Abstract
Dopamine D2 autoreceptors (D2ARs), located in somatodendritic and axon terminal compartments of dopamine (DA) neurons, function to provide a negative feedback regulatory control on DA neuron firing, DA synthesis, reuptake and release. Dysregulation of D2AR-mediated DA signaling is implicated in vulnerability to substance use disorder (SUD). Due to the extreme low abundance of D2ARs compared to postsynaptic D2 receptors (D2PRs) and the lack of experimental tools to differentiate the signaling of D2ARs from D2PRs, the regulation of D2ARs by drugs of abuse is poorly understood. The recent availability of conditional D2AR knockout mice and newly developed virus-mediated gene delivery approaches have provided means to specifically study the function of D2ARs at the molecular, cellular and behavioral levels. There is a growing revelation of novel mechanisms and new proteins that mediate D2AR activity, suggesting that D2ARs act cooperatively with an array of membrane and intracellular proteins to tightly control DA transmission. This review highlights D2AR-interacting partners including transporters, G-protein-coupled receptors, ion channels, intracellular signaling modulators, and protein kinases. The complexity of the D2AR interaction network illustrates the functional divergence of D2ARs. Pharmacological targeting of multiple D2AR-interacting partners may be more effective to restore disrupted DA homeostasis by drugs of abuse.
Keywords: Dopamine D2 autoreceptors, dopamine, psychostimulants
1. Introduction
Substance use disorder (SUD) is a chronic relapse disease characterized by compulsive and impulsive drug-seeking and taking behavior, inability to limit drug intake and emergence of negative affectivity during withdrawal (Koob & Volkow, 2010). SUD is a serious public health problem with devastating consequences to society. Opioid abuse has emerged as the deadliest drug crisis in the American history. There is an urgent need to improve our understanding of the neurobiological complexity underpinning SUD so that new, better pharmacotherapies can be developed.
Drugs of abuse exert their effects by disrupting signal transduction of multiple neurotransmitter systems and thus producing profound changes in the expression, localization, and function of many G-protein coupled receptors (GPCRs), transporters and ion channels [see review in (Ross & Peselow, 2009; Vetulani, 2001)]. The growing revelation of new proteins and novel mechanisms implicated in SUD has expanded the repertoire of pharmacological targets. However, monodrug therapies targeting individual proteins have not been effective. In recent years it has become apparent that receptors, transporters and ion channels do not operate alone in native tissues. It remains debatable whether or not these membrane proteins are assembled into functional and stable heterodimers or oligomers on the plasma membrane (Frederick, et al., 2015; Fuxe, et al., 2012; Kasai, et al., 2018; Lambert, 2010; Milligan, 2009). However, they do form functional complexes with other membrane proteins as well as cytoplasmic proteins including scaffold proteins and protein kinases to regulate intracellular signal transduction (Natarajan & Berk, 2006; Sager & Torres, 2011; Seagar & Takahashi, 1998). Disruption of the protein-protein interaction network induces aberrant cellular activity and signaling of specific proteins within the network. For example, it has been demonstrated in cultured cells and in vivo that there are reciprocal interactions between adenosine A2 receptors (A2ARs) and dopamine (DA) D2 receptors (D2Rs) (Kull, et al., 1999; Salim, et al., 2000; Shen, et al., 2013; Taura, et al., 2018). Chronic cocaine exposure abolishes the functional interaction between D2Rs and A2ARs in rat striatum resulting in abnormal signaling for both DA and adenosine (Borroto-Escuela, et al., 2017; Pintsuk, et al., 2016). Moreover, human methamphetamine (METH) abusers show persistent deficits in DA function including reduced striatal D2R availability (Volkow, et al., 1999). Interestingly, genetic variations in human ADORA2A, the gene encoding A2ARs, are associated with METH dependence (Kobayashi, et al., 2010) and anxiety following amphetamine (AMPH) consumption (Hohoff, et al., 2005). These data suggest that responses to METH are at least in part dependent on the balance between DA and adenosine signaling mediated by D2Rs and A2ARs, respectively. Understanding the function of protein complexes at the molecular, cellular and behavioral levels will potentially provide valuable information for applying drug combinations to target multiple components of the protein complexes and their signaling pathways for future treatment of SUD.
A major neurotransmitter system involved in SUD is the DA system, which consists the mesocorticolimbic, nigrostriatal and tuberoinfundibular DA pathways. The mesocorticolimbic DA pathway originates from the ventral tegmental area (VTA) and projects to the nucleus accumbens (NAc), amygdala and prefrontal cortex (PFC). Altered DA signaling in the mesocorticolimbic DA pathway has been classically associated with the actions of drugs of abuse especially psychostimulants (Di Chiara & Imperato, 1988; Wise, 1996). The ability of cocaine and AMPH to increase midbrain DA neuron firing and augment synaptic DA concentrations in the NAc and PFC is the primary underlying mechanism for their reinforcing and rewarding properties. In addition, the mesocorticolimbic DA pathway regulates cognitive function and emotional behavior. The nigrostriatal DA pathway, projecting from the substantia nigra (SN) to the caudate putamen (CPu), is not only critically involved in motor function but is also implicated in brain-stimulation reward, intravenous drug self-administration and reward learning [see review in (Wise, 2009)]. Although these two DA pathways share common roles in regulation of reward function, the VTA/NAc and SN/CPu regions are interconnected with distinct subpopulations of neurons, and thus contribute differentially to DA-mediated activity (Haber, 2014; Wise, 2009). Lastly, DA neurons in the hypothalamus project via the tuberoinfundibular tract to the infundibulum and anterior pituitary, and DA within this pathway inhibits the release of prolactin (Moore, 1992).
Extracellular DA binds to DA receptors to induce diverse intracellular signaling events that control DA-related physiological functions including DA synthesis, locomotor activity, reward, learning and memory (Dalley & Everitt, 2009). The DA receptors are categorized into two classes with distinct pharmacological properties and signal transduction pathways: the D1-like receptors (D1Rs and D5Rs) and the D2-like receptors (D2Rs, D3Rs and D4Rs). The D1-like receptors are coupled to the heterotrimeric G-proteins Gαs or Golf to stimulate the activity of adenylyl cyclase (AC) and increase the synthesis of cyclic adenosine monophosphate (cAMP). The D1R-like receptors are located on non-DA neurons, and modulate neuron excitability and neurotransmitter release via various mechanisms including cAMP-mediated activation of the second messengers including protein kinase A (PKA) and phosphoinositide, calcium mobilization, activation of calcium and sodium channels and induced trafficking of ionotropic glutamate receptors (Sidhu, 1998; Undieh, 2010). The D2-like receptors are coupled to the Gαi/o-protein to inhibit the activity of AC and thus reduce intracellular cAMP production (Beaulieu, et al., 2015). The D2-like receptors are present on DA and non-DA neurons and control neuron excitability and the release of neurotransmitters including DA, glutamate, GABA and acetylcholine.
Exposure to drugs of abuse such as cocaine produces marked changes in the expression, localization and function of D1- and D2-like receptors and these changes are linked to vulnerability to relapse (Anderson & Pierce, 2005; Ashok, et al., 2017). Thus, both D1Rs and D2Rs are potential therapeutic targets for treatment of SUD. The D2Rs are categorized into two subtypes: D2 autoreceptors (D2ARs) and postsynaptic D2 receptors (D2PRs; or heteroreceptors) based on their cellular localization and functionality. Tremendous efforts have been devoted to delineate the role of striatal D2PRs in the regulation of SUD [see review in (Ashok, et al., 2017)]; however, emerging evidence sheds light on an equivalent, important contribution of D2ARs to SUD. The important role of D2ARs in regulation of DA signaling has been elegantly reviewed (Ford, 2014). The objective of the current review is two-fold: a) to summarize the association of dysfunctional D2AR with addiction-like behavior; and b) to describe the diverse functional interactions of D2ARs with other membrane and intracellular proteins that mediate DA signaling and behavioral responses to drugs.
2. D2ARs: Distribution and Function
2.1. D2ARs: Cellular and Anatomic Distribution
The D2ARs are abundantly expressed in the somatodendritic compartments of midbrain DA neurons and presynaptically on their projections to the ventral and dorsal striatum. However, the D2ARs are absent at terminal sites of DA neurons projected from the midbrain to the cortex (Chiodo, et al., 1984; Lammel, et al., 2008; Talmaciu, et al., 1986). The D2PRs are concentrated in neurons receiving DA afferents in various brain regions including GABA neurons and acetylcholinergic interneurons in the striatum and cortex (Sesack, et al., 1994).
The D2Rs exist in the long (D2LR) and short (D2SR) form by an alternative splicing at exon 6 of the same gene (Tan, et al., 2003). The D2LRs differ from D2SRs by the addition of 29 amino acids in the third intracellular loop, which contributes to their differential coupling to Gα subunits and downstream signal transduction shown in COS-7 cells (Montmayeur, et al., 1991). Because they are splice variants, the mRNA for both D2SR and D2LR is postulated and further confirmed to be expressed in the same DA neurons (Dal Toso, et al., 1989; Jang, et al., 2011; Montmayeur, et al., 1991; Neve, et al., 2013; Neve, et al., 1991) and non-DA neurons (Neve, et al., 2013). However, it is technically challenging to determine whether the proteins for D2SRs and D2LRs are equally expressed in the same neurons due to a lack of distinguishable antibodies and radioactive ligands for these two isoforms.
2.2. D2ARs: Physiological Function
The D2Rs belong to a subfamily of 7-transmembrane domain GPCRs that couple to Gαi/o proteins. Activation of the D2Rs induces diverse downstream signaling via both Gαi/o and Gβγ subunits. Inhibition of AC activity, potassium channel activity, and mitogen-activated protein kinase activity is mediated by Gαi/o subunits whereas activity of ion channels, protein kinases and phospholipases is regulated by dissociated Gβγ subunits (Neve, et al., 2004). Due to their differences in cellular localization in vivo, the D2ARs and D2PRs have distinct functions in regulation of DA neuron firing, DA release and synthesis. The D2ARs operate through a negative feedback mechanism to tightly control DA transmission. Somatodendritic D2ARs directly couple to G-protein-coupled inwardly rectifying potassium (GIRK) channels via Gβγ subunits (Pillai, et al., 1998). Activation of D2ARs opens GIRK channels, which increases the membrane potassium conductance and thus inhibits DA neuron firing (Beckstead, et al., 2004; Lacey, et al., 1987; Pucak & Grace, 1994). Axon terminal D2ARs regulate exocytotic DA release via the voltage-gated Kv1 potassium channels. Activation of D2ARs increases Kv1 currents, and inhibits neuron terminal excitability and probability for DA release (Cubeddu & Hoffmann, 1982; Schmitz, et al., 2003; Wolf & Roth, 1990). The D2ARs modulate the opening of mouse striatal Kv1 channels via Gβγ subunits as blockade of Gβγ subunits abolishes D2AR-mediated DA overflow and potentiation of Kv1 channel conductance (Fulton, et al., 2011). For D2PRs on non-DA neurons, receptor activation regulates the release of neurotransmitters including GABA (Centonze, et al., 2002), glutamate (Bamford, et al., 2004) and acetylcholine (Bamford, et al., 2004), which in turn influences presynaptic DA release. It is important to note that D2PRs act synergistically with D1Rs to regulate cellular and behavioral responses to direct and indirect DA receptor agonists (Hasbi, et al., 2011); however, this is not the case for D2ARs because D1Rs are not expressed in DA neurons.
DA synthesis is regulated by D2ARs through its inhibition of the activity of tyrosine hydroxylase (TH), the rate-limiting enzyme in DA synthesis (Bunney, et al., 1973). Although axon terminal D2ARs have been considered to play a major role in DA synthesis in the striatum, somatodendritic D2ARs are also implicated (Argiolas, et al., 1982). TH activity is primarily determined by its phosphorylation (Haycock & Haycock, 1991; Lindgren, et al., 2000). Activation of D2ARs by quinpirole reduces basal and forskolin-stimulated TH phosphorylation at Ser40 but not Ser19 and Ser31, suggesting that D2ARs can inhibit TH phosphorylation via decreasing AC activity (Lindgren, et al., 2001). However, a recent study shows that quinpirole stimulates TH phosphorylation at Ser40 in mice that have either D2AR deletion in DA neurons or D2PR deletion in striatal GABA neurons (Anzalone, et al., 2012), indicating that both D2ARs and D2PRs are involved in the feedback mechanism in regulation of DA synthesis.
It appears that the D2SRs and D2LRs function equivalently as autoreceptors. Both isoforms have similar binding affinities for DA and share the same canonical signaling pathways in HEK293 cells (Leysen, et al., 1993). Electrophysiological recordings on Xenopus oocytes co-expressing GIRKs and D2SRs or D2LRs are similar in DA inhibition of GIRK activation (Sahlholm, et al., 2008). Moreover, in rat primary mesencephalic neurons transfected with D2SRs or D2LRs, both isoforms inhibit neuronal firing and neurotransmitter release with similar potencies (Jomphe, et al., 2006). Lastly, these two isoforms are equally functional as autoreceptors in inhibition of GIRK currents in the SN of D2R knockout mice expressing either D2SRs or D2LRs (Neve, et al., 2013). However, several lines of evidence also suggest that D2SRs and D2LRs are molecularly and functionally distinct from each other. First, D2SRs are more susceptible to agonist-induced internalization than D2LRs in cultured cell lines and primary cultured mouse DA neurons (Ito, et al., 1999; Itokawa, et al., 1996; Liu, et al., 1992; Morris, et al., 2007; Thibault, et al., 2011). Second, these two isoforms differ in their posttranslational modifications and intracellular transport in CHO cells (Fishburn, et al., 1995). The D2LRs are processed to the matured form (fully glycosylated, ~70 kDa) much slower than are D2SRs. A notable portion of the intracellular D2LR pool is partially glycosylated and remains in the endoplasmic reticulum and Golgi without reaching to the plasma membrane. Therefore, these two isoforms are coupled to different proteins that mediate post-translational processing and intracellular compartmental transport, which may contribute to their differential responses to agonist-induced signaling transduction and internalization. Third, D2SRs are more susceptible to desensitization than D2LRs. Acute cocaine exposure reduces D2SR inhibition of GIRK currents in the SN of D2LR knockout mice but has no effect on D2LR activity in D2SR knockout mice (Robinson, et al., 2017b). Moreover, D2SR-, but not D2LR-mediated desensitization of GIRK currents is calcium dependent (Gantz, et al., 2015). Finally, using D2LR and D2SR knockout mice, the Borrelli group elegantly delineates the differential roles of these two isoforms in regulation of pre- and post-synaptic DA signaling (Radl, et al., 2018). While D2SRs function as autoreceptors on DA neurons to control DA synthesis via TH phosphorylation, D2LRs are necessary for postsynaptic D2R-mediated locomotor response to stimuli (e.g. cocaine) and appear to act through regulation of striatal acetylcholine and activity of medium spiny neurons. These observations are in agreement with a previous report that the D2SRs are predominantly expressed in the cell bodies and proximal and distal dendrites of DA neurons whereas the D2LRs are postsynaptic, mostly present in the striatal neurons innervated by DA fibers (Khan, et al., 1998)
3. D2ARs and Vulnerability to SUD
It has been well established that there is an inverse relationship between the availability of striatal D2PRs and the vulnerability for drug intake in humans (Martinez, et al., 2004; Shumay, et al., 2012; Volkow, et al., 1990; Volkow, et al., 1999), non-human primates (Nader & Czoty, 2005; Nader, et al., 2006), rats (Maggos, et al., 1998; Tsukada, et al., 1996), and mice (Bailey, et al., 2008). However, accumulating evidence also suggests an apparent association of D2AR expression and activity with vulnerability to drug abuse. A recent publication clearly shows that a partial Drd2 deletion in rat VTA causes a global elevation in functional brain activity (Martin, et al., 2020), demonstrating the significant impact of altered activity of D2Rs in the midbrain on brain networks that are involved in processing reward, motor, cognition and emotional information.
3.1. D2ARs and Novelty-seeking
Novelty seeking is commonly used to assess individual vulnerability for drug-taking behavior (Piazza, et al., 1989). Animals can be characterized as high- or low-responders based on novelty-induced locomotor activity. High-responders exhibit a greater and longer duration of locomotor activity than low-responders do, and are more likely self-administer abused drugs including cocaine (Flagel, et al., 2016; Marinelli & White, 2000), AMPH (Piazza, et al., 1989; Piazza, et al., 1998), morphine (Xigeng, et al., 2004), and ethanol (Nadal, et al., 2002).
Novelty-seeking behavior is mediated in part by D2AR-mediated DA transmission. In humans, individuals high on novelty-seeking possess low availability of midbrain D2Rs/D3Rs (mostly autoreceptors) measured by positron emission tomography (PET) of [18F]fallypride, a D2R/D3R antagonist (Zald, et al., 2008). Ex vivo autoradiography of [18F]Fallypride binding shows that high-responding rats display lower levels of D2Rs in the midbrain when compared to low-responding rats (Tournier, et al., 2013). Moreover, D2AR knockout mice exhibit hyperlocomotor activity in a novel environment (Bello, et al., 2011). Low D2AR activity in high novelty-seeking animals contributes to high basal firing and bursting activity of DA neurons in these animals (Bello, et al., 2011; Marinelli & White, 2000). There is no difference in the binding of the D3R-preferring antagonist [3H]PHNO in rat midbrain between high- and low-responders (Tournier, et al., 2013), suggesting novelty-seeking behavior is primarily mediated by D2ARs instead of D3ARs.
High novelty-seekers are associated with increased axon terminal DA release presumably mediated by subsensitive presynaptic D2ARs. Low doses of quinpirole preferentially activate D2AR on DA terminals resulting in inhibition of DA release and suppression of locomotor activity induced by novel environment (Eilam & Szechtman, 1989; Hu & Wang, 1988; White & Wang, 1986). Thus, novelty-induced locomotor suppression by quinpirole is commonly used as a measure for presynaptic D2AR activity. High-responders are less sensitive to quinpirole inhibition of novelty-induced locomotor activity than are low-responders (Tournier, et al., 2013). High-responders also display higher striatal basal DA levels and greater DA release by AMPH than low responders do (Bradberry, et al., 1991; Braff, et al., 1992; Tournier, et al., 2013). Furthermore, in drug naïve healthy humans, the amount of striatal DA release induced by AMPH treatments correlates with the intensity of novelty-seeking, drug craving and cognition deficits (Leyton, et al., 2002; Riccardi, et al., 2006). Collectively, these data suggest that high novelty-seeking is associated with low levels and/or reduced activity of D2ARs. The D2AR subsensitivity confers elevated DA signaling, which likely subserves novelty-seeking and propensity to drug-taking behavior.
3.2. D2ARs and Impulsivity for Drugs
A prominent behavioral phenotype of drug addicts is impulsive behavior or lack of self-control. Impulsivity is characterized by inability to wait, altered time perception and insensitivity to long-term rewards despite the negative consequence (de Wit, 2009). Impulsivity is considered to be an important factor contributing to relapse (Volkow, et al., 2010). Experimentally, impulsivity is frequently measured by delay discounting task which assesses preference for smaller immediate rewards than larger delayed rewards (Bickel & Marsch, 2001). High impulsivity is associated with choice for an immediate reward. Human patients addicted to cocaine (Garcia-Rodriguez, et al., 2013), METH (Hoffman, et al., 2006), nicotine (Bickel, et al., 1999), opioids (Kirby, et al., 1999), and alcohol (Mitchell, et al., 2005) all favor smaller immediate rewards over larger delayed rewards as compared to healthy subjects. High impulsivity measured by delay discounting is a clinical relevant model for understanding why drug addicts choose the transient effect of drug reward without thinking about the negative long-term consequences.
