Abstract
Background
The objective of the current study was to provide insight into the effect of coronavirus disease 2019 (COVID‐19) on breast cancer screening, breast surgery, and genetics consultations.
Methods
User data from a risk assessment company were collected from February 2 to April 11, 2020. The use of risk assessment was used as a proxy for the use of 3 breast cancer services, namely, breast imaging, breast surgery, and genetics consultation. Changes in the use of these services during the study period were analyzed.
Results
All 3 services experienced significant declines after the COVID‐19 outbreak. The decline in breast surgery began during the week of March 8, followed by breast imaging and genetics consultation (both of which began during the week of March 15). Breast imaging experienced the most significant reduction, with an average weekly decline of 61.7% and a maximum decline of 94.6%. Breast surgery demonstrated an average weekly decline of 20.5%. When surgical consultation was stratified as breast cancer versus no breast cancer, the decrease among in non–breast cancer patients was more significant than that of patients with breast cancer (a decline of 66.8% vs 11.5% from the pre‐COVID average weekly volume for non–breast cancer patients and patients with breast cancer, respectively). During the week of April 5, use of genetics consultations dropped to 39.9% of the average weekly volumes before COVID‐19.
Conclusions
COVID‐19 has had a significant impact on the number of patients undergoing breast cancer prevention, screening, diagnosis, and treatment.
Keywords: breast cancer, breast imaging, breast surgery, cancer genetics, coronavirus disease 2019 (COVID‐19)
Short abstract
Coronavirus disease 2019 (COVID‐19) has had a significant impact on the number of patients undergoing breast cancer prevention, screening, diagnosis, and treatment in the United States. In the current study, user data from a risk assessment company were collected from February 2 to April 11, 2020, with the use of risk assessment used as a proxy to analyze changes in the use of 3 breast cancer services: breast imaging, breast surgery, and genetics consultation.
Introduction
Coronavirus disease 2019 (COVID‐19), also known as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), is the third coronavirus infection to present within the past 20 years, its predecessors being the severe acute respiratory syndrome (SARS) in 2003 and the Middle East respiratory syndrome (MERS) in 2012. 1 Since the beginning of the COVID‐19 pandemic, health care delivery around the globe has been transformed. As the number of COVID‐19–positive patients began to increase, so did concerns that patients would outstrip hospital bed capacity and overwhelm health care providers. As a result, many health care systems began to discourage elective visits and procedures, and to adopt other aggressive approaches to minimize resource use. 2 , 3 , 4 Fearing exposure to COVID‐19, patients also grew reluctant to seek medical care, 5 and the number of individuals presenting to the emergency department with serious problems, such as stroke and myocardial infarction, similarly declined. 6 , 7 , 8 , 9 , 10 The situation for patients with cancer is not dissimilar to these scenarios. 11 Viewed through the lens of breast cancer screening, the ramifications of the COVID‐19 pandemic may provide lessons for future management.
Preventing, screening, diagnosing, and treating breast cancer involves several interdependent types of care, including but not limited to screening breast imaging, diagnostic breast imaging, surgical consultation for radiographic findings, genetics testing and the management of genetics test results, and treatment. Breast imaging is categorized further either as screening, which is considered elective, or diagnostic, which remains an essential service for women with signs or symptoms of breast cancer. 12 , 13 Hence, the cessation of “elective” breast cancer screening due to the emergence of COVID‐19 is amplified further by the concomitant decline in surgical consultations and genetics counseling sessions. All told, these events most likely will result in a significant decrease in screen‐detected, early‐stage breast cancer over the next several months. 14 , 15 , 16 Quantifying these changes may alter the management of future outbreaks and illuminate the current path to normalization. Although previous studies have focused on inpatient care, to our knowledge only limited data are available in the outpatient cancer setting. 11 The objective of the current study was to analyze a unique set of data to provide insight into the effect of COVID‐19 on breast cancer care in the United States.
