Abstract
Background
Acral inflammatory lesions that have some resemblance to idiopathic or autoimmune‐associated perniosis (chilblains) have been described in multiple countries during the COVID‐19 pandemic.
Methods
We examined histopathologic findings in six consecutive such cases from five patients received in mid‐May to mid‐June of 2020, evaluating immunohistochemical staining for the SARS‐CoV‐2 nucleocapsid protein. We compared these six cases to eight cases diagnosed as perniosis between January and June of 2019.
Results
Five of six lesions with perniosis‐like histopathology during the COVID‐19 pandemic had distinctive tight cuffing of lymphocytes; intravascular material was present in one case. SARS‐CoV‐2 immunohistochemical staining using an antibody directed at the nucleocapsid protein was negative in all six cases. Only one of eight specimens with microscopic findings of perniosis received prior to the COVID‐19 pandemic had tightly cuffed perivascular lymphocytes, and none had obvious intravascular occlusion.
Conclusions
A tightly cuffed pattern of perivascular lymphocytes is a feature of perniosis during the COVID‐19 pandemic. The absence of SARS‐CoV‐2 nucleocapsid protein in these cases suggests against the virus being directly present in these lesions.
Keywords: chilblain lupus, chilblains, coronavirus, COVID‐19, pandemic, perniosis, SARS‐CoV‐2
1. INTRODUCTION
Skin findings resembling perniosis or chilblains, most commonly affecting children's toes, have been reported from geographic areas where COVID‐19 has been prevalent. 1 , 2 , 3 , 4 Soles and heels, as well as fingers, 4 , 5 can be involved. 1 , 3 , 6 Affected individuals may manifest mild fever or respiratory symptoms in the weeks preceding the skin lesions. 1 , 3 , 7 The observation that the majority of reported individuals have been negative for detectable SARS‐CoV‐2 from nasopharyngeal swabs has precluded drawing a direct link between this manifestation and COVID‐19. 1 , 2 , 4 However, the typical manifestation of lesions at least 1 week after any systemic symptoms (when present), as well as other observations summarized in Tables 1 and 3 ,7‐9 suggests that perniosis‐like lesions are somehow related to the COVID‐19 pandemic. 8
TABLE 1.
Support for perniosis during the COVID‐19 pandemic being related to SARS‐CoV2
| Clustering of perniosis‐like cases in cities with a high incidence of COVID‐19 |
| Some affected individuals with associated mild symptoms that could be attributed to COVID‐19 |
| Many affected individuals with documented prior exposure to an individual with COVID‐19 |
| Prevalence in children > adults (unlike perniosis prior to the emergence of COVID‐19) |
| Absence of prior history of chilblains or associated disease (eg, lupus erythematosus) |
| Absence of a consistent laboratory abnormality (eg, antiphospholipid antibodies or cryoproteins) |
| Positive COVID‐19 testing in a small subset (either by RT‐PCR from nasopharyngeal swabs or positive serology for anti‐IgG, anti‐IgM, or anti‐IgA against SARS‐CoV2 proteins) |
TABLE 3.
Histopathological comparison between perniosis during the COVID‐19 pandemic and perniosis diagnosed in 2019
| Perniosis during the COVID‐19 pandemic n = 6 except where indicated (number, %) | Perniosis diagnosed in 2019 n = 8 (number, %) | ||
|---|---|---|---|
| Interface change | 2 (33) | 4 (50) | |
| Papillary dermal edema | 2 (33) | 2 (25) | |
| Lymphocytic infiltrate | Tight cuffing | 4 (66) | 1 (13) |
| Superficial perivascular | 6 (100) | 8 (100) | |
| Deep perivascular | 2 (100) a | 8 (100) | |
| Interstitial | 1 (16) | 1 (13) | |
| Perieccrine | 1 (33) a | 5 (63) | |
| Extravasated erythrocytes | 2 (33) | 1 (13) | |
| Vascular occlusion | 1 (16) | 0 (0) | |
Four specimens were shave biopsies and did not include the deeper dermis; eccrine glands were present in three specimens.