DA signaling in the PFC is implicated in impulsivity. During the delay discounting performance, there is an increase in the DA metabolite 3,4-dihydroxyphenylacetic acid in rat orbitofrontal cortex (Winstanley, et al., 2006), suggesting DA utilization in decision making of delay discounting. In agreement with this notion, acute AMPH treatment to healthy humans deceases discounting of delayed reward when compared to placebo treatment (de Wit, et al., 2002). However, high impulsivity in humans is also associated with greater AMPH-induced DA release in the striatum (Buckholtz, et al., 2010). Thus, DA transmission in both the mesolimbic and mesocortical pathways appears to mediate impulsivity although through different mechanisms. Low levels of D2ARs in the midbrain are linked to high impulsivity and drug craving in human (Buckholtz, et al., 2010), presumably due to diminished control of axon terminal DA release and midbrain DA neuron excitability. A recent study further reveals that there is a direct causal, inverse relationship between midbrain D2AR levels and impulsivity (Bernosky-Smith, et al., 2018). When Drd2 was decreased in the VTA by an adeno-associated virus (AAV) encoding shRNAs against Drd2, rats exhibited greater choice impulsivity for the smaller, immediate reward as compared to their corresponding control animals.
3.3. D2AR, Drug Self-administration and Reinstatement
A deficit in D2AR activity results in increased DA release and enhanced response to cocaine. Conditional Drd2 autoreceptor knockout mice are more sensitive to cocaine-induced locomotor activity and cocaine-conditioned place preference as compared to controls (Bello, et al., 2011). These mice acquire cue-triggered self-administration of cocaine faster and show enhanced salience of cocaine- but not sucrose-paired cues. However, conditional knockout of Drd2 does not change the total cocaine intake in responding to 1 mg/kg/infusion cocaine in these mice (Holroyd, et al., 2015). In contrast, Drd2 knockdown in rat VTA via lentivirus-mediated shRNA delivery dose-dependently increases cocaine self-administration at doses between 0.02–0.56 mg/kg/infusion (Chen, et al., 2018). The differences in the animal species, the potential compensatory changes resulting from the complete Drd2 knockout, and the doses of cocaine may contribute to the observed discrepancy between these two studies. Interestingly, Drd2 knockdown in only the NAc increases cocaine intake at lower doses (0.02–0.07 m/kg/infusion) and has no effect when cocaine doses are higher than 0.07 m/kg/infusion. These data suggest the dissociable roles of D2Rs in the VTA and the NAc in limiting high doses of cocaine consumption.
Relapse to drug-seeking behavior is in part mediated by neuroadaptations in mesocorticolimbic DA signaling following chronic drug exposure (Koob & Volkow, 2010). The activity of VTA DA neurons controls the strength of DA signaling in the mesocorticolimbic circuit and plays a key role in drug reinstatement. Blockade of ionic glutamate receptor-mediated DA neuron activation in the VTA prevents cocaine reinstatement (Sun, et al., 2005; Xue, et al., 2011). Because DA neuron activity is tightly controlled by D2AR, deficiency in D2AR activity would precipitate relapse. Microinjection of quinpirole into rat VTA dose-dependently decreases cocaine reinstatement and this process is reversed by co-administration of the D2R/D3R antagonist eticlopride (Xue, et al., 2011). Furthermore, prior exposure to AMPH in rat VTA desensitizes D2ARs and potentiates AMPH-seeking behavior under a progressive ratio self-administration schedule (Vezina, et al., 2002). Interestingly, the persistency of D2AR adaptation to chronic drug exposure is brain region- and function-specific. The reduced presynaptic D2AR activity in inhibition of striatal DA release following chronic AMPH treatment appears to be transient because it only lasts 3–4 days (Wolf, et al., 1993). In contrast, AMPH treatment (i.p. 5 mg/kg, once or twice daily for 7 days) produces a marked reduction in D2AR-mediated inhibition of DA neuron firing in rat VTA after 8 days of abstinence although to a lesser degree when compared to 24–32 hrs withdrawal from the final administration of AMPH (White & Wang, 1984).
Drug-induced midbrain D2AR subsensitivity is likely the first step in a cascade of signaling events which, when repeated, might produce enduring changes in DA transmission in the midbrain and DA neuron projection areas. The D2AR subsensitivity is attributed by many factors. Because D2ARs are coupled to Gαi/o to signal, chronic drug exposure may attenuate the coupling strength between D2ARs and Gαi/o proteins. For example, AMPH self-administration diminishes D2R (mostly D2AR)-stimulated G-protein activation in rat midbrain assessed by an antibody-capture [35S]GTPγS scintillation proximity assay (Calipari, et al., 2014). Chronic exposure to drugs of abuse disrupts the lipid composition of plasma membrane and alters the compartmentalization of membrane proteins [see review in (Luessen & Chen, 2016)], which likely contributes to decoupling of D2ARs from their cognate G-protein subunits. Alternatively, chronic drug exposure reduces D2AR activity by accelerating agonist-induced receptor internalization and delaying receptor recycling. Although it is not feasible to investigate D2AR internalization in vivo, it has been shown that D2Rs undergo robust β-arrestin dependent internalization upon agonist stimulation in cultured cells (Kim, et al., 2004; Lan, et al., 2009; Namkung, et al., 2009a). However, a recent study also demonstrates that GFP-D2ARs in mouse DA neurons desensitize but do not internalize in response to agonist treatments (Robinson, et al., 2017a). It would be interesting to investigate if chronic exposure to drugs of abuse (e.g. cocaine & AMPH) preferentially targets Gα-mediated over β-arrestin-mediated signal transduction of D2ARs, which many explain the commonly observed D2AR desensitization as a consequence of drug exposure.
4. The D2AR Interactome
In recent years, many proteins have been identified to interact with D2ARs including transporters, GPCRs, ion channels, intracellular signaling modulators and protein kinases. These interactions suggest that the function, trafficking and compartmentalization of D2ARs can be regulated via a wide variety of molecular mechanisms (see Fig.1). Understanding these regulations will have important implications for appreciation of reward, motor, cognitive and affective functions tied to D2AR-mediated DA signaling.
Figure 1.
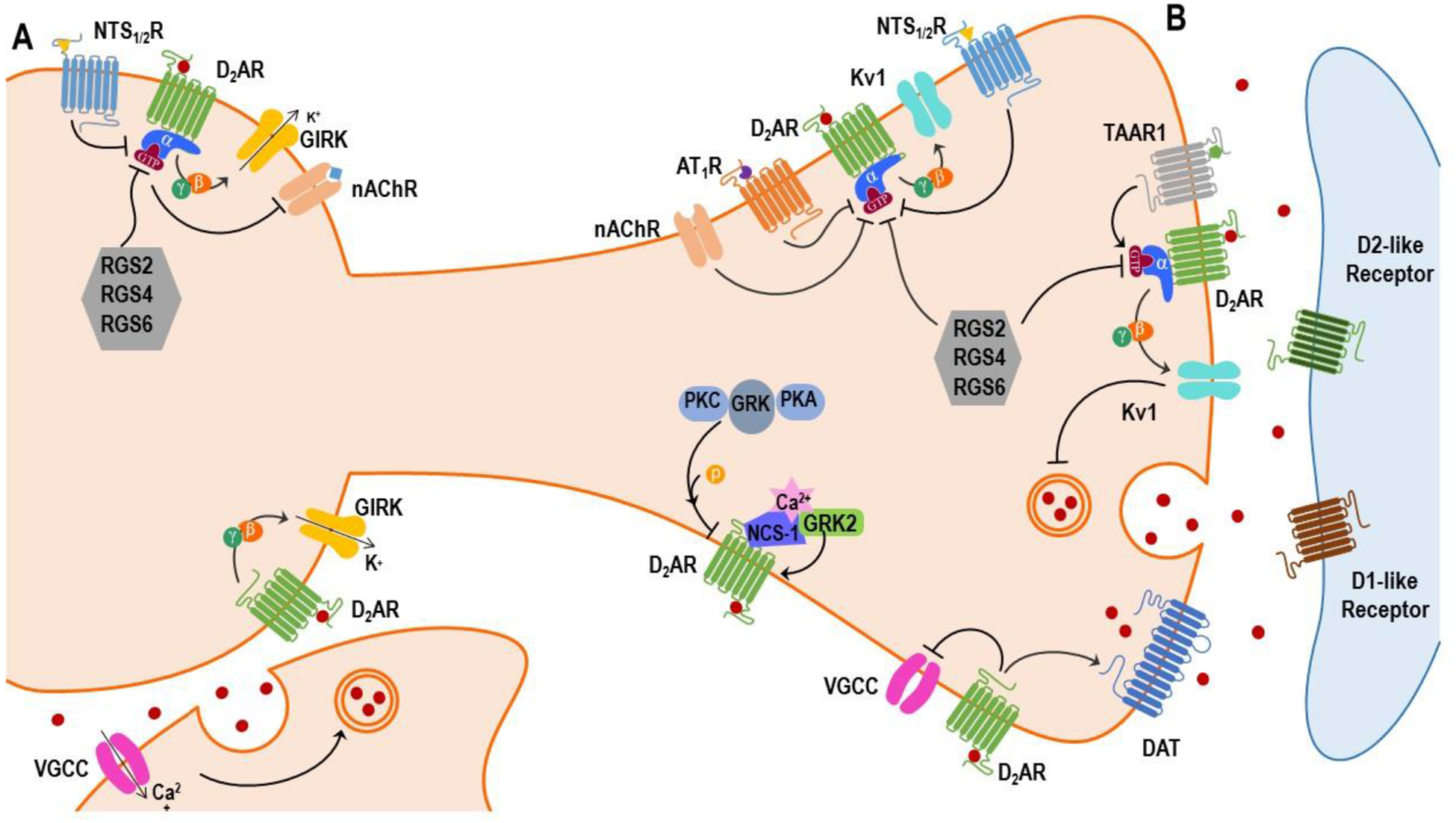
The dopamine D2 autoreceptor interactome. (A) In the somatodendritic compartment of dopamine (DA) neurons, the dopamine D2 autoreceptor (D2AR) inhibits DA neuron firing by activating GIRK channel activity via Gβγ subunit. Activation of the neurotensin receptor type 1/2 (NTS1/2R) inhibits D2AR-mediated GIRK currents. Activation of the nicotinic receptor (nAChR) increases DA neuron firing which is antagonized by D2ARs. When DA neurons are depolarized, DA released via exocytosis in the midbrain produces an inhibitory postsynaptic current that is dependent on voltage-sensitive calcium channel (VGCC). (B) In the axon terminal compartment of DA neurons, D2AR inhibits Kv1 channel-mediated DA release via Gβγ subunits. Activation of nAChR, angiotensin receptor type 1 (AT1R) and NTS1/2R attenuate D2AR inhibition of evoked DA release. In contrast, activation of trace amine-associated receptor1 (TAAR1) promotes D2AR activity. The VGCC-mediated neuron excitability is inhibited by D2AR. Activation of D2AR increases dopamine transporter (DAT) surface expression and reuptake function. In both the somatodendritic and axon terminal compartment of DA neurons, D2AR is subject to phosphorylation by G-protein coupled receptor kinase (GRK), protein kinase C (PKC) and PKA. The D2AR couples to calcium-sensitive proteins including neuronal calcium sensor-1 (NCS-1) which recruits GRK2 to the receptor in the presence of calcium. Lastly, D2AR-mediated G protein signaling is regulated by regulator of G-protein signaling (RGS) proteins including RGS2, RGS4 and RGS6.
4.1. Dopamine Transporters (DATs)
The D2ARs and DATs are the two primary mechanisms to terminate synaptic DA transmission and maintain synaptic DA homeostasis. The D2Rs (either D2SRs or D2LRs) and DATs are both expressed in DA neurons and can physically and functionally interact with each other to control DA signaling. The DAT N-terminus directly couples to the third intracellular loop of D2Rs (Lee, et al., 2007). Co-expression of D2Rs and DATs in cultured cells increases DAT surface expression and DA reuptake (Bolan, et al., 2007; Lee, et al., 2007). Moreover, activation of D2Rs by agonists increases surface DAT levels and DAT reuptake function in cultured cells (Bolan, et al., 2007; Chen, et al., 2013; Lee, et al., 2007). In vitro activation of D2ARs in mouse dorsal striatum (Gowrishankar, et al., 2018) or whole striatum (Chen, et al., 2013) also increases DAT surface expression and function. In agreement with these observations, mice carrying a mutant DAT construct A599V exhibit constitutive D2AR hyperactivity as well as increased basal surface DAT expression in the dorsal striatum (Gowrishankar, et al., 2018). This A599V mutation in DAT is associated with increased phosphorylation of Thr53 at the N-terminus of DATs, suggesting that altered DAT phosphorylation may underlie the disrupted interaction between D2ARs and DATs.
The D2R-stimulated increase in DAT surface expression results from accelerated DAT recycling instead of decreased DAT internalization and this process involves protein kinase Cβ (PKCβ) and extracellular signal-regulated kinase (ERK) (Chen, et al., 2013). In neuroblastoma 2a (N2A) cells, inhibition of PKCβ activity abolishes D2R-activated DAT trafficking to the surface. Moreover, quinpirole-stimulated DAT trafficking is completely abolished in PKCβ knockout mice. D2R stimulation increases phosphorylation of PKCβII but not PKCβI in striatal synaptosomes that are Percoll-purified to eliminate postsynaptic membranes. These data suggest that D2AR-mediated DAT trafficking requires activation of PKCβ activity. Significantly, the laboratory of Gnegy has shown that activation of PKC, especially PKCβ, promotes rapid DAT trafficking to the surface in N2A cells upon short-term exposure to DAT substrates including DA and AMPH (Chen, et al., 2009; Furman, et al., 2009; Johnson, et al., 2005a). Therefore, PKCβ appears to act as a functional linker to transduce and propagate the signaling of D2ARs to DAT in control of D2R-mediated DAT trafficking and activity. In addition, inhibition of ERK activity prevents D2R-stimulated DAT trafficking to surface in cultured cells (Bolan, et al., 2007; Chen, et al., 2013; Lee, et al., 2007) and mouse striatum (Chen, et al., 2013). Interestingly, deletion of PKCβ diminishes quinpirole-stimulated ERK phosphorylation whereas ERK inhibition does not influence PKCβII phosphorylation by quinpirole (Chen, et al., 2013), indicating that PKCβ is upstream of ERK. To summarize, these data suggest the PKCβ-ERK signaling cascade is required for D2R-medidated DAT insertion on the plasma membrane.
Disruption of the D2AR-DAT interaction in vivo alters D2AR activity and DA-related behavior. The Dat1 knockout mice have a complete loss of D2AR-mediated inhibition of DA neuron firing, evoked striatal DA release, and DA synthesis, and display hyperlocomotor activity (Jones, et al., 1999). Moreover, treatment with a membrane penetrant peptide that disrupts the D2AR-DAT interaction in mice decreases synaptosomal DA reuptake and increases locomotor activity (Lee, et al., 2007). Accordingly, acute depletion of Drd2 in rat SN decreases DAT reuptake function, increases basal locomotor activity, and reduces locomotor responsiveness to high doses of cocaine (Budygin, et al., 2016). However, these observations are in contrast to a report that conditional Drd2 knockout mice do not show a change in DA reuptake kinetics (Bello, et al., 2011), suggesting potential compensatory changes resulting from a complete, persistent deletion of Drd2.
To date, most studies have focused on understanding D2AR regulation of DAT trafficking and reuptake function; however, whether and how D2AR influences the ability of DAT to efflux DA remain largely unknown. Because phosphorylation of DAT N-terminus is required for reverse transport of DA mediated by DAT (Guptaroy, et al., 2009; Khoshbouei, et al., 2004), stimulation of protein kinases by D2AR activation can alter DAT N-terminus phosphorylation and thus may influence DA efflux. Mice carrying the DAT-A559V mutation display hyperactive D2AR activity and increased DA efflux that can be blocked by the D2R antagonist raclopride (Bowton, et al., 2010). Interestingly, D2AR hyperactivity in this DAT variant parallels an increase in the basal activity of Ca2+/calmodulin-dependent protein kinase II (CaMKII) and DAT N-terminus phosphorylation. These data indicate that the anomalous DA efflux in the DAT-A559V mutant is mediated by CaMKII activity via phosphorylation of DAT and a change in DAT conformation from outward- to inward-facing. It would be interesting to further elucidate the role of D2AR in regulation of DAT-mediated DA efflux and identify additional protein kinases involved. The tight interaction between D2ARs and DAT is an important mechanism by which extracellular DA is regulated. An abnormal interaction between D2ARs and DATs has been implicated not only in drug abuse (Buckholtz, et al., 2010) but also in several other brain disorders including attention deficit and hyperactivity disorder (Mazei-Robison, et al., 2008), Parkinson’s disease (Seeman & Niznik, 1990), and schizophrenia (Lee, et al., 2009). Thus, intracellular modulators that can strengthen this D2AR-DAT interaction may be beneficial to restore abnormal DA signaling in these disease states.
4.2. GPCRs
4.2.1. Neurotensin type 1 and 2 Receptors (NTS1/2Rs)
Neurotensin is a neuropeptide abundantly expressed throughout the brain (Hokfelt, et al., 1984). Neurotensin binds to G-protein coupled neurotensin type 1 (NTS1Rs, couple to Gαq, Gαi/o and Gαs) and 2 receptors (NTS2Rs, couple to Gαq) and non-G-protein coupled NTS3R (Vincent, et al., 1999). The action of brain neurotensin is mostly mediated by NTS1Rs and NTS2Rs. These receptors are widely distributed in the brain and notably localized in midbrain DA cell bodies and dendrites and in striatal DA axon terminals (Nicot, et al., 1994; Palacios & Kuhar, 1981; Quirion, et al., 1987). Midbrain DA neurons are also innervated by neurotensin-positive fibers projected from the forebrain (Geisler & Zahm, 2006). The neurotensin system has long been known to modulate DA transmission via diverse mechanisms [see review in (Tschumi & Beckstead, 2019)]. Neurotensin depolarizes midbrain DA neurons through activation of a non-selective cation conductance (Jiang, et al., 1994). The effect of neurotensin on DA neuron excitability is mediated by the glutamate, GABA and DA systems. Neurotensin exerts a NTS1R-dependent, biphasic effect on glutamate signaling in VTA DA neurons in that that low concentrations augment and high concentrations reduce the currents (Kempadoo, et al., 2013). Genetic deletion of Ntsr1, encoding NTS1Rs, decreases DA neuron firing (Dominguez-Lopez, et al., 2019), suggests an excitatory role of NTS1Rs on neurotensin-mediated DA neuron activity. Neurotensin also increases DA neuron excitability by decreasing GABA signaling at GABAB receptor synapses in the midbrain, and this effect is mediated by NTS2Rs (Stuhrman & Roseberry, 2015; Tschumi & Beckstead, 2018). Finally, neurotensin excites midbrain DA neurons through depression of D2AR-mediated synaptic currents (Piccart, et al., 2015; Shi & Bunney, 1990; Shi & Bunney, 1991). Neurotensin enhances DA release at both somatodendritic and axon terminal sites of DA neurons (Fawaz, et al., 2009; Okuma, et al., 1983). A direct application of neurotensin to midbrain slices increases calcium-dependent DA neuron firing (Jiang, et al., 1994; Seutin, et al., 1989; Werkman, et al., 2000), which can be blocked by a NTS1R antagonist (St-Gelais, et al., 2004). In addition, blockade of D2Rs/D3Rs by sulpride prevents neurotensin-induced DA release in rat NAc (Fawaz, et al., 2009), suggesting the functional antagonism of neurotensin on D2AR activity.
