Abstract
As severe acute respiratory syndrome coronavirus 2 continues to spread worldwide, there have been increasing reports from Europe, North America, Asia, and Latin America describing children and adolescents with COVID-19-associated multisystem inflammatory conditions. However, the association between multisystem inflammatory syndrome in children and COVID-19 is still unknown. We review the epidemiology, causes, clinical features, and current treatment protocols for multisystem inflammatory syndrome in children and adolescents associated with COVID-19. We also discuss the possible underlying pathophysiological mechanisms for COVID-19-induced inflammatory processes, which can lead to organ damage in paediatric patients who are severely ill. These insights provide evidence for the need to develop a clear case definition and treatment protocol for this new condition and also shed light on future therapeutic interventions and the potential for vaccine development.
Translations
For the French, Chinese, Arabic, Spanish and Russian translations of the abstract see Supplementary Materials section.
Introduction
Since a cluster of pneumonia cases arising from unknown causes was first reported in Wuhan (Hubei province, China) in December, 2019, the COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly spread worldwide. As of Aug 5, 2020, there are more than 18 million confirmed cases of COVID-19 and over 690 000 deaths.1
Children and adolescents make up a small proportion of COVID-19 cases. National statistics from countries in Asia, Europe, and North America show that paediatric cases account for 2·1–7·8% of confirmed COVID-19 cases.2, 3, 4, 5 However, because of asymptomatic infections, the underdiagnosis of clinically silent or mild cases (typically occurring in younger people), and the availability, validity, and targeted strategies of current testing methods (eg, viral testing instead of serological testing), there is still uncertainty about the actual disease burden among children and adolescents. Although the manifestations of the disease are generally milder in children than in adults, a small proportion of children require hospitalisation and intensive care.6, 7
In the past 3 months, there have been increasing reports from Europe, North America, Asia, and Latin America describing children and adolescents with COVID-19-associated multisystem inflammatory conditions, which seem to develop after the infection rather than during the acute stage of COVID-19. The clinical features of these paediatric cases are both similar and distinct from other well described inflammatory syndromes in children, including Kawasaki disease, Kawasaki disease shock syndrome, and toxic shock syndrome.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 This COVID-19-associated multisystem inflammatory syndrome in children and adolescents is referred to interchangeably as paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) or multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19, and herein is referred to as MIS-C. MIS-C can lead to shock and multiple organ failure requiring intensive care. The European and US Centers for Disease Prevention and Control (CDC), Australian Government Department of Health, and WHO have released scientific briefs or advisories for MIS-C in response to this emerging challenge.6, 9, 37, 38
Much remains unknown regarding the epidemiology, pathogenesis, clinical spectrum, and long-term outcomes of MIS-C. In this Review, we critically appraise and summarise the available evidence to provide insights into current clinical practice and implications for future research directions.
Case definitions and clinical spectrum
Different terminology and case definitions for this COVID-19-associated multisystem inflammatory phenotype in children are used depending on the country and region. An internationally accepted case definition for MIS-C is still evolving. The UK has used PIMS-TS as their preliminary case definition for this disease, with criteria that include clinical manifestations (eg, persistent inflammation), organ dysfunction, SARS-CoV-2 PCR testing, which might be positive or negative, and exclusion of any other microbial cause.9, 39 The US CDC case definition is based on clinical presentation, evidence of severe illness and multisystem (two or more) organ involvement, no plausible alternative diagnoses, and a positive test for current or recent SARS-CoV-2 infection or COVID-19 exposure within 4 weeks before the onset of symptoms.37 WHO has developed a similar preliminary case definition and a case report form for multisystem inflammatory disorder in children and adolescents. This case definition for MIS-C includes clinical presentation, elevated markers of inflammation, evidence of infection or contact with patients who have COVID-19, and exclusion of other obvious microbial causes of inflammation (table 1 ).6
Table 1.
Preliminary case definitions for MIS-C
| MIS-C associated with COVID-19 | PIMS-TS | MIS-C associated with COVID-19 | Complete Kawasaki disease | Incomplete Kawasaki disease | Kawasaki disease shock syndrome | |
|---|---|---|---|---|---|---|
| Organisation or publication | WHO6 | Royal College of Pediatrics and Child Health39 | US Centers for Disease Control and Prevention37 | American Heart Association40 | American Heart Association40 | Kanegaye et al,41 |
| Age | 0–19 years | Child (age not specified) | <21 years | Child (age not specified) | Child (age not specified) | Child (age not specified) |
| Inflammation | Fever and elevated inflammatory markers for 3 days or more | Fever and elevated inflammatory markers | Fever and elevated inflammatory markers | Fever lasting 5 days or more* | Fever lasting 5 days or more* | Fever |
| Main features | Two of the following: (A) rash or bilateral non-purulent conjunctivitis or mucocutaneous inflammation signs (oral, hands, or feet); (B) hypotension or shock; (C) features of myocardial dysfunction, pericarditis, valvulitis, or coronary abnormalities (including echocardiogram findings or elevated troponin or N-terminal pro B-type natriuretic peptide); (D) evidence of coagulopathy (elevated prothrombin time, partial thromboplastin time, and elevated D-dimers); and (E) acute gastrointestinal problems (diarrhoea, vomiting, or abdominal pain) | Single or multiple organ dysfunction (shock or respiratory, renal, gastrointestinal, or neurological disorder; additional features (appendix 6 pp 3–4) | Clinically severe illness requiring hospitalisation; and multisystem (two or more) organ involvement (cardiac, renal, respiratory, haematological, gastrointestinal, dermatological, or neurological) | Four or more principal clinical features: (A) erythema and cracking of lips, strawberry tongue or oral and pharyngeal mucosa; (B) bilateral bulbar conjunctival injection without exudate; (C) rash; (D) erythema and oedema of the hands and feet in acute phase and periungual desquamation in subacute phase; and (E) cervical lymphadenopathy | Two or three principal clinical features or a positive echocardiogram | Kawasaki disease-like clinical features and any of the following causing initiation of volume expansion, vasoactive agents, or transfer to the intensive care unit: systolic hypotension based on age, or a decrease in systolic blood pressure from baseline by 20% or more, or clinical signs of poor perfusion |
| Exclusion | Other microbial cause of inflammation | Any other microbial cause | Other plausible alternative diagnoses | .. | .. | Other microbial cause |
| SARS-CoV-2 status | Positive RT-PCR, antigen test, or serology; or any contact with patients with COVID-19 | RT-PCR positive or negative | Positive RT-PCR, serology, or antigen test; or COVID-19 exposure within the past 4 weeks before symptom onset | .. | .. | .. |
MIS-C=multisystem inflammatory syndrome in children. PIMS-TS=paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
In the presence of four or more principal clinical features, particularly when redness and swelling of the hands and feet are present, the diagnosis of Kawasaki disease can be made with only 4 days of fever.
Key messages.
