Abstract
Background:
Cannabidiol (CBD) is being investigated as a potential treatment for several medical indications, many of which are characterised by altered memory processing. However, the mechanisms underlying these effects are unclear.
Aims:
Our primary aim was to investigate how CBD influences cerebral blood flow (CBF) in regions involved in memory processing. Our secondary aim was to determine if the effects of CBD on CBF were associated with differences in working and episodic memory task performance.
Methods:
We used a randomised, crossover, double-blind design in which 15 healthy participants were administered 600 mg oral CBD or placebo on separate days. We measured regional CBF at rest using arterial spin labelling 3 h after drug ingestion. We assessed working memory with the digit span (forward, backward) and n-back (0-back, 1-back, 2-back) tasks, and we used a prose recall task (immediate and delayed) to assess episodic memory.
Results:
CBD increased CBF in the hippocampus (mean (95% confidence intervals) = 15.00 (5.78–24.21) mL/100 g/min, t14 = 3.489, Cohen’s d = 0.75, p = 0.004). There were no differences in memory task performance, but there was a significant correlation whereby greater CBD-induced increases in orbitofrontal CBF were associated with reduced reaction time on the 2-back working memory task ( r= −0.73, p = 0.005).
Conclusions:
These findings suggest that CBD increases CBF to key regions involved in memory processing, particularly the hippocampus. These results identify potential mechanisms of CBD for a range of conditions associated with altered memory processing, including Alzheimer’s disease, schizophrenia, post-traumatic stress disorder and cannabis-use disorders.
Keywords: ASL, cannabidiol, hippocampus, memory, MRI, perfusion
Introduction
Cannabidiol (CBD) is one of the main constituents of cannabis and is gaining interest for its broad therapeutic potential (Campos et al., 2016; Devinsky et al., 2014; Freeman et al., 2019; Zuardi, 2008). In addition to antipsychotic (Leweke et al., 2012; McGuire et al., 2018; Zuardi et al., 2012) and anxiolytic properties (Bergamaschi et al., 2011; Blessing et al., 2015; Crippa et al., 2011; Soares and Campos, 2017), there is some evidence to suggest that CBD may improve memory impairment across multiple domains, including working and episodic memory, as demonstrated in several preclinical models (Avraham et al., 2011; Barichello et al., 2012; Campos et al., 2015; Cassol et al., 2010; Cheng et al., 2014a, 2014b; Fagherazzi et al., 2012; Magen et al., 2009, 2010; Martin-Moreno et al., 2011; Pazos et al., 2012; Schiavon et al., 2014; Wright et al., 2013), cannabis users (Morgan et al., 2010, 2012), and in cognitive impairment caused by the other main constituent of cannabis, ∆9-tetrahydrocannabinol (THC) (Englund et al., 2013; Hindocha et al., 2015), although this has not been found in all studies (Boggs et al., 2018; Hindocha et al., 2018a; Morgan et al., 2018). Additionally, CBD modulates emotional memory processing (Bitencourt and Takahashi, 2018; Das et al., 2013; de Carvalho and Takahashi, 2017; Hindocha et al., 2015; Hudson et al., 2018; Lee et al., 2017; Stern et al., 2017; Uhernik et al., 2018), which may help to explain its putative therapeutic effects in post-traumatic stress disorder (PTSD; Hindocha et al., 2019; Shannon and Opila-Lehman, 2016) and anxiety disorders. However, the precise mechanisms underlying the effects of CBD on memory are unclear.
There is evidence that CBD alters cerebral blood flow (CBF) (Crippa et al., 2004, 2011) and this offers one possible mechanism through which it may influence memory function. CBD has been widely described as an arterial vasodilator (Sultan et al., 2017), and increases CBF in mouse models of stroke (England et al., 2015). In human single-photon emission computed tomography (SPECT) studies of resting state, 400 mg of oral CBD modulated resting CBF in key limbic and paralimbic regions involved in memory processing, including decreased CBF in the left amygdala-hippocampal complex and increased CBF in the left parahippocampal gyrus in healthy volunteers (Crippa et al., 2004). A similar study from the same laboratory later found that CBD decreased resting CBF in the left hippocampus and parahippocampal gyrus in patients with anxiety disorder (Crippa et al., 2011). Several functional neuroimaging studies using blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) have also demonstrated haemodynamic effects of CBD (Bhattacharyya et al., 2012a), including reductions in medial temporal lobe structures whilst viewing fearful faces (Fusar-Poli et al., 2009) and during attentional salience processing (Bhattacharyya et al., 2012b).
However, the proposed effects of CBD on regional CBF in humans have been disputed (Sultan et al., 2017). Previous studies (Crippa et al., 2004, 2011) have directly measured CBD-related changes in regional CBF using SPECT, an imaging modality with relatively low resolution to investigate regional effects. Additionally, no study has investigated the association between regional CBF and memory task performance under acute CBD. Our primary aim was therefore to investigate the acute effects of CBD on CBF in regions involved in memory processing in healthy individuals at rest using arterial spin labelling (ASL), a non-invasive, direct measure of CBF. Our secondary aim was to investigate the relationship between CBF and memory performance in episodic and working memory tasks. We defined regions of interest (ROIs) in the medial temporal lobe (MTL) and prefrontal cortex (PFC) a priori, which are differentially involved in both memory domains, including the hippocampus (Leszczynski, 2011; Squire and Zolamorgan, 1991), parahippocampal gyrus (Luck et al., 2010; Zolamorgan et al., 1989), amygdala (Hamann et al., 1999; Peinadomanzano, 1990; Phelps, 2004), dorsolateral PFC (Mars and Grol, 2007; Nyberg et al., 1996), orbitofrontal cortex (OFC) (Barbey et al., 2011; Brand and Markowitsch, 2006) and ventromedial PFC (Bechara et al., 1998; Bonnici et al., 2012). Based on previous studies, we hypothesised that CBD would decrease resting CBF in the hippocampus. We also sought to explore the differences in CBF in each of the other ROIs described above. Finally, we explored the relationship between regional CBF and memory performance.
Materials and methods
This study was conducted in accordance with Good Clinical Practice and the Helsinki Declaration (UCL Research Ethics Committee 3325/002). Participants provided written informed consent and received an honorarium for participation (£10 per hour).
