Vascularized composite allotransplantation (VCA) is a promising new treatment for patients with severe soft-tissue damage, which cannot be restored by conventional methods. VCA has been applied clinically in over 10 countries, and used for many types of organs, such as the hand, forearm, face, abdominal wall and larynx [1–4]. However, unlike solid organ transplantation, VCA is still considered by many to be its experimental and not “standard of care” and many questions in this field remain unanswered. VCAs are life-improving rather than life-saving procedures. The associated immunosuppressive therapy and respective side effects must be weighed against the benefits of VCA. Therefore, more research is required to decrease the risks of VCA to a level at which the clinical use of this non-life-saving procedure can be justified.
Murine models are commonly used in VCA research because of their affordability and relatively fixed genetic backgrounds. A variety of such models has been established.5–8 However, although the majority of VCAs that have been performed thus far have involved upper extremity transplantations, no rat model of paw or forelimb VCA has been established. In this report, we present an orthotopic forelimb allotransplantation model in rat.
Materials and Methods
Eight inbred male Brown Norway (BN) rats weighing 250–275 g that expressed the RT1n gene were used as donors. Eight inbred male Lewis rats weighing 250–275 g that expressed the RT1l gene were used as recipients (Charles River Laboratories, Madison, WI). The experiment was approved by the institutional animal care and use committee of the Shanghai Jiaotong University School of Medicine.
Surgical Procedures
The rats were anesthetized via isoflurane inhalation (doses: 3% for induction anesthesia, and 2% for maintenance anesthesia). After anesthesia, a circular incision around the elbow joint, three midline incisions on the anterior aspect of the upper arm, and one incision each on the anterior and posterior aspects of the upper half of the donor forelimb. The ulnar, radial, and median nerves were identified and isolated. The axillary vessels (brachial vessels) were isolated from their origins in the chest wall and were followed to the elbow joint. The extensor and flexor muscles were divided at their origins. All muscles, together with the vascular pedicle and the three nerves, were peeled off of the subperiosteal surfaces of the ulna and radius down to the insertion of the biceps. Next, 0.35-mm drills were used to drill two small holes into the ulna. A rotary saw was used to amputate the ulna and radius just proximal to the interosseous membrane. The harvested forelimb was draped with salinesoaked gauze and stored at 48C until transplantation. Following forelimb procurement, the donor rats were sacrificed (Figs. 1A and 1B).
Figure 1.
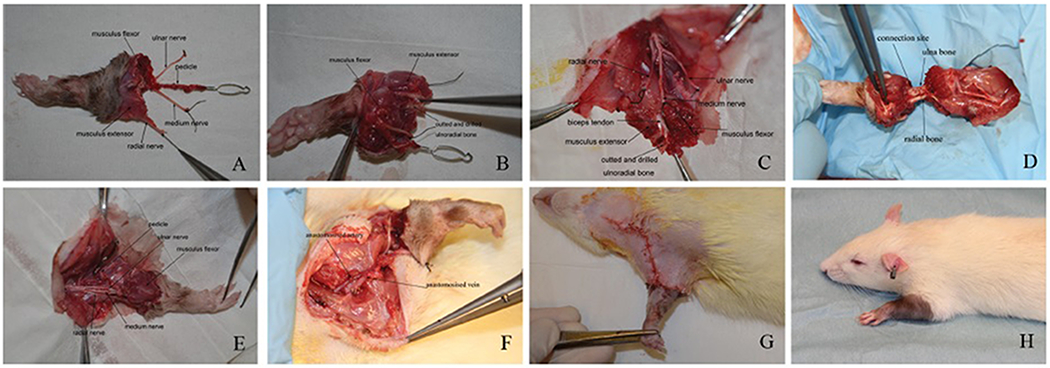
A, B: The harvested forelimb; C: recipient preparation; D: bone fixation; E: nerve and muscle repair F: vascular anastomoses; G: immediately after transplantation; H: one month after transplantation.
