Summary
Achieving most of the UN Sustainable Development Goals requires a strong focus on addressing the double burden of malnutrition, which includes both diet-related maternal and child health (MCH) and non-communicable diseases (NCDs). Although, the most optimal dietary metric for assessing malnutrition remains unclear. Our aim was to review available global dietary quality metrics (hereafter referred to as dietary metrics) and evidence for their validity to assess MCH and NCD outcomes, both separately and together. A systematic search of PubMed was done to identify meta-analyses or narrative reviews evaluating validity of diet metrics in relation to nutrient adequacy or health outcomes. We identified seven dietary metrics aiming to address MCH and 12 for NCDs, no dietary metrics addressed both together. Four NCD dietary metrics (Mediterranean Diet Score, Alternative Healthy Eating Index, Healthy Eating Index, and Dietary Approaches to Stop Hypertension) had convincing evidence of protective associations with specific NCD outcomes, mainly mortality, cardiovascular disease, type 2 diabetes, and total cancer. The remaining NCD dietary metrics and all MCH dietary metrics were not convincingly validated against MCH or NCD health outcomes. None of the dietary metrics had been validated against both MCH and NCD outcomes. These findings highlight major gaps in assessing and addressing diet to achieve global targets and effective policy action.
Introduction
Poor diet quality is a leading and preventable cause of adverse health globally, which includes both maternal and child health (MCH) and non-communicable diseases (NCDs).1, 2 The UN Sustainable Development Goals (SDGs) outline global consensus on social, economic, environmental, and health targets to be met by 2030, with most goals concerned with nutrition including one goal to end malnutrition.3 Yet rates of progress toward achieving the SDGs have been slow, and accelerated momentum is needed.4, 5, 6, 7, 8 To develop sound strategies and monitor progress toward these goals, the assessment of global dietary quality is essential.
Although a variety of dietary metrics have been developed and used to summarise various components of a diet (eg, adequacy, quality, diversity),9, 10 there remains an absence of widely used, validated metrics to define the double burden of malnutrition, and to compare effectively across country settings. This absence at least partly relates to a historical distinction between global nutrition efforts for MCH versus NCDs.11, 12, 13 Malnutrition for MCH has traditionally been considered as insufficient caloric or other nutrient intake leading to insufficient physical growth (stunting), rapid weight loss or failure to gain weight (wasting), cognitive impairment, exacerbation of anaemia and blindness, or weakening of the immune system resulting in increased risk of infectious diseases and mortality.14 Malnutrition for NCDs has historically been considered as excess consumption of certain nutrients such as fat, sugar, and salt as well as calories.12, 15 Yet, malnutrition for MCH and NCDs are frequently coexisting consequences of poor diet quality within populations, households, or individuals across the lifespan.16, 17 A unified global dietary quality metric (hereafter referred to as dietary metric) would aid in policy and programme decision making around improving diet in which components that contribute to both MCH and NCDs would be represented in the same assessments, and that the relative contributions of malnutrition for MCH and NCDs could be characterised and compared across settings in a standardised way.
The optimal dietary metric for assessing malnutrition for both MCH and NCDs remains uncertain. Previous reviews have described the development and characteristics of dietary metrics.9, 18, 19, 20, 21, 22, 23, 24 Some reviews qualitatively summarised the evidence for dietary metrics in relation to a single health outcome (eg, type 2 diabetes, obesity)25, 26 and others focused on several MCH10 or NCD outcomes.20, 27, 28, 29, 30 However, to our knowledge, the comprehensive reviews of dietary metrics in relation to several MCH or NCD outcomes10, 20, 27, 28, 29, 30 were done more than a decade ago, and no review has focused on both MCH and NCD outcomes together. To understand whether any existing dietary metrics are validated against MCH or NCDs, or both, and what researchers and health organisations are using to measure various aspects of the dietary contribution to MCH and NCDs, we extensively reviewed and assessed the validity of existing multinational dietary metrics for MCH and NCD health outcomes.
Key messages.
-
•
Many global efforts are focused on tackling the double burden of malnutrition including the UN Sustainable Development Goals. However, these targets might not be achieved without a practical and valid dietary metric to reduce malnutrition across global contexts.
-
•
We identified 19 dietary metrics, including seven developed for maternal and child health (MCH), 12 developed for non-communicable diseases (NCDs) and none developed or applied for both.
-
•
All MCH dietary metrics used foods only, while most NCD metrics included foods and nutrients. The most frequent components for both MCH and NCD dietary metrics were vegetables, fruits, grains, roots, and tubers.
-
•
When the validity was assessed, we found that four NCD metrics had convincing evidence of protective associations with specific NCD outcomes, primarily mortality, cardiovascular disease, type 2 diabetes, and total cancer. The remaining eight NCD dietary metrics and all seven MCH dietary metrics did not.
-
•
We found that few dietary metrics have been validated against MCH or NCD health outcomes, and none for both indicates. More work is needed to validate existing and novel dietary metrics for MCH and NCD, an approach that is likely critical to the achievement of global nutrition and health targets.
Dietary metric definition and inclusion
We defined a dietary metric as a metric derived from nutrients or food or food groups, or both, with the aim of measuring dietary diversity (number of different foods consumed), or nutrient adequacy (achievement of recommended intakes of energy or essential nutrients).10 We included dietary metrics with the reported intended use of relating diet to MCH (micronutrient adequacy, mortality in children <5 years, maternal mortality, underweight, stunting, wasting, infectious diseases, diarrhoeal disease) and NCD health outcomes (all-cause mortality, cardiovascular disease, type 2 diabetes, gestational diabetes; total cancer and subtypes, and anthropometrics in adults and children), and that quantified the level of intake of foods or nutrients consumed or the achievement of recommended intakes. A detailed description of the health outcomes is shown in the appendix (p 1). We selected the health outcomes in accordance with WHO's categories of malnutrition.31 Given the interest in dietary metrics that can be used globally, we included dietary metrics that were used for this purpose in at least three countries. We did not include dietary metrics that included non-dietary factors as a core component, that only summarised a single nutrient or food, or that were developed with a primary focus on non-health outcomes (eg, sustainability). We did not include dietary metrics derived primarily from statistical data clustering approaches, such as cluster or factor analysis, because these are often not generalisable.32 We also did not include dietary metrics at the population level (eg, food supply) because we were interested in individual-level and household-level dietary metrics.
Literature searches
Given the abundance of potential dietary metrics, we did not systematically identify all possible metrics. First, we compiled a list of dietary metrics through expert contacts, who were faculty members at the Friedman School of Nutrition Science, Tufts University, with expertise in food policy, agriculture, economics, international nutrition programming, humanitarian emergency relief, clinical nutrition, and epidemiology. This initial list was then used to search PubMed and Google to identify studies and development reports from government agencies, health organisations, and non-profit organisations up until Sept 21, 2018, that included these dietary metrics, met our inclusion criteria, and were published in English. These searches were complemented by hand-searching of the citations of all identified articles and reports to identify additional potential dietary metrics. We documented all identified dietary metrics that both did and did not meet the scope of our inclusion criteria (appendix p 5).
To assess validity of each relevant dietary metric, we systematically searched PubMed from Oct 11, 2000, to April 17, 2020, to identify meta-analyses or narrative reviews (including systematic reviews without a meta-analysis, but not umbrella reviews) evaluating these dietary metrics in relation to MCH and NCD health outcomes. The search terms and restrictions are described in full in the appendix (pp 2–4).
Data extraction
VM did the literature searches, assessed titles and abstracts of all identified studies and reviewed and extracted relevant data by hand using a standardised electronic spreadsheet. The intended purpose, food and nutrient components, scoring, reference period, validity, and reliability was tabulated on each dietary metric. For each published meta-analysis or narrative review, data were extracted on the number of studies included, pooled relative risks and corresponding uncertainty, sample sizes, number of events, follow-up durations, unit of exposures, and population characteristics. In most cases, the total number of participants was not reported in the original meta-analyses and was computed from summary tables of the individual studies. Because narrative reviews do not provide pooled estimates, the extractions were done for each individual study included in the narrative review. When more than one meta-analysis or narrative review was identified for each dietary metric-health outcome relationship, we included all published meta-analyses or narrative reviews. Questions or uncertainties related to article screening and extraction were resolved by discussion with another investigator (DM).
Assessment and grading of validity
Two investigators (VM and then either PW, RM, or DM), independently and in duplicate, used the most comprehensive (with the greatest number of participants and studies) or recent meta-analysis when available, followed by the most comprehensive (with the greatest number of participants and studies) or recent narrative review to assess and grade the evidence for validity of dietary metrics in relation to MCH and NCD health outcomes. In cases in which the most recent meta-analysis or narrative review was not the most comprehensive, we selected the most comprehensive. Each assessor extracted data on the number of studies and the direction of effects across studies (positive association, null association, negative association). If the number of studies with null associations was equal or greater to the number of studies with positive or negative associations, the relationship was classified as no association. For both meta-analyses and narrative reviews, we considered the direction of effects from each included individual study. The validity of each dietary metric was assessed using two criteria: first, the number of studies, and second, the consistency of evidence from meta-analyses and narrative reviews of prospective cohort studies or randomised controlled trials. Consistency was defined as the association is repeatedly observed in different populations and circumstances. For a consistent relationship, at least half of the associations were in the same direction and for an inconsistent relationship, fewer than half of the associations were in the same direction. Three assessment categories were established: consistent evidence for the dietary metric-health outcome relationship (positive, null, negative) from five or more studies; inconsistent evidence for the dietary metric-health outcome relationship from five or more studies; and little evidence from less than five studies. For assessing validity of each dietary metric against foods, nutrients, and other non-dietary metrics (eg, biomarkers, food insecurity indicators) we used the published papers or development reports that described the development process of the dietary metric. All disagreements among reviewing investigators regarding the assessment and grading of validity were resolved through discussion.
Identified dietary metrics
In total, we identified 19 dietary metrics used in three or more countries each to assess diet quality in relation to various health outcomes (Table 1, Table 2, Table 3). Seven dietary metrics were primarily used for MCH: Dietary Diversity Score (DDS), Food Consumption Score (FCS), Food Variety Score (FVS), Household Dietary Diversity Score (HDDS), Infant and Young Child Minimum Dietary Diversity (IYCMDD), Minimum Dietary Diversity for Women (MDD-W), and Women's Dietary Diversity Score (WDDS) and Individual Dietary Diversity Score (IDDS). Twelve dietary metrics were most commonly used for NCDs: Alternative Healthy Eating Index (AHEI), Dietary Approaches to Stop Hypertension (DASH), Dietary Guidelines for Americans Adherence Index (DGAI), Dietary Inflammatory Index (DII), Dietary Quality Index International (DQI-I), Healthy Eating Index-2010 (HEI), Mediterranean Diet Quality Index for Children and Teenagers (KIDMED), Mediterranean Diet Score (MED), Prospective Urban Rural Epidemiology Diet Score (PURE), Recommended Foods Score (RFS), WHO Healthy Diet Metric (WHO-HDI), and World Cancer Research Fund and American Institute for Cancer Research Dietary Recommendations (WCRF-AICR). No dietary metrics were identified that were used to assess both MCH and NCDs.
Table 1.
Dietary metrics used for assessing maternal and child health
|
Foods or nutrients and reference period |
Scoring and cutoffs |
||||
|---|---|---|---|---|---|
| Item list | Reference period | Calculation | Range | Cutoffs or classification | |
| DDS*33, 34 | |||||
| Metric focuses on the mean number of major food groups consumed; the original DDS was developed using data from the National Health and Nutrition Examination Survey; multiple versions of the DDS have been used, including adaptations for children35 | Dairy; meat; grain; fruit; vegetable | 24 h | Count of food groups consumed | 0 to 5 | No cutoff |
| FCS36, 37, 38 | |||||
| Metric focuses on predicting adequate food quantity or calorie consumption per capita in households from low-income and middle-income countries | Main staples (cereals and cereal products, roots and tubers); pulses; vegetables; fruit; meat or fish; milk; sugar; oil | 7 days | Frequency-weighted score calculated using the frequency of consumption of food groups consumed by a household during reference period; data on food frequency are grouped into food groups and the consumption frequencies for each food items within a group are summed to yield a score for the food group; any food group score >7 is truncated at 7; values obtained for each food group are multiplied by weights (weights range from 0·5 to 4·0 and are based on nutrient density) to create weighted food group scores; the weights for each food group are sugar and oil (0·5), vegetables and fruit (1·0), staples (2·0), pulses (3·0), and meat or fish and milk (4·0); the weights are summed | 0 to 112 | Usual cutoffs are 0 to 21 (poor), 21·5 to 35·0 (borderline), and >35 (acceptable); cutoff for locations where oil and sugar are consumed daily are 0 to 28 (poor), 28·5 to 42·0 (borderline), and >42 (acceptable) |
| FVS35, 39, 40 | |||||
| Metric focuses on the number of unique foods consumed during the reference period; commonly used among children ≤5 years as a measure of dietary diversity | Not applicable | 24 h | Count of food groups consumed | Not applicable | Not specified |
| HDDS41, 42, 43 | |||||
| Metric focuses on whether the number of unique foods consumed over a given period is a good measure of household food access in urban and rural areas; HDDS is typically measured in the person primarily responsible for food preparation in the household | Cereals; roots and tubers; legumes, nuts, and seeds; dairy; meat; fish; eggs; vegetables; fruit; oils and fats; sweets; spices, condiments, and beverages (Food and Nutrition Technical Assistance Project); and cereals; white roots and tubers; legumes, nuts, and seeds; milk and milk products; organ meat; flesh meat; fish and seafood; eggs; vitamin A-rich vegetables and tubers; dark green leafy vegetables; other vegetables; vitamin A-rich fruits; other fruits; oils and fats; sweets; spices, condiments, and beverages (Food and Agriculture Organization) | 24 h | Count of food groups consumed | 0 to 12 | Not specified |
| IYCMDD44, 45 | |||||
| Metric focuses on dietary diversity as a marker of micronutrient adequacy for ten nutrients (thiamin, riboflavin, vitamin B-6, folate, vitamin C, vitamin A, calcium, zinc, iron, and without iron separately) in children aged 6 to 23 months (both breastfed and non-breastfed) in low-income and middle-income countries; WHO recommended metric of infant and young child feeding practices | Grains, roots, and tubers; legumes and nuts; dairy; flesh foods (meat, fish, poultry and liver or organ meats); eggs; vitamin A-rich fruits and vegetables; other fruits and vegetables | 24 h | Count of food groups consumed | 0 to 7 | WHO guidelines on infant and young child feeding practices defines minimum dietary diversity ≥4 food groups consumed |
| MDD-W46, 47 | |||||
| Metric focuses on dietary diversity as a marker of micronutrient adequacy for 11 nutrients (thiamin, riboflavin, niacin, vitamin B-6, folate, vitamin B-12, vitamin C, vitamin A, calcium, iron, zinc) in women of reproductive age (15 to 49 years; both non-pregnant non-lactating and lactating women) in low-income and middle-income countries | Grains, white roots and tubers, and plantains; pulses; nuts and seeds; dairy; meat, poultry, and fish; eggs; dark green leafy vegetables; other vitamin A-rich fruits and vegetables; other vegetables; other fruits | 24 h | Count of food groups consumed | 0 to 10 | Recommendation is ≥5 food groups consumed |
| WDDS and IDDS42, 47 | |||||
| Metric focuses on the probability of micronutrient density in the diet of women of reproductive age (15 to 49 years; WDDS) and most commonly used in children aged 6–23 months (IDDS) in low-income and middle-income countries | Cereals; white roots and tubers; legumes, nuts, and seeds; dairy; organ meat; flesh meat; fish; eggs; vitamin A-rich vegetables and tubers; dark green leafy vegetables; other vegetables; vitamin A-rich fruits; other fruits; oil and fats; sweets; condiments that are aggregated into the following food groups of starchy staples; legumes, nuts, and seeds; milk and milk products; organ meat; meat and fish; eggs; dark green leafy vegetables; other vitamin A-rich fruits and vegetables; other fruits and vegetables | 24 h | Count of food groups consumed | 0 to 9 or 0 to 16 depending on whether further aggregation occurs | No universal cutoff; Recommendation is to use mean value or distribution to identify cutoff for the specific population |
The modifications and adaptations to dietary metrics noted are not exhaustive. DDS=Dietary Diversity Score. FCS=World Food Programme's Food Consumption Score. FVS=Food Variety Score. HDDS=Household Dietary Diversity Score. IYCMDD=Infant and Young Child Minimum Dietary Diversity. MDD-W=Minimum Dietary Diversity for Women. WDDS=Women's Dietary Diversity Score. IDDS=Individual Dietary Diversity Score.
