Abstract
In December 2019, the world was introduced to a new betacoronavirus, referred to as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) for its propensity to cause rapidly progressive lung damage, resulting in high death rates. As fast as the virus spread, it became evident that the novel coronavirus causes a multisystem disease (COVID‐19) that may involve multiple organs and has a high risk of thrombosis associated with striking elevations in pro‐inflammatory cytokines, D‐dimer, and fibrinogen, but without disseminated intravascular coagulation. Postmortem studies have confirmed the high incidence of venous thromboembolism, but also notably revealed diffuse microvascular thrombi with endothelial swelling, consistent with a thrombotic microangiopathy, and inter‐alveolar endothelial deposits of complement activation fragments. The clinicopathologic presentation of COVID‐19 thus parallels that of other thrombotic diseases, such as atypical hemolytic uremic syndrome (aHUS), that are caused by dysregulation of the complement system. This raises the specter that many of the thrombotic complications arising from SARS‐CoV‐2 infections may be triggered and/or exacerbated by excess complement activation. This is of major potential clinical relevance, as currently available anti‐complement therapies that are highly effective in protecting against thrombosis in aHUS, could be efficacious in COVID‐19. In this review, we provide mounting evidence for complement participating in the pathophysiology underlying the thrombotic diathesis associated with pathogenic coronaviruses, including SARS‐CoV‐2. Based on current knowledge of complement, coagulation and the virus, we suggest lines of study to identify novel therapeutic targets and the rationale for clinical trials with currently available anti‐complement agents for COVID‐19.
Keywords: complement, covid‐19, microvascular, thrombotic microangiopathy, tissue factor
1. INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic1 continues to spread, a rising cause of morbidity and mortality.2 Although the course of the resultant disease, COVID‐19, varies, the most severe manifestations are catastrophic multiorgan failure with widespread inflammation, microvascular thrombosis, and increased risk of venous thromboembolism.3., 4., 5., 6., 7., 8., 9. In spite of comprehensive intensive care with cardiorespiratory support, anti‐inflammatory agents, antibiotics where needed, and anticoagulants,10 there are still no proven effective therapies,11., 12. and death rates remain high. This highlights the critical need to expand our knowledge of the pathophysiological mechanisms underlying the dramatic host response to SARS‐CoV‐2 infection toward the design of urgently needed diagnostic and therapeutic strategies.
The aggressive nature of COVID‐19 is reminiscent of other rapidly progressive, organ‐damaging, thrombotic disorders where innate immune host responses escalate out of control. This is exemplified by the thrombotic microangiopathy (TMA), atypical hemolytic uremic syndrome (aHUS), where the major multiprotein, bloodborne component of the innate immune system, complement, is overactivated because of loss‐of‐function or gain‐of‐function mutations in complement regulatory genes.13 Before the introduction of the anticomplement drug, eculizumab,14., 15. aHUS episodes, often triggered by a preceding viral illness, commonly resulted in accelerated microvascular thrombosis and multiorgan damage, with a high mortality rate, similar to what is all too often seen with COVID‐19. There are compelling arguments to support the notion that complement overactivation also participates in SARS‐CoV‐2‐triggered thrombosis and multiorgan failure. We hypothesize that many of the manifestations of COVID‐19 are mediated in part by excess activation of complement, initiated directly by virus surface constituents and infected cell modulation, overall contributing to the thromboinflammatory tempest. If this is true, administration of available and/or newly developed anti‐complement therapies might be beneficial toward improved outcomes for COVID‐19.
In this review, we describe the links between complement and coagulation, the evidence that supports a role for complement in COVID‐19, potential mechanisms by which SARS‐CoV‐2 triggers complement activation and thromboinflammation, underline some of the challenges and inconsistencies to this hypothesis, and propose several therapeutic strategies using existing drugs and new potential targets.
2. COMPLEMENT ACTIVATION
The complement system comprises >40 soluble and membrane‐bound proteins, eliciting innate immune responses via opsonization, destruction and elimination of pathogens and damaged/apoptotic/infected host cells, and recruitment of inflammatory cells and adaptive immunity.16., 17. Following recognition of pathogen‐ and/or danger‐associated molecular patterns (DAMPs), complement is activated in a cascade‐like fashion via three pathways. These have in common the generation of potent biological effectors, including the covalent deposition of C3b onto foreign particles for macrophage targeting; the production of chemotaxins and anaphylatoxins, C3a, C4a and C5a; and the membrane insertion of a lytic membrane attack complex (MAC). These trigger an inflammatory response and destruction of the invading pathogen (Figure 1 ).
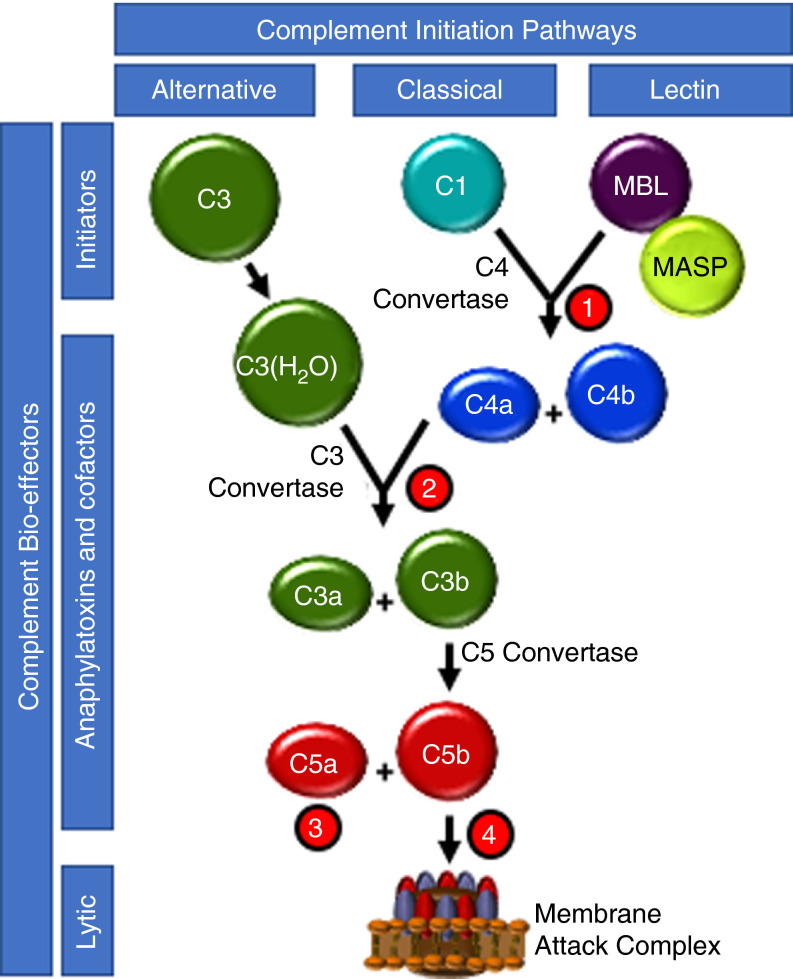
FIGURE 1.
