Summary
Mantle cell lymphoma (MCL) is an incurable mature B cell proliferation, combining the unfavourable clinical features of aggressive and indolent lymphomas. The blastic variant of MCL has an even worse prognosis and new treatment options are clearly needed. We analysed the effects of BL22, an immunotoxin composed of the Fv portion of an anti- CD22 antibody fused to a 38-kDa Pseudomonas exotoxin-A fragment on four MCL cell lines as well as on primary cells of four MCL patients. Apoptosis induction by BL22 was much more pronounced in MCL cell lines with low Bcl-2 expression (NCEB-1, JeKo-1 and JVM-2) compared to Granta-519 cells with high Bcl-2 expression. While the expression of the antiapoptotic protein Mcl-1 declined (NCEB-1, Granta-519), Bcl-2 levels remained unchanged in Granta-519 cells. However transfection of BCL2 cDNA into NCEB-1, JeKo-1 and JVM-2 cells significantly reduced BL22-mediated toxicity. Accordingly we examined the effects of Bcl-2 inactivation in Granta-519 cells using siRNA. Indeed, apoptosis induction was strongly enhanced in Granta-519 cells with silenced Bcl-2. Our results were confirmed in freshly isolated MCL-cells from patients with leukaemic MCL. We conclude that Bcl-2 expression is important for mediating resistance against the immunotoxin BL22 in MCL cells.
Keywords: apoptosis, Bcl-2, lymphoma drugs, non-Hodgkin lymphoma, drug resistance
Mantle cell lymphoma (MCL) is recognized as a distinct entity of non-Hodgkin lymphoma (NHL) in the revised European–American classification of lymphoid neoplasms (REAL) and World Health Organization classifications (Harris et al, 1994) (Harris et al, 1999). The hallmark of MCL is the translocation t(11;14)(q23,q32), which results in overexpression of CCND1 (Bosch et al, 1994). The clinical course of MCL patients is aggressive and is characterized by a poor response to conventional chemotherapy with a mean overall survival of approximately 3–4 years (Campo et al, 1999) (Leonard et al, 2001).
More recently, new therapeutic approaches, including stem cell transplantation and monoclonal antibody-based therapies, have shown increased response rates but only minor effects on overall survival (Freedman et al, 1998) (Howard et al, 2002). Therefore, new therapies are clearly needed for patients with MCL.
Immunotoxins are composed of an antibody or antibody fragment and a toxin portion. Most immunotoxins administered in the clinic are derived from Pseudomonas exotoxin-A, diphtheria toxin or ricin. These agents mediate their cytotoxicity by inhibiting protein synthesis due to inactivation of elongation factor-2 (EF-2) or binding to ribosomes, respectively (Pastan & Kreitman, 2002) (Siegall et al, 1989).
BL22 is a recombinant protein composed of the variable portion of monoclonal antibody RFB4, which binds to the CD22 glycoprotein on the surface of B cells, and of PE38, a truncated Pseudomonas exotoxin derivative (Mansfield et al, 1997). Lacking the cell-binding domain of Pseudomonas exotoxin, PE38 is only incorporated into target cells expressing a Fv domain (Mansfield et al, 1997). To exert its cytotoxicity, internalization and processing of the toxin within its translocation domain is required (Ogata et al, 1992) followed by binding of the 35-kDa carboxyterminus of the toxin intracellularly to the KDEL receptor that carries it to the endoplasmic reticulum (Kreitman & Pastan, 1995). Finally, the toxin is translocated to the cytosol, where its adenosine diphosphate (ADP)–ribosylating enzyme domain inactivates elongation factor 2 (EF-2), thereby inhibiting protein synthesis (Siegall et al, 1989) (Mozola et al, 1984). Cell death mediated by PE38 is often facilitated by apoptosis, although immunotoxins can kill cells without inducing caspase-dependent programmed cell death, and other mechanisms may play a role in certain cell types (Keppler-Hafkemeyer et al, 1998) (Keppler-Hafkemeyer et al, 2000).
CD22 is an inhibitory coreceptor of the B cell receptor expressed on normal and malignant lymphocytes. (Walker & Smith, 2008). It is also expressed on MCL cells at higher numbers than needed for the cytotoxic effect of immunotoxins (D’Arena et al, 2000) (Kreitman & Pastan, 1998). In-vitro and in-vivo activity of BL22 has been observed against a variety of haematological malignancies, including MCL (Mansfield et al, 1997), (Decker et al, 2004), (Kreitman et al, 1999). Importantly, the recombinant immunotoxin BL22 has demonstrated clinical activity, inducing long lasting remissions in hairy cell leukaemia and NHL (Kreitman et al, 2001) (Kreitman et al, 2005).
We have previously reported that BL22 promotes caspase-mediated programmed cell death in chronic-lymphocytic leukaemia (CLL) (Decker et al, 2004).
The present work investigated the effect of BL22 in MCL cell lines (NCEB-1, Granta-519, JeKo-1 and JVM-2). Whereas NCEB-1, Granta-519 and JVM-2 carry the t(11;14)(q23,q32), JeKo-1 cells lack t(11;14)(q23,q32). However, BCL1/IGHJ@ gene rearrangement in JeKo-1 cells was confirmed at the molecular level, and overexpression of PRAD1/cyclin D1 protein was revealed in JeKo-1 cells (Jeon et al, 1998). All of the cell lines except JVM-2 are characterized by complex karyotypes and additional genetic alterations of cell cycle regulatory genes, including deletion of CDKN2B and CDKN2A (Granta-519) (Jadayel et al, 1997), and mutation of TP53 (NCEB-1) (Saltman et al, 1988). Of note, NCEB-1 cells carry several stable mouse chromosomes. Therefore this cell line had to be considered a human-mouse hybrid cell line (Camps et al, 2006).
While apoptosis was induced in all four cell lines, the cell lines with low Bcl-2 expression were more sensitive to apoptosis induction by BL22 compared to Granta-519 cells, which display high positivity for Bcl-2. Apoptosis was accompanied by membrane flipping, loss of the mitochondrial membrane potential Δψm, increased DNA fragmentation as well as caspase 9 and 3 cleavage (NCEB-1 and Granta-519 cells). After transfection of human BCL2 cDNA to the BL22-sensitive Bcl-2 negative cell lines (NCEB-1, JeKo-1, JVM-2), apoptosis was strongly inhibited in response to BL22 treatment. Accordingly, we observed increased sensitivity to BL22 treatment in Granta-519 cells upon inactivation of Bcl-2 using BCL2 siRNA. Importantly, Bcl-2 expression was also associated with resistance to BL22 in purified primary MCL cells.
Material and methods
Cell samples and cell lines
After informed consent, peripheral blood was obtained from four patients with a diagnosis of leukaemic MCL, according to clinical and immunophenotypic criteria and cyclin D1 expression. Patients were either untreated or had not received cytoreductive chemotherapy for a period of at least 3 months prior to investigation. At the time of analysis, all patients were free from infectious complications. The cell lines Granta-519 and NCEB-1 were kindly provided by Dr Mark Raffeld, Laboratory of Pathology, National Cancer Institute, National Institutes of Health, Bethesda, USA. The cell lines JeKo-1 and JVM-2 were kindly provided by Dr Dolors Colomer, Hematopathology Unit, Hospital Clínic, Villarroel, Barcelona, Spain.
Separation procedures
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood samples by centrifugation over a Ficoll-Hypaque layer (Biochrom KG, Berlin, Germany) of 1·077 g/ml density. For separation of MCL cells, PBMC were incubated with anti-CD2 and anti-CD14 magnetic beads (Dynabeads M450, Dynal, Oslo, Norway) according to the manufacturer’s instructions. After separation, B-cells from MCL patients were >98% pure as assessed by direct immunofluorescence.
Culture conditions
Granta-519, NCEB-1, JeKo-1 and JVM-2 cells were maintained in Roswell Park Memorial Institute (RPMI) – 1640 medium (Gibco, Berlin, Germany) supplemented with 20% fetal calf serum (seromed®; Biochrom KG), penicillin/streptomycin 50 U/ml, Na-pyruvate 1 mmol/l, l-glutamine 2 mmol/l, l-asparagine 20 μg/ml, 2-mercaptoethanol 0·05 mmol/l, HE-PES 10 mmol/l, and minimal essential medium (MEM) non-essential amino acids 0·7× (Biochrom KG) at 37°C and 5% CO2 in a fully humidified atmosphere.
Flow cytometric analysis
Flow cytometric analysis was performed on purified B cells of MCL patients or on MCL cell lines (NCEB-1, Granta-519, JeKo-1, JVM2 cells) cultured at 1 × 106cells/ml either in medium alone or together with BL22 at a concentration of 100 ng/ml for 24–48 h.
