Abstract
Background
Enterohemorrhagic Escherichia coli O157:H7 (EHEC) is a significant foodborne pathogen that resides asymptomatically within cattle and other ruminants. The EHEC genome harbors an extensive collection of mobile genetic elements (MGE), including multiple prophage, prophage-like elements, plasmids, and insertion sequence (IS) elements.
Results
A chronological collection of EHEC strains (FRIK804, FRIK1275, and FRIK1625) isolated from a Wisconsin dairy farm (farm X) comprised a closely related clade genetically differentiated by structural alterations to the chromosome. Comparison of the FRIK804 genome with a reference EHEC strain Sakai found a unique prophage like element (PLE, indel 1) and an inversion (1.15 Mb) situated symmetrically with respect to the terminus region. Detailed analysis determined the inversion was due to homologous recombination between repeat sequences in prophage. The three farm X strains were distinguished by the presence or absence of indel 3 (61 kbp) and indel 4 (48 kbp); FRIK804 contained both of these regions, FRIK1275 lacked indel 4, and indels 3 and 4 were both absent in FRIK1625. Indel 3 was the stx2 prophage and indel 4 involved a deletion between two adjacent prophage with shared repeat sequences. Both FRIK804 and FRIK1275 produced functional phage while FRIK1625 did not, which is consistent with indel 3. Due to their involvement in recombination events, direct and inverted repeat sequences were identified, and their locations mapped to the chromosome. FRIK804 had a greater number and overall length of repeat sequences than E. coli K12 strain MG1655. Repeat sequences were most commonly associated with MGE.
Conclusions
This research demonstrated that three EHEC strains from a Wisconsin dairy farm were closely related and distinguished by variability within prophage regions and other MGE. Chromosome alterations were associated with recombination events between repeat sequences. An inventory of direct and inverted repeat sequences found a greater abundance and total length of repeat sequences in the EHEC strains compared to E. coli strain MG1655. The locations of the repeat sequences were biased towards MGE. The findings from this study expand our understanding of the precise molecular events and elements that contributed to genetic diversification of wild-type EHEC in the bovine and farm environments.
Keywords: E. coli O157, stx2, Recombination, Prophage, Direct and inverted repeats
Background
Enterohemorrhagic E. coli O157:H7 (EHEC) is a significant zoonotic pathogen that causes hemorrhagic colitis and abdominal cramping. In some cases, patients develop hemolytic uremic syndrome (HUS) and kidney failure, particularly in young children [1–3]. Cattle are the primary reservoir of EHEC where residence is asymptomatic [4, 5]. Contaminated ground beef has been associated with transmission from cattle to humans, but an increasing array of foods including leafy greens [6–8], sprouts [9–11], in-shell hazelnuts [12], and cookie dough [13] have been implicated as vehicles in recent outbreaks.
Genomic comparisons of EHEC with nonpathogenic E. coli strain MG1655 found a common core sequence interrupted by hundreds of genomic islands [14, 15]. Many of these islands are recognized mobile genetic elements (MGE) including prophage, prophage-like elements (PLE), and insertion sequence (IS) elements. EHEC usually harbor pO157, a ~ 92 kbp F-like plasmid with some genes encoding for virulence factors (i.e., hemolysin) [16, 17]. Other smaller plasmids have been found in some strains [18–20]. EHEC strain Sakai possesses a typical complement of mobile MGE: 18 prophage, 6 PLE, and 80 identified IS, including 19 IS629 elements [15]. By length, prophage account for 11% of the Sakai chromosome and a majority of MGE. Most of the identified prophage elements are considered incapable of excision or replication and are regarded as cryptic [21]. The genes encoding for Shiga-like toxins Stx1 and Stx2 are located within separate prophage. Stx2 possesses greater cytotoxicity in comparison to Stx1, and Stx2 production is correlated with the incidence of HUS [22–24]. The stx2-prophage is typically the only functional phage present [21]. Virulence factors located in other MGE also contribute to EHEC pathogenesis [25, 26].
EHEC have been divided into distinct lineages based upon octamer-based genome scanning, amplification of lineage-specific polymorphisms, and microarray-based comparative genome hybridization techniques [27–31]. Lineages I (LI) and I/II (LI/II) are isolated from clinical and bovine/environmental sources while lineage II (LII) strains are confined to bovine/environmental sources. This suggests that LII has lower human virulence potential with respect to LI and LI/II. In a previous study, the prophage content of EHEC strains isolated from a Wisconsin dairy farm (farm X) was characterized using phage-based PCR markers [32]. Prophage polymorphism profiles (PPP) of strains showed an initial resident LII population supplanted by LI (FRIK804, FRIK1275, and FRIK1625) with strain-specific PPP. Originally distinguished on the basis of differing PFGE profiles, the differences between these strains included the insertional inactivation of stx2 by IS629 in FRIK1275 and the absence of the stx2-prophage in FRIK1625. FRIK804 contained the stx2 prophage without IS629. Based on the genomic differences and the date of isolation, FRIK804 likely was the original LI strain on farm X followed by genomic alterations that resulted in strains FRIK1275 and FRIK1625.
In the current study, whole-genome restriction site mapping and DNA sequencing were used to confirm that the LI strains isolated from farm X were closely related and to discern the molecular events leading to the formation of FRIK1275 and FRIK1625. Prophage and PLE, containing repeat sequences, occupied the sites of chromosomal alterations that distinguished the farm X strains in most cases. A greater number and overall length of repeat sequences were present in FRIK804 than E. coli strain MG1655. The distribution of repeats was skewed towards MGE. Results from this study highlight the prevalence of repeat sequences, particularly within prophage and PLE, and their role in EHEC diversification in the bovine-farm ecosystem.
Results
de novo sequence assembly of the FRIK804 genome
Sequence assembly using Illumina short-read data was hampered by an inability to resolve DNA sequence repeats longer than read length. Draft genomes produced using only short-read data produced fragmented assemblies. Crucially, these assemblies failed to completely capture the assortment of MGE present in the EHEC genome. A high-quality de novo assembly of the FRIK804 was produced using single molecule real-time (SMRT) sequence data in conjunction with Illumina paired-end data and confirmation using whole-genome mapping (i.e., optical mapping). The gapless assembly of the FRIK804 genome was required to provide a reference for the other strains analyzed in this study.
Initial assembly of the FRIK804 genome used SPAdes and both SMRT and Illumina data [33]; however, the substitution of two prophage regions was identified and a new assembly was produced using Canu and SMRT data only that lacked this assembly error [34]. Assembly improvement and correction was performed using Pilon [35]. Contigs representing the chromosome and pO157 were identified in the Canu assembly (Table 1). Three small plasmids (pFRIK804–1, pFRIK804–2, and pFRIK804–3) present in the former assembly were absent in the latter suggesting that multiple assembly approaches are useful. pFRIK804–1 was 6.73 kbp and carried genes encoding for colicin D and associated immunity and lysis genes [19]. pFRIK804–2 was 4.09 kbp in length and possessed no predicted phenotype. pFRIK804–3 was 3.31 kbp in length and featured 100% sequence similarity with pOSAK1, a plasmid previously reported in the genome of EHEC strain Sakai.
Table 1.
FRIK804 genome assembly statistics
| Contig name | Size (kbp) | GC% | ORFs |
|---|---|---|---|
| Chromosome | 5554.24 | 50.52 | 5836 |
| pO157 | 92.70 | 47.59 | 99 |
| pFRIK804–1 | 6.73 | 50.19 | 6 |
| pFRIK804–2 | 4.09 | 49.57 | 3 |
| pFRIK804–3 | 3.31 | 43.42 | 4 |
Comparative analysis of FRIK804 and Sakai chromosomes
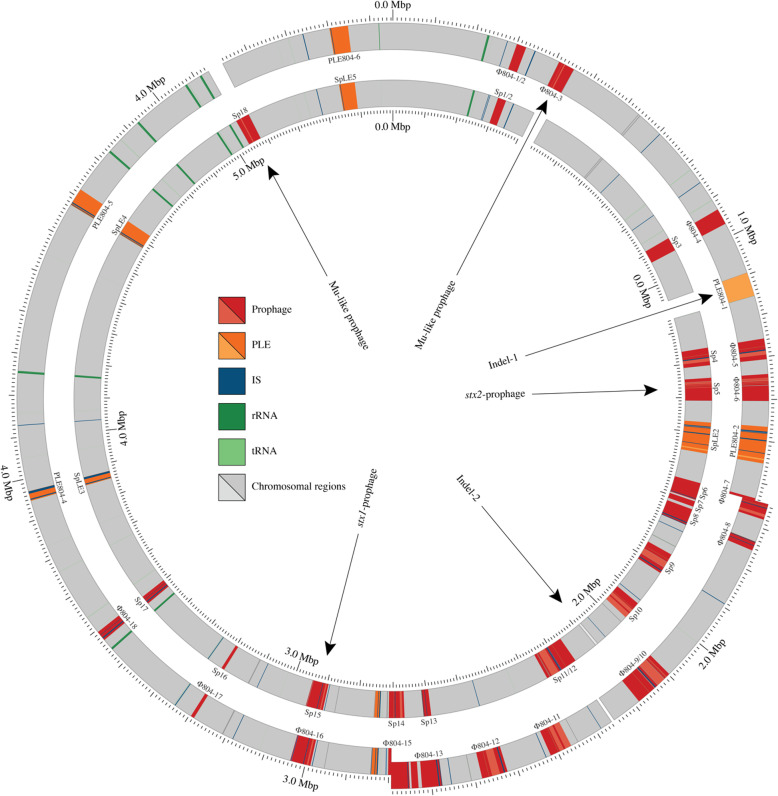
The EHEC strain Sakai was used as a reference for comparison with FRIK804 [15]. The extensive synteny of the two chromosomes was interrupted by a few structural differences. Non-conserved regions consisted of Mu-like prophage with distinct strain-specific integration sites, an inverted segment of the chromosome that included the terminus, and two indels (Fig. 1). Both strains harbored 18 prophage (Φ804–1 – Φ804–18 for FRIK804) (Sp1 – Sp18 for Sakai) while FRIK804 contained 7 PLE (PLE804–1 – PLE804–7) and Sakai 6 PLE (SpLE1 – SpLE6)(Table 2 and Fig. 1). Both strains harbored the pO157 plasmid and a 3.31 kbp plasmid pFRIK804–3 (FRIK804) and pOSAK1 (Sakai). IS629 and ISEc8 were the predominate IS in both genomes. Twenty-one IS629 elements were present in FRIK804 and 17 in Sakai (Table S6). Fifteen integration sites for IS629 were shared by the two strains. Nine ISEc8 elements were present in both strains with 8 common sites of integration (Table S7).