The DDS was classified as a metric for maternal and child health because it has primarily been used for this purpose despite being originally developed for chronic diseases.
Table 2.
Dietary metrics used for assessing non-communicable disease risk
|
Foods or nutrients and reference period |
Scoring and cutoffs |
||||
|---|---|---|---|---|---|
| Item list | Reference period | Calculation | Range | Cutoffs or classification | |
| AHEI-201048 | |||||
| Metric is an alternative version of the HEI that focuses on adherence to a dietary pattern associated with chronic disease risk; the AHEI was revised in 2010 to incorporate new scientific evidence on diet and health and is based on a comprehensive literature review and expert discussions to identify foods and nutrients robustly associated with low risk of chronic diseases | Vegetables; fruit; whole grains; sugar-sweetened beverages; nuts and legumes; red and processed meat; trans fat; long-chain (n-3) fats (eicosapentaenoic acid and docosahexaenoic acid); polyunsaturated fat; sodium; alcohol | Food frequency questionnaire | Components are scored from 0 (worst) to 10 (best) based on specified recommended intake for each component; the scoring for intermediate intake is not well described; recommended intake was determined a priori using the HEI recommendations, upper range of dietary guidelines (US and American Heart Association), and population distributions | 0 to 110 | Not specified |
| DASH49 | |||||
| Metric developed to measure adherence to the DASH diet, a dietary pattern used in randomised controlled feeding trials to lower blood pressure in people with hypertension; multiple variations of the DASH score have been used in the literature and the DASH score described by Fung et al (2008)50 is the most commonly used in the literature among US populations51 | Fruits; vegetables; nuts and legumes; low-fat dairy products; whole grains; sodium; sweetened beverages; red and processed meats (2008 version) | Food frequency questionnaire | For each component, sex-specific intake quintiles (Q) are computed, and a component score is assigned for each quintile; for fruits, vegetables, nuts and legumes, low-fat dairy products, and whole grains Q1 is assigned a value of 1, Q2 a value of 2, Q3 a value of 3, Q4 a value of 4, and Q5 a value of 5; alternatively for sodium, red and processed meats, and sweetened beverages Q1 is assigned a value of 5, Q2 a value of 4, Q3 a value of 3, Q4 a value of 2 and Q5 a value of 1; the component scores are summed (2008 version) | 5 to 40 | Not specified |
| DGAI52, 53, 54, 55, 56, 57, 58 | |||||
| Metric describes adherence to the key dietary recommendations in the 2005 Dietary Guidelines for Americans except for two recommendations for special populations (eg, individuals who should not consume alcohol) | Dark green vegetable; orange vegetable; legume; starchy vegetable; other vegetable; fruit; variety of fruits and vegetables; meat and legume; milk and milk products; grain; discretionary energy (food intake subscore); whole grain; fibre; low-fat choices; total fat; saturated fat; trans-fat; cholesterol; alcohol; sodium (healthy choice subscore) | Food frequency questionnaire | A score of 1 is assigned when intake meets the recommendation, 0·5 for intake >33% of the recommendation, and 0 for intake <33% of the recommendation | 0 to 20 | Not specified |
| DII59, 60, 61 | |||||
| Metric classifies an individuals' diet from pro-inflammatory to anti-inflammatory based on six inflammatory markers (IL-1β, IL-4, IL-6, IL-10, TNF-α, CRP) | Alcohol; vitamin B12; vitamin B6; β-carotene; caffeine; carbohydrate; cholesterol; energy; eugenol; total fat; fibre; folic acid; garlic; ginger; iron; magnesium; monounsaturated fat; niacin; n-3 fatty acids; n-6 fatty acids; onion; protein; polyunsaturated fat; riboflavin; saffron; saturated fat; selenium; thiamin; trans-fat; turmeric; vitamin A; vitamin C; vitamin D; vitamin E; zinc; green or black tea; flavan-3-ol; flavones; flavonones; anthocyanidins; isoflavones; pepper; thyme or oregano; rosemary | Food frequency questionnaire or 24 h | Dietary data are linked to the globally representative world database and the mean and standard deviation for each component are used as multipliers; the standard global mean is subtracted from each individual's reported amount, divided by the standard deviation and converted to a centred percentile score; the centred percentile score for each component for each individual is multiplied by the respective food parameter effect score (obtained from a literature review) to obtain a food parameter-specific score, which are summed to create an overall score; more negative scores represent anti-inflammatory diet, whereas more positive score represent pro-inflammatory diet | Approximate −10 to 10 | Not specified |
| DQI-I62, 63, 64 | |||||
| Metric was designed to promote aspects of a healthy diet in relation to major, diet-related chronic diseases and allow for international comparisons; DQI-I is a modified version of existing dietary metrics including the DQI, Institute of Nutrition and Food Hygiene-University of North Carolina at Chapel Hill Diet Quality Index, DQI-Revised, and HEI; other versions include: Med-DQI, Aussie-DQI, DQI-K, C-DQI, RC-DQI, DQI-CH | Meat, poultry, fish, or egg; dairy or beans; grains; fruits and vegetables (variety food groups); meat; poultry; fish; dairy; beans; eggs (variety protein sources); vegetables; fruit; grain; fibre; protein; iron; calcium and vitamin A (adequacy); total fat; saturated fat; cholesterol; sodium; empty calorie foods (moderation); macronutrient ratio; fatty acid ratio (overall balance) | Usual diet measured through multiple 24 h reference periods or food frequency questionnaire, or both | Variety is scored from 0 to 20, a score of 20 is allocated if at least one serving of food per day from all five food groups is consumed, if any of the food groups are not consumed, each food group consumed is scored 3 points each, maximum score of 15; adequacy is scored based on the percentage attainment of recommended intakes of eight components on a continuous scale, scoring ranges from 0 points for 0% to 5 points for 100% for each component, score range of 0 to 40; moderation is scored from 0 to 30 with a maximum of 6 points for each of the five components, intake of the components is scored as tiers with 0 points for the bottom tier, 3 points for the middle tier and 6 points for the highest tier; overall balance is scored from 0 to 10 and consists of macronutrient ratio, which is scored from 0 to 6 points based on four tiers in 2-point increments and fatty acid ratio, which is scored on three tiers in 2-point increments | 0 to 100 | No cutoff |
| HEI-201065, 66, 67, 68, 69 | |||||
| Metric describes adherence to the 2010 Dietary Guidelines for Americans; Other variations are HEI-2005 and HEI-2015 based on the corresponding year of US Dietary Guidelines, Chinese HEI, and HEI-Canada | Total fruit (includes fruit juice); whole fruit (includes all forms except juice); total vegetables; greens and beans; whole grains; refined grains; dairy; total protein foods; seafood and plant proteins; fatty acids (polyunsaturated, monounsaturated, and saturated); sodium; empty calories (energy from solid fats, alcohol, and added sugar) | Food frequency questionnaire | Maximum 5 points for total fruit, whole fruit, total vegetables, greens and beans, total protein foods, seafood and plant protein; maximum 10 points for whole grains, dairy, fatty acids, refined grains, sodium; maximum 20 points for empty calories; maximum score for each component is based on 2010 US dietary guidelines; the component scores are summed | 0 to 100 | Not specified |
| KIDMED70, 71, 72, 73, 74, 75, 76, 77 | |||||
| Metric describes adherence to the Mediterranean diet pattern in adolescents | Components grouped into favourable and non-favourable; favourable components are daily fruit or fruit juice, eats second fruit serving daily, one daily serving of fresh or cooked vegetables, >1 daily serving of fresh or cooked vegetables, fish (2 to 3/week), legumes consumed >1/week, pasta or rice ≥5/week, cereals or grains consumed for breakfast, nuts >2 to 3/week, uses olive oil at home, dairy for breakfast (eg, yoghurt, milk), or ≥2 daily yoghurt or cheese (40 g); non-favourable components are fast food consumed >1/week, skips breakfast, commercial baked goods or pastries for breakfast, and sweets and candy several times per day | Food frequency questionnaire | Beneficial items are assigned a value of 1 when met or 0 when not met, and non-beneficial items are assigned a value of −1 when met and 0 when not met; the component scores are summed | 0 to 12 | Poor adherence is 0 to 3, average adherence is 4 to 7, and good adherence is 8 to 12 |
| MED78, 79, 80, 81 | |||||
| Metric describes adherence to the Mediterranean diet pattern in adults; variations of the MED (MDS [an alternative published abbreviation for MED], rMED, MSDPS, aMDS) exist and have been used in populations including Denmark, France, Germany, UK, Spain, Netherlands, Norway, Sweden, Italy, Switzerland, Belgium, Portugal, Hungary, Canada, USA, Japan, China and Australia23, 82, 83 | Fruits, vegetables, legumes, cereals, meat and meat products, dairy, monounsaturated fatty acids-saturated fatty acids (MUFA-SFA) ratio, and alcohol (1999 version); fruits and nuts, vegetables, legumes, cereals, meat and meat products, dairy, MUFA-SFA ratio, alcohol, and fish (2003 version) | Food frequency questionnaire | Scoring not described; for 1999 version; for the 2003 version calculate sex-specific medians; intake below the median for beneficial components (vegetables, legumes, fruits and nuts, cereal, and fish) are assigned a value of 0, and intake above or at the median is assigned a value of 1; components assumed to be detrimental (meat, poultry, and dairy products) intake below the median is assigned a value of 1 and at or above the median a value of 0; for ethanol, a value of 1 is assigned to men who consume between 10 and 50 g per day and to women who consume between 5 and 25 g per day; for MUFA-SFA ratio intake at or above the median is assigned a value of 1 and 0 for below the median | 0 to 9 | Not specified |
| PURE84 | |||||
| Metric focuses on the specific food groups found to be beneficially associated with the risk of mortality in a multinational prospective cohort study | Fruits; vegetables; legumes; nuts; dairy; unprocessed red meat; fish | Food frequency questionnaire | For each component, intake quintiles are computed, and a component score is assigned for each quintile; Q1 is assigned a value of 1, Q2 a value of 2, Q3 a value of 3, Q4 a value of 4, and Q5 a value of 5; the component scores are summed | 7 to 35 | Not specified |
| RFS85, 86, 87, 88, 89 | |||||
| Metric measures diet quality as the consumption of foods recommended by several US dietary guidelines (US National Research Council, Surgeon General and US Department of Agriculture and Health and Human Services) | Apples, pears; oranges; cantaloupe; orange juice, grapefruit juice; grapefruit; other fruit juices; dried beans; tomatoes; broccoli; spinach; mustard, turnip, collard greens; carrots, mixed vegetables with carrots; green salad; sweet potatoes, yams; other potatoes; baked or stewed chicken or turkey; baked or broiled fish; dark breads (eg, whole wheat, rye, pumpernickel); cornbread, tortillas, grits; high-fibre cereals (eg, bran, granola, shredded wheat); cooked cereals; 2% milk and beverages with 2% milk; 1% or skim milk | Food frequency questionnaire | For each component, 1 point is allocated if consumed at least once per week; the component scores are summed | 0 to 23 | Not specified |
| WHO-HDI90, 91, 92, 93, 94 | |||||
| Metric describes adherence to the WHO dietary guidelines (initially 1990 guidelines and revised to the 2003 guidelines) in European populations | Saturated fatty acids; polyunsaturated fatty acids; protein; complex carbohydrates; dietary fibre; fruits and vegetables; pulses, nuts, seeds; monosaccharides and disaccharides; cholesterol (WHO 1990 guidelines); saturated fatty acids; monosaccharides and disaccharides, cholesterol; protein; total dietary fibre; fruits and vegetables; n3-polyunsaturated fatty acids; n6-polyunsaturated fatty acids; trans fatty acids; sodium (WHO 2003 guidelines); saturated fatty acids; free sugar; total fat; total dietary fibre; fruits and vegetables; polyunsaturated fatty acids; potassium (WHO 2015 guidelines) | Food frequency questionnaire | For each component, a value of 1 is assigned if intake is in the recommended range and a value of 0 if not in the recommended range; the components are summed | 0 to 7 for the WHO 2015 version | Not specified |
| WCRF-AICR95, 96, 97 | |||||
| Metric describes adherence to the WCRF-AICR dietary recommendations | Limit consumption of energy-dense foods and sugary drinks; eat mostly foods of plant origin; limit red meat intake and avoid processed meat; limit alcoholic drinks; recommendation to limit consumption of salt and avoid mouldy cereals (grains) or pulses (legumes) was not included | Food frequency questionnaire | Each component is scored with 1 point for complete adherence, 0·5 for moderate adherence, and 0 for non-adherence for each recommendation specific cutoff; the component scores are summed | 0 to 4 | Not specified |
The modifications and adaptations to dietary metrics noted are not exhaustive. AHEI=Alternative Healthy Eating Index. aMDS=Alternative Mediterranean Diet Score. Aussie-DQI=Australian Diet Quality Index. C-DQI=Children's Diet Quality Index. DASH=Dietary Approaches to Stop Hypertension. DGAI=Dietary Guidelines for Americans Adherence Index. DII=Dietary Inflammatory Index. DQI-CH=Dietary Quality Index for China. DQI-I=Diet Quality Index-International. DQI-K=Diet Quality Index for Koreans. HEI=Healthy Eating Index. KIDMED=Mediterranean Diet Quality Index for Children and Teenagers. MDS=Mediterranean Diet Score. MED=Mediterranean Diet Score. Med-DQI=Mediterranean Diet Quality Index. MSDPS=Mediterranean-Style Dietary Pattern Score. PURE=Prospective Urban Rural Epidemiology Diet Score. RC-DQI=Revised Children's Diet Quality Index. RFS=Recommended Foods Score. rMED=Revised Mediterranean Diet Score. WHO-HDI=WHO Healthy Diet Indicator. WCRF-AICR=World Cancer Research Fund and American Institute for Cancer Research.