Materials and Methods
Use of a Risk Assessment Service
Risk assessment companies provide cancer risk assessment services to breast imaging centers, cancer genetics clinics, and surgeons. Patient data are provided to the service provider through a central application programming interface (API). Multiple standard breast cancer risk assessment models and guidelines then are applied to the data to determine the patient's individual cancer risk. The risk assessment analysis for the current study was conducted by CRA Health LLC (Waltham, Massachusetts), and the users included 3 types of clinics: 1) breast imaging centers; 2) surgeons who participate in the American Society of Breast Surgeons (ASBrS) Mastery of Breast Surgery program; and 3) cancer genetics clinics. Institutional review board approval was not needed for the current study because it did not involve identified human participants.
Among the users of CRA Health, breast imaging centers usually perform a risk assessment for all patients undergoing breast imaging, although the chances of undergoing risk assessment could be different between non–breast cancer patients and patients with a personal history of breast cancer. Thus, the number of risk assessments submitted to the API is representative of the number of imaging studies. The risk assessment usually is not repeated at the time of a callback, but if this occurs, it would lead to double counting of an individual.
The Mastery of Breast Surgery program of the ASBrS is used by a subset of ASBrS members (surgeons) to perform risk assessment on their patients. Although some of these surgeons perform risk assessment on all new patients, others do so only if the patient appears to be at high risk. The number of risk assessments most likely is proportional to the number of new patients seen. Although cancer genetics clinics provide care for individuals with any cancer susceptibility gene, the majority of patients are seen for possible breast cancer genes. 18 , 19 Thus, the number of genetic risk assessments most likely is proportionately representative of breast genetics care.
Cancer genetics clinics perform risk assessment on all new patients, and thus the number of risk assessments submitted to the API is representative of the number of new patients being managed. One can assume that the risk assessment would be performed regardless of whether the patients were seen in person or using telehealth.
Therefore, in the current study, the use of risk assessment in each service was used as a proxy for the use of breast imaging, breast surgery, and genetics counseling, respectively.
Data Collection
We identified data from February 2, 2020, through April 11, 2020, (total of 10 weeks) for each of 3 clinic types.
For the risk assessment data, we collected the clinic type (breast imaging, breast surgery, and cancer genetics clinic) and the breast cancer status (with or without breast cancer) of each request to the API. We counted the number of requests per week from each type of clinic. Sites that performed both breast imaging and cancer genetics clinic services were excluded because we were unable to separate the appointment types.
In total, we identified 55 breast imaging centers from 27 states (New Jersey, Missouri, Massachusetts, Oregon, Washington, Minnesota, Colorado, Ohio, California, Indiana, North Carolina, Illinois, New Mexico, Wisconsin, Pennsylvania, New York, Mississippi, Oklahoma, Virginia, Iowa, Florida, Louisiana, South Carolina, Maryland, Georgia, Michigan, and Texas). The states were consolidated into 4 geographical regions based on the US Census Bureau–defined regions: Northeast, Midwest, West, and South. 20 Because the numbers per state for risk clinic and surgery sites were sparse, we opted not to analyze them by state or region.
Data Analysis
Using line charts, we plotted the weekly volume of breast imaging, breast surgery, and cancer genetics clinics. We then plotted the changing trends in breast surgery stratified by disease types (breast cancer vs benign breast diseases).
The starting point of the decline was defined as the week in which the number of cases decreased by at least 15% compared with that of the previous week. We identified the starting point of the drop and calculated the average and maximum weekly decline rates (volume of week N minus the volume of week N+1, divided by the volume of week N).
We next fitted a linear regression to assess the smooth relationship between the number of days since the beginning of the study period (February 2, 2020) and the number of cases of breast imaging, breast surgery, and cancer genetics consultations, in which the number of days was modeled by a natural cubic spline to capture the potential nonlinear trend over the study period. 21
Among patients who underwent breast imaging (mammograms), the likelihood of receiving risk assessment could be different between non–breast cancer patients and patients with a personal history of breast cancer. Therefore, we performed a sensitivity analysis to evaluate the changing trend by excluding those patients with a personal history of breast cancer.
