Abstract
Follicle-stimulating hormone (FSH) is a glycoprotein hormone produced by gonadotropes in the anterior pituitary that plays a central role in controlling ovarian folliculogenesis and steroidogenesis in females. Moreover, recent studies strongly suggest that FSH exerts extragonadal actions, particularly regulating bone mass and adiposity. Despite its crucial role, the mechanisms regulating FSH secretion are not completely understood. It is evident that hypothalamic, ovarian, and pituitary factors are involved in the neuroendocrine, paracrine, and autocrine regulation of FSH production. Large animal models, such as the female sheep, represent valuable research models to investigate specific aspects of FSH secretory processes. This review: (i) summarizes the role of FSH controlling reproduction and other biological processes; (ii) discusses the hypothalamic, gonadal, and pituitary regulation of FSH secretion; (iii) considers the biological relevance of the different FSH isoforms; and (iv) summarizes the distinct patterns of FSH secretion under different physiological conditions.
I. INTRODUCTION
Follicle-stimulating hormone (FSH) is a heterodimeric glycoprotein hormone secreted by gonadotropes in the anterior pituitary that plays a central role in reproduction. In female mammals, FSH stimulates antrum formation in secondary ovarian follicles, growth and maturation of antral follicles, and proliferation of granulosa cells and estradiol production (Hunzicker-Dunn and Maizels 2006; Richards 1994). The requirement for FSH in female reproduction is evidenced by clinical and animal studies. Women with loss-of-function mutations in the genes encoding the FSH beta subunit (FSHB) or the FSH receptor (FSHR) manifest arrest in follicle development at the preantral stage and associated amenorrhea (Huhtaniemi and Themmen 2005). Transgenic mouse models with deficiency (knockout) in those genes exhibit similar ovarian perturbations (Danilovich, et al. 2000; Kumar, et al. 1997).
In males, FSH mediates induction of aromatase and acts upon Sertoli cells to support spermatogenesis (Pomerantz, 1979; Ramaswamy and Weinbauer 2014). Despite these important roles, the absolute requirement for FSH on male reproduction has been a topic of debate. Men with loss-of-function mutation in the FSHR gene present clinically with different levels of oligozoospermia and may manifest normal fertility, subfertility, or complete infertility (Tapanainen, et al. 1997). In male mice, deficiency in the FSHβ gene does not impair fertility despite reduced testes size and sperm counts (Kumar et al. 1997). Therefore, these observations suggest that FSH contributes to spermatogenesis, however, it may not be critically required for fertility in males.
In addition to the classical regulation of reproductive organs, recent evidence indicates that FSH also exerts important extragonadal effects (Sun et al. 2006; Kumar 2017). The FSHR is expressed on different extragonadal tissues, including bone (Sun, et al. 2006) and adipose tissue (Liu, et al. 2017). Epidemiological and clinical observations suggest that FSH directly regulates bone mass. During perimenopausal transition, women experience a drastic increase in bone turnover, which is highly correlated to elevated circulating concentrations of FSH independent of estrogen levels (Sowers, et al. 2003). Similarly, there is a strong correlation between elevated concentrations of FSH and low bone mass in women with amenorrhea (Devleta, et al. 2004). Moreover, women with polymorphisms in FSHR that result in constitutively active FSHR exhibit rapid reduction in bone density and higher prevalence of osteoporosis (Rendina, et al. 2010). In female mice, deletion of the FSHR prevents the negative effects of ovariectomy on bone density (Sun et al. 2006), further indicating that the rise in FSH levels after ovariectomy (or menopause in women) drives bone loss. This premise was corroborated recently by observations that immunoneutralization of FSH prevents the ovariectomy-induced bone loss in mice (Zhu, et al. 2012). Interestingly, FSH immunoneutralization also results in a reduction in total, visceral, and subcutaneous fat volume in wild-type mice (Liu et al. 2017). Administration of the FSH antibody also prevents the increase in adiposity seen after ovariectomy in female mice (Liu et al. 2017). Therefore, FSH immunoneutralization could act as a dual-purpose intervention with promising future clinical applications for treating both obesity and osteoporosis in women during the perimenopausal transition (Liu et al. 2017).
While the studies mentioned above provide evidence that FSH may contribute directly to the regulation of bone resorption and, thereby, bone mass, it is important to note that several studies present contradicting results. More recently, Allan et al. (2010) reported no detectable FSHR mRNA in mouse bone or cultured osteoblasts or osteoclasts, suggesting that FSH regulates bone mass indirectly, likely via ovary-dependent mechanisms. Moreover, Danilovich et al. (2000) reported that FSHR knockout mice have elevated androgen levels, raising the possibility that changes in bone mass reported by Sun et al. (2006) could result from local aromatization of these androgens. Therefore, future studies are required to confirm the putative direct effect of FSH on bone mass and other extragonadal targets. For additional information regarding the extragonadal actions of FSH, readers are referred to Kumar (2017) and Zhu, et al. (2018).
II. FSH STRUCTURE, ISOFORMS, AND BIOLOGICAL ACTIVITY
FSH is a heterodimeric glycoprotein comprised of a common α subunit noncovalently linked with a hormone-specific β subunit (FSH-β) (Baenziger and Green, 1988; Ryan et al. 1988). The α subunit (chorionic gonadotropin alpha [CGA]) is common to 3 pituitary glycoprotein hormones, FSH, luteinizing hormone (LH) and thyroid stimulating hormone (TSH), whereas the β chain is unique to each hormone and confers specific biological function (Baenziger and Green 1988). Separately, the two FSH chains are not able to bind and activate receptors and need to be associated in a dimeric structure to exhibit biological activity (Andersen 2002). After the dimeric structure is formed and before release into the circulation, oligosaccharide structures are added to two N-linked glycosylation sites present on each subunit resulting in the glycosylation of FSH (Andersen 2002). The structure of oligosaccharides attached to the FSH peptide backbone is highly variable resulting in a variety of different hormone isoforms (Creus, et al. 2001). Additionally, each carbohydrate branch may or may not terminate in a negatively charged sialic acid residue, which results in a wide array of isoforms with different isoelectric points (Andersen 2002). Consequently, more acidic isoforms have higher numbers of sialic acid residues, reflecting a more complex branching pattern, whereas less acidic isoforms have fewer sialic acid residues (Ulloa-Aguirre and Timossi 2000). As discussed below in the section “IV. Pattern of FSH secretion during different physiological states”, different regulators and physiological states control the pattern of FSH glycosylation, thus modulating the availability of the different FSH isoforms.
The metabolism and biological activity of different FSH isoforms are influenced by the glycosylation pattern and content of sialic acid residues. FSH molecules with higher number of sialic acid residues (acidic isoforms) have lower metabolic clearance rates compared to isoforms with lower sialic acid content (less acidic isoforms) (Blum et al. 1985; Padmanabhan, et al. 1999; Ulloa-Aguirre and Timossi 2000). Consequently, the plasma half-life of less acidic isoforms is markedly shorter than that of more acidic isoforms (Blum et al. 1985; Andersen 2002). However, despite the shorter half-life, the less acidic isoforms of FSH have been shown to have higher binding affinity for the FSHR and greater efficiency to stimulate proliferation of granulosa cells and rapid preantral follicular growth (Barrios-De-Tomasi et al. 2002). Additionally, less acidic FSH isoforms are significantly more effective than more acidic isoforms in stimulating ovarian synthesis and secretion of estrogens in vivo and in vitro (Barrios-De-Tomasi, et al. 2002). In contrast, more acidic FSH isoforms induce higher ovarian synthesis of inhibin-A when compared to less acidic isoforms (Ulloa-Aguirre, et al. 2003). Collectively, most in vitro an in vivo studies indicate that less acidic FSH isoforms exhibit higher potency compared to more acidic isoforms, although there are a few exceptions in which the reverse occurs. This may occur because in addition to sialic acid composition, the complexity of oligosaccharide branching also plays a role in determining the biological activity of the different FSH isoforms (Creus et al. 2001). For additional information regarding FSH structure, glycobiology, and the biological activity of the different FSH isoforms, readers are referred to previously published reviews (Bousfield and Harvey 2019; Padmanabhan et al. 1999; Smitz, et al. 2016).
III. REGULATION OF FSH SECRETION
Regulation of FSH production and release is an intricate process that involves hypothalamic (neuroendocrine), gonadal (endocrine), and pituitary (autocrine and paracrine) factors (Figure 1). Importantly, the different levels of FSH regulation not only control synthesis and secretion, but also posttranslational modifications of FSH, such and glycosylation and a sialylation (Sairam et al. 1985; Ulloa-Aguirre, et al. 1995). Several factors pose challenges for investigators to better understand the mechanisms underlying the regulation of FSH secretion. First, as discussed previously, FSH is secreted as a mixture of numerous isoforms with different half-life (Padmanabhan et al. 1999; Padmanabhan and Sharma 2001). Second, while different stimulus promotes FSH secretion, most FSH present in the peripheral circulation is secreted soon after synthesis in a constitutive manner (Padmanabhan et al. 1997; McNeilly, et al. 2003; Nicol et al., 2004; Wang et al. 2014). This differential secretion of gonadotropins is possible, at least in part, because FSH and LH are stored into different secretory granules within gonadotropes (Nicol et al. 2004; McNeilly et al. 2003). The relatively long half-life coupled with the constitutive mode of FSH secretion result in sustained levels of FSH in the peripheral circulation that hinder the detection of FSH secretory pulses, which are predominantly comprised of short-lived less acidic FSH isoforms (Padmanabhan, et al. 2002). Third, existing assays to measure FSH are unable to discriminate between the different FSH isoforms (Padmanabhan et al. 2002; Stanton, et al. 1996). Therefore, unlike LH, pulsatile patterns of FSH secretion from the anterior pituitary cannot be characterized effectively based on peripheral FSH measurements. In this regard, large animal models, such as the female sheep, represent valuable biological models to investigate the neuroendocrine control of FSH secretion. This is primarily because these animals are suitable for surgical procedures that allow parallel monitoring of hypothalamic and pituitary hormone levels near the site of their release (e.g., portal vasculature cannulation) and allow collection of repetitive blood samples due to their large blood volume (Padmanabhan et al. 2002).