Fig. 1.
Comparison of FRIK804 and Sakai chromosomes. Alignment of the FRIK804 (outer) and Sakai (inner) chromosome found disruption of synteny by large-scale structural alterations. To evaluate each dissimilarity, the locations of relevant genomic features (prophage, PLE, IS, rRNA and tRNA) were identified. Non-conserved regions (e.g. indel-1 and indel-2) of each chromosome are shaded lighter relative to conserved regions. Evidence of mosaicism in otherwise conserved prophage was evident in several pairs of homologs, including the stx2-prophage. The majority of dissimilarities that distinguished each chromosome were associated with MGE. A 1.15 Mb inversion is denoted by the offset region in FRIK804. Indel-1 was classified as a PLE and indel-2 was not associated with any MGE. Mu-like prophage were integrated at different loci. The locations of rRNA and tRNA regions are denoted by dark green and light green regions, respectively
Table 2.
Designation and chromosomal locations of corresponding prophage and PLE in EHEC strains FRIK804 and Sakai. Prophage and PLE were numerically designated in clockwise order (see Fig. 1). The locations of prophage in Sakai were used to identify and locate most prophage and PLE in FRIK804
| FRIK804 | Sakai | |||||||
|---|---|---|---|---|---|---|---|---|
| Name | Start | End | Length (bp) | Name | Start | End | Length (bp) | Notes |
| Φ804–1 | 300,040 | 310,625 | 10,586 | Sp1 | 300,041 | 310,626 | 10,586 | |
| Φ804–2 | 310,626 | 323,512 | 12,887 | Sp2 | 310,627 | 323,513 | 12,887 | |
| Φ804–3 | 409,092 | 448,271 | 39,180 | Mu-like prophagea | ||||
| Φ804–4 | 929,716 | 968,301 | 38,586 | Sp3 | 891,123 | 929,708 | 38,586 | |
| PLE804–1 | 1,095,506 | 1,152,526 | 57,021 | |||||
| Φ804–5 | 1,256,797 | 1,306,445 | 49,649 | Sp4 | 1,161,091 | 1,210,740 | 49,650 | |
| Φ804–6 | 1,341,717 | 1,403,616 | 61,900 | Sp5 | 1,246,012 | 1,308,719 | 62,708 | stx2-prophageb |
| PLE804–2 | 1,465,380 | 1,552,938 | 87,559 | SpLE1 | 1,370,456 | 1,456,704 | 86,249 | |
| Φ804–7 | 1,637,715 | 1,679,972 | 42,258 | Sp6 | 1,541,470 | 1,589,892 | 48,423 | Inversion terminus |
| Φ804–8 | 1,733,959 | 1,755,078 | 21,120 | Sp7 | 1,594,570 | 1,610,032 | 15,463 | |
| Φ804–9 | 2,097,886 | 2,142,115 | 44,230 | Sp8 | 1,618,153 | 1,665,049 | 46,897 | Indel-4 |
| Φ804–10 | 2,142,116 | 2,187,895 | 45,780 | Sp9 | 1,757,506 | 1,815,680 | 58,175 | Indel-4 |
| Φ804–11 | 2,366,081 | 2,417,801 | 51,721 | Sp10 | 1,921,414 | 1,972,525 | 51,112 | |
| Φ804–12 | 2,523,534 | 2,583,605 | 60,072 | Sp11 | 2,158,174 | 2,203,951 | 45,778 | |
| Φ804–13 | 2,675,768 | 2,722,663 | 46,896 | Sp12 | 2,203,952 | 2,250,093 | 46,142 | |
| Φ804–14 | 2,730,784 | 2,746,246 | 15,463 | Sp13 | 2,592,901 | 2,614,020 | 21,120 | |
| Φ804–15 | 2,750,924 | 2,801,116 | 50,193 | Sp14 | 2,668,007 | 2,712,035 | 44,029 | Inversion terminus |
| PLE804–3 | 2,828,472 | 2,843,243 | 14,772 | SpLE2 | 2,738,079 | 2,751,537 | 13,459 | |
| Φ804–16 | 2,987,650 | 3,036,836 | 49,187 | Sp15 | 2,895,926 | 2,943,804 | 47,879 | stx1-prophagec |
| Φ804–17 | 3,287,328 | 3,295,878 | 8551 | Sp16 | 3,192,983 | 3,201,533 | 8551 | |
| Φ804–18 | 3,570,310 | 3,594,556 | 24,247 | Sp17 | 3,475,965 | 3,500,163 | 24,199 | |
| PLE804–4 | 3,946,431 | 3,969,884 | 23,454 | SpLE3 | 3,852,036 | 3,875,489 | 23,454 | |
| PLE804–5 | 4,675,258 | 4,718,713 | 43,456 | SpLE4 | 4,580,864 | 4,624,313 | 43,450 | |
| Sp18 | 5,040,843 | 5,079,601 | 38,759 | Mu-like prophagea | ||||
| PLE804–6 | 5,402,757 | 5,412,991 | 10,235 | SpLE5 | 5,347,085 | 5,357,319 | 10,235 | |
| PLE804–7 | 5,413,043 | 5,447,190 | 34,148 | SpLE6 | 5,357,371 | 5,391,518 | 34,148 | |
aThe Mu-like prophage is capable of transposition
bFunctional phage
cDoes not produce functional phage
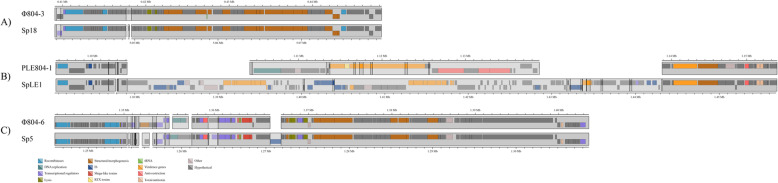
Temperate prophage Mu exhibits transposable activity within the host chromosome [36]. The Mu-like prophage in Sakai (Sp18) is 38.76 kbp in length and is integrated within a putative sorbose operon, disrupting the sorbose operon and specifically locus sorM [37]. Mu-like prophage Φ804–3 was 39.18 kbp in length and was integrated in an intergenic region separating loci prpD and prpE. The Mu-like prophage shared 37.97 kbp of (96.52%) sequence identity (Fig. 2).
Fig. 2.
Alignment of distinctive prophage and the functions of predicted genes in EHEC strains FRIK804 and Sakai. a Alignment of Mu-like prophage Φ804–3 and Sp18. b 804PLE-1 and SpLE1 showed conserved flanking regions and divergent core sequences. c Alignment of stx2-prophage Φ804–6 and Sp5 found overall sequence homology disrupted by several regions of low sequence similarity
Indel-1 (PLE804–1) was a 57.02 kbp region present in FRIK804 and absent in Sakai. Indel-1 disrupted serW encoding for serine tRNA. Alignment of the nucleotide sequence of indel-1 from FRIK804 with the nucleotide sequences of PLE in the Sakai genome (SpLE1-SPLE6) identified common flanking regions shared with SpLE1 (Fig. S1). On this basis, indel-1 was classified as a PLE and designated as PLE804–1. Indel-2 was a 7.46 kbp region present in Sakai but absent in FRIK804 and was not recognized as a MGE. A majority of the ddp operon and dosP were within this region. The ddp operon contains genes encoding for D-ala-D-ala transport and a dipeptididase [38, 39]. dosP is a predicted pseudogene.
Comparison of the stx2-prophage in FRIK804 (Φ804–6) and Sakai (Sp5) was conducted due to its central role in human pathogenesis. Sp5 measured 62.71 kbp in length while Φ804–6 was 61.90 kbp in length. The prophage shared 58.10 kbp (90.4%) of common sequence (Fig. 2). Alignment of the prophage was interrupted at several locations; including key phage regulatory regions encoding for repressors CI and Cro, replication proteins O and P, and anti-terminator N found in non-conserved regions. Strain Sakai had an IS629 element inserted downstream of stx2 in Sp5 that was absent in Φ804–6. A broader comparison of Φ804–6 with other stx2-prophage identified closest sequence homology with phage 933 W, the stx2-prophage present in the genome of EHEC strain EDL933 [40].
An inversion measuring 1.15 Mbp disrupted the alignment of the FRIK804 and Sakai chromosomes. The inverted segment in FRIK804 relative to strain Sakai centered around the terminus of replication region. Sequence motifs associated with termination of replication within the inversion included dif and four Ter sites (TerA, TerB, TerC, and TerD) (Table S1). dif was medially situated with respect to the inversion, resulting in approximate symmetry with respect to both replichores. Replichores 1 and 2 were 2894.4 kbp and 2603.8 kbp in length in Sakai while replichores 1 and 2 in FRIK804 were 2970.9 kbp and 2583.0 kbp, respectively. The inversion terminated bilaterally within prophage in both strains. Termini were present within prophage regions Φ804–7 and Φ804–15 in FRIK804, and their chimeric counterparts Sp6 and Sp14 in Sakai (Fig. 3). The sequences of Φ804–7 and Φ804–15 were searched for the presence of repeat sequences greater than 100 bp in length. Sixteen inverted repeat sequences were shared between the prophage (Table S2). A 174 bp repeat sequence precisely flanking the boundaries of the inversion in both Φ804–7/Φ804–15 and Sp6/Sp14 was identified. To confirm the precise boundaries of the inversion, two pairs of oligonucleotide primers were designed to amplify the repeat sequence and flanking regions in Sp6 (ECs_1507-F/ ECs_1508-R) and Sp14 (ECs_2759-F/ECs_2760-R) using PCR (Fig. S1). No amplification was observed using gDNA extracted from FRIK804. Exchange of primers specific to sequences within the inversion (ECs_2760-R/ECs_1508-R and ECs_1507-F /ECs_2759-F) resulted in amplification of appropriate size amplicons when using gDNA extracted from FRIK804 only.
Fig. 3.
