Abstract
The COVID‐19 pandemic caused by SARS‐Cov‐2 demands rapid, safe and effective therapeutic options. In the last decades, the endogenous gasotransmitter hydrogen sulfide (H2S) has emerged as modulator of several biological functions and its deficiency has been associated with different disorders. Therefore, many H2S‐releasing agents have been developed as potential therapeutic tools for diseases related with impaired H2S production and/or activity. Some of these compounds are in advanced clinical trials. Presently, the pivotal role of H2S in modulating the inflammatory response and pro‐inflammatory cytokine cascade is well recognized, and the usefulness of some H2S‐donors for the treatment of acute lung inflammation has been reported. Recent data is elucidating several mechanisms of action, which may account for antiviral effects of H2S. Noteworthy, some preliminary clinical results suggest an inverse relationship between endogenous H2S levels and severity of COVID‐19. Therefore, repurposing of H2S‐releasing drugs may be a potential therapeutic opportunity for treatment of COVID‐19.
Linked Articles
This article is part of a themed issue on The Pharmacology of COVID‐19. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.21/issuetoc
Keywords: COVID‐1, drug repurposing, hydrogen sulfide, H2S‐donor, SARS‐CoV‐2
Abbreviations
- 3CLpro
3‐chymotrypsin‐like protease
- 3‐MST
3‐mercaptopyruvate sulfurtransferase
- AnxA1
annexin A1
- CAT
cysteine aminotransferase
- CBS
cystathionine β‐synthase
- CMV
cytomegalovirus
- COVID‐19
coronavirus disease 2019
- CSE
cystathionine γ‐lyase
- DADS
diallyl disulfide
- DATS
diallyl trisulfide
- DIC
disseminated intravascular coagulation
- FPR2
formyl peptide receptor 2
- HSV‐1
herpes simplex virus type 1
- IKκβ
I‐kappa‐β kinase‐β
- IRF‐3
IFN regulatory factor 3
- IκBα
inhibitor of NF‐κB
- KATP
ATP‐sensitive potassium channels
- MERS‐CoV
Middle East respiratory syndrome coronavirus
- MPO
myeloperoxidase
- NiV
Nipah virus
- Nrf2
nuclear factor erythroid 2‐related factor 2
- PLAs
platelet–leukocyte aggregates
- RSV
respiratory syncytial virus
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SFN
sulforaphane
- TMPRSS2
transmembrane serine protease 2
- TSMT
thiol‐S‐methyltransferase
1. INTRODUCTION
The current pandemic caused bythe new betacoronavirus SARS‐CoV‐2 (COVID‐19) has placed the global healthcare system in such an unprecedented emergency state that the situation demands the rapid implementation of effective and safe pharmacological strategies to extinguish viral infection or, at least, to mitigate its pathogenic effects and to slow down its contagion. In this perspective, early SARS‐CoV‐2 clinical studies evaluated the effects of many drugs, both old and new, known for their broad antiviral spectrum, such as favipiravir, ribavirin, galidesivir, remdesivir, and hydroxychloroquine (Harrison, 2020; Li & De Clercq, 2020). Moreover, the repurposing of available drugs developed for different therapeutic indications offers a promising strategy to take advantage of the safety and therapeutic window of such drugs. This should lead to a more rapid evaluation of their use against SARS‐CoV‐2, considering the current health emergency and the lack of therapeutic solutions.
The “cytokine storm” syndrome, with subsequent acute lung injury (and other systemic disorders), is a frequent feature of many infectious diseases, including those caused by many viral pathogens (Tisoncik et al., 2012). Indeed, rapid virus replication, massive infiltration of immune cells and uncontrolled production of pro‐inflammatory cytokines were already observed in severe acute respiratory syndromes previously caused by other betacoronaviruses, such as SARS‐CoV (which caused the severe acute respiratory syndrome epidemic in 2002–2003) and MERS‐CoV (which caused the epidemic of Middle East respiratory syndrome in 2012–2013) (Channappanavar & Perlman, 2017). Noteworthy, a cytokine storm is also observed in the most serious cases of COVID‐19 (Mehta et al., 2020). Indeed, patients with severe clinical conditions exhibit pulmonary distress syndrome, lung oedema, and respiratory failure. In addition, liver, heart, and kidney injury, as well as impaired haemostatic regulation, are also frequently observed. In particular, intensive care patients display elevated levels of pro‐inflammatory cytokines. Many cytokines detected in these patients derive from a Th17‐type immune response (as already reported for MERS‐CoV and SARS‐CoV patients). The corresponding IL‐17‐related pathway triggers a wide pro‐inflammatory cascade through the induction of specific cytokines, such as IL‐6 and TNF, chemokines, and matrix metalloproteinases, the latter responsible for tissue remodelling and damage (Wu & Yang, 2020). The production of IL‐1β and IL‐6 is also induced by direct interaction of SARS‐CoV‐2 viral components with host toll‐like receptors (Russell et al., 2020) and indirectly through the activation of the NF‐κB signalling pathway, as demonstrated by Wang et al. (2007) in murine RAW264.7 cells The SARS‐CoV‐2 spike protein is associated with increased degradation of the inhibitor of NF‐κB (IκBα), leading to the activation of the NF‐κB signalling pathway. Once activated and translocated into the nucleus, NF‐κB promotes the transcription of a large variety of genes encoding stress‐response proteins, chemokines and pro‐inflammatory cytokines. Notably, the excessive NF‐κB activation is involved in the lung inflammatory process induced by respiratory viruses, including SARS‐CoV (Catanzaro et al., 2020; Dosch, Mahajan, & Collins, 2009).
To date, the powerful and uncontrolled inflammatory response promoted by the “cytokine storm” is considered the main pathogenic factor of SARS‐CoV‐2 infections, accounting for the most severe symptomatic features of the disease and fatal outcomes (Mehta et al., 2020). Accordingly, effective strategies able to mitigate the force of this inflammatory storm are considered a valuable option for reducing the severity of the disease. For instance, tocilizumab, a monoclonal anti‐IL‐6 antibody, is taken as a promising candidate for fighting against the “cytokine storm” and elevated levels of IL‐6 in patients who show serious lung inflammation and organ damage (Mehta et al., 2020). Furthermore, blockade of the NF‐κB pathway reduces lung damage and inflammation and significantly increases mouse survival after SARS‐CoV infection. These results confirm that the NF‐κB signalling pathway can be a major mediator of the inflammation induced by SARS‐CoV infection and that NF‐κB inhibition may be considered a promising antiviral strategy for SARS‐CoV infections and other pathogenic human coronaviruses (DeDiego et al., 2014).
Recent insights point out that the changes in the vascular endothelium are likely to be pivotal elements in facilitating the diffusion of the inflammatory damage in the lung and other organs associated with SARS‐CoV‐2 infection. Endothelial cells are responsible for the endogenous production of vasoactive factors and for preventing immune cell adhesion to the vascular wall (Citi, Martelli, et al., 2020; Park & Park, 2015). Diffuse endothelial dysfunction is a hallmark of many cardiovascular and metabolic pathologies, such as hypertension, diabetes, and atherosclerosis. Noteworthy, clinical and preclinical studies support the hypothesis that COVID‐19 leads to more severe systemic complications in patients with cardiovascular or metabolic pathologies (Sardu et al., 2020). In particular, hypertension and diabetes are the most common co‐morbidities associated with a worsening prognosis in COVID‐19 patients. Furthermore, dysregulation of the immune response leads to marked increases in permeability of lung endothelium, with subsequent acute respiratory distress syndrome and thrombotic events, especially in late‐stage COVID‐19 infection.