All analyses were performed using R language statistical software (version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria); a P < .05 was to be considered statistically significant.
Results
Breast Imaging
Breast imaging (mammograms) was plotted in Supporting Figure 1. The starting point of the drop in mammograms occurred during the week of March 15, which demonstrated a decrease of 51.3% compared with that of the previous week (from 24,969 to 12,166 mammograms).
Measuring from the starting point of the decline (week of March 15), the average weekly decline rate was 61.7%, with a maximum weekly decline rate of 72.1% (week of March 15 to the week of March 22). The overall downward trend was found to be statistically significant (P = .001) (Fig. 1). Only 1355 mammograms were performed during the week of April 5, representing only 5.4% of the average weekly volume before the drop.
Figure 1.
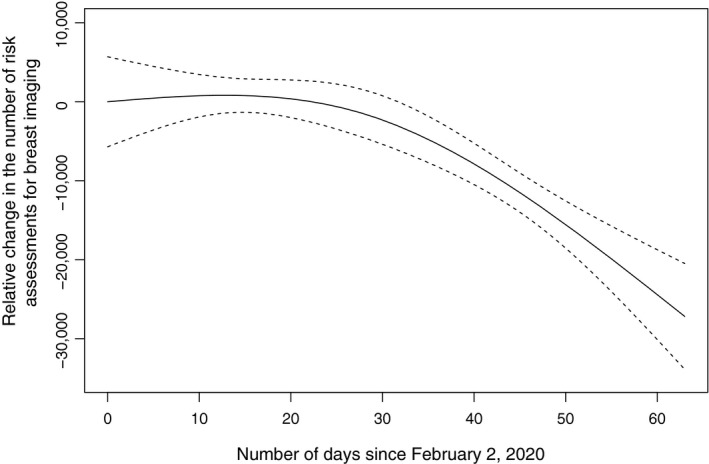
The relative change in the number of risk assessments for breast imaging since February 2, 2020. Dashed lines indicate the 95% confidence interval.
We further stratified the breast imaging centers by their regions and found that all regions appeared to decline maximally during the week of March 15. There also was a similar drop proportionally for all regions.
During the study period, 7304 of 161,932 patients (4.5%) who received a risk assessment for breast imaging had a personal history of breast cancer. The downward trend was similar in the sensitivity analysis when excluding patients with a personal history of breast cancer (P = .001) (see Supporting Fig. 2).
Breast Surgery
The change in breast surgery was plotted in Supporting Figure 3. The drop in total breast surgical consultations started during the week of March 8 when it decreased from 880 to 720 consultations (an 18.2% drop), which was 1 week earlier than the drop in breast imaging.
After the week of March 8, the average weekly rate of decline was 20.5%, with a maximum weekly decline of 35.5% (week of March 8 to the week of March 15). The overall downward trend was statistically significant (P = .003) (Fig. 2). Only 340 surgical consultations were conducted during the week of April 5, accounting for approximately 40.1% of the average weekly volume before the drop.
Figure 2.
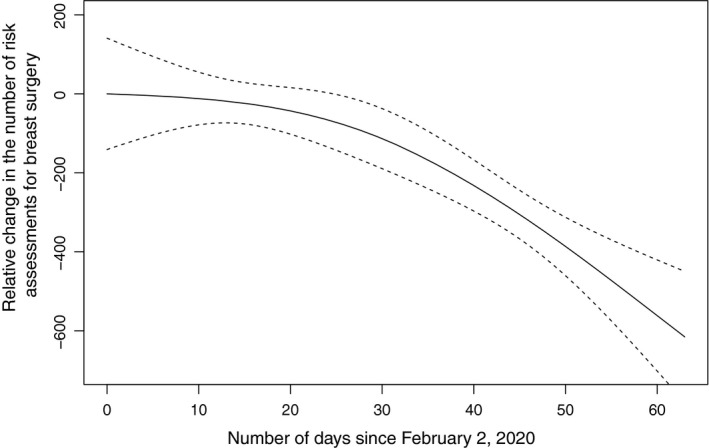
The relative change in the number of risk assessments for breast surgery since February 2, 2020. Dashed lines indicate the 95% confidence interval.