The samples were washed with phosphate-buffered saline (PBS) and resuspended in 500 μl binding buffer (annexin V–fluorescein isothiocyanate (FITC) kit, Immunotech, Marseille, France), containing 1 μl of annexin V–FITC stock and 5 μl of 20 μg/ml propidium iodide (PI) for determination of phosphatidylserine exposure on the outer plasma membrane. After incubation for 10 min at room temperature in the dark, the specimens were quantified by flow cytometry, using a Coulter Epics XL cytofluorometer, acquiring 5000 events. Viable cells were both annexin V and PI negative.
For measurement of the mitochondrial membrane potential, the cells were incubated with 3,3′-dihexyloxacarbocyanine iodide for 30 min at 37°C, washed in PBS, resuspended in 500 μl binding buffer (annexin V–FITC kit, Immunotech) containing 5 μl of 20 μg/ml PI and were analysed via flow cytometry, acquiring 5000 events.
To identify fragmented DNA, a TdT-mediated dUTP nick-end labelling (TUNEL) assay was performed and assessed by flow cytometry (Boehringer Mannheim, Indianapolis, USA). 106cells per sample were washed in PBS and fixed in 2% paraformaldehyde for 30 min at room temperature. After fixation, the cells were washed twice in PBS containing 0·01% bovine serum albumin (BSA) and resuspended in TUNEL reaction mixture containing fluorescein dUTP. Fluorescein incorporated in DNA strand breaks was detected by flow cytometry.
Transfection procedures
The MCL cell line NCEB-1, JeKo-1 and JVM-2, all of which expressed low levels of Bcl-2 protein in immunoblot and flow cytometry analysis, were transfected with the mammalian expression vector pEF-BOS/BCL2 (Mizushima & Nagata, 1990), containing human BCL2 cDNA (pEF-BOS contains puromycin resistance marker). Therefore 1 × 106 cells in a total volume of 400 μl volume were electroporated together with pEF-BOS/BCL2 at 400 V for 4 ms (Impulse generator, Dr. Fischer, Heidelberg, Germany). For control analysis the cell lines were transfected with control pEF-BOS lacking BCL2 cDNA. After transfection the cells were cultured in 2 ml RPMI medium containing 20% of FCS. After 2 d of culture, puromycin was added at rising concentrations (0·2–2 μg/ml). The cells were then selected over a period of approximately 2 weeks. After selection they were tested for CD22 and Bcl-2 expression and used for further experiments.
siRNA experiments
Granta-519 cells were transfected with human BCL2 siRNA or control siRNA. 2 × 106 cells in a total volume of 400 μl volume were electroporated together with 3 μg high performance validated BCL2 siRNA (Qiagen GmbH-Germany, Hilden, Germany) at 280 V, 950 μF (Gene Pulser II; Bio-Rad Laboratories, Richmond, CA, USA). For control analysis, the cells were transfected with non-binding siRNA (Qiagen). Transfection efficacy was tested by flow cytometry using non-binding AlexaFlour 488 siRNA (Qiagen). Immunoblots were performed to evaluate the effects on protein level. For further experiments, we assumed a transfection efficacy higher than 90%.
Preparation of immunotoxins
Development and production of the disulfide-linked, recombinant immunotoxin BL22 (RFB4(dsFv)-PE38), which reacts with the CD22 antigen, has been described (Mansfield et al, 1997). BL22 was a generous gift of Ira Pastan, NCI-Bethesda MD, USA.
Immunoblotting
Cells grown in the presence or in the absence of immunotoxin were harvested from cultures and were washed twice with PBS. They were then lysed in ice-cold 10 mmol/l Tris–HCl buffer (pH 7·4) containing 5 mmol/l EDTA (ethylenediaminetetra-acetic acid), 130 mmol/l NaCl, 1% Triton X-100, 1 mmol/l phenylmethylsulfonyl fluoride (PMSF), 1 mmol/l sodium vanadate, and 10 mg/ml each phenanthrolene, aprotinin, leupeptin, and pepstatin. Cell lysates were spun at 16 090 g (Hettich Mikro 22R, Hettich GmbH, Tuttlingen, Germany) for 20 min, and supernatants were collected. After determination of the protein content with a commercially available assay (Bio-Rad Laboratories), 50 μg protein was separated by 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and was transferred onto polyvinylidene difluoride (PVDF) membranes (Immobilon-P; Millipore Schwalbach, Germany). Membranes were hybridized with antibodies to Bax (purchased from BD PharMingen, San Diego, CA, USA), to Mcl-1 (from Santa Cruz Biotechnology, Santa Cruz, CA), to Bcl-2 (from BD Biosciences Transduction Laboratory, San Diego, CA, USA), or to βactin (from Sigma-Aldrich, Hannover, Germany). Antibodies to procaspase-9 were from Cell Signalling (Frankfurt, Germany). Blots were developed using Super Signal chemo luminescent substrates (Pierce, KMF GmbH, St Augustin, Germany).
Statistical analysis
All analyses were performed at least three times. Statistical significances were determined using a two-tailed paired t -test. A P-value <0·05 was considered to be statistically significant.
Results
CD22 is equally expressed on NCEB-1 and Granta-519 cells
A representative flow cytometric result of a CD22 expression analysis on NCEB-1 and Granta-519 cells is shown in Fig 1A. Both cell lines showed equally basic CD22 expression levels, which was not altered after coincubation with BL22 (data not shown).
Fig 1.
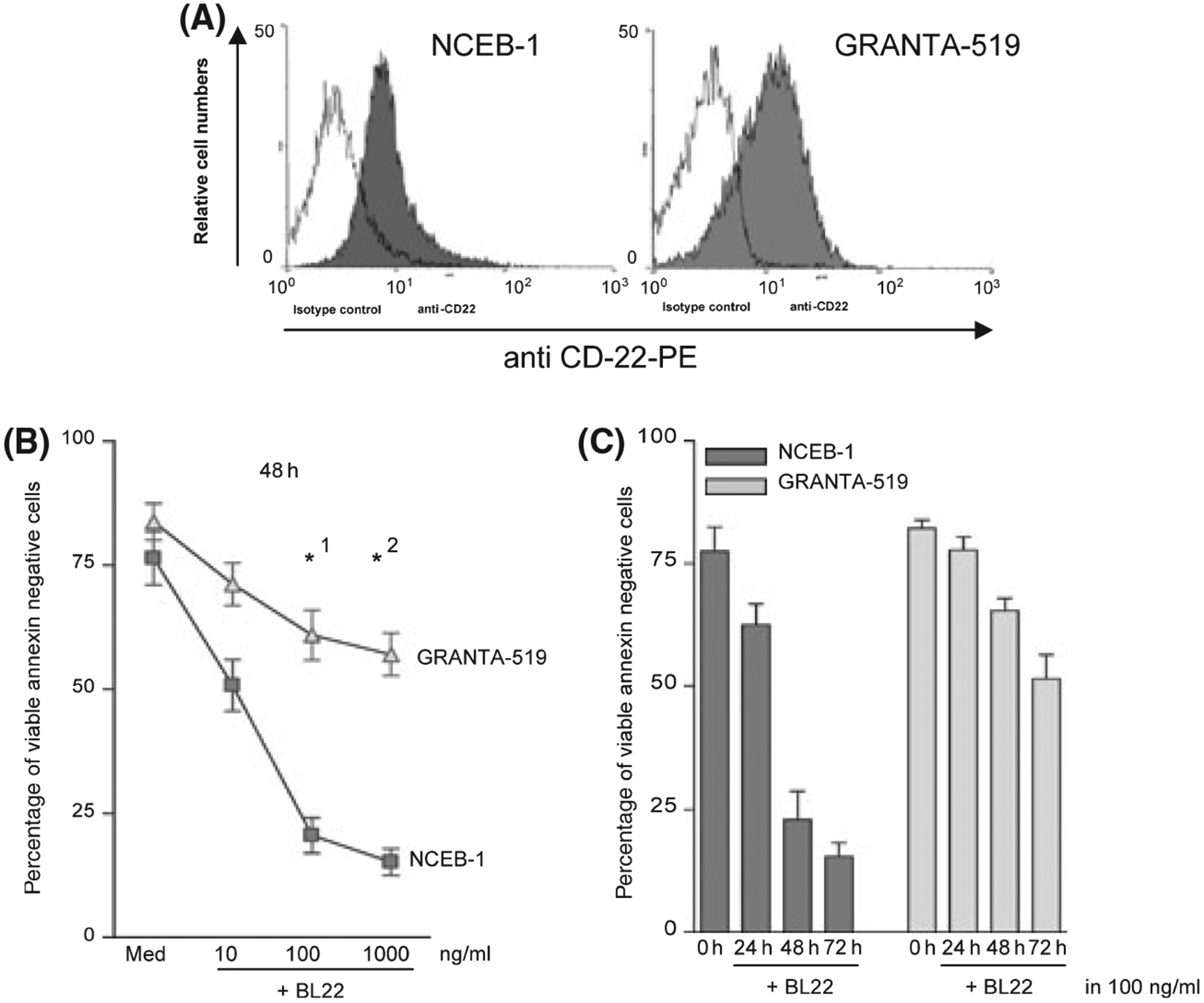
(A) CD22 expression of NCEB-1 and GRANTA-519 cells was tested using phycoerythrin (PE) labelled anti CD-22 FACS antibody. (A) shows one representative result of a flow cytometric analysis. (B) NCEB-1 and GRANTA-519 cells were cultured in the presence of BL22 at the indicated concentration for 48 h. Phosphatidylserine exposure was measured using the annexin V assay. The mean percentage of viable cells [annexin- and propidium iodide (PI)- negative] ± SEM of three different experiments is shown. Paired t-tests were performed to test for differences between GRANTA-519 and NCEB-1 cells. *indicates a statistically significant difference between GRANTA-519 and NCEB-1 cells (P < 0·05) (*1: P 0·020; *2: P 0·0018). (C) Apoptosis induction is displayed as the mean percentage of viable cells (annexin- and PI- negative) ± SEM of three different experiments performed on NCEB-1 and GRANTA-519 cells in medium or together with BL22 at a concentration of 100 ng/ml for 24, 48 and 72 h.