Figure 1.
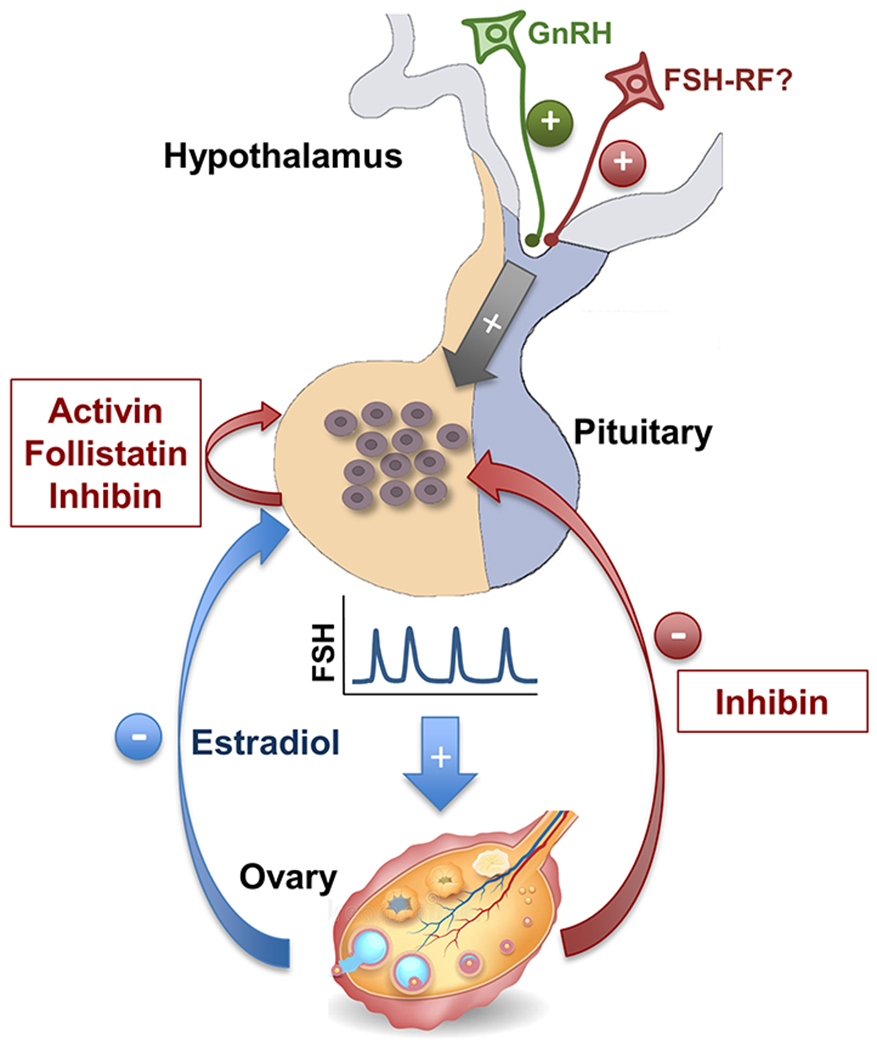
Schematic diagram of the hypothalamic, pituitary (local), and ovarian regulation of FSH secretion in female mammals. At the hypothalamic level, GnRH pulses are transported to the anterior pituitary by the hypothalamic-hypophyseal portal vasculature to stimulate FSH synthesis and secretion by gonadotrope cells. There is also evidence suggesting the existence of a hypothalamic FSH-releasing factor (FSH-RF). At the anterior pituitary level, a local loop involving activin, inhibin, and follistatin regulates FSH secretion in an autocrine/paracrine fashion. At the ovarian level, estradiol and inhibin are two key negative feedback regulators of FSH secretion. While the ovary also produces activin and follistatin, these hormones are not believed to play an endocrine role in controlling FSH secretion.
The following sections will discuss: (i) the hypothalamic regulation of FSH; (ii) the ovarian factors controlling FSH; (iii) the autocrine and paracrine regulation of FSH at the pituitary level; and (iv) the differential regulation of LH and FSH, the two gonadotropins co-synthesized within the gonadotropes, by these hypothalamic-pituitary-gonadal factors. While relevant findings from different species will be presented, these sections will highlight important contributions that the sheep model has made to advance our understanding of the regulation of FSH secretion and the potential benefits that the use of large animal models can provide to this research field. Additionally, this review will focus primarily on FSH secretory aspects; detailed information regarding FSH synthesis can be found elsewhere (Bernard, et al. 2010; Bousfield & Dias 2011; Das and Kumar 2018; Thompson & Kaiser, 2014).
a. Hypothalamic Regulation of FSH Secretion
Gonadotropin-releasing hormone (GnRH).
Since its discovery, GnRH, a hypothalamic decapeptide secreted into the pituitary portal vasculature, is arguably the most important regulator of FSH secretion (Schally, et al. 1971). With the exception of the preovulatory GnRH/gonadotropin surge, GnRH is secreted in a pulsatile fashion to stimulate LH and FSH secretion by gonadotrope cells. The pattern of GnRH pulsatile secretion, including pulse frequency and amplitude, changes throughout the reproductive cycle and is largely modulated by ovarian steroids (Karsch, et al. 1987). Perifusion studies have clearly shown that administration of GnRH pulses induces the pulsatile secretion of both LH and FSH from cultured pituitary cells from rodents (Ishizaka, et al. 1992; Weiss, et al. 1990) and sheep (Padmanabhan et al. 2002). However, a clear association between GnRH and FSH pulsatile secretion was not evident in whole-animal studies in which blood samples were collected from the peripheral circulation. The primary reasons for this lack of clear association are discussed above and include the relative long half-life of FSH and the predominantly constitutive pattern of FSH release.
The development of surgical procedures to collect blood samples directly from the hypothalamic-hypophyseal portal system of conscious and physiologically uncompromised sheep (Caraty, et al. 1982; Clarke, et al. 1983) has proved invaluable in elucidating the associations between GnRH and FSH secretion. With this approach, portal blood vessels in the anterior limit of the pituitary gland are cut and blood containing undiluted, freshly secreted hypothalamic and pituitary hormones can be sampled. Using this surgical approach, studies in in the female sheep have shown that FSH is indeed secreted in a pulsatile manner and that a clear temporal association exists between GnRH and FSH pulses (Padmanabhan, et al. 1997). While studies monitoring peripheral concentrations of gonadotropins in GnRH-immunoneutralized rats had shown similar findings (Culler and Negro-Vilar 1987), characterization of GnRH and FSH secretory patterns in the sheep pituitary portal vasculature confirmed the interrelationship between GnRH and FSH pulses.
FSH-releasing factor.
Despite the well-characterized association between GnRH and FSH pulsatile secretion, anatomical, physiological, and biochemical data suggest that a second hypothalamic factor may regulate the secretion of FSH. Evidence for the existence of an FSH-releasing factor (FSH-RF) is briefly discussed below. For detailed information on this topic, readers are referred to earlier reviews (McCann, et al. 2001; Padmanabhan and McNeilly 2001).
Evidence supporting the existence of FSH-RF derives from neuroanatomical studies that demonstrated that hypothalamic regions that do not contain GnRH neurons are involved in the control of FSH secretion. Ablation (Lumpkin and McCann 1984) or deafferentation (Lamperti and Hill 1987) of the dorsal anterior hypothalamic area (DAHA) selectively suppressed FSH secretion. A subsequent study confirmed that radiofrequency lesions of the DAHA suppressed FSH secretion in female rats (Lumpkin, et al. 1989). Collectively, these data suggest that separate mechanisms regulate LH and FSH secretion and that the DAHA is an important brain region controlling the latter.
Physiological evidence that supports the existence of FSH-RF derives from animal studies that report the secretion of FSH pulses that are not associated with GnRH pulses. Studies demonstrating episodic pattern of FSH in the peripheral circulation after blockage of GnRH action with GnRH antagonists in rats or immunization against GnRH in ovariectomized rabbits provide strong evidence for the existence of GnRH-independent FSH pulses (Culler and Negro-Vilar 1987; Pau, et al. 1991). While the persistence of FSH pulses in peripheral blood might result from different clearance rates of the various FSH isoforms, our studies in the female sheep demonstrate the existence of GnRH-independent FSH pulses also in the pituitary portal blood, providing evidence to the contrary (Padmanabhan et al. 1997). These studies in portal-vasculature cannulated sheep demonstrate: 1) a clear one-to-one relationship between GnRH and LH pulses; 2) that all GnRH pulses are associated with FSH pulses; and 3) the existence of additional FSH pulses that are not associated with GnRH pulses (GnRH-independent pulses of FSH). The existence of a GnRH-independent component of episodic FSH secretion was further confirmed by the observation that administration of Nal-Glu, a GnRH antagonist, eliminated LH but not FSH pulsatility in the sheep pituitary portal vasculature (Padmanabhan, et al. 2003).
