I read with great interest the recently published article by Li et al. 1 which suggests that a risk of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection may be significantly higher in subjects with blood group A and significantly lower in those with blood group O. This case‐control study compared the ABO blood‐group distribution in 265 cases with coronavirus disease 2019 (COVID‐19) at the Central Hospital of Wuhan with that in 3 694 healthy controls. 1 To verify the clinical findings by Li et al. 1 I herein would like to epidemiologically analyze the relationship between blood‐group distribution (i.e. proportion of subjects with blood‐group O, and A, B, and AB ) and SARS‐CoV‐2 infection (i.e. COVID‐19 prevalence) in nations around the world.
Blood‐group distribution in 101 different nations was available on Rhesus Negative (http://www.rhesusnegative.net/themission/bloodtypefrequencies/). For these nations, I extracted total confirmed COVID‐19 cases and deaths on 25 June 2020 from the website of the World Health Organization (WHO) (https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/situation‐reports/); total population, total pure‐alcohol consumption, life expectancy at birth, medical‐doctor and nursing/midwifery personnel density, hypertension and obesity prevalence, and annual PM2.5 [particulate matter 2.5] concentrations from WHO (https://www.who.int/gho/publications/world_health_statistics/2020/en/); population ages 0–14 and ≥65, GDP (Gross Domestic Product) and GNI (Gross National Income) per capita, PPP (Purchasing Power Parity), and diabetes prevalence from World Bank (https://data.worldbank.org/indicator/); and daily‐ambient UV (ultraviolet) radiation from WHO (https://apps.who.int/gho/data/view.main.35300) (Table SI). Random‐effects meta‐regression (i.e. inverse variance‐weighted regression), dealing with a nation as a study (in a meta‐analysis), was performed using OpenMetaAnalyst (http://www.cebm.brown.edu/openmeta/index.html). No comparable data regarding COVID‐testing regimens, death timing (post a positive test), and imposed‐lockdown timing/extent/duration were available. All the above‐mentioned covariates were together entered in the multivariate model. A meta‐regression graph depicted the COVID‐19 prevalence/fatality (plotted as the logarithm‐transformed prevalence/fatality on the y‐axis) as a function of a given covariate (plotted on the x‐axis). Because of relatively low proportions of subjects with blood‐group Rh(−), only blood‐group Rh(+) was investigated.
The present analysis included a total of 8·9‐million COVID‐19 cases and 465 000 deaths on a total of 6·8‐billion populations. Results of the univariate and multivariate meta‐regression were summarized in Table I. The univariate model for COVID‐19 prevalence indicated a significant association of higher blood‐group B‐Rh(+) (coefficient, −0·089; P <0·001; Fig 1A) with lower prevalence and no correlation of O‐Rh(+), A‐Rh(+), and AB‐Rh(+) to prevalence. In the multivariate regression, however, there was no association of B‐Rh(+) with prevalence. The univariate model for COVID‐19 fatality indicated significant correlations of higher O‐Rh(+) (coefficient, −0·030; P < 0·001; Fig 1B) and B‐Rh(+) (coefficient, −0·037; P = 0·004; Fig 1C) to lower fatality, a significant association of higher A‐Rh(+) (coefficient, 0·073; P = 0·001; Fig 1D) with higher fatality, and no correlation of AB‐Rh(+) to fatality. In the multivariate regression, there was no association of A‐Rh(+) and B‐Rh(+) with fatality but a significant correlation of higher O‐Rh(+) (coefficient, −0·024; P = 0·022) to lower fatality.
Table I.
Meta‐regression summary.
| Covariate | COVID‐19 prevalence | COVID‐19 fatality | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | Lower limit of 95% CI | Upper limit of 95% CI | P value | Figure | Coefficient | Lower limit of 95% CI | Upper limit of 95% CI | P value | Figure | |
| Univariate model | ||||||||||
| Blood‐group O‐Rh(+) (%) | −0·008 | −0·041 | 0·024 | 0·615 | −0·030 | −0·047 | −0·014 | <0·001 | Fig 1B | |
| Blood‐group A‐Rh(+) (%) | 0·032 | −0·022 | 0·086 | 0·247 | 0·073 | 0·047 | 0·099 | <0·001 | Fig 1D | |
| Blood‐group B‐Rh(+) (%) | −0·089 | −0·134 | −0·043 | <·001 | Fig 1A | −0·037 | −0·063 | −0·012 | 0·004 | Fig 1C |
| Blood‐group AB‐Rh(+) (%) | −0·096 | −0·254 | 0·062 | 0·233 | −0·003 | −0·088 | 0·082 | 0·945 | ||
| Multivariate model* | ||||||||||
| Blood‐group O‐Rh(+) (%) | Not performed | −0·024 | −0·044 | −0·003 | 0·022 | |||||
| Blood‐group A‐Rh(+) (%) | Not performed | 0·028 | −0·003 | 0·059 | 0·081 | |||||
| Blood‐group B‐Rh(+) (%) | −0·573 | −1·439 | 0·293 | 0·195 | −0·001 | −0·035 | 0·034 | 0·970 | ||
| Blood‐group AB‐Rh(+) (%) | Not performed | Not performed | ||||||||
CI, confidence interval.
