Abstract
Pseudomonas aeruginosa is an important opportunistic pathogen responsible for the cause of acute lung injury and acute respiratory distress syndrome. P. aeruginosa isthe leading species isolated from patients with nosocomial infection and is detected in almost all the patients with long term ventilation in critical care units. P. aeruginosa infection is also the leading cause of deleterious chronic lung infections in patients suffering from cystic fibrosis as well as the major reason for morbidity in people with chronic obstructive pulmonary disease. P. aeruginosa infections are linked to diseases with high mortality rates and are challenging for treatment, for which no effective remedies have been developed. Massive lung epithelial cell death is a hallmark of severe acute lung injury and acute respiratory distress syndrome caused by P. aeruginosa infection. Lung epithelial cell death poses serious challenges to air barrier and structural integrity that may lead to edema, cytokine secretion, inflammatory infiltration, and hypoxia. Here we review different types of cell death caused by P. aeruginosa serving as a starting point for the diseases it is responsible for causing. We also review the different mechanisms of cell death and potential therapeutics in countering the serious challenges presented by this deadly bacterium.
Keywords: Pseudomonas aeruginosa, cell death, apoptosis, ferroptosis, acute lung injury/acute respiratory distress syndrome, lung infection
1. Introduction
Acute lung injury and acute respiratory distress syndrome (ALI/ARDS) are a severe public concern worldwide. Pneumonia from viral and bacterial infection remains a major cause of infectious death. The current pandemic of COVID-19, a viral infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), mainly causes ALI/ARDS and affects almost all countries worldwide. More than 10 million patients have been diagnosed with COVID-19 with half of million deaths, and the numbers of confirmed cases and deaths are continuously increasing [1]. Viral infection destroys host defense with exhausted immune response, that is easily followed by a secondary bacterial infection. The secondary bacterial infection always renders poor prognosis of the patients. Among the microbial pathogens involving secondary infection, Pseudomonas aeruginosa, a Gram-negative rod-shaped bacterium is the leading opportunistic pathogen isolated from patients with nosocomial infection. P. aeruginosa is present in the respiratory tract of many of the patients with long-term ventilation more than 7 days [2]. It is also the leading cause of deleterious chronic lung infections in patients suffering from cystic fibrosis (CF) [3]. P. aeruginosa infection is the major reason for morbidity in people with chronic obstructive pulmonary disease (COPD) as well [4]. Patients with viral infection, trauma, and cancer leads to host immunosuppression that largely enhances secondary infection with P. aeruginosa. P. aeruginosa is in most of the cases multi-drug resistant and no effective cure has been developed. Diseases linked with P. aeruginosa infection display high mortality rates and are particularly challenging for treatment in patients with compromised immunity [2].
P. aeruginosa is able to adapt to a specific functional role to escape the host defense using highly complex intracellular and intercellular signaling networks. Besides, P. aeruginosa releases a large number of virulence effectors [5,6]. This adaptive ability however has variances according to the types of clones and strains in the P. aeruginosa population worldwide [7].The Pathogenicity of P. aeruginosa is a result of long-time adaption and evolution. Pathological changes include inflammatory response, cytokine secretion, immune cell infiltration, air-blood barrier dysfunction, leakiness, and cell death.
Once P. aeruginosa enters into the airway tract, the bacteria replicate and colonize in the lumen of the airway and alveolar sacs. Part of the bacteria will be engulfed and cleared by lung residential macrophages. Meanwhile, P. aeruginosa activated macrophages release inflammatory factors, result in immune cell infiltration, and subsequent hyper-reacted cytokine storm may damage the respiratory system. On the other side, part of the bacteria will invade into lung epithelial cells through the damaged physical protection barrier of mucin or surfactant films. The bacteria adhere on the surface of epithelial cell membrane and penetrate the membrane to replicate and colonize in the cells [8,9,10,11,12]. The flagella and Type IV pili (TFP) play the role of adhesins for host cells [9]. Other adhesins such as cup fimbrial adhesins [13], lectins PA-IL (LecA) and PA-IIL (LecB) [14] have also been identified. In the study by Hayashi N. et al. [15], it was observed that the bacteria are associated with Caco-2 cells even in the absence of intact pili, suggesting that P. aeruginosa can bind to Caco-2 cells using other adhesion systems. Firstly, the replication and colonization of the invaded bacteria may physically damage the integrity of the lung epithelial cells. Secondly, the bacteria cause host inflammatory response via multiple channels of molecular mechanisms that induce lung epithelial cell death. Furthermore, P. aeruginosa may directly release a number of toxins that directly destroy cellular membranes to lead to cell death. In addition, the dead cells and debris may release toxic cellular components that may further augment inflammation and autoimmune responses. P. aeruginosa infection mediated lung epithelial cell death destroys the integrity of the air–blood barrier, and leads to leaks and edema, that will attract peripheral circulating neutrophils, microphages, and lymphocyte infiltration and enhance inflammatory response. Therefore, P. aeruginosa mediated lung epithelial cell death is a major mechanism in the pathogenesis of ALI/ARDS. Our understanding on the underlying molecular mechanisms of P. aeruginosa induced lung epithelial cell death is yet to be studied for the development of an effective therapy against the pathogen and the related illnesses.
2. Distinct Cell Death Mechanisms
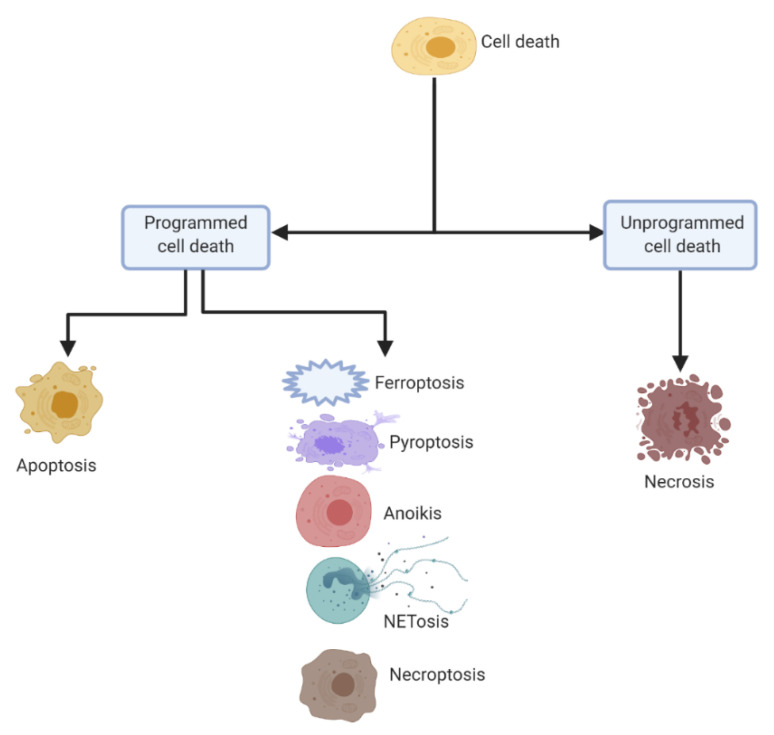
Cell death is a phenomenon exclusively existing in multicellular organisms, both in physiological processes and in responding to pathological stimulus. In order to maintain homeostasis of tissues as well as eliminate potentially harmful stimulus, cell death is a crucial process [16]. Mounting studies have dissected the molecular mechanisms of cell death and a range of distinct types of cell death have been reported. Cell death can be classified into two major classes—programmed cell death and unprogrammed cell death (Figure 1). Mechanistically, programmed cell death is subclassified as apoptotic programmed, consisting of apoptosis, and non-apoptotic programmed, consisting of ferroptosis, pyroptosis, anoikis, NETosis, and necroptosis. The unprogrammed cell death on the other hand mainly refers to necrosis as the mechanism.