Finally, biochemical evidence also supports the notion that a hypothalamic factor other than GnRH controls FSH secretion by the anterior pituitary. Using pig and sheep hypothalamic tissues, McCann, et al. (1983) were able to partially separate different hypothalamic extracts that had more FSH-releasing activity than could be accounted for by the content of GnRH. A preparation with strong FSH-releasing biological activity free of GnRH was obtained subsequently after ion exchange chromatography of ovine hypothalamic extracts (Lumpkin, et al. 1987). Identification of different GnRH isoforms in lower vertebrates (Lin, et al. 1998) raised the possibility that one of the GnRH variants could be the long sought after FSH-RF. This possibility was supported by the findings that receptors for a second isoform of GnRH (GnRH-II) were present in gonadotropes and that GnRH-II does not potently stimulate LH release (Millar, et al. 2001; Padmanabhan et al. 2003). In sheep, GnRH-II administration produced a higher ratio of FSH to LH secretion than that achieved with GnRH treatment, although GnRH was markedly more effective than GnRH-II in stimulating secretion of either of the gonadotropins (Millar et al. 2001). However, more recent studies contradict these earlier findings. Studies in sheep and rhesus monkeys reported no selective FSH-releasing activity for GnRH-II (Densmore and Urbanski 2003; Gault, et al. 2003). Similar observations were reported in vitro using cultured primate pituitaries (Okada, et al. 2003). Collectively, these observations suggest that GnRH-II has only weak selective actions inducing FSH secretion and, unlike GnRH, the primary role of GnRH-II in the brain is not to stimulate gonadotropin secretion (Kauffman and Rissman 2006). While other studies implicated a third GnRH isoform (lamprey GnRH-III) as a FSH-RF candidate (Wen, et al. 1997; Yu, et al. 2002), several studies have reported no selective FSH-releasing action of lamprey GnRH-III (Amstalden, et al. 2004; Kovacs, et al. 2002), thus leaving this issue currently unresolved.
b. Ovarian Regulation of FSH Secretion
Gonadal Steroids.
Gonadotropins stimulate sex steroid production by the gonads, which in turn feedback to the hypothalamus and pituitary to regulate gonadotropin secretion. In general, androgens, progestogens, and estrogens have the potential to exert negative feedback effects and suppress FSH release, either via direct effects in the pituitary (estrogens) or indirectly by suppressing GnRH secretion by the hypothalamus (Gharib, et al. 1990; Nett et al., 2002). Estrogens also have positive feedback actions on GnRH secretion prior to ovulation. In the female sheep, a large body of work demonstrated that sex steroids exert negative feedback effects on the GnRH neurosecretory system resulting in diminished GnRH release and subsequent reduced gonadotropin secretion (Clarke, et al. 1989; Karsch, et al. 1997). In addition to hypothalamic actions, gonadal steroids act directly at the anterior pituitary level in both males and females (Gharib et al. 1990; Martin, et al. 1988). The importance of direct steroid actions in the pituitary are clearly demonstrated by the infertile phenotype observed in female mice lacking the estrogen receptor α specifically in gonadotropes (Gieske, et al. 2007). The effects of gonadal steroids on the pituitary are mediated via several processes, including modifications in the expression of the GnRH receptor (Gregg and Nett 1989), transcription of FSHβ mRNA and FSH secretion (Bernard, et al. 2010), and FSH posttranslational modifications (Bousfield and Harvey 2019; Ulloa-Aguirre et al. 1992). For detailed information regarding the steroid regulation of FSH biosynthesis, readers are referred to Gharib et al. (1990) and Bernard et al. (2010).
Activins, Inhibins, and Follistatins.
In addition to gonadal steroids, regulatory proteins secreted by the gonads, specifically activin, inhibin, and follistatin are also important contributors to the amount of FSH secreted (Carroll, et al. 1989; Ling et al. 1986; Ueno, et al. 1987; Vale, et al. 1986; Vale, et al. 1988). Activin and inhibin are members of the transforming growth factor β superfamily and are structurally related (Ling et al. 1986; Ying 1988). Inhibin α, βA, and βB subunits are encoded by different genes and these subunits dimerize to form inhibin A (α-βA), inhibin B (α-βB), activin A (βA-βA), activin B (βB-βB), and activin AB (βA-βB) (Ying 1988). Follistatin is a monomeric protein that binds to the β subunit of both activin and inhibin (Phillips and de Kretser 1998). Activin stimulates intracellular signaling in gonadotropes that results in enhanced expression of FSHB mRNA and subsequent FSH release primarily due to its constitutive pattern of secretion (Ling, et al. 1986; Pangas and Woodruff 2000). Inhibin and follistatin appear to regulate FSH secretion primarily by antagonizing activin’s action rather than by directly initiating signaling events (DePaolo, et al. 1991; Padmanabhan and West 2001). Inhibins, which are secreted by granulosa and luteal cells in the ovary, act in an endocrine manner to suppress synthesis and consequently the amount of FSH secreted (Padmanabhan and West 2001; Woodruff, et al. 1996). Inhibins bind to activin receptors on gonadotropes and, via competitive antagonism, prevent activins from triggering intracellular signaling pathways (Cook, et al. 2004). Follistatins are structurally different from activins and inhibins, but bind to activins with high affinity preventing their receptor binding (Thompson, et al. 2005). Follistatins also inhibit FSH secretion by promoting internalization and degradation of activins, thus reducing their bioavailability (Cash, et al. 2009).
The endocrine role of these regulatory proteins has been demonstrated in different studies. A negative relationship between inhibin and FSH concentrations in the peripheral circulation supports this premise (Padmanabhan and West 2001). Moreover, at the time of menopause, a decrease in inhibin B is associated with the hallmark increase in circulating concentrations of FSH, consistent with a negative feedback relationship between inhibin and FSH (Klein and Soules 1998). In support for an endocrine role for follistatin are the observations that administration of recombinant human follistatin to sheep result in a marked suppression in FSH but not in LH concentrations (Padmanabhan and Sharma 2001). Additionally, these studies in the female sheep reported that after administration of follistatin the clearance of total follistatin was slower than that of free follistatin, providing evidence for activin-bound follistatin and thus indirect evidence for the presence of free activin in the circulation (Padmanabhan and Sharma 2001). Additional information on the ovarian regulatory proteins that control FSH synthesis can be found elsewhere (Bernard et al. 2010; Bilezikjian, et al. 2006; Das and Kumar 2018; DePaolo et al. 1991).
c. Pituitary Regulation of FSH Secretion
Studies in the last two decades have confirmed that inhibin, activin, and follistatin are produced in many tissues including the anterior pituitary gland (Bilezikjian, et al. 2004; Padmanabhan et al. 2002). Therefore, these proteins have been postulated to act in an autocrine and paracrine fashion to locally control FSH production and secretion (Besecke, et al. 1996; DePaolo, et al. 1991; Padmanabhan and West 2001). Moreover, changes in the gonadal steroid profile have been shown to result in modifications in the expression patterns of activin, inhibin, and follistatin in the anterior pituitary (Bilezikjian, et al. 2001).
One of the first evidences supporting local pituitary modulation of FSH secretion came from studies that showed that exposure of cultured pituitary cells from rats to an antiserum that neutralized the effects of activin B resulted in marked suppression of FSH secretion (Corrigan, et al. 1991). Additional support for a paracrine control of FSH came from studies that showed a negative relationship between the expression pattern of follistatin and FSHβ mRNA expression, and a positive relationship between activin and FSHβ expression (Besecke et al. 1996; Dalkin, et al. 1999). Using follistatin to neutralize locally produced activin, our studies in perifused ovine pituitary cells provide direct evidence that FSH secretion can be considerably modulated by changes in activin tone in the pituitary (Padmanabhan et al. 2002). In agreement, other studies have found that activin stimulates FSHβ mRNA expression and FSH secretion in fetal human pituitary cultures as well as in some pituitary adenomas (Blumenfeld and Ritter 2001; Takano, et al. 1992).
d. Differential control of LH and FSH
The numerous endocrine and local regulators of both constitutive and pulsatile secretion of FSH act in concert to mediate selective release of FSH. Different endocrine and molecular mechanisms have been proposed to explain the differential regulation of FSH and LH in different physiological conditions. As mentioned previously, GnRH is secreted in pulses and the nature of these pulses (both frequency and amplitude) impacts the relative synthesis and secretion of both gonadotropins (Burger, et al. 2004). Rapid GnRH pulses (every 30 to 60 min) tend to favor LH release, whereas slower GnRH pulses (every 2 to 4 hours) preferentially stimulate synthesis and secretion of FSH (Kaiser, et al. 1997). Therefore, physiological changes in GnRH pulse frequency may explain situations in which FSH and LH are differently regulated. Notably, FSH does not appear to depend on GnRH pulsatile stimulation to the extent required for LH secretion, since daily injections of GnRH are sufficient to stimulate marked increases in FSH content in the pituitary and plasma concentrations, but not LH in GnRH-deficient mice (Charlton, et al. 1983). Studies in the rhesus monkey also indicate that higher frequency of GnRH pulses favor LH secretion, while lower frequency favors FSH secretion (Wildt, et al. 1981). While changes in GnRH pulse characteristics may explain changes in the overall amount of FSH released, they do not explain the presence of FSH pulses that are not associated with GnRH, particularly after pharmacological blockage of GnRH actions. There are two plausible explanations for the persistent episodic pattern of FSH secretion in the absence of GnRH pulses. First, as discussed previously, the existence of other hypothalamic factors that selectively regulate FSH secretion (e.g., FSH-RF) could explain the presence of FSH pulses in the absence of GnRH and LH pulses (Padmanabhan and McNeilly 2001). Second, a time lag in the response of activin, inhibin, and follistatin to GnRH input could result in increased or decreased FSH secretion in the absence of changes in LH secretion that would culminate in what appears to be GnRH-independent pulses of FSH (Padmanabhan et al. 2002).