Adjusted for population ages 0–14/≥65, hypertension, obesity, diabetes, tobacco‐use, life expectancy at birth, medical‐doctor/nursing/midwifery personnel density, GDP (Gross Domestic Product)/GNI (Gross National Income) per capita–PPP (Purchasing Power Parity), annual PM2·5 (particulate matter 2·5) concentration, and daily ambient UV (ultraviolet) radiation. Bold values mean statically significant.
Fig 1.
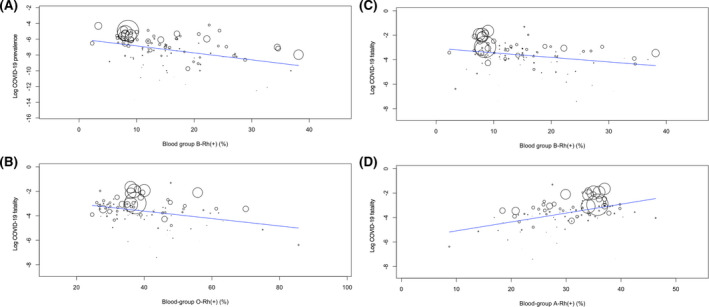
Metaregression graphs depicting the COVID‐19 prevalence/fatality (plotted as the logarithm‐transformed prevalence/fatality on the y‐axis) as a function of a given covariate (plotted on the x‐axis). Logarithm‐transformed prevalence on blood group B‐Rh(+) (A) and logarithm‐transformed fatality on O‐Rh(+) (B), B‐Rh(+) (C), and A‐Rh(+) (D).
In summary, although blood groups may not be associated with SARS‐CoV‐2 infection (i.e. COVID‐19 prevalence), O‐Rh(+) may be independently and protectively correlated to COVID‐19 fatality. The present findings do not mean directly that COVID‐19 patients with blood group O‐Rh(+) are at lower risk of death, which should be heeded. The present results denote simply that COVID‐19 fatality was lower in nations with higher blood group O‐Rh(+) prevalence because not patients' but populations' blood groups were analyzed. It should be also mentioned that COVID‐testing regimens, death timing (post a positive test), and imposed‐lockdown timing/extent/duration were not considered as covariates in the present multivariate regression, which may have brought about inconsistence of COVID prevalence and fatality. The absence of an association of blood groups with SARS‐CoV‐2 infection suggested in the present analysis does not correspond with the findings by Li et al. 1 which may be explained by the following. First, the present analysis applied meta‐regression (i.e. inverse variance‐weighted regression) with the multivariable model adjusting for 14 potential confounders, whereas Li et al. 1 simply compared the COVID‐19 cases and the healthy controls. Second, sample size of the study by Li et al. 1 was only 3 959 (265 cases and 3 694 controls), which is far smaller than the total of 6·8‐billion people (including a total of 8·9‐million cases) in the present analysis. Several previous findings, 2 , 3 , 4 however, may strengthen the results of the study by Li et al. 1 Furthermore, the Severe Covid‐19 GWAS [genome‐wide association study] Group 5 recently found, in their meta‐analysis adjusting for age and sex, a higher and lower risk for respiratory failure due to COVID‐19, respectively, in blood groups A and O than in other blood groups. Because of the nation‐level epidemiological design, the present results should be confirmed by further experimental and clinical investigations.
Conflict of interest
The author declares no competing interests.
Supporting information
Table SI. Data from 101 nations across the world.
References
- 1. Li J, Wang X, Chen J, Cai Y, Deng A, Yang M. Association between ABO blood groups and risk of SARS‐CoV‐2 pneumonia. Br J Haematol. 2020;190:24–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Batool Z, Durrani SH, Tariq S. Association of Abo and Rh blood group types to hepatitis B, hepatitis C, HIV and syphilis infection, a five year' experience in healthy blood donors in a tertiary care hospital. J Ayub Med Coll Abbottabad. 2017;29:90–2. [PubMed] [Google Scholar]
- 3. Guillon P, Clément M, Sébille V, Rivain JG, Chou CF, Ruvoën‐Clouet N, et al. Inhibition of the interaction between the SARS‐CoV spike protein and its cellular receptor by anti‐histo‐blood group antibodies. Glycobiology. 2008;18:1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng Y, Cheng G, Chui CH, Lau FY, Chan PK, Ng MH, et al. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA. 2005;293:1450–1. [DOI] [PubMed] [Google Scholar]
- 5. Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, Invernizzi P, et al. Genomewide association study of severe covid‐19 with respiratory failure. N Engl J Med. 2020:NEJMoa2020283. 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Data from 101 nations across the world.