Alignment of prophage Φ804–7 and Φ804–15 and the locations of 16 inverted and direct repeat sequences, including the flanking ends of the inversion. Inverted and direct repeat sequences ≥100 bp in length were evaluated as potential sites of recombination (blue). The inverted segment of the FRIK804 chromosome relative to the Sakai chromosome was flanked by a pair of repeats, the site of the crossover of the inversion is shown in red. To better show alignment, the orientation of Φ804–15 is inverted relative to Φ804–7
Whole-genome mapping
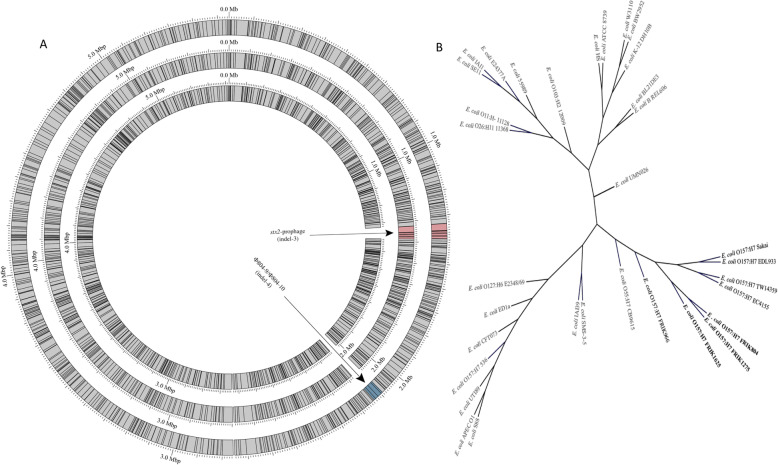
Whole-genome mapping (also known as optical mapping) produced ordered restriction maps of each farm X strain. Mapping of the chromosome provided a better understanding of the chromosome rearrangements that distinguished each strain. Whole genome mapping was also valuable for verification of genome assembly of the FRIK804 chromosome. Maps were prepared using the restriction enzyme NcoI. FRIK804, FRIK1275, and FRIK1625 had 559, 548, and 542 fragments, respectively, that were greater than 2.0 kbp in length (Fig. 4a). Based on the sum of the length of the fragments, the chromosome lengths were estimated to be 5.494 (FRIK804), 5.440 (FRIK1275), and 5.349 (FRIK1625) Mbp. A side-by-side comparison of mapping data from each strain revealed collinear chromosomes disrupted by two indels (indel-3 and indel-4). The presence or absence of these indels served to distinguish each strain. Indel-3 and indel-4 were estimated to be 61 and 48 kbp in length, respectively. Both indels were present in FRIK804 and absent in FRIK1625. FRIK1275 possessed indel-3 but lacked indel-4. Guided by the nucleotide sequence of FRIK804, the position of indel-3 in FRIK1625 was consistent with the absence of the stx2-prophage. The location of indel-4 corresponded with portions of two adjacent prophage in FRIK804, Φ804–9 and Φ804–10. Pairwise alignment scoring of the ordered restriction maps of the three farm X strains and maps of 30 other E. coli strains was used to assess similarity via hierarchical clustering. Farm X strains clustered in a single clade (Fig. 4b).
Fig. 4.
a NcoI restriction site maps of the chromosomes from FRIK804 (outer), FRIK1275 (middle), and FRIK1625 (inner). An alignment and comparison of farm X strains detected the presence of two indels which distinguished each strain. The identity of each indel was determined using the nucleotide sequence of FRIK804. The location of indel-3 was consistent with the absence of the stx2-prophage in FRIK1625. Indel-4 was determined to overlap portions of two adjacent prophage, Φ804–9 and Φ804–10. b Hierarchical clustering and pairwise alignment scoring of NcoI chromosome restriction maps was used to assess relative similarity of the three farm X strains with 30 other E. coli. EHEC O157:H7 strains grouped together (black), and farm X strains (underlined) formed a cluster (bold), indicating that these strains were closely related to one another. FRIK966 is a lineage group II strain included for comparative purposes
Plasmid content
All three farm X strains contained pO157. FRIK804 also contained three smaller plasmids: pFRIK804–1, pFRIK804–2, and pFRIK804–3. Draft genome assemblies were produced using SPAdes with Illumina sequencing data and iteratively polished using Pilon. The FRIK1275 and FRIK1625 assemblies had contigs representing pO157 and pFRIK804–3, but contigs for pFRIK804–1 and pFRIK804–2 were absent.
Inter-prophage deletion in FRIK1275 and FRIK1625
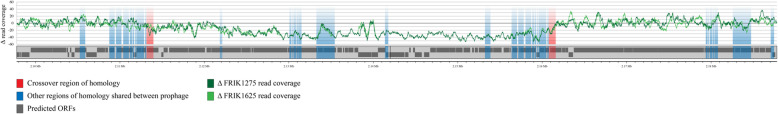
Whole-genome mapping identified an inter-prophage deletion in adjacent prophage Φ804–9 and Φ804–10 (indel 4) in FRIK1275 and FRIK1625. To precisely determine the boundaries of the absent prophage region in FRIK1275 and FRIK1625, Illumina sequencing data from each strain (including FRIK804) were aligned to the nucleotide sequence of Φ804–9 and Φ804–10 using Bowtie (Fig. 5). Divergence in read coverage was calculated between FRIK804 and both FRIK1275 and FRIK1625. Read coverage found a 47-kbp deletion that spanned prophage Φ804–9 and Φ804–10 in both strains.
Fig. 5.
Detection of the boundaries of inter-prophage deletion (indel-4) in Φ804–9/Φ804–10 present in FRIK1275 and FRIK1625. Short-read Illumina sequencing data from each strain was aligned to the nucleotide sequence of Φ804–9 and Φ804–10 from FRIK804. The difference in read coverage in FRIK1275 (dark green) and FRIK1625 (light green) relative to FRIK804 was determined at each location. Additionally, repeat sequences shared between Φ804–9 and Φ804–10 that were ≥ 100 bp (blue) were determined and mapped to identify potential sites of recombination. The difference in read coverage in ΔFRIK1275 and ΔFRIK1625 was below zero in the region of the deletion and terminated in direct repeats (shaded red) that flanked the deleted 47.7 kbp fragment. Predicted ORFs in Φ804–9 and Φ804–10 are shown in dark gray
Twenty-three direct repeat sequences of 100 bp or greater in length were shared between the two adjacent prophage Φ804–9 and Φ804–10 (Table S3). An 822 bp direct repeat was situated at both ends of the region missing in FRIK1275 and FRIK1625. This suggested that homologous recombination between the two repeat sequences was responsible for the deleted region in FRIK1275 and FRIK1625 (indel 4, Fig. 4a). The predicted location and function of the remaining Φ804–9 and Φ804–10 genes, in FRIK1275 and FRIK1625, aligned with those in FRIK804 (Fig. S3). The 822 bp repeat overlapped with a gene predicted to encode for a phage anti-repressor protein (Table S4). PCR amplification of the region was performed using oligonucleotide primers specific to sequences flanking the repeat sequence (ECs_2180-int-F and ECs_2272-R). Amplification was observed using gDNA extracted from FRIK1275 and FRIK1625 (Fig. S2). Because of the excessive length, an amplicon was not observed using gDNA extracted from FRIK804 (> 47.7 kbp).
FRIK804 harbors a greater number and overall length of repetitive sequences than nonpathogenic E. coli K12 strain MG1655
The abundance of repeat sequences in the chromosome of FRIK804 was quantified using a custom program written in Perl. Briefly, a sliding-window of 75-mer nucleotide sequences were iteratively hashed to the chromosome coordinate occupied by that sequence. Sequences present in only one location or those lacking a reverse complement in the hash table were discarded. The distribution of repeat sequences was determined using the start and end coordinates of chromosome regions and repeat sequence(s). The categories of chromosome elements were prophage, PLE, IS, rRNA, tRNA, and rearrangement hot spot (Rhs) elements. There were 5,402,917 unique 75-mer sequences in the FRIK804 chromosome (5,554,243 bp in length) in which 112,206 were present two or more times irrespective of orientation. The majority (67.81%) of 75-mer repeats were present in prophage and PLE (Fig. 7a), followed by IS (14.58%) and rRNA (9.01%). MG1655 possessed fewer repeat sequences overall. The MG1655 chromosome had 38,188 75-mer sequences present more than once and 458,4562 unique sequences (4,641,652 bp in length). There were 24,249 repeat sequences present two or more times, irrespective of orientation. The greatest number of repeats were located within IS (40.45%) followed by rRNA (30.18%), prophage (5.42%), and tRNA (0.15%).
Fig. 7.
The abundance, complexity, and total length of repeats in FRIK804 compared with nonpathogenic E. coli strain MG1655. Repeat sequences that were ≥ 75 bp were identified and included in analyses. a Repeat sequence abundance was categorized by location and genetic element. Repeats located outside of the designated genetic elements were listed under chromosome. b Complexity was measured by binning repeat sequences according to copy number. c Merged direct (above) and inverted (below) repeat sequences were used to measure both complexity and length. d The length of repetitive regions, areas of the chromosome featuring one or more repeats, was calculated and classified by genetic element
Repeat sequence complexity was a measure of the repeat copy number irrespective of orientation, i.e. the more times repeat sequences appeared in a chromosome the greater the complexity. Measurement of the copy number of each 75-mer repeat sequence (and disregarding sequence orientation) in each strain found a greater number in FRIK804 compared to MG1655 (Fig. 7b). To further evaluate repeat sequence complexity, the locations of pairs of direct and inverted repeats were defined and termed as links. The number of links for a given direct repeat sequence was a function of where nd is the number of direct repeats, and the number of inverted links (reverse complement sequences) was ndni I, where ni is the number of inverted repeats. The pairs of start and end locations that defined each link were then aligned with their chromosome location. In FRIK804, there were 289,610 direct and 303,420 inverted links. IS accounted for the greatest number of direct links (42.68%) followed by links within prophage/PLE (34.37%) and rRNA (15.50%). IS also accounted for the greatest number of inverted (46.28%) links followed by prophage/PLE and rRNA (37.65 and 12.96%, respectively) (Fig. 7b). MG1655 possessed fewer direct (113,733) and inverted (96,478) links. IS accounted for the locations of most direct (51.46%) and inverted (55.34%) links followed by rRNA genes (direct 36.23% and inverted 38.39% inverted).