Complement pathway overview and therapeutic intervention. The three pathways of complement initiation are depicted. The alternative pathway (AP) is initiated by spontaneous hydrolysis of the intramolecular thioester within C3, thus providing a continuous source of the C3b‐like cofactor C3(H2O) that accelerates the proteolytic generation of the cofactor C3b for pathway propagation and anaphylatoxin C3a generation. The classical pathway (CP) is initiated by association of the C1q/r/s complex with antibody bound to a multivalent foreign particle, resulting in generation of the cofactor C4b, which accelerates enzymatic generation of C3a and C3b and mild anaphylatoxin C4a. Like the CP, initiation of the lectin pathway (LP) by recognition of foreign lectins by the MBL/MASP complex results in C3b/C3a production via C3 convertase. Once enough C3b accumulates, its cofactor activity produces the cofactor C5b, which triggers MAC assembly and foreign membrane permeabilization. The most potent complement anaphylatoxin, C5a, is a by‐product. Several complement loci have been targeted for therapeutic intervention: ①, CP/LP pathway initiation is inhibited by the serpin, CI‐INH, at the level of MASP and C1r; ②, C3a and C3b product formation is inhibited by the compstatin derivatives AMY‐101 and APL‐2; ③, the anaphylatoxin C5a interaction with its cellular receptor is prevented by the humanized monoclonal antibody, BDB001; and ④ C5b and C5a generation is inhibited by the humanized monoclonal antibodies, eculizumab and ravulizumab, thus preventing MAC assembly
The lectin and classical pathways (LP and CP, respectively) are triggered by interactions of mannose binding lectin (MBL) and C1q, respectively, to pathogens and damaged cells. This leads to generation of MBL‐associated serine proteases (MASPs)18 for the LP, and C1r and C1s for the CP. MASP‐2 and C1s have in common that they cleave C2 and C4 to generate the CP/LP C3 convertase, C4b2b, with liberation of C3a and C4a. The alternative pathway (AP) is constitutively "on" by virtue of continuous hydrolysis of C3 to C3(H2O), generating the C3 convertase, C3bBb, when factor B is cleaved by factor D. Thus, all pathways converge to form a C3‐convertase, which proteolyses C3 into C3a and C3b, subsequently shifting in specificity to C5‐convertase, proteolyzing C5 to liberate C5b and C5a. C5b assembles with C6, C7, C8, and C9 molecules to yield the MAC comprising C5b‐9. Mediated primarily by binding and signaling via their respective cognate receptors, C3aR and C5aR1/2, the anaphylatoxins C3a and C5a are potent biological effectors of the complement system. A thrombo‐inflammatory response may be elicited by a broad range of effects, including recruiting leukocytes, and activating platelets and endothelial cells.19., 20.
Several fluid‐phase and membrane‐anchored regulators strictly moderate and localize complement, including, for example, factor H (FH), C4bBP, CD46, CD55, CD59, and thrombomodulin.21., 22., 23., 24., 25., 26., 27. Excess complement activation from loss‐of‐function or gain‐of‐function mutations in these factors notably causes thrombosis and resultant tissue damage as seen in aHUS and paroxysmal nocturnal hemoglobinuria (PNH).28., 29., 30., 31. This is relevant to COVID‐19, which is similarly strongly associated with vascular thrombosis and inflammation.32., 33., 34., 35.
3. SPIRALLING ACTIVATION OF COMPLEMENT AND COAGULATION
Several molecular and cellular mechanisms by which complement activation promotes coagulation have been delineated36., 37. (Figure 2 ). For example, the MASPs activate prothrombin, cleave fibrinogen to fibrin, enhance factor XIIIa activity, and activate protease activated receptors (PARs)38., 39., 40., 41. (Figure 3 ). C5a triggers release of prothrombotic factors from platelets, induces expression of the coagulation initiator tissue factor (TF) on endothelial cells and monocytes,42., 43. and suppresses expression of natural anticoagulant proteins. C5a furthermore induces P‐selectin exposure, which is a C3b receptor,20 thus providing a site on the platelet for C3/C5 convertase assembly, amplifying complement activation. C5b‐7 activates TF on monocytes,44 and C5b‐9 activates platelets and endothelial cells and promotes TF procoagulant function.45., 46., 47. Notably, the key complement‐induced procoagulant factors, thrombin and TF,48., 49. feed back and amplify complement activation, thereby sustaining a vicious, escalating, and tissue‐damaging cycle. This is further exacerbated by the virus itself, which may acquire and express host TF.50., 51., 52., 53. Overall, there is an often‐overlooked but relevant bidirectional interplay between complement and coagulation, which may help explain the high incidence of thrombosis in COVID‐19.
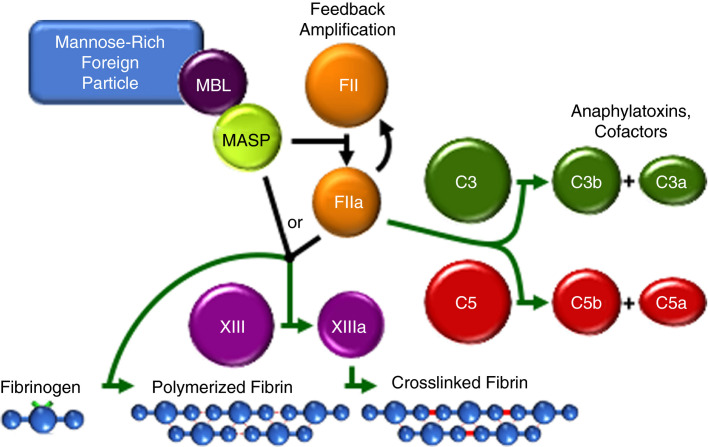
FIGURE 2.
The humoral MASP‐thrombin coagulation amplification cycle and reciprocal thrombin‐complement crossover. When associated with a mannose‐rich foreign particle (such as a virus‐infected cell surface), MBL triggers MASP‐mediated prothrombin (FII) activation to thrombin (FIIa), which feedback amplifies its own generation via the coagulation cascade (black lines). MASP and thrombin share substrate specificity resulting in cell modulation and crosslink‐stabilized clot formation. Thrombin crosses over into complement by cleaving C3 and C5, propagating inflammation and anaphylaxis
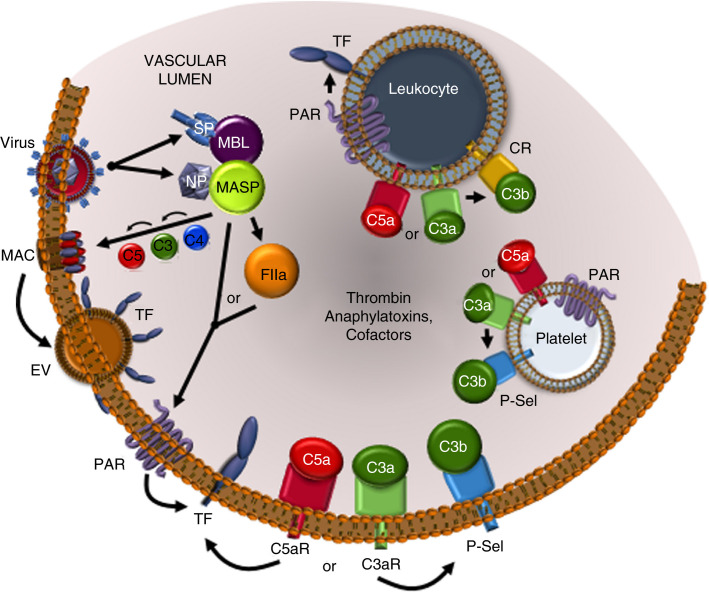
FIGURE 3.
