Abstract
Despite tremendous research efforts at every level, globally, there is still a lack of effective drugs for the treatment of Alzheimer′s disease (AD). The biochemical mechanisms of this devastating neurodegenerative disease are not yet clearly understood. This review analyses the relevance of multiple ligands in drug discovery for AD as a versatile toolbox for a polypharmacological approach to AD. Herein, we highlight major targets associated with AD, ranging from acetylcholine esterase (AChE), beta-site amyloid precursor protein cleaving enzyme 1 (BACE-1), glycogen synthase kinase 3 beta (GSK-3β), N-methyl-d-aspartate (NMDA) receptor, monoamine oxidases (MAOs), metal ions in the brain, 5-hydroxytryptamine (5-HT) receptors, the third subtype of histamine receptor (H3 receptor), to phosphodiesterases (PDEs), along with a summary of their respective relationship to the disease network. In addition, a multitarget strategy for AD is presented, based on reported milestones in this area and the recent progress that has been achieved with multitargeted-directed ligands (MTDLs). Finally, the latest publications referencing the enlarged panel of new biological targets for AD related to the microglia are highlighted. However, the question of how to find meaningful combinations of targets for an MTDLs approach remains unanswered.
Keywords: multitarget drug discovery, MTDLs, tacrine, donepezil, AChE inhibitors, BACE-1 inhibitors, GSK-3β inhibitors
1. Introduction
In 1906, Alois Alzheimer presented his first signature case and the pathological features of the disease which, from 1910, became known as Alzheimer’s disease (AD). AD is clinically characterized by a loss of memory, the retardation of thinking and reasoning, and changes in personality and behaviours [1,2]. Nowadays, approximately 40 million people over the age of 60 suffer from AD worldwide, and the number of patients is increasing, with the perspective of cases doubling every 20 years [3]. AD is a progressive and irreversible neurological disorder occurring in the central nervous system (CNS) mainly confined within the hippocampus and the cerebral cortex, domains of the forebrain related to memory and higher cognitive functions. The histological manifestation of AD presents extracellular deposits of β-amyloid peptide (Aβ) and the intracellular formation of neurofibrillary tangles consisting of paired helical filaments of hyperphosphorylated tau protein [4,5]. AD is a complex and multifactorial disease, which means that it is influenced by a combination of multiple genes and environmental/risk factors. In the early 1990s, mutations in the genes of amyloid-beta A4 precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2) were determined for familial AD [6,7,8]. Presenilins are components of the γ-secretase complex which, when mutated, can affect amyloid precursor protein (APP) processing to form toxic forms of Aβ. In addition to genes, genetic risk loci for AD were determined and one of them, apolipoprotein E, type ε4 allele (APOE ε4), is associated with late-onset familial AD [9,10]. Studies on the binding of apoE (a peptide corresponding to the low-density lipoprotein receptor binding domain) to APP, showed that blocking of the interaction of apoE with N-terminal APP reduces Alzheimer′s-associated Aβ accumulation and tau pathologies in the brain [11]. Known risk factors include age, having a family history of AD, APOE ε4, vascular problems (heart disease, stroke, high blood pressure), diabetes, and obesity [12,13]. However, the reasons why sporadic AD occurs is still unknown. There are various descriptive hypotheses regarding the causes of sporadic AD, including the cholinergic hypothesis [13], amyloid hypothesis [14,15,16], tau propagation hypothesis [17,18], mitochondrial cascade hypothesis [19,20], calcium homeostasis hypothesis [21,22], inflammatory hypothesis [23,24,25], neurovascular hypothesis [26], metal ion hypothesis [27,28,29], and lymphatic system hypothesis [30,31]. Moreover, there are many factors that may associated with AD, such as various microbes (triggering amyloidosis), viral pathogens (Herpesviridae family) [32,33], decreased expression of microRNAs-107 (miRNA-107) [34,35,36,37,38] and RAS-RAF-MEK signalling pathway (atrophy of neurons) [39], regional hypometabolism [40,41], and mitochondrial dysfunction [42,43,44]. In summary, the aetiology of AD is recognized but not fully understood.
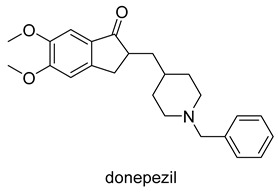
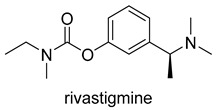
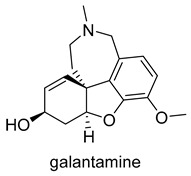
Currently, there are a total of five therapies in the clinic, and four drugs approved by the FDA for AD (Table 1), which mostly aim at restoring physiological ACh levels. The inhibition of the enzyme acetylcholinesterase (AChE), responsible for the hydrolysis of Ach, is the main biochemical mechanism of action of donepezil, rivastigmine and galantamine. In turn, memantine, through noncompetitive antagonism of the N-methyl-d-aspartate (NMDA) receptor, blocks current flow (especial calcium) and reduces the excitotoxic effect of glutamate [45].
Table 1.
Drugs approved for AD therapy.
| Drug Structure | Targets | Therapeutic Effects |
|---|---|---|
|
-AChE inhibitor -5-HT2A inducer -ChE inducer -NOS inhibitor/inducer -TNF inhibitor -IL-1β inhibitor/inducer -NMDAR downregulator |
-selectively and reversibly inhibits AChE -improves the cognitive and behavioral signs and symptoms of AD -neuroprotective |
|
-AChE inhibitor -ChE inhibitor |
-parasympathomimetic and a reversible cholinesterase inhibitor -inhibits both BuChE and AChE -enhances cholinergic function |
|
-AChE competitive and reversible inhibitor -AChR subunit alpha-7 allosteric modulator -N AChR allosteric modulator -ChE inhibitor |
-enhances cholinergic function -improve cognitive performance in AD -not considered as a disease-modifying drug |
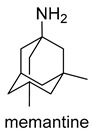
|
-NMDAR uncompetitive (open-channel) antagonist -Alpha-7 nicotinic cholinergic receptor subunit antagonist |
-inhibits calcium influx into cells that is normally caused by chronic NMDAR activation by glutamate -enhances neuronal synaptic plasticity |
Drugs only provide temporary symptomatic relief among patients with mild-to-moderate symptoms of AD, but do not provide a cure or protection. Thus, one of the largest unmet medical needs is a modifying treatment for AD. The complicated pathogenesis of AD, in association with the various descriptive hypotheses involved in the onset and development of the disease and along with the imperfect single-target drugs available, form an excellent rational basis for the implementation of multi-target strategies as part of the drug discovery pipeline against AD. The multi-target-directed ligands (MTDLs) strategy foresees the development of a single molecule able to affect several key targets/pathways, which can have a synergistic effect on the AD network, leading to superior improvement on memory and cognition. While the idea of MDTLs is simple at a glance, the rational design of a compound in which two or more pharmacophores are combined in a single molecular entity is a challenge. Herein, we highlight major targets associated with AD and collate the latest publications which have resulted in an enlarged panel of drug targets.
2. AD-Related Targets
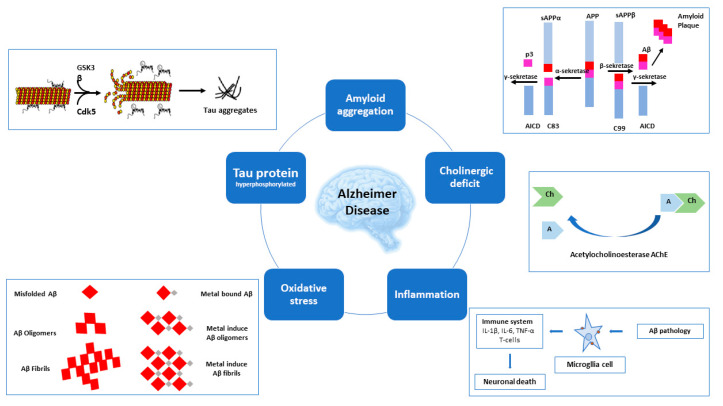
Exploration of hypotheses regarding the causes of AD focus on major pathogenesis factors and pathways (Figure 1). Firstly, cholinergic deficit, which led to the discovery of the primary AD targets of acetylcholinesterase (AChE) and butyrylocholinosterase (BuChE). Next, for amyloid aggregation, where the main target is beta-secretase 1 (BACE-1), whereas for the hyperphosphorylation of tau protein, glycogen synthase kinase 3 beta (GSK-3β) and cyclin dependent kinase 5 (Cdk5) are the key targets. Increased oxidative damage and inflammation and unbalanced homeostasis of biometals in the course of AD led to the discovery of further potential targets for AD treatment.
Figure 1.
Multiple pathological pathways of AD.
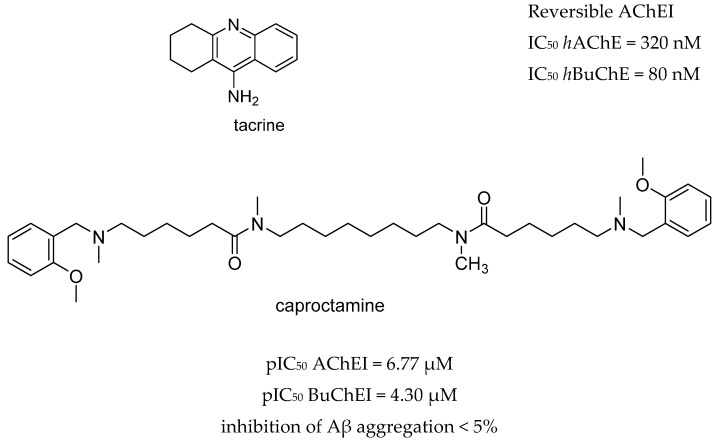
Moreover, the pathogenesis of AD involves numerous receptors, namely, (N-methyl-d-aspartate (NMDA), 5-hydroxytryptamine (5-HT) serotonin, the third subtype of histamine receptor (H3 receptor), and enzymes, namely monoaminoxidases (MAOs), and phosphodiesterases (PDEs). The first AChE inhibitor (AChEI) for AD treatment, tacrine, was approved in 1993. However it was withdrawn shortly after release due to liver toxicity [46] (Figure 2). Currently, the inhibition of AChE is a fundamental property of drugs approved by the FDA which are principally AChE inhibitors (AChEIs), such as donepezil, galantamine, and rivastigmine. Even so, tacrine and donepezil are still the targets for modifications or are used as positive controls in enzyme or pharmacological activity tests. The cholinergic hypothesis, combining ACh and AChE as a common modality is emerging as a promising approach in designing MTDLs for AD. The first clinical example of this approach was caproctamine, which was reported in 1998 as a compound with noncovalent inhibitory activity against the cholinergic system and acetylcholinesterase [47].
Figure 2.
Tacrine and captopramine.
Later, the amyloid cascade hypothesis was proposed to explore the mechanism of AD and has been the gold-standard-beta-amyloid dogma for almost 30 years [48] and has become one of the most dominant research focuses conducted in academia and the pharmaceutical industry.
The typical hallmarks of AD-synaptic dysfunction and senile plaques—are consequences of the production, oligomerization and self-aggregation of beta-amyloid (Aβ). Amyloid precursor protein (APP) is degraded via the non-amyloidogenic (catalyzed by α-secretase and γ-secretase) and amyloidogenic pathways, where the degradation by β-secretase (BACE-1) and γ-secretase generate Aβ species. The Aβ species are composed of 37–49 amino acid residues, with the major species being Aβ40, whereas the major type from the minor species is Aβ42. Aβ40 is the more common metabolite and may actually be anti-amyloidogenic [49], whereas Aβ42 and other longer peptides are highly self-aggregating and lead to profound Aβ deposition [50,51]. Studies of AD-causing mutations in APP, presenilin 1 (PSEN1), and presenilin 2 (PSEN2) genes demonstrate that the vast majority of these mutations alter APP processing in a manner that either increases the absolute or relative levels of Aβ42 [52].
The key histopathological hallmark of AD is the senile plaque-intracellular neurofibrillary tangles in the brain. Tangles are composed of paired helical filaments and straight filaments which are mainly caused by hyperphosphorylated tau protein [53]. The tau protein hypothesis of AD is based on hyperphosphorylation of the tau protein (from the 2–3 to the 5–9 phosphate groups) by the threonine-serine kinase, GSK-3β. Such hyperphosphorylated tau protein is separated from microtubules and subsequently aggregates into insoluble intracellular neurofibrillary tangles which ultimately cause cell death [54,55]. GSK-3β is a pivotal kinase in neurodevelopment and involved in both physiological and pathological aging. In the non-amyloidogenic pathway, GSK-3β may down-regulate the activity of the α-secretase complex through inhibition of metalloproteinase (ADAM) activity [56] and regulates Aβ production by interfering with APP cleavage at the γ-secretase complex [57]. In turn, in the amyloidogenic pathway, GSK-3β inhibition reduces BACE1-mediated cleavage of APP through a nuclear factor kappa-light-chain-enhancer of an activated B cell (NF-κB) signaling-mediated mechanism. This observation thus suggests that the inhibition of GSK-3β reduces Aβ pathology [58,59,60] and GSK-3β plays a key role in choline metabolism, which involves the regulation of choline acetyltransferase (ChAT) and AChE [61,62]. Further, GSK-3β has the capacity to phosphorylate several mitogen-activated protein kinases (MAPKs), thus regulating axonal stability. Inhibitors of GSK-3β provide protection from intrinsic apoptotic signaling, but potentiate that of extrinsic apoptosis [63].
GSK3β mediates an interaction between two major forms of synaptic plasticity in the brain, N-methyl-d-aspartate (NMDA) receptor-dependent long-term potentiation (LTP) and NMDA receptor-dependent long-term depression (LTD). LTP and LTD of hippocampal synaptic transmission represent the principal experimental models underlying learning and memory. In mouse models of AD, early impairments in synaptic transmission were caused, among other factors, by Aβ, which leads to impairment of LTP via tau protein [64]. In the normal brain, activation of GSK3β is essential for NMDA receptor-dependent LTD, and its activity can be regulated by LTP. Following the induction of LTP, there is inhibition of GSK3β activity, whereas GSK3β inhibitors block the induction of LTD. In addition, GSK-3β has been identified as a prominent regulator of inflammation via the promotion of the production of proinflammatory cytokines (interleukin-6(IL-6), IL-1β) and tumor necrosis factor (TNF), or by decreasing the production of the anti-inflammatory cytokine, IL-10 [65,66]. To conclude, GSK-3β inhibition is a popular target for small molecule compounds, mainly based on the three therapeutic approaches, namely the inhibition of phosphorylation, prevention of the aggregation of tau protein, and stabilization of microtubules.
NMDA receptor plays a crucial role in modifying major forms of synaptic plasticity, certain types of learning and memory formation, as well as consolidation of short-term memory into long-term memory under physiological conditions [67]. The glutamatergic (NMDA) hypothesis of AD is based on the observation that inhibition of the NMDA receptor would ameliorate the overall condition of AD patients. However, NMDA cannot be fully antagonized since it exerts important bio-functions in normal synaptic transmission, whereas glutamate-related excitotoxicity and cell death could be caused when NMDA receptors are overstimulated by excess glutamate [68]. Thus, establishing an optimal balance between glutamate stimulation and glutamate-related excitotoxicity is crucial to achieve the most effective treatment of AD.
Other targets for AD are the monoamine oxidase (MAO) enzymes, a group of enzymes consisting of two distinct isoforms (MAO-A and MAO-B) which, by deamination, lead to the metabolism of amine neurotransmitters (e.g., monoamine neurotransmitters). In AD patients, the activity and gene expression of MAO-A is up-regulated in different brain areas [69,70] as well as MAO-B [71]. High levels of MAOs catalyze oxidative deamination, increasing the production of hydrogen peroxide and reactive oxygen species (ROS), which are responsible for oxidative injuries and the toxic environment characteristic of neurodegeneration [72,73] and increased MAO-B levels can enhance astrogliosis in the brain [74,75].