Study participants
Participants were recruited through online adverts, posters and word-of-mouth. All participants included were right-handed and aged 18–70 (see Table 1 for demographic and clinical characteristics). Exclusion criteria were: (a) current use of psychotropic agents; (b) current or past use of cannabis or CBD; (c) previous use of any psychoactive (recreational) drug on >5 occasions; (d) current or previous mood disorder, psychosis, anxiety disorder, or substance abuse disorder according to Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) criteria; (e) current nicotine dependence (defined by Fagerström Test for Nicotine Dependence (Heatherton et al., 1991)); (f) score >7 on the Alcohol Use Disorders Identification Test (Saunders et al., 1993); (g) pregnancy; (h) impaired mental capacity; (i) allergy to CBD or placebo excipients; (j) claustrophobia or other contraindications to MRI.
Table 1.
Demographic and baseline clinical characteristics of study participants (n = 15).
| Characteristic | n |
|---|---|
| Sex | nine female, six male |
| Mean (±SD) | |
| Age (years) | 24.1 (±5.0) |
| BMI (kg/m2) | 22.6 (±4.1) |
| AUDIT score (0–40) | 1.5 (±1.7) |
| FDNT score (0–10) | 0.0 (±0.0) |
| BAI score (0–63) | 2.5 (±3.9) |
| BDI score (0–63) | 1.4 (±2.0) |
| ASI score (0–29) | 2.1 (±3.1) |
ASI: addiction severity index (averaged across four readings over the two sessions); AUDIT: alcohol use disorders identification test; BAI: beck anxiety inventory; BDI: beck depression inventory; BMI: body mass index; FDNT: Fagerström test for nicotine dependence.
Study design
We used a within-subjects, randomised, double-blind, placebo-controlled design. Participants received single doses of either 600 mg of CBD (pure synthetic (-)-CBD) or placebo in identical capsules at two sessions, separated by at least one week. Synthetic CBD (99.9% purity) was obtained from STI Pharmaceuticals (Brentwood, UK) and manufactured by Nova Laboratories (Leicester, UK). Size 2 gelatin capsules contained microcrystalline cellulose filler and CBD. Matched placebo capsules contained lactose filler. Whilst earlier SPECT studies used a lower dose of 400 mg (Crippa et al., 2004, 2011), more recent fMRI studies demonstrate that 600 mg is safe and exhibits measurable perfusion changes (Bhattacharyya et al., 2012a); a larger dose was opted on the assumption that it would be associated with larger, more measurable effects. The order of drug was randomised and stratified for sex. Following drug administration, brain scanning occurred at +180 min post capsule ingestion, which coincides with previously described peak plasma concentrations (Haney et al., 2016), and memory tasks were performed in succession roughly from between +275 and +340 min (see Supplemental material). Participants underwent three memory tasks: a prose recall task, N-back task and digit span task, and completed other tasks to be reported elsewhere. Matched versions of the tasks were used in the two study sessions. In order to control for variation in the absorption of CBD, participants were instructed to fast from midnight (excluding water, and caffeine if part of their morning routine) until after the brain scanning session. All participants underwent neuroimaging at least 12 h since their last meal; time of last meal was confirmed as part of pre-test screening conducted on the morning of testing. Drug administration and neuroimaging occurred at the same time each session. Drug administration was at 09:00 and neuroimaging at 12:00.
Power calculation
Two previous human studies have found acute effects of CBD v. placebo on resting CBF using a sample size of n = 10 in a crossover design (Crippa et al., 2004, 2011). This study has a sample size of n = 15, providing a 50% increase in sample size from these previous studies to adjust for Winner’s Curse (Button et al., 2013; Hindocha et al., 2018b). A sensitivity power analysis conducted using G*Power 3 (Faul et al., 2007) indicated that our sample size would provide 80% power to detect a large effect size (Cohen’s d = 0.8) at an alpha of 0.05.
Memory assessments
Prose recall task
The prose recall subtest of the Rivermead Behavioural Memory test (Wilson et al., 1991) taps episodic memory. Participants heard 30 s of prose (a news bulletin) and recalled it immediately and following a 25 min delay filled with other assessments, which included other tasks to compete as a distractor. The number of idea units recalled out of 21 was recorded.
N-back task
The N-back task (Freeman et al., 2012; Hindocha et al., 2017; van der Wee et al., 2003[TS: please link van der Wee to reference]), a spatial working memory (WM) task, required participants to observe sets of visual stimuli in one of six locations in a sequential order, and then record when the current stimulus corresponds with the stimulus seen in a pre-defined region (0-back), one step earlier (1-back), and two steps earlier (2-back). This order was fixed, and participants had a practice session before each N-back stage. Reaction time (RT) and accuracy were recorded automatically.
Digit span task
In the digit span task (Wechsler, 1997), which taps WM, participants read a series of digit strings to participants, who were then required to recall the digits in the same order in which they appeared, both forwards and backwards. Forwards recall taps maintenance of digits, where backwards recall taps both maintenance and manipulation of digits (Aben et al., 2012). The number of items increased every two strings starting from three for forwards and two for backwards. If a participant failed both strings at each level (i.e. strings of four numbers), the task was terminated, and moved on to backward or ended. The number of digits correctly recalled was recorded. There were a maximum of 12 forwards and 12 backwards series.
Image acquisition
We conducted image acquisition and data analysis blind to drug condition. We used a Siemens Magnetom Prisma 3T scanner to perform 3D axial pulsed ASL using the FAIR-QUIPSS II (Flow-sensitive Alternating Inversion Recovery–QUantitative Imaging of Perfusion Using a Single Subtraction) acquisition method (Wong et al., 1998). Scanner parameters were as follows: background suppression (grey-white) was on, bolus length (BL) 700 ms, inversion time (TI) 1990 ms, TR 4600 ms, TE 13.36 ms, slice thickness 4 mm, flip angle 180°, voxel size (mm) 1.9 × 1.9 × 4.0, bandwidth 3256 Hz/pixel, presumed tissue blood partition coefficient of water (λ) 0.9 mL/g, presumed relaxation time of blood (T1b) 1650 ms, inversion fraction (α) 0.98. Movement-corrected perfusion maps were calculated from the control and label images, and separately acquired Mo maps, using a MATLAB (Mathworks, Inc.) script written in-house (see Supplemental material).
Structural images were acquired using a T1 MPRAGE with 1 mm3 isotropic voxels, TR = 2300 ms, TE = 2.91, TI = 900, flip angle = 9°, parallel imaging factor = 2, bandwidth = 140 Hz/pixel. Using fMRIB Linear Image Registration Tool (FLIRT) within fMRIB Software Library (FSL) perfusion maps were registered to structural images and converted into standard Montreal Neurological Institute (MNI) space. Masks were generated using the Harvard–Oxford probabilistic atlas and applied to the specified regions of interest (ROI). Mean values of CBF (mL/100 mL/min) were then extracted using FSL.
