Abstract
Respiratory virus infections are among the most prevalent diseases in humans and contribute to morbidity and mortality in all age groups. Moreover, since they can evolve fast and cross the species barrier, some of these viruses, such as influenza A and coronaviruses, have sometimes caused epidemics or pandemics and were associated with more serious clinical diseases and even mortality. The recently identified Coronavirus Disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a Public Health Emergency of International concern and has been associated with rapidly progressive pneumonia. To ensure protection against emerging respiratory tract infections, the development of new strategies based on modulating the immune responses is essential. The use of probiotic components has substantially increased due to their effects on immune responses, in particular on those that occur in the upper/lower respiratory tract. Superinduction of inflammatory reaction, known as a cytokine storm, has been correlated directly with viral pneumonia and serious complications of respiratory infections. In this review, probiotics, as potential immunomodulatory agents, have been proposed to improve the host's response to respiratory viral infections. In addition, the effects of probiotics on different aspects of immune responses and their antiviral properties in both pre-clinical and clinical contexts have been described in detail.
Keywords: COVID-19, SARS-CoV-2, Probiotics, Immunomodulatory, Respiratory virus, Influenza
Highlights
-
•
Immunomodulatory effects of probiotics on different aspects of immune responses has been presented.
-
•
Antiviral properties effects of probiotics in both pre-clinical and clinical contexts have been described in detail.
-
•
Probiotics' mechanism of action on immunity in respiratory virus infections has been elucidated.
Abbreviation
- COVID-19
Coronavirus disease 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- WHO
World Health Organization
- FAO
Food and Agriculture Organization
- MFs
Macrophages
- DCs
Dendritic cells
- M cells
Microfold cells
- IECs
Intestinal epithelial cells
- GALT
Gut-associated lymphoid tissue
- TLRs
Toll-like receptors
- PRRs
Pattern recognition receptors
- PAMPs
Pathogen-associated molecular patterns
- TP
Transmembrane protein
- MyD88
Myeloid differentiation protein 88
- RTIs
Respiratory tract infections
- HRV
Human rhinovirus
- IFV
Influenza virus
- RSV
Respiratory syncytial virus
- IAV
Influenza A virus
- IFN- α
Interferon α
- IFN-β
interferon β
- TNF-α
Tumor necrosis factor α
- IFN-γ
Interferon gamma
- IL-1
Interleukin 1
- IL-6
Interleukin 6
- IL-4
Interleukin 4
- IL-8
Interleukin 8
- IL-10
Interleukin 10
- IL-12
Interleukin 12
- Th1
T helper type 1
- Th2
T helper type 2
- MIPs
Macrophage inflammatory proteins
- MCPs
Monocyte chemoattractant proteins
- BAL
Bronchoalveolar lavage
- NK cell
Natural killer cells
- EPSs
Exopolysaccharides
- IgA
Immunoglobulin A
- IgG
Immunoglobulin G
- SIgA
Secretory Immunoglobulin A
- PPs
Peyer's patches
- Tfh
Follicular helper T
- ACOT
acyl-CoA thioesterase
- CYR61
Cysteine-rich angiogenic inducer 61
- Egr1
Early Growth Response 1
- FOS
Fos Proto-Oncogene
- Rsad2
Radical S-Adenosyl Methionine Domain Containing 2
- Klrk1
Killer Cell Lectin like Receptor K1
- ILC
Innate lymphoid cells
- MLN
Mediastina lymph node
- BALF
Bronchoalveolar lavage fluid
- IP10
interferon-inducible protein 10
- PEDV
Porcine epidemic diarrhea virus
- CFS
cell-free supernatants
- CPE
Cytopathic effect
- DCpep
DC-targeting peptide
- COE
Core neutralizing epitope
- OAS
Oligoadenylate synthetase
- ISG15
Interferon-stimulated gene 1
- SRCAP
SWI2/SNF2-related CREB-binding protein activator protein
- PFU
A plaque-forming unit
- mPIV1
Murine parainfluenza virus
- CRP
C-reactive protein
- CXCL8
C-X-C motif chemokine ligand 8
1. Introduction to probiotics general attitudes and functions
Since the first observation of probiotic bacteria by Elie Metchnikoff, there have been several studies on the immunological effects of probiotics on the host immune system. According to WHO and FAO, probiotics are defined as “live microorganisms which, when administered in proper amounts, confer a health benefit on the host” [1]. Among several genera of bacteria (and yeasts) that identified and defined as probiotics, health benefits of Lactobacillus and Bifidobacterium on the host have been proved and are generally consumed as a part of fermented foods like those in dietary supplements [2]. There are some reports about probiotics potential in promoting health benefits by regulating allergic reactions [[3], [4], [5]], protecting the hosts against bacterial and viral infection [1,[6], [7], [8], [9]], and even reducing the tumor growth in some cancer models [[10], [11], [12]]. The probiotics-conferred health benefits are attributable to their effects on the immune system. Recognition and stimulation of immune system in the gut lumen is followed through three distinct pathways: (1) engulfment of probiotics by macrophages (Mfs) or dendritic cells (DCs) present immediately below M cells (Specialized epithelial cells); (2) DCs-directed sampling and processing of probiotics in the gut lumen; and (3) direct stimulation of intestinal epithelial cells (IECs) by probiotics to secrete an array of cytokines, modulating the immune functions of DCs, T cells, and B cells in the gut-associated lymphoid tissue (GALT) [13,14]. Briefly, the regulatory effects of probiotics on host immune responses are followed through activation of the function of dendritic cells, macrophages, and T and B lymphocytes [15,16]. In addition, probiotics have proved to modulate and regulate innate and adaptive immune responses partly through the activation of toll-like receptors (TLRs) [17].
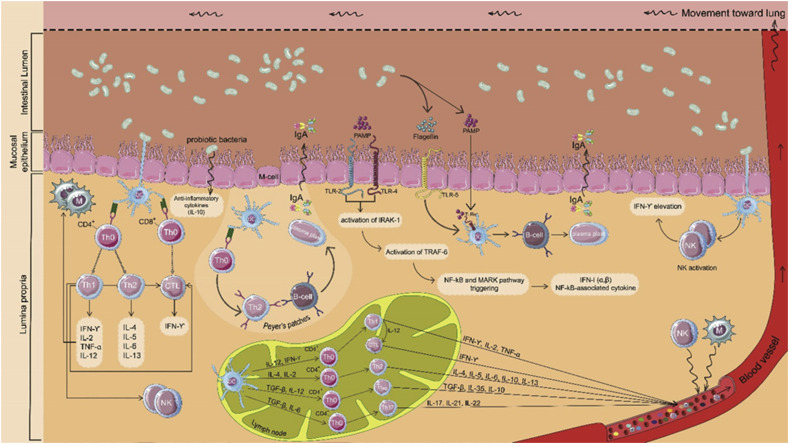
As the role of the intestinal epithelium is to form a physiological barrier against pathogenic microbes, and detrimental substances available in the intestinal lumen, this monolayer is responsible for distinguishing between pathogens and commensal bacteria as well as regulation of intestinal immune responses. It has been shown that probiotics can regulate immunomodulatory responses of intestinal epithelial cells [18] (Fig. 1 ).
Fig. 1.
Schematic presentation of possible mechanisms of probiotic immunomodulation effects in the intestine. Probiotics trigger immunomodulation through direct and indirect interaction with intestinal epithelial cells. Dendritic cells extend their dendrites between intestinal epithelial cells (IECs) and might directly sample and process probiotics in the gut lumen, leading to activation of innate and adaptive immune responses. Dendritic cells, present immediately below M cells, engulf probiotics, resulting in the maturation of DCs and may derive B cells into plasma cells. Additionally, after the interaction of probiotics with macrophages and dendritic cells presented in lamina propria, these cells are activated and induce NK cell activation, which leads to IFN-γ elevation to defend against viruses. Upon the interaction of probiotics' PAMPs with different types of toll-like receptors (TLRs), nuclear factor-κB (NF-κB)-mediated antiviral gene expression is stimulated. Eventually, active immune cells migrate to sites of infection through lymphatic and circulatory systems to defend against respiratory viruses.