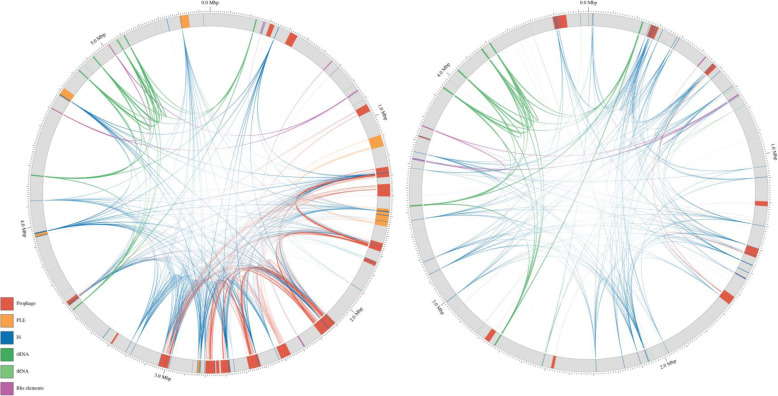
The extent and topography of repeat sequences in the chromosome were examined by merging pairs of direct and inverted links that were adjacent to one another, mapping their chromosome locations and connecting links by lines that were plotted using Circos (Fig. 6). Merged links were both more abundant and longer in FRIK804 compared to MG1655 (Fig. 7c). There were 1075 direct and 1241 inverted merged links in FRIK804. The maximum and median direct repeat lengths were 10,011 and 134 bp, respectively, and for inverted repeats, the maximum length was 4729 and the median length was 141 bp. In MG1655, there were 407 direct and 234 inverted merged links identified. The maximum repeat length for direct repeats was 2816 bp with a median of 144 bp, and for inverted repeats, the maximum length was 3024 bp and median was 245 bp.
Fig. 6.
Locations of direct and inverted contiguous repeats ≥75 bp in length in the chromosomes of FRIK804 (left) and E. coli strain MG1655 (right). The chromosome element in which the repeat sequence is located is denoted by color; prophage (red), PLE (orange), IS (blue), rRNA (dark green), tRNA (light green), and rhs elements (purple)
Repetitive regions of the chromosome were defined as areas containing one or more repeat sequences. To evaluate repeat sequences on the basis of length rather than copy number (e.g., complexity), the length of each annotated chromosome region occupied by repetitive regions were determined. A total of 417,747 bp (7.52%) of the FRIK804 chromosome consisted of repetitive regions. These regions were predominantly located within prophage/PLE (5.22%) (Fig. 7d) followed by IS (0.97%). Strain MG1655 had a total length of 117,294 bp (2.53%) of repetitive regions that were most commonly associated with IS (0.92%) and rRNA genes (0.69%).
stx2-prophage excision site in FRIK1625
The site of integration of the stx2-prophage is specific in each EHEC lineage [41, 42], with the stx2-prophage integrating into wrbA in LI and I/II strains. Prophage excision requires both Int and excisionase (Xis) activity, resulting in restoration of attP and attB sites [40]. A putative attB site within wrbAEDL933 was previously identified by Plunkett et al. [40]. Comparison of the nucleotide sequence of wrbA from the FRIK1625 with wrbA from a LI/II strain (without stx2 prophage) found 100% sequence identity (data not shown). This shows that if the stx2-prophage was present in FRIK1625, excision was mediated by Int/Xis activity rather than homologous recombination, and excision occurred without subsequent lysis of the host.
Detection of stx2 transcript in FRIK1275 (stx2::IS629)
Identification of different EHEC strains from farm X was previously determined using XbaI restriction enzyme digest profiles (REDP) generated using PFGE [43]. A majority of EHEC isolates from farm X during the last year of visits to this farm had a common REDP profile, and FRIK1275 is a representative isolate from this group [43]. PCR amplification of stx2 from strains with this common REDP (80 samples) had IS629 inserted in stx2 [32]. Since Stx2 production and release is linked with prophage induction [44], the farm X strains were tested for transcript of stx2 and a downstream gene encoding for a putative terminase. Three RT-PCR targets were designed. Primers stx2-US-RT-F/R and stx2-DS-RT-F/R targeted regions of stx2 immediately upstream and downstream of IS629. The identification of suitable targets downstream of stx2::IS629 was hampered by repeat sequences shared between the stx2-prophage and other prophage and PLE in the chromosome; however, a suitable target was identified in a gene annotated as a terminase (primers ECs_1220-RT-F/R). Amplification of a portion of the 16S rRNA gene (primers 16S-RT-F/R) was included as a control. Following prophage induction with MMC, amplification of both stx2-prophage targets and the downstream terminase was detected in RNA extracted from FRIK804 and FRIK1275, demonstrating that IS629 in stx2 did not abolish the production of transcript from stx2 and the downstream terminase in FRIK1275 (Table 3). Amplification using RNA extracted from cultures of FRIK1625 did not result in amplification of targets since it lacked the stx2-prophage.
Table 3.
RT-PCR amplification of stx2-prophage markers following induction with mitomycin C
| Strain | |||
|---|---|---|---|
| Target | FRIK804 | FRIK1275 | FRIK16215 |
| stx2-US-RT-F/R | + | + | – |
| stx2-DS-RT-F/R | + | + | – |
| ECs_1220-RT-F/R | + | + | – |
| 16S-F/R | + | + | + |
Discussion
Epidemiological investigations of EHEC outbreaks have noted REDP variations in strains isolated from implicated foods and clinical stool samples [45, 46]. The presence of multiple cryptic prophage regions in the EHEC genome are thought to serve as recombination hotspots; however, a detailed understanding of the underlying molecular event(s) that lead to the observed chromosomal alterations is lacking, particularly in isolates from the bovine reservoir [47, 48]. In this study, a precise examination of chromosome modifications in a chronological set of E. coli O157:H7 strains from a Wisconsin dairy farm (farm X) was conducted. The three strains, each with a unique REDP, belonged to LI and were isolated over a period of approximately 2 years from farm X. FRIK804 was the first E. coli O157:H7 strain isolated from the farm and was found in multiple cattle fecal samples over a two-month period [49]. FRIK1275 was isolated roughly 2 years later than FRIK804 over a 7-month period and was recovered from feed, water, and cattle [43, 49]. FRIK1625 was isolated from a single fecal sample in the last year of the study. Findings from these analyses found that the presence, absence, and location of MGE, (i.e., plasmids, prophage, and IS elements) accounted for the genomic differences among the strains. Furthermore, direct and inverted repeat sequences commonly found in prophage and PLE in EHEC played a central role in the chromosome changes in the farm X strains.
Analysis of MGE in draft E. coli O157:H7 genomes assembled using short-read DNA sequence data (Illumina) was complicated by repeat sequences found in multiple regions of the chromosome. The assembly of the FRIK804 genome was accomplished using SMRT long-read sequencing data and improved using short-read data. Validation of the finished sequence assembly was conducted using whole-genome mapping data (optical mapping). Pairwise alignment of the ordered restriction maps and hierarchical clustering determined the farm X strains comprised a single clade of strains (Fig. 4b).
Genome diversity in EHEC is associated with MGE [37, 50], particularly prophage and PLE. By length, the largest difference between FRIK804 and strain Sakai was a 1.15 Mb inversion in which inverted repeat sequences were identified at the boundaries in a pair of chimeric prophages. The inversion was nearly symmetrical with respect to the axis of replication (defined by dif and oriC). This is important since inversion of the Ter (terminus of replication) region can stall or stop replication forks and induce the SOS response in E. coli [51, 52]. Inversions spanning the terminus of replication region have been found in the chromosomes of EHEC and other enterics and linked to pairs of inverted repeats [48, 53, 54]. The persistence of this clade of strains on farm X, with the inversion relative to strain Sakai, indicates the inversion likely had no or little impact. Other differences were the integration sites of a Mu-like prophage, the presence of an additional PLE in FRIK804, and a 7.46 kbp region not associated with MGE that was present in Sakai and absent in FRIK804 (Fig. 1). Comparison of prophage homologs occupying the same chromosomal site in the two strains identified regions of reduced sequence similarity in otherwise conserved prophage. Exchange of portions of phage genomes by homologous recombination has been previously observed and attributed to phage-encoded recombinases with relaxed fidelity [55–57]. Both FRIK804 and Sakai harbored the pO157 virulence plasmid and a small plasmid (pFRIK804–3) sharing 100% sequence similarity. FRIK804 possessed two other plasmids (pFRIK804–1 and pFRIK804–2). pFRIK804–1 carried genes for production and immunity to colicin D. No predicted phenotype was ascribed to pFRIK804–2. IS629 was the most numerous recognized IS in both chromosomes. Although the locations of a majority of IS629 elements were conserved between the two chromosomes, variability in copy number and location was in agreement with previous reports suggesting relatively high frequencies of transpositional activity [58, 59].
Analysis of the three farm X strains determined that FRIK1275 and FRIK1625 shared a common plasmid profile with Sakai. In addition, FRIK1275 and FRIK1625 shared a common deletion (47.7 Kbp) in two adjacent prophage Φ804–9/Φ804–10 in comparison to FRIK804 (indel 4, Fig. 4a). The IS629 content of the farm X strains was similar. One important difference noted in FRIK1275 was the insertion of IS629 in stx2 (stx2::IS629). FRIK1625 lacked the stx2-prophage (indel 3) suggesting non-lethal excision of the stx2-prophage. Loss of the stx2-prophage has been observed before during laboratory passage [60, 61].
Detailed analysis of the 47.7-kbp deletion in FRIK1275 and FRIK1625 was conducted by alignment of short-read sequence data to the intact sequence of adjacent prophage Φ804–9 and Φ804–10 from FRIK804. A comparison of the difference in read coverage between strains FRIK1275 and FRIK1625 with that of FRIK804 (no deletion) enabled demarcation of the deletion boundaries (Fig. 5). The difference in read coverage relative to FRIK804 (< 0) terminated in direct repeats that flanked the deletion boundaries. Similar deletions in Sakai involving Sp11 and Sp12 in Sakai have been observed in laboratory conditions [62]. The propensity for deletions in this region may be due to the proximity of the two prophages.
Homologous recombination is a process fundamental to DNA replication, repair, and horizontal gene transfer. The frequency of recombination between homologous repeat sequences increases with the length of the repeat in a biphasic manner [63]. The inflection point in this curve is 74 bp, below which there is a dramatic decrease in recombination frequency. Based on these findings, Perl scripts were written to detect repeat sequences ≥75 bp in length. We did not address approximate repeats in DNA sequences because of the extensive number of homologous sequences present in the O157:H7 genome and the dramatic decrease in the frequency of recombination when mismatches are present within the repeats [63].