Regarding the molecular mechanism that likely contribute to the differential regulation of LH and FSH, studies have shown that the GnRH receptor has several regulatory elements and binding of GnRH to its receptor can activate multiple intracellular signaling pathways that could result in differential transcription of FSHβ and LHβ (Bernard et al. 2010). Additionally, while intracellular calcium influx is well known to regulate LH release by gonadotropes, it does not appear to be involved in FSH secretion (Kile and Nett 1994). While the exact mechanisms involved remain unknown, it is possible that FSH-bound sialic acid residues may target translocation of FSH to different secretory granules than those containing LH, thereby providing a regulatory mechanisms for the differential secretion of both gonadotropins (Baenziger and Green 1988). For additional information regarding the molecular regulation of FSH synthesis and secretion, readers are referred to Das and Kumar (2018).
IV. PATTERN OF FSH SECRETION DURING DIFFERENT PHYSIOLOGICAL STATES
Pubertal Development.
The beginning of puberty is associated with increased GnRH activity and subsequent gonadotropin secretion in girls (Sizonenko, et al. 1970). The pattern of gonadotropin response to exogenous administration of GnRH also changes during pubertal development. Initially, the FSH response is relatively greater, but as puberty advances, the LH response increases and the relative FSH decreases, leading up to the adult pattern (Cumming 1990). This change in pattern of gonadotropin response to GnRH likely reflects the increased ovarian feedback at the pituitary level by sex steroids and protein regulators of FSH. In support of this, are the observations that the circulating concentrations of inhibin A and total follistatin, two negative regulators of FSH secretion, change in opposite directions during pubertal maturation in girls (Foster, et al. 2000). Concentrations of inhibin increase while follistatin levels decrease during pubertal development (Foster et al. 2000). Total concentrations of activin A, a positive regulator of FSH, remain unchanged during pubertal progression in girls. Therefore, the reduction in follistatin, a binding neutralizer of activin, in the face of relatively constant concentrations of total activin A suggests that the bioavailability of activin (free activin A) increases with pubertal maturation, such that activin could override the inhibin increase and contribute to the rise in FSH secretion that occurs during puberty (Foster et al. 2000).
In addition to changes in FSH secretory pattern, the proportion of the different FSH isoforms also changes during pubertal progression. While no changes are observed for LH isoforms, FSH composition shifts to more acidic isoforms during pubertal progression in girls (Phillips, et al. 1997). Although the underlying mechanisms regulating the changes in FSH isoforms during puberty remain unclear, changes in GnRH secretory pattern, circulating concentrations of gonadal steroids, and endocrine or paracrine effects of protein regulators are all suspects (Ulloa-Aguirre et al. 1995).
Menstrual Cycle.
The reduction in circulating levels of estradiol, progesterone and inhibin A in the beginning of the menstrual cycle due to the demise of the corpus luteum reduces the inhibitory effects on the pituitary and allows FSH to rise in the early follicular phase and stimulate follicular growth and selection (Lee, et al. 1988; Mishell, et al. 1971; Yding Andersen 2017). At approximately Day 7 of the menstrual cycle, when selection of the dominant follicle occurs, FSH concentrations have already peaked and started to decline, with a continued slow decline until ovulation occurs (Lee, et al. 1988; Yding Andersen 2017). The general view is that this down regulation of FSH in the follicular phase of the menstrual cycle occurs due to increased estradiol production by the selected follicle, which in turn exerts a negative feedback at the anterior pituitary level. However, there appears to be a spatial-temporal issue with this premise since levels of FSH begin to decline several days prior to the rise in circulating concentrations of estradiol, suggesting that other ovarian factors, such as inhibin, activin, and follistatin may also play a role (Schneyer, et al. 2000; Yding Andersen 2017). The observations that inhibin B concentrations increase gradually during the follicular phase to reach a mid-cycle peak coincident with the pre-ovulatory gonadotrophin surge are supportive of this premise (Muttukrishna, et al. 1994).
During the midcycle, FSH and LH concentrations rise rapidly in response to the increased estradiol production by the preovulatory follicle (Reed and Carr 2015; Yding Andersen 2017). While a GnRH surge is critical to drive the preovulatory gonadotropin surge in rodents (Sarkar et al. 1976) and sheep (Moenter et al. 1991),the gonadotropin surge in women appears to unfold in the absence of a midcycle GnRH discharge being generated instead by the interaction between a pulsatile GnRH input to the pituitary and an action of estradiol (Martin et al. 1998; Plant 2012). After falling immediately after the pre-ovulatory gonadotropin surge, inhibin A increases in parallel with serum progesterone to reach a peak during the mid-luteal phase (Muttukrishna et al. 1994). While concentrations of inhibin B rise immediately after the gonadotropin surge, they rapidly decrease during the luteal phase (Groome et al. 1996; Welt, 2004). Concentrations of FSH remain relatively low through most of the luteal phase primarily due to the inhibitory effects of inhibin A and the suppressive effects of progesterone on GnRH secretion (Lee, et al. 1988; Reed and Carr 2015). Activin A concentrations vary in a biphasic manner during the menstrual cycle, with highest levels occurring around the midcycle and the late luteal phase (Muttukrishna, et al. 1996). While it is possible that activin A plays an endocrine role during the menstrual cycle, findings that virtually all detectable activin A in the peripheral circulation is associated with binding proteins raise questions about its relative bioavailability for acting at the pituitary level (Muttukrishna et al. 1996).
During the luteal phase, progesterone also plays a role modulating FSH heterogeneity. In the presence of high progesterone levels, estradiol fails to increase the presence of less acidic isoforms of FSH in the circulation (Wide, et al. 1996). Moreover, during the luteal phase of the menstrual cycle, the predominant circulating isoform of FSH is acidic (Padmanabhan, et al. 1988). Contrarily, during the follicular phase of the menstrual cycle, when estradiol levels are relatively high, the less acidic FSH isoforms predominate (Padmanabhan et al. 1988). Findings that estradiol decreases the expression of pituitary α 2,3-sialyltransferase, which incorporates sialic acid residues into the FSH molecule, provide a potential mechanism by which estradiol can stimulate increased production and secretion of less acidic isoforms of FSH (Damian-Matsumura, et al. 1999). As discussed in detail in other review articles (Bousfield and Harvey 2019; Padmanabhan et al. 1988; Ulloa-Aguirre et al. 2003), these changes in FSH heterogeneity are likely to play important biological roles in controlling ovarian processes during the menstrual cycle.
Perimenopause Transition.
A hallmark alteration observed during the perimenopause period is a continuous rise in FSH levels in the face of normal basal concentrations of LH, presumably due to declining estrogenic effects in the neuroendocrine system (Bäckström, et al. 1982; Reame, et al. 1996). It is believed that FSH secretion, due to its elevated sensitivity to the inhibitory effects of estradiol and inhibin, increases first in response to the decline in negative feedback from the aging ovary as the total number of responsive follicles markedly reduces during the perimenopause transition (Ferin, et al. 1993). While a reduced ovarian steroidogenic capacity could be involved in this FSH rise, enhanced FSH concentrations are observed in perimenopausal women with normal levels of ovarian steroids (Reame et al. 1996), suggesting that other ovarian regulators could be involved. In agreement, a substantial decrease in circulating levels of inhibin-B with no significant changes in estradiol or inhibin-A were observed in women during the early perimenopausal phase (Burger, et al. 1998). Later during the perimenopausal period, inhibin-A and estradiol also fall markedly, further contributing to the FSH rise (Burger et al. 1998). An age-dependent reduction in follistatin was also reported in women during the perimenopause transition (Reame, et al. 2007). Collectively, these findings suggest that changes in the secretory pattern of these regulatory proteins, which occur before significant changes in circulating levels of estradiol, are consistent with enhanced activin bioavailability and may contribute to the perimenopausal rise in concentrations of FSH (Burger et al. 1998; Reame et al. 2007).
In addition to a rise in FSH concentrations, the perimenopausal transition is also characterized by changes in the FSH heterogeneity. The predominant FSH glycoforms during the perimenopausal transition are the least complex and more simple, yet very acidic isoforms (Anobile, et al. 1998). Because the perimenopausal phase is characterized by decreased circulating levels of estradiol, these findings further support the notion that estradiol increases the presence of less acidic FSH isoforms in the circulation (Wide et al. 1996). In conjunction with the observations during the normal menstrual cycle, these findings in perimenopausal women suggest that changes in the steroidal milieu and ovarian production of protein regulators of FSH (e.g., inhibin and activin) modulate the carbohydrate complexity and charge of FSH (Figure 2). Therefore, this FSH heterogeneity may provide a secondary level of control by which FSH regulates ovarian function (Padmanabhan and Sharma 2001).
Figure 2.