One family of pattern recognition receptors (PRRs) in the innate immune system are toll-like receptors, which play a pivotal role in the linking of innate and adaptive immunity. TLRs can specifically recognize pathogen-associated molecular patterns (PAMPs) and convey pathogen-related molecular signals into cells by transmembrane (TM) protein. Afterward, TLR-mediated multistep signaling cascades are initiated, leading to the activation of transcriptional pathways, such as NF-κB, against the invader pathogens [19]. This signal transmission activates both immune system arms aimed at the pathogenic microorganism through a cascade reaction, which is severely dependent on signaling pathway directed by toll-like receptor 7 (TLR7) and myeloid differentiation protein 88 (MyD88) [20]. Interestingly, it has been determined that TLR7 expression considerably reduces after influenza infection. In this context, Wu et al. revealed that after consumption of probiotics by neomycin-treated mice, the balance of intestinal flora restored and thereby TLR7 pathway up-regulated [21]. This evidence presents promise for the regulatory role of probiotics in host innate and adaptive immune responses as underlying mechanisms for protection from viral infection.
2. Pathology of influenza virus; the most common respiratory virus infection
Influenza virus belonging to the orthomyxoviruses family is among viruses that cause respiratory tract infections (RTIs). Several human viruses can cause RTI, and due to hospitalizations, medical costs, sick leave, and school or daycare absences, viral respiratory diseases can pose a considerable social and economic burden [22]. Human rhinovirus (HRV), enterovirus [23], influenza virus (IFV), respiratory syncytial virus (RSV), and adenovirus are common etiological agents of acute respiratory disease [24].
Influenza A virus (IAV) initiates pulmonary inflammation and intensifies chronic lung diseases in response to the infiltration of inflammatory cells and augmentation of airway hyperresponsiveness [25]. The main target and host for IAV is the bronchial epithelial cell, which plays a key role in influenza pathogenesis [26]. Infection occurs following 4–6 h of influenza virus replication for the first cycle, and then initial high titers of virus are shed during this period. IFV infection can result in several symptoms like fever, cough, headache, and pneumonia, which may become immunologically incompetent [27]. While the induction of inflammatory cytokines by influenza infection is attributed to its systemic feature, it is unlikely that the virus to be propagated outside the respiratory tract during an uncomplicated infection [28].
One of the key components of the influenza virus in pathogenesis is HA domain, which is recognized by the host's neutralizing antibodies. The emerged HA is directed to the cell membrane in an infected host cell, fastening to the cell membrane by means of a short transmembrane region at the C-terminal, and once this domain attached to terminal sialic acid residues on the cell, it facilitates entry and fusion of the virus. Due to the acidification of host cells by proton pumps, HA rearranges so that the highly conserved N-terminal of HA2 is exposed. This exposure leads to the fusion of viral membrane with cell membranes, and thus activation of the replication complex [29].
Despite all known clinical and pathogenesis descriptions of the influenza virus, the mechanism through which influenza virus disease being developed has not precisely understood. However, it is thought that local non-immune cells, which release early cytokines, are the cause of many of the clinical signs [30,31]. Some cytokines including IFN-α, TNF-α, and IL-1 (α and β) located at the site of infection are responsible for local inflammatory reactions as well as some systemic effects [32,33]. Afterward, IL-6 and many other chemotactic cytokines like the neutrophil attracting interleukin-8 (IL-8), macrophage inflammatory proteins (MIPs), and monocyte chemoattractant proteins (MCPs) are rapidly produced [34]. Fever, excessive sleepiness, and anorexia are attributed to the activation of IFN-α, TNF-α, IL-1, and IL-6 after influenza infection. Neutrophil and macrophage functions are stimulated by TNF-α and IL-1 and both cytokines potently up-regulate leukocyte adhesion molecules on the vascular endothelium, therefore, mediating the first indispensable step for sequestration of neutrophils and (or) macrophages into the respiratory tract. A study by Van Reeth demonstrated that there is a correlation between BAL fluid levels of some cytokines (IFN-α, TNF-α, and IL-1) and virus titers, neutrophil infiltration, and influenza disease [35]. Additionally, Lee et al. showed that IFN-α, TNF-α, IL-1, and IL-6 all participate in non-specific and specific antiviral immune responses [36].
3. Immunomodulatory role of probiotics on influenza virus in the context of pre-clinical studies
Since the manifestation of probiotics impacts on several diseases from non-viral to viral ones [12,[37], [38], [39], [40], [41]], several studies have surveyed the probiotic roles in immune responses of influenza-infected animal models. It has been fully demonstrated that upon infection with influenza, many cytokines such as IL-12 (one of the mediators of Th1 immune-response), interferon (IFN)-γ (representative of Th1 cytokine), IL-4 and IL-10 (Th2 cytokines), IL-1α, IL-1β, IL-6, and tumor necrosis factor (TNF)-α (pro-inflammatory cytokines), and IFN-α and IFN-β are produced in the respiratory tract [36,[42], [43], [44]]. Studies on ameliorating influenza infection as well as alleviating influenza symptoms have been trying to redress the imbalance attributed to runaway cytokines production (namely cytokine storm) after IFV infection.
Kawahara et al. demonstrated that probiotic Bifidobacterium longum MM-2 can significantly reduce influenza-elicited pro-inflammatory cytokines such as IL-6 and TNF-α. Moreover, a slightly elevated IFN-α level in the BALF indicated the impact of this probiotic on the enhancement of NK cell activity. These results along with the reduction of pulmonary mRNA levels of NK cell activators including pro-inflammatory cytokine IL-1β and chemokines MIP-2 and MCP-1 suggest the modulating effect of this probiotic on influenza infection [2,45].
In another study, continuous oral administration of Lactobacillus plantarum 06CC2 led to an elevation in the production of IFN-α and Th1 cytokines (IL-12 and IFN-γ) and reduction in the production of TNF-α and IL-6 cytokines in BALF. This probiotic could also control the number of total infiltrated cells such as macrophages and neutrophils in the BALF of infected mice [46]. Similarly, Nagai et al. revealed that 4 days after oral administration of the yogurt fermented with L. bulgaricus OLL1073R-1 or its exopolysaccharides (EPSs), influenza virus infection ameliorated, which attributed to the development of NK cell activity of splenocytes [47]. Assessment of kimchi-derived Lactobacillus plantarum and Leuconostoc mesenteroides has confirmed their effectiveness against lethal influenza viruses H1N1 and H7N9, by decreasing the sizes of viral plaques, both in vitro and in vivo [48].
In addition, it has been shown that lactococcal strains or their EPS induced weight regain and also reduced viral titer in the lung of mice infected with influenza virus H1N1 [49]. Starosila et al. investigated the antiviral ability of Bacillus subtilis and showed that after a single dose administration of the probiotic bacteria, the survival rate of mice challenged with the IFV increased [50]. Song et al. assessed the impact of oral intake of Lactobacillus rhamnosus M21 on lethally IFV-infected mice. An increase in the level of IFN-γ and IL-12 and a decline in IL-4 level suggested that this probiotic can modulate some disease outcomes attributed to changes in cytokine profiles such as that happens in the lung after influenza infection [51].
In our very recent study, we showed that Bifidobacterium bifidum can increase the level of both Th1 (IFN–Y and IL-12) and Th2 (IL-4) cytokines. An increase in the level of total IgG antibodies in pooled sera of treated mice and IgG1 and IgG2a isotypes demonstrated the efficacy of the probiotic in eliciting humoral immune responses and Th1/Th2 responses, respectively. Moreover, it revealed that the level of inflammatory cytokines like IL-6, which increases upon influenza infection, decreased in the probiotic group, suggesting the ameliorating potential of this probiotic in influenza-infected mice [52]. Based on the results of body weight changes, survival rates, and viral titre among treatment groups of 3 different influenza viruses, Park et al. showed that lactobacillus plantarum has anti-influenza effects that are not virus type- or strain-dependent, revealing that regular intake of that probiotic can help to alleviate the influenza symptoms [53].