-
•
Although severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections in children are generally mild and non-fatal, there is increasing recognition of a paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2, also known as multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19, herein referred to as MIS-C, which can lead to serious illness and long-term side-effects
-
•
Clinical and laboratory features of MIS-C are similar to those of Kawasaki disease, Kawasaki disease shock syndrome, and toxic shock syndrome, but the disorder has some distinct features, and it needs a clear clinical and pathophysiological definition
-
•
MIS-C might be distinct from Kawasaki disease, with features including an age at onset of more than 7 years, a higher proportion of African or Hispanic children affected, and diffuse cardiovascular involvement suggestive of a generalised immune-mediated disease
-
•
Pathophysiology of MIS-C is still unclear and possible mechanisms include antibody or T-cell recognition of self-antigens (viral mimicry of the host) resulting in autoantibodies, antibody or T-cell recognition of viral antigens expressed on infected cells, formation of immune complexes which activate inflammation, and viral superantigen sequences which activate host immune cells
-
•
Most cases of MIS-C associated with COVID-19 were managed following the standard protocols for Kawasaki disease, with inotropic or vasoactive agents often required in patients with cardiac dysfunction and hypotension and anticoagulation also used frequently; clinical research is required to prove the effectiveness and safety of these treatments
-
•
The medium-term to long-term outcomes of MIS-C, such as the sequelae of coronary artery aneurysm formation, remain unknown and close follow-up is important
Cases reported in the past 3 months, which met the current diagnostic criteria, most likely represent a small proportion of MIS-C cases, and those individuals were severely affected by the illness. A broader UK definition of MIS-C describes this illness as a spectrum ranging from persistent fever and inflammation, to characteristic features of Kawasaki disease in children, and to children who are severely ill with shock and multiple organ failure.39, 40 In the study by Dufort and colleagues,21 a third of the reported cases did not meet the US CDC case definition for MIS-C but presented with similar clinical and laboratory features to those seen in confirmed cases.
Despite overlap in clinical presentation, the initially speculated relationship between MIS-C and toxic shock syndrome seems implausible because most MIS-C cases had negative blood cultures (appendix 6 pp 3–4); thus, there is no evidence that staphylococcal or streptococcal toxins are involved in the cause of MIS-C. However, studies to exclude infection with superantigen-producing organisms are scarce. Overlap has also been observed between the diagnostic criteria of Kawasaki disease, Kawasaki disease shock syndrome, and the newly emerged MIS-C. According to criteria developed by the American Heart Association,42 the diagnosis of complete Kawasaki disease includes the presence of a high fever for 5 days or more and at least four of the five principle clinical features, whereas incomplete Kawasaki disease is diagnosed when children present with unexplained fever for 5 days or more and two to three of the principle clinical features supported by laboratory findings or cardiac lesions (table 1).
Kawasaki disease shock syndrome is a severe form of Kawasaki disease,41 defined as complete or incomplete Kawasaki disease complicated by haemodynamic instability, resulting in the patient requiring intensive care, without evidence of another bacterial infection such as group A streptococcus or staphylococcus. The cause and factors contributing to the development of Kawasaki disease shock syndrome are still unclear, but a contributory role for underlying inflammation and more intense vasculitis has been suggested on the basis of laboratory results, progression, and the disease outcome.43, 44, 45, 46, 47 Researchers have suggested several possible explanations for Kawasaki disease shock syndrome including a superantigen-mediated response,48 overexpression of proinflammatory cytokines,49 and gut bacteria involvement.50 A large number of MIS-C cases present with Kawasaki-like clinical symptoms, and cardiac impairment and shock similar to Kawasaki disease shock syndrome. Gastrointestinal symptoms, hyponatremia, hypoalbuminemia, and intravenous immunoglobulin resistance are also common in Kawasaki disease shock syndrome and MIS-C (appendix 6 pp 3–4).
Although features of MIS-C overlap with those of Kawasaki disease, a study from Whittaker and colleagues18 found a wider spectrum of MIS-C symptoms. Despite differences in severity, coronary aneurysms have occurred in all three groups of patients, including those with shock, those who meet the criteria for Kawasaki disease, and those with fever and inflammation but who do not have shock or meet the criteria for Kawasaki disease. In addition to a wider clinical spectrum, there are several other distinct features of MIS-C compared with Kawasaki disease, including the age and ethnic groups affected. Patients with MIS-C are typically older than 7 years, of African or Hispanic origin, and show greater elevation of inflammatory markers.10, 13, 15, 18 Over 80% of patients with MIS-C also present with an unusual cardiac injury shown by high concentrations of troponin and brain natriuretic peptide, whereas others develop arrhythmia, left ventricle dysfunction, and unusual coronary dilatation or aneurysms (appendix 6 pp 3–4).10, 12, 13, 15, 16, 17, 18, 19
Blondiaux and colleagues28 examined cardiac MRI findings in four patients who had MIS-C with cardiovascular involvement, and found a diffuse myocardial oedema on T2-weighted short-tau inversion recovery sequences and native T1 mapping, with no evidence of late gadolinium enhancement suggestive of replacement fibrosis or focal necrosis. These findings favour the hypothesis of an immune response to an antigen rather than a direct complication secondary to SARS-CoV-2 infection.
COVID-19 causes and link with MIS-C
Risk factors for developing severe disease among children infected with SARS-CoV-2 include age, viral load, and chronic comorbidities.51, 52, 53 There is a U-shaped curve of severity in children diagnosed with COVID-19, and babies younger than 1 year are at a higher risk of developing severe COVID-19,54 although these infections are infrequent. After the first year of life, most younger patients appear to be asymptomatic or have milder symptoms of SARS-CoV-2 infection.4, 55, 56 Data suggest a genetic locus is partly associated with more severe disease,57 and some ethnic groups (eg, African) might have a strong association with MIS-C.13, 31, 33
The relationship between coronaviruses and multisystem inflammatory diseases, such as Kawasaki disease, has been studied previously. Kawasaki disease is a systemic vasculitis in children and one of the leading causes of childhood-acquired heart disease.58 Although its exact cause remains unknown, Kawasaki disease is thought to be triggered by a response to an infectious agent in genetically predisposed individuals, and research has focused on identifying host factors and specific triggers associated with the development of Kawasaki disease. Coronaviruses have a large genome, which might explain the varied pathogenicity and ability to affect multiple organs. In 2005, Esper and colleagues59 reported a possible association of the New Haven coronavirus (previously identified as HCoV-NL63) with Kawasaki disease. However, five subsequent studies60, 61, 62, 63, 64 showed negative results for this association. The results from newer studies remain inconclusive.65, 66, 67 A South Korean study65 from 2012, did not find a significant association between coronavirus strains OC43, 229E, and NL63 and Kawasaki disease. However, a Japanese study66 published in 2014 found possible involvement of strain 229E in Kawasaki disease, but not strain NL63. Another South Korean study67 from 2014 showed a non-significant correlation between monthly Kawasaki disease occurrence and monthly coronavirus infection.
In the current COVID-19 pandemic, there have been increasing observations of an inflammatory illness occurring in children; most reports were 4–6 weeks after the peak of COVID-19 infections in the affected population.22, 68 On April 7, 2020, Jones and colleagues8 first reported a case of a 6-month-old infant in the USA, presenting with persistent fever and minor respiratory symptoms, who was diagnosed with Kawasaki disease and had a positive RT-PCR result for SARS-CoV-2. On April 24, 2020, the UK National Health Service had issued an alert on an emerging paediatric inflammatory multisystem disorder. On May 1, 2020, the UK Royal College of Paediatrics and Child Health published guidance39 on the clinical management of children with MIS-C and proposed a case definition. Since then, several other countries have reported that the multisystem inflammatory disease temporally associated with SARS-CoV-2 infection (appendix 6 pp 1–2).
COVID-19 pathophysiology and link with MIS-C
Coronaviruses are a large family of positive-sense single-stranded RNA viruses. There are four described genera of coronaviruses (α, β, δ, and γ).69 Six species of human coronaviruses are known, with one species subdivided into two different strains. The β coronavirus genus includes SARS-CoV, SARS-CoV-2, and Middle East respiratory syndrome. SARS-CoV-2, similarly to other coronaviruses, is transmitted between humans primarily through close contact with the infected individual or through contaminated surfaces—eg, dispersing droplets when coughing or sneezing. The virus enters a cell mainly by binding to the angiotensin-converting enzyme 2, which is highly expressed in lung cells, alveolar cells, cardiac myocytes, the vascular endothelium, and a small subset of immune cells.70, 71, 72, 73, 74 The pathogenesis of COVID-19 is still being studied. Evidence has shown that a dysregulated innate immune response and a subsequent cytokine storm,70, 74, 75, 76, 77, 78, 79, 80 and endothelial damage,81, 82 might play a role in the clinical manifestation of severe COVID-19 cases, leading to acute lung injury, acute respiratory distress syndrome, and multiple organ failure.
