Abstract
Background
Mammography is not effective in detecting breast cancer in dense breasts.
Methods
A search in Medline, Cochrane, EMBASE and Google Scholar databases was conducted from January 1, 1980 to April 10, 2019 to identify women with dense breasts screened by mammography (M) and/or ultrasound (US). Meta-analysis was performed using the random-effect model.
Results
A total of 21 studies were included. The pooled sensitivity values of M alone and M + US in patients were 74% and 96%, while specificity of the two methods were 93% and 87%, respectively. Screening sensitivity was significantly higher in M + US than M alone (risk ratio: M alone vs. M + US = 0.699, P < 0.001), but the slight difference in specificity was statistically significant (risk ratio = 1.060, P = 0.001). Pooled diagnostic performance of follow-up US after initial negative mammography demonstrated a high pooled sensitivity (96%) and specificity (88%). The findings were supported by subgroup analysis stratified by study country, US method and timing of US.
Conclusions
Breast cancer screening by supplemental US among women with dense breasts shows added detection sensitivity compared with M alone. However, US slightly decreased the diagnostic specificity for breast cancer. The cost-effectiveness of supplemental US in detecting malignancy in dense breasts should be considered additionally.
Subject terms: Breast cancer, Cancer
Background
Mammography has been established as the primary method of screening for breast cancer, and since its introduction, the diagnosis of early-stage disease was significantly enhanced.1,2 The overall sensitivity of mammography for the detection of non-palpable cancers is approximately 85%,1,2 but the density of breast tissue can markedly reduce the mammography detection rate of early-stage disease.3–5 Breast density can be classified according to the Breast Imaging-Reporting and Data System (BI-RADS) American College of Radiology (ACR) categories and/or quantification: BI-RADS A: almost entirely fat (low density of mammary gland parenchyma), BI-RADS B: scattered fibroglandular densities (average density of gland parenchyma), BI-RADS C: heterogeneously dense (high density of gland parenchyma) and BI-RADS D: extremely dense (very high density of gland parenchyma).6,7 In women with >75% breast parenchymal dense tissue, the sensitivity of mammography for detecting early-stage cancer can be as low as 48%.4,5 Dense breast tissue is an independent marker associated with increased breast cancer risk, especially in women who are at higher risk due to other factors such as family history.8 Women with dense breast tissue who develop carcinoma in one breast are also at higher risk of developing cancer in the contralateral breast.9 It is estimated that approximately two-thirds of premenopausal women and one-third of elderly women aged 75–79 years have a breast density of 50% or higher.4 Furthermore, ethnic differences also exist as dense breasts are more prevalent in Asian than in Caucasian women.10,11 Although breast cancer incidence rates in the Asian population were found to be lower than those in the Western population according to a large-scale epidemiology study, the incidence in Asia is quickly increasing and surpassing than in the Western countries.12 This highlights an urgent need for more efficient breast cancer prevention and management strategies in the Asian population.
In light of the limitations of mammography in women with dense breasts, a study has suggested that ultrasound (US) is more sensitive than mammography, and can identify mammography- occult breast cancers in dense breasts, especially of younger women aged 30–39.13 Other studies have indicated that adjunctive US and mammography in women with dense breasts resulted in a significant increase in the cancer detection rate as compared with mammography alone.3,14 Some authors have therefore suggested that mammography with supplemental US screening can be beneficial for women with dense breasts, specific female groups prone to have dense breast tissue as previously described and women in resource-poor healthcare systems.15 In the prospective J-START study, improved sensitivity in breast cancer detection was found in mammography with ultrasound compared with without (91.1% vs. 77.0%) in asymptomatic Japanese women aged 40–49 years unlimited to breast density, albeit with a concurrent lower specificity.16 In particular, breast cancers detected by US are likely to be different characteristically than those detected by mammography, in which breast cancers detected by US are more likely to be smaller-sized, invasive and of the luminal A subtype compared with those detected by mammogram.17 Nevertheless, the use of adjunctive US may increase the number of false-positive findings and unnecessary biopsy recommendations,18,19 and the added diagnostic benefit of the screening strategy should be reconsidered as a whole.
Therefore, the added clinical benefit of US to mammography has been the interest of many systematic reviews, which provides only qualitative evaluation of published clinical evidence. In contrast, quantitative analysis that pooled and compared the diagnostic yield of conjunctive or sequential mammography and US screening strategies is limited. Moreover, it was suggested by a systematic review that the overall available evidence regarding the detection rate of breast cancer by screening with mammography and adjunct US may be low based on the Grades of Recommendation Assessment Development and Evaluation (GRADE) system.18
Thus, the objective of this study was to perform a systematic review and meta-analysis examining the diagnostic performance of mammography alone plus US for breast cancer in women with dense breasts, as well as that of follow-up US in women with dense breasts and negative mammography results.
Methods
Literature search and study selection
This meta-analysis was performed in accordance with the PRISMA guidelines,20 and the PICO model for clinical questions (P: Patient, Population or Problem [Female patients with heterogeneously or extremely dense breasts], I: Intervention, Prognostic Factor or Exposure [Screening mammography in combination with ultrasonography or ultrasonography as adjuvant for mammography-negative women], C: Comparison or Intervention [Only screening mammography or no comparative group] and O: Outcome Measures [sensitivity, specificity, positive predictive value [PPV], negative predictive value [NPV] and accuracy for detecting early-stage breast cancer). The review protocol had not been registered or published previously.
Medline, Cochrane, EMBASE and Google Scholar databases were searched for studies published between January 1, 1980 and April 10, 2019 using the keywords as follows: breast, dense, density, breast cancer, breast density, mammography, ultrasonography, ultrasound, specificity, sensitivity, screening and comparison. The search strategies included “mammography and ultrasound and breast and (dense OR density) with search filters of abstract availability, publications English, and clinical trials”, and “(ultrasound) AND (mammography) AND (breast cancer) AND (screening) AND (sensitivity) AND (specificity) AND (comparison)”. Literature inclusion criteria for meta-analysis were (1) randomised controlled trials (RCTs), 2-arm prospective studies, retrospective studies and cohort studies; (2) participants were women with dense breasts with BI-RADS categories ≥2; (3) study design included either mammography with adjunctive ultrasonography or additional ultrasonography following a negative mammography; (4) quantitative outcome data for outcomes of interest (i.e. PPV, NPV, sensitivity and specificity); (5) full-text studies published in English. Letters, comments, editorials, case reports, proceedings and personal communication were excluded. Studies of patients without dense breasts, and those that did not provide direct comparisons of the outcomes of interest, were further excluded. Studies designed for the detection of microcalcifications were excluded due to the technical nature of ultrasound, which is limited to detect breast microcalcifications.21,22 The reference lists of articles included for qualitative review were searched for studies that fit the above criteria. Literature searches were performed by two independent reviewers who were breast cancer specialists, and a third reviewer, also a breast cancer specialist, was consulted for resolutions of any disagreements.
Quality assessment
The quality of the included studies was assessed using QUADAS-2, a revised tool for the quality assessment of diagnostic accuracy studies.23 Briefly, QUADAS-2 comprises four domains: patient selection, index test, reference standard and flow and timing. Each domain was assessed for risk of bias, and the first three domains were subsequently assessed regarding topic relevance.
Data extraction and statistical analysis
Studies’ characteristics, including the number of total enrolled patients and number of patients with confirmed cancer, mean ± standard deviation (SD), mean or median with range (minimum–maximum) for age, detection rate per 1000 patients screened in cancer detection or added cancer detection benefit, were extracted. The dispersion of density categories, definition of dense breast, recall rate, biopsy rate per 1000 patients screened, reference standard and PPV were also extracted and summarised in preformed data forms accordingly. PPV1 was defined as the malignancy rate among cases with positive results; PPV2 was defined as the malignancy rate among positive cases with biopsy recommendations; PPV3 was defined as the malignancy rate of positive cases with a performed biopsy. The diagnostic outcomes, including sensitivity and specificity for the detection of early-stage breast cancer, were extracted according to full-text reviewing, and summarised as % (TP/TP + FN) and % (TN/FP + TN), respectively, where TP, FP, TN and FN indicated the number of patients with true positivity, false positivity, true negativity and false negativity predicted. Specificity, sensitivity or the difference between these outcomes where available were further evaluated by meta-analysis.
Through Meta-DiSc analysis, sensitivity and specificity of cancer detection from either test arm were then calculated and summarised as a forest plot presenting values of each study with the corresponding 95% confidence interval (CI, lower and upper limit), and then a pooled effect among those studies with completed measurements was calculated. Furthermore, a summary receiver-operating characteristic (SROC) curve was graphed along with the area under SROC curve (AUC) with standard error (SE).