Concerning the effect of long-term probiotic administration, Kiso et al. orally injected Lactobacillus pentosus b240 to mice for 5 weeks and evaluated its inhibitory properties against influenza challenge. Assessment of 34 different cytokines/chemokines in the lungs of infected animals revealed that excluding IL-5, administration of that probiotic did not affect the immune system regarding cytokines/chemokines secretion. However, A(H1N1) pdm infected mice survived, probably due to protecting effects of the probiotic by down-regulation of Acots (Acot1, Acot2, and Acot5), Cyr61, Egr1, and Fos, as well as upregulation of Stfa1, and antiviral Rsad2 genes in the lungs of uninfected mice [54].
In agreement with all aforementioned results, Harata et al. revealed that oral administration of probiotics Lactobacillus GG and L. gasseri TMC035 in mice infected with a lethal dose of influenza A(H1N1) pdm prompted the secretion of IL‐12, IL‐6, IFN‐γ, and IgA from isolated PP cells in vitro. However, unlike Lactobacillus GG, the oral administration of L. gasseri had no impact on the production of IFN-γ, IL-6, as well as total IgA in vivo, proving the vital role of probiotic interaction with the component cells of GALT in the protection against influenza [55].
The investigation of the effects of L. casei strain Shirota on aged mice showed that this probiotic can enhance not only the level of IFN-γ and TNF-α, but also pulmonary and spleen NK cells activity, and thereby ameliorates IFV infection [56]. In another study, oral administration of Bifidobacterium longum BB536 could significantly reduce the loss of body weight, inhibit viral proliferation in the lungs, and improve the symptoms of influenza-infected mice, which may be related to the decreased level of IL-6 [57,58]. Belkacem et al. observed that while administration of probiotic L. paracasei induced significantly higher levels of pro-inflammatory cytokines in probiotic-fed influenza mice models, this trend was reversed seven days upon influenza challenge except for IL-33. The number of all tissue-resident or circulatory myeloid cells and B cells after the probiotic consumption and before viral infection increased; and the probiotic administration generated more IFN-γ-producing ILC1 (mainly NK cells) and Th2 cells during the late phase of influenza infection. Additionally, L. paracasei peptidoglycans administration before influenza infection increased dendritic cells, but did not affect other cell types, and significantly reduced viral loads [59].
Besides the effectiveness of oral administration of probiotics, intranasal administration of Lactobacillus pentosus S-PT84 to mice proved to induce the production of IL-12 and IFN-γ in mediastinal lymph node (MLN) cells, and IL-12 and IFN-α in BALF, thereby improved the survival rates of mice, reduced the IFV titer in BALF, and subsequently suppressed influenza infection in mice [60]. Employing the novel sublingual route, Lee et al. showed that, in contrary to pro-inflammatory cytokines, the level of IL-12 in the lung homogenates of mice treated with Lactobacillus rhamnosus significantly increased. In addition, besides the increase in NK cell activities and anti-influenza virus IgA, the expression of CD25 by both CD8+ and CD4+ lymphocytes highly increased in the lungs of mice. These results recommend that compared to the traditional methods, sublingual delivery is a more effective way for the administration of probiotics against seasonal and pandemic influenza [61].
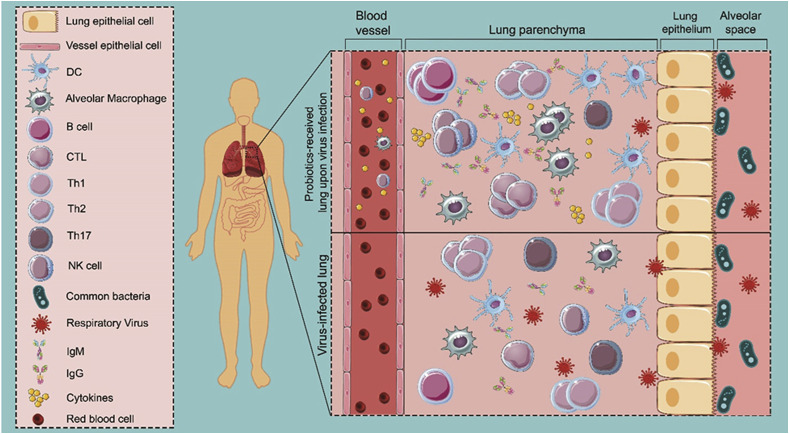
Regarding other animal models, Poorbaghi et al. showed that microencapsulated Lactobacillus acidophilus probiotic and its symbiotic form with inulin decreased faecal shedding of H9N2 avian influenza virus (AIV) in both non-vaccinated and vaccinated broiler chicks [62]. In another study, Enterococcus faecalis-1 has been proved to improve the body weight and feed conversion ratio of treated broilers, and also significantly elevated the total IgY serum level, resulting in efficient modulation of the cecal microbiota and decrease in the mortality percentage of broilers [63]. An investigation on the possible effect of interaction between lactobacilli and chicken macrophages on eliciting antiviral responses against the AIV showed that certain probiotic species such as L. acidophilus and L. salivarius, when administered as live bacteria either alone or in combination, can induce an antiviral response in chicken macrophages [64]. In another study, Seo et al. reported that live Leuconostoc mesenteroides YML003 significantly restored the body weight and increased the IFN-γ levels in splenic cells of low-pathogenic AIV H9N2-infected chickens [65]. Examining the effectiveness of Enterococcus faecium NCIMB 10415 and Zinc Oxide in modulating the immune system of piglets in confronting with swine influenza virus (SIV) revealed that the body weights of piglets fed with the probiotic and vaccinated with trivalent influenza vaccine significantly increased, and noticeably higher H3N2-specific antibodies were detected among them [66]. Based on these considerations, probiotics administration is effective in the secretion of high concentration of cytokines from immune cells, located in the airway, leading to the migration of immune cells to the lung space and thereby amelioration of influenza infection (Fig. 2 ).
Fig. 2.
Model of the interaction of active immune cells triggered by probiotics with respiratory viruses in the lung. Following virus infection, immune cells in the airway, such as dendritic cells and macrophages, secrete cytokines to defend against viruses. In a probiotic-received subject, the high concentration of cytokines leads to the migration of immune cells to the lung space through the gut–lung axis, resulting in rapid recruitment of activated T and B cells in the lung that eventually promote upregulation of virus-specific immunoglobulins and cytokines in probiotic-received subject; whereas, in the absence of activated immune cells, the respiratory virus can cause severe lung damage due to the lack of immediate immune responses.
4. The probiotic effects on coronavirus infections
The current outbreak of coronavirus disease (COVID-19) reported from Wuhan, China, has again gained global attention to taking a new measure that could work out as fast as possible against such an outbreak of viruses. Interestingly, accumulated data obtained from clinical investigations on 41 patients who suffered from severe COVID-19 in a hospital in Wuhan demonstrated the presence of signs for cytokine storm, especially among patients in severe stages of the disease. Particularly, the levels of cytokines and chemokines involved in both Th1 (such as IL-1B, IFN-γ) and Th2 (IL-4 and IL-10) immune responses were promoted in studied patients. Moreover, the levels of IP-10, MCP-1, MIP-1a, and TNF-α were in direct correlation with the severity of patients' symptoms [67]. On the other hand, it has been determined that the cytokine storm may lead to a rise in platelets and longer hospitalization of COVID-19 patients [68]. Other studies also have revealed other aspects of virally-driven manipulation of immune responses by human coronaviruses [[69], [70], [71]]. Therefore, addressing the cytokine storm may be the key for the treatment of patients infected with SARS-CoV-2. While some reagents such as steroids can be considered as hyperinflammation suppressors, their side-effects impede to count on them as a trustworthy medicine for COVID-19 [72]. Alternatively, addressing the urgent need for standing against the increasing rate of morbidity and mortality related to the current pandemic requires employing previously approved therapies harnessing safety profiles. Probiotics as a safe, available treatment with the ability to modulate immune responses and manipulate cytokines production have been considered to be studied against different strains of coronavirus in some studies soon [73,74]. Moreover, a clinical survey has reported an intestine microbiota imbalance, in particular a decline in the level of probiotics such as Lactobacillus and Bifidobacterium, among some COVID-19 patients, which may result in secondary infection in response to bacterial translocation [75].