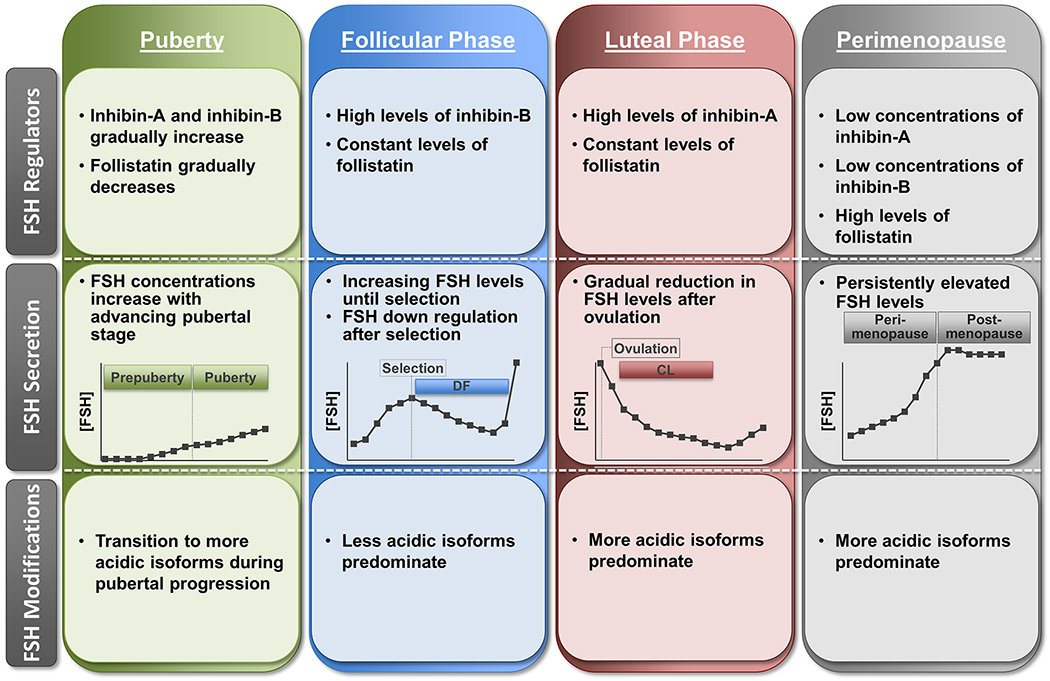
Schematic summarizing the general changes in circulating levels of FSH regulators, FSH secretory patterns, and posttranslational modifications of FSH during pubertal progression, menstrual cycle, and perimenopause transition in women (reviewed in Cumming, 1990; Padmanabhan et al., 2002; Ulloa-Aguirre et al., 2003; and Yding Andersen, 2017). CL, corpus luteum; DF, dominant follicle.
V. CONCLUSIONS
This review provides an overview of the neuroendocrine, endocrine, paracrine, and autocrine regulation of FSH secretion and heterogeneity during different physiological states. At the hypothalamic level (neuroendocrine), GnRH pulses clearly stimulate FSH pulsatile secretion, particularly detectable when FSH is monitored close to the site of secretion (e.g., portal vasculature in sheep). Yet, GnRH-independent pulses of FSH suggest that other neuroendocrine factors, such as a putative FSH-RF, may also regulate the episodic release of FSH. At the ovarian level, sex steroids and inhibin act in an endocrine fashion to provide the primary negative feedback control of FSH biosynthesis and consequently to the constitutive release of FSH. It remains uncertain whether activin and follistatin produced by the ovary play an endocrine role in controlling FSH secretion. At the pituitary level, activin, inhibin (Bilezikjian et al. 1996; Peeters et al. 1997; Roberts et al. 1989), and follistatin produced locally act to form a paracrine/autocrine loop that contributes to the amount of FSH released. Hypothalamic, ovarian, and pituitary factors act in concert to control not only secretion of FSH but also heterogeneity, and changes in these regulators during different physiological states can ultimately modulate FSH biological activity and its effects in the gonads and extragonadal targets.
While it is well established that FSH has great therapeutic potential and it represents an indispensable part of fertility treatment in women, there are numerous FSH preparations commercially available or in development that have some differences related to the glycosylation patterns and biological activity (Smitz et al. 2016). Both urinary-derived products and FSH produced through recombinant techniques are currently available. Future studies are needed to provide additional information regarding their source, purity, potency, and biological activity and to guide clinicians to choose which preparation or combination of preparations will be administered to women undergoing assisted reproductive technologies and/or fertility treatment. Additionally, a better understanding of the extragonadal actions of FSH is warranted, particularly in light of the recent findings that suggest a potential causal link between FSH hypersecretion, bone loss, and increased adiposity in perimenopausal women (Kumar 2017; Liu et al. 2017; Sun et al. 2006).
REFERENCES
- Allan CM, Kalak R, Dunstan CR, McTavish KJ, Zhou H, Handelsman DJ. & Seibel MJ. 2010. Follicle-stimulating hormone increases bone mass in female mice. Proceedings of the National Academy of Sciences, 107 22629–22634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstalden M, Zieba D, Garcia M, Stanko R, Welsh T, Hansel W & Williams G 2004. Evidence that lamprey GnRH-III does not release FSH selectively in cattle. Reproduction 127 35–43. [DOI] [PubMed] [Google Scholar]
- Andersen CY 2002. Effect of FSH and its different isoforms on maturation of oocytes from pre-ovulatory follicles. Reproductive BioMedicine Online 5 232–239. [DOI] [PubMed] [Google Scholar]
- Anobile C, Talbot J, McCann S, Padmanabhan V & Robertson W 1998. Glycoform composition of serum gonadotrophins through the normal menstrual cycle and in the post-menopausal state. Molecular Human Reproduction 4 631–639. [DOI] [PubMed] [Google Scholar]
- Bäckström C, McNeilly A, Leask R & Baird D 1982. Pulsatile secretion of LH, FSH, prolactin, oestradiol and progesterone during the human menstrual cycle. Clinical Endocrinology 17 29–42. [DOI] [PubMed] [Google Scholar]
- Baenziger JU & Green ED 1988. Pituitary glycoprotein hormone oligosaccharides: structure, synthesis and function of the asparagine-linked oligosaccharides on lutropin, follitropin and thyrotropin. Biochimica et Biophysica Acta (BBA)-Reviews on Biomembranes 947 287–306. [DOI] [PubMed] [Google Scholar]
- Barrios-De-Tomasi J, Timossi C, Merchant H, Quintanar A, Avalos J, Andersen CY & Ulloa-Aguirre A 2002. Assessment of the in vitro and in vivo biological activities of the human follicle-stimulating isohormones. Molecular and Cellular Endocrinology 186 189–198. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Fortin J, Wang Y & Lamba P 2010. Mechanisms of FSH synthesis: what we know, what we don’t, and why you should care. Fertility and Sterility 93 2465–2485. [DOI] [PubMed] [Google Scholar]
- Besecke LM, Guendner MJ, Schneyer AL, Bauer-Dantoin AC, Jameson JL & Weiss J 1996. Gonadotropin-releasing hormone regulates follicle-stimulating hormone-beta gene expression through an activin/follistatin autocrine or paracrine loop. Endocrinology 137 3667–3673. [DOI] [PubMed] [Google Scholar]
- Bilezikjian LM, Corrigan AZ, Blount AL & Vale WW. 1996. Pituitary follistatin and inhibin subunit messenger ribonucleic acid levels are differentially regulated by local and hormonal factors. Endocrinology 137 4277–4284. [DOI] [PubMed] [Google Scholar]
- Bilezikjian LM, Blount AL, Corrigan AZ, Leal A, Chen Y & Vale WW 2001. Actions of activins, inhibins and follistatins: implications in anterior pituitary function. Clinical and Experimental Pharmacology and Physiology 28 244–248. [DOI] [PubMed] [Google Scholar]
- Bilezikjian LM, Blount AL, Donaldson CJ & Vale WW 2006. Pituitary actions of ligands of the TGF-β family: activins and inhibins. Reproduction 132 207–215. [DOI] [PubMed] [Google Scholar]
- Bilezikjian LM, Blount AL, Leal AM, Donaldson CJ, Fischer WH & Vale WW 2004. Autocrine/paracrine regulation of pituitary function by activin, inhibin and follistatin. Molecular and Cellular Endocrinology 225 29–36. [DOI] [PubMed] [Google Scholar]
- Blum WFP & Gupta D 1985. Heterogeneity of rat FSH by chromatofocusing: studies on serum FSH, hormone released in vitro and metabolic clearance rates of its various forms [ill]. Journal of Endocrinology, 105 29–37. [DOI] [PubMed] [Google Scholar]
- Blumenfeld Z & Ritter M 2001. Inhibin, activin, and follistatin in human fetal pituitary and gonadal physiology. Annals of the New york Academy of Sciences 943 34–48. [DOI] [PubMed] [Google Scholar]
- Bousfield GR & Dias JA 2011. Synthesis and secretion of gonadotropins including structure-function correlates. Reviews in Endocrine and Metabolic Disorders 12 289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousfield GR & Harvey DJ 2019. Follicle-Stimulating Hormone Glycobiology. Endocrinology 160 1515–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger H, Cahir N, Robertson D, Groome N, Dudley E, Green A & Dennerstein L 1998. Serum inhibins A and B fall differentially as FSH rises in perimenopausal women. Clinical Endocrinology 48 809–813. [DOI] [PubMed] [Google Scholar]
- Burger L, Haisenleder D, Dalkin A & Marshall J 2004. Regulation of gonadotropin subunit gene transcription. Journal of Molecular Endocrinology 33 559–584. [DOI] [PubMed] [Google Scholar]
- Caraty A, Orgeur P & Thiery J 1982. Demonstration of the pulsatile secretion of LH-RH into hypophysial portal blood of ewes using an original technic for multiple samples. Comptes rendus des seances de l’Academie des sciences. Serie III, Sciences de la vie 295 103–106. [PubMed] [Google Scholar]
- Carroll RS, Corrigan AZ, Gharib SD, Vale W & Chin WW 1989. Inhibin, activin, and follistatin: regulation of follicle-stimulating hormone messenger ribonucleic acid levels. Molecular Endocrinology 3 1969–1976. [DOI] [PubMed] [Google Scholar]
- Cash JN, Rejon CA, McPherron AC, Bernard DJ & Thompson TB 2009. The structure of myostatin: follistatin 288: insights into receptor utilization and heparin binding. The EMBO Journal 28 2662–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton H, Halpin D, Iddon C, Rosie R, Levy G, McDowell I, Megson A, Morris J, Bramwell A & Speight A 1983. The effects of daily administration of single and multiple injections of gonadotropin-releasing hormone on pituitary and gonadal function in the hypogonadal (hpg) mouse. Endocrinology 113 535–544. [DOI] [PubMed] [Google Scholar]