Plasma CBD concentrations
We performed venepuncture immediately after scanning to measure CBD concentrations. Blood samples were collected in EDTA vacutainers and were immediately centrifuged to plasma for storage at −80°C. Samples were analysed using Gas Chromatography coupled with Mass Spectrometry with a lower limit of quantification of 0.5 ng/mL.
Statistical analysis
Two-tailed paired t-tests (significance threshold p <0.05, Holm–Bonferroni corrected for family-wise errors across six bilateral regions-of-interest) were performed to compare CBF between CBD and placebo sessions (i.e. both drug conditions) for each region. Repeated measures ANOVAs with a factor of drug (CBD v. placebo) were used to compare performance on memory tasks. For the N-back, an additional factor of load (0-back, 1-back, 2-back) was included for the outcomes of accuracy and RT. For the digit span, the additional factor was direction (forward, backward). For the prose recall task, the additional factor was delay (immediate, delayed). All post-hoc pairwise comparisons were Bonferroni-corrected. To investigate the relationship between differences in regional CBF and memory task performance, correlations were performed using the Pearson correlation coefficient at a two-tailed significance threshold of p <0.005 adjusted for 10 simultaneous comparisons using Bonferroni correction. This was performed for regions which demonstrated statistically significant differences in CBF (p <0.05). The 10 simultaneous comparisons were correlations between differences in hippocampal or orbitofrontal perfusion and differences in N-back accuracy and reaction time at 0-back, 1-back and 2-back, differences in forward and backward digit span scores, and differences in delayed and immediate prose recall scores. To assess differences in plasma CBD concentrations between the CBD and placebo groups, the non-parametric Wilcoxon signed ranks test was used. Levels of plasma CBD were correlated against hippocampal and orbitofrontal CBF during the CBD session using the Pearson correlation coefficient. Each analysis was repeated with a between subject factor of order of drug which did not significantly change the results; therefore, results are displayed without this factor.
Results
Demographic and clinical characteristics
Participant demographics and baseline clinical characteristics are in Table 1. From an original cohort of 17 healthy participants, we excluded two due to excessive rotation or motion artefact (voxel shift >9 mm and rotation >2°) resulting in 15 participants (nine female, six male).
Regional cerebral blood flow
See Table 2 and Figure 1 for differences in regional CBF between CBD and placebo. CBD caused a significant increase in CBF to the hippocampus (mean difference 15.00 mL/100 g/min (95% confidence intervals (CI) 5.78–24.21, t14 = 3.489, p = 0.004, Cohen’s d = 0.75, Figure 2) which survived Holm–Bonferroni correction. CBD did not cause significant differences in CBF in other regions of the MTL. In the PFC, CBD caused a significant increase in CBF in the OFC by a mean difference of 10.04 mL/100 g/min (95% CI 1.90–18.19, t14 = 2.644, p = 0.019, Cohen’s d = 0.55), however, this did not survive Holm-Bonferroni correction. There were no significant differences in other PFC areas. There were no significant effects of controlling for drug order on CBF in all regions.
Table 2.
Differences in regional cerebral blood flow between CBD and placebo.
| Region of interest | Δ Perfusion (mL/100 g/min (CI)) | Δ Perfusion (% (CI)) | t14 statistic | Cohen’s d | p | p # |
|---|---|---|---|---|---|---|
| Hippocampus | 15.00 (5.78–24.21) | 12.69 (5.75–19.63) | 3.489 | 0.75 | 0.004** | 0.024* |
| Parahippocampal gyrus | 2.52 (−14.33–19.38) | −0.32 (−13.87–13.23) | 0.792 | 0.09 | 0.441 | 1.000 |
| Amygdala | −3.26 (−13.80–7.29) | −4.31 (−16.48–7.86) | −0.663 | 0.15 | 0.518 | 1.000 |
| Orbitofrontal cortex | 10.04 (1.90–18.19) | 7.02 (1.06–12.97) | 2.644 | 0.55 | 0.019* | 0.095 |
| Ventromedial prefrontal cortex | 5.95 (−1.69–13.60) | 4.55 (−2.76–11.85) | 1.671 | 0.20 | 0.117 | 0.468 |
| Dorsolateral prefrontal cortex | 0.60 (−6.51–7.70) | −0.73 (−7.55–6.08) | 0.180 | 0.02 | 0.860 | 0.860 |
CI: confidence interval.
Significant at the p <0.05 level.
Significant at the p <0.01 level.
p# = Holm–Bonferroni corrected (using alpha 0.05).
Figure 1.
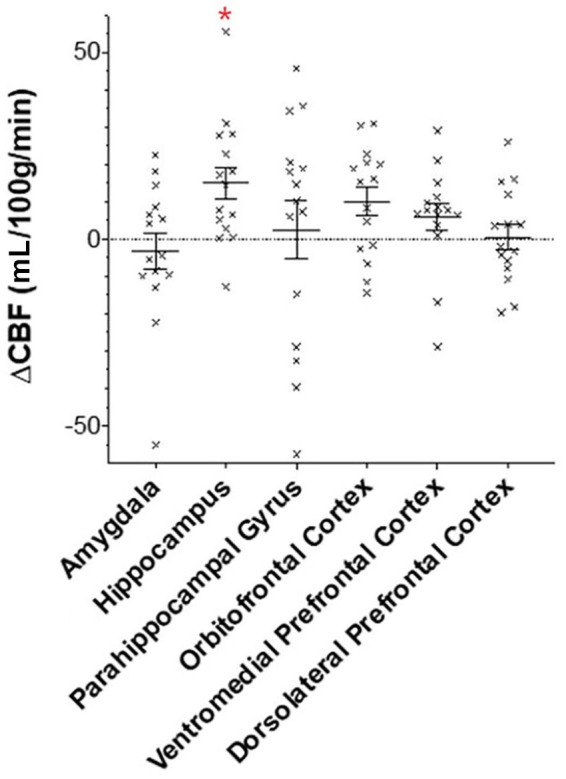
Differences in regional CBF between CBD and placebo. ΔCBF is the mean difference in cerebral blood flow (mL/100 g/min ± standard error of the mean) after CBD v. placebo in regions within the medial temporal lobe and prefrontal cortex.
The asterisk indicates regions with statistically significant change in CBF after correcting for multiple comparisons.
Figure 2.