One report has shown that the recent outbreak of porcine epidemic diarrhea virus (PEDV) can be prevented through the use of either cell-free supernatants (CFS) or live lactic acid bacteria (LAB). It demonstrated that probiotics, though the precise mechanism is not clear, could be effective against the pandemic strain of PEDV in a strain-specific manner using CPE reduction assays that further confirmed by qualitative immunofluorescence [76]. In another investigation, Lactobacillus casei was used as a carrier for the DC-targeting peptide (DCpep) fused with the PEDV core neutralizing epitope (COE) antigen. This survey demonstrated that this genetically engineered Lactobacillus casei oral vaccine is able to induce systemic IgG and mucosal SIgA antibody responses in mice models [77]. There have been other articles using different types of probiotics for displaying the desired genes or antigens against PEDV [[77], [78], [79], [80]]. For instance, Liu et al. demonstrated that their modified Lactobacillus plantarum has the property to act like a strong antiviral agent against coronavirus infection in the intestinal porcine epithelial cell line [80].
5. Probiotic impacts on other viral respiratory infections
Eguchi et al. demonstrated that Lactobacillus gasseri SBT2055 (LG2055), when administered orally to mice before infection with a human RSV, could suppress the virus titre in lung tissue homogenates, RSV replication, and the intensity of the symptoms. Moreover, a decrease in the expression level of pro-inflammatory cytokines and an increase in the mRNA level of IFN-β, IFN-γ, OAS1a, and ISG15 in the mice lung upon probiotic administration, are satisfactory evidence for antiviral properties of this probiotic. Also, SWI2/SNF2-related CREB-binding protein activator protein (SRCAP) introduced as a candidate for the antiviral activity of LG2055 against RSV [81].
To investigate whether probiotics can control the inflammatory pathway and modulate the coagulation system upon respiratory viral infection, rhamnosus CRL1505 was orally administered in RSV or IFV mice models. The results elucidated that this probiotic could successfully modulate TLR3-triggered immune coagulation reaction in the lung upon viral infection and prevent exacerbated respiratory injuries. Notably, this study substantiated the vital role of probiotic-provoked secretion of IL-10 in taming the coagulation system after the viral attack [82]. Additionally, in a study conducted by Tomosada et al., nasal administration of Lactobacillus rhamnosus CRL1505 (and CRL1506) has proved to modulate elevated respiratory levels of the pro-inflammatory mediators caused by administration of the viral pathogen-associated molecular pattern poly(I:C). Moreover, a nasal administration of the probiotic prior to 106 PFU of RSV challenge improved resistance against RSV infection [83].
Considering the effect of probiotics on the para-influenza virus, there is only one study evaluating the antiviral effects of oral administration of Lactococcus lactis subsp. Lactis JCM5805 in a mouse model of murine parainfluenza virus (mPIV1) infection. The probiotic administration resulted in a rise in the survival rate of treated mice without any weight loss and also a decline in the lung histopathology scores compared to the non-treated group, which was attributed to the incorporation of JCM5805 into CD11c+ immune cells in PP, and thereafter activation of PP pDCs and ultimately elevation of IFNs expression. It is of note that although no activated local pDCs were observed at lung, upregulation in IFNs-induced antiviral factors in the lung may be due to the delivery of IFNs from the intestine of JCM5805-fed mice into the lung [84]. Studies reporting the effects of probiotic bacterias on respiratory viruses have been demonstrated in Table (supplementary section).
6. Clinical evidence of probiotic immunomodulation
In a pilot study, intake of Lactobacillus brevis KB290 has shown to curtail the incidence of influenza infection among schoolchildren with no adverse effects associated with consuming the probiotic-containing drinks [85]. Hu et al. demonstrated that H7N9 IFV infection led to a decrease in intestinal microbial diversity and species richness among patients. They observed that although administration of C. butyricum probiotic was unable to alleviate the antibiotic-related disturbances in the gut microbiome of H7N9-infected patients, an increase in microbiota diversity and evenness gradually appeared through continuous administration of probiotics after antibiotic cessation. Additionally, based on the evaluation of CRP levels or bacteremia and pneumonia in the patients treated with probiotics, the safety of probiotic administration was approved and no inflammatory effects were observed [86].
In another study conducted by Wang et al., the impact of Lactobacillus rhamnosus GG administration on nursing home residents aged 65 and older was assessed. It revealed that probiotic administration reduced the risk of influenza and other respiratory viral infections among the elderly received probiotics compared to those receiving a placebo. Although not statistically significant, the trial provided a framework to assess the effectiveness of probiotics in reducing respiratory infections among senescent individuals [87]. Similarly, it has been shown that there is no connection between intaking the yogurt fermented with probiotic Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 and incidence rate of influenza in humans. However, the immunological study showed an increase in the level of IFN-γ in the probiotic-treated group [88].
While there are some available treatments for HRV, the most frequent cause of the common cold [89], most of them have failed to be efficient in clinical trials due to their drawbacks [90]. In this regard, probiotics have shown to prevent or treat common colds and upper respiratory infections [91]. Several studies have revealed that the rhinovirus-related common cold pathogenesis is associated to the innate inflammatory response to the virus [92]. Therefore, more attempts have done to incorporate probiotics to modulate immune responses, consequently leading to balanced responses and optimal outcomes in combating viral infection [93]. In this regard, an investigation on the impact of Bifidobacterium animalis ssp. Lactis Bl-04 on healthy adults showed a modest modulation of innate immune host responses upon infection with HRV, particularly reduction of CXCL8 response in the nasal lavage, resulting in a decline in the rhinovirus replication approved by a decrease in virus shedding in the nasal secretions [92].
Tapiovaara et al. demonstrated that adults consumed juice enriched with live or heat-inactivated L. rhamnosus GG before intranasal inoculation of HRV showed no significant differences in nasopharyngeal HRV loads compared to the placebo group [94]. Another survey has illustrated that consumption of probiotic is a good strategy to prevent viral RTIs in the first year of life in a cohort of preterm infants. The results showed that the probiotic-driven change in microbiota leads to the induction of long-lasting effects, which can reduce the risk of viral RTIs [95]. In agreement with that study, it was demonstrated that live L. rhamnosus GG might be more effective in reducing the rhinovirus infection rate than the inactivated form of the same strain [96].
Respiratory syncytial virus from the Paramyxoviridae family is considered as the major cause of lower respiratory tract infection in infants and children around the world and is becoming an important pathogen of the elderly. Although most children have experienced a first RSV infection by two years of age, some cases suffering premature birth, bronchopulmonary dysplasia, immunodeficiency, and congenital heart disease are vulnerable to symptoms worsening and hospitalization as well. However, the probiotic administration has proved to be effective in developing protection against virally-induced inflammation [97]. Besides, there is a study demonstrating that while probiotic consumption significantly reduced the number of days with respiratory symptoms during the intervention, no significant effect was neither observed on the occurrence of viruses in the nasopharynx nor on the symptoms during viral episodes among daycare children [98]. Clinical studies reporting regulation of immune responses by probiotic bacterias have been presented in Table 1 .
Table 1.