Table 3.
Estimates of aetiologic effects of dietary metrics and risk of health outcomes
| Meta-analysis search date | Studies included | Source | Number of participants | Countries | Unit of exposure* | Relative risk (95% CI) | I2† | p value for heterogeneity | |
|---|---|---|---|---|---|---|---|---|---|
| AHEI | |||||||||
| All-cause mortality | May 15, 2017 | 7 | Schwingshackl et al (2018)98 | 975 639 | USA, China, UK | High vs low | 0·76 (0·74 to 0·79) | 71% | 0·003 |
| All-cause mortality among cancer survivors | May 15, 2017 | 3 | Schwingshackl et al (2018)98 | 9508 | USA | High vs low | 0·85 (0·70 to 1·03) | 65% | 0·03 |
| Cardiovascular disease | May 15, 2017 | 13 | Schwingshackl et al (2018)98 | 1 296 276 | USA, China, UK | High vs low | 0·75 (0·72 to 0·77) | 39% | 0·05 |
| Cardiovascular mortality | Dec 14, 2015 | 7 | Onvani et al (2017)99 | 820 778 | USA, China, UK | High vs low | 0·74 (0·71 to 0·78) | NR | NR |
| Type 2 diabetes | May 15, 2017 | 9 | Schwingshackl et al (2018)98 | 605 077 | USA, Denmark, France, Germany, Italy, Spain, Sweden, UK, Netherlands | High vs low | 0·80 (0·74 to 0·86) | 76% | <0·001 |
| Cancer | May 15, 2017 | 18 | Schwingshackl et al (2018)98 | 3 013 168 | USA, Great Britain, China, Australia | High vs low | 0·88 (0·85 to 0·91) | 54% | 0·001 |
| Cancer mortality | June 2017 | 9 | Milajerdi et al (2018)100 | 964 740 | USA, England | High vs low | 0·90 (0·85 to 0·95) | 62% | 0·003 |
| Cancer mortality among cancer survivors | May 15, 2017 | 3 | Schwingshackl et al (2018) 98 | 9508 | USA | High vs low | 0·95 (0·79 to 1·13) | 20% | 0·29 |
| DASH | |||||||||
| All-cause mortality | May 15, 2017 | 8 | Schwingshackl et al (2018)98 | 1 353 039 | USA, China, Denmark, France, Germany, Greece, Italy, Netherlands, Spain, Sweden, Norway, UK | High vs low | 0·80 (0·79 to 0·82) | 9% | 0·36 |
| All-cause mortality among cancer survivors | May 15, 2017 | 3 | Schwingshackl et al (2018)98 | 9508 | USA | High vs low | 0·94 (0·82 to 1·08) | 27% | 0·25 |
| Cardiovascular disease | May 15, 2017 | 18 | Schwingshackl et al (2018)98 | 1 745 815 | USA, Taiwan, China, UK, Denmark, France, Germany, Greece, Italy, Netherlands, Spain, Sweden and Norway | High vs low | 0·80 (0·77 to 0·84) | 49% | 0·006 |
| Coronary heart disease | January 2012 | 3 | Salehi-Abargouei et al (2013)101 | 144 337 | USA | High vs low | 0·79 (0·71 to 0·88) | 0% | 0·583 |
| Coronary artery disease | June 2019 | 7 | Yang et al (2019)102 | 377 725 | USA, UK, Netherlands | High vs low | 0·82 (0·78 to 0·87) | 0% | 0·53 |
| Total stroke | May 2018 | 11 | Feng et al (2018)103 | 474 228 | USA, Hong Kong, Taiwan, Italy, Sweden, Germany, UK, Netherlands | High vs low | 0·88 (0·83 to 0·93) | 4% | NR |
| Type 2 diabetes | May 15, 2017 | 8 | Schwingshackl et al (2018)98 | 258 893 | USA, Denmark, France, Germany, Italy, Spain, Sweden, UK, Netherlands | High vs low | 0·80 (0·74 to 0·86) | 61% | 0·01 |
| Cancer | May 15, 2017 | 14 | Schwingshackl et al (2018)98 | 2 987 645 | USA, Sweden, China, Denmark, France, German, Greece, Italy, Netherlands, Spain, Norway, UK | High vs low | 0·82 (0·80 to 0·86) | 48% | 0·007 |
| Cancer mortality | July 2018 | 9 | Ali Mohsenpour et al (2019)104 | 1 414 944 | USA, China, Denmark, France, Germany, Greece, Italy, Netherlands, Norway, Spain, UK, Sweden, Singapore | High vs low | 0·84 (0·81 to 0·86) | 13% | 0·323 |
| Cancer mortality among cancer survivors | May 15, 2017 | 3 | Schwingshackl et al (2018)98 | 9508 | USA | High vs low | 0·93 (0·79 to 1·10) | 0% | 0·73 |
| Colorectal cancer | April 2019 | 6 | Mohseni et al (2020)105 | 836 218 | USA, Canada | High vs low | 0·81 (0·75 to 0·88) | 54% | 0·017 |
| Colon cancer | July 2018 | 2 | Ali Mohsenpour et al (2019)104 | 624 587 | USA | High vs low | 0·80 (0·74 to 0·87) | 0% | 0·922 |
| Rectal cancer | July 2018 | 2 | Ali Mohsenpour et al (2019)104 | 624 287 | USA | High vs low | 0·84 (0·74 to 0·96) | 16% | 0·274 |
| Weight loss in adults, kg | December 2015 | 10 | Soltani et al (2016)106 | 1291 | USA, Australia, Iran | DASH diet vs control diet | −1·42 (−2·03 to −0·82) | 71% | <0·001 |
| Body-mass index in adults, kg/m2 | December 2015 | 6 | Soltani et al (2016)106 | 1157 | USA, Iran and China | DASH diet vs control diet | −0·42 (−0·64 to −0·20) | 82% | 0·01 |
| Waist circumference in adults, cm | December 2015 | 2 | Soltani et al (2016)106 | 511 | USA, Iran | DASH diet vs control diet | −1·05 (−1·61 to −0·49) | 80% | <0·001 |
| DDS | |||||||||
| Cancer mortality | June 2017 | 2 | Milajerdi et al (2018)100 | 12 080 | USA, Taiwan | High vs low | 1·03 (0·59 to 1·82) | 63% | 0·068 |
| DII | |||||||||
| All-cause mortality | NR | 5 | Shivappa et al (2017)59 | 99 147 | UK, USA, Sweden, France | High vs low | 1·04 (1·03 to 1·05) | 53% | 0·074 |
| Cardiovascular mortality | NR | 4 | Shivappa et al (2017)59 | 91 260 | UK, USA, Sweden | High vs low | 1·05 (1·03 to 1·07) | 15% | 0·319 |
| Cancer mortality | NR | 5 | Shivappa et al (2017)59 | 99 142 | UK, USA, Sweden, France | High vs low | 1·05 (1·03 to 1·07) | 30% | 0·22 |
| Breast cancer | February 2017 | 5 | Zahedi et al (2018)107 | 279 402 | USA, Sweden, France | High vs low | 1·04 (0·98 to 1·10) | 31% | 0·218 |
| Gastric cancer‡ | December 2018 | 3 | Du et al (2019)108 | 2118 | Italy, Korea, Iran | Low vs high | 2·11 (1·41 to 3·15) | 41% | 0·19 |
| DQI | |||||||||
| Cancer mortality | June 2017 | 5 | Milajerdi et al (2018)100 | 599 041 | Sweden, USA, Spain, England | High vs low | 0·91 (0·89 to 0·93) | 2% | 0·420 |
| HEI | |||||||||
| All-cause mortality | May 15, 2017 | 8 | Schwingshackl et al (2018)98 | 1 328 413 | USA, Denmark, France, Germany, Greece, Italy, Netherlands, Norway, Spain, Sweden, UK | High vs low | 0·78 (0·76 to 0·80) | 37% | 0·11 |
| All-cause mortality among cancer survivors | May 15, 2017 | 5 | Schwingshackl et al (2018) 98 | 12 040 | USA | High vs low | 0·85 (0·75 to 0·96) | 26% | 0·24 |
| Cardiovascular disease | May 15, 2017 | 11 | Schwingshackl et al (2018) 98 | 1 600 121 | USA, Denmark, France, Germany, Greece, Italy, Netherlands, Norway, Spain, Sweden, UK | High vs low | 0·79 (0·77 to 0·82) | 16% | 0·28 |
| Cardiovascular mortality | Dec 14, 2015 | 5 | Onvani et al (2017)99 | 740 455 | USA | High vs low | 0·79 (0·76 to 0·83) | NR | NR |
| Type 2 diabetes | May 15, 2017 | 3 | Schwingshackl et al (2018)98 | 303 213 | USA | High vs low | 0·87 (0·82 to 0·93) | 61% | 0·05 |
| Cancer | May 15, 2017 | 21 | Schwingshackl et al (2018)98 | 5 048 954 | USA, Denmark, France, Germany, Greece, Italy, Netherlands, Norway, Spain, Sweden, UK | High vs low | 0·83 (0·79 to 0·87) | 73% | <0·001 |
| Cancer mortality | Dec 14, 2015 | 6 | Onvani et al (2017)99 | 741 091 | USA, China | High vs low | 0·80 (0·76 to 0·83) | NR | NR |
| Cancer mortality among cancer survivors | May 15, 2017 | 5 | Schwingshackl et al (2018)98 | 12 040 | USA | High vs low | 0·84 (0·73 to 0·97) | 18% | 0·30 |
| IYCMDD | |||||||||
| Stunting‡ | November 2017 | 5 | Berhe et al (2019)109 | NR | Ethiopia | <4 score vs ≥4 score | 1·95 (1·31 to 2·92) | 72% | 0·006 |
| MED | |||||||||
| All-cause mortality | NR | 7 | Bonaccio et al (2018)110 | 11 738 | Australia, Greece, Sweden, UK, Italy, Belgium, Denmark, France, Netherlands, Portugal, Spain, Switzerland | 1-point increase | 0·95 (0·93 to 0·96) | 0% | 0·47 |
| Cardiovascular disease | August 2016 | 11 | Rosato et al (2017)111 | 758 280 | USA, Spain, Sweden, UK, Netherlands, Italy, Finland | High vs low | 0·81 (0·74 to 0·88) | 80% | <0·0001 |
| Cardiovascular mortality | May 7, 2018 | 21 | Becerra-Tomas et al (2019)112 | 883 878 | USA, UK, Denmark, Spain, Switzerland, Italy, Australia, Sweden | High vs low | 0·79 (0·77 to 0·82) | 0% | 0·64 |
| Coronary heart disease | August 2016 | 11 | Rosato et al (2017)111 | 379 473 | USA, Spain, Sweden, Netherlands, Greece, Italy, Finland | High vs low | 0·70 (0·62 to 0·80) | 45% | 0·06 |
| Coronary heart disease mortality | May 7, 2018 | 6 | Becerra-Tomas et al (2019)112 | 270 565 | USA, UK, Australia, Sweden | High vs low | 0·73 (0·59 to 0·89) | 63% | 0·02 |
| Myocardial infarction | June 2014 | 3 | Grosso et al (2017)113 | 44 428 | USA, Germany, Sweden | High vs low | 0·67 (0·54 to 0·83) | NR | NR |
| Total stroke§ | August 2016 | 6 | Rosato et al (2017)111 | 181 353 | USA, China, Netherlands, Greece, Italy, Australia | High vs low | 0·73 (0·59 to 0·91) | 46% | 0·10 |
| Ischaemic stroke | August 2016 | 5 | Rosato et al (2017)111 | 206 562 | USA, Sweden, Italy | High vs low | 0·82 (0·73 to 0·92) | 0% | 0·46 |
| Haemorrhagic stroke | August 2016 | 4 | Rosato et al (2017)111 | 203 994 | USA, Sweden, Italy | High vs low | 1·01 (0·74 to 1·27) | 36% | 0·20 |
| Stroke mortality | May 7, 2018 | 4 | Becerra-Tomas et al (2019)112 | 195 644 | Greece, USA, UK, Denmark, Sweden | High vs low | 0·87 (0·80 to 0·96) | 0% | 0·74 |
| Type 2 diabetes | Dec 31, 2015 | 6 | Jannasch et al (2017)25 | 196 772 | USA, Spain, Greece, Denmark, France, Germany, Italy, Sweden, UK, Netherlands | High vs low | 0·87 (0·82 to 0·93) | 26% | 0·24 |
| Cancer mortality | June 2017 | 6 | Milajerdi et al (2018)100 | 789 104 | USA | High vs low | 0·81 (0·78 to 0·83) | 2% | 0·420 |
| Breast cancer | August 2016 | 5 | Van den Brandt et al (2017)114 | 58 923 | USA, UK, Sweden, Netherlands, Denmark, France, Germany, Greece, Italy, Norway, Spain | High vs low | 0·94 (0·88 to 1·01) | 13% | 0·33 |
| Gastric cancer | December 2018 | 2 | Du et al (2019)108 | 956 518 | USA, Denmark, UK, France, Sweden, Germany, Italy, Spain, Netherlands, Norway, Greece | High vs low | 0·89 (0·68 to 1·17) | 52% | 0·10 |
| Weight loss in adults, kg | June 2010 | 12 | Esposito et al (2011)115 | 2683 | Italy, USA, France, Israel, Greece, Spain, Germany, Netherlands | MED diet vs control diet | −1·75 (−2·86 to −0·64) | 95% | 0·001 |
| Body-mass index in adults, kg/m2 | June 2010 | 15 | Esposito et al (2011)115 | 3337 | Italy, USA, France, Israel, Greece, Spain, Germany | MED diet vs control diet | −0·57 (−0·93 to −0·21) | 92% | <0·001 |
| Waist circumference in adults, cm | Feb 9, 2016 | 29 | Garcia et al (2016)116 | 4133 | Canada, Algeria, Netherlands, UK, Spain, Italy, USA, Greece, Chile, Sweden, Australia, Romania, South Africa | MED vs control diet | −0·44 (−0·48 to −0·41) | 96% | <0·0001 |
Summary of the meta-analyses finding used for grading the evidence for associations. AHEI=Alternative Healthy Eating Index. DASH=Dietary Approaches to Stop Hypertension. DDS=Dietary Diversity Score. DII=Dietary Inflammatory Index. DQI-I=Diet Quality Index-International. HEI=Healthy Eating Index. IYCMDD=Infant and Young Child Minimum Dietary Diversity. MED=Mediterranean Diet Score. NR=not reported.