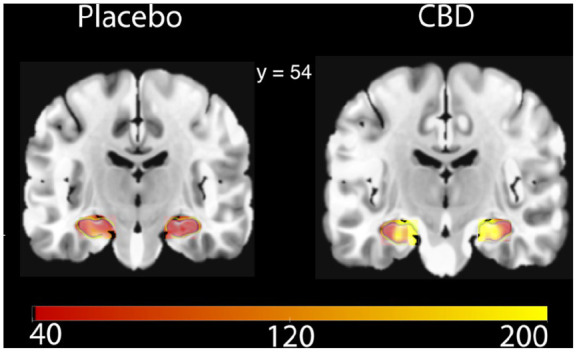
Differences in hippocampal CBF after CBD or placebo.
This coronal slice highlights differences in grey matter perfusion in the hippocampus (in green). Scale bar units = mL/100 g/min. MNI coordinate displayed.
Memory task performance
For the prose recall task, there was no main effect of drug (F1,14 = 3.701, η2 = 0.184, p = 0.075, mean difference −0.517, 95% CI −1.126–0.092, Figure 3a), or task (immediate or delayed; F1,14 = 3.311 η2 = 0.014, p = 0.090), and there was no significant drug*task interaction (F1,14 = 0.037, η2 = 0.000, p = 0.850).
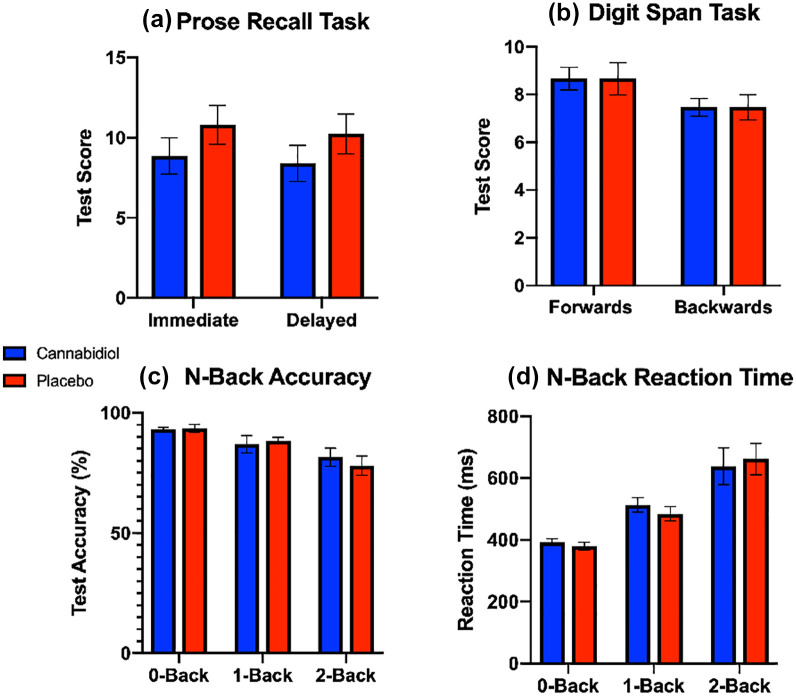
Figure 3.
Differences in memory task performance in the (a) prose recall, (b) digit span, and (c, d) N-back task (mean ± standard error of the mean) post-CBD or placebo.
For the digit span task, there was no main effect of drug (F1,14 = 0.312, η2 = 0.007, p = 0.585), there was a significant effect of task (forwards vs backwards; F1,14 = 9.333, η2 = 0.182. p = 0.009) reflecting the standard decreased score in backwards digit span score compared with forwards (see Figure 3b), but there was no significant drug–task interaction (F1,14 = 0.497, η2 = 0.007, p= 0.492).
Two participants did not complete all parts of the N-back task and were therefore removed from analysis. For accuracy scores in the N-back task, there was no main effect of drug (F1,12 = 0.026, η2 = 0.000, p = 0.875), there was a significant effect of task (0-back vs. 1-back v. 2-back; F2,24 = 10.180, η2 = 0.305, p = 0.001) shown by decreasing accuracy across increasing WM load (Figure 3c), but there was no drug*task interaction (F2,24=0.693, η2 =0.016, p=0.510). For RTs in the N-back task, there was no main effect of drug (F1,12=0.168, η2=0.000, p=0.689), there was a main effect of task (0-back vs. 1-back vs. 2-back; F1,12=25.642, η2=0.619, p<0.001) as shown by increasing RTs across increasing WM load (Figure 3d) but there was no drug–task interaction (F2,24 = 1.420, η2 = 0.006, p = 0.261). All analyses for memory task performance were repeated with a between subjects’ factor of order of drug administration and the results did not change.
Correlational analysis showed that CBD-induced differences in OFC CBF were correlated with 2-back task performance after correcting for multiple comparisons, such that increased CBF in the OFC following CBD was associated with decreased RT and thus better working memory performance (r11 = −0.73, p = 0.005; Supplemental Figure 1). There were no other statistically significant relationships between OFC CBF and 0-back and 1-back conditions, digit span, or prose recall tasks. There were no statistically significant correlations between changes in hippocampal CBF and memory performance after correcting for multiple comparisons. There was no significant effect of correcting for order of drug administration.
Plasma CBD levels
Analysis of plasma CBD levels using Wilcoxon Signed-Ranks Test showed that the CBD session ranks (median 6.010 ng/mL) were significantly higher than the placebo session ranks (median 0.000 ng/mL; Z = 3.124, p= 0.002; Supplemental Figure 2). There was a significant correlation between hippocampal CBF and CBD plasma levels during the CBD session (r = 0.623, p = 0.01), but this relationship was driven by an outlier. Once removed, this correlation was non-significant (r = 0.075, p = 0.798). Removal of this outlier still yielded a significant difference in hippocampal CBF under CBD v. placebo conditions (tdf = 3.5413, p= 0.004). There was no significant correlation between orbitofrontal CBF and CBD plasma levels during the CBD session (r = 0.445, p = 0.09). We note that one participant had an elevated cannabidiol level (4.94 ng/mL) during their placebo session. Considering that sessions were conducted at least one week apart, and the elimination half-life of cannabidiol is approximately 18–32 h (Devinsky et al., 2014), it is likely that this participant consumed CBD from a third-party source before their placebo session which may have included consuming cannabis. This participant was excluded, and analysis was re-conducted with no significant changes: there remained a significant difference in hippocampal CBF (tdf = 3.21513, p = 0.007), and a significant correlation between OFC CBF and 2-back reaction times (r = 0.760, p = 0.004).