Clinical studies reporting regulatory effect of probiotic bacteria on immune responses.
| Study design | Subjects | Probiotics used | Main findings |
|---|---|---|---|
| An open-label, parallel-group trial [85] | 2783 schoolchildren (6–12 years of age) | Lactobacillus brevis KB290 (KB290), 5 days per week for 8 weeks in the form of test drink containing 6 × 109 CFU | The risk of infection ↓ |
| A retrospective study [86] | 15 patients | Clostridium butyricum, three times per day at the dose of 108 CFU/tablet prior to H7N9 infection | Microbiota diversity after antibiotic cessation ↑ |
| A randomized, double-blind, placebo-controlled pilot trial [88] | 209 nursing home residents (65 years of age and older) | Lactobacillus rhamnosus GG, twice a day for 6 months in the form of capsule containing 1010 CFU | The risk of influenza infection ↓not statically significance (NS) The risk of other respiratory viral infections ↓(NS) |
| A randomized controlled, open labeled study [89] | 982 women (aged 20 or older) | Lactobacillus bulgaricus OLL1073R-1 and Streptococcus thermophiles, daily for 16 weeks in the form of yoghurt containing 109 CFU | IFN-γ production in serum ↑ |
| A randomized, double-blind, placebo-controlled trial [92] | 190 adult volunteers | Bifidobacterium animalis, daily for 33 days in the form of a sachet containing a minimum of 2 × 109 CFU | Nasal lavage viral titers ↓ virus shedding in the nasal secretions ↓ |
| A randomized, double-blind, placebo-controlled study [94] | 94 preterm infants(Aged between days 3 and 60 of life) | Lactobacillus rhamnosus GG, daily for 30 days in the form of capsule at the dose of 1 × 109/2 × 109 | Incidence of RTIs ↓ |
| The clinical and experimental randomized, double-blind, placebo-controlled, pilot study [96] | 59 healthy subjects (aged 18–65 years) | Lactobacillus rhamnosus GG, daily for 6 weeks in the form of juice containing 109 CFU | Rhinovirus infection rate ↓(NS) |
| A randomized, double-blinded, and placebo-controlled parallel group intervention study [98] | 523 children attending day care (aged 2–6 years) | Lactobacillus rhamnosus GG, daily for 25 weeks in the form of milk at the dose of 108 CFU | The number of days with respiratory symptoms ↓ |
7. Conclusion
In this review, we presented the current advances in the administration of probiotics to alleviate and cure respiratory virus infections. There is a key point that may correlate respiratory virus diseases to each other, the emergence of imbalanced immune responses as a result of virus-host interactions. Employment of probiotics for modulating the inflammatory immune responses upon virus infection has shown promising results. Although most studies have conducted on influenza virus, elucidation of probiotics' mechanism of action is helpful to conclude the effectiveness of probiotics in other respiratory virus infections. Nowadays, we are confronting with the biggest pandemic of the contemporary era, COVID-19, with highly rapid expansion and increasing rate of mortality, which at the moment has estimated to be 10 times more than the seasonal H1N1 influenza virus infection. The newly emerged SARS-CoV-2, the agent of COVID-19, has shown to induce inflammatory responses, which is in direct correlation with the severity of symptoms and inpatient time. Based on this observation, and although there are not any available data substantiating the effectiveness of probiotics on SARS-CoV-2 infection, previously proven antiviral properties of probiotics against different respiratory viruses may suggest probiotics as a safe and available complementary medicine against COVID-19 disease.
Authors contribution statement
M.M, S.M.M, and A.G. drafted the study concepts and design; M.M, E.A and A.G accomplished the literature research. All authors read and approved the final manuscript.
Funding
This study was supported by Pasteur Institute of Iran (Grant No:1029).
Ethical statement
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgements
We thank all the physician, nurses, scientists and researchers who have fought against the coronavirus on the epidemic. This research did not receive a specific grant.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.micpath.2020.104452.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Corcionivoschi N., Drinceanu D., Stef L., Luca I., Julean C. Probiotics-identification and ways of action. Innov. Rom. Food Biotechnol. 2010;6:1. [Google Scholar]
- 2.Kawahara T., Takahashi T., Oishi K., Tanaka H., Masuda M., Takahashi S. Consecutive oral administration of Bifidobacterium longum MM‐2 improves the defense system against influenza virus infection by enhancing natural killer cell activity in a murine model. Microbiol. Immunol. 2015;59:1–12. doi: 10.1111/1348-0421.12210. [DOI] [PubMed] [Google Scholar]
- 3.Ohno H., Tsunemine S., Isa Y., Shimakawa M., Yamamura H. Oral administration of Bifidobacterium bifidum G9-1 suppresses total and antigen specific immunoglobulin E production in mice. Biol. Pharm. Bull. 2005;28:1462–1466. doi: 10.1248/bpb.28.1462. [DOI] [PubMed] [Google Scholar]
- 4.Cross M., Gill H. Can immunoregulatory lactic acid bacteria be used as dietary supplements to limit allergies? Int. Arch. Allergy Immunol. 2001;125:112–119. doi: 10.1159/000053804. [DOI] [PubMed] [Google Scholar]
- 5.Hajavi J., Esmaeili S.A., Varasteh A.R., Vazini H., Atabati H., Mardani F. The immunomodulatory role of probiotics in allergy therapy. J. Cell. Physiol. 2019;234:2386–2398. doi: 10.1002/jcp.27263. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda S., Toh H., Hase K., Oshima K., Nakanishi Y., Yoshimura K. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 7.Isolauri E., Kirjavainen P., Salminen S. Probiotics: a role in the treatment of intestinal infection and inflammation? Gut. 2002;50 doi: 10.1136/gut.50.suppl_3.iii54. iii54-iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park M.-K., Ngo V., Kwon Y.-M., Lee Y.-T., Yoo S., Cho Y.-H. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PloS One. 2013;8 doi: 10.1371/journal.pone.0075368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasui H., Kiyoshima J., Hori T. Reduction of influenza virus titer and protection against influenza virus infection in infant mice fed Lactobacillus casei Shirota. Clin. Diagn. Lab. Immunol. 2004;11:675–679. doi: 10.1128/CDLI.11.4.675-679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C.-C., Lin W.-C., Kong M.-S., Shi H.N., Walker W.A., Lin C.-Y. Oral inoculation of probiotics Lactobacillus acidophilus NCFM suppresses tumour growth both in segmental orthotopic colon cancer and extra-intestinal tissue. Br. J. Nutr. 2012;107:1623–1634. doi: 10.1017/S0007114511004934. [DOI] [PubMed] [Google Scholar]
- 11.Commane D., Hughes R., Shortt C., Rowland I. The potential mechanisms involved in the anti-carcinogenic action of probiotics. Mutat. Res. Fund Mol. Mech. Mutagen. 2005;591:276–289. doi: 10.1016/j.mrfmmm.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 12.Shin R., Itoh Y., Kataoka M., Iino-Miura S., Miura R., Mizutani T. Anti-tumor activity of heat-killed lactobacillus plantarum BF-LP284 on Meth-A tumor cells in BALB/c mice. Int. J. Food Sci. Nutr. 2016;67:641–649. doi: 10.1080/09637486.2016.1185771. [DOI] [PubMed] [Google Scholar]
- 13.Both E., György É., Ábrahám B., Lányi S. Beneficial effects of probiotic microorganisms. A review. Acta Universitatis Sapientiae, Alimentaria. 2011;4:44–58. [Google Scholar]