Figure 1.
General classification of cell death. The broad classification is programmed and unprogrammed. The programmed cell death is subclassified as apoptotic and non-apoptotic, with apoptotic consisting of apoptosis, while non-apoptotic consisting of ferroptosis, pyroptosis, anoikis, NETosis, and necroptosis. Unprogrammed cell death has necrosis as the mechanism (all figures were created using Biorender.com).
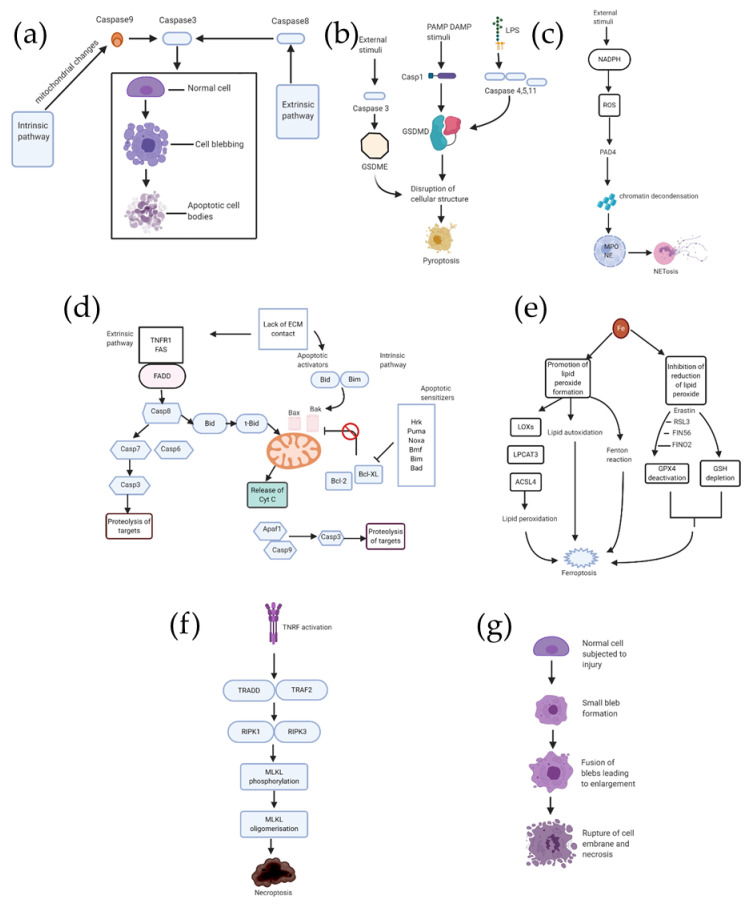
2.1. Apoptosis (Figure 2a)
Figure 2.
Schema for the mechanisms for different types of cell deaths. Part (a) displays two important pathways for apoptosis, namely intrinsic and extrinsic pathways, both converging at caspase-3 as the executioner pathway. Part (b) shows the three underlying pathways for pyroptosis involving caspases 1,3,4,5, and 11. Part (c) is the schematic representation for NETosis. Part (d) exhibits the two pathways for anoikis, intrinsic and extrinsic, respectively. Part (e) outlines the mechanistic pathways associated with ferroptosis, promotion of lipid peroxide formation being one path and inhibition of reduction of lipid peroxide being the other. TNF/TNFR signaling pathway for necroptosis via MLKL phosphorylation is displayed in part (f). Finally, part (g) represents the different stages in a general necrosis mechanism.
Apoptosis, also called programmed cell death, is the result of an orderly cascade of a multistep enzymatic activity. Histologically, the characteristic features of apoptosis are (i) cell shrinkage, (ii) membrane blebbing, and (iii) condensation of the chromatin [17]. A molecular hallmark of apoptosis is DNA fragmentation, the nuclear genomic DNA is cleaved into ≈180 bp in length. Mechanistic studies reveal that apoptosis can be classified into two distinct pathways: the intrinsic pathway and extrinsic pathway. Both the intrinsic pathway and extrinsic pathway share caspase protease activation [18]. In the extrinsic pathway, apoptosis is initiated via death receptors, a group of cellular membrane proteins including tumor necrosis factor receptor (TNFR) and Fas/CD95. Once the legitimate ligands bind to the receptors on the surface of the cellular membrane, the receptors will be activated and downstream signaling will be culminated. By interacting with Fas-associated death domain protein (FADD) and other such adaptor proteins, the Death-inducing signaling complex (DISC) activates caspase 8, which then activates caspases 6, 7, and subsequently caspase 3, resulting finally in proteolysis of substrate and eventual cell death [19] (Figure 2a). The intrinsic pathway, also known as the mitochondrial pathway, is activated via responding to cellular stresses. Cellular stresses activate Bad/Bax, that release cytochrome C from the membrane of mitochondria to activate caspase 9, and caspase 9 cleaves caspase 3 to induce cell death [16] (Figure 2a).Apoptosis could be observed in physiological processes such as in embryonic development, and it extensively exists in the pathogenesis of various diseases as well.
2.2. Pyroptosis (Figure 2b)
Pyroptosis is a type of programmed cell death involving breakage of plasma-membrane, resulting in the release of proinflammatory intracellular contents [20]. Caspases 1, 4, 5, and 11 play an important role in pyroptosis [21]. Three different pathways for pyroptosis have been proposed so far(Figure 2b): the caspase 1 dependent pathway also called the canonical inflammasome pathway, the noncanonical inflammasome pathway consisting of caspases 4, 5, and 11, and most recently proposed the caspase 3 dependent pathway [21,22].In the canonical inflammasome pathway, intracellular pattern recognition receptors (PRR) receive signal stimulation in the form of pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs). Subsequently, the inflammatory body is assembled and an intracellular macromolecular protein complex is produced, leading to the activation of caspase 1, which thereby causes secretion ofIL-1β and IL-18 on one hand, and cleavage of gasdermin-D (GSDMD) to generate two types of ends–reactive amino (N) and carboxyl (C). All this leads to the destruction of cellular structure and subsequent cell lysis and cell death [21].
In the noncanonical inflammasome pathway, caspase-11, derived from mice, and caspases 4 and 5, both derived from humans, can induce pyroptosis. In this pathway, binding of the caspase to LPS occurs, leading to the cleavage of GSDMD which promotes pyroptosis [21]. The third pathway, which is caspase-3 dependent, is similar to the canonical inflammasome pathway, with the only difference being that it involves the cleavage of gasdermin E (GSDME), instead of GSDMD. This cleavage leads to the cell structure destruction and eventual pyroptosis [21].
2.3. NETosis (Figure 2c)
The dynamic process of activation and release of neutrophil extracellular traps (NETs) which may result in cellular death is termed as NETosis [23]. NADPH-oxidase production of reactive oxygen species (ROS) is suggested to be the starting point of the pathway, leading to the activation of protein-arginine deiminase 4 (PAD4), an enzyme responsible for chromatin decondensation in the neutrophil nucleus. The subsequent rupture of the nuclear envelope occurs when myeloperoxidase (MPO) and neutrophil elastase (NE) enters the nucleus. Suicidal NETosis and vital NETosis are the two forms, with the key differences being in the stimuli, timing, and the end result (Figure 2c). NETosis is observed in many cases of infection, and it is also observed in no-infectious conditions.