Complement‐ and coagulation‐mediated cell activation in COVID‐19. SARS‐CoV‐2‐encoded spike (SP) and nucleocapsid (NP) proteins can directly induce LP stimulation via MBL and MASP, respectively, triggering the complement‐coagulation crossover pathways and deposition of membrane attack complex (MAC) on infected cell surfaces. Perturbation of the cell membrane by MAC is known to induce extracellular vesicles (EV), which contain TF, thus further amplifying thrombin generation. Thrombin and MASP directly cleave protease activated receptors (PAR) leading to activation of endothelial cells, leukocytes, and platelets and additional production of thrombin. Ligand engagement by C5a (C5aR) and C3a (C3aR) receptors induces numerous cell changes including P‐selectin (P‐Sel) stimulation, which can localize complement cofactors C3b and C3(H2O) on the endothelium and platelets. C5aR and C3aR on leukocytes also trigger activation of complement receptors (CR), which bind C3b and its degradation products that modulate cell function. The stimulation of anaphylatoxin receptors and PARs can cause release of pro‐inflammatory intracellular granule contents. Thus, virus‐induced provocation of complement cofactors and anaphylatoxins and thrombin, cause cellular amplification of thromboinflammation
4. SARS‐COV‐1 AND MERS‐COV: TRIGGERS FOR COMPLEMENT‐MEDIATED THROMBOPATHY?
Serious epidemics have recently been caused by betacoronaviruses, with significant homology to SARS‐CoV‐2, these being SARS‐CoV‐1 and Middle East respiratory syndrome (MERS)‐CoV. A review of the clinical and pathological findings in survivors and nonsurvivors of SARS‐CoV‐1 infection revealed a high incidence of venous thromboembolism (VTE), as well as microvascular thrombosis involving multiple organs,3 like COVID‐19. Although data are more limited, the epidemic resulting from MERS‐CoV was also associated with a high risk of VTE.3 Interestingly, transgenic overexpression in mice of the receptor responsible for MERS‐CoV entry into cells, resulted in a virus‐triggered, rapidly progressive pulmonary disease, with diffuse microvascular thrombosis.54 Combined with SARS‐CoV‐2, these findings imply that thrombosis of large and small vessels are typical clinical characteristics of the betacoronavirus genus. But does complement participate, and if so, how?
Preclinical studies with SARS‐CoV‐1 and MERS‐CoV support the notion that complement activation is not only associated with virus‐related organ damage, but also is likely causative. In mouse models, intranasal administration of MERS‐CoV55 triggered inflammatory lung damage, with increased deposition of C5b‐9, and elevated circulating levels of C5a, IL‐1β, tumor necrosis factor‐α (TNF‐α) and interferon‐γ. Inhibition of C5aR with specific antibodies, reduced complement activation, alleviated the lung damage, and limited viral replication. By a similar approach, Gralinski et al56 administered SARS‐CoV‐1 to mice, inducing lung damage with increased tissue deposition of C5b‐9, C3b, and C4d, amounts of which correlated with severity of injury. A role for complement activation in this adverse viral response, was confirmed by the marked reduction in lung pathology and cytokine release when the virus was administered to mice deficient in C3. Protection was also afforded to mice lacking C4 and factor B, but to a lesser extent than with C3 deficiency. These data implicated both the AP and the CP/LP as mediating the SARS‐CoV‐1‐triggered lung injury.56
Other studies have revealed direct interactions of these coronaviruses with components of the LP. In vitro, MBL binds to immobilized SARS‐CoV‐1 likely via surface glycans, and enhances C4 deposition on the virus in a mannan‐dependent manner.57 The group of Gao et al58 has performed the most extensive studies (albeit reported in a non‐peer‐reviewed publication) that underscore the role of the LP. Expanding on the work of Ip et al,57 they showed that a site of N‐glycosylation within the spike protein of SARS‐CoV‐1 binds to and activates MBL, thereby enhancing the cleavage of C3 via the LP. They further demonstrated that there is a highly conserved motif within the nucleocapsid (N) protein of SARS‐CoV‐1, MERS‐CoV, and SARS‐CoV‐2 that binds to MASP‐2 and triggers its dimerization and autoactivation, resulting in enhanced C4 cleavage and generation of the LP C3 convertase in the presence of mannan and MBL (Figure 3). They also showed that the N‐protein resides in close proximity to MASP‐2 on SARS‐CoV‐1‐infected pulmonary cells. Thus, these complement‐linked adaptations of the N‐protein may have evolved to contribute to the virulence of the betacoronaviruses.
That the N‐protein has been found in the serum of patients acutely infected with SARS‐CoV‐159., 60. raises the likelihood that the preceding observations are pathophysiologically relevant. In an LPS‐triggered lung injury model in mice, gene delivery of the SARS‐CoV‐1 N‐protein caused profound damage/inflammation, with increased neutrophil accumulation, and C3 fragment and C4b deposition.58 Consequently, Masp‐2 deficient mice were relatively resistant to this LPS‐N‐protein challenge, experiencing less severe pneumonia and a shorter course of illness. Mortality and lung damage were also significantly reduced by coadministration of anti‐N antibodies, anti‐MASP‐2 antibodies, or C1‐esterase inhibitor (C1‐INH), all consistent with the LP mediating the N‐protein triggered disease. Similar findings were obtained when the MERS‐CoV N‐protein was administered, with partial rescue of the mice achieved with C1‐INH and anti‐MASP‐2 antibodies, indicating a general response to betacoronaviruses by the LP.
5. EVIDENCE SUPPORTING A ROLE FOR COMPLEMENT IN COVID‐19‐ASSOCIATED THROMBOSIS
Taken together, the preceding evidences strongly suggest that there is a direct correlation between the complement overactivation and thrombopathies associated with infections with SARS‐CoV‐1 and MERS‐CoV. Similarly, the data for complement participating in the pathogenesis of thrombosis and end‐organ damage associated with COVID‐19, albeit less abundant, are nonetheless supportive. In the few postmortem studies of COVID‐19 patients that have been published, there were increased inter‐alveolar endothelial deposits of MBL, MASP‐2, C4b, C3b, and C5b‐9,33., 61. as well as C5b‐9 deposits in the glomeruli of kidneys.62 These reports are in line with excess activation of the LP, as appears to occur with the other pathogenic coronaviruses.58 This is not surprising, as the spike protein of SARS‐CoV‐2 is heavily glycosylated with L‐fucose and mannose,63 thereby providing recognition sites for MBL binding and activation of the LP. Providing a direct link to the thrombotic diathesis of patients with COVID‐19, complement hyperactivation was accompanied by histopathologic evidence, particularly in the lungs, comprising diffuse microvascular thrombi and endothelial swelling consistent with TMA, which is a hallmark of aHUS.33., 61. This was not an isolated finding, as similar small vessel changes with microthrombi were also observed in the largest autopsy series reported (12 patients).9 These disturbances of the vasculature may also be caused by direct endothelial invasion by SARS‐CoV‐2 via the ACE‐2 receptor,64 which would promote a prothrombotic and pro‐inflammatory phenotype, with exposure of DAMPs and activation of the CP. Interestingly, although anemia, thrombocytopenia, and particularly elevations in lactate hydrogenase are frequently observed in patients with COVID‐19, fragmentation hemolysis is not a major feature. This does not, however, exclude a diagnosis of complement‐mediated TMA, as even in active aHUS, schistocytes are not always detected on peripheral blood smear examination.65., 66.