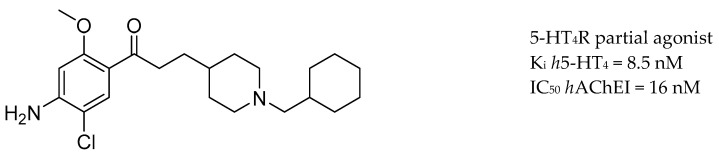
AD may be implicated by high levels and dysregulation of Cu2+, Fe2+, Zn2+, and Ca2+, which are important biometal ions [76,77]. Cu2+ and Zn2+ are known to induce the generation of toxic Aβ oligomers by binding to Aβ peptides and influencing the Aβ aggregation pathway [78,79], the redox-active metals, Cu(I/II) and Fe(II/III) generate cytotoxic reactive oxygen species (ROS) [80]. Thus, the use of biometal chelators that can down-regulate high levels of biometals could be a potential therapeutic strategy for the treatment of AD. The serotoninergic neurotransmitter and histaminergic systems play a critical role in the regulation of the CNS. Brain functions mediated by 5-HT4R and 5-HT6R require a synergistic effect from cholinergic neurotransmission. Activation of 5-HT4R can enhance the release of ACh in the hippocampus, whereas 5-HT6R blockade enhance cholinergic neurotransmission [81,82]. Moreover, 5-HT4R agonists could promote the nonamyloidogenic cleavage of APP, which releases a soluble sAPPα fragment which, in contrast to Aβ, has putative neurotrophic and neuroprotective properties [83]. The selective partial 5-HT4 agonist, RS-67333, which is a potent cognitive and learning function enhancer, may reduce Aβ production and has a neuroprotective activity in a cellular model of AD [84,85,86]. Activation of the histamine H3 receptor decreases the presynaptic release of ACh, and its blockade augments the presynaptic release of Ach, resulting in improved cholinergic neurotransmission in the cortex. However, in clinical trials, the H3 receptor antagonists failed to achieve cognitive improvement in AD patients [87].
Impaired signaling pathways of cyclic-3′,5′- adenosine monophosphate (cAMP) and cyclic-3′,5′-guanosine monophosphate (cGMP) may contribute to the development and progression of AD. Thus, phosphodiesterase inhibitors (PDEIs), such as rolipram and roflumilast (PDE4Is), vinpocetine (PDE1I), cilostazol and milrinone (PDE3Is), sildenafil and tadalafil (PDE5Is) were found to be involved in the phosphorylation of tau, aggregation of Aβ, neuroinflammation as well as regulation of cognition, mood, and emotion processing. Despite rational arguments, the clinical data do not demonstrate efficacy of selective PDEIs in improving cognition in patients with prodromal and mild AD, and a number of these have been discontinued due to failure to meet efficacy endpoints in a phase I/II clinical trial [88,89,90]. However, the combination therapy of donepezil with cilostazol showed positive effects on patients with mild or moderate-to-severe Alzheimer’s patients [91,92]. To conclude, there are many biological targets and signalling pathways involved in AD pathology [93,94]. However, the complex interactions between them is unclear.
3. Multi-Target Strategy for AD
The complex nature of AD has led to the development of a multitarget approach in the design and development of new potential anti-AD drugs. At present, the MTDLs strategy can be divided into two main categories based on the biological targets, namely, involving AChE or distinct to AchE. The first category is mainly based on inhibitors of AChE/BuChE and β-amyloid aggregation. The success of memoquin and ferulic acid-memoquin hybrids (Figure 3), which can inhibit both AChE- and self-induced Aβ aggregation, open exciting new opportunities for MTDLs drug discovery [95,96,97].
Figure 3.
Memoquin and 17d, the most active ferulic acid-memoquin hybrid, from [97].
The MTDLs involving AChE consist mainly of tacrine- or donepezil-related compounds. Tacrine and donepezil are non-competitive and reversible ChE inhibitors and alleviate neuronal degeneration caused by damage of cholinergic transmission. On the other hand, AChE has become to be regarded as an inducer to trigger the aggregation of Aβ. Interaction of a peripheral anionic site of AChE with Aβ could facilitate fibril formation. Blocking the peripheral anionic site of AChE has been identified as an effective method for inhibition of Aβ aggregation [98]. The simultaneous binding to a catalytic active and a peripheral anionic side of AChE (dual-site inhibitors) is an example of successful adaptation of an MTDLs design strategy applied in the most prevalent and dominant class of tacrine hybrids. However, various donepezil-based cholinesterase inhibitors could also show additional inhibition of Aβ aggregation, oxidative stress, and MAOs. MTDLs based on tacrine and donepezil scaffolds are described in Table 2.
Table 2.
Examples of MTDLs based on tacrine and donepezil scaffolds.
| Scaffold | Lead Fragment | Targets | Ref. |
|---|---|---|---|
| Tacrine | xanomeline, iperoxo-fragment | AChE Is and MRs | [99] |
| valmerin | AChE Is and GSK-3α/βIs | [100] | |
| deferasirox | AChE Is and metal chelators | [101] | |
| indole-3acetic acid | AChE/BuChE Is | [102] | |
| pulmonarin B | AChE/BuChE Is | [103] | |
| d-xylose, d-ribose, d-galactose | AChE/BuChE Is | [104] | |
| acridine | AChE/BuChE Is | [105] | |
| 1,2,3-thiadiazole | AChE/BuChE Is | [106] | |
| ferulic acid | AChE/BuChE Is | [107] | |
| flavonoid quercetin | AChE/BuChE Is and metal chelators | [108] | |
| quinolone carboxylic acids | AChE/BuChE Is, M1R Is | [109] | |
| Donepezil | hydroxyphenyl-benzimidazole | AChE Is and metal chelators | [110] |
| hydroxybenzimidazole | AChE Is and metal chelators | [111] | |
| butylated hydroxytoluene hybrids | AChE Is, MAO-B Is, antioxidants | [112] | |
| trolox | AChE Is, MAO-B Is, metal chelators | [113] | |
| chromone | AChE Is, MAO-B Is | [114,115] | |
| chalcones | AChE/BuChE Is | [116] | |
| 2-acetylphenol | AChE/BuChE Is, MAO-A/B Is, metal chelators |
[117] | |
| deoxyvasicinone | AChE Is and BACE-1 Is | [118] | |
| introduction of amide bonds | AChE Is and BACE-1 Is | [119] | |
| conjugation of benzylpiperidine moiety benzimidazole or benzofuran | AChE Is, antioxidant, metal chelators |
[120] | |
| melatonin | AChE/BuChE Is, antioxydants, metal chelators | [121] |
3.1. AChE and BACE-1 Inhibitors
The analysis of the SciFinder database showed that there were almost 567 articles (original and review) related to combining the functionality of AChE drugs and BACE-1 inhibitor pharmacophores over the last decade. MTDLs which contain a pharmacophoric moiety derived from tacrine are potent BACE-1 inhibitors, inhibitors of tau-protein aggregation and possess additional properties (antioxidative, neuroprotective or metal chelating ability) [122,123,124,125,126,127] as well as another major chemical group consisting of donepezil-related compounds [121,128,129,130,131,132,133,134,135,136]. Moreover, miscellaneous compounds based on the cyanopyridine, quinazoline, quinoline, benzo-chromene, pyrimidinimine, and thiazole cores are inhibitors of both AChE and β-amyloid aggregation and some of them possess antioxidative properties and inhibitory potency against BACE-1 [137].
A family of tacrine and 4-oxo-4H-chromene hybrids was reported by Fernandez-Bachiller and co-workers. The most promising compound (Figure 4) exhibited potent inhibition activity against human AChE and BACE-1, antioxidant activity (1.3-fold more potent than a vitamin E analogue–trolox) and good CNS permeability [138].
Figure 4.
The most active tacrine and 4-oxo-4H-chromene hybrid from [138].
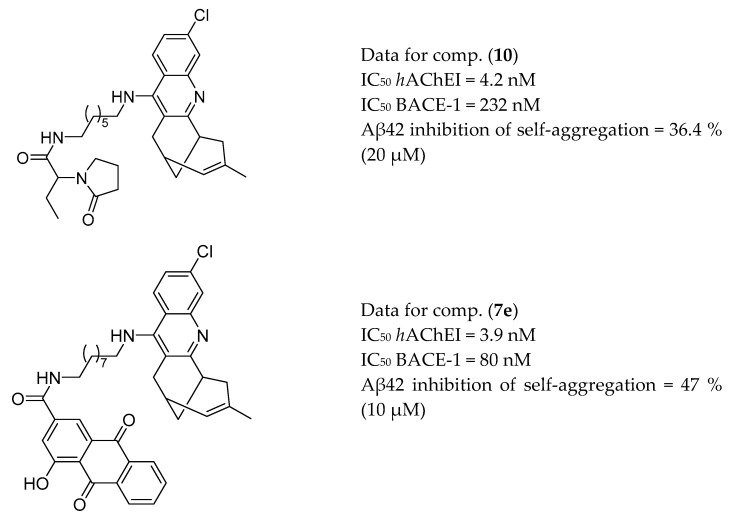
Muñoz-Torrero et al. synthesized a series of heptamethylene-linked levetiracetam-huprine and levetiracetam-6-chloro-tacrine hybrids with potent inhibitory activities against human AChE and BACE-1 and Aβ42 anti-aggregating activity. The most interesting compound, (10, Figure 5), reduced the frequency of spontaneous convulsions, prevented memory impairment and reduced the Aβ burden in the cortex of APP/PS1 mice [125]. The presented hybrid protects transgenic mice from cognitive deficits and, thereby, can be considered a multifunctional disease-modifying anti-Alzheimer agent. Additionally, the group has reported on second-generation anti-AD rhein-huprine hybrids with potent inhibitory activities against human AChE and BACE-1 and Aβ42 anti-aggregating activity [139]. The most potent compound (7e, Figure 5), after intraperitoneal administration in APP-PS1 transgenic mice, showed a central effect in lowering soluble Aβ [140].
Figure 5.
The most active levetiracetam-huprine and rhein-huprine hybrids from [125,140].
Zha et al. designed and synthesized novel tacrine–benzofuran derivatives containing amino or amido linkages. Most hybrids exhibited good inhibitory activities on AChEs and β-amyloid self-aggregation. The most promising compound (2e, Figure 6) showed an interesting profile as a subnanomolar selective inhibitor of hAChE and a good inhibitor of both β-amyloid aggregation (hAChE- and self-induced, 61.3% and 58.4%, respectively) and hBACE-1 activity. This hybrid also exhibited lower hepatotoxicity than tacrine and in vivo studies confirmed its ability to penetrate the BBB. Next, in scopolamine-induced memory impairment studies in knock-out mice, the hybrid significantly ameliorated memory performance of mice in a Morris water maze test [126].
Figure 6.
The most active tacrine–benzofuran hybrid from [126].
3.2. AChE and GSK-3β Inhibitors
Two important AD-related targets influencing both ACh concentration modulation and tau protein phosphorylation, are AChE and GSK-3β. MTDLs possessing inhibitory potency for both enzymes have been rarely reported so far. Potent AChE and GSK-3β dual-target inhibitors were obtained by hybridizing the pharmacophores of GSK-3βI with AChEI. Thus, tacrine was incorporated at the thiazolyl ring of potent GSK-3βI with an appropriate linker. The most promising hybrid showed the most interesting profile as a nanomolar dual enzyme inhibitor, good inhibitory effect on Aβ self-aggregation, inhibition of tau protein hyperphosphorylation, and significant in vivo cognitive improvement in mice tests [141]. Hui et al. linked tacrine (AChEI) with phenothiazine via an alkylenediamine-type spacer. Phenothiazine is a core of methylthioninium chloride, also known as methylene blue or Rember®, which has been shown to reduce tau levels in vitro and in vivo in several different mechanisms of action [142,143]. In addition, an appropriate linker containing a 1,2,3-triazole moiety for two scaffolds, tacrine and valmerin (GSK-3α/β), was designed based on molecular docking and crystallography [100] (Figure 7).
Figure 7.
Tacrine and valmerin hybrids from [100].
3.3. AChE and MAO Inhibitors
MTDLs strategies involving the AChE and MAOs targets in the SciFinder data base showed over 400 articles (original and review) related to combining the functionality of AChE drugs and MAOIs [144,145,146,147,148,149,150,151]. Ladostigil (Figure 8) was designed on the basis of the structures of rivastigmine and rasagiline. However, clinical trials revealed that the drug failed in its primary endpoint of curbing progression from mild cognitive impairment to AD [152,153]. Homo isoflavonoid Mannich-based derivatives, the selective dual inhibitors of AChE and MAO-B, exhibited Aβ(1–42) aggregation inhibitory efficacy with antioxidant activity and biometal chelating ability [154].
Figure 8.
Ladostigil.
3.4. AChE Inhibitors and NMDA Antagonists
The degenerative process of cholinergic neurons in AD are implicated by excessive activation of the NMDA receptor. Thus, NMDA receptor antagonists can confront neurodegeneration and AChEIs can recover memory and cognition. Namzaric was approved in 2015 for the treatment of AD, using a once-daily fixed-dose drug combination comprised of memantine hydrochloride and donepezil hydrochloride. The first rationally-designed multifunctional ligand, carbacrine, was obtained by integrating synergistic fragments of the chloro-substituted tetrahydroacridine moiety of 6-chlorotacrine and carbachol as the starting lead components [155]. Carbacrine showed dual inhibitory activity against AChE and the NMDA receptor, blocking in vitro Aβ self-aggregation and aggregation mediated by AChE, antagonizes NMDA receptors, and reduces oxidative stress [156] (Figure 9).
Figure 9.
Carbacrine.
More recently, a new class of multi-target compounds that combine the structures of galantamine and memantine have been designed. The most potent drug candidate, memagal (Figure 10), showed a remarkable inhibitory potency against AChE and NMDA receptor inhibition which was tested by a [3H] MK-801 binding assay.
Figure 10.
Memagal.
Optimization of the dimebion structure (a multi-target antihistamine drug) led to compounds with a multi-target profile against both the AChE and NMDA receptors and self-induced Aβ aggregation inhibitory potency [157]. Makhaeva and co-workers fused dimebon with phenothiazine, and obtained compounds with dual BChE and NMDA receptor-inhibition potency [158].
3.5. AChE Inhibitors and 5-HTR
Modulating neurotransmission of ACh and 5-HT could be achieved through 5-HT4 and 5-HT6 receptors (5-HTR). Activation of 5-HT4R can enhance the release of ACh in the hippocampus, 5-HT4R agonists can promote the nonamyloidogenic cleavage of APP, forming neurotrophic human soluble amyloid precursor protein α (sAPP-α) fragments and decrease Aβ secretion in primary neurons [81]. Moreover, 5-HT6R antagonists are thought to have the ability to enhance cholinergic neurotransmission [82,159,160]. RS67333, a partial 5-HT4R agonist well-known for its precognitive effect, showed a synergistic effect with donepezil and lowered Aβ levels by directly inhibiting the activity of AChE [161]. In addition, the key pharmacophores of RS67333 and donepezil were merged into a single novel chemical entity, leading to the discovery of donecopride [86], a dual-action AChEI and 5-HT4 receptor agonist. Donecopride (Figure 11) stimulates the nonamyloidogenic 5-HT4 receptor-mediated cleavage of APP and has a greater potency in promoting neurotrophic sAPP-a release compared with RS67333.