2.4. Anoikis (Figure 2d)
Anoikis is a particular type of apoptosis occurring in the cells, either in absence of attachment to extracellular matrix (ECM) or the cells getting adhered to an inappropriate location [19]. Two apoptotic pathways are responsible for inducing the anoikis program, namely the intrinsic pathway, involving the perturbation of mitochondria, and the extrinsic pathway, consisting of cell surface death receptors getting triggered. The proteins of the Bcl-2 family are critical to both the pathways [19]. There are three categorizations of Bcl-2 proteins: anti-apoptotic proteins, multidomain pro-apoptotic proteins, and BH3-only proteins, which are also pro-apoptotic. Anoikis shares common downstream pathways of apoptosis (Figure 2d). In the intrinsic pathway, BH3-only proteins, including Bim, Hrk, Bmf, Bad, Bik, Puma, and Noxa, promote the activation of Bax/Bak. Among them, the direct promotion is done by Bid and Bim (activators), while the other members also known as sensitizers, promote indirectly by counteracting the anti-apoptotic functions of Bcl–2. The release of cytochrome c to the cytoplasm is the final step, leading to apoptosome formation, and thereby activation of executioner caspases [19]. The extrinsic pathway, which is the death receptor pathway, is initiated by members of TNFR, such as Fas and TNFR1, which leads to the apoptotic signal transductions.
2.5. Ferroptosis (Figure 2e)
Ferroptosis can be defined as a death program which is executed by selective oxidation of arachidonic acid-phosphatidylethanolamines (AA-PE) by 15-lipoxygenases [24]. It has been identified that the substrates and the products of this process are arachidonoyl-phosphatidylethanoamine (AA-PE) and 15-hydroperoxy-AA-PE (15-HOO-AA-PE), respectively [25,26]. Since lipid peroxide is an important participant in ferroptosis (Figure 2e), this categorizes two processes, one which promotes lipid peroxide formation and thereby ROS generation, and other which inhibits the reduction of lipid peroxides [27].
Inhibition of reduction of lipid peroxide is achieved either by deactivating GPX4 or by depleting its cofactor glutathione (GSH) [28,29].The inhibitors which directly or indirectly deactivate GPX4 are Erastin, RSL3, FIN56, and FINO2, while GSH depletion is done by Erastin alone [27]. On the other hand, the enzymes which promote the lipid peroxide formation are lysophosphatidylcholine acyltransferase 3 (LPCAT3), lipoxygenases (LOXs), and acyl-CoA synthetase long-chain family 4 (ACSL4) [25,30,31,32,33]. Additionally, production of ROS by fenton reaction and lipid autoxidation are also the pathways for ferroptosis [34,35].
2.6. Necroptosis (Figure 2f)
Necroptosis is a programmed form of necrosis [36]. Distinct with unregulated necrosis, cells can execute necrosis in a programmed manner independent of caspase activation. The tumor necrosis factor/tumor necrosis factor receptor (TNF/TNFR) signaling pathway has revealed to initiate necroptosis. Activation of TNFR signals TNFR-associated death protein (TRADD) and TNFR-associated factor 2 (TRAF2) that recruits Receptor-interacting protein kinase 1/ Receptor-interacting protein kinase 3 (RIPK1/RIPK3) to form a necrosome. The necrosome promotes mixed lineage kinase domain-like protein (MLKL) phosphorylation, allowing the MLKL to insert into and permeabilize plasma membranes and organelles that result in cell death (Figure 2f).
2.7. Necrosis (Figure 2g)
Necrosis is a form of cell death due to an external or internal injury that results in a premature death of the cell in the living tissues with autolysis. A variety of injury factors including microbial infection, toxins, or trauma induce necrosis by a digestion of the cell components. Necrosis is detrimental and can be fatal. Morphologically, necrosis is characterized by the nuclei of the cells fading away and shrinking and disruption of the membrane of the cells or organelle. It is in general believed that necrosis is an unregulated process (Figure 2g).
3. P. aeruginosa Induced Lung Epithelial Cell Death
3.1. P. aeruginosa Triggers Apoptosis in Lung Epithelial Cells (Figure 3a)
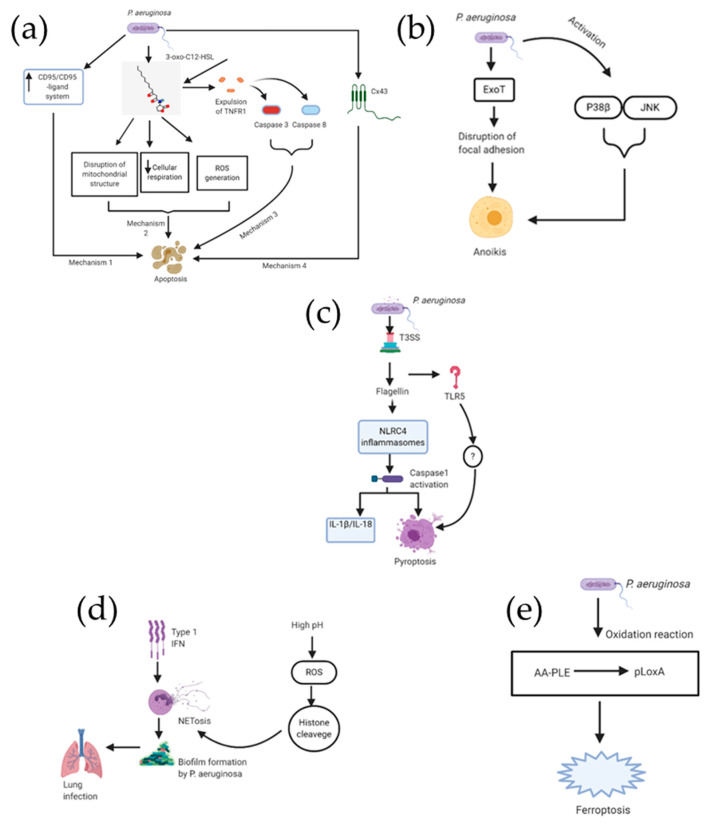
Figure 3.
Schematic representation for the different cell death mechanisms caused due to P. aeruginosa infection. Part (a) consists of four important pathways for apoptosis, part (b) displays ExoT induced as well as activated P38-JNK induced anoikis, part (c) focuses on the two pathways associated with pyroptosis, part (d) is a cartoon representation of NEtosis pathway. Finally, part (e) is a single mechanism for P. aeruginosa induced ferroptosis involving peroxide formation and reactive oxygen species (ROS) generation, since ferroptosis is a newer cell death mechanism still being investigated for additional pathways.