For comparing the differences in diagnostic performance between mammography alone (M alone) and mammography with conjunctive ultrasound (M + US) in dense breast patients, an effect size defined as risk ratio (RR) was adopted and presented with 95% CI for each study, and a combined effect was subsequently calculated using the Comprehensive Meta-Analysis software, version 2.0 (Biostat, Englewood, NJ). An RR > 1 indicated that M alone might provide a higher diagnostic value than M + US, while an RR < 1 indicated that M + US provided a higher diagnostic value than M alone. An RR = 1 indicated that the results were similar between M alone and M + US.
The heterogeneity test was evaluated according to a χ2-based statistic and I2 statistic with a p value. For the Q statistic (or otherwise indicated as chi-square), P values <0.10 were considered statistically significant for heterogeneity. For the I2 statistic, heterogeneity was assessed as follows: no heterogeneity (I2 = 0–25%), moderate heterogeneity (I2 = 25–50%), large heterogeneity (I2 = 50–75%) and extreme heterogeneity (I2 = 75–100%).24 A random-effect model was used in the current meta-analysis, assuming substantial heterogeneity present among the studies.25
Subgroup analyses were performed with regard to study country, US method and available data obtained during first-round US. Sensitivity analysis was conducted using a leave-one-out approach. Publication bias analysis by funnel plot was not performed in the current meta-analysis due to the limited number of studies included (<10 studies).26 In all analyses, a two-sided P value <0.05 was considered statistically significant. The statistical analyses were performed using Meta-DiSc analysis software, version 1.4 and Comprehensive Meta-Analysis software, version 2.0 (Biostat, Englewood, NJ).
Results
Literature search
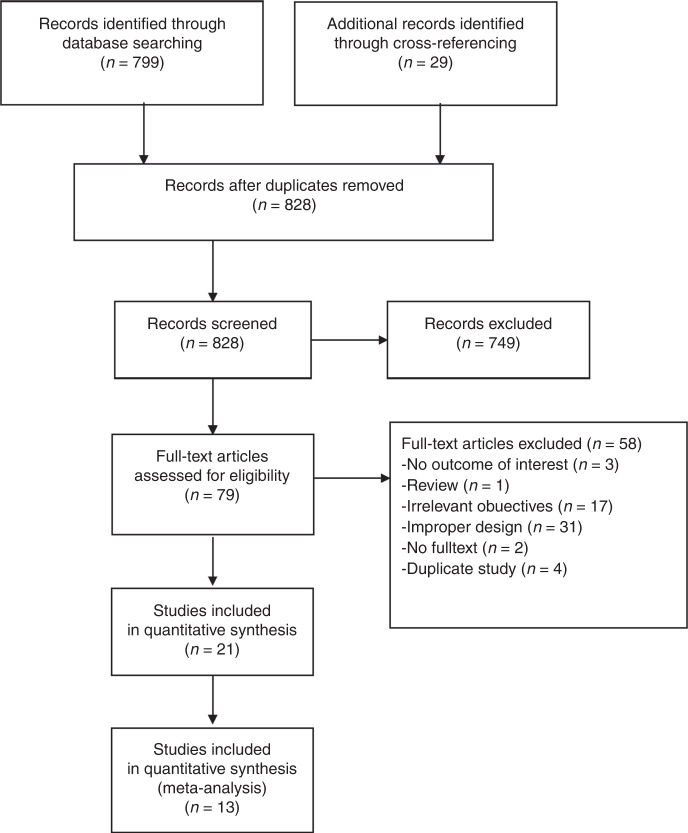
A flow diagram of study selection is shown in Fig. 1. After initially identifying 828 articles, 749 articles were excluded based on the exclusion criteria. The full text of 79 articles was then reviewed, and 58 articles were excluded; the reasons for exclusion are shown in Fig. 1. Three sets of studies (Corsetti et al.,27,28 Berg et al.29,30 and Weigert et al.31–33) were series reports of three individual patient cohorts. The earlier papers were excluded due to data duplication or lack of data on sensitivity and specificity. Thus, 21 studies were finally included in a systematic review.3,28,30,33–50
Fig. 1. Flow diagram of study selection for systematic review and meta-analysis.
Twenty-one studies with quantitative synthesis were included for systematic review and 13 studies with complete diagnostic results were included for conducting meta-analysis.
Study characteristics
Study characteristics are summarised in Table 1; recall rate, biopsy rate, PPV1 and PPV3 are summarised in Table 2. The risk factors considered in each of the studies were summarised in Supplementary Table S1. Eight studies included women with dense breasts who received M alone or M + US screening for breast cancer,3,30,34,36,40,42,47,49 and five of these performed US using the automated breast US (ABUS).3,34,40,42,49 US was done in a whole-breast screening fashion in all included studies. In these studies, 443 out of 69,096 participants were diagnosed with malignancies confirmed by biopsy. Most of the studies that compared M alone with M + US were performed in countries highly populated by Caucasians (United States and Sweden).3,30,34,40,42,49 Five of the eight studies comparing M alone and M + US in patients with dense breasts provided data for the presence of common breast cancer risk factor, which included BRCA1/2 mutations, family history, personal breast cancer history, use of hormone therapy, etc.3,30,34,42,49 (Supplementary Table S1).
Table 1.
Characteristic of studies included in the meta-analysis.
| First author | Study design | Country | Ethnicity (%) | US method | Comparison | Number of patients | Age (years)a | Cancer detection rate (per 1000 screened) | Added cancer detection rate (per 1000 screened) | Number of patients with confirmed cancer |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients with dense breasts | ||||||||||
| Wilczek et al.49 | Retro | Sweden | Not specified | ABUS (Rg) | M alone | 1668 | 49.5 ± 7.9 | 4.2 | 2.4 | 11 |
| (Rg) | M + US | 1668 | 6.6 | |||||||
| Brem et al.34 | Pro | USA |
White 11915 (77.8) Hispanics, Latinas or Spanish 810 (5.3) American Indians or Alaskan natives 33 (0.2) Asians 657 (4.3) Blacks, African Americans or Haitians only 1645 (10.7) Native Hawaiians or Pacific Islanders 27 (0.2) Mixed race or ethnicity 108 (0.7) Unknown or other race or ethnicity 123 (0.8) |
ABUS | M alone | 15,318 | 53.3 ± 10.0 | 5.4 | 1.9 | 82 |
| (T) | M + US | 7.3 | 112 | |||||||
| Giger et al.40b | Retro | USA | Not specified | ABUS | M alone | 185 | N/A | N/A | N/A | 52 |
| (R) | M + US | 185 | N/A | N/A | ||||||
| Giuliano42 | Pro | USA | Not specified | ABUS | M alone | 4076 | Median 54c | 4.6 | 7.7 | 19 |
| (T) | M + US | 3418 | Median 57c | 12.3 | 42 | |||||
| Korpraphong et al.47 | Retro | Thailand | Not specified | HHUS (R) | M alone | 14,483 | Mean 49.4 | 6.5 | 1.4 | 115 |
| M + US | 7.9 | |||||||||
| Chae et al.36 | Retro | Korea | Asian | HHUS (R) | M alone | 12,505 | Mean = 52 (range 22–91) | 0.48 | 2.391 | 11 |
| M + US | 8359 | 2.871 | 24 | |||||||
| Berg et al.30 | RCT | USA |
White 2467 (92.8) Hispanic or Latino 265 (10.0) Black or African American 91 (3.4) Native Hawaiian or other Pacific Islander 4 (0.2) Asian 90 (3.4) American Indian or Alaskan Native 4 (0.2) Unknown 11 (0.4) |
HHUS (P) | M alone | 2659 | 55.2 ± 10.1 | 7.5 | 5.3 | N/A |
| M + US | 12.8 | |||||||||
| Kelly et al.3 | Pro | USA |
White 3843 (87) Hispanics 248 (6) Asians 128 (3) Blacks 61 (1) |
ABUS (T) | M alone | 6425 | Median = 53 (range, 24–89) | 3.6 | 3.6 | 57 |
| M + US | 6425 | 7.2 | ||||||||
| Patients with negative mammogram and dense breasts | ||||||||||
| Destounis et al.39 | Retro | USA | Not specified | HHUS | M (–) → US | 4898 | Mean 55.8 | 3.3 | N/A | 18 |
| Klevos et al.46 | Retro | USA | Not specified | HHUS (T followed by a radiologist) | M (–) → US | 394 | NA | N/A | N/A | 0 |
| Kim et al.45 | Pro | Korea | Asian | HHUS (R) | M (–) → US |
Total: 3,171 Initial US: 998 Non-initial US: 2173 |
51.2 ± 7.7 |
Total: 2.8 Initial US: 4 Non-initial US: 2.3 |
N/A |
Total: 9 Initial US: 4 Non-initial US: 5 |
| Weigert33 | Retro | USA | Not specified |
HHUS (T, in some substances, radiologist-rescanned) |
M (–) → US |
Year 1: 2706 Year 2: 3351 Year 3: 4128 Year 4: 3331 |
Range 45–77 |
Year 1: 4.1 Year 2: 2.7 Year 3: 2.7 Year 4: 3.0 |
N/A |
Year 1: 11 Year 2: 9 Year 3: 11 Year 4: 10 |
| Chang et al.37 | Retro | Korea | Asian | HHUS (R) | M (–) → US | 990 | Median = 47 (range, 27–79) | 5.1 | N/A | 5 |
| Girardi et al.41 | Retro | Italy | Not specified | HHUS (R) | M (–) → US | 9960 | NA | 2.21 | N/A | 22 |
| Hooley et al.43 | Retro | USA | Not specified | HHUS (T) | M (–) → US | 935 | 52 (range, 29–86) | 3.2 | N/A | 3 |
| Leong et al.48 | Pro | Singapore |
Chinese (94) Indian (4) Malay (1) Eurasian (1) |
HHUS (S, radiologist verified) | M (–) → US | 141 | Mean = 45.1 (range, 30–64) | US: 14 | N/A | 2 |
| Corsetti et al.28 | Retro | Italy | Not specified | HHUS | M (–) → US | 3356 | N/A | US: 4.4 | N/A | N/A |
| Youk et al.50 | Retro | Korea | Asian | HHUS (R) | M (–) → US | 1507 | 47.5 ± 7.8 | 29 | N/A | 43 |
| Crystal et al.38 | Retro | Israel | N/A | HHUS (R) | M (–) → US | 1517 | 52.1 ± 8.1 | 4.6 | N/A | 7 |
| Kaplan44 | Pro | USA | Not specified | HHUS (T or R) | M (–) → US | 1862 | Range 35–87 | 3 | N/A | 6 |
| Buchberger et al.35 | Retro | Austria | Not specified | HHUS (R) | M (–) → US | 8103 | Mean 47.6 | 4.1 | N/A | 40 |
ABUS automated breast US, HHUS handheld US, M mammogram, N/A not available, Pro prospective study, Retro retrospective study, RCT randomised controlled trial, US ultrasound, USA United States of America, R radiologist-performed, Rg radiographer-performed, P physician-performed, S sonographer-performed, T technologist-performed.