By further stratifying the breast surgical consultations by disease type (see Supporting Fig. 4), we found that the drop in non–breast cancer diseases was greater than that in breast cancer surgery (declined by 66.8% vs 11.5% pre‐COVID average weekly volume for non–breast cancer patients and patients with breast cancer, respectively). Since the starting point of the drop (week of March 8), the average weekly decline rate for non–breast cancer diseases was 23.1%, with a maximum weekly decline rate of 42.8% (week of March 8 to the week of March 15). In contrast, the total number of patients with breast cancer seen by surgeons did not appear to decrease substantially, with a maximum weekly decline noted between the weeks of March 1 and March 8 (from 87 patients to 57 patients, a decline of 34.4%), followed by a slight increment in the number of cases in the following weeks. Before the starting point of the drop (ie, the week of March 8), the average number of weekly consultations for breast cancer was 94, which annualizes to 4888 patients with breast cancer per year, representing approximately 1.8% of newly diagnosed breast cancers in the United States. 22 During the week of April 5, a total of 77 patients with breast cancer underwent consultation, which was 81.9% of the average weekly volume before March 8.
Risk Clinic
The change in the number of genetics consultations performed at genetics clinics was plotted in Supporting Figure 5. Similar to breast imaging, the starting point of the decline was the week of March 15, with a 29.1% decrease noted compared with that of the previous week (from 392 consultations to 278 consultations).
Before the drop (week of February 2 to the week of March 5), the average number of genetics consultations done per week at the genetics centers was 391, which annualizes to 20,332 genetics consultations per year. Because approximately 600,000 genetics tests are performed annually in the United States (data collected through personal communications with several genetic testing companies in 2019), this set of centers represents approximately 3.4% of the total genetics consultations.
Since the starting point of the decline (week of March 15), the average weekly decline rate was 26.4%, with a maximum weekly decline rate of 29.1% (week of March 8 to the week of March 15). The overall downward trend was found to be statistically significant (P < .001) (Fig. 3). Only 156 consultations were done during the week of April 5, accounting for approximately 39.9% of the average weekly volume before the drop.
Figure 3.
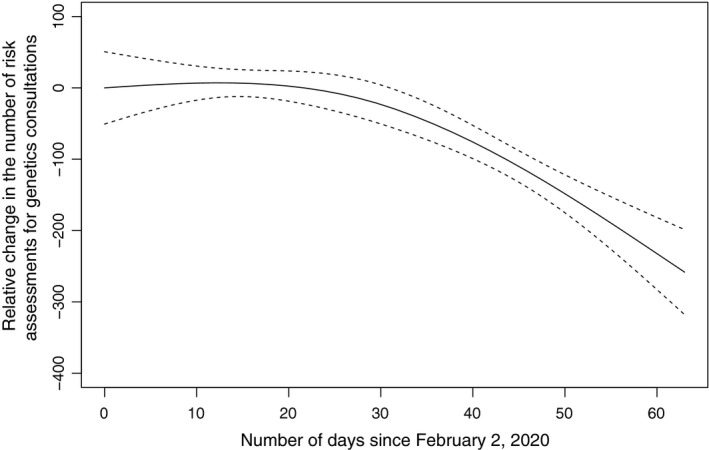
The relative change in the number of risk assessments for genetics consultations since February 2, 2020. Dashed lines indicate the 95% confidence interval.
Discussion
Although it is obvious that the volume of patients with breast cancer has decreased substantially during the COVID‐19 pandemic, to the best of our knowledge the current study is the first to quantify the magnitude of the decline using a large, unique data set.
