Abstract
As the coronavirus disease 2019 (COVID-19, also called severe acute respiratory syndrome coronavirus-2) outbreak accelerates, vigorous and diverse efforts were made in developing treatment strategies. In addition to direct acting agents, increasing evidence showed some potential adjuvant therapies with promising efficacy against COVID-19. These therapies include immunomodulators (i.e. intravenous immunoglobulin, thymosin α-1, interleukin [IL]-6, tocilizumab, cyclosporine, thalidomide, fingolimod), Chinese medicines (i.e. glycyrrhizin, baicalin, Xuebijing), anti–vascular endothelial growth factors (bevacizumab), estrogen modulating drugs, statins, and nutritional supplements (i.e. vitamins A, B, C, D, E and zinc). This article reviewed the pharmacological development of potential adjuvants for COVID-19 treatment.
Keywords: Adjuvant therapy, COVID-19, Pharmacological development, SARS-CoV-2
1. INTRODUCTION
Based on the World Health Organization (WHO) report on COVID-19 pandemic, there are a total of 6 164 784 confirmed cases, 371 987 deaths, and 2 641 068 recovered until June 1, 2020. The novel coronavirus (severe acute respiratory syndrome coronavirus-2 [SARS-CoV-2]) is structurally similar to SARS-CoV-1 and Middle East respiratory syndrome coronavirus (MERS-CoV).1 The most common symptoms of COVID-19 infection include fever (83%-98.6%), cough (59.4%-82%), shortness of breath (31%), myalgia (11%-34.8%), accompanied by sore throats, rhinorrhea, and diarrhea in a few patients.2 Although the potential cause of COVID-19 is still unknown, initial reports predicted that it is possible for the virus to have a zoonotic origin.3 Respiratory droplets are believed to be the main route of transmission. However, transmission via the ocular surface should also be carefully prevented because the conjunctival epithelium is vulnerable to the infectious droplets and body fluid. COVID-19 virus can live on common surfaces (i.e. in air for 3 hours, on copper for 4 hours, on cardboard for 24 hours, on stainless steel for 2-3 days, and on polypropylene plastic for 3 days).4 Furthermore, vertical transmission between mothers and infants was reported as a potential transmission route due to the case of a 30-hour-old newborn tested positive for COVID-19 infection.5 However, observations in a small number of infected pregnant women showed that there was no mother–child transmission.6 It is still controversial regarding whether COVID-19 can be transmitted in utero from an infected mother to her infant before birth.
In the current review, other than the drug acting directly to COVID-19,7 we discussed the therapeutic options and focused on the pharmacological development of the potential adjuvants for clinical COVID-19 treatment.
2. PHARMACOLOGICAL DEVELOPMENT OF THE POTENTIAL ADJUVANTS
2.1. Immune modulators
Intravenous immunoglobulin (IVIG) has been widely applied in the clinical specialty fields including neurology, dermatology, rheumatology, and so on. IVIG exerts diverse effects on the immune system in a dose-dependent manner.8 Generally, at low doses (0.2-0.4 g/kg), IVIG is used in replacement therapy for antibody deficiency. At higher doses (up to 2 g/kg), however, IVIG exhibits its immunomodulatory functions such as suppressing inflammatory cells proliferation, inhibiting phagocytosis, and interfering with antibody-dependent-cytotoxicity. Current trial focus on the supplementary effects of low-dose IVIG for the COVID-19 patients are still ongoing (0.5 g/kg for 5 days, NCT04261426; 2 g/kg over 4 days, NCT04350580; 0.8 g/kg for 2 days, NCT04403269).