Figure 11.
Donecopride.
3.6. AChE Inhibitors and H3R
The H3 histamine receptors (H3R) regulate the release of histamine, acetylcholine and other monoamines. Activation of H3R decreases the presynaptic release of ACh, and its blockade augments the presynaptic release of ACh and improves cholinergic neurotransmission in the cortex. Evaluation of H3R antagonists or inverse agonists revealed their promnesic properties and ability to modulate learning by improving memory consolidation [162]. One of these antagonists or inverse agonists of H3R, pitolisant, has been approved for clinical use in patients with narcolepsy [163,164]. Thus, synergistic effects on up-regulating synaptic levels of ACh could be achieved by involving AChE and H3R. Bajda et al. applied docking-based virtual screening for novel multifunctional compounds in a non-imidazole histamine H3R ligand library. The most promising derivatives combined the flavone moiety via a six-carbon atom linker with a heterocyclic moiety, such as azepane, piperidine or 3-methylpiperidine (Figure 12). Compound 17 showed the highest inhibitory activities toward cholinesterases as well as well-balanced potencies against H3R and both ChE enzymes [165]. Huang and co-workers reported a series of compounds with a quinoxaline scaffold that showed related inhibitory activities against AChE, H3R, and BACE-1 targets [166] (Figure 12).
Figure 12.
3.7. AChE Inhibitors and Biometal Chelators
Starting with the hypothesis that biometal chelators that can down-regulate high levels of biometals could form a potential therapeutic strategy for the treatment of AD, Fernandez-Bachiller and co-workers reported a hybrid fused with tacrine and the metal chelator drug, clioquinol (Figure 13) [167].
Figure 13.
Hybrid of tacrine-clioquinol.
In turn, hybrids of tacrine and a flavone, namely coumarin, chromone or donepezil, and curcumin, exhibited a significant ability to inhibit AChE. Additionally, hybrids of tacrine and a flavone, such as coumarin, chromone or donepezil and curcumin, exhibited significant β-amyloid-reducing and metal (Cu2+ and Fe2+) chelating properties [168,169,170] (Figure 14).
Figure 14.
Hybrids of tacrine-flavone and donepezil-curcumin from [168,169,170].
Wichur et al. designed and synthesized novel 1-benzylpyrrolidine-3-amine-based BuChE and BACE-1 inhibitors with activities towards Aβ and tau protein aggregation, as well as concomitant antioxidant and metal-chelating properties [171] (Figure 15).
Figure 15.
Inhibitor of BuChE with antioxidant and metal-chelating properties from [172].
3.8. BACE-1 and GSK-3β Inhibitors
Even though the importance of AChE in the AD signalling network is prominent, MTDLs strategies are also under consideration in the treatment of specific and multiple protein pockets of AD-associated targets. Anti-amyloid based strategies not only focus on Aβ alone, but also on effective modulation of the robust network of Aβ-mediated events. Thus, dual inhibitors of BACE-1 and GSK-3β show promise as drug candidates based on the inhibition of Aβ and tau protein aggregation [172] (Figure 16).
Figure 16.
Oligo heteroaromatic hybrid with BACE-1 and GSK-3β inhibitory activity from [172].
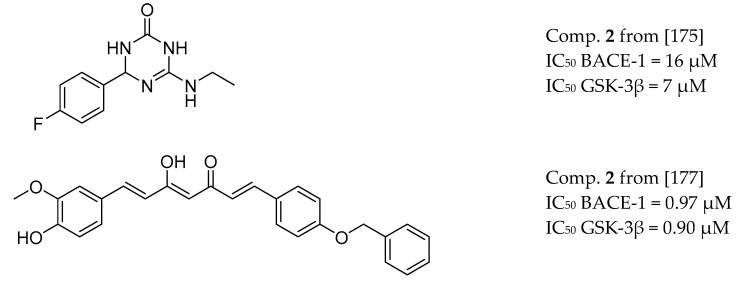
Table 1. GSK-3β dual inhibitors was obtained based on a 3,4-dihydro-1,3,5-triazin-2(1H)-one skeleton. Subsequently, triazinones (6-amino-4-phenyl-3,4-dihydro-1 and 3,5-triazin-2(1H)-ones), with a critical pharmacophore comprising the cyclic amide group and guanidino motif, have been shown to constitute a promising class of multifunctional fragments able to modulate tau and amyloid cascades simultaneously (Figure 17) [173]. Bottegoni et al. reported an optimized virtual screening method to identify fragments with multifunctional activities against BACE-1 and GSK-3β [174]. Another approach described a series of dual inhibitors targeting BACE-1 and GSK-3β, based on curcumin derivatives [175] (Figure 17).
Figure 17.
BACE-1 and GSK-3β inhibitors.
3.9. Other Targets
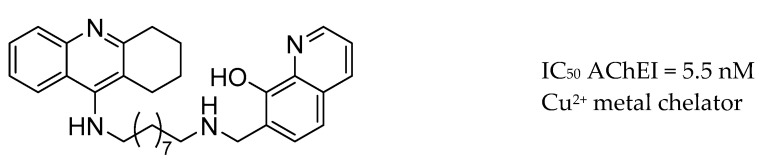
The approved antifungal drug, clioquinol, with an 8-hydroxyquinoline scaffold, was shown to extract metal ions from extracellular Aβ aggregates. Thus, a series of novel hybrids fused with the main pharmacophores of selegiline and clioquinol, showed MAO-B inhibitory potency, antioxidant activity, biometal chelating ability, and effective inhibition against Cu(II)-induced Aβ aggregation [176].Clioquinol was then fused with the PDE9A inhibitor, PF-04447943, and the resulting hybrid showed inhibitory potency against PDE9 with high selectivity over other PDEs, notable Cu2+-induced Aβ aggregation inhibition, and favorable blood–brain barrier (BBB) permeability [177]. New perspectives were presented by novel hybrids endowed with anti-inflammatory and anticholinesterase activity via triple-targeting properties. Hybrids obtained through the merger of triazoles and thiosemicarbazides simultaneously impact on cholinesterases, cyclooxygenase-2 (COX-2) and 15-lipoxygenase (15-LOX), and have emerged as promising new hits [178].
Epigenetics involves the study of heritable and reversible changes in gene expression that cannot be explained by changes in the sequence of bases in genomic DNA but through covalent modifications to the cytosine residues of DNA, covalent chemical modifications in histones and chromatin remodeling, and noncoding RNAs. Such transcriptional dysregulation plays an important role in the progression and development of AD mainly through transcription of immediate early genes (IEGs), important for neuronal plasticity, memory and behavior [179,180]. Thus, there are interesting targets concerning epigenetics, especially lysine (K)-specific demethylase 1A (KDM1A or LSD1) and histone deacetylase (HDAC). KDM1A is a flavin adenine dinucleotide (FAD) dependent amine oxidase that acts primarily as a histone demethylase and has been implicated in the control of IEG transcription. HDAC is involved in the deacetylation of histone proteins which promote heterochromatin, leading to silenced gene transcription and expression. KDM1A and HDAC inhibitors have a promising therapeutic potential in recovering memory defects and cognitive disabilities through eliminating these diverse molecular and cellular functions of HDAC enzymes in the brain.
Vafidemstat (ORY-2001) (Figure 18), an oral, brain penetrating, dual KDM1A/MAO-B inhibitor active at doses suitable for long-term treatment, corrects memory deficit in the Senescence Accelerated Mouse Prone 8 (SAMP8) model for accelerated aging and Alzheimer′s disease. Moreover, multiple genes modulated by ORY-2001 are differentially expressed in late onset Alzheimer’s disease. Vafidemstat is currently in multiple Phase IIa studies [181].
Figure 18.
Vafidemstat (ORY-2001).
Cuadrado-Tejedor et al. reported CM-414, a first-in class small-molecule that acts as a dual inhibitor of HDAC and PDE5 (Figure 19). Inhibition of PDE5 induces an increase in the activation of cAMP/cGMP- responsive element-binding protein (CREB) which combined with moderate HDAC class I inhibition, leads to efficient histone acetylation. CM-414 rescued the impaired long-term potentiation evident in hippocampal slices from APP/PS1 mice, diminished brain Aβ and tau phosphorylation (pTau) levels in chronic treatment of Tg2576 mice [182].
Figure 19.
CM-414—first-in class dual inhibitor of HDAC and PDE5.
The combined treatment of GSK-3β and HDAC inhibition induces synergistic neuroprotective effects in various in vitro and in vivo models of neurological diseases. De Simone et al. discovered the first-in-class GSK-3β/HDAC dual inhibitor as disease-modifying agent for AD (Figure 20). Compound 11 induces an increase in histone acetylation and a reduction of tau phosphorylation. Moreover, it promotes neurogenesis and displays immunomodulatory effects. It is nontoxic and protective against H2O2 and 6-OHDA stimuli in SH-SY5Y and in CGN cell lines, respectively [183].
Figure 20.
GSK-3β/HDAC dual inhibitor [184].
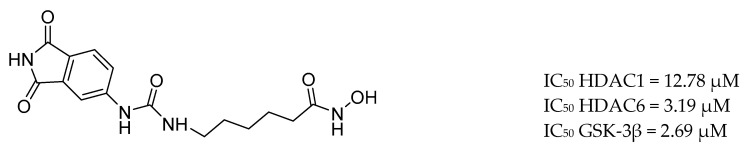
Transglutaminase 2 (TG2) is the most studied and expressed isoform of the TG family which is important in cross-linking proteins in Alzheimer’s disease. Next, the genetic elimination of TG2 in Huntington’s disease (HD) mice models ameliorated the symptoms and restored the message and protein levels of brain-derived neurotrophic factor (BDNF). Moreover, the inhibition or genetic suppression of TG2 halts oxidative stress-induced cell loss in cortical neurons [184,185]. Thus, neuroprotective effects can be achieved by the TG2 and HDAC inhibition, which synergistically protects against toxic stimuli mediated by glutamate. The most active compound (3) [(E)-N-hydroxy-5-(3-(4-(3-oxo-3-(pyridin-3-yl)prop-1-en-1-yl)phenyl)thioureido)pentanamide] inhibit TG2 and HDACs both in vitro and in cell-based assays (Figure 21). Furthermore, this nontoxic compound protects cortical neurons against toxic insults induced by glutamate [185].
Figure 21.
Dual TG2 and HDAC inhibitor from [185].
In summary, a multitarget strategy for AD is emerging, involving a broad spectrum of potential therapeutic strategies from modulating neurotransmission, tau-based therapies, amyloid based therapies, modulating intracellular signalling cascades, oxidative stress reduction, modulation of cellular calcium homeostasis, to anti-inflammatory therapy. Research in the field of application of MTDLs strategy in the field of AD has been extensively reviewed elsewhere [118,186,187,188].
4. Physicochemical Properties of MTDLs for AD
MTDLs are designed by combining two (or more) pharmacophoric structural scaffolds, thus physicochemical properties such as molecular weight, solubility, permeability and the ability of MTDLs to permeate cell membranes have to optimized for absorption and distribution within the body [189]. The ‘Lipinski rule of five’ (RO5) [190] defined the optimal physicochemical properties for perorally administered drugs, which are preferred for perspective long-term treatment of AD patients. RO5 postulated desirable physicochemical property space by setting up limits for molecular weight < 500Da, logarithm of partition coefficient (logP; a value classifying the lipophilicity of a compound) < 5, number of hydrogen bond donors (HBDs)< 5, and number of hydrogen bond acceptors (HBAs)< 10. Although RO5 indicates possibility of absorption of drug after oral administration, the rule is not specific for CNS drugs. It turns out that AD drugs need to fulfil strict physicochemical criteria defined for blood–brain barrier (BBB) penetration by transcellular passive diffusion, which is typical for most CNS drugs. Blood-to-brain influx is mediated by the receptor for advanced glycation end products (RAGE). The BBB efflux pumps are transmembrane P-glycoprotein (P-gp), which mediate brain-to-blood transport. Cordon-Cardo et al. first suggested that P-gp might play a role in vivo in limiting brain penetration of xenobiotics [191]. The high expression of P-gp is one of the main reasons that a lot of lipophilic drugs could not penetrate CNS for brain disease therapy.
Extensive studies on physicochemical properties for CNS drugs laid out that molecular weight should not exceed 430–450 Da, logP values should be < 4 and the number of HBD and HBA must be < 2 and < 7, respectively [192]. Next, characterisation has been completed with topological polar surface area (TPSA), Log D and acid dissociation constant (pKa). TPSA counts the surface sum over all polar atoms in molecule, and for most CNS drugs to be < 70 Å2. LogD is a pH dependent lipophilicity indicator displaying the distribution of a chemical compound between the lipid and aqueous phase. For CNS drugs logD is expected to be <3. The strength of acidic functional groups in the molecule expressed by pKa values for CNS drugs should be 7–9. For streamlining the design of proper physicochemical properties of MTDLs for AD, several powerful predicting techniques, such as BBB-score and the CNS multiparameter optimization approach, have been developed. However, the most of MTDLs have been tested in a parallel artificial membrane permeation assay (PAMPA), which experimentally determine permeability values which would enable them to cross the BBB by passive diffusion [125,127,132,193,194]. The current chemical methods for crossing the BBB could be divided into chemical modification of the drug to form a prodrug, coupling the drugs with mannitol or aromatic substances or using appropriate chemical drug delivery system or drug carrier with the ability to cross BBB [195].
5. Perspectives for MTDLs in the Treatment of AD
At present, only symptomatic treatments exist for AD, namely 3 cholinesterase inhibitors and memantine, which mainly block the progression of the disease and are supposed to interfere with the pathogenic steps responsible for the clinical symptoms. AD is a complex disease, thus the rational way for searching for pathway-target-drug-disease relationships seemed to be a genome-based analysis [196,197]. Kwok et al. [198] used a gene-based test for the genetic validation of potential AD drugs, to provide an initial screening tool for the identification of drugs that are unlikely to be successful. This study, however, provides no evidence that any approved or investigational AD drugs target products of genes strongly associated with late-onset AD, which might explain the lack of efficacy to date.
MTDLs toward AD have to confirm their simultaneous multitarget engagement in both in vitro and in vivo assay systems. Thus, the use of cellular models (cell-screening systems) of AD, based on human induced pluripotent stem cell (hiPSCs) technologies could change preclinical research [199]. In addition, the animal models currently available cannot fully reflect the multifactorial nature of human AD for multiple ligand testing. Age-dependent neurodegeneration mouse models that mimic AD rely mostly on the neuronal overexpression of human proteins carrying a familial AD-causing mutation in presenilin, which increases Aβ42 production and oligomerization [200,201]. In fact, with respect to neurodegenerative diseases generally, mouse mutations corresponding to human disease-linked mutations rarely result in neurodegenerative phenotypes, mostly because age is the single greatest risk factor for neurodegeneration, and mice have much shorter lifespans than humans.
Up to 2019, over 2000 clinical trials in AD drug development had been reported in which various hypotheses for AD were tested. Liu et al. [25] reported that the amyloid hypothesis was the most heavily tested (22.3% of trials), with the neurotransmitter hypothesis being the second most tested (19.0% of trials). In turn, a systematic review of the AD drug development pipeline in 2019, showed that there were 132 agents in clinical trials and 90 agents in trials targeting cognitive enhancement, and a further 14 trials intended to treat neuropsychiatric and behavioural aspects of AD [202]. Results suggested that there is a conceptual supremacy for disease-modifying therapies, as opposed to symptomatic-disease approaches [48]. From 96 agents reported in disease modification trials, 40% have amyloid as the primary target, or as one of several effects, whereas seven small molecules and 10 biologics have tau as a primary or combination, target (18%). Until recently, AD drug development has been largely focused on beta amyloid plaques and tau tangles in the brain. These attempts have yielded less successful results than had been anticipated. Currently, some of the most novel non-amyloid approaches were put to the discovery and development of drugs for Alzheimer’s and related dementias. Firstly, repurposing an existing drug accelerates the drug development timeline. An example of this is the repurposing of a Parkinson’s drug, rasagiline, that holds promise for slowing the progression of Alzheimer’s disease in patients with mild cognitive impairment [203]. In addition, new drug candidates could restore lost cognitive function and lead to neuroprotective therapies for AD and other forms of dementia [204].