Diagnostic criteria suggested for classic perniosis include the presence of persistent, localized erythema and swelling on acral sites with at least one of the following: (a) onset in November to March in the Northern hemisphere, (b) typical histopathology (eg, dermal edema and superficial plus deep perivascular lymphocytes), or (c) response to conservative treatments (eg, warming). 10 Acral lesions described during the COVID‐19 pandemic could therefore be classified as perniosis through March of 2020, given their morphology and persistence for over 7 days with histopathologic findings of perniosis. 1 , 4 , 7 While cases have fulfilled the suggested diagnostic criteria for perniosis, cases continue to present after March, 2020, and the purpuric and eroded lesions reported in the literature would not be expected to resolve with warming. 2 , 7 , 8 Additionally, although thrombi have been described in idiopathic perniosis and perniosis in the setting of systemic disease, 11 , 12 thrombi present in perniosis lesions during the COVID‐19 pandemic have been proposed as a distinctive finding. 13
There are several possible explanations for the apparent increase in perniosis during the COVID‐19 pandemic (henceforth abbreviated PDC for ease of discussion). PDC could be (a) a manifestation of direct infection of skin with SARS‐CoV‐2, 8 (b) an inflammatory reaction secondary to viral host response, 1 or (c) a coincidental rise of true idiopathic and autoimmune‐associated perniosis. Polymerase chain reaction (PCR) testing of skin lesions has not detected SARS‐CoV‐2, supporting the latter two possibilities. 14 , 15 In contrast, positive IHC for the spike protein (SARS‐CoV/SARS‐CoV‐2 spike 1A9, GeneTex, Inc, Irvine, CA 16 , 17 ) in endothelial cells and eccrine glands of perniosis‐like lesions suggests that virus is present in the skin. Given the conflicting data, we stained six consecutive cases of PDC with an antibody against SARS‐CoV‐2 nucleocapsid protein and compared and contrasted eight cases with perniosis‐like histopathologic findings received in the first half of 2019.
2. MATERIALS AND METHODS
Six consecutive cases from five different patients during a 4‐week period from mid‐May to mid‐June, 2020, with clinical and histopathological findings compatible with PDC were stained with anti‐SARS‐CoV‐2 nucleocapsid protein antibody (Thermofisher, mouse monoclonal antibody clone B46F, dilution 1:200). Staining was validated using appropriate positive and negative controls. The nucleocapsid proteins of SARS‐CoV and SARS‐CoV‐2 have 70% homology, and the antibody cross‐reacts with both SARS coronaviruses but not human coronaviruses 229E and OC43. 18 Antibodies against the SARS‐CoV nucleocapsid protein have shown immunoreactivity in organs including the skin, lung, kidneys, and liver but not bone marrow, heart, or reproductive organs on autopsies of patients who died of SARS‐CoV. 19
The nucleocapsid antibody was validated using placental tissue from a patient known to have COVID‐19. 20 PCR testing of the placenta was positive for SARS‐CoV‐2, and this tissue stained positively with the nucleocapsid protein antibody from Thermofisher in two different laboratories, with two different spike protein antibodies (Sino Biological, 40 150‐T62‐COV; and GeneTex, SARS‐CoV/SARS‐CoV‐2 spike antibody clone 1A9), and with RNA in situ hybridization (ISH) 21 using a probe directed against the spike protein (V‐nCoV2019‐S; Advanced Cell Diagnostics). Placental tissue that was negative with PCR testing for SARS‐CoV‐2 did not stain positively for the antibodies or the RNA ISH probe. Other tissue specimens from non‐COVID‐19 patients stained negatively with these antibodies and probes, as expected. Owing to the paucity of tissue specimens from COVID‐19‐positive patients, the sensitivity of the nucleocapsid stain is currently unknown. The current skin lesions, unlike the placental control, were not tested by PCR for SARS‐CoV‐2; as the specimens were formalin‐fixed, ultrastructural studies and direct immunofluorescence studies were also not performed.
Using the terms “perniosis” and “chilblains,” we searched the Yale Dermatopathology database for cases with perniosis‐like histopathologic findings, in which the clinical differential diagnosis included perniosis, received from January to June, 2019.
All cases were re‐examined microscopically by three fellowship‐trained dermatopathologists, one of whom has not yet taken the dermatopathology subspecialty examination.
