Structured Abstract
Stress is a pervasive component of the human experience. While often considered an adversity to be ignored, chronic stress has important pathologic consequences, including cardiovascular disease (CVD). Stress also increases the prevalence and severity of several CVD risk factors, including hypertension, diabetes, and obesity. Yet even after adjustment, stress’ attributable CVD risk is similar to those risk factors, suggesting it is a particularly potent contributor. Nevertheless, there has been insufficient study of mechanisms linking stress to CVD or of methods to attenuate stress’ pathologic impact. This review covers the current concepts of how stress impacts CVD and emerging approaches to mitigate stress-attributable CVD risk.
Keywords: psychosocial stress, PET imaging, amygdalar activity, inflammation, imaging, lifestyle, cardiovascular disease
Introduction
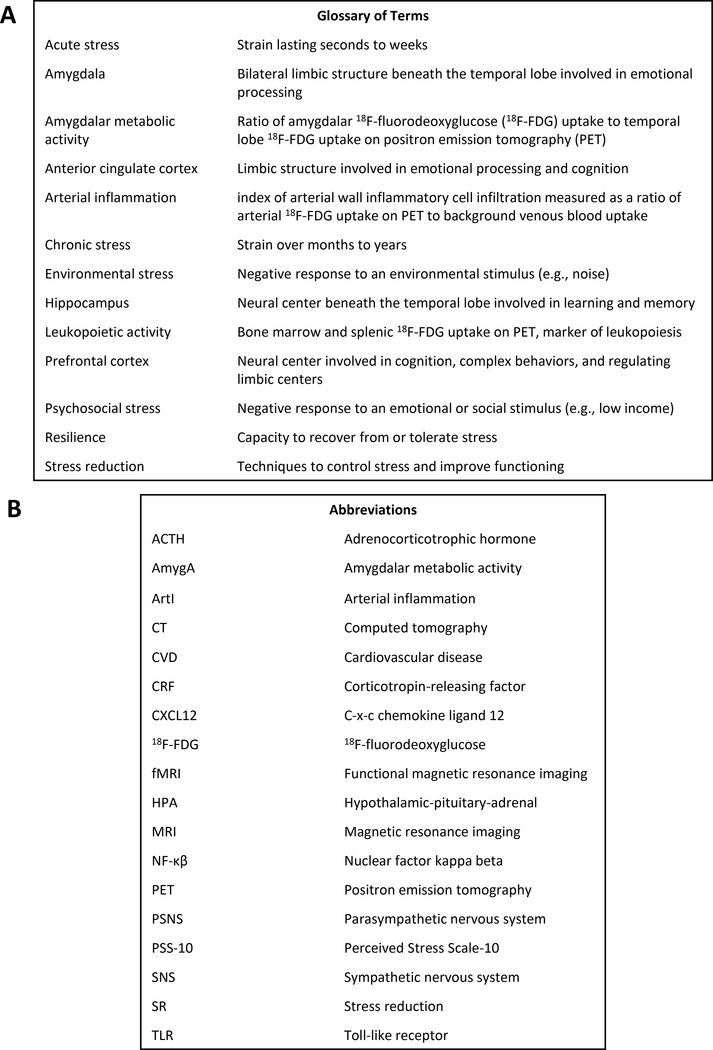
Throughout antiquity, the heart was believed to be the source of emotions. However, the link between emotions and the heart became understood to be metaphorical after 17th century neuroanatomist Thomas Willis discovered that sentiments arise from the brain. Persisting beyond Willis’ discovery was the recognition that emotional stressors precipitate physical ailments. Research into stress’ physical impact has been complicated by difficulty in measuring it objectively and in isolating it from confounding factors with which it clusters, including socioeconomic factors, health behaviors, and environmental exposures.1 However, several recent studies demonstrated that stress independently associates with the risk of several diseases, including cardiovascular disease (CVD). This review seeks to delineate the emerging links between stress and CVD from epidemiology to pathobiological mechanisms. A glossary of terms and abbreviations are provided (Figure 1A and 1B).
Figure 1:
Glossary of Terms and Abbreviations
Commonly used terms (A) and abbreviations (B) are defined.
Epidemiology
Psychological stress is a fundamental component of life, afflicting all humans with a range of frequencies and intensities. Stressors can manifest acutely or chronically and take numerous forms, including life changes (e.g., marital discord, natural disasters), adverse socioeconomic conditions (e.g., low income, high crime), and can link to chronic psychiatric conditions (e.g., depression, anxiety). Moreover, each individual’s physiologic response to a given stress exposure determines a stressor’s health consequences.2
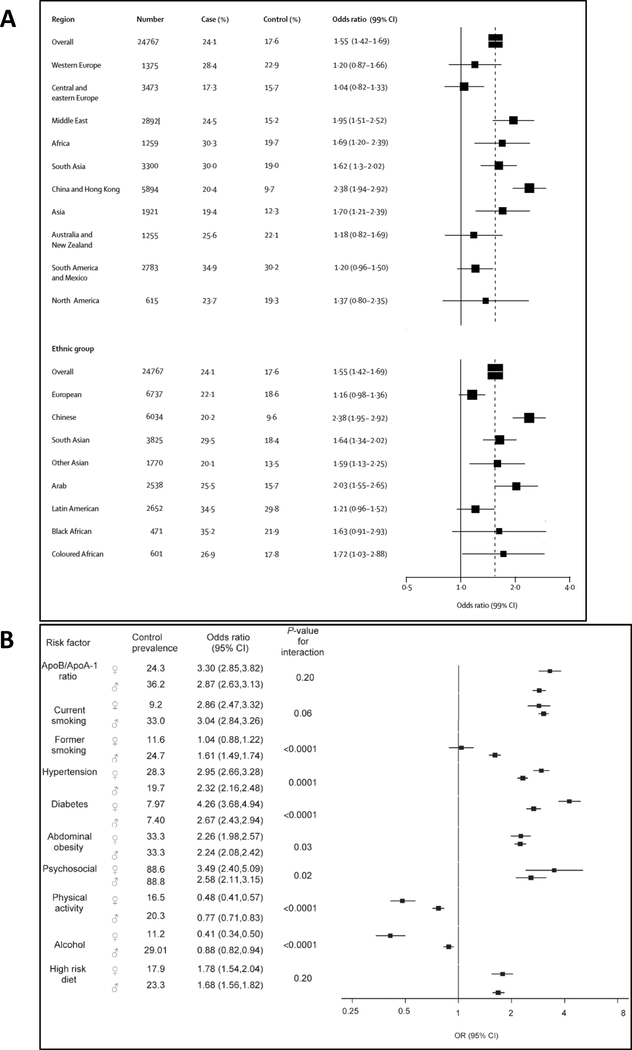
Chronic stress occurs over months to years and can result in cumulative adverse health consequences. The relationship between chronic stress and disease has been studied extensively; most studies confirm an association between stress and adverse health consequences despite heterogeneity in study design, stressor type, measurement of stress, outcomes, assessment of confounders, and subject demographics.1–3 Recent large studies have demonstrated that chronic stress associates with heightened risk for several diseases, including various cancers and CVD, leading to its recognition in clinical guidelines.1, 4, 5 The seminal INTERHEART study was a large case-control study that evaluated the relationship between modifiable risk factors and coronary disease in 24,767 patients from 52 countries.5 In that study, heightened psychosocial stress over the previous year (questionnaire-based) associated with a >2-fold increased myocardial infarction (MI) risk after adjusting for CVD risk factors (Figure 2A). Further, this association was independent of socioeconomic status and lifestyle factors and was largely consistent across geographic regions, ages, and sexes. Similarly, chronic stress is associated with increased stroke risk,6 and chronic stress-related conditions (e.g., depression) associate with heightened CVD risk and worse outcomes in CVD patients.1
Figure 2:
Stress and CVD Risk
