Abstract
Background
Selenium is a key component of a number of selenoproteins which protect against oxidative stress and have the potential to prevent chronic diseases including cardiovascular disease (CVD). However, observational studies have shown inconsistent associations between selenium intake and CVD risk; in addition, there is concern around a possible increased risk of type 2 diabetes with high selenium exposure.
Objectives
To determine the effectiveness of selenium only supplementation for the primary prevention of CVD and examine the potential adverse effect of type 2 diabetes.
Search methods
The following electronic databases were searched: the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 10 of 12, October 2012) on The Cochrane Library; MEDLINE (Ovid) (1946 to week 2 October 2012); EMBASE Classic + EMBASE (Ovid) (1947 to 2012 Week 42); CINAHL (EBSCO) (to 24 October 2012); ISI Web of Science (1970 to 24 October 2012); PsycINFO (Ovid) (1806 to week 3 October 2012); Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment Database and Health Economics Evaluations Database (Issue 4 of 4, October 2012) on The Cochrane Library. Trial registers and reference lists of reviews and articles were searched and experts in the field were approached. No language restrictions were applied.
Selection criteria
Randomised controlled trials on the effects of selenium only supplementation on major CVD end‐points, mortality, changes in CVD risk factors, and type 2 diabetes were included both in adults of all ages from the general population and in those at high risk of CVD. Trials were only considered where the comparison group was placebo or no intervention. Only studies with at least three months follow‐up were included in the meta‐analyses, shorter term studies were dealt with descriptively.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. Study authors were contacted for additional information.
Main results
Twelve trials (seven with duration of at least three months) met the inclusion criteria, with 19,715 participants randomised. The two largest trials that were conducted in the USA (SELECT and NPC) reported clinical events. There were no statistically significant effects of selenium supplementation on all cause mortality (RR 0.97, 95% CI 0.88 to 1.08), CVD mortality (RR 0.97, 95% CI 0.79 to 1.2), non‐fatal CVD events (RR 0.96, 95% CI 0.89 to 1.04) or all CVD events (fatal and non‐fatal) (RR 1.03, 95% CI 0.95 to 1.11). There was a small increased risk of type 2 diabetes with selenium supplementation but this did not reach statistical significance (RR 1.06, 95% CI 0.97 to 1.15). Other adverse effects that increased with selenium supplementation, as reported in the SELECT trial, included alopecia (RR 1.28, 95% CI 1.01 to 1.62) and dermatitis grade 1 to 2 (RR 1.17, 95% CI 1.0 to 1.35). Selenium supplementation reduced total cholesterol but this did not reach statistical significance (WMD ‐ 0.11 mmol/L, 95% CI ‐ 0.3 to 0.07). Mean high density lipoprotein (HDL) levels were unchanged. There was a statistically significant reduction in non‐HDL cholesterol (WMD ‐ 0.2 mmol/L, 95% CI ‐ 0.41 to 0.00) in one trial of varying selenium dosage. None of the longer term trials examined effects on blood pressure. Overall, the included studies were regarded as at low risk of bias.
Authors' conclusions
The limited trial evidence that is available to date does not support the use of selenium supplements in the primary prevention of CVD.
Keywords: Adult; Humans; Dietary Supplements; Antioxidants; Antioxidants/administration & dosage; Cardiovascular Diseases; Cardiovascular Diseases/prevention & control; Cholesterol; Cholesterol/blood; Food, Fortified; Primary Prevention; Primary Prevention/methods; Randomized Controlled Trials as Topic; Selenium; Selenium/administration & dosage
Plain language summary
Selenium supplements for the prevention of cardiovascular disease
Use of selenium enriched foods, supplements and fertilizers has increased in recent years in many countries because of the perception that selenium may reduce the risk of cardiovascular disease and other chronic conditions. Therefore, it is important to understand the effects of a nutrient that is frequently supplemented on common conditions such as cardiovascular disease or diabetes. This review assessed the effects of providing selenium supplements to healthy adults in order to prevent the occurrence of cardiovascular disease. Whether selenium supplements would reduce the risk factors associated with heart disease was also examined. We found 12 trials in which 19,715 healthy adults were randomly assigned to receive selenium supplements or placebo. The vast majority of participants involved in these trials were male individuals from the US, where people are already well nourished and take large amounts of selenium from natural foods. Overall, the included studies were regarded as at low risk of bias. In our review, providing selenium supplements to healthy adults did not prevent the occurrence of major cardiovascular disease. The increased risk of developing type 2 diabetes when taking selenium supplements, as suggested in some previous studies, could not definitely be ruled out in our review. In summary, this review of the available evidence to date suggests that taking selenium supplements is neither beneficial nor harmful for cardiovascular disease, but it is probably unnecessary for those who are already well nourished and who take large amounts of selenium from natural foods.
Background
Description of the condition
Cardiovascular disease (CVD) is still the number one cause of death and disability worldwide (WHO 2011). In 2008 it accounted for 30% of total global deaths, with 6.2 million deaths the consequence of stroke and 7.2 million due to coronary heart disease (CHD) (WHO 2011). The burden of disease will increase with an aging population and increasing levels of obesity and sedentary lifestyles. Prevention of CVD by targeting modifiable factors remains a key public health priority. Diet plays a major role in the aetiology of many chronic diseases, including CVD, thereby contributing to a significant geographical variability in morbidity and mortality rates across different countries and populations worldwide (WHO 2003).
Description of the intervention
Selenium is a trace element that is essential to humans, and is currently the focus of major scientific debate and investigation (Rayman 2009; Stranges 2010a). A recent Cochrane systematic review (Dennert 2011) examining the effect of selenium in the prevention of cancer found from observational studies that people with higher selenium levels or intake had a lower frequency of certain cancers (such as bladder or prostate cancer) but results from trials of selenium supplementation were inconsistent. For CVD, a number of observational studies have examined the association between selenium status and risk of CHD and other CVD end‐points across different populations (Bleys 2008; Bleys 2009; Flores‐Mateo 2006; Salonen 1982; Virtamo 1985; Wei 2004). Although some of the early studies suggest possible inverse associations, especially in populations with relatively low dietary selenium intakes (Flores‐Mateo 2006; Salonen 1982; Virtamo 1985; Wei 2004), more recent observational evidence is suggestive of a possible U‐shaped association between selenium status and CVD risk, at least in selenium‐replete populations such as in the United States (US) (Bleys 2008; Bleys 2009).
Results from randomised controlled trials of selenium supplementation do not, however, provide conclusive evidence to support a role for selenium in CVD disease prevention (Brown 2001; Flores‐Mateo 2006; Hercberg 2004; Korpela 1989; Kuklinski 1994; Stranges 2006; You 2001). In a post hoc analysis from the Nutritional Prevention of Cancer (NPC) trial in the US (Stranges 2006), selenium supplementation alone (200 μg/day as high‐selenium yeast) was not significantly associated with any of the CVD end‐points after 7.6 years of follow‐up. Other randomised controlled trials that have examined the effect of selenium in combination with other vitamins or minerals on CVD end‐points have also yielded inconclusive findings (Brown 2001; Hercberg 2004; Korpela 1989; Kuklinski 1994; You 2001).
The intervention to be examined in the current review is selenium supplementation as a single ingredient. Selenium is a key component of a number of selenoproteins involved in essential enzymatic functions such as redox homeostasis, thyroid hormone metabolism, immunity and reproduction (Burk 2002; Papp 2007). There is a longstanding recognition that selenium deficiency, as originally observed in North‐eastern provinces of China in the 1970s, is associated with the occurrence of diseases such as cardiomyopathy (Keshan disease) and athropathy (Kashim‐Beck disease), with potential reversal of these conditions with selenium supplementation (Keshan Disease Research Group; Rayman 2000). In addition, because of the potential of these selenoproteins to protect against oxidative stress, significant expectations have been raised for a role for selenium in the prevention of several chronic diseases commonly associated with oxidative stress including cancer, CVD and type 2 diabetes (Combs 1998; Neve 1996; Rayman 2000).
However, there is some evidence of potential adverse effects of selenium supplementation. Recent findings from observational studies and randomised controlled trials have raised concerns that high selenium exposure may lead to adverse cardio‐metabolic effects, at least in selenium‐replete populations, such as an increased risk of diabetes, hypertension and hyperlipidemia (Laclaustra 2009a; Stranges 2007; Stranges 2010b). For example, in a post hoc analysis of the NPC trial in the Eastern US (Stranges 2007), supplementation with selenium alone (200 μg/day as high‐selenium yeast) increased the risk of type 2 diabetes compared to placebo, particularly in men and in participants with high baseline plasma selenium (hazard ratio of 2.70 in the highest tertile of plasma selenium, that is > 121.6 ng/ml). In addition, the recent Cochrane review examining the effect of selenium for the prevention of cancer also highlighted the potential adverse effects of selenium supplementation notably gastrointestinal upset, alopecia and an increased risk of type 2 diabetes (Dennert 2011).
How the intervention might work
In theory, potential CVD benefits of selenium supplements are supported by the ability of selenoproteins such as glutathione peroxidase (GPx) and selenoprotein S to combat the oxidative modification of lipids, inhibit platelet aggregation and reduce inflammation (Blankenberg 2003; Brigelius‐Flohe 2003; Curran 2005; Gao 2006; Neve 1996; Salonen 1988; Sattler 1994; Vunta 2007). However, selenium has a narrow therapeutic window and there is considerable individual variability in terms of metabolic sensitivity and optimal selenium intake (Whanger 1996). Part of the inconsistencies in the effect of selenium supplements on cardio‐metabolic outcomes in different studies might be explained by the variability of selenium status and selenium intake across countries and population subgroups (Rayman 2009; Stranges 2010a). In this view, the association between selenium and cardio‐metabolic outcomes is likely to be U‐shaped, with potential harm occurring at selenium levels both below and above the physiological range for optimal activity of selenoproteins. For example, recent findings from the UK PRECISE Pilot trial among 501 elderly volunteers with a relatively low selenium status showed that supplementation with selenium alone at 100 and 200 μg/day significantly decreased total and non‐HDL cholesterol concentrations, and that the ratio of total‐to‐HDL cholesterol decreased progressively with increasing selenium doses (Rayman 2011).
Why it is important to do this review
Selenium deficiency, as observed in North‐eastern provinces of China in the 1970s, is associated with the occurrence of diseases such as cardiomyopathy (Keshan disease) and athropathy (Kashim‐Beck disease), with potential reversal of these conditions with selenium supplementation (Keshan Disease Research Group; Rayman 2000). On the other hand, there is some suggestive evidence of potential adverse effects of selenium supplementation, such as an increased diabetes risk, in populations with high‐selenium status. The association between selenium and cardio‐metabolic outcomes is likely to be U‐shaped, with potential harms occurring at selenium levels both below and above the physiological range for optimal activity of selenoproteins (Stranges 2010a).
Use of selenium enriched foods, supplements and fertilizers has increased markedly in recent years in the US and other Western countries (Broadley 2006; Millen 2004; Rayman 1997) because of the perception that the anti‐oxidant properties of selenium could potentially reduce the risk of cancer, CVD and other chronic diseases. Given the recent findings on potential adverse effects of high selenium exposure (Laclaustra 2009a; Stranges 2007; Stranges 2010b), from a public health perspective the relationship between selenium status and CVD health should be clarified in order to help guide consumers in their choices of nutritional supplements and enriched food products. There has been no published systematic review on the effect of selenium only supplements on the primary prevention of CVD.
Objectives
1. To determine the effectiveness of selenium only supplementation to prevent cardiovascular disease (CVD) events.
2. To determine the effects of selenium only supplementation on cardiovascular risk factors (blood pressure, lipid levels) and adverse effects including type 2 diabetes.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs)
Types of participants
Adults of all ages from the general population and those at high risk of CVD were included. The review focused on the effects of selenium supplementation on the primary prevention of CVD, and we have therefore excluded those people who have experienced a previous myocardial infarction (MI), stroke, revascularisation procedure (coronary artery bypass grafting (CABG) or percutaneous transluminal coronary angioplasty (PTCA)), those with angina or angiographically defined coronary heart disease (CHD).
Types of interventions
The intervention was selenium only supplementation, as a single ingredient. Multivitamin and mineral preparations including selenium were excluded as it would be impossible to disentangle selenium‐specific effects from those derived from other micronutrients. Baseline selenium status is likely to influence the effect of selenium supplementation on cardiovascular outcomes (Rayman 2009; Rayman 2012; Stranges 2010a), so results were analysed by baseline selenium status and country, where possible, as well as by selenium dosage and duration of the intervention.
Trials were only considered for inclusion where the comparison group was placebo or no intervention. We focused on follow‐up periods of six months or more as these are most relevant for public health interventions, but length of follow‐up was not an exclusion criteria. Only studies with at least three months follow‐up were included in the meta‐analyses. Very short term studies (less than three months follow‐up) were dealt with descriptively.
Types of outcome measures
Primary outcomes
Major CVD end‐points: CVD, non‐fatal myocardial infarction (MI), non‐fatal stroke, and revascularisation procedures (CABG or PTCA).
Secondary outcomes
All cause mortality
CHD composite end‐point: fatal CHD, non‐fatal MI, or CABG or PTCA
Stroke composite end‐point: fatal and non‐fatal stroke
Peripheral artery disease
Type 2 diabetes*
Changes in levels of blood pressure and blood lipids
* This outcome was used as a potential side effect of selenium. Other adverse effects were noted and data were collected on costs where available.
Search methods for identification of studies
Electronic searches
The following electronic databases were searched:
Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 10 of 12, October 2012) on The Cochrane Library;
MEDLINE (Ovid) (1946 to week 2 October 2012);
EMBASE Classic + EMBASE (Ovid) (1947 to 2012 Week 42);
CINAHL (EBSCO) (to 24 October 2012);
ISI Web of Science (1970 to 24 October 2012);
PsycINFO (Ovid) (1806 to week 3 October 2012);
Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment Database and Health Economics Evaluations Database (Issue 4 of 4, October 2012) on The Cochrane Library.