High versus low dietary metrics (categorical), point increase in score (continuous), or trial experimental and control groups.
Values are rounded to the nearest whole number.
Odds ratio and 95% CI reported.
Unspecified stroke considered total stroke.
Intended purpose of the dietary metric
Of the seven MCH dietary metrics, two were assessed at the household-level (FCS, HDDS) and five at the individual level (DDS, FVS, IYCMDD, MDD-W, and WDDS and IDDS). All 12 NCD dietary metrics were assessed at the individual level. Most of the MCH dietary metrics were developed for describing micronutrient or caloric adequacy (FCS, IYCMDD, MDD-W, and WDDS and IDDS). Metrics measuring dietary diversity (DDS, FVS) or household food access (HDDS) were less common. NCD dietary metrics were designed to describe adherence to US (DGAI, HEI, RFS) or international (WHO-HDI, WCRF-AICR) dietary guidelines, diet patterns (AHEI, DASH, KIDMED, MED), or foods associated with chronic disease risk (DQI-I, PURE). One NCD dietary metric was designed to measure inflammatory potential (DII).
Included food and nutrient components
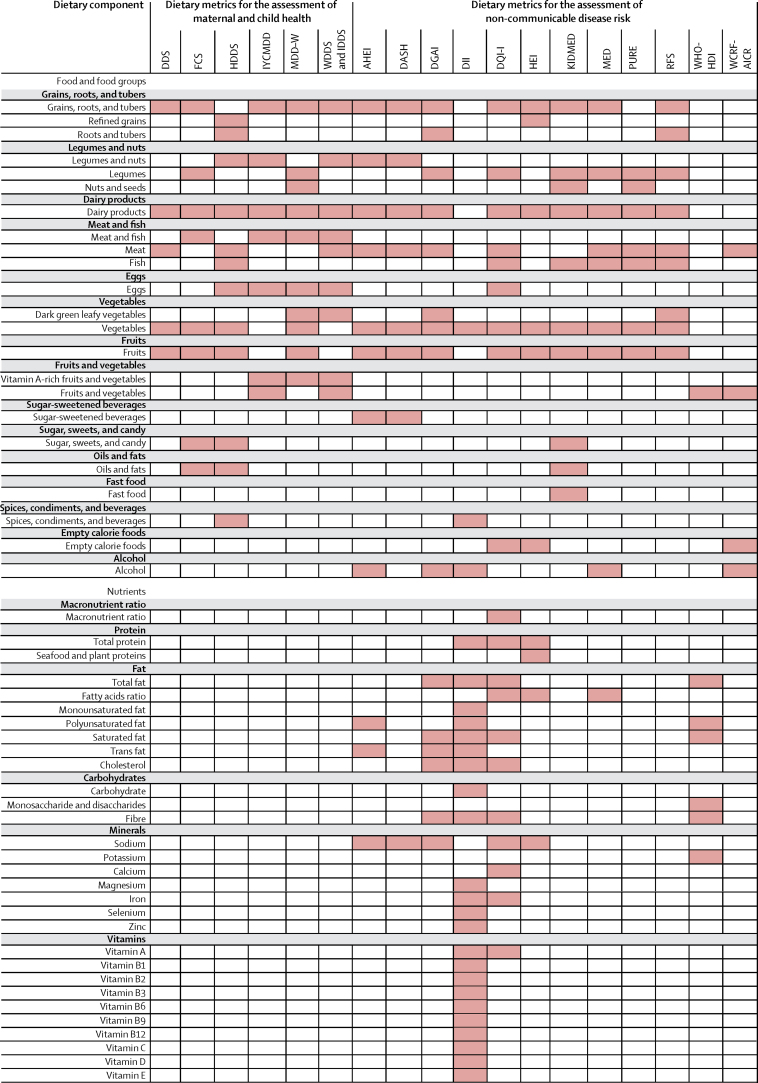
The foods and nutrients included in each dietary metric is shown in figure 1. The median number of included food groups or nutrients per dietary metric is 9·5 (IQR 7·25–12·0). The MCH dietary metrics (8·5, IQR 7·25–9·75) included fewer food groups or nutrients compared with the NCD dietary metrics (11·5, IQR 7·75–20·25). All MCH dietary metrics (excluding the FVS which counts the number of unique foods eaten without specifying food groups or nutrients) used only food groups. Alternatively, six NCD dietary metrics used food groups only, and six used foods and nutrients. None of the identified dietary metrics used only nutrients.
Figure 1.
Foods and nutrients included in the dietary metrics
Shading indicates that the food or nutrient is included in the dietary metric (appendix pp 22–23). DDS=Dietary Diversity Score. FCS=World Food Programme's Food Consumption Score. FVS=Food Variety Score. HDDS=Household Dietary Diversity Score. IYCMDD=Infant and Young Child Minimum Dietary Diversity. MDD-W=Minimum Dietary Diversity for Women. WDDS=Women's Dietary Diversity Score. IDDS=Individual Dietary Diversity Score. AHEI=Alternative Healthy Eating Index. DASH=Dietary Approaches to Stop Hypertension. DGAI=Dietary Guidelines for Americans Adherence Index. DII=Dietary Inflammatory Index. DQI-I=Diet Quality Index-International. HEI=Healthy Eating Index. KIDMED=Mediterranean Diet Quality Index for Children and Teenagers. MED=Mediterranean Diet Score. PURE=Prospective Urban Rural Epidemiology Diet Score. RFS=Recommended Foods Score. WHO-HDI=WHO Healthy Diet Indicator. WCRF-AICR World Cancer Research Fund and American Institute for Cancer Research.
Among all dietary metrics (excluding the FVS), the most frequent foods or nutrients included were vegetables (18 metrics); fruits (17 metrics); dairy products (15 metrics); and grains, roots, and tubers (14 metrics). Among MCH dietary metrics, grains, roots, and tubers, fruits, vegetables, dairy products, and meat and fish were included in all dietary metrics; other frequent foods were legumes and nuts (five metrics), and eggs (four metrics). The most common foods in NCD dietary metrics were vegetables (12 metrics) and fruits (11 metrics), followed by dairy products (nine metrics), grains, roots, and tubers (eight metrics), legumes and nuts (eight metrics), and meat (eight metrics). Uncommon food groups included spices, condiments, and beverages; fast foods; and sugar-sweetened beverages. Unhealthy food groups (eg, sugar-sweetened beverages, sugar, sweets, candy, fast food, or empty calorie foods) were included in two MCH dietary metrics (FCS, HDDS) and six NCD dietary metrics (DASH, AHEI, DQI, HEI, KIDMED, WCRF-AICR). Of the eight NCD dietary metrics that included nutrients (AHEI, DASH, DGAI, DII, DQI-I, HEI, MED, WHO-HDI), the most frequent nutrients were sodium (six of eight metrics), total fat, saturated fat, and fibre (four of eight each).
Reference period, scoring, and cutoffs
All dietary metrics were intended to assess habitual diet, five MCH dietary metrics asked about intake in the past 24 hours, and all NCD dietary metrics asked about diet over an extended period, often the past year.
Methods of scoring varied considerably. Five MCH dietary metrics (FVS, HDDS, IYCMDD, MDD-W, and WDDS and IDDS) and four NCD dietary metrics (DDS, KIDMED, RFS, WHO-HDI) assigned a binary value if the foods or nutrients were consumed during the reference period or recommended intakes were met. Several other NCD dietary metrics (AHEI, DGAI, WCRF-AICR) scored with more than two categories. To identify scoring thresholds, three NCD dietary metrics (MED, DASH, PURE) used the intake distributions in the population in which the metric was being used (ie, using medians and quintiles). One MCH (FCS) and three NCD dietary metrics (DII, DQI-I, HEI) used complex, component-specific scoring.
Positive value only scores were used by all MCH dietary metrics and most NCD dietary metrics, except the KIDMED and DII. Four NCD dietary metrics (DASH, KIDMED, MED, PURE) assigned scores based on the perceived directional benefit of the foods or nutrients, or both, with both lower intake of unhealthy items and greater intake of healthy items receiving higher scores. Unequal scoring weights were used by one MCH dietary metric (FCS) and three NCD dietary metrics (DQI-I, DII, HEI).
Once the overall dietary metric score was determined, assessment of the score varied. Most dietary metrics (15 of 19) used continuous assessments (ie, higher is better). Two MCH dietary metrics (ICYMDD, MDD-W) specified binary cutoffs, while one MCH (FCS) and one NCD dietary metric (KIDMED) provided ordinal (multi-category) cutoffs.
Dietary metric reliability and validity
Reliability, defined as repeated measure validity, was assessed for only two NCD dietary metrics (appendix pp 6–13). The HEI metric showed reasonable reliability for all components except sodium and dairy. The KIDMED metric showed moderate to excellent test-retest reliability.
From their development reports, three MCH dietary metrics were validated against foods or nutrients (IYCMDD, MDD-W, and WDDS and IDDS) and two MCH metrics against other non-dietary metrics (FCS, HDDS; appendix pp 6–13). Three NCD dietary metrics were validated against foods or nutrients (HEI, DGAI) or biomarkers (DII).
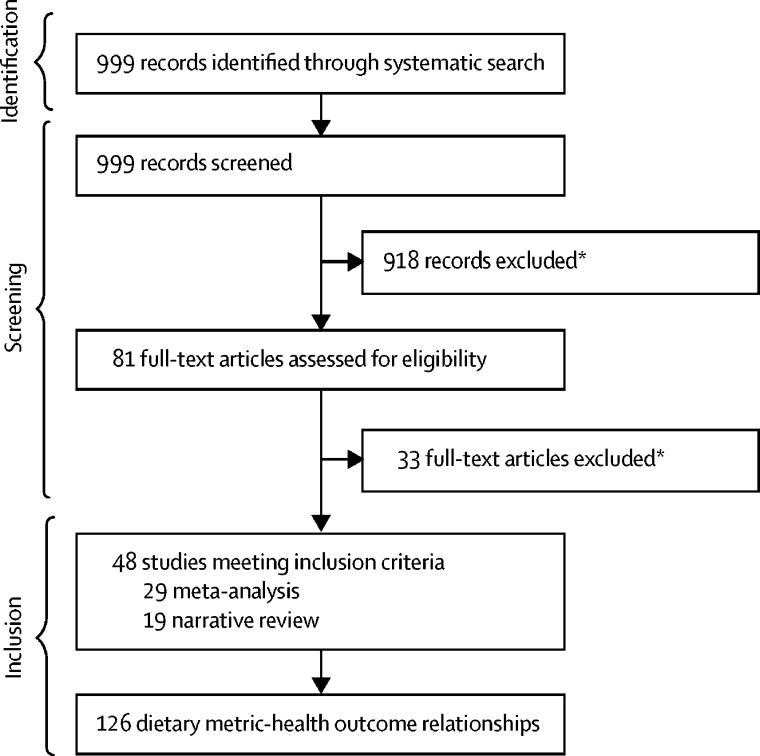
Our systematic search identified 48 meta-analyses or narrative reviews25, 26, 59, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141 that assessed validity of these dietary metrics against health outcomes (figure 2). These reports included 126 dietary metric-health outcome relationships for 13 of the 19 dietary metrics. Nearly all dietary metric-health outcome relationships (116 of 126) were for NCD dietary metrics, with only ten for MCH dietary metrics. Table 3 describes the identified associations of each dietary metric and MCH and NCD health outcome relationship from published meta-analyses, and the evidence for associations based on both meta-analyses and narrative reviews is shown in figure 3.
Figure 2.
Screening and selection process of meta-analyses evaluating dietary metric-disease relationships
*Study design or not relevant outcome or exposure.