The chromosome inversion present in farm X strains relative to the Sakai strain and the partial deletion of Φ804–9/Φ804–10 present FRIK1275 and FRIK1625 both involved repeat sequences. Analysis of direct and inverted repeat sequences ≥75 bp was conducted using Perl scripts written to evaluate the abundance, location, and complexity of repeat sequences [GitHub (http://github.com/eliotstanton/)]. There was a greater abundance of repeat sequences in FRIK804 in comparison to non-pathogenic E. coli K-12 strain MG1655 (Figs. 6 and 7). In FRIK804, the abundance of 75mer repeat sequences was most prominent in prophage/PLE regions. The complexity of repeat sequences (includes copy number of both direct and inverted repeat sequences) was most commonly associated with IS elements. Analysis of areas of the chromosome containing one or more repeats (repeat regions) found that most repeat regions were located within prophage/PLE. In MG1655, the abundance and complexity of repeat sequences were mostly associated with IS elements. PLE were not identified and comparatively few repeat sequences were located in prophage regions.
IS integration can result in polar mutations [64]. The production of functional phage by FRIK1275 (stx2::IS629) indicated that genes downstream of stx2::IS629 (encoding for lysis, head, and tail proteins) were expressed. Transcripts from genes upstream and downstream of the stx2::IS629 were detected by RT-PCR although Stx2 was not detected by Western blot [32]. Phage from FRIK1275 (stx2::IS629) formed plaques on host strain MG1655, and PCR amplification of material from individual plaques generated amplicons with a size consistent with the presence of stx2:: IS629. This indicated that phage production and plaque formation was not the result of excision of IS629 and the restoration of phage function. FRIK1275 (stx2::IS629) was the dominant strain isolated from farm X [43] over a 7-month period of time indicating that Stx2 production was not required for dominance or persistence of EHEC within cattle and the farm environment.
Conclusion
The results of this study support and illustrate the contribution of MGE (i.e., plasmids, prophage, PLE, and IS) to genome diversity in EHEC from cattle and the farm environment. Detailed analysis of an inversion and inter-prophage deletion provided evidence that homologous recombination between pairs of repeat sequences in prophage were involved in structural alterations to the chromosome. Analysis of repeat sequences in the genome found a greater number and complexity in FRIK804 compared to E. coli K12 strain MG1655 with a preponderance of the repetitive sequences present in MGE. The abundance and location of repeat sequences in FRIK804 may be a driver of chromosome rearrangements in EHEC.
This study contributes to our understanding of the precise molecular events contributing to genomic diversity in wild-type EHEC strains from the bovine and farm environments.
Methods
Strains
The EHEC strain Sakai (RIMD 0559952) is a well characterized lineage group I strain that was used as a standard reference for comparison purposes (Accession: BA000007.2)(10.1093/dnares/8.1.11). EHEC strains FRIK804, FRIK1275 and FRIK1625 also belong to lineage group I and were isolated from bovine fecal samples on farm X (PMCID: PMC106160). FRIK966 was used as a representative lineage group II strain isolated from farm R in Wisconsin [49]. E. coli K-12 strain MG1655 was from Dr. Tricia Kiley. Stocks of all strains were maintained at − 70 °C in LB (Luria broth, BD Difco, Houston TX) with 20% glycerol.
Media and buffers
LB was used for propagation of E. coli strains. LB agar was used for resuscitation of strains from frozen storage. LB soft agar consisted of LB, agar (6.0 g/L) and CaCl2 (10 mM). SM buffer (100 mM NaCl, 8 mM MgSO4, and 50 mM Tris-HCl) was used to serially dilute phage lysates. For SMRT sequencing of FRIK804, cells were grown in M9 medium (BD Difco, Houston, TX).
Whole-genome mapping of farm X strains
Ordered restriction maps (also known as optical maps) of the chromosomes from farm X strains were conducted by OpGen (Gaithersburg, MD) using restriction enzyme NcoI as outlined by Zhou et al. [65]. Structural differences in the chromosome of each strain were first resolved by map alignment using Argus MapSolver software. Alignment scoring data of in silico maps of other E. coli and the farm X strains was obtained from MapSolver and used to create a similarity matrix. Hierarchical clustering was performed using UPGMA in R to create an unrooted tree illustrating the relative similarity of maps from each strain [66].
Illumina sequencing of farm X strains
Strains were individually inoculated into LB directly from frozen stock cultures maintained at − 70 °C. Following incubation overnight at 37 °C, cells were harvested by centrifugation. Genomic DNA was prepared using MasterPure Complete DNA and RNA Purification Kit (Epicentre, Madison, WI). Samples were treated with RNAse A (Thermo Fisher Scientific, Waltham, MA) and incubated for 30 min at 37 °C to remove RNA. The manufacturer’s protocol was modified with regards to precipitation of DNA to include an overnight incubation in 70% ethanol at − 20 °C. DNA samples were then submitted to the University of Wisconsin-Madison Biotechnology Center. DNA concentration was verified using the Qubit® dsDNA HS Assay Kit (Life Technologies, Grand Island, NY). Samples were prepared according to the TruSeq Nano DNA LT Library Prep Kit (Illumina Inc., San Diego, CA) with minor modifications. Samples were sheared using a Covaris M220 Ultrasonicator (Covaris Inc., Woburn, MA), and were size selected for an average insert size of 550 bp using SPRI bead-based size exclusion. The quality and quantity of the finished libraries were assessed using an Agilent High Sensitivity DNA kit and Qubit® dsDNA HS Assay Kit, respectively. Libraries were standardized to 2 nM, and paired-end 250 bp sequencing was performed using the Illumina MiSeq Sequencer and a MiSeq 500 bp (v2) sequencing cartridge. Images were analyzed using the standard Illumina Pipeline, version 1.8.2.
SMRT sequencing of FRIK804
FRIK804 was inoculated into M9 media from a single colony on a LB agar plate and incubated overnight at 37 °C. Cells were harvested by centrifugation and washed 4 times using sterile 10% glycerol. gDNA from washed cell pellets was purified using the method “bacterial genomic DNA isolation using CTAB” from JGI protocol (version 3) (https://jgi.doe.gov/user-programs/pmo-overview/protocols-sample-preparation-information/jgi-bacterial-dna-isolation-ctab-protocol-2012/). The gDNA sample was submitted to the University of Wisconsin-Milwaukee Great Lakes Genomic Center. A standard Pacific Biosciences large insert library was prepared by fragmenting DNA to approximately 20 kb using g-TUBEs (Covaris, Woburn, MA). Fragmented DNA was enzymatically repaired and ligated to a PacBio adapter to form the SMRTbell Template. Templates larger than 10 kb were size selected using BluePippin (Sage Science, Beverly, MA). Templates were annealed to a sequence primer, bound to polymerase (P6), and then bound to PacBio Mag-beads and SMRTcell sequenced using a RSII sequencer and C4 chemistry.
Genome assembly
Draft genome assemblies of each farm X strain were produced using Illumina short-read data and the genome assembler SPAdes 3.11.1 [33]. Corrected paired-end reads were aligned to the assembly using Bowtie 1.1.2 [67]. SAM files were reformatted using Sequence Alignment/Map (SAM) tools (http://samtools.sourceforge.net), and Pilon 1.22 [35] was used to identify and resolve sequence variants. Improvement of the draft assemblies was iteratively performed until no sequence variants were found by Pilon. Contigs smaller than 1.0 kb or with kmer coverage less than 20 were excluded from final draft assemblies.
The FRIK804 genome was also assembled using PacBio long-read data and Canu 1.7 [34]. Iterative improvement of the assembly was performed as previously outlined. Circularization of the chromosome was performed manually using BLASTn [68–70] to identify overlapping regions. Validation of the assembly was confirmed by generating an in silico whole-genome map of NcoI restriction sites and comparing it to map generated from the FRIK804 chromosome to ensure that the two maps were congruent.
Genome annotation and prophage identification
Contigs from the complete FRIK804 genome and draft genomes of FRIK1275 and FRIK1625 were automatically annotated using RAST [71, 72]. Prophage and PLE regions in FRIK804 were identified using the published start and end locations of prophage and PLE in strain Sakai and BLASTn [65].
Nucleotide accession sequence numbers
The genome sequences of the E. coli O157:H7 strains have been deposited in GenBank; FRIK804 under the accession numbers CP034384-CP034388, FRIK1275 under RWJR00000000 and FRIK1625 under RWJQ00000000.
Whole genome alignment and comparisons
Alignment of the FRIK804 and Sakai chromosomes was performed using progressiveMauve [73] and BLASTn [68–70]. To better identify common and divergent regions, alignment data from progressiveMauve was formatted using custom Perl scripts to format data for visualization using Circos 0.69 [74]. Common sequence identity shared between genome regions was calculated using the BLAST global alignment interface (Needleman-Wunch). All custom Perl scripts written for this study are available on GitHub (http://github.com/eliotstanton/).
PCR amplification of inversion termini
The boundaries of the inversion present in strains of the farm X clade, with respect to Sakai, were verified using oligonucleotide primers ECs_2759-F, ECs_22760-R, ECs_1507-R, and ECs_1508-R. All primers used in this study were manufactured by Integrated DNA technologies (Coralville, IA) and are listed in Table S5. The individual primer pairs ECs_2759-F/ECs_2760-R, ECs_1507-F/ECs_1508-R, ECs_2759/ECs_1507-R, and ECs_2760-R/ECs_1508-R were separately mixed with gDNA extracted from Sakai, FRIK804, FRIK1275, and FRIK1625. DNA was amplified using rTaq DNA polymerase (Bulldog, Portsmouth, NH) and PCR conditions used were 94 °C for 5 min, followed by 35 cycles consisting of 94 °C for 30 s, 51 °C for 30 s, and 72 °C for 3 min, and concluded by 72 °C for 5 min. Amplicons were visualized using agarose (1.0%) gel electrophoresis and ethidium bromide staining.