The diversification of targets and the entry of combination therapies into the potential therapeutic pipeline is particularly noticeable between phase 3 and 2 of clinical trials. The using of new biomarkers allow early assessments of the impact of candidate interventions on disease biology [202], however there is lack of accurate biomarkers to identify and track the progression of AD, which has slowed therapeutic development in tauopathies.
So far, a wide range of new biological targets are under consideration as microglial targets (Table 3). Microglia are a class of innate immune cells (macrophages) within the CNS. Microglia fulfil a number of varied roles within the CNS including the immune response, maintenance of homeostasis, extracellular signalling, phagocytosis, antigen presentation and synaptic pruning [205]. In AD, microglia reaction was initially thought to be incidental and triggered by Aβ deposits and dystrophic neurites. Moreover, recent genome-wide association studies have established that the majority of AD risk loci are found in or near genes that are highly and sometimes uniquely expressed in microglia [206]. On the other hand, a multitude of independent sources have suggested that neuroinflammation contributes to the pathogenesis of AD. Therefore, a hypothesis has been proposed that microglia could be critically involved in the early steps of AD and microglial targets are now being considered as the next important potential therapeutic targets.
Table 3.
| Pathway | Targets | Evidence |
|---|---|---|
| Purinergic signalling | -ionotropic P2XRs -metabotropic P2Y -adenosine P1Rs -P2YR-dependent calcium signaling |
-P2 × 7R is up-regulated in AD -P2Y2R protective role -P2Y6R—small molecule from GliaCure claims to promote microglial phagocytosis through binding of the microglial purinergic P2Y6 receptor [209] -P2Y12 plays an important role in homeostatic microglia |
| NOD-like receptor family pyrin domain containing 3 (NLRP3) | -triggers TREM2 receptor expressed in myeloid cells 2 | -NLRP3 inflammasome activation is a pathophysiological pathway in AD |
| Toll-like receptors (TLRs) |
-TLR2 -TLR4 |
-triggers the neuroinflammatory response -downstream TLR signalling through NFκB, activator protein 1 and IFN regulatory factor (IRF) pathways lead to proinflammatory gene transcription |
| microglial fractalkine receptor | -CX3CL1/CX3CR1 | -CX3CL1 exerts an inhibitory signal, maintaining microglia in a resting state |
| Receptor- interacting serine/threonine-protein kinase 1 (RIPK1) |
-RIPK1 inhibitor | -enzyme downstream of TNFα signalling, has been shown to mediate microglial responses in AD |
It is also worth mentioning the innovative nanotechnology-based approaches in the treatment of AD, an approach that is seen as a great hope for developing new treatment strategies. The achievements of nanotechnology are particularly encouraging because they provide many benefits while overcoming the limitations of conventional formulations.
Recently, Chen et al. [210] reported the preparation of a methylene blue loaded multifunctional nanocomposite. The constructed nanocomposite has ultra-small ceria nanocrystals and iron oxide nanocrystals assembled onto the surface of mesoporous silica nanoparticles, methylene blue loaded into its pores and, additionally, a tau tracer, Amino-T807, grafted onto the surface. The adopted strategy allowed for the achievement of a promising synergistic effect as a result of ROS scavenging ability of ultra-small ceria nanocrystals, ameliorating mitochondrial oxidative stress-induced damage and use of a tau aggregation inhibitor, methylene blue, which could be released in neurons to prevent hyperphosphorylated tau aggregation. On the other hand, Guo et al. [211] designed and optimized a novel fusion peptide (TPL) comprising a BBB-penetrating peptide, TGN, and a neuron binding peptide, Tet1, through a four-glycine linker. Interestingly, TPL-modified nanoparticles led to an increase in BBB-penetration and neuron targeting efficacy, in comparison to the nanoparticles co-decorated with the two mono-ligands. Moreover, after the encapsulation of a neuroprotective peptide, NAP, the TPL nanoparticles reduced the intracellular ROS, showed protection of microtubules against Aβ25-35-induced toxicity, and rescued the OA-induced tau aggregation and neuronal apoptosis.
Another interesting study concerns the development of a polymeric micelle drug delivery system consisting of the RAGE targeting peptide (Ab) derived from Aβ protein, an amphiphilic polymer with ROS responsiveness and scavenging ability, and curcumin, a natural compound which has been described to target to Aβ aggregation [212]. Such a type of nanosystem has the ability to accumulate in the AD brain and initiate subsequent microglia-based microenvironment modulation. More importantly, it is possible that multiple targets within the AD microenvironment could be controlled to reeducate the hyperactive microglia and protect damaged neurons. The examples listed above confirm the need for further intensive studies to obtain better clinical translation of multifunctional nanosized drug delivery systems.
6. Conclusions
The increasing number of potential biological targets for AD raises the question of how to find meaningful combinations of targets for an MTDLs approach. A pioneering study of a human pharmacology interaction network revealed relationships between proteins in chemical space and assessed the polypharmacology profile of ligands [213]. Next, a computational model to map the polypharmacology network was assembled by connecting targets according to the structural similarity of their binders (similarity ensemble approach, SEA) [214]. Thus far, the computationally driven multitarget hit discovery has been successfully applied to the discovery of MTDLs combining galantamine and memantine [215] and triazinones as BACE-1 and GSK-3β inhibitors [216]. Besnard et al. outlined a strategy for the automated design of MTDLs for the optimization process of multitarget hits [217].
Modern drug discovery has the tools to screen millions of compounds or fragments to determine potential association with AD. Oset-Gasque and Marco-Contelles have proposed that there is a need to find universal and polyvalent pharmacophoric groups able to modulate diverse receptors or enzymatic systems [218]. For novel MTDL hits against AD, two applicable strategies have been used, namely, the framework combination approach or fragment-based drug discovery (FBDD) [219,220,221]. Meanwhile, Prati et al. proposed the adoption of both strategies, in parallel [222]. The identification of a high-quality starting hit is crucial for next steps towards lead optimization. A high-quality starting hit is characterized by a balanced modulation of several targets, but it is still a challenge to choose the right combination of targets. The main goal of MTDL design is to choose such combinations of targets that can provide a superior therapeutic effect and side-effect profile, compared to the action of a selective ligand on its own. It requires a good understanding of target-disease associations, pathway-target-drug-disease relationships and adverse events profiling. However, the complex interactions among potential AD targets is complicated and the precise mechanisms are unclear. The application of a big-data research approach should be able to identify the biological pathways and key molecules involved in the aetiology of AD through the generation and analysis of large-scale transcriptome, epigenetic, proteome, and clinical data, thereby providing a rational and validated basis for extending the MTDL approach in AD [223].
Author Contributions
Conceptualization, A.Z.; writing—original draft preparation, A.Z.; writing—review and editing, A.J.; visualization, A.J.; supervision, A.Z.; All authors have read and agreed to the published version of the manuscript.
Funding
The publication of this article was funded by the Priority Research Area qLife under the program “Excellence Initiative—Research University” at the Jagiellonian University in Krakow. (application number 06/IDUB/2019/94).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Blennow K., de Leon M.J., Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 2.Nelson P.T., Braak H., Markesbery W.R. Neuropathology and cognitive impairment in alzheimer disease: A complex but coherent relationship. J. Neuropathol. Exp. Neurol. 2009;68:1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichols E., Szoeke C.E.I., Vollset S.E., Abbasi N., Abd-Allah F., Abdela J., Aichour M.T.E., Akinyemi R.O., Alahdab F., Asgedom S.W., et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kandimalla R., Reddy P.H. Therapeutics of Neurotransmitters in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017;57:1049–1069. doi: 10.3233/JAD-161118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knopman D.S. Lowering of amyloid-beta by β-secretase inhibitors—Some informative failures. N. Engl. J. Med. 2019;380:1476–1478. doi: 10.1056/NEJMe1903193. [DOI] [PubMed] [Google Scholar]
- 6.Goate A., Chartier-Harlin M.C., Mullan M., Brown J., Crawford F., Fidani L., Giuffra L., Haynes A., Irving N., James L., et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 7.Canter R.G., Penney J., Tsai L.H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature. 2016;539:187–196. doi: 10.1038/nature20412. [DOI] [PubMed] [Google Scholar]
- 8.Cuyvers E., Sleegers K. Genetic variations underlying Alzheimer’s disease: Evidence from genome-wide association studies and beyond. Lancet Neurol. 2016;15:857–868. doi: 10.1016/S1474-4422(16)00127-7. [DOI] [PubMed] [Google Scholar]
- 9.Saunders A.M., Strittmatter W.J., Schmechel D., St. George-Hyslop P.H., Pericak-Vance M.A., Joo S.H., Rosi B.L., Gusella J.F., Crapper-Mac Lachlan D.R., Alberts M.J., et al. Association of apolipoprotein E allele ϵ4 with late-onset familial and sporadic alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/WNL.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki Y., Zhao N., Caulfield T.R., Liu C.C., Bu G. Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat. Rev. Neurol. 2019;15:501–518. doi: 10.1038/s41582-019-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawmiller D., Habib A., Hou H., Mori T., Fan A., Tian J., Zeng J., Giunta B., Sanberg P.R., Mattson M.P., et al. A Novel Apolipoprotein E Antagonist Functionally Blocks Apolipoprotein E Interaction With N-terminal Amyloid Precursor Protein, Reduces β-Amyloid-Associated Pathology, and Improves Cognition. Biol. Psychiatry. 2019;86:208–220. doi: 10.1016/j.biopsych.2019.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Reitz C., Mayeux R. Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 2014;88:640–651. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bateman R.J., Xiong C., Benzinger T.L.S., Fagan A.M., Goate A., Fox N.C., Marcus D.S., Cairns N.J., Xie X., Blazey T.M., et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease. N. Engl. J. Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selkoe D.J. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 15.Hardy J., Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-V. [DOI] [PubMed] [Google Scholar]
- 16.Hardy J.A., Higgins G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 17.Frost B., Jacks R.L., Diamond M.I. Propagation of Tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kfoury N., Holmes B.B., Jiang H., Holtzman D.M., Diamond M.I. Trans-cellular propagation of Tau aggregation by fibrillar species. J. Biol. Chem. 2012;287:19440–19451. doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swerdlow R.H., Khan S.M. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med. Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 20.Hauptmann S., Keil U., Scherping I., Bonert A., Eckert A., Müller W.E. Mitochondrial dysfunction in sporadic and genetic Alzheimer’s disease. Exp. Gerontol. 2006;41:668–673. doi: 10.1016/j.exger.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Mattson M.P., Cheng B., Davis D., Bryant K., Lieberburg I., Rydel R.E. β-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J. Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.KIM H.-S. Carboxyl-terminal fragment of Alzheimer’s APP destabilizes calcium homeostasis and renders neuronal cells vulnerable to excitotoxicity. FASEB J. 2000;14:1508–1517. doi: 10.1096/fj.14.11.1508. [DOI] [PubMed] [Google Scholar]
- 23.McGeer P.L., Rogers J. Anti–inflammatory agents as a therapeutic approach to Alzheimer’s disease. Neurology. 1992;42:447–448. doi: 10.1212/WNL.42.2.447. [DOI] [PubMed] [Google Scholar]
- 24.Fan L., Mao C., Hu X., Zhang S., Yang Z., Hu Z., Sun H., Fan Y., Dong Y., Yang J., et al. New Insights Into the Pathogenesis of Alzheimer’s Disease. Front. Neurol. 2019;10:1312. doi: 10.3389/fneur.2019.01312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu P.-P., Xie Y., Meng X.-Y., Kang J.-S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct. Target. Ther. 2019;4 doi: 10.1038/s41392-019-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 27.Bush A.I., Pettingell W.H., Multhaup G., Paradis M.D., Vonsattel J.P., Gusella J.F., Beyreuther K., Masters C.L., Tanzi R.E. Rapid induction of Alzheimer Aβ amyloid formation by zinc. Science. 1994;265:1464–1467. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- 28.Lyubartseva G., Lovell M.A. A potential role for zinc alterations in the pathogenesis of Alzheimer’s disease. Biofactors. 2012;38:98–106. doi: 10.1002/biof.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abelein A., Gräslund A., Danielsson J. Zinc as chaperone-mimicking agent for retardation of amyloid β peptide fibril formation. Proc. Natl. Acad. Sci. USA. 2015;112:5407–5412. doi: 10.1073/pnas.1421961112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deane R., Wu Z., Sagare A., Davis J., Du Yan S., Hamm K., Xu F., Parisi M., LaRue B., Hu H.W., et al. LRP/amyloid β-peptide interaction mediates differential brain efflux of Aβ isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Papadopoulos Z., Herz J., Kipnis J. Meningeal Lymphatics: From Anatomy to Central Nervous System Immune Surveillance. J. Immunol. 2020;204:286–293. doi: 10.4049/jimmunol.1900838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itzhaki R.F., Lathe R., Balin B.J., Ball M.J., Bearer E.L., Braak H., Bullido M.J., Carter C., Clerici M., Cosby S.L., et al. Microbes and Alzheimer’s disease. J. Alzheimer’s Dis. 2016;51:979–984. doi: 10.3233/JAD-160152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itzhaki R.F. Herpes simplex virus type 1 and Alzheimer’s disease: Possible mechanisms and signposts. FASEB J. 2017;31:3216–3226. doi: 10.1096/fj.201700360. [DOI] [PubMed] [Google Scholar]