aAge was summarised as mean ± standard deviation, or mean or median with range (minimum, maximum).
bEnriched-reader study.
cPatients with cancer.
Table 2.
Summary of recall rate, biopsy rate and biopsy-referenced PPV.
| First author | Comparison | Recall rate | Biopsy rate (per 1000) | Reference standard | PPV3 | PPV1 |
|---|---|---|---|---|---|---|
| Patients with dense breasts | ||||||
| Wilczek et al.49 | M alone | 13.8/1000 | 6.6 | Biopsy result | 63.6% (7/11) | 30.4% (7/23) |
| M plus US | 22.8/1000 | 13.8 | 47.8% (11/23) | 28.9% (11/38) | ||
| Brem et al.34 | M alone | 2301/15,318 | 38.3 | Biopsy result | 14% (82/586) | 3.6% (82/2301) |
| M plus US | 4364/15,318 | 74.3 | 9.8% (112/1138) | 2.6% (112/4364) | ||
| Giger et al.40 | M alone | N/A | Biopsy result | N/A | 50.85% (30/59) | |
| M plus US | N/A | N/A | 55.07% (38/69) | |||
| Giuliano42 | M alone | 4.6 | Biopsy result | N/A | 20.43% (19/93) | |
| M plus US | 12.3 | N/A | 80.77% (42/52) | |||
| Korpraphong et al.47 | M alone | 6.5 | Biopsy result | N/A | 35% (105/300) | |
| M plus US | 7.9 | N/A | 20.2% (115/569) | |||
| Chae et al.36 | M alone | 4.20% | 1 | Biopsy result and 2-year follow-up | 50% (6/12)a | 1.14% (6/526) |
| M plus US | 5.50% | 26 | 11.1% (24/216)a | 5.30% (24/452) | ||
| Berg et al.30 | M alone | 11.5% (306/2659) | 24 | Biopsy result and 12-month follow-up | 29.2% (19/65) | 6.5% (20/306) |
| M plus US | 26.6% (707/2659) | 102 | 11.4% (31/272) | 4.8% (34/707) | ||
| Kelly et al.3 | M alone | 4.20% | 9.18 | Biopsy result and 1-year follow-up | 39% (23 / 59) | N/A |
| M plus US | 9.60% | 20.85 | 34.3% (46/134) | N/A | ||
| Patients with dense breasts and negative mammography | ||||||
| Destounis et al.39 | M (–) → US | 20 | Biopsy result | 18% (18/100) | N/A | |
| Klevos et al.46 | M (–) → US | 66 | Biopsy result and 12-month follow-up | N/A | N/A | |
| Kim et al.45 | M (–) → US |
Total: 46 Initial US: 96 Non-initial US: 23 |
Biopsy result |
Total: 6.9% (9/131) Initial US: 4.5% (4/89) Non-initial US: 11.9% (5/42) |
Total: 1.1% (9/831) Initial US: 0.9% (4/471) Non-initial US: 1.4% (5/360) |
|
| Weigert et al.33 | M (–) → US | 15.9 | Biopsy result |
Year 1: 7.3% (11/151)a Year 2: 5.0% (9/180)a Year 3: 7.4% (11/148)a Year 4: 18.9% (10/53)a |
Total: 2.9% (41/1400) Year 1: 3.4% (11/325) Year 2: 2.6% (9/348) Year 3: 3.5% (11/316) Year 4: 2.4% (10/411) |
|
| Chang et al.37 | M (–) → US | 84.8 | Biopsy result and 12-month follow-up | 5.95% (5/84)a | N/A | |
| Girardi et al.41 | M (–) → US | 19 | Biopsy result and at least 12-month follow-up | N/A | N/A | |
| Hooley et al.43 | M (–) → US | 56.7 (BI-RADS category 3 + 4) | Biopsy result |
5.6% (3/54 lesions) 6.5% (3/46 patients) (BI-RADS category 4) |
N/A | |
| Leong et al.48 | M (–) → US | 99 | Biopsy result | 14.3% (2/14) | N/A | |
| Corsetti et al.28 | M (–) → US | Biopsy result | N/A | 7.5% (32/427) | ||
| Youk et al.50 | M (–) → US | 96.9 | Biopsy or at least 2-year follow-up | 33.9% (38/112) | N/A | |
| Crystal et al.38 | M (–) → US | 25 | Biopsy result | 18.4% (7/38) | 7.8% (7/90) | |
| Kaplan44 | M (–) → US | 30 | Biopsy result | 11.8% (6/51) | N/A | |
| Buchberger35 | M (–) → US | Biopsy result | N/A | 29.6% (37/125) | ||
M mammogram, US ultrasound, PPV3 positive predictive value of all biopsies performed, PPV1 positive predictive value among cases that have positive results, N/A not available.
aPPV2, positive predictive value of all biopsy recommendations.
On the other hand, thirteen other studies included women with dense breasts and negative results on initial mammogram, and subsequently received additional US examination by handheld US (HHUS).28,33,35,37–39,41,43–46,48,50 In these studies, 196 out of 50,350 participants were diagnosed with malignancies confirmed by biopsy. Four of the studies that evaluated follow-up US were conducted in Far Eastern countries (Korea and Singapore) (Table 1).37,45,48,50 While Giuliano et al.42 adopted the Wolf classification of 50% or greater breast density for the definition of dense breasts, patients of all other studies were of BI-RADS categories 2–4, with 17 studies focusing on patients with BI-RADS categories 3 or 4. Six of the 13 studies that focused on follow-up US in patients with initial negative mammography reported the presence of breast cancer risk factors among their patients38,39,43,46,48,50 (Supplementary Table S1). Four studies focused on patients with definite negative mammography results prior to follow-up US,35,37,38,48 while patients with suspicious mammography results were included additionally by other studies (Supplementary Table S2).
The specificity and sensitivity of the different methods reported in the studies are summarised in Table 3. To achieve homogeneity in screening strategy among studies, the included studies were stratified for those comparing M alone versus M + US, and those with follow-up US during meta-analysis evaluations.
Table 3.