By using the number of risk assessments as a proxy for the number of breast images, breast surgeries, and genetics consultations, the results of the current study demonstrated that before the drop (measured from the week of February 2 to the week of March 8), the average number of mammograms performed per week at the imaging centers included in the current study was 24,926, which annualizes to 1,296,152 mammograms per year. The number of mammograms performed in the United States each year as recorded by the US Food and Drug Administration is 39,792,833 mammograms. 23 This suggests that the group of mammography centers represented in the current study is responsible for an estimated 3.3% of the total annual screening mammograms performed in the United States.
During this COVID‐19 crisis, breast imaging has decreased significantly (Fig. 1) (see Supporting Figs. 1 and 2). We can assume that the drop is related to some extent to the percentage of imaging that accounts for screening, which is estimated to be approximately 80.7% using the most recent national data. 24 , 25 If so, the percentage that continues to be performed should be approximately 19.3% (ie, the percentage of diagnostic mammograms). However, the percentage noted in the current study (during the week of April 5, approximately 5.4% of the average weekly volume before the drop) was much lower than the expected number of diagnostic studies. This may be related to the fact that many diagnostic imaging studies are callbacks from screening, and many are performed for women presenting with breast signs and symptoms. If screening mammography does not take place, the callbacks also will not take place and a decline in diagnostic imaging will occur if women with signs and symptoms choose not to be seen at the breast center.
The performance of breast surgery has dropped to approximately 40.1% of the pre‐COVID average weekly volume during this crisis, with a significant effect noted with regard to non–breast cancer surgeries and a minimal influence on breast cancer surgeries (Fig. 2) (see Supporting Figs. 3 and 4). Because breast surgeons treat women whose screening mammography identifies lesions requiring surgical intervention, this decrease is expected to occur as rates of screening decrease. Surgeons also treat women with signs and symptoms that may represent breast cancer. These women likely are presenting in lower numbers out of fear of interacting with the health care system, as evidenced by emergency room statistics for even acute issues such as stroke and myocardial infarction. 6 , 7 , 8 , 9 , 10 It is interesting to note that the impact of COVID‐19 on breast cancer surgeries appears to have been minimal because these patients require consultation even if surgery is deferred. Although currently not declining, a decrease is expected in the near future as fewer screen‐detected cancers are found.
Cancer genetics consultations also were found to have decreased to 39.9% of the pre‐COVID average weekly volume during this crisis (Fig. 3) (see Supporting Fig. 5). Although the identification of women with pathogenic variants in cancer susceptibility genes is critical, this always has been treated as an elective consultation except in cases in which the result will determine the surgical approach. As such, the dropoff initially is likely due to the lack of urgency perceived by clinicians and patients regarding this consultation, in which the patient's fear of coming to the hospital outweighs the perceived need for testing, and even arranging a telemedicine visit is not treated as urgent. In addition, because many patients are tested once the cancer is diagnosed, the decrease in cancer diagnoses by either screening or the surgical management of signs and symptoms is partly responsible for the drop. Cancer genetics is unique in that the initial consultation and testing can be done via telemedicine, with the saliva sample kit being sent to the patient's home. This most likely has mitigated the drop in consultations but has not prevented it entirely.
In terms of timing, surgery began to drop first during the week of March 8, followed by breast imaging and genetics consultations (both began during the week of March 15). This chronology makes sense because the initial response of hospital systems was to conserve acute care beds and physician time to meet the demands of an expected surge of patients with COVID‐19. Surgeons would have been asked to curtail their practices early. The next phase was to decrease the number of elective patients coming into outpatient facilities (breast imaging and cancer genetics).
We acknowledge that the current study has several limitations. First, the data we analyzed were derived from a single risk assessment company. Although the volume was large, the decreasing trends we identified might not reflect the actual changes nationwide. Second, with regard to breast imaging, we were unable to differentiate screening from diagnostic studies. In addition, mammography use could not be estimated from these data for patients with a personal history of breast cancer because risk assessment may not be performed routinely in this population at the time of surveillance mammography. Third, with regard to genetics consultations, we could not be sure how many patient visits were related to breast disease. With regard to surgery, we were taking a convenience sample of patients before and after COVID‐19, but were unable to determine whether the same surgeons had the same proportional use before and after the COVID‐19 outbreak.