Activation of NTS1Rs by neurotensin reduces the potency of D2AR agonists that may contribute to the antagonism of neurotensin on D2ARs. In HEK293 cells co-expressing D2LRs and NTS1Rs, the D2R agonist FAUC326 displays a 34-fold reduction in the binding affinity for D2Rs when compared to cells expressing D2LRs alone (Koschatzky, et al., 2011). Moreover, a neurotensin receptor agonist (e.g. JMV449) markedly reduces quinpirole inhibition of forskolin-induced stimulation of cAMP response element-binding protein in HEK293T cells expressing D2LRs and NTS1Rs (Borroto-Escuela, et al., 2013). Recent evidence indicates the existence of a constitutive NTS1R-D2R (D2SRs or D2LRs) complex on the membrane in cultured cells and animal brain tissues measured by Bioluminiscence Resonance Energy Transfer (BRET), confocal microscopy and coimmunoprecipitation assays (Borroto-Escuela, et al., 2013; Koschatzky, et al., 2011). Although it has yet to be determined whether there is a direct physical interaction between these two receptors, the existence of the NTS1R-D2R complex suggests that the antagonistic action of neurotensin on D2AR activity is likely modulated by neurotensin-stimulated, NTS1R-mediated intracellular signaling, resulting in reduced D2AR activity such as decoupling of D2ARs from G-proteins and rapid receptor internalization. Acute treatment of HEK293 cells expressing either D2SRs or D2LRs with neurotensin produces receptor phosphorylation, internalization and heterologous desensitization through activation of PKC (Thibault, et al., 2011). The involvement of PKC activation in neurotensin signaling may underlie the increased potency of quinpirole to activate the intracellular ERK signaling pathway in the presence of neurotensin (Borroto-Escuela, et al., 2013). These data collectively suggest an antagonistic interaction on the plasma membrane and a synergistic cytoplasmic, PKC-dependent interaction between D2ARs and NTS1/2Rs.
Aberrant neurotensin signaling has been associated with drugs of abuse through modulation of DA signaling, especially D2AR-mediated signaling. Microinjection of neurotensin in rat midbrain reduces intracranial self-stimulation threshold, suggesting the neurotensin peptide promotes motivated behavior (Rompre, et al., 1992). Both NTS1Rs and NTS2Rs are involved in neurotensin-mediated drug-seeking and taking behavior. Microinjection of the NTS1R/NTS2R antagonist SR142948A into mouse VTA delays METH acquisition, attenuates METH intake during the training and maintenance of self-administration, and reduces drug-seeking behavior during extinction, cue-induced reinstatement, and METH intake under the progressive ratio schedule (Dominguez-Lopez, et al., 2018). Genetic deletion of NtsR1 reduces METH intake and motivation for METH in mice (Dominguez-Lopez, et al., 2019). Additionally, intra-VTA pretreatment with or systemic administration of the selective NTS1R antagonist SR142948A prevents AMPH-induced behavioral sensitization (Panayi, et al., 2005; Rompre & Perron, 2000). Systemic treatment with PD149136, a NTS1R agonist, decreases METH self-administration (Sharpe, et al., 2017). These data suggest that neurotensin-mediated activation of NTS1/2R signaling promotes drug-seeking and taking behavior whereas inhibition of neurotensin signaling diminishes it. Modulation of the neurotensin signaling is a mechanism by which drugs of abuse exert their effects. For example, METH self-administration increases neurotensin levels in the midbrain and striatum (Hanson, et al., 2012; Wagstaff, et al., 1996). The increased neurotensin content may dampen D2AR activity resulting in an imbalance between DA and neurotensin signaling and increased vulnerability to drug-seeking and taking behavior. Future studies are necessary to establish the functional relationship between D2ARs and NTS1Rs/NTS2Rs in animal models of drug abuse. It would be also interesting to investigate whether there is a functional reciprocal relationship between D2ARs and NTS1/2Rs.Thus, targeting both the neurotensin-NTS1R and DA-D2AR signaling could be a promising approach to normalize DA signaling.
4.2.2. Trace Amine-Associated Receptors 1
Trace amines including p-tyramine, β-phenylethylamine, octopamine and tryptamine are endogenous amines present in the brain with low concentrations (Pei, et al., 2016). They bind primarily to trace amine-associated receptors 1 (TAAR1s), which couple to Gαs and Gαq (Berry, 2004; Borowsky, et al., 2001; Bunzow, et al., 2001; Lindemann, et al., 2008). The Taar1 mRNA is ubiquitous but with notable presence in the mesolimbic and nigrostriatal DA pathways (Borowsky, et al., 2001; Lindemann, et al., 2008). In contrast to majority of GPCRs that are localized on the membrane, TAAR1s are predominantly intracellular with an abundant expression within the presynaptic terminals of monoamine neurons (Barak, et al., 2008; Bunzow, et al., 2001; Miller, 2011; Xie, et al., 2007). The TAAR1s may traffic to the membrane to respond to agonist stimulation. Alternatively, TAAR1s may functionally interact with amines intracellularly.
The TAAR1s play an important role in regulation of DA signaling. Deletion of Taar1 or blockade of TAAR1s with antagonists increases the DA neuron firing rate in mouse midbrain and augments evoked DA release in the NAc (Bradaia, et al., 2009; Leo, et al., 2014; Lindemann, et al., 2008). In contrast, activation of TAAR1s reduces the firing frequency of mouse DA neurons (Revel, et al., 2011). Interestingly, TAAR1 activation does not alter basal DA release and reuptake in rat NAc slices (Asif-Malik, et al., 2017), suggesting that TAAR1-mediated DA signaling is absent when DA tone is low. Moreover, TAAR1s regulate DA-related behavioral responses to psychostimulants. Activation of TAAR1s in rodents decreases cocaine-stimulated locomotor activity (Revel, et al., 2012), blocks cocaine self-administration (Revel, et al., 2012), prevents cocaine reinstatement (Pei, et al., 2014; Pei, et al., 2015), and attenuates cocaine-induced increase in intracranial self-stimulation (Pei, et al., 2015). Moreover, Taar1 gene variants are directly associated with METH-induced effects including consumption, hyperthermia and toxicity (Miner, et al., 2017; Mootz, et al., 2020; Shi, et al., 2016; Stafford, et al., 2019).
The TAAR1s modulate DA transmission via D2ARs. The TAAR1-D2R complex is confirmed in rat midbrain as TAAR1s are immunoprecipitated by an antibody against D2Rs (D2ARs) (Harmeier, et al., 2015). In HEK293 cells overexpressing TAAR1s and D2LRs, the presence of D2LRs promotes the trafficking of TAAR1s from the cytosol to the membrane (Harmeier, et al., 2015). This observation is consistent with a report showing co-localization of these two receptors on the plasma member in HEK293 cells (Espinoza, et al., 2011). Moreover, the presence of D2Rs is reported to have no influence on the overall expression of TAAR1s (Harmeier, et al., 2015), which contradicts to the reported reduction in the total expression of TAAR1s in the presence of D2Rs (Espinoza, et al., 2011). The discrepancy may result from the variation in the expression levels of TAAR1s relative to D2Rs between these two experiments. Moreover, the TAAR1-D2R complex modulates the signaling of D2Rs. When TAAR1s are stimulated, β-arrestin is recruited from D2Rs to TAAR1s to initiate TAAR1-activated, β-arrestin-mediated intracellular signaling including Akt phosphorylation and subsequent activation of glycogen synthase kinase-3β (GSK3β) activity (Harmeier, et al., 2015). Because of a lesser association with β-arrestin, application of the D2R agonist results in increased Gαi/o signaling, which explains the increased affinity of D2Rs for agonists when both receptors are expressed.
A functional TAAR1-D2R (D2AR) interaction has been confirmed in vitro and in vivo. A TAAR1 agonist potentiates quinpirole inhibition of DA release in wildtype mice but not in Taar1 knockout mice (Leo, et al., 2014). When D2AR activation is measured by paired-pulse stimulation in the same study, Taar1 deletion results in a reduction in D2AR activity, confirming that TAAR1s mediate DA signaling through D2ARs. Furthermore, application of RO5256390, a TAAR1 agonist, blocks cocaine-induced inhibition of DA clearance in rat NAc, and this blockade is reversed by co-treatment with L-741,626, a D2R antagonist, and GSK3 inhibitors (Asif-Malik, et al., 2017). Collectively, these data suggest that D2AR activation is necessary for TAAR1 modulation of cocaine’s effects.
4.2.3. Angiotensin II type 1 Receptors
Although angiotensin II (AngII) is present in the brain and the peripheral tissue, the AngII levels in the brain are much higher than in the peripheral circulation (Hermann, et al., 1984). AngII is notably present in the somatodendritic and axon terminal compartments of DA neurons (Garrido-Gil, et al., 2013). AngII binds to Gαq-coupled angiotensin II type 1 receptors (AT1Rs), which form a complex with D2Rs in HEK293 cells (Martinez-Pinilla, et al., 2015). This AT1R-D2R complex appears to be functional. In HEK293 cells co-expressing AT1Rs and D2LRs, the AT1R antagonist candesartan blocks D2LR-mediated cAMP production, ERK activation and β-arrestin recruitment whereas the D2LR antagonist prevents AT1R-mediated Gαq signaling (Martinez-Pinilla, et al., 2015). The observation of the cross-antagonism for AT1Rs and D2LRs suggest that these two receptors may share some common signaling pathways. However, Martinez-Pinilla et al. (2015) also reported that co-expression of D2LRs with AT1Rs does not alter D2LR signaling but influences AT1R signaling. In the presence of D2LRs, the coupling efficiency between AT1Rs and Gαq is attenuated and the potency of AngII to stimulate intracellular calcium release via AT1Rs is reduced. This reduction is reversed by the D2LR antagonist raclopride, suggesting that D2LRs antagonize the activity of AT1Rs likely through the intracellular signaling. This mechanism may be applicable in vivo given that both AT1Rs and D2ARs are expressed in the axon terminals of DA neurons. The AT1Rs plays a role in regulation of presynaptic DA signaling. Perfusion of AngII to rat striatum in vivo or treatment with AngII to striatal slices triggers DA release that is reversed by AT1R antagonists (Brown, et al., 1996; Mendelsohn, et al., 1993). AngII-stimulated DA release may be mediated through inhibition of striatal presynaptic D2AR activity given the functional antagonism shown in HEK293 cells. It would be interesting to investigate the functional interaction between AT1Rs and D2ARs on DA release in vivo. The AT1Rs are also involved in METH-seeking and taking behavior. Blockade of AT1Rs attenuates METH self-administration and reinstatement in rats (Xu, et al., 2019), implicating increased AT1R activity resulting from METH treatment. Whether increased AT1R activity following METH self-administration is due to attenuated presynaptic D2AR activity warrants further investigation.
4.3. Channels
4.3.1. G-protein-coupled Inwardly-rectifying Potassium Channels
Activation of the D2ARs inhibits midbrain neuron firing and decreases somatodendritic DA release primarily through activation of GIRK channels (Inanobe, et al., 1999; Lacey, et al., 1987; Uchida, et al., 2000). The D2ARs directly couple to GIRK channels via G-protein βγ subunits (Pillai, et al., 1998). Although midbrain DA neurons express both D2ARs and D3 autoreceptors (D3ARs), D3ARs play a minimum role in GIRK channel activation because D2R knockout mice exhibit the absence of quinpirole-mediated GIRK currents in the SN DA neurons (Davila, et al., 2003). This notion is further demonstrated in Xenopus oocytes that DA activation of GIRK currents is mediated by either D2SRs or D2LRs, but not D3Rs, and this regulation is voltage-dependent (Sahlholm, et al., 2008). Midbrain DA neurons express transcripts for GIRKs1–4; however, GIRK2 and GIRK3 channel subunits are the most abundant (Davila, et al., 2003). It appears that GIRK2 subunits are primarily responsible for most of the DA receptor-mediated synaptic currents (Beckstead, et al., 2004; McCall, et al., 2017). In contrast, GIRK3 subunits are critical for GABAB-mediated GIRK currents in DA neurons (Munoz, et al., 2016).
The D2ARs modulate GIRK channel activity and behavioral response to drugs. Decreasing GIRK current in VTA DA neurons increases cocaine-induced locomotor activity (McCall, et al., 2019) and cocaine self-administration (McCall, et al., 2017). Accumulating evidence indicates that chronic exposure to drugs of abuse alters GIRK currents in midbrain DA neurons, which is partially attributed to disrupted somatodendritic D2AR activity. For example, withdrawal from chronic ethanol exposure (2 g/kg, i.p., three times daily for 7 days) enhances D2AR-mediated GIRK current in a calcium-dependent manner in C57BL/6J mice (Perra, et al., 2011). This may contribute to the hypodopaminergic state during withdrawal. In contrast, 2 weeks of short-access (1hr/day) and long-access (4 hrs/day) METH self-administration (0.0015–0.15 mg/kg/infusion) markedly reduce D2AR inhibition of GIRK currents in both VTA and SN DA neurons from DBA/2J mice (Sharpe, et al., 2014). Similarly, chronic phencyclidine administration (5 mg/kg, i.p., twice/day for 7 days) to DBA/2J mice attenuates D2AR inhibition of GIRK currents in SN DA neurons (Piccart, et al., 2019). One likely underlying mechanism for the desensitization of D2AR-mediated GIRK currents following METH and phencyclidine treatment is the reduced coupling efficiency between D2ARs and GIRK channels mediated by Gβγ subunits. Chronic exposure to drugs alters the expression of G-proteins (Luessen, et al., 2017; Sun, et al., 2015), which in turn can influence the strength of the coupling between D2ARs and GIRK channels. Alternatively, chronic exposure to drugs of abuse may change the affinity of DA for D2ARs. Binge pattern cocaine administration (15 mg/kg/day, i.p., 7 or 14 days) reduces the apparent affinity of the substrates for striatal D2Rs but not the number of binding sites (Tsukada, et al., 1996), suggesting that chronic cocaine treatment alters the conformation or stability of membrane D2Rs and thus attenuates substrate binding affinity. The D2AR-dependent activation of GIRK channel conductance is desensitized following exposure to other DA-related drugs including cocaine (Dragicevic, et al., 2014; Gantz, et al., 2015) and L-DOPA (Dragicevic, et al., 2014) in rodents. Furthermore, acute cocaine treatment induces desensitization of D2SRs, but not the D2LRs, in mouse SN DA neurons (Robinson, et al., 2017b). These data suggest that desensitization of D2AR-GIRK currents in midbrain DA neurons may be a common mechanism underlying drugs of abuse.
4.3.2. Voltage-gated Potassium Channels
The presynaptic DA release is unlikely to be regulated by GIRK channels because they are not commonly detected on axon terminals (Luscher, et al., 1997). Instead, voltage-gated potassium channels such as Kv channels are localized at these sites and are involved in DA release. The wide spectrum Kv channel blockers such as 4-aminopyridine (4-AP) and tetraethylammonium (TEA) enhance electrically evoked [3H]DA release from rat striatal slices (Cass & Zahniser, 1991), confirming the functional presence of voltage-gated Kv-type potassium channel at DA neuron terminals. The action of these voltage-gated potassium channels is mediated by D2ARs because 4-AP-and TEA-evoked DA release is attenuated in the presence of quinpirole in rat striatum (Cass & Zahniser, 1991). Moreover, in primary cultured DA neurons, action-potential evoked glutamate-mediated excitatory postsynaptic currents are inhibited by D2ARs and this effect is largely abolished by 4-AP but not the GIRK channel blocker barium (Congar, et al., 2002), implying that terminal DA release is primarily mediated by Kv channels.
Members of Kv1 subfamily are abundantly expressed in various brain regions (Veh, et al., 1995) and function to regulate neuron excitability and neurotransmitter release (Robertson, 1997). The laboratory of Dr. Trudeau has elegantly delineated the distribution and the function of Kv1 subunits in DA neurons (Fulton, et al., 2011). The Kv1.1, Kv1.2, Kv1.6 are all expressed on DAT-positive mouse DA neuron axons. Moreover, these channels regulate D2AR activity because α-dendrotoxin, a Kv1.1, Kv1.2 or Kv1.6 subunit-containing potassium channel blocker, diminishes D2AR inhibition of evoked DA release. The Kv1.2 subunits and D2Rs can be coimmunoprecipitated in cultured COS cells and primary cultured DA neurons. Maurotoxin (10 nM), which inhibits Kv1.2-containing channels at this dose, attenuates D2AR inhibition of evoked DA release. The Kv1.2 knockout mice exhibit a pronounced reduction in D2AR-mediated inhibition of evoked DA release in striatal slices, further implying a specific role of Kv1.2 in regulation of DA release. Furthermore, the Gβγ subunit contributes to the coupling between axon D2ARs and Kv1 channels. In DA neurons, Kv3 family subunits are also expressed (Dufour, et al., 2014); however, blockade of Kv3.1, Kv3.2 and Kv3.4 channels fail to modulate D2AR-mediated inhibition of DA release (Martel, et al., 2011).
The effects of drugs of abuse on the expression, trafficking and function of voltage-gated potassium channels are largely unknown. A recent report shows that withdrawal from METH self-administration increases the gene expression and membrane protein localization of Kv1.1 and Kv1.3 in rat NAc (Jayanthi, et al., 2020). These changes are also accompanied by altered DNA methylation at the CpG-rich sites near the promoter region of Kcna1 and Kcna3, genes encoding Kv1.1 and Kv1.3. Additionally, some reports indicate that members of Kv1 channels are involved in behavioral and neurochemical responses to cocaine (Kourrich, et al., 2013), AMPH (Ghelardini, et al., 2003), METH (Qu, et al., 2014), and sucrose (O’Donovan, et al., 2019). Future research is necessary to further delineate the direct role of each Kv1 isoforms in regulation of D2AR-mediated DA signaling and behavioral responses to drugs of abuse.
4.3.3. Calcium and Calcium Channels
The internalization, phosphorylation and function of D2Rs are regulated either directly or indirectly by calcium or calcium channels. D2Rs can physically and functionally interact with the neuronal calcium sensor-1 (NCS-1), a member of the neuronal calcium sensor family of EF hand calcium-binding proteins. The N-terminus of NCS-1 directly binds to the C-terminus of D2Rs demonstrated in the yeast two-hybrid, GST-pulldown and coimmunoprecipitation assays (Kabbani, et al., 2002). Functionally, overexpression of NCS-1 in HEK293 cells markedly reduces basal D2R phosphorylation, and attenuates DA-induced D2R internalization without altering the basal surface receptor levels. The D2Rs form a complex with NCS-1 in a calcium-independent manner in HEK293 cells and primary neurons; however, GRK2 is recruited to this complex in response to receptor activation in a calcium-dependent manner. Structural biology analysis suggests that NCS-1 has structural flexibility at its C-terminus and acts as a small scaffold protein by allowing both D2Rs and NCS-1 to bind simultaneously in the presence of calcium (Pandalaneni, et al., 2015). This notion is further validated in vitro. Activation of Cav1.3, a L-type calcium channel expressed in SN DA neurons (Olson, et al., 2005; Sinnegger-Brauns, et al., 2009), increases intracellular calcium levels, resulting in recruitment of GRK2 to the NCS-1-D2R complex to modulate D2R phosphorylation and internalization (Dragicevic, et al., 2014). Since NCS-1 modulates calcium-dependent neurotransmitter release (Chen, et al., 2001; Guild, et al., 2001; McFerran, et al., 1998) and calcium channel activity (Tsujimoto, et al., 2002; Weiss, et al., 2000; Weiss & Burgoyne, 2001), this physical and functional interaction between NCS-1 and D2Rs mediates D2AR-mediated, calcium-dependent DA release and DA neuron excitability. It would be interesting to investigate if chronic exposure to drugs of abuse alters this D2AR-NCS-1 interaction and related DA signaling. In addition to NCS-1, D2Rs form a complex with other calcium-sensitive proteins including S100B (Dempsey & Shaw, 2011; Liu, et al., 2008) and calmodulin (Bofill-Cardona, et al., 2000; Liu, et al., 2007), and these complexes regulate D2R signaling although through different mechanisms.
When DA neurons are depolarized, calcium influx through voltage-gated channels promotes somatodendritic DA release, which in turn activates D2ARs and inhibits GIRK currents. Prolonged activation desensitizes D2AR and thus increases D2AR-dependent GIRK currents. When intracellular calcium is chelated in mouse midbrain DA neurons, the amplitude of the D2AR-GIRK current is greater and the rate of D2AR desensitization is slower (Beckstead & Williams, 2007; Perra, et al., 2011; Sharpe, et al., 2014). Moreover, the calcium-dependent D2AR desensitization involves activation of phospholipase C and CaMKII (Perra, et al., 2011). It is further revealed that D2SR-, but not D2LR-, mediated inhibition of GIRK currents in DA neurons requires calcium (Gantz, et al., 2015; Robinson, et al., 2017b). Drug exposure dysregulates calcium-dependent D2AR activity. For example, desensitization of the D2AR-GIRK current in mouse DA neurons is abolished by METH self-administration (Sharpe, et al., 2014) and repeated ethanol exposure (Perra, et al., 2011). Moreover, a single cocaine injection reduces calcium-dependent desensitization of D2SRs, not D2LRs, when each D2R isoform is overexpressed in D2R knockout mice (Robinson, et al., 2017b).