Thymosin α-1 is a thymic peptide hormone responsible for restoring the homeostasis of the immune system. It not only plays a critical role in thymocyte development but also increases thymocytes’ resistance to glucocorticoid-induced death.9,10 Evidence suggested that thymosin α-1 was used as an immune enhancer to SARS patients and was effective in controlling the spread of infection.11,12 According to the COVID-19 treatment guidelines from the National Health Commission (NHC), China, using thymosin α-1 might be an alternative treatment options for COVID-19 patients with low lymphocyte counts or are immunodeficient.13
A well-coordinated cytokine response is essential for a normal host immune response. A deregulated immune system leads to a hyper-inflammatory state which was the condition found in some COVID-19 patients. Evidence showed that the COVID-19 patients in the intensive care units (ICUs) had high levels of cytokines in their plasma compared to the non-ICU patients, indicating that cytokine storms might be associated with the disease severity.2 In addition, a larger population of CD4 T cells positive for granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-6 were found in COVID-19 patients in the ICU compared with the non-ICU patients (BioRxiv doi: 10.1101/2020.02.12.945576). However, due to the controversy of suppressing vigorous and unregulated systematic inflammation, the current guideline from NHC of China emphasizes that the routine usage of systematic corticosteroids is not recommended unless indicated for other reasons. Moreover, there were no available results showing that the SRAS-CoV or MERS patients can benefit from receiving regular corticosteroid treatment, which might be attributed to the suppression of original immune response against the virus.14 However, a low dose of corticosteroids treatment may provide potential benefit in a subgroup of critically ill SRAS-CoV-2-infected patients.15 Based on these results, treatment comprising the inhibition of excessive inflammatory response may be an adjuvant treatment for COVID-19 patients.
Tocilizumab is a humanized monoclonal antibody that inhibits both membrane-bound and soluble IL-6 receptors and was approved for giant cell arteritis, rheumatoid arthritis, and cytokine release syndrome. Recently, several case studies showed that tocilizumab can rapidly relief fever, suppress C-reactive proteins, and decrease the oxygen demands, improving lung capacity on computerized tomography imaging (chinaXiv:202003.00026).16 A multicenter, randomized, controlled clinical trial is ongoing to examine the efficacy and safety of tocilizumab for cytokine storm in COVID-19 patients (ChiCTR2000029765). The optimal administration of tocilizumab is not yet fully defined, nor is there a well-known IL-6 threshold level to define the progression of severe COVID-19 cases. It is imminent to proceed with a long-term observation and large clinical trials to assess the risks and benefits of tocilizumab for COVID-19 patients in the future.
Cyclosporine, a popular immunosuppressive drug used for many transplantation and autoimmune disorders, was identified to bind to the N protein of SARS-CoV and potentially affect the assembly and release process of the viral particles.17 Cyclophilin, which is a target of cyclosporine, is not only a key subunit of immunophilins but also plays a key role in viral infection by regulating their replication.18 Cyclosporine might target cyclophilin and interfere with CoVs’ replication such as SARS-CoV and avian infectious bronchitis viruses.19 Therefore, cyclosporine and its non-immunosuppressive derivatives might serve as potential drugs for broad-spectrum coronavirus inhibitors against the emerging COVID-19. However, the clinical results should be carefully interpreted due to immune-suppressive effects.
Thalidomide, an immunomodulatory and anti-inflammatory medication approved for multiple myeloma and erythema nodosum leprosum, was investigated for its clinical effects on T-cell stimulation, cell proliferation inhibition, and lung injury and pulmonary fibrosis reduction. Its anti-inflammatory action is originated from the ability to speed up the degradation of messenger RNA in blood cells and thus reduce tumor necrosis factor-α (TNFα). Moreover, it increases the secretion of interleukins, such as IL-12, and activates natural killer cells. Previous studies revealed that thalidomide can improve lung injury, increase survival, reduce inflammatory cell infiltration, and inhibit cytokines against H1N1 influenza virus in vivo.20 Recently, thalidomide, combined with low-dose glucocorticoid, was proved to have beneficial outcomes in a patient with severe COVID-19 pneumonia via its immunomodulatory effect. It inhibited the inflammatory cytokine storm, decreased oxygen demand by relieving anxiety, and mitigated vomiting and pleural effusion (Preprints 2020, 2020020395). Therefore, thalidomide may serve as a potential adjuvant treatment for COVID-19 patients in a critical condition. Further clinical trial on the efficacy of thalidomide for COVID-19 is still ongoing (NCT04273529).