- Clarke I, Cummins J, Crowder M & Nett T 1989. Long-term negative feedback effects of oestrogen and progesterone on the pituitary gland of the long-term ovariectomized ewe. Journal of Endocrinology 120 207–214. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT & de Kretser DM 1983. Pituitary gland function after disconnection from direct hypothalamic influences in the sheep. Neuroendocrinology 36 376–384. [DOI] [PubMed] [Google Scholar]
- Cook RW, Thompson TB, Jardetzky TS & Woodruff TK 2004. Molecular biology of inhibin action In Seminars in Reproductive Medicine, pp 269–276: Copyright© 2004 by Thieme Medical Publishers, Inc., 333 Seventh Avenue, New: …. [DOI] [PubMed] [Google Scholar]
- Corrigan AZ, Bilezikjian LM, Carroll RS, Bald LN, Schmelzer CH, Fendly BM, Mason AJ, Chin WW, Schwall RH & Vale W 1991. Evidence for an autocrine role of activin B within rat anterior pituitary cultures. Endocrinology 128 1682–1684. [DOI] [PubMed] [Google Scholar]
- Creus S, Chaia Z, Pellizzari EH, Cigorraga SB, Ulloa-Aguirre A & Campo S 2001. Human FSH isoforms: carbohydrate complexity as determinant of in-vitro bioactivity. Molecular and Cellular Endocrinology 174 41–49. [DOI] [PubMed] [Google Scholar]
- Culler MD & Negro-Vilar A 1987. Pulsatile follicle-stimulating hormone secretion is independent of luteinizing hormone-releasing hormone (LHRH): pulsatile replacement of LHRH bioactivity in LHRH-immunoneutralized rats. Endocrinology 120 2011–2021. [DOI] [PubMed] [Google Scholar]
- Cumming DC 1990. Menarche, menses and menopause: a brief review. Cleveland Clinical Journal of Medicine 57 169–175. [DOI] [PubMed] [Google Scholar]
- Dalkin A, Haisenleder D, Gilrain J, Aylor K, Yasin M & Marshall J 1999. Gonadotropin-releasing hormone regulation of gonadotropin subunit gene expression in female rats: actions on follicle-stimulating hormoneβ messenger ribonucleic acid (mRNA) involve differential expression of pituitary activin (β-B) and follistatin mRNAs. Endocrinology 140 903–908. [DOI] [PubMed] [Google Scholar]
- Damian-Matsumura P, Zaga V, Maldonado A, Sanchez-Hernandez C, Timossi C & Ulloa-Aguirre A 1999. Oestrogens regulate pituitary α2, 3-sialyltransferase messenger ribonucleic acid levels in the female rat. Journal of Molecular Endocrinolology 23 153–165. [DOI] [PubMed] [Google Scholar]
- Danilovich N, Babu PS, Xing W, Gerdes M, Krishnamurthy H & Sairam MR 2000. Estrogen deficiency, obesity, and skeletal abnormalities in follicle-stimulating hormone receptor knockout (FORKO) female mice. Endocrinology 141 4295–4308. [DOI] [PubMed] [Google Scholar]
- Das N & Kumar TR 2018. Molecular regulation of follicle-stimulating hormone synthesis, secretion and action. Journal of Molecular Endocrinology 60 R131–R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Densmore VS & Urbanski HF 2003. Relative effect of gonadotropin-releasing hormone (GnRH)-I and GnRH-II on gonadotropin release. The Journal of Clinical Endocrinology & Metabolism 88 2126–2134. [DOI] [PubMed] [Google Scholar]
- DePaolo LV, Bicsak TA, Erickson GF, Shimasaki S & Ling N 1991. Follistatin and activin: a potential intrinsic regulatory system within diverse tissues. Proceedings of the Society for Experimental Biology and Medicine 198 500–512. [DOI] [PubMed] [Google Scholar]
- Devleta B, Adem B. & Senada S. 2004. Hypergonadotropic amenorrhea and bone density: new approach to an old problem. Journal of Bone and Mineral Metabolism, 22 360–364. [DOI] [PubMed] [Google Scholar]
- Ferin M, Jewelewicz R & Warren M 1993. The menstrual cycle: physiology, reproductive disorders, and infertility: Oxford University Press, USA. [Google Scholar]
- Foster CM, Phillips DJ, Wyman T, Evans LW, Groome NP & Padmanabhan V 2000. Changes in serum inhibin, activin and follistatin concentrations during puberty in girls. Human Reproduction 15 1052–1057. [DOI] [PubMed] [Google Scholar]
- Gault P, Maudsley S & Lincoln G 2003. Evidence that gonadotropin‐releasing hormone II is not a physiological regulator of gonadotropin secretion in mammals. Journal of Neuroendocrinology 15 831–839. [DOI] [PubMed] [Google Scholar]
- Gharib SD, Wierman ME, Shupnik MA & Chin WW 1990. Molecular biology of the pituitary gonadotropins. Endocrine Reviews 11 177–199. [DOI] [PubMed] [Google Scholar]
- Gieske MC, Kim HJ, Legan SJ, Koo Y, Krust A, Chambon P & Ko C 2007. Pituitary gonadotroph estrogen receptor-α is necessary for fertility in females. Endocrinology 149 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg DW & Nett TM 1989. Direct effects of estradiol-17β on the number of gonadotropin-releasing hormone receptors in the ovine pituitary. Biology of Reproduction 40 288–293. [DOI] [PubMed] [Google Scholar]
- Groome NP, Illingworth PJ, O’Brien M, Pai R, Rodger FE, Mather JP & McNeilly AS 1996. Measurement of dimeric inhibin B throughout the human menstrual cycle. The Journal of Clinical Endocrinology & Metabolism 81 1401–1405. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I & Themmen A 2005. Mutations in human gonadotropin and gonadotropin-receptor genes. Endocrine 26 207–217. [DOI] [PubMed] [Google Scholar]
- Hunzicker-Dunn M & Maizels ET 2006. FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cellular Signalling 18 1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka K, Kitahara S, Oshima H, Troen P, Attardi B & Winters SJ 1992. Effect of gonadotropin-releasing hormone pulse frequency on gonadotropin secretion and subunit messenger ribonucleic acids in perifused pituitary cells. Endocrinology 130 1467–1474. [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Jakubowiak A, Steinberger A & Chin WW 1997. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology 138 1224–1231. [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Bowen JM, Caraty A, Evans NP & Moenter SM 1997. Gonadotropin-releasing hormone requirements for ovulation. Biology of Reproduction 56 303–309. [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Cummins JT, Thomas GB & Clarke IJ 1987. Steroid feedback inhibition of pulsatile secretion of gonadotropin-releasing hormone in the ewe. Biology of Reproduction 36 1207–1218. [DOI] [PubMed] [Google Scholar]
- Kauffman AS & Rissman EF 2006. Role of gonadotropin-releasing hormone II in the mammalian nervous system. Expert Review of Endocrinology & Metabolism 1 133–145. [DOI] [PubMed] [Google Scholar]
- Kile JP & Nett TM 1994. Differential secretion of follicle-stimulating hormone and luteinizing hormone from ovine pituitary cells following activation of protein kinase A, protein kinase C, or increased intracellular calcium. Biology of Reproduction 50 49–54. [DOI] [PubMed] [Google Scholar]
- Klein NA & Soules MR 1998. Endocrine changes of the perimenopause. Clinical Obstetrics and Gynecology 41 912–920. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Seprodi J, Koppan M, Horvath J, Vincze B, Teplan I & Flerko B 2002. Lamprey gonadotropin hormone‐releasing hormone‐III has no selective follicle‐stimulating hormone‐releasing effect in rats. Journal of Neuroendocrinology 14 647–655. [DOI] [PubMed] [Google Scholar]
- Kumar TR 2017. Extragonadal actions of FSH: a critical need for novel genetic models. Endocrinology 159 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N & Matzuk MM 1997. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nature Genetics 15 201. [DOI] [PubMed] [Google Scholar]
- Lamperti A & Hill L 1987. The effects of anterior hypothalamic deafferentation on FSH-releasing activity in the intact female hamster. Experimental Brain Research 68 189–194. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lenton EA, Sexton L. & Cooke ID 1988. The effect of age on the cyclical patterns of plasma LH, FSH, oestradiol and progesterone in women with regular menstrual cycles. Human Reproduction 3 851–855. [DOI] [PubMed] [Google Scholar]
- Ling N, Ying SY, Ueno N, Shimasaki S, Esch F, Hotta M & Guillemin R. 1986. Pituitary FSH is released by a heterodimer of the β-subunits from the two forms of inhibin. Nature 321 779. [DOI] [PubMed] [Google Scholar]
- Lin X-W, Otto CJ & Peter RE 1998. Evolution of neuroendocrine peptide systems: gonadotropin-releasing hormone and somatostatin. Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology 119 375–388. [DOI] [PubMed] [Google Scholar]
- Ling N, Ying S-Y, Ueno N, Shimasaki S, Esch F, Hotta M & Guillemin R 1986. Pituitary FSH is released by a heterodimer of the β-subunits from the two forms of inhibin. Nature 321 779. [DOI] [PubMed] [Google Scholar]