In the midbrain, DA neurons form dendrodendritic synapses with other DA neurons (Groves & Linder, 1983; Nirenberg, et al., 1996; Wilson, et al., 1977). When DA neurons are excited, exocytotically released DA in the midbrain produces the inhibitory postsynaptic current (IPSC) that is tetrodotoxin sensitive, calcium dependent and inhibited by D2Rs but not D3Rs (Beckstead, et al., 2004). Moreover, the DA-mediated IPSC is dependent on voltage-sensitive calcium channels as cadmium (a blocker for all types, 300 μM) completely blocks the IPSC. Although application of ω-agatoxin IVA (blocker for P/Q-type) has no effect on IPSC, a combination of blockers of P/Q-type and N-type (ω-conotoxin GVIA) in the presence or absence of nimodipine (a blocker for L-type) reduces the amplitude of IPSC. These data suggest that more than one voltage-sensitive calcium channel is involved in IPSC. Further, prolonged DA exposure produces a calcium-dependent, long-term depression of IPSC and this depression is mediated through desensitization of postsynaptic D2ARs in midbrain DA neurons (Beckstead & Williams, 2007).
Ample evidence also suggests that intracellular calcium levels and voltage-gated calcium channels control DA volume transmission [see review in (Rice & Patel, 2015)]. The extent of calcium and calcium channel regulation is brain region-dependent, with nearly a full order of magnitude difference between the values of EC50 for somatodendritic regulation of DA release (μM Ca2+ range) in the midbrain vs axon terminal DA release (mM Ca2+ range) in the striatum (Chen, et al., 2011). However, voltage-gated calcium channel blockers alone or in combination are less effective at reducing somatodendritic DA release in the SN compared to the striatal axonal release (Bergquist, et al., 1998; Chen, et al., 2006). Moreover, calcium and calcium channels can indirectly determine the activation of D2ARs in both somatodendritic and axonal release region via control of DA volume transmission. As already discussed, the amount of DA in these regions determines activation of D2ARs, which themselves are differentially distributed in expression (Hurd, et al., 1994) and function (Cragg & Greenfield, 1997) across regions for corresponding control of DA neuron activity and DA release.
4.3.4. Ionotropic Nicotinic Acetylcholine Receptors
Nicotine binds to nicotinic acetylcholine receptors (nAChRs), which are ligand-gated cationic channels. Neuronal nAChRs are heteromeric pentamers made of a heterogeneous family of eight different α (α3–7) and β (β2–4) subunits. These receptors are categorized into homomeric α7-containing nAChRs (α7nAChRs) and heteromeric non-α7 nAChRs. The mRNA of nAChR subunits is expressed throughout the VTA and other brain regions (Azam, et al., 2002; Collins, et al., 2009). The β2-containing nAChRs are present in the cell bodies and axon terminals of DA neurons in the SN (Jones, et al., 2001; Wonnacott, et al., 2000; Zoli, et al., 2002). The nAChRs comprising exclusively of the α7 subunit (α7nAChRs) are also present in DA neurons of rat midbrain (Pidoplichko, et al., 1997; Wu, et al., 2004; Yang, et al., 2009). Electron microscopic imaging of dual immunolabeling of α7-containing nAChRs and D2ARs in the VTA reveals that these two receptors co-localize in the soma, axon and terminal of DA neuron as well as in other types of neurons (Garzon, et al., 2013). The α6-containing nAChRs are the other major nAChR subtype that are strongly expressed in midbrain DA neurons (Azam, et al., 2002; Mackey, et al., 2012). Activation of these nAChRs on DA neurons increase neuron firing which is antagonized by D2ARs.
Selective activation of striatal cholinergic interneurons elicits DA release via nAChRs located on DA cell bodies and does not require VTA mediated cell firing (Cachope, et al., 2012; Threlfell, et al., 2012). This nAChR-mediated facilitation of DA release is inherent to ex vivo voltammetry work that uses electrical stimulation to elicit DA because of simultaneous activation of cholinergic interneurons and a supply of ACh at DA axon terminals. This explains how nAChR antagonists, or desensitizing concentrations of nicotine, reduce DA release to single pulse or low frequency electrical stimulations in this preparation. The magnitude of DA release is restored (and sometimes exceeds pre-drug, baseline levels) when higher frequency stimulations are applied to the slice. Notably, this nAChR-mediated, frequency-dependent release of DA is similar to what is observed following application of the D2R agonist quinpirole (Zhang & Sulzer, 2004). Thus, D2R agonism and nAChR antagonism facilitate paired-pulse ratios by selectively reducing the first stimulation in slice preparations, and may model amplification of phasic bursts relative to tonic DA release.
Perfusion of nicotine to rat NAc persistently increases the extracellular level of DA [see review in (Subramaniyan & Dani, 2015)]. Mice lacking the β2 subunit of nAChRs display striking hyperactivity in the open field, which suggests disrupted DA neurotransmission (Avale, et al., 2008). Given the prominent role of D2AR in regulation of axon terminal DA release, there might be a functional crosstalk between D2ARs and nAChRs in striatal DA neuron terminals. Nicotine-stimulated striatal DA release is mediated by both presynaptic nAChRs and D2ARs as dihydro-β-erythroidine (DHβE), a non-α7 nAChR antagonist, or the D2R/D3R agonist quinpirole blocks DA release (Quarta, et al., 2007), suggesting that D2ARs modulate nicotine-induced, non-α7 nAChR-mediated DA release. Moreover, DhβE abolishes the D2R/D3R antagonist raclopride-induced DA release, indicating that D2ARs inhibit nicotine-stimulated DA release by blockade of non-α7 nAChR activity. This functional interaction is further supported by the existence of a protein complex between D2ARs and β2 subunit of the nAChRs in HEK293 cells and rat brain (Quarta, et al., 2007). Immunocytochemistry analysis show that D2ARs and non-α7 nAChRs are co-localized in the terminals of DA cells. Additionally, in rat striatal tissues, D2Rs can be immunoprecipitated by α4 subunit in the presence of the β2 subunit of nAChRs and vise verse. It is worth noting that D2ARs and the β2 subunit of nAChRs are also co-localized in the soma and dendrites of DA neurons, suggesting that these heteromeric receptor complexes in the VTA might play a functional role in regulation of DA neuron excitability.
Repeated activation of nAChRs via nicotine influences D2AR activity. For example, somatodendritic D2AR availability is increased in female, but not male, human smokers (Okita, et al., 2016). In contrast, chronic nicotine exposure to mice has shown no effect on D2AR sensitivity determined by D2AR-mediated locomotor suppression (Tammimaki, et al., 2006), or decreased D2AR sensitivity in ex vivo studies measuring stimulated [3H]DA release (Harsing, et al., 1992). Most research that has been published does not differentiate between nicotine’s effect on presynaptic D2ARs and postsynaptic D2PRs on GABA or cholinergic interneurons. For example, either nicotine self-administration (0.5 mg/kg/day) or administration via osmatic minipumps (1.5 mg/kg/day) for 14 days increases high-affinity D2R expression in the striatum (Novak, et al., 2010). These receptor levels are normalized and even drop below the baseline following a period of prolonged withdrawal. Moreover, Drd2 knockout mice showed no conditioned place preference or locomotor stimulation to nicotine (0.5 mg/kg) (Wilar, et al., 2019). These effects likely describe the interaction of nAChRs with D2PRs rather than D2ARs.
4.4. Regulator of G-protein Signaling (RGS) Proteins
All RGS proteins contain a RGS domain which binds directly to activated Gα subunits to accelerate the rate of GTP hydrolysis, rapidly terminating G-protein signaling and receptor activation (Hepler, 1999; Watson, et al., 1996). More than 20 subtypes of RGS proteins are distributed in a brain region- and neuron-dependent manner (Gold, et al., 1997; Hooks, et al., 2008), suggesting that modulation of GPCR signaling by RGS proteins is receptor-type and brain region-specific. Accumulating evidence shows that drugs of abuse alter the expression of RGS proteins, which likely contributes to disrupted GPCR signaling and aberrant behavioral responses to drugs [see review in (Traynor, 2010)]. Due to a lack of specific antibodies and inhibitors for majority of RGS subtypes, the in vivo functions of most RGS proteins are largely unknown. Among RGS subtypes, the long splice isoform of RGS9 (RGS9–2) is selectively expressed in striatal medium spiny neurons and co-localizes with D2Rs (Kovoor, et al., 2005; Rahman, et al., 2003; Zachariou, et al., 2003). RGS9–2 controls the Gαi/o signaling activated by striatal D2PRs (Cabrera-Vera, et al., 2004; Rahman, et al., 2003), and deficiency in RGS9–2 is associated with aberrant DA transmission, enhanced cocaine reward and motor dysfunction in mice (Kovoor, et al., 2005; Rahman, et al., 2003). Although there is yet a report on the direct regulation of D2AR activity by RGS proteins, a few RGS subtypes (e.g. RGS2, RGS4 and RGS6) are present in DA neurons and may potentially regulate D2AR function.
RGS2 was hypothesized to have a selective affinity for Gαq when single-turnover GTPase assays are performed in solutions with purified proteins (Heximer, et al., 1997; Nance, et al., 2013). Although putative residues within the RGS domain of RGS2 proteins are necessary for RGS2 specificity towards Gαq or Gαi/o, the structural property of the Gα helical domain critically governs the Gα-RGS2 specificity and is influenced by the experimental environment (Kasom, et al., 2018). Accumulating evidence strongly suggests that RGS2 also has an affinity for activated Gαi/o in biological complex systems. For example, RGS2 stimulates GTPase activity of activated Gαi1 only when Gαi/o-coupled M2 muscarinic acetylcholine receptors (mAChR) are reconstituted in phospholipid vesicles (Ingi, et al., 1998). Additionally, RGS2 couples to activated Gαi/o proteins (e.g. Gαi2) by aluminum fluoride in N2A cells and rat striatum in an immunoprecipitation assay (Luessen, et al., 2016). Functionally, RGS2 inhibits ERK phosphorylation mediated by M1 and M2 mAChRs in CHO cells (Ingi, et al., 1998). Moreover, RGS2 suppresses β2 adrenergic receptor-mediated Gαi/o signaling in HEK293 cells and cardiomyocytes (Chakir, et al., 2011). Lastly, RGS2 negatively modulates D2R-mediated G-protein signaling (Luessen, et al., 2016). Knockdown of RGS2 via siRNAs in N2A cells stably expressing D2SRs increases quinpirole-stimulated [35S]GTPγS binding and enhances inhibition of forskolin-stimulated cAMP production confirming the ability of RGS2 to antagonize D2R-mediated Gαi/o signaling in a biological relevant system. Single-cell gene analysis shows that Rgs2 mRNA is predominantly expressed in DA neurons over GABA neurons in rat VTA (Labouebe, et al., 2007). Immunocytochemistry further confirms the presence of RGS2 proteins in TH-positive neurons in rat VTA (Calipari, et al., 2014). Although it has yet to be investigated if RGS2 directly modulates D2AR activity in DA neurons in vivo, AMPH self-administration causes increased RGS2 membrane translocation and reduced D2AR inhibition of evoked DA release (Calipari, et al., 2014). The increased RGS2 membrane translocation likely dampens D2AR-mediated G-protein signaling and thus reduces D2AR activity. Given that AMPH self-administration produces the most pronounced change in RGS2 mRNA levels, compared to other RGS subtypes, in rat VTA (Sun, et al., 2015), it would be interesting to investigate if RGS2 proteins are a potential target to restore D2AR activity disrupted by drugs of abuse and thus prevent drug-seeking and taking behavior.
RGS4 has been identified as a key mediator of opioid reward and analgesia primarily through interactions with Gαi/o-coupled μ-opioid receptors (Traynor, 2012). However, RGS4 also controls Gαi/o signaling stimulated by D2Rs in HEK293 cells (Min, et al., 2012). The N-terminus of RGS4 proteins binds to the third intracellular loop of D2Rs and this direct coupling is required for RGS4 to inhibit quinpirole-stimulated Gαi/o activation and downstream signaling. The Rgs4 mRNA is present in midbrain DA neurons as well as in GABA neurons (Labouebe, et al., 2007). Whole body deletion of Rgs4 attenuates cocaine-induced conditioned place preference in male mice (Rorabaugh, et al., 2018) although this effect might be mediated by both D2ARs and D2PRs.
RGS6 is present in both SN and VTA DA neurons, albeit with a greater abundance in the SN (Bifsha, et al., 2014; Luo, et al., 2019). The Rgs6 knockout mice exhibit enhanced sensitivity to quinpirole-induced suppression of locomotor activity in the novel environment (Luo, et al., 2019), suggesting hyperactive D2AR activity. The increased D2AR activity is also consistent with enhanced TH phosphorylation and DA synthesis in these mice. Moreover, Rgs6 knockout mice consume less ethanol when given free access, show reduced ethanol-induced reward measured by conditioned place preference, and are less susceptible to alcohol-induced withdrawal (Stewart, et al., 2015). Given that ethanol increases or decreases D2AR activity in DA neurons depending on the species, doses and treatment regimen (Karkhanis, et al., 2015; Mrejeru, et al., 2015), RGS6 may regulate the action of ethanol in part through modulation of D2AR activity.
In addition to the canonical role as a GTPase accelerator for many RGS proteins, newly emerging evidence suggests that RGS proteins can regulate GPCR trafficking via their non-canonical functions. For example, RGS9–2 inhibits DA-mediated internalization of D2Rs in HEK293 cells via its RGS and DEP (Dishevelled, Egl-10 and Pleckstrin) domains (Celver, et al., 2010). RGS2 siRNA knockdown in N2A cells abolishes quinpirole-induced D2R internalization in parallel with persistent membrane localization of β-arrestin (Luessen, et al., 2016). RGS2 GTPase activity may influence receptor phosphorylation and binding to β-arrestin. Alternatively, RGS2 may alter phosphorylation of β-arrestin which is necessary for β-arrestin to be released from the membrane (Alloway & Dolph, 1999). This is an important question to address in the future because D2AR endocytosis is critical for receptor desensitization.
4.5. Protein Kinases
The canonical mechanism underlying D2R internalization and desensitization involves receptor phosphorylation by protein kinases. In heterologous expression systems, stimulation of D2Rs by agonists results in receptor phosphorylation followed by receptor uncoupling from activated G-proteins, arresting binding, receptor internalization and recycling [see review in (Lachowicz & Sibley, 1997)]. However, D2R desensitization can be independent of receptor internalization. In primary cultured DA neurons, quinpirole stimulation of D2ARs produces desensitization without altering receptor internalization (Robinson, et al., 2017a). Regardless, D2R phosphorylation is an early event that triggers receptor internalization, desensitization and resensitization. The D2Rs are phosphorylated primarily by G-protein-coupled receptor kinases (GRKs) and the second messenger activated protein kinases including PKC, PKA and CaMKII. The functional consequence of D2R phosphorylation on receptor internalization, desensitization, recycling and resensitization relies on protein kinase-specific phosphorylation residues on D2Rs.
4.5.1. GRKs
In heterologous expression systems, GRK2 and GRK3 can phosphorylate D2Rs when activated by agonists (Ito, et al., 1999; Kim, et al., 2001; Li, et al., 2015b; Namkung, et al., 2009a); however, GRK4, GRK5 and GRK6 have no effect (Li, et al., 2015a; Namkung, et al., 2009a). Mutation of the serine/threonine residues within the third intracellular loop of D2Rs that are substrates for GRK2/3, abolishes agonist-stimulated D2R phosphorylation (Namkung, et al., 2009a), further suggesting that GRK2 and GRK3 are the primary GRKs to regulate D2R phosphorylation.
GRKs play a role in D2R trafficking including internalization and recycling. GRK2 promotes DA-induced D2R internalization in HEK293 and COS-7 cells (Cho, et al., 2010; Iwata, et al., 1999; Namkung, et al., 2009b). GRK5 also facilitates D2R internalization and may act in a phosphorylation-independent manner given that there is no evidence demonstrating direct phosphorylation of D2Rs by GRK5 (Ito, et al., 1999). Furthermore, elimination of GRK-mediated phosphorylation residues within the third intracellular loop of D2Rs has no influence on agonist-induced receptor internalization and functional coupling to G-proteins; instead, this mutation accelerates degradation of internalized D2Rs and thus prevents receptor recycling to the membrane (Cho, et al., 2010; Namkung, et al., 2009a). Although these observations seem contradictory to the canonical role of GRK2 in regulation of D2R phosphorylation and subsequent internalization, elimination of multiple GRK phosphorylation sites within D2Rs may change structural properties of D2Rs and thus alter the sensitivity of the receptors to the effects of receptor agonists. The slow D2R recycling in GRK phosphorylation-deficient mutants is believed to be mediated by dissociation of receptors with β-arrestin because receptor phosphorylation and β-arrestin are necessary for receptor resensitization once desensitized and internalized (Cho, et al., 2010). These data collectively suggest that GRK-dependent phosphorylation via GRK2/3 promotes the recycling of internalized D2Rs to the membrane and thus responsible for resensitization.
Among GRK isoforms, GRK2, GRK3 and GRK6 are ubiquitously expressed in the brain whereas GRK5 is limited to the limbic and mesolimbic regions (Arriza, et al., 1992; Erdtmann-Vourliotis, et al., 2001). Given that GRK5 and GRK6 are not active in D2R phosphorylation shown in cultured cells and the fact that GRK3 expression is notably weak in the brain, GRK2 has been considered as a major player in regulation of D2R activity in vivo. Mice with selective GRK2 deletion in DA neurons exhibit increased basal locomotor activity and reduced sensitivity to acute and chronic cocaine-induced locomotor stimulation (Daigle, et al., 2014). Moreover, mice with GRK2 deficiency in D2R-containing medium spiny neurons show increased basal locomotor activity and a marked reduction in sensitivity to cocaine stimulation. Interestingly, these mice show decreased basal DA release and hyperactive presynaptic D2AR activity that can be blocked by sulpride, suggesting that the reduced cocaine effects are likely mediated through presynaptic D2ARs instead of D2PRs. The increased basal locomotor activity in both conditional GRK2 knockout mice is most likely mediated by dysregulated DAT function resulting from altered D2AR and D2PR activity in the absence of GRK2. In contrast to the regulation of D2ARs primarily by GRK2, striatal D2PR activity is critically modulated by GRK6 as GRK6 knockout mice show a greater coupling of striatal D2PRs to G-proteins and enhanced sensitivity to the locomotor-stimulating effect by direct D2R agonists, cocaine and AMPH (Gainetdinov, et al., 2003).
4.5.2. PKC
The phosphorylation of D2Rs can occur in the absence of GRKs in HEK293 cells (Kim, et al., 2001), suggesting that other mechanisms are involved. Activation of PKC by treatment with phorbol 12-myristate 13-acetate (PMA) or 12-O-tetradecanoyl 4beta-phorbol 13 alpha-acetate produces a marked increase in D2R phosphorylation and this effect is reversed by PKC inhibition (Morris, et al., 2007; Namkung & Sibley, 2004). Moreover, PKCβ plays a significant role in regulation of D2R phosphorylation as overexpression of PKCβ increases basal and PMA-stimulated receptor phosphorylation (Namkung & Sibley, 2004). In contrast, other PKC isoforms including PKCδ, PKCμ and PKCζ have no roles in D2R phosphorylation when overexpressed. Multiple sites within the second and third intracellular loops of D2Rs have been identified as the substrate for PKC and deletion of these sites abolishes PMA-mediated D2R phosphorylation (Morris, et al., 2007; Namkung & Sibley, 2004). In addition, PKC-mediated, site-specific phosphorylation of D2Rs is involved in receptor internalization (Namkung & Sibley, 2004).