Recently, inhalation of NO gas was investigated as a strategy to prevent COVID‐19 (e.g., clinical trials NCT04306393, NCT04312243, NCT04338828, and NCT04305457) and to improve arterial oxygenation against respiratory distress (Teman et al., 2015). NO diffuses into the lungs and bronchi where it promotes vasorelaxant and bronchodilatory effects. NO also promotes ciliary movements, which help to remove viral particles from the airways, and in a pig experimental model, NO inhibits pulmonary viral replication (Xu, Zheng, Dweik, & Erzurum, 2006). In humans, higher levels of exhaled NO correlate with a lower incidence of respiratory illness, suggesting that endogenous NO represents a crucial defence against viruses in the airways (Keyaerts et al., 2004).
Noteworthy, NO is a well‐known endothelium‐derived factor, and it belongs to the class of endogenous gasotransmitters. Together with NO, H2S is another important member of this class of endogenous mediators, and while it exhibits many NO‐like effects, the mechanisms of action are quite different. Physiological levels of H2S have systemic anti‐inflammatory effects, prevent endothelial dysfunction in cardiovascular‐related pathologies, and act as a scavenger of ROS and peroxynitrite (Calderone, Martelli, Testai, Citi, & Breschi, 2016). Furthermore, it is widely known that exposure to low H2S levels significantly improves respiratory function by regulating mucolytic activity (Bazhanov, Ansar, et al., 2017) and through the up‐regulation of endothelial NOS and increased NO bioavailability, thus indirectly protecting the airways from viral infection disease (King et al., 2014).
In this paper, the pharmacological basis for a potential beneficial role of H2S against COVID‐19 and the potential repurposing of H2S donor drugs in the management of this disease are discussed.
2. ENDOGENOUS H2S
Abe and Kimura (1996) first discovered that H2S is endogenously produced and is endowed with relevant physiological roles. Thereafter, H2S was recognized as the third gasotransmitter, after carbon monoxide (CO) and NO, and its biosynthesis and metabolism pathways were widely investigated (Martelli et al., 2012). In mammalian cells, H2S is produced by non‐enzymic and enzymic routes (Yang et al., 2019). Cystathionine γ‐lyase (CSE) and cystathionine β‐synthase (CBS), which act within the “transsulfuration pathway”, are the main H2S biosynthesizing enzymes (Miles & Kraus, 2004; Pan, Liu, Gong, Yang, & Zhu, 2012). A further biosynthetic pathway involves the tandem cooperation of cysteine aminotransferase (CAT) and 3‐mercaptopyruvate sulfurtransferase (3‐MST) (Beltowski, 2019). A major source of H2S production in the brain derives from 3‐MST, which is localized into neuronal cytosol and mitochondria. 3‐MST produces H2S and bound sulfane sulfur more efficiently than CBS, which was previously believed to be the only H2S‐producing enzyme in the CNS. Furthermore, the 3MST–CAT pathway produces polysulfides as well as H2S. In particular, levels of polysulfides measured as bound sulfane sulfur in 3‐MST overexpressing cells were more than double those in control cells (Kimura et al., 2017; Shibuya et al., 2009).
Regarding the degradation of H2S, several pathways have been described. H2S is a reducing agent and thus can be oxidized by several circulating oxidants. However, one of the main catabolic pathways for H2S operates in the mitochondria, leading to the formation of thiosulfate, which is then converted by rhodanese enzyme into sulfite and finally sulfate. In addition, H2S can be methylated through thiol‐S‐methyltransferase (TSMT) with the formation of dimethyl sulfide. Finally, H2S interacts with Hb to produce sulfhaemoglobin (Guo, Cheng, & Zhu, 2013).
S‐sulfhydration, a post‐translational modification of cysteine residues of several target proteins, is considered the most plausible molecular mechanism for the pleiotropic effects of H2S (Banerjee, 2011; Meng, Zhao, Xie, Han, & Ji, 2018). Noteworthy, H2S coexists in biological systems with sulfane sulfur species. They are heterogeneous compounds containing a reactive, labile sulfur atom, which is covalently bonded to other sulfur atoms or to a sulfur and a hydrogen atom to form reactive sulfur compounds, including persulfides, polysulfides, and some forms of elemental sulfur (Iciek, Bilska‐Wilkosz, & Gorny, 2019; Toohey, 2011). Sulfane sulfur species can be viewed as a form of “H2S storage”, to maintain a low‐grade toxicity and to allow gasotransmitter release in response to biological signals (Ishigami et al., 2009; Toohey, 2011). Furthermore, sulfane sulfur species may act themselves through S‐sulfhydration and therefore may be largely responsible for those biological activities attributed to H2S (Toohey, 2012). Indeed, it has been demonstrated that alterations in sulfane sulfur levels reflect some pathophysiological conditions related to H2S‐altered biosynthesis, strongly suggesting the close reciprocal relationships.
Although intense research activity has been carried out and many roles of H2S in the homoeostatic regulation of different systems and in the pathogenesis of several disorders have been elucidated, some mechanisms and pathways of H2S signalling are not yet completely understood. As defective endogenous production of H2S has been associated with many systemic disorders, great efforts have been directed to the development of effective pharmacological agents able to increase H2S levels. The pharmacological modulation of H2S is a dynamic field in recent drug discovery research, which has been well‐examined and reported in some seminal reviews (Gojon & Morales, 2020; Szabo & Papapetropoulos, 2017; Zheng et al., 2018). Presently, a large number of natural and synthetic compounds have been recognized as effective H2S donors (Keyaerts et al., 2004; Sardu et al., 2020), and some of them are in clinical trials for the treatment of cardiovascular diseases (SG‐1002 for heart failure) (Polhemus et al., 2015) and cancer disease (sulforaphane [SFN]) (Jiang et al., 2018).
3. H2S IN INFLAMMATORY LUNG DISEASES
The pro‐inflammatory response and cytokine storm are involved in the most severe cases of SARS‐CoV‐2 infection disease (Coperchini, Chiovato, Croce, Magri, & Rotondi, 2020), and consistently, high levels of pro‐inflammatory cytokines (IL‐1β, IL‐6, and TNF) and chemokines (CCL2, CCL3, and CCL5) have been detected in COVID‐19 patients. In addition, haemopoietic growth factors such as G‐CSF and GM‐CSF are also elevated in COVID‐19 patients (Schett, Sticherling, & Neurath, 2020). Interestingly, the exacerbation of NF‐κB activation described above is also peculiar to SARS‐CoV infection disease (Dediego et al., 2014).
Very recently, Renieris et al. (2020) investigated the role of H2S in COVID‐19 respiratory disease, evaluating H2S plasma levels during progression of the disease and its association with final outcome, in a cohort of patients with COVID‐19 pneumonia. In this study, a correlation between the severity of SARS‐CoV‐2 infection, cytokine production, and H2S plasma levels has been described, suggesting a potential predictive role of H2S in the outcome of pneumonia caused by SARS‐CoV‐2. Indeed, the kinetics of circulating H2S revealed that patients with favourable outcome display levels of the gasotransmitter higher than those found in patients with severe COVID‐19 pneumonia. This evidence suggests that the reduction of H2S bioavailability may be considered as an indicator of enhanced pro‐inflammatory response and that the administration of exogenous H2S may be viewed as a pharmacological strategy to restore H2S plasma levels in order to counteract the severe consequences of COVID‐19 infection (Renieris et al., 2020).