Overall, we propose that SARS‐CoV‐2 triggers complement activation through its recognition by the host as a foreign pathogen, by its acting as a cofactor to enhance LP activation, and by its invading and injuring ACE‐2 receptor‐expressing host cells. This inevitably promotes a thromboinflammatory response, which in turn, cycles back to further amplify complement activation and clotting. Proving this hypothesis will require clinical trials with anti‐complement therapies, several of which are under way. These will be discussed in the following section.
6. OTHER CONSIDERATIONS
Despite the evidence that rapid onset of microvascular thrombosis and multiorgan failure following SARS‐CoV‐2 infections may at least in part, be mediated via hyperactivation of complement, major gaps remain in our understanding of the mechanisms and the reasons for the varied manifestations. For example, is the CP also involved? Is the increased risk of VTE and large vessel arterial thrombosis34 in COVID‐19 patients also complement‐mediated? Does SARS‐CoV‐2 cause direct dysregulation of complement and is this an advantage to the virus? Can the often‐profound pro‐inflammatory response that accompanies thrombosis in COVID‐19 be explained by complement activation? Why do children appear to either be spared from disease or sometimes follow a different course? Are there genetic variants in complement regulatory genes that determine severity of outcome? Finally, which therapeutic anti‐complement interventions should be considered to best prevent/treat the thromboinflammatory response to SARS‐CoV‐2 infection?
6.1. CP, coagulation, and COVID‐19
It has been speculated that following exposure to SARS‐CoV‐2, antiviral IgM and IgG antibodies may be generated, resulting in immune complexes on the virus or the infected cell(s), thereby triggering complement activation via the CP.67 Although reasonable, to our knowledge, this has not yet been demonstrated with any of the pathogenic coronaviruses, using available in vitro or in vivo models. This would be important to determine, as there are clinically tested, safe and available anti‐complement therapies that specifically target C1s,68 or both C1s and the MASPs, the latter through the use of C1‐INH.69
6.2. Does complement activation trigger venous thromboembolism and arterial thrombosis in COVID‐19?
The incidence of VTE in COVID‐19 ranges from ~10% to 35%70, with autopsies indicating that it may exceed 50%.9 The mechanisms underlying the thrombosis are entirely unknown. Although many of these patients have elevated D‐dimer and fibrinogen, they also commonly have increased C‐reactive protein and IL‐6, consistent with a vigorous inflammatory response.35 The thrombopathy may be a response to direct virus‐induced damage to vascular endothelial cells64 and/or the virus acquiring TF from host cells, both triggering coagulation activation.50., 52., 53., 71. However, it is also possible that complement contributes. In contrast to aHUS, which causes a TMA with microvascular thrombi, the complement‐mediated disorder, PNH, is strongly associated with VTE and arterial thrombosis.30., 31. PNH is caused by somatic mutations in the PIGA gene that result in loss of glycosylphosphatidylinositol‐anchored proteins, including complement negative regulators CD55 and CD59 on hematopoietic stem cell clones. There are multiple mechanisms by which thrombosis in PNH may occur, but the strong protection afforded by eculizumab is overwhelming evidence of the central role of complement.30., 31.
SARS‐CoV‐2 infection might impart a PNH‐like thrombotic phenotype through one of several proteases encoded by the viral genome, including the nonstructural protein Mpro (also known as 3CLpro or Nsp5) that has chymotrypsin‐like activity. Mpro in turn, generates multiple additional nonstructural proteins from the virus, several of which also possess proteolytic properties.72 Complement activation products have been detected on erythrocytes of patients with COVID‐19,73 but the relevance of these findings have not been explored. It is conceivable that one or more of the SARS‐CoV‐2 proteases cleave glycosylphosphatidylinositol‐anchored complement regulatory proteins from the surface of circulating or vascular endothelial cells, resulting in a PNH‐like phenotype predisposing to large vessel thrombosis.
6.3. SARS‐CoV‐2 proteases, complement, coagulation, and the cytokine storm
The detection of SARS‐CoV‐1 proteins in the serum of acutely infected patients,59., 60. and the proteolytic activities of some of the SARS‐CoV‐2‐encoded proteins, raises other considerations that may help explain heightened cyclical activation of complement and coagulation. It is possible that one or more SARS‐CoV‐2‐encoded proteins released into the blood following lysis of infected cells, augment complement activation and the resultant risk of TMA and/or large vessel thrombosis by altering the function of complement factors and/or coagulation factors, similar to what may occur when the N‐protein interacts with MASP‐2.58 Like thrombin, plasmin, and other serine proteases, it is also possible that SARS‐CoV‐2‐encoded proteases cleave and activate C3 and/or C5,49., 74. liberating more of the pro‐inflammatory/pro‐coagulant C5a and augmenting the amount and/or activity of the prothrombotic MAC. Alternatively, SARS‐CoV‐2 proteins may bind to and/or cleave/inactivate negative regulators of complement, such as occurs with FH in its interactions with multiple microorganisms.75 Such phenomena may not be restricted to the extracellular environment.
The long‐held view that complement activation occurs only in the circulation and on the surface of cells, pathogens, and foreign surfaces has recently been supplanted by recent studies that reveal roles also for intracellular complement (referred to as the complosome) in immune cell regulation.76 Indeed, most complement components, including C3, C5, and FH, may be synthesized by many immune and nonimmune cells, including epithelial and endothelial cells. Intracellular activation of complement via cleavage of C3 and C5 can be triggered by pathogens such as viruses, leading to a strong proinflammatory cytokine response, with the release of, for example, IL‐6, IL‐1β. and TNF‐α.77 Notably, it is these pro‐inflammatory cytokines that are characteristically elevated in severe COVID‐19, eliciting the so‐called "cytokine storm."35
The mechanisms underlying the prominent COVID‐19‐associated hyper‐inflammatory response are not well understood. However, excess complement activation by itself is not a plausible explanation, as this phenomenon is not observed in aHUS. Extensively reviewed by others,78., 79. it has been proposed that SARS‐CoV‐2 invasion of ACE‐2 receptor expressing cells (airway epithelial cells, vascular endothelial cells, circulating monocytes), causes cellular damage, exposure of DAMPs, release of pro‐inflammatory chemokines and chemoattractants (including C3a and C5a), resulting in recruitment of inflammatory leukocytes that release for example, IL‐1β, IL‐6, IL‐18, TNF‐α, and interferon‐γ, all of which work in concert to trigger a self‐amplifying pro‐inflammatory cascade with massive release of cytokines. In addition to promoting widespread vascular and organ damage, these cytokines also exhibit prominent prothrombotic properties, triggering platelet activation, the release of reactive oxygen species by neutrophils, upregulated expression of monocyte TF, and reduced synthesis and function of vasculoprotective molecules, such as thrombomodulin.80., 81. Although suppressing complement alone may not be sufficient to halt this virus‐triggered cyclone of organ‐damaging events, such a therapeutic strategy may have value when given in conjunction with other therapeutic modalities.82
6.4. Age‐dependent differences in COVID‐19: thrombosis in children?
The clinical spectrum following SARS‐CoV‐2 infection ranges from asymptomatic to critically ill, with severity highest in the very elderly, particularly in those with preexisting comorbidities, and lowest in children. Although children and adolescents have a low incidence of symptomatic infections compared to adults, those who do require hospitalization, may suffer from what has been termed a "multisystem inflammatory syndrome in children (MIS‐C)."83 Sharing features with other rare disorders, including Kawasaki disease, toxic shock syndrome, and hemophagocytic lymphohistiocytosis,84 the presentation is distinct from adult COVID‐19. Although varied, these patients suffer from cardiovascular shock with myocardial dysfunction, fever, mucocutaneous lesions, rash, gastrointestinal disorders, thrombocytopenia, neutrophilia, evidence of acute kidney injury, and biochemical evidence of hyperinflammation. Strikingly, these younger individuals often lack severe pulmonary manifestations, and equally enigmatic, thromboembolic events are rarely seen.83 Complement activation has not reportedly been measured in these patients. Among various proposed explanations for the differing responses in children and adults, it is possible that age‐dependent differences in circulating ACE‐2 and expression of the ACE‐2 receptor by host cells may contribute to the lower incidence of lung disease and thrombosis.85., 86., 87. Elucidating the pathogenic mechanisms for MIS‐C will be key for optimal treatment and prevention.