For preparation of recipients, a midline anterior incision and a circular incision were made on the proximal one-third of the forelimb. The ulnar, median, and radial nerves were identified, traced to the elbow joint, and transected. The flexor and extensor muscles were divided at the level of the proximal one-third of the forelimb. Next, two 0.35-mm holes were drilled in the ulna just proximal to the proximal edge of the interosseous membrane, and the ulna and radius were amputated at this level (Fig. 1C). An incision was made in the cervical region. The external jugular vein was exposed and prepared as a recipient vein. The common carotid artery was prepared as a recipient artery. Prior to transplantation, the donor forelimb and the amputated recipient forelimb were held side by side, and when required, the lengths of the donor bones were adjusted to create a properly fitting forelimb.
At transplantation, a 27-G needle of appropriate length was used as an intramedullary rod to connect the radial bones of both donor and recipient. Two wires (0.2-mm diameters) were used to connect the ulnar bones (Fig. 1D). The recipient extensor and flexor muscles were attached to their corresponding donor counterparts with nonabsorbable 7-0 monofilament sutures. The nerves were repaired and pedicle vessels were anastomosed in an end-to-end pattern using 11-0 microsurgical sutures (Figs. 1E and 1F). Finally, the wounds were closed using 4-0 absorbable sutures (Fig. 1G).
After surgery, a tapered doses of cyclosporine (Sandimmun, Novartis Pharma Schweiz AG, Switzerland) was administered to five of the animals (16 mg/kg/day in the first week, 8 mg/kg/day in the second week, 4 mg/kg/day in the third week, and 2 mg/kg/day thereafter). The other three rats did not receive any immunosuppressive treatment.
All transplanted forelimbs were assessed daily for surgery-related complications, perfusion, and signs of rejection transplanted forelimb. The forelimb movement and sensation were assessed weekly after surgery. Sensory recovery in the transplanted forelimb was confirmed with the finger pinch test9. On the 90th postoperative day, the recipients underwent X-ray and micro-CT examinations. The forelimbs were then harvested, fixed in 10% formalin solution for 3 days, and decalcified in Immunocal solution (Decal Chemical Corporation, Tallman, NY) for 14 days. The specimens were then embedded in paraffin and 5-mm cross-sections were taken from the midforelimb, wrist, and paw. The sections were stained with hematoxylin–eosin for histological study.
RESULTS
One rat in the group with CsA treatment failed to recover from surgery and subsequently died. All other recipients tolerated the transplantation procedure and returned to their activities on the second day after surgery. In the rats treated with cyclosporine, severe edema was observed in the first 7 days after surgery but all of the transplanted forelimbs were pink and pliable. Nail and new hair growth were observed within 20–25 days after transplantation (Fig. 1H). No auto-cannibalization was observed. The shoulder and elbow joints regained normal function and range of motion within 10 days after the operation. No active wrist or finger movements were noticed. However, normal ranges of passive motion were seen throughout the observation period. Muscle twitching and sensory recovery were observed in all rats beginning 4–6 weeks after surgery. Sensation was confirmed with the finger pinch test. In one rat, signs of rejection, including skin redness, peeling, and hair loss were observed in transplanted forelimb on the 75th postoperative day. In the rats without receiving cyclosporine, the transplanted forelimbs showed epidermal necrosis and the nail felling off, and the limbs ultimately exhibited necrosis around the 12th postoperative day.
In the rats treated with cyclosporine, X-rays and Micro-CT revealed good positions, good fixations, and bone healing in the transplanted forelimbs on the 90th postoperative day (Fig. 2). The histological samples revealed viable bone with bone marrow on the 90th postoperative day. The skin, subcutaneous connective tissue, vessels, tendons, and muscles exhibited normal structures without signs of rejection in all of rats except one (Fig. 3).
Figure 2.