- 34.Wang W.X., Rajeev B.W., Stromberg A.J., Ren N., Tang G., Huang Q., Rigoutsos I., Nelson P.T. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of β-site amyloid precursor protein-cleaving enzyme 1. J. Neurosci. 2008;28:1213–1223. doi: 10.1523/JNEUROSCI.5065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu C.-G., Wang J.-L., Li L., Wang P.-C. MicroRNA-384 regulates both amyloid precursor protein and β-secretase expression and is a potential biomarker for Alzheimer’s disease. Int. J. Mol. Med. 2014;34:160–166. doi: 10.3892/ijmm.2014.1780. [DOI] [PubMed] [Google Scholar]
- 36.Long J.M., Ray B., Lahiri D.K. MicroRNA-339-5p down-regulates protein expression of β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) in human primary brain cultures and is reduced in brain tissue specimens of Alzheimer disease subjects. J. Biol. Chem. 2014;289:5184–5198. doi: 10.1074/jbc.M113.518241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohr A.M., Mott J.L. Overview of microRNA biology. Semin. Liver Dis. 2015;35:3–11. doi: 10.1055/s-0034-1397344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woldemichael B.T., Mansuy I.M. Micro-RNAs in cognition and cognitive disorders: Potential for novel biomarkers and therapeutics. Biochem. Pharmacol. 2016;104:1–7. doi: 10.1016/j.bcp.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z.D., Zhang S., Hao J.J., Xie T.R., Kang J.S. Cellular model of neuronal atrophy induced by DYNC1I1 deficiency reveals protective roles of RAS-RAF-MEK signaling. Protein Cell. 2016;7:638–650. doi: 10.1007/s13238-016-0301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.La Joie R., Perrotin A., Barré L., Hommet C., Mézenge F., Ibazizene M., Camus V., Abbas A., Landeau B., Guilloteau D., et al. Region-specific hierarchy between atrophy, hypometabolism, and 2-amyloid (Aβ) load in Alzheimer’s disease dementia. J. Neurosci. 2012;32:16265–16273. doi: 10.1523/JNEUROSCI.2170-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dyrba M., Mohammadi R., Grothe M.J., Kirste T., Teipel S.J. Gaussian Graphical Models Reveal Inter-Modal and Inter-Regional Conditional Dependencies of Brain Alterations in Alzheimer’s Disease. Front. Aging Neurosci. 2020;12 doi: 10.3389/fnagi.2020.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grimm A., Mensah-Nyagan A.G., Eckert A. Alzheimer, mitochondria and gender. Neurosci. Biobehav. Rev. 2016;67:89–101. doi: 10.1016/j.neubiorev.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Cardoso S., Seiça R.M., Moreira P.I. Mitochondria as a target for neuroprotection: Implications for Alzheimer’s disease. Expert Rev. Neurother. 2017;17:77–91. doi: 10.1080/14737175.2016.1205488. [DOI] [PubMed] [Google Scholar]
- 44.Grimm A., Lejri I., Hallé F., Schmitt M., Götz J., Bihel F., Eckert A. Mitochondria modulatory effects of new TSPO ligands in a cellular model of tauopathies. J. Neuroendocrinol. 2020;32 doi: 10.1111/jne.12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson J.W., Glasgow N.G., Povysheva N.V. Recent insights into the mode of action of memantine and ketamine. Curr. Opin. Pharmacol. 2015;20:54–63. doi: 10.1016/j.coph.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hung S.Y., Fu W.M. Drug candidates in clinical trials for Alzheimer’s disease. J. Biomed. Sci. 2017;24 doi: 10.1186/s12929-017-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melchiorre C., Andrisano V., Bolognesi M.L., Budriesi R., Cavalli A., Cavrini V., Rosini M., Tumiatti V., Recanatini M. Acetylcholinesterase noncovalent inhibitors based on a polyamine backbone for potential use against Alzheimer’s disease. J. Med. Chem. 1998;41:4186–4189. doi: 10.1021/jm9810452. [DOI] [PubMed] [Google Scholar]
- 48.Marco-Contelles J. Facts, Results, and Perspectives of the Current Alzheimer’s Disease Research. ACS Chem. Neurosci. 2019;10:1127–1128. doi: 10.1021/acschemneuro.9b00034. [DOI] [PubMed] [Google Scholar]
- 49.Kim J., Onstead L., Randle S., Price R., Smithson L., Zwizinski C., Dickson D.W., Golde T., McGowan E. Aβ40 inhibits amyloid deposition in vivo. J. Neurosci. 2007;27:627–633. doi: 10.1523/JNEUROSCI.4849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghezzi L., Scarpini E., Galimberti D. Disease-modifying drugs in Alzheimer’s disease. Drug Des. Devel. Ther. 2013;7:1471–1478. doi: 10.2147/DDDT.S41431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graham W.V., Bonito-Oliva A., Sakmar T.P. Update on Alzheimer’s Disease Therapy and Prevention Strategies. Annu. Rev. Med. 2017;68:413–430. doi: 10.1146/annurev-med-042915-103753. [DOI] [PubMed] [Google Scholar]
- 52.Price D.L., Tanzi R.E., Borchelt D.R., Sisodia S.S. ALZHEIMER’S DISEASE: Genetic Studies and Transgenic Models. Annu. Rev. Genet. 1998;32:461–493. doi: 10.1146/annurev.genet.32.1.461. [DOI] [PubMed] [Google Scholar]
- 53.Panza F., Solfrizzi V., Seripa D., Imbimbo B.P., Lozupone M., Santamato A., Zecca C., Barull M.R., Bellomo A., Pilotto A., et al. Tau-Centric Targets and Drugs in Clinical Development for the Treatment of Alzheimer’s Disease. Biomed Res. Int. 2016;2016 doi: 10.1155/2016/3245935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Avila J., Wandosell F., Hernández F. Role of glycogen synthase kinase-3 in Alzheimer’s disease pathogenesis and glycogen synthase kinase-3 inhibitors. Expert Rev. Neurother. 2010;10:703–710. doi: 10.1586/ern.10.40. [DOI] [PubMed] [Google Scholar]
- 55.Iba M., Guo J.L., McBride J.D., Zhang B., Trojanowski J.Q., Lee V.M.Y. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of alzheimer’s-like tauopathy. J. Neurosci. 2013;33:1024–1037. doi: 10.1523/JNEUROSCI.2642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H., Ma Q., Zhang Y.W., Xu H. Proteolytic processing of Alzheimer’s β-amyloid precursor protein. J. Neurochem. 2012;120:9–21. doi: 10.1111/j.1471-4159.2011.07519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai Z., Zhao Y., Zhao B. Roles of Glycogen Synthase Kinase 3 in Alzheimer’s Disease. Curr. Alzheimer Res. 2012;9:864–879. doi: 10.2174/156720512802455386. [DOI] [PubMed] [Google Scholar]
- 58.Rossner S., Sastre M., Bourne K., Lichtenthaler S.F. Transcriptional and translational regulation of BACE1 expression--implications for Alzheimer’s disease. Prog. Neurobiol. 2006;79:95–111. doi: 10.1016/j.pneurobio.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Sun X., Bromley-Brits K., Song W. Regulation of β-site APP-cleaving enzyme 1 gene expression and its role in Alzheimer’s disease. J. Neurochem. 2012;120:62–70. doi: 10.1111/j.1471-4159.2011.07515.x. [DOI] [PubMed] [Google Scholar]
- 60.Ly P.T.T., Wu Y., Zou H., Wang R., Zhou W., Kinoshita A., Zhang M., Yang Y., Cai F., Woodgett J., et al. Inhibition of GSK3β-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J. Clin. Invest. 2013;123:224–235. doi: 10.1172/JCI64516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Godridge H., Reynolds G.P., Czudek C., Calcutt N.A., Benton M. Alzheimer-like neurotransmitter deficits in adult Down’s syndrome brain tissue. J. Neurol. Neurosurg. Psychiatry. 1987;50:775–778. doi: 10.1136/jnnp.50.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.H. Ferreira-Vieira T., M. Guimaraes I., R. Silva F., M. Ribeiro F. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016;14:101–115. doi: 10.2174/1570159X13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beurel E., Jope R.S. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog. Neurobiol. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shipton O.A., Leitz J.R., Dworzak J., Acton C.E.J., Tunbridge E.M., Denk F., Dawson H.N., Vitek M.P., Wade-Martins R., Paulsen O., et al. Tau protein is required for amyloid β-induced impairment of hippocampal long-term potentiation. J. Neurosci. 2011;31:1688–1692. doi: 10.1523/JNEUROSCI.2610-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin M., Rehani K., Jope R.S., Michalek S.M. Toll-like receptor—Mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hofmann C., Dunger N., Schölmerich J., Falk W., Obermeier F. Glycogen synthase kinase 3-β: A master regulator of toll-like receptor-mediated chronic intestinal inflammation. Inflamm. Bowel Dis. 2010;16:1850–1858. doi: 10.1002/ibd.21294. [DOI] [PubMed] [Google Scholar]
- 67.Shimizu E., Tang Y.P., Rampon C., Tsien J.Z. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290:1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- 68.Geerts H., Grossberg G.T. Pharmacology of acetylcholinesterase inhibitors and N-methyl-D-aspartate receptors for combination therapy in the treatment of Alzheimer’s disease. J. Clin. Pharmacol. 2006;46 doi: 10.1177/0091270006288734. [DOI] [PubMed] [Google Scholar]
- 69.Naoi M., Maruyama W., Inaba-Hasegawa K. Type A and B monoamine oxidase in age-related neurodegenerative disorders: Their distinct roles in neuronal death and survival. Curr. Top. Med. Chem. 2012;12:2177–2188. doi: 10.2174/156802612805219950. [DOI] [PubMed] [Google Scholar]
- 70.Naoi M., Riederer P., Maruyama W. Modulation of monoamine oxidase (MAO) expression in neuropsychiatric disorders: Genetic and environmental factors involved in type A MAO expression. J. Neural Transm. 2016;123:91–106. doi: 10.1007/s00702-014-1362-4. [DOI] [PubMed] [Google Scholar]
- 71.Hirvonen J., Kailajärvi M., Haltia T., Koskimies S., Någren K., Virsu P., Oikonen V., Sipilä H., Ruokoniemi P., Virtanen K., et al. Assessment of MAO-B Occupancy in the Brain With PET and [11C]-L-Deprenyl-D2: A Dose-Finding Study With a Novel MAO-B Inhibitor, EVT 301. Clin. Pharmacol. Ther. 2009;85:506–512. doi: 10.1038/clpt.2008.241. [DOI] [PubMed] [Google Scholar]
- 72.Youdim M.B.H., Fridkin M., Zheng H. Bifunctional drug derivatives of MAO-B inhibitor rasagiline and iron chelator VK-28 as a more effective approach to treatment of brain ageing and ageing neurodegenerative diseases. Mech. Ageing Dev. 2005;126:317–326. doi: 10.1016/j.mad.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 73.Youdim M.B.H., Bakhle Y.S. Monoamine oxidase: Isoforms and inhibitors in Parkinson’s disease and depressive illness. Br. J. Pharmacol. 2006;147 doi: 10.1038/sj.bjp.0706464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Riederer P., Danielczyk W., Grünblatt E. Monoamine Oxidase-B Inhibition in Alzheimer’s Disease. Neurotoxicology. 2004;25:271–277. doi: 10.1016/S0161-813X(03)00106-2. [DOI] [PubMed] [Google Scholar]
- 75.Gökhan-Kelekçi N., Yabanoǧlu S., Küpeli E., Salgin U., Özgen Ö., Uçar G., Yeşilada E., Kendi E., Yeşilada A., Bilgin A.A. A new therapeutic approach in Alzheimer disease: Some novel pyrazole derivatives as dual MAO-B inhibitors and antiinflammatory analgesics. Bioorg. Med. Chem. 2007;15:5775–5786. doi: 10.1016/j.bmc.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 76.Crouch P.J., White A.R., Bush A.I. The modulation of metal bio-availability as a therapeutic strategy for the treatment of Alzheimer’s disease. FEBS J. 2007;274:3775–3783. doi: 10.1111/j.1742-4658.2007.05918.x. [DOI] [PubMed] [Google Scholar]
- 77.Kepp K.P. Bioinorganic chemistry of Alzheimer’s disease. Chem. Rev. 2012;112:5193–5239. doi: 10.1021/cr300009x. [DOI] [PubMed] [Google Scholar]
- 78.Li X., Wang H., Lu Z., Zheng X., Ni W., Zhu J., Fu Y., Lian F., Zhang N., Li J., et al. Development of Multifunctional Pyrimidinylthiourea Derivatives as Potential Anti-Alzheimer Agents. J. Med. Chem. 2016;59:8326–8344. doi: 10.1021/acs.jmedchem.6b00636. [DOI] [PubMed] [Google Scholar]
- 79.Basha S.J., Mohan P., Yeggoni D.P., Babu Z.R., Kumar P.B., Rao A.D., Subramanyam R., Damu A.G. New Flavone-Cyanoacetamide Hybrids with a Combination of Cholinergic, Antioxidant, Modulation of β-Amyloid Aggregation, and Neuroprotection Properties as Innovative Multifunctional Therapeutic Candidates for Alzheimer’s Disease and Unraveling Their Mechan. Mol. Pharm. 2018;15:2206–2223. doi: 10.1021/acs.molpharmaceut.8b00041. [DOI] [PubMed] [Google Scholar]
- 80.Braidy N., Poljak A., Marjo C., Rutlidge H., Rich A., Jayasena T., Inestrosa N.C., Sachdev P. Metal and complementary molecular bioimaging in alzheimer’s disease. Front. Aging Neurosci. 2014;6 doi: 10.3389/fnagi.2014.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matsumoto M., Togashi H., Mori K., Ueno K., Ohashi S., Kojima T., Yoshioka M. Evidence for Involvement of Central 5-HT4 Receptors in Cholinergic Function Associated with Cognitive Processes: Behavioral, Electrophysiological, and Neurochemical Studies. J. Pharmacol. Exp. Ther. 2001;296:676–682. [PubMed] [Google Scholar]
- 82.Upton N., Chuang T.T., Hunter A.J., Virley D.J. 5-HT6 Receptor Antagonists as Novel Cognitive Enhancing Agents for Alzheimer’s Disease. Neurotherapeutics. 2008;5:458–469. doi: 10.1016/j.nurt.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turner P.R., O’Connor K., Tate W.P., Abraham W.C. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog. Neurobiol. 2003;70:1–32. doi: 10.1016/S0301-0082(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 84.Lezoualc’h F. 5-HT4 receptor and Alzheimer’s disease: The amyloid connection. Exp. Neurol. 2007;205:325–329. doi: 10.1016/j.expneurol.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 85.Lecoutey C., Hedou D., Freret T., Giannoni P., Gaven F., Since M., Bouet V., Ballandonne C., Corvaisier S., Fréon A.M., et al. Design of donecopride, a dual serotonin subtype 4 receptor agonist/acetylcholinesterase inhibitor with potential interest for Alzheimer’s disease treatment. Proc. Natl. Acad. Sci. USA. 2014;111:E3825–E3830. doi: 10.1073/pnas.1410315111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rochais C., Lecoutey C., Gaven F., Giannoni P., Hamidouche K., Hedou D., Dubost E., Genest D., Yahiaoui S., Freret T., et al. Novel Multitarget-Directed Ligands (MTDLs) with Acetylcholinesterase (AChE) Inhibitory and Serotonergic Subtype 4 Receptor (5-HT4R) Agonist Activities As Potential Agents against Alzheimer’s Disease: The Design of Donecopride. J. Med. Chem. 2015;58:3172–3187. doi: 10.1021/acs.jmedchem.5b00115. [DOI] [PubMed] [Google Scholar]