P. aeruginosa uses distinct mechanisms for initiating the cell death of the infected host cells [37]. The most studied cell death is apoptosis (Table 1). A well-studied paradigm is the extrinsic apoptotic pathway Fas (CD95)/Fas ligand signaling in lung epithelial cells. Following the infection of epithelial cells, either by in vitro or in vivo route, P. aeruginosa causes an upregulation of Fas/Fas ligand on the cell surface [38], an endogenous receptor ligand pair considered to be one of the most important ones responsible for the triggering of apoptosis. Bacteria derived type III secretion system (T3SS) upregulates the Fas/Fas ligand pair in P. aeruginosa infected cells and bacteria who do not possess a functional T3SS almost fail to cause apoptosis in epithelial cells. Upon upregulation, ligation of Fas by Fas ligand leads to induction of caspase 8 and caspase 3 activation, release of mitochondrial cytochrome C, as well asc-Jun N-terminal protein kinase(JNK) activation [37]. It is also found that reactive oxygen intermediates seem to be crucial for inducing P. aeruginosa triggered death [39]. Genetic studies using cells or mice genetically deficient for functional Fas or Fas ligand have shown the significance of the Fas/Fas ligand system for P. aeruginosa triggered cell death [38]. There was no response to P. aeruginosa infections resulting in induction of apoptosis in epithelial cells obtained in vivo from mice deficient in Fas or Fas ligand or ex vivo fibroblasts deficient in either Fas or Fas ligand. Apoptosis of lung epithelial cells upon getting infected with P. aeruginosa forms a crucial part of the host defense against infection from P. aeruginosa, which is contrary to the role of apoptosis due to infection from some other bacteria such as Shigella flexneri [37]. This was demonstrated by comparative in vivo pulmonary infection of Fas or Fas ligand deficient mice and normal mice. The former rapidly developed sepsis and died, while the latter completely cleared the infection within a period of few days. Another study has also demonstrated the protective effect of apoptosis triggered by bacteria [40]. The finding of that study is that a ced 3 and ced 4 regulated death of worm gonad cells is triggered when the worm Caenorhabditis elegans (C. elegans) is infected with Salmonella Typhimurium (S. typhimurium). However, apoptosis inhibition in those cells caused by mutations of ced3 and ced4, respectively, leads to hyper-sensitization of C. elegans to the S. typhimurium infection [37].
Table 1.
A summary of different mechanisms underlying the different types of epithelial cell death.
| Type of Cell Death | Description | Mechanism | Reference Numbers |
|---|---|---|---|
| Apoptosis | CD95/CD95-ligand system for P. aeruginosa triggered apoptosis | P. aeruginosa causes an up-regulation of CD95/CD95ligand on the cell surface, responsible for the triggering of apoptosis | [37,38,39,40] |
| Apoptosis | Disruption of mitochondrial morphology using 3-oxo-C12-HSL | Quorum sensing molecule 3-oxo-C12-HSL activates the apoptosis by disrupting the mitochondrial structure, attenuating cellular respiration and inducing ROS generation | [41] |
| Apoptosis | Caspase 3-caspase 8-mediated apoptosis | Necrosis factor receptor 1 expelled into the disordered lipid phase triggers cell death | [42] |
| Apoptosis | Apoptosis due to Cx43-mediated cell-to-cell communication | Cx43-mediated gap junctional communication enhances apoptosis in PAO1-infected airway epithelial cells, while on the other hand JNK signaling inhibits Cx43 function | [43] |
| Pyroptosis | PAO1 flagellin induced CASP1-dependent neutrophil pyroptosis | PAO1-induced pyroptosis depends on NLRC4 and Toll-like receptor 5 (TLR5) in neutrophils | [44] |
| NETosis | Type I interferon associated NETosis | Excessive activation of neutrophils by type I IFNs causes aboost in NETosis which triggers biofilm formation by P. aeruginosa, thereby supporting its persistence in the infected lung. | [45,46,47,48] |
| NETosis | NADPH Oxidase-Dependent NETosis | Increase in pH in neutrophils stimulates Nox activity and ROS production requiredl for NETosis | [49] |
| Anoikis | Pseudomonas aeruginosa ExoT induced atypical anoikis | GAP domain of ExoT is responsible for triggering the mitochondrial intrinsic pathway of anoikis apoptosis | [50,51,52] |
| Ferroptosis | P. aeruginosa produced biofilm induces ferroptosis | Caused by enhancing expression of pLoxA as well as oxidising host cell AA-PE to 15-HOOAA-PE | [24] |
P. aeruginosa may induce extrinsic apoptosis without ligand binding by directly polymerizing the death receptors within lipid rafts, that process activates the death receptor. A recent study reported that the quorum-sensing autoinducers N-(3-oxo-dodecanoyl) homoserine lactone in P. aeruginosa triggers significant cell death in B-lymphocytes, T-lymphocytes, dendritic cells, microphages, and monocytes. N-(3-oxo-dodecanoyl) homoserine lactone is able to incorporate into the host cell plasma membrane, plasma membrane lipid components containing cholesterol, sphingomyelin, and 1-2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) efficiently retain 3-oc [42]. Addition of cholesterol inhibitor methyl-β-cyclodextrin dissolved cholesterol into the solution, thus disrupted the retention of 3-oc in the lipid domains. Retention of 3-oc in the plasma membrane dramatically changed the appearance of the presumed solid ordered lipid domains with a clear dissolution of the elevated plains of lipid domains to form smaller sized lipid rafts and collapse of the cholesterol and sphingomyelin-rich lipid domains, which was unrelated to membrane leakiness. However, domains formed with a mixture of DOPC and DPPG, unrelated to lipid rafts, were minimally affected. This event expels TNFR1 into the disordered lipid phase for its spontaneous trimerization without its ligand and drives Caspase 8-Caspase 3 mediated apoptosis [42].
P. aeruginosa activates cell surface receptors, thereby eliciting a cascade for intracellular signaling [43]. This leads to an increase in the communication at gap junction of the protein Cx43. It is observed that the expression of Cx43 displayed regulation in opposite directions exerted by JNK and p38 MAPKs. JNK inhibitor caused an increase in the PAO1-induced apoptosis, but the apoptosis was prevented by lentiviral expression of a Cx43-specific short hairpin RNA. Interestingly it is found that JNK activity undergoes upregulation by pharmacological restriction of CFTR in Calu-3 cells, however, when the CF airway cell line (CF15 cells) is corrected by adenoviral expression of CFTR, it causes a reduction in this MAPK being activated. This CTFR inhibition is also linked to the downregulation of Cx43 and thereby reduction in apoptosis. These results indicate that Cx43 expression forms a part of the response of airway epithelial cells by maintaining a balance of survival and apoptosis [43].
Another study demonstrated that P. aeruginosa induces the intrinsic apoptotic pathway via mitochondria. It has identified an important pathway responsible for the pathogenesis of lung injury caused by a P. aeruginosa linked virulence factor [41]. 3-oxo-C12-HSL is a principal quorum sensing molecule of P. aeruginosa, which causes disruption of mitochondrial morphology and promotes mitochondrial DNA oxidative injury. 3-oxo-C12-HSL downregulates the expression of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), which is responsible for regulating biogenesis of mitochondria, acting as an antioxidant defense, as well as playing a role in cellular respiration. Overexpression of PGC-1α reduces the inhibition in cellular respiration produced due to 3-oxo-C12-HSL. It is also observed that the pharmacologic activation of PGC-1α causes restoration of barrier integrity in cells treated with 3-oxo-C12-HSL.
P. aeruginosa mediated apoptosis is stringently regulated in a few ways. Oral bacteria may be responsible for modulating the adhesion and invasion of respiratory pathogens to epithelial cells [53,54]. The presence of oral pathogenic bacteria leads to more P. aeruginosa infected epithelial cells via augmented mucosal surface invasion and lung epithelial cell apoptosis. Furthermore, oral as well as respiratory bacteria appear to induce the proinflammatory cytokines release from respiratory epithelial cell lines in vitro [54]. Oral bacteria may be responsible for altering the local microenvironment, thereby facilitating the onset and/or progression of respiratory disease in susceptible individuals [54].
It has been demonstrated that physiological levels of acidosis cause enhancement of epithelial cell cytotoxicity during a P. aeruginosa infection [55]. It has been shown in the study that the increase in epithelial cytotoxicity during acidosis is caused due to reduction in antimicrobial activity. Additionally, it has also been established that the epithelial-derived bactericidal activity is dependent on pH. Overall, these findings have provided key insights into the contribution of changes in extracellular pH to the pathogenesis of P. aeruginosa infections.