BL22 induces apoptosis in MCL cell lines
To assess apoptosis induction by BL22 in Granta-519 and NCEB-1 cells, phosphatidylserine exposure to the outer plasma membrane was analysed by the Annexin V assay, changes of mitochondrial membrane potential ΔΨ with DiOC(C6) staining, as well as induction of DNA strand breaks using the TUNEL assay upon incubation with BL22.
Figure 1B shows the percentage of viable Annexin V negative NCEB-1 cells 48h after incubation with BL22 compared to Granta-519 cells. NCEB-1 cells were much more susceptible to apoptosis-induction by BL22 compared to Granta-519 cells. Maximal cell death was observed in both cell lines at 100 ng/ml BL22. Although apoptosis was detected 24 h after incubation, the effect was more pronounced after 48 and 72 h of culture. Results (mean ± SEM of three independent experiments) are shown in Fig 1C. Cell death was accompanied by loss of intact mitochondrial membrane potential ΔΨ (Fig 2A). This effect was again more pronounced in NCEB-1 cells where a loss of mitochondrial membrane potential ΔΨ was detected in 79·3% of NCEB-1 cells compared to 25·1% in Granta-519 cells (Fig 2A). In addition, the amount of DNA strand breaks, the common endpoint of caspase-dependent apoptosis, was clearly higher in NCEB-1 cells than in Granta-519 cells. One representative experiment is shown in Fig 2B, with 65·3% UTP FITC positive NCEB-1 cells compared to 38·7% UTP FITC positive Granta-519 cells.
Fig 2.
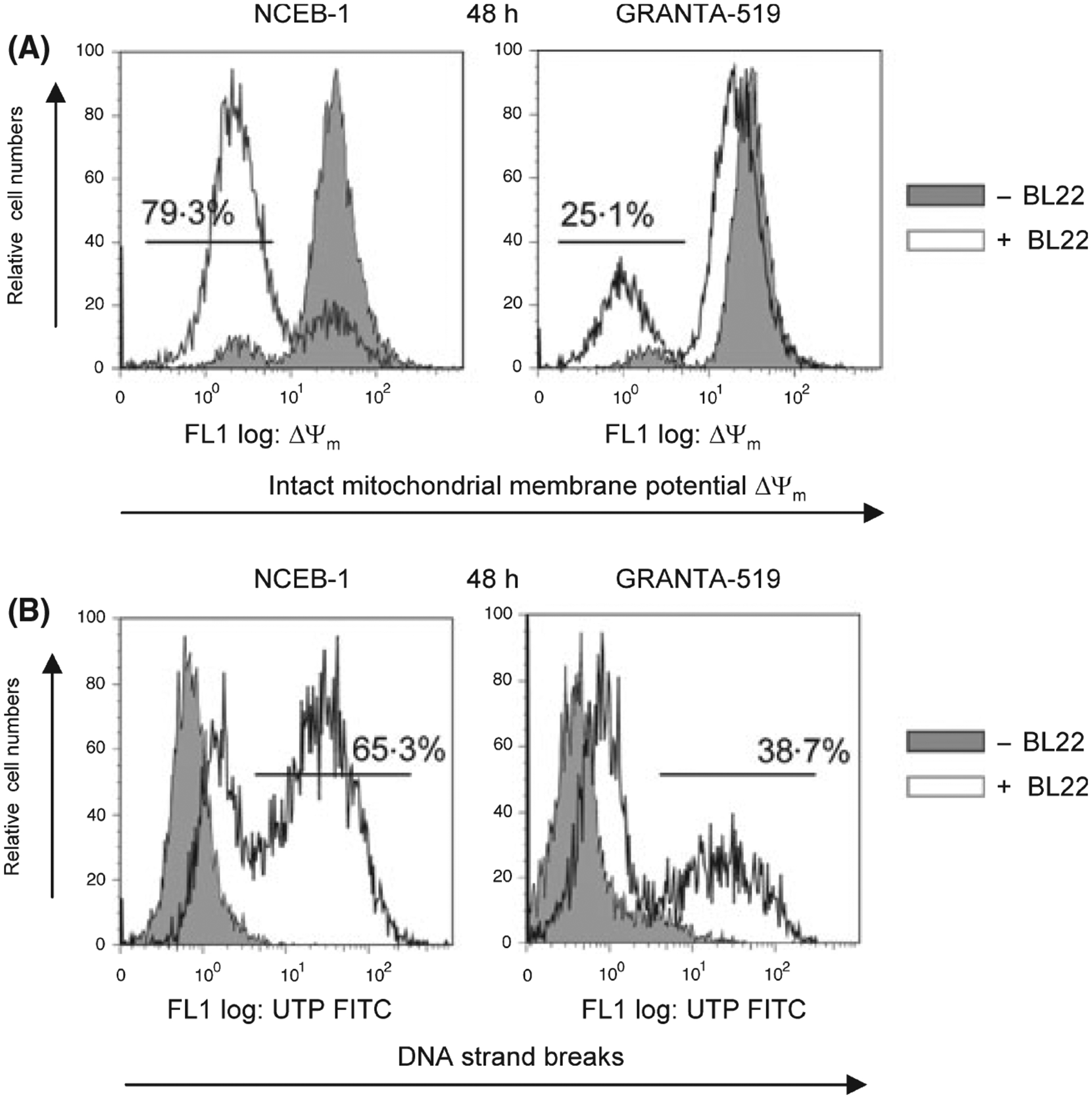
(A) DiOC(C6) staining of NCEB-1 and GRANTA-519 cells upon incubation with BL22 for 48 h in a concentration of 100 ng/ml. The figure shows the differences in the loss of intact mitochondrial membrane potential Δψm for NCEB-1 and GRANTA-519 cells. (B) DNA strand breaks for NCEB-1- and GRANTA-519 cells are shown as a representative TUNEL assay analysis of NCEB-1 and GRANTA-519 cells upon incubation with BL22 for 48 h at a concentration of 100 ng/ml.
To further investigate the effects of BL22 treatment on apoptosis in MCL cells, the expression of Bcl-2 family proteins and caspase cleavage was analysed in NCEB-1 and Granta-519 cells cultured with or without BL22.
Only a very low expression of Bcl-2 was observed in BL22-sensitive NCEB-1 cells but strong Bcl-2 expression was detected in Granta-519 cells (Fig 3A). Bcl-2 expression remained unchanged in BL22-resistant Granta-519 cells in the presence of BL22 (Fig 3B).
Fig 3.