- 14.Shida K., Nanno M. Probiotics and immunology: separating the wheat from the chaff. Trends Immunol. 2008;29:565–573. doi: 10.1016/j.it.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Vanderpool C., Yan F., Polk B.D. Mechanisms of probiotic action: implications for therapeutic applications in inflammatory bowel diseases. Inflamm. Bowel Dis. 2008;14:1585–1596. doi: 10.1002/ibd.20525. [DOI] [PubMed] [Google Scholar]
- 16.Yan F., Polk D.B. Probiotics and immune health. Curr. Opin. Gastroenterol. 2011;27:496–501. doi: 10.1097/MOG.0b013e32834baa4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan F., Polk D. Probiotics and immune health. Curr. Opin. Gastroenterol. 2011;27:496. doi: 10.1097/MOG.0b013e32834baa4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zanello G., Berri M., Dupont J., Sizaret P.-Y., d'Inca R., Salmon H. Saccharomyces cerevisiae modulates immune gene expressions and inhibits ETEC-mediated ERK1/2 and p38 signaling pathways in intestinal epithelial cells. PloS One. 2011;6 doi: 10.1371/journal.pone.0018573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unterholzner L., Bowie A.G. The interplay between viruses and innate immune signaling: recent insights and therapeutic opportunities. Biochem. Pharmacol. 2008;75:589–602. doi: 10.1016/j.bcp.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 20.Davidson S., Kaiko G., Loh Z., Lalwani A., Zhang V., Spann K. Plasmacytoid dendritic cells promote host defense against acute pneumovirus infection via the TLR7–MyD88-dependent signaling pathway. J. Immunol. 2011;186:5938–5948. doi: 10.4049/jimmunol.1002635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S., Jiang Z.-Y., Sun Y.-F., Yu B., Chen J., Dai C.-Q. Microbiota regulates the TLR7 signaling pathway against respiratory tract influenza A virus infection. Curr. Microbiol. 2013;67:414–422. doi: 10.1007/s00284-013-0380-z. [DOI] [PubMed] [Google Scholar]
- 22.Fendrick A.M., Monto A.S., Nightengale B., Sarnes M. The economic burden of non–influenza-related viral respiratory tract infection in the United States. Arch. Intern. Med. 2003;163:487–494. doi: 10.1001/archinte.163.4.487. [DOI] [PubMed] [Google Scholar]
- 23.Ermolenko E., Desheva Y., Kolobov A., Kotyleva M., Sychev I., Suvorov A. Anti–influenza activity of enterocin B in vitro and protective effect of bacteriocinogenic enterococcal probiotic strain on influenza infection in mouse model. Probiotics and antimicrobial proteins. 2019;11:705–712. doi: 10.1007/s12602-018-9457-0. [DOI] [PubMed] [Google Scholar]
- 24.Lehtoranta L., Pitkäranta A., Korpela R. Probiotics in respiratory virus infections. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:1289–1302. doi: 10.1007/s10096-014-2086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillot L., Le Goffic R., Bloch S., Escriou N., Akira S., Chignard M. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 2005;280:5571–5580. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- 26.Message S.D., Johnston S.L. Host defense function of the airway epithelium in health and disease: clinical background. J. Leukoc. Biol. 2004;75:5–17. doi: 10.1189/jlb.0703315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang J.W., Shetty N., Lam T.T., Hon K.E. Emerging, novel, and known influenza virus infections in humans. Infect. Dis. Clin. 2010;24:603–617. doi: 10.1016/j.idc.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zambon M.C. Epidemiology and pathogenesis of influenza. J. Antimicrob. Chemother. 1999;44:3–9. doi: 10.1093/jac/44.suppl_2.3. [DOI] [PubMed] [Google Scholar]
- 29.de Lima M.C.P., Ramalho-Santos J., Flasher D., Slepushkin V.A., Nir S., Düzguneş N. Target cell membrane sialic acid modulates both binding and fusion activity of influenza virus. Biochim. Biophys. Acta Biomembr. 1995;1236:323–330. doi: 10.1016/0005-2736(95)00067-d. [DOI] [PubMed] [Google Scholar]
- 30.Fotouhi F., Shaffifar M., Farahmand B., Shirian S., Saeidi M., Tabarraei A. Adjuvant use of the NKT cell agonist alpha-galactosylceramide leads to enhancement of M2-based DNA vaccine immunogenicity and protective immunity against influenza A virus. Arch. Virol. 2017;162:1251–1260. doi: 10.1007/s00705-017-3230-7. [DOI] [PubMed] [Google Scholar]
- 31.Shokouhi H., Farahmand B., Ghaemi A., Mazaheri V., Fotouhi F. Vaccination with three tandem repeats of M2 extracellular domain fused to Leismania major HSP70 protects mice against influenza A virus challenge. Virus Res. 2018;251:40–46. doi: 10.1016/j.virusres.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Fotouhi F., Shaffifar M., Farahmand B., Shirian S., Saeidi M., Tabarraei A. Adjuvant use of the NKT cell agonist alpha-galactosylceramide leads to enhancement of M2-based DNA vaccine immunogenicity and protective immunity against influenza A virus. Arch. Virol. 2017;162:1251–1260. doi: 10.1007/s00705-017-3230-7. [DOI] [PubMed] [Google Scholar]
- 33.Shokouhi H., Farahmand B., Ghaemi A., Mazaheri V., Fotouhi F. Vaccination with three tandem repeats of M2 extracellular domain fused to Leismania major HSP70 protects mice against influenza A virus challenge. Virus Res. 2018;251:40–46. doi: 10.1016/j.virusres.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Bielefeldt-Ohmann H. Marcel Dekker; New York: 1995. Role of Cytokines in the Pathogenesis and Treatment of Respiratory Disease. Cytokines in Animal Health and Disease; pp. 291–332. [Google Scholar]
- 35.Van Reeth K. Cytokines in the pathogenesis of influenza. Vet. Microbiol. 2000;74:109–116. doi: 10.1016/s0378-1135(00)00171-1. [DOI] [PubMed] [Google Scholar]
- 36.Junming L. Tumor necrosis factor and interleukin-1: cytokines with multiple overlapping biological activities. Lab. Invest. 1987;56:234–248. [PubMed] [Google Scholar]
- 37.Philippe D., Heupel E., Blum-Sperisen S., Riedel C.U. Treatment with Bifidobacterium bifidum 17 partially protects mice from Th1-driven inflammation in a chemically induced model of colitis. Int. J. Food Microbiol. 2011;149:45–49. doi: 10.1016/j.ijfoodmicro.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 38.Ventola H., Lehtoranta L., Madetoja M., Simonen-Tikka M.-L., Maunula L., Roivainen M. Effects of the viability of Lactobacillus rhamnosus GG on rotavirus infection in neonatal rats. World J. Gastroenterol.: WJG. 2012;18:5925. doi: 10.3748/wjg.v18.i41.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendes M.C.S., Paulino D.S., Brambilla S.R., Camargo J.A., Persinoti G.F., Carvalheira J.B.C. Microbiota modification by probiotic supplementation reduces colitis associated colon cancer in mice. World J. Gastroenterol. 2018;24:1995–2008. doi: 10.3748/wjg.v24.i18.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdolalipour E., Mahooti M., Salehzadeh A., Torabi A., Mohebbi S.R., Gorji A. Evaluation of the antitumor immune responses of probiotic Bifidobacterium bifidum in human papillomavirus-induced tumor model. Microb. Pathog. 2020:145. doi: 10.1016/j.micpath.2020.104207. [DOI] [PubMed] [Google Scholar]
- 41.Abdolalipour E., Mahooti M., Salehzadeh A., Torabi A., Mohebbi S.R., Gorji A. Evaluation of the antitumor immune responses of probiotic Bifidobacterium bifidum in human papillomavirus-induced tumor model. Microb. Pathog. 2020:104207. doi: 10.1016/j.micpath.2020.104207. [DOI] [PubMed] [Google Scholar]
- 42.Hennet T., Ziltener H.J., Frei K., Peterhans E. A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J. Immunol. 1992;149:932–939. [PubMed] [Google Scholar]