Medical subject headings (MeSH) or equivalent and text word terms were used. The Cochrane sensitive‐maximising RCT filter (Lefebvre 2011) was used for MEDLINE and adaptations of it were used for EMBASE, Web of Science and PsycINFO.
There were no language restrictions.
Searches were tailored to individual databases (Appendix 1).
Searching other resources
In addition, reference lists of reviews and retrieved articles were checked for additional studies.
We searched the metaRegister of controlled trials (mRCT) (www.controlled‐trials.com/mrct), Clinicaltrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/) for ongoing trials (search date 5 November 2012).
Citation searches were performed on key articles. Google Scholar was also used to search for further studies. Experts in the field were contacted for unpublished and ongoing trials. Authors were contacted, where necessary, for additional information.
Data collection and analysis
Selection of studies
From the searches, the title and abstract of each paper were reviewed by two review authors (KR, LH, NF or CD) and potentially relevant references retrieved. Following this initial screening, the full text reports of potentially relevant studies were obtained, and two review authors (KR, LH, NF or CD) independently selected studies to be included in the review using predetermined inclusion criteria. In all cases disagreements about any study inclusions were resolved by consensus and a third review author (SS) was consulted if disagreement persisted.
Data extraction and management
Data were extracted independently by two review authors (KR, LH) using a proforma and chief investigators were contacted to provide additional relevant information if necessary. Details of the study design, participant characteristics, study setting, intervention (including dose and duration), and outcome data including details of outcome assessment, adverse effects, and methodological quality (randomisation, blinding, attrition) were extracted from each of the included studies. Disagreements about extracted data were resolved by consensus.
Assessment of risk of bias in included studies
Risk of bias was assessed by examining the quality of the random sequence generation and allocation concealment, the description of drop‐outs and withdrawals (including analysis by intention to treat), blinding (participants, personnel and outcome assessment) and selective outcome reporting (Higgins 2011). The risk of bias in included studies was assessed independently by two review authors (KR, LH).
Measures of treatment effect
Data were processed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Dichotomous outcomes were expressed as relative risks (RR), and 95% confidence intervals (CI) were calculated for each study. For continuous variables net changes were compared (that is intervention group minus control group differences) and a weighted mean difference (WMD) and 95% CI were calculated for each study.
Assessment of heterogeneity
For each outcome tests of heterogeneity were carried out (using the Chi² test of heterogeneity and I² statistic). In the situation of no heterogeneity a fixed‐effect model meta‐analysis was performed. If substantial heterogeneity was detected the review authors looked for possible explanations for this (for example participants and intervention). If the heterogeneity could not be explained, the review authors considered the following options: to provide a narrative overview and not aggregate the studies at all, or use a random‐effects model with appropriate cautious interpretation.
Subgroup analysis and investigation of heterogeneity
If there were sufficient trials that met the inclusion criteria, it was our intention to stratify results according to baseline selenium status and country, and selenium dosage. There were not sufficient trials for us to perform these analyses.
Sensitivity analysis
If there were sufficient trials that met the inclusion criteria, it was our intention to perform sensitivity analyses excluding studies of low methodological quality and to undertake funnel plots and tests of asymmetry (Egger 1997) to assess possible publication bias. There were not sufficient trials for us to perform these analyses.
Results
Description of studies
Results of the search
The searches generated 2225 hits, and 1656 after de‐duplication. Screening the titles and abstracts, we identified 65 papers for formal inclusion or exclusion. One further trial was identified following contact with experts. Of these, 12 RCTs (14 papers) met the inclusion criteria; seven RCTs had a duration of three months or more and contributed to the meta‐analyses. Five short term trials of selenium supplementation (less than three months) were dealt with descriptively. We did not identify any ongoing trials. The study flow diagram is presented in Figure 1.
1.

Study flow diagram.
Included studies
Details of the methods, participants, intervention, comparison group and outcome measures for each of the studies included in the review are shown in the Characteristics of included studies table. Twelve trials were included, with 19,715 participants randomised. Six trials recruited only male participants (17,843 randomised). Four trials (18,954 participants randomised) were conducted in the USA (Algotar 2010; Hawkes 2008; NCP; SELECT) and included the two largest trials, the Selenium and Vitamin E Cancer Prevention Trial (SELECT) with 17,448 participants randomised and the Nutritional Prevention of Cancer trial (NCP) with 1312 participants randomised. The remaining studies were conducted in Australia (Wu 2009), China (Yu 1990), Denmark (Ravn‐Haren 2008), Finland (Luoma 1984), Norway (Meltzer 1994; Meltzer 1997), Spain (Navas‐Carretero 2011) and the UK (UK PRECISE).
The duration of the intervention and follow‐up periods varied enormously from a very short one and two weeks (Luoma 1984; Ravn‐Haren 2008) to long term follow‐up for the largest trials SELECT (SELECT) and NCP (NCP). Similarly, the dose of selenium supplementation that was used varied from 100 to 800 μg/day. Baseline selenium status varied by country, being lowest in China and highest in the USA. The country of recruitment, baseline plasma selenium level, dose of selenium supplementation and duration of the intervention for each study are shown in Table 1.
1. Country, baseline selenium status and dose of selenium supplementation.
| Study | References | Country | Baseline plasma selenium level (μg/L) | Dose of selenium supplementation studied | Duration of the intervention |
| Watchful waiting study | Agotar 2009 | USA | 128‐146 | 200μg/day and 800μg/day | 5 years |
| Hawkes 2008 | Hawkes 2008 | USA | 142‐146 | 300μg/day | 48 weeks |
| SELECT | Lippman 2009 Klein 2011 |
USA | 135‐137.6 | 200μg/day | 7‐12 years |
| Luoma 1984 | Luoma 1984 | Finland | 73.7 (intervention group) | 96μg/day | 2 weeks |
| Meltzer 1994 | Meltzer 1994 | Norway | 109 (14) in the intervention group, 104 (15) in the control group. | Dietary intake 135(25)μg/day | 6 weeks |
| Meltzer 1997 | Meltzer 1997 | Norway | 1.4 μmol/L | Dietary intake 185(27)μg/day | 6 weeks |
| Navas‐Carretero 2011 | Navas‐Carretero 2011 | Spain | 142‐146 | 36.4 μg/day | 10 weeks |
| Ravn‐Haren 2008 | Ravn‐Haren 2008 | Denmark | 107.8‐114.5 | 300μg/day | 1 week |
| UK PRECISE | Rayman 2011 | UK | 88.1‐90.2 | 100μg/day and 200μg/day and 30μg/day | 6 months |
| NCP | Stranges 2006 Stranges 2007 |
USA | 113.3‐113.8 | 200μg/day | 7.6 years |
| Wu 2009 | Wu 2009 | Australia | 121‐122.3 | increases from 100μg/day to 200μg/day to 300μg/day | 24 weeks |
| Yu 1990 | Yu 1990 | China | No baseline values, selenium level in control group 50 | 300μg/day | 12 months |
Studies were also variable in the participants recruited. One study was conducted in patients with prostate cancer, the Watchful Waiting Study (Algotar 2010). Two trials were conducted in participants at high risk of cancer, where CVD outcomes were secondary end‐points (NCP; Yu 1990). Three trials were conducted in healthy populations where the focus was cancer prevention and CVD outcomes were secondary end‐points (SELECT; UK PRECISE; Wu 2009). Six small trials focused on vascular function and oxidative defence (Hawkes 2008; Luoma 1984; Meltzer 1994; Meltzer 1997; Navas‐Carretero 2011; Ravn‐Haren 2008) with five of these being very short term (Luoma 1984; Meltzer 1994; Meltzer 1997; Navas‐Carretero 2011; Ravn‐Haren 2008).
Excluded studies
Details and reasons for exclusion for the studies that most closely missed the inclusion criteria are presented in the Characteristics of excluded studies table. Reasons for exclusion for the majority of studies included the use of multivitamin preparations including selenium rather than single selenium supplements, and no relevant outcomes.
Risk of bias in included studies
Details are provided for each of the included studies in the risk of bias tables in Characteristics of included studies.
Allocation
The methods of random sequence generation and allocation concealment were unclear in nine of the included studies. In the three studies where they were clear, the methods used were judged to be of low risk of bias (Hawkes 2008; NCP; UK PRECISE).
Blinding
Eleven of the 12 included studies stated that they were double blind (participants and personnel were blind to treatment allocation, as were outcome assessors) and were regarded at low risk of bias. In the remaining trial this was unclear (Meltzer 1994).
Incomplete outcome data
Most studies reported losses to follow‐up and these were judged to have low risk of bias as the number of losses and reasons for loss to follow‐up were similar across treatment arms. In three of nine studies this was judged unclear as losses to follow‐up had not been reported (Algotar 2010) or a total number had been recorded and it was unclear from which treatment arm losses occurred (Hawkes 2008), or there was some differential loss due to adverse effects associated with higher selenium doses (UK PRECISE).
Selective reporting
For most studies the risk of bias associated with selective reporting was unclear, with the exception of two studies that clearly stated the primary and secondary outcomes and reported the results for these (Algotar 2010; Wu 2009). The largest trial focused on the effects of selenium in preventing the onset of cancer, where cardiovascular outcomes were secondary endpoints (SELECT). The two reports of the NCP trial reported secondary analyses and examined the outcomes of cardiovascular events and type 2 diabetes (NCP). A further trial stated that the non‐specified outcomes were informed by the literature (UK PRECISE). It is unclear how these factors may have influenced reporting bias.
Other potential sources of bias
In most cases there was insufficient information to judge the risk of bias in other sources of bias not covered above, and all were categorised as unclear.
Effects of interventions
Clinical events
Major CVD end‐points
The two largest trials had long term follow‐up and reported clinical events (NCP; SELECT). The analyses were dominated by the SELECT trial, which carried over 80% of the weight. There were no statistically significant effects of selenium supplementation on CVD mortality (RR 0.97, 95% CI 0.79 to 1.2), non‐fatal CVD events (RR 0.96, 95% CI 0.89 to 1.04) or all CVD events (fatal and non‐fatal) (RR 1.03, 95% CI 0.95 to 1.11).
Non‐fatal strokes
One study examined the effects of selenium supplementation on non‐fatal strokes (SELECT). There was a reduction in all non‐fatal strokes (ischaemic and haemorrhagic) with the intervention but this did not reach statistical significance (RR 0.79, 95% CI 0.58 to 1.07). Similarly, no statistically significant effects were seen with selenium supplementation for ischaemic and haemorrhagic strokes reported separately.
All cause mortality
The two largest studies also reported all cause mortality (NCP; SELECT). There were no statistically significant effects of selenium supplementation on all cause mortality (RR 0.97, 95% CI 0.88 to 1.08).
Cardiovascular risk factors
Nine of 12 trials measured lipid levels, but only three trials (six trial arms) contributed to the meta‐analysis (Hawkes 2008; Wu 2009; UK PRECISE). The five short term trials of less than three months (Luoma 1984; Meltzer 1994; Meltzer 1997; Navas‐Carretero 2011; Ravn‐Haren 2008) were not included in the pooled analysis, and a further trial had follow‐up data and did not include change from baseline (Yu 1990). Results from these studies have been dealt with in a narrative fashion.
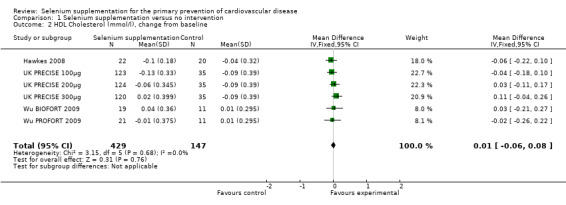
From the pooled analysis, selenium supplementation reduced total cholesterol but this did not reach statistical significance (WMD ‐ 0.11 mmol/L, 95% CI ‐ 0.3 to 0.07). There was no effect of selenium supplementation on high density lipoprotein (HDL) levels (WMD 0.01 mmol/L, 95% CI ‐ 0.006 to 0.08). Non‐HDL cholesterol was measured in one trial of varying doses of selenium supplementation (UK PRECISE). Pooling these data showed a statistically significant reduction in non‐HDL cholesterol (WMD ‐ 0.2 mmol/L, 95% CI ‐ 0.41 to 0.00). Similarly there was a reduction in low density lipoprotein (LDL) cholesterol in the one trial where this was measured (Hawkes 2008) but this did not reach statistical significance (WMD ‐ 0.18 mmol/L, 95% CI ‐ 0.54 to 0.18). There was no effect of selenium supplementation on triglycerides in the one trial that measured this outcome (Hawkes 2008).
Four short term studies showed no effect of short term supplementation on lipid levels (Luoma 1984; Meltzer 1994; Navas‐Carretero 2011; Ravn‐Haren 2008). A further short term study showed no change in total and LDL cholesterol or triglycerides with selenium supplementation, but a 12% (P < 0.05) increase in HDL cholesterol (Meltzer 1997). In the study in Chinese tin miners where only follow‐up data were available, there was no effect of selenium supplementation on total cholesterol levels (Yu 1990).
One short term study measured blood pressure and found no effect of selenium supplementation (Navas‐Carretero 2011). None of the longer term (three months plus) studies examined the effects of selenium supplementation on blood pressure levels.
Adverse effects
The effect of selenium supplementation on the risk of type 2 diabetes was measured in three trials (four trial arms, 18,790 participants randomised). There was a small increased risk of type 2 diabetes with selenium supplementation but this did not reach statistical significance (RR 1.06, 95% CI 0.97 to 1.15). The results were dominated by the SELECT trial, which carried 95% of the weight. The UK PRECISE trial measured plasma adiponectin as an independent risk factor of type 2 diabetes. After six months of supplementation, they found no effect of selenium supplementation on adiponectin levels.