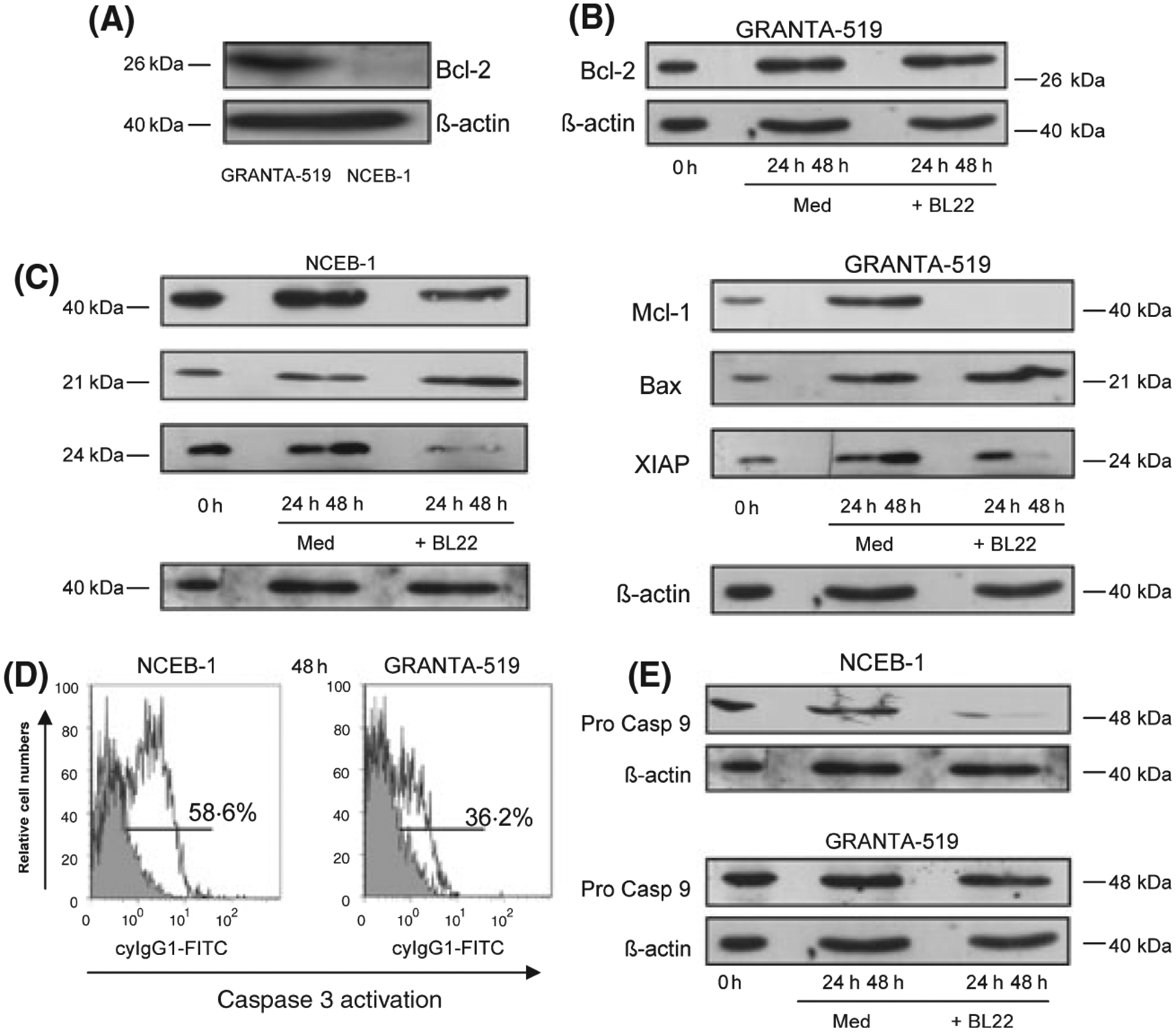
(A) Representative Bcl-2 immunoblot of GRANTA-519 cells and NCEB-1 cells. (B) Coincubation with BL22 for 24–48 h did not change Bcl-2 levels in Granta-519 cells compared to medium control, shown as a representative result. (C) Mcl-1, bax and XIAP immunoblots of NCEB-1- and GRANTA-519 cells were performed in medium (control) and together with BL22 in a concentration of 100 ng/ml for 24–48 h. (D) Representative results of flow cytometric analysis of caspase 3 activation in NCEB-1 and GRANTA-519 cells after incubation with BL22 at a concentration of 100 ng/ml for 48 h and (E) immunoblot results of procaspase 9 after BL22 incubation for the indicated time points. Anti β-actin blotting was used as loading control.
Both the expression of Mcl-1, as an antiapoptotic Bcl-2 member protein, as well as the expression of XIAP, a known inhibitor of caspase 3 activation, was clearly reduced in both cell lines after 24 and 48 h, respectively, of co incubation with BL22 (Fig 3C). In contrast, we detected a slight increase in the expression of the proapoptotic Bcl-2 familiy member Bax upon incubation with BL22 in both cell lines (Fig 3C).
Strong caspase 3 activation was detected in NCEB-1 cells upon BL22 incubation (58·6%) with less activation in Granta-519 cells (36·2%), representatively shown in Fig 3D. Caspase 9 immunoblots demonstrated procaspase 9 cleavage in NCEB-1 cells (Fig 3E). Anti β-actin blotting was used as loading control (Fig 3A–E).
Transfection of human BCL2 cDNA to NCEB-1 increases resistance to BL22
A prominent difference between sensitive NCEB-1 cells and resistant Granta-519 cells was the expression of Bcl-2.
Therefore, we further analysed the importance of Bcl-2 expression for BL22 resistance by transfecting BCL2 cDNA into NCEB-1 cells and into another Bcl-2 low expressing MCL-derived cell line, JeKo-1.
After transfection, NCEB-1/tBCL2 cells had the same CD22 expression as NCEB-1 cells and JeKo-1/tBCL2 cells had the same CD22 expression as JeKo-1 cells (data not shown). The same was true for NCEB-1/tcontrol and JeKo-1/tcontrol cells (data not shown). The overall CD22 expression was higher in NCEB-1 cells compared to JeKo-1 cells (Fig S1B). A representative immunoblot result of Bcl-2 protein expression of NCEB-1 cells, NCEB-1/t control cells, NCEB-1/tBCL2 cells and JeKo-1 cells, JeKo-1/t control cells and JeKo-1/tBCL2 cells is shown in Fig 4A. Granta-519 cells were used as positive control. A flow cytometric result of the expression of Bcl-2 in NCEB-1/tBCL2 cells and JeKo-1/tBCL2 cells is shown in Fig 4B. Comparable Bcl-2 expression levels were detected in both transfected cell lines (59·6% vs. 63·3%).
Fig 4.
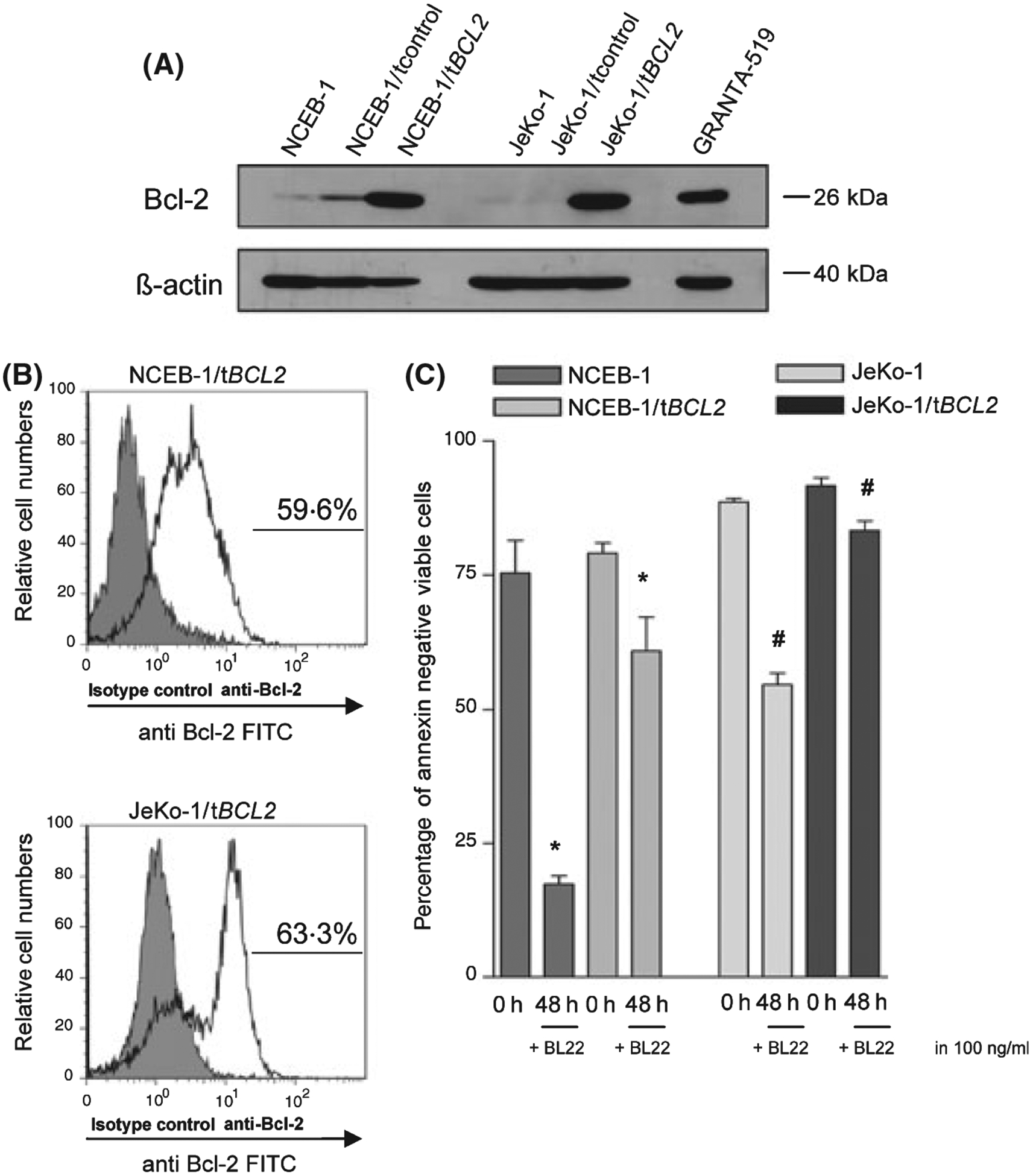
(A) Representative Bcl-2 immunoblot of GRANTA-519 cells (positive control), NCEB-1 cells, control or BCL2 cDNA transfected NCEB-1 cells as well as JeKo-1 cells, control and BCL2 cDNA transfected JeKo-1 cells. Anti β-actin blotting was used as loading control. (B) Flow cytometric Bcl-2 detection in BCL-2 transfected NCEB-1 cells (59·6%) and BCL-2 transfected JeKo-1 cells (63·3%). (C) NCEB-1, NCEB-1/tBCL2 cells as well as JeKo-1 and JeKo-1/tBCL2 cells were cultured in the presence of BL22 at the indicated concentrations for 48 h. Phosphatidylserine exposure was measured using the annexin V assay. The mean percentage of viable cells (annexin – and PI- negative) ± SEM of three different experiments is shown. Paired t-tests were performed to test for differences between NCEB-1 and NCEB-1/tBCL2 cells (*) and JeKo-1 and JeKo-1/tBCL2 cells (#). */# indicates when the difference between the cells was statistically significant (P < 0·05) (*: P 0·0217), (#: P 0·0175).