Fingolimod (FTY720) is an immunosuppressive natural product derived from myriocin. It was isolated from the culture broth a type of entomopathogenic fungus (Isaria sinclairii) that is an eternal youth nostrum in traditional Chinese medicine through chemical modification.21 FTY720, an approved immunomodulating medication for clinical treatment of multiple sclerosis, together with ventilation support might be considered for critical patients to prevent the progress of acute respiratory distress syndrome (ARDS). Recently, it is investigated for COVID-19 patients (NCT04280588). However, it may increase the risk of infections due to fingolimod acting as an immunomodulator which might restrict the ability of the immune system to respond to viral infections. Hence, the clinical application of FTY720 for COVID-19 patients remains to be carefully investigated.
2.2. Chinese medicine
Glycyrrhizin, an active component of liquorice roots in Chinese medicine, was proposed to inhibit the replication of SARS-associated viruses in vitro and was suggested as an alternative treatment option for SARS.22 In addition, baicalin, another Chinese medicine and a flavonoid from Radix Scutellaria, was also found to inhibit SARS-CoV in vitro.23 Recent evidence demonstrated that Xuebijing, a Chinese herbal medicine extract infusion formulation, can reduce mortality in severe community-acquired pneumonia patients in China. It was suggested as a “may consider” alternative treatment for severe/critical COVID-19 cases in the NHC treatment guidelines.24 Furthermore, according to the Chinese clinical guidelines for COVID-19 pneumonia diagnosis and treatment (7th edition), several traditional Chinese medicines such as Huoxiang Zhengqi capsules, Jinhua Qinggan granules, Lianhua Qingwen capsules, and Shufeng Jiedu capsules might be used for the medical observation period under doctors’ instructions. Therefore, Chinese medicine might be considered as an adjuvant to enhance the host immunity against COVID-19.
Evidence revealed that Chinese medicines which were used to treat respiratory tract infections might be helpful for SARS-CoV-2 treatment.25,26 Recently, it was proposed that a combination of Western and Chinese medicines might be useful for COVID-19 patients.27 However, few published studies on Chinese medicine products for COVID-19 treatment, especially those of high quality and good scientific rigor, are available. Well-defined researches are urgently required to confirm the therapeutic effect of Chinese medicines. The mechanisms of their antiviral actions might need to be further investigated to elucidate their potential roles in COVID-19 clinical treatment.
2.3. Anti–vascular endothelial growth factor
Bevacizumab (Avastin) is a recombinant humanized monoclonal antibody which works as an anti–vascular endothelial growth factor (anti-VEGF) medication. It is approved for a number of cancers including colorectal cancer, non-small-cell lung cancer, kidney cancer, cervical cancer, ovarian cancer, and recurrent glioblastoma. Since pulmonary edema caused by inflammatory exudate process was a distinguishable feature of COVID-19, specific pharmacotherapeutic development targeting potent inducing factors that increase vascular permeability was proposed. Based on bevacizumab’s ability to decrease the VEGF levels caused by hypoxia and severe inflammation and maintain the infected respiratory tract epithelium, it might help relieve edema in COVID-19 patients.28 Evidence revealed that VEGF was a key factor and a potential therapeutic target in acute lung injuries (ALI) and ARDS. The efficacy and safety of bevacizumab in critical patients with COVID-19 are currently investigated (NCT04275414; NCT04305106).
2.4. Estrogen modulating drugs
Estrogen modulating drugs might be a repurposed remedy for CoVs.29 Epidemiological results indicated a higher incidence rate and fatalities in males than in females with SARS-CoV infections.30,31 The disease occurrence rate in men was about twofold higher than that in women during the MERS outbreak as well.32 Besides, the mortality of SARS-CoV infection was higher in ovariectomized females or those receiving estrogen receptor antagonist treatment in vivo.33 Interestingly, estrogen modulating compounds were only found to inhibit the replication of influenza A virus in primary human nasal epithelial cells derived from female donors.34 Estrogen receptors were suggested to be involved in inhibiting the viral replication,35 and selective estrogen receptor modulators (SERMs) were proposed to play a wide range of roles in inhibiting viral replication through the non-classical estrogen receptor pathways. In addition, SERMs could interfere with the viral entry/fusion steps even in the absence of estrogen receptors.36 Toremifene, a first generation SERM, exhibits potential antiviral effects against MERS-CoV, SARS-CoV, and Ebola virus in vitro,37,38 while other popular SERMs used for breast cancer, such as tamoxifen, also have potent anti-CoVs activities.39 Moreover, resveratrol, a phytoestrogen from grape seeds and red wine, was reported to have potent activity against MERS in vitro.40 In conclusion, estrogen modulating medications or phytoestrogen might be a potential alternative for the COVID-19 treatment.