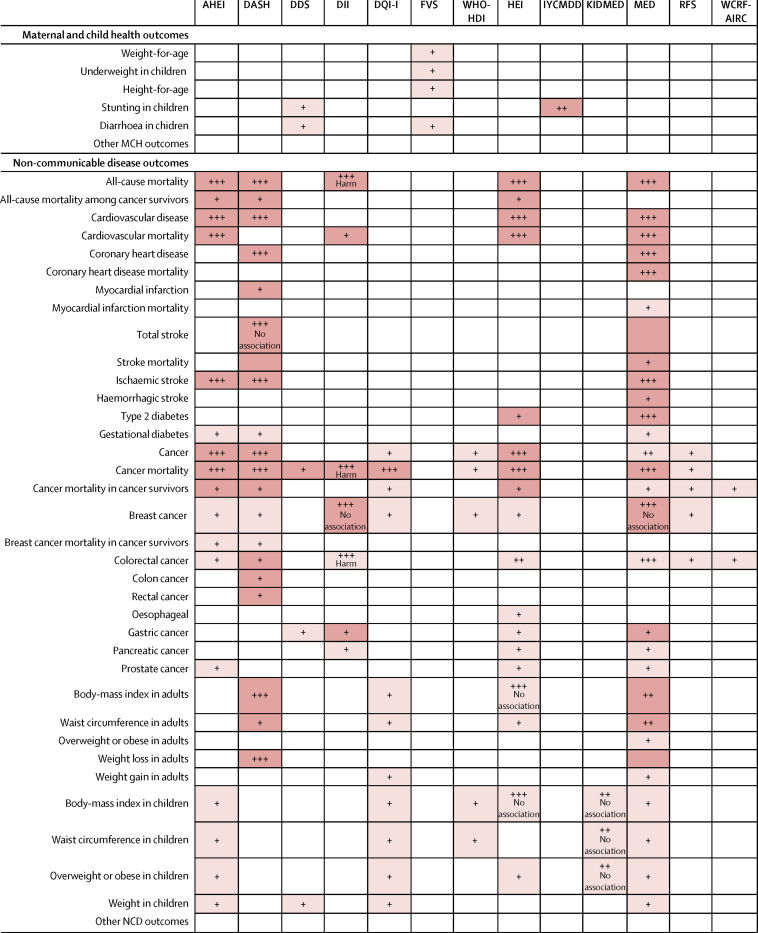
Figure 3.
Grading of evidence for associations of dietary metrics with maternal and child health (MCH) and non-communicable diseases (NCDs) based on meta-analyses and narrative reviews
Dark shading indicates a meta-analysis, light shading indicates a narrative review, and no shading indicates that no review was identified. One plus sign indicates little evidence from few studies (<5), two plus signs indicate inconsistent results from a moderate number of studies (≥5), and three plus signs indicate consistent evidence from multiple high-quality studies (≥5). The relationship between a higher dietary metric and the health outcome was protective, unless stated otherwise. AHEI=Alternative Healthy Eating Index. DASH=Dietary Approaches to Stop Hypertension. DDS=Dietary Diversity Score. DII=Dietary Inflammatory Index. DQI-I=Diet Quality Index-International. FVS=Food Variety Score. WHO-HDI=WHO Healthy Diet Indicator. HEI=Healthy Eating Index. IYCMDD=Infant and Young Child Minimum Dietary Diversity. KIDMED=Mediterranean Diet Quality Index for Children and Teenagers. MED=Mediterranean Diet Score. RFS=Recommended Foods Score. WCRF-AICR World Cancer Research Fund and American Institute for Cancer Research.
The most commonly studied NCD dietary metrics were the MED (29 dietary metric-relationships), DASH (19 dietary metric-relationships), HEI (18 dietary metric-relationships), and AHEI (17 dietary metric-relationships). The most frequently studied outcomes were cancer (53 relationships), anthropometrics in children (21 relationships), cardiovascular disease (18 relationships), and anthropometrics in adults (13 relationships). Among our outcomes of interest, we did not find meta-analyses or narrative reviews of any of the dietary metrics in relation to major MCH outcomes including micronutrient adequacy, mortality for children younger than 5 years, maternal mortality, wasting, or infectious diseases. We also did not find meta-analyses or narrative reviews evaluating any dietary metric against both MCH and NCD outcomes.
Evidence synthesis and grading for dietary metrics
Four of the MCH dietary metrics (FCS, HHDS, MDD-W, and WDDS and IDDS) did not have any identified meta-analyses or narrative reviews assessing their relationship with MCH or NCD health outcomes (figure 3). We found little evidence from few studies for the DDS and stunting and diarrhoea, the IYCMDD and stunting, and the FVS and child underweight.
Four of the NCD dietary metrics had consistent evidence for associations with NCD health outcomes (figure 3). The MED was found to be inversely associated with all-cause mortality, cardiovascular disease, cardiovascular disease mortality, coronary health disease, total and ischaemic stroke, type 2 diabetes, and cancer mortality. Both AHEI and HEI were associated with lower risk of all-cause mortality, cardiovascular disease, cardiovascular disease mortality, cancer, and cancer mortality, while DASH was inversely associated with all-cause mortality, cardiovascular disease, type 2 diabetes, cancer, cancer mortality, body-mass index (BMI), and weight loss in adults.
We found inconsistent evidence for MED and cancer, BMI, waist circumference, and weight loss in adults; for KIDMED and BMI, waist circumference and overweight or obesity in children; and for HEI and colorectal cancer. Six NCD dietary metrics (AHEI, DASH, DQI-I, WHO-HDI, HEI, RFS) had little evidence for associations with breast cancer. For two NCD dietary metrics (DGAI, PURE), no meta-analyses or narrative reviews were identified of relationships with MCH or NCD health outcomes.
Several dietary metrics had evidence (consistent or inconsistent) showing no association with NCD health outcomes including: DASH with total stroke; DII with breast cancer; HEI with BMI in adults and children; KIDMED with BMI, waist circumference, and overweight or obesity in children; and MED with breast cancer (figure 3).
When we looked at what characteristics of the dietary metrics might be more predictive of validity of health outcomes we found that the four NCD metrics (AHEI, DASH, HEI, MED) with consistent evidence of associations were developed to describe adherence to dietary guidelines or diet patterns, consisted of food and nutrient components, all included healthy plant foods (fruits, vegetables, whole grains, nuts and legumes) and dairy, and most included red and processed meat, or sodium.
Discussion
In this comprehensive review of dietary metrics for assessing diet quality globally, we identified 19 dietary metrics, including seven for MCH and 12 for NCD outcomes; and none developed or applied for both. The dietary metrics varied substantially in their composition and scoring, with MCH dietary metrics generally focused on a few key foods (grains, fruits, vegetables, dairy products, meat, and fish) and NCD dietary metrics incorporating a more diverse mix of foods and nutrients, or both. Importantly, most of these dietary metrics were not validated against health outcomes in meta-analyses or narrative reviews. Only four NCD dietary metrics (MED, AHEI, HEI, DASH) had convincing evidence of protective associations, mainly for all-cause mortality, cardiovascular diseases, type 2 diabetes, total cancer, and cancer mortality. These four dietary metrics had little evidence of associations for anthropometrics in adults or children, and were not validated against MCH outcomes (micronutrient adequacy, mortality in children younger than 5 years, stunting, wasting, and infectious and diarrhoeal disease) in published meta-analyses or narrative reviews. The remaining eight NCD dietary metrics and all seven MCH dietary metrics were either not convincingly validated against most MCH and NCD health outcomes or published meta-analyses and narrative reviews assessing their validity were not identified. Additionally, our findings show that no dietary metrics currently exist designed or validated to characterise the double burden of malnutrition. To our knowledge, these findings provide the most current and extensive synthesis of specific multinational dietary metrics for risk of MCH and NCDs.
Among MCH dietary metrics, the IYCMDD is the most widely used and is routinely collected in studies in low-income and middle-income countries, such as the Demographic and Health Surveys142 and UNICEF Multiple Indicator Cluster Surveys,143 as a component of the WHO's Minimum Acceptable Diet for children aged 6–23 months.144, 145, 146 Despite its frequent use, we identified one meta-analysis assessing the association of the IYCMDD with one MCH health outcome, and no meta-analyses or narrative reviews with NCD outcomes. We are aware of selected individual studies that have reported beneficial associations of IYCMDD with child growth outcomes (eg, stunting, wasting, height-for-age Z score, and weight-for-height Z score).147, 148, 149, 150, 151, 152, 153, 154, 155 However, such evidence has not been systematically reviewed nor summarised. The IYCMDD assesses binary recall of seven broad food groups over the previous 24 hours. Although data collection is quick and undemanding for research staff and participants, the simplicity of the IYCMDD is also a potential limiting factor in predicting health outcomes. Ironically, the existing widespread use of the IYCMDD could impede development and validation of new metrics for MCH or the double burden of malnutrition, because a substantial proportion of available dietary data in low-income and middle-income countries is restricted to IYCMDD's broad food groups. Our findings show that much work is needed to validate the IYCMDD for uses other than its intended purpose of measuring micronutrient adequacy. In the absence of robust validation of the IYCMDD against health outcomes, studies and organisations should consider more rigorously validated questions on diet quality.
Among the other MCH dietary metrics, we identified only two narrative reviews for the DDS,100, 118 and four for the FVS.118, 123, 131, 141 No meta-analyses or narrative reviews were identified for the remaining MCH dietary metrics (FCS, HDDS, MDD-W, and WDDS and IDDS). We expect that individual studies will have assessed the relationship between these dietary metrics and MCH outcomes, and our findings highlight the need to pool and synthesise available individual studies of these dietary metrics in relation to both MCH and NCD outcomes in adults and children.
Our investigation also found that although numerous dietary metrics are used to assess the dietary risks of NCDs, most do not have convincing evidence of associations that have been summarised in published narrative reviews or meta-analyses. Only four metrics (MED, AHEI, HEI, DASH) had generally consistent evidence of associations with specific NCD outcomes. For outcomes such as anthropometrics and certain cancer subtypes, further validation is needed. Additionally, none of these four NCD dietary metrics have been assessed in meta-analyses or systematic reviews of important MCH outcomes.
Previous reviews of dietary metrics have focused solely on describing dietary metric features and dietary metric development,9, 19, 20, 21, 22, 23, 24 or the evidence for dietary metrics in relation to a single disease.25, 26 Other reviews looked more broadly at dietary metrics and MCH10 or NCDs,20, 27, 28, 29, 30 but these are more than a decade old and did not also consider both MCH and NCDs together. Consistent with our findings, these previous studies generally found that MCH dietary metrics were not validated against MCH or NCD health outcomes, and that only a modest number of NCD dietary metrics predicted some chronic diseases. Our findings build upon and greatly extend these previous reviews by providing the most extensive contemporary summary of the evidence for associations of dietary metrics with both MCH and NCD outcomes.
Future studies should consider analyses to validate existing dietary metrics with both MCH and NCD outcomes. Additionally, our findings suggest the need for development of novel dietary metrics designed from the start to assess and monitor both MCH and NCDs. Novel dietary metrics could be entirely new, or adaptations of existing dietary metrics and should be developed with several key considerations. First, comprehensive reviews of existing dietary metrics and health outcome relationships can be used to inform the features of new metrics (eg, foods or nutrients, scoring, cutoffs). We found that the four NCD dietary metrics with convincing evidence for associations were comprised of food and nutrient components, included healthy plant foods and dairy, and often included red and processed meat, or sodium. This finding might suggest that these components are important aspects of dietary metrics. However, the validity of most remaining dietary metrics has not been assessed against health outcomes, and it is not known whether dietary metrics without these components are less predictive of validity than those with these components. Second, systematic evaluations to summarise the aetiologic effects and optimal levels of dietary factors for MCH outcomes might provide hypotheses to inform the selection of foods and nutrients in new dietary metrics. In the past 10 years the growing availability of standardised, global dietary data has provided greater clarity on actual food and nutrient intakes,156, 157 and the relationships between foods and nutrients, and cardiovascular disease, type 2 diabetes158 and cancer159 are well documented, but similar associations for many MCH outcomes have not been systematically assessed. Third, new dietary metrics need to acknowledge and reconcile potentially opposing effects of some foods and nutrients on MCH and NCD outcomes. For example, animal source foods (eg, meat, fish, eggs, dairy) have been shown to have beneficial effects on micronutrient deficiencies, child growth outcomes, and cognitive function,148, 160, 161 but some of these foods, such as processed meats, are negatively associated with NCDs.158, 159 Although we did not include sustainability metrics in our Review, an important consideration for future dietary metric development is the incorporation of the measurable effect of different food production systems on the environment in dietary metrics. Lastly, an influential consideration for the widespread adoption of existing dietary metrics by health agencies and governments is likely dependent on cultural appropriateness and ease of collecting and scoring these dietary metrics.
Several challenges have likely limited validation of dietary metrics against both MCH and NCD. Ideally, prospective cohort studies with detailed and accurately measured data on both dietary habits and health outcomes should be leveraged. However, populations with MCH outcomes often do not have strong dietary assessment methods and longitudinal data. Most studies on MCH dietary metrics collect information on dietary intake over the previous 24 hour period because the priority is on identifying trends, comparisons, and rankings of outcomes within and between groups, which is generally of interest in setting and evaluating programme targets, and a longer reference period is not necessarily appropriate for these purposes and might be more difficult to operationalise. Moreover, these studies frequently assess diet qualitatively and the absence of quantitative data limit opportunities to construct and validate existing NCD dietary metrics. Ecological analyses are an alternative study design, which bring their own limitations but could address both MCH and NCD across low-income, middle-income, and high-income countries. In addition to these practical data challenges, one of the most important limitations of previous work might have been the general segregation of diet related MCH versus NCD research and policy.
Our Review has several strengths. We did systematic searches for meta-analyses and narrative reviews of validation against health outcomes, making it less likely that we missed any existing syntheses of dietary metric and health outcome relationships. We included a broad range of health outcomes for MCH and NCDs, informing the modern priorities for the double burden of malnutrition. We evaluated the evidence for health associations independently and in duplicate, including the grading of consistency, reducing the potential for bias.
Limitations should be considered. First, we focused on major MCH and NCD health outcomes, and there might be other relevant health outcomes that were not included, such as cognitive function, bone and joint disorders, and NCD risk factors (hypertension, dyslipidaemia, and glucose control). Second, our systematic reviews for each dietary metric in relation to MCH and NCD health outcomes was limited to published meta-analyses and narrative reviews and did not separately assess individual studies. However, meta-analyses and narrative review are more likely to provide accurate and reliable conclusions compared to single studies, minimising publication bias. Lastly, the evidence for validity came mostly from observational studies and some trials, and these are subject to residual confounding, which could overestimate associations, and measurement error in both diet assessment methods and outcome ascertainment, which might result in an underestimation of the relationships between the dietary metrics and MCH and NCD health outcomes.