BL22 still induced cell death in BCL2 cDNA transfected NCEB-1 and JeKo-1cells although significantly less compared to non-transfected NCEB-1 (P = 0·0217) or JeKo-1 (P = 0·0175) cells. The results of three annexin V experiments for NCEB-1 and JeKo-1 cells are shown in Fig 4C. The decrease in apoptosis induction in BCL2 cDNA transfected NCEB-1 and JeKo-1 cells was confirmed by TUNEL analysis and DiOC6(3) staining (data not shown). In contrast, apoptosis induction in control transfected NCEB-1 and JeKo-1 cells was equal to that of untransfected NCEB-1 or JeKo-1 cells (data not shown).
To further confirm our findings we transfected another Bcl-2 low expressing MCL-derived cell line (JVM-2) with human BCL2 cDNA. Transfection efficacy was controlled by FACS analysis (Fig S1B). Again we detected a significant drop in apoptosis induction upon BL22 incubation within the BCL2 cDNA transfected JVM-2 cells compared to the control transfected cells (P 0·0408) (Fig S1A). JVM-2 cells had a higher CD22 expression than NCEB-1 cells (Fig S1C).
Bcl-2 inactivation causes increased apoptosis induction by BL22
We transfected Granta cells with BCL2 siRNA as described in Material and Methods. The transfection efficacy was higher than 90%. This approach resulted in a strong down-regulation of Bcl-2 expression in Granta-519 cells when compared to Granta-519 cells transfected with a non-binding control siRNA (Fig 5A). Although spontaneous cell death was not increased in Granta-519 cells with down-regulated Bcl-2, co incubation with BL22 resulted in a strong induction of apoptosis in these cells as confirmed by Annexin assays and analysis of the mitochondrial membrane potential (Fig 5B). In contrast, BL22 induced only low levels of apoptosis in Granta-519 cells transfected with the control si-RNA (Fig 5B, C). CD22 expression was comparable in BCL2 siRNA transfected and control siRNA transfected cells (data not shown).
Fig 5.
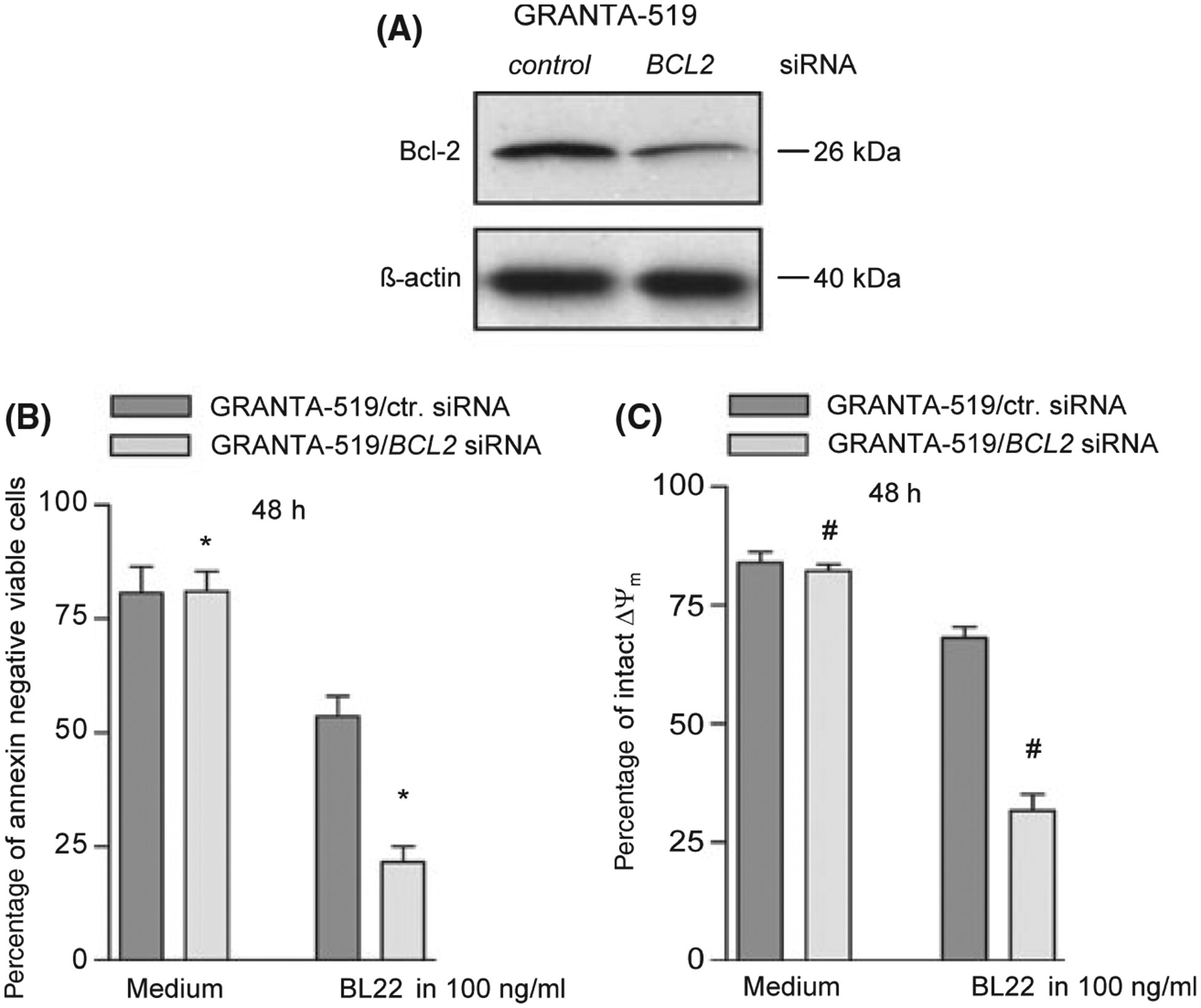
(A) Representative Bcl-2 immunoblot of GRANTA-519 cells 48 h after transfection with BCL2 siRNA or non binding control siRNA. Anti ß actin blotting was used as control. (B) 48 h after transfection of non binding control siRNA (GRANTA-519/ctr. siRNA) and BCL2 siRNA (GRANTA-519/BCL-2 siRNA) GRANTA-519 cells were cultured in the presence of BL22 at the indicated concentration for 48 h. Phosphatidylserine exposure was measured using the annexin V assay. The mean percentage of viable cells (annexin – and PI- negative) ± SEM of three different experiments is shown. Paired t-tests were performed to test for differences between GRANTA-519/t control and GRANTA-519/t BCL2 siRNA cells (*). *indicates when the difference between the cells was statistically significant (P < 0·05) (*: P 0·012). (C) GRANTA-519 cells transfected with non binding control siRNA and BCL2 siRNA were analysed by DIOC(C6) staining upon incubation with BL22 in a concentration of 100 ng/ml for 48 h. The amount of intact mitochondrial membrane potential is shown as the mean percentage of viable cells (annexin – and PI- negative) ± SEM of three different experiments (#: P 0·003).
BL22 induction of apoptosis in primary MCL cells is dependent on Bcl-2 status
To investigate our findings in primary cells of patients with the diagnosis of MCL, annexin assays were performed in four samples from patients with leukaemic MCL. The cells were purified as described in Material and Methods, and cultured for 24 h with or without BL22 at 100 ng/ml. In all experiments, MNC were more than 75% CD5+ CD19+ prior to further depletion of remaining T lymphocytes and monocytes. The cells were purified to more than 90% CD20+ and CD5+ cells after depletion (data not shown). Annexin/PI staining was performed to calculate viable (annexin/PI-negative) cells. The clinical data and source of cells are provided in Table I. All four patients were highly pretreated. The patients had not received cytoreductive therapy for at least 3 months prior to investigation.
Table I.
