–84. [DOI] [PMC free article] [PubMed]
- 71.van Maanen A, Meijer AM, van der Heijden KB, Oort FJ. The effects of light therapy on sleep problems: a systematic review and meta-analysis. Sleep Med Rev. 2016;29:52–62. doi: 10.1016/j.smrv.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 72.Trauer JM, Qian MY, Doyle JS, Rajaratnam SMW, Cunnington D. Cognitive behavioral therapy for chronic insomnia. Ann Intern Med. 2015;163:191–205. [DOI] [PubMed]