IL‐6 has been proposed as the main pro‐inflammatory mediator involved in the cytokine storm that leads to severe lung injury, respiratory failure, and death in COVID‐19 patients (Gubernatorova, Gorshkova, Polinova, & Drutskaya, 2020). Noteworthy, H2S is likely to be an effective down‐regulator of IL‐6 and an inhibitor of the NF‐κB pathway (Kodela et al., 2015; Rios, Szczesny, Soriano, Olah, & Szabo, 2015). Generally, the role of H2S in inflammatory response in lung function and disease has been widely studied, and recent evidence highlights that H2S positively correlates with lung function and improves peak expiratory and inspiratory flow rate (Tian et al., 2012).
Both exogenous and endogenous H2S exert beneficial effects in the respiratory system by regulating mucolytic activity. H2S is able to make the mucus less viscous, because it promotes the breakage of mucins via interactions with disulfide bonds (Costantino, Lampa, & Nappi, 2006). Furthermore, in human bronchiolar epithelia, H2S triggers electrolyte absorption through the activation of ATP‐sensitive potassium channels (KATP) and inhibition of Na+/K+–ATPase and calcium‐sensitive potassium channels (Pouokam & Althaus, 2016). These properties enhance mucociliary clearance, promoting the expulsion of foreign microorganisms.
Disturbances of the endogenous production of H2S are related to pathological processes and progression of several diseases, including hypertension, hypoxic pulmonary hypertension, myocardial injury, and viral infection (Calderone et al., 2016; Chen et al., 2020; Citi et al., 2018). The role of exogenous H2S in lung disease has been studied by administering H2S donor agents. The importance of the “nature” of fast or slow H2S‐releasing molecules in inflammatory responses was mostly assessed by using molecules able to generate H2S with slow and sustained release kinetics. Indeed, the administration of NaHS, a “fast releasing” H2S donor, induced an important inflammatory reaction in mice, underlined by an increased myeloperoxidase (MPO) activity (a marker for tissue leukocyte infiltration) and by the presence of accumulated leukocytes in the lung (Bhatia, 2012). In contrast, the slow H2S‐releasing compound GYY4137 showed anti‐inflammatory activity in vivo and reduced plasma pro‐inflammatory cytokines (TNF, IL‐1β, and IL‐6) in an experimental model of LPS‐induced pulmonary inflammation in rats. Furthermore, GYY4137 treatment was related to a marked antioxidant effect, by restoring the activity of the antioxidant enzymes catalase and SOD in lung tissues, and leading to normalization of the balancing between reduced and oxidized GSH (GSH/GSSG ratio) (Faller et al., 2018). GYY4137 also inhibited the expression of pro‐inflammatory genes by modulating the activation of NF‐кB and IFN regulatory factor 3 (IRF‐3) (Li et al., 2015). Mechanistically, Zhang et al. (2019) revealed that H2S blocks activation of the NF‐κB pathway in a model of monocrotaline pyrrole‐induced inflammatory response in pulmonary artery endothelial cells, through the sulfhydration of IKκβ at Cys179 residue, thus inhibiting IKκβ activity. Such a mechanism of action leads to protective effects in vivo against pulmonary vascular inflammation, vascular remodelling, and pulmonary arterial hypertension, suggesting that the post‐transcriptional regulation of IKκβ is a novel target of H2S to prevent vascular inflammation (Zhang et al., 2019).
In a mouse model of LPS‐induced acute lung injury, GYY4137 prevented lung injury and neutrophil transmigration, by reducing chemoattractant signalling molecules in vitro in endothelial cells and in vivo in lung tissue (Faller et al., 2018). Indeed, neutrophil infiltration in lungs is a key event in the exacerbation of pulmonary disease associated with COVID‐19. Interestingly, H2S modulates the egress of leukocytes, namely, neutrophils, from the bloodstream to inflamed tissues (Zanardo et al., 2006), and this effect is dependent upon activation of annexin A1 (AnxA1) pro‐resolving pathway (Brancaleone, Mitidieri, Flower, Cirino, & Perretti, 2014). Indeed, AnxA1 is exposed to the neutrophil surface and prevents them from transmigration into the subendothelial space, thus suppressing cytokine production in inflamed tissues (Perretti & D'acquisto, 2009). This mechanism is associated with a reduction of adhesion molecule expression and occurs through the engagement of a specific receptor called formyl peptide receptor 2 (FPR2) (Dufton et al., 2010). It is feasible that this overall mechanism could operate in the lung of COVID‐19 patients, where the use of H2S‐releasing molecules would limit the passage of leukocytes into the underlying tissue. On one hand, this effect could down‐regulate the release of cytokines by activated neutrophils and macrophages; on the other hand, the production of growth factors (G‐CSF and GM‐CSF) responsible for increase in circulating neutrophils could be suppressed, thus inhibiting the generation of the “cytokine storm”. It is noteworthy to underline that H2S donors impair the stability of thrombi formed into the vessels, thus facilitating thrombolysis (Grambow et al., 2017). This effect has been associated with a reduced formation of platelet–leukocyte aggregates (PLAs), which normally facilitate both leukocyte recruitment and extravasation to sites of inflammation and thrombus formation (Badrnya et al., 2014; Diacovo, Roth, Buccola, Bainton, & Springer, 1996; Finsterbusch, Schrottmaier, Kral‐Pointner, Salzmann, & Assinger, 2018). In the case of COVID‐19 patients, there is a high incidence of coagulative disorders, generating a disseminated intravascular coagulation (DIC),which is lethal in severe conditions (Marietta, Coluccio, & Luppi, 2020). The possibility of modulating PLA formation by H2S is therefore of therapeutic interest, as PLAs promote both vascular inflammation and coagulation. Indeed, the inhibition of such aggregates could attenuate, at the same time, both events and achieve amelioration of the patient's conditions.