The international INTERHEART study showed that psychosocial stress associates with a heightened CVD risk across ethnic groups (A), and the attributable risk is similar to that of major CVD risk factors (B). Reused with permission.5, 17
Similarly, acute stress, which has typically been studied following natural disasters or emotional events, associates with increased short-term CVD risk, especially among those with pre-existing risk. The 2004 Northridge earthquake triggered a nearly 3.5-fold increase in sudden cardiac death on the day it occurred.7 This finding, along with the unusually low incidence of SCD in the following week, suggests that sudden stress precipitates sudden cardiac death in at-risk individuals. Acute stress distributed over several days also increases CVD risk. During the month-long 2006 FIFA World Cup in Germany, local acute coronary syndrome rates increased 2.7-fold on days that Germany played and 6-fold on days that Germany played elimination or close games.8 Importantly, there was no increase in acute coronary syndrome on days that Germany did not play. Acute stress is also a commonly-described precipitant of Takotsubo Syndrome, an acute heart failure syndrome.9–11 While acute stress undoubtably contributes to CVD, the remainder of this review will focus on chronic stress unless specified.
Further complicating the relationship between stress and CVD is the fact that chronic stress also promotes CVD risk factors. Chronic stress triggers unhealthy eating habits and a preference for unhealthy foods.12 It also associates with hypertension and greater adiposity (independent of diet and physical activity).13, 14 Similarly, stress increases diabetes risk and worsens glycemic control among diabetics.15 Furthermore, stress-related conditions associate with a higher smoking rate.16 While this complicated interplay presents a significant challenge to evaluating the impact of stress in isolation on CVD, these risk factors alone do not explain the relationship between chronic stress and CVD. Notably, even after adjusting for them, psychosocial stress’ attributable CVD risk is similar to that for smoking, dyslipidemia, hypertension, and diabetes (Figure 2B).5, 17 Accordingly, important pathological mechanisms beyond increased risk factors link chronic stress to CVD.
Pathological Mechanisms
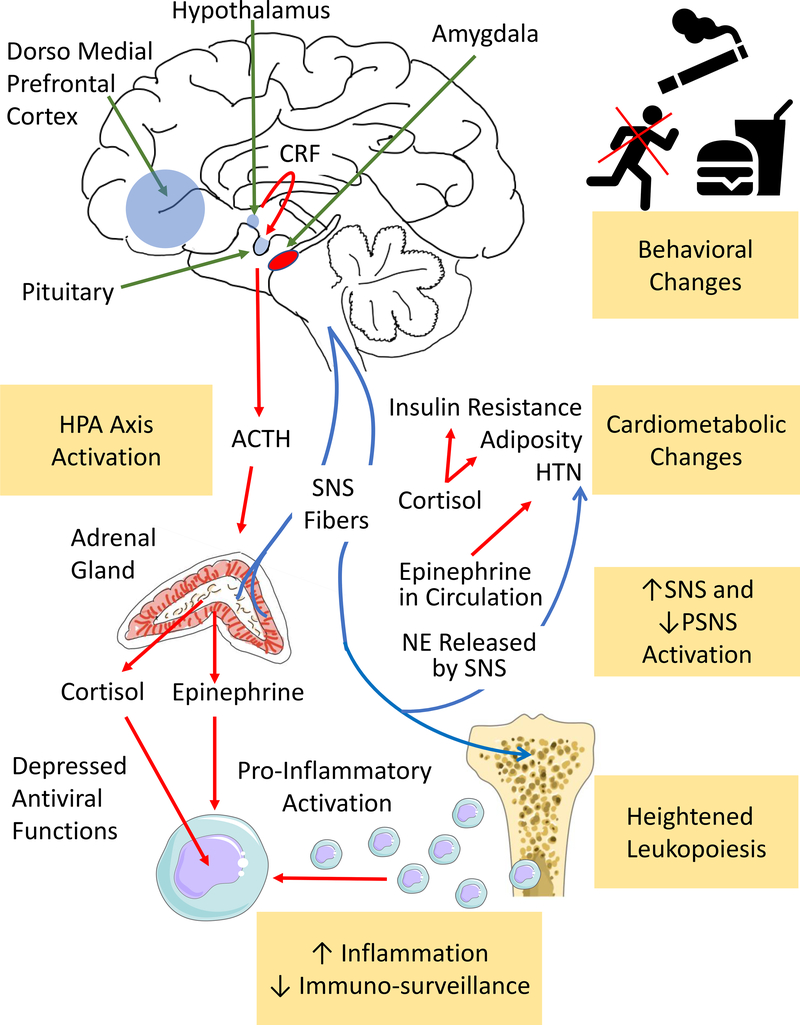
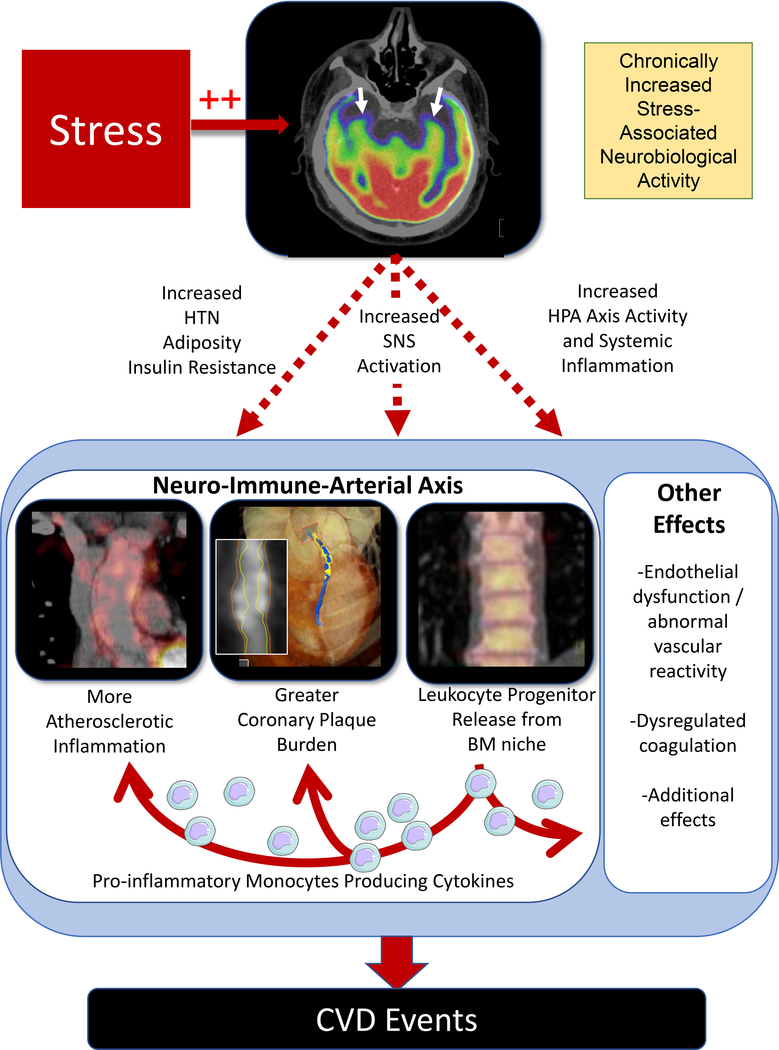
An understanding of mechanisms linking chronic stress to disease logically begins with the central nervous system (Figure 3). The brain processes a constant stream of sensory stimuli and actively selects specific stimuli for additional processing. Much of that processing is done by the brain’s salience network, a collection of centers involved in complex functions such as cognition and emotion. The limbic system serves a critical function within that network, and the amygdala plays a prominent role.18 To actuate the stress response, the amygdala, through efferent neurons, directs the hypothalamus to increase sympathetic nervous system (SNS) and decrease parasympathetic nervous system (PSNS) activity and initiate neurohormonal output through the hypothalamic-pituitary-adrenal (HPA) axis. Simultaneously, the amygdala’s efferent neurons to the periaqueductal gray alter behavior.19 The prefrontal cortex, through cognitive evaluation of stimuli, can provide regulatory signals that attenuate the stress response via so called “top-down” regulation. The prefrontal cortex’s downstream projections include the amygdala and brainstem, providing regulation of autonomic and HPA axis activity.20 Additional signaling from the anterior cingulate cortex and hippocampus contributes to stress-related neural activity. Under threatening environmental circumstances, this activity yields an adaptive response of fear and anxiety, accompanied by the “fight or flight” response.21
Figure 3:
Physiological Consequences of Stress