Other adverse effects of selenium supplementation were noted in the included studies and are presented in Table 2. The SELECT trial found a significantly increased risk of alopecia (RR 1.28, 95% CI 1.01 to 1.62) and dermatitis grade 1 to 2 (RR 1.17, 95% CI 1.0 to 1.35) with selenium supplementation.
2. Adverse effects reported.
| Study | References | Adverse effects reported |
| Watchful waiting study | Agotar 2009 | Type 2 diabetes recorded but not as an adverse event |
| Hawkes 2008 | Hawkes 2008 | Not recorded |
| SELECT | Lippman 2009 Klein 2011 |
Type 2 diabetes, Alopecia*, Dermatitis*, Halitosis, Nail changes, Fatigue, Nausea |
| Luoma 1984 | Luoma 1984 | Not recorded |
| Meltzer 1994 | Meltzer 1994 | Not recorded |
| Meltzer 1997 | Meltzer 1997 | Not recorded |
| Navas‐Carretero 2011 | Navas‐Carretero 2011 | Not recorded |
| Ravn‐Haren 2008 | Ravn‐Haren 2008 | Not recorded |
| UK PRECISE | Rayman 2011 | 12 adverse events, principally stomach and abdominal discomfort, no differences between the selenium and placebo groups |
| NCP | Stranges 2006 Stranges 2007 |
Type 2 diabetes* (measured in Stranges 2007, not as an adverse event) |
| Wu 2009 | Wu 2009 | 1 adverse event, not described. |
| Yu 1990 | Yu 1990 | Not recorded |
* statistically significant increased risk of adverse effect with selenium supplementation
Discussion
The aim of this review was to evaluate the effectiveness of selenium supplementation, as a single ingredient, for the primary prevention of CVD. We also examined the effects of selenium only supplementation on major CVD risk factors, including blood lipids and blood pressure, as well as on potential adverse cardio‐metabolic effects such as type 2 diabetes, which has been previously indicated by two individual trials (NCP; SELECT).
Summary of main results
There were no statistically significant effects of selenium supplementation on major CVD clinical end‐points, such as all cause mortality and cardiovascular mortality, or on overall non‐fatal cardiovascular disease including CHD and stroke. There was a suggestion of a potential reduction in all non‐fatal strokes (ischaemic and haemorrhagic) with the selenium supplementation in one trial (SELECT), which however did not reach statistical significance.
With regard to CVD risk factors, current trial evidence suggests a potential beneficial effect of selenium supplementation on blood lipids, namely non‐HDL cholesterol; for total and HDL cholesterol as well as for triglycerides the findings did not reach statistical significance. Surprisingly, no trial has to date examined the effect of selenium supplementation alone on blood pressure end‐points.
Findings from the pooled analysis did not show a statistically significant increased risk of type 2 diabetes with single selenium supplements. These analyses were largely dominated by the SELECT trial (SELECT), which only recruited male participants and did not provide results by the baseline selenium status, unlike the NPC findings (NCP). In subgroup analyses within the NPC trial, there was a significant increased risk of type 2 diabetes only among participants with high baseline plasma selenium (hazard ratio of 2.70 in the highest tertile of plasma selenium, that is > 121.6 ng/ml).
However, results of this review also highlight major gaps in the published literature. There is still a lack of definitive evidence on the effects of selenium only supplementation on CVD clinical events, lipid levels and type 2 diabetes, and for the primary prevention of CVD. More trial evidence is especially needed to clarify potential benefits of selenium supplementation on blood lipids, as shown by the PRECISE trial findings (UK PRECISE), as well as potential adverse effects of selenium supplementation on development of type 2 diabetes, as suggested by secondary analyses of the NPC trial (NCP).
Overall completeness and applicability of evidence
Twelve trials (seven of at least three months duration and contributing to the meta‐analyses) were included, with 19,715 participants randomised. However, only the two largest trials (NCP; SELECT) contributed data to our primary outcomes, that is major CVD clinical end‐points. Also, the vast majority of participants that were included were from these two trials, whereas the other included studies comprised a much smaller number of participants and only examined the effect of selenium supplementation on CVD risk factors or its potential adverse effects on fasting glucose levels.
Six trials recruited only male participants, including SELECT, which provided the vast majority of data to this review. Therefore, the applicability of these findings to the female population is uncertain given the suggested gender differences in the response of selenoprotein biomarkers to selenium supplementation (Méplan 2007; Rayman 2012) as well as the well‐established differences in cardio‐metabolic risk factor profiles between women and men (Mosca 2011).
The large majority of individuals randomised (18,954 out of 19,715, over 96% of total participants) came from studies based in the US, a selenium‐replete population. Hence, the applicability and relevance of these findings to the effect of selenium supplements in populations with lower dietary selenium intakes and suboptimal or deficient selenoprotein status are uncertain. Indeed, current recommendations for dietary selenium intake (55 to 75 µg/day) are based on optimising the activity of selenoproteins, namely glutathione peroxidises (GPx) (Rayman 2012), which require a plasma selenium concentration around 90 μg/L (Burk 2006; Duffield 1999; Xia 2005). However, the selenium dietary intake and selenium concentration required for optimal selenoprotein P activity are likely to be higher (Hurst 2010; Xia 2010). The vast majority of the US population (99%) have blood selenium concentration well above 95 μg/L based on data from the National Health and Nutrition Examination Survey (NHANES), 2003 to 2004 (Laclaustra 2009b; Laclaustra 2010). It is therefore plausible that the average American participant randomised in these selenium supplementation trials would have had replete selenoprotein status, at least as far as GPx activities. Health benefits of additional selenium intake in the US population are questionable and adverse effects, such as an increase in diabetes risk, are possible (Stranges 2010a). In fact, accumulating evidence seems to point out that the association between selenium and cardio‐metabolic health is likely to be U‐shaped, with potential harms occurring at levels of selenium status both below and above the physiological range for optimal activity of selenoproteins (Rayman 2012; Stranges 2010a).
Furthermore, the duration of selenium supplementation and follow‐up periods varied largely across studies, from the very short intervention of one and two weeks (Luoma 1984; Ravn‐Haren 2008) to long term supplementation and follow‐ups for the largest trials, SELECT (Klein 2011; Lippman 2009) and NCP (Stranges 2006; Stranges 2007). Both short and long term health effects of selenium supplements are plausible given the relatively quick response of selenium biomarkers and selenoproteins to selenium supplementation within the time range of 12 to 20 weeks (Burk 2006; Duffield 1999; Hurst 2010; Xia 2005; Xia 2010) as well as the observed beneficial effect of a relatively short term (six months) selenium supplementation on blood lipid profiles (UK PRECISE). However, it is likely that any beneficial or detrimental effect of selenium supplements in terms of major chronic disease end‐points, such as mortality, CVD and type 2 diabetes, would represent the outcome of a long term process linked to a sustained effect by the interplay of selenium status with genetic and environmental factors (Rayman 2012; Stranges 2010a). Therefore, the public health relevance of selenium supplementation trials with extremely short term interventions or follow‐up periods is highly questionable in this context. However, this review was largely dominated by two trials, SELECT and NPC, which also had the longest periods of intervention and follow‐up (NCP; SELECT), hence their results can be considered reliable in terms of potential health impact of selenium supplementation.
In the included trials, the dose of selenium supplementation used varied from 36 to 800 μg/day, the baseline selenium status varied by country, being lowest in China and highest in the US, and the chemical forms of selenium supplements used varied. The largest trials used either selenomethionine (SELECT) or selenium yeast (NPC; PRECISE), which are considered to be the most effective forms of selenium supplementation. Indeed, selenomethionine, the organic form of selenium, has been shown to have nearly twice the bioavailability of selenium as compared to inorganic selenium compounds (Burk 2006; Xia 2005). Again, the null results from both SELECT and NPC with regard to CVD endpoints come from selenium‐replete populations, in which most selenoproteins would already have been optimised at baseline. Therefore their participants would have been unlikely to experience any additional increase in selenoprotein activities as a result of selenium supplementation (Rayman 2012; Stranges 2010a).
Finally, studies were also variable in the nature of participants recruited. The NPC trial was conducted among participants with a confirmed history of non‐melanoma skin cancer (NCP); the Watchful Waiting Study was based on prostate cancer patients (Algotar 2010); and one trial recruited participants at high risk of cancer (Yu 1990). Other trials were conducted in healthy populations with a focus on cancer prevention, or were pilot studies to assess the feasibility of larger trials (SELECT; UK PRECISE; Wu 2009). In all these included trials, CVD clinical outcomes or risk factors were secondary end‐points. Six small trials focused on vascular function and oxidative defence (Hawkes 2008; Luoma 1984; Meltzer 1994; Meltzer 1997; Navas‐Carretero 2011; Ravn‐Haren 2008) with five of these being very short term (Luoma 1984; Meltzer 1994; Meltzer 1997; Navas‐Carretero 2011; Ravn‐Haren 2008). Therefore, the overall applicability and relevance of these findings to the effectiveness of selenium supplements for CVD primary prevention in the general population or in high risk individuals for CVD is questionable, given that no trial has been specifically designed to examine the effect of selenium supplements on major CVD outcomes as the primary end‐points.
Quality of the evidence
The two largest trials contributing to this review, SELECT and NPC, were primarily cancer prevention trials conducted in the US, a selenium‐replete population; CVD clinical outcomes or potential adverse effects such as type 2 diabetes were secondary end‐points (NCP; SELECT). Furthermore, the largest trial examining the effect of selenium supplementation on blood lipids was conducted in a group of relatively healthy elderly people, aged 60 to 74 years, recruited from four general practices in different parts of the UK; blood lipids were measured in frozen non‐fasting blood samples as secondary non‐prespecified outcomes (UK PRECISE). The other studies included were small trials which only marginally contributed to this review. Obviously, there are concerns about the robustness of results from secondary end‐points or subgroup analyses of clinical trials, especially with regard to the potential usefulness of these results to inform public health guidelines or clinical recommendations (Brookes 2004; Freemantle 2001). Therefore, caution is needed in the interpretation of findings from post hoc analyses of clinical trials and secondary end‐points, such as CVD outcomes and type 2 diabetes in SELECT and NPC or blood lipids in the UK‐PRECISE trial, given the intrinsic limitations and potential biases of such an approach.
Specifically, in both NPC and SELECT diagnosis of type 2 diabetes was based on self‐report or use of diabetes medication rather than on biomarker data. This may have led to some misclassification (under‐diagnosis) of diabetes at baseline or during the trials. However, given the randomised design and blinding, differential misclassification according to treatment assignment is unlikely. It should be noted that the effect of non‐differential misclassification would probably be to underestimate the true relative risk and decrease the statistical power of these studies (Copeland 1977).
Likewise, CVD incidence and mortality were not primary end‐points in either the SELECT or the NPC trial. Therefore, findings must be cautiously interpreted as they result from secondary analyses. However, in both studies the ascertainment of the CVD end‐points did not change throughout the entire blinded phase of the trial. In addition, the selected CVD end‐points are all hard clinical outcomes and should be less subject to diagnostic misclassification. With regard to the results from the PRECISE trial (UK PRECISE), blood lipids were measured in frozen plasma samples that were collected in the non‐fasting state so that only total and HDL cholesterol concentrations could be measured, while triglyceride levels were not.
Furthermore, although CVD incidence and mortality risk estimates as well as other selected outcomes in these trials were adjusted for a wide range of potential confounders, there was a lack of information on some important variables at baseline, such as family history of diabetes or other CVD risk factors, in some of these trials (NPC, SELECT). However, randomisation should have minimized the impact of potential confounding by unmeasured risk factors.
A further limitation of the evidence to date concerns the generalisability of results from the major trials contributing to this review (NPC; PRECISE; SELECT) to the general public and specific population subgroups, because of the selective nature of the participants randomised in these trials. Specifically, SELECT recruited only male participants from a selenium‐replete population. Therefore, the applicability of SELECT findings to the female gender or to populations with lower dietary selenium intakes and suboptimal or deficient selenoprotein status is uncertain. The NPC trial sample consisted of elderly individuals (mean age: 63.2 years) from the eastern USA who had a history of non‐melanoma skin cancer. The generalisability of the NPC findings to other population subgroups may therefore be limited. Finally, the PRECISE trial sample was based on a group of relatively healthy elderly, mostly white volunteers, aged 60 to 74 years, recruited from four general practices in different parts of the UK. Again, the generalisability of PRECISE findings to younger adults or to other population subgroups is uncertain. Altogether, the relatively selective nature of participants in these trials makes the generalisability of the evidence to date questionable.
Potential biases in the review process
We decided to restrict our review to clinical trials in which the intervention was selenium only supplementation, as a single ingredient. Multivitamin and mineral preparations including selenium were excluded as it would have been impossible to disentangle selenium‐specific effects from those derived from other micronutrients. While this restriction avoids the potential impact of confounding by other ingredients in multivitamin and mineral supplements, it does not allow us to examine the potential interaction of selenium with other micronutrients, which is plausible both statistically and biologically (SELECT).
It should be noted, however, that other RCTs which have examined the effect of selenium in combination with other vitamins or minerals on CVD end‐points have also yielded inconclusive findings (Brown 2001; Hercberg 2004; Korpela 1989; Kuklinski 1994; You 2001).
Obviously, the restriction to trials using selenium only supplementation prevented the inclusion of important studies, primarily the large SU.VI.MAX (SUpplementation en VItamines et Minéraux AntioXydants) trial in France, which would have boosted the statistical power of our pooled analyses for the selected outcomes.