Other studies have shown that administration of H2S donors in ovalbumin‐treated rats significantly reduced IL‐6 and IL‐8 and increased anti‐inflammatory IL‐10 levels in the lung and plasma. H2S directly suppressed the pro‐inflammatory response and the production of ROS in neutrophils, underlining the beneficial potential of H2S‐releasing compounds in the prophylaxis of acute lung injury. Moreover, H2S promoted anti‐inflammatory effects through epigenetic alterations. In particular, it modulated the acetylation and methylation of histones involved in the regulation of pro‐inflammatory factor production and contributed to reduce cytokine release following stimulation with LPS in mice (Faller et al., 2018). The administration of diallyl disulfide (DADS; a natural, slow‐releasing, H2S donor) promoted a protective effect in naphthalene‐induced lung injury (Benavides et al., 2007; Martelli et al., 2013). DADS treatment increased GSH levels in the lung tissue, inhibited TNF, IL‐6, and IL‐8 release, and was associated with suppression of lung inflammatory cell recruitment, in particular neutrophil infiltration (Liu et al., 2018). Sulforaphane, a naturally occurring isothiocyanate able to release H2S (Lucarini et al., 2018), also decreased pro‐inflammatory mediator release in a mouse model of LPS‐induced acute lung injury. In particular, the transcription factor Nrf2 was involved in SFN‐mediated lung protection through the improvement of mitochondrial function and energy metabolism. Nrf2 is a nuclear factor responsible for promoting the expression of multiple antioxidant genes and preventing oxidative damage. Such a mechanism of action has been described also for synthetic isothiocyanates whose H2S donor profile has been widely described (Citi, Corvino, et al., 2020; Citi et al., 2014, 2019; Martelli, Citi, et al., 2020; Martelli, Piragine, et al., 2020; Martelli et al., 2014; Prawan et al., 2009; Sestito, Daniele, et al., 2019; Sestito, Pruccoli, et al., 2019; Testai et al., 2016). Anethole dithiolethione, another H2S donor, has been widely used as H2S donor or as a moiety for the development of H2S‐releasing hybrid drugs, including non‐steroidal anti‐inflammatory agents, such as H2S‐aspirin and H2S‐diclofenac. These novel hybrid drugs have been shown to have better anti‐inflammatory effects than the corresponding “parent drugs” aspirin and diclofenac, and the administration of H2S‐diclofenac was shown to be more effective in reducing lung MPO activity in a rat model of LPS‐induced septic shock, compared with diclofenac alone (Takayama et al., 2011) (Figure 1).
FIGURE 1.
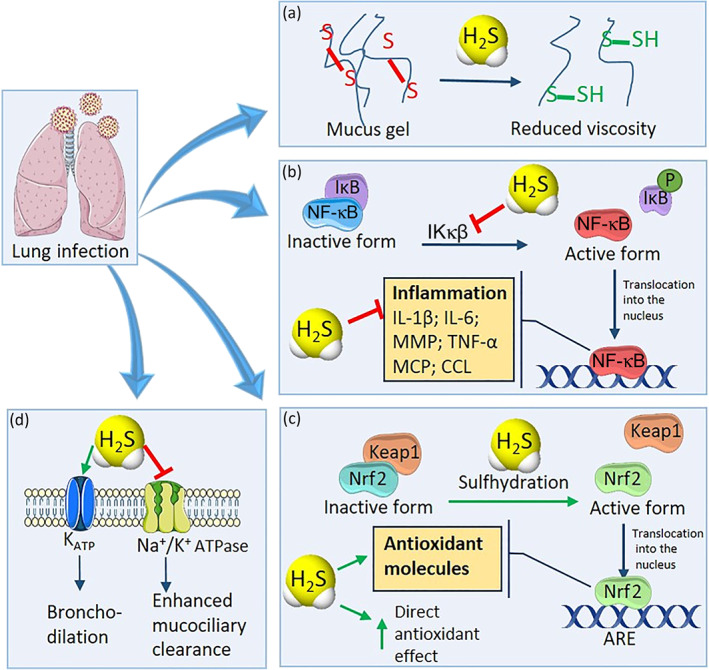
Role of H2S in lung inflammation. H2S shows many beneficial effects in lung inflammation disease. In particular, (a) H2S is able to break mucin disulfide bonds, making the mucus less viscous and easier to be expelled by the respiratory ciliary apparatus, facilitating the elimination of potentially harmful viruses or extraneous particles. (b) H2S blocks activation of the NF‐κB pathway, through the sulfhydration and inhibition of IKκβ enzyme, preventing the translocation of NF‐κB into the nucleus, thus preventing generation of the cytokine storm. (c) H2S promotes the activation of Nrf2, enhancing the expression of antioxidant molecules and enzymes. Moreover, H2S is endowed with direct antioxidant activity, thus protecting the tissues from the oxidative stress. (d) H2S activates KATP channels expressed on the cell membrane of bronchial smooth muscle cells, promoting bronchodilation, and blocks the Na+/K+ ATPase pump, triggering electrolyte absorption and enhancing mucociliary clearance
4. H2S AND VIRAL INFECTIONS
The effect of H2S on viral infections represents a promising research field, which is presently still poorly investigated. Many pulmonary viral infections increase ROS levels and impair antioxidant enzymes, including those associated with the Nrf2–ARE pathway, with consequent reduction of antioxidant response. This leads to the suggestion that agents able to activate the Nrf2 system and induce sustained antioxidant activity may represent a useful strategy to counteract the viral infection by respiratory viruses (Komaravelli & Casola, 2014). As reported above, many H2S‐releasing agents evoke significant protective effects against oxidative stress in the host cells, which are mediated by H2S and/or related sulfane sulfur through well‐clarified mechanisms, such as increased nuclear translocation of Nrf2 and inhibition of NADPH oxidase enzymes (Gojon & Morales, 2020).
Besides the triggering of the “antioxidant machinery” directly attributable to H2S, other “indirect” effects are also involved in antioxidant activity of H2S. For instance, H2S contributes to the maintenance of elevated levels of GSH, which is one of the main intracellular antioxidant compounds and an effective scavenger of reactive species (Gojon & Morales, 2020). GSH can itself be another player with antiviral effects. Indeed, GSH ability to limit viral infections has been long reported. In particular, GSH levels were dramatically reduced after 24 h of herpes simplex virus type 1 (HSV‐1) viral infection and exogenous administration of GSH almost totally abolished HSV‐1 replication (Palamara et al., 1995). Experiments carried out on human small airway epithelial cells and on homogenates of BALB/c mouse lung and trachea (following intranasal inoculation of mouse‐adapted influenza strain A/X‐31 virus) showed that GSH reduced viral replication in the in vitro model and viral titer in organs explanted after the in vivo experiments (Cai et al., 2003). Very recently, GSH was also proposed as a potentially useful agent against SARS‐CoV‐2, since high‐throughput artificial intelligence‐based binding affinity prediction indicated a possible GSH interaction with ACE2 or transmembrane serine protease 2 (TMPRSS2), two human proteins closely involved in the process of viral adhesion and entry into the host cell (Kim et al., 2020).