- 87.Kubo M., Kishi T., Matsunaga S., Iwata N. Histamine H3 Receptor Antagonists for Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. J. Alzheimer’s Dis. 2015;48:667–671. doi: 10.3233/JAD-150393. [DOI] [PubMed] [Google Scholar]
- 88.Prickaerts J., Heckman P.R.A., Blokland A. Investigational phosphodiesterase inhibitors in phase I and phase II clinical trials for Alzheimer’s disease. Expert Opin. Investig. Drugs. 2017;26:1033–1048. doi: 10.1080/13543784.2017.1364360. [DOI] [PubMed] [Google Scholar]
- 89.Wu Y., Li Z., Huang Y.-Y., Wu D., Luo H.-B. Novel Phosphodiesterase Inhibitors for Cognitive Improvement in Alzheimer’s Disease. J. Med. Chem. 2018;61:5467–5483. doi: 10.1021/acs.jmedchem.7b01370. [DOI] [PubMed] [Google Scholar]
- 90.Nabavi S.M., Talarek S., Listos J., Nabavi S.F., Devi K.P., Roberto de Oliveira M., Tewari D., Argüelles S., Mehrzadi S., Hosseinzadeh A., et al. Phosphodiesterase inhibitors say NO to Alzheimer’s disease. Food Chem. Toxicol. 2019;134 doi: 10.1016/j.fct.2019.110822. [DOI] [PubMed] [Google Scholar]
- 91.Arai H., Takahashi T. A combination therapy of Donepezil and Cilostazol for patients with moderate Alzheimer disease: Pilot follow-up study. Am. J. Geriatr. Psychiatry. 2009;17:353–354. doi: 10.1097/JGP.0b013e31819431ea. [DOI] [PubMed] [Google Scholar]
- 92.Ihara M., Nishino M., Taguchi A., Yamamoto Y., Hattori Y., Saito S., Takahashi Y., Tsuji M., Kasahara Y., Takata Y., et al. Cilostazol add-on therapy in patients with mild dementia receiving donepezil: A retrospective study. PLoS ONE. 2014;9:e89516. doi: 10.1371/journal.pone.0089516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chaudhary A., Maurya P.K., Yadav B.S., Singh S., Mani A. Current Therapeutic Targets for Alzheimer’s Disease. J. Biomed. 2018;3:74–84. doi: 10.7150/jbm.26783. [DOI] [Google Scholar]
- 94.Loera-Valencia R., Goikolea J., Parrado-Fernandez C., Merino-Serrais P., Maioli S. Alterations in cholesterol metabolism as a risk factor for developing Alzheimer’s disease: Potential novel targets for treatment. J. Steroid Biochem. Mol. Biol. 2019;190:104–114. doi: 10.1016/j.jsbmb.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 95.Bolognesi M.L., Cavalli A., Melchiorre C. Memoquin: A Multi-Target-Directed Ligand as an Innovative Therapeutic Opportunity for Alzheimer’s Disease. Neurotherapeutics. 2009;6:152–162. doi: 10.1016/j.nurt.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Capurro V., Busquet P., Lopes J.P., Bertorelli R., Tarozzo G., Bolognesi M.L., Piomelli D., Reggiani A., Cavalli A. Pharmacological Characterization of Memoquin, a Multi-Target Compound for the Treatment of Alzheimer’s Disease. PLoS ONE. 2013;8:e56870. doi: 10.1371/journal.pone.0056870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pan W., Hu K., Bai P., Yu L., Ma Q., Li T., Zhang X., Chen C., Peng K., Liu W., et al. Design, synthesis and evaluation of novel ferulic acid-memoquin hybrids as potential multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2016;26:2539–2543. doi: 10.1016/j.bmcl.2016.03.086. [DOI] [PubMed] [Google Scholar]
- 98.Li Q., He S., Chen Y., Feng F., Qu W., Sun H. Donepezil-based multi-functional cholinesterase inhibitors for treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2018;158:463–477. doi: 10.1016/j.ejmech.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 99.Maspero M., Volpato D., Cirillo D., Yuan Chen N., Messerer R., Sotriffer C., De Amici M., Holzgrabe U., Dallanoce C. Tacrine-xanomeline and tacrine-iperoxo hybrid ligands: Synthesis and biological evaluation at acetylcholinesterase and M1 muscarinic acetylcholine receptors. Bioorg. Chem. 2020;96:103633. doi: 10.1016/j.bioorg.2020.103633. [DOI] [PubMed] [Google Scholar]
- 100.Oukoloff K., Coquelle N., Bartolini M., Naldi M., Le Guevel R., Bach S., Josselin B., Ruchaud S., Catto M., Pisani L., et al. Design, biological evaluation and X-ray crystallography of nanomolar multifunctional ligands targeting simultaneously acetylcholinesterase and glycogen synthase kinase-3. Eur. J. Med. Chem. 2019;168:58–77. doi: 10.1016/j.ejmech.2018.12.063. [DOI] [PubMed] [Google Scholar]
- 101.Wang Y., Yang Y., Hong K.H., Ning Y., Yu P., Ren J., Ji M., Cai J. Design, synthesis and evaluation of a novel metal chelator as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Chem. 2019;87:720–727. doi: 10.1016/j.bioorg.2019.03.064. [DOI] [PubMed] [Google Scholar]
- 102.Cheng Z.Q., Zhu K.K., Zhang J., Song J.L., Muehlmann L.A., Jiang C.S., Liu C.L., Zhang H. Molecular-docking-guided design and synthesis of new IAA-tacrine hybrids as multifunctional AChE/BChE inhibitors. Bioorg. Chem. 2019;83:277–288. doi: 10.1016/j.bioorg.2018.10.057. [DOI] [PubMed] [Google Scholar]
- 103.Cheng Z.Q., Song J.L., Zhu K., Zhang J., Jiang C.S., Zhang H. Total synthesis of pulmonarin B and design of brominated phenylacetic acid/tacrine hybrids: Marine pharmacophore inspired discovery of new CHE and Aβ aggregation inhibitors. Mar. Drugs. 2018;16:293. doi: 10.3390/md16090293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lopes J.P.B., Silva L., da Costa Franarin G., Antonio Ceschi M., Seibert Lüdtke D., Ferreira Dantas R., de Salles C.M.C., Paes Silva-Jr F., Roberto Senger M., Alvim Guedes I., et al. Design, synthesis, cholinesterase inhibition and molecular modelling study of novel tacrine hybrids with carbohydrate derivatives. Bioorg. Med. Chem. 2018;26:5566–5577. doi: 10.1016/j.bmc.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 105.Chufarova N., Czarnecka K., Skibiński R., Cuchra M., Majsterek I., Szymański P. New tacrine-acridine hybrids as promising multifunctional drugs for potential treatment of Alzheimer’s disease. Arch. Pharm. (Weinheim.) 2018;351:1800050. doi: 10.1002/ardp.201800050. [DOI] [PubMed] [Google Scholar]
- 106.Makhaeva G.F., Kovaleva N.V., Boltneva N.P., Lushchekina S.V., Rudakova E.V., Stupina T.S., Terentiev A.A., Serkov I.V., Proshin A.N., Radchenko E.V., et al. Conjugates of tacrine and 1,2,4-thiadiazole derivatives as new potential multifunctional agents for Alzheimer’s disease treatment: Synthesis, quantum-chemical characterization, molecular docking, and biological evaluation. Bioorg. Chem. 2020;94:103387. doi: 10.1016/j.bioorg.2019.103387. [DOI] [PubMed] [Google Scholar]
- 107.Zhu J., Yang H., Chen Y., Lin H., Li Q., Mo J., Bian Y., Pei Y., Sun H. Synthesis, pharmacology and molecular docking on multifunctional tacrine-ferulic acid hybrids as cholinesterase inhibitors against Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2018;33:496–506. doi: 10.1080/14756366.2018.1430691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Habibpour R., Eslami M., Amani P., Bagheri Novir S. Tacrine-Flavonoid Quercetin Hybride as a MTDL ligand against Alzheimer’s disease with metal chelating and AChE, BChE, AChE-induced Aβ aggregation inhibition properties: A computational study. Phys. Chem. Res. 2019;7:561–579. doi: 10.22036/pcr.2019.183077.1624. [DOI] [Google Scholar]
- 109.Hepnarova V., Korabecny J., Matouskova L., Jost P., Muckova L., Hrabinova M., Vykoukalova N., Kerhartova M., Kucera T., Dolezal R., et al. The concept of hybrid molecules of tacrine and benzyl quinolone carboxylic acid (BQCA) as multifunctional agents for Alzheimer’s disease. Eur. J. Med. Chem. 2018;150:292–306. doi: 10.1016/j.ejmech.2018.02.083. [DOI] [PubMed] [Google Scholar]
- 110.Costa M., Josselin R., Silva D.F., Cardoso S.M., May N.V., Chaves S., Santos M.A. Donepezil-based hybrids as multifunctional anti-Alzheimer’s disease chelating agents: Effect of positional isomerization. J. Inorg. Biochem. 2020;206:111039. doi: 10.1016/j.jinorgbio.2020.111039. [DOI] [PubMed] [Google Scholar]
- 111.Chaves S., Resta S., Rinaldo F., Costa M., Josselin R., Gwizdala K., Piemontese L., Capriati V., Pereira-Santos A.R., Cardoso S.M., et al. Design, synthesis, and in vitro evaluation of hydroxybenzimidazole-donepezil analogues as multitarget-directed ligands for the treatment of Alzheimer’s disease. Molecules. 2020;25:985. doi: 10.3390/molecules25040985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cai P., Fang S.Q., Yang H.L., Yang X.L., Liu Q.H., Kong L.Y., Wang X.B. Donepezil-butylated hydroxytoluene (BHT) hybrids as Anti-Alzheimer’s disease agents with cholinergic, antioxidant, and neuroprotective properties. Eur. J. Med. Chem. 2018;157:161–176. doi: 10.1016/j.ejmech.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 113.Cai P., Fang S.Q., Yang X.L., Wu J.J., Liu Q.H., Hong H., Wang X.B., Kong L.Y. Rational Design and Multibiological Profiling of Novel Donepezil-Trolox Hybrids against Alzheimer’s Disease, with Cholinergic, Antioxidant, Neuroprotective, and Cognition Enhancing Properties. ACS Chem. Neurosci. 2017;8:2496–2511. doi: 10.1021/acschemneuro.7b00257. [DOI] [PubMed] [Google Scholar]
- 114.Wang X.B., Yin F.C., Huang M., Jiang N., Lan J.S., Kong L.Y. Chromone and donepezil hybrids as new multipotent cholinesterase and monoamine oxidase inhibitors for the potential treatment of Alzheimer’s disease. RSC Med. Chem. 2020;11:225–233. doi: 10.1039/C9MD00441F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Malek R., Refouvelet B., Benchekroun M., Iriepa I., Moraleda I., Andrys R., Musilek K., Marco-Contelles J., Ismaili L. Synthesis and Biological Evaluation of Novel Chromone+Donepezil Hybrids for Alzheimer’s Disease Therapy. Curr. Alzheimer Res. 2019;16:815–820. doi: 10.2174/1567205016666191011112624. [DOI] [PubMed] [Google Scholar]
- 116.Thamban Chandrika N., Fosso M.Y., Tsodikov O.V., LeVine H., Garneau-Tsodikova S. Combining Chalcones with Donepezil to Inhibit Both Cholinesterases and Aβ Fibril Assembly. Molecules. 2019;25:77. doi: 10.3390/molecules25010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhu G., Wang K., Shi J., Zhang P., Yang D., Fan X., Zhang Z., Liu W., Sang Z. The development of 2-acetylphenol-donepezil hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2019;29:126625. doi: 10.1016/j.bmcl.2019.126625. [DOI] [PubMed] [Google Scholar]
- 118.Du H., Liu X., Xie J., Ma F. Novel Deoxyvasicinone-Donepezil Hybrids as Potential Multitarget Drug Candidates for Alzheimer’s Disease. ACS Chem. Neurosci. 2019;10:2397–2407. doi: 10.1021/acschemneuro.8b00699. [DOI] [PubMed] [Google Scholar]
- 119.Gabr M.T., Abdel-Raziq M.S. Design and synthesis of donepezil analogues as dual AChE and BACE-1 inhibitors. Bioorg. Chem. 2018;80:245–252. doi: 10.1016/j.bioorg.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 120.Piemontese L., Tomás D., Hiremathad A., Capriati V., Candeias E., Cardoso S.M., Chaves S., Santos M.A. Donepezil structure-based hybrids as potential multifunctional anti-Alzheimer’s drug candidates. J. Enzyme Inhib. Med. Chem. 2018;33:1212–1224. doi: 10.1080/14756366.2018.1491564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang J., Wang Z.M., Li X.M., Li F., Wu J.J., Kong L.Y., Wang X.B. Synthesis and evaluation of multi-target-directed ligands for the treatment of Alzheimer’s disease based on the fusion of donepezil and melatonin. Bioorg. Med. Chem. 2016;24:4324–4338. doi: 10.1016/j.bmc.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 122.Girek M., Szymański P. Tacrine hybrids as multi-target-directed ligands in Alzheimer’s disease: Influence of chemical structures on biological activities. Chem. Pap. 2019;73:269–289. doi: 10.1007/s11696-018-0590-8. [DOI] [Google Scholar]
- 123.Spilovska K., Korabecny J., Nepovimova E., Dolezal R., Mezeiova E., Soukup O., Kuca K. Multitarget Tacrine Hybrids with Neuroprotective Properties to Confront Alzheimer’s Disease. Volume 17. Bentham Science Publishers; Sharjah, UAE: 2017. [DOI] [PubMed] [Google Scholar]
- 124.Mahdavi M., Hariri R., Mirfazli S.S., Lotfian H., Rastergari A., Firuzi O., Edraki N., Larijani B., Akbarzadeh T., Saeedi M. Synthesis and Biological Activity of Some Benzochromenoquinolinones: Tacrine Analogs as Potent Anti-Alzheimer’s Agents. Chem. Biodivers. 2019;16 doi: 10.1002/cbdv.201800488. [DOI] [PubMed] [Google Scholar]
- 125.Sola I., Aso E., Frattini D., López-González I., Espargaró A., Sabaté R., Di Pietro O., Luque F.J., Clos M.V., Ferrer I., et al. Novel Levetiracetam Derivatives That Are Effective against the Alzheimer-like Phenotype in Mice: Synthesis, in Vitro, ex Vivo, and in Vivo Efficacy Studies. J. Med. Chem. 2015;58:6018–6032. doi: 10.1021/acs.jmedchem.5b00624. [DOI] [PubMed] [Google Scholar]
- 126.Zha X., Lamba D., Zhang L., Lou Y., Xu C., Kang D., Chen L., Xu Y., Zhang L., De Simone A., et al. Novel Tacrine-Benzofuran Hybrids as Potent Multitarget-Directed Ligands for the Treatment of Alzheimers Disease: Design, Synthesis, Biological Evaluation, and X-ray Crystallography. J. Med. Chem. 2016;59:114–131. doi: 10.1021/acs.jmedchem.5b01119. [DOI] [PubMed] [Google Scholar]
- 127.Liao S., Deng H., Huang S., Yang J., Wang S., Yin B., Zheng T., Zhang D., Liu J., Gao G., et al. Design, synthesis and evaluation of novel 5,6,7-trimethoxyflavone-6-chlorotacrine hybrids as potential multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2015;25:1541–1545. doi: 10.1016/j.bmcl.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 128.Green K.D., Fosso M.Y., Garneau-Tsodikova S. Multifunctional donepezil analogues as cholinesterase and BACE1 inhibitors. Molecules. 2018;23:3252. doi: 10.3390/molecules23123252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ismaili L., Refouvelet B., Benchekroun M., Brogi S., Brindisi M., Gemma S., Campiani G., Filipic S., Agbaba D., Esteban G., et al. Multitarget compounds bearing tacrine- and donepezil-like structural and functional motifs for the potential treatment of Alzheimer’s disease. Prog. Neurobiol. 2017;151:4–34. doi: 10.1016/j.pneurobio.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 130.Irannejad H., Amini M., Khodagholi F., Ansari N., Tusi S.K., Sharifzadeh M., Shafiee A. Synthesis and in vitro evaluation of novel 1,2,4-triazine derivatives as neuroprotective agents. Bioorg. Med. Chem. 2010;18:4224–4230. doi: 10.1016/j.bmc.2010.04.097. [DOI] [PubMed] [Google Scholar]