In summary, we identified seven international dietary metrics for MCH, none of which have been validated against health outcomes in meta-analyses or narrative reviews, and 12 dietary metrics for NCDs, of which only four have convincing evidence for validation against specific NCD outcomes. These findings highlight important gaps and major opportunities in global analyses of diet quality relating to malnutrition, which are highly relevant to the achievement of the UN SDGs, other global nutrition targets, and corresponding effective policy actions.
Search strategy and selection criteria
We searched PubMed for articles published between Oct 11, 2000, and April 17, 2020, using the terms “diet quality”, “diet metric”, or “diet score” with “malnutrition”, “undernutrition”, “maternal health”, “child health”, “stunting”, “wasting”, “mortality”, “non-communicable disease”, “chronic disease”, “cardiovascular disease”, “diabetes”, “cancer”, or “obesity” with “meta-analysis”, “systematic review”, or “narrative review”. We used search terms in English but did not apply any language restrictions. We screened articles by title and abstract to identify full-text reports that were relevant to the study aims. We also screened citation lists for these full-text reports to identify other relevant articles. Articles were considered relevant if they reported the relation between dietary metrics and malnutrition. Several previous reviews qualitatively summarised the evidence for dietary metrics in relation to a single health outcome, and other reviews focused on several maternal and child health (MCH) or non-communicable disease (NCD) health outcomes. A few comprehensive reviews of dietary metrics in relation to several MCH or NCD outcomes were completed more than a decade ago, and no review has focused on both MCH and NCD outcomes.
Acknowledgments
Acknowledgments
This study was funded by The Bill & Melinda Gates Foundation. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Contributors
VM, PW, and DM conceived of the study and designed the methods. VM did the article screening, data extraction, and data synthesis. All authors graded the evidence for associations. VM, PW, and DM wrote the first and subsequent drafts of the manuscript. RM reviewed and edited the manuscript.
Declaration of interests
VM reports a grant from the American Heart Association, outside the submitted work. PW reports research grants and contracts from the United States Agency for International Development and UNICEF and personal fees from the Global Panel on Agriculture and Food Systems for Nutrition, outside the submitted work. RM reports grants from National Institute of Health, Nestle, Danone, and personal fees from Bunge and Development Initiatives, outside the submitted work. DM reports research funding from the National Institutes of Health and the Bill & Melinda Gates Foundation; personal fees from GOED, Bunge, Indigo Agriculture, Motif FoodWorks, Amarin, Acasti Pharma, Cleveland Clinic Foundation, America's Test Kitchen, and Danone; scientific advisory board member for Brightseed, DayTwo, Elysium Health, Filtricine, HumanCo, and Tiny Organics; and chapter royalties from UpToDate, all outside the submitted work.
Supplementary Material
References
- 1.Stanaway JD, Afshin A, Gakidou E. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afshin A, Sur PJ, Fay KA. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393:1958–1972. doi: 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United Nations Statistical Commission . UN Statistical Commission; New York, NY: 2017. Report of the inter-agency and expert group on Sustainable Development Goals Indicators. [Google Scholar]
- 4.Fullman N, Barber RM, Abajobir AA. Measuring progress and projecting attainment on the basis of past trends of the health-related Sustainable Development Goals in 188 countries: an analysis from the Global Burden of Disease Study 2016. Lancet. 2017;390:1423–1459. doi: 10.1016/S0140-6736(17)32336-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sachs J, Schmidt-Traub G, Kroll C, Durand-Delacre D, Teksoz K. Bertelsmann Stiflung and Sustainable Development Solutions Network; New York, NY: 2017. SDG index and dashboards report 2017. [Google Scholar]
- 6.WHO . World Health Organization; Geneva, Switzerland: 2017. World health statistics 2017: monitoring health for the SDGs. [Google Scholar]
- 7.WHO . WHO; Geneva, Switzerland: 2018. World health statistics 2018: monitoring health for the SDGs, sustainable development goals. [Google Scholar]
- 8.World Bank . World Bank; Washington, D.C.: 2017. Atlas of the Sustainable Development Goals 2017: from the World Development Indicators. [Google Scholar]
- 9.Gil Á, Martinez de Victoria E, Olza J. Indicators for the evaluation of diet quality. Nutr Hosp. 2015;31(suppl 3):128–144. doi: 10.3305/nh.2015.31.sup3.8761. [DOI] [PubMed] [Google Scholar]
- 10.Ruel MT. Operationalizing dietary diversity: a review of measurement issues and research priorities. J Nutr. 2003;133(suppl 2):3911. doi: 10.1093/jn/133.11.3911S. 326S. [DOI] [PubMed] [Google Scholar]
- 11.Mozaffarian D, Angell SY, Lang T, Rivera JA. Role of government policy in nutrition-barriers to and opportunities for healthier eating. BMJ. 2018;361 doi: 10.1136/bmj.k2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mozaffarian D, Rosenberg I, Uauy R. History of modern nutrition science-implications for current research, dietary guidelines, and food policy. BMJ. 2018;361 doi: 10.1136/bmj.k2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawkes C, Ruel MT, Salm L, Sinclair B, Branca F. Double-duty actions: seizing programme and policy opportunities to address malnutrition in all its forms. Lancet. 2020;395:142–155. doi: 10.1016/S0140-6736(19)32506-1. [DOI] [PubMed] [Google Scholar]
- 14.Caulifield LE, Richard SA, Rivera RJ, Musgrove P, Black RE. Stunting, wasting, and micronutrient deficiency disorders. In: Jamison DT, Breman JG, Measham AR, editors. Disease Control Priorities in Developing Countries. 2nd edn. World Bank and Oxford University Press; Washington, DC: 2006. pp. 551–567. [Google Scholar]
- 15.Davis C, Saltos E. Dietary recommendations and how they have changed over time. America's eating habits: changes and consequences. 1999. https://www.ers.usda.gov/webdocs/publications/42215/5831_aib750b_1_.pdf
- 16.Shrimpton R, Rokx C. World Bank's Human Development Network; Washington, DC: 2012. The double burden of malnutrition: a review of global evidence. [Google Scholar]
- 17.Romieu I, Dossus L, Barquera S. Energy balance and obesity: what are the main drivers? Cancer Causes Control. 2017;28:247–258. doi: 10.1007/s10552-017-0869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arvaniti F, Panagiotakos DB. Healthy indexes in public health practice and research: a review. Crit Rev Food Sci Nutr. 2008;48:317–327. doi: 10.1080/10408390701326268. [DOI] [PubMed] [Google Scholar]
- 19.Mertens E, Van't Veer P, Hiddink GJ, Steijns JM, Kuijsten A. Operationalising the health aspects of sustainable diets: a review. Public Health Nutr. 2017;20:739–757. doi: 10.1017/S1368980016002664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kant AK. Indexes of overall diet quality: a review. J Am Diet Assoc. 1996;96:785–791. doi: 10.1016/S0002-8223(96)00217-9. [DOI] [PubMed] [Google Scholar]
- 21.Fransen HP, Ocké MC. Indices of diet quality. Curr Opin Clin Nutr Metab Care. 2008;11:559–565. doi: 10.1097/MCO.0b013e32830a49db. [DOI] [PubMed] [Google Scholar]
- 22.Alkerwi A. Diet quality concept. Nutrition. 2014;30:613–618. doi: 10.1016/j.nut.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Zaragoza-Martí A, Cabañero-Martínez MJ, Hurtado-Sánchez JA, Laguna-Pérez A, Ferrer-Cascales R. Evaluation of Mediterranean diet adherence scores: a systematic review. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-019033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smithers LG, Golley RK, Brazionis L, Lynch JW. Characterizing whole diets of young children from developed countries and the association between diet and health: a systematic review. Nutr Rev. 2011;69:449–467. doi: 10.1111/j.1753-4887.2011.00407.x. [DOI] [PubMed] [Google Scholar]
- 25.Jannasch F, Kröger J, Schulze MB. Dietary patterns and type 2 diabetes: a systematic literature review and meta-analysis of prospective Studies. J Nutr. 2017;147:1174–1182. doi: 10.3945/jn.116.242552. [DOI] [PubMed] [Google Scholar]
- 26.Asghari G, Mirmiran P, Yuzbashian E, Azizi F. A systematic review of diet quality indices in relation to obesity. Br J Nutr. 2017;117:1055–1065. doi: 10.1017/S0007114517000915. [DOI] [PubMed] [Google Scholar]
- 27.Kant AK. Dietary patterns and health outcomes. J Am Diet Assoc. 2004;104:615–635. doi: 10.1016/j.jada.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Waijers PM, Feskens EJ, Ocké MC. A critical review of predefined diet quality scores. Br J Nutr. 2007;97:219–231. doi: 10.1017/S0007114507250421. [DOI] [PubMed] [Google Scholar]
- 29.Wirt A, Collins CE. Diet quality—what is it and does it matter? Public Health Nutr. 2009;12:2473–2492. doi: 10.1017/S136898000900531X. [DOI] [PubMed] [Google Scholar]
- 30.Kourlaba G, Panagiotakos DB. Dietary quality indices and human health: a review. Maturitas. 2009;62:1–8. doi: 10.1016/j.maturitas.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 31.WHO Malnutrition. 2018. https://www.who.int/news-room/fact-sheets/detail/malnutrition
- 32.Edefonti V, De Vito R, Dalmartello M, Patel L, Salvatori A, Ferraroni M. Reproducibility and validity of a posteriori dietary patterns: a systematic review. Adv Nutr. 2020;11:293–326. doi: 10.1093/advances/nmz097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kant AK, Schatzkin A, Ziegler RG. Dietary diversity and subsequent cause-specific mortality in the NHANES I epidemiologic follow-up study. J Am Coll Nutr. 1995;14:233–238. doi: 10.1080/07315724.1995.10718501. [DOI] [PubMed] [Google Scholar]
- 34.Kant AK, Schatzkin A, Harris TB, Ziegler RG, Block G. Dietary diversity and subsequent mortality in the First National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am J Clin Nutr. 1993;57:434–440. doi: 10.1093/ajcn/57.3.434. [DOI] [PubMed] [Google Scholar]
- 35.Hatløy A, Torheim LE, Oshaug A. Food variety - a good indicator of nutritional adequacy of the diet? A case study from an urban area in Mali, West Africa. Eur J Clin Nutr. 1998;52:891–898. doi: 10.1038/sj.ejcn.1600662. [DOI] [PubMed] [Google Scholar]
- 36.Coates J, Rogers BL, Webb P, Maxwell D, Houser R, McDonald C. Tufts University; Boston, MA: 2007. Diet diversity study. [Google Scholar]
- 37.Wiesmann D, Bassett L, Benson T, Hoddinott J. IFPRI; Washington, DC: 2009. Validation of the World Food Programme's food consumption score and alternative indicators of household food security. [Google Scholar]
- 38.World Food Programme Vulnerability Analysis and Mapping . World Food Programme; Rome, Italy: 2008. Food consumption analysis: calculation and use of the food consumption score in food security analysis. [Google Scholar]
- 39.Hatløy A, Hallund J, Diarra MM, Oshaug A. Food variety, socioeconomic status and nutritional status in urban and rural areas in Koutiala (Mali) Public Health Nutr. 2000;3:57–65. doi: 10.1017/s1368980000000628. [DOI] [PubMed] [Google Scholar]
- 40.Rathnayake KM, Madushani P, Silva K. Use of dietary diversity score as a proxy indicator of nutrient adequacy of rural elderly people in Sri Lanka. BMC Res Notes. 2012;5:469. doi: 10.1186/1756-0500-5-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swindale A, Bilinsky P. Household dietary diversity score (HDDS) for measurement of household food access: indicator guide. Washington, DC: FHI 360/FANTA. 2006.