Some studies reported the potential antiviral effects of organosulfur molecules. However, only subsequent studies have shown that these compounds act as H2S donors, letting us hypothesize a posteriori that the antiviral effect can be linked to the release of this gas. For instance, Fang, Li, Cui, and Dong (1999) evaluated the effect of diallyl trisulfide (DATS) in cytomegalovirus (CMV)‐induced hepatitis. In their research, the authors reported that the polysulfide DATS, derived from Allium sativum L., reduced CMV viral load in infected organs in human and mice (Fang, Li, Cui, & Dong, 1999; Liu et al., 2011; Liu, Fang, Dong, Li, & Zhen, 2004) through the inhibition of gene transcription and promotion of CMV‐induced T‐regulatory helper cells amplification, with the consequent immunosuppressive effect against CMV observed in murine models (Li et al., 2013; Yi, Feng, Xiang, & Ge, 2005; Zhen et al., 2006). H2S accounts (at least in part) for the above antiviral effects, since Benavides et al. (2007) demonstrated that garlic polysulfides (e.g., DADS and DATS) are effective H2S donors. Similarly, Lin et al. (2005) studied the antiviral properties of the root extract of Isatis indigotica L. (also called Isatis tinctoria L.), a plant belonging to the Brassicaceae or Crucifers and widely used in Chinese traditional medicine. Among the various compounds contained in the extract, sinigrin (precursor of the H2S donor allyl isothiocyanate) was the most effective compound in inhibiting the function of 3‐chymotrypsin‐like protease (3CLpro; also called main protease or Mpro) of SARS‐CoV (responsible for the 2002–2003 epidemic). Notably, SARS‐CoV 3CLpro is the protease responsible for the proteolytic processing of polypeptides into functional proteins, necessary for viral replication. Thus, sinigrin can affect SARS‐CoV infection by inhibiting 3CLpro (Lin et al., 2005). The results obtained using the natural isothiocyanate sulforaphane (present in several plants of Brassicaceae family) indicate that the antiviral effect of Brassicaceae extracts can be mainly attributed to their isothiocyanate metabolites. As reported above, isothiocyanates have been recently described as smart H2S donors by Calderone and colleagues (Calderone et al., 2016; Citi et al., 2014, 2019; Lucarini et al., 2018; Marino et al., 2016; Martelli, Citi, et al., 2020; Martelli, Piragine, et al., 2020; Martelli et al., 2014; Testai et al., 2016). In particular, sulforaphane demonstrated antiviral activity against the influenza A/WSN/33 (H1N1) virus by reducing virus replication in vitro using canine kidney cells at low micromolar concentrations (Zhansheng et al., 2019). These results seem to find a confirmation in a randomized, double‐blind, placebo‐controlled clinical study. In this trial, healthy volunteers were treated with SFN or placebo and received a standard nasal vaccine dose of live attenuated influenza virus. Their peripheral blood immune cell population was analysed, paying particular attention to NK cells. Blood samples were examined at day 1 (before vaccine dosing) and at days 2 and 21 (after vaccine dosing). The results showed that sulforaphane increased virus‐induced granzyme B production by NK cells. Granzyme B is a protease released by NK or T cells in order to induce cell apoptosis, and this increase in granzyme B levels was negatively associated with influenza RNA levels in nasal lavage fluid cells, demonstrating again an antiviral role for sulforaphane (Muller et al., 2016). Concerning other H2S donors, some studies were focused on the phosphinodithioate GYY4137. GYY4137 is a well‐known slow H2S donor, and it was used by the Casola group for a thorough investigation on H2S and its antiviral activity against respiratory syncytial virus (RSV). Indeed, the researchers observed that the infection with RSV was associated with low levels of CSE mRNA and protein expression in airway epithelial cells, with consequent reduction of H2S generation (Li et al., 2015). Moreover, in RSV‐infected CSE knockout mice, they recorded increased viral replication and airway inflammation, compared with wild‐type mice (Ivanciuc et al., 2016). Based on these observations, they used GYY4137 to evaluate the role of H2S on several paramyxovirus infections (RSV, human metapneumovirus, and Nipah virus [NiV]) and recorded a significant reduction of viral replication and of pro‐inflammatory mediator production. The mechanism of GYY4137‐induced antiviral effect was not linked to genome replication or to the synthesis of viral mRNA or proteins, but to the inhibition of syncytia formation and viral assembly and release. As reported above, GYY4137 showed also anti‐inflammatory effects due to regulation of NF‐κB and IRF‐3 activation after their nuclear translocation (Li et al., 2015). This GYY4137 effect on RSV was also confirmed using in vivo experiments where intranasal administration of GYY4137 to infected mice, within 24 h of infection, inhibited viral replication, reduced pro‐inflammatory mediators, and improved airway dysfunction (Ivanciuc et al., 2016). Recent studies showed that GYY4137 also exhibited similar effects (reduced viral replication and decrease of pro‐inflammatory mediators) in in vitro models using other types of highly pathogenic RNA viruses, such as influenza A and B, Far Eastern subtype tick‐borne flavivirus, Crimean–Congo hemorrhagic fever virus, Rift Valley fever virus, and Ebola virus (Bazhanov, Escaffre, et al., 2017). Other thiol‐activated gem‐dithiol‐based H2S donors, while structurally different from GYY4137 yet sharing the ability to release H2S, also inhibited viral replication, deceased pro‐inflammatory mediators and improved airways dysfunction, both in in vitro and in vivo models. This provides strong evidence to suggest that H2S is the actual player responsible for the observed antiviral effects (Bazhanov et al., 2018).
The antiviral activity of a library of H2S‐releasing molecules, together with reference H2S donors (e.g., GYY4137 and sodium hydrosulfide), was initially tested on pseudotyped NiV. This preliminary screening showed that most sulfur molecules produced significant effect and a disulfide compound (XM‐01) was selected for further evaluation on both enveloped and non‐enveloped viral strains, such as RSV, influenza virus (A/WSN/33 strain), and rotavirus. The H2S‐releasing effects of XM‐01 (a cysteine‐based perthiol derivative) were previously reported in a study aimed at identifying cardioprotective H2S donors (Zhao et al., 2013). As observed in previous GYY4137 studies, XM‐01 exhibited antiviral effects on enveloped viruses. Noteworthy, no activity was observed on non‐enveloped viruses, such as rotavirus. Thus, as the antiviral action may be due to alterations of the viral membrane, GYY4137 and its analogues could be useful against enveloped viruses at the entry phase of host cell infiltration. However, the author hypothesized that the antiviral activity can also rely on mechanisms other than H2S (Pacheco & Chemistry WSUDO, 2017). Finally, in a very recent paper, it was hypothesized that H2S may also exhibit antiviral activity against SARS‐CoV‐2 by interfering with ACE2 and TMPRSS2 (Yang, 2020) (Figure 2).
FIGURE 2.
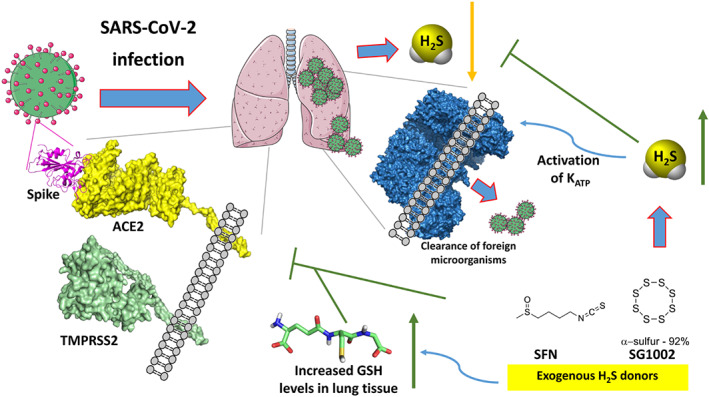
Potential mechanisms of H2S donors as anti‐COVID‐19 agents
5. CONCLUSIONS
The severity of COVID‐19 pandemic is mainly based on two relevant aspects: high degree of contagiousness and high frequency of massive inflammatory reactions, which lead to serious and life‐threatening disease outcomes. In this perspective, the pathophysiological role of the gasotransmitter H2S provides opportunities for additional investigation. This endogenous mediator can modulate the inflammatory response in a complex regulation of the cytokine cascade. Moreover, preliminary studies point to a role of H2S in regulating the host response to viral infections (Yang, 2020). Such evidence provides a strong case for the potential antiviral benefits offered by different classes and chemotypes of H2S donor molecules as therapeutic agents, as recently proposed also by other pioneering hypotheses (Evgen'ev & Frenkel, 2020; Yang, 2020). Several compounds already in clinical development for other therapeutic indications, such as SG1002 or sulforaphane, can be viewed as potential high‐value candidates for rapid repurposing for antiviral therapy against COVID‐19.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (http://www.guidetopharmacology.org), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos, et al., 2019; Alexander, Fabbro, et al., 2019; Alexander et al., 2019a, 2019b; Alexander, Mathie, et al., 2019; Harding et al., 2018).