Neutrophils play a major role in the innate immune response. One of their functional mechanisms is the formation of neutrophil extracellular traps (NETs).83 NETs are a lattice-like web of cell-free DNA, histones, and neutrophil granule content including microbicidal proteins and enzymes. NETs have been involved in the pathophysiology of a wide range of inflammatory and prothrombotic states such as sepsis, thrombosis, and respiratory failure. The generation of NETs by neutrophils, called NETosis, can be stimulated by many viruses. Although their major function is to trap the virus, virus-induced NETs can trigger inflammatory and immunological reactions in an uncontrolled manner, leading to an exaggerated systemic inflammatory response,84 similar to hyperinflammation seen in MIS-C. Zuo and colleagues85 have shown that NETs are increased in the plasma of patients infected with SARS-CoV-2, and higher concentrations of NETs are seen in those with respiratory failure. Thrombotic complications have been reported in severe COVID-19 cases. Abnormal coagulopathy (eg, elevated D-dimer or fibrinogen) has also been observed in many cases of MIS-C. NETosis plays a crucial part in promoting thrombosis;86, 87, 88 therefore, the role of NETs in MIS-C is highly plausible. Although NETosis might be an important mechanism linking neutrophil activation, cytokine release, and thrombosis in COVID-19, they have not yet been reported to be involved in MIS-C.
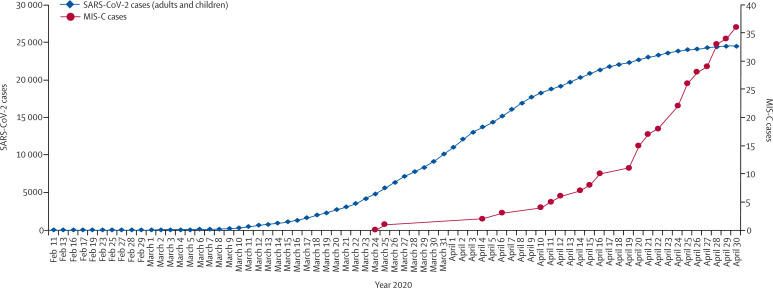
Children form only a small portion of confirmed COVID-19 cases. Most children have had minor symptoms or an asymptomatic SARS-CoV-2 infection.4, 55, 56 Unlike in adults, severe respiratory illness such as acute respiratory distress syndrome is rare in children. The newly emerging MIS-C might lead to severe clinical manifestations; however, its distinct characteristics are different from other severe complications seen in paediatric COVID-19 cases. First, MIS-C cases start appearing around 1 month after a COVID-19 peak in the population. According to data from Public Health England, the number of MIS-C cases increased drastically around April 16, 2020, approximately 4 weeks after the substantial increase in COVID-19 cases in the UK (figure 1 ).89 Epidemiological studies from the USA22 and France68 revealed similar trends. Second, children often show previous rather than a current infection with SARS-CoV-2. Only a third of reported MIS-C cases are positive by RT-PCR for SARS-CoV-2, whereas most cases are positive with an antibody test, indicating past infection. The delay in presentation of this condition relative to the pandemic curve, a low proportion of cases who were SARS-CoV-2 positive by RT-PCR, and a high proportion who were antibody positive suggest that this inflammatory syndrome is not mediated by direct viral invasion but coincides with the development of acquired immune responses to SARS-CoV-2.
Figure 1.
Time course of MIS-C in PCR-positive COVID-19 cases
Only incudes PCR-positive cases in London, UK. Data taken from Public Health England.89 Figure courtesy of Alasdair Bamford and Myrsini Kaforou. MIS-C=multisystem inflammatory syndrome in children. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Selva and colleagues90 compared the antibodies produced by children and adults against coronavirus proteins and found marked differences between the antibody responses in patients with COVID-19. The varying responses were linked to different Fcγ receptor binding properties and antibody subgroup concentrations. Although studies in patients with MIS-C are needed, these research findings suggest that differences in antibody response might contribute to the hyperinflammatory response seen in adults with COVID-19. Considering the similarities between the adult hyperinflammatory response and MIS-C, antibodies might play a role in both conditions. In a preprint study, Gruber and colleagues91 have reported that patients with MIS-C had neutralising antibodies against SARS-CoV-2, which are associated with interleukin-18 (IL-18) and IL-6 activation, myeloid chemotaxis, and activation of lymphocytes, monocytes, and natural killer cells. Upregulation of the intercellular adhesion molecule 1 and Fc-γ receptor 1 on neutrophils and macrophages suggests enhanced antigen presentation and Fc-mediated responses. Gruber and colleagues91 also reported the presence of autoantibodies against endothelial, gastrointestinal, and immune cells in patients with MIS-C.
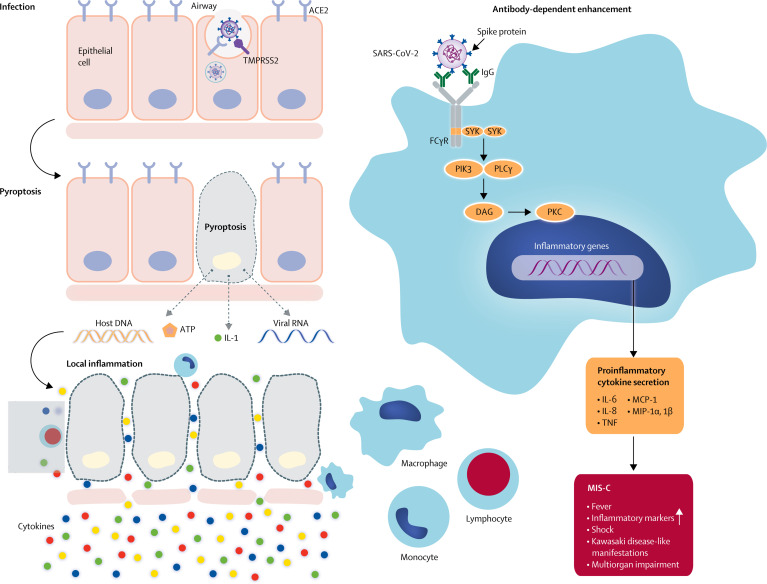
Antibodies to SARS-CoV might accentuate disease through antibody-dependent enhancement of viral entry and amplification of viral replication, as observed in dengue,92, 93, 94 or by triggering a host inflammatory response through the formation of immune complexes or direct anti-tissue antibody activation or cellular activation, or both. Similar mechanisms might be involved in the inflammatory disorder associated with SARS-CoV-2. SARS-CoV-2 is not usually detected in patients with MIS-C; thus the antibody-dependent enhancement of inflammation is more likely to occur through an acquired immune response rather than increased viral replication. Anti-spike antibodies against SARS-CoV have been shown to accentuate inflammation in primates and in human macrophages;95 therefore, the anti-spike antibodies against SARS-CoV-2 might also be able to trigger inflammation through a similar mechanism (figure 2 ). Hoepel and colleagues96 have reported, in a preprint study, that immune complexes generated by linking patient anti-spike antibodies with spike protein cause macrophage activation, which supports the proposed mechanism for SARS-CoV-2.
Figure 2.