3. RESULTS
Patient information is summarized in Table 2. The average patient age was 62, which is older than the pediatric cohorts previously described with PDC. Fingers were also predominantly affected, rather than the toes. None of the patients had a prior history of perniosis or connective tissue disease. Lesions were persistent over at least 6 weeks for four of five patients, without improvement with warming. There were no known contacts with COVID‐19, and patients did not have systemic symptoms of COVID‐19. Two tested negatively for antinuclear antibodies; one of these patients also tested negative for antiphospholipid antibodies. One of the patients had a history of breast cancer and another had a history of colorectal carcinoma; all others had no history of malignancy.
TABLE 2.
Clinical data on six consecutive specimens with perniosis‐like histopathologic findings received mid‐May to mid‐June, 2020
| Case | Age | Gender | Biopsy site | Distribution | SARS‐CoV2 testing |
|---|---|---|---|---|---|
| 1 | 82 | Female | Right ring finger | Finger only | IgG, IgM antibody testing negative |
| 2 | 62 | Female | Right second toe | Toe only | Not performed |
| 3 | 76 | Male | Right fifth finger | Finger only | Patient refused testing |
| 4 | 61 | Female | Left knee | Knees, dorsal right foot, right great toe, wrists | IgG, IgM antibody testing negative |
| 56 | 31 | Female | Right fifth finger inferiorRight fifth finger superior | Finger only | IgG, IgM antibody testing negative |
All six cases of PDC lacked immunohistochemical staining using an antibody against the SARS‐CoV‐2 nucleocapsid protein; all had findings of perivascular lymphocytes. Subtle nuances included tightly cuffed lymphocytes around vessels in five of six cases of PDC (Figures 1 and 2), and very focal amorphous, nonpolarizing material within a small vessel in one case (see Figure 2). This material did not appear to be foreign in origin and could represent fibrin or vessel wall damage. Tight cuffing was defined visually as well‐organized, wreath‐like arrangements of lymphocytes, with uniform intercellular spacing, involving 50%‐90% of the vessels in the specimen (Figure 3). Papillary dermal edema was present in two cases of PDC, with subtle interface change in two cases. Biopsies in only three cases of PDC were deep enough to include eccrine glands, with focal perieccrine lymphocytes in one of the three.
FIGURE 1.
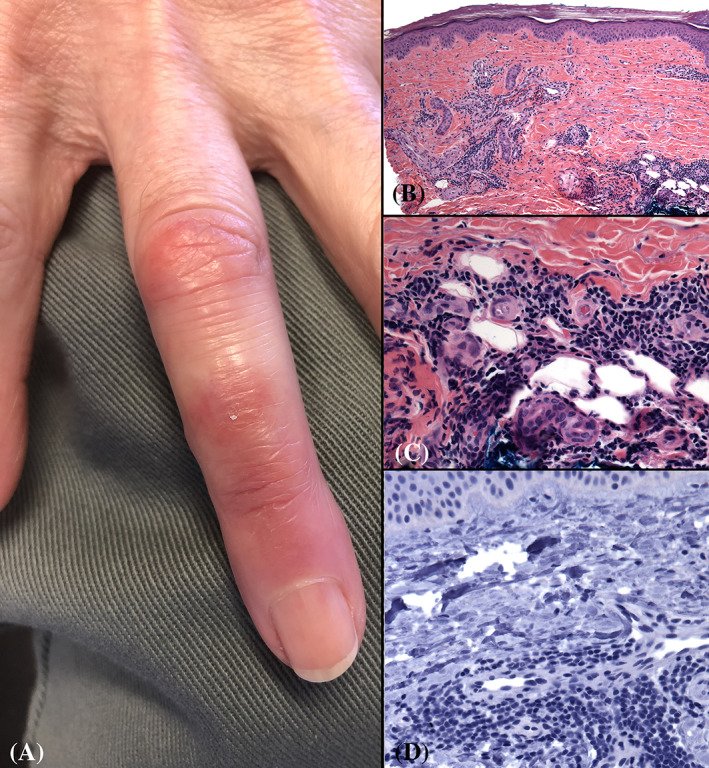
Perniosis on the finger; presenting in May, 2020, during the COVID‐19 pandemic. A, There are pink plaques on the dorsal right ring finger; no other lesions were present. B and C, Biopsy findings include tightly cuffed lymphocytes around vessels and eccrine glands near the base of the biopsy (B, original magnification 40×; C,D, original magnification 200×). D, Absent immunohistochemical staining using an antibody against SARS‐CoV‐2 nucleocapsid protein. Original magnification 200×. Clinical photo courtesy of Robert Patrignelli, MD
FIGURE 2.