2.5. Statins
Until now, no clear clinical evidence suggested that statins are beneficial for COVID-19 patients. However, several studies suggesting statin’s usage in COVID-19 were proposed. Statins may be helpful for the patients via regulating innate immune response. The myeloid differentiation primary response 88 (MYD88) gene, which is one of the highly inducible genes during SARS-CoV infection. It may activate the NF-kB pathway and induce inflammation.41,42 Statins can target the MYD88 pathway and may therefore have a protective effect in COVID-19 patients.41 Furthermore, statins reduce the mortality of chronic obstructive pulmonary diseases (COPD) and influenza infections.43,44 Hence, the usage of statins for those who have an indication might be recommended for COVID-19 patients. However, patients with liver enzymes elevation might influence the appropriate of statins usage.45 More attention should therefore be paid to evaluate clinical risks and benefits of statins and monitor its safety in COVID-19 patients.
2.6. Nutritional supplements
Evidence revealed that vitamin A supplements could reduce morbidity and mortality in different infectious diseases such as measles, diarrhea, measles-related pneumonia, human immunodeficiency virus (HIV) infection, and malaria.46 In addition, vitamin A supplements also provide some protection against the complications in lung diseases, malaria, and HIV infections.47 Adequate vitamin A in the diet can ameliorate the effects of infectious bronchitis virus, a kind of coronavirus commonly found in chickens fed with a vitamin A–deficient diet.48 The antiviral mechanism of vitamin A might be through the upregulation of the innate immune responses, inhibiting invasion of the virus during subsequent rounds of replications.49 Therefore, vitamin A supplement may be a potential alternative for the treatment of novel coronaviruses such as COVID-19. Vitamin B is a water-soluble vitamin and works with coenzymes in physiological homeostasis. Evidence showed that vitamin B2 and ultraviolet light effectively reduced the titer of MERS-CoV in human plasma products.50 Vitamin B deficiency weakens the host immune responses, which means that vitamin B supplement can be a remedy for COVID-19.
Vitamin C supports the integrity of the immune system and may be used against the coronavirus.51 Previous studies revealed that vitamin C can increase the resistance of chicken embryo tracheal organ cultures to avian coronaviruses.52 Several trials showed that vitamin C supplement could significantly lower the incidence of pneumonia, suggesting that vitamin C might reduce the susceptibility to lower respiratory tract infections.53 Lower respiratory tract infections were reported in the COVID-19 patients. Hence, vitamin C can be an alternative adjuvant agent for the treatment of COVID-19. The vitamin C transporters coupled with sodium transporter in human cells and their activities are strictly controlled by the blood vitamin C level.54 High dosage of vitamin C administered via the enteral route (>400 mg) may saturate the intestinal absorption and its plasma concentrations may not be high enough to scavenge the excessive reactive oxygen species.55 Hence, IV vitamin C infusion may be the preferred administration route to achieve adequate plasma concentration and prevent undesirable effects.56 Recently, there is an ongoing clinical trial in China using vitamin C 12 g IV twice per day for the treatment of COVID-19 (NCT04264533). Vitamin C might be a therapy option but is not recommended to be used individually against COVID-19 at this time.
Vitamin D is synthesized in the human body with sunlight. It maintains bone integrity and induces the maturation of immune cells. COVID-19 was reported to mostly affect middle-aged to elderly people who might have insufficient vitamin D due to their lack of exposure to the sunlight. The lack of sunlight may be due to the cohort being housebound or institutionalized.57,58 Vitamin E is a lipid-soluble vitamin responsible for reducing oxidative species via reaction with the free radicals. Reports showed that low vitamin D and E levels in calves might cause them to suffer from bovine coronavirus infections.58 Therefore, vitamin D and E supplements might work as another adjuvant therapy for the treatment of COVID-19.