CONFLICT OF INTEREST
G.G. is a founder and CSO of Sulfagenix, Inc. G.M. is CEO and president of Sulfagenix, Inc. All the other authors declare no conflict of interest.
Citi V, Martelli A, Brancaleone V, et al. Anti‐inflammatory and antiviral roles of hydrogen sulfide: Rationale for considering H2S donors in COVID‐19 therapy. Br J Pharmacol. 2020;177:4931–4941. 10.1111/bph.15230
REFERENCES
- Abe, K. , & Kimura, H. (1996). The possible role of hydrogen sulfide as an endogenous neuromodulator. The Journal of Neuroscience, 16, 1066–1071. 10.1523/JNEUROSCI.16-03-01066.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176(Suppl 1), S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176(Suppl 1), S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , … CGTP Collaborators . (2019a). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Introduction and other protein targets. British Journal of Pharmacology, 176(Suppl 1), S1–S20. 10.1111/bph.14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , … CGTP Collaborators . (2019b). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Transporters. British Journal of Pharmacology, 176(Suppl 1), S397–S493. 10.1111/bph.14753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , … CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 1(Suppl 1), S142–S228. 10.1111/bph.14749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrnya, S. , Schrottmaier, W. C. , Kral, J. B. , Yaiw, K. C. , Volf, I. , Schabbauer, G. , & Assinger, A. (2014). Platelets mediate oxidized low‐density lipoprotein‐induced monocyte extravasation and foam cell formation. Arteriosclerosis, Thrombosis, and Vascular Biology, 34, 571–580. 10.1161/ATVBAHA.113.302919 [DOI] [PubMed] [Google Scholar]
- Banerjee, R. (2011). Hydrogen sulfide: Redox metabolism and signaling. Antioxidants & Redox Signaling, 15, 339–341. 10.1089/ars.2011.3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazhanov, N. , Ansar, M. , Ivanciuc, T. , Garofalo, R. P. , & Casola, A. (2017). Hydrogen sulfide: A novel player in airway development, pathophysiology of respiratory diseases, and antiviral defenses. American Journal of Respiratory Cell and Molecular Biology, 57, 403–410. 10.1165/rcmb.2017-0114TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazhanov, N. , Escaffre, O. , Freiberg, A. N. , Garofalo, R. P. , & Casola, A. (2017). Broad‐range antiviral activity of hydrogen sulfide against highly pathogenic RNA viruses. Scientific Reports, 7, 41029 10.1038/srep41029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazhanov, N. , Ivanciuc, T. , Wu, H. , Garofalo, M. , Kang, J. , Xian, M. , & Casola, A. (2018). Thiol‐activated hydrogen sulfide donors antiviral and anti‐inflammatory activity in respiratory syncytial virus infection. Viruses, 10 10.3390/v10050249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltowski, J. (2019). Synthesis, metabolism, and signaling mechanisms of hydrogen sulfide: An overview. Methods in Molecular Biology, 2007, 1–8. 10.1007/978-1-4939-9528-8_1 [DOI] [PubMed] [Google Scholar]
- Benavides, G. A. , Squadrito, G. L. , Mills, R. W. , Patel, H. D. , Isbell, T. S. , Patel, R. P. , … Kraus, D. W. (2007). Hydrogen sulfide mediates the vasoactivity of garlic. Proceedings of the National Academy of Sciences of the United States of America, 104, 17977–17982. 10.1073/pnas.0705710104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia, M. (2012). Role of hydrogen sulfide in the pathology of inflammation. Scientifica (Cairo), 2012, 159680–159612. 10.6064/2012/159680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancaleone, V. , Mitidieri, E. , Flower, R. J. , Cirino, G. , & Perretti, M. (2014). Annexin A1 mediates hydrogen sulfide properties in the control of inflammation. The Journal of Pharmacology and Experimental Therapeutics, 351, 96–104. 10.1124/jpet.114.217034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, J. , Chen, Y. , Seth, S. , Furukawa, S. , Compans, R. W. , & Jones, D. P. (2003). Inhibition of influenza infection by glutathione. Free Radical Biology & Medicine, 34, 928–936. 10.1016/s0891-5849(03)00023-6 [DOI] [PubMed] [Google Scholar]
- Calderone, V. , Martelli, A. , Testai, L. , Citi, V. , & Breschi, M. C. (2016). Using hydrogen sulfide to design and develop drugs. Expert Opinion on Drug Discovery, 11, 163–175. 10.1517/17460441.2016.1122590 [DOI] [PubMed] [Google Scholar]
- Catanzaro, M. , Fagiani, F. , Racchi, M. , Corsini, E. , Govoni, S. , & Lanni, C. (2020). Immune response in COVID‐19: Addressing a pharmacological challenge by targeting pathways triggered by SARS‐CoV‐2. Signal Transduction and Targeted Therapy, 5, 84 10.1038/s41392-020-0191-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar, R. , & Perlman, S. (2017). Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Seminars in Immunopathology, 39, 529–539. 10.1007/s00281-017-0629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Zhang, H. , Yu, W. , Chen, L. , Wang, Z. , & Zhang, T. (2020). Expression of pulmonary arterial elastin in rats with hypoxic pulmonary hypertension using H2S. Journal of Receptor and Signal Transduction Research, 40, 1–5. 10.1080/10799893.2020.1738482 [DOI] [PubMed] [Google Scholar]
- Citi, V. , Corvino, A. , Fiorino, F. , Frecentese, F. , Magli, E. , Perissutti, E. , … Severino, B. (2020). Structure–activity relationships study of isothiocyanates for H2S releasing properties: 3‐Pyridyl‐isothiocyanate as a new promising cardioprotective agent. Journal of Advanced Research. 10.1016/j.jare.2020.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi, V. , Martelli, A. , Gorica, E. , Brogi, S. , Testai, L. , & Calderone, V. (2020). Role of hydrogen sulfide in endothelial dysfunction: Pathophysiology and therapeutic approaches. Journal of Advanced Research. 10.1016/j.jare.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi, V. , Martelli, A. , Testai, L. , Marino, A. , Breschi, M. C. , & Calderone, V. (2014). Hydrogen sulfide releasing capacity of natural isothiocyanates: Is it a reliable explanation for the multiple biological effects of Brassicaceae? Planta Medica, 80, 610–613. 10.1055/s-0034-1368591 [DOI] [PubMed] [Google Scholar]
- Citi, V. , Piragine, E. , Pagnotta, E. , Ugolini, L. , Di Cesare, M. L. , Testai, L. , … Martelli, A. (2019). Anticancer properties of erucin, an H2S‐releasing isothiocyanate, on human pancreatic adenocarcinoma cells (AsPC‐1). Phytotherapy Research, 33, 845–855. 10.1002/ptr.6278 [DOI] [PubMed] [Google Scholar]