Agreements and disagreements with other studies or reviews
A number of observational studies have examined the association between selenium status and risk of CVD across different populations. The overall observational evidence is suggestive of a possible U‐shaped association between selenium and CVD risk (Bleys 2008; Bleys 2009; Salonen 1982; Virtamo 1985; Wei 2004), but with a large degree of inconsistency across studies. This is likely to be explained by the large variability in dietary selenium intakes and selenium status in different regions and populations around the world (Rayman 2012; Stranges 2010a). Moreover, a previous meta‐analysis of 14 cohort studies found a modest inverse association between biomarkers of selenium status, such as blood or toenail selenium concentrations, and the risk for coronary heart disease (Flores‐Mateo 2006). However, results from this early meta‐analysis of the few randomised trials examining the effect of selenium supplementation on cardiovascular outcomes were inconclusive. Likewise, other randomised trials which have examined the effect of selenium supplements in combination with other vitamins or minerals on CVD end‐points have been inconclusive (Hercberg 2004; You 2001). Results from the present review, which incorporates recent findings from the large SELECT trial, are in agreement with previous evidence and do not support a role for selenium supplementation in the primary prevention of CVD at the present time, especially among those individuals with adequate‐to‐high selenium status.
With regard to the effect of selenium supplementation on CVD risk factors, in addition to the studies included in this review, few other trials have examined the effect of selenium supplementation in combination with other micronutrients on blood lipids. For example, in the SU.VI.MAX trial, long term daily supplementation with a combination of antioxidants including selenium (100 µg/day as high‐selenium yeast) increased serum triglyceride levels compared to supplementation with placebo (Hercberg 2005). Likewise, in a randomised trial in a rural Chinese population with a low dietary intake of selenium, long term combined supplementation with selenium (37.5 μg), vitamin C and vitamin E resulted in small but significant increases in total and LDL cholesterol levels, although HDL concentrations were not affected (Zhang 2006). In partial disagreement with this previous evidence, results from the PRECISE trial suggest a potential beneficial effect of selenium supplementation on blood lipids, with a significant reduction in non‐HDL cholesterol concentrations. However, the clinical significance and potential implications of these findings for CVD prevention are unclear given the overall lack of effect of selenium supplementation on major CVD end‐points, as also shown by this review. Data from randomised studies on the effect of selenium only supplementation on other CVD risk factors, such as blood pressure, are lacking.
Evidence from observational studies and RCTs on selenium and diabetes is conflicting. Early findings from the NPC trial and observational cross‐sectional studies (NHANES) from the selenium‐replete US population were suggestive of a potential increased risk of type 2 diabetes with high selenium status or selenium supplementation (Bleys 2007; Laclaustra 2009b; NCP). However, results from the SELECT trial did not show a significant increased risk of type 2 diabetes with single selenium supplements, with a tendency to null findings with increasing duration of follow‐up (SELECT). Recently, a pooled longitudinal analysis from two US cohorts showed inverse associations between toenail selenium levels and the incidence of type 2 diabetes, with a reducing diabetes risk across quintiles of toenail selenium (Park 2012).
In other populations with dietary selenium intakes or selenium status lower than in the USA, such as in Europe, the evidence linking selenium to diabetes risk is also inconsistent (Rayman 2012; Stranges 2010a). For example, in the French SU.VI.MAX trial after 7.5 years of follow‐up, no effect on fasting plasma glucose was observed for a combined supplementation with anti‐oxidant micronutrients including selenium (100 µg/day as high‐selenium yeast) despite a positive association between glucose and selenium concentrations at baseline in the whole population (Czernichow 2006). Overall, these discrepant results remain unexplained although there is convincing biological evidence on the potential role of selenoproteins, such as glutathione peroxidises (GPx1) and selenoprotein P (SEPP1), in glucose metabolism and insulin resistance (Rayman 2012; Stranges 2010a). Both low and high levels of expression of these selenoproteins have been shown to promote development of type 2 diabetes in animal models (Labunskyy 2011), which might partly explain the apparent U‐shaped association between selenium status and diabetes risk (Rayman 2012; Stranges 2010a). In line with recent evidence, namely from the SELECT trial, pooled analyses from the present review did not show a significant increased risk of type 2 diabetes with single selenium supplements.
Authors' conclusions
Implications for practice.
Selenium is commonly added to several multivitamin and mineral preparations and enriched foods that are widely used by the general public in many Western countries because it is recognised as an essential trace element for maintaining optimal health status. Its use is also a result of aggressive marketing. Results from the present review, based on a small number of available clinical trials, do not support a role for selenium supplementation in the primary prevention of CVD at the present time, especially in those individuals and populations with adequate‐to‐high selenium status. Current trial evidence mostly comes from US‐based studies, where the average selenium status and dietary selenium intakes are above the levels recommended for optimal activities of selenoproteins (that is a selenium concentration around 90 μg/L and dietary selenium intake of 55 to 75 µg/day, respectively) (Rayman 2012). Health benefits of additional selenium intake from supplementation are therefore unlikely in such populations. In line with this notion, recent findings from the SELECT trial did not support a role of selenium supplementation in cancer prevention among healthy male individuals from the US and Canada, two selenium‐replete populations, with a median baseline serum selenium concentration of 136 μg/L (SELECT).
Unfortunately, there is very limited trial evidence on the effect of selenium only supplementation on CVD outcomes in populations with lower selenium status and selenium dietary intakes than in the US. The few non‐US trials are based on small samples or short duration of intervention and follow‐up; not one of these trials has examined the effect of selenium supplements on CVD clinical end‐points. Only the UK‐PRECISE trial, with a fairly sizable sample, examined the effect of a six month supplementation with 100, 200 or 300 µg selenium/day as high‐selenium yeast, compared to placebo, among 501 elderly volunteers with a mean plasma selenium concentration at baseline of 88.8 ng/ml (equivalent to 91.2 μg/L) (UK PRECISE). In this trial, supplementation at 100 and 200 µg selenium/day lowered total serum cholesterol and non‐HDL cholesterol; the 300 µg/day dose had no significant effect on total or non‐HDL cholesterol but raised HDL cholesterol significantly. In addition, the total–HDL cholesterol ratio decreased progressively with increasing selenium dose (UK PRECISE). Pooled analyses from the present review showed a statistically significant reduction in non‐HDL cholesterol with selenium supplementation. While the potential implications of these findings for cardiovascular disease prevention are unclear, potential benefits of selenium supplementation are plausible only in populations with suboptimal or insufficient selenoprotein status. Moreover, these findings corroborate the notion that the association between selenium and cardio‐metabolic outcomes is likely to be U‐shaped, with potential harms occurring at selenium levels both below and above the range for optimal activity of selenoproteins (Rayman 2012; Stranges 2010a).
Results from the present review did not show a statistically significant increased risk of type 2 diabetes with single selenium supplements but, given the limited evidence to date, an increased risk of diabetes with selenium supplementation cannot be ruled out. The limited trial evidence to date synthesised in this review does not support the use of selenium supplements in the primary prevention of cardiovascular disease. In particular, the indiscriminate and widespread use of selenium supplements in individuals and populations with adequate‐to‐high selenium status is not justified and should not be encouraged.
Obviously, there are specific population subgroups affected by conditions predisposing to selenium deficiency that might therefore benefit from selenium supplementation. For example, the potential effectiveness of selenium supplementation among individuals in areas with endemic low selenium in the soil, or among certain genetic subgroups with poor anti‐oxidative capacity, or in patients with chronic conditions (for example HIV, chronic kidney disease) warrants further investigation (Fairweather 2011).
Implications for research.
There is a lack of trial evidence on potential effects of selenium only supplementation specifically designed to mitigate cardiovascular disease (CVD) outcomes across a wider range of selenium concentration, especially in populations with suboptimal or insufficient selenoprotein status. Randomised trials in participants across a wider range of selenium status would help determine the optimal levels of selenium intake in the general population, to maximize health benefits whilst avoiding potential chronic toxic effects. Also, optimal intake for any individual is likely to depend on polymorphisms in selenoprotein genes that may also affect the risk of disease, including coronary heart disease and ischaemic stroke (Alanne 2007; Rayman 2012). Future work in the field examining the effect of selenium supplements on CVD risk should also give attention to the potential interaction between genetic make‐up and selenium intake or status. Further trial evidence with a larger representation of women is also desirable given the well‐known differences in cardio‐metabolic risk factor profiles between women and men (Mosca 2011) and the suggested gender differences in the response of selenoprotein biomarkers to selenium supplementation (Méplan 2007). Finally, additional evidence is needed to clarify the link between selenium status and supplementation with metabolic effects on blood lipids and diabetes risk, and in more detail across different ranges of selenium concentration and dietary selenium intakes, as well as potential underlying mechanisms.
Acknowledgements
We are grateful for Nicole Martin and Jo Abbot for conducting the searches for this review. We would also like to acknowledge Dr Hawkes for providing additional data on lipid levels from his trial (Hawkes 2008).
Appendices
Appendix 1. Search strategies October 2012
CENTRAL, DARE, HTA, HEE
#1 MeSH descriptor: [Selenium] this term only #2 MeSH descriptor: [Selenium Compounds] explode all trees #3 selen* #4 selepen #5 80Se #6 SeO3 #7 SeO4 #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 #9 MeSH descriptor: [Cardiovascular Diseases] explode all trees #10 cardio* #11 cardia* #12 heart* #13 coronary* #14 angina* #15 ventric* #16 myocard* #17 pericard* #18 isch?em* #19 emboli* #20 arrhythmi* #21 thrombo* #22 atrial next fibrillat* #23 tachycardi* #24 endocardi* #25 (sick near/2 sinus) #26 MeSH descriptor: [Stroke] explode all trees #27 (stroke or stokes) #28 cerebrovasc* #29 cerebral next vascular #30 apoplexy #31 (brain near/2 accident*) #32 ((brain* or cerebral or lacunar) near/2 infarct*) #33 MeSH descriptor: [Hypertension] explode all trees #34 hypertensi* #35 peripheral next arter* next disease* #36 ((high or increased or elevated) near/2 blood near/2 pressure) #37 MeSH descriptor: [Hyperlipidemias] explode all trees #38 hyperlipid* #39 hyperlip?emia* #40 hypercholesterol* #41 hypercholester?emia* #42 hyperlipoprotein?emia* #43 hypertriglycerid?emia* #44 MeSH descriptor: [Diabetes Mellitus] explode all trees #45 diabet* #46 MeSH descriptor: [Arteriosclerosis] explode all trees #47 MeSH descriptor: [Cholesterol] explode all trees #48 cholesterol #49 "coronary risk factor*" #50 MeSH descriptor: [Blood Pressure] this term only #51 "blood pressure" #52 #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 #53 #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 #54 #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 #55 #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 #56 #52 or #53 or #54 or #55 #57 #8 and #56
MEDLINE (Ovid)
1. Selenium/ 2. exp Selenium Compounds/ 3. selen*.tw. 4. selepen.tw. 5. 80Se.tw. 6. SeO3.tw. 7. SeO4.tw. 8. or/1‐7 9. exp Cardiovascular Diseases/ 10. cardio*.tw. 11. cardia*.tw. 12. heart*.tw. 13. coronary*.tw. 14. angina*.tw. 15. ventric*.tw. 16. myocard*.tw. 17. pericard*.tw. 18. isch?em*.tw. 19. emboli*.tw. 20. arrhythmi*.tw. 21. thrombo*.tw. 22. atrial fibrillat*.tw. 23. tachycardi*.tw. 24. endocardi*.tw. 25. (sick adj sinus).tw. 26. exp Stroke/ 27. (stroke or stokes).tw. 28. cerebrovasc*.tw. 29. cerebral vascular.tw. 30. apoplexy.tw. 31. (brain adj2 accident*).tw. 32. ((brain* or cerebral or lacunar) adj2 infarct*).tw. 33. exp Hypertension/ 34. hypertensi*.tw. 35. peripheral arter* disease*.tw. 36. ((high or increased or elevated) adj2 blood pressure).tw. 37. exp Hyperlipidemias/ 38. hyperlipid*.tw. 39. hyperlip?emia*.tw. 40. hypercholesterol*.tw. 41. hypercholester?emia*.tw. 42. hyperlipoprotein?emia*.tw. 43. hypertriglycerid?emia*.tw. 44. exp Diabetes Mellitus/ 45. diabet*.tw. 46. exp Arteriosclerosis/ 47. exp Cholesterol/ 48. cholesterol.tw. 49. "coronary risk factor*".tw. 50. Blood Pressure/ 51. blood pressure.tw. 52. or/9‐51 53. 8 and 52 54. randomized controlled trial.pt. 55. controlled clinical trial.pt. 56. randomized.ab. 57. placebo.ab. 58. drug therapy.fs. 59. randomly.ab. 60. trial.ab. 61. groups.ab. 62. 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 63. exp animals/ not humans.sh. 64. 62 not 63 65. 53 and 64