Sensitivity and specificity of indicated screening strategy in the included studies.
| First author | Comparison | Number of patients | Sensitivity | Specificity |
|---|---|---|---|---|
| Patients with dense breasts | ||||
| Wilczek et al.49 | M alone | 1668 | 63.6% (7/11) | 99% (1641/1657) |
| M plus US | 1668 | 100% (11/11) | 98.4% (1630/1657) | |
| Brem et al.34 | M alone | 15,318 | 73.2% (82/112) | 85.4% (12,987/15,206) |
| M plus US | 100% (112/112) | 72% (10,954/15,206) | ||
| Giger et al.40 | M alone | 185 | 57.5% (30/52) | 78.1% (104/133) |
| M plus US | 185 | 74.1% (38/52) | 76.2% (102/133) | |
| Giuliano42 | M alone | 4076 | 76% (19/25) | 98.21% (3977/4051) |
| M plus US | 3418 | 97.67% (42/43) | 99.7%(3365/3375) | |
| Korpraphong et al.47 | M alone | 14,483 | 91.3% (105/115) | 98.6% (14173/14,368) |
| M plus US | 100% (115/115) | 96.8% (13914/14,368) | ||
| Chae et al.36 | M alone | 12,505 | 54.55% (6/11) | 95.85% (11974/12,494) |
| M plus US | 8359 | 100% (24/24) | 94.8% (7908/8335) | |
| Berg et al.30 | M alone | 2659 | 55.6% (20/36) | 89.1% (2337/2623) |
| M plus US | 94.4% (34/36) | 74.3% (1950/2623) | ||
| Kelly et al.3a | M alone | 6425 | 40% (23/57) | 95.15% |
| M plus US | 6425 | 81% (46/57) | 98.7% | |
| Patients with negative mammogram and dense breasts | ||||
| Destounis et al.39 | M (–) → US | 4898 | 100% (18/18) | N/A |
| Klevos et al.46 | M (–) → US | 394 | N/A | N/A |
| Kim et al.45 | M (–) → US |
Total: 3,171 Initial US: 998 Non-initial US: 2173 |
Total: 100% (9/9) Initial US: 100% (4/4) Non-initial US: 100% (5/5) |
Total: 74% (2340/3162) Initial US: 53% (527/994) Non-initial US: 83.6% (1813/2168) |
| Weigert et al.33 | M (–) → US |
Year 1: 2706 Year 2: 3351 Year 3: 4128 Year 4: 3331 |
Total: 97.6% (41/42) Year 1: 91.7% (11/12) Year 2: 100% (9/9) Year 3: 100% (11/11) Year 4: 100% (10/10) |
Total: 89.9% (12,115/13,474) Year 1: 88.3% (2380/2694) Year 2: 89.9% (3003/3342) Year 3: 92.6% (3812/4117) Year 4: 87.9% (2920/3321) |
| Chang et al.37 | M (–) → US | 990 | 100% (5/5) | 91.9% (906/985) |
| Girardi et al.41 | M (–) → US | 9960 | N/A | N/A |
| Hooley et al.43 | M (–) → US | 935 | 100% (3/3) | N/A |
| Leong et al.48 | M (–) → US | 141 | 100% (2/2) | 88.5% (92/104) |
| Corsetti et al.28 | M (–) → US | 3356 | 86.7%b | N/A |
| Youk et al.50 | M (–) → US | 1507 | 88.4% (38/43) | N/A |
| Crystal et al.38 | M (–) → US | 1517 | 100% (7/7) | 94.5% (1427/1510) |
| Kaplan et al.44 | M (–) → US | 1862 | N/A | N/A |
| Buchberger35 | M (–) → US | 8103 | 92.5% (37/40) | 75.9% (277/365) |
M mammogram, US ultrasound, N/A not available.
aThis study was not included for meta-analysis because the specificity was derived based on recalls.
bScreening sensitivity = cancers detected at screening/cancers detected at screening plus interval cancers.
Meta-analysis
M alone versus M+US in patients with dense breasts
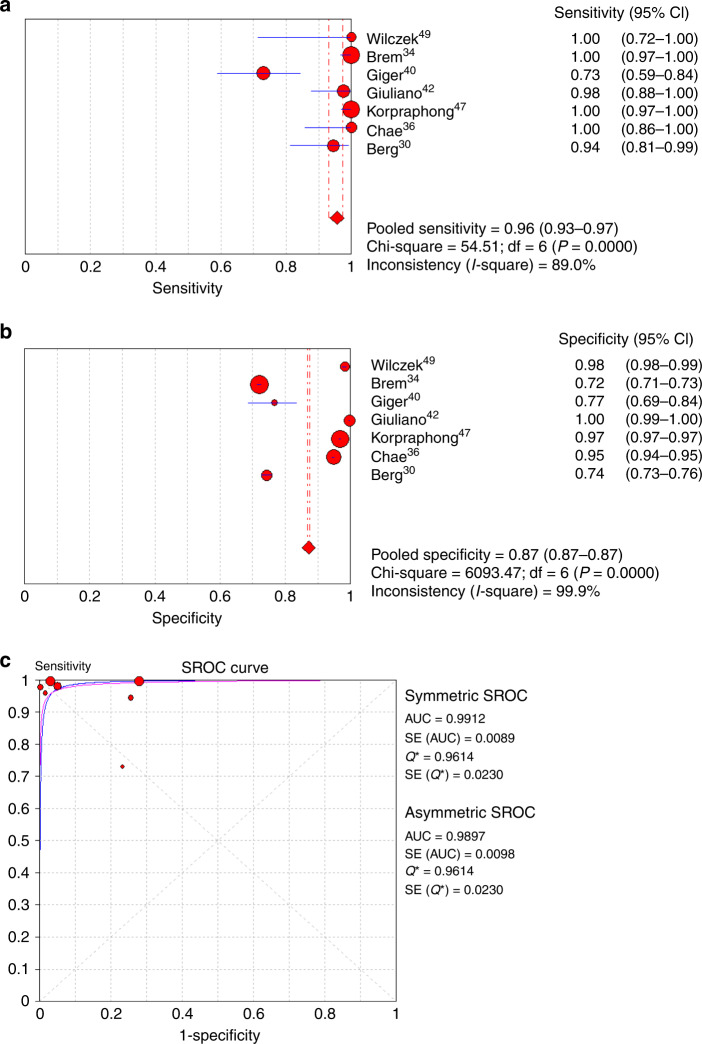
Seven of the eight studies provided complete sensitivity and specificity data.30,34,36,40,42,47,49 The sensitivity of M alone for cancer detection ranged from 40% to 91.3%, and the specificity ranged from 78.1% to 99.0% (Table 3). High heterogeneity was found among studies reporting sensitivity or specificity of either methods (I2 ranged from 83.8% to 99.9%, all P < 0.001, Figs. 2 and 3). For this reason, a random-effect model was used for meta-analysis. For M + US, the sensitivity for cancer detection ranged from 74.1% to 100.0%, and the specificity ranged from 72% to 99.7%. For all studies combined, the pooled sensitivity and specificity of M alone for cancer detection was 74% (95% CI: 0.69–0.79) and 93% (95% CI: 0.93–0.94), respectively (Fig. 2). On the other hand, the pooled sensitivity and specificity for M + US was 96% (95% CI: 0.93–0.97) and 87% (95% CI: 0.87–0.87), respectively (Fig. 3).
Fig. 2. Meta-analysis of cancer diagnostic yield of mammography alone in patients with dense breast.
a Sensitivity, b specificity and c summary of the ROC curve. ROC receiver-operating characteristics, SROC summary ROC, CI confidence interval, AUC area under SROC, SE standard error.
Fig. 3. Meta-analysis of cancer diagnostic yield of mammography plus ultrasound in patients with dense breast.
a Sensitivity, b specificity and c summary of the ROC curve. ROC receiver-operating characteristics, SROC summary ROC, CI confidence interval, AUC area under SROC, SE standard error.