Chronic stress triggers several disease-promoting physiological changes, including: HPA axis activation, behavioral and cardiometabolic changes, increased SNS and decreased PSNS activity, heightened leukopoiesis, and immune dysregulation. ACTH-adrenocorticotropic hormone, CRF-corticotropin-releasing factor, HPA-hypothalamic-pituitary-adrenal, NE-norepinephrine, SNS and PNS-sympathetic and parasympathetic nervous systems, respectively.
The hypothalamic-pituitary-adrenal axis
Stress-induced hypothalamic activation triggers a cascade involving the pituitary and adrenal glands known as the HPA axis. Under stress, the hypothalamus synthesizes corticotropin-releasing factor (CRF) and vasopressin. CRF stimulates the anterior pituitary to release adrenocorticotropic hormone (ACTH), which induces the adrenal cortex to produce glucocorticoids. While glucocorticoids play an important counter-regulatory role (i.e., reducing HPA activation through negative feedback), they also contribute to increased adiposity, hypertension, and insulin resistance.22
Autonomic nervous system
Stress-induced changes in SNS and PSNS tone produce important physiological effects. SNS stimulation causes vasoconstriction and increased peripheral vascular resistance and promotes higher blood pressure and heart rate as well as lower heart rate variability. Activation of the skin sympathetic pathway precipitates perspiration and flushing. Furthermore, SNS activation of the adrenal medulla elicits the systemic release of epinephrine and norepinephrine, further heightening the sympathetic response.23
Stress-induced immune dysregulation
Another key physiological consequence of chronic stress is immune system dysregulation. Several stress-elicited pathways impact the transcription of immune response genes, including toll-like receptors (TLRs) and those governed by nuclear factor kappa beta (NF-κβ).24 Stress also increases bone marrow leukopoietic proliferation and cytokine release (e.g., interleukin-6) through beta-adrenergic signaling via increased norepinephrine and the β3-adrenergic receptor on progenitor inflammatory cells and macrophages.25–28 These newly-released inflammatory cells are primed to manifest heightened expression of pro-inflammatory immune response genes and production of pro-inflammatory cytokines in a feed-forward pattern.24 Furthermore, increased norepinephrine binds to bone marrow stromal cells via the β3-adrenergic receptor to attenuate production of C-x-c chemokine ligand 12 (CXCL12), which typically functions to retain leukocytes within the bone marrow.25, 26, 29 This stress-accentuated innate immune cell output and cytokine production potentiates atherosclerosis.25, 28, 30 Simultaneously, stress decreases anti-viral actions.23 Glucocorticoids suppress anti-viral gene programs (e.g., interferon regulatory factors), and catecholamines repress interferon regulatory factors by stimulating leukocyte adrenergic receptors, thereby increasing the risk of acquiring viral infections.31 Thus, stress-induced immune dysregulation results in greater inflammation with decreased immunosurveillance, which subsequently promote several downstream maladies.
Stress-induced vascular effects
Consequent to its effects on neurohormonal activity and inflammation, chronic stress is also associated with downstream endothelial dysfunction, altered vascular reactivity, and heightened coagulation. Additionally, the neurohormonal and inflammatory effects of stress promote greater atherosclerotic inflammation. Together these factors further potentiate atherosclerotic risk and importantly contribute to its link with CVD.2, 32
Measurement of Stress
Several methods can be used to measure stress. Psychometric questionnaires are among the most widely used tools in stress research given their ready availability, low cost, and association with adverse outcomes.6 Such questionnaires include the Perceived Stress Scale-10 (PSS-10) and the Perceived Stress Questionnaire. However, while recognized as the gold standard, questionnaires are limited because they measure solely one’s perceived emotional response to stress, which may diverge from the neuropsychiatric, behavioral, and physical manifestations of stress.