6.5. Molecular determinants of outcome in COVID‐19
As is evident, the determinants of susceptibility to infection, and the pattern and severity of illness remain poorly understood. Ramlall et al88 evaluated the linkage between severe COVID‐19 and genetic variations associated with complement and coagulation pathways by performing a candidate‐driven analysis of the United Kingdom Biobank89 that comprises genetic, physical, and health data from ~500 000 individuals. Their analyses identified variants in CD55, FH, and C4bBP,88 all of which conferred a significantly increased risk of an adverse clinical outcome. Interestingly, these negative regulators of complement have also been linked to age‐related macular degeneration, another disorder that is most commonly manifest in the elderly.90 Such information is not only further evidence of the important role that complement likely plays in COVID‐19, but if validated, may be of value in risk assessments which in turn will impact on clinical decision making.
6.6. Therapeutic considerations
While the world searches for effective means of halting the spread of SARS‐CoV‐2, an array of strategic therapeutic approaches are being tested to shorten the course of COVID‐19 and to limit the complications, including for VTE, inflammation and end‐organ damage from microvascular thrombosis. Because of the complexity of the disease, it is readily apparent that multiple interventional strategies will be necessary to abrogate progression and to effect recovery and healing. It is also evident that current anticoagulant strategies will not be adequate to fully protect these patients from thrombosis; however, anti‐complement drugs might be of benefit.
There are numerous potential sites in the complement system that may be targeted to help treat COVID‐19 (Figure 1). Several anti‐complement drugs are currently in the clinic, being evaluated or used for a variety of complement mediated disorders. One or more of these may be well‐suited for evaluation of efficacy for COVID‐19.
Activation of the primary effector of complement, C3, would seemingly be a logical target to attenuate the thrombopathology in COVID‐19. Compstatin derivatives that interfere with C3 convertase activity, include, for example APL‐2 (Apellis Pharmaceuticals) and AMY‐101 (Amyndas Pharmaceuticals). The safety of an approach that would suppress activation of all three pathways91 in the setting of a viral infection has been reasonably questioned. However, such fears may be partly allayed by several lines of evidence: First, although children with inherited deficiency of C3 are more susceptible to infections, for unexplained reasons, they do not carry that risk into adulthood.92 Second, pharmacologic suppression of C3 in nonhuman primates did not result in adverse effects. In fact, skin wounds of the C3‐deficient animals tended to heal more rapidly.93 Third, C3‐deficient mice were protected against tissue damage triggered by SARS‐CoV‐1 infection.56 Finally, AMY‐101 has been used safely and apparently with benefit in a single adult patient with COVID‐19.94 It may be prudent to avoid using this approach in children with COVID‐19.
The apparent prominent role of the LP in mediating the lung damage and thromboinflammatory response to all three of the pathogenic coronaviruses, suggests that this pathway might also be an ideal therapeutic target.95 MASP‐2 is the only LP protease that cleaves C4, and as noted previously, also has direct procoagulant properties. Anti‐MASP‐2 monoclonal antibodies (OMS721, Narsoplimab) have been deemed to be safe for aHUS, and are being evaluated by Omeros Corporation in phase 3 studies.95 It would be interesting to test its efficacy in COVID‐19.
A naturally occurring LP inhibitor, C1‐INH, has broader specificity and is additionally a potent suppressor of the CP. It is widely and safely used for patients with C1‐INH deficiency, the basis of hereditary angioedema.69 For treatment of COVID‐19 and other thrombo‐inflammatory diseases, C1‐INH may also have the added benefit of reducing activation of the contact pathway of coagulation and generation of kallikrein and bradykinin, and thus controlling pulmonary vascular permeability.96
Anti‐C5a and anti‐C5aR blocking antibodies are also therapeutic options, inhibiting downstream in the complement cascade, but without affecting assembly of the MAC. Administration of anti‐C5a antibodies (BDB001) to two patients with COVID‐19 was associated with rapid resolution of fever, respiratory symptoms, hepatic dysfunction, and systemic inflammation (C‐reactive protein).58
Eculizumab and its long‐acting form, ravulizumab, are well‐established for their efficacy in aHUS and PNH and are the only anti‐complement drugs for which clinical trials have been initiated for patients with severe COVID‐19. These target the terminal pathway of complement, interfering with cleavage of C5, thereby reducing the generation of the most potent anaphylatoxin, C5a, and the MAC, both of which have pro‐inflammatory and pro‐thrombotic properties, while allowing the formation of opsonic C3 and C4 by‐products. In an uncontrolled trial, complicated by other therapies, four patients with severe COVID‐19 appeared to derive benefit.97 Too early to draw conclusions, eculizumab has the advantage of retaining generation of upstream C3 activation products, and several years of clinical experience with the drug for aHUS and PNH.
The anecdotal nature of all of the reports to date necessitates cautious optimism that dampening complement activation is a valid therapeutic strategy to abrogate the thromboinflammatory sequelae of SARS‐CoV‐2 infections. And whichever anti‐complement drug is selected, one must consider the potential increased risk of infection from loss of one or more components of the complement system, with early introduction of appropriate antibiotic and/or vaccination protocols.98
In terms of when to treat, we are of the opinion that early intervention for COVID‐19 with anti‐complement agents may be important to limit cell/tissue damage. Although a systematic evaluation of complement activation during COVID‐19 has not yet been performed, preliminary data from our laboratory (unpublished) and others99 indicate that complement activation occurs early, with elevations in C5a and C5b‐9 in patients with moderate disease. Could early dampening of complement activation, and thus the innate immune response to the virus, worsen the disease? Theoretically, this could occur if intervention is too early (ie, when SARS‐CoV‐2‐infected individuals are asymptomatic or have mild disease). This should certainly be a consideration. At the least, when the disease is at a mild stage, it might be a good opportunity to vaccinate against encapsulated organisms or start the appropriate antibiotic coverage, in the case that more aggressive interventions, including anti‐complement therapies, become necessary.
Overall, the question as to when to administer anti‐complement agents and which ones to use, will only be answered with well‐controlled clinical trials, informed in part by comprehensive studies that correlate severity of disease and outcomes, with biological markers of complement activation.
7. CONCLUSION
There is overwhelming evidence that the complement‐thrombosis axis is a successful therapeutic target to mitigate disease in aHUS. Although the pathogenesis and progression of the multiorgan damage resulting from SARS‐CoV‐2 are different from aHUS, the clinicopathologic similarities justify consideration of complement as a therapeutic target to help to ameliorate the thromboinflammatory and organ‐damaging manifestations of COVID‐19. The deployment of existing anti‐complement drugs with the aim of dampening the severity of COVID‐19 may have obvious advantages. Using investigations with other betacoronaviruses and preliminary evidence gathered from SARS‐CoV‐2 as a paradigm, will furthermore spur the emergence of novel therapies. These may interfere with interactions between the SARS‐CoV‐2‐encoded N‐protein and MASP‐2, the spike protein and MBL, or various other SARS‐CoV‐2‐encoded proteins that are found to promote complement activation. The development of small animal models to quickly assess efficacy in abrogating virus‐induced thromboinflammation will move these rapidly into the clinic for careful evaluation.