- 43.Kurokawa M., Imakita M., Kumeda C.A., Shiraki K. Cascade of fever production in mice infected with influenza virus. J. Med. Virol. 1996;50:152–158. doi: 10.1002/(SICI)1096-9071(199610)50:2<152::AID-JMV8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 44.Cheung C., Poon L., Lau A., Luk W., Lau Y., Shortridge K. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831–1837. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 45.Stein-Streilein J., Guffee J. In vivo treatment of mice and hamsters with antibodies to asialo GM1 increases morbidity and mortality to pulmonary influenza infection. J. Immunol. 1986;136:1435–1441. [PubMed] [Google Scholar]
- 46.Takeda S., Takeshita M., Kikuchi Y., Dashnyam B., Kawahara S., Yoshida H. Efficacy of oral administration of heat-killed probiotics from Mongolian dairy products against influenza infection in mice: alleviation of influenza infection by its immunomodulatory activity through intestinal immunity. Int. Immunopharm. 2011;11:1976–1983. doi: 10.1016/j.intimp.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Nagai T., Makino S., Ikegami S., Itoh H., Yamada H. Effects of oral administration of yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 and its exopolysaccharides against influenza virus infection in mice. Int. Immunopharm. 2011;11:2246–2250. doi: 10.1016/j.intimp.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Bae J.-Y., Kim J.I., Park S., Yoo K., Kim I.-H., Joo W. Effects of lactobacillus plantarum and Leuconostoc mesenteroides probiotics on human seasonal and avian influenza viruses. J. Microbiol. Biotechnol. 2018;28:893–901. doi: 10.4014/jmb.1804.04001. [DOI] [PubMed] [Google Scholar]
- 49.Maruo T., Gotoh Y., Nishimura H., Ohashi S., Toda T., Takahashi K. Oral administration of milk fermented with Lactococcus lactis subsp. cremoris FC protects mice against influenza virus infection. Lett. Appl. Microbiol. 2012;55:135–140. doi: 10.1111/j.1472-765X.2012.03270.x. [DOI] [PubMed] [Google Scholar]
- 50.Starosila D., Rybalko S., Varbanetz L., Ivanskaya N., Sorokulova I. Anti-influenza activity of a Bacillus subtilis probiotic strain. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.00539-17. e00539-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song J.A., Kim H.J., Hong S.K., Lee D.H., Lee S.W., Song C.S. Oral intake of Lactobacillus rhamnosus M21 enhances the survival rate of mice lethally infected with influenza virus. J. Microbiol. Immunol. Infect. 2016;49:16–23. doi: 10.1016/j.jmii.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Mahooti M., Abdolalipour E., Salehzadeh A., Mohebbi S.R., Gorji A., Ghaemi A. Immunomodulatory and prophylactic effects of Bifidobacterium bifidum probiotic strain on influenza infection in mice. World J. Microbiol. Biotechnol. 2019;35:91. doi: 10.1007/s11274-019-2667-0. [DOI] [PubMed] [Google Scholar]
- 53.Park S., Kim J.I., Bae J.-Y., Yoo K., Kim H., Kim I.-H. Effects of heat-killed Lactobacillus plantarum against influenza viruses in mice. J. Microbiol. 2018;56:145–149. doi: 10.1007/s12275-018-7411-1. [DOI] [PubMed] [Google Scholar]
- 54.Kiso M., Takano R., Sakabe S., Katsura H., Shinya K., Uraki R. Protective efficacy of orally administered, heat-killed Lactobacillus pentosus b240 against influenza A virus. Sci. Rep. 2013;3:1563. doi: 10.1038/srep01563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harata G., He F., Kawase M., Hosono A., Takahashi K., Kaminogawa S. Differentiated implication of Lactobacillus GG and L. gasseri TMC0356 to immune responses of murine Peyer's patch. Microbiol. Immunol. 2009;53:475–480. doi: 10.1111/j.1348-0421.2009.00146.x. [DOI] [PubMed] [Google Scholar]
- 56.Hori T., Kiyoshima J., Shida K., Yasui H. Augmentation of cellular immunity and reduction of influenza virus titer in aged mice fed Lactobacillus casei strain Shirota. Clin. Diagn. Lab. Immunol. 2002;9:105–108. doi: 10.1128/CDLI.9.1.105-108.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iwabuchi N., Xiao J.-Z., Yaeshima T., Iwatsuki K. Oral administration of Bifidobacterium longum ameliorates influenza virus infection in mice. Biol. Pharm. Bull. 2011;34:1352–1355. doi: 10.1248/bpb.34.1352. [DOI] [PubMed] [Google Scholar]
- 58.Kozak W., Poli V., Soszynski D., Conn C.A., Leon L.R., Kluger M.J. Sickness behavior in mice deficient in interleukin-6 during turpentine abscess and influenza pneumonitis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997;272:R621–R630. doi: 10.1152/ajpregu.1997.272.2.R621. [DOI] [PubMed] [Google Scholar]
- 59.Belkacem N., Serafini N., Wheeler R., Derrien M., Boucinha L., Couesnon A. Lactobacillus paracasei feeding improves immune control of influenza infection in mice. PloS One. 2017;12 doi: 10.1371/journal.pone.0184976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Izumo T., Maekawa T., Ida M., Noguchi A., Kitagawa Y., Shibata H. Effect of intranasal administration of Lactobacillus pentosus S-PT84 on influenza virus infection in mice. Int. Immunopharm. 2010;10:1101–1106. doi: 10.1016/j.intimp.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 61.Lee Y.-N., Youn H.-N., Kwon J.-H., Lee D.-H., Park J.-K., Yuk S.-S. Sublingual administration of Lactobacillus rhamnosus affects respiratory immune responses and facilitates protection against influenza virus infection in mice. Antivir. Res. 2013;98:284–290. doi: 10.1016/j.antiviral.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 62.Poorbaghi S., Dadras H., Gheisari H., Mosleh N., Firouzi S., Roohallazadeh H. Effects of L actobacillus acidophilus and inulin on faecal viral shedding and immunization against H 9 N 2 A vian influenza virus. J. Appl. Microbiol. 2014;116:667–676. doi: 10.1111/jam.12390. [DOI] [PubMed] [Google Scholar]
- 63.Shehata A., Tarabees R., Basiouni S., ElSayed M., Gaballah A., Krueger M. Effect of a potential probiotic candidate Enterococcus faecalis-1 on growth performance, intestinal microbiota, and immune response of commercial broiler chickens. Probiotics Antimicrob. Proteins. 2019:1–10. doi: 10.1007/s12602-019-09557-2. [DOI] [PubMed] [Google Scholar]
- 64.Shojadoost B., Kulkarni R.R., Brisbin J.T., Quinteiro-Filho W., Alkie T.N., Sharif S. Interactions between lactobacilli and chicken macrophages induce antiviral responses against avian influenza virus. Res. Vet. Sci. 2019;125:441–450. doi: 10.1016/j.rvsc.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 65.Seo B., Rather I., Kumar V., Choi U., Moon M., Lim J. Evaluation of Leuconostoc mesenteroides YML003 as a probiotic against low‐pathogenic avian influenza (H9N2) virus in chickens. J. Appl. Microbiol. 2012;113:163–171. doi: 10.1111/j.1365-2672.2012.05326.x. [DOI] [PubMed] [Google Scholar]