3.2. Epithelial Anoikis Caused by P. aeruginosa Infection (Figure 3b)
It has been reported that the GAP as well as the ADPRT domains of Exoenzyme T (ExoT) derived from P. aeruginosa contribute to ExoT-induced apoptosis in epithelial cells [50]. Crk adaptor protein gets transformed into a cytotoxin due to the ADPRT domain activity, inducing atypical anoikis apoptosis due to interference in the signaling of integrin survival [51]. However, the mechanism for the GAP-induced apoptosis was unknown until a more recent study shed light on this. It has been demonstrated in this study that the GAP domain of ExoT is responsible for triggering the mitochondrial intrinsic pathway of apoptosis [52]. The data from the study shows that intoxication of GAP leads to (i) Bax, Bid, and to a lesser extent Bim, getting activated and accumulated in the mitochondrial membrane; (ii) mitochondrial membrane losing its potential as well as cytochrome c release; (iii) activation of the initiator caspase-9 getting activated, resulting in the activation of the executioner caspase-3 thereby culminating in cell demise [52].
3.3. P. aeruginosa Induced Pyroptosis (Figure 3c)
Neutrophil pyroptosis is caused due to acute P. aeruginosa infection via inflammasome signaling. It has been reported that flagellin within P. aeruginosa and mitochondrial reactive oxygen species (ROS) cause neutrophil pyroptosis upon acute lung infection through CASP1-dependent signaling in Nox2 mice, resulting in enhanced lung inflammation and injury [44]. It has been demonstrated that PAO1-induced pyroptosis depends on NLRC4 and Toll-like receptor 5 (TLR5) in neutrophils. The authors suggest that mitochondrial ROS plays a role as a bridge between NOX2-mediated signaling and inflammasome activation in neutrophils, caused due to bacterial infection.
3.4. P. aeruginosa Associated NETosis (Figure 3d)
Type I interferons (IFNs) regulate neutrophil activity, and therefore act as the first line of anti-bacterial host defense [45,46]. Infections with bacteria, such as P. aeruginosa, are often linked to the increase in type I IFN signaling in lung epithelial cells [47]. It has been demonstrated that type I IFN-mediated activation of neutrophils in lungs causes increase in NETosis, leads to the prominent tissue damage, and supports biofilm formation by P. aeruginosa and its persistence in the lung [48]. The role of PH in mediation of NETosis is also an important aspect [49]. It shows that increasing pH in neutrophils caused stimulation in Nox activity and ROS production, both of which are essential agonist-induced Nox-dependent NETosis. At higher pH, neutrophil proteases, considered to be important for NETosis [50,51], could better cleave the histones after entering NETotic nuclei. Hence, high pH enables NETosis while low pH suppresses NETosis. It is also mentioned in the study that compounds such as sodium bicarbonate and THAM, which are clinically used, effectively increase pH and promote NETosis, which suggests the possible role of these compounds in correcting defective NETosis in vivo.
3.5. Epithelial Ferroptosis in Context of P. aeruginosa (Figure 3e)
In a new study, it has been reported that a mutant of P. aeruginosa producing biofilm induces ferroptosis in human bronchial epithelial (HBE) cells [24]. This is done by enhancing expression of pLoxA as well as oxidizing host cell AA-PE to 15-HOOAA-PE. It was observed that clinical P. aeruginosa isolates from patients suffering from prolonged lower respiratory infection resulted in pLoxA-dependent ferroptosis of HBE cells. High levels of 15-HOO-AA-PE in airway tissues from cystic fibrosis patients were detected using global redox phosphorlipidomics. However, in CF patients without P. aeruginosa in airway cultures, such elevated levels were not detected. Based on the assumption that disruption of epithelial barrier and immune-regulatory functions play a key role for pathogenesis of P. aeruginosa-related respiratory diseases, pLoxA as a potential new therapeutic target was proposed.
4. Cell Death Mediation and Therapeutic Strategies
4.1. Role of IL-15 in Apoptosis Prevention
Interleukin-15 (IL-15) belongs to the γ-chain family of cytokines, with IL-2, IL-4, IL-7, IL-9, and IL-21 being the other members [56,57,58,59,60]. IL-15 is unique among all of its family members on account of its pattern of receptor expression, which is observed in DC, NK, and CD8 T cells [61]. Due to this fact, IL-15 performs coordination of the response of these innate and adaptive immune cells for protecting the host [62,63,64,65,66]. IL-15 blocks sepsis-induced apoptosis in NK cells, dendritic cells, and CD8 T cells [61]. Sepsis induced gut epithelial apoptosis also decreases on account of IL-15. Furthermore IL-15 therapy increases antiapoptotic Bcl-2 and decreases proapoptotic Bim and PUMA. IL-15 increases circulating IFN-γ, along with the percentage of NK cells that produced IFN-γ. Lastly, IL-15 is responsible for increase in survival in both models of cecal ligation and puncture and P. aeruginosa pneumonia. Thus IL-15 is a potential novel therapy for the deadly P. aeruginosa caused disease.
4.2. Platelets Inhibit Epithelial Cell Apoptosis
A study has found that platelets are responsible for attenuating pathogen induced lung injury and protecting from lung epithelial apoptosis [7]. Platelet deficiency was identified to associate with severe disruption of alveolar-capillary barrier in live bacteria as well as bacterial exoproduct models representing P. aeruginosa infection. Platelets play a role in alveolar-capillary barrier homeostasis, but can also enter the airspace and release protective factors at the time of infection, thereby limiting alveolar epithelial cell death and reducing the further effect of lung injury. Lastly, the study also identified a potential protective role for platelet granule factors against lung injury triggered by pathogen as well as showed that platelet releasates are responsible for attenuating in vitro lung epithelial cell death as well as disrupting, independent of the whole platelets, the lung vascular barrier in mice deficient in platelets.
4.3. Mediation of Epithelial Cell Death in P. aeruginosa Pneumonia by Morf4l1
Mortality factor 4 like 1 (Morf4l1) is a protein playing a role in chromatin remodeling [67]. Its fundamentally low level of expression in the lung is on account of its short life via continuous ubiquitin proteasomal degradation, which is mediated by Fbxl18, an orphan ubiquitin E3 ligase subunit [67]. There is however an increase in the expression of Morf4l1 in humans with pneumonia and an upregulation in lung epithelia on getting exposed to P. aeruginosa or lipopolysaccharide (LPS) [67]. In a mouse model of pneumonia induced by P. aeruginosa, it was observed that Morf4l1 is stabilized due to acetylation that protects it from Fbxl18-mediated degradation. After the mice were infected with P. aeruginosa, overexpression of Morf4l1 enhanced lung epithelial cell death, in contrast to restored cell viability when it was depleted. A U.S. Food and Drug Administration-approved thrombin inhibitor argatroban, was identified to be a Morf411 antagonist using in-silico modeling as well as drug-target interaction studies. It was observed that Morf4l1-dependent histone acetylation, inhibited by argatroban, caused a reduction in its cytotoxicity and thereby improved the survival of mice with experimental lung injury at doses where the anticoagulant activity was none. This study uncovered a new biological mechanism, but also identified a potential molecular target for non-antibiotic pharmacological therapy in severe pulmonary infection.