Possible mechanisms of inflammatory processes for MIS-C
Antibodies might enhance disease by increasing viral entry into cells. Alternative mechanisms include antibody or T-cell-mediated cell damage or activation of inflammation. Antibodies or T cells attack cells expressing viral antigens or attack host antigens which cross-react or mimic viral antigens. The low rate of virus detection in MIS-C would favour this second mechanism rather than the classic antibody-dependent enhancement. ACE2=angiotensin-converting enzyme 2. DAG=diacylglycerol. FcγR=Fc-gamma receptor. IL=interleukin. MCP=monocyte chemoattractant protein. MIS-C=multisystem inflammatory syndrome in children. MIP=macrophage inflammatory protein. PIK3=phosphoinositide 3 kinase. PKC=protein kinase C. PLCγ=phospholipase C gamma. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. SYK=tyrosine protein kinase SYK. TMPRSS2=transmembrane serine protease 2. TNF=tumour necrosis factor.
The inflammatory disorders triggered by SARS-CoV-2 have features similar to Kawasaki disease and can also result in coronary aneurysms. This finding suggests that the virus might be acting as the immune trigger and causing a similar immune-mediated injury to the heart and coronary arteries as the one seen in Kawasaki disease. Immune complexes have been well documented in Kawasaki disease,97, 98, 99, 100 and might mediate vascular injury by activation of inflammatory responses through the Fc-γ receptor or complement activation. This theory is supported by the fact that genetic variants associated with Kawasaki disease include FCGR2A, B-lymphoid tyrosine kinase, and the CD40 ligand gene,101, 102, 103 which are genes involved in antibody production or clearance of immune complexes. The development of T-cell responses to SARS-CoV-2 might also play a role in organ damage and inflammatory processes since increased T-cell responses were seen in Kawasaki disease. Genetic variants in the inositol 1,4,5-triphosphate 3-kinase C (ITPKC) gene, regulating T-cell activation,104 are associated with increased susceptibility to Kawasaki disease, and treatment with cyclosporin, which works by lowering T-cell activity, might have beneficial effects in the treatment of Kawasaki disease.105
The possible mechanisms for an acquired immune response to accentuate SARS-CoV-2 include: (1) antibody or T-cell recognition of self-antigens (viral mimicry of the host) resulting in autoantibodies; (2) antibody or T-cell recognition of viral antigens expressed on infected cells; (3) formation of immune complexes which activate inflammation; and (4) viral superantigen sequences which activate host immune cells.106
Management of MIS-C
To date, there are no widely accepted guidelines on the management of MIS-C, but several organisations have published their own guidelines (table 2 ). Physicians at various centres have created treatment protocols based on specific symptoms, previous treatment of similar conditions such as Kawasaki disease, or COVID-19 treatment guidelines for adult patients. If MIS-C is suspected or diagnosed, a multidisciplinary team approach should be taken, including a paediatric infectious diseases unit, and cardiology, immunology, rheumatology, and intensive care unit teams to consider antiviral therapy (if PCR positive for SARS-CoV-2) or immunotherapy, or both. General supportive care is crucial, especially attention to vital signs, hydration, electrolytes, and metabolic status. Few children present with respiratory compromise or hypoxia, but they should be closely monitored for potential compromise.
Table 2.
Published guidance on the management of multisystem inflammatory syndrome in children associated with COVID-19
| Royal College of Paediatrics and Child Health39 | US Centers for Disease Control and Prevention37 | |
|---|---|---|
| Supportive care | Only recommended for mild to moderate disease; discuss early with paediatric intensive care unit and paediatric infectious disease, immunology, and rheumatology team; if clinically deteriorating or in cases of severe disease, discuss transfer with paediatric intensive care unit retrieval teams | Fluid resuscitation, inotropic support, respiratory support, and in rare cases, extracorporeal membranous oxygenation |
| Directed care against underlying inflammatory process | Immunotherapy should be discussed with a paediatric infectious diseases unit and experienced clinicians on a case-by-case basis and used in the context of a trial if eligible and available | Intravenous immunoglobulin, steroids, aspirin, and anticoagulation treatment |
| Antiviral therapy | Should be given only in the context of a clinical trial and should be discussed at multidisciplinary team meetings with a clinician from an external trust | .. |
| Antibiotics for sepsis | .. | Given while waiting for bacterial cultures |
| Other | All children treated as if they have COVID-19 and all should be considered for recruitment in research studies | .. |
Standard protocol for Kawasaki disease
Because many cases met the diagnostic criteria of classic or incomplete Kawasaki disease, most reported MIS-C cases were treated using the standard protocol for Kawasaki disease, which is intravenous immunoglobulin with or without aspirin (appendix 6 pp 3–4).8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 107, 108 A large proportion of MIS-C cases (67%) have a similar presentation to Kawasaki disease shock syndrome, mainly shock, so supportive and inotropic or vasoactive treatment should also be applied. Steroids have also been used to treat MIS-C. Because clinical and laboratory features of MIS-C overlap with those of Kawasaki disease, Kawasaki disease shock syndrome, and macrophage activation syndromes, patients with severe MIS-C have received immunomodulatory agents such as infliximab (anti-tumour necrosis factor drug),18, 25 tocilizumab (IL-6 antagonist),16, 17, 22, 24, 25 and anakinra (IL-1 receptor antagonist),15, 17, 18, 20, 22, 24, 25, 26, 27 which have been shown to be effective in similar diseases. There is no consensus on which of these agents is optimal, and the choice of drug is dependent on clinician preference, cytokine panel results, and availability. Randomised clinical trials are needed to establish which treatment is beneficial and effective at preventing or reversing shock and cardiac failure, or the development of coronary artery aneurysms. However, because clinical trials take a long time to complete, an international best available treatment study109 has been initiated. This study will invite paediatricians to collaborate globally and provide information on the treatment administered to children with inflammatory diseases temporally associated with COVID-19. Propensity score matching will be used to compare the rate of inflammation resolution with other outcomes such as length of hospital stay, overall survival, and frequency and severity of coronary artery aneurysms, which will help to inform the design of randomised trials.
The role of remdesivir and dexamethasone
Remdesivir is a nucleoside analogue that inhibits the action of viral RNA polymerase resulting in the termination of RNA transcription, which decreases viral RNA production and has been shown to shorten COVID-19 illness duration in adults.110 However, because remdesivir inhibits the actively replicating virus and most children with MIS-C are not in the acute phase of illness and the virus is not detectable by PCR, the role of remdesivir in the treatment of MIS-C is limited. In rare cases in which the PCR test is positive and the child is severely ill, the use of remdesivir could be considered.
The recent UK RECOVERY trial,111 has shown that dexamethasone might reduce death by a third in patients who are on mechanical ventilation as a result of severe respiratory complications from COVID-19. In line with these new findings, administration of low-dose dexamethasone to patients with MIS-C could be beneficial to suppress the immune response and subsequent inflammatory disorders. Other steroids such as methylprednisolone or prednisolone have become extensively used for MIS-C; thus, prospective clinical trials are needed to identify the role of steroids, the optimal dose, and the appropriate agent.