Discussion
To our knowledge, this is the first study to find that acute CBD increases CBF in the hippocampus. This supports the view that CBD has region-specific haemodynamic effects in the human brain, which has previously been disputed (Sultan et al., 2017).
Relation to previous studies
Our finding of increased hippocampal CBF after CBD contrasts with two previous SPECT studies (Crippa et al., 2004, 2011). These found a decrease in CBF in healthy (Crippa et al., 2004) and anxiety disorder participants (Crippa et al., 2011). There are several possible explanations for this difference. Our study had higher statistical power than the previous two studies, with increased sample size of 50% to account for inflation of effect size (Button et al., 2013; Hindocha et al., 2018b). Moreover, our study used ASL which has better resolution to detect regional effects than SPECT. Our findings are consistent with a meta-analysis reporting that CBD increased CBF in mouse models of stroke (Sultan et al., 2017). Additionally, we administered a higher dose (600 mg CBD) than previous studies (400 mg CBD; (Crippa et al., 2004, 2011) which may have accounted for different findings due to the complex dose-effect profile of CBD (Zuardi et al., 2017).
Implications for hippocampal disorders
If replicated, the finding that acute CBD increases CBF in the hippocampus may be relevant for hippocampal disorders, since higher resting hippocampal blood flow is associated with better memory performance (Heo et al., 2010; Nishimura et al., 1998; Suzuki et al., 2016), although this relationship is not entirely clear (Finkelmeyer et al., 2016; Rane et al., 2013). With its key role in learning and memory (Sweatt, 2004), the hippocampus is an important therapeutic target across multiple neuropsychiatric disorders including schizophrenia (Green et al., 2000), depression (Rock et al., 2014), PTSD (Scott et al., 2015) and Alzheimer’s disease. Regional CBF is strongly associated with brain volume change and has a complex bidirectional relationship (Appelman et al., 2008; Zonneveld et al., 2015). Since acute CBD may increase hippocampal CBF, further studies are required to investigate whether CBD can attenuate the hippocampal structural alterations, including atrophy, and hippocampal-dependent memory impairments associated with these disorders (Ott et al., 2019). This notion is supported by findings that CBD can rescue hippocampal atrophy (Beale et al., 2018) and improve episodic memory performance (Englund et al., 2013) in chronic cannabis users. This finding may be particularly relevant to Alzheimer’s disease, where there are defects in blood flow control (Girouard and Iadecola, 2006).
The precise mechanisms through which CBD may modulate memory processing are unclear. In addition to causing endothelial vasodilatation through modulation of the endocannabinoid system (Stanley et al., 2015), CBD has numerous neuronal targets (Bih et al., 2015; Elsaid and Le Foll, 2020), which may underlie its pro-cognitive, anxiolytic, and antipsychotic effects (Premoli et al., 2019). Regarding memory, preclinical data suggest that CBD promotes hippocampal neurogenesis and facilitates synaptic plasticity (Campos et al., 2017). In addition, human spectroscopy studies demonstrate that CBD modulates glutamate and GABA levels across several limbic and prefrontal regions (Pretzsch et al., 2019). Through its cerebrovascular and neuronal activity, CBD may therefore alter the dysfunctional prefrontal-hippocampal circuitry associated with impaired memory in several neuropsychiatric disorders (Jin and Maren, 2015).
Although we found no differences in memory performance following CBD, improvements in hippocampal-dependent memory tasks with CBD have been described elsewhere, including improved performance in prose recall during cannabis use (Morgan et al., 2010), and delayed verbal memory following THC administration (Englund et al., 2013). However, findings have also been mixed in this area. CBD has previously not demonstrated improvements in memory performance following THC-challenge (Morgan et al., 2018), nicotine abstinence (Hindocha et al., 2018b), and in schizophrenia (Boggs et al., 2018). As our study was in healthy participants, it is possible that ceiling effects account for this lack of CBD-induced differences in memory task performance, and the lack of relationship between hippocampal CBF and memory task performance. This variability may additionally be due to drug dose, route of administration, memory test timing, and methodological variation across studies. A number of the studies on CBD-related improved memory performance were conducted on models of cognitive impairment due to systemic insult (e.g. sepsis), Alzheimer’s, or current cannabis users which may not emerge in studies of healthy volunteers.
We found a moderately sized, negative correlation between the effects of CBD on OFC CBF and 2-back RT. Among other functions (Rolls, 2004), the OFC is involved in WM, particularly emotion and/or face processing working memory (LoPresti et al., 2008; Ross et al., 2013). The direction of this correlation and its specificity to the 2-back task suggests that the effects of CBD on CBF in the OFC might be related to an increase in performance in manipulation of working memory. However, given that the effects of CBD were weak for increasing OFC CBF and did not show an improvement in 2-back task overall, further evidence would be needed to support this hypothesis.
Strengths and limitations
Major strengths of our study are the use of robust methodology including double-blinding, within-subject design, randomization of CBD and placebo order, and the inclusion of plasma CBD levels. ASL has good reproducibility within subjects (Jiang et al., 2010), and suffers from less inter-subject variability than BOLD signal in fMRI (Liu and Brown, 2007). Furthermore, ASL is a more direct neuroimaging modality for measuring regional CBF than other MRI methods, such as fMRI, which may detect reactive hyperaemia driven by astrocyte signaling (Attwell et al., 2010). ASL also offers better spatial resolution than other neuroimaging modalities used for similar studies such as SPECT.
Although our study had higher statistical power than previous studies (Crippa et al., 2004, 2011) it was not well powered to detect medium or small effect sizes. This study used a single dose of CBD in healthy volunteers, which may not translate to the effects of repeated CBD dosing and the use of CBD for psychiatric disorders or cognitive impairments. Finally, as the memory tasks were conducted outside of the scanner, we were unable to investigate the effects of CBD on fMRI measures of task performance, and correlations between these measures and CBF.
Conclusion
We found evidence that acute CBD causes a significant increase in regional CBF to the hippocampus. These findings may have implications for the potential use of CBD across a range of disorders associated with hippocampal dysfunction including Alzheimer’s disease, PTSD and depression.
Supplemental Material
Supplemental material, JOP_CBD_ASL_Supplementary_Material for The effects of acute cannabidiol on cerebral blood flow and its relationship to memory: An arterial spin labelling magnetic resonance imaging study by Michael A P Bloomfield, Sebastian F Green, Chandni Hindocha, Yumeya Yamamori, Jocelyn Lok Ling Yim, Augustus P M Jones, Hannah R Walker, Pawel Tokarczuk, Ben Statton, Oliver D Howes, H Valerie Curran and Tom P Freeman in Journal of Psychopharmacology
Acknowledgments
We are extremely grateful to all the radiographers at the Robert Steiner MRI Unit. We are also grateful to Professor Glyn Lewis. We would also like to thank all our participants for taking part in this study. We are grateful to Ting-Yun Chang for her help with preparing this manuscript.