Patient characteristics.
| No. | Age (years) | Immunophenotype | Source | Prior therapy |
|---|---|---|---|---|
| M1 | 65 | CD5+, CD23−, cyclin D1+ | Peripheral blood | 3× R-CHOP, 3× R-DHAP, 1× HAM |
| M2 | 73 | CD5+, CD23−, cyclin D1+ | Peripheral blood | 3× Chlorambucil, 6× COP, 1× R-FCM, 1× IEV, 1× D-BEAM, 2× R- DHAP, non myeloablative Radioimmunotherapy, Bendamustine, Bortezomib |
| M3 | 71 | CD5+, CD23−, cyclin D1+ | Leukapheresis | 7× Chlorambucil, 5× Fludarabine, 2× Cyclophosphamide/Prednisone, Fludarabine/Mitoxantrone, 3× R-CHOP |
| M4 | 66 | CD5+, CD23−, cyclin D1+ | Peripheral blood | 6× R-CHOP, 3× R-DHAP, R BEAM, autol. stem cell transplantation |
R, rituximab; CHOP, cyclophosphamide, hydroxdaunorubicine, vincristine (Oncovin®), predniso(lo)ne; DHAP, dexamethasone, cytarabine, cis-platin; HAM, high dose cytarabine, mitoxantrone; COP, cyclophosphamide, vincristine (Oncovin®), predniso(lo)ne; FCM, fludarabine, cyclophosphamide, mitoxantrone; IEV, ifosfamide, epirubicin, etoposide; BEAM, BCNU (carmustine), etoposide, cytarabine, melphalan.
The Bcl-2 protein status of these patients was analysed by immunoblot (patients M1-M3) and FACS analysis (patient M4). Bcl-2 expression was detectable in three samples (M1, M2, M4) compared to one sample (M3) with low Bcl-2 expression (Fig 6A). The changes in viability upon BL22 treatment were comparably low in the Bcl-2 positive samples M1, M2 and M4 compared to a high induction of apoptosis in the Bcl-2 negative patient M3 (Fig 6B).
Fig 6.
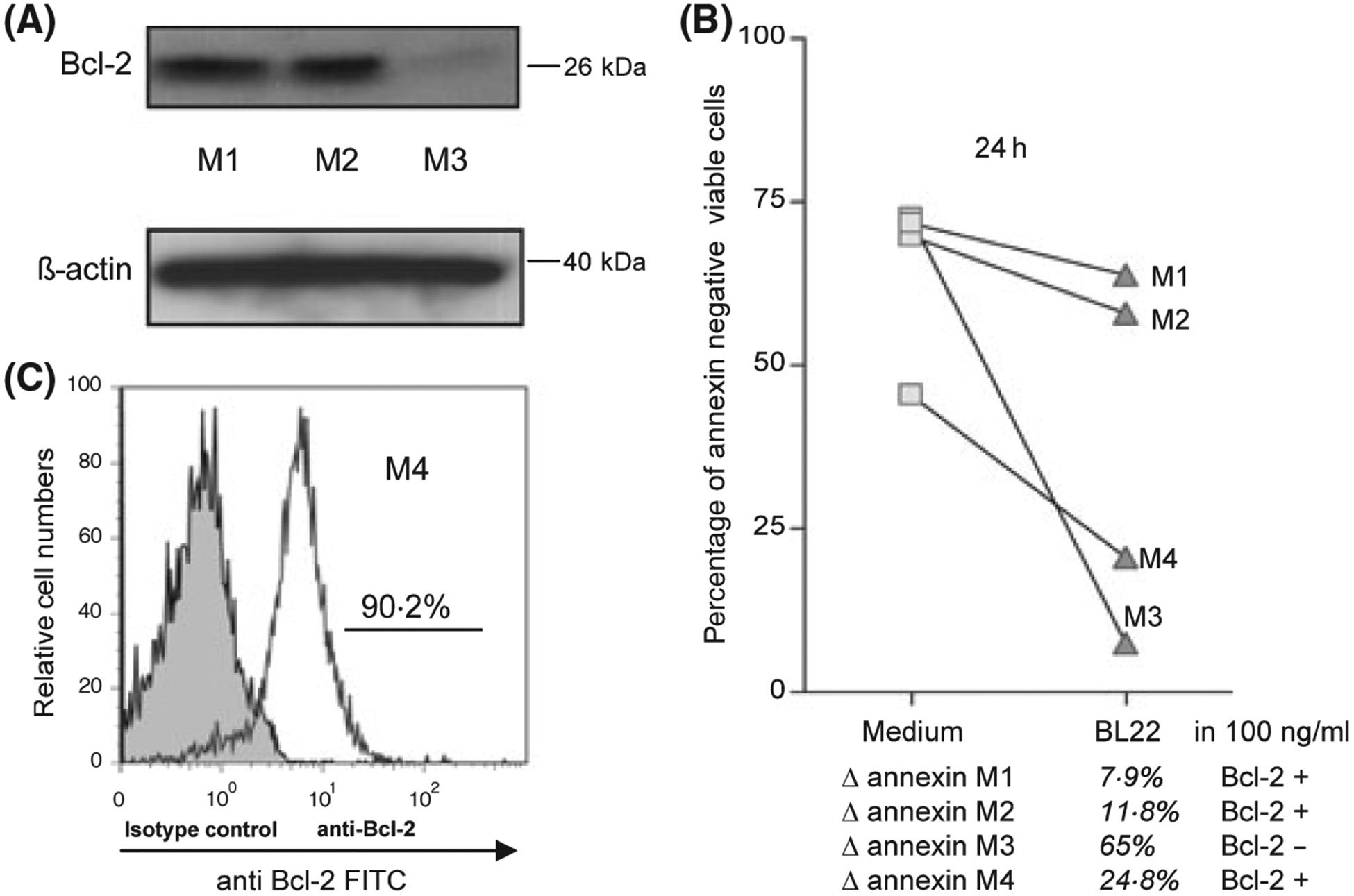
(A) Representative Bcl-2 immunoblot of cells of three patients with a diagnosis of Mantle Cell Lymphoma. Anti β-actin blotting was used as control. (B) Representative results of Annexin V assays of cell samples of four MCL patients M1-M4 upon coincubation with BL22 at a concentration of 100 ng/ml for 24 h. Δ annexin = differences in viable cells upon BL22 incubation. (C) Bcl-2 status of patient M4 was detected by FACS analysis.
Discussion
MCL remains a disease which is incurable for most patients with current treatment modalities. Though immunochemotherapy regimens incorporating the monoclonal antibody rituximab have improved response rates, the remission duration remains short and median survival is in the range of 3–4 years (Forstpointner, et al 2006) (Dreyling et al, 2008) (Popat et al, 2004). Incorporation of rituximab has undoubtedly improved lymphoma therapy, but ‘naked’ antibodies (including rituximab) by themselves are often only weakly cytotoxic. Attaching potent toxins to these antibodies can improve results by combining antibody selectivity with toxin cell-killing potency (Pastan et al, 2006). Indeed, the immunotoxin BL22 has been reported to be very effective in hairy cell leukaemia (Kreitman et al, 2001). Responses have also been observed in B-CLL patients (Kreitman et al, 2005), but very limited data are available in NHL patients.
MCL express CD22 at intermediate levels well above the amount needed for a sufficient cytotoxic effect of an immunotoxin (D’Arena et al, 2000) (Kreitman & Pastan, 1998). Therefore we analysed the effect of the immunotoxin BL22 on MCL cells. Culture of Granta-519 or NCEB-1 cells together with immunotoxin BL22 for 24–48 h resulted in apoptosis induction in both cell lines but NCEB-1 cells were significantly more susceptible to BL22 than Granta-519 cells. Importantly, both cell lines expressed CD22 at comparable levels (Fig 1). Therefore different expression of the target molecule did not account for the observed difference.
We have reported previously that Immunotoxins induce caspase-dependent cell death in B-CLL cells. Cell death was associated with reduced expression of the antiapoptotic proteins Mcl-1 and XIAP (Decker et al, 2004). XIAP and Mcl-1 expression have both been associated with chemotherapy and rituximab resistance in NHL. (Hussain et al, 2007) (Byrd et al, 2002). However, the present study demonstrated that expression of Mcl-1 and XIAP was strongly reduced in both cell lines upon BL22 co incubation, making both proteins unlikely to account for the differences in apoptosis induction.
Bcl-2 alters the activity of a variety of cell signalling proteins involved in proliferation, cell survival and apoptosis (Borner, 2003). Overexpression of prosurvival Bcl-2 together with other antiapoptotic members of the Bcl-2 family is commonly associated with tumour progression and chemotherapy resistance (Danial & Korsmeyer, 2004) (Hanahan & Weinberg, 2000). Bcl-2 overexpression is the consequence of t(14;18) in follicular lymphoma and is frequently overexpressed in lymphoproliferative diseases including MCL, where the frequency of Bcl-2 overexpression has been described to be higher than 50% (Menendez et al, 2004). Bcl-2 silencing in two MCL cell lines has recently been reported to interfere with cyclin D1, p27 and p53 expression (Tucker et al, 2008). However, the importance of Bcl-2 for MCL progression or chemotherapy resistance remains largely unknown (Jares et al, 2007). Previously Bcl-2 has been reported to be involved in mediating chemotherapy resistance in NHL (Mounier et al, 2003) (Wilson, 2006).