The D2R function is modulated by PKC in heterologous expression systems. PKC phosphorylation of D2Rs desensitizes the receptors by decreasing the potency of agonists to inhibit cAMP accumulation in HEK293T cells (Namkung & Sibley, 2004). PKC activation switches the preferential coupling of D2Rs from inhibition of AC to facilitation of arachidonic acid release in CHO cells (Di Marzo, et al., 1993), suggesting that D2R phosphorylation by PKC may alter the efficacy of D2R coupling to different Gαi/o subunits and recruitment of different downstream effectors. Moreover, prolonged activation of D2Rs leads to heterologous sensitization of AC responsiveness by decoupling of Gαi/o from AC and this sensitization is further enhanced when PKC is activated in HEK293 cells (Beazely & Watts, 2005). Lastly, PKC differentially modulates desensitization of D2SRs and D2LRs in the fibroblast and GH4 cells (Morris, et al., 2007). Activation of D2Rs promotes Gβγ-mediated, phospholipase C-dependent calcium mobilization and this effect is inhibited by PKC when D2SRs, but not D2LRs, are activated. Because D2SRs function primarily as autoreceptors and D2LRs are postsynaptic (Radl, et al., 2018), the greater sensitivity of D2SRs to PKC-mediated calcium immobilization likely renders the autoreceptors more susceptible to desensitization.
The D2AR function is modulated by PKC, especially by PKCβ, both in vitro and in vivo. In rat striatal membranes treated with purified PKC, D2R-mediated [35S]GTPγS binding is abolished (Rogue, et al., 1990), although both D2ARs and D2PRs are activated. Regardless, these data suggest that PKC-dependent mechanisms can regulate the coupling between D2Rs and G-proteins. Accordingly, downregulation of PKC activity by a prolonged treatment with a PKC activator attenuates D2AR inhibition of the [3H]DA release from rat striatal slices (Iannazzo, et al., 1997). D2AR inhibition and PKC activation can synergistically increase [3H]DA release from the striatum of rats and rabbits (Dong, et al., 1995). Moreover, PKCβ knockout increases D2AR inhibition of endogenous DA release evoked by electrical stimulation and treatment with the potassium channel blocker 4-AP (Luderman, et al., 2015), indicating that the PKCβ isoform is involved in D2AR-stimulated Kv1 channel activity to regulate evoked DA release. The enhanced D2AR activity in PKCβ knockout mice is accompanied by an increase in surface receptor expression. In addition, ruboxistaurin, a selective inhibitor of PKCβ, attenuates cocaine-mediated DA overflow and locomotor activity, and this effect is abolished in the presence of the D2R/D3R antagonist raclopride (Zestos, et al., 2019). Lastly, because PKCβ is responsible for D2R-stimulated DAT recycling shown in both cultured cells and mouse striatal synaptosomes (Chen, et al., 2013), these data suggest that PKC, especially PKCβ, modulates DA signaling in vivo by targeting both D2AR and DAT activity.
The D2ARs are also subject to regulation by a few other protein kinases. There are multiple consensus recognition sites for PKA within D2Rs. PKA can directly phosphorylate purified D2R proteins (Elazar & Fuchs, 1991), but has no effect on D2R phosphorylation in HEK293 cells expressing D2Rs (Namkung & Sibley, 2004). PKA phosphorylation of D2Rs from striatal membranes and purified proteins reduces the efficacy of agonist binding by decoupling of D2Rs from G-proteins (Elazar & Fuchs, 1991). Moreover, prolonged stimulation-induced D2AR desensitization in rat SN is attenuated when AMP-activated protein kinase (AMPK) is activated (Yang, et al., 2019). Lastly, withdrawal from repeated ethanol exposure causes desensitization of D2AR-dependent activation of GIRK channel conductance in mouse VTA DA neurons, which is mediated by IP3-induced calcium release from intracellular store and activation of CaMKII (Perra, et al., 2011). The structural biology modeling suggests that CaMKII interacts with D2Rs and modulates DA binding affinity for D2Rs. (Laoye, et al., 2016).
5. Conclusion & Perspectives
This review highlights many D2AR-interacting partners including transporters, GPCRs, ion channels, signaling molecules and protein kinases (Fig.1), suggesting the functional divergence of the receptors. It is important to note that the D2AR-interacting proteins involved in regulating midbrain DA neuron firing and axon terminal DA release are distinct. Although chronic drug exposure often alters D2AR activity in both somatodendritic and axon terminal compartments of DA neurons, it appears that changes in somatodendritic D2AR-mediated DA neuron firing are more enduring than the changes in axon terminal D2AR-mediated DA release. The more persistent reduction of somatodendritic D2AR activity induced by chronic exposure to drugs perhaps results from an irreversible disruption of the cellular D2AR interaction network in the midbrain. Additionally, chronic exposure to drugs of abuse often downregulates surface receptor expression, which will likely diminish the efficacy and potency of receptor agonists/antagonists because of the reduced accessibility of the receptors by these drugs. This is particularly interesting because monodrug therapy has been the main approach for treatment of SUD and ineffective. Thus, concurrent targeting of D2AR-interacting proteins at the membrane and intracellular levels along with direct receptor agonists or antagonists are a more promising approach to effectively normalize disrupted DA transmission. Future research on spatiotemporal analysis of the D2AR interaction network will open up avenues to discover novel, dynamic D2AR-interacting proteins, define new signal transduction pathways, and improve our understanding of the actions of drugs of abuse.
Acknowledgements
This work is supported by National Institute of Drug Abuse grants R01DA042862 and P50DA006634.
Abbreviations:
- AAV
adeno-associated virus
- A2AR
adenosine A2 receptor
- AC
adenylyl cyclase
- 4-AP
4-aminopyridine
- AMPK
AMP-activated protein kinase
- AMPH
amphetamine
- AT1Rs
angiotensin II type 1 receptors
- BRET
bioluminescence resonance energy transfer
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- CPu
caudate putamen
- cAMP
cyclic adenosine monophosphate
- DA
dopamine
- D2R
dopamine D2 receptors
- D2AR
dopamine D2 autoreceptor
- D2PR
postsynaptic D2 receptor
- DAT
dopamine transporter
- EPSCs
glutamate-mediated excitatory postsynaptic currents
- ERK
extracellular signal-regulated kinase
- GPCRs
G-protein coupled receptors
- GIRK
G-protein-coupled inwardly rectifying potassium
- GSK-3
glycogen synthase kinase-3
- METH
methamphetamine
- mAChR
muscarinic acetylcholinergic receptors
- NAc
nucleus accumbens
- nAChR
nicotinic acetylcholinergic receptors
- NCS-1
neuronal calcium sensor 1
- NTS1R
neurotensin type 1 receptors
- PFC
prefrontal cortex
- PKA
protein kinase A
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- RGS
regulator of G-protein signaling
- RGS9–2
the long splice form of RGS9
- SN
substantia nigra
- SUD
substance use disorder
- TAAR1
trace amine-associated receptor 1
- TEA
tetraethylammonium
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no conflict of interest.
References
- Alloway PG, & Dolph PJ (1999). A role for the light-dependent phosphorylation of visual arrestin. Proc Natl Acad Sci U S A, 96, 6072–6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SM, & Pierce RC (2005). Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther, 106, 389–403. [DOI] [PubMed] [Google Scholar]
- Anzalone A, Lizardi-Ortiz JE, Ramos M, De Mei C, Hopf FW, Iaccarino C, Halbout B, Jacobsen J, Kinoshita C, Welter M, Caron MG, Bonci A, Sulzer D, & Borrelli E (2012). Dual Control of Dopamine Synthesis and Release by Presynaptic and Postsynaptic Dopamine D2 Receptors. J Neurosci, 32, 9023–9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argiolas A, Melis MR, Fadda F, & Gessa GL (1982). Evidence for dopamine autoreceptors controlling dopamine synthesis in the substantia nigra. Brain Res, 234, 177–181. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Dawson TM, Simerly RB, Martin LJ, Caron MG, Snyder SH, & Lefkowitz RJ (1992). The G-protein-coupled receptor kinases beta ARK1 and beta ARK2 are widely distributed at synapses in rat brain. J Neurosci, 12, 4045–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashok AH, Mizuno Y, Volkow ND, & Howes OD (2017). Association of Stimulant Use With Dopaminergic Alterations in Users of Cocaine, Amphetamine, or Methamphetamine: A Systematic Review and Meta-analysis. JAMA Psychiatry, 74, 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asif-Malik A, Hoener MC, & Canales JJ (2017). Interaction Between the Trace Amine-Associated Receptor 1 and the Dopamine D2 Receptor Controls Cocaine’s Neurochemical Actions. Sci Rep, 7, 13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avale ME, Faure P, Pons S, Robledo P, Deltheil T, David DJ, Gardier AM, Maldonado R, Granon S, Changeux JP, & Maskos U (2008). Interplay of beta2* nicotinic receptors and dopamine pathways in the control of spontaneous locomotion. Proc Natl Acad Sci U S A, 105, 15991–15996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan UH, Chen Y, & Leslie FM (2002). Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol, 444, 260–274. [DOI] [PubMed] [Google Scholar]
- Bailey A, Metaxas A, Yoo JH, McGee T, & Kitchen I (2008). Decrease of D2 receptor binding but increase in D2-stimulated G-protein activation, dopamine transporter binding and behavioural sensitization in brains of mice treated with a chronic escalating dose ‘binge’ cocaine administration paradigm. Eur J Neurosci, 28, 759–770. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, & Meshul CK (2004). Dopamine modulates release from corticostriatal terminals. J Neurosci, 24, 9541–9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak LS, Salahpour A, Zhang X, Masri B, Sotnikova TD, Ramsey AJ, Violin JD, Lefkowitz RJ, Caron MG, & Gainetdinov RR (2008). Pharmacological characterization of membrane-expressed human trace amine-associated receptor 1 (TAAR1) by a bioluminescence resonance energy transfer cAMP biosensor. Mol Pharmacol, 74, 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Espinoza S, & Gainetdinov RR (2015). Dopamine receptors - IUPHAR Review 13. Br J Pharmacol, 172, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beazely MA, & Watts VJ (2005). Activation of a novel PKC isoform synergistically enhances D2L dopamine receptor-mediated sensitization of adenylate cyclase type 6. Cellular signalling, 17, 647–653. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, & Williams JT (2004). Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron, 42, 939–946. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, & Williams JT (2007). Long-term depression of a dopamine IPSC. J Neurosci, 27, 2074–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, & Rubinstein M (2011). Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci, 14, 1033–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist F, Jonason J, Pileblad E, & Nissbrandt H (1998). Effects of local administration of L-, N-, and P/Q-type calcium channel blockers on spontaneous dopamine release in the striatum and the substantia nigra: a microdialysis study in rat. J Neurochem, 70, 1532–1540. [DOI] [PubMed] [Google Scholar]
- Bernosky-Smith KA, Qiu YY, Feja M, Lee YB, Loughlin B, Li JX, & Bass CE (2018). Ventral tegmental area D2 receptor knockdown enhances choice impulsivity in a delay-discounting task in rats. Behav Brain Res, 341, 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MD (2004). Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J Neurochem, 90, 257–271. [DOI] [PubMed] [Google Scholar]
- Bickel WK, & Marsch LA (2001). Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction, 96, 73–86. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, & Madden GJ (1999). Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology (Berl), 146, 447–454. [DOI] [PubMed] [Google Scholar]
- Bifsha P, Yang J, Fisher RA, & Drouin J (2014). Rgs6 is required for adult maintenance of dopaminergic neurons in the ventral substantia nigra. PLoS Genet, 10, e1004863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bofill-Cardona E, Kudlacek O, Yang Q, Ahorn H, Freissmuth M, & Nanoff C (2000). Binding of calmodulin to the D2-dopamine receptor reduces receptor signaling by arresting the G protein activation switch. J Biol Chem, 275, 32672–32680. [DOI] [PubMed] [Google Scholar]
- Bolan EA, Kivell B, Jaligam V, Oz M, Jayanthi LD, Han Y, Sen N, Urizar E, Gomes I, Devi LA, Ramamoorthy S, Javitch JA, Zapata A, & Shippenberg TS (2007). D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol Pharmacol, 71, 1222–1232. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, & Gerald C (2001). Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A, 98, 8966–8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Narvaez M, Wydra K, Pintsuk J, Pinton L, Jimenez-Beristain A, Di Palma M, Jastrzebska J, Filip M, & Fuxe K (2017). Cocaine self-administration specifically increases A2AR-D2R and D2R-sigma1R heteroreceptor complexes in the rat nucleus accumbens shell. Relevance for cocaine use disorder. Pharmacol Biochem Behav, 155, 24–31. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Ravani A, Tarakanov AO, Brito I, Narvaez M, Romero-Fernandez W, Corrales F, Agnati LF, Tanganelli S, Ferraro L, & Fuxe K (2013). Dopamine D2 receptor signaling dynamics of dopamine D2-neurotensin 1 receptor heteromers. Biochem Biophys Res Commun, 435, 140–146. [DOI] [PubMed] [Google Scholar]
- Bowton E, Saunders C, Erreger K, Sakrikar D, Matthies HJ, Sen N, Jessen T, Colbran RJ, Caron MG, Javitch JA, Blakely RD, & Galli A (2010). Dysregulation of dopamine transporters via dopamine D2 autoreceptors triggers anomalous dopamine efflux associated with attention-deficit hyperactivity disorder. The Journal of neuroscience : the official journal of the Society for Neuroscience, 30, 6048–6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradaia A, Trube G, Stalder H, Norcross RD, Ozmen L, Wettstein JG, Pinard A, Buchy D, Gassmann M, Hoener MC, & Bettler B (2009). The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc Natl Acad Sci U S A, 106, 20081–20086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW, Gruen RJ, Berridge CW, & Roth RH (1991). Individual differences in behavioral measures: correlations with nucleus accumbens dopamine measured by microdialysis. Pharmacol Biochem Behav, 39, 877–882. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, & Geyer MA (1992). Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry, 49, 206–215. [DOI] [PubMed] [Google Scholar]
- Brown DC, Steward LJ, Ge J, & Barnes NM (1996). Ability of angiotensin II to modulate striatal dopamine release via the AT1 receptor in vitro and in vivo. Br J Pharmacol, 118, 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, & Zald DH (2010). Dopaminergic network differences in human impulsivity. Science, 329, 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budygin EA, Oleson EB, Lee YB, Blume LC, Bruno MJ, Howlett AC, Thompson AC, & Bass CE (2016). Acute Depletion of D2 Receptors from the Rat Substantia Nigra Alters Dopamine Kinetics in the Dorsal Striatum and Drug Responsivity. Front Behav Neurosci, 10, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney BS, Aghajanian GK, & Roth RH (1973). Comparison of effects of L-dopa, amphetamine and apomorphine on firing rate of rat dopaminergic neurones. Nat New Biol, 245, 123–125. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, Olson SB, Magenis RE, Amara SG, & Grandy DK (2001). Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol, 60, 1181–1188. [DOI] [PubMed] [Google Scholar]
- Cabrera-Vera TM, Hernandez S, Earls LR, Medkova M, Sundgren-Andersson AK, Surmeier DJ, & Hamm HE (2004). RGS9–2 modulates D2 dopamine receptor-mediated Ca2+ channel inhibition in rat striatal cholinergic interneurons. Proc Natl Acad Sci U S A, 101, 16339–16344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, Lovinger DM, & Cheer JF (2012). Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep, 2, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Sun H, Eldeeb K, Luessen DJ, Feng X, Howlett AC, Jones SR, & Chen R (2014). Amphetamine self-administration attenuates dopamine D2 autoreceptor function. Neuropsychopharmacology, 39, 1833–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass WA, & Zahniser NR (1991). Potassium channel blockers inhibit D2 dopamine, but not A1 adenosine, receptor-mediated inhibition of striatal dopamine release. J Neurochem, 57, 147–152. [DOI] [PubMed] [Google Scholar]
- Celver J, Sharma M, & Kovoor A (2010). RGS9–2 mediates specific inhibition of agonist-induced internalization of D2-dopamine receptors. J Neurochem, 114, 739–749. [DOI] [PubMed] [Google Scholar]
- Centonze D, Picconi B, Baunez C, Borrelli E, Pisani A, Bernardi G, & Calabresi P (2002). Cocaine and amphetamine depress striatal GABAergic synaptic transmission through D2 dopamine receptors. Neuropsychopharmacology, 26, 164–175. [DOI] [PubMed] [Google Scholar]
- Chakir K, Zhu W, Tsang S, Woo AY, Yang D, Wang X, Zeng X, Rhee MH, Mende U, Koitabashi N, Takimoto E, Blumer KJ, Lakatta EG, Kass DA, & Xiao RP (2011). RGS2 is a primary terminator of beta(2)-adrenergic receptor-mediated G(i) signaling. J Mol Cell Cardiol, 50, 1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Moran KA, Avshalumov MV, & Rice ME (2006). Limited regulation of somatodendritic dopamine release by voltage-sensitive Ca channels contrasted with strong regulation of axonal dopamine release. J Neurochem, 96, 645–655. [DOI] [PubMed] [Google Scholar]
- Chen BT, Patel JC, Moran KA, & Rice ME (2011). Differential calcium dependence of axonal versus somatodendritic dopamine release, with characteristics of both in the ventral tegmental area. Front Syst Neurosci, 5, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Daining CP, Sun H, Fraser R, Stokes SL, Leitges M, & Gnegy ME (2013). Protein kinase Cbeta is a modulator of the dopamine D2 autoreceptor-activated trafficking of the dopamine transporter. J Neurochem, 125, 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Furman CA, Zhang M, Kim MN, Gereau R. W. t., Leitges M, & Gnegy ME (2009). Protein kinase Cbeta is a critical regulator of dopamine transporter trafficking and regulates the behavioral response to amphetamine in mice. J Pharmacol Exp Ther, 328, 912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, McIntosh S, Hemby SE, Sun H, Sexton T, Martin TJ, & Childers SR (2018). High and low doses of cocaine intake are differentially regulated by dopamine D2 receptors in the ventral tegmental area and the nucleus accumbens. Neurosci Lett, 671, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XL, Zhong ZG, Yokoyama S, Bark C, Meister B, Berggren PO, Roder J, Higashida H, & Jeromin A (2001). Overexpression of rat neuronal calcium sensor-1 in rodent NG108–15 cells enhances synapse formation and transmission. J Physiol, 532, 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo LA, Bannon MJ, Grace AA, Roth RH, & Bunney BS (1984). Evidence for the absence of impulse-regulating somatodendritic and synthesis-modulating nerve terminal autoreceptors on subpopulations of mesocortical dopamine neurons. Neuroscience, 12, 1–16. [DOI] [PubMed] [Google Scholar]
- Cho D, Zheng M, Min C, Ma L, Kurose H, Park JH, & Kim KM (2010). Agonist-induced endocytosis and receptor phosphorylation mediate resensitization of dopamine D(2) receptors. Mol Endocrinol, 24, 574–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AC, Salminen O, Marks MJ, Whiteaker P, & Grady SR (2009). The road to discovery of neuronal nicotinic cholinergic receptor subtypes. Handb Exp Pharmacol, 85–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congar P, Bergevin A, & Trudeau LE (2002). D2 receptors inhibit the secretory process downstream from calcium influx in dopaminergic neurons: implication of K+ channels. J Neurophysiol, 87, 1046–1056. [DOI] [PubMed] [Google Scholar]