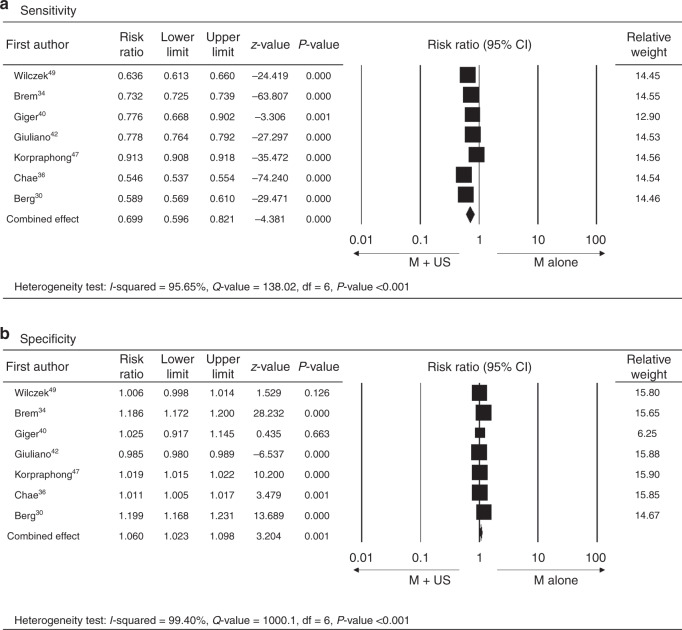
When comparing the diagnostic accuracy of cancer detection between M alone and M + US, the AUC value of the SROC curve from the combined effects among those studies showed that the M + US had better diagnostic efficacy of pooled sensitivity and specificity as compared with M alone (M + US vs. M alone, asymmetric SROC AUC value = 0.989 vs. 0.741) (Figs. 2c and 3c). In reflection to this finding, the meta-analysis of differences in the diagnostic yield of the two methods also showed that M + US might have higher sensitivity in cancer detection compared with mammography alone (M alone vs. M + US, RR = 0.699, 95% CI = 0.569–0.821, P < 0.001) (Fig. 4). The difference in specificity between M + US and M alone was shown significantly. However, the RR is represented close to 1 between two groups (RR = 1.060, 95% CI = 1.023–1.098, P = 0.001) (Fig. 4). The meta-analysis was performed by a random-effect model again, as high heterogeneity was found in the differences in diagnostic yield (difference in sensitivity: I2 = 95.65%, P < 0.001; difference in specificity: I2 = 99.4%, P < 0.001).
Fig. 4. Meta-analysis of differences in cancer diagnostic yield between mammography alone and mammography plus ultrasound in patients with dense breast.
a Sensitivity and b specificity. M alone, mammography alone, M + US mammography plus ultrasound, lower and upper limit, lower and upper bound of 95% confidence intervals (CI).
Follow-up ultrasound in patients with dense breasts and negative mammography
Six out of 13 studies with complete sensitivity and specificity data for the detection of malignancy by follow-up US in patients with negative mammography and dense breasts were included in the analysis.33,35,37,38,45,48 The sensitivity for cancer detection by follow-up US ranged from 88.4% to 100%, and specificity ranged from 74% to 94.5% (Table 3). A fixed-effect model was used for sensitivity, and a random-effect model used as high heterogeneity was found for specificity (sensitivity: I2 = 0%, P = 0.665; specificity: I2 = 99.2%, P < 0.001) (Fig. 5). Upon meta-analysis, the pooled sensitivity of cancer detection was found to be 96% (95% CI: 0.91–0.99) (Fig. 5a), and the pooled specificity was 88% (95% CI: 0.87–0.88) (Fig. 5b). The diagnostic accuracy (AUC) was derived as 0.962 (SE = 0.02) by asymmetric SROC (Fig. 5c).
Fig. 5. Meta-analysis of cancer diagnostic yield of follow-up ultrasound in patients with dense breast and negative mammography.
a Sensitivity, b specificity and c summary of the ROC curve. ROC receiver-operating characteristics, SROC summary ROC, CI confidence interval, AUC area under SROC, SE standard error.
Subgroup analyses
To address potential confounding imposed by disease prevalence, US method and timing of follow-up US, subgroup analyses were conducted and summarised in Table 4.
Table 4.
Subgroup analysis of studies given countries or US methods, respectively.
| Statistics | ||||||
|---|---|---|---|---|---|---|
| Number of studies | Sensitivity | Specificity | Asymmetric AUC (SE) of SROC | Risk ratio (95% CI) of sensitivity | Risk ratio (95% CI) of specificity | |
| Western countries | ||||||
| M alone | 5 | 0.67 (0.61–0.73) | 0.89 (0.88–0.89) | 0.643 (0.118) | 0.693 (0.636–0.755)* | 1.079 (1.001–1.164)* |
| M + US | 5 | 0.93 (0.90–0.96) | 0.78 (0.78–0.79) | 0.982 (0.023) | Reference | Reference |
| M (–) → US | 3 | 0.95 (0.98–0.99) | 0.90 (0.89–0.90) | 0.970 (0.041) | – | |
| Far Eastern countries | ||||||
| M alone | 2 | 0.88 (0.81–0.93) | 0.97 (0.97–0.98) | N/A | 0.706 (0.426–1.169) | 1.015 (1.008–1.023)* |
| M + US | 2 | 1.00 (0.97–1.00) | 0.96 (0.95–0.96) | N/A | Reference | Reference |
| M (–) → US | 3 | 1.00 (0.79–1.00) | 0.79 (0.77–0.80) | 0.950 (0.035) | – | |
| ABUS | ||||||
| M alone | 4 | 0.69 (0.62–0.75) | 0.89 (0.88–0.89) | 0.671 (0.120) | 0.722 (0.673–0.774)* | 1.05 (0.969–1.137) |
| M + US | 4 | 0.93 (0.89–0.96) | 0.79 (0.78–0.79) | 0.987 (0.021) | Reference | Reference |
| M (–) → US | 0 | – | ||||
| HHUS | ||||||
| M alone | 3 | 0.81 (0.74–0.87) | 0.97 (0.96–0.97) | 0.961 (0) | 0.665 (0.446–0.99)* | 1.067 (1.029–1.106)* |
| M + US | 3 | 0.99 (0.96–1.00) | 0.94 (0.94–0.94) | 0.972 (0.333) | Reference | Reference |
| M (–) → US | 6 | 0.96 (0.91–0.99) | 0.88 (0.87–0.88) | 0.962 (0.02) | – | |
| First-round US screening | ||||||
| M alone | 2 | 0.72 (0.64–0.80) | 0.87 (0.86–0.87) | N/A | 0.683 (0.595–0.784)* | 1.092 (0.930–1.284) |
| M + US | 2 | 1.00 (0.97–1.00) | 0.75 (0.74–0.75) | N/A | Rreference | Reference |
| M (–) → US | 3 | 1.00 (0.71–1.00) | 0.73 (0.71–0.75) | 0.944 (0.039) | – | |
M mammogram, US ultrasound, ABUS automated breast US, HHUS handheld US, N/A not available, ROC receiver-operating characteristic, SROC summary ROC, CI confidence interval, AUC area under SROC, SE standard error.
*P value <0.05.
In studies conducted in either Western3,10,30,34,40,42,49 or Far Eastern36,47 countries, the diagnostic accuracy suggested that M + US had higher sensitivity but lower specificity compared with M alone (sensitivity: 0.93 vs. 0.67 in Western countries and 1.00 vs. 0.88 in the Far East; specificity: 0.78 vs. 0.89 in Western countries and 0.96 vs. 0.97 in Far East). The RR also showed that the M + US method had significantly higher sensitivity rate than M alone only in studies conducted in Western countries (RR = 0.69, 95% CI = 0.64–0.76, P < 0.001), while M + US method had a lower specificity rate than M alone in both Western and in Far Eastern countries (RR = 1.08, 95% CI = 1.00–1.16, P = 0.048 in Western countries; RR = 1.015, 95% CI = 1.008–1.023, P < 0.001 in the Far East). With regard to the US method, the diagnostic outcomes suggest that among studies adopting either ABUS34,40,42,49 or HHUS,30,36,47 M + US had higher sensitivity and lower specificity than M alone (sensitivity for ABUS: 0.93 vs. 0.69 for M + US vs. M alone; sensitivity for HHUS: 0.99 vs. 0.81 for M + US vs. M alone; specificity for ABUS: 0.79 vs. 0.89 for M + US vs. M alone; specificity for HHUS: 0.94 vs. 0.97 for M + US vs. M alone). The RR showed that the M + US method had significantly higher sensitivity rate than M alone, given that either ABUS or HHUS method was adopted (ABUS method: RR = 0.72, 95% CI = 0.67–0.77, P < 0.001; HHUS method: RR = 0.67, 95% CI = 0.45–0.99, P = 0.045), and significantly lower specificity rate than M alone only when HHUS method was performed (RR = 1.07, 95% CI = 1.03–1.11, P < 0.001) (Table 4). In studies that had data available specifically during the first-round US screening34,49 (Supplementary Table S2), M + US again had higher sensitivity and lower specificity than M alone (sensitivity: 1.00 vs. 0.72, RR = 0.683, P < 0.05; specificity: 0.75 vs. 0.87, RR = 1.09, P value insignificant).
For the six studies adopting follow-up US in patients with negative mammography and dense breasts, the sensitivity and specificity of the screening strategy were 0.95 and 0.90, respectively, for studies conducted in Western countries,33,35,38 and 1.00 and 0.79, respectively, for Far Eastern countries.37,45,48 The asymmetric AUC of SROC for Western countries was 0.97 (SE = 0.041); an asymmetric AUC of SROC = 0.950 (SE = 0.035) was found for studies conducted in Far Eastern countries. Regarding the US method, all six eligible studies were conducted using HHUS method,33,35,37,38,45,48 and the data were in line with the main results. Three studies evaluating follow-up US presented specific results for first-round screening37,45,48 (Supplementary Table S2), and the pooled sensitivity and specificity were 1.00 and 0.73, respectively, with an asymmetric AUC of SROC of 0.94 (Table 4).