Stress’ physiologic impacts can be captured through several validated measures. For example, SNS and PSNS activities can be assessed by measuring the skin conductance response and heart rate variability, respectively. Similarly, blood or urine catecholamines provide reproducible measures of SNS activity. HPA axis activity is also used as an index of stress by measuring hormone levels (e.g., glucocorticoids, CRF, ACTH, and vasopressin) in blood, urine, hair, or saliva. Vascular reactivity also allows quantification of the cardiovascular impact of a stressor (i.e., cold pressor test, mental stress) through changes in hemodynamics and ultrasound-based measures of vascular function. While the above are useful tools to assess the physiologic response to stress, these measures are not specific for perceived stress and are impacted by numerous confounders.2, 32
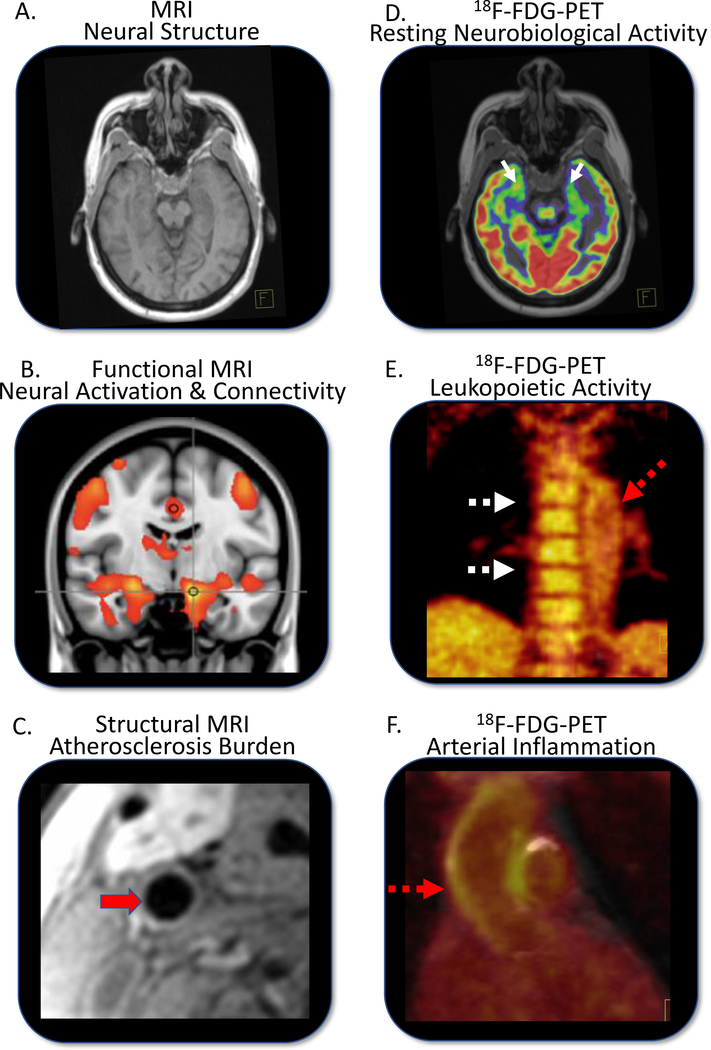
Advanced neuroimaging, which can directly measure stress’ neurobiological impact, has revolutionized neuropsychiatry and stress research. Functional magnetic resonance imaging (fMRI) assesses neural activity and connectivity between brain regions. fMRI can instantaneously measure the neural response to rapidly changing stimuli (e.g., emotional facial expressions). Human PET activation and fMRI studies show that stressful or threatening stimuli activate the amygdala and elicit the hormonal, autonomic, and behavioral changes associated with fear and stress.33, 34 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) is used to measure resting metabolic activity in various body tissues including brain. Large series studies in primates show that amygdalar 18F-FDG uptake associates with anxious temperament in adults35 and predicts future temperament among juveniles.36 In humans, amygdalar metabolic activity (AmygA; amygdalar activity adjusted for temporal lobe or prefrontal cortex activity) associates with circulating inflammatory markers, leukopoietic activity, and atherosclerosis.37, 38 Moreover, it associates with stress syndromes and independently predicts subsequent cardiometabolic diseases and CVD events.37–39 While costly and limited in availability, PET/MRI systems allow concurrent measurement of structural MRI, fMRI, and PET signals, allowing simultaneous quantification of neurobiological activity,16–19,21 leukopoietic tissue activity (e.g., bone marrow), and arterial inflammation (ArtI),12,22 thereby making this modality uniquely suitable for investigating stress-related atherogenic mechanisms (Figure 4).
Figure 4:
Multi-System Imaging of Tissue Biology
Advanced imaging using 18F-Fluorodeoxyglucose positron emission tomography/magnetic resonance imaging (18F-FDG-PET/MRI) provides a unique opportunity to measure stress-associated neurobiology as it relates to cardiovascular disease. Multiparametric-multi-tissue imaging can be obtained in a single session. MRI data include measurements of neural structures (A), stressor-stimulated neural activation and connectivity using functional MRI (B), and structural cardiovascular measures (C). 18F-FDG-PET/MRI is used to image resting neurobiological metabolic activity (D), leukopoietic activity (E) and arterial inflammation (F). Red solid arrow=carotid artery, red hatched arrows=aorta, white solid arrows=amygdalar activity, white hatched arrows=bone marrow.
Insights from Human Imaging Studies
Human neuroimaging studies have illuminated a multi-system mechanism linking chronic stress to CVD (Figure 5). In an fMRI study of 36 individuals, heightened amygdalar activation and positive connectivity between the amygdala and anterior cingulate cortex associated with a greater burden of subclinical atherosclerosis.40 In an 18F-FDG-PET/CT imaging study of 293 individuals without baseline CVD or active malignancy, AmygA robustly associated with subsequent CVD events, and a serial mechanism linking AmygA to CVD was identified: ↑AmygA→↑leukopoietic activity→↑ArtI→↑CVD events.37 This neural-immune-arterial mechanism was subsequently corroborated and extended in a prospective study of patients who underwent 18F-FDG-PET/CT and coronary CT angiography. In that study, increased AmygA associated with greater bone marrow leukopoietic activity and atherosclerosis (both ArtI and non-calcified coronary plaque volume).38 Further, the relationship between heightened AmygA and atherosclerosis was again mediated by increased leukopoietic activity. A third study also observed significant associations between increased amygdalar activity and heightened leukopoietic activity, abnormal myocardial perfusion, and decreased left ventricular function among women.41 These findings collectively implicate a neural-immune mechanism linking stress to CVD and suggest novel therapeutic targets.