Footnotes
Declaration of conflicting interests: Dr Bloomfield has undertaken consultancy work at an advisory panel for Spectrum Therapeutics. Otherwise, the authors declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this study was funded by a British Medical Association Foundation for Medical Research Margaret Temple Award to Dr Bloomfield. Dr Bloomfield is funded by a UCL Excellence Fellowship. Drs Bloomfield and Hindocha and Professor Curran are supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre. Dr Freeman was funded by a senior academic fellowship from the Society for the Study of Addiction.
ORCID iDs: Michael Andrew Pinho Bloomfield  https://orcid.org/0000-0002-1972-4610
https://orcid.org/0000-0002-1972-4610
Chandni Hindocha  https://orcid.org/0000-0003-1692-7401
https://orcid.org/0000-0003-1692-7401
Supplemental material: Supplemental material for this article is available online.
References
- Aben B, Stapert S, Blokland A. (2012) About the distinction between working memory and short-term memory. Front Psychol 3: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelman APA, Van der Graaf Y, Vincken KL, et al. (2008) Total cerebral blood flow, white matter lesions and brain atrophy: The SMART-MR study. J Cereb Blood Flow Metab 28: 633–639. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, et al. (2010) Glial and neuronal control of brain blood flow. Nature 468: 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham Y, Grigoriadis NC, Poutahidis T, et al. (2011) Cannabidiol improves brain and liver function in a fulminant hepatic failure-induced model of hepatic encephalopathy in mice. Br J Pharmacol 162: 1650–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Koenigs M, Grafman J. (2011) Orbitofrontal contributions to human working memory. Cereb Cortex 21: 789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barichello T, Ceretta RA, Generoso JS, et al. (2012) Cannabidiol reduces host immune response and prevents cognitive impairments in Wistar rats submitted to pneumococcal meningitis. Eur J Pharmacol 697: 158–164. [DOI] [PubMed] [Google Scholar]
- Beale C, Broyd S, Chye Y, et al. (2018) Prolonged cannabidiol treatment effects on hippocampal subfield volumes in current cannabis users. Cannabis Cannabinoid Res 3: 94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, et al. (1998) Dissociation of working memory from decision making within the human prefrontal cortex. J Neurosci 18: 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi MM, Queiroz RHC, Chagas MHN, et al. (2011) Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology 36: 1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Atakan Z, Martin-Santos R, et al. (2012. a) Neural mechanisms for the cannabinoid modulation of cognition and affect in man: A critical review of neuroimaging studies. Curr Pharm Des 18: 5045–5054. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Crippa JA, Allen P, et al. (2012. b) Induction of psychosis by Δ9-tetrahydrocannabinol reflects modulation of prefrontal and striatal function during attentional salience processing. Arch Gen Psychiatry 69: 27–36. [DOI] [PubMed] [Google Scholar]
- Bih CI, Chen T, Nunn AVW, et al. (2015) Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics 12: 699–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitencourt RM, Takahashi RN. (2018) Cannabidiol as a therapeutic alternative for post-traumatic stress disorder: From bench research to confirmation in human trials. Front Neurosci 12: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing EM, Steenkamp MM, Manzanares J, et al. (2015) Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics 12: 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs DL, Surti T, Gupta A, et al. (2018) The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology 235: 1923–1932. [DOI] [PubMed] [Google Scholar]
- Bonnici HM, Chadwick MJ, Lutti A, et al. (2012) Detecting representations of recent and remote autobiographical memories in vmPFC and hippocampus. J Neurosci 32: 16982–16991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Markowitsch H. (2006) Memory processes and the orbitofrontal cortex. In: Zald David H, Rauch Scott L. (eds) The Orbitofrontal Cortex. Oxford: Oxford University Press, pp. 285–306. [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, et al. (2013) Power failure: Why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14: 365. [DOI] [PubMed] [Google Scholar]
- Campos AC, Brant F, Miranda AS, et al. (2015) Cannabidiol increases survival and promotes rescue of cognitive function in a murine model of cerebral malaria. Neuroscience 289: 166–180. [DOI] [PubMed] [Google Scholar]
- Campos AC, Fogaca MV, Scarante FF, et al. (2017) Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front Pharmacol 8: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AC, Fogaca MV, Sonego AB, et al. (2016) Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res 112: 119–127. [DOI] [PubMed] [Google Scholar]
- Cassol OJ, Comim CM, Silva BR, et al. (2010) Treatment with cannabidiol reverses oxidative stress parameters, cognitive impairment and mortality in rats submitted to sepsis by cecal ligation and puncture. Brain Res 1348: 128–138. [DOI] [PubMed] [Google Scholar]
- Cheng D, Low JK, Logge W, et al. (2014. a) Chronic cannabidiol treatment improves social and object recognition in double transgenic APP(swe)/PS1ΔE9 mice. Psychopharmacology 231: 3009–3017. [DOI] [PubMed] [Google Scholar]
- Cheng D, Spiro AS, Jenner AM, et al. (2014. b) Long-term cannabidiol treatment prevents the development of social recognition memory deficits in Alzheimer’s disease transgenic mice. J Alzheimers Dis 42: 1383–1396. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Zuardi AW, Garrido GE, et al. (2004) Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology 29: 417. [DOI] [PubMed] [Google Scholar]
- Crippa JAS, Derenusson GN, Ferrari TB, et al. (2011) Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report. J Psychopharmacol 25: 121–130. [DOI] [PubMed] [Google Scholar]
- Das RK, Kamboj SK, Ramadas M, et al. (2013) Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology 226: 781–792. [DOI] [PubMed] [Google Scholar]
- de Carvalho CR, Takahashi RN. (2017) Cannabidiol disrupts the reconsolidation of contextual drug-associated memories in Wistar rats. Addict Biol 22: 742–751. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Cilio MR, Cross H, et al. (2014) Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 55: 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaid S, Le Foll B. (2020) The complexity of pharmacology of cannabidiol (CBD) and its implications in the treatment of brain disorders. Neuropsychopharmacology 45: 229–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England TJ, Hind WH, Rasid NA, et al. (2015) Cannabinoids in experimental stroke: A systematic review and meta-analysis. J Cereb Blood Flow Metab 35: 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund A, Morrison PD, Nottage J, et al. (2013) Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol 27: 19–27. [DOI] [PubMed] [Google Scholar]