Treatment for children with hypotension
Many children with MIS-C also present with hypotension. If signs of shock are present, patients should be resuscitated with volume expansion using buffered or balanced crystalloids112 (ie, Plasma-Lyte B or Ringers lactate) and patients should stay under close monitoring. Hypotension in children with MIS-C is often fluid resistant and vasopressors should be added if necessary. Epinephrine is recommended as the first-line treatment for children and norepinephrine is added if the shock persists. Using dobutamine has also been suggested in patients with severe myocardial dysfunction, because of its selective inotropic effect.17, 113 Because some patients might have severe myocardial dysfunction, caution is needed to avoid fluid overload. Initiation of broad-spectrum antibiotics is also appropriate because the clinical presentation (eg, high C-reactive protein, increased neutrophils) makes it difficult to exclude bacterial infection; however, antibiotic treatment should be stopped once the infection has been excluded and the patient is improving. Most children with MIS-C do not require respiratory support for pulmonary disease; however, some children have required intubation and extracorporeal membrane oxygenation as a result of cardiovascular collapse.10, 15, 16, 17
Cardiac monitoring and follow-up
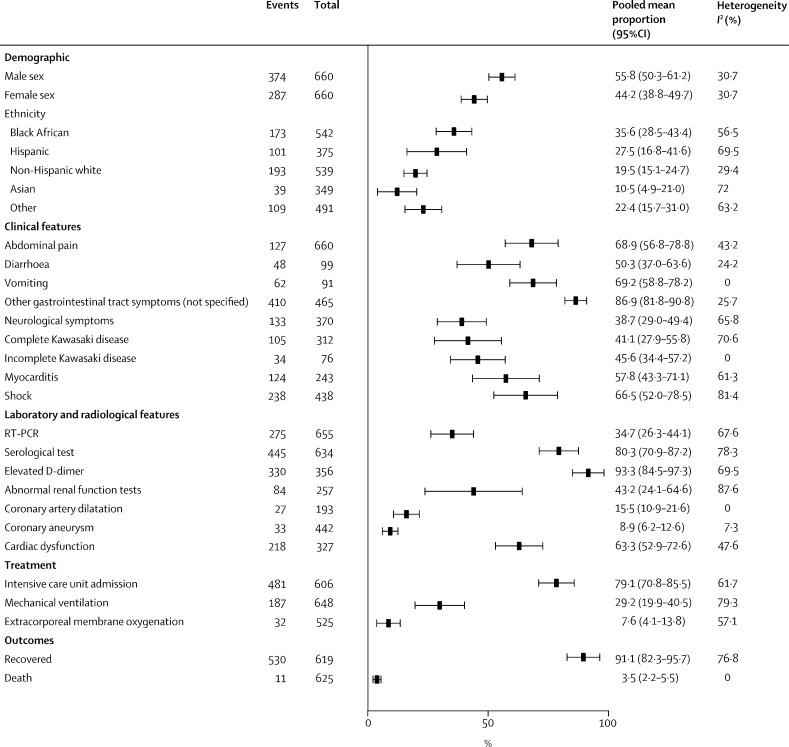
The first animal model to support the hypothesis that viruses belonging to the coronavirus family were able to induce acute myocarditis and congestive heart failure was shown in a study in 1992.114 The heart also appears to be a major target of injury in MIS-C. Many patients present with significantly elevated troponin (80·9%, 95% CI 70·2–88·4) or brain natriuretic peptide (84·9%, 77·3–90·3), or both, which indicates myocardial cell injury, and some patients also develop arrhythmia and left ventricle dysfunction (63·3%, 52·9–72·6).10, 12, 13, 15, 16, 17, 18, 19 Coronary artery dilatation was observed in 8·9% (95% CI 6·2–12·6) of patients, whereas aneurysm formation was seen in 15·5% (10·9–21·6) of patients at presentation (figure 3 , appendix 6 pp 3–4), and a smaller proportion have shown persistent coronary artery aneurysms at discharge from hospital.8, 10, 12, 13, 15, 17, 18, 19 The incidence of coronary artery aneurysms that might develop after discharge from hospital is unknown. Arrhythmia, myocardial injury, or conduction injury have also been detected by an electrocardiogram in some cases of MIS-C.17, 18 Coronary artery aneurysms have not only been reported in children with severe MIS-C and those with Kawasaki disease, but also in children showing only fever and inflammation;18 therefore, cardiac assessment and follow-up is essential in all cases. All patients need echocardiographic assessment on presentation and daily electrocardiogram monitoring in severe cases. To establish if coronary artery injury has occurred, follow-up echocardiograms are needed at discharge from hospital and after 2–6 weeks. A cardiac MRI assessment should be considered to investigate whether persistent myocardial damage was induced by a viral infection or mediated by a cytokine storm.115, 116, 117, 118 However, a cardiac MRI is difficult and time-consuming, especially when young patients are intubated. Given that there are many unknowns about the long-term cardiovascular morbidity in children with MIS-C, a cardiology follow-up is recommended for all cases.
Figure 3.
Pooled meta-analysis of patient characteristics in multisystem inflammatory syndrome in children associated with COVID-198, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36
Three case series were not included in the meta-analysis because of the overlap in cases. Cases reported in two studies34, 35 were also included in the case series reported by Feldstein and colleagues.22 Cases reported by Riphagen and colleagues10 were also included in the study by Whittaker and colleagues.18 The random-effect model is applied.
Coagulopathy prevention and management
A hallmark of COVID-19 in adult and paediatric patients has been the striking coagulopathy. Some patients have developed major vessel thrombosis. Although mechanisms underlying the coagulopathy in COVID-19 are still unknown, anticoagulant therapy (mainly heparin or low-molecular-weight heparin) is currently recommended for patients with severe COVID-19.17, 18, 19, 56 Many children with MIS-C have elevated D-dimers which, in some institutions, is used as a guide for giving anticoagulants, especially for those with a high concentration of D-dimers. Overall, there is substantial variability and a lack of consensus on anticoagulants. Low-dose aspirin, used in Kawasaki disease, has also been used for MIS-C. In patients who are severely ill with COVID-19-associated inflammatory syndrome and with marked inflammation, raised D-dimers, and a high fibrinogen concentration, anticoagulation therapy and antiplatelet therapy are generally recommended depending on the risk of thrombosis in adults. Dose, duration, and the choice of anticoagulants should be decided during consultation with paediatric haematologists and should be closely monitored throughout the illness. Low-dose aspirin is given until the follow-up echocardiograms exclude persisting coronary artery aneurysms or injury. Further research is needed on the mechanisms and treatment of coagulopathy in COVID-19.
Follow-up after discharge from hospital
Paediatric patients diagnosed with MIS-C often require special care and aggressive treatment; however, most patients have shown favourable outcomes (appendix 6 pp 1–2). Children can be discharged from hospital once their inflammatory laboratory markers have normalised; they are afebrile, normotensive, and well hydrated; and they do not require supplementary oxygen. Close follow-up is very important because the natural history of MIS-C is still unclear; in most centres the follow-up occurs with the child's primary care provider and subspecialists from infectious diseases, rheumatology, cardiology, and haematology. The medium-term to long-term outcomes, such as the sequelae of coronary artery aneurysm formation following MIS-C, remain unknown and represent an important area of future research.
Treatment choices for resource-limited countries
Cases of MIS-C have also been reported in low-income and middle-income countries (LMICs).11, 14 Because many therapeutic agents used to treat MIS-C are unavailable or unaffordable in most LMICs, the choices for immunomodulation are limited. Steroids are a cheap and more accessible option in LMICs, but their potential to induce broad immunosuppression might be hazardous in countries in which tuberculosis and HIV infection are highly prevalent and where diagnostic facilities (to exclude other types of infection) are scarce. Therefore, steroid use needs to be restricted to short-term courses in children who have been hospitalised with MIS-C and who are severely ill. Trials to establish the optimal treatment in high-income countries, that would also include agents which are available and affordable in LMICs, are needed. The ongoing international study comparing the best available treatment depending on clinician preference and drug availability,109 might provide information on the treatment options available in LMICs.