- 131.Sinha A., Tamboli R.S., Seth B., Kanhed A.M., Tiwari S.K., Agarwal S., Nair S., Giridhar R., Chaturvedi R.K., Yadav M.R. Neuroprotective Role of Novel Triazine Derivatives by Activating Wnt/β Catenin Signaling Pathway in Rodent Models of Alzheimer’s Disease. Mol. Neurobiol. 2015;52:638–652. doi: 10.1007/s12035-014-8899-y. [DOI] [PubMed] [Google Scholar]
- 132.Shidore M., Machhi J., Shingala K., Murumkar P., Sharma M.K., Agrawal N., Tripathi A., Parikh Z., Pillai P., Yadav M.R. Benzylpiperidine-Linked Diarylthiazoles as Potential Anti-Alzheimer’s Agents: Synthesis and Biological Evaluation. J. Med. Chem. 2016;59:5823–5846. doi: 10.1021/acs.jmedchem.6b00426. [DOI] [PubMed] [Google Scholar]
- 133.Więckowska A., Więckowski K., Bajda M., Brus B., Sałat K., Czerwińska P., Gobec S., Filipek B., Malawska B. Synthesis of new N-benzylpiperidine derivatives as cholinesterase inhibitors with β-amyloid anti-aggregation properties and beneficial effects on memory in vivo. Bioorg. Med. Chem. 2015;23:2445–2457. doi: 10.1016/j.bmc.2015.03.051. [DOI] [PubMed] [Google Scholar]
- 134.Panek D., Więckowska A., Wichur T., Bajda M., Godyń J., Jończyk J., Mika K., Janockova J., Soukup O., Knez D., et al. Design, synthesis and biological evaluation of new phthalimide and saccharin derivatives with alicyclic amines targeting cholinesterases, beta-secretase and amyloid beta aggregation. Eur. J. Med. Chem. 2017;125:676–695. doi: 10.1016/j.ejmech.2016.09.078. [DOI] [PubMed] [Google Scholar]
- 135.Wang Z.M., Cai P., Liu Q.H., Xu D.Q., Yang X.L., Wu J.J., Kong L.Y., Wang X.B. Rational modification of donepezil as multifunctional acetylcholinesterase inhibitors for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2016;123:282–297. doi: 10.1016/j.ejmech.2016.07.052. [DOI] [PubMed] [Google Scholar]
- 136.Mishra C.B., Kumari S., Manral A., Prakash A., Saini V., Lynn A.M., Tiwari M. Design, synthesis, in-silico and biological evaluation of novel donepezil derivatives as multi-target-directed ligands for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2017;125:736–750. doi: 10.1016/j.ejmech.2016.09.057. [DOI] [PubMed] [Google Scholar]
- 137.Panek D., Wichur T., Godyń J., Pasieka A., Malawska B. Advances toward multifunctional cholinesterase and β-amyloid aggregation inhibitors. Future Med. Chem. 2017;9:1835–1854. doi: 10.4155/fmc-2017-0094. [DOI] [PubMed] [Google Scholar]
- 138.Fernández-Bachiller M.I., Pérez C., Monjas L., Rademann J., Rodríguez-Franco M.I. New tacrine-4-oxo-4H-chromene hybrids as multifunctional agents for the treatment of Alzheimer’s disease, with cholinergic, antioxidant, and β-amyloid-reducing properties. J. Med. Chem. 2012;55:1303–1317. doi: 10.1021/jm201460y. [DOI] [PubMed] [Google Scholar]
- 139.Pérez-Areales F.J., Betari N., Viayna A., Pont C., Espargaró A., Bartolini M., De Simone A., Rinaldi Alvarenga J.F., Pérez B., Sabate R., et al. Design, synthesis and multitarget biological profiling of second-generation anti-Alzheimer rhein-huprine hybrids. Future Med. Chem. 2017;9:965–981. doi: 10.4155/fmc-2017-0049. [DOI] [PubMed] [Google Scholar]
- 140.Viayna E., Sola I., Bartolini M., De Simone A., Tapia-Rojas C., Serrano F.G., Sabaté R., Juárez-Jiménez J., Pérez B., Luque F.J., et al. Synthesis and multitarget biological profiling of a novel family of rhein derivatives as disease-modifying anti-Alzheimer agents. J. Med. Chem. 2014;57:2549–2567. doi: 10.1021/jm401824w. [DOI] [PubMed] [Google Scholar]
- 141.Sivaprakasam P., Han X., Civiello R.L., Jacutin-Porte S., Kish K., Pokross M., Lewis H.A., Ahmed N., Szapiel N., Newitt J.A., et al. Discovery of new acylaminopyridines as GSK-3 inhibitors by a structure guided in-depth exploration of chemical space around a pyrrolopyridinone core. Bioorg. Med. Chem. Lett. 2015;25:1856–1863. doi: 10.1016/j.bmcl.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 142.Wu W.Y., Dai Y.C., Li N.G., Dong Z.X., Gu T., Shi Z.H., Xue X., Tang Y.P., Duan J.A. Novel multitarget-directed tacrine derivatives as potential candidates for the treatment of alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2016;32:572–587. doi: 10.1080/14756366.2016.1210139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Congdon E.E., Wu J.W., Myeku N., Figueroa Y.H., Herman M., Marinec P.S., Gestwicki J.E., Dickey C.A., Yu W.H., Duff K.E. Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo. Autophagy. 2012;8:609–622. doi: 10.4161/auto.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bolea I., Juárez-Jiménez J., De Los Ríos C., Chioua M., Pouplana R., Luque F.J., Unzeta M., Marco-Contelles J., Samadi A. Synthesis, biological evaluation, and molecular modeling of donepezil and N-[(5-(Benzyloxy)-1-methyl-1H-indol-2-yl)methyl]-N-methylprop-2-yn-1-amine hybrids as new multipotent cholinesterase/monoamine oxidase inhibitors for the treatment of Alzheimer’s di. J. Med. Chem. 2011;54:8251–8270. doi: 10.1021/jm200853t. [DOI] [PubMed] [Google Scholar]
- 145.Wang L., Esteban G., Ojima M., Bautista-Aguilera O.M., Inokuchi T., Moraleda I., Iriepa I., Samadi A., Youdim M.B.H., Romero A., et al. Donepezil + propargylamine + 8-hydroxyquinoline hybrids as new multifunctional metal-chelators, ChE and MAO inhibitors for the potential treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014;80:543–561. doi: 10.1016/j.ejmech.2014.04.078. [DOI] [PubMed] [Google Scholar]
- 146.Wu M.Y., Esteban G., Brogi S., Shionoya M., Wang L., Campiani G., Unzeta M., Inokuchi T., Butini S., Marco-Contelles J. Donepezil-like multifunctional agents: Design, synthesis, molecular modeling and biological evaluation. Eur. J. Med. Chem. 2016;121:864–879. doi: 10.1016/j.ejmech.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 147.Estrada M., Herrera-Arozamena C., Pérez C., Viña D., Romero A., Morales-García J.A., Pérez-Castillo A., Rodríguez-Franco M.I. New cinnamic—N-benzylpiperidine and cinnamic—N,N-dibenzyl(N-methyl)amine hybrids as Alzheimer-directed multitarget drugs with antioxidant, cholinergic, neuroprotective and neurogenic properties. Eur. J. Med. Chem. 2016;121:376–386. doi: 10.1016/j.ejmech.2016.05.055. [DOI] [PubMed] [Google Scholar]
- 148.Lu C., Zhou Q., Yan J., Du Z., Huang L., Li X. A novel series of tacrine-selegiline hybrids with cholinesterase and monoamine oxidase inhibition activities for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2013;62:745–753. doi: 10.1016/j.ejmech.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 149.Wang Y., Sun Y., Guo Y., Wang Z., Huang L., Li X. Dual functional cholinesterase and MAO inhibitors for the treatment of Alzheimers disease: Synthesis, pharmacological analysis and molecular modeling of homoisoflavonoid derivatives. J. Enzyme Inhib. Med. Chem. 2016;31:389–397. doi: 10.3109/14756366.2015.1024675. [DOI] [PubMed] [Google Scholar]
- 150.Benek O., Soukup O., Pasdiorova M., Hroch L., Sepsova V., Jost P., Hrabinova M., Jun D., Kuca K., Zala D., et al. Design, Synthesis and in vitro Evaluation of Indolotacrine Analogues as Multitarget-Directed Ligands for the Treatment of Alzheimer’s Disease. ChemMedChem. 2016;11:1264–1269. doi: 10.1002/cmdc.201500383. [DOI] [PubMed] [Google Scholar]
- 151.Xu Y., Wang H., Li X., Dong S., Liu W., Gong Q., Wang T., Tang Y., Zhu J., Li J., et al. Discovery of novel propargylamine-modified 4-aminoalkyl imidazole substituted pyrimidinylthiourea derivatives as multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2018;143:33–47. doi: 10.1016/j.ejmech.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 152.Weinreb O., Amit T., Bar-Am O., Youdim M.B.H. Neuroprotective effects of multifaceted hybrid agents targeting MAO, cholinesterase, iron and β-amyloid in ageing and Alzheimer’s disease. Br. J. Pharmacol. 2016;173:2080–2094. doi: 10.1111/bph.13318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Safety and Efficacy Study of Ladostigil in Mild to Moderate Probable Alzheimer’s Disease—Full Text View—ClinicalTrials.gov. [(accessed on 28 May 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01354691.
- 154.Li Y., Qiang X., Luo L., Yang X., Xiao G., Zheng Y., Cao Z., Su F., Deng Y., Sang Z. Multitarget drug design strategy against Alzheimer’s disease: Homoisoflavonoid Mannich base derivatives serve as acetylcholinesterase and monoamine oxidase B dual inhibitors with multifunctional properties. Bioorg. Med. Chem. 2017;25:714–726. doi: 10.1016/j.bmc.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 155.Rosini M., Simoni E., Minarini A., Melchiorre C. Multi-target Design Strategies in the Context of Alzheimer’s Disease: Acetylcholinesterase Inhibition and NMDA Receptor Antagonism as the Driving Forces. Neurochem. Res. 2014;39:1914–1923. doi: 10.1007/s11064-014-1250-1. [DOI] [PubMed] [Google Scholar]
- 156.Rosini M., Simoni E., Bartolini M., Cavalli A., Ceccarini L., Pascu N., McClymont D.W., Tarozzi A., Bolognesi M.L., Minarini A., et al. Inhibition of acetylcholinesterase, β-amyloid aggregation, and NMDA receptors in Alzheimer’s disease: A promising direction for the multi-target-directed ligands gold rush. J. Med. Chem. 2008;51:4381–4384. doi: 10.1021/jm800577j. [DOI] [PubMed] [Google Scholar]
- 157.Rosini M., Simoni E., Bartolini M., Soriano E., Marco-Contelles J., Andrisano V., Monti B., Windisch M., Hutter-Paier B., Mcclymont D.W., et al. The bivalent ligand approach as a tool for improving the in vitro anti-alzheimer multitarget profile of dimebon. ChemMedChem. 2013;8:1276–1281. doi: 10.1002/cmdc.201300263. [DOI] [PubMed] [Google Scholar]
- 158.Makhaeva G.F., Lushchekina S.V., Boltneva N.P., Sokolov V.B., Grigoriev V.V., Serebryakova O.G., Vikhareva E.A., Aksinenko A.Y., Barreto G.E., Aliev G., et al. Conjugates of γ 3-Carbolines and Phenothiazine as new selective inhibitors of butyrylcholinesterase and blockers of NMDA receptors for Alzheimer Disease. Sci. Rep. 2015;5 doi: 10.1038/srep13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yun H.-M., Rhim H. The Serotonin-6 Receptor as a Novel Therapeutic Target. Exp. Neurobiol. 2011;20:159–168. doi: 10.5607/en.2011.20.4.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Claeysen S., Bockaert J., Giannoni P. Serotonin: A new hope in Alzheimer’s disease? ACS Chem. Neurosci. 2015;6:940–943. doi: 10.1021/acschemneuro.5b00135. [DOI] [PubMed] [Google Scholar]
- 161.Freret T., Bouet V., Quiedeville A., Nee G., Dallemagne P., Rochais C., Boulouard M. Synergistic effect of acetylcholinesterase inhibition (donepezil) and 5-HT 4 receptor activation (RS67333) on object recognition in mice. Behav. Brain Res. 2012;230:304–308. doi: 10.1016/j.bbr.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 162.Charlier Y., Brabant C., Serrano M.E., Lamberty Y., Tirelli E. The prototypical histamine H3 receptor inverse agonist thioperamide improves multiple aspects of memory processing in an inhibitory avoidance task. Behav. Brain Res. 2013;253:121–127. doi: 10.1016/j.bbr.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 163.Inocente C., Arnulf I., Bastuji H., Thibault-Stoll A., Raoux A., Reimão R., Lin J.S., Franco P. Pitolisant, an inverse agonist of the histamine H3 receptor: An alternative stimulant for narcolepsy-cataplexy in teenagers with refractory sleepiness. Clin. Neuropharmacol. 2012;35:55–60. doi: 10.1097/WNF.0b013e318246879d. [DOI] [PubMed] [Google Scholar]
- 164.Syed Y.Y. Pitolisant: First Global Approval. Drugs. 2016;76:1313–1318. doi: 10.1007/s40265-016-0620-1. [DOI] [PubMed] [Google Scholar]
- 165.Bajda M., Łażewska D., Godyń J., Zaręba P., Kuder K., Hagenow S., Łątka K., Stawarska E., Stark H., Kieć-Kononowicz K., et al. Search for new multi-target compounds against Alzheimer’s disease among histamine H3 receptor ligands. Eur. J. Med. Chem. 2020;185:111785. doi: 10.1016/j.ejmech.2019.111785. [DOI] [PubMed] [Google Scholar]
- 166.Huang W., Tang L., Shi Y., Huang S., Xu L., Sheng R., Wu P., Li J., Zhou N., Hu Y. Searching for the Multi-Target-Directed Ligands against Alzheimer’s disease: Discovery of quinoxaline-based hybrid compounds with AChE, H 3R and BACE 1 inhibitory activities. Bioorg. Med. Chem. 2011;19:7158–7167. doi: 10.1016/j.bmc.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 167.Fernández-Bachiller M.I., Pérez C., González-Muñoz G.C., Conde S., López M.G., Villarroya M., García A.G., Rodríguez-Franco M.I. Novel tacrine-8-hydroxyquinoline hybrids as multifunctional agents for the treatment of Alzheimers disease, with neuroprotective, cholinergic, antioxidant, and copper-complexing properties. J. Med. Chem. 2010;53:4927–4937. doi: 10.1021/jm100329q. [DOI] [PubMed] [Google Scholar]
- 168.Li S.Y., Wang X.B., Xie S.S., Jiang N., Wang K.D.G., Yao H.Q., Sun H.B., Kong L.Y. Multifunctional tacrine-flavonoid hybrids with cholinergic, β-amyloid-reducing, and metal chelating properties for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2013;69:632–646. doi: 10.1016/j.ejmech.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 169.Xie S.S., Wang X.B., Li J.Y., Yang L., Kong L.Y. Design, synthesis and evaluation of novel tacrine-coumarin hybrids as multifunctional cholinesterase inhibitors against Alzheimer’s disease. Eur. J. Med. Chem. 2013;64:540–553. doi: 10.1016/j.ejmech.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 170.Yan J., Hu J., Liu A., He L., Li X., Wei H. Design, synthesis, and evaluation of multitarget-directed ligands against Alzheimer’s disease based on the fusion of donepezil and curcumin. Bioorg. Med. Chem. 2017;25:2946–2955. doi: 10.1016/j.bmc.2017.02.048. [DOI] [PubMed] [Google Scholar]