- 42.Kennedy G, Ballard T, Dop MC. FANTA; Rome, Italy: 2011. Guidelines for measuring household and individual dietary diversity. [Google Scholar]
- 43.Hoddinott J, Yohannes I. FANTA; Washington, DC: 2002. Dietary diversity as a household food security indicator. [Google Scholar]
- 44.Working Group on Infant and Young Child Feeding Indicators . FANTA; Washington, DC: 2006. Developing and validating simple indicators of dietary quality and energy intake of infants and young children in developing countries: summary of findings from analysis of 10 data sets. [Google Scholar]
- 45.Working Group on Infant and Young Child Feeding Indicators . FANTA; Washington, DC: 2007. Developing and validating simple indicators of infants and young children in developing countries: additional analysis of 10 data sets. [Google Scholar]
- 46.Arimond M, Wiesmann D, Becquey E. Simple food group diversity indicators predict micronutrient adequacy of women's diets in 5 diverse, resource-poor settings. J Nutr. 2010;140:2059. doi: 10.3945/jn.110.123414. 269S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin-Prevel Y, Allemand P, Wiesmann D. FAO; Rome, Italy: 2015. Moving forward on choosing a standard operational indicator of women's dietary diversity. [Google Scholar]
- 48.Chiuve SE, Fung TT, Rimm EB. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 50.Izadi V, Tehrani H, Haghighatdoost F, Dehghan A, Surkan PJ, Azadbakht L. Adherence to the DASH and Mediterranean diets is associated with decreased risk for gestational diabetes mellitus. Nutrition. 2016;32:1092–1096. doi: 10.1016/j.nut.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Liese AD, Krebs-Smith SM, Subar AF. The Dietary Patterns Methods Project: synthesis of findings across cohorts and relevance to dietary guidance. J Nutr. 2015;145:393–402. doi: 10.3945/jn.114.205336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fogli-Cawley JJ, Dwyer JT, Saltzman E, McCullough ML, Troy LM, Jacques PF. The 2005 Dietary Guidelines for Americans Adherence Index: development and application. J Nutr. 2006;136:2908–2915. doi: 10.1093/jn/136.11.2908. [DOI] [PubMed] [Google Scholar]
- 53.Fogli-Cawley JJ, Dwyer JT, Saltzman E. The 2005 Dietary Guidelines for Americans and risk of the metabolic syndrome. Am J Clin Nutr. 2007;86:1193–1201. doi: 10.1093/ajcn/86.4.1193. [DOI] [PubMed] [Google Scholar]
- 54.Fogli-Cawley JJ, Dwyer JT, Saltzman E. The 2005 Dietary Guidelines for Americans and insulin resistance in the Framingham Offspring Cohort. Diabetes Care. 2007;30:817–822. doi: 10.2337/dc06-1927. [DOI] [PubMed] [Google Scholar]
- 55.Mohseni-Takalloo S, Hosseini-Esfahani F, Mirmiran P, Azizi F. Associations of pre-defined dietary patterns with obesity associated phenotypes in tehranian adolescents. Nutrients. 2016;8:E505. doi: 10.3390/nu8080505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hosseini-Esfahani F, Jessri M, Mirmiran P, Bastan S, Azizi F. Adherence to dietary recommendations and risk of metabolic syndrome: Tehran Lipid and Glucose Study. Metabolism. 2010;59:1833–1842. doi: 10.1016/j.metabol.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 57.Jessri M, Lou WY, L'Abbé MR. The 2015 Dietary Guidelines for Americans is associated with a more nutrient-dense diet and a lower risk of obesity. Am J Clin Nutr. 2016;104:1378–1392. doi: 10.3945/ajcn.116.132647. [DOI] [PubMed] [Google Scholar]
- 58.Fresan U, Sabate J, Martinez-Gonzalez MA, Segovia-Siapco G, de la Fuente-Arrillaga C, Bes-Rastrollo M. Adherence to the 2015 Dietary Guidelines for Americans and mortality risk in a Mediterranean cohort: the SUN project. Prev Med. 2019;118:317–324. doi: 10.1016/j.ypmed.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 59.Shivappa N, Hebert JR, Kivimaki M, Akbaraly T. Alternative Healthy Eating Index 2010, Dietary Inflammatory Index and risk of mortality: results from the Whitehall II cohort study and meta-analysis of previous Dietary Inflammatory Index and mortality studies. Br J Nutr. 2017;118:210–221. doi: 10.1017/S0007114517001908. [DOI] [PubMed] [Google Scholar]
- 60.Deng FE, Shivappa N, Tang Y, Mann JR, Hebert JR. Association between diet-related inflammation, all-cause, all-cancer, and cardiovascular disease mortality, with special focus on prediabetics: findings from NHANES III. Eur J Nutr. 2017;56:1085–1093. doi: 10.1007/s00394-016-1158-4. [DOI] [PubMed] [Google Scholar]
- 61.Shivappa N, Blair CK, Prizment AE, Jacobs DR, Jr, Steck SE, Hébert JR. Association between inflammatory potential of diet and mortality in the Iowa Women's Health study. Eur J Nutr. 2016;55:1491–1502. doi: 10.1007/s00394-015-0967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim S, Haines PS, Siega-Riz AM, Popkin BM. The Diet Quality Index-International (DQI-I) provides an effective tool for cross-national comparison of diet quality as illustrated by China and the United States. J Nutr. 2003;133:3476–3484. doi: 10.1093/jn/133.11.3476. [DOI] [PubMed] [Google Scholar]
- 63.Tur JA, Romaguera D, Pons A. The Diet Quality Index-International (DQI-I): is it a useful tool to evaluate the quality of the Mediterranean diet? Br J Nutr. 2005;93:369–376. doi: 10.1079/bjn20041363. [DOI] [PubMed] [Google Scholar]
- 64.Shin MK, Kim YS, Kim JH, Kim SH, Kim Y. Dietary patterns and their associations with the Diet Quality Index-International (DQI-I) in Korean women with gestational diabetes mellitus. Clin Nutr Res. 2015;4:216–224. doi: 10.7762/cnr.2015.4.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guenther PM, Reedy J, Krebs-Smith SM, Reeve BB. Evaluation of the Healthy Eating Index-2005. J Am Diet Assoc. 2008;108:1854–1864. doi: 10.1016/j.jada.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 66.Guenther PM, Reedy J, Krebs-Smith SM, Reeve BB, Basiotis PP. Center for Nutrition Policy and Promotion, US Department of Agriculture; 2007. Development and evaluation of the healthy eating index-2005: technical report. [Google Scholar]
- 67.Guenther PM, Kirkpatrick SI, Reedy J. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J Nutr. 2014;144:399–407. doi: 10.3945/jn.113.183079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guenther PM, Casavale KO, Reedy J. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113:569–580. doi: 10.1016/j.jand.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Britten P, Cleveland LE, Koegel KL, Kuczynski KJ, Nickols-Richardson SM. Updated US Department of Agriculture Food Patterns meet goals of the 2010 dietary guidelines. J Acad Nutr Diet. 2012;112:1648–1655. doi: 10.1016/j.jand.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 70.Lazarou C, Panagiotakos DB, Matalas AL. Physical activity mediates the protective effect of the Mediterranean diet on children's obesity status: the CYKIDS study. Nutrition. 2010;26:61–67. doi: 10.1016/j.nut.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 71.Durá Travé T, Castroviejo Gandarias A. Adherence to a Mediterranean diet in a college population. Nutr Hosp. 2011;26:602–608. doi: 10.1590/S0212-16112011000300025. (in Spanish). [DOI] [PubMed] [Google Scholar]
- 72.Sahingoz SA, Sanlier N. Compliance with Mediterranean Diet Quality Index (KIDMED) and nutrition knowledge levels in adolescents. A case study from Turkey. Appetite. 2011;57:272–277. doi: 10.1016/j.appet.2011.05.307. [DOI] [PubMed] [Google Scholar]
- 73.Mariscal-Arcas M, Rivas A, Velasco J, Ortega M, Caballero AM, Olea-Serrano F. Evaluation of the Mediterranean Diet Quality Index (KIDMED) in children and adolescents in Southern Spain. Public Health Nutr. 2009;12:1408–1412. doi: 10.1017/S1368980008004126. [DOI] [PubMed] [Google Scholar]
- 74.Štefan L, Prosoli R, Juranko D. The Reliability of the Mediterranean Diet Quality Index (KIDMED) Questionnaire. Nutrients. 2017;9:E419. doi: 10.3390/nu9040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Serra-Majem L, Ribas L, Ngo J. Food, youth and the Mediterranean diet in Spain. Development of KIDMED, Mediterranean Diet Quality Index in children and adolescents. Public Health Nutr. 2004;7:931–935. doi: 10.1079/phn2004556. [DOI] [PubMed] [Google Scholar]
- 76.García Cabrera S, Herrera Fernández N, Rodríguez Hernández C, Nissensohn M, Román-Viñas B, Serra-Majem L. KIDMED test; prevalence of low adherence to the Mediterranean diet in children and young; a systematic review. Nutr Hosp. 2015;32:2390–2399. doi: 10.3305/nh.2015.32.6.9828. [DOI] [PubMed] [Google Scholar]
- 77.Martin-Calvo N, Chavarro JE, Falbe J, Hu FB, Field AE. Adherence to the Mediterranean dietary pattern and BMI change among US adolescents. Int J Obes (Lond) 2016;40:1103–1108. doi: 10.1038/ijo.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 79.Trichopoulou A, Kouris-Blazos A, Wahlqvist ML. Diet and overall survival in elderly people. BMJ. 1995;311:1457–1460. doi: 10.1136/bmj.311.7018.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rumawas ME, Dwyer JT, McKeown NM, Meigs JB, Rogers G, Jacques PF. The development of the Mediterranean-style dietary pattern score and its application to the American diet in the Framingham Offspring Cohort. J Nutr. 2009;139:1150–1156. doi: 10.3945/jn.109.103424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Buckland G, González CA, Agudo A. Adherence to the Mediterranean diet and risk of coronary heart disease in the Spanish EPIC Cohort Study. Am J Epidemiol. 2009;170:1518–1529. doi: 10.1093/aje/kwp282. [DOI] [PubMed] [Google Scholar]
- 82.Sofi F, Dinu M, Pagliai G, Marcucci R, Casini A. Validation of a literature-based adherence score to Mediterranean diet: the MEDI-LITE score. Int J Food Sci Nutr. 2017;68:757–762. doi: 10.1080/09637486.2017.1287884. [DOI] [PubMed] [Google Scholar]
- 83.Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014;17:2769–2782. doi: 10.1017/S1368980013003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mente A. Association of dietary quality and risk of cardiovascular disease and mortality in 218,000 people from over 50 countries. 2018. https://www.acc.org/~/media/Clinical/PDF-Files/Approved-PDFs/2018/08/21/ESC-2018-Slides/Aug28-Tue/515amET_PURE.pdf
- 85.Kant AK, Schatzkin A, Graubard BI, Schairer C. A prospective study of diet quality and mortality in women. JAMA. 2000;283:2109–2115. doi: 10.1001/jama.283.16.2109. [DOI] [PubMed] [Google Scholar]
- 86.Collins CE, Burrows TL, Rollo ME. The comparative validity and reproducibility of a diet quality index for adults: the Australian Recommended Food Score. Nutrients. 2015;7:785–798. doi: 10.3390/nu7020785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burrows TL, Collins K, Watson J. Validity of the Australian Recommended Food Score as a diet quality index for Pre-schoolers. Nutr J. 2014;13:87. doi: 10.1186/1475-2891-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marshall S, Watson J, Burrows T, Guest M, Collins CE. The development and evaluation of the Australian child and adolescent recommended food score: a cross-sectional study. Nutr J. 2012;11:96. doi: 10.1186/1475-2891-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaluza J, Håkansson N, Brzozowska A, Wolk A. Diet quality and mortality: a population-based prospective study of men. Eur J Clin Nutr. 2009;63:451–457. doi: 10.1038/sj.ejcn.1602968. [DOI] [PubMed] [Google Scholar]
- 90.Huijbregts P, Feskens E, Räsänen L. Dietary pattern and 20 year mortality in elderly men in Finland, Italy, and The Netherlands: longitudinal cohort study. BMJ. 1997;315:13–17. doi: 10.1136/bmj.315.7099.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stefler D, Pikhart H, Jankovic N. Healthy diet indicator and mortality in Eastern European populations: prospective evidence from the HAPIEE cohort. Eur J Clin Nutr. 2014;68:1346–1352. doi: 10.1038/ejcn.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jankovic N, Geelen A, Streppel MT. WHO guidelines for a healthy diet and mortality from cardiovascular disease in European and American elderly: the CHANCES project. Am J Clin Nutr. 2015;102:745–756. doi: 10.3945/ajcn.114.095117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jennings A, Welch A, van Sluijs EM, Griffin SJ, Cassidy A. Diet quality is independently associated with weight status in children aged 9–10 years. J Nutr. 2011;141:453–459. doi: 10.3945/jn.110.131441. [DOI] [PubMed] [Google Scholar]
- 94.Haveman-Nies A, Tucker KL, de Groot LC, Wilson PW, van Staveren WA. Evaluation of dietary quality in relationship to nutritional and lifestyle factors in elderly people of the US Framingham Heart Study and the European SENECA study. Eur J Clin Nutr. 2001;55:870–880. doi: 10.1038/sj.ejcn.1601232. [DOI] [PubMed] [Google Scholar]
- 95.Jankovic N, Geelen A, Winkels RM. Adherence to the WCRF/AICR dietary recommendations for cancer prevention and risk of cancer in elderly from Europe and the United States: a meta-analysis within the CHANCES project. Cancer Epidemiol Biomarkers Prev. 2017;26:136–144. doi: 10.1158/1055-9965.EPI-16-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Romaguera D, Vergnaud AC, Peeters PH. Is concordance with World Cancer Research Fund/American Institute for Cancer Research guidelines for cancer prevention related to subsequent risk of cancer? Results from the EPIC study. Am J Clin Nutr. 2012;96:150–163. doi: 10.3945/ajcn.111.031674. [DOI] [PubMed] [Google Scholar]
- 97.Vergnaud AC, Romaguera D, Peeters PH. Adherence to the World Cancer Research Fund/American Institute for Cancer Research guidelines and risk of death in Europe: results from the European Prospective Investigation into Nutrition and Cancer cohort study1,4. Am J Clin Nutr. 2013;97:1107–1120. doi: 10.3945/ajcn.112.049569. [DOI] [PubMed] [Google Scholar]
- 98.Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2018;118:74–100.e11. doi: 10.1016/j.jand.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 99.Onvani S, Haghighatdoost F, Surkan PJ, Larijani B, Azadbakht L. Adherence to the Healthy Eating Index and Alternative Healthy Eating Index dietary patterns and mortality from all causes, cardiovascular disease and cancer: a meta-analysis of observational studies. J Hum Nutr Diet. 2017;30:216–226. doi: 10.1111/jhn.12415. [DOI] [PubMed] [Google Scholar]
- 100.Milajerdi A, Namazi N, Larijani B, Azadbakht L. The association of dietary quality indices and cancer mortality: a systematic review and meta-analysis of cohort studies. Nutr Cancer. 2018;70:1091–1105. doi: 10.1080/01635581.2018.1502331. [DOI] [PubMed] [Google Scholar]
- 101.Salehi-Abargouei A, Maghsoudi Z, Shirani F, Azadbakht L. Effects of dietary approaches to stop hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases--incidence: a systematic review and meta-analysis on observational prospective studies. Nutrition. 2013;29:611–618. doi: 10.1016/j.nut.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 102.Yang ZQ, Yang Z, Duan ML. Dietary approach to stop hypertension diet and risk of coronary artery disease: a meta-analysis of prospective cohort studies. Int J Food Sci Nutr. 2019;70:668–674. doi: 10.1080/09637486.2019.1570490. [DOI] [PubMed] [Google Scholar]