Conclusion
SARS-CoV-2 is a novel virus, and currently only scarce scientific evidence is available to understand its association with multisystem inflammatory syndrome in paediatric patients. Although there has been an increasing number of case reports and case series, the global and population-specific incidence of MIS-C remains unknown, and the causal relationship and pathogenesis of Kawasaki disease and MIS-C remain unclear. Although there is some evidence that the development of MIS-C is a post-viral immunological reaction to COVID-19, understanding of the immune response induced by SARS-CoV-2 remains poor.
There are many questions currently emerging that need to be answered—for example, how the pathophysiology of MIS-C differs from Kawasaki disease, Kawasaki disease shock syndrome, toxic shock syndrome, and macrophage activation syndromes. Genetic factors are well recognised contributors to Kawasaki disease susceptibility, but it is unknown whether the same or different genetic factors influence MIS-C. Another question is whether patients with fever and inflammation following SARS-CoV-2 infection progress to Kawasaki disease, shock, or organ failure if left untreated. Clinical trials are needed to establish which treatment is optimal and could possibly reverse inflammatory processes and prevent coronary artery aneurysms. Other emerging questions include: whether infection at a different stage of childhood and adolescence influences the severity of disease progression and prognosis; whether there are differences in clinical features or underlying immunology of MIS-C when further stratified by age (neonates, children, and adolescents); and whether MIS-C is associated with an increased risk of medium-term to long-term adverse paediatric outcomes. Importantly, future trials need to investigate whether the pathophysiology and mechanisms for the immune response of MIS-C will help to inform the development of safe and effective SARS-CoV-2 vaccines for use in children.
As the COVID-19 outbreak evolves, the scientific community needs to generate good evidence for the diagnosis and treatment of MIS-C. A recent report119 from the US CDC describes in detail the clinical characteristics and treatment modalities available for patients with MIS-C in a large case series of the US population. However, epidemiological data using cohort or case-control designs are urgently needed to establish the cause and causality between COVID-19 and MIS-C. Clinical management and potential treatment protocols should be tested in randomised controlled trials or cohort designs to compare clinical outcomes and changes in inflammatory markers. It is also important to understand whether Kawasaki disease-type morbidities, including coronary artery dilatation, occur in patients with MIS-C and how frequently they occur, and whether the use of aspirin or other interventions can reduce this risk and long-term morbidities. Laboratory investigations into the pathophysiological and immunological mechanisms of the disease are urgently needed to provide insights into potential treatment targets and to inform strategies for vaccine development. Finally, with the small number of cases globally, establishing an international research collaboration is vital to rapidly conduct these studies in a coordinated and effective way.
Search strategy and selection criteria
References for this Review were identified through searches of PubMed for articles published from Jan 1, 1985, to July 7, 2020, using the Medical Subject Headings terms “SARS virus”, “coronavirus”, “systemic inflammatory response syndrome”, “mucocutaneous lymph node syndrome (Kawasaki disease)”, “infant, newborn”, “child”, “adolescent”, and any relevant entry terms and supplementary concepts. Relevant articles and data were also identified through searches in Google Scholar, WHO, Centers for Disease Control and Prevention, UK National Health Service, and other websites. Articles resulting from these searches and relevant references cited in those articles were reviewed. Articles published in English were identified and included.
This online publication has been corrected. The corrected version first appeared at thelancet.com/infection on August 5, 2022
Acknowledgments
Acknowledgments
We thank Rajiv Bahl (Maternal, Child, and Adolescent Health Department, WHO, Geneva, Switzerland) for his comments on the manuscript. We also thank Tyler Vaivada and Yiqian Xin for their contribution in graphic illustration.
Contributors
ZAB is the guarantor. ZAB conceptualised the paper and established the writing consortium. LJ, KT, and OI developed the first draft under supervision by ZAB. ML wrote the sections on pathophysiology and diagnosis. LJ, KT, ML, OI, SKM, KW, JDK, and ZAB contributed to the writing and the review process.
Declaration of interests
We declare no competing interests.
Supplementary Materials
References
- 1.WHO WHO coronavirus disease (COVID-19) dashboard. 2020. https://covid19.who.int
- 2.Government of Canada Coronavirus disease 2019 (COVID-19): epidemiology update. https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html
- 3.European Centre for Disease Prevention and Control COVID-19. https://qap.ecdc.europa.eu/public/extensions/COVID-19/COVID-19.html [DOI] [PubMed]
- 4.Epidemiology Working Group for NCIP Epidemic Response The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 5.Government of Pakistan Pakistan cases details. http://covid.gov.pk/stats/pakistan
- 6.WHO Multisystem inflammatory syndrome in children and adolescents with COVID-19. https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19
- 7.Sanna G, Serrau G, Bassareo PP, Neroni P, Fanos V, Marcialis MA. Children's heart and COVID-19: up-to-date evidence in the form of a systematic review. Eur J Pediatr. 2020;179:1079–1087. doi: 10.1007/s00431-020-03699-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones VG, Mills M, Suarez D, et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020;10:537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 9.European Centre for Disease Prevention and Control Rapid risk assessment: paediatric inflammatory multisystem syndrome and SARS-CoV-2 infection in children. 2020. https://www.ecdc.europa.eu/en/publications-data/paediatric-inflammatory-multisystem-syndrome-and-sars-cov-2-rapid-risk-assessment
- 10.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balasubramanian S, Nagendran TM, Ramachandran B, Ramanan AV. Hyper-inflammatory syndrome in a child with COVID-19 treated successfully with intravenous immunoglobulin and tocilizumab. Indian Pediatr. 2020 doi: 10.1016/S0140-6736(20)31094-1. published online May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the COVID-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369 doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rauf A, Vijayan A, John ST, Krishnan R, Latheef A. Multisystem inflammatory syndrome with features of atypical Kawasaki disease during COVID-19 pandemic. Indian J Pediatr. 2020 doi: 10.21203/rs.3.rs-29369/v1. published online May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiotos K, Bassiri H, Behrens EM, et al. Multisystem inflammatory syndrome in children during the coronavirus 2019 pandemic: a case series. J Pediatric Infect Dis Soc. 2020;9:393–398. doi: 10.1093/jpids/piaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greene AG, Saleh M, Roseman E, Sinert R. Toxic shock-like syndrome and COVID-19: a case report of multisystem inflammatory syndrome in children (MIS-C) Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.05.117. published online June 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimaud M, Starck J, Levy M, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10:69. doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020 doi: 10.1001/jama.2020.10369. published online June 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung EW, Zachariah P, Gorelik M, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020 doi: 10.1001/jama.2020.10374. published online June 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belhadjer Z, M'ot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.048360. published online May 17. [DOI] [PubMed] [Google Scholar]