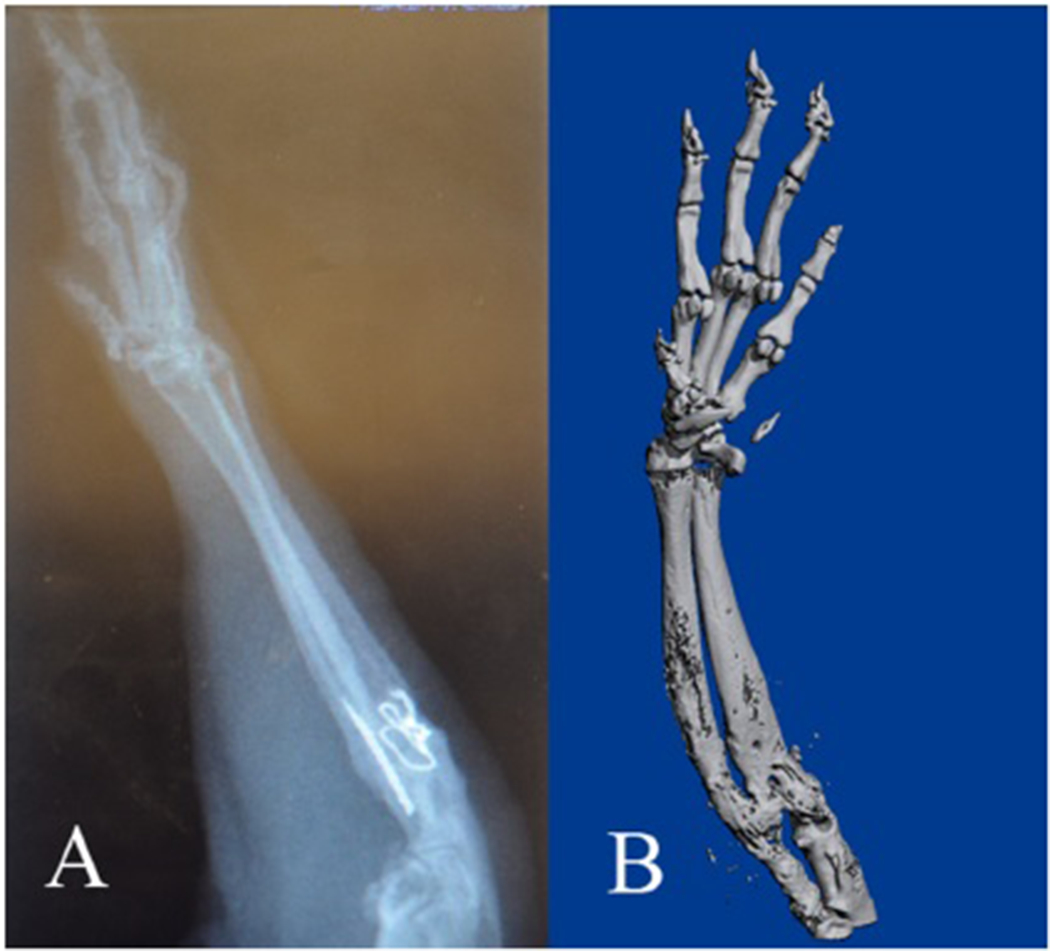
X-ray (A) and microCT (B) results of the transplanted forelimb showing bone healing on 90th postoperative day.
Figure 3.
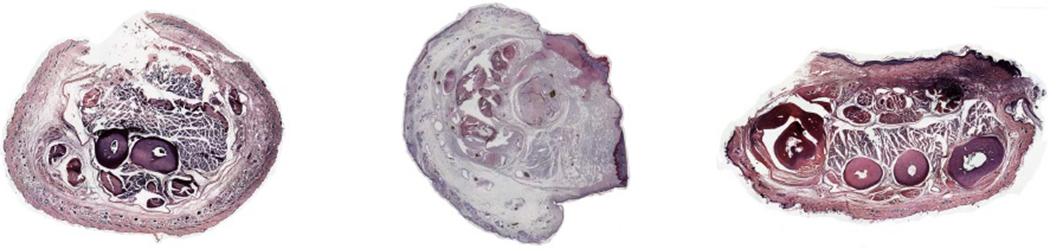
Histological results of the proximal part (left), wrist part (center), and paw part (right) of transplanted forelimb. All showed normal bone, muscle, and skin structures.
DISCUSSION
Dubernard et al. performed the first successful hand transplantation in Lyon, France in 1998. Since then, over 100 hand transplantations have been performed all over the world. However, the need for lifetime immunosuppressive therapy continues to limit the use of VCA, which is a nonlife-saving procedure, in the clinical setting.10 To overcome the immunological concerns associated with upper limb allotransplantation, researchers have developed a variety of experimental animal models that serve as vital tools for investigating transplantation and the related rejection phenomena. In this experimental study, we attempted to develop a rat model of forelimb allotransplantation.