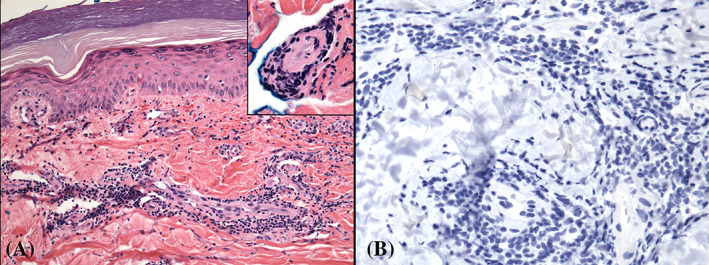
Perniosis from the fifth finger; presenting May, 2020, during the COVID‐19 pandemic. No other lesions were present. A, There is tight cuffing of lymphocytes around vessels. The biopsy was transected through the mid‐dermis, and near the base, one vessel appeared to have intraluminal pink‐purple material (A, original magnification ×200; inset; original magnification ×400). B, Absent immunohistochemical staining using an antibody against SARS‐CoV‐2 nucleocapsid protein. Original magnification ×400
FIGURE 3.
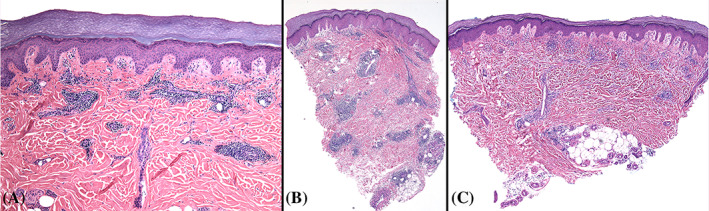
Perniosis: Histopathologic findings in a case presenting during the COVID‐19 pandemic (A) and 2 cases diagnosed in 2019 (B and C). A There is tight cuffing of lymphocytes around vessels. Original magnification ×100. B, Tight cuffing of perivascular lymphocytes around most of the vessels in a case of idiopathic perniosis, diagnosed in the early half of 2019. There is also perieccrine inflammation. C, More loosely distributed perivascular lymphocytes in idiopathic perniosis, diagnosed in the early half of 2019. B and C, Original magnification ×40
For the cases of perniosis diagnosed in 2019, the average age was 54 (range 24‐90); seven of eight biopsies were from a finger. Five patients were female and three were male. Microscopic findings are summarized in Table 3. Vascular occlusion was not evident. Tight cuffing of lymphocytes was present in just one of the eight cases (see Figure 3B).
4. DISCUSSION
In six cases of PDC in Connecticut, SARS‐CoV‐2 nucleocapsid protein was not detectable in skin biopsies by IHC. These cases were received from mid‐May to mid‐June, 2020; the peak incidence of COVID‐19 hospitalizations in Connecticut was in mid‐April, 2020. The lack of SARS‐CoV‐2 nucleocapsid staining, the older average age of patients in this cohort, the absence of a history of autoimmune disease, and the fingers being affected more than the toes would all support that these cases represent a manifestation of idiopathic perniosis rather than perniosis secondary to direct infection of the skin by SARS‐CoV‐2. This conclusion is in line with the absence of detectable SARS‐CoV‐2 by PCR testing of lesional PDC tissue 14 , 15 and negative IgM and IgG SARS‐CoV‐2 serologic studies in this series and others, 1 , 2 , 3 , 4 , 22 but conflicts with reports of positive immunohistochemical staining for the spike protein in PDC. 16 , 17 IgM and IgG serologies may not be the best way to test for COVID‐19, with evidence that IgA serologies may be better in detecting relatively asymptomatic patients in later stages of the disease. 2 , 23 Similarly, the negative nucleocapsid staining in this series vs spike protein staining in the literature deserves further study and warrants continued validation of different antibodies in the skin.
A pattern of very tightly cuffed perivascular lymphocytes was present more commonly in PDC (5/6 cases) than in perniosis diagnosed in early 2019. The histopathologic differential diagnosis of tightly cuffed lymphocytes includes erythema annulare centrifugum (EAC), erythema chronicum migrans (ECM), and chronic lymphocytic leukemia (CLL) infiltrates involving the skin; neither EAC nor ECM typically present on acral sites, and none of these patients had known CLL.