Figure 5:
Mechanisms Linking Stress to CVD
Chronic stress upregulates stress-associated neurobiological activity, leading to increased CVD risk factors (e.g., obesity, hypertension, and insulin resistance) and heightened inflammation via a neural-immune axis. This in turn drives increased arterial inflammation and non-calcified coronary plaque burden, resulting in higher CVD risk independent of traditional risk factors. BM-bone marrow, CVD-cardiovascular disease, HTN-hypertension, SNS-sympathetic nervous system, HPA-hypothalamic-pituitary-adrenal.
Neuroimaging studies have similarly illuminated mechanistic insights into Takotsubo Syndrome. Among individuals with prior Takotsubo Syndrome, there is altered structure and connectivity of stress-associated brain centers (including the amygdala) and increased systemic inflammation.9, 10, 42 Notably, a recent retrospective case-control 18F-FDG-PET/CT study showed that heightened AmygA is present years before Takotsubo Syndrome onset, suggesting that vulnerable individuals are primed prior to developing Takotsubo Syndrome after an acute stressor.11
Social and Environmental Stressors
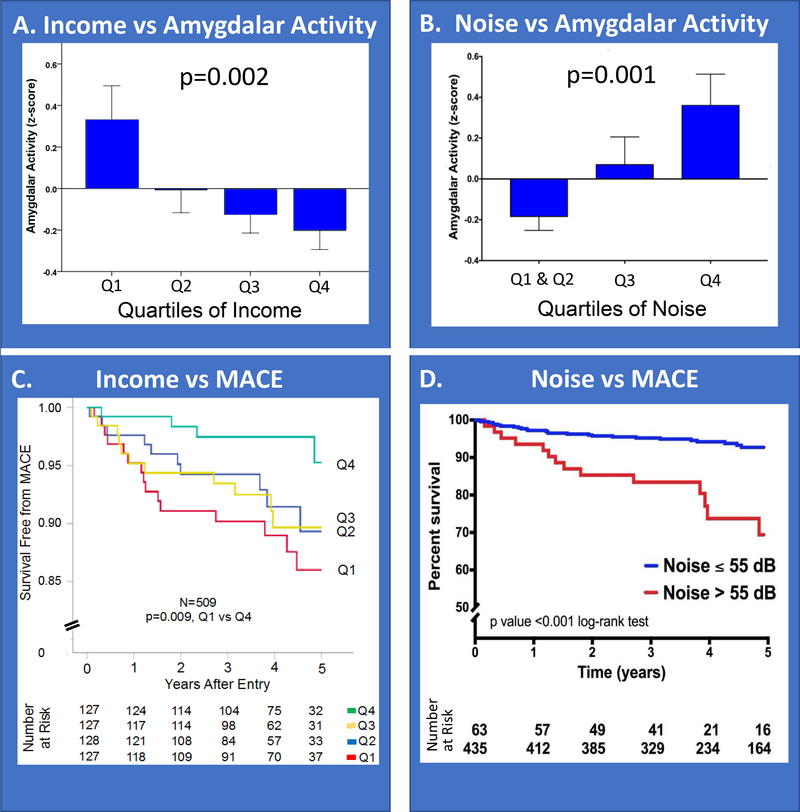
Modern humans are increasingly subjected to chronic socioeconomic and environmental stressors (e.g., economic stress, noise exposure). Such stressors have repeatedly been associated with higher systemic inflammation, CVD risk, and reduced life expectancy beyond differences in risk factors, healthcare access, or health behaviors.43–45 A recent study evaluated whether heightened AmygA contributes to the mechanism linking adverse socioeconomic status to CVD. That study derived neighborhood-level socioeconomic status indices among 509 individuals who had undergone 18F-FDG-PET/CT imaging. Lower neighborhood socioeconomic status (i.e., lower income or higher crime) associated with increased AmygA (Figure 6A), ArtI, and subsequent CVD events (Figure 6C). Furthermore, path analysis demonstrated that socioeconomic status acts at the front end of the aforementioned neural-immune-arterial mechanism: lower socioeconomic status→↑AmygA→↑leukopoietic activity→↑ArtI →↑CVD events.46
Figure 6:
Socioeconomic and Environmental Stressors vs. Stress-Associated Neurobiological Activity and CVD Events.
Individuals were categorized according to quartiles of neighborhood median income and transportation noise exposure. Amygdalar activity decreased as neighborhood median income increased (A) and increased as transportation noise exposure increased (B). Error bars represent standard error of the mean. Similarly, CVD event-free survival was lower among individuals with lower neighborhood income (C) or higher chronic noise exposure (D, >55 dBA, a level deemed unhealthy by the World Health Organization). Reused with permission.46, 49 MACE-major adverse cardiovascular event.
Similarly, chronic environmental noise exposure associates with heightened systemic inflammation, oxidative stress, and risk for CVD and metabolic diseases.47 Since the amygdala is intimately involved in the perception and response to noise,48 increased noise exposure’s impact on the amygdala and CVD was also evaluated with 18F-FDG-PET/CT imaging. Transportation noise exposure (average 24-hour exposure at each individual’s home address) independently associated with AmygA (Figure 6B), ArtI, and incident CVD events (Figure 6D). Moreover, the mechanism linking noise exposure and CVD events was serially mediated by increased AmygA and ArtI,49 further implicating the neural-immune-arterial pathway in the pathogenesis of stress-associated CVD.
Importantly, not all individuals exposed to socioeconomic and environmental stressors have higher AmygA. In fact, among those with relatively higher levels of chronic stress exposure (e.g., lowest neighborhood income tertile) there was a range of AmygA that remained independently predictive of ArtI and CVD events. Notably, individuals with lower AmygA in spite of increased stress exposure (i.e., neurobiologically resilient individuals) were relatively protected from CVD events.50 As such, the assessment of AmygA may provide a construct that offers insight into resilience and merits additional study to identify factors (e.g., behaviors, genetics) that may underlie this finding.