PCR amplification of regions of inter-prophage deletions
The boundaries of the inter-prophage region present in FRIK804 but absent in FRIK1275 and FRIK1625 was verified using oligonucleotide primers (Table S5). ECs_2183-F and ECs_2261-int-R. gDNA extracted from FRIK804, FRIK1275, and FRIK1625 was amplified using Phusion DNA polymerase (New England Biolabs, Ipswich, MA). PCR conditions used were 98 °C for 30 s followed by 30 cycles consisting of 98 °C for 15 s, 66 °C for 20 s, and 72 °C for 60 s. PCR was concluded by 72 °C for 5 min. Amplicons were visualized using agarose (0.8%) gel electrophoresis and ethidium bromide staining.
RNA extraction
In three separate trials, overnight cultures of FRIK804, FRIK1275, and FRIK1625 were incubated overnight at 37 °C. OD600 of overnight cultures was measured and inoculated into fresh LB at OD600 = 0.01. Cultures were inoculated in duplicate, to provide a negative control, at 37 °C with shaking (100 RPM) for 2.25 h. At this point OD600 of cultures was measured prior to addition of mitomycin C (Dot Scientific, Burton, MI) at a final concentration of 1.0 μg/ml. Cultures were incubated for one additional hour prior to measuring OD600 of cultures, collection of cells by centrifugation at 4 °C, and disruption of cells by the addition of TRIzol (Thermo Fisher, Waltham, MA). Samples containing TRIzol were stored at − 70 °C until RNA extraction.
RNA from each frozen TRIzol sample was extracted according to the manufacturer’s instructions. Extracted RNA quality and quantity was inspected by measurement of absorbance at 230 nm, 260 nm, and 280 nm. Residual DNA contamination was removed using RQ1 DNase (Promega, Madison, WI) in accordance with manufacture’s protocol. Following DNase treatment nucleic acid concentration of samples was adjusted to 10 ng/μl.
RT-PCR
Primers (Table S5) targeting regions immediately upstream (stx2-US-RT-F/R) and downstream (stx2-DS-RT-F/R) of the IS629 insertion in the FRIK1275 copy of stx2 were used. Primers targeting an additional gene annotated as a phage terminase that was located downstream of stx2 were also used (ECs_1220-RT-F/R). Amplification of 16S rRNA (16S-RT-F/R) was used to provide positive and negative controls. One-step RT-PCR using AccessQuick RT-PCR System (Promega, Madison, WI) was performed consisting of cDNA synthesis at 45 °C for 45 min followed by DNA synthesis consisting of 94 °C for 2 min, and the following cycle conditions 94 °C for 30 s and 57 °C for 30 s. 16S-RT-F/R marker was amplified for 19 cycles and stx2-US-RT-F/R, stx2-US-RT-F/R, and ECs_1220-RT-F/R markers were amplified for 23–25 cycles. A final extension step consisting of 68 °C for 5 min was included for all reactions performed. Amplicons were visualized using agarose (1.5%) gel electrophoresis and ethidium bromide staining.
Analysis of IS629 stability during stx2-phage propagation
In three separate trials, FRIK1275 was incubated overnight at 37 °C. One mL of overnight culture was transferred into 9.0 ml of LB broth in 250 mL Erlenmeyer flasks and incubated at 37 °C with shaking (100 RPM). Following incubation for 4 h, supernatant containing spontaneously produced phage was collected following centrifugation. Supernatant was sterilized using 0.22 μm PVDF filters (Millipore, Burlingame, MA). Concurrently, MG1655 was prepared as a host cell suspension. Upon reaching mid-log phase (OD600 = 0.4–0.6), MG1655 was centrifuged, washed with SM buffer, and resuspended to an OD600 = 2.5 using SM buffer before storage at 4 °C. Serial dilution of phage lysate was performed using SM buffer. In triplicate, 100 μL of each diluted sample was co-incubated with an equal volume of MG1655 cell suspension at 37 °C for 20 min. Three ml of soft agar (48 °C) was mixed with each sample and immediately poured onto pre-warmed LB agar plates. Plates were allowed to cool on the bench for 15 min before overnight incubation at 37 °C.
Twenty-four plaques were picked at random from each trial and material from the plaque was transferred to 10 μL of nuclease-free H2O. DNA was amplified using rTaq DNA polymerase (Bulldog, Portsmouth, NH) and stx2a-F/R primers (Table S5). PCR conditions were 94 °C for 10 min, followed by 30 cycles consisting of 94 °C for 30 s, 53 °C for 30 s, and 72 °C for 1 min, amplification was concluded by 72 °C for 5 min. Amplicons were visualized using agarose (1.0%) gel electrophoresis and ethidium bromide staining. The presence or absence of IS629 was determined by amplicon size.
Supplementary information
Additional file 1: Fig. S1. PCR confirmation of inverted repeats present at the flanking ends of the inversion in farm X strains (FRIK804, FRIK1275, and FRIK1625) and control strain Sakai. a Primer pairs ECs_1507-F/ECs_1508-R and ECs_2759-F/ECs_2760-R were specific to Sp6 and Sp14 in Sakai. Primer pairs ECs_1507-F/ECs_2759-F and ECs_1508-R/ECs_2760-R were specific to regions of Φ804–7 and Φ804–15. b Amplification was observed using primer pairs ECs_1507-F/ECs_1508-R (lane 15) and ECs_2759-F/ECs_2760-R (lane 16) using gDNA extracted from Sakai. Amplification was observed using primer pairs ECs_1507-F/ECs_2759-F (lanes 4, 8, and 13) and ECs_1508-R/ECs_2760-R (lanes 5, 9, and 14) using gDNA extracted from farm X strains. Lanes 1 and 10, 1.0-kb ladder.
Additional file 2: Fig. S2. PCR confirmation of regions flanking inter-prophage deletion in FRIK1275 and FRIK1625 using PCR amplification. Lane 1: 1.0 kb ladder. gDNA in lane 2 (FRIK804), lane 3 (FRIK1275), lane 4 (FRIK1625), and lane 5 (Sakai). Amplification was observed only in strains with the inter-prophage deletion between the identified direct repeats.
Additional file 3: Fig. S3. Predicted function and location of genes in Φ804–9 and Φ804–10. The portions of the two adjacent phage in all farm X strain has a shaded grey background. The region in FRIK804 but absent in FRIK1275 and FRIK1625 has a white background.
Additional file 4: Table S1. Chromosomal locations of corresponding replication motifs in EHEC FRIK804 and Sakai. Highlighted motifs (light orange) located within the segment of the FRIK804 chromosome that is inverted relative to strain Sakai.
Additional file 5: Table S2. Location and length of inverted repeats in Φ804–7 and Φ804–15. Crossover region highlighted in light orange.
Additional file 6: Table S3. Location and length of direct repeats in Φ804–9 and Φ804–10. Crossover region of highlighted in light orange.
Additional file 7: Table S4. Location, classification, and predicted functions of genes in Φ804–9 and Φ804–10. Highlighted region is present in FRIK804 but absent in FRIK1275 and FRIK1625.
Additional file 8: Table S5. Oligonucleotide primers used in this study.
Additional file 9: Table S6. Locations of IS629 elements in FRIK804 and Sakai chromosomes.
Additional file 10: Table S7. Locations of ISEc8 locations in FRIK804 and Sakai chromosomes.
Acknowledgements
We thank Jared Godfrey for technical assistance with PCR analyses and helpful discussions, Dr. Garret Suen for guidance in genome assembly and the development of program scripts, UW-Madison Biotechnology Center for Illumina sequencing, and UW-Milwaukee Great Lakes Genomics Center for Pacific Biosciences RSII Sequencing. Ordered restriction maps (optical maps) of the chromosomes of each farm X strain were determined by OpGen (Gaithersburg, MD).
Abbreviations
- BLAST
Basic local alignment search tool
- EHEC
Enterohemorrhagic Escherichia coli
- FRIK
Food research institute-Kaspar
- gDNA
Genomic DNA
- LB
Luria broth
- MGE
Mobile genetic element
- ORF
Open reading frame
- PFGE
Pulsed field gel electrophoresis
- PacBio
Pacific biosciences
- PCR
Polymerase chain reaction
- PLE
Prophage-like element
- PPP
Prophage polymorphism profile
- REDP
Restriction endonuclease digestion profile
- RT-PCR
Reverse transcriptase-polymerase chain reaction
- SMRT
Single molecule real-time
- TER
Terminus of replication
Authors’ contributions
ES designed the study, preformed experiments, and prepared the draft of the manuscript; TW contributed to gDNA extraction, analysis of DNA sequence data and genome assembly, and contributed to manuscript preparation; DP helped design the experiments and contributed to manuscript preparation; CK coordinated study, analyzed data, wrote portions and reviewed the manuscript. All authors reviewed and approved the manuscript.
Funding
This work was supported by HATCH grant 142-AAA3661, the Food Research Institute, and the College of Agricultural and Life Sciences at the University of Wisconsin-Madison. E. Stanton was the recipient of the E. Michael and Winona Foster Distinguished Graduate Fellowship.
Availability of data and materials
The genome sequences of the E. coli O157:H7 strains have been deposited in GenBank; FRIK804 under the accession numbers CP034384-CP034388, FRIK1275 under RWJR00000000 and FRIK1625 under RWJQ00000000. All custom Perl scripts written for this study are available on GitHub (http://github.com/eliotstanton/).
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12864-020-06943-x.