Table 1.
Abbreviations
| ACE‐2 | Angiotensin converting enzyme‐2 |
| aHUS | Atypical hemolytic uremic syndrome |
| AP | Alternative pathway of complement |
| C1‐INH | C1 esterase inhibitor |
| C1s, C1r | CP serine proteases |
| C3a, C4a, C5a | Complement anaphylatoxic peptides |
| C4b2b | CP/LP C3 convertase |
| C5aR | Receptor for C5a |
| CP | Classical pathway of complement |
| DAMP | Danger associated molecular pattern |
| FB | FACTOR B |
| FH | Factor H |
| IFN | Interferon |
| IL | Interleukin |
| LDH | Lactate dehydrogenase |
| LP | Lectin pathway of complement |
| LPS | Lipopolysaccharide |
| MAC | Membrane attack complex (C5b‐9) |
| MASP | MBL associated serine protease |
| MBL | Mannose binding lectin |
| MIS‐C | Multisystem inflammatory syndrome in children |
| N‐protein | Nucleoplasmid protein of SARS‐CoV‐2 |
| PAMP | Pathogen associated molecular pattern |
| PAR | Protease activated receptor |
| PNH | Paroxysmal nocturnal hemoglobinuria |
| ROS | Reactive oxygen species |
| SARS‐CoV‐2 | Severe acute respiratory syndrome coronavirus‐ 2 |
| TF | Tissue factor |
| TMA | Thrombotic microangiopathy |
| TNF | Tumor necrosis factor |
| VTE | Venous thromboembolism |
CONFLICT OF INTERESTS
The authors have no competing interests or conflicts.
AUTHOR CONTRIBUTIONS
Edwin M. Conway and Edward L.G. Pryzdial were equal contributors to writing and editing the manuscript. Both bear equal responsibility for the content.
ACKNOWLEDGMENTS
E.M.C. is supported by grants from the Canadian Institutes of Health Research (CIHR), the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canada Foundations for Innovation (CFI), the Heart and Stroke Foundation of Canada (HSFC) and CanVECTOR. He holds a Tier 1 Canada Research Chair in Endothelial Cell Biology and is an adjunct Scientist with the Canadian Blood Services (CBS). E.L.G.P. was supported by grants from the CIHR and HSFC.
Canada Research Chairs
Natural Sciences and Engineering Research Council of Canada
CanVECTOR
Heart and Stroke Foundation of Canada
Canadian Institutes of Health Research
Footnotes
Manuscript handled by: Patricia Liaw
Final decision: Patricia Liaw and 31‐Jul‐2020
REFERENCES
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID‐19, SARS‐CoV‐1, MERS‐CoV and lessons from the past. J Clin Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lodigiani C., Iapichino G., Carenzo L., et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok F.A., Kruip M., van der Meer N.J.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID‐19: an updated analysis. Thromb Res. 2020;19:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demelo‐Rodriguez P., Cervilla‐Munoz E., Ordieres‐Ortega L., et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID‐19 pneumonia and elevated D‐dimer levels. Thromb Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wichmann D., Sperhake J.P., Lutgehetmann M., et al. Autopsy findings and venous thromboembolism in patients with COVID‐19: a prospective cohort study. Ann Intern Med. 2020 doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID‐19 patients: emerging evidence and call for action. Br J Haematol. 2020;189(5):846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander S.P.H., Armstrong J., Davenport A.P., et al. A rational roadmap for SARS‐CoV‐2/COVID‐19 pharmacotherapeutic research and development. IUPHAR review. Br J Pharmacol. 2020 doi: 10.1111/bph.15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 13.Noris M., Caprioli J., Bresin E., et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5(10):1844–1859. doi: 10.2215/CJN.02210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruppo R.A., Rother R.P. Eculizumab for congenital atypical hemolytic‐uremic syndrome. N Engl J Med. 2009;360(5):544–546. doi: 10.1056/NEJMc0809959. [DOI] [PubMed] [Google Scholar]
- 15.Licht C., Greenbaum L.A., Muus P., et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2‐year extensions of phase 2 studies. Kidney Int. 2015;87(5):1061–1073. doi: 10.1038/ki.2014.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan B.P. The complement system: an overview. Methods Mol Biol. 2000;150:1–13. doi: 10.1385/1-59259-056-X:1. [DOI] [PubMed] [Google Scholar]
- 17.Ricklin D., Lambris J.D. Complement‐targeted therapeutics. Nat Biotechnol. 2007;25(11):1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawal N., Rajagopalan R., Salvi V.P. Stringent regulation of complement lectin pathway C3/C5 convertase by C4b‐binding protein (C4BP) Mol Immunol. 2009;46(15):2902–2910. doi: 10.1016/j.molimm.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monsinjon T., Gasque P., Chan P., Ischenko A., Brady J.J., Fontaine M.C. Regulation by complement C3a and C5a anaphylatoxins of cytokine production in human umbilical vein endothelial cells. FASEB J. 2003;17(9):1003–1014. doi: 10.1096/fj.02-0737com. [DOI] [PubMed] [Google Scholar]
- 20.Del Conde I., Cruz M.A., Zhang H., Lopez J.A., Afshar‐Kharghan V. Platelet activation leads to activation and propagation of the complement system. J Exp Med. 2005;201(6):871–879. doi: 10.1084/jem.20041497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gasser C., Gautier E., Steck A., Siebenmann R.E., Oechslin R. Hemolytic‐uremic syndrome: bilateral necrosis of the renal cortex in acute acquired hemolytic anemia. Schweiz Med Wochenschr. 1955;85(38–39):905–909. [PubMed] [Google Scholar]
- 22.Hofer J., Rosales A., Fischer C., Giner T. Extra‐renal manifestations of complement‐mediated thrombotic microangiopathies. Front Pediatr. 2014;2:97. doi: 10.3389/fped.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unsworth D.J. Complement deficiency and disease. J Clin Pathol. 2008;61(9):1013–1017. doi: 10.1136/jcp.2008.056317. [DOI] [PubMed] [Google Scholar]
- 24.Sim R.B., Tsiftsoglou S.A. Proteases of the complement system. Biochem Soc Trans. 2004;32(Pt 1):21–27. doi: 10.1042/bst0320021. [DOI] [PubMed] [Google Scholar]
- 25.Noris M., Remuzzi G. Hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16(4):1035–1050. doi: 10.1681/ASN.2004100861. [DOI] [PubMed] [Google Scholar]
- 26.Licht C., Pluthero F.G., Li L., et al. Platelet‐associated complement factor H in healthy persons and patients with atypical HUS. Blood. 2009;114(20):4538–4545. doi: 10.1182/blood-2009-03-205096. [DOI] [PubMed] [Google Scholar]
- 27.Delvaeye M., Noris M., De Vriese A., et al. Thrombomodulin mutations in atypical hemolytic‐uremic syndrome. N Engl J Med. 2009;361(4):345–357. doi: 10.1056/NEJMoa0810739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cofiell R., Kukreja A., Bedard K., et al. Eculizumab reduces complement activation, inflammation, endothelial damage, thrombosis, and renal injury markers in aHUS. Blood. 2015;125(21):3253–3262. doi: 10.1182/blood-2014-09-600411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang K., Lu Y., Harley K.T., Tran M.H. Atypical hemolytic uremic syndrome: a brief review. Hematol Rep. 2017;9(2):7053. doi: 10.4081/hr.2017.7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill A., DeZern A.E., Kinoshita T., Brodsky R.A. Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Primers. 2017;3:17028. doi: 10.1038/nrdp.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill A., Kelly R.J., Hillmen P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood. 2013;121(25):4985–4996. doi: 10.1182/blood-2012-09-311381. [DOI] [PubMed] [Google Scholar]