- Cragg SJ, & Greenfield SA (1997). Differential autoreceptor control of somatodendritic and axon terminal dopamine release in substantia nigra, ventral tegmental area, and striatum. J Neurosci, 17, 5738–5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubeddu LX, & Hoffmann IS (1982). Operational characteristics of the inhibitory feedback mechanism for regulation of dopamine release via presynaptic receptors. J Pharmacol Exp Ther, 223, 497–501. [PubMed] [Google Scholar]
- Daigle TL, Ferris MJ, Gainetdinov RR, Sotnikova TD, Urs NM, Jones SR, & Caron MG (2014). Selective deletion of GRK2 alters psychostimulant-induced behaviors and dopamine neurotransmission. Neuropsychopharmacology, 39, 2450–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Toso R, Sommer B, Ewert M, Herb A, Pritchett DB, Bach A, Shivers BD, & Seeburg PH (1989). The dopamine D2 receptor: two molecular forms generated by alternative splicing. EMBO J, 8, 4025–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, & Everitt BJ (2009). Dopamine receptors in the learning, memory and drug reward circuitry. Seminars in cell & developmental biology, 20, 403–410. [DOI] [PubMed] [Google Scholar]
- Davila V, Yan Z, Craciun LC, Logothetis D, & Sulzer D (2003). D3 dopamine autoreceptors do not activate G-protein-gated inwardly rectifying potassium channel currents in substantia nigra dopamine neurons. J Neurosci, 23, 5693–5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H (2009). Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol, 14, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, & Richards JB (2002). Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology, 27, 813–825. [DOI] [PubMed] [Google Scholar]
- Dempsey BR, & Shaw GS (2011). Identification of calcium-independent and calcium-enhanced binding between S100B and the dopamine D2 receptor. Biochemistry, 50, 9056–9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, & Imperato A (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A, 85, 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Vial D, Sokoloff P, Schwartz JC, & Piomelli D (1993). Selection of alternative G-mediated signaling pathways at the dopamine D2 receptor by protein kinase C. J Neurosci, 13, 4846–4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Lopez S, Piccart E, Lynch WB, Wollet MB, Sharpe AL, & Beckstead MJ (2018). Antagonism of Neurotensin Receptors in the Ventral Tegmental Area Decreases Methamphetamine Self-Administration and Methamphetamine Seeking in Mice. Int J Neuropsychopharmacol, 21, 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Lopez S, Sharma R, & Beckstead MJ (2019). Neurotensin receptor 1 deletion decreases methamphetamine self-administration and the associated reduction in dopamine cell firing. Addict Biol, e12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong ZJ, Jin GZ, & Huang HY (1995). Augmentation of dopamine release by (−)-stepholidine from rabbit and rat caudate slices. Zhongguo Yao Li Xue Bao, 16, 497–501. [PubMed] [Google Scholar]
- Dragicevic E, Poetschke C, Duda J, Schlaudraff F, Lammel S, Schiemann J, Fauler M, Hetzel A, Watanabe M, Lujan R, Malenka RC, Striessnig J, & Liss B (2014). Cav1.3 channels control D2-autoreceptor responses via NCS-1 in substantia nigra dopamine neurons. Brain, 137, 2287–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour MA, Woodhouse A, & Goaillard JM (2014). Somatodendritic ion channel expression in substantia nigra pars compacta dopaminergic neurons across postnatal development. J Neurosci Res, 92, 981–999. [DOI] [PubMed] [Google Scholar]
- Eilam D, & Szechtman H (1989). Biphasic effect of D-2 agonist quinpirole on locomotion and movements. Eur J Pharmacol, 161, 151–157. [DOI] [PubMed] [Google Scholar]
- Elazar Z, & Fuchs S (1991). Phosphorylation by cyclic AMP-dependent protein kinase modulates agonist binding to the D2 dopamine receptor. J Neurochem, 56, 75–80. [DOI] [PubMed] [Google Scholar]
- Erdtmann-Vourliotis M, Mayer P, Ammon S, Riechert U, & Hollt V (2001). Distribution of G-protein-coupled receptor kinase (GRK) isoforms 2, 3, 5 and 6 mRNA in the rat brain. Brain Res Mol Brain Res, 95, 129–137. [DOI] [PubMed] [Google Scholar]
- Espinoza S, Salahpour A, Masri B, Sotnikova TD, Messa M, Barak LS, Caron MG, & Gainetdinov RR (2011). Functional interaction between trace amine-associated receptor 1 and dopamine D2 receptor. Mol Pharmacol, 80, 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawaz CS, Martel P, Leo D, & Trudeau LE (2009). Presynaptic action of neurotensin on dopamine release through inhibition of D(2) receptor function. BMC Neurosci, 10, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishburn CS, Elazar Z, & Fuchs S (1995). Differential glycosylation and intracellular trafficking for the long and short isoforms of the D2 dopamine receptor. J Biol Chem, 270, 29819–29824. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Chaudhury S, Waselus M, Kelly R, Sewani S, Clinton SM, Thompson RC, Watson SJ Jr., & Akil H (2016). Genetic background and epigenetic modifications in the core of the nucleus accumbens predict addiction-like behavior in a rat model. Proc Natl Acad Sci U S A, 113, E2861–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP (2014). The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick AL, Yano H, Trifilieff P, Vishwasrao HD, Biezonski D, Meszaros J, Urizar E, Sibley DR, Kellendonk C, Sonntag KC, Graham DL, Colbran RJ, Stanwood GD, & Javitch JA (2015). Evidence against dopamine D1/D2 receptor heteromers. Mol Psychiatry, 20, 1373–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton S, Thibault D, Mendez JA, Lahaie N, Tirotta E, Borrelli E, Bouvier M, Tempel BL, & Trudeau LE (2011). Contribution of Kv1.2 voltage-gated potassium channel to D2 autoreceptor regulation of axonal dopamine overflow. J Biol Chem, 286, 9360–9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman CA, Chen R, Guptaroy B, Zhang M, Holz RW, & Gnegy M (2009). Dopamine and amphetamine rapidly increase dopamine transporter trafficking to the surface: live-cell imaging using total internal reflection fluorescence microscopy. J Neurosci, 29, 3328–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Borroto-Escuela DO, Marcellino D, Romero-Fernandez W, Frankowska M, Guidolin D, Filip M, Ferraro L, Woods AS, Tarakanov A, Ciruela F, Agnati LF, & Tanganelli S (2012). GPCR heteromers and their allosteric receptor-receptor interactions. Curr Med Chem, 19, 356–363. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Bohn LM, Sotnikova TD, Cyr M, Laakso A, Macrae AD, Torres GE, Kim KM, Lefkowitz RJ, Caron MG, & Premont RT (2003). Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice. Neuron, 38, 291–303. [DOI] [PubMed] [Google Scholar]
- Gantz SC, Robinson BG, Buck DC, Bunzow JR, Neve RL, Williams JT, & Neve KA (2015). Distinct regulation of dopamine D2S and D2L autoreceptor signaling by calcium. Elife, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rodriguez O, Secades-Villa R, Weidberg S, & Yoon JH (2013). A systematic assessment of delay discounting in relation to cocaine and nicotine dependence. Behav Processes, 99, 100–105. [DOI] [PubMed] [Google Scholar]
- Garrido-Gil P, Valenzuela R, Villar-Cheda B, Lanciego JL, & Labandeira-Garcia JL (2013). Expression of angiotensinogen and receptors for angiotensin and prorenin in the monkey and human substantia nigra: an intracellular renin-angiotensin system in the nigra. Brain structure & function, 218, 373–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon M, Duffy AM, Chan J, Lynch MK, Mackie K, & Pickel VM (2013). Dopamine D(2) and acetylcholine alpha7 nicotinic receptors have subcellular distributions favoring mediation of convergent signaling in the mouse ventral tegmental area. Neuroscience, 252, 126–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, & Zahm DS (2006). Neurotensin afferents of the ventral tegmental area in the rat: [1] re-examination of their origins and [2] responses to acute psychostimulant and antipsychotic drug administration. Eur J Neurosci, 24, 116–134. [DOI] [PubMed] [Google Scholar]
- Ghelardini C, Quattrone A, Galeotti N, Livi S, Banchelli G, Raimondi L, & Pirisino R (2003). Antisense knockdown of the Shaker-like Kv1.1 gene abolishes the central stimulatory effects of amphetamines in mice and rats. Neuropsychopharmacology, 28, 1096–1105. [DOI] [PubMed] [Google Scholar]
- Gold SJ, Ni YG, Dohlman HG, & Nestler EJ (1997). Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J Neurosci, 17, 8024–8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar R, Gresch PJ, Davis GL, Katamish RM, Riele JR, Stewart AM, Vaughan RA, Hahn MK, & Blakely RD (2018). Region-Specific Regulation of Presynaptic Dopamine Homeostasis by D2 Autoreceptors Shapes the In Vivo Impact of the Neuropsychiatric Disease-Associated DAT Variant Val559. J Neurosci, 38, 5302–5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves PM, & Linder JC (1983). Dendro-dendritic synapses in substantia nigra: descriptions based on analysis of serial sections. Exp Brain Res, 49, 209–217. [DOI] [PubMed] [Google Scholar]
- Guild SB, Murray AT, Wilson ML, Wiegand UK, Apps DK, Jin Y, Rindler M, Roder J, & Jeromin A (2001). Over-expression of NCS-1 in AtT-20 cells affects ACTH secretion and storage. Mol Cell Endocrinol, 184, 51–63. [DOI] [PubMed] [Google Scholar]
- Guptaroy B, Zhang M, Bowton E, Binda F, Shi L, Weinstein H, Galli A, Javitch JA, Neubig RR, & Gnegy ME (2009). A juxtamembrane mutation in the N terminus of the dopamine transporter induces preference for an inward-facing conformation. Mol Pharmacol, 75, 514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN (2014). The place of dopamine in the cortico-basal ganglia circuit. Neuroscience, 282, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson GR, Hoonakker AJ, Alburges ME, McFadden LM, Robson CM, & Frankel PS (2012). Response of limbic neurotensin systems to methamphetamine self-administration. Neuroscience, 203, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmeier A, Obermueller S, Meyer CA, Revel FG, Buchy D, Chaboz S, Dernick G, Wettstein JG, Iglesias A, Rolink A, Bettler B, & Hoener MC (2015). Trace amine-associated receptor 1 activation silences GSK3beta signaling of TAAR1 and D2R heteromers. Eur Neuropsychopharmacol, 25, 2049–2061. [DOI] [PubMed] [Google Scholar]
- Harsing LG Jr., Sershen H, & Lajtha A (1992). Dopamine efflux from striatum after chronic nicotine: evidence for autoreceptor desensitization. J Neurochem, 59, 48–54. [DOI] [PubMed] [Google Scholar]
- Hasbi A, O’Dowd BF, & George SR (2011). Dopamine D1-D2 receptor heteromer signaling pathway in the brain: emerging physiological relevance. Mol Brain, 4, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock JW, & Haycock DA (1991). Tyrosine hydroxylase in rat brain dopaminergic nerve terminals. Multiple-site phosphorylation in vivo and in synaptosomes. J Biol Chem, 266, 5650–5657. [PubMed] [Google Scholar]
- Hepler JR (1999). Emerging roles for RGS proteins in cell signalling. Trends Pharmacol Sci, 20, 376–382. [DOI] [PubMed] [Google Scholar]
- Hermann K, McDonald W, Unger T, Lang RE, & Ganten D (1984). Angiotensin biosynthesis and concentrations in brain of normotensive and hypertensive rats. J Physiol (Paris), 79, 471–480. [PubMed] [Google Scholar]
- Heximer SP, Watson N, Linder ME, Blumer KJ, & Hepler JR (1997). RGS2/G0S8 is a selective inhibitor of Gqalpha function. Proc Natl Acad Sci U S A, 94, 14389–14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, & Mitchell SH (2006). Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology (Berl), 188, 162–170. [DOI] [PubMed] [Google Scholar]
- Hohoff C, McDonald JM, Baune BT, Cook EH, Deckert J, & de Wit H (2005). Interindividual variation in anxiety response to amphetamine: possible role for adenosine A2A receptor gene variants. Am J Med Genet B Neuropsychiatr Genet, 139B, 42–44. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Everitt BJ, Theodorsson-Norheim E, & Goldstein M (1984). Occurrence of neurotensinlike immunoreactivity in subpopulations of hypothalamic, mesencephalic, and medullary catecholamine neurons. J Comp Neurol, 222, 543–559. [DOI] [PubMed] [Google Scholar]
- Holroyd KB, Adrover MF, Fuino RL, Bock R, Kaplan AR, Gremel CM, Rubinstein M, & Alvarez VA (2015). Loss of feedback inhibition via D2 autoreceptors enhances acquisition of cocaine taking and reactivity to drug-paired cues. Neuropsychopharmacology, 40, 1495–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks SB, Martemyanov K, & Zachariou V (2008). A role of RGS proteins in drug addiction. Biochem Pharmacol, 75, 76–84. [DOI] [PubMed] [Google Scholar]
- Hu XT, & Wang RY (1988). Disinhibition of nucleus accumbens neurons by the dopamine D2 receptor agonist LY-141865: prevented by 6-OHDA pretreatment. Brain Res, 444, 389–393. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Pristupa ZB, Herman MM, Niznik HB, & Kleinman JE (1994). The dopamine transporter and dopamine D2 receptor messenger RNAs are differentially expressed in limbic- and motor-related subpopulations of human mesencephalic neurons. Neuroscience, 63, 357–362. [DOI] [PubMed] [Google Scholar]
- Iannazzo L, Sathananthan S, & Majewski H (1997). Modulation of dopamine release from rat striatum by protein kinase C: interaction with presynaptic D2-dopamine-autoreceptors. Br J Pharmacol, 122, 1561–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inanobe A, Yoshimoto Y, Horio Y, Morishige KI, Hibino H, Matsumoto S, Tokunaga Y, Maeda T, Hata Y, Takai Y, & Kurachi Y (1999). Characterization of G-protein-gated K+ channels composed of Kir3.2 subunits in dopaminergic neurons of the substantia nigra. J Neurosci, 19, 1006–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingi T, Krumins AM, Chidiac P, Brothers GM, Chung S, Snow BE, Barnes CA, Lanahan AA, Siderovski DP, Ross EM, Gilman AG, & Worley PF (1998). Dynamic regulation of RGS2 suggests a novel mechanism in G-protein signaling and neuronal plasticity. J Neurosci, 18, 7178–7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Haga T, Lameh J, & Sadee W (1999). Sequestration of dopamine D2 receptors depends on coexpression of G-protein-coupled receptor kinases 2 or 5. Eur J Biochem, 260, 112–119. [DOI] [PubMed] [Google Scholar]
- Itokawa M, Toru M, Ito K, Tsuga H, Kameyama K, Haga T, Arinami T, & Hamaguchi H (1996). Sequestration of the short and long isoforms of dopamine D2 receptors expressed in Chinese hamster ovary cells. Mol Pharmacol, 49, 560–566. [PubMed] [Google Scholar]
- Iwata K, Ito K, Fukuzaki A, Inaki K, & Haga T (1999). Dynamin and rab5 regulate GRK2-dependent internalization of dopamine D2 receptors. Eur J Biochem, 263, 596–602. [DOI] [PubMed] [Google Scholar]
- Jang JY, Jang M, Kim SH, Um KB, Kang YK, Kim HJ, Chung S, & Park MK (2011). Regulation of dopaminergic neuron firing by heterogeneous dopamine autoreceptors in the substantia nigra pars compacta. Journal of neurochemistry, 116, 966–974. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Torres OV, Ladenheim B, & Cadet JL (2020). A Single Prior Injection of Methamphetamine Enhances Methamphetamine Self-Administration (SA) and Blocks SA-Induced Changes in DNA Methylation and mRNA Expression of Potassium Channels in the Rat Nucleus Accumbens. Mol Neurobiol, 57, 1459–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZG, Pessia M, & North RA (1994). Neurotensin excitation of rat ventral tegmental neurones. J Physiol, 474, 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Furman CA, Zhang M, Guptaroy B, & Gnegy ME (2005a). Rapid delivery of the dopamine transporter to the plasmalemmal membrane upon amphetamine stimulation. Neuropharmacology, 49, 750–758. [DOI] [PubMed] [Google Scholar]
- Jomphe C, Tiberi M, & Trudeau LE (2006). Expression of D2 receptor isoforms in cultured neurons reveals equipotent autoreceptor function. Neuropharmacology, 50, 595–605. [DOI] [PubMed] [Google Scholar]
- Jones IW, Bolam JP, & Wonnacott S (2001). Presynaptic localisation of the nicotinic acetylcholine receptor beta2 subunit immunoreactivity in rat nigrostriatal dopaminergic neurones. J Comp Neurol, 439, 235–247. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Hu XT, Cooper DC, Wightman RM, White FJ, & Caron MG (1999). Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat Neurosci, 2, 649–655. [DOI] [PubMed] [Google Scholar]
- Kabbani N, Negyessy L, Lin R, Goldman-Rakic P, & Levenson R (2002). Interaction with neuronal calcium sensor NCS-1 mediates desensitization of the D2 dopamine receptor. J Neurosci, 22, 8476–8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Rose JH, Huggins KN, Konstantopoulos JK, & Jones SR (2015). Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug Alcohol Depend, 150, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai RS, Ito SV, Awane RM, Fujiwara TK, & Kusumi A (2018). The Class-A GPCR Dopamine D2 Receptor Forms Transient Dimers Stabilized by Agonists: Detection by Single-Molecule Tracking. Cell Biochem Biophys, 76, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasom M, Gharra S, Sadiya I, Avital-Shacham M, & Kosloff M (2018). Interplay between negative and positive design elements in Galpha helical domains of G proteins determines interaction specificity toward RGS2. Biochem J, 475, 2293–2304. [DOI] [PubMed] [Google Scholar]
- Kempadoo KA, Tourino C, Cho SL, Magnani F, Leinninger GM, Stuber GD, Zhang F, Myers MG, Deisseroth K, de Lecea L, & Bonci A (2013). Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J Neurosci, 33, 7618–7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan ZU, Mrzljak L, Gutierrez A, de la Calle A, & Goldman-Rakic PS (1998). Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A, 95, 7731–7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, Galli A, & Javitch JA (2004). N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol, 2, E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Valenzano KJ, Robinson SR, Yao WD, Barak LS, & Caron MG (2001). Differential regulation of the dopamine D2 and D3 receptors by G protein-coupled receptor kinases and beta-arrestins. J Biol Chem, 276, 37409–37414. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim MY, Lee EJ, Ahn YS, & Baik JH (2004). Distinct regulation of internalization and mitogen-activated protein kinase activation by two isoforms of the dopamine D2 receptor. Mol Endocrinol, 18, 640–652. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, & Bickel WK (1999). Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen, 128, 78–87. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Ujike H, Iwata N, Inada T, Yamada M, Sekine Y, Uchimura N, Iyo M, Ozaki N, Itokawa M, & Sora I (2010). The adenosine A2A receptor is associated with methamphetamine dependence/psychosis in the Japanese population. Behav Brain Funct, 6, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology, 35, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschatzky S, Tschammer N, & Gmeiner P (2011). Cross-receptor interactions between dopamine D2L and neurotensin NTS1 receptors modulate binding affinities of dopaminergics. ACS Chem Neurosci, 2, 308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Hayashi T, Chuang JY, Tsai SY, Su TP, & Bonci A (2013). Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine. Cell, 152, 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovoor A, Seyffarth P, Ebert J, Barghshoon S, Chen CK, Schwarz S, Axelrod JD, Cheyette BN, Simon MI, Lester HA, & Schwarz J (2005). D2 dopamine receptors colocalize regulator of G-protein signaling 9–2 (RGS9–2) via the RGS9 DEP domain, and RGS9 knock-out mice develop dyskinesias associated with dopamine pathways. J Neurosci, 25, 2157–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kull B, Ferre S, Arslan G, Svenningsson P, Fuxe K, Owman C, & Fredholm B (1999). Reciprocal interactions between adenosine A2A and dopamine D2 receptors in Chinese hamster ovary cells co-transfected with the two receptors. Biochem Pharmacol, 58, 1035–1045. [DOI] [PubMed] [Google Scholar]