- 21.Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hameed S, Elbaaly H, Reid CEL, et al. Spectrum of imaging findings on chest radiographs, US, CT, and MRI images in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. Radiology. 2020 doi: 10.1148/radiol.2020202543. published online June 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaushik S, Aydin SI, Derespina KR, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection: a multi-institutional study from New York City. J Pediatr. 2020 doi: 10.1016/j.jpeds.2020.06.045. published online June 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capone CA, Subramony A, Sweberg T, et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory disease of childhood (MIS-C) associated with SARS-CoV-2 infection. J Pediatr. 2020 doi: 10.1016/j.jpeds.2020.06.044. published online June 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller J, Cantor A, Zachariah P, Ahn D, Martinez M, Margolis K. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children (MIS-C) that is related to COVID-19: a single center experience of 44 cases. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.079. published online June 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riollano-Cruz M, Akkoyun E, Briceno-Brito E, et al. Multisystem inflammatory syndrome in children (MIS-C) related to COVID-19: a New York City experience. J Med Virol. 2020 doi: 10.1002/jmv.26224. published online June 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blondiaux E, Parisot P, Redheuil A, et al. Cardiac MRI of children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19: case series. Radiology. 2020 doi: 10.1148/radiol.2020202288. published online June 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng KF, Kothari T, Bandi S, et al. COVID-19 multisystem inflammatory syndrome in three teenagers with confirmed SARS-CoV-2 infection. J Med Virol. 2020 doi: 10.1002/jmv.26206. published online June 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouldali N, Pouletty M, Mariani P, et al. Emergence of Kawasaki disease related to SARS-CoV-2 infection in an epicentre of the French COVID-19 epidemic: a time-series analysis. Lancet Child Adolesc Health. 2020 doi: 10.1016/S2352-4642(20)30175-9. published online July 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramcharan T, Nolan O, Lai CY, et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol. 2020 doi: 10.1007/s00246-020-02391-2. published online June 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schupper AJ, Yaeger KA, Morgenstern PF. Neurological manifestations of pediatric multi-system inflammatory syndrome potentially associated with COVID-19. Childs Nerv Syst. 2020;36:1579–1580. doi: 10.1007/s00381-020-04755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pouletty M, Borocco C, Ouldali N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79:999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivera-Figueroa EI, Santos R, Simpson S, Garg P. Incomplete Kawasaki disease in a child with COVID-19. Indian Pediatr. 2020 doi: 10.1007/s13312-020-1900-0. published online May 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waltuch T, Gill P, Zinns LE, et al. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.05.058. published online May 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Licciardi F, Pruccoli G, Denina M, et al. SARS-CoV-2-induced Kawasaki-like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics. 2020 doi: 10.1542/peds.2020-1711. published online July 28. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention Multisystem inflammatory syndrome. 2020. https://www.cdc.gov/mis-c/hcp/
- 38.Australian Government Department of Health Australian Health Protection Principal Committee (AHPPC) coronavirus (COVID-19) statements on 14 May 2020. https://www.health.gov.au/news/australian-health-protection-principal-committee-ahppc-coronavirus-covid-19-statements-on-14-may-2020
- 39.The Royal College of Paediatrics and Child Health Guidance–paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS) 2020. https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims
- 40.Levin M. Childhood multisystem inflammatory syndrome—a new challenge in the pandemic. N Engl J Med. 2020;383:393–395. doi: 10.1056/NEJMe2023158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanegaye JT, Wilder MS, Molkara D, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123:e783–e789. doi: 10.1542/peds.2008-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 43.Gámez-González LB, Murata C, Muñoz-Ramírez M, Yamazaki-Nakashimada M. Clinical manifestations associated with Kawasaki disease shock syndrome in Mexican children. Eur J Pediatr. 2013;172:337–342. doi: 10.1007/s00431-012-1879-1. [DOI] [PubMed] [Google Scholar]
- 44.Chen P-S, Chi H, Huang F-Y, Peng C-C, Chen M-R, Chiu N-C. Clinical manifestations of Kawasaki disease shock syndrome: a case-control study. J Microbiol Immunol Infect. 2015;48:43–50. doi: 10.1016/j.jmii.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Lin Y-J, Cheng M-C, Lo M-H, Chien S-J. Early differentiation of Kawasaki disease shock syndrome and toxic shock syndrome in a pediatric intensive care unit. Pediatr Infect Dis J. 2015;34:1163–1167. doi: 10.1097/INF.0000000000000852. [DOI] [PubMed] [Google Scholar]
- 46.Yim D, Ramsay J, Kothari D, Burgner D. Coronary artery dilatation in toxic shock-like syndrome: the Kawasaki disease shock syndrome. Pediatr Cardiol. 2010;31:1232–1235. doi: 10.1007/s00246-010-9771-0. [DOI] [PubMed] [Google Scholar]
- 47.Gamez-Gonzalez LB, Moribe-Quintero I, Cisneros-Castolo M, et al. Kawasaki disease shock syndrome: unique and severe subtype of Kawasaki disease. Pediatr Int (Roma) 2018;60:781–790. doi: 10.1111/ped.13614. [DOI] [PubMed] [Google Scholar]
- 48.Matsubara K, Fukaya T. The role of superantigens of group A Streptococcus and Staphylococcus aureus in Kawasaki disease. Curr Opin Infect Dis. 2007;20:298–303. doi: 10.1097/QCO.0b013e3280964d8c. [DOI] [PubMed] [Google Scholar]
- 49.Gatterre P, Oualha M, Dupic L, et al. Kawasaki disease: an unexpected etiology of shock and multiple organ dysfunction syndrome. Intensive Care Med. 2012;38:872–878. doi: 10.1007/s00134-012-2473-8. [DOI] [PubMed] [Google Scholar]
- 50.Nagata S, Yamashiro Y, Ohtsuka Y, et al. Heat shock proteins and superantigenic properties of bacteria from the gastrointestinal tract of patients with Kawasaki disease. Immunology. 2009;128:511–520. doi: 10.1111/j.1365-2567.2009.03135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y, Yan L-M, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020 doi: 10.1016/S2352-4642(20)30177-2. published online June 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.WHO Clinical management of COVID-19 interim guidance. 2020. https://apps.who.int/iris/handle/10665/332196
- 57.Ellinghaus D, Degenhardt F, Bujanda L, et al. Genomewide association study of severe COVID-19 with respiratory failure. N Engl J Med. 2020 doi: 10.1056/NEJMoa2020283. published online June 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 59.Esper F, Shapiro ED, Weibel C, Ferguson D, Landry ML, Kahn JS. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis. 2005;191:499–502. doi: 10.1086/428291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ebihara T, Endo R, Ma X, Ishiguro N, Kikuta H. Lack of association between New Haven coronavirus and Kawasaki disease. J Infect Dis. 2005;192:351–352. doi: 10.1086/430797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belay ED, Erdman DD, Anderson LJ, et al. Kawasaki disease and human coronavirus. J Infect Dis. 2005;192:352–353. doi: 10.1086/431609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shimizu C, Shike H, Baker SC, et al. Human coronavirus NL63 is not detected in the respiratory tracts of children with acute Kawasaki disease. J Infect Dis. 2005;192:1767–1771. doi: 10.1086/497170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang L-Y, Chiang B-L, Kao C-L, et al. Lack of association between infection with a novel human coronavirus (HCoV), HCoV-NH, and Kawasaki disease in Taiwan. J Infect Dis. 2006;193:283–286. doi: 10.1086/498875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dominguez SR, Anderson MS, Glod' MP, Robinson CC, Holmes KV. Blinded case-control study of the relationship between human coronavirus NL63 and Kawasaki syndrome. J Infect Dis. 2006;194:1697–1701. doi: 10.1086/509509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim JH, Yu JJ, Lee J, et al. Detection rate and clinical impact of respiratory viruses in children with Kawasaki disease. Korean J Pediatr. 2012;55:470–473. doi: 10.3345/kjp.2012.55.12.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shirato K, Imada Y, Kawase M, Nakagaki K, Matsuyama S, Taguchi F. Possible involvement of infection with human coronavirus 229E, but not NL63, in Kawasaki disease. J Med Virol. 2014;86:2146–2153. doi: 10.1002/jmv.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim GB, Park S, Kwon BS, Han JW, Park YW, Hong YM. Evaluation of the temporal association between Kawasaki disease and viral infections in South Korea. Korean Circ J. 2014;44:250–254. doi: 10.4070/kcj.2014.44.