- 32.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID‐19 pneumonia: a random association? Eur Heart J. 2020;41(19):1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox S., Akmatbekov A., Harbert J., Li G., Quincy Brown J., Vander H.R. Pulmonary and cardiac pathology in Covid‐19: the first autopsy series from New Orleans. MedRxiv (preprint, not peer‐reviewed) 2020 doi: 10.1101/2020.04.06.20050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klok F.A., Kruip M., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calabrese L.H. Cytokine storm and the prospects for immunotherapy with COVID‐19. Cleve Clin J Med. 2020;87(7):389–393. doi: 10.3949/ccjm.87a.ccc008. [DOI] [PubMed] [Google Scholar]
- 36.Conway E.M. Reincarnation of ancient links between coagulation and complement. J Thromb Haemost. 2015;13(Suppl 1):S121–S132. doi: 10.1111/jth.12950. [DOI] [PubMed] [Google Scholar]
- 37.Conway E.M. Complement‐coagulation connections. Blood Coagul Fibrinolysis. 2018;29(3):243–251. doi: 10.1097/MBC.0000000000000720. [DOI] [PubMed] [Google Scholar]
- 38.La Bonte L.R., Pavlov V.I., Tan Y.S., et al. Mannose‐binding lectin‐associated serine protease‐1 is a significant contributor to coagulation in a murine model of occlusive thrombosis. J Immunol. 2012;188(2):885–891. doi: 10.4049/jimmunol.1102916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi K., Chang W.C., Takahashi M., et al. Mannose‐binding lectin and its associated proteases (MASPs) mediate coagulation and its deficiency is a risk factor in developing complications from infection, including disseminated intravascular coagulation. Immunobiology. 2011;216(1–2):96–102. doi: 10.1016/j.imbio.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krarup A., Gulla K.C., Gal P., Hajela K., Sim R.B. The action of MBL‐associated serine protease 1 (MASP1) on factor XIII and fibrinogen. Biochim Biophys Acta. 2008;1784(9):1294–1300. doi: 10.1016/j.bbapap.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Krarup A., Wallis R., Presanis J.S., Gal P., Sim R.B. Simultaneous activation of complement and coagulation by MBL‐associated serine protease 2. PLoS One. 2007;2(7) doi: 10.1371/journal.pone.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davie E.W., Ratnoff O.D. Waterfall sequence for intrinsic blood clotting. Science. 1964;145:1310–1312. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 43.Seshan S.V., Franzke C.W., Redecha P., Monestier M., Mackman N., Girardi G. Role of tissue factor in a mouse model of thrombotic microangiopathy induced by antiphospholipid (aPL) antibodies. Blood. 2009;114(8):1675–1683. doi: 10.1182/blood-2009-01-199117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langer F., Spath B., Fischer C., et al. Rapid activation of monocyte tissue factor by antithymocyte globulin is dependent on complement and protein disulfide isomerase. Blood. 2013;121(12):2324–2335. doi: 10.1182/blood-2012-10-460493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiedmer T., Esmon C.T., Sims P.J. Complement proteins C5b–9 stimulate procoagulant activity through platelet prothrombinase. Blood. 1986;68(4):875–880. [PubMed] [Google Scholar]
- 46.Zelaya H., Rothmeier A., Ruf W. Tissue factor at the crossroad of coagulation and cell signaling. J Thromb Haemost. 2018;16(10):1941–1952. doi: 10.1111/jth.14246. [DOI] [PubMed] [Google Scholar]
- 47.Subramaniam S., Jurk K., Hobohm L., et al. Distinct contributions of complement factors to platelet activation and fibrin formation in venous thrombus development. Blood. 2017;129(16):2291–2302. doi: 10.1182/blood-2016-11-749879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huber‐Lang M., Sarma J.V., Zetoune F.S., et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12(6):682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 49.Krisinger M.J., Goebeler V., Lu Z., et al. Thrombin generates previously unidentified C5 products that support the terminal complement activation pathway. Blood. 2012;120(8):1717–1725. doi: 10.1182/blood-2012-02-412080. [DOI] [PubMed] [Google Scholar]
- 50.Pryzdial E.L., Sutherland M.R., Ruf W. The procoagulant envelope virus surface: contribution to enhanced infection. Thromb Res. 2014;133(Suppl 1):S15–17. doi: 10.1016/j.thromres.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sutherland M.R., Raynor C.M., Leenknegt H., Wright J.F., Pryzdial E.L. Coagulation initiated on herpesviruses. Proc Natl Acad Sci USA. 1997;94(25):13510–13514. doi: 10.1073/pnas.94.25.13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sutherland M.R., Ruf W., Pryzdial E.L.G. Tissue factor and glycoprotein C on herpes simplex virus type 1 are protease‐activated receptor 2 cofactors that enhance infection. Blood. 2012;119:3638–3645. doi: 10.1182/blood-2011-08-376814. [DOI] [PubMed] [Google Scholar]
- 53.Sutherland M.R., Simon A.Y., Shanina I., Horwitz M.S., Ruf W., Pryzdial E.L.G. Virus envelope tissue factor promotes infection in mice. J Thromb Haemost. 2019;17(3):482–491. doi: 10.1111/jth.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li K., Wohlford‐Lenane C., Perlman S., et al. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis. 2016;213(5):712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang Y., Zhao G., Song N., et al. Blockade of the C5a–C5aR axis alleviates lung damage in hDPP4‐transgenic mice infected with MERS‐CoV. Emerg Microbes Infect. 2018;7(1):77. doi: 10.1038/s41426-018-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gralinski L.E., Sheahan T.P., Morrison T.E., et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. MBio. 2018;9(5) doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ip W.K., Chan K.H., Law H.K., et al. Mannose‐binding lectin in severe acute respiratory syndrome coronavirus infection. J Infect Dis. 2005;191(10):1697–1704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao T., Hu M., Zhang X., et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP‐2‐mediated complement over‐activation. medRxiv. 2020 doi: 10.1101/2020.03.29.20041962. [DOI] [Google Scholar]
- 59.Che X.Y., Qiu L.W., Pan Y.X., et al. Sensitive and specific monoclonal antibody‐based capture enzyme immunoassay for detection of nucleocapsid antigen in sera from patients with severe acute respiratory syndrome. J Clin Microbiol. 2004;42(6):2629–2635. doi: 10.1128/JCM.42.6.2629-2635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Che X.Y., Hao W., Wang Y., et al. Nucleocapsid protein as early diagnostic marker for SARS. Emerg Infect Dis. 2004;10(11):1947–1949. doi: 10.3201/eid1011.040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Magro C., Mulvey J.J., Berlin D., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diao B., Wang C., Wang R., et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. medRxiv. 2020 doi: 10.1101/2020.03.04.20031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watanabe Y., Allen J.D., Wrapp D., McLellan J.S., Crispin M. Site‐specific glycan analysis of the SARS‐CoV‐2 spike. Science. 2020;369(6501):330–333. doi: 10.1126/science.abb9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee H., Kang E., Kang H.G., et al. Consensus regarding diagnosis and management of atypical hemolytic uremic syndrome. Korean J Intern Med. 2020;35(1):25–40. doi: 10.3904/kjim.2019.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fox L.C., Cohney S.J., Kausman J.Y., et al. Consensus opinion on diagnosis and management of thrombotic microangiopathy in Australia and New Zealand. Intern Med J. 2018;48(6):624–636. doi: 10.1111/imj.13804. [DOI] [PubMed] [Google Scholar]