Although we recognize its weaknesses, the strength of the current study lies within the volume of data analyzed. The data sets used for the current study were derived from multiple institutions and represented a significant percentage of breast imaging studies (approximately 3.3% of the total annual cases in the US), a significant percentage of patients with breast cancer who were managed surgically (approximately 1.8% of the total annual cases in the US), and a significant percentage of those patients receiving the results of genetics tests (approximately 3.4% the total annual cases in the US). Because it would be hard to represent this volume of data in a single health care system for this wide area of breast disease, we believe the results of the current analysis are valuable.
Although the current study results demonstrated significant decreases in breast cancer screening and treatment during the COVID‐19 outbreak, we are fully aware that the actual impact on diagnosis and treatment among patients with breast cancer still is unknown. The interruption of breast cancer screening for 3 to 4 months may not necessarily influence the cancer stage, especially when medical services have started to catch up alongside the phased reopening nationwide. Further evaluation of the impact of COVID‐19 on the survival outcomes of patients with breast cancer is warranted.
Conclusions
The cessation of elective health care services due to the emergence of COVID‐19 may have far‐reaching consequences on health care delivery in the United States. To our knowledge, the current study is the first to quantify the magnitude of this effect through the lens of outpatient services for breast cancer screening and treatment. To our knowledge, to date, the pandemic has exerted significant influence over the number of patients undergoing management for breast cancer prevention, screening, and diagnosis. The decline in screening for the detection of early‐stage breast cancer is particularly ominous because it may alter disease progression not only for patients with cancers diagnosed during this time frame, but also going forward as health care providers attempt to ramp up services to accommodate normal patient volumes in addition to the backlog of cases created by COVID‐19.
Funding Support
No specific funding was disclosed.
Conflict of Interest Disclosures
Brian Drohan is an employee of CRA Health LLC, which provided the data for the current study, and he assisted in the analysis. Kevin S. Hughes received honoraria from Hologic Inc and Myriad Genetics and is a founder of and has a financial interest in CRA Health LLC (formerly Hughes RiskApps), which develops risk assessment models/software with a particular focus on breast cancer and colorectal cancer, but he receives no payments from them to either himself or Massachusetts General Hospital. In addition, Dr. Hughes is a cocreator of Ask2Me.Org, which is freely available for clinical use and is licensed for commercial use by the Dana‐Farber Cancer Institute and the Massachusetts General Hospital. Dr. Hughes's interests were reviewed and are managed by Massachusetts General Hospital and Partners Health Care in accordance with their conflict of interest policies. Kanhua Yin and Preeti Singh made no disclosures.
Author Contributions
Kanhua Yin: Investigation, formal analysis, writing–original draft, and writing–review and editing. Preeti Singh: Investigation, formal analysis, writing–original draft, and writing–review and editing. Brian Drohan: Conceptualization and writing–review and editing. Kevin S. Hughes: Conceptualization, methodology, supervision, and writing–review and editing.
Supporting information
Supplementary Material
Yin K, Singh P, Drohan B, Hughes KS. Breast imaging, breast surgery, and cancer genetics in the age of COVID-19. Cancer. 2020:126:4466–4472. 10.1002/cncr.33113
The first 2 authors contributed equally to this article.
We thank Ann S. Adams of the Department of Surgery at Massachusetts General Hospital for editorial consultation.