- 66.Wang Z., Burwinkel M., Chai W., Lange E., Blohm U., Breithaupt A. Dietary Enterococcus faecium NCIMB 10415 and zinc oxide stimulate immune reactions to trivalent influenza vaccination in pigs but do not affect virological response upon challenge infection. PloS One. 2014;9 doi: 10.1371/journal.pone.0087007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qu R., Ling Y., Zhang Yh, Ly Wei, Chen X., Li X. Platelet‐to‐lymphocyte ratio is associated with prognosis in patients with Corona Virus Disease‐19. J. Med. Virol. 2020 Mar 17 doi: 10.1002/jmv.25767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P. Coronavirus infections and immune responses. J. Med. Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intens. Care Med. 2020:1–3. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Channappanavar R., Perlman S. Springer; 2017. Pathogenic Human Coronavirus Infections: Causes and Consequences of Cytokine Storm and Immunopathology. Seminars in Immunopathology; pp. 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuen K.-S., Ye Z.-W., Fung S.-Y., Chan C.-P., Jin D.-Y. SARS-CoV-2 and COVID-19: the most important research questions. Cell Biosci. 2020;10:1–5. doi: 10.1186/s13578-020-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morais A.H., Passos T.S., Maciel B.L., da Silva-Maia J.K. Can probiotics and diet promote beneficial immune modulation and purine control in coronavirus infection? Nutrients. 2020;12(6):1737. doi: 10.3390/nu12061737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang X., Hou X., Tang L., Jiang Y., Ma G., Li Y. A phase trial of the oral Lactobacillus casei vaccine polarizes Th2 cell immunity against transmissible gastroenteritis coronavirus infection. Appl. Microbiol. Biotechnol. 2016;100:7457–7469. doi: 10.1007/s00253-016-7424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu K., Cai H., Shen Y., Ni Q., Chen Y., Hu S. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. J. Zhejiang Univ. (Med. Sci.) 2020;49 doi: 10.3785/j.issn.1008-9292.2020.02.02. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sirichokchatchawan W., Temeeyasen G., Nilubol D., Prapasarakul N. Protective effects of cell-free supernatant and live lactic acid bacteria isolated from Thai pigs against a pandemic strain of porcine epidemic diarrhea virus. Probiotics Antimicrob. Proteins. 2018;10:383–390. doi: 10.1007/s12602-017-9281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang X., Wang L., Huang X., Ma S., Yu M., Shi W. Oral delivery of probiotics expressing dendritic cell-targeting peptide fused with porcine epidemic diarrhea virus COE antigen: a promising vaccine strategy against PEDV. Viruses. 2017;9:312. doi: 10.3390/v9110312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma S., Wang L., Huang X., Wang X., Chen S., Shi W. Oral recombinant Lactobacillus vaccine targeting the intestinal microfold cells and dendritic cells for delivering the core neutralizing epitope of porcine epidemic diarrhea virus. Microb. Cell Factories. 2018;17:20. doi: 10.1186/s12934-018-0861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang X., Wang L., Zheng D., Chen S., Shi W., Qiao X. Oral immunization with a Lactobacillus casei‐based anti‐porcine epidemic diarrhoea virus (PEDV) vaccine expressing microfold cell‐targeting peptide Co1 fused with the COE antigen of PEDV. J. Appl. Microbiol. 2018;124:368–378. doi: 10.1111/jam.13652. [DOI] [PubMed] [Google Scholar]
- 80.Liu Y.S., Liu Q., Jiang Y.L., Yang W.T., Huang H.B., Shi C.W. Surface-displayed porcine IFN-λ3 in Lactobacillus plantarum inhibits porcine enteric coronavirus infection of porcine intestinal epithelial cells. J. Microbiol. Biotechnol. 2020;30(4):515–525. doi: 10.4014/jmb.1909.09041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eguchi K., Fujitani N., Nakagawa H., Miyazaki T. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci. Rep. 2019;9:4812. doi: 10.1038/s41598-019-39602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zelaya H., Tsukida K., Chiba E., Marranzino G., Alvarez S., Kitazawa H. Immunobiotic lactobacilli reduce viral-associated pulmonary damage through the modulation of inflammation–coagulation interactions. Int. Immunopharm. 2014;19:161–173. doi: 10.1016/j.intimp.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 83.Tomosada Y., Chiba E., Zelaya H., Takahashi T., Tsukida K., Kitazawa H. Nasally administered Lactobacillus rhamnosus strains differentially modulate respiratory antiviral immune responses and induce protection against respiratory syncytial virus infection. BMC Immunol. 2013;14:40. doi: 10.1186/1471-2172-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jounai K., Sugimura T., Ohshio K., Fujiwara D. Oral administration of Lactococcus lactis subsp. lactis JCM5805 enhances lung immune response resulting in protection from murine parainfluenza virus infection. PloS One. 2015;10 doi: 10.1371/journal.pone.0119055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Waki N., Matsumoto M., Fukui Y., Suganuma H. Effects of probiotic Lactobacillus brevis KB 290 on incidence of influenza infection among schoolchildren: an open‐label pilot study. Lett. Appl. Microbiol. 2014;59:565–571. doi: 10.1111/lam.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu X., Zhang H., Lu H., Qian G., Lv L., Zhang C. The effect of probiotic treatment on patients infected with the H7N9 influenza virus. PloS One. 2016;11 doi: 10.1371/journal.pone.0151976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang B., Hylwka T., Smieja M., Surrette M., Bowdish D.M., Loeb M. Probiotics to prevent respiratory infections in nursing homes: a pilot randomized controlled trial. J. Am. Geriatr. Soc. 2018;66:1346–1352. doi: 10.1111/jgs.15396. [DOI] [PubMed] [Google Scholar]
- 88.Kinoshita T., Maruyama K., Suyama K., Nishijima M., Akamatsu K., Jogamoto A. The effects of OLL1073R-1 yogurt intake on influenza incidence and immunological markers among women healthcare workers: a randomized controlled trial. Food Funct. 2019;10:8129–8136. doi: 10.1039/c9fo02128k. [DOI] [PubMed] [Google Scholar]
- 89.Jacobs S.E., Lamson D.M., George K.S., Walsh T.J. Human rhinoviruses. Clin. Microbiol. Rev. 2013;26:135–162. doi: 10.1128/CMR.00077-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Palma A.M., Vliegen I., De Clercq E., Neyts J. Selective inhibitors of picornavirus replication. Med. Res. Rev. 2008;28:823–884. doi: 10.1002/med.20125. [DOI] [PubMed] [Google Scholar]
- 91.Olivares M., Díaz-Ropero M.P., Sierra S., Lara-Villoslada F., Fonollá J., Navas M. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition. 2007;23:254–260. doi: 10.1016/j.nut.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 92.Turner R., Woodfolk J., Borish L., Steinke J., Patrie J., Muehling L. Effect of probiotic on innate inflammatory response and viral shedding in experimental rhinovirus infection–a randomised controlled trial. Benef. Microbes. 2017;8:207. doi: 10.3920/BM2016.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pang I.K., Iwasaki A. Control of antiviral immunity by pattern recognition and the microbiome. Immunol. Rev. 2012;245:209–226. doi: 10.1111/j.1600-065X.2011.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tapiovaara L., Kumpu M., Mäkivuokko H., Waris M., Korpela R., Pitkäranta A. Human rhinovirus in experimental infection after peroral Lactobacillus rhamnosus GG consumption, a pilot study. Int. Forum Allergy Rhinol. 2016;6:848–853. doi: 10.1002/alr.21748. [DOI] [PubMed] [Google Scholar]
- 95.Luoto R., Ruuskanen O., Waris M., Kalliomäki M., Salminen S., Isolauri E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2014;133:405–413. doi: 10.1016/j.jaci.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kumpu M., Kekkonen R., Korpela R., Tynkkynen S., Järvenpää S., Kautiainen H. Effect of live and inactivated Lactobacillus rhamnosus GG on experimentally induced rhinovirus colds: randomised, double blind, placebo-controlled pilot trial. Benef. Microbes. 2015;6:631–639. doi: 10.3920/BM2014.0164. [DOI] [PubMed] [Google Scholar]
- 97.Rosenberg H F., B Domachowske J. Inflammatory responses to respiratory syncytial virus (RSV) infection and the development of immunomodulatory pharmacotherapeutics. Curr. Med. Chem. 2012;19:1424–1431. doi: 10.2174/092986712799828346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kumpu M., Lehtoranta L., Roivainen M., Rönkkö E., Ziegler T., Söderlund‐Venermo M. The use of the probiotic Lactobacillus rhamnosus GG and viral findings in the nasopharynx of children attending day care. J. Med. Virol. 2013;85:1632–1638. doi: 10.1002/jmv.23623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.