References
- 1.Rangel JM, Sparling PH, Crowe C, Griffin PM, Swerdlow DL. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982-2002. Emerg Infect Dis. 2005;11:603–609. doi: 10.3201/eid1104.040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wachsmuth IK, Griffin PM, Wells JG. Escherichia coli O157:H7, a cause of hemorrhagic colitis and hemolytic uremic syndrome. Acta Paediatr Jpn. 1991;33:603–612. doi: 10.1111/j.1442-200x.1991.tb01872.x. [DOI] [PubMed] [Google Scholar]
- 3.Riley LW, Remis RS, Helgerson SD, Mcgee HB, Wells JG, Davis BR, et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 4.Borczyk AA, Karmali MA, Lior H, Duncan LMC. Bovine reservoir for Verotoxin-producing Escherichia Coli 0157:H7. Lancet. 1987;329:98. doi: 10.1016/s0140-6736(87)91928-3. [DOI] [PubMed] [Google Scholar]
- 5.Ferens WA, Hovde CJ. Escherichia coli O157:H7: animal reservoir and sources of human infection. Foodborne Pathog Dis. 2011;8:465–487. doi: 10.1089/fpd.2010.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marder EP, Garman KN, Ingram LA, Dunn JR. Multistate outbreak of escherichia coli O157:H7 associated with bagged salad. Foodborne Pathog Dis. 2014;11:593–595. doi: 10.1089/fpd.2013.1726. [DOI] [PubMed] [Google Scholar]
- 7.Wendel AM, Hoang Johnson D, Sharapov U, Grant J, Archer JR, Monson T, et al. Multistate outbreak of Escherichia coli O157:H7 infection associated with consumption of packaged spinach, august–September 2006: the Wisconsin investigation. Clin Infect Dis. 2009;48:1079–1086. doi: 10.1086/597399. [DOI] [PubMed] [Google Scholar]
- 8.Grant J, Wendelboe AM, Wendel A, Jepson B, Torres P, Smelser C, et al. Spinach-associated Escherichia coli O157:H7 outbreak, Utah and New Mexico, 2006. Emerg Infect Dis. 2008;14:1633–1636. doi: 10.3201/eid1410.071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michino H, Araki K, Minami S, Takaya S, Sakai N, Miyazaki M, et al. Massive outbreak of Escherichia coli O157: H7 infection in schoolchildren in Sakai City, Japan, associated with consumption of white radish sprouts. Am J Epidemiol. 1999;150:787–796. doi: 10.1093/oxfordjournals.aje.a010082. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson DD, Scheftel J, Cronquist A, Smith K, Woo-Ming A, Anderson E, et al. Temporally distinct Escherichia coli O157 outbreaks associated with alfalfa sprouts linked to a common seed source - Colorado and Minnesota, 2003. Epidemiol Infect. 2005;133:439–447. doi: 10.1017/s0950268804003589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breuer T, Benkel DH, Shapiro RL, Hall WN, Winnett MM, Linn MJ, et al. A Multistate Outbreak of Escherichia coli O157:H7 Infections Linked to Alfalfa Sprouts Grown from Contaminated Seeds. Emerg Infect Dis J - CDC. 2001;7:6. doi: 10.3201/eid0706.010609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller BD, Rigdon CE, Ball J, Rounds JM, Klos RF, Brennan BM, et al. Use of traceback methods to confirm the source of a multistate escherichia coli O157:H7 outbreak due to in-shell hazelnuts. J Food Prot. 2012;75:320–327. doi: 10.4315/0362-028X.JFP-11-309. [DOI] [PubMed] [Google Scholar]
- 13.Neil KP, Biggerstaff G, MacDonald JK, Trees E, Medus C, Musser KA, et al. A novel vehicle for transmission of Escherichia coli O157:H7 to humans: multistate outbreak of E. coli O157:H7 infections associated with consumption of ready-to-bake commercial prepackaged cookie dough--United States, 2009. Clin Infect Dis. 2012;54:511–8 10.1093/cid/cir831. [DOI] [PubMed]
- 14.Perna NT, Plunkett G, Burland V, Mau B, Glasner JD, Rose DJ, et al. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature. 2001;409:529–533. doi: 10.1038/35054089. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi T, Makino K, Ohnishi M, Kurokawa K, Ishii K, Yokoyama K, et al. Complete genome sequence of enterohemorrhagic Eschelichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 2001;8:11–22. doi: 10.1093/dnares/8.1.11. [DOI] [PubMed] [Google Scholar]
- 16.Bauer ME, Welch RA. Characterization of an RTX toxin from enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1996;64:167–175. doi: 10.1128/iai.64.1.167-175.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt H, Beutin L, Karch H. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect Immun. 1995;63:1055–1061. doi: 10.1128/iai.63.3.1055-1061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makino K. Complete Nucleotide Sequences of 93-kb and 3.3-kb Plasmids of an Enterohemorrhagic Escherichia coli O157:H7 Derived from Sakai Outbreak. DNA Res. 1998;5:1–9. doi: 10.1093/dnares/5.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Hofinger C, Karch H, Schmidt H. Structure and function of plasmid pColD157 of enterohemorrhagic Escherichia coli O157 and its distribution among strains from patients with diarrhea and hemolytic-uremic syndrome. J Clin Microbiol. 1998;36:24–29. doi: 10.1128/jcm.36.1.24-29.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratnam S, March SB, Ahmed R, Bezanson GS, Kasatiya S. Characterization of Escherichia coli serotype O157:H7. J Clin Microbiol. 1988;26:2006–2012. doi: 10.1128/jcm.26.10.2006-2012.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asadulghani M, Ogura Y, Ooka T, Itoh T, Sawaguchi A, Iguchi A, et al. The defective prophage pool of Escherichia coli O157: prophage-prophage interactions potentiate horizontal transfer of virulence determinants. PLoS Pathog. 2009;5:e1000408. doi: 10.1371/journal.ppat.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boerlin P, McEwen SA, Boerlin-Petzold F, Wilson JB, Johnson RP, Gyles CL. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J Clin Microbiol. 1999;37:497–503. doi: 10.1128/jcm.37.3.497-503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donohue-Rolfe A, Kondova I, Oswald S, Hutto D, Tzipori S, Donohue-Rolfe A, et al. Escherichia coli 0157:H7 strains that express Shiga toxin (Stx) 2 alone are more neurotropic for Gnotobiotic piglets than are Isotypes producing only Stx1 or both Stx1 and Stx2. J Infect Dis. 2000;181:1825–1829. doi: 10.1086/315421. [DOI] [PubMed] [Google Scholar]
- 24.Fuller CA, Pellino CA, Flagler MJ, Strasser JE, Weiss AA. Shiga toxin subtypes display dramatic differences in potency. Infect Immun. 2011;79:1329–1337. doi: 10.1128/IAI.01182-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruenheid S, Sekirov I, Thomas NA, Deng W, O’Donnell P, Goode D, et al. Identification and characterization of NleA, a non-LEE-encoded type III translocated virulence factor of enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 2004;51:1233–1249. doi: 10.1046/j.1365-2958.2003.03911.x. [DOI] [PubMed] [Google Scholar]
- 26.Tobe T, Beatson SA, Taniguchi H, Abe H, Bailey CM, Fivian A, et al. An extensive repertoire of type III secretion effectors in Escherichia coli O157 and the role of lambdoid phages in their dissemination. Proc Natl Acad Sci U S A. 2006;103:14941–14946. doi: 10.1073/pnas.0604891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J, Nietfeldt J, Benson AK. Octamer-based genome scanning distinguishes a unique subpopulation of Escherichia coli O157:H7 strains in cattle. Proc Natl Acad Sci U S A. 1999;96:13288–13293. doi: 10.1073/pnas.96.23.13288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Nietfeldt J, Ju J, Wise J, Fegan N, Desmarchelier P, et al. Ancestral divergence, genome diversification, and phylogeographic variation in subpopulations of sorbitol-negative, β-glucuronidase-negative enterohemorrhagic Escherichia coli O157. J Bacteriol. 2001;183:6885–6897. doi: 10.1128/JB.183.23.6885-6897.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z, Kovar J, Kim J, Nietfeldt J, Smith DR, Moxley RA, et al. Identification of common subpopulations of non-sorbitol-fermenting, beta-glucuronidase-negative Escherichia coli O157:H7 from bovine production environments and human clinical samples. Appl Environ Microbiol. 2004;70:6846–6854. doi: 10.1128/AEM.70.11.6846-6854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Laing C, Steele M, Ziebell K, Johnson R, Benson AK, et al. Genome evolution in major Escherichia coli O157:H7 lineages. BMC Genomics. 2007;8:121. doi: 10.1186/1471-2164-8-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laing CR, Buchanan C, Taboada EN, Zhang Y, Karmali MA, Thomas JE, et al. In silico genomic analyses reveal three distinct lineages of Escherichia coli O157:H7, one of which is associated with hyper-virulence. BMC Genomics. 2009;10:287. doi: 10.1186/1471-2164-10-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park D, Stanton E, Ciezki K, Parrell D, Bozile M, Pike D, et al. Evolution of the Stx2-encoding Prophage in persistent bovine Escherichia coli O157:H7 strains. Appl Environ Microbiol. 2013;79:1563–1572. doi: 10.1128/AEM.03158-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, Phillippy AM. Canu: scalable and accurate long-read assembly via adaptive κ-mer weighting and repeat separation. Genome Res. 2017;27:722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 2014;9. [DOI] [PMC free article] [PubMed]
- 36.Harshey RM. Transposable Phage Mu. Microbiol Spectr. 2014;2. 10.1128/microbiolspec.MDNA3-0007-2014. [DOI] [PMC free article] [PubMed]