Therapeutic Insights
Behavioral interventions may reduce perceived stress and attenuate the neural-immune-arterial axis. Ideal treatments should be widely available, safe, and inexpensive in order to benefit as broad a population as possible. Accordingly, the impact of stress reduction (SR) on CVD has been of substantial interest. Nevertheless, existing studies have often been limited by small sizes, varied patient populations (e.g., different ages, ethnicities), use of different SR techniques (e.g., mindfulness-based SR, yoga, transcendental meditation), and diverse endpoints (e.g., carotid wall measures, inflammatory biomarkers, symptoms).51 Given these limitations, meaningful conclusions about SR’s impact on CVD cannot yet be drawn; however, findings remain encouraging.52
While SR significantly reduces perceived stress,53 several recent studies show that SR has additional important physiological benefits. SR modifies the structure and connectivity of stress-associated neural centers, including the amygdala.54, 55 Its downstream effects include reduced systemic inflammation56 and improved blood pressure and health behaviors (e.g., reduced smoking).57, 58 SR also favorably alters gene expression by amplifying genetic pathways governing ATP and insulin production, while attenuating pro-inflammatory pathways (e.g., NF-κβ pathway).59, 60 Notably, its anti-inflammatory impact may be greatest among individuals with inflammatory conditions or CVD.56, 61
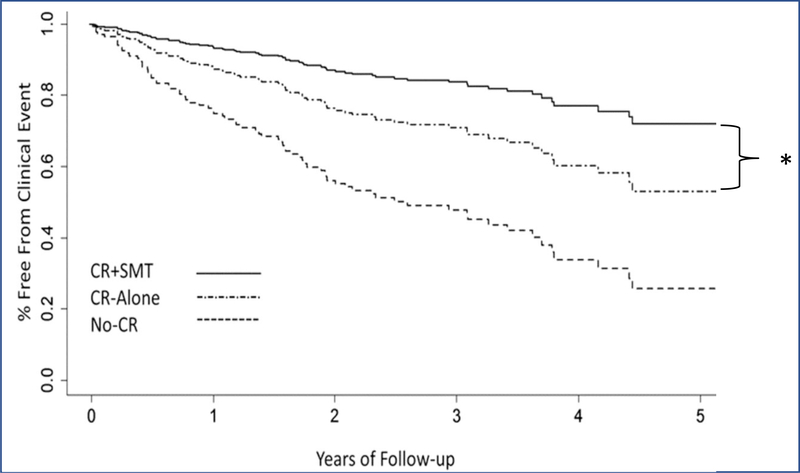
Data on SR’s impact on atherosclerosis are also promising. Small studies have demonstrated carotid plaque regression and improved myocardial blood flow after SR.62, 63 Furthermore, a randomized controlled trial showed fewer adverse CVD events when SR was added to traditional cardiac rehabilitation versus cardiac rehabilitation alone (Figure 7). This finding has catalyzed SR’s broad incorporation into cardiac rehabilitation programs.64
Figure 7:
Impact of Stress Reduction on CVD Events
Individuals randomized to cardiac rehabilitation (CR) plus stress management training (SMT) had fewer CVD events versus CR-alone (18% vs. 33%, HR [95% CI]=0.49 [0.25, 0.95], p=0.035,*), and both CR groups had lower event rates versus the No-CR group (47%, HR [95% CI]=0.44 [0.27, 0.71], p<0.001). Reused with permission.64 CI-confidence interval, HR-hazard ratio.
Given these findings, the American Heart Association released a statement that concluded that the body of evidence, although inconsistent, suggests a potential benefit for SR in CVD and recommended further research. Given its low cost and potential for widespread application, the statement called for larger randomized trials to evaluate SR’s effect on CVD.51 Additionally, further research is needed to study the mechanisms by which SR yields these benefits, evaluate SR’s impact on neurobiological resilience, and identify which SR methods have the greatest impact in different populations.
Salutary modification of health behaviors may also mitigate the relationship between chronic stress and CVD.29 Habitual exercise reduces stress and systemic inflammation.65 Poor sleep increases stress and triggers several stress-linked inflammatory atherogenic mechanisms.66 Accordingly, SR programs that also address lifestyle may potentially dampen stress’ adverse biological impacts more than SR alone.67 Modern tools such as wearable technologies and remote monitoring provide opportunities to better address and monitor these behaviors.
Lastly, several pharmacologic therapies targeting the neural-immune-arterial axis may attenuate stress’ association with CVD. Increased amygdalar activity and its downstream effects may be reduced by antidepressants or ketamine, a dissociative anesthetic with efficacy in treating depression.68 In individuals with clinical depression and known CVD, selective serotonin reuptake inhibitors attenuate depressive symptoms and may lower secondary CVD risk.1 Beyond the brain, beta blockers may decrease SNS-driven leukopoiesis.25, 27 Anti-inflammatory agents (e.g., canakinumab) may also prove beneficial by directly targeting elevated pro-inflammatory cytokines.69 Finally, agents that decrease ArtI (e.g., statins) may reduce consequent CVD events.70 Further development and testing of novel targeted pharmacologic therapies (e.g., those targeting interleukin-6, CXCL12, TLRs, or the NF-κβ pathway) along with additional research into the durability of therapies and the comparative effectiveness of medications and lifestyle changes is required. Prospective studies are needed to evaluate the changes in the neural-inflammatory-arterial axis effected by both behavioral and pharmacologic therapies to improve our treatment of stress-associated CVD and evaluate a causative role for the neural-immune mechanism by inducing an upstream change to reduce downstream CVD.
While not part of most CVD treatment guidelines, it may be appropriate to address stress in individuals at risk for CVD given the low cost and low risk of intervention. Beyond encouraging treatment of underlying stress disorders, cardiologists should be aware of chronic stress’ impact on patients’ health. Questionnaire-based screening and appropriate therapies for patients at risk for CVD could be considered.1, 51
Conclusion
Chronic stress is a pervasive, under-appreciated CVD risk factor. Recent research has helped to clarify the neurobiological pathways through which this occurs. More studies are needed to understand the underlying mechanisms and to develop appropriate treatments. Moreover, clinicians should consider the role of chronic stress when evaluating individuals with or at risk for CVD and recommend approaches to reduce stress among those with high perceived stress or greater exposure.
Supplementary Material
Acknowledgments
Sources of Funding: This work is partially supported by the following United States NIH grants: KL2TR002542 (MTO), R01HL137913 (AT), R33HL141047 (AT), and P01HL131478 (AT, LMS, ZF).
Disclosures: AT reports institutional research grants from Genentech and personal fees from Actelion and Esperion and MTO reports consulting fees from Intrinsic Imaging, LLC for unrelated work. The other authors have no disclosures.