- Liu P, Ji Y, Yuen T, Rendina-Ruedy E, DeMambro VE, Dhawan S, Abu-Amer W, Izadmehr S, Zhou B & Shin AC 2017. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature 546 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin M, Moltz J, Yu W, Samson W & McCann S 1987. Purification of FSH-releasing factor: its dissimilarity from LHRH of mammalian, avian, and piscian origin. Brain Research Bulletin 18 175–178. [DOI] [PubMed] [Google Scholar]
- Lumpkin MD & McCann SM 1984. Effect of destruction of the dorsal anterior hypothalamus on follicle-stimulating hormone secretion in the rat. Endocrinology 115 2473–2480. [DOI] [PubMed] [Google Scholar]
- Lumpkin MD, McDonald JK, Samson WK & McCann SM 1989. Destruction of the dorsal anterior hypothalamic region suppresses pulsatile release of follicle stimulating hormone but not luteinizing hormone. Neuroendocrinology 50 229–235. [DOI] [PubMed] [Google Scholar]
- Martin G, Price C, Thiery J & Webb R 1988. Interactions between inhibin, oestradiol and progesterone in the control of gonadotrophin secretion in the ewe. Reproduction 82 319–328. [DOI] [PubMed] [Google Scholar]
- Martin KA, Welt CK, Taylor AE, Smith JA, Crowley WF Jr & Hall JE 1998. Is GnRH Reduced at the Midcycle Surge in the Human? Neuroendocrinology 67 363–369. [DOI] [PubMed] [Google Scholar]
- McCann S, Mizunuma H, Samson W & Lumpkin M 1983. Differential hypothalamic control of FSH secretion: a review. Psychoneuroendocrinology 8 299–308. [DOI] [PubMed] [Google Scholar]
- McCann SM, Karanth S, Mastronardi CA, Les Dees W, Childs G, Miller B, Sower S & Wen HY 2001. Control of gonadotropin secretion by follicle-stimulating hormone-releasing factor, luteinizing hormone-releasing hormone, and leptin. Archives of Medical Research 32 476–485. [DOI] [PubMed] [Google Scholar]
- McNeilly A, Crawford J, Taragnat C, Nicol L & McNeilly J 2003. The differential secretion of FSH and LH: regulation through genes, feedback and packaging. Reproduction 463–476. [PubMed] [Google Scholar]
- Millar R, Lowe S, Conklin D, Pawson A, Maudsley S, Troskie B, Ott T, Millar M, Lincoln G & Sellar R 2001. A novel mammalian receptor for the evolutionarily conserved type II GnRH. Proceedings of the National Academy of Sciences 98 9636–9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell DR Jr, Nakamura RM, Crosignani PG, Stone S, Kharma K, Nagata Y & Thorneycroft IH 1971. Serum gonadotropin and steroid patterns during the normal menstrual cycle. American Journal of Obstetrics and Gynecology, 111 60–65. [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Locatelli A & Karsch FJ 1991. Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology 129 1175–1182. [DOI] [PubMed] [Google Scholar]
- Muttukrishna S, Fowler P, George L, Groome N & Knight PG 1996. Changes in peripheral serum levels of total activin A during the human menstrual cycle and pregnancy. The Journal of Clinical Endocrinology & Metabolism 81 3328–3334. [DOI] [PubMed] [Google Scholar]
- Muttukrishna S, Fowler P, Groome N, Mitchell G, Robertson W & Knight P 1994. Endocrinology: Serum concentrations of dimeric inhibin during the spontaneous human menstrual cycle and after treatment with exogenous gonadotrophin. Human Reproduction 9 1634–1642. [DOI] [PubMed] [Google Scholar]
- Nett TM, Turzillo AM, Baratta M & Rispoli LA 2002. Pituitary effects of steroid hormones on secretion of follicle-stimulating hormone and luteinizing hormone. Domestic Animal Endocrinology, 23 33–42. [DOI] [PubMed] [Google Scholar]
- Nicol L, Stridsberg M & McNeilly AS 2004. Differential secretion of gonadotrophins: investigation of the role of secretogranin II and chromogranin A in the release of LH and FSH in LbetaT2 cells. Journal of Molecular Endocrinology 32 467–480. [DOI] [PubMed] [Google Scholar]
- Okada Y, Murota-Kawano A, Kakar SS & Winters SJ 2003. Evidence that gonadotropin-releasing hormone (GnRH) II stimulates luteinizing hormone and follicle-stimulating hormone secretion from monkey pituitary cultures by activating the GnRH I receptor. Biology of Reproduction 69 1356–1361. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Brown MB, Dahl GE, Evans NP, Karsch FJ, Mauger DT, Neill JD & Van Cleeff J 2003. Neuroendocrine control of follicle-stimulating hormone (FSH) secretion: III. Is there a gonadotropin-releasing hormone-independent component of episodic FSH secretion in ovariectomized and luteal phase ewes? Endocrinology 144 1380–1392. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Karsch F & Lee J 2002. Hypothalamic, pituitary and gonadal regulation of FSH. Reproduction 59 67–82. [PubMed] [Google Scholar]
- Padmanabhan V, Lang LL, Sonstein J, Kelch RP & Beitins IZ 1988. Modulation of serum follicle-stimulating hormone bioactivity and isoform distribution by estrogenic steroids in normal women and in gonadal dysgenesis. The Journal of Clinical Endocrinology & Metabolism 67 465–473. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Lee J & Beitins I 1999. Follicle-stimulating isohormones: regulation and biological significance. Journal of Reproduction and Fertility. 54 87–99. [PubMed] [Google Scholar]
- Padmanabhan V, McFadden K, Mauger DT, Karsch FJ & Midgley AR Jr 1997. Neuroendocrine control of follicle-stimulating hormone (FSH) secretion. I. Direct evidence for separate episodic and basal components of FSH secretion. Endocrinology 138 424–432. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V & McNeilly AS 2001. Is there an FSH-releasing factor? Reproduction 121 21–30. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V & Sharma TP 2001. Neuroendocrine vs. paracrine control of follicle-stimulating hormone. Archives of Medical Research 32 533–543. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V & West C 2001. Endocrine, autocrine and paracrine actions of inhibin, activin and follistatin on follicle-stimulating hormone In Inhibin, activin and follistatin in human reproductive physiology, pp 61–90: World Scientific. [Google Scholar]
- Pangas SA & Woodruff TK 2000. Activin signal transduction pathways. Trends in Endocrinology & Metabolism 11 309–314. [DOI] [PubMed] [Google Scholar]
- Pau K-YF, Gliessman PM, Oyama T & Spies HG 1991. Disruption of GnRH pulses by anti-GnRH serum and phentolamine obliterates pulsatile LH but not FSH secretion in ovariectomized rabbits. Neuroendocrinology 53 382–391. [DOI] [PubMed] [Google Scholar]
- Peeters R, Vanmontfort D, Van Isterdael J, Verhoeven G, Rombauts L & Decuypere E. 1997. Evidence for the presence of immunoreactive inhibin in extragonadal tissues of ovariectomized ewes. Animal Reproduction Science, 48 257–268. [DOI] [PubMed] [Google Scholar]
- Phillips DJ, Albertsson-Wikland K, Eriksson K & Wide L 1997. Changes in the isoforms of luteinizing hormone and follicle-stimulating hormone during puberty in normal children. The Journal of Clinical Endocrinology & Metabolism 82 3103–3106. [DOI] [PubMed] [Google Scholar]
- Phillips DJ & de Kretser DM 1998. Follistatin: a multifunctional regulatory protein. Frontiers in Neuroendocrinology 19 287–322. [DOI] [PubMed] [Google Scholar]
- Plant TM 2012. A comparison of the neuroendocrine mechanisms underlying the initiation of the preovulatory LH surge in the human, Old World monkey and rodent. Frontiers in Neuroendocrinology 33 160–168. [DOI] [PubMed] [Google Scholar]
- Pomerantz DK 1979. Effects of in vivo gonadotropin treatment on estrogen levels in the testis of the immature rat. Biology of Reproduction 21 1247–1255. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S & Weinbauer GF 2014. Endocrine control of spermatogenesis: Role of FSH and LH/testosterone. Spermatogenesis 4 e996025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reame NE, Kelche R, Beitins I, Yu M, Zawacki CM & Padmanabhan V 1996. Age effects of follicle-stimulating hormone and pulsatile luteinizing hormone secretion across the menstrual cycle of premenopausal women. The Journal of Clinical Endocrinology & Metabolism 81 1512–1518. [DOI] [PubMed] [Google Scholar]
- Reame NE, Lukacs JL, Olton P, Ansbacher R & Padmanabhan V 2007. Differential effects of aging on activin A and its binding protein, follistatin, across the menopause transition. Fertility and Sterility 88 1003–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BG & Carr BR 2015. The normal menstrual cycle and the control of ovulation In Endotext: MDText. com, Inc. [Google Scholar]
- Rendina D, Gianfrancesco F, De Filippo G, Merlotti D, Esposito T, Mingione A, Nuti R, Strazzullo P, Mossetti G & Gennari L 2010. FSHR gene polymorphisms influence bone mineral density and bone turnover in postmenopausal women. European Journal of Endocrinology 163 165–172. [DOI] [PubMed] [Google Scholar]
- Richards JS 1994. Hormonal control of gene expression in the ovary. Endocrine Reviews 15 725–751. [DOI] [PubMed] [Google Scholar]