- 103.Feng Q, Fan S, Wu Y. Adherence to the dietary approaches to stop hypertension diet and risk of stroke: A meta-analysis of prospective studies. Medicine (Baltimore) 2018;97 doi: 10.1097/MD.0000000000012450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ali Mohsenpour M, Fallah-Moshkani R, Ghiasvand R. Adherence to dietary approaches to stop hypertension (DASH)-style diet and the risk of cancer: a systematic review and meta-analysis of cohort studies. J Am Coll Nutr. 2019;38:513–525. doi: 10.1080/07315724.2018.1554460. [DOI] [PubMed] [Google Scholar]
- 105.Mohseni R, Mohseni F, Alizadeh S, Abbasi S. The association of dietary approaches to stop hypertension (DASH) diet with the risk of colorectal cancer: a meta-analysis of observational studies. Nutr Cancer. 2020;72:778–790. doi: 10.1080/01635581.2019.1651880. [DOI] [PubMed] [Google Scholar]
- 106.Soltani S, Shirani F, Chitsazi MJ, Salehi-Abargouei A. The effect of dietary approaches to stop hypertension (DASH) diet on weight and body composition in adults: a systematic review and meta-analysis of randomized controlled clinical trials. Obes Rev. 2016;17:442–454. doi: 10.1111/obr.12391. [DOI] [PubMed] [Google Scholar]
- 107.Zahedi H, Djalalinia S, Sadeghi O. Dietary inflammatory potential score and risk of breast cancer: systematic review and meta-analysis. Clin Breast Cancer. 2018;18:e561–e570. doi: 10.1016/j.clbc.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 108.Du S, Li Y, Su Z. Index-based dietary patterns in relation to gastric cancer risk: a systematic review and meta-analysis. Br J Nutr. 2019 doi: 10.1017/S0007114519002976. published online Nov 26. [DOI] [PubMed] [Google Scholar]
- 109.Berhe K, Kidanemariam A, Gebremariam G, Gebremariam A. Prevalence and associated factors of adolescent undernutrition in Ethiopia: a systematic review and meta-analysis. BMC Nutr. 2019;5:49. doi: 10.1186/s40795-019-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bonaccio M, Di Castelnuovo A, Costanzo S. Mediterranean diet and mortality in the elderly: a prospective cohort study and a meta-analysis. Br J Nutr. 2018;120:841–854. doi: 10.1017/S0007114518002179. [DOI] [PubMed] [Google Scholar]
- 111.Rosato V, Temple NJ, La Vecchia C, Castellan G, Tavani A, Guercio V. Mediterranean diet and cardiovascular disease: a systematic review and meta-analysis of observational studies. Eur J Nutr. 2019;58:173–191. doi: 10.1007/s00394-017-1582-0. [DOI] [PubMed] [Google Scholar]
- 112.Becerra-Tomás N, Blanco Mejía S, Viguiliouk E. Mediterranean diet, cardiovascular disease and mortality in diabetes: a systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit Rev Food Sci Nutr. 2020;60:1207–1227. doi: 10.1080/10408398.2019.1565281. [DOI] [PubMed] [Google Scholar]
- 113.Grosso G, Marventano S, Yang J. A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: are individual components equal? Crit Rev Food Sci Nutr. 2017;57:3218–3232. doi: 10.1080/10408398.2015.1107021. [DOI] [PubMed] [Google Scholar]
- 114.van den Brandt PA, Schulpen M. Mediterranean diet adherence and risk of postmenopausal breast cancer: results of a cohort study and meta-analysis. Int J Cancer. 2017;140:2220–2231. doi: 10.1002/ijc.30654. [DOI] [PubMed] [Google Scholar]
- 115.Esposito K, Kastorini CM, Panagiotakos DB, Giugliano D. Mediterranean diet and weight loss: meta-analysis of randomized controlled trials. Metab Syndr Relat Disord. 2011;9:1–12. doi: 10.1089/met.2010.0031. [DOI] [PubMed] [Google Scholar]
- 116.Garcia M, Bihuniak JD, Shook J, Kenny A, Kerstetter J, Huedo-Medina TB. The effect of the traditional Mediterranean-style diet on metabolic risk factors: a meta-analysis. Nutrients. 2016;8:168. doi: 10.3390/nu8030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schwingshackl L, Missbach B, König J, Hoffmann G. Adherence to a Mediterranean diet and risk of diabetes: a systematic review and meta-analysis. Public Health Nutr. 2015;18:1292–1299. doi: 10.1017/S1368980014001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Potter J, Brown L, Williams RL, Byles J, Collins CE. Diet quality and cancer outcomes in adults: a systematic review of epidemiological studies. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17071052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Steck SE, Guinter M, Zheng J, Thomson CA. Index-based dietary patterns and colorectal cancer risk: a systematic review. Adv Nutr. 2015;6:763–773. doi: 10.3945/an.115.009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Anton SD, Hida A, Heekin K. Effects of popular diets without specific calorie targets on weight loss outcomes: systematic review of findings from clinical trials. Nutrients. 2017;9:E822. doi: 10.3390/nu9080822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Iaccarino Idelson P, Scalfi L, Valerio G. Adherence to the Mediterranean Diet in children and adolescents: a systematic review. Nutr Metab Cardiovasc Dis. 2017;27:283–299. doi: 10.1016/j.numecd.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 122.Aljefree N, Ahmed F. Association between dietary pattern and risk of cardiovascular disease among adults in the Middle East and North Africa region: a systematic review. Food Nutr Res. 2015;59 doi: 10.3402/fnr.v59.27486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marshall S, Burrows T, Collins CE. Systematic review of diet quality indices and their associations with health-related outcomes in children and adolescents. J Hum Nutr Diet. 2014;27:577–598. doi: 10.1111/jhn.12208. [DOI] [PubMed] [Google Scholar]
- 124.Aljadani HMAMSD, Patterson AP, Sibbritt DP, Collins CEP. The association between dietary patterns and weight change in adults over time: a systematic review of studies with follow up. JBI Database Syst Rev Implement Reports. 2013;11:272–316. doi: 10.11124/01938924-201109161-00013. [DOI] [PubMed] [Google Scholar]
- 125.Mijatovic-Vukas J, Capling L, Cheng S. Associations of diet and physical activity with risk for gestational diabetes mellitus: a systematic review and meta-analysis. Nutrients. 2018;10:E698. doi: 10.3390/nu10060698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zheng J, Guinter MA, Merchant AT. Dietary patterns and risk of pancreatic cancer: a systematic review. Nutr Rev. 2017;75:883–908. doi: 10.1093/nutrit/nux038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schwedhelm C, Boeing H, Hoffmann G, Aleksandrova K, Schwingshackl L. Effect of diet on mortality and cancer recurrence among cancer survivors: a systematic review and meta-analysis of cohort studies. Nutr Rev. 2016;74:737–748. doi: 10.1093/nutrit/nuw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pourmasoumi M, Karimbeiki R, Vosoughi N. Healthy eating index/alternative healthy eating index and breast cancer mortality and survival: a systematic review and meta-analysis. Asia Pac J Oncol Nurs. 2016;3:297–305. doi: 10.4103/2347-5625.189819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kontogianni MD, Panagiotakos DB. Dietary patterns and stroke: a systematic review and re-meta-analysis. Maturitas. 2014;79:41–47. doi: 10.1016/j.maturitas.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 130.Sherzai A, Heim LT, Boothby C, Sherzai AD. Stroke, food groups, and dietary patterns: a systematic review. Nutr Rev. 2012;70:423–435. doi: 10.1111/j.1753-4887.2012.00490.x. [DOI] [PubMed] [Google Scholar]
- 131.Yusof AS, Isa ZM, Shah SA. Dietary patterns and risk of colorectal cancer: a systematic review of cohort studies (2000–2011) Asian Pac J Cancer Prev. 2012;13:4713–4717. doi: 10.7314/apjcp.2012.13.9.4713. [DOI] [PubMed] [Google Scholar]
- 132.Maghsoudi Z, Azadbakht L. How dietary patterns could have a role in prevention, progression, or management of diabetes mellitus? Review on the current evidence. J Res Med Sci. 2012;17:694–709. [PMC free article] [PubMed] [Google Scholar]
- 133.Foroughi M, Akhavanzanjani M, Maghsoudi Z, Ghiasvand R, Khorvash F, Askari G. Stroke and nutrition: a review of studies. Int J Prev Med. 2013;4(suppl 2):S165–S179. [PMC free article] [PubMed] [Google Scholar]
- 134.Godos J, Bella F, Torrisi A, Sciacca S, Galvano F, Grosso G. Dietary patterns and risk of colorectal adenoma: a systematic review and meta-analysis of observational studies. J Hum Nutr Diet. 2016;29:757–767. doi: 10.1111/jhn.12395. [DOI] [PubMed] [Google Scholar]
- 135.Esposito K, Chiodini P, Maiorino MI, Bellastella G, Panagiotakos D, Giugliano D. Which diet for prevention of type 2 diabetes? A meta-analysis of prospective studies. Endocrine. 2014;47:107–116. doi: 10.1007/s12020-014-0264-4. [DOI] [PubMed] [Google Scholar]
- 136.Ge L, Sadeghirad B, Ball GDC. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: systematic review and network meta-analysis of randomised trials. BMJ. 2020;369:m696. doi: 10.1136/bmj.m696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tangestani H, Salari-Moghaddam A, Ghalandari H, Emamat H. Adherence to the Dietary Approaches to Stop Hypertension (DASH) dietary pattern reduces the risk of colorectal cancer: a systematic review and meta-analysis. Clin Nutr. 2020 doi: 10.1016/j.clnu.2020.02.002. published online Feb 7. [DOI] [PubMed] [Google Scholar]
- 138.Coletta AM, Peterson SK, Gatus LA. Diet, weight management, physical activity and ovarian & breast cancer risk in women with BRCA1/2 pathogenic Germline gene variants: systematic review. Hered Cancer Clin Pract. 2020;18:5. doi: 10.1186/s13053-020-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Paula Bricarello L, Poltronieri F, Fernandes R, Retondario A, de Moraes Trindade EBS, de Vasconcelos FAG. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on blood pressure, overweight and obesity in adolescents: a systematic review. Clin Nutr ESPEN. 2018;28:1–11. doi: 10.1016/j.clnesp.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 140.Uusitupa M, Khan TA, Viguiliouk E. Prevention of type 2 diabetes by lifestyle changes: a systematic review and meta-analysis. Nutrients. 2019;11 doi: 10.3390/nu11112611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tabung FK, Brown LS, Fung TT. Dietary patterns and colorectal cancer risk: a review of 17 years of evidence (2000–2016) Curr Colorectal Cancer Rep. 2017;13:440–454. doi: 10.1007/s11888-017-0390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Demographic and Health Survey Programme Demographic and Health Survey. https://dhsprogram.com/data/available-datasets.cfm
- 143.UNICEF Multiple Indicator Cluster Survey. 2014. https://www.unicef.org/statistics/index_24302.html
- 144.WHO . World Health Organization; Geneva, Switzerland: 2008. Indicators for assessing infant and young child feeding practices (Part 1 definitions) [Google Scholar]
- 145.WHO . World Health Organization; Geneva, Switzerland: 2010. Indicators for assessing infant and young child feeding practices (Part 2 measurement) [Google Scholar]
- 146.WHO . World Health Organization; Geneva, Switzerland: 2017. Global nutrition monitoring framework: operational guidance for tracking progress in meeting targets for 2015. [Google Scholar]
- 147.Jones AD, Ickes SB, Smith LE. World Health Organization infant and young child feeding indicators and their associations with child anthropometry: a synthesis of recent findings. Matern Child Nutr. 2014;10:1–17. doi: 10.1111/mcn.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Krasevec J, An X, Kumapley R, Begin F, Frongillo EA. Diet quality and risk of stunting among infants and young children in low- and middle-income countries. Matern Child Nutr. 2017;13(suppl 2) doi: 10.1111/mcn.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mallard SR, Houghton LA, Filteau S. Dietary diversity at 6 months of age is associated with subsequent growth and mediates the effect of maternal education on infant growth in urban Zambia. J Nutr. 2014;144:1818–1825. doi: 10.3945/jn.114.199547. [DOI] [PubMed] [Google Scholar]
- 150.Marriott BP, White A, Hadden L, Davies JC, Wallingford JC. World Health Organization (WHO) infant and young child feeding indicators: associations with growth measures in 14 low-income countries. Matern Child Nutr. 2012;8:354–370. doi: 10.1111/j.1740-8709.2011.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zongrone A, Winskell K, Menon P. Infant and young child feeding practices and child undernutrition in Bangladesh: insights from nationally representative data. Public Health Nutr. 2012;15:1697–1704. doi: 10.1017/S1368980012001073. [DOI] [PubMed] [Google Scholar]
- 152.Saaka M, Wemakor A, Abizari AR, Aryee P. How well do WHO complementary feeding indicators relate to nutritional status of children aged 6–23 months in rural northern Ghana? BMC Public Health. 2015;15 doi: 10.1186/s12889-015-2494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Menon P, Bamezai A, Subandoro A, Ayoya MA, Aguayo VM. Age-appropriate infant and young child feeding practices are associated with child nutrition in India: insights from nationally representative data. Matern Child Nutr. 2015;11:73–87. doi: 10.1111/mcn.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Heidkamp RA, Ayoya MA, Teta IN, Stoltzfus RJ, Marhone JP. Complementary feeding practices and child growth outcomes in Haiti: an analysis of data from Demographic and Health Surveys. Matern Child Nutr. 2015;11:815–828. doi: 10.1111/mcn.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kamenju P, Liu E, Hertzmark E. Nutritional status and complementary feeding among HIV-exposed infants: a prospective cohort study. Matern Child Nutr. 2017;13 doi: 10.1111/mcn.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Micha R, Khatibzadeh S, Shi P, Andrews KG, Engell RE, Mozaffarian D. Global, regional and national consumption of major food groups in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys worldwide. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-008705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Singh GM, Micha R, Khatibzadeh S. Global, regional, and national consumption of sugar-sweetened beverages, fruit juices, and milk: a systematic assessment of beverage intake in 187 countries. PLoS One. 2015;10 doi: 10.1371/journal.pone.0124845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Micha R, Shulkin ML, Peñalvo JL. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: Systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE) PLoS One. 2017;12 doi: 10.1371/journal.pone.0175149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.WCRF/AICR Diet, nutrition, physical activity and cancer: a global perspective. 2018. https://www.wcrf.org/dietandcancer
- 160.Dror DK, Allen LH. The importance of milk and other animal-source foods for children in low-income countries. Food Nutr Bull. 2011;32:227–243. doi: 10.1177/156482651103200307. [DOI] [PubMed] [Google Scholar]
- 161.Headey D, Hirvonen K, Hoddinott J. Animal source foods and child stunting. Am J Agric Econ. 2018;100:1302–1319. doi: 10.1093/ajae/aay053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.