- Labouebe G, Lomazzi M, Cruz HG, Creton C, Lujan R, Li M, Yanagawa Y, Obata K, Watanabe M, Wickman K, Boyer SB, Slesinger PA, & Luscher, (2007). RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci, 10, 1559–1568. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, & North RA (1987). Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol, 392, 397–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachowicz JE, & Sibley DR (1997). Molecular characteristics of mammalian dopamine receptors. Pharmacol Toxicol, 81, 105–113. [DOI] [PubMed] [Google Scholar]
- Lambert NA (2010). GPCR dimers fall apart. Sci Signal, 3, pe12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, & Roeper J (2008). Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron, 57, 760–773. [DOI] [PubMed] [Google Scholar]
- Lan H, Liu Y, Bell MI, Gurevich VV, & Neve KA (2009). A dopamine D2 receptor mutant capable of G protein-mediated signaling but deficient in arrestin binding. Mol Pharmacol, 75, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laoye BJ, Okurumeh OA, Obagaye OV, Olagunju MO, Bankole OO, Olubiyi OO, & Ogundele OM (2016). Dopamine binds calmodulin during autoregulation of dopaminergic D2 receptor signaling through CaMKIIalpha-calmodulin complex. J Recept Signal Transduct Res, 36, 271–277. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Pei L, & Liu F (2009). Disruption of the dopamine transporter-dopamine D2 receptor interaction in schizophrenia. Synapse, 63, 710–712. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Pei L, Moszczynska A, Vukusic B, Fletcher PJ, & Liu F (2007). Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J, 26, 2127–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo D, Mus L, Espinoza S, Hoener MC, Sotnikova TD, & Gainetdinov RR (2014). Taar1-mediated modulation of presynaptic dopaminergic neurotransmission: role of D2 dopamine autoreceptors. Neuropharmacology, 81, 283–291. [DOI] [PubMed] [Google Scholar]
- Leysen JE, Gommeren W, Mertens J, Luyten WH, Pauwels PJ, Ewert M, & Seeburg P (1993). Comparison of in vitro binding properties of a series of dopamine antagonists and agonists for cloned human dopamine D2S and D2L receptors and for D2 receptors in rat striatal and mesolimbic tissues, using [125I] 2’-iodospiperone. Psychopharmacology (Berl), 110, 27–36. [DOI] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, & Dagher A (2002). Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology, 27, 1027–1035. [DOI] [PubMed] [Google Scholar]
- Li B, Liu ML, Wu XP, Jia J, Cao J, Wei ZW, & Wang YJ (2015a). Effects of ketamine exposure on dopamine concentrations and dopamine type 2 receptor mRNA expression in rat brain tissue. Int J Clin Exp Med, 8, 11181–11187. [PMC free article] [PubMed] [Google Scholar]
- Li L, Homan KT, Vishnivetskiy SA, Manglik A, Tesmer JJ, Gurevich VV, & Gurevich EV (2015b). G Protein-coupled Receptor Kinases of the GRK4 Protein Subfamily Phosphorylate Inactive G Protein-coupled Receptors (GPCRs). J Biol Chem, 290, 10775–10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H, Bettler B, Wettstein JG, Borroni E, Moreau JL, & Hoener MC (2008). Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther, 324, 948–956. [DOI] [PubMed] [Google Scholar]
- Lindgren N, Xu ZQ, Herrera-Marschitz M, Haycock J, Hokfelt T, & Fisone G (2001). Dopamine D(2) receptors regulate tyrosine hydroxylase activity and phosphorylation at Ser40 in rat striatum. Eur J Neurosci, 13, 773–780. [DOI] [PubMed] [Google Scholar]
- Lindgren N, Xu ZQ, Lindskog M, Herrera-Marschitz M, Goiny M, Haycock J, Goldstein M, Hokfelt T, & Fisone G (2000). Regulation of tyrosine hydroxylase activity and phosphorylation at Ser(19) and Ser(40) via activation of glutamate NMDA receptors in rat striatum. J Neurochem, 74, 2470–2477. [DOI] [PubMed] [Google Scholar]
- Liu Y, Buck DC, Macey TA, Lan H, & Neve KA (2007). Evidence that calmodulin binding to the dopamine D2 receptor enhances receptor signaling. J Recept Signal Transduct Res, 27, 47–65. [DOI] [PubMed] [Google Scholar]
- Liu Y, Buck DC, & Neve KA (2008). Novel interaction of the dopamine D2 receptor and the Ca2+ binding protein S100B: role in D2 receptor function. Mol Pharmacol, 74, 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YF, Civelli O, Grandy DK, & Albert PR (1992). Differential sensitivity of the short and long human dopamine D2 receptor subtypes to protein kinase C. J Neurochem, 59, 2311–2317. [DOI] [PubMed] [Google Scholar]
- Luderman KD, Chen R, Ferris MJ, Jones SR, & Gnegy ME (2015). Protein kinase C beta regulates the D(2)-like dopamine autoreceptor. Neuropharmacology, 89, 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luessen DJ, & Chen R (2016). Psychostimulants, brain membrane lipids and dopamine transmission. Biomolecular Research & Therapeutics, 5, 1–5. [Google Scholar]
- Luessen DJ, Hinshaw TP, Sun H, Howlett AC, Marrs G, McCool BA, & Chen R (2016). RGS2 modulates the activity and internalization of dopamine D2 receptors in neuroblastoma N2A cells. Neuropharmacology, 110, 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luessen DJ, Sun H, McGinnis MM, McCool BA, & Chen R (2017). Chronic intermittent ethanol exposure selectively alters the expression of Galpha subunit isoforms and RGS subtypes in rat prefrontal cortex. Brain Res, 1672, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Ahlers-Dannen KE, Spicer MM, Yang J, Alberico S, Stevens HE, Narayanan NS, & Fisher RA (2019). Age-dependent nigral dopaminergic neurodegeneration and alpha-synuclein accumulation in RGS6-deficient mice. JCI Insight, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, & Nicoll RA (1997). G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron, 19, 687–695. [DOI] [PubMed] [Google Scholar]
- Mackey ED, Engle SE, Kim MR, O’Neill HC, Wageman CR, Patzlaff NE, Wang Y, Grady SR, McIntosh JM, Marks MJ, Lester HA, & Drenan RM (2012). alpha6* nicotinic acetylcholine receptor expression and function in a visual salience circuit. J Neurosci, 32, 10226–10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggos CE, Tsukada H, Kakiuchi T, Nishiyama S, Myers JE, Kreuter J, Schlussman SD, Unterwald EM, Ho A, & Kreek MJ (1998). Sustained withdrawal allows normalization of in vivo [11C]N-methylspiperone dopamine D2 receptor binding after chronic binge cocaine: a positron emission tomography study in rats. Neuropsychopharmacology, 19, 146–153. [DOI] [PubMed] [Google Scholar]
- Marinelli M, & White FJ (2000). Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci, 20, 8876–8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel P, Leo D, Fulton S, Berard M, & Trudeau LE (2011). Role of Kv1 potassium channels in regulating dopamine release and presynaptic D2 receptor function. PLoS One, 6, e20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Smith HR, Luessen DJ, Chen R, & Porrino LJ (2020). Functional brain activity is globally elevated by dopamine D2 receptor knockdown in the ventral tegmental area. Brain Res, 1727, 146552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pinilla E, Rodriguez-Perez AI, Navarro G, Aguinaga D, Moreno E, Lanciego JL, Labandeira-Garcia JL, & Franco R (2015). Dopamine D2 and angiotensin II type 1 receptors form functional heteromers in rat striatum. Biochem Pharmacol, 96, 131–142. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, Perez A, Frankle WG, Cooper T, Kleber HD, Fischman MW, & Laruelle M (2004). Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology, 29, 1190–1202. [DOI] [PubMed] [Google Scholar]
- Mazei-Robison MS, Bowton E, Holy M, Schmudermaier M, Freissmuth M, Sitte HH, Galli A, & Blakely RD (2008). Anomalous dopamine release associated with a human dopamine transporter coding variant. J Neurosci, 28, 7040–7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall NM, Kotecki L, Dominguez-Lopez S, Marron Fernandez de Velasco E, Carlblom N, Sharpe AL, Beckstead MJ, & Wickman K (2017). Selective Ablation of GIRK Channels in Dopamine Neurons Alters Behavioral Effects of Cocaine in Mice. Neuropsychopharmacology, 42, 707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall NM, Marron Fernandez de Velasco E, & Wickman K (2019). GIRK Channel Activity in Dopamine Neurons of the Ventral Tegmental Area Bidirectionally Regulates Behavioral Sensitivity to Cocaine. J Neurosci, 39, 3600–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFerran BW, Graham ME, & Burgoyne RD (1998). Neuronal Ca2+ sensor 1, the mammalian homologue of frequenin, is expressed in chromaffin and PC12 cells and regulates neurosecretion from dense-core granules. J Biol Chem, 273, 22768–22772. [DOI] [PubMed] [Google Scholar]
- Mendelsohn FA, Jenkins TA, & Berkovic SF (1993). Effects of angiotensin II on dopamine and serotonin turnover in the striatum of conscious rats. Brain Res, 613, 221–229. [DOI] [PubMed] [Google Scholar]
- Miller GM (2011). The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J Neurochem, 116, 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G (2009). G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br J Pharmacol, 158, 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min C, Cheong SY, Cheong SJ, Kim M, Cho DI, & Kim KM (2012). RGS4 exerts inhibitory activities on the signaling of dopamine D2 receptor and D3 receptor through the N-terminal region. Pharmacol Res, 65, 213–220. [DOI] [PubMed] [Google Scholar]
- Miner NB, Elmore JS, Baumann MH, Phillips TJ, & Janowsky A (2017). Trace amine-associated receptor 1 regulation of methamphetamine-induced neurotoxicity. Neurotoxicology, 63, 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Fields HL, D’Esposito M, & Boettiger CA (2005). Impulsive responding in alcoholics. Alcohol Clin Exp Res, 29, 2158–2169. [DOI] [PubMed] [Google Scholar]
- Montmayeur JP, Bausero P, Amlaiky N, Maroteaux L, Hen R, & Borrelli E (1991). Differential expression of the mouse D2 dopamine receptor isoforms. FEBS Lett, 278, 239–243. [DOI] [PubMed] [Google Scholar]
- Moore KE (1992). Differential regulation of dopaminergic neurons in the mammalian brain: a brief review. Chin J Physiol, 35, 67–76. [PubMed] [Google Scholar]
- Mootz JRK, Miner NB, & Phillips TJ (2020). Differential genetic risk for methamphetamine intake confers differential sensitivity to the temperature-altering effects of other addictive drugs. Genes Brain Behav, e12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SJ, Van H II, Daigle M, Robillard L, Sajedi N, & Albert PR (2007). Differential desensitization of dopamine D2 receptor isoforms by protein kinase C: the importance of receptor phosphorylation and pseudosubstrate sites. Eur J Pharmacol, 577, 44–53. [DOI] [PubMed] [Google Scholar]
- Mrejeru A, Marti-Prats L, Avegno EM, Harrison NL, & Sulzer D (2015). A subset of ventral tegmental area dopamine neurons responds to acute ethanol. Neuroscience, 290, 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz MB, Padgett CL, Rifkin R, Terunuma M, Wickman K, Contet C, Moss SJ, & Slesinger PA (2016). A Role for the GIRK3 Subunit in Methamphetamine-Induced Attenuation of GABAB Receptor-Activated GIRK Currents in VTA Dopamine Neurons. J Neurosci, 36, 3106–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal R, Armario A, & Janak PH (2002). Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology (Berl), 162, 333–338. [DOI] [PubMed] [Google Scholar]
- Nader MA, & Czoty PW (2005). PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation. Am J Psychiatry, 162, 1473–1482. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, & Mach RH (2006). PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci, 9, 1050–1056. [DOI] [PubMed] [Google Scholar]
- Namkung Y, Dipace C, Javitch JA, & Sibley DR (2009a). G protein-coupled receptor kinase-mediated phosphorylation regulates post-endocytic trafficking of the D2 dopamine receptor. J Biol Chem, 284, 15038–15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkung Y, Dipace C, Urizar E, Javitch JA, & Sibley DR (2009b). G protein-coupled receptor kinase-2 constitutively regulates D2 dopamine receptor expression and signaling independently of receptor phosphorylation. J Biol Chem, 284, 34103–34115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkung Y, & Sibley DR (2004). Protein kinase C mediates phosphorylation, desensitization, and trafficking of the D2 dopamine receptor. The Journal of biological chemistry, 279, 49533–49541. [DOI] [PubMed] [Google Scholar]
- Nance MR, Kreutz B, Tesmer VM, Sterne-Marr R, Kozasa T, & Tesmer JJ (2013). Structural and functional analysis of the regulator of G protein signaling 2-galphaq complex. Structure, 21, 438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan K, & Berk BC (2006). Crosstalk coregulation mechanisms of G protein-coupled receptors and receptor tyrosine kinases. Methods Mol Biol, 332, 51–77. [DOI] [PubMed] [Google Scholar]
- Neve KA, Ford CP, Buck DC, Grandy DK, Neve RL, & Phillips TJ (2013). Normalizing Dopamine D2 Receptor-Mediated Responses in D2 Null Mutant Mice by Virus-Mediated Receptor Restoration: Comparing D2 and D2. Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve KA, Neve RL, Fidel S, Janowsky A, & Higgins GA (1991). Increased abundance of alternatively spliced forms of D2 dopamine receptor mRNA after denervation. Proc Natl Acad Sci U S A, 88, 2802–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, & Trantham-Davidson H (2004). Dopamine receptor signaling. J Recept Signal Transduct Res, 24, 165–205. [DOI] [PubMed] [Google Scholar]
- Nicot A, Rostene W, & Berod A (1994). Neurotensin receptor expression in the rat forebrain and midbrain: a combined analysis by in situ hybridization and receptor autoradiography. J Comp Neurol, 341, 407–419. [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Chan J, Liu Y, Edwards RH, & Pickel VM (1996). Ultrastructural localization of the vesicular monoamine transporter-2 in midbrain dopaminergic neurons: potential sites for somatodendritic storage and release of dopamine. J Neurosci, 16, 4135–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak G, Seeman P, & Le Foll B (2010). Exposure to nicotine produces an increase in dopamine D2(High) receptors: a possible mechanism for dopamine hypersensitivity. Int J Neurosci, 120, 691–697. [DOI] [PubMed] [Google Scholar]
- O’Donovan B, Adeluyi A, Anderson EL, Cole RD, Turner JR, & Ortinski PI (2019). Altered gating of Kv1.4 in the nucleus accumbens suppresses motivation for reward. Elife, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Petersen N, Robertson CL, Dean AC, Mandelkern MA, & London ED (2016). Sex Differences in Midbrain Dopamine D2-Type Receptor Availability and Association with Nicotine Dependence. Neuropsychopharmacology, 41, 2913–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuma Y, Fukuda Y, & Osumi Y (1983). Neurotensin potentiates the potassium-induced release of endogenous dopamine from rat striatal slices. Eur J Pharmacol, 93, 27–33. [DOI] [PubMed] [Google Scholar]
- Olson PA, Tkatch T, Hernandez-Lopez S, Ulrich S, Ilijic E, Mugnaini E, Zhang H, Bezprozvanny I, & Surmeier DJ (2005). G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J Neurosci, 25, 1050–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios JM, & Kuhar MJ (1981). Neurotensin receptors are located on dopamine-ontaining neurones in rat midbrain. Nature, 294, 587–589. [DOI] [PubMed] [Google Scholar]
- Panayi F, Colussi-Mas J, Lambas-Senas L, Renaud B, Scarna H, & Berod A (2005). Endogenous neurotensin in the ventral tegmental area contributes to amphetamine behavioral sensitization. Neuropsychopharmacology, 30, 871–879. [DOI] [PubMed] [Google Scholar]
- Pandalaneni S, Karuppiah V, Saleem M, Haynes LP, Burgoyne RD, Mayans O, Derrick JP, & Lian LY (2015). Neuronal Calcium Sensor-1 Binds the D2 Dopamine Receptor and G-protein-coupled Receptor Kinase 1 (GRK1) Peptides Using Different Modes of Interactions. J Biol Chem, 290, 18744–18756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Asif-Malik A, & Canales JJ (2016). Trace Amines and the Trace Amine-Associated Receptor 1: Pharmacology, Neurochemistry, and Clinical Implications. Front Neurosci, 10, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Lee J, Leo D, Gainetdinov RR, Hoener MC, & Canales JJ (2014). Activation of the trace amine-associated receptor 1 prevents relapse to cocaine seeking. Neuropsychopharmacology, 39, 2299–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Mortas P, Hoener MC, & Canales JJ (2015). Selective activation of the trace amine-associated receptor 1 decreases cocaine’s reinforcing efficacy and prevents cocaine-induced changes in brain reward thresholds. Prog Neuropsychopharmacol Biol Psychiatry, 63, 70–75. [DOI] [PubMed] [Google Scholar]
- Perra S, Clements MA, Bernier BE, & Morikawa H (2011). In vivo ethanol experience increases D(2) autoinhibition in the ventral tegmental area. Neuropsychopharmacology, 36, 993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, & Simon H (1989). Factors that predict individual vulnerability to amphetamine self-administration. Science, 245, 1511–1513. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Rouge-Pont F, & Le Moal M (1998). Behavioral and biological factors associated with individual vulnerability to psychostimulant abuse. NIDA Res Monogr, 169, 105–133. [PubMed] [Google Scholar]
- Piccart E, Courtney NA, Branch SY, Ford CP, & Beckstead MJ (2015). Neurotensin Induces Presynaptic Depression of D2 Dopamine Autoreceptor-Mediated Neurotransmission in Midbrain Dopaminergic Neurons. J Neurosci, 35, 11144–11152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccart E, Tschumi CW, & Beckstead MJ (2019). Acute and subchronic PCP attenuate D2 autoreceptor signaling in substantia nigra dopamine neurons. Eur Neuropsychopharmacol, 29, 444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, & Dani JA (1997). Nicotine activates and desensitizes midbrain dopamine neurons. Nature, 390, 401–404. [DOI] [PubMed] [Google Scholar]
- Pillai G, Brown NA, McAllister G, Milligan G, & Seabrook GR (1998). Human D2 and D4 dopamine receptors couple through betagamma G-protein subunits to inwardly rectifying K+ channels (GIRK1) in a Xenopus oocyte expression system: selective antagonism by L-741,626 and L-745,870 respectively. Neuropharmacology, 37, 983–987. [DOI] [PubMed] [Google Scholar]
- Pintsuk J, Borroto-Escuela DO, Pomierny B, Wydra K, Zaniewska M, Filip M, & Fuxe K (2016). Cocaine self-administration differentially affects allosteric A2A-D2 receptor-receptor interactions in the striatum. Relevance for cocaine use disorder. Pharmacol Biochem Behav, 144, 85–91. [DOI] [PubMed] [Google Scholar]
- Pucak ML, & Grace AA (1994). Evidence that systemically administered dopamine antagonists activate dopamine neuron firing primarily by blockade of somatodendritic autoreceptors. J Pharmacol Exp Ther, 271, 1181–1192. [PubMed] [Google Scholar]
- Qu YH, Leung KP, Qiao DF, Li DR, Liu C, Yue X, & Wang HJ (2014). Remodeling of ion channel expression may contribute to electrophysiological consequences caused by methamphetamine in vitro and in vivo. Biochem Biophys Res Commun, 443, 441–446. [DOI] [PubMed] [Google Scholar]
- Quarta D, Ciruela F, Patkar K, Borycz J, Solinas M, Lluis C, Franco R, Wise RA, Goldberg SR, Hope BT, Woods AS, & Ferre S (2007). Heteromeric nicotinic acetylcholine-dopamine autoreceptor complexes modulate striatal dopamine release. Neuropsychopharmacology, 32, 35–42. [DOI] [PubMed] [Google Scholar]