Overall, the results of the subgroup analyses and the main meta-analysis exhibited similar trends.
Sensitivity analysis among studies
Sensitivity analyses were performed using the leave-one-out approach in which the meta-analyses of cancer detection outcomes were performed with each study removed in turn. The results are summarised in Supplementary Tables S3 and S4. The direction and magnitude of combined estimates did not vary markedly with the removal of most of the studies, indicating that each of the meta-analyses had good reliability, and the data were not overly influenced by each study.
Quality assessment
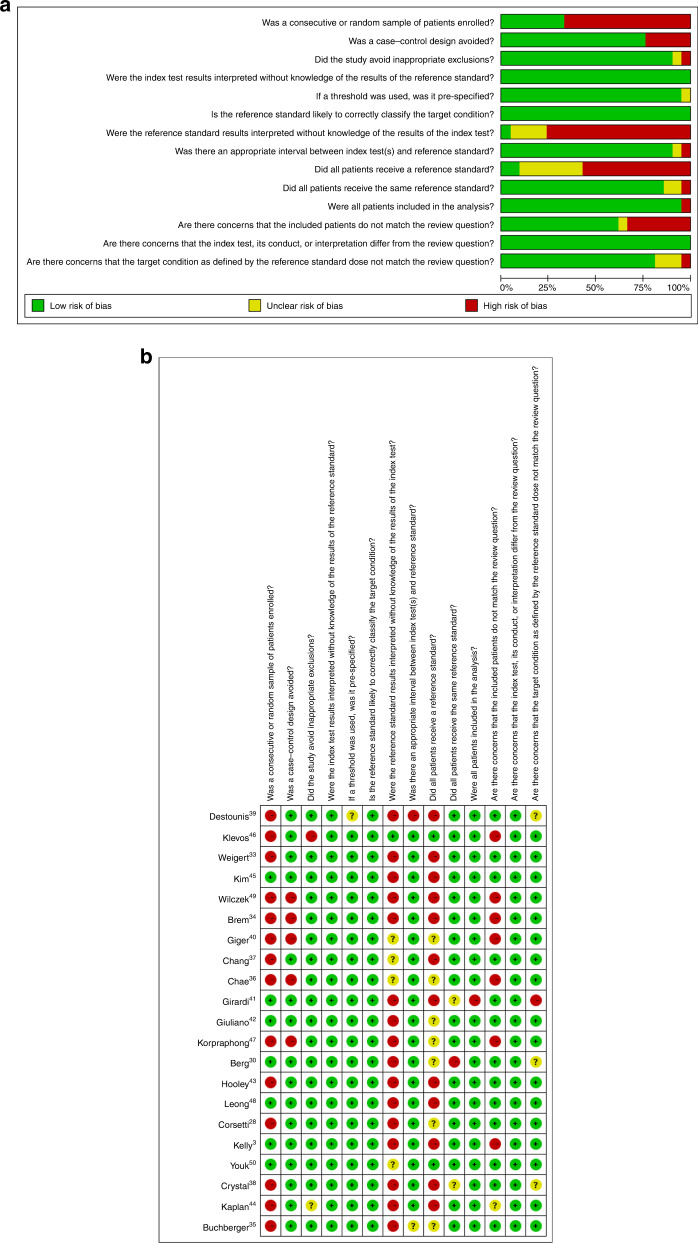
The quality assessments of included diagnostic accuracy studies are shown in Fig. 6. The quality assessment of the included studies indicated that the quality of the studies was acceptable, except for the retrospective design and the reference standard used by studies; the risk of bias mainly resulted from lack of enrolled-patient randomisation, index test masking and available reference standard in a number of studies.
Fig. 6. Quality assessment.
a The summary of bias of the 21 studies; b risk of bias for each included study.
Discussion
This systematic review and meta-analysis examined and compared the diagnostic yield and accuracy of US as an adjunct to mammography with mammography alone for the screening of breast cancer in women with dense breasts. For general participants with dense breasts, the combined sensitivity of M + US for breast cancer was significantly higher than that of M alone (96% and 74%, respectively; RR = 0.699, P < 0.001). The combined specificity of M + US for breast cancer in the general female population with dense breasts was slightly lower than that of M alone (87% vs. 93%, respectively; RR = 1.06, P = 0.001). In contrast, in women with dense breasts and initially negative in mammography, the follow-up ultrasonography had high sensitivity (96%) and specificity (88%). Subgroup analyses with data stratified by study country, US method and first-round US further supported the main findings, suggesting that adjunctive US is beneficial for detecting breast cancer in women with dense breasts, albeit with an expected but tolerable sacrifice in detection specificity.
One meta-analysis published by Rebolj et al.51 examined the rate of breast cancer detected only by US versus that detected by multimodal screening methods (mammography with or without US). The authors found that the proportion of cancers detected only by US was 0.29 (95% CI: 0.27–0.31) of all detected cancers, and this translated to approximately 40% increased breast cancer detection compared with other screening methods. Furthermore, follow-up US additionally contributed to 3.8 (95% CI: 3.4–4.2) screen-detected cases per 1000 mammography-negative women. Despite these findings, US was not recommended by the authors to be a stand-alone screening method, but rather as a supplemental tool. It was difficult to correlate the findings reported by Rebolj et al.51 to our study, as neither the comparisons nor the outcomes of interests (M alone vs. M + US, and diagnostic yield in our case) were comparable between the two studies. Moreover, a fixed-effect model was adopted by Rebolj et al.51 disregarding the varied screening strategy and target population among their included studies, while a random-effect model was preferred in the current meta-analysis accompanied by study stratification.
Previously published systematic reviews have examined the usefulness of adding US to mammography screening for women with dense breasts. A 2009 review by Nothacker et al.52 only identified 6 cohort studies of intermediate-level evidence (3b) (no RCTs or other systematic reviews were identified). A more recent systematic review by Scheel et al.53 identified 12 studies, and concluded that there was consistent evidence that adjunctive US screening detects more invasive cancers compared with mammography alone in women with dense breasts, but there was no evidence to support that adjunctive US screening was associated with reduced long-term breast cancer mortality.53 In contrast to our study, the diagnostic outcomes of M + US did not receive individual review from follow-up US by Scheel et al.53. Furthermore, Scheel et al.53 study did not evaluate diagnostic yield by meta-analyses, which was also likely due to the disparate screening methodology adopted by studies included in the systematic review.53 A 2016 systematic review of supplemental screening for breast cancer in women with dense breasts done for the United States Preventive Services Task Force concluded that supplemental US screening increases the cancer detection rate, but was associated with an increase in the false-positive rate, and the impact on long-term breast cancer outcomes was unclear.54
The detection and differentiation of malignant microcalcifications in dense breast tissue are a particular issue of concern, but traditional radiologist-based interpretation of US imaging remains limited in providing an immediate solution.21,22 Computer-aided automatic reporting systems have been enthusiastically evaluated,55,56 and their implementation in future mammography screening may achieve greater diagnostic accuracy of microlesions in dense breasts. Furthermore, the adjunctive use of tomosynthesis in mammography-negative patients has been tested prospectively, and shown to have exhibited less false-positive results in contrast to supplemental US.57 In reflection, supplemental US screening for women with dense breasts was found to produce relatively small survival benefits, despite substantial increase in costs in a review using data from large medical databases and extensive literature search.58 Although outside the context of the current meta-analysis, the cost-effectiveness of US performed in the present fashion as a supplemental or follow-up screening for breast cancer should be carefully considered.
In the current meta-analysis, the subjective disparity and observer variability of US in each study could not be clearly distinguished, and thus may confound the findings. The image acquisition and interpretation of US are highly operator-dependent, and for this reason, computer-aided diagnosis systems have been rigorously developed in order to facilitate efficient interpretation, and improve the diagnostic accuracy in identifying malignant breast lesions.59 In addition, the observed differences among studies may depend on differences in learning curves, individual radiologic experience and the way protocols and reports are filled out. The low PPV reported by Brem et al.34 could be a result of the ABUS readout protocol, where the radiologist interpretation time was 2.9 min and evidently lower than that of Wilczek et al.49 In particular, low breast cancer rates in the Asian population may explain why Chae et al. reported low PPV values.36
Apart from the bias presented in the risk evaluations, the findings in the current meta-analyses may also be subjected to influence from heterogeneity among study design, patient characteristics, follow-up period and other details in the respective studies. Giger et al.40 performed an enriched-reader study involving 17 radiologists from different types of health facilities; thus, the readout performance or enrolled population may not be comparable to the real-world scenario.10,60 Leong et al.48 involved only one medical centre in their study, reporting a sensitivity of 100% since no false-negative cases were found after 1 year of follow-up of participants with BI-RADS assessment category 1 or 2 under mammogram and categories U1–U4 under US assessment.48 In Corsetti’s study in 2011,28 all subjects with negative screening mammograms and with dense breasts had bilateral breast US, and reported a screening sensitivity (86.7%) calculated by dividing cancers detected at screening with cancers detected at screening plus interval cancers occurring over 365 days for this study. Therefore, the variation of sensitivity of additional US ranging from 86.7% to 100% in the subgroup of patients with dense breasts and negative in mammography might result from heterogeneity in sample size and definition of true-positive cases.