Importantly, only very low Bcl-2 expression was observed in BL22-sensitive NCEB-1, JeKo-1 and JVM-2 cells but strong Bcl-2 expression was detected in resistant Granta-519 cells. Bcl-2 expression in Granta-519 cells remained unchanged in the presence of BL22 (Fig 3B). Therefore, the absence of Bcl-2 in NCEB-1, JeKo-1 and JVM-2 cells seems to render cells more susceptible to immunotoxin BL22 treatment. This hypothesis is supported by the finding that inactivation of Bcl-2 results in a strong increase in the susceptibility of Bcl-2 positive MCL cell lines (Tucker et al, 2008) including Granta-519 cells (Fig 5). We are aware that the cell line NCEB-1 contains mouse chromosomes (Camps et al, 2006). Though expressing these chromosomes at low levels this cell line represents a human-mouse hybridoma. Nevertheless transfection of human BCL2 cDNA into NCEB-1 cells and additionally into JeKo-1 (homo sapiens, (Jeon et al, 1998)) and JVM-2 cells (homo sapiens, (Melo et al, 1986)) strongly reduced apoptosis induction in response to BL22 in all three cell lines (Figs 4, S1).
Our findings were confirmed in purified MCL cells from patients with leukaemic MCL. Cells from three patients with high Bcl-2 expression showed low apoptosis induction upon BL22 treatment while cells from one patient without detectable Bcl-2 expression showed a strong response (Fig 6).
Cell death may result from an imbalance of pro- and antiapoptotic proteins. It is known that the balance of pro- and antiapoptotic proteins within the Bcl-2 family is of particular importance for cell survival or apoptosis induction (Adams & Cory, 2007; Brooks & Dong, 2007). In this context the presence or absence of cell survival protein Bcl-2 with concomitant reduction of Mcl-1 expression and upregulation of proapototic Bcl-2 family members like Bax may contribute to the discovered differences of apoptosis induction (van Delft et al, 2006), (Fig 3C).
In summary, treatment with the immunotoxin BL22 induces apoptosis in MCL cell lines and primary MCL cells from patients with leukaemic disease. However, BL22 seems to be less efficacious in Bcl-2 expressing MCL-cells. Therefore, a promising treatment strategy in MCL cells might be a combination of BL22 and drugs that target Bcl-2. Indeed, drugs like the Bcl-2 antisense oblimersen or the new class of BH3-mimics are currently in clinical evaluation and have demonstrated the potential to be combined with chemotherapy or rituximab. (Pro et al, 2008) Therefore, BL22 treatment in Bcl-2 negative and combination regimen in Bcl-2 positive MCL may be interesting therapeutic approaches in this incurable disease.
Supplementary Material
Fig S1. (A) NCEB-1, NCEB-1/t BCL-2 cells, JeKo-1, JeKo-1/t-BCL2 cells as well as JVM-2 and JVM-2/tBCL2 cells were cultured in the presence of BL22 at the indicated concentration for 48 h. Phosphatidylserine exposure was measured using the annexin V assay. The mean percentage of viable cells (annexin - and PI- negative) ± SEM of three different experiments is shown. Paired t-tests were performed to test for differences between NCEB-1 and NCEB-1/tBCL2 cells (*), JeKo-1 and JeKo-1/tBCL2 cells (#), as well as JVM-2 and JVM-2/tBCL2 cells (+). */#/+ indicates when the difference between the cells was statistically significant (P < 0.05) (*: P 0·0217), (#: P 0·0175), (+: P 0·0408). (B) Bcl-2 expression in JVM-2 cells measured via FACS analysis. (C) Representative data of a CD22 expression analysis for JeKo-1, NCEB-1 and JVM-2 cells. Also shown are the isotype controls.
Acknowledgement
This work was supported by the research grant R05/17 DJCLS from the Jose Carreras Leukaemia fund.
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article:
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Adams JM & Cory S (2007) The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene, 26, 1324–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner C (2003) The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Molecular Immunology, 39, 615–647. [DOI] [PubMed] [Google Scholar]
- Bosch F, Jares P, Campo E, Lopez-Guillermo A, Piris MA, Villamor N, Tassies D, Jaffe ES, Montserrat E, Rozman C & Cardesa A (1994) PRAD-1/cyclin D1 gene overexpression in chronic lymphoproliferative disorders: a highly specific marker of mantle cell lymphoma. Blood, 84, 2726–2732. [PubMed] [Google Scholar]
- Brooks C & Dong Z (2007) Regulation of mitochondrial morphological dynamics during apoptosis by Bcl-2 family proteins: a key in Bak? Cell Cycle, 6, 3043–3047. [DOI] [PubMed] [Google Scholar]
- Byrd JC, Kitada S, Flinn IW, Aron JL, Pearson M, Lucas D & Reed JC (2002) The mechanism of tumor cell clearance by rituximab in vivo in patients with B-cell chronic lymphocytic leukemia: evidence of caspase activation and apoptosis induction. Blood, 99, 1038–1043. [DOI] [PubMed] [Google Scholar]
- Campo E, Raffeld M & Jaffe ES (1999) Mantle-cell lymphoma. Seminars in Hematology, 36, 115–127. [PubMed] [Google Scholar]
- Camps J, Salaverria I, Garcia MJ, Prat E, Bea S, Pole JC, Hernandez L, Del Rey J, Cigudosa JC, Bernues M, Caldas C, Colomer D, Miro R & Campo E (2006) Genomic imbalances and patterns of karyotypic variability in mantle-cell lymphoma cell lines. [see comment]. Leukemia Research, 30, 923–934. [DOI] [PubMed] [Google Scholar]
- D’Arena G, Musto P, Cascavilla N, Dell’Olio M, Di Renzo N & Carotenuto M (2000) Quantitative flow cytometry for the differential diagnosis of leukemic B-cell chronic lymphoproliferative disorders. American Journal of Hematology, 64, 275–281. [DOI] [PubMed] [Google Scholar]
- Danial NN & Korsmeyer SJ (2004) Cell death: critical control points. Cell, 116, 205–219. [DOI] [PubMed] [Google Scholar]
- Decker T, Oelsner M, Kreitman RJ, Salvatore G, Wang QC, Pastan I, Peschel C & Licht T (2004) Induction of caspase-dependent programmed cell death in B-cell chronic lymphocytic leukemia by anti-CD22 immunotoxins. Blood, 103, 2718–2726. [DOI] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, Adams JM, Roberts AW & Huang DC (2006) The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized.[see comment]. Cancer Cell, 10, 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyling M, Weigert O, Hiddemann W & European MCLN (2008) Current treatment standards and future strategies in mantle cell lymphoma. Annals of Oncology, 19(Suppl. 4), iv41–44. [DOI] [PubMed] [Google Scholar]
- Forstpointner R, Unterhalt M, Dreyling M, Bock HP, Repp R, Wandt H, Pott C, Seymour JF, Metzner B, Hanel A, Leh-mann T, Hartmann F, Einsele H, Hiddemann W & German Low Grade Lymphoma Study, G (2006) Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG). Blood, 108, 4003–4008. [DOI] [PubMed] [Google Scholar]
- Freedman AS, Neuberg D, Gribben JG, Mauch P, Soiffer RJ, Fisher DC, Anderson KC, Andersen N, Schlossman R, Kroon M, Ritz J, Aster J & Nadler LM (1998) High-dose chemoradiotherapy and anti-B-cell monoclonal antibody-purged autologous bone marrow transplantation in mantle-cell lymphoma: no evidence for long-term remission. Journal of Clinical Oncology, 16, 13–18. [DOI] [PubMed] [Google Scholar]
- Hanahan D & Weinberg RA (2000) The hallmarks of cancer. Cell, 100, 57–70. [DOI] [PubMed] [Google Scholar]
- Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Muller-Hermelink HK, Pileri SA, Piris MA, Ralfkiaer E & Warnke RA (1994) A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood, 84, 1361–1392. [PubMed] [Google Scholar]
- Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA & Bloomfield CD (1999) World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. Journal of Clinical Oncology, 17, 3835–3849. [DOI] [PubMed] [Google Scholar]
- Howard OM, Gribben JG, Neuberg DS, Grossbard M, Poor C, Janicek MJ & Shipp MA (2002) Rituximab and CHOP induction therapy for newly diagnosed mantle-cell lymphoma: molecular complete responses are not predictive of progression-free survival. Journal of Clinical Oncology, 20, 1288–1294. [DOI] [PubMed] [Google Scholar]