- 37.Ohnishi M, Terajima J, Kurokawa K, Nakayama K, Murata T, Tamura K, et al. Genomic diversity of enterohemorrhagic Escherichia coli O157 revealed by whole genome PCR scanning. Proc Natl Acad Sci U S A. 2002;99:17043–17048. doi: 10.1073/pnas.262441699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magasanik B. Global regulation of gene expression. Proc Natl Acad Sci U S A. 2000;97:14044–14045. doi: 10.1073/pnas.97.26.14044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimmer DP, Soupene E, Lee HL, Wendisch VF, Khodursky AB, Peter BJ, et al. Nitrogen regulatory protein C-controlled genes of Escherichia coli: scavenging as a defense against nitrogen limitation. Proc Natl Acad Sci U S A. 2000;97:14674–14679. doi: 10.1073/pnas.97.26.14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plunkett G, Rose DJ, Durfee TJ, Blattner FR. Sequence of Shiga toxin 2 phage 933W from Escherichia coli O157:H7: Shiga toxin as a phage late-gene product. J Bacteriol. 1999;181:1767–1778. doi: 10.1128/jb.181.6.1767-1778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaikh N, Tarr PI. Escherichia coli O157:H7 Shiga toxin-encoding bacteriophages: integrations, excisions, truncations, and evolutionary implications. J Bacteriol. 2003;185:3596–3605. doi: 10.1128/JB.185.12.3596-3605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eppinger M, Mammel MK, Leclerc JE, Ravel J, Cebula TA. Genomic anatomy of Escherichia coli O157:H7 outbreaks. Proc Natl Acad Sci U S A. 2011;108:20142–20147. doi: 10.1073/pnas.1107176108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shere JA, Bartlett KJ, Kaspar CW. Longitudinal study of Escherichia coli O157:H7 dissemination on four dairy farms in Wisconsin. Appl Envir Microbiol. 1998;64:1390–1399. doi: 10.1128/aem.64.4.1390-1399.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner PL, Neely MN, Zhang X, Acheson DW, Waldor MK, Friedman DI. Role for a phage promoter in Shiga toxin 2 expression from a pathogenic Escherichia coli strain. J Bacteriol. 2001;183:2081–2085. doi: 10.1128/JB.183.6.2081-2085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gouveia S, Proctor ME, Lee MS, Luchansky JB, Kaspar CW. Genomic comparisons and Shiga toxin production among Escherichia coli O157:H7 isolates from a day care center outbreak and sporadic cases in southeastern Wisconsin. J Clin Microbiol. 1998;36:727–733. doi: 10.1128/jcm.36.3.727-733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welinder-Olsson C, Stenqvist K, Badenfors M, Brandberg Å, Florén K, Holm M, et al. EHEC outbreak among staff at a children’s hospital - use of PCR for verocytotoxin detection and PFGE for epidemiological investigation. Epidemiol Infect. 2004;132:43–49. doi: 10.1017/s0950268803001444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kotewicz ML, Jackson SA, LeClerc JE, Cebula TA. Optical maps distinguish individual strains of Escherichia coli O157 : H7. Microbiology. 2007;153:1720–1733. doi: 10.1099/mic.0.2006/004507-0. [DOI] [PubMed] [Google Scholar]
- 48.Iguchi A, Iyoda S, Terajima J, Watanabe H, Osawa R. Spontaneous recombination between homologous prophage regions causes large-scale inversions within the Escherichia coli O157:H7 chromosome. Gene. 2006;372:199–207. doi: 10.1016/j.gene.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Faith NG, Shere JA, Brosch R, Arnold KW, Ansay SE, Lee MS, et al. Prevalence and clonal nature of Escherichia coli O157:H7 on dairy farms in Wisconsin. Appl Environ Microbiol. 1996;62. [DOI] [PMC free article] [PubMed]
- 50.Kudva IT, Evans PS, Perna NT, Barrett TJ, Ausubel FM, Blattner FR, et al. Strains of Escherichia coli O157:H7 differ primarily by insertions or deletions, not single-nucletide polymorphisms. J Bacteriol. 2002;184:1873–1879. doi: 10.1128/JB.184.7.1873-1879.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma B, Hill TM. Insertion of inverted Ter sites into the terminus region of the Escherichia coli chromosome delays completion of DNA replication and disrupts the cell cycle. Mol Microbiol. 1995;18:45–61. doi: 10.1111/j.1365-2958.1995.mmi_18010045.x. [DOI] [PubMed] [Google Scholar]
- 52.Bidnenko V. Replication fork collapse at replication terminator sequences. EMBO J. 2002;21:3898–3907. doi: 10.1093/emboj/cdf369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alokam S, Liu S-L, Said K, Sanderson KE. Inversions over the terminus region in Salmonella and Escherichia coli: IS200s as the sites of homologous recombination inverting the chromosome of Salmonella enterica serovar typhi. J Bacteriol. 2002;184:6190–6197. doi: 10.1128/JB.184.22.6190-6197.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang D, Li S, Guo F, Ning K, Wang L. Core-genome scaffold comparison reveals the prevalence that inversion events are associated with pairs of inverted repeats. BMC Genomics. 2017;18 10.1186/s12864-017-3655-0. [DOI] [PMC free article] [PubMed]
- 55.De Paepe M, Hutinet G, Son O, Amarir-Bouhram J, Schbath S, Petit M-A. Temperate phages acquire DNA from defective prophages by relaxed homologous recombination: the role of rad52-like recombinases. PLoS Genet. 2014;10:e1004181. doi: 10.1371/journal.pgen.1004181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johansen BK, Wasteson Y, Granum PE, Brynestad S. Mosaic structure of Shiga-toxin-2-encoding phages isolated from Escherichia coli O157:H7 indicates frequent gene exchange between lambdoid phage genomes. Microbiology. 2001;147:1929–1936. doi: 10.1099/00221287-147-7-1929. [DOI] [PubMed] [Google Scholar]
- 57.Juhala RJ, Ford ME, Duda RL, Youlton A, Hatfull GF, Hendrix RW. Genomic sequences of bacteriophages HK97 and HK022: pervasive genetic mosaicism in the lambdoid bacteriophages. J Mol Biol. 2000;299:27–51. doi: 10.1006/jmbi.2000.3729. [DOI] [PubMed] [Google Scholar]
- 58.Stanton E, Park D, Döpfer D, Ivanek R, Kaspar CWCW. Phylogenetic characterization of Escherichia coli O157:H7 based on IS629 distribution and Shiga toxin genotype. Microbiology. 2014;160(PART 3):502–5013. doi: 10.1099/mic.0.073437-0. [DOI] [PubMed] [Google Scholar]
- 59.Rump LV, Fischer M, González-Escalona N. Different IS629 transposition frequencies exhibited by Escherichia coli O157:H7 strains in the stepwise evolutionary model. Appl Environ Microbiol. 2011;77:5030–5033. doi: 10.1128/AEM.00249-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kulow MJ, Gonzales TK, Pertzborn KM, Dahm J, Miller BA, Park D, et al. Differences in colonization and shedding patterns after oral challenge of cattle with three Escherichia coli O157:H7 strains. Appl Environ Microbiol. 2012;78:8045–8055. doi: 10.1128/AEM.02363-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karch H, Meyer T, Russmann H, Heesemann J. Frequent loss of Shiga-like toxin genes in clinical isolates of Escherichia coli upon subcultivation downloaded from. 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen C, Lewis CR, Goswami K, Roberts EL, DebRoy C, Dudley EG. Identification and characterization of spontaneous deletions within the Sp11-Sp12 prophage region of Escherichia coli O157:H7 Sakai. Appl Environ Microbiol. 2013;79:1934–1941. doi: 10.1128/AEM.03682-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watt VM, Ingles CJ, Urdea MS, Rutter WJ. Homology requirements for recombination in Escherichia coli. Proc Natl Acad Sci U S A. 1985;82:4768–4722. doi: 10.1073/pnas.82.14.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hutchison CA, Merryman C, Sun L, Assad-Garcia N, Richter RA, Smith HO, et al. Polar effects of transposon insertion into a minimal bacterial genome. J Bacteriol. 2019;201. [DOI] [PMC free article] [PubMed]
- 65.Zhou Z, Li X, Liu B, Beutin L, Xu J, Ren Y, et al. Derivation of Escherichia coli O157:H7 from its O55:H7 precursor. PLoS One. 2010;5:e8700. 10.1371/journal.pone.0008700. [DOI] [PMC free article] [PubMed]
- 66.Team RC . R: a language and environment for statistical computing. 2017. [Google Scholar]
- 67.Langmead B, Trapnell C, Pop M, Salzberg SL, Down T, Rakyan V, et al. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agarwala R, Barrett T, Beck J, Benson DA, Bollin C, Bolton E, et al. Database resources of the National Center for biotechnology information. Nucleic Acids Res. 2017;45:D12–D17. doi: 10.1093/nar/gkw1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Madden T. The BLAST sequence analysis tool. 2003. [Google Scholar]
- 71.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, et al. The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST). Nucleic Acids Res. 2014;42(Database issue):D206–14. 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed]
- 72.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, et al. The RAST server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75. 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed]
- 73.Darling ACE, Mau B, Blattner FR, Perna NT. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004;14:1394–403 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed]
- 74.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. PCR confirmation of inverted repeats present at the flanking ends of the inversion in farm X strains (FRIK804, FRIK1275, and FRIK1625) and control strain Sakai. a Primer pairs ECs_1507-F/ECs_1508-R and ECs_2759-F/ECs_2760-R were specific to Sp6 and Sp14 in Sakai. Primer pairs ECs_1507-F/ECs_2759-F and ECs_1508-R/ECs_2760-R were specific to regions of Φ804–7 and Φ804–15. b Amplification was observed using primer pairs ECs_1507-F/ECs_1508-R (lane 15) and ECs_2759-F/ECs_2760-R (lane 16) using gDNA extracted from Sakai. Amplification was observed using primer pairs ECs_1507-F/ECs_2759-F (lanes 4, 8, and 13) and ECs_1508-R/ECs_2760-R (lanes 5, 9, and 14) using gDNA extracted from farm X strains. Lanes 1 and 10, 1.0-kb ladder.
Additional file 2: Fig. S2. PCR confirmation of regions flanking inter-prophage deletion in FRIK1275 and FRIK1625 using PCR amplification. Lane 1: 1.0 kb ladder. gDNA in lane 2 (FRIK804), lane 3 (FRIK1275), lane 4 (FRIK1625), and lane 5 (Sakai). Amplification was observed only in strains with the inter-prophage deletion between the identified direct repeats.
Additional file 3: Fig. S3. Predicted function and location of genes in Φ804–9 and Φ804–10. The portions of the two adjacent phage in all farm X strain has a shaded grey background. The region in FRIK804 but absent in FRIK1275 and FRIK1625 has a white background.
Additional file 4: Table S1. Chromosomal locations of corresponding replication motifs in EHEC FRIK804 and Sakai. Highlighted motifs (light orange) located within the segment of the FRIK804 chromosome that is inverted relative to strain Sakai.
Additional file 5: Table S2. Location and length of inverted repeats in Φ804–7 and Φ804–15. Crossover region highlighted in light orange.
Additional file 6: Table S3. Location and length of direct repeats in Φ804–9 and Φ804–10. Crossover region of highlighted in light orange.
Additional file 7: Table S4. Location, classification, and predicted functions of genes in Φ804–9 and Φ804–10. Highlighted region is present in FRIK804 but absent in FRIK1275 and FRIK1625.
Additional file 8: Table S5. Oligonucleotide primers used in this study.
Additional file 9: Table S6. Locations of IS629 elements in FRIK804 and Sakai chromosomes.
Additional file 10: Table S7. Locations of ISEc8 locations in FRIK804 and Sakai chromosomes.
Data Availability Statement
The genome sequences of the E. coli O157:H7 strains have been deposited in GenBank; FRIK804 under the accession numbers CP034384-CP034388, FRIK1275 under RWJR00000000 and FRIK1625 under RWJQ00000000. All custom Perl scripts written for this study are available on GitHub (http://github.com/eliotstanton/).