4.4. Inhibition of Cellular Apoptosis and Autophagy Due to Tremella Polysaccharides
The endotoxin LPS derived from P. aeruginosa is able to induce apoptosis and autophagy, as well as increases the production of reactive oxygen species (ROS) in a time dependent manner in human epithelial A549 lung cancer cells [68]. It was also observed that LPS treatment suppressed sirtuin 1 (SIRT1) protein expression in the cells. The notable aspect of the study was the demonstration of SIRT1 activation due to Tremella polysaccharides activating SIRT1, resulting in an increase in the p62 expression, decrease of p53 acetylation as well as B-cell lymphoma 2-associated X protein expression, which subsequently attenuated LPS-induced apoptotic cell death and autophagy. These results open several potential avenues for treating the P. aeruginosa infection.
5. Conclusions and Future Directions
P. aeruginosa is a highly versatile microorganism, which continues to astonish us by evolving and acquiring new and unexplored modes of niche adaptation, lifestyle, as well as pathogenicity. A major mechanism of the pathogenicity of P. aeruginosa is causing host cell death. Among distinct cell death pathways that P. aeruginosa elicited, apoptosis is well studied. It may use a range of mechanisms to cause host cell apoptosis both in intrinsic and extrinsic apoptotic pathways. Meanwhile, a few studies focused on the regulation of P. aeruginosa induced apoptosis. A number of cell death pathways other than apoptosis have been reported (tableure 3). The importance of each pathway should be weighted in the pathogenesis of cell death by P. aeruginosa. Several attempts have been thrown into targeting P. aeruginosa induced cell death, these include IL–15, platelet factors, chromatin modulator Morf4l1, or LPS antagonists. Continued focus needs to be given on understanding the mechanisms of cell death that may lead to the identification of novel targeting therapeutics. Preclinical trials are required to verify the efficacy of the discovered targets. P. aeruginosa is the leading pathogen isolated in patients within critical care units. Secondary infection with P. aeruginosa worsens the prognosis of the patients. In the current pandemic of COVID-19, it is estimated that approximately 5% of the total patients are treated within critical care units [69,70]. Among these patients, sepsis has been noticed as a major contributor to the poor prognosis. The importance of the secondary infection with P. aeruginosa in the severe COVID-19 patients with long-term ventilation has yet to be understood. Prevention of the nosocomial P. aeruginosa infection may be critical to reduce the mortality of COVID-19.
Abbreviations
| AA-PE | Arachidonic acid-phosphatidylethanolamines |
| ACSL4 | Acyl-CoA synthetase long-chain family 4 |
| ADPRT | (ADP-ribosyl) transferase |
| ALI | Acute lung injury |
| ARDS | Acute respiratory distress syndrome |
| BAD | BCL2 associated agonist of cell death |
| BAX | Bcl-2-associated X protein |
| BIK | BCL2 Interacting Killer |
| BIM | Bcl-2-like protein 11 |
| BMF | Bcl2 Modifying Factor |
| C. elegans | Caenorhabditis elegans |
| CD8 | Cluster of differentiation 8 |
| CD95 | Cluster of differentiation 95 |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| COPD | Chronic obstructive pulmonary disease |
| COVID–19 | Coronavirus disease of 2019 |
| CX43 | Connexin 43 |
| DAMPs | Danger-associated molecular patterns |
| DC | Dendritic cells |
| DISC | Death-inducing signaling complex |
| DOPC | 1-2-dioleoyl-sn-glycero–3-phosphocholine |
| DPPG | 1,2-Dipalmitoyl-sn-glycero–3-phosphoglycerol |
| ExoT | Exoenzyme T |
| FADD | Fas-associated death domain protein |
| FBXL18 | F-Box and Leucine Rich Repeat Protein 18 |
| GAP | GTPase-accelerating protein |
| GPX4 | Glutathione peroxidase 4 |
| GSDMD | Gasdermin-D |
| GSDME | Gasdermin E |
| GSH | Glutathione |
| HBE | Human bronchial epithelial |
| HRK | Harakiri |
| IFNs | Interferons |
| IL–15 | Interleukin-15 |
| JNK | c-Jun N-terminal kinases |
| LecA | Lectins PA-IL |
| LecB | Lectins PA-IIL |
| LOXs | Lipoxygenases |
| LPCAT3 | Lysophosphatidylcholine acyltransferase 3 |
| LPS | Lipopolysaccharide |
| MLKL | Mixed lineage kinase domain-like protein |
| Morf4l1 | Mortality factor 4 like 1 |
| MPO | Myeloperoxidase |
| NE | Neutrophil elastase |
| NK | Natural killer cells |
| NLRC4 | NLR Family CARD Domain Containing 4 |
| NOXA | Phorbol-12-myristate-13-acetate-induced protein 1 |
| P. aeruginosa | Pseudomonas aeruginosa |
| PAMPs | Pathogen-associated molecular patterns |
| PAMPs | Pathogen-associated molecular patterns |
| PGC-1α | Proliferator-activated receptor-γ coactivator-1α |
| PRR | Pattern recognition receptors |
| PUMA | p53 upregulated modulator of apoptosis |
| RIPK1 | Receptor-interacting protein kinase 1 |
| RIPK3 | Receptor-interacting protein kinase 3 |
| ROS | Reactive oxidation species |
| SARS-CoV2 | Severe acute respiratory syndrome coronavirus 2 |
| SIRT1 | Sirtuin 1 |
| T3SS | Type III secretion system |
| TFP | Type IV pili |
| THAM | Tris-hydroxymethyl aminomethane |
| TLR5 | Toll-like receptor 5 |
| TNF | Tumor necrosis factor |
| TNFR | Tumor necrosis factor receptor |
| TRADD | TNFR-associated death protein |
| TRAF2 | TNFR-associated factor 2 |
| 3-oc | (3-oxo-dodecanoyl) homoserine lactone |
Funding
This work is supported, in part, with R01 grants (HL125435 and HL142997) from National Institute of Health at The United States to C.Z.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.COVID-19 Map. [(accessed on 6 July 2020)]; Available online: https://coronavirus.jhu.edu/map.html.