Prior to the emergence of SARS‐CoV‐2, differences have been described in idiopathic perniosis vs chilblain lupus. Features that are supportive of the latter include absent papillary dermal edema and perieccrine infiltrates and the presence of vacuolar interface change and fibrin thrombi within vessels of the reticular dermis. 11 , 12 , 24 CD123 staining does not reliably distinguish idiopathic perniosis from chilblain lupus, 25 but myxovirus resistance protein A (MxA) does stain chilblain lupus more diffusely than idiopathic perniosis. 26 Chilblain lupus 27 and familial chilblain lupus 28 may be the end manifestation of increased interferon expression in acral sites, 27 and similarly, PDC have been hypothesized to have a similar pathogenesis reflecting high levels of type I interferons. 9
Limitations include the small number of PDC cases tested and testing with just one immunohistochemical antibody. As acute purpuric lesions in severely ill, hospitalized patients with known COVID‐19 are also reported as positive for detectable SARS‐CoV‐2 spike protein in endothelial cells, 29 further testing of multiple antibodies on more cases of PDC as well as other skin manifestations of COVID‐19 would be important. In the current study, a nucleoprotein antibody was chosen given previously reported sensitivity and specificity of this protein across organ systems. 19 Comprehensive study of different testing modalities (PCR, electron microscopy, RNA ISH, direct immunofluorescence, and comparative IHC against different SARS‐CoV‐2 proteins) is warranted. This is more difficult to achieve than might be expected given that the COVID‐19 pandemic caused many outpatient clinics (including dermatology) to be closed, with inpatient consults being performed remotely; thus, biopsy material from patients with COVID‐19 is in short supply.
In conclusion, we present a series of six consecutive cases of PDC which are negative for immunohistochemical staining for SARS‐CoV‐2 nucleocapsid protein, and it is possible that the cases in this series represent a form of idiopathic perniosis not directly related to acute or subacute SARS‐CoV‐2 infection of the skin. We emphasize a characteristic histopathologic nuance of tightly cuffed perivascular lymphocytes in PDC compared to cases of perniosis prior to the COVID‐19 pandemic.
Ko CJ, Harigopal M, Damsky W, et al. Perniosis during the COVID‐19 pandemic: Negative anti‐SARS‐CoV‐2 immunohistochemistry in six patients and comparison to perniosis before the emergence of SARS‐CoV‐2. J Cutan Pathol. 2020;47(11):997–1002. 10.1111/cup.13830
REFERENCES
- 1. Cordoro KM, Reynolds SD, Wattier R, McCalmont TH. Clustered cases of acral perniosis: clinical features, histopathology and relationship to COVID‐19. Pediatr Dermatol. 2020;37(3):419‐423. 10.1111/pde.14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. El Hachem M, Diociaiuti A, Concato C, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain‐like lesions: lights and shadows on the relationship with COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andina D, Noguera‐Morel L, Bascuas‐Arribas M, et al. Chilblains in children in the setting of COVID‐19 pandemic. Pediatr Dermatol. 2020;37(3):406‐411. 10.1111/pde.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kanitakis J, Lesort C, Danset M, Jullien D. Chilblain‐like acral lesions during the COVID‐19 pandemic (“COVID toes”): histologic, immunofluorescence and immunohistochemical study of 17 cases. J Am Acad Dermatol. 2020. 10.1016/j.jaad.2020.05.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alramthan A, Aldaraji W. A case of COVID‐19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol. 2020;45(6):746‐748. 10.1111/ced.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Landa N, Mendieta‐Eckert M, Fonda‐Pascual P, Aguirre T. Chilblain‐like lesions on feet and hands during the COVID‐19 pandemic. Int J Dermatol. 2020;59(6):739‐743. 10.1111/ijd.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernandez‐Nieto D, Jimenez‐Cauhe J, Suarez‐Valle A, et al. Characterization of acute acro‐ischemic lesions in non‐hospitalized patients: a case series of 132 patients during the COVID‐19 outbreak. J Am Acad Dermatol. 2020;83(1):e61‐e63. 10.1016/j.jaad.2020.04.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71‐77. 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection‐induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6(6):489‐492. 10.1016/j.jdcr.3020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89(2):207‐215. [DOI] [PubMed] [Google Scholar]