- 171.Wichur T., Więckowska A., Więckowski K., Godyń J., Jończyk J., Valdivieso Á.D.R., Panek D., Pasieka A., Sabaté R., Knez D., et al. 1-Benzylpyrrolidine-3-amine-based BuChE inhibitors with anti-aggregating, antioxidant and metal-chelating properties as multifunctional agents against Alzheimer’s disease. Eur. J. Med. Chem. 2020;187:111916. doi: 10.1016/j.ejmech.2019.111916. [DOI] [PubMed] [Google Scholar]
- 172.Fuse S., Matsumura K., Fujita Y., Sugimoto H., Takahashi T. Development of dual targeting inhibitors against aggregations of amyloid-β and tau protein. Eur. J. Med. Chem. 2014;85:228–234. doi: 10.1016/j.ejmech.2014.07.095. [DOI] [PubMed] [Google Scholar]
- 173.Prati F., De Simone A., Armirotti A., Summa M., Pizzirani D., Scarpelli R., Bertozzi S.M., Perez D.I., Andrisano V., Perez-Castillo A., et al. 3,4-Dihydro-1,3,5-triazin-2(1H)-ones as the First Dual BACE-1/GSK-3β Fragment Hits against Alzheimer’s Disease. ACS Chem. Neurosci. 2015;6:1665–1682. doi: 10.1021/acschemneuro.5b00121. [DOI] [PubMed] [Google Scholar]
- 174.Bottegoni G., Veronesi M., Bisignano P., Kacker P., Favia A.D., Cavalli A. Development and Application of a Virtual Screening Protocol for the Identification of Multitarget Fragments. ChemMedChem. 2016;11:1259–1263. doi: 10.1002/cmdc.201500521. [DOI] [PubMed] [Google Scholar]
- 175.Di Martino R.M.C., De Simone A., Andrisano V., Bisignano P., Bisi A., Gobbi S., Rampa A., Fato R., Bergamini C., Perez D.I., et al. Versatility of the Curcumin Scaffold: Discovery of Potent and Balanced Dual BACE-1 and GSK-3β Inhibitors. J. Med. Chem. 2016;59:531–544. doi: 10.1021/acs.jmedchem.5b00894. [DOI] [PubMed] [Google Scholar]
- 176.Xie S., Chen J., Li X., Su T., Wang Y., Wang Z., Huang L., Li X. Synthesis and evaluation of selegiline derivatives as monoamine oxidase inhibitor, antioxidant and metal chelator against Alzheimer’s disease. Bioorg. Med. Chem. 2015;23:3722–3729. doi: 10.1016/j.bmc.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 177.Wang Z., Wang Y., Wang B., Li W., Huang L., Li X. Design, synthesis, and evaluation of orally available clioquinol-moracin M hybrids as multitarget-directed ligands for cognitive improvement in a rat model of neurodegeneration in Alzheimer’s disease. J. Med. Chem. 2015;58:8616–8637. doi: 10.1021/acs.jmedchem.5b01222. [DOI] [PubMed] [Google Scholar]
- 178.AlFadly E.D., Elzahhar P.A., Tramarin A., Elkazaz S., Shaltout H., Abu-Serie M.M., Janockova J., Soukup O., Ghareeb D.A., El-Yazbi A.F., et al. Tackling neuroinflammation and cholinergic deficit in Alzheimer’s disease: Multi-target inhibitors of cholinesterases, cyclooxygenase-2 and 15-lipoxygenase. Eur. J. Med. Chem. 2019;167:161–186. doi: 10.1016/j.ejmech.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 179.Castanho I., Lunnon K. Chromatin Signaling and Neurological Disorders. Elsevier; Amsterdam, The Netherlands: 2019. Epigenetic Processes in Alzheimer’s Disease; pp. 153–180. [Google Scholar]
- 180.Tomaselli D., Lucidi A., Rotili D., Mai A. Epigenetic polypharmacology: A new frontier for epi-drug discovery. Med. Res. Rev. 2020;40:190–244. doi: 10.1002/med.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Maes T., Mascaró C., Rotllant D., Lufino M.M.P., Estiarte A., Guibourt N., Cavalcanti F., Griñan-Ferré C., Pallàs M., Nadal R., et al. Modulation of KDM1A with vafidemstat rescues memory deficit and behavioral alterations. PLoS ONE. 2020;15:e0233468. doi: 10.1371/journal.pone.0233468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cuadrado-Tejedor M., Garcia-Barroso C., Sánchez-Arias J.A., Rabal O., Pérez-González M., Mederos S., Ugarte A., Franco R., Segura V., Perea G., et al. A First-in-Class Small-Molecule that Acts as a Dual Inhibitor of HDAC and PDE5 and that Rescues Hippocampal Synaptic Impairment in Alzheimer’s Disease Mice. Neuropsychopharmacology. 2016;42:524–539. doi: 10.1038/npp.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.De Simone A., La Pietra V., Betari N., Petragnani N., Conte M., Daniele S., Pietrobono D., Martini C., Petralla S., Casadei R., et al. Discovery of the First-in-Class GSK-3β/HDAC Dual Inhibitor as Disease-Modifying Agent to Combat Alzheimer’s Disease. ACS Med. Chem. Lett. 2019;10:469–474. doi: 10.1021/acsmedchemlett.8b00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Selkoe D.J. Introducing transglutaminase into the study of Alzheimer’s disease. A personal look back. Neurochem Int. 2002;40:13–16. doi: 10.1016/S0197-0186(01)00057-2. [DOI] [PubMed] [Google Scholar]
- 185.Basso M., Chen H.H., Tripathy D., Conte M., Apperley K.Y.P., De Simone A., Keillor J.W., Ratan R., Nebbioso A., Sarno F., et al. Designing Dual Transglutaminase 2/Histone Deacetylase Inhibitors Effective at Halting Neuronal Death. ChemMedChem. 2018;13:227–230. doi: 10.1002/cmdc.201700601. [DOI] [PubMed] [Google Scholar]
- 186.Benek O., Korabecny J., Soukup O. A Perspective on Multi-target Drugs for Alzheimer’s Disease. Trends Pharmacol. Sci. 2020;41:434–445. doi: 10.1016/j.tips.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 187.Savelieff M.G., Nam G., Kang J., Lee H.J., Lee M., Lim M.H. Development of multifunctional molecules as potential therapeutic candidates for Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis in the last decade. Chem. Rev. 2018;119:1221–1322. doi: 10.1021/acs.chemrev.8b00138. [DOI] [PubMed] [Google Scholar]
- 188.Mishra P., Kumar A., Panda G. Anti-cholinesterase hybrids as multi-target-directed ligands against Alzheimer’s disease (1998–2018) Bioorg. Med. Chem. 2019;27:895–930. doi: 10.1016/j.bmc.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 189.Rankovic Z. CNS Drug Design: Balancing Physicochemical Properties for Optimal Brain Exposure. J. Med. Chem. 2015;58:2584–2608. doi: 10.1021/jm501535r. [DOI] [PubMed] [Google Scholar]
- 190.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997;23:3–25. doi: 10.1016/S0169-409X(96)00423-1. [DOI] [PubMed] [Google Scholar]
- 191.Cordon-Cardo C., O’Brien J.P., Casals D., Rittman-Grauer L., Biedler J.L., Melamed M.R., Bertino J.R. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc. Natl. Acad. Sci. USA. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pajouhesh H., Lenz G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx. 2005;2:541–553. doi: 10.1602/neurorx.2.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lan J.S., Xie S.S., Li S.Y., Pan L.F., Wang X.B., Kong L.Y. Design, synthesis and evaluation of novel tacrine-(β-carboline) hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2014;22:6089–6104. doi: 10.1016/j.bmc.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 194.Tang H., Zhao H.-T., Zhong S.-M., Wang Z.-Y., Chen Z.-F., Liang H. Novel oxoisoaporphine-based inhibitors of acetyl- and butyrylcholinesterase and acetylcholinesterase-induced beta-amyloid aggregation. Bioorg. Med. Chem. Lett. 2012;22:2257–2261. doi: 10.1016/j.bmcl.2012.01.090. [DOI] [PubMed] [Google Scholar]
- 195.He Q., Liu J., Liang J., Liu X., Li W., Liu Z., Ding Z., Tuo D. Towards Improvements for Penetrating the Blood–Brain Barrier—Recent Progress from a Material and Pharmaceutical Perspective. Cells. 2018;7:24. doi: 10.3390/cells7040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Llorens-Martín M., Jurado J., Hernández F., Ávila J., Wang H., Cieslikiewicz-bouet M., Naldi M., Bartolini M., Pérez B., Servent D., et al. Epigenetic dysregulation of enhancers in neurons is associated with Alzheimer’s disease pathology and cognitive symptoms. Eur. J. Med. Chem. 2019;10:1–8. doi: 10.1039/c8ra03620a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tavana J.P., Rosene M., Jensen N.O., Ridge P.G., Kauwe J.S.K., Karch C.M. RAB10: An alzheimer’s disease resilience locus and potential drug target. Clin. Interv. Aging. 2019;14:73–79. doi: 10.2147/CIA.S159148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kwok M.K., Lin S.L., Schooling C.M. Re-thinking Alzheimer’s disease therapeutic targets using gene-based tests. EBioMedicine. 2018;37:461–470. doi: 10.1016/j.ebiom.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Penney J., Ralvenius W.T., Tsai L.H. Modeling Alzheimer’s disease with iPSC-derived brain cells. Mol. Psychiatry. 2020;25:148–167. doi: 10.1038/s41380-019-0468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Esquerda-Canals G., Montoliu-Gaya L., Güell-Bosch J., Villegas S. Mouse Models of Alzheimer’s Disease. J. Alzheimer’s Dis. 2017;57:1171–1183. doi: 10.3233/JAD-170045. [DOI] [PubMed] [Google Scholar]
- 201.Sasaguri H., Nilsson P., Hashimoto S., Nagata K., Saito T., De Strooper B., Hardy J., Vassar R., Winblad B., Saido T.C. APP mouse models for Alzheimer’s disease preclinical studies. EMBO J. 2017;36:2473–2487. doi: 10.15252/embj.201797397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cummings J., Lee G., Ritter A., Sabbagh M., Zhong K. Alzheimer’s disease drug development pipeline: 2019. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019;5:272–293. doi: 10.1016/j.trci.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rasagiline Rescue in Alzheimer’s Disease Clinical Trial—Full Text View—ClinicalTrials.gov. [(accessed on 24 June 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02359552.
- 204.Study of LM11A-31-BHS in Mild-moderate AD Patients—Full Text View—ClinicalTrials.gov. [(accessed on 24 June 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03069014.
- 205.Hemonnot A.L., Hua J., Ulmann L., Hirbec H. Microglia in Alzheimer disease: Well-known targets and new opportunities. Front. Cell. Infect. Microbiol. 2019;9:233. doi: 10.3389/fnagi.2019.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Paudel Y.N., Angelopoulou E., Piperi C., Othman I., Aamir K., Shaikh M.F., Moore Z., Taylor J.M., Crack P.J., Hemonnot A.L., et al. Microglia in Alzheimer disease: Well-known targets and new opportunities. Cells. 2019;9:3533–3543. doi: 10.1111/bph.14546. [DOI] [Google Scholar]
- 207.Nizami S., Hall-Roberts H., Warrier S., Cowley S.A., Di Daniel E. Microglial inflammation and phagocytosis in Alzheimer’s disease: Potential therapeutic targets. Br. J. Pharmacol. 2019;176:3515–3532. doi: 10.1111/bph.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Moore Z., Taylor J.M., Crack P.J. The involvement of microglia in Alzheimer’s disease: A new dog in the fight. Br. J. Pharmacol. 2019;176:3533–3543. doi: 10.1111/bph.14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Safety, Tolerability, and Pharmacokinetic Study of Single Ascending Doses of GC021109 in Healthy Subjects—Full Text View—ClinicalTrials.gov. [(accessed on 20 June 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02254369.
- 210.Chen Q., Du Y., Zhang K., Liang Z., Li J., Yu H., Ren R., Feng J., Jin Z., Li F., et al. Tau-Targeted Multifunctional Nanocomposite for Combinational Therapy of Alzheimer’s Disease. ACS Nano. 2018;12:1321–1338. doi: 10.1021/acsnano.7b07625. [DOI] [PubMed] [Google Scholar]
- 211.Guo Q., Xu S., Yang P., Wang P., Lu S., Sheng D., Qian K., Cao J., Lu W., Zhang Q. A dual-ligand fusion peptide improves the brain-neuron targeting of nanocarriers in Alzheimer’s disease mice. J. Control. Release. 2020;320:347–362. doi: 10.1016/j.jconrel.2020.01.039. [DOI] [PubMed] [Google Scholar]
- 212.Lu Y., Guo Z., Zhang Y., Li C., Zhang Y., Guo Q., Chen Q., Chen X., He X., Liu L., et al. Microenvironment Remodeling Micelles for Alzheimer’s Disease Therapy by Early Modulation of Activated Microglia. Adv. Sci. 2019;6 doi: 10.1002/advs.201970024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Paolini G.V., Shapland R.H.B., Van Hoorn W.P., Mason J.S., Hopkins A.L. Global mapping of pharmacological space. Nat. Biotechnol. 2006;24:805–815. doi: 10.1038/nbt1228. [DOI] [PubMed] [Google Scholar]
- 214.Keiser M.J., Roth B.L., Armbruster B.N., Ernsberger P., Irwin J.J., Shoichet B.K. Relating protein pharmacology by ligand chemistry. Nat. Biotechnol. 2007;25:197–206. doi: 10.1038/nbt1284. [DOI] [PubMed] [Google Scholar]
- 215.Simoni E., Daniele S., Bottegoni G., Pizzirani D., Trincavelli M.L., Goldoni L., Tarozzo G., Reggiani A., Martini C., Piomelli D., et al. Combining galantamine and memantine in multitargeted, new chemical entities potentially useful in Alzheimer’s disease. J. Med. Chem. 2012;55:9708–9721. doi: 10.1021/jm3009458. [DOI] [PubMed] [Google Scholar]
- 216.Prati F., De Simone A., Bisignano P., Armirotti A., Summa M., Pizzirani D., Scarpelli R., Perez D.I., Andrisano V., Perez-Castillo A., et al. Multitarget drug discovery for Alzheimer’s disease: Triazinones as BACE-1 and GSK-3β inhibitors. Angew. Chemie—Int. Ed. 2015;54:1578–1582. doi: 10.1002/anie.201410456. [DOI] [PubMed] [Google Scholar]
- 217.Besnard J., Ruda G.F., Setola V., Abecassis K., Rodriguiz R.M., Huang X.P., Norval S., Sassano M.F., Shin A.I., Webster L.A., et al. Automated design of ligands to polypharmacological profiles. Nature. 2012;492:215–220. doi: 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Oset-Gasque M.J., Marco-Contelles J. Alzheimer’s Disease, the “one-Molecule, One-Target” Paradigm, and the Multitarget Directed Ligand Approach. ACS Chem. Neurosci. 2018;9:401–403. doi: 10.1021/acschemneuro.8b00069. [DOI] [PubMed] [Google Scholar]
- 219.Morphy R., Kay C., Rankovic Z. From magic bullets to designed multiple ligands. Drug Discov. Today. 2004;9:641–651. doi: 10.1016/S1359-6446(04)03163-0. [DOI] [PubMed] [Google Scholar]
- 220.Morphy R., Rankovic Z. The physicochemical challenges of designing multiple ligands. J. Med. Chem. 2006;49:4961–4970. doi: 10.1021/jm0603015. [DOI] [PubMed] [Google Scholar]
- 221.Ramsay R.R., Popovic-Nikolic M.R., Nikolic K., Uliassi E., Bolognesi M.L. A perspective on multi-target drug discovery and design for complex diseases. Clin. Transl. Med. 2018;7 doi: 10.1186/s40169-017-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Prati F., Cavalli A., Bolognesi M. Navigating the Chemical Space of Multitarget-Directed Ligands: From Hybrids to Fragments in Alzheimer’s Disease. Molecules. 2016;21:466. doi: 10.3390/molecules21040466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ertekin-Taner N. Identifying therapeutic targets for Alzheimer’s disease with big data. Neurodegener. Dis. Manag. 2017;7:101–105. doi: 10.2217/nmt-2017-0008. [DOI] [PubMed] [Google Scholar]