- Citi, V. , Piragine, E. , Testai, L. , Breschi, M. C. , Calderone, V. , & Martelli, A. (2018). The role of hydrogen sulfide and H2S‐donors in myocardial protection against ischemia/reperfusion injury. Current Medicinal Chemistry, 25, 4380–4401. 10.2174/0929867325666180212120504 [DOI] [PubMed] [Google Scholar]
- Coperchini, F. , Chiovato, L. , Croce, L. , Magri, F. , & Rotondi, M. (2020). The cytokine storm in COVID‐19: An overview of the involvement of the chemokine/chemokine‐receptor system. Cytokine & Growth Factor Reviews, 53, 25–32. 10.1016/j.cytogfr.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino, M. , Lampa, E. , & Nappi, G. (2006). Effectiveness of sulphur spa therapy with politzer in the treatment of rhinogenic deafness. Acta Otorhinolaryngologica Italica, 26, 7–13. [PMC free article] [PubMed] [Google Scholar]
- Dediego, M. L. , Nieto‐Torres, J. L. , Regla‐Nava, J. A. , Jimenez‐Guardeno, J. M. , Fernandez‐Delgado, R. , Fett, C. , … Enjuanes, L. (2014). Inhibition of NF‐κB‐mediated inflammation in severe acute respiratory syndrome coronavirus‐infected mice increases survival. Journal of Virology, 88, 913–924. 10.1128/JVI.02576-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacovo, T. G. , Roth, S. J. , Buccola, J. M. , Bainton, D. F. , & Springer, T. A. (1996). Neutrophil rolling, arrest, and transmigration across activated, surface‐adherent platelets via sequential action of P‐selectin and the beta 2‐integrin CD11b/CD18. Blood, 88, 146–157. 10.1182/blood.V88.1.146.146 [DOI] [PubMed] [Google Scholar]
- Dosch, S. F. , Mahajan, S. D. , & Collins, A. R. (2009). SARS coronavirus spike protein‐induced innate immune response occurs via activation of the NF‐κB pathway in human monocyte macrophages in vitro. Virus Research, 142, 19–27. 10.1016/j.virusres.2009.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufton, N. , Hannon, R. , Brancaleone, V. , Dalli, J. , Patel, H. B. , Gray, M. , … Flower, R. J. (2010). Anti‐inflammatory role of the murine formyl‐peptide receptor 2: Ligand‐specific effects on leukocyte responses and experimental inflammation. J Immunol, 184, 2611–2619. 10.4049/jimmunol.0903526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evgen'ev, M. B. , & Frenkel, A. (2020). Possible application of H2S‐producing compounds in therapy of coronavirus (COVID‐19) infection and pneumonia. Cell Stress & Chaperones. 10.1007/s12192-020-01120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller, S. , Hausler, F. , Goeft, A. , Von Itter, M. A. , Gyllenram, V. , Hoetzel, A. , & Spassov, S. G. (2018). Hydrogen sulfide limits neutrophil transmigration, inflammation, and oxidative burst in lipopolysaccharide‐induced acute lung injury. Scientific Reports, 8, 14676 10.1038/s41598-018-33101-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, F. , Li, H. , Cui, W. , & Dong, Y. (1999). Treatment of hepatitis caused by cytomegalovirus with allitridin injection—An experimental study. Journal of Tongji Medical University, 19, 271–274. 10.1007/BF02886960 [DOI] [PubMed] [Google Scholar]
- Finsterbusch, M. , Schrottmaier, W. C. , Kral‐Pointner, J. B. , Salzmann, M. , & Assinger, A. (2018). Measuring and interpreting platelet–leukocyte aggregates. Platelets, 29, 677–685. 10.1080/09537104.2018.1430358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gojon, G. , & Morales, G. A. (2020). SG1002 and catenated divalent organic sulfur compounds as promising hydrogen sulfide prodrugs. Antioxidants & Redox Signaling. 10.1089/ars.2020.8060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grambow, E. , Leppin, C. , Leppin, K. , Kundt, G. , Klar, E. , Frank, M. , & Vollmar, B. (2017). The effects of hydrogen sulfide on platelet–leukocyte aggregation and microvascular thrombolysis. Platelets, 28, 509–517. 10.1080/09537104.2016.1235693 [DOI] [PubMed] [Google Scholar]
- Gubernatorova, O. , Gorshkova, E. A. , Polinova, A. I. , & Drutskaya, M. S. (2020). IL‐6: Relevance for immunopathology of SARS‐CoV‐2. Cytokine & Growth Factor Reviews., in Press, 53, 13–24. 10.1016/j.cytogfr.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, W. , Cheng, Z. Y. , & Zhu, Y. Z. (2013). Hydrogen sulfide and translational medicine. Acta Pharmacologica Sinica, 34, 1284–1291. 10.1038/aps.2013.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, C. (2020). Coronavirus puts drug repurposing on the fast track. Nature Biotechnology, 38, 379–381. 10.1038/d41587-020-00003-1 [DOI] [PubMed] [Google Scholar]
- Iciek, M. , Bilska‐Wilkosz, A. , & Gorny, M. (2019). Sulfane sulfur—New findings on an old topic. Acta Biochimica Polonica, 66, 533–544. 10.18388/abp.2019_2909 [DOI] [PubMed] [Google Scholar]
- Ishigami, M. , Hiraki, K. , Umemura, K. , Ogasawara, Y. , Ishii, K. , & Kimura, H. (2009). A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxidants and Redox Signaling, 11, 205–214. 10.1089/ars.2008.2132 [DOI] [PubMed] [Google Scholar]
- Ivanciuc, T. , Sbrana, E. , Ansar, M. , Bazhanov, N. , Szabo, C. , Casola, A. , & Garofalo, R. P. (2016). Hydrogen sulfide is an antiviral and antiinflammatory endogenous gasotransmitter in the airways. Role in respiratory syncytial virus infection. American Journal of Respiratory Cell and Molecular Biology, 55, 684–696. 10.1165/rcmb.2015-0385OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X. , Liu, Y. , Ma, L. , Ji, R. , Qu, Y. , Xin, Y. , & Lv, G. (2018). Chemopreventive activity of sulforaphane. Drug Design, Development and Therapy, 12, 2905–2913. 10.2147/DDDT.S100534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyaerts, E. , Vijgen, L. , Chen, L. , Maes, P. , Hedenstierna, G. , & Van Ranst, M. (2004). Inhibition of SARS‐coronavirus infection in vitro by S‐nitroso‐N‐acetylpenicillamine, a nitric oxide donor compound. International Journal of Infectious Diseases, 8, 223–226. 10.1016/j.ijid.2004.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. Z. J. , Cha, Y. , Kolitz, S. , Funt, J. , Escalante Chong, R. , Barrett, S. , … Kaufman, H. (2020). Advanced bioinformatics rapidly identifies existing therapeutics for patients with coronavirus disease‐2019 (COVID‐19). ChemRxivpreprint. 10.26434/chemrxiv.12037416.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, Y. , Koike, S. , Shibuya, N. , Lefer, D. , Ogasawara, Y. , & Kimura, H. (2017). 