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belot A, Antona D, Renolleau S, et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.22.2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woo PC, Huang Y, Lau SK, Yuen K-Y. Coronavirus genomics and bioinformatics analysis. Viruses. 2010;2:1804–1820. doi: 10.3390/v2081803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamming I, Timens W, Bulthuis M, Lely A, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;39:10. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 73.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hedrich CM. COVID-19—considerations for the paediatric rheumatologist. Clin Immunol. 2020;214 doi: 10.1016/j.clim.2020.108420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McGonagle D, Sharif K, O'Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Atkin-Smith GK, Duan M, Chen W, Poon IKH. The induction and consequences of influenza A virus-induced cell death. Cell Death Dis. 2018;9 doi: 10.1038/s41419-018-1035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sardu C, Gambardella J, Morelli MB, Wang X, Marfella R, Santulli G. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J Clin Med. 2020;9 doi: 10.3390/jcm9051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mozzini C, Girelli D. The role of neutrophil extracellular traps in Covid-19: only an hypothesis or a potential new field of research? Thromb Res. 2020;191:26–27. doi: 10.1016/j.thromres.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brill A, Fuchs TA, Savchenko AS, et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–144. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Borissoff JI, Joosen IA, Versteylen MO, et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol. 2013;33:2032–2040. doi: 10.1161/ATVBAHA.113.301627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martinod K, Wagner DD. Thrombosis: tangled up in NETs. Blood. 2014;123:2768–2776. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Public Health England Coronavirus (COVID-19) statistics and analysis. 2020. https://www.gov.uk/guidance/coronavirus-covid-19-statistics-and-analysis
- 90.Selva KJ, van de Sandt CE, Lemke MM, et al. Distinct systems serology features in children, elderly and COVID patients. medRxiv. 2020 doi: 10.1101/2020.05.11.20098459. published online May 18. (preprint) [DOI] [Google Scholar]
- 91.Gruber C, Patel R, Trachman R, et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C) medRxiv. 2020 doi: 10.1101/2020.07.04.20142752. published online July 6. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Katzelnick LC, Gresh L, Halloran ME, et al. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358:929–932. doi: 10.1126/science.aan6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Endy TP, Nisalak A, Chunsuttitwat S, et al. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J Infect Dis. 2004;189:990–1000. doi: 10.1086/382280. [DOI] [PubMed] [Google Scholar]
- 94.Waggoner JJ, Katzelnick LC, Burger-Calderon R, et al. Antibody-dependent enhancement of severe disease is mediated by serum viral load in pediatric dengue virus infections. J Infect Dis. 2020;221:1846–1854. doi: 10.1093/infdis/jiz618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang S-F, Tseng S-P, Yen C-H, et al. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun. 2014;451:208–214. doi: 10.1016/j.bbrc.2014.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hoepel W, Chen HJ, Allahverdiyeva S, et al. Anti-SARS-CoV-2 IgG from severely ill COVID-19 patients promotes macrophage hyper-inflammatory responses. bioRxiv. 2020 doi: 10.1101/2020.07.13.190140. published online July 13. (preprint) [DOI] [Google Scholar]
- 97.Menikou S, Langford PR, Levin M. Kawasaki disease: the role of immune complexes revisited. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.01156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mason WH, Jordan SC, Sakai R, Takahashi M, Bernstein B. Circulating immune complexes in Kawasaki syndrome. Pediatr Infect Dis. 1985;4:48–51. doi: 10.1097/00006454-198501000-00012. [DOI] [PubMed] [Google Scholar]
- 99.Ono S, Onimaru T, Kawakami K, Hokonohara M, Miyata K. Impaired granulocyte chemotaxis and increased circulating immune complexes in Kawasaki disease. J Pediatr. 1985;106:567–570. doi: 10.1016/s0022-3476(85)80073-1. [DOI] [PubMed] [Google Scholar]
- 100.Levin M, Holland PC, Nokes TJ, et al. Platelet immune complex interaction in pathogenesis of Kawasaki disease and childhood polyarteritis. Br Med J (Clin Res Ed) 1985;290:1456–1460. doi: 10.1136/bmj.290.6480.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khor CC, Davila S, Breunis WB, et al. Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nat Genet. 2011;43:1241–1246. doi: 10.1038/ng.981. [DOI] [PubMed] [Google Scholar]
- 102.Lee Y-C, Kuo H-C, Chang J-S, et al. Two new susceptibility loci for Kawasaki disease identified through genome-wide association analysis. Nat Genet. 2012;44:522–525. doi: 10.1038/ng.2227. [DOI] [PubMed] [Google Scholar]
- 103.Onouchi Y, Onoue S, Tamari M, et al. CD40 ligand gene and Kawasaki disease. Eur J Hum Genet. 2004;12:1062–1068. doi: 10.1038/sj.ejhg.5201266. [DOI] [PubMed] [Google Scholar]
- 104.Onouchi Y, Gunji T, Burns JC, et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. 2008;40:35–42. doi: 10.1038/ng.2007.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tremoulet AH, Pancoast P, Franco A, et al. Calcineurin inhibitor treatment of intravenous immunoglobulin-resistant Kawasaki disease. J Pediatr. 2012;161:506. doi: 10.1016/j.jpeds.2012.02.048. 12.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheng MH, Zhang S, Porritt RA, Arditi M, Bahar I. An insertion unique to SARS-CoV-2 exhibits superantigenic character strengthened by recent mutations. bioRxiv. 2020 doi: 10.1101/2020.05.21.109272. published online May 21. (preprint) [DOI] [Google Scholar]
- 107.Deza Leon MP, Redzepi A, McGrath E, et al. COVID-19-associated pediatric multisystem inflammatory syndrome. J Pediatric Infect Dis Soc. 2020;9:407–408. doi: 10.1093/jpids/piaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hennon TR, Penque MD, Abdul-Aziz R, et al. COVID-19 associated multisystem inflammatory syndrome in children (MIS-C) guidelines; a Western New York approach. Prog Pediatr Cardiol. 2020 doi: 10.1016/j.ppedcard.2020.101232. published online May 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Levin M. Best available treatment study for inflammatory conditions associated with COVID-19. 2020. http://www.isrctn.com/ISRCTN69546370
- 110.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. published online May 22. [DOI] [PubMed] [Google Scholar]
- 111.Horby P, Lim WS, Emberson J, et al. Dexamethasone in hospitalized patients with COVID-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. published online July 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Levin M, Cunnington AJ, Wilson C, et al. Effects of saline or albumin fluid bolus in resuscitation: evidence from re-analysis of the FEAST trial. Lancet Respir Med. 2019;7:581–593. doi: 10.1016/S2213-2600(19)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Crit Care Med. 2020;48:e440–e469. doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Edwards S, Small JD, Geratz JD, Alexander LK, Baric RS. An experimental model for myocarditis and congestive heart failure after rabbit coronavirus infection. J Infect Dis. 1992;165:134–140. doi: 10.1093/infdis/165.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mahmud E, Dauerman HL, Welt FGP, et al. Management of acute myocardial infarction during the COVID-19 pandemic. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.039. published online April 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Caforio ALP, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 117.Veronese G, Ammirati E, Cipriani M, Frigerio M. Fulminant myocarditis: characteristics, treatment, and outcomes. Anatol J Cardiol. 2018;19:279–286. doi: 10.14744/AnatolJCardiol.2017.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Imazio M, Klingel K, Kindermann I, et al. COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart. 2020;106:1127–1131. doi: 10.1136/heartjnl-2020-317186. [DOI] [PubMed] [Google Scholar]
- 119.Godfred-Cato S, Bryant B, Leung J, et al. COVID-19–associated multisystem inflammatory syndrome in children — United States, March–July 2020. MMWR Morb Mortal Wkly Rep. 2020 doi: 10.15585/mmwr.mm6932e2. published online August 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.