- 2.Vincent J. International Study of the Prevalence and Outcomes of Infection in Intensive Care Units. JAMA. 2009;302:2323. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 3.Høiby N., Frederiksen B. Cystic Fibrosis. Arnold Publishers-International Book and Journal Publishers; London, UK: 2000. Microbiology of cystic fibrosis; pp. 83–107. [Google Scholar]
- 4.Murphy T.F. Pseudomonas aeruginosa in adults with chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 2009;15:138–142. doi: 10.1097/MCP.0b013e328321861a. [DOI] [PubMed] [Google Scholar]
- 5.Balasubramanian D., Schneper L., Kumari H., Mathee K. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res. 2012;41:1–20. doi: 10.1093/nar/gks1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinhart A.A., Oglesby-Sherrouse A.G. Regulation of Pseudomonas aeruginosa Virulence by Distinct Iron Sources. Genes. 2016;7:126. doi: 10.3390/genes7120126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bain W., Olonisakin T., Yu M., Qu Y., Hulver M., Xiong Z., Li H., Pilewski J., Mallampalli R.K., Nouraie M., et al. Platelets inhibit apoptotic lung epithelial cell death and protect mice against infection-induced lung injury. Blood Adv. 2019;3:432–445. doi: 10.1182/bloodadvances.2018026286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rashid M.H., Kornberg A. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 2000;97:4885–4890. doi: 10.1073/pnas.060030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bucior I., Pielage J.F., Engel J. Pseudomonas aeruginosa Pili and Flagella Mediate Distinct Binding and Signaling Events at the Apical and Basolateral Surface of Airway Epithelium. PLoS Pathog. 2012;8:e1002616. doi: 10.1371/journal.ppat.1002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burrows L.L. Pseudomonas aeruginosa Twitching Motility: Type IV Pili in Action. Annu. Rev. Microbiol. 2012;66:493–520. doi: 10.1146/annurev-micro-092611-150055. [DOI] [PubMed] [Google Scholar]
- 11.Irvin R.T., Doig P., Lee K.K., Sastry P.A., Paranchych W., Todd T., Hodges R.S. Characterization of the Pseudomonas aeruginosa pilus adhesin: Confirmation that the pilin structural protein subunit contains a human epithelial cell-binding domain. Infect. Immun. 1989;57:3720–3726. doi: 10.1128/IAI.57.12.3720-3726.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S.K., Berk R.S., Masinick S., Hazlett L.D. Pili and lipopolysaccharide of Pseudomonas aeruginosa bind to the glycolipid asialo GM1. Infect. Immun. 1994;62:4572–4579. doi: 10.1128/IAI.62.10.4572-4579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallet I., Olson J.W., Lory S., Lazdunski A., Filloux A. The chaperone/usher pathways of Pseudomonas aeruginosa: Identification of fimbrial gene clusters (cup) and their involvement in biofilm formation. Proc. Natl. Acad. Sci. USA. 2001;98:6911–6916. doi: 10.1073/pnas.111551898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chemani C., Imberty A., De Bentzmann S., Pierre M., Wimmerová M., Guery B., Faure K. Role of LecA and LecB Lectins in Pseudomonas aeruginosa-Induced Lung Injury and Effect of Carbohydrate Ligands. Infect. Immun. 2009;77:2065–2075. doi: 10.1128/IAI.01204-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi N., Nishizawa H., Kitao S., Deguchi S., Nakamura T., Fujimoto A., Shikata M., Gotoh N. Pseudomonas aeruginosainjects type III effector ExoS into epithelial cells through the function of type IV pili. FEBS Lett. 2015;589:890–896. doi: 10.1016/j.febslet.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Green D.R., Llambi F. Cell Death Signaling. Cold Spring Harb. Perspect. Biol. 2015:7. doi: 10.1101/cshperspect.a006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerr J.F.R., Wyllie A.H., Currie A.R. Apoptosis: A Basic Biological Phenomenon with Wideranging Implications in Tissue Kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galluzzi L., Vitale I., Abrams J.M., Alnemri E.S., Baehrecke E.H., Blagosklonny M.V., Dawson T.M., Dawson V.L., El-Deiry W.S., Fulda S., et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2011;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paoli P., Giannoni E., Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta (BBA) Bioenerg. 2013;1833:3481–3498. doi: 10.1016/j.bbamcr.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Bergsbaken T., Fink S.L., Cookson B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Genet. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong W., Shi Y., Ren J. Research progresses of molecular mechanism of pyroptosis and its related diseases. Immunobiology. 2020;225:151884. doi: 10.1016/j.imbio.2019.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Platnich J.M., Muruve D.A. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch. Biochem. Biophys. 2019;670:4–14. doi: 10.1016/j.abb.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Jorch S.K., Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017;23:279–287. doi: 10.1038/nm.4294. [DOI] [PubMed] [Google Scholar]
- 24.Dar H., Tyurina Y.Y., Mikulska-Ruminska K., Shrivastava I., Ting H.-C., Tyurin V.A., Krieger J., Croix C.M.S., Watkins S., Bayir E., et al. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium. J. Clin. Investig. 2018;128:4639–4653. doi: 10.1172/JCI99490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kagan V., Mao G., Qu F., Angeli J.P.F., Doll S., Croix C.S., Dar H.H., Liu B., Tyurin V.A., Ritov V.B., et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Methods. 2016;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wenzel S.E., Tyurina Y.Y., Zhao J., Croix C.M.S., Dar H., Mao G., Tyurin V.A., Anthonymuthu T.S., Kapralov O., Amoscato A.A., et al. PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals. Cell. 2017;171:628–641.e26. doi: 10.1016/j.cell.2017.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei P., Bai T., Sun Y. Mechanisms of Ferroptosis and Relations with Regulated Cell Death: A Review. Front. Physiol. 2019;10 doi: 10.3389/fphys.2019.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brigelius-Flohé R., Maiorino M. Glutathione peroxidases. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 29.Yang W.S., SriRamaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V., Cheah J.H., Clemons P.A., Shamji A.F., Clish C., et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dixon S.J., Winter G.E., Musavi L.S., Lee E.D., Snijder B., Rebsamen M., Superti-Furga G., Stockwell B.R. Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem. Biol. 2015;10:1604–1609. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang W.S., Kim K.J., Gaschler M.M., Patel M., Shchepinov M.S., Stockwell B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA. 2016;113:E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doll S., Proneth B., Tyurina Y.Y., Panzilius E., Kobayashi S., Ingold I., Irmler M., Beckers J., Aichler M., Walch A., et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Methods. 2016;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shintoku R., Takigawa Y., Yamada K., Kubota C., Yoshimoto Y., Takeuchi T., Koshiishi I., Torii S. Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci. 2017;108:2187–2194. doi: 10.1111/cas.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toyokuni S., Ito F., Yamashita K., Okazaki Y., Akatsuka S. Iron and thiol redox signaling in cancer: An exquisite balance to escape ferroptosis. Free Radic. Biol. Med. 2017;108:610–626. doi: 10.1016/j.freeradbiomed.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 35.Shah R., Shchepinov M.S., Pratt D.A. Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Central Sci. 2018;4:387–396. doi: 10.1021/acscentsci.7b00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nirmala J.G., Lopus M. Cell death mechanisms in eukaryotes. Cell Biol. Toxicol. 2019;36:145–164. doi: 10.1007/s10565-019-09496-2. [DOI] [PubMed] [Google Scholar]
- 37.Grassmé H., Jendrossek V., Gulbins E. Molecular mechanisms of bacteria induced apoptosis. Apoptosis. 2001;6:441–445. doi: 10.1023/a:1012485506972. [DOI] [PubMed] [Google Scholar]