- Fagherazzi EV, Garcia VA, Maurmann N, et al. (2012) Memory-rescuing effects of cannabidiol in an animal model of cognitive impairment relevant to neurodegenerative disorders. Psychopharmacology 219: 1133–1140. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, et al. (2007) G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191. [DOI] [PubMed] [Google Scholar]
- Finkelmeyer A, Nilsson J, He J, et al. (2016) Altered hippocampal function in major depression despite intact structure and resting perfusion. Psychol Med 46: 2157–2168. [DOI] [PubMed] [Google Scholar]
- Freeman TP, Hindocha C, Green SF, et al. (2019) Medicinal use of cannabis based products and cannabinoids. BMJ 365: l1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman TP, Morgan CJA, Vaughn-Jones J, et al. (2012) Cognitive and subjective effects of mephedrone and factors influencing use of a ‘new legal high’. Addiction 107: 792–800. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Crippa JA, Bhattacharyya S, et al. (2009) Distinct effects of Δ9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry 66: 95–105. [DOI] [PubMed] [Google Scholar]
- Girouard H, Iadecola C. (2006) Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol 100: 328–335. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, et al. (2000) Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the ‘right stuff’? Schizophr Bull 26: 119–136. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, et al. (1999) Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci 2: 289–293. [DOI] [PubMed] [Google Scholar]
- Haney M, Malcolm RJ, Babalonis S, et al. (2016) Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology 41: 1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, et al. (1991) The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86: 1119–1127. [DOI] [PubMed] [Google Scholar]
- Heo S, Prakash RS, Voss MW, et al. (2010) Resting hippocampal blood flow, spatial memory and aging. Brain Res 1315: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindocha C, Cousijn J, Rall M, et al. (2019) The effectiveness of cannabinoids in the treatment of posttraumatic stress disorder (PTSD): A systematic review. J Dual Diagn 16: 120–139. [DOI] [PubMed] [Google Scholar]
- Hindocha C, Freeman TP, Grabski M, et al. (2018. a) The effects of cannabidiol on impulsivity and memory during abstinence in cigarette dependent smokers. Sci Rep 8: 7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindocha C, Freeman TP, Grabski M, et al. (2018. b) Cannabidiol reverses attentional bias to cigarette cues in a human experimental model of tobacco withdrawal. Addiction 113: 1696–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindocha C, Freeman TP, Schafer G, et al. (2015) Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: A randomised, double-blind, placebo-controlled study in cannabis users. Eur Neuropsychopharmacol 25: 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindocha C, Freeman TP, Xia JX, et al. (2017) Acute memory and psychotomimetic effects of cannabis and tobacco both ‘joint’ and individually: A placebo-controlled trial. Psychol Med 47: 2708–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson R, Rushlow W, Laviolette SR. (2018) Phytocannabinoids modulate emotional memory processing through interactions with the ventral hippocampus and mesolimbic dopamine system: Implications for neuropsychiatric pathology. Psychopharmacology 235: 447–458. [DOI] [PubMed] [Google Scholar]
- Jiang L, Kim M, Chodkowski B, et al. (2010) Reliability and reproducibility of perfusion MRI in cognitively normal subjects. Magn Reson Imaging 28: 1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JJ, Maren S. (2015) Prefrontal-hippocampal interactions in memory and emotion. Front Syst Neurosci 9: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JLC, Bertoglio LJ, Guimaraes FS, et al. (2017) Cannabidiol regulation of emotion and emotional memory processing: Relevance for treating anxiety-related and substance abuse disorders. Br J Pharmacol 174: 3242–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszczynski M. (2011) How does hippocampus contribute to working memory processing? Front Hum Neurosci 5: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leweke FM, Piomelli D, Pahlisch F, et al. (2012) Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2: e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT, Brown GG. (2007) Measurement of cerebral perfusion with arterial spin labeling: Part 1. Methods. J Int Neuropsychol Soc 13: 517–525. [DOI] [PubMed] [Google Scholar]
- LoPresti ML, Schon K, Tricarico MD, et al. (2008) Working memory for social cues recruits orbitofrontal cortex and amygdala: A functional magnetic resonance imaging study of delayed matching to sample for emotional expressions. J Neurosci 28: 3718–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck D, Danion JM, Marrer C, et al. (2010) The right parahippocampal gyrus contributes to the formation and maintenance of bound information in working memory. Brain Cogn 72: 255–263. [DOI] [PubMed] [Google Scholar]
- Magen I, Avraham Y, Ackerman Z, et al. (2009) Cannabidiol ameliorates cognitive and motor impairments in mice with bile duct ligation. J Hepatol 51: 528–534. [DOI] [PubMed] [Google Scholar]
- Magen I, Avraham Y, Ackerman Z, et al. (2010) Cannabidiol ameliorates cognitive and motor impairments in bile-duct ligated mice via 5-HT1A receptor activation. Br J Pharmacol 159: 950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Grol MJ. (2007) Dorsolateral prefrontal cortex, working memory, and prospective coding for action. J Neurosci 27: 1801–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Moreno AM, Reigada D, Ramirez BG, et al. (2011) Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: Relevance to Alzheimer’s disease. Mol Pharmacol 79: 964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire P, Robson P, Cubala WJ, et al. (2018) Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: A multicenter randomized controlled trial. Am J Psychiatry 175: 225–231. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Freeman TP, Hindocha C, et al. (2018) Individual and combined effects of acute delta-9-tetrahydrocannabinol and cannabidiol on psychotomimetic symptoms and memory function. Transl Psychiatry 8: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Schafer G, Freeman TP, et al. (2010) Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: Naturalistic study. Br J Psychiatry 197: 285–290. [DOI] [PubMed] [Google Scholar]
- Morgan CJA, Gardener C, Schafer G, et al. (2012) Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychol Med 42: 391–400. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Fukuchi K, Hayashida K, et al. (1998) Decreased hippocampal blood flow related to memory impairment after cardiovascular surgery: Assessment by reconstructed SPECT parallel to the longitudinal axis of the hippocampal formations. Clin Nucl Med 23: 356–360. [DOI] [PubMed] [Google Scholar]