- Hussain SR, Cheney CM, Johnson AJ, Lin TS, Grever MR, Caligiuri MA, Lucas DM & Byrd JC (2007) Mcl-1 is a relevant therapeutic target in acute and chronic lymphoid malignancies: down-regulation enhances rituximab-mediated apoptosis and complement-dependent cytotoxicity. Clinical Cancer Research, 13, 2144–2150. [DOI] [PubMed] [Google Scholar]
- Jadayel DM, Lukas J, Nacheva E, Bartkova J, Stranks G, De Schouwer PJ, Lens D, Bartek J, Dyer MJ, Kruger AR & Catovsky D (1997) Potential role for concurrent abnormalities of the cyclin D1, p16CDKN2 and p15CDKN2B genes in certain B cell non-Hodgkin’s lymphomas. Functional studies in a cell line (Granta 519). Leukemia, 11, 64–72. [DOI] [PubMed] [Google Scholar]
- Jares P, Colomer D & Campo E (2007) Genetic and molecular pathogenesis of mantle cell lymphoma: perspectives for new targeted therapeutics. Nature Reviews. Cancer, 7, 750–762. [DOI] [PubMed] [Google Scholar]
- Jeon HJ, Kim CW, Yoshino T & Akagi T (1998) Establishment and characterization of a mantle cell lymphoma cell line. British Journal of Haematology, 102, 1323–1326. [DOI] [PubMed] [Google Scholar]
- Keppler-Hafkemeyer A, Brinkmann U & Pastan I (1998) Role of caspases in immunotoxin-induced apoptosis of cancer cells. Bio-chemistry, 37, 16934–16942. [DOI] [PubMed] [Google Scholar]
- Keppler-Hafkemeyer A, Kreitman RJ & Pastan I (2000) Apoptosis induced by immunotoxins used in the treatment of hematologic malignancies. International Journal of Cancer, 87, 86–94. [PubMed] [Google Scholar]
- Kreitman RJ & Pastan I (1995) Importance of the glutamate residue of KDEL in increasing the cytotoxicity of Pseudomonas exotoxin derivatives and for increased binding to the KDEL receptor. Bio-chemical Journal, 307, (Pt 1), 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitman RJ & Pastan I (1998) Accumulation of a recombinant immunotoxin in a tumor in vivo: fewer than 1000 molecules per cell are sufficient for complete responses. Cancer Research, 58, 968–975. [PubMed] [Google Scholar]
- Kreitman RJ, Wang QC, FitzGerald DJ & Pastan I (1999) Complete regression of human B-cell lymphoma xenografts in mice treated with recombinant anti-CD22 immunotoxin RFB4(dsFv)-PE38 at doses tolerated by cynomolgus monkeys. International Journal of Cancer, 81, 148–155. [DOI] [PubMed] [Google Scholar]
- Kreitman RJ, Wilson WH, Bergeron K, Raggio M, Stetler-Stevenson M, FitzGerald DJ & Pastan I (2001) Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. New England Journal of Medicine, 345, 241–247. [DOI] [PubMed] [Google Scholar]
- Kreitman RJ, Squires DR, Stetler-Stevenson M, Noel P, Fitz-Gerald DJ, Wilson WH & Pastan I (2005) Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. Journal of Clinical Oncology, 23, 6719–6729. [DOI] [PubMed] [Google Scholar]
- Leonard JP, Schattner EJ & Coleman M (2001) Biology and management of mantle cell lymphoma. Current Opinion in Oncology, 13, 342–347. [DOI] [PubMed] [Google Scholar]
- Mansfield E, Amlot P, Pastan I & FitzGerald DJ (1997) Recombinant RFB4 immunotoxins exhibit potent cytotoxic activity for CD22-bearing cells and tumors. Blood, 90, 2020–2026. [PubMed] [Google Scholar]
- Melo JV, Brito-Babapulle V, Foroni L, Robinson DS, Luzzatto L & Catovsky D (1986) Two new cell lines from B-prolymphocytic leukaemia: characterization by morphology, immunological markers, karyotype and Ig gene rearrangement. International Journal of Cancer, 38, 531–538. [DOI] [PubMed] [Google Scholar]
- Menendez P, Vargas A, Bueno C, Barrena S, Almeida J, De Santiago M, Lopez A, Roa S, San Miguel JF & Orfao A (2004) Quantitative analysis of bcl-2 expression in normal and leukemic human B-cell differentiation. Leukemia, 18, 491–498. [DOI] [PubMed] [Google Scholar]
- Mizushima S & Nagata S (1990) pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Research, 18, 5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier N, Briere J, Gisselbrecht C, Emile JF, Lederlin P, Seb-ban C, Berger F, Bosly A, Morel P, Tilly H, Bouabdallah R, Reyes F, Gaulard P & Coiffier B (2003) Rituximab plus CHOP (R-CHOP) overcomes bcl-2–associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL). Blood, 101, 4279–4284. [DOI] [PubMed] [Google Scholar]
- Mozola MA, Wilson RB, Jordan EM, Draper RK & Clowes RC (1984) Cloning and expression of a gene segment encoding the enzymatic moiety of Pseudomonas aeruginosa exotoxin A. Journal of Bacteriology, 159, 683–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M, Fryling CM, Pastan I & FitzGerald DJ (1992) Cell-mediated cleavage of Pseudomonas exotoxin between Arg279 and Gly280 generates the enzymatically active fragment which translocates to the cytosol. Journal of Biological Chemistry, 267, 25396–25401. [PubMed] [Google Scholar]
- Pastan I & Kreitman RJ (2002) Immunotoxins in cancer therapy. Current Opinion in Investigational Drugs, 3, 1089–1091. [PubMed] [Google Scholar]
- Pastan I, Hassan R, Fitzgerald DJ & Kreitman RJ (2006) Immunotoxin therapy of cancer. Nature Reviews. Cancer, 6, 559–565. [DOI] [PubMed] [Google Scholar]
- Popat U, Hosing C, Saliba RM, Anderlini P, van Besien K, Przepiorka D, Khouri IF, Gajewski J, Claxton D, Giralt S, Rodriguez M, Romaguera J, Hagemeister F, Ha C, Cox J, Cabanillas F, Andersson BS & Champlin RE (2004) Prognostic factors for disease progression after high-dose chemotherapy and autologous hematopoietic stem cell transplantation for recurrent or refractory Hodgkin’s lymphoma. Bone Marrow Transplantation, 33, 1015–1023. [DOI] [PubMed] [Google Scholar]
- Pro B, Leber B, Smith M, Fayad L, Romaguera J, Hagemeister F, Rodriguez A, McLaughlin P, Samaniego F, Zwiebel J, Lopez A, Kwak L & Younes A (2008) Phase II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide, in combination with rituximab in patients with recurrent B-cell non-Hodgkin lymphoma. British Journal of Haematology, 143, 355–360. [DOI] [PubMed] [Google Scholar]
- Saltman DL, Cachia PG, Dewar AE, Ross FM, Krajewski AS, Ludlam C & Steel CM (1988) Characterization of a new non-Hodgkin’s lymphoma cell line (NCEB-1) with a chromosomal (11:14) translocation [t(11:14)(q13;q32)]. Blood, 72, 2026–2030. [PubMed] [Google Scholar]
- Siegall CB, Chaudhary VK, FitzGerald DJ & Pastan I (1989) Functional analysis of domains II, Ib, and III of Pseudomonas exotoxin. Journal of Biological Chemistry, 264, 14256–14261. [PubMed] [Google Scholar]
- Tucker CA, Kapanen AI, Chikh G, Hoffman BG, Kyle AH, Wilson IM, Masin D, Gascoyne RD, Bally M & Klasa RJ (2008) Silencing Bcl-2 in models of mantle cell lymphoma is associated with decreases in cyclin D1, nuclear factor-kappaB, p53, bax, and p27 levels. Molecular Cancer Therapeutics, 7, 749–758. [DOI] [PubMed] [Google Scholar]
- Walker JA & Smith KG (2008) Dependence of surface monoclonal antibody binding on dynamic changes in FcgammaRIIb expression. [erratum appears in Immunology. 2008 Jul;124(3):417]. Immunology, 124, 412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WH (2006) Drug resistance in diffuse large B-cell lymphoma. [see comment]. Seminars in Hematology, 43, 230–239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. (A) NCEB-1, NCEB-1/t BCL-2 cells, JeKo-1, JeKo-1/t-BCL2 cells as well as JVM-2 and JVM-2/tBCL2 cells were cultured in the presence of BL22 at the indicated concentration for 48 h. Phosphatidylserine exposure was measured using the annexin V assay. The mean percentage of viable cells (annexin - and PI- negative) ± SEM of three different experiments is shown. Paired t-tests were performed to test for differences between NCEB-1 and NCEB-1/tBCL2 cells (*), JeKo-1 and JeKo-1/tBCL2 cells (#), as well as JVM-2 and JVM-2/tBCL2 cells (+). */#/+ indicates when the difference between the cells was statistically significant (P < 0.05) (*: P 0·0217), (#: P 0·0175), (+: P 0·0408). (B) Bcl-2 expression in JVM-2 cells measured via FACS analysis. (C) Representative data of a CD22 expression analysis for JeKo-1, NCEB-1 and JVM-2 cells. Also shown are the isotype controls.