Zinc is a dietary trace mineral and plays a crucial role for the maintenance and development of the innate and adaptive immune responses.59 In addition, increasing intracellular zinc with zinc-ionophores-like pyrithione can efficiently disrupt the replication of many RNA viruses.60 Moreover, the combination of zinc and pyrithione inhibits the replication of SARS-CoV.60 Therefore, zinc supplement may not only increase immune activity but also be an active antiviral agent against COVID-19.
In conclusion, we presented several adjuvant therapies for COVID-19 treatment and summarized the possible therapeutic options currently under investigation and the future outlook for the disease (Fig. 1). We also speculated on several mechanisms contributing to the novel profile of COVID-19 pneumonia. According to the current knowledge of COVID-19, it is believed that the combination of novel ideas and follow-up experiments for the potential antiviral agents can help us to have more remedies to combat COVID-19. Also, the healthcare professionals should pay more attention to ensuring the safety and efficacy of the novel treatments.
Fig. 1.
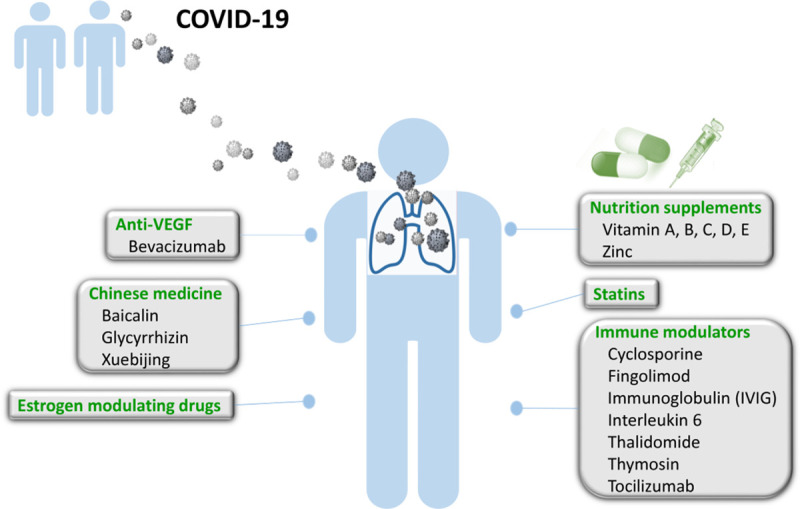
Potential adjuvants for COVID-19. COVID-19 = coronavirus disease 2019.
Footnotes
Author Contributions: Dr. Kuan-Hsuan Chen and Dr. Sheng-Fan Wang contributed equally to this work.
Conflicts of interest: The authors declare that they have no conflicts of interest related to the subject matter or materials discussed in this article.
REFERENCES
- 1.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 2020;5:536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. ; China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 2020;382:1564–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong L, Tian J, He S, Zhu C, Wang J, Liu C, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA 2020;323:1846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020;395:809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang SF, Chen KH, Wang SY, Yarmishyn AA, Lai WY, Lin YY, et al. The pharmacological development of direct acting agents for emerging needed therapy against SARS-CoV-2. J Chin Med Assoc 2020Doi:10.1097/JCMA.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez EE, Orange JS, Bonilla F, Chinen J, Chinn IK, Dorsey M, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol 2017;1393SS1–S46. [DOI] [PubMed] [Google Scholar]
- 9.Matteucci C, Grelli S, Balestrieri E, Minutolo A, Argaw-Denboba A, Macchi B, et al. Thymosin alpha 1 and HIV-1: recent advances and future perspectives. Future Microbiol 2017;12:141–55. [DOI] [PubMed] [Google Scholar]
- 10.Baumann CA, Badamchian M, Goldstein AL. Thymosin alpha 1 antagonizes dexamethasone and CD3-induced apoptosis of CD4+ CD8+ thymocytes through the activation of cAMP and protein kinase C dependent second messenger pathways. Mech Ageing Dev 1997;94:85–101. [DOI] [PubMed] [Google Scholar]
- 11.Kumar V, Jung YS, Liang PH. Anti-SARS coronavirus agents: a patent review (2008–present). Expert Opin Ther Pat 2013;23:1337–48. [DOI] [PubMed] [Google Scholar]
- 12.Gao ZC, Zhu JH, Sun Y, Ding XL, Ma JS, Cui YX, et al. Clinical investigation of outbreak of nosocomial severe acute respiratory syndrome. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2003;15:332–5.[In Chinese] [PubMed] [Google Scholar]
- 13.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol 2020;92:479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020;395:473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou W, Liu Y, Tian D, Wang C, Wang S, Cheng J, et al. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct Target Ther 2020;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol 2020Doi:10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo C, Luo H, Zheng S, Gui C, Yue L, Yu C, et al. Nucleocapsid protein of SARS coronavirus tightly binds to human cyclophilin A. Biochem Biophys Res Commun 2004;321:557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawar FU, Tu J, Khattak MN, Mei J, Lin L. Cyclophilin A: a key factor in virus replication and potential target for anti-viral therapy. Curr Issues Mol Biol 2017;21:1–20. [DOI] [PubMed] [Google Scholar]