- Nyberg L, McIntosh AR, Cabeza R, et al. (1996) General and specific brain regions involved in encoding and retrieval of events: What, where, and when. Proc Natl Acad Sci USA 93: 11280–11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott CV, Johnson CB, Macoveanu J, et al. (2019) Structural changes in the hippocampus as a biomarker for cognitive improvements in neuropsychiatric disorders: A systematic review. Eur Neuropsychopharmacol 29: 319–329. [DOI] [PubMed] [Google Scholar]
- Pazos MR, Cinquina V, Gomez A, et al. (2012) Cannabidiol administration after hypoxia-ischemia to newborn rats reduces long-term brain injury and restores neurobehavioral function. Neuropharmacology 63: 776–783. [DOI] [PubMed] [Google Scholar]
- Peinadomanzano MA. (1990) The role of the amygdala and the hippocampus in working memory for spatial and nonspatial information. Behav Brain Res 38: 117–134. [DOI] [PubMed] [Google Scholar]
- Phelps EA. (2004) Human emotion and memory: Interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol 14: 198–202. [DOI] [PubMed] [Google Scholar]
- Premoli M, Aria F, Bonini SA, et al. (2019) Cannabidiol: Recent advances and new insights for neuropsychiatric disorders treatment. Life Sci 224: 120–127. [DOI] [PubMed] [Google Scholar]
- Pretzsch CM, Freyberg J, Voinescu B, et al. (2019) Effects of cannabidiol on brain excitation and inhibition systems: A randomised placebo-controlled single dose trial during magnetic resonance spectroscopy in adults with and without autism spectrum disorder. Neuropsychopharmacology 44: 1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane S, Ally BA, Hussey E, et al. (2013) Inverse correspondence between hippocampal perfusion and verbal memory performance in older adults. Hippocampus 23: 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock PL, Roiser JP, Riedel WJ, et al. (2014) Cognitive impairment in depression: A systematic review and meta-analysis. Psychol Med 44: 2029–2040. [DOI] [PubMed] [Google Scholar]
- Rolls ET. (2004) The functions of the orbitofrontal cortex. Brain Cogn 55: 11–29. [DOI] [PubMed] [Google Scholar]
- Ross RS, LoPresti ML, Schon K, et al. (2013) Role of the hippocampus and orbitofrontal cortex during the disambiguation of social cues in working memory. Cogn Affect Behav Neurosci 13: 900–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, et al. (1993) Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption. Addiction 88: 791–804. [DOI] [PubMed] [Google Scholar]
- Schiavon AP, Soares LM, Bonato JM, et al. (2014) Protective effects of cannabidiol against hippocampal cell death and cognitive impairment induced by bilateral common carotid artery occlusion in mice. Neurotox Res 26: 307–316. [DOI] [PubMed] [Google Scholar]
- Scott JC, Matt GE, Wrocklage KM, et al. (2015) A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull 141: 105–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon S, Opila-Lehman J. (2016) Effective of cannabidiol oil for pediatric anxiety and insomnia as part of posttraumatic stress disorder: A case report. Perm J 20: 108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares VP, Campos AC. (2017) Evidences for the anti-panic actions of cannabidiol. Curr Neuropharmacol 15: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zolamorgan S. (1991) The medial temporal lobe memory system. Science 253: 1380–1386. [DOI] [PubMed] [Google Scholar]
- Stanley CP, Hind WH, Tufarelli C, et al. (2015) Cannabidiol causes endothelium-dependent vasorelaxation of human mesenteric arteries via CB1 activation. Cardiovasc Res 107: 568–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CAJ, da Silva TR, Raymundi AM, et al. (2017) Cannabidiol disrupts the consolidation of specific and generalized fear memories via dorsal hippocampus CB1 and CB2 receptors. Neuropharmacology 125: 220–230. [DOI] [PubMed] [Google Scholar]
- Sultan SR, Millar SA, England TJ, et al. (2017) A systematic review and meta-analysis of the haemodynamic effects of cannabidiol. Front Pharmacol 8: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Matsumoto Y, Ota H, et al. (2016) Hippocampal blood flow abnormality associated with depressive symptoms and cognitive impairment in patients with chronic heart failure. Circ J 80: 1773–1780. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. (2004) Hippocampal function in cognition. Psychopharmacology 174: 99–110. [DOI] [PubMed] [Google Scholar]
- Uhernik AL, Montoya ZT, Balkissoon CD, et al. (2018) Learning and memory is modulated by cannabidiol when administered during trace fear-conditioning. Neurobiol Learn Mem 149: 68–76. [DOI] [PubMed] [Google Scholar]
- van der Wee NJA, Ramsey NF, Jansma JM, et al. (2003) Spatial working memory deficits in obsessive compulsive disorder are associated with excessive engagement of the medial frontal cortex. Neuroimage 20: 2271–2280. [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1997) Wechsler Adult Intelligence Scale: Administration and Scoring Manual. 3rd ed. San Antonio: Psychological Corporation. [Google Scholar]
- Wilson B, Cockburn J, Baddeley A. (1991) The Rivermead Behavioural Memory Test. Bury St. Edmunds, Suffolk: Thames Valley Test Company. [Google Scholar]
- Wong EC, Buxton RB, Frank LR. (1998) Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II). Magn Reson Med 39: 702–708. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Vandewater SA, Taffe MA. (2013) Cannabidiol attenuates deficits of visuospatial associative memory induced by Δ9tetrahydrocannabinol. Br J Pharmacol 170: 1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolamorgan S, Squire LR, Amaral DG, et al. (1989) Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J Neurosci 9: 4355–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonneveld HI, Loehrer EA, Hofman A, et al. (2015) The bidirectional association between reduced cerebral blood flow and brain atrophy in the general population. J Cereb Blood Flow Metab 35: 1882–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW. (2008) Cannabidiol: From an inactive cannabinoid to a drug with wide spectrum of action. Braz J Psychiatry 30: 271–280. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Crippa JAS, Hallak JEC, et al. (2012) A critical review of the antipsychotic effects of cannabidiol: 30 Years of a translational investigation. Curr Pharm Des 18: 5131–5140. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Rodrigues NP, Silva AL, et al. (2017) Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front Pharmacol 8: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, JOP_CBD_ASL_Supplementary_Material for The effects of acute cannabidiol on cerebral blood flow and its relationship to memory: An arterial spin labelling magnetic resonance imaging study by Michael A P Bloomfield, Sebastian F Green, Chandni Hindocha, Yumeya Yamamori, Jocelyn Lok Ling Yim, Augustus P M Jones, Hannah R Walker, Pawel Tokarczuk, Ben Statton, Oliver D Howes, H Valerie Curran and Tom P Freeman in Journal of Psychopharmacology