- 11. Crowson AN, Magro CM. Idiopathic perniosis and its mimics: a clinical and histological study of 38 cases. Hum Pathol. 1997;28(4):478‐484. [DOI] [PubMed] [Google Scholar]
- 12. Cribier B, Djeridi N, Peltre B, Grosshans E. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45(6):924‐929. [DOI] [PubMed] [Google Scholar]
- 13. Sigal AC, Zirn JR, Lipper GM, Shapiro PE. A case of pernio‐like lesions (“covid toes”) with histologic confirmation of microthrombi. https://www.facebook.com/pages/category/Medical-Lab/Dermatopathology-Laboratory-of-New-England-100846528289317/
- 14. Battesti G, El Khalifa J, Abdelhedi N, et al. New insights in COVID‐19‐associated chilblains: a comparative study with chilblain lupus erythematosus. J Am Acad Dermatol. 2020. 10.1016/j.jaad.2020.06.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herman A, Peeters C, Verroken A, et al. Evaluation of chilblains as a manifestation of the COVID‐19 pandemic. JAMA Dermatol. 2020. 10.1001/jamadermatol.2020.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Colmenero I, Noguera‐Morel L, Hernandez‐Martin A, et al. SARS‐CoV‐2 endothelial infection causes COVID‐19 chilblains: histopathological, immunohistochemical and ultrastructural study of 7 paediatric cases. Br J Dermatol. 2020. 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Santonja C, Requena L. COVID‐19 chilblain‐like lesion: immunohistochemical demonstration of SARs‐CoV‐2 spike protein in blood vessel endothelium and sweat gland epithelium in a PCR‐negative patient. Br J Dermatol. 10.1111/bjd.19338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SARS/SARS‐CoV‐2 coronavirus nucleocapsid monoclonal antibody (B46F). https://www.thermofisher.com/antibody/product/SARS-SARS-CoV-2-Coronavirus-Nucleocapsid-Antibody-clone-B46F-Monoclonal/MA1-7404
- 19. Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS‐CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203(2):622‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hosier H, Farhadian SF, Morotti RA, et al. SARS‐CoV‐2 infection of the placenta. J Clin Invest. 2020;139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang F, Flanagan J, Su N, et al. RNAscope: a novel in situ RNA analysis platform for formalin‐fixed, paraffin‐embedded tissues. J Mol Diagn. 2012;14(1):22‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Freeman EE, McMahon DE, Lipoff JB, et al. Pernio‐like skin lesions associated with COVID‐19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83(2):486‐492. 10.1016/j.jaad.2020.05.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hubiche T, LeDuff F, Chiaverini C, Giordanegro V, Passeron T. Negative SARS‐CoV‐2 PCR in patients with chilblain‐like lesions. Lancet Infect Dis. 2020. S1473‐3099(20)30518‐1. https://10.1016/S1473‐3099(20)30518‐1. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boada A, Bielsa I, Fernandez‐Figueras M, Ferrandiz C. Perniosis: clinical and histopathological analysis. Am J Dermatopathol. 2010;32(1):19‐23. [DOI] [PubMed] [Google Scholar]
- 25. Wang ML, Chan MP. Comparative analysis of chilblain lupus erythematosus and idiopathic perniosis. Am J Dermatopathol. 2018;40(4):265‐271. [DOI] [PubMed] [Google Scholar]
- 26. https://my.clevelandclinic.org/departments/dermatology-plastic-surgery/outcomes/894-use-of-mxa-immunohistochemistry-for-distinguishing-chilblain-lupus-erythematosus-skin-lesions-from-idiopathic-chilblains-perniosis
- 27. Crow MK. Type I interferon in the pathogenesis of lupus. J Immunol. 2014;192(12):5459‐5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Günther C, Berndt N, Wolf C, Lee‐Kirsch MA. Familial chilblain lupus due to a novel mutation in the exonuclease III domain of 3′ repair exonuclease 1 (TREX1). JAMA Dermatol. 2015;151(4):426‐431. [DOI] [PubMed] [Google Scholar]
- 29. Magro C, Mulvey J, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res. 2020;220:1‐13. 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