EMBASE Classic + EMBASE (Ovid)
1. selenium/ 2. selenium derivative/ 3. sodium selenite/ 4. selenious acid/ 5. selen*.tw. 6. selepen.tw. 7. 80Se.tw. 8. SeO3.tw. 9. SeO4.tw. 10. or/1‐9 11. exp cardiovascular disease/ 12. cardio*.tw. 13. cardia*.tw. 14. heart*.tw. 15. coronary*.tw. 16. angina*.tw. 17. ventric*.tw. 18. myocard*.tw. 19. pericard*.tw. 20. isch?em*.tw. 21. emboli*.tw. 22. thrombo*.tw. 23. arrhythmi*.tw. 24. atrial fibrillat*.tw. 25. tachycardi*.tw. 26. endocardi*.tw. 27. (sick adj sinus).tw. 28. exp cerebrovascular disease/ 29. (stroke or stokes).tw. 30. cerebrovasc*.tw. 31. cerebral vascular.tw. 32. apoplexy.tw. 33. (brain adj2 accident*).tw. 34. ((brain* or cerebral or lacunar) adj2 infarct*).tw. 35. exp hypertension/ 36. hypertensi*.tw. 37. peripheral arter* disease*.tw. 38. ((high or increased or elevated) adj2 blood pressure).tw. 39. exp hyperlipidemia/ 40. hyperlipid*.tw. 41. hyperlip?emia*.tw. 42. hypercholesterol*.tw. 43. hypercholester?emia*.tw. 44. hyperlipoprotein?emia*.tw. 45. hypertriglycerid?emia*.tw. 46. exp diabetes mellitus/ 47. diabet*.tw. 48. exp Arteriosclerosis/ 49. exp Cholesterol/ 50. cholesterol.tw. 51. "coronary risk factor*".tw. 52. Blood Pressure/ 53. blood pressure.tw. 54. or/11‐53 55. 10 and 54 56. random$.tw. 57. factorial$.tw. 58. crossover$.tw. 59. cross over$.tw. 60. cross‐over$.tw. 61. placebo$.tw. 62. (doubl$ adj blind$).tw. 63. (singl$ adj blind$).tw. 64. assign$.tw. 65. allocat$.tw. 66. volunteer$.tw. 67. crossover procedure/ 68. double blind procedure/ 69. randomized controlled trial/ 70. single blind procedure/ 71. 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 72. (animal/ or nonhuman/) not human/ 73. 71 not 72 74. 55 and 73
CINAHL
S30 S4 and S29 S29 S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 S28 TI "Blood Pressure" OR AB "Blood Pressure" S27 (MH "Blood Pressure+") S26 TI "coronary risk factor*" OR AB "coronary risk factor*" S25 TI cholesterol OR AB cholesterol S24 (MH "Cholesterol+") S23 (MH "Arteriosclerosis+") S22 TI diabet* OR AB diabet* S21 (MH "Diabetes Mellitus+") S20 AB (hyperlipid* OR hyperlip?emia* OR hypercholesterol* OR hypercholester?emia* OR hyperlipoprotein?emia* OR hypertriglycerid?emia*) S19 TI (hyperlipid* OR hyperlip?emia* OR hypercholesterol* OR hypercholester?emia* OR hyperlipoprotein?emia* OR hypertriglycerid?emia*) S18 (MH "Hyperlipidemia+") S17 TI "high blood pressure" OR AB "high blood pressure" S16 AB (hypertensi* OR "peripheral arter* disease*") S15 TI (hypertensi * OR "peripheral arter* disease*") S14 (MH "Hypertension+") S13 TI (stroke OR stokes OR cerebrovasc* OR cerebral N2 vascular OR apoplexy OR brain N2 accident* OR brain N2 infarct*) S12 (MH "Stroke") S11 AB ("atrial fibrillat*" OR tachycardi* OR endocardi* OR sick N2 sinus) S10 TI ("atrial fibrillat*" OR tachycardi* OR endocardi* OR sick N2 sinus) S9 AB (pericard* OR isch?em* OR emboli* OR arrhythmi* OR thrombo*) S8 TI (pericard* OR isch?em* OR emboli* OR arrhythmi* OR thrombo*) S7 AB (cardio* OR cardia* OR heart* OR coronary* OR angina* OR ventric* OR myocard*) S6 TI (cardio* OR cardia* OR heart* OR coronary* OR angina* OR ventric* OR myocard*) S5 (MH "Cardiovascular Diseases+") S4 S1 OR S2 OR S3 S3 TI (selen* OR selepen OR 80Se or SeO3 or SeO4) OR AB (selen* OR selepen OR 80Se OR SeO3 OR SeO4) S2 (MH "Selenium Compounds") S1 (MH "Selenium")
Web of Science
#17 #16 AND #15 #16 TS=(random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*) #15 #14 AND #4 #14 #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 #13 TS=(arteriosclerosis or cholesterol or "coronary risk factor*" or "blood pressure") #12 TS=diabet* #11 TS=(hyperlipid* OR hyperlip?emia* OR hypercholesterol* OR hypercholester?emia* OR hyperlipoprotein?emia* OR hypertriglycerid?emia*) #10 TS=("high blood pressure") #9 TS=(hypertensi* OR "peripheral arter* disease*") #8 TS=(stroke OR stokes OR cerebrovasc* OR cerebral OR apoplexy OR (brain SAME accident*) OR (brain SAME infarct*)) #7 TS=("atrial fibrillat*" OR tachycardi* OR endocardi*) #6 TS=(pericard* OR isch?em* OR emboli* OR arrhythmi* OR thrombo*) #5 TS=(cardio* OR cardia* OR heart* OR coronary* OR angina* OR ventric* OR myocard*) #4 #3 OR #2 OR #1 #3 TS=(80Se OR SEO3 OR SEO4) #2 TS=selepen #1 TS=selen*
PsycINFO
1. selen*.tw. 2. selepen.tw. 3. 80Se.tw. 4. SeO3.tw. 5. SeO4.tw. 6. or/1‐5 7. exp Cardiovascular Disorders/ 8. cardio*.tw. 9. cardia*.tw. 10. heart*.tw. 11. coronary*.tw. 12. angina*.tw. 13. ventric*.tw. 14. myocard*.tw. 15. pericard*.tw. 16. isch?em*.tw. 17. emboli*.tw. 18. arrhythmi*.tw. 19. thrombo*.tw. 20. atrial fibrillat*.tw. 21. tachycardi*.tw. 22. endocardi*.tw. 23. (sick adj sinus).tw. 24. exp Stroke/ 25. (stroke or stokes).tw. 26. cerebrovasc*.tw. 27. cerebral vascular.tw. 28. apoplexy.tw. 29. (brain adj2 accident*).tw. 30. ((brain* or cerebral or lacunar) adj2 infarct*).tw. 31. exp Hypertension/ 32. hypertensi*.tw. 33. peripheral arter* disease*.tw. 34. ((high or increased or elevated) adj2 blood pressure).tw. 35. hyperlipid*.tw. 36. hyperlip?emia*.tw. 37. hypercholesterol*.tw. 38. hypercholester?emia*.tw. 39. hyperlipoprotein?emia*.tw. 40. hypertriglycerid?emia*.tw. 41. exp Diabetes Mellitus/ 42. diabet*.tw. 43. exp Arteriosclerosis/ 44. exp Cholesterol/ 45. cholesterol.tw. 46. "coronary risk factor*".tw. 47. exp blood pressure/ 48. blood pressure.tw. 49. or/7‐48 50. 6 and 49 51. random$.tw. 52. factorial$.tw. 53. crossover$.tw. 54. cross‐over$.tw. 55. placebo$.tw. 56. (doubl$ adj blind$).tw. 57. (singl$ adj blind$).tw. 58. assign$.tw. 59. allocat$.tw. 60. volunteer$.tw. 61. control*.tw. 62. "2000".md. 63. or/51‐62 64. 50 and 63
Data and analyses
Comparison 1. Selenium supplementation versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Cholesterol (mmol/l), change from baseline | 6 | 576 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.30, 0.07] |
| 2 HDL Cholesterol (mmol/l), change from baseline | 6 | 576 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.06, 0.08] |
| 3 Non‐HDL Cholesterol (mmol/l), change from baseline | 3 | 472 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.41, 0.00] |
| 4 LDL Cholesterol (mmol/l), change from baseline | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.54, 0.18] |
| 5 Triglycerides (mmol/l), change from baseline | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.28, 0.36] |
| 6 All cause mortality | 2 | 18452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.88, 1.08] |
| 7 CVD mortality | 2 | 18452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.79, 1.20] |
| 8 All CVD events (fatal and non fatal) | 2 | 18452 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.95, 1.11] |
| 9 Non fatal CVD events | 2 | 18452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.89, 1.04] |
| 10 Non fatal strokes | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 All non fatal strokes | 1 | 17453 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.58, 1.07] |
| 10.2 Hemorrhagic strokes | 1 | 17453 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.43, 2.29] |
| 10.3 Ischemic strokes | 1 | 17453 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.62, 1.32] |
| 11 Type 2 diabetes | 4 | 18790 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.97, 1.15] |
1.1. Analysis.

Comparison 1 Selenium supplementation versus no intervention, Outcome 1 Total Cholesterol (mmol/l), change from baseline.
1.2. Analysis.
Comparison 1 Selenium supplementation versus no intervention, Outcome 2 HDL Cholesterol (mmol/l), change from baseline.
1.3. Analysis.

Comparison 1 Selenium supplementation versus no intervention, Outcome 3 Non‐HDL Cholesterol (mmol/l), change from baseline.
1.4. Analysis.

Comparison 1 Selenium supplementation versus no intervention, Outcome 4 LDL Cholesterol (mmol/l), change from baseline.
1.5. Analysis.

Comparison 1 Selenium supplementation versus no intervention, Outcome 5 Triglycerides (mmol/l), change from baseline.
1.6. Analysis.

Comparison 1 Selenium supplementation versus no intervention, Outcome 6 All cause mortality.
1.7. Analysis.

Comparison 1 Selenium supplementation versus no intervention, Outcome 7 CVD mortality.
1.8. Analysis.

Comparison 1 Selenium supplementation versus no intervention, Outcome 8 All CVD events (fatal and non fatal).
1.9. Analysis.

Comparison 1 Selenium supplementation versus no intervention, Outcome 9 Non fatal CVD events.
1.10. Analysis.

Comparison 1 Selenium supplementation versus no intervention, Outcome 10 Non fatal strokes.
1.11. Analysis.

Comparison 1 Selenium supplementation versus no intervention, Outcome 11 Type 2 diabetes.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Algotar 200μg 2010.
| Methods | Details as Algotar 2010 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Algotar 2010.
| Methods | RCT of parallel group design | |
| Participants | 140 men from the US with biopsy proven non‐metastatic prostate cancer who had elected to be followed by active surveillance (watchful waiting) for their disease, and so were not on chemotherapy. Men were randomised to 200μg selenium daily, 800μg selenium daily or placebo. Mean age 72.8 years, mean BMI 26.9. Baseline selenium status 134.5 (41.5) µg/L. | |
| Interventions | Daily selenium supplementation of 200μg (n = 47) or 800 μg (n = 47) high selenium yeast or placebo (n = 46) for up to 5 years (progression of cancer or therapy for cancer were study endpoints). Participants were followed up every 3 months. | |
| Outcomes | Type 2 diabetes (adverse event) | |
| Notes | Secondary analysis of a pre‐existing RCT. The focus of trial was to examine the effect of selenium on prostate cancer progression. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details regarding losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Study protocol clearly prespecifies primary and secondary outcomes. Type 2 diabetes is an adverse effect |
| Other bias | Unclear risk | Insufficient information to judge |
Algotar 800μg 2010.
| Methods | Details as Algotar 2010 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Hawkes 2008.
| Methods | RCT of parallel group design | |
| Participants | Healthy men from the US aged 18 ‐ 45 years free of self‐reported hypertension, diabetes, cancer and were not taking in excess of 50 μg of selenium as a supplement daily. 54 men randomised to 300 μg selenium daily or placebo. Mean age 31 years. Baseline selenium status 142 (19) µg/L intervention group, 146 (19) µg/L placebo group. | |
| Interventions | Daily selenium supplementation of 300 μg of high selenium yeast for 48 weeks. Placebos were identical yeast tablets without selenium. | |
| Outcomes | Serum triacylglycerol. Authors provided additional data on total, LDL and HDL cholesterol. | |
| Notes | Focus of study was on endothelial function | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin flip |
| Allocation concealment (selection bias) | High risk | Randomised in pairs, one from each pair assigned by coin flip |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some losses to follow‐up (12/54), unclear from which group |
| Selective reporting (reporting bias) | Unclear risk | Not all outcomes are presented in the results |
| Other bias | Unclear risk | Insufficient information to judge |
Luoma 1984.
| Methods | RCT of parallel group design | |
| Participants | 27 healthy medical students from Finland (9 males, 18 females), mean age 24 years. Baseline selenium status 73.7 (14) µg/L in the intervention group, 75.1 (15.4) µg/L in the control group. | |
| Interventions | Daily selenium supplementation of 96 µg selenium yeast tablets for 2 weeks. Control group received yeast tablets without selenium. Follow‐up at the end of the intervention period of 2 weeks. | |
| Outcomes | Total cholesterol, HDL cholesterol, triglycerides | |
| Notes | Focus of the trial was to examine the relationship between serum selenium levels and glutathione peroxidase activity and lipids connected with atherogenesis. Very short term trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 out of 12 subjects (8%) in the intervention group did not complete the trial |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Insufficient information to judge |
Meltzer 1994.
| Methods | RCT of parallel group design | |
| Participants | Healthy students or employees at the Institute of Nutrition Research, University of Oslo. Participants were not taking medication, were not pregnant or lactating, and had not taken mineral containing supplements within 3 months of the study. 32 participants (2 male) were randomised to 3 arms. Age ranged from 21‐56 years. | |
| Interventions | The intervention group (n=11) ate selenium enriched bread (70µg selenium per 90g of bread ‐ 3 slices). Mean selenium intake was 135 (25)µg/day. The control group (n=10) ate their normal diet (mean selenium intake 77(25) µg/day). The intervention period was 6 weeks. | |
| Outcomes | LDL and HDL cholesterol. | |
| Notes | 3 arm trial, fish (containing selenium, arsenic and mercury), selenium enriched bread and a control arm. We have just used the selenium enriched bread as the intervention group. Short term study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Behavioural intervention (different diets) so difficult to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No losses to follow‐up reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Insufficient information to judge |
Meltzer 1997.