Technically, all of the upper arm muscles were preserved to avoid impairing elbow joint function. Therefore, the recipient forelimb was amputated distal to the insertion of the biceps. The bone fixation methods in rat transplantation models include small Kirschner pins,11,12 pins prepared by modifying injection needles,13,14 and stainless steel wires.15 Considering the small size of the bones in this forelimb transplantation model, we selected combination fixation with intramedullary rods and wires.
The rat brachial vessels are very thin (~0.4–0.5 mm) with the exception of the segments near the chest wall (which are approximately 1 mm) before the branching of the infrascapular artery. We observed that this artery was well matched to the common carotid artery. The proper pedicle tension was attained by precisely controlling the length of the transplanted forelimb and dissecting the vascular pedicles of recipient site and donor forelimb as long as possible. Because the common carotid artery is situated deep within the cervical muscles, the arterial pedicle of forelimb was tunneled through the cervical muscles to approach the recipient.
Bone union was achieved ~3 months after the operation, which is similar to the healing times that have reported for other fractures in experimental studies.16,17 Functional recovery after VCA in rats has invariably been difficult.18 However, elbow joint function was fully maintained in our model based on our surgical design. Sensory recovery was similar to the report in the literature.19
Some similar and classic models in rats and other species have been published, such as the rat hindlimb transplant model, the rat iliac osseous myocutaneous flap model, and the swine gracilis myocutaneous free flap model. 5,20–24 Compared to these existing VCA models, our model requires a technically more demanding, which is the weakness of model.
In conclusion, this experimental study showed the feasibility of orthotopic forelimb allotransplantation in the rat model.
Reference:
- 1.Petruzzo P, Dubernard JM. The International Registry on hand and composite tissue allotransplantation. Clin Transpl 2011;25:247–253. [PubMed] [Google Scholar]
- 2.Strome M, Stein J, Esclamado R, Hicks D, Lorenz RR, Braun W, Yetman R, Eliachar I, Mayes J. Laryngeal transplantation and 40-month follow-up. N Engl J Med 2001;344:1676–1679 [DOI] [PubMed] [Google Scholar]
- 3.Zhu H, Wei X, Lineaweaver W, Li Q. Perioperative risk factors for vascularized composite allotransplantation: A systematic review and proposal of identity-defining VCA. Microsurgery 2014;34:240–244. [DOI] [PubMed] [Google Scholar]
- 4.Devauchelle B, Badet L, Lengele B, Morelon E, Testelin S, Michallet M, D’Hauthuille C, Dubernard JM. First human face allograft: Early report. Lancet 2006;368:203–209. [DOI] [PubMed] [Google Scholar]
- 5.Nasir S, Klimczak A, Sonmez E, Bozkurt M, Gibson S, Siemionow M. New composite tissue allograft model of vascularized bone marrow transplant: The iliac osteomyocutaneous flap. Transpl Int 2010; 23:90–100. [DOI] [PubMed] [Google Scholar]
- 6.Larsen M, Friedrich PF, Bishop AT. A modified vascularized whole knee joint allotransplantation model in the rat. Microsurgery 2010; 30:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siemionow M, Gozel-Ulusal B, Engin Ulusal A, Ozmen S, Izycki D, Zins JE. Functional tolerance following face transplantation in the rat. Transplantation 2003; 75: 1607. [DOI] [PubMed] [Google Scholar]
- 8.Yazici I, Unal S, Siemionow M. Composite hemiface/calvaria transplantation model in rats. Plast Reconstr Surg 2006;118:1321. [DOI] [PubMed] [Google Scholar]
- 9.Temporin K1, Tanaka H, Kuroda Y, Okada K, Yachi K, Moritomo H, Murase, Yoshikawa H. Interleukin-1 beta promotes sensory nerve regeneration after sciatic nerve injury. Neurosci Lett 2008; 440:130–133. [DOI] [PubMed] [Google Scholar]