References
- 1.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635–1701 [DOI] [PubMed] [Google Scholar]
- 2.Dar T, Radfar A, Abohashem S, Pitman RK, Tawakol A, Osborne MT. Psychosocial stress and cardiovascular disease. Curr Treat Options Cardiovasc Med. 2019;21:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backe EM, Seidler A, Latza U, Rossnagel K, Schumann B. The role of psychosocial stress at work for the development of cardiovascular diseases: A systematic review. Int Arch Occup Environ Health. 2012;85:67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batty GD, Russ TC, Stamatakis E, Kivimaki M. Psychological distress in relation to site specific cancer mortality: Pooling of unpublished data from 16 prospective cohort studies. BMJ. 2017;356:j108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi-amorn C, Sato H, Yusuf S, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): Case-control study. Lancet. 2004;364:953–962 [DOI] [PubMed] [Google Scholar]
- 6.Booth J, Connelly L, Lawrence M, Chalmers C, Joice S, Becker C, Dougall N. Evidence of perceived psychosocial stress as a risk factor for stroke in adults: A meta-analysis. BMC Neurol. 2015;15:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med. 1996;334:413–419 [DOI] [PubMed] [Google Scholar]
- 8.Wilbert-Lampen U, Leistner D, Greven S, Pohl T, Sper S, Volker C, Guthlin D, Plasse A, Knez A, Kuchenhoff H, et al. Cardiovascular events during World Cup soccer. N Engl J Med. 2008;358:475–483 [DOI] [PubMed] [Google Scholar]
- 9.Hiestand T, Hanggi J, Klein C, Topka MS, Jaguszewski M, Ghadri JR, Luscher TF, Jancke L, Templin C. Takotsubo syndrome associated with structural brain alterations of the limbic system. J Am Coll Cardiol. 2018;71:809–811 [DOI] [PubMed] [Google Scholar]
- 10.Templin C, Hanggi J, Klein C, Topka MS, Hiestand T, Levinson RA, Jurisic S, Luscher TF, Ghadri JR, Jancke L. Altered limbic and autonomic processing supports brain-heart axis in Takotsubo syndrome. Eur Heart J. 2019;40:1183–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radfar A, Wang Y, Osborne MT, Patrich T, Tung B, Oberfeld B, Neilan T, Wood M, Tawakol A. Associations between amygdalar activity and the subsequent development of Takotsubo syndrome: Novel mechanistic insights. Circulation. 2018;138:A15767 [Google Scholar]
- 12.Morera LP, Marchiori GN, Medrano LA, Defago MD. Stress, dietary patterns and cardiovascular disease: A mini-review. Front Neurosci 2019;13:1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: Prospective study. BMJ. 2006;332:521–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markovitz JH, Matthews KA, Whooley M, Lewis CE, Greenlund KJ. Increases in job strain are associated with incident hypertension in the CARDIA study. Ann Behav Med. 2004;28:4–9 [DOI] [PubMed] [Google Scholar]
- 15.Kumari M, Head J, Marmot M. Prospective study of social and other risk factors for incidence of type 2 diabetes in the Whitehall II study. Arch Intern Med. 2004;164:1873–1880 [DOI] [PubMed] [Google Scholar]
- 16.Kearns NT, Carl E, Stein AT, Vujanovic AA, Zvolensky MJ, Smits JAJ, Powers MB. Posttraumatic stress disorder and cigarette smoking: A systematic review. Depress Anxiety. 2018;35:1056–1072 [DOI] [PubMed] [Google Scholar]
- 17.Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, Keltai M, Diaz R, Rangarajan S, Yusuf S, et al. Risk factors for myocardial infarction in women and men: Insights from the INTERHEART study. Eur Heart J. 2008;29:932–940 [DOI] [PubMed] [Google Scholar]
- 18.Wang SS, Yan XB, Hofman MA, Swaab DF, Zhou JN. Increased expression level of corticotropin-releasing hormone in the amygdala and in the hypothalamus in rats exposed to chronic unpredictable mild stress. Neurosci Bull. 2010;26:297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koutsikou S, Watson TC, Crook JJ, Leith JL, Lawrenson CL, Apps R, Lumb BM. The periaqueductal gray orchestrates sensory and motor circuits at multiple levels of the neuraxis. J Neurosci. 2015;35:14132–14147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McEwen BS, Morrison JH. The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girod JP, Brotman DJ. Does altered glucocorticoid homeostasis increase cardiovascular risk? Cardiovasc Res. 2004;64:217–226 [DOI] [PubMed] [Google Scholar]
- 23.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A. 2014;111:16136–16141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernberg E, Ulleryd MA, Johansson ME, Bergstrom GM. Social disruption stress increases IL-6 levels and accelerates atherosclerosis in ApoE−/− mice. Atherosclerosis. 2012;221:359–365 [DOI] [PubMed] [Google Scholar]
- 29.Nahrendorf M, Swirski FK. Lifestyle effects on hematopoiesis and atherosclerosis. Circ Res. 2015;116:884–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emami H, Singh P, MacNabb M, Vucic E, Lavender Z, Rudd JH, Fayad ZA, Lehrer-Graiwer J, Korsgren M, Figueroa AL, et al. Splenic metabolic activity predicts risk of future cardiovascular events: Demonstration of a cardiosplenic axis in humans. JACC Cardiovasc Imaging. 2015;8:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen S, Tyrrell DAJ, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325:606–612 [DOI] [PubMed] [Google Scholar]
- 32.Steptoe A, Marmot M. The role of psychobiological pathways in socio-economic inequalities in cardiovascular disease risk. Eur Heart J. 2002;23:13–25 [DOI] [PubMed] [Google Scholar]
- 33.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281 [DOI] [PubMed] [Google Scholar]
- 35.Oler JA, Fox AS, Shelton SE, Rogers J, Dyer TD, Davidson RJ, Shelledy W, Oakes TR, Blangero J, Kalin NH. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466:864–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fox AS, Oler JA, Shelton SE, Nanda SA, Davidson RJ, Roseboom PH, Kalin NH. Central amygdala nucleus (Ce) gene expression linked to increased trait-like Ce metabolism and anxious temperament in young primates. Proc Natl Acad Sci U S A. 2012;109:18108–18113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, Truong QA, Solomon CJ, Calcagno C, Mani V, et al. Relation between resting amygdalar activity and cardiovascular events: A longitudinal and cohort study. Lancet. 2017;389:834–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goyal A, Dey AK, Chaturvedi A, Elnabawi YA, Aberra TM, Chung JH, Belur AD, Groenendyk JW, Lerman JB, Rivers JP, et al. Chronic stress-related neural activity associates with subclinical cardiovascular disease in psoriasis: A prospective cohort study. JACC Cardiovasc Imaging. 2020; 13:465–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osborne MT, Ishai A, Hammad B, Tung B, Wang Y, Baruch A, Fayad ZA, Giles JT, Lo J, Shin LM, et al. Amygdalar activity predicts future incident diabetes independently of adiposity. Psychoneuroendocrinology. 2018;100:32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gianaros PJ, Hariri AR, Sheu LK, Muldoon MF, Sutton-Tyrrell K, Manuck SB. Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biol Psychiatry. 2009;65:943–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiechter M, Haider A, Bengs S, Maredziak M, Burger IA, Roggo A, Portmann A, Schade K, Warnock GI, Treyer V, et al. Sex-dependent association between inflammation, neural stress responses, and impaired myocardial function. Eur J Nucl Med Mol Imaging. 2019; 10.1007/s00259-019-04537-8 [DOI] [PubMed] [Google Scholar]