- Roberts V, Meunier H, Vaughan J, Rivier J, Rivier C, Vale W. & Sawchenko P. 1989. Production and regulation of inhibin subunits in pituitary gonadotropes. Endocrinology 124 552–554. [DOI] [PubMed] [Google Scholar]
- Ryan RJ, Charlesworth MC, McCormick DJ, Milius RP & Keutmann HT 1988. The glycoprotein hormones: recent studies of structure-function relationships. The FASEB Journal, 2 2661–2669. [DOI] [PubMed] [Google Scholar]
- Sairam MR & Bhargavi GN 1985. A role for glycosylation of the alpha subunit in transduction of biological signal in glycoprotein hormones. Science, 229 65–67. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Chiappa SA, Fink G & Sherwood NM 1976. Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature 264 461. [DOI] [PubMed] [Google Scholar]
- Schally AV, Arimura A, Kastin A, Matsuo H, Baba Y, Redding T, Nair R, Debeljuk L & White W 1971. Gonadotropin-releasing hormone: one polypeptide regulates secretion of luteinizing and follicle-stimulating hormones. Science 173 1036–1038. [DOI] [PubMed] [Google Scholar]
- Schneyer AL, Fujiwara T, Fox J, Welt CK, Adams J, Messerlian GM & Taylor AE 2000. Dynamic changes in the intrafollicular inhibin/activin/follistatin axis during human follicular development: relationship to circulating hormone concentrations. The Journal of Clinical Endocrinology & Metabolism 85 3319–3330. [DOI] [PubMed] [Google Scholar]
- Sizonenko PC, Burr IM, Kaplan SL & Grumbach MM 1970. Hormonal changes in puberty II. Correlation of serum luteinizing hormone and follicle stimulating hormone with stages of puberty and bone age in normal girls. Pediatric Research 4 36. [DOI] [PubMed] [Google Scholar]
- Smitz J, Wolfenson C, Chappel S & Ruman J 2016. Follicle-stimulating hormone: a review of form and function in the treatment of infertility. Reproductive Sciences 23 706–716. [DOI] [PubMed] [Google Scholar]
- Sowers MR, Greendale GA, Bondarenko I, Finkelstein JS, Cauley JA, Neer RM & Ettinger B. 2003. Endogenous hormones and bone turnover markers in pre-and perimenopausal women: SWAN. Osteoporosis International 14 191–197. [DOI] [PubMed] [Google Scholar]
- Stanton P, Burgon P, Hearn M & Robertson D 1996. Structural and functional characterisation of hFSH and hLH isoforms. Molecular and Cellular Endocrinology 125 133–141. [DOI] [PubMed] [Google Scholar]
- Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu L-L, Yaroslavskiy BB & Zhou H 2006. FSH directly regulates bone mass. Cell 125 247–260. [DOI] [PubMed] [Google Scholar]
- Takano K, Yamashita N, Kojima I, Kitaoka M, Teramoto A & Ogata E 1992. Effects of activin A and somatostatin on intact FSH secretion and intracellular Ca2+ concentration in human FSH-secreting pituitary adenoma cells. Biochemical and Biophysical Research Communications 182 1408–1415. [DOI] [PubMed] [Google Scholar]
- Tapanainen JS, Aittomäki K, Min J, Vaskivuo T & Huhtaniemi IT 1997. Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nature Genetics 15 205. [DOI] [PubMed] [Google Scholar]
- Thompson IR & Kaiser UB 2014. GnRH pulse frequency-dependent differential regulation of LH and FSH gene expression. Molecular and Cellular Endocrinology 385 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson TB, Lerch TF, Cook RW, Woodruff TK & Jardetzky TS 2005. The structure of the follistatin: activin complex reveals antagonism of both type I and type II receptor binding. Developmental Cell 9 535–543. [DOI] [PubMed] [Google Scholar]
- Ueno N, Ling N, Ying S-Y, Esch F, Shimasaki S & Guillemin R 1987. Isolation and partial characterization of follistatin: A single-chain Mr 35.000 monomeric protein that inhibits the release of follicle-stimulating hormone. Proceedings of the National Academy of Sciences of the United States of America 84 8282–8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Schwall R, Cravioto A, Zambrano E. & Damian-Matsumura P. 1992. Effects of gonadotrophin-releasing hormone, recombinant human activin-A and sex steroid hormones upon the follicle-stimulating isohormones secreted by rat anterior pituitary cells in culture. Journal of Endocrinology, 134, 97–106. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Midgley AR Jr, Beitins IZ & Padmanabhan V 1995. Follicle-stimulating isohormones: characterization and physiological relevance. Endocrine Reviews 16 765–787. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A & Timossi C 2000. Biochemical and functional aspects of gonadotrophin-releasing hormone and gonadotrophins. Reproductive BioMedicine Online 1 48–62. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Timossi C, Barrios-de-Tomasi J, Maldonado A & Nayudu P 2003. Impact of carbohydrate heterogeneity in function of follicle-stimulating hormone: studies derived from in vitro and in vivo models. Biology of Reproduction 69 379–389. [DOI] [PubMed] [Google Scholar]
- Vale W, Rivier J, Vaughan J, McClintock R, Corrigan A, Woo W, Karr D & Spiess J 1986. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature 321 776. [DOI] [PubMed] [Google Scholar]
- Vale W, Rivier C, Hsueh A, Campen C, Meunier H, Bicsak T, Vaughan J, Corrigan A, Bardin W, Sawchenko P, Petraglia F, Yu J, Plotsky P, Spiess J, Rivier J 1988. Chemical and biological characterization of the inhibin family of protein hormones. Recent Progress in Hormone Research 44 1–34. [DOI] [PubMed] [Google Scholar]
- Wang H, Larson M, Jablonka-Shariff A, Pearl CA, Miller WL, Conn PM, Boime I & Kumar TR 2014. Redirecting intracellular trafficking and the secretion pattern of FSH dramatically enhances ovarian function in mice. Proceedings of the National Academy of Sciences, 111, 5735–5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J, Duca KA & Crowley WF Jr 1990. Gonadotropin-releasing hormone-induced stimulation and desensitization of free α-subunit secretion mirrors luteinizing hormone and follicle-stimulating hormone in perifused rat pituitary cells. Endocrinology 127 2364–2371. [DOI] [PubMed] [Google Scholar]
- Welt CK 2004. Regulation and function of inhibins in the normal menstrual cycle In Seminars in Reproductive Medicine 22 187–193. Copyright© 2004 by Thieme Medical Publishers, Inc., 333 Seventh Avenue, New York, NY 10001, USA.. [DOI] [PubMed] [Google Scholar]
- Wen HY, Karanth S, Walczewska A, Sower SA & McCann SM 1997. A hypothalamic follicle-stimulating hormone-releasing decapeptide in the rat. Proceedings of the National Academy of Sciences 94 9499–9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wide L, Naessén T, Eriksson K & Rune C 1996. Time-related effects of a progestogen on the isoforms of serum gonadotrophins in 17β-oestradiol treated post-menopausal women. Clinical Endocrinology 44 651–658. [DOI] [PubMed] [Google Scholar]
- Wildt L, Häusler A, Marshall G, Hutchison J, Plant T, Belchetz P & Knobil E 1981. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109 376–385. [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Besecke LM, Groome N, Draper LB, Schwartz NB & Weiss J 1996. Inhibin A and inhibin B are inversely correlated to follicle-stimulating hormone, yet are discordant during the follicular phase of the rat estrous cycle, and inhibin A is expressed in a sexually dimorphic manner. Endocrinology 137 5463–5467. [DOI] [PubMed] [Google Scholar]
- Yding Andersen C 2017. Inhibin-B secretion and FSH isoform distribution may play an integral part of follicular selection in the natural menstrual cycle. MHR: Basic Science of Reproductive Medicine 23 16–24. [DOI] [PubMed] [Google Scholar]
- Ying S-Y 1988. Inhibins, activins, and follistatins: gonadal proteins modulating the secretion of follicle-stimulating hormone. EndocrineRreviews 9 267–293. [DOI] [PubMed] [Google Scholar]
- Yu W, Karanth S, Mastronardi C, Sealfon S, Dean C, Dees W & McCann S 2002. Lamprey GnRH-III acts on its putative receptor via nitric oxide to release follicle-stimulating hormone specifically. Experimental Biology and Medicine 227 786–793. [DOI] [PubMed] [Google Scholar]
- Zhu D, Li X, Macrae VE, Simoncini T & Fu X. 2018. Extragonadal effects of follicle-stimulating hormone on osteoporosis and cardiovascular disease in women during menopausal transition. Trends in Endocrinology & Metabolism 29 571–580. [DOI] [PubMed] [Google Scholar]
- Zhu L-L, Blair H, Cao J, Yuen T, Latif R, Guo L, Tourkova IL, Li J, Davies TF & Sun L 2012. Blocking antibody to the β-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proceedings of the National Academy of Sciences 109 14574–14579. [DOI] [PMC free article] [PubMed] [Google Scholar]