- 10.Dubernard JM, Lengele B, Morelon E, Testelin S, Badet L, Moure C, Beziat JL, Dakpe S, Kanitakis J, D’Hauthuille C, El Jaafari A, Petruzzo P, Lefrancois N, Taha F, Sirigu A, Di Marco G, Carmi E, Bachmann D, Cremades S, Giraux P, Burloux G, Hequet O, Parquet N, Francès C, Michallet M, Martin X, Devauchelle B. Outcomes 18 months after the first human partial face transplantation. N Engl J Med 2007;357:2451–2460. [DOI] [PubMed] [Google Scholar]
- 11.Kim SK, Aziz S, Oyer P, Hentz VR. Use of cyclosporin A in allotransplantation of rat limbs. Ann Plast Surg 1984;12:249–255. [DOI] [PubMed] [Google Scholar]
- 12.Kuroki H, Bean MA, Ikuta Y, Akiyama M, Burgess EM. Effect of FK 506 and donor-specific blood transfusion on the rat composite tissue limb allograft and the mechanism of long-term graft survival. Transplant Proc 1993;25(1 Part 1):658–661. [PubMed] [Google Scholar]
- 13.Klimczak A, Agaoglu G, Carnevale KA, Siemionow M. Applications of bilateral vascularized femoral bone marrow transplantation for chimerism induction across the major histocompatibility (MHC) barrier. II. Ann Plast Surg 2006;57:422–430. [DOI] [PubMed] [Google Scholar]
- 14.Fealy MJ, Umansky WS, Bickel KD, Nino JJ, Morris RE, Press BH. Efficacy of rapamycin and FK506 in prolonging rat hind limb allograft survival. Ann Surg 1994;219:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hewitt CW, Black KS, Fraser LA, Howard EB, Martin DC, Achauer BM, Furnas DW. Composite tissue (limb) allografts in rats. I. Dosedependent increase in survival with cyclosporine. Transplantation 1985; 39:360–364. [DOI] [PubMed] [Google Scholar]
- 16.Hao Y, Ma Y, Wang X, Jin F, Ge S. Short-term muscle atrophy caused by botulinum toxin-A local injection impairs fracture healing in the rat femur. J Orthop Res 2012;30: 574–580. [DOI] [PubMed] [Google Scholar]
- 17.Mølster AO. Effects of rotational instability on healing of femoral osteotomies in the rat. Acta Orthop Scand 1984; 55:632–636. [DOI] [PubMed] [Google Scholar]
- 18.Yeh LS, Gregory CR, Theriault BR, Hou SM, Lecouter RA. A functional model for whole limb transplantation in the rat. Plast Reconstr Surg 2000; 105:1704–1711. [DOI] [PubMed] [Google Scholar]
- 19.Moore AM, Borschel GH, Santosa KA, Flagg ER, Tong AY, Kasukurthi R, Newton P, Yan Y, Hunter DA, Johnson PJ, Mackinnon SE. A transgenic rat expressing green fluorescent protein (GFP) in peripheral nerves provides a new hindlimb model for the study of nerve injury and regeneration. Neurosci Methods 2012; 204:19–27. [DOI] [PubMed] [Google Scholar]
- 20.Zor F, Bozkurt M, Nair D, Siemionow M. A new composite midface allotransplantation model with sensory and motor reinnervation. Transpl Int 2010; 23: 649–656. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro RI, Cerra FB. A model for reimplantation and transplantation of a complex organ: The rat hind limb. J Surg Res 1978; 24: 501–506. [DOI] [PubMed] [Google Scholar]
- 22.Kiermeir DM, Meoli M, uller S, Abderhalden S, ogelin E, Constantinescu MA. Evaluation of a porcine whole-limb heterotopic autotransplantation model. Microsurgery 2013; 33: 141–147. [DOI] [PubMed] [Google Scholar]
- 23.Leto Barone AA, Leonard DA, Torabi R, Mallard C, Glor T, Scalea JR, Randolph MA, Sachs DH, Cetrulo CL Jr. The gracilis myocutaneous free flap in swine: An advantageous preclinical model for vascularized composite allograft transplantation research. Microsurgery 2013; 33:51–55. [DOI] [PubMed] [Google Scholar]
- 24.Bozkurt M, Klimczak A, Nasir S, Zor F, Krokowicz L, Siemionow M. Composite osseomusculocutaneous sternum, ribs, thymus, pectoralis muscles, and skin allotransplantation model of bone marrow transplantation. Microsurgery 2013; 33:43–50. [DOI] [PubMed] [Google Scholar]