- Quirion R, Welner S, Gauthier S, & Bedard P (1987). Neurotensin receptor binding sites in monkey and human brain: autoradiographic distribution and effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment. Synapse, 1, 559–566. [DOI] [PubMed] [Google Scholar]
- Radl D, Chiacchiaretta M, Lewis RG, Brami-Cherrier K, Arcuri L, & Borrelli E (2018). Differential regulation of striatal motor behavior and related cellular responses by dopamine D2L and D2S isoforms. Proc Natl Acad Sci U S A, 115, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman Z, Schwarz J, Gold SJ, Zachariou V, Wein MN, Choi KH, Kovoor A, Chen CK, DiLeone RJ, Schwarz SC, Selley DE, Sim-Selley LJ, Barrot M, Luedtke RR, Self D, Neve RL, Lester HA, Simon MI, & Nestler EJ (2003). RGS9 modulates dopamine signaling in the basal ganglia. Neuron, 38, 941–952. [DOI] [PubMed] [Google Scholar]
- Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, Durkin S, Zbinden KG, Norcross R, Meyer CA, Metzler V, Chaboz S, Ozmen L, Trube G, Pouzet B, Bettler B, Caron MG, Wettstein JG, & Hoener MC (2011). TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc Natl Acad Sci U S A, 108, 8485–8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel FG, Moreau JL, Gainetdinov RR, Ferragud A, Velazquez-Sanchez C, Sotnikova TD, Morairty SR, Harmeier A, Groebke Zbinden K, Norcross RD, Bradaia A, Kilduff TS, Biemans B, Pouzet B, Caron MG, Canales JJ, Wallace TL, Wettstein JG, & Hoener MC (2012). Trace amine-associated receptor 1 partial agonism reveals novel paradigm for neuropsychiatric therapeutics. Biol Psychiatry, 72, 934–942. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Li R, Ansari MS, Zald D, Park S, Dawant B, Anderson S, Doop M, Woodward N, Schoenberg E, Schmidt D, Baldwin R, & Kessler R (2006). Amphetamine-induced displacement of [18F] fallypride in striatum and extrastriatal regions in humans. Neuropsychopharmacology, 31, 1016–1026. [DOI] [PubMed] [Google Scholar]
- Rice ME, & Patel JC (2015). Somatodendritic dopamine release: recent mechanistic insights. Philos Trans R Soc Lond B Biol Sci, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B (1997). The real life of voltage-gated K+ channels: more than model behaviour. Trends Pharmacol Sci, 18, 474–483. [DOI] [PubMed] [Google Scholar]
- Robinson BG, Bunzow JR, Grimm JB, Lavis LD, Dudman JT, Brown J, Neve KA, & Williams JT (2017a). Desensitized D2 autoreceptors are resistant to trafficking. Sci Rep, 7, 4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BG, Condon AF, Radl D, Borrelli E, Williams JT, & Neve KA (2017b). Cocaine-induced adaptation of dopamine D2S, but not D2L autoreceptors. Elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogue P, Zwiller J, Malviya AN, & Vincendon G (1990). Phosphorylation by protein kinase C modulates agonist binding to striatal dopamine D2 receptors. Biochem Int, 22, 575–582. [PubMed] [Google Scholar]
- Rompre P, & Perron S (2000). Evidence for a role of endogenous neurotensin in the initiation of amphetamine sensitization. Neuropharmacology, 39, 1880–1892. [DOI] [PubMed] [Google Scholar]
- Rompre PP, Bauco P, & Gratton A (1992). Facilitation of brain stimulation reward by mesencephalic injections of neurotensin-(1–13). Eur J Pharmacol, 211, 295–303. [DOI] [PubMed] [Google Scholar]
- Rorabaugh BR, Rose MJ, Stoops TS, Stevens AA, Seeley SL, & D’Souza MS (2018). Regulators of G-protein signaling 2 and 4 differentially regulate cocaine-induced rewarding effects. Physiology & behavior, 195, 9–19. [DOI] [PubMed] [Google Scholar]
- Ross S, & Peselow E (2009). Pharmacotherapy of addictive disorders. Clin Neuropharmacol, 32, 277–289. [DOI] [PubMed] [Google Scholar]
- Sager JJ, & Torres GE (2011). Proteins interacting with monoamine transporters: current state and future challenges. Biochemistry, 50, 7295–7310. [DOI] [PubMed] [Google Scholar]
- Sahlholm K, Marcellino D, Nilsson J, Fuxe K, & Arhem P (2008). Differential voltage-sensitivity of D2-like dopamine receptors. Biochem Biophys Res Commun, 374, 496–501. [DOI] [PubMed] [Google Scholar]
- Salim H, Ferre S, Dalal A, Peterfreund RA, Fuxe K, Vincent JD, & Lledo PM (2000). Activation of adenosine A1 and A2A receptors modulates dopamine D2 receptor-induced responses in stably transfected human neuroblastoma cells. J Neurochem, 74, 432–439. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Benoit-Marand M, Gonon F, & Sulzer D (2003). Presynaptic regulation of dopaminergic neurotransmission. J Neurochem, 87, 273–289. [DOI] [PubMed] [Google Scholar]
- Seagar M, & Takahashi M (1998). Interactions between presynaptic calcium channels and proteins implicated in synaptic vesicle trafficking and exocytosis. J Bioenerg Biomembr, 30, 347–356. [DOI] [PubMed] [Google Scholar]
- Seeman P, & Niznik HB (1990). Dopamine receptors and transporters in Parkinson’s disease and schizophrenia. FASEB J, 4, 2737–2744. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Aoki C, & Pickel VM (1994). Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci, 14, 88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seutin V, Massotte L, & Dresse A (1989). Electrophysiological effects of neurotensin on dopaminergic neurones of the ventral tegmental area of the rat in vitro. Neuropharmacology, 28, 949–954. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Varela E, & Beckstead MJ (2017). Systemic PD149163, a neurotensin receptor 1 agonist, decreases methamphetamine self-administration in DBA/2J mice without causing excessive sedation. PLoS One, 12, e0180710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AL, Varela E, Bettinger L, & Beckstead MJ (2014). Methamphetamine self-administration in mice decreases GIRK channel-mediated currents in midbrain dopamine neurons. Int J Neuropsychopharmacol, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HY, Canas PM, Garcia-Sanz P, Lan JQ, Boison D, Moratalla R, Cunha RA, & Chen JF (2013). Adenosine A(2)A receptors in striatal glutamatergic terminals and GABAergic neurons oppositely modulate psychostimulant action and DARPP-32 phosphorylation. PLoS One, 8, e80902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WS, & Bunney BS (1990). Neurotensin attenuates dopamine D2 agonist quinpirole-induced inhibition of midbrain dopamine neurons. Neuropharmacology, 29, 1095–1097. [DOI] [PubMed] [Google Scholar]
- Shi WX, & Bunney BS (1991). Neurotensin modulates autoreceptor mediated dopamine effects on midbrain dopamine cell activity. Brain Res, 543, 315–321. [DOI] [PubMed] [Google Scholar]
- Shi X, Walter NA, Harkness JH, Neve KA, Williams RW, Lu L, Belknap JK, Eshleman AJ, Phillips TJ, & Janowsky A (2016). Genetic Polymorphisms Affect Mouse and Human Trace Amine-Associated Receptor 1 Function. PLoS One, 11, e0152581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumay E, Fowler JS, Wang GJ, Logan J, Alia-Klein N, Goldstein RZ, Maloney T, Wong C, & Volkow ND (2012). Repeat variation in the human PER2 gene as a new genetic marker associated with cocaine addiction and brain dopamine D2 receptor availability. Transl Psychiatry, 2, e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu A (1998). Coupling of D1 and D5 dopamine receptors to multiple G proteins: Implications for understanding the diversity in receptor-G protein coupling. Mol Neurobiol, 16, 125–134. [DOI] [PubMed] [Google Scholar]
- Sinnegger-Brauns MJ, Huber IG, Koschak A, Wild C, Obermair GJ, Einzinger U, Hoda JC, Sartori SB, & Striessnig J (2009). Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol Pharmacol, 75, 407–414. [DOI] [PubMed] [Google Scholar]
- St-Gelais F, Legault M, Bourque MJ, Rompre PP, & Trudeau LE (2004). Role of calcium in neurotensin-evoked enhancement in firing in mesencephalic dopamine neurons. J Neurosci, 24, 2566–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford AM, Reed C, Baba H, Walter NA, Mootz JR, Williams RW, Neve KA, Fedorov LM, Janowsky AJ, & Phillips TJ (2019). Taar1 gene variants have a causal role in methamphetamine intake and response and interact with Oprm1. Elife, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A, Maity B, Anderegg SP, Allamargot C, Yang J, & Fisher RA (2015). Regulator of G protein signaling 6 is a critical mediator of both reward-related behavioral and pathological responses to alcohol. Proc Natl Acad Sci U S A, 112, E786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhrman K, & Roseberry AG (2015). Neurotensin inhibits both dopamine- and GABA-mediated inhibition of ventral tegmental area dopamine neurons. J Neurophysiol, 114, 1734–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniyan M, & Dani JA (2015). Dopaminergic and cholinergic learning mechanisms in nicotine addiction. Ann N Y Acad Sci, 1349, 46–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Calipari ES, Beveridge TJ, Jones SR, & Chen R (2015). The brain gene expression profile of dopamine D2/D3 receptors and associated signaling proteins following amphetamine self-administration. Neuroscience, 307, 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Akins CK, Mattingly AE, & Rebec GV (2005). Ionotropic glutamate receptors in the ventral tegmental area regulate cocaine-seeking behavior in rats. Neuropsychopharmacology, 30, 2073–2081. [DOI] [PubMed] [Google Scholar]
- Talmaciu RK, Hoffmann IS, & Cubeddu LX (1986). Dopamine autoreceptors modulate dopamine release from the prefrontal cortex. J Neurochem, 47, 865–870. [DOI] [PubMed] [Google Scholar]
- Tammimaki A, Pietila K, Raattamaa H, & Ahtee L (2006). Effect of quinpirole on striatal dopamine release and locomotor activity in nicotine-treated mice. Eur J Pharmacol, 531, 118–125. [DOI] [PubMed] [Google Scholar]
- Tan S, Hermann B, & Borrelli E (2003). Dopaminergic mouse mutants: investigating the roles of the different dopamine receptor subtypes and the dopamine transporter. Int Rev Neurobiol, 54, 145–197. [DOI] [PubMed] [Google Scholar]
- Taura J, Valle-Leon M, Sahlholm K, Watanabe M, Van Craenenbroeck K, Fernandez-Duenas V, Ferre S, & Ciruela F (2018). Behavioral control by striatal adenosine A2A -dopamine D2 receptor heteromers. Genes Brain Behav, 17, e12432. [DOI] [PubMed] [Google Scholar]
- Thibault D, Albert PR, Pineyro G, & Trudeau LE (2011). Neurotensin triggers dopamine D2 receptor desensitization through a protein kinase C and beta- arrestin1-dependent mechanism. J Biol Chem, 286, 9174–9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, & Cragg SJ (2012). Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron, 75, 58–64. [DOI] [PubMed] [Google Scholar]
- Tournier BB, Steimer T, Millet P, Moulin-Sallanon M, Vallet P, Ibanez V, & Ginovart N (2013). Innately low D2 receptor availability is associated with high novelty-seeking and enhanced behavioural sensitization to amphetamine. Int J Neuropsychopharmacol, 16, 1819–1834. [DOI] [PubMed] [Google Scholar]
- Traynor J (2010). Regulator of G protein-signaling proteins and addictive drugs. Ann N Y Acad Sci, 1187, 341–352. [DOI] [PubMed] [Google Scholar]
- Traynor J (2012). mu-Opioid receptors and regulators of G protein signaling (RGS) proteins: from a symposium on new concepts in mu-opioid pharmacology. Drug Alcohol Depend, 121, 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschumi CW, & Beckstead MJ (2018). Neurotensin speeds inhibition of dopamine neurons through temporal modulation of GABAA and GABAB receptor-mediated synaptic input. Neuropharmacology, 131, 414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschumi CW, & Beckstead MJ (2019). Diverse actions of the modulatory peptide neurotensin on central synaptic transmission. Eur J Neurosci, 49, 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto T, Jeromin A, Saitoh N, Roder JC, & Takahashi T (2002). Neuronal calcium sensor 1 and activity-dependent facilitation of P/Q-type calcium currents at presynaptic nerve terminals. Science, 295, 2276–2279. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Kreuter J, Maggos CE, Unterwald EM, Kakiuchi T, Nishiyama S, Futatsubashi M, & Kreek MJ (1996). Effects of binge pattern cocaine administration on dopamine D1 and D2 receptors in the rat brain: an in vivo study using positron emission tomography. J Neurosci, 16, 7670–7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Akaike N, & Nabekura J (2000). Dopamine activates inward rectifier K+ channel in acutely dissociated rat substantia nigra neurones. Neuropharmacology, 39, 191–201. [DOI] [PubMed] [Google Scholar]
- Undieh AS (2010). Pharmacology of signaling induced by dopamine D(1)-like receptor activation. Pharmacol Ther, 128, 37–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veh RW, Lichtinghagen R, Sewing S, Wunder F, Grumbach IM, & Pongs O (1995). Immunohistochemical localization of five members of the Kv1 channel subunits: contrasting subcellular locations and neuron-specific co-localizations in rat brain. Eur J Neurosci, 7, 2189–2205. [DOI] [PubMed] [Google Scholar]
- Vetulani J (2001). Drug addiction. Part III. Pharmacotherapy of addiction. Pol J Pharmacol, 53, 415–434. [PubMed] [Google Scholar]
- Vezina P, Lorrain DS, Arnold GM, Austin JD, & Suto N (2002). Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci, 22, 4654–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JP, Mazella J, & Kitabgi P (1999). Neurotensin and neurotensin receptors. Trends Pharmacol Sci, 20, 302–309. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, & Swanson JM (2010). Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage, 49, 2536–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D, & et al. (1990). Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry, 147, 719–724. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, & Pappas N (1999). Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry, 156, 1440–1443. [DOI] [PubMed] [Google Scholar]
- Wagstaff JD, Gibb JW, & Hanson GR (1996). Microdialysis assessment of methamphetamine-induced changes in extracellular neurotensin in the striatum and nucleus accumbens. J Pharmacol Exp Ther, 278, 547–554. [PubMed] [Google Scholar]
- Watson N, Linder ME, Druey KM, Kehrl JH, & Blumer KJ (1996). RGS family members: GTPase-activating proteins for heterotrimeric G-protein alpha-subunits. Nature, 383, 172–175. [DOI] [PubMed] [Google Scholar]
- Weiss JL, Archer DA, & Burgoyne RD (2000). Neuronal Ca2+ sensor-1/frequenin functions in an autocrine pathway regulating Ca2+ channels in bovine adrenal chromaffin cells. J Biol Chem, 275, 40082–40087. [DOI] [PubMed] [Google Scholar]
- Weiss JL, & Burgoyne RD (2001). Voltage-independent inhibition of P/Q-type Ca2+ channels in adrenal chromaffin cells via a neuronal Ca2+ sensor-1-dependent pathway involves Src family tyrosine kinase. J Biol Chem, 276, 44804–44811. [DOI] [PubMed] [Google Scholar]
- Werkman TR, Kruse CG, Nievelstein H, Long SK, & Wadman WJ (2000). Neurotensin attenuates the quinpirole-induced inhibition of the firing rate of dopamine neurons in the rat substantia nigra pars compacta and the ventral tegmental area. Neuroscience, 95, 417–423. [DOI] [PubMed] [Google Scholar]
- White FJ, & Wang RY (1984). Electrophysiological evidence for A10 dopamine autoreceptor subsensitivity following chronic D-amphetamine treatment. Brain Res, 309, 283–292. [DOI] [PubMed] [Google Scholar]
- White FJ, & Wang RY (1986). Electrophysiological evidence for the existence of both D-1 and D-2 dopamine receptors in the rat nucleus accumbens. J Neurosci, 6, 274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilar G, Shinoda Y, Sasaoka T, & Fukunaga K (2019). Crucial Role of Dopamine D2 Receptor Signaling in Nicotine-Induced Conditioned Place Preference. Mol Neurobiol. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM, & Fifkova E (1977). Monoaminergic synapses, including dendro-dendritic synapses in the rat substantia nigra. Exp Brain Res, 30, 161–174. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Cardinal RN, & Robbins TW (2006). Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex, 16, 106–114. [DOI] [PubMed] [Google Scholar]
- Wise RA (1996). Neurobiology of addiction. Curr Opin Neurobiol, 6, 243–251. [DOI] [PubMed] [Google Scholar]
- Wise RA (2009). Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci, 32, 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, & Roth RH (1990). Autoreceptor regulation of dopamine synthesis. Ann N Y Acad Sci, 604, 323–343. [DOI] [PubMed] [Google Scholar]
- Wolf ME, White FJ, Nassar R, Brooderson RJ, & Khansa MR (1993). Differential development of autoreceptor subsensitivity and enhanced dopamine release during amphetamine sensitization. J Pharmacol Exp Ther, 264, 249–255. [PubMed] [Google Scholar]
- Wonnacott S, Kaiser S, Mogg A, Soliakov L, & Jones IW (2000). Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. Eur J Pharmacol, 393, 51–58. [DOI] [PubMed] [Google Scholar]
- Wu J, George AA, Schroeder KM, Xu L, Marxer-Miller S, Lucero L, & Lukas RJ (2004). Electrophysiological, pharmacological, and molecular evidence for alpha7-nicotinic acetylcholine receptors in rat midbrain dopamine neurons. J Pharmacol Exp Ther, 311, 80–91. [DOI] [PubMed] [Google Scholar]
- Xie Z, Westmoreland SV, Bahn ME, Chen GL, Yang H, Vallender EJ, Yao WD, Madras BK, & Miller GM (2007). Rhesus monkey trace amine-associated receptor 1 signaling: enhancement by monoamine transporters and attenuation by the D2 autoreceptor in vitro. J Pharmacol Exp Ther, 321, 116–127. [DOI] [PubMed] [Google Scholar]
- Xigeng Z, Yonghui L, Xiaojing L, Lin X, Dongmei W, Jie L, Xiaoyan Y, & Nan S (2004). Social crowding sensitizes high-responding rats to psychomotor-stimulant effects of morphine. Pharmacol Biochem Behav, 79, 213–218. [DOI] [PubMed] [Google Scholar]
- Xu X, Pan J, Li X, Cui Y, Mao Z, Wu B, Xu H, Zhou W, & Liu Y (2019). Inhibition of Methamphetamine Self-Administration and Reinstatement by Central Blockade of Angiotensin II Receptor in Rats. J Pharmacol Exp Ther, 369, 244–258. [DOI] [PubMed] [Google Scholar]
- Xue Y, Steketee JD, Rebec GV, & Sun W (2011). Activation of D(2)-like receptors in rat ventral tegmental area inhibits cocaine-reinstated drug-seeking behavior. Eur J Neurosci, 33, 1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Hu J, Lucero L, Liu Q, Zheng C, Zhen X, Jin G, Lukas RJ, & Wu J (2009). Distinctive nicotinic acetylcholine receptor functional phenotypes of rat ventral tegmental area dopaminergic neurons. J Physiol, 587, 345–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Munhall AC, & Johnson SW (2019). AMP-activated protein kinase slows D2 dopamine autoreceptor desensitization in substantia nigra neurons. Neuropharmacology, 158, 107705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Georgescu D, Sanchez N, Rahman Z, DiLeone R, Berton O, Neve RL, Sim-Selley LJ, Selley DE, Gold SJ, & Nestler EJ (2003). Essential role for RGS9 in opiate action. Proc Natl Acad Sci U S A, 100, 13656–13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, Shelby ES, Smith CE, McHugo M, & Kessler RM (2008). Midbrain dopamine receptor availability is inversely associated with novelty-seeking traits in humans. J Neurosci, 28, 14372–14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zestos AG, Carpenter C, Kim Y, Low MJ, Kennedy RT, & Gnegy ME (2019). Ruboxistaurin Reduces Cocaine-Stimulated Increases in Extracellular Dopamine by Modifying Dopamine-Autoreceptor Activity. ACS Chem Neurosci, 10, 1960–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, & Sulzer D (2004). Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci, 7, 581–582. [DOI] [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, & Gotti C (2002). Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci, 22, 8785–8789. [DOI] [PMC free article] [PubMed] [Google Scholar]