References
- 1. Morens DM, Daszak P, Taubenberger JK. Escaping Pandora's box–another novel coronavirus. N Engl J Med. 2020;382:1293‐1295. [DOI] [PubMed] [Google Scholar]
- 2. Moghadas SM, Shoukat A, Fitzpatrick MC, et al. Projecting hospital utilization during the COVID‐19 outbreaks in the United States. Proc Natl Acad Sci U S A. 2020;117:9122‐9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. AHA/ASA Stroke Council Leadership . Temporary Emergency Guidance to US Stroke Centers During the Coronavirus Disease 2019 (COVID‐19) Pandemic: on Behalf of the American Heart Association/American Stroke Association Stroke Council Leadership. Stroke. 2020;51:1910‐1912. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention , US Department of Health and Human Services . Coronavirus disease 2019 (COVID‐19): cases in the U.S. Published 2020. Accessed May 8, 2020. https://www.cdc.gov/coronavirus/2019‐ncov/cases‐updates/cases‐in‐us.html
- 5. Gong K, Xu Z, Cai Z, Chen Y, Wang Z. Internet hospitals help prevent and control the epidemic of COVID‐19 in China: multicenter user profiling study. J Med Internet Res. 2020;22:e18908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST‐segment elevation cardiac catheterization laboratory activations in the United States during COVID‐19 pandemic. J Am Coll Cardiol. 2020;75:2871‐2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morelli N, Rota E, Terracciano C, et al. The baffling case of ischemic stroke disappearance from the casualty department in the COVID‐19 era. Eur Neurol. 2020;83(2):213‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Markus HS, Brainin M. EXPRESS: COVID‐19 and stroke–a Global World Stroke Organisation perspective. Int J Stroke. 2020;15:361‐364. [DOI] [PubMed] [Google Scholar]
- 9. Tam CF, Cheung KS, Lam S, et al. Impact of coronavirus disease 2019 (COVID‐19) outbreak on ST‐segment‐elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13:e006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carter P, Anderson M, Mossialos E. Health system, public health, and economic implications of managing COVID‐19 from a cardiovascular perspective. Eur Heart J. 2020;41(27):2516‐2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al‐Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID‐19) pandemic: an international collaborative group. Oncologist. 2020;25:e936‐e945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: systematic review and meta‐analysis to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164:244‐255. [DOI] [PubMed] [Google Scholar]
- 13. Matsen CB, Neumayer LA. Breast cancer: a review for the general surgeon. JAMA Surg. 2013;148:971‐979. [DOI] [PubMed] [Google Scholar]
- 14. Schrag D, Hershman DL, Basch E. Oncology practice during the COVID‐19 pandemic. JAMA. Published online April 13, 2020;323(20):2005‐2006. [DOI] [PubMed] [Google Scholar]
- 15. Soran A, Gimbel M, Diego E. Breast cancer diagnosis, treatment and follow‐up during COVID‐19 pandemic. Eur J Breast Health. 2020;16:86‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coles CE, Aristei C, Bliss J, et al. International guidelines on radiation therapy for breast cancer during the COVID‐19 pandemic. Clin Oncol (R Coll Radiol). 2020;32:279‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. The American Society of Breast Surgeons . Mastery of Breast Surgery. Accessed May 8, 2020. https://www.breastsurgeons.org/new_layout/programs/mastery/
- 18. LaDuca H, Polley EC, Yussuf A, et al. A clinical guide to hereditary cancer panel testing: evaluation of gene‐specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high‐risk patients. Genet Med. 2020;22:407‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rummel SK, Lovejoy LA, Turner CE, Shriver CD, Ellsworth RE. Should genetic testing for cancer predisposition be standard‐of‐care for women with invasive breast cancer? The Murtha Cancer Center experience. Cancers (Basel). 2020;12:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. US Census Bureau . Census regions and divisions of the United States. Published 2013. Accessed May 8, 2020. https://www2.census.gov/geo/pdfs/maps‐data/maps/reference/us_regdiv.pdf
- 21. Wood SN. Generalized Additive Models: An Introduction With R. Chapman & Hall/CRC; 2006. [Google Scholar]
- 22. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7‐30. [DOI] [PubMed] [Google Scholar]
- 23. US Food and Drug Administration . MQSA National Statistics. Accessed May 8, 2020. https://www.fda.gov/radiation‐emitting‐products/mqsa‐insights/mqsa‐national‐statistics
- 24. Sprague BL, Arao RF, Miglioretti DL, et al. National performance benchmarks for modern diagnostic digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283:59‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283:49‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