- 67.Risitano A.M., Mastellos D.C., Huber‐Lang M., et al. Complement as a target in COVID‐19? Nat Rev Immunol. 2020;20(6):343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jager U., D'Sa S., Schorgenhofer C., et al. Inhibition of complement C1s improves severe hemolytic anemia in cold agglutinin disease: a first‐in‐human trial. Blood. 2019;133(9):893–901. doi: 10.1182/blood-2018-06-856930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Henry Li H., Riedl M., Kashkin J. Update on the use of C1‐esterase inhibitor replacement therapy in the acute and prophylactic treatment of hereditary angioedema. Clin Rev Allergy Immunol. 2019;56(2):207–218. doi: 10.1007/s12016-018-8684-1. [DOI] [PubMed] [Google Scholar]
- 70.Connors J.M., Levy J.H. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pryzdial E.L.G., Sutherland M.R., Lin B.H., Horwitz M.S. Antiviral anticoagulation. Res Pract Thromb Haemost. 2020;4(5):774–788. doi: 10.1002/rth2.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID‐19: their roles in pathogenesis. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lam L.M., Murphy S.J., Kuri‐Cervantes L., et al. Erythrocytes reveal complement activation in patients with COVID‐19. medRxiv. 2020 doi: 10.1101/2020.05.20.20104398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Foley J.H., Walton B.L., Aleman M.M., et al. complement activation in arterial and venous thrombosis is mediated by plasmin. EBioMedicine. 2016;5:175–182. doi: 10.1016/j.ebiom.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jozsi M., Factor H. Family proteins in complement evasion of microorganisms. Front Immunol. 2017;8:571. doi: 10.3389/fimmu.2017.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arbore G., Kemper C., Kolev M. Intracellular complement ‐ the complosome ‐ in immune cell regulation. Mol Immunol. 2017;89:2–9. doi: 10.1016/j.molimm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tam J.C., Bidgood S.R., McEwan W.A., James L.C. Intracellular sensing of complement C3 activates cell autonomous immunity. Science. 2014;345(6201):1256070. doi: 10.1126/science.1256070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Girija A.S.S., Shankar E.M., Larsson M. Could SARS‐CoV‐2‐induced hyperinflammation magnify the severity of coronavirus disease (CoViD‐19) leading to acute respiratory distress syndrome? Front Immunol. 2020;11:1206. doi: 10.3389/fimmu.2020.01206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McKechnie J.L., Blish C.A. The innate immune system: fighting on the front lines or fanning the flames of COVID‐19? Cell Host Microbe. 2020;27(6):863–869. doi: 10.1016/j.chom.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bester J., Pretorius E. Effects of IL‐1beta, IL‐6 and IL‐8 on erythrocytes, platelets and clot viscoelasticity. Sci Rep. 2016;6:32188. doi: 10.1038/srep32188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Najem M.Y., Couturaud F., Lemarie C.A. Cytokine and chemokine regulation of venous thromboembolism. J Thromb Haemost. 2020;18(5):1009–1019. doi: 10.1111/jth.14759. [DOI] [PubMed] [Google Scholar]
- 82.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020 doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 83.Nakra N.A., Blumberg D.A., Herrera‐Guerra A., Lakshminrusimha S. Multi‐system inflammatory syndrome in children (MIS‐C) following SARS‐CoV‐2 infection. review of clinical presentation, hypothetical pathogenesis, and proposed management. Children (Basel) 2020;7(7):E69. doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feldstein L.R., Rose E.B., Horwitz S.M., et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ciaglia E., Vecchione C., Puca A.A. COVID‐19 infection and circulating ACE2 levels: protective role in women and children. Front Pediatr. 2020;8:206. doi: 10.3389/fped.2020.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bunyavanich S., Do A., Vicencio A. Nasal gene expression of angiotensin‐converting enzyme 2 in children and adults. JAMA. 2020;323(23):2427. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cristiani L., Mancino E., Matera L., et al. Will children reveal their secret? The coronavirus dilemma. Eur Respir J. 2020;55(4) doi: 10.1183/13993003.00749-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ramlall V., Thangaraj P., Tatonetti N., Shapira S. Identification of immune complement function as a determinant of adverse SARS‐CoV‐2 infection outcome. medRxiv. 2020 doi: 10.1101/2020.05.05.20092452. [DOI] [Google Scholar]
- 89.Bycroft C., Freeman C., Petkova D., et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maugeri A., Barchitta M., Mazzone M.G., Giuliano F., Agodi A. Complement system and age‐related macular degeneration: implications of gene‐environment interaction for preventive and personalized medicine. Biomed Res Int. 2018;2018 doi: 10.1155/2018/7532507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Noris M., Benigni A., Remuzzi G. The case of complement activation in COVID‐19 multiorgan impact. Kidney Int. 2020;98(2):314–322. doi: 10.1016/j.kint.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reis E.S., Falcao D.A., Isaac L. Clinical aspects and molecular basis of primary deficiencies of complement component C3 and its regulatory proteins factor I and factor H. Scand J Immunol. 2006;63(3):155–168. doi: 10.1111/j.1365-3083.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- 93.Reis E.S., Berger N., Wang X., et al. Safety profile after prolonged C3 inhibition. Clin Immunol. 2018;197:96–106. doi: 10.1016/j.clim.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mastaglio S., Ruggeri A., Risitano A.M., et al. The first case of COVID‐19 treated with the complement C3 inhibitor AMY‐101. Clin Immunol. 2020;215 doi: 10.1016/j.clim.2020.108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dobo J., Kocsis A., Gal P. Be on target: strategies of targeting alternative and lectin pathway components in complement‐mediated diseases. Front Immunol. 2018;9:1851. doi: 10.3389/fimmu.2018.01851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaplan A.P., Joseph K. Complement, kinins, and hereditary angioedema: mechanisms of plasma instability when C1 inhibitor is absent. Clin Rev Allergy Immunol. 2016;51(2):207–215. doi: 10.1007/s12016-016-8555-6. [DOI] [PubMed] [Google Scholar]
- 97.Diurno F., Numis F.G., Porta G., et al. Eculizumab treatment in patients with COVID‐19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. 2020;24(7):4040–4047. doi: 10.26355/eurrev_202004_20875. [DOI] [PubMed] [Google Scholar]
- 98.Hillmen P., Muus P., Roth A., et al. Long‐term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2013;162(1):62–73. doi: 10.1111/bjh.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cugno M., Meroni P.L., Gualtierotti R., et al. Complement activation in patients with COVID‐19: A novel therapeutic target. J Allergy Clin Immunol. 2020;146(1):215–217. doi: 10.1016/j.jaci.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]