3‐Mercaptopyruvate sulfurtransferase produces potential redox regulators cysteine‐ and glutathione‐persulfide (Cys‐SSH and GSSH) together with signaling molecules H2S2, H2S3 and H2S. Scientific Reports, 7(10459), 2017 10.1038/s41598-017-11004-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, A. L. , Polhemus, D. J. , Bhushan, S. , Otsuka, H. , Kondo, K. , Nicholson, C. K. , … Lefer, D. J. (2014). Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase‐nitric oxide dependent. Proceedings of the National Academy of Sciences of the United States of America, 111, 3182–3187. 10.1073/pnas.1321871111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodela, R. , Nath, N. , Chattopadhyay, M. , Nesbitt, D. E. , Velazquez‐Martinez, C. A. , & Kashfi, K. (2015). Hydrogen sulfide‐releasing naproxen suppresses colon cancer cell growth and inhibits NF‐κB signaling. Drug Design, Development and Therapy, 9, 4873–4882. 10.2147/DDDT.S91116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaravelli, N. , & Casola, A. (2014). Respiratory viral infections and subversion of cellular antioxidant defenses. Journal Pharmacogenomics Pharmacoproteomics, 5 10.4172/2153-0645.1000141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. , & De Clercq, E. (2020). Therapeutic options for the 2019 novel coronavirus (2019‐nCoV). Nature Reviews. Drug Discovery, 19, 149–150. 10.1038/d41573-020-00016-0 [DOI] [PubMed] [Google Scholar]
- Li, H. , Ma, Y. , Escaffre, O. , Ivanciuc, T. , Komaravelli, N. , Kelley, J. P. , … Casola, A. (2015). Role of hydrogen sulfide in paramyxovirus infections. Journal of Virology, 89, 5557–5568. 10.1128/JVI.00264-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. N. , Huang, F. , Liu, X. L. , Shu, S. N. , Huang, Y. J. , Cheng, H. J. , & Fang, F. (2013). Allium sativum‐derived allitridin inhibits Treg amplification in cytomegalovirus infection. Journal of Medical Virology, 85, 493–500. 10.1002/jmv.23480 [DOI] [PubMed] [Google Scholar]
- Lin, C. W. , Tsai, F. J. , Tsai, C. H. , Lai, C. C. , Wan, L. , Ho, T. Y. , … Chao, P. D. (2005). Anti‐SARS coronavirus 3C‐like protease effects of Isatis indigotica root and plant‐derived phenolic compounds. Antiviral Research, 68, 36–42. 10.1016/j.antiviral.2005.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. L. , Wang, H. , Li, Y. N. , Ge, H. X. , Shu, S. N. , & Fang, F. (2011). Effects of allitridin on acute and chronic mouse cytomegalovirus infection. Archives of Virology, 156, 1841–1846. 10.1007/s00705-011-1025-9 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Li, A. , Feng, X. , Sun, X. , Zhu, X. , & Zhao, Z. (2018). Pharmacological investigation of the anti‐inflammation and anti‐oxidation activities of diallyl disulfide in a rat emphysema model induced by cigarette smoke extract. Nutrients, 10, 10 10.3390/nu10010079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. F. , Fang, F. , Dong, Y. S. , Li, G. , & Zhen, H. (2004). Experimental study on the prevention and treatment of murine cytomegalovirus hepatitis by using allitridin. Antiviral Research, 61, 125–128. 10.1016/s0166-3542(03)00087-1 [DOI] [PubMed] [Google Scholar]
- Lucarini, E. , Micheli, L. , Trallori, E. , Citi, V. , Martelli, A. , Testai, L. , … Di Cesare, M. L. (2018). Effect of glucoraphanin and sulforaphane against chemotherapy‐induced neuropathic pain: Kv7 potassium channels modulation by H2S release in vivo. Phytotherapy Research, 32, 2226–2234. 10.1002/ptr.6159 [DOI] [PubMed] [Google Scholar]
- Marietta, M. , Coluccio, V. , & Luppi, M. (2020). More on: ‘COVID‐19 coagulopathy in Caucasian patients’. British Journal of Haematology, 189, 1059–1060. 10.1111/bjh.16772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino, A. , Martelli, A. , Citi, V. , Fu, M. , Wang, R. , Calderone, V. , & Levi, R. (2016). The novel H2S donor 4‐carboxy‐phenyl isothiocyanate inhibits mast cell degranulation and renin release by decreasing intracellular calcium. British Journal of Pharmacology, 173, 3222–3234. 10.1111/bph.13583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli, A. , Citi, V. , Testai, L. , Brogi, S. , & Calderone, V. (2020). Organic isothiocyanates as hydrogen sulfide donors. Antioxidants & Redox Signaling, 32, 110–144. 10.1089/ars.2019.7888 [DOI] [PubMed] [Google Scholar]
- Martelli, A. , Piragine, E. , Citi, V. , Testai, L. , Pagnotta, E. , Ugolini, L. , … Calderone, V. (2020). Erucin exhibits vasorelaxing effects and antihypertensive activity by H2S‐releasing properties. British Journal of Pharmacology, 177, 824–835. 10.1111/bph.14645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli, A. , Testai, L. , Breschi, M. C. , Blandizzi, C. , Virdis, A. , Taddei, S. , & Calderone, V. (2012). Hydrogen sulphide: Novel opportunity for drug discovery. Medicinal Research Reviews, 32, 1093–1130. 10.1002/med.20234 [DOI] [PubMed] [Google Scholar]
- Martelli, A. , Testai, L. , Citi, V. , Marino, A. , Bellagambi, F. G. , Ghimenti, S. , … Calderone, V. (2014). Pharmacological characterization of the vascular effects of aryl isothiocyanates: Is hydrogen sulfide the real player? Vascular Pharmacology, 60, 32–41. 10.1016/j.vph.2013.11.003 [DOI] [PubMed] [Google Scholar]
- Martelli, A. , Testai, L. , Citi, V. , Marino, A. , Pugliesi, I. , Barresi, E. , … Calderone, V. (2013). Arylthioamides as H2S donors: L‐cysteine‐activated releasing properties and vascular effects in vitro and in vivo. ACS Medicinal Chemistry Letters, 4, 904–908. 10.1021/ml400239a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, P. , Mcauley, D. F. , Brown, M. , Sanchez, E. , Tattersall, R. S. , Manson, J. J. , & Hlh across Speciality Collaboration UK . (2020). COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet, 395, 1033–1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, G. , Zhao, S. , Xie, L. , Han, Y. , & Ji, Y. (2018). Protein S‐sulfhydration by hydrogen sulfide in cardiovascular system. British Journal of Pharmacology, 175, 1146–1156. 10.1111/bph.13825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles, E. W. , & Kraus, J. P. (2004). Cystathionine beta‐synthase: Structure, function, regulation, and location of homocystinuria‐causing mutations. The Journal of Biological Chemistry, 279, 29871–29874. 10.1074/jbc.R400005200 [DOI] [PubMed] [Google Scholar]
- Muller, L. , Meyer, M. , Bauer, R. N. , Zhou, H. , Zhang, H. , Jones, S. , … Jaspers, I. (2016). Effect of broccoli sprouts and live attenuated influenza virus on peripheral blood natural killer cells: A randomized, double‐blind study. PLoS ONE, 11, e0147742 10.1371/journal.pone.0147742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco, A. , & Chemistry WSUDO . (2017). Sulfur‐