There are limitations of this analysis that need to be considered in the interpretation of the results. The study design of most included studies in the analyses was retrospective rather than randomised head-to-head comparisons. Although the quality of the studies was found to be adequate, and the sensitivity analysis indicated that the results were robust, heterogeneity was detected among the studies. A number of studies evaluating the diagnostic effectiveness of follow-up US included patients who had initial suspicious rather than negative mammography results (Supplementary Table S2), and thus the effect imposed by prevalent cases could not be completely ruled out. In addition, we relied on breast-density results reported by the individual studies, and did not examine or stratify patients based on the actual breast density in the participants of the individual studies. Moreover, we did not take into account the mammography and US technical or instrumental differences among individual studies.
Conclusions
The results of this systematic review and meta-analysis suggest that the addition of US to mammography screening of women with dense breasts improves the sensitivity for the detection of breast cancer, despite a slightly decreased specificity. Follow-up US also had good diagnostic sensitivity and specificity for screening women with dense breasts and negative mammogram findings. Future prospective studies designed to evaluate US as an adjunct or follow-up screening method to mammography in women with dense breasts are needed to confirm the results from our meta-analysis. Enrolment of specific high-risk populations should be further considered to identify those that may benefit from adjunctive US screening for breast cancers most cost-effectively, and reduce the number of recall or false-negative biopsies performed.
Supplementary information
Acknowledgements
We thank Convergence CT, Taiwan for statistical analysis and English language editing.
Author contributions
W.H.Y. and H.C.H. participated in the conception, design and implementation of the study. H.C.H. and C.H.W. participated in literature research. H.C.H., Y.Y.C. and C.H.W. extracted the data. All of the authors analysed and interpreted the data. W.H.Y. and H.C.H. wrote the paper. Y.Y.C. and C.H.W. revised the paper. W.H.Y. and H.C.H. guaranteed the integrity of the entire study, performed the statistical analysis and definition of intellectual content. All authors reviewed and approved the final paper.
Ethics approval and consent to participate
Ethical approval was not sought as the study was based entirely on previously published data.
Consent to publish
Not applicable.
Data availability
All data generated within this study are available from the corresponding author on request.
Competing interests
The authors declare no competing interests.
Funding information
None.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41416-020-0928-1.
References
- 1.Swedish Organised Service Screening Evaluation Group. Reduction in breast cancer mortality from organized service screening with mammography: 1. Further confirmation with extended data. Cancer Epidemiol. Biomark. Prev. 2006;15:45–51. doi: 10.1158/1055-9965.EPI-05-0349. [DOI] [PubMed] [Google Scholar]
- 2.Drukteinis JS, Mooney BP, Flowers CI, Gatenby RA. Beyond mammography: new frontiers in breast cancer screening. Am. J. Med. 2013;126:472–479. doi: 10.1016/j.amjmed.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur. Radio. 2010;20:734–742. doi: 10.1007/s00330-009-1588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology. 2004;230:29–41. doi: 10.1148/radiol.2301020870. [DOI] [PubMed] [Google Scholar]
- 5.Ciatto S, Visioli C, Paci E, Zappa M. Breast density as a determinant of interval cancer at mammographic screening. Br. J. Cancer. 2004;90:393–396. doi: 10.1038/sj.bjc.6601548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American College of Radiology. BI-RADS Committee. ACR BI-RADS Atlas: Breast Imaging Reporting and Data System, 5th edn. (American College of Radiolog, Reston, VA, 2013).
- 7.Winkler NS, Raza S, Mackesy M, Birdwell RL. Breast density: clinical implications and assessment methods. Radiographics. 2015;35:316–324. doi: 10.1148/rg.352140134. [DOI] [PubMed] [Google Scholar]
- 8.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. N. Engl. J. Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 9.Raghavendra A, Sinha AK, Le-Petross HT, Garg N, Hsu L, Patangan MJ, et al. Mammographic breast density is associated with the development of contralateral breast cancer. Cancer. 2017;123:1935–1940. doi: 10.1002/cncr.30573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae JM, Kim EHBreast. Density and risk of breast cancer in Asian women: a meta-analysis of observational studies. J. Prev. Med. Public Health. 2016;49:367–375. doi: 10.3961/jpmph.16.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajaram N, Mariapun S, Eriksson M, Tapia J, Kwan PY, Ho WK, et al. Differences in mammographic density between Asian and Caucasian populations: a comparative analysis. Breast Cancer Res. Treat. 2017;161:353–362. doi: 10.1007/s10549-016-4054-y. [DOI] [PubMed] [Google Scholar]
- 12.Sung H, Rosenberg PS, Chen WQ, Hartman M, Lim WY, Chia KS, et al. Female breast cancer incidence among Asian and Western populations: more similar than expected. J. Natl Cancer Inst. 2015;107:djv107. doi: 10.1093/jnci/djv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehman CD, Lee CI, Loving VA, Portillo MS, Peacock S, DeMartini WB. Accuracy and value of breast ultrasound for primary imaging evaluation of symptomatic women 30-39 years of age. AJR Am. J. Roentgenol. 2012;199:1169–1177. doi: 10.2214/AJR.12.8842. [DOI] [PubMed] [Google Scholar]
- 14.Okello J, Kisembo H, Bugeza S, Galukande M. Breast cancer detection using sonography in women with mammographically dense breasts. BMC Med. Imaging. 2014;14:41. doi: 10.1186/s12880-014-0041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burkett BJ, Hanemann CW. A review of supplemental screening ultrasound for breast cancer: certain populations of women with dense breast tissue may benefit. Acad. Radio. 2016;23:1604–1609. doi: 10.1016/j.acra.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Ohuchi N, Suzuki A, Sobue T, Kawai M, Yamamoto S, Zheng YF, et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387:341–348. doi: 10.1016/S0140-6736(15)00774-6. [DOI] [PubMed] [Google Scholar]
- 17.Bae MS, Han W, Koo HR, Cho N, Chang JM, Yi A, et al. Characteristics of breast cancers detected by ultrasound screening in women with negative mammograms. Cancer Sci. 2011;102:1862–1867. doi: 10.1111/j.1349-7006.2011.02034.x. [DOI] [PubMed] [Google Scholar]
- 18.Health Quality O. Ultrasound as an adjunct to mammography for breast cancer screening: a health technology assessment. Ont. Health Technol. Assess. Ser. 2016;16:1–71. [PMC free article] [PubMed] [Google Scholar]
- 19.Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N. Engl. J. Med. 2016;375:1438–1447. doi: 10.1056/NEJMoa1600249. [DOI] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann. Intern. Med. 2009;151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 21.Ouyang YL, Zhou ZH, Wu WW, Tian J, Xu F, Wu SC, et al. A review of ultrasound detection methods for breast microcalcification. Math. Biosci. Eng. 2019;16:1761–1785. doi: 10.3934/mbe.2019085. [DOI] [PubMed] [Google Scholar]
- 22.Sadoughi F, Kazemy Z, Hamedan F, Owji L, Rahmanikatigari M, Azadboni TT. Artificial intelligence methods for the diagnosis of breast cancer by image processing: a review. Breast Cancer (Dove Med Press) 2018;10:219–230. doi: 10.2147/BCTT.S175311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 24.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int. J. Epidemiol. 2008;37:1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JP. Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int. J. Epidemiol. 2008;37:1158–1160. doi: 10.1093/ije/dyn204. [DOI] [PubMed] [Google Scholar]