| Methods | RCT of parallel group design | |
| Participants | Healthy female students from the Institute of Nutrition Research, University of Oslo. Participants were not taking medication, were not pregnant or lactating, and had not taken vitamin, mineral or fatty acid containing supplements within 3 months of the study. 32 participants were randomised to 3 arms. Mean age 23.5 (3.4) years. | |
| Interventions | The intervention group (n=7) ate selenium enriched bread (115µg selenium per 90g of bread ‐ 3 slices) and placebo oil capsules. Mean selenium intake was 185 (27) µg/day. The control group (n=8) ate their normal diet with placebo oli capsules and placebo bread (mean selenium intake 77(25) µg/day). The intervention period was 6 weeks. | |
| Outcomes | Total, LDL and HDL cholesterol and triglycerides. | |
| Notes | Two intervention arms ‐ PUFA capsules and selenium enriched bread. We have just used the selenium enriched bread group. Short term study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One participant withdrew (unclear from which group) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Insufficient information to judge |
Navas‐Carretero 2011.
| Methods | RCT of parallel group design | |
| Participants | Healthy participants, men and women aged 20‐45 years with a BMI between 18.5 and 30. Participants were not taking any medication and were not following any dietary treatment, and had maintained their weight for the last 3 months (±‐3 kg). Exclusion criteria included metabolic diseases such as diabetes, thyroid impairments and other endocrine disturbances, hypertension, gastric and peptic ulcers, constipation or diarrhoea. 24 participants randomised. | |
| Interventions | The intervention group received an isocalorific moderately high protein diet (30% of energy) including consumption of 200g chicken breasts enriched with selenium four times a week (25.5µg/100g). The control group ate the same diet but the chicken breasts were not enriched with selenium (6.5µg/100g). The intervention period lasted for 10 weeks. | |
| Outcomes | Lipid levels, blood pressure. | |
| Notes | The focus of the study was on high protein diets for weight loss and antioxidant support from selenium. Short term study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 31% drop out in the intervention group, 19% drop out in the control group. Reasons for drop‐out not given |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Insufficient information to judge |
NCP.
| Methods | Stranges 2006 and Stranges 2007 ‐ Secondary analysis of the blinded phase (1983‐96) of the Nutritional Prevention of Cancer (NCP) trial among participants free of CVD or type 2 diabetes at baseline respectively. The NCP was a double blind parallel group RCT. | |
| Participants | Inclusion criteria for the NCP trial: participants recruited from 7 dermatology clinics in low selenium areas in Eastern United States. Subjects were eligible if they had confirmed histories of non‐melanoma skin cancers within the year prior to randomisation, an estimated 5 year life expectancy and had no reported internal cancer within the previous 5 years. Exclusion criteria were kidney and liver disorders. Stranges 2006 ‐ 1004 participants from 1316 had a valid baseline selenium value and were free of CVD at baseline (504 from the selenium group and 500 from the placebo group). Stranges 2007 ‐ 1202 participants from 1316 had a valid baseline selenium value and were free of type 2 diabetes at baseline (600 from the selenium group and 602 from the placebo group). 71% of participants were males and baseline selenium status was 113 µg/L. |
|
| Interventions | Daily supplementation of 200 µg high selenium yeast or a yeast placebo. Mean follow‐up period was 7.6 years. | |
| Outcomes | Stranges 2006 ‐ all cardiovascular disease, all coronary heart disease, all cerebrovascular disease, cardiovascular disease mortality, total mortality. Stranges 2007 ‐ type 2 diabetes |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Unique sequential treatment number |
| Allocation concealment (selection bias) | Low risk | Allocation assignment made centrally using sealed identical pill bottles |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to vital follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Secondary analysis of a cancer prevention study, so not the original pre‐specified outcomes of the NCP trial |
| Other bias | Unclear risk | Secondary analysis of a cancer prevention study, so not the original pre‐specified outcomes of the NCP trial |
Ravn‐Haren 2008.
| Methods | RCT crossover design (analysed as parallel group 1 week for each intervention, 4 weeks follow‐up, 8 weeks washout between interventions) | |
| Participants | 20 healthy men aged 18‐40 years (mean 26.8) recruited by advertising at local Universities in the Copenhagen area. Smoking, obesity (BMI>30), family history of chronic disease, use of any medication, heavy physical activity (>10 hours per week), high intake of fish (>2 times per week), and use of vitamin and mineral supplements 2 weeks before the trial or selenium supplements 8 weeks before the trial were exclusion criteria. Baseline selenium status across the 3 intervention groups and placebo group ranged between 107 and 114 µg/L. | |
| Interventions | Subjects supplemented their usual diet with one of 4 test meals 8 weeks apart. On the first day of each intervention period the subjects were served a standardised breakfast (with the test meal) and a standardised lunch at the University. Subjects were not allowed to consume other foods during their stay except water. On the second day they were served the test meal with breakfast and were supplied with 5 L of frozen milk and 5 tablets for the last days of the intervention week. The 4 test meals consisted of 1 L of milk (selenium enriched or control milk) and a tablet (selenium enriched yeast, selenate or placebo). Selenium enriched milk (480 µg selenium/day) was given with a placebo tablet and control milk with selenium enriched yeast (300 µg selenium/day), selenate (300µg selenium/day) or placebo tablets. The follow‐up period for each intervention was 4 weeks. | |
| Outcomes | Total cholesterol, LDL and HDL cholesterol and triacylglyceride (TAG). | |
| Notes | Very short term study ‐ 1 week intervention period for each intervention, 4 weeks follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Just states given in random order |
| Allocation concealment (selection bias) | Unclear risk | Test meals were coded with different colours and code not broken until the results were analysed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up ‐ very short term trial |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Insufficient information to judge |
SELECT.
| Methods | Multicentre RCT of parallel group design ‐ The Selenium and Vitamin E Cancer Prevention Trial (SELECT). | |
| Participants | 35,533 men from 427 participating sites in the US, Canada and Puerto Rico randomised to 4 arms ‐ selenium, vitamin E, selenium and vitamin E, placebo. Eligibility criteria: men aged 55 years or more (50 plus for African Americans) with no prior prostate cancer and digital rectal examination not suspicious for cancer. No current use of anticoagulation therapy other than 175mg/day or less of aspirin or 81mg/day aspirin with clopidogrel bisulphate. No history of stroke and normal BP also required. 8910 men were randomised to receive selenium and 8856 men were randomised to receive placebo. Median age 62.6 years (range 58‐68), 79% white, 12% African American, 7% Hispanic, 2% other. Baseline selenium status 135 (range 123.4 to 145.9) µg/L in the intervention group, 137.6 (range 124.7 to 151.8) µg/L in the placebo group. | |
| Interventions | Daily selenium supplementation of 200μg L‐selenomethionine (1 selenium tablet, one placebo tablet) or placebo (2 placebo tablets). Mean follow‐up period 5.46 years (Lippman 2009) and 7‐12 years (Klein 2011). | |
| Outcomes | Lippman 2009 ‐ CVD mortality, CVD events (any including death), non‐fatal stroke Klein 2011 ‐ all cause mortality, non‐fatal CVD events, diabetes. |
|
| Notes | Data only used from the selenium only and placebo arms. Focus of the trial was to examine the effects of selenium and vitamin E in the prevention of prostate cancer and other diseases in relatively healthy men. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | States double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Insufficient information to judge |
UK PRECISE.
| Methods | RCT of parallel group design | |
| Participants | Healthy older people recruited from 4 general practices in the UK affiliated with the MRC General Practice Research Framework. Recruited similar numbers of men and women from each of 3 age groups 60‐64, 65‐69 and 70‐74 years. Exclusion criteria: Southwest Oncology Group performance score of >1 (i.e. incapable of carrying out light housework or office work), active liver or kidney disease, a previous diagnosis of cancer, diagnosed HIV infection, receipt of immunosuppressive therapy at recruitment, diminished mental capacity, or receipt of selenium supplements 50µg selenium/day, in the previous 6 months. 501 participants were randomised, mean age 67.4 years and mean baseline selenium of 88µg/L. | |
| Interventions | After a 4 week placebo run in phase, 501 participants were randomised in equal numbers to receive one of 4 treatment regimes: placebo or 100, 200 or 300μg per day for at least 6 months. The intervention agent was the high selenium yeast SelanoPrecise or an identical placebo yeast. Participants were followed up at 6 months. | |
| Outcomes | Lipid levels | |
| Notes | Pilot study to determine recruitment, retention and adherence (prespecified primary outcomes) to inform the PRECISE trial which was never funded. Non specified outcomes (lipid levels) were informed by the literature. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computer random number generator |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel blinded to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Similar numbers of withdrawals from treatment across different treatment arms, although there were more adverse events in the higher dose selenium group 300 µg selenium/day |
| Selective reporting (reporting bias) | Unclear risk | The primary outcomes were recruitment, retention and adherence to assess the viability of conducting the main PRECISE trial which was never funded, and secondary outcomes were mood and thyroid function. The non‐specified outcomes later examined were based on the literature (e.g. cholesterol) |
| Other bias | Unclear risk | Insufficient information to judge |
UK PRECISE 100μg.
| Methods | Details as UK PRECISE | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
UK PRECISE 200μg.
| Methods | Details as UK PRECISE | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
UK PRECISE 300μg.
| Methods | Details as for UK PRECISE | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Wu 2009.
| Methods | RCT of parallel group design | |
| Participants | Healthy older males aged 40‐70 yrs recruited in or near Adelaide Australia via adverts in local papers and electronic media in 2004. Exclusion criteria were cancer patients undergoing chemotherapy or radiotherapy, those with a sensitivity to the study foods (e.g. gluten/wheat intolerance), those unable to comply with the protocol and those not available for all follow‐up periods. Men currently supplementing with selenium or supplementing above recommended daily intakes for folate and/or vitamin B12 and/or vitamin C were also excluded. 179 men were eligible and were screened for plasma selenium levels, the 81 men with the lowest selenium levels were randomised into 3 dietary groups: CONTROL, BIOFORT and PROFORT, depending on the wheat source of the biscuits they were required to consume. 27 men randomised to each group, mean age 56 years, mean baseline selenium 122 µg/L. | |
| Interventions | Trial participants were required to consume 1 biscuit per day for the first 8 weeks, 2 biscuits per day for the second 8 weeks and 3 biscuits per day for the third 8 weeks. Each BIOFORT and PROFORT biscuit would provide approximately 75 µg/day so the daily amount of selenium from the biscuits would increase from 75 to 150 to 225µg/day in the two intervention arms. The control group received low selenium biscuits. The BIOFORT biscuits used bio fortified wheat and the PROFORT biscuits acted as a positive control and used process fortified wheat. Follow‐up was at the end of the intervention period of 24 weeks. | |
| Outcomes | Lipid levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data reasonably balanced across groups with similar reasons for drop‐out |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes clearly stated and reported |
| Other bias | Unclear risk | Insufficient information to judge |
Wu BIOFORT 2009.
| Methods | Details as Wu 2009 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Wu PROFORT 2009.
| Methods | Details as for Wu 2009 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Yu 1990.
| Methods | RCT of parallel group design | |
| Participants | Healthy male tin miners aged 40‐64 years with at least 10 years of underground experience. 40 men randomised, baseline selenium status not measured, but this was 50µg/L in the control group at follow‐up. | |
| Interventions | Selenium cakes made of selenium enriched malt providing 300µg/day and identical placebo cakes. Follow‐up after 1 year at the end of the trial. | |
| Outcomes | Final levels of total blood cholesterol (baseline values not recorded) | |
| Notes | Pilot study to evaluate the feasibility and effectiveness of conducting a double blind clinical trial for the prevention of lung cancer with selenium in Tin miners in China where the incidence of lung cancer is very high. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Insufficient information to judge |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alfthan 1991 | Not a RCT. |
| Asfour 2006 | Selenium supplementation was given in conjunction with chemotherapy and there were no relevant outcomes. |
| Karp 2010 | Selenium supplementation was given in conjunction with chemotherapy. |
| Kruger 1998 | The intervention was multivitamin and mineral supplementation including selenium rather than selenium only supplementation. |
| Linxian trials | The intervention was multivitamin and mineral supplementation including selenium rather than selenium only supplementation. |
| Mundal 1994 | The focus of the trial is fish consumption and its effects on triglyceride levels. The comparison group was split into 2 where followed their normal diet and half had selenium enriched bread to approximate the selenium levels in the fish (70 μg daily). We have contacted the authors twice to see if they have separate estimates for each of the control groups with no response. |
| Mutanen 1989 | No relevant outcomes. |
| Ravn‐Haren 2008b | No relevant outcomes. |
| SU.VI.MAX | The intervention was multivitamin and mineral supplementation including selenium rather than selenium only supplementation. |
| Wolters 2003 | The intervention was multivitamin and mineral supplementation including selenium rather than selenium only supplementation. |
Differences between protocol and review
It was our intention to examine effects of interventions by stratified analyses to explore the impact of different selenium doses, duration of the intervention, baseline selenium status and country, but there are currently insufficient available trials to do this. Similarly there are insufficient trials to date to perform sensitivity analyses and funnel plots to explore the effects of methodological quality and publication bias. In the protocol we stated that we would focus on studies of six months or more follow‐up as these are most relevant for public health interventions. We did not have a minimum period of follow‐up for inclusion. We have revised this to examine studies of three months or more follow‐up in meta‐analyses, where appropriate, and have dealt with shorter term studies descriptively.
Contributions of authors
All authors contributed to the protocol development. The trials search co‐ordinators of the Cochrane Heart Group ran the searches; KR, LH, NF or CD screened titles and abstracts, assessed studies for formal inclusion or exclusion and abstracted data. KR and LH assessed methodological quality. KR conducted the analysis, and wrote the first draft of the methods and results sections; SS wrote the first draft of the background and discussion sections. All authors contributed to later drafts.