- 38.Grassmé H. CD95/CD95 Ligand Interactions on Epithelial Cells in Host Defense to Pseudomonas aeruginosa. Science. 2000;290:527–530. doi: 10.1126/science.290.5491.527. [DOI] [PubMed] [Google Scholar]
- 39.Valente E., Assis M.C., Alvim I.M., Pereira G.M., Plotkowski M.C. Pseudomonas aeruginosa induces apoptosis in human endothelial cells. Microb. Pathog. 2000;29:345–356. doi: 10.1006/mpat.2000.0400. [DOI] [PubMed] [Google Scholar]
- 40.Aballay A., Ausubel F.M. Programmed cell death mediated by ced-3 and ced-4 protects Caenorhabditis elegans from Salmonella typhimurium-mediated killing. Proc. Natl. Acad. Sci. USA. 2001;98:2735–2739. doi: 10.1073/pnas.041613098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maurice N.M., Bedi B., Yuan Z., Goldberg J.B., Koval M., Hart C.M., Sadikot R.T. Pseudomonas aeruginosa Induced Host Epithelial Cell Mitochondrial Dysfunction. Sci. Rep. 2019;9:1–15. doi: 10.1038/s41598-019-47457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song D., Meng J., Cheng J., Fan Z., Chen P., Ruan H., Tu Z., Kang N., Li N., Xu Y., et al. Pseudomonas aeruginosa quorum-sensing metabolite induces host immune cell death through cell surface lipid domain dissolution. Nat. Microbiol. 2018;4:97–111. doi: 10.1038/s41564-018-0290-8. [DOI] [PubMed] [Google Scholar]
- 43.Losa D., Köhler T., Bellec J., Dudez T., Crespin S., Bacchetta M., Boulanger P., Hong S.S., Morel S., Nguyen T.H., et al. Pseudomonas aeruginosa–Induced Apoptosis in Airway Epithelial Cells Is Mediated by Gap Junctional Communication in a JNK-Dependent Manner. J. Immunol. 2014;192:4804–4812. doi: 10.4049/jimmunol.1301294. [DOI] [PubMed] [Google Scholar]
- 44.Ryu J.-C., Kim M.-J., Kwon Y., Oh J.-H., Yoon S.S., Shin S.J., Yoon J.-H., Ryu J.-H. Neutrophil pyroptosis mediates pathology of P. aeruginosa lung infection in the absence of the NADPH oxidase NOX2. Mucosal Immunol. 2016;10:757–774. doi: 10.1038/mi.2016.73. [DOI] [PubMed] [Google Scholar]
- 45.Pylaeva E., Lang S., Jablonska J. The Essential Role of Type I Interferons in Differentiation and Activation of Tumor-Associated Neutrophils. Front. Immunol. 2016;7 doi: 10.3389/fimmu.2016.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.González-Navajas J.M., Lee J., David M., Raz E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 2012;12:125–135. doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parker D., Cohen T.S., Alhede M., Harfenist B.S., Martin F.J., Prince A. Induction of Type I Interferon Signaling by Pseudomonas aeruginosa Is Diminished in Cystic Fibrosis Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2012;46:6–13. doi: 10.1165/rcmb.2011-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pylaeva E., Bordbari S., Spyra I., Decker A.S., Häussler S., Vybornov V., Lang S., Jablonska J. Detrimental Effect of Type I IFNs During Acute Lung Infection With Pseudomonas aeruginosa Is Mediated Through the Stimulation of Neutrophil NETosis. Front. Immunol. 2019;10:2190. doi: 10.3389/fimmu.2019.02190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan M.A., Philip L.M., Cheung G., Vadakepeedika S., Grasemann H., Sweezey N., Palaniyar N. Regulating NETosis: Increasing pH Promotes NADPH Oxidase-Dependent NETosis. Front. Med. 2018;5 doi: 10.3389/fmed.2018.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shafikhani S.H., Morales C., Engel J. The Pseudomonas aeruginosa type III secreted toxin ExoT is necessary and sufficient to induce apoptosis in epithelial cells. Cell. Microbiol. 2008;10:994–1007. doi: 10.1111/j.1462-5822.2007.01102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wood S., Goldufsky J., Shafikhani S.H. Pseudomonas aeruginosa ExoT Induces Atypical Anoikis Apoptosis in Target Host Cells by Transforming Crk Adaptor Protein into a Cytotoxin. PLoS Pathog. 2015;11:e1004934. doi: 10.1371/journal.ppat.1004934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wood S.J., Goldufsky J.W., Bello D., Masood S., Shafikhani S.H. Pseudomonas aeruginosaExoT Induces Mitochondrial Apoptosis in Target Host Cells in a Manner That Depends on Its GTPase-activating Protein (GAP) Domain Activity. J. Biol. Chem. 2015;290:29063–29073. doi: 10.1074/jbc.M115.689950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scannapieco F., Wang B., Shiau H.J. Oral Bacteria and Respiratory Infection: Effects on Respiratory Pathogen Adhesion and Epithelial Cell Proinflammatory Cytokine Production. Ann. Periodontol. 2001;6:78–86. doi: 10.1902/annals.2001.6.1.78. [DOI] [PubMed] [Google Scholar]
- 54.Pan Y., Teng D., Burke A.C., Haase E., Scannapieco F. Oral bacteria modulate invasion and induction of apoptosis in HEp-2 cells by Pseudomonas aeruginosa. Microb. Pathog. 2009;46:73–79. doi: 10.1016/j.micpath.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 55.Torres I.M., Demirdjian S., Vargas J., Goodale B.C., Berwin B. Acidosis increases the susceptibility of respiratory epithelial cells to Pseudomonas aeruginosa-induced cytotoxicity. Am. J. Physiol. Cell. Mol. Physiol. 2017;313:L126–L137. doi: 10.1152/ajplung.00524.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma A., Boone D.L., Lodolce J.P. The Pleiotropic Functions of Interleukin 15. J. Exp. Med. 2000;191:753–756. doi: 10.1084/jem.191.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fehniger T.A., Caligiuri M.A. Interleukin 15: Biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 58.Li X.C., Demirci G., Ferrari-Lacraz S., Groves C., Coyle A., Malek T.R., Strom T.B. IL-15 and IL-2: A matter of life and death for T cells in vivo. Nat. Med. 2001;7:114–118. doi: 10.1038/83253. [DOI] [PubMed] [Google Scholar]
- 59.Waldmann T.A. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 60.Lucas M., Schachterle W., Oberle K., Aichele P., Diefenbach A. Dendritic Cells Prime Natural Killer Cells by trans-Presenting Interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Inoue S., Unsinger J., Davis C.G., Muenzer J.T., Ferguson T.A., Chang K., Osborne D.F., Clark A.T., Coopersmith C.M., McDunn J.E., et al. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J. Immunol. 2009;184:1401–1409. doi: 10.4049/jimmunol.0902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kennedy M.K., Glaccum M., Brown S.N., Butz E.A., Viney J.L., Embers M., Matsuki N., Charrier K., Sedger L.M., Willis C.R., et al. Reversible Defects in Natural Killer and Memory Cd8 T Cell Lineages in Interleukin 15–Deficient Mice. J. Exp. Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma A., Koka R., Burkett P. DIVERSE FUNCTIONS OF IL-2, IL-15, AND IL-7 IN LYMPHOID HOMEOSTASIS. Annu. Rev. Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 64.Maeurer M., Trinder P., Hommel G., Walter W., Freitag K., Atkins D., Storkel S. Interleukin-7 or Interleukin-15 Enhances Survival of Mycobacterium tuberculosis-Infected Mice. Infect. Immun. 2000;68:2962–2970. doi: 10.1128/IAI.68.5.2962-2970.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Motegi A., Kinoshita M., Inatsu A., Habu Y., Saitoh D., Seki S. IL-15-induced CD8+CD122+T cells increase antibacterial and anti-tumor immune responses: Implications for immune function in aged mice. J. Leukoc. Biol. 2008;84:1047–1056. doi: 10.1189/jlb.0807530. [DOI] [PubMed] [Google Scholar]
- 66.Hiromatsu T., Yajima T., Matsuguchi T., Nishimura H., Wajjwalku W., Arai T., Nimura Y., Yoshikai Y. Overexpression of Interleukin-15 Protects againstEscherichia coli–Induced Shock Accompanied by Inhibition of Tumor Necrosis Factor-α-Induced Apoptosis. J. Infect. Dis. 2003;187:1442–1451. doi: 10.1086/374643. [DOI] [PubMed] [Google Scholar]
- 67.Zou C., Li J., Xiong S., Chen Y., Wu Q., Li X., Weathington N.M., Han S.H., Snavely C., Chen B.B., et al. Mortality factor 4 like 1 protein mediates epithelial cell death in a mouse model of pneumonia. Sci. Transl. Med. 2015;7:311ra171. doi: 10.1126/scitranslmed.aac7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi X., Wei W., Wang N. Tremella polysaccharides inhibit cellular apoptosis and autophagy induced by Pseudomonas aeruginosa lipopolysaccharide in A549 cells through sirtuin 1 activation. Oncol. Lett. 2018;15:9609–9616. doi: 10.3892/ol.2018.8554. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]