- 26.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 27.Corsetti V, Houssami N, Ferrari A, Ghirardi M, Bellarosa S, Angelini O, et al. Breast screening with ultrasound in women with mammography-negative dense breasts: evidence on incremental cancer detection and false positives, and associated cost. Eur. J. Cancer. 2008;44:539–544. doi: 10.1016/j.ejca.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Corsetti V, Houssami N, Ghirardi M, Ferrari A, Speziani M, Bellarosa S, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur. J. Cancer. 2011;47:1021–1026. doi: 10.1016/j.ejca.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Bohm-Velez M, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. J. Am. Med. Assoc. 2008;299:2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. J. Am. Med. Assoc. 2012;307:1394–1404. doi: 10.1001/jama.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weigert J, Steenbergen S. The connecticut experiment: the role of ultrasound in the screening of women with dense breasts. Breast J. 2012;18:517–522. doi: 10.1111/tbj.12003. [DOI] [PubMed] [Google Scholar]
- 32.Weigert J, Steenbergen S. The connecticut experiments second year: ultrasound in the screening of women with dense breasts. Breast J. 2015;21:175–180. doi: 10.1111/tbj.12386. [DOI] [PubMed] [Google Scholar]
- 33.Weigert JM. The connecticut experiment; the third installment: 4 years of screening women with dense breasts with bilateral ultrasound. Breast J. 2017;23:34–39. doi: 10.1111/tbj.12678. [DOI] [PubMed] [Google Scholar]
- 34.Brem RF, Tabar L, Duffy SW, Inciardi MF, Guingrich JA, Hashimoto BE, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight Study. Radiology. 2015;274:663–673. doi: 10.1148/radiol.14132832. [DOI] [PubMed] [Google Scholar]
- 35.Buchberger W, Niehoff A, Obrist P, DeKoekkoek-Doll P, Dunser M. Clinically and mammographically occult breast lesions: detection and classification with high-resolution sonography. Semin Ultrasound CT MR. 2000;21:325–336. doi: 10.1016/s0887-2171(00)90027-1. [DOI] [PubMed] [Google Scholar]
- 36.Chae EY, Kim HH, Cha JH, Shin HJ, Kim H. Evaluation of screening whole-breast sonography as a supplemental tool in conjunction with mammography in women with dense breasts. J. Ultrasound Med. 2013;32:1573–1578. doi: 10.7863/ultra.32.9.1573. [DOI] [PubMed] [Google Scholar]
- 37.Chang JM, Koo HR, Moon WK. Radiologist-performed hand-held ultrasound screening at average risk of breast cancer: results from a single health screening center. Acta Radio. 2014;56:652–658. doi: 10.1177/0284185114538252. [DOI] [PubMed] [Google Scholar]
- 38.Crystal P, Strano SD, Shcharynski S, Koretz MJ. Using sonography to screen women with mammographically dense breasts. AJR Am. J. Roentgenol. 2003;181:177–182. doi: 10.2214/ajr.181.1.1810177. [DOI] [PubMed] [Google Scholar]
- 39.Destounis S, Arieno A, Morgan R. New York state breast density mandate: follow-up data with screening sonography. J. Ultrasound Med. 2017;36:2511–2517. doi: 10.1002/jum.14294. [DOI] [PubMed] [Google Scholar]
- 40.Giger ML, Inciardi MF, Edwards A, Papaioannou J, Drukker K, Jiang Y, et al. Automated breast ultrasound in breast cancer screening of women with dense breasts: reader study of mammography-negative and mammography-positive cancers. AJR Am. J. Roentgenol. 2016;206:1341–1350. doi: 10.2214/AJR.15.15367. [DOI] [PubMed] [Google Scholar]
- 41.Girardi V, Tonegutti M, Ciatto S, Bonetti F. Breast ultrasound in 22, 131 asymptomatic women with negative mammography. Breast. 2013;22:806–809. doi: 10.1016/j.breast.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Giuliano V, Giuliano C. Improved breast cancer detection in asymptomatic women using 3D-automated breast ultrasound in mammographically dense breasts. Clin. Imaging. 2013;37:480–486. doi: 10.1016/j.clinimag.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 43.Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology. 2012;265:59–69. doi: 10.1148/radiol.12120621. [DOI] [PubMed] [Google Scholar]
- 44.Kaplan SS. Clinical utility of bilateral whole-breast US in the evaluation of women with dense breast tissue. Radiology. 2001;221:641–649. doi: 10.1148/radiol.2213010364. [DOI] [PubMed] [Google Scholar]
- 45.Kim SY, Kim MJ, Moon HJ, Yoon JH, Kim EK. Application of the downgrade criteria to supplemental screening ultrasound for women with negative mammography but dense breasts. Medicine. 2016;95:e5279. doi: 10.1097/MD.0000000000005279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klevos GA, Collado-Mesa F, Net JM, Yepes MM. Utility of supplemental screening with breast ultrasound in asymptomatic women with dense breast tissue who are not at high risk for breast cancer. Indian J. Radio. Imaging. 2017;27:52–58. doi: 10.4103/0971-3026.202962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korpraphong P, Limsuwarn P, Tangcharoensathien W, Ansusingha T, Thephamongkhol K, Chuthapisith S. Improving breast cancer detection using ultrasonography in asymptomatic women with non-fatty breast density. Acta Radio. 2014;55:903–908. doi: 10.1177/0284185113507711. [DOI] [PubMed] [Google Scholar]
- 48.Leong LC, Gogna A, Pant R, Ng FC, Sim LS. Supplementary breast ultrasound screening in Asian women with negative but dense mammograms-a pilot study. Ann. Acad. Med. Singap. 2012;41:432–439. [PubMed] [Google Scholar]
- 49.Wilczek B, Wilczek HE, Rasouliyan L, Leifland K. Adding 3D automated breast ultrasound to mammography screening in women with heterogeneously and extremely dense breasts: report from a hospital-based, high-volume, single-center breast cancer screening program. Eur. J. Radio. 2016;85:1554–1563. doi: 10.1016/j.ejrad.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Youk JH, Kim EK, Kim MJ, Kwak JY, Son EJ. Performance of hand-held whole-breast ultrasound based on BI-RADS in women with mammographically negative dense breast. Eur. Radio. 2011;21:667–675. doi: 10.1007/s00330-010-1955-8. [DOI] [PubMed] [Google Scholar]
- 51.Rebolj M, Assi V, Brentnall A, Parmar D, Duffy SW. Addition of ultrasound to mammography in the case of dense breast tissue: systematic review and meta-analysis. Br. J. Cancer. 2018;118:1559–1570. doi: 10.1038/s41416-018-0080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nothacker M, Duda V, Hahn M, Warm M, Degenhardt F, Madjar H, et al. Early detection of breast cancer: benefits and risks of supplemental breast ultrasound in asymptomatic women with mammographically dense breast tissue. A systematic review. BMC Cancer. 2009;9:335. doi: 10.1186/1471-2407-9-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scheel JR, Lee JM, Sprague BL, Lee CI, Lehman CD. Screening ultrasound as an adjunct to mammography in women with mammographically dense breasts. Am. J. Obstet. Gynecol. 2015;212:9–17. doi: 10.1016/j.ajog.2014.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melnikow J, Fenton JJ, Whitlock EP, Miglioretti DL, Weyrich MS, Thompson JH, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the U.S. preventive services task force. Ann. Intern. Med. 2016;164:268–278. doi: 10.7326/M15-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fanizzi A, Basile TMA, Losurdo L, Amoroso N, Bellotti R, Bottigli U, et al. Hough transform for the detection of cluster microcalcifications in full-field digital mammograms. SPIE Acts. 2017;10396:1039616–1039612. [Google Scholar]
- 56.Losurdo, L., Fanizzi, A., Basile, T. M. A., Bellotti, R., Bottigli, U., Dentamaro, R. et al. A combined approach of multiscale texture analysis and interest point/corner detectors for microcalcifications diagnosis. in Rojas, I., Ortuno, F. (eds). International Conference on Bioinformatics and Biomedical Engineering. 302–313 (Springer International Publishing AG, New York, NY, 2018).
- 57.Tagliafico AS, Mariscotti G, Valdora F, Durando M, Nori J, La Forgia D, et al. A prospective comparative trial of adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts (ASTOUND-2) Eur. J. Cancer. 2018;104:39–46. doi: 10.1016/j.ejca.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 58.Sprague BL, Stout NK, Schechter C, van Ravesteyn NT, Cevik M, Alagoz O, et al. Benefits, harms, and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann. Intern. Med. 2015;162:157–166. doi: 10.7326/M14-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park HJ, Kim SM, La Yun B, Jang M, Kim B, Jang JY, et al. A computer-aided diagnosis system using artificial intelligence for the diagnosis and characterization of breast masses on ultrasound: added value for the inexperienced breast radiologist. Medicine. 2019;98:e14146. doi: 10.1097/MD.0000000000014146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gur D, Bandos AI, Cohen CS, Hakim CM, Hardesty LA, Ganott MA, et al. The “laboratory” effect: comparing radiologists’ performance and variability during prospective clinical and laboratory mammography interpretations. Radiology. 2008;249:47–53. doi: 10.1148/radiol.2491072025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated within this study are available from the corresponding author on request.