Sources of support
Internal sources
Warwick Medical School, University of Warwick, UK.
External sources
NIHR Cochrane Programme Grant, UK.
Declarations of interest
None known
New
References
References to studies included in this review
Algotar 200μg 2010 {published data only}
- Algotar AM, Stratton MS, Stratton PS, Hsu CH, Ahmann FR. No effect of selenium supplementation on serum glucose levels in men with prostate cancer. American Journal of Medicine 2010;123(8):765‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Algotar 2010 {published data only}
- Algotar AM, Stratton MS, Stratton PS, Hsu CH, Ahmann FR. No effect of selenium supplementation on serum glucose levels in men with prostate cancer. American Journal of Medicine 2010;123(8):765‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Algotar 800μg 2010 {published data only}
- Algotar AM, Stratton MS, Stratton PS, Hsu CH, Ahmann FR. No effect of selenium supplementation on serum glucose levels in men with prostate cancer. American Journal of Medicine 2010;123(8):765‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawkes 2008 {published and unpublished data}
- Hawkes WC, Laslett LJ. Selenium supplementation does not improve vascular responsiveness in healthy North American men. American Journal of Physiology‐Heart and Circulatory Physiology 2009;296(2):H256‐62. [DOI] [PubMed] [Google Scholar]
Luoma 1984 {published data only}
- Luoma PV, Korpela H, Sotaniem EA, Kumpulainen J. Serum selenium, glutathione peroxidase, lipids, and human liver microsomal enzyme activity. A double‐blind controlled trial of selenium supplementation. Biological Trace Element Research 1985;8:113‐121. [DOI] [PubMed] [Google Scholar]
- Luoma PV, Sotaniemi EA, Korpela H, Kumpulainen J. Serum selenium, glutathione peroxidase activity and high‐density cholesterol ‐ Effect of selenium supplementation. Research Communications in Chemical Pathology and Pharmacology 1984;46(3):469‐72. [PubMed] [Google Scholar]
Meltzer 1994 {published data only}
- Meltzer HM, Mundal HH, Alexander J, Bibow K, Ydersbond TA. Does dietary arsenic and mercury affect cutaneous bleeding time and blood lipids in humans?. Biological Trace Element Research 1994;46:135‐53. [DOI] [PubMed] [Google Scholar]
Meltzer 1997 {published data only}
- Meltzer HM, Folmer M, Wang S, Lie O, Maage A, Mundal HH, Ydersbond TA. Supplementary selenium influences the response to fatty acid‐induced oxidative stress in humans. Biological Trace Element Research 1997;60:51‐68. [DOI] [PubMed] [Google Scholar]
Navas‐Carretero 2011 {published data only}
- Navas‐Carretero S, Cuervo M, Abete I, Zulet MA, Martínez JA. Frequent consumption of selenium‐enriched chicken meat by adults causes weight loss and maintains their antioxidant status. Biological Trace Element Research 2011;143:8‐19. [DOI: 10.1007/s12011-010-8831-x] [DOI] [PubMed] [Google Scholar]
NCP {published data only}
- Stranges S, Marshall JR, Trevisan M, Natarajan R, Donahue RP, Combs GF, et al. Effects of long‐term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Annals of Internal Medicine 2007;147(4):217‐23. [DOI] [PubMed] [Google Scholar]
- Stranges S, Marshall JR, Trevisan M, Natarajan R, Donahue RP, Combs GF, et al. Effects of selenium supplementation on cardiovascular disease incidence and mortality: secondary analyses in a randomized clinical trial. American Journal of Epidemiology 2006;163(8):694‐9. [DOI] [PubMed] [Google Scholar]
Ravn‐Haren 2008 {published data only}
- Ravn‐Haren G, Bugel S, Krath BN, Hoac T, Stagsted J, Jorgensen K, et al. A short‐term intervention trial with selenate, selenium‐enriched yeast and selenium‐enriched milk: effects on oxidative defence regulation. British Journal of Nutrition 2008;99(4):883‐92. [DOI] [PubMed] [Google Scholar]
SELECT {published data only}
- Klein EA, Thompson IM Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011;306(14):1549‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers. The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009;301(1):39‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
UK PRECISE {published data only}
- Rayman MP, Blundell‐Pound G, Pastor‐Barriuso R, Guallar E, Steinbrenner H, Stranges S. A randomized trial of selenium supplementation and risk of type‐2 diabetes, as assessed by plasma adiponectin. PLoS ONE 2012;7(9):e45269. [DOI: 10.1371/journal.pone.0045269] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayman MP, Stranges S, Griffin BA, Pastor‐Barriuso R, Guallar E. Effect of supplementation with high‐selenium yeast on plasma lipids. A randomized trial. Annals of Internal Medicine 2011;154:656‐65 (Appendix W‐236‐40). [DOI] [PubMed] [Google Scholar]
UK PRECISE 100μg {published data only}
- Rayman MP, Stranges S, Griffin BA, Pastor‐Barriuso R, Guallar E. Effect of supplementation with high‐selenium yeast on plasma lipids. A randomized trial. Annals of Internal Medicine 2011;154:656‐65 (Appendix W‐236‐40). [DOI] [PubMed] [Google Scholar]
UK PRECISE 200μg {published data only}
- Rayman MP, Stranges S, Griffin BA, Pastor‐Barriuso R, Guallar E. Effect of supplementation with high‐selenium yeast on plasma lipids. A randomized trial. Annals of Internal Medicine 2011;154:656‐65 (Appendix W‐236‐40). [DOI] [PubMed] [Google Scholar]
UK PRECISE 300μg {published data only}
- Rayman MP, Stranges S, Griffin BA, Pastor‐Barriuso R, Guallar E. Effect of supplementation with high‐selenium yeast on plasma lipids. A randomized trial. Annals of Internal Medicine 2011;154:656‐65 (Appendix W‐236‐40). [DOI] [PubMed] [Google Scholar]
Wu 2009 {published data only}
- Wu J, Salisbury C, Graham R, Lyons G, Fenech. Increased consumption of wheat biofortified with selenium does not modify biomarkers of cancer risk, oxidative stress, or immune function in healthy Australian males. Environmental & Molecular Mutagenesis 2009;50(6):489‐501. [DOI] [PubMed] [Google Scholar]
Wu BIOFORT 2009 {published data only}
- Wu J, Salisbury C, Graham R, Lyons G, Fenech. Increased consumption of wheat biofortified with selenium does not modify biomarkers of cancer risk, oxidative stress, or immune function in healthy Australian males. Environmental & Molecular Mutagenesis 2009;50(6):489‐501. [DOI] [PubMed] [Google Scholar]
Wu PROFORT 2009 {published data only}
- Wu J, Salisbury C, Graham R, Lyons G, Fenech. Increased consumption of wheat biofortified with selenium does not modify biomarkers of cancer risk, oxidative stress, or immune function in healthy Australian males. Environmental & Molecular Mutagenesis 2009;50(6):489‐501. [DOI] [PubMed] [Google Scholar]
Yu 1990 {published data only}
- Yu SY, Mao BL, Xiao P, Yu WP, Wang YL, Huang CZ, et al. Intervention trial with selenium for the prevention of lung cancer among tin miners in Yunnan, China. A pilot study. Biological Trace Element Research 1990;24(2):105‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alfthan 1991 {published data only}
- Alfthan G, Aro A, Arvilommi H, Huttunen JK. Selenium metabolism and platelet glutathione peroxidase activity in healthy Finnish men: Effects of selenium yeast, selenite, and selenate. American Journal of Clinical Nutrition 1991;53(1):120‐5. [DOI] [PubMed] [Google Scholar]
Asfour 2006 {published data only}
- Asfour IA, El SS, Fayek MH, Hegab HM, Raouf S, Moussa MA. Effect of high‐dose sodium selenite therapy on polymorphonuclear leukocyte apoptosis in non‐Hodgkin's lymphoma patients. Biological Trace Element Research 2006;110(1):19‐32. [DOI] [PubMed] [Google Scholar]
Karp 2010 {published data only}
- Karp DD, Lee SJ, Shaw Wright GL, Johnson DH, Johnston MR, Goodman GE, et al. A phase III, intergroup, randomized, double‐blind, chemoprevention trial of selenium (Se) supplementation in resected stage I non‐small cell lung cancer (NSCLC). Journal of Clinical Oncology 2010;28(18 Suppl):CRA7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kruger 1998 {published data only}
- Kruger HS, Veldman FJ, Vorster HH, Vermaak WJH, Barnard HC. Combination anti‐oxidant supplement increases resistance of low‐density lipoprotein to oxidation and improves lipoprotein profile of hypercholesterolaemic men. South African Medical Journal 1998;88(5):646‐52. [Google Scholar]
Linxian trials {published data only}
- Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, et al. Nutrition intervention trials in Linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease‐specific mortality in the general population. Journal of the National Cancer Institute 1993;85(18):1483‐92. [DOI] [PubMed] [Google Scholar]
- Blot WJ, Li JY, Taylor PR, Guo W, Dawsey SM, Li B. The Linxian trials: mortality rates by vitamin‐mineral intervention group. American Journal of Clinical Nutrition 1995;62(6):1424S‐6S. [DOI] [PubMed] [Google Scholar]
- Li JY, Taylor PR, Li B, Dawsey S, Wang GQ, Ershow AG, et al. Nutrition intervention trials in Linxian, China: Multiple vitamin/mineral supplementation, cancer incidence, and disease‐specific mortality among adults with esophageal dysplasia. Journal of the National Cancer Institute 1993;85(18):1492‐8. [DOI] [PubMed] [Google Scholar]
Mundal 1994 {published data only}
- Mundal HH, Meltzer HM, Aursnes I. Bleeding times related to serum triglyceride levels in healthy young adults. Thrombosis Research 1994;75(3):285‐91. [DOI] [PubMed] [Google Scholar]
Mutanen 1989 {published data only}
- Mutanen M, Viita L, Mykkanen HM. Selenium supplementation does not alter platelet activation in subjects with normal selenium status. Vitamin & Nutrition Research 1989;59(3):309‐13. [PubMed] [Google Scholar]
Ravn‐Haren 2008b {published data only}
- Ravn‐Haren G, Krath BN, Overvad K, Cold S, Moesgaard S, Larsen EH, et al. Effect of long‐term selenium yeast intervention on activity and gene expression of antioxidant and xenobiotic metabolising enzymes in healthy elderly volunteers from the Danish Prevention of Cancer by Intervention by Selenium (PRECISE) pilot study. British Journal of Nutrition 2008;99(6):1190‐8. [DOI] [PubMed] [Google Scholar]
SU.VI.MAX {published data only}
- Czernichow S, Couthouis A, Bertrais S, Vergnaud A, Dauchet L, Galan P, Hercberg S. Antioxidant supplementation does not affect fasting plasma glucose in the Supplementation with Antioxidant Vitamins and Minerals (SU.VI.MAX) study in France: association with dietary intake and plasma concentrations. American Journal of Clinical Nutrition 2006;84(2):395‐9. [DOI] [PubMed] [Google Scholar]
- Czernichow S, Vergnaud AC, Galan P, Arnaud J, Favier A, Faure H, et al. Effects of long‐term antioxidant supplementation and association of serum antioxidant concentrations with risk of metabolic syndrome in adults. American Journal of Clinical Nutrition 2009;90(2):329‐35. [DOI] [PubMed] [Google Scholar]
- Hercberg S, Bertrais S, Czernichow S, Noisette N, Galan P, Jaouen A, et al. Alterations of the lipid profile after 7.5 years of low‐dose antioxidant supplementation in the SU.VI.MAX study. Lipids 2005;40(4):335‐42. [DOI] [PubMed] [Google Scholar]
- Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, et al. The SU.VI.MAX study: A randomized, placebo‐controlled trial of the health effects of antioxidant vitamins and minerals. Archives of Internal Medicine 2004;164(21):2335‐42. [DOI] [PubMed] [Google Scholar]
- Hercberg S, Preziosi P, Briancon S, Galan P, Triol I, Malvy D, et al. A primary prevention trial using nutritional doses of antioxidant vitamins and minerals in cardiovascular diseases and cancers in a general population: The SU.Vi.Max study ‐ Design, methods, and participant characteristics. Controlled Clinical Trials 1998;19(4):336‐51. [DOI] [PubMed] [Google Scholar]
Wolters 2003 {published data only}
- Wolters M, Hahn A. Plasma ubiquinone status and response to six‐month supplementation combined with multivitamins in healthy elderly women ‐ Results of a randomized, double‐blind, placebo‐controlled study. International Journal for Vitamin and Nutrition Research 2003;73(3):207‐14. [DOI] [PubMed] [Google Scholar]
Additional references
Alanne 2007
- Alanne M, Kristiansson K, Auro K, Silander K, Kuulasmaa K, Peltonen L, et al. Variation in the selenoprotein S gene locus is associated with coronary heart disease and ischemic stroke in two independent Finnish cohorts.. Hum Genet 2007;122:355‐65. [DOI] [PubMed] [Google Scholar]
Blankenberg 2003
- Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, et al. AtheroGene Investigators. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. New England Journal of Medicine 2003;349:1605‐13. [DOI] [PubMed] [Google Scholar]
Bleys 2007
- Bleys J, Navas‐Acien A, Guallar E. Serum selenium and diabetes in U.S. Adults. Diabetes Care 2007;30:829‐34. [DOI] [PubMed] [Google Scholar]
Bleys 2008
- Bleys J, Navas‐Acien A, Guallar E. Serum Selenium levels and all‐cause, cancer and cardiovascular mortality among US adults. Archives of Internal Medicine 2008;168:404‐10. [DOI] [PubMed] [Google Scholar]
Bleys 2009