- 19.Pfefferle S, Schöpf J, Kögl M, Friedel CC, Müller MA, Carbajo-Lozoya J, et al. The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. Plos Pathog 2011;7:e1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu H, Shi X, Ju D, Huang H, Wei W, Dong X. Anti-inflammatory effect of thalidomide on H1N1 influenza virus-induced pulmonary injury in mice. Inflammation 2014;37:2091–8. [DOI] [PubMed] [Google Scholar]
- 21.Adachi K, Chiba K. FTY720 story. Its discovery and the following accelerated development of sphingosine 1-phosphate receptor agonists as immunomodulators based on reverse pharmacology. Perspect Medicin Chem 2007;1:11–23. [PMC free article] [PubMed] [Google Scholar]
- 22.Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003;361:2045–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen F, Chan KH, Jiang Y, Kao RY, Lu HT, Fan KW, et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol 2004;31:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song Y, Yao C, Yao Y, Han H, Zhao X, Yu K, et al. XueBiJing injection versus placebo for critically ill patients with severe community-acquired pneumonia: a randomized controlled trial. Crit Care Med 2019;47:e735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends 2020;14:69–71. [DOI] [PubMed] [Google Scholar]
- 26.Ren JL, Zhang AH, Wang XJ. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res 2020;155:104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends 2020;14:64–8. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Fei D, Vanderlaan M, Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis 2004;7:335–45. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov 2020;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlberg J, Chong DS, Lai WY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol 2004;159:229–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leong HN, Earnest A, Lim HH, Chin CF, Tan C, Puhaindran ME, et al. SARS in Singapore—predictors of disease severity. Ann Acad Med Singapore 2006;35:326–31. [PubMed] [Google Scholar]
- 32.Alghamdi IG, Hussain II, Almalki SS, Alghamdi MS, Alghamdi MM, El-Sheemy MA. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int J Gen Med 2014;7:417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol 2017;198:4046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peretz J, Pekosz A, Lane AP, Klein SL. Estrogenic compounds reduce influenza A virus replication in primary human nasal epithelial cells derived from female, but not male, donors. Am J Physiol Lung Cell Mol Physiol 2016;310:L415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lasso G, Mayer SV, Winkelmann ER, Chu T, Elliot O, Patino-Galindo JA, et al. A structure-informed atlas of human-virus interactions. Cell 2019;178:1526–41.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansen LM, Brannan JM, Delos SE, Shoemaker CJ, Stossel A, Lear C, et al. FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection. Sci Transl Med 2013;5:190ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dyall J, Coleman CM, Hart BJ, Venkataraman T, Holbrook MR, Kindrachuk J, et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother 2014;58:4885–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Wilde AH, Jochmans D, Posthuma CC, Zevenhoven-Dobbe JC, van Nieuwkoop S, Bestebroer TM, et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother 2014;58:4875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pillaiyar T, Meenakshisundaram S, Manickam M. Recent discovery and development of inhibitors targeting coronaviruses. Drug Discov Today 2020;25:668–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin SC, Ho CT, Chuo WH, Li S, Wang TT, Lin CC. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis 2017;17:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan S. Statins may decrease the fatality rate of Middle East respiratory syndrome infection. mBio 2015;6:e01120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeDiego ML, Nieto-Torres JL, Regla-Nava JA, Jimenez-Guardeño JM, Fernandez-Delgado R, Fett C, et al. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J Virol 2014;88:913–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frost FJ, Petersen H, Tollestrup K, Skipper B. Influenza and COPD mortality protection as pleiotropic, dose-dependent effects of statins. Chest 2007;131:1006–12. [DOI] [PubMed] [Google Scholar]
- 44.Vandermeer ML, Thomas AR, Kamimoto L, Reingold A, Gershman K, Meek J, et al. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: a multistate study. J Infect Dis 2012;205:13–9. [DOI] [PubMed] [Google Scholar]
- 45.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int 2020;40:998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semba RD. Vitamin A and immunity to viral, bacterial and protozoan infections. Proc Nutr Soc 1999;58:719–27. [DOI] [PubMed] [Google Scholar]
- 47.Villamor E, Mbise R, Spiegelman D, Hertzmark E, Fataki M, Peterson KE, et al. Vitamin A supplements ameliorate the adverse effect of HIV-1, malaria, and diarrheal infections on child growth. Pediatrics 2002;109:E6. [DOI] [PubMed] [Google Scholar]
- 48.West CE, Sijtsma SR, Kouwenhoven B, Rombout JH, van der Zijpp AJ. Epithelia-damaging virus infections affect vitamin A status in chickens. J Nutr 1992;122:333–9. [DOI] [PubMed] [Google Scholar]
- 49.Trottier C, Colombo M, Mann KK, Miller WH, Jr, Ward BJ. Retinoids inhibit measles virus through a type I IFN-dependent bystander effect. FASEB J 2009;23:3203–12. [DOI] [PubMed] [Google Scholar]
- 50.Keil SD, Bowen R, Marschner S. Inactivation of Middle East respiratory syndrome coronavirus (MERS-CoV) in plasma products using a riboflavin-based and ultraviolet light-based photochemical treatment. Transfusion 2016;56:2948–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hemilä H. Vitamin C and SARS coronavirus. J Antimicrob Chemother 2003;52:1049–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Atherton JG, Kratzing CC, Fisher A. The effect of ascorbic acid on infection chick-embryo ciliated tracheal organ cultures by coronavirus. Arch Virol 1978;56:195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hemilä H. Vitamin C intake and susceptibility to pneumonia. Pediatr Infect Dis J 1997;16:836–7. [DOI] [PubMed] [Google Scholar]
- 54.Rodrigo R, Guichard C, Charles R. Clinical pharmacology and therapeutic use of antioxidant vitamins. Fundam Clin Pharmacol 2007;21:111–27. [DOI] [PubMed] [Google Scholar]
- 55.Graumlich JF, Ludden TM, Conry-Cantilena C, Cantilena LR, Jr, Wang Y, Levine M. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm Res 1997;14:1133–9. [DOI] [PubMed] [Google Scholar]
- 56.Jackson TS, Xu A, Vita JA, Keaney JF., Jr Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res 1998;83:916–22. [DOI] [PubMed] [Google Scholar]
- 57.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 2004;806 Suppl1678S–88S. [DOI] [PubMed] [Google Scholar]
- 58.Nonnecke BJ, McGill JL, Ridpath JF, Sacco RE, Lippolis JD, Reinhardt TA. Acute phase response elicited by experimental bovine diarrhea virus (BVDV) infection is associated with decreased vitamin D and E status of vitamin-replete preruminant calves. J Dairy Sci 2014;97:5566–79. [DOI] [PubMed] [Google Scholar]
- 59.Maares M, Haase H. Zinc and immunity: an essential interrelation. Arch Biochem Biophys 2016;611:58–65. [DOI] [PubMed] [Google Scholar]
- 60.te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. Plos Pathog 2010;6:e1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]