- 42.Scally C, Abbas H, Ahearn T, Srinivasan J, Mezincescu A, Rudd A, Spath N, Yucel-Finn A, Yuecel R, Oldroyd K, et al. Myocardial and systemic inflammation in acute stress-induced (Takotsubo) cardiomyopathy. Circulation. 2019;139:1581–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, Quyyumi AA, Taylor HA, Gulati M, Harold JG, et al. Socioeconomic status and cardiovascular outcomes: Challenges and interventions. Circulation. 2018;137:2166–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chetty R, Stepner M, Abraham S, Lin S, Scuderi B, Turner N, Bergeron A, Cutler D. The association between income and life expectancy in the United States, 2001–2014. JAMA. 2016;315:1750–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ranjit N, Diez-Roux AV, Shea S, Cushman M, Ni H, Seeman T. Socioeconomic position, race/ethnicity, and inflammation in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2007;116:2383–2390 [DOI] [PubMed] [Google Scholar]
- 46.Tawakol A, Osborne MT, Wang Y, Hammed B, Tung B, Patrich T, Oberfeld B, Ishai A, Shin LM, Nahrendorf M, et al. Stress-associated neurobiological pathway linking socioeconomic disparities to cardiovascular disease. J Am Coll Cardiol. 2019;73:3243–3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munzel T, Schmidt FP, Steven S, Herzog J, Daiber A, Sorensen M. Environmental noise and the cardiovascular system. J Am Coll Cardiol. 2018;71:688–697 [DOI] [PubMed] [Google Scholar]
- 48.Spreng M Central nervous system activation by noise. Noise Health. 2000;2:49–58 [PubMed] [Google Scholar]
- 49.Osborne MT, Radfar A, Hassan MZO, Abohashem S, Oberfeld B, Patrich T, Tung B, Wang Y, Ishai A, Scott JA, et al. A neurobiological mechanism linking transportation noise to cardiovascular disease in humans. Eur Heart J. 2020;41:772–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dar T, Abohashem S, Ghoneem A, Naddaf N, Osborne MT, Tawakol A. Neurobiological resilience associates with a reduced risk of mace. Circulation. 2019;140:A16096 [Google Scholar]
- 51.Levine GN, Lange RA, Bairey-Merz CN, Davidson RJ, Jamerson K, Mehta PK, Michos ED, Norris K, Ray IB, Saban KL, et al. Meditation and cardiovascular risk reduction: A scientific statement from the American Heart Association. J Am Heart Assoc. 2017;6:e002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Younge JO, Gotink RA, Baena CP, Roos-Hesselink JW, Hunink MG. Mind-body practices for patients with cardiac disease: A systematic review and meta-analysis. Eur J Prev Cardiol. 2015;22:1385–1398 [DOI] [PubMed] [Google Scholar]
- 53.Klatt MD, Buckworth J, Malarkey WB. Effects of low-dose mindfulness-based stress reduction (MBSR-ld) on working adults. Health Educ Behav. 2009;36:601–614 [DOI] [PubMed] [Google Scholar]
- 54.Desbordes G, Negi LT, Pace TW, Wallace BA, Raison CL, Schwartz EL. Effects of mindful-attention and compassion meditation training on amygdala response to emotional stimuli in an ordinary, non-meditative state. Front Hum Neurosci. 2012;6:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, Pitman RK, Lazar SW. Stress reduction correlates with structural changes in the amygdala. Soc Cogn Affect Neurosci. 2010;5:11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malarkey WB, Jarjoura D, Klatt M. Workplace based mindfulness practice and inflammation: A randomized trial. Brain Behav Immun. 2013;27:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rainforth MV, Schneider RH, Nidich SI, Gaylord-King C, Salerno JW, Anderson JW. Stress reduction programs in patients with elevated blood pressure: A systematic review and meta-analysis. Curr Hypertens Rep. 2007;9:520–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oikonomou MT, Arvanitis M, Sokolove RL. Mindfulness training for smoking cessation: A meta-analysis of randomized-controlled trials. J Health Psychol. 2017;22:1841–1850 [DOI] [PubMed] [Google Scholar]
- 59.Bhasin MK, Dusek JA, Chang BH, Joseph MG, Denninger JW, Fricchione GL, Benson H, Libermann TA. Relaxation response induces temporal transcriptome changes in energy metabolism, insulin secretion and inflammatory pathways. PLoS One. 2013;8:e62817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Creswell JD, Irwin MR, Burklund LJ, Lieberman MD, Arevalo JM, Ma J, Breen EC, Cole SW. Mindfulness-based stress reduction training reduces loneliness and pro-inflammatory gene expression in older adults: A small randomized controlled trial. Brain Behav Immun. 2012;26:1095–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun 2007;21:1038–1049 [DOI] [PubMed] [Google Scholar]
- 62.Fields JZ, Walton KG, Schneider RH, Nidich S, Pomerantz R, Suchdev P, Castillo-Richmond A, Payne K, Clark ET, Rainforth M. Effect of a multimodality natural medicine program on carotid atherosclerosis in older subjects: A pilot trial of Maharishi Vedic medicine. Am J Cardiol. 2002;89:952–958 [DOI] [PubMed] [Google Scholar]
- 63.Bokhari S, Schneider RH, Salerno JW, Rainforth MV, Gaylord-King C, Nidich SI. Effects of cardiac rehabilitation with and without meditation on myocardial blood flow using quantitative positron emission tomography: A pilot study. J Nucl Cardiol. 2019; 10.1007/s12350-019-01884-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blumenthal JA, Sherwood A, Smith PJ, Watkins L, Mabe S, Kraus WE, Ingle K, Miller P, Hinderliter A. Enhancing cardiac rehabilitation with stress management training: A randomized, clinical efficacy trial. Circulation. 2016;133:1341–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Keefe EL, O’Keefe JH, Lavie CJ. Exercise counteracts the cardiotoxicity of psychosocial stress. Mayo Clin Proc. 2019;94:1852–1864 [DOI] [PubMed] [Google Scholar]
- 66.McAlpine CS, Kiss MG, Rattik S, He S, Vassalli A, Valet C, Anzai A, Chan CT, Mindur JE, Kahles F, et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature. 2019;566:383–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park ER, Traeger L, Vranceanu AM, Scult M, Lerner JA, Benson H, Denninger J, Fricchione GL. The development of a patient-centered program based on the relaxation response: The relaxation response resiliency program (3RP). Psychosomatics. 2013;54:165–174 [DOI] [PubMed] [Google Scholar]
- 68.Scheidegger M, Henning A, Walter M, Lehmann M, Kraehenmann R, Boeker H, Seifritz E, Grimm S. Ketamine administration reduces amygdalo-hippocampal reactivity to emotional stimulation. Hum Brain Mapp. 2016;37:1941–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131 [DOI] [PubMed] [Google Scholar]
- 70.Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, Subramanian SS, Abdelbaky A, Rudd JH, Farkouh ME, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: Results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62:909–917 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.