- Bleys J, Navas‐Acien A, Laclaustra M, Pastor‐Barriuso R, Menke A, Ordovas JM, et al. Serum selenium and peripheral arterial disease: Results from the National Health and Nutrition Examination Survey (NHANES) 2003 ‐ 2004. American Journal of Epidemiology 2009;169:996‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brigelius‐Flohe 2003
- Brigelius‐Flohe R, Banning A, Schnurr K. Selenium‐dependent enzymes in endothelial function. Antioxidants & Redox Signaling 2003;5:205‐15. [DOI] [PubMed] [Google Scholar]
Broadley 2006
- Broadley MR, White PJ, Bryson RJ, Meacham MC, Bowen HC, Johnson SE, et al. Biofortification of UK food crops with selenium. Proceedings of the Nutrition Society 2006;65:169‐81. [DOI] [PubMed] [Google Scholar]
Brookes 2004
- Brookes ST, Whitely E, Egger M, Smith GD, Mulheran PA, Peters TJ. Subgroup analyses in randomized trials: risks of subgroup‐specific analyses; power and sample size for the interaction test. Journal of Clinical Epidemiology 2004;57:229‐36. [DOI] [PubMed] [Google Scholar]
Brown 2001
- Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. New England Journal of Medicine 2001;345:1583‐92. [DOI] [PubMed] [Google Scholar]
Burk 2002
- Burk RF. Selenium, an antioxidant nutrient. Nutrition in Clinical Care 2002;5:75‐9. [DOI] [PubMed] [Google Scholar]
Burk 2006
- Burk RF, Norsworthy BK, Hill KE, Motley AK, Byrne DW. Effects of chemical form of selenium on plasma biomarkers in a high‐dose human supplementation trial. Cancer Epidemiology, Biomarkers & Prevention 2006;15:804‐10. [DOI] [PubMed] [Google Scholar]
Combs 1998
- Combs GF Jr, Gray WP. Chemopreventive agents: selenium. Pharmacology & Therapeutics 1998;79:179‐92. [DOI] [PubMed] [Google Scholar]
Copeland 1977
- Copeland KT, Checkoway H, McMichael AJ, Holbrook RH. Bias due to misclassification in the estimation of relative risk. American Journal of Epidemiology 1977;105:488‐95. [DOI] [PubMed] [Google Scholar]
Curran 2005
- Curran JE, Jowett JB, Elliott KS, Gao Y, Gluschenko K, Wang J, et al. Genetic variation in selenoprotein S influences Inflammatory response. Nature Genetics 2005;37:1234‐41. [DOI] [PubMed] [Google Scholar]
Czernichow 2006
- Czernichow S, Couthouis A, Bertrais S, Vergnaud A, Dauchet L, Galan P, Hercberg S. Antioxidant supplementation does not affect fasting plasma glucose in the Supplementation with Antioxidant Vitamins and Minerals (SU.VI.MAX) study in France: association with dietary intake and plasma concentrations. American Journal of Clinical Nutrition 2006;84(2):395‐9. [DOI] [PubMed] [Google Scholar]
Dennert 2011
- Dennert G, Zwahlen M, Brinkman M, Vinceti M, Zeegers MPA, Horneber M. Selenium for preventing cancer. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duffield 1999
- Duffield AJ, Thomson CD, Hill KE, et al. An estimation of selenium requirements for New Zealanders. American Journal of Clinical Nutrition 1999;70:896‐903. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fairweather 2011
- Fairweather‐Tait SJ, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE, Hurst R. Selenium in human health and disease. Antioxidants & Redox Signaling 2011;14:1337‐83. [DOI] [PubMed] [Google Scholar]
Flores‐Mateo 2006
- Flores‐Mateo G, Navas‐Acien A, Pastor‐Barriuso R, et al. Selenium and coronary heart disease: a meta‐analysis. American Journal of Clinical Nutrition 2006;84:762‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freemantle 2001
- Freemantle N. Interpreting the results of secondary end points and subgroup analyses in clinical trials: should we lock the crazy aunt in the attic?. BMJ 2001;322:989‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gao 2006
- Gao Y, Hannan NR, Wanyonyi S, Konstantopolous N, Pagnon J, Feng HC, et al. Activation of the selenoprotein SEPS1 gene expression by pro‐inflammatory cytokines in HepG2 cells. Cytokine 2006;33:246‐51. [DOI] [PubMed] [Google Scholar]
Hercberg 2004
- Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX study: a randomized, placebo‐controlled trial of the health effects of antioxidant vitamins and minerals. Archives of Internal Medicine 2004;164:2335‐42. [DOI] [PubMed] [Google Scholar]
Hercberg 2005
- Hercberg S, Bertrais S, Czernichow S, et al. Alterations of the lipid profile after 7.5 years of low‐dose antioxidant supplementation in the SU.VI.MAX Study. Lipids 2005;40:335‐42. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systmatic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. Available from www.cochrane‐handbook.org.
Hurst 2010
- Hurst R, Armah CN, Dainty JR, Hart DJ, Teucher B, Goldson AJ, et al. Establishing optimal selenium status: results of a randomized, double‐blind, placebo‐controlled trial. American Journal of Clinical Nutrition 2010;91:923‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keshan Disease Research Group
- Keshan Disease Research Group. Observations on effect of sodium selenite in prevention of Keshan disease. Chinese Medical Journal 1979;92:471‐6. [PubMed] [Google Scholar]
Korpela 1989
- Korpela H, Kumpulainen J, Jussila E, et al. Effect of selenium supplementation after acute myocardial infarction. Research Communications in Chemical Pathology and Pharmacology 1989;65:249‐52. [PubMed] [Google Scholar]
Kuklinski 1994
- Kuklinski B, Weissenbacher E, Fahnrich A. Coenzyme Q10 and antioxidants in acute myocardial infarction. Molecular Aspects of Medicine 1994;15:S143‐7. [DOI] [PubMed] [Google Scholar]
Labunskyy 2011
- Labunskyy VM, Lee BC, Handy DE, Loscalzo J, Hatfield DL, Gladyshev VN. Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes‐like phenotype in mice. Antioxidants & Redox Signaling 2011;14:2327‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laclaustra 2009a
- Laclaustra M, Navas‐Acien A, Stranges S, Ordovas JM, Guallar E. Serum selenium levels and hypertension in the US population. Circulation, Cardiovascular Quality and Outcomes 2009;2:369‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laclaustra 2009b
- Laclaustra M, Navas‐Acien A, Stranges S, Ordovas JM, Guallar E. Serum selenium concentrations and diabetes in US adults: National Health and Nutrition Examination Survey (NHANES) 2003‐2004. Environmental Health Perspectives 2009;117:1409‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laclaustra 2010
- Laclaustra M, Stranges S, Navas‐Acien A, Ordovas JM, Guallar E. Serum selenium and plasma lipids in US adults: National Health and Nutrition Examination Survey (NHANES) 2003‐2004. Atherosclerosis 2010;210:643‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for Studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. [updated March 2011]. The Cochrane Collaboration, 2011.
Millen 2004
- Millen AE, Dodd KW, Subar AF. Use of vitamin, mineral, nonvitamin, and nonmineral supplements in the United States: The 1987, 1992, and 2000 National Health Interview Survey results. Journal of the American Dietetic Association 2004;104:942‐50. [DOI] [PubMed] [Google Scholar]
Mosca 2011
- Mosca L, Barrett‐Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation 2011;124:2145‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Méplan 2007
- Méplan C, Crosley LK, Nicol F, Beckett GJ, Howie AF, Hill KE, et al. Genetic polymorphisms in the human selenoprotein P gene determine the response of selenoprotein markers to selenium supplementation in a gender‐specific manner (the SELGEN study). FASEB J 2007;21:3063‐74. [DOI] [PubMed] [Google Scholar]
Neve 1996
- Neve J. Selenium as a risk factor for cardiovascular diseases. Journal of Cardiovascular Risk 1996;3:42‐4. [PubMed] [Google Scholar]
Papp 2007
- Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxidants & Redox Signaling 2007;9:775‐806. [DOI] [PubMed] [Google Scholar]
Park 2012
- Park K, Rimm EB, Siscovick DS, Spiegelman D, Manson JE, Morris JS, et al. Toenail selenium and incidence of type 2 diabetes in US men and women. Diabetes Care 2012;35:1544‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rayman 1997
- Rayman MP. Dietary selenium: time to act. BMJ 1997;314:387‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rayman 2000
- Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233‐41. [DOI] [PubMed] [Google Scholar]
Rayman 2009
- Rayman MP. Selenoproteins and human health: Insights from epidemiological data. Biochimica et Biophysica Acta 2009;1790:1533‐40. [DOI] [PubMed] [Google Scholar]
Rayman 2011
- Rayman MP, Stranges S, Griffin B, Pastor‐Barriuso R, Guallar E. Effect of supplementation with high‐selenium yeast on plasma lipids: a randomized, controlled trial. Annals of Internal Medicine 2011;154:656‐65. [DOI] [PubMed] [Google Scholar]
Rayman 2012
- Rayman MP. Selenium and human health. Lancet 2012;379:1256‐68. [DOI] [PubMed] [Google Scholar]
Salonen 1982
- Salonen JT, Alfthan G, Huttunen JK, Pikkarainen J, Puska P. Association between cardiovascular death and myocardial infarction and serum selenium in a matched‐pair longitudinal study. Lancet 1982;2:175‐9. [DOI] [PubMed] [Google Scholar]
Salonen 1988
- Salonen JT, Salonen R, Seppaenen K, Kantola M, Parviainen M, Alfthan G, et al. Relationship of serum selenium and antioxidants to plasma lipoproteins, platelet aggregability and prevalent ischemic heart disease in Eastern Finnish men. Atherosclerosis 1988;70:155‐65. [DOI] [PubMed] [Google Scholar]
Sattler 1994
- Sattler W, Maiorino M, Stocker R. Reduction of HDL‐and LDL‐associated cholesterylester and phospholipid hydroperoxides by phospholipid hydroperoxide glutathione peroxidase and Ebselen (PZ 51). Archives of Biochemistry and Biophysics 1994;309:214‐21. [DOI] [PubMed] [Google Scholar]
Stranges 2006
- Stranges S, Marshall JR, Trevisan M, Natarajan R, Donahue RP, Combs GF, et al. Effects of selenium supplementation on cardiovascular disease incidence and mortality: Secondary analyses in a randomized clinical trial. American Journal of Epidemiology 2006;163:694‐9. [DOI] [PubMed] [Google Scholar]
Stranges 2007
- Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, Combs GF, et al. Effects of long‐term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Annals of Internal Medicine 2007;147:217‐23. [DOI] [PubMed] [Google Scholar]
Stranges 2010a
- Stranges S, Navas‐Acien A, Rayman MP, Guallar E. Selenium status and cardio‐metabolic health: State of the evidence. Nutrition, Metabolism, and Cardiovascular Diseases 2010;20:754‐60. [DOI] [PubMed] [Google Scholar]
Stranges 2010b
- Stranges S, Laclaustra M, Ji C, Cappuccio FP, Navas‐Acien A, Ordovas JM, et al. High‐normal selenium status is associated with adverse lipid profile in British adults. Journal of Nutrition 2010;140:81‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Virtamo 1985
- Virtamo J, Valkeila E, Alfthan G, Punsar S, Huttunen JK, Karvonen MJ. Serum selenium and the risk of coronary heart disease and stroke. Am J Epidemiol. 1985;122:276‐82. [DOI] [PubMed] [Google Scholar]
Vunta 2007
- Vunta H, Davis F, Palempalli UD, Bhat D, Arner RJ, Thompson JT, et al. The anti‐inflammatory effects of selenium are mediated through 15‐deoxy‐{Delat} 12,14‐prostaglandin J2 in macrophages. Journal of Biological Chemistry 2007;282:17964‐73. [DOI] [PubMed] [Google Scholar]
Wei 2004
- Wei WQ, Abnet CC, Qiao YL, et al. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke and total death. American Journal of Clinical Nutrition 2004;79:80‐5. [DOI] [PubMed] [Google Scholar]
Whanger 1996
- Whanger P, Vendeland S, Park YC, Xia Y. Metabolism of sub‐toxic levels of selenium in animals and humans. Annals of Clinical and Laboratory Science 1996;26:99‐113. [PubMed] [Google Scholar]
WHO 2003
- WHO Study Group. Diet, nutrition and the prevention of chronic diseases. World Health Organization, Geneva 2003; Vol. Report Series 916. [PubMed]
WHO 2011
- Mendis S, Puska P, Norrving B editors. Global Atlas on Cardiovascular Disease Prevention and Control. World Health Organization, Geneva 2011.
Xia 2005
- Xia Y, Hill KE, Byrne DW, Xu J, Burk RF. Effectiveness of selenium supplements in a low selenium area of China. American Journal of Clinical Nutrition 2005;81:829‐34. [DOI] [PubMed] [Google Scholar]
Xia 2010
- Xia Y, Hill KE, Li P, Xu J, Zhou D, Motley AK, Wang L, et al. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: a placebo‐controlled, double‐blind study of selenomethionine supplementation in selenium‐deficient Chinese subjects. American Journal of Clinical Nutrition 2010;92:525‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
You 2001
- You WC, Chang YS, Heinrich J, et al. An intervention trial to inhibit the progression of precancerous gastric lesions: compliance, serum micronutrients and S‐allyl cysteine levels, and toxicity. European Journal of Cancer Prevention 2001;10:257‐63. [DOI] [PubMed] [Google Scholar]
Zhang 2006
- Zhang L, Gail MH, Wang YQ, et al. A randomized factorial study of the effects of long‐term garlic and micronutrient supplementation and of 2‐wk antibiotic treatment for Helicobacter pylori infection on serum cholesterol and lipoproteins. American Journal of Clinical Nutrition 2006;84:912‐9. [DOI] [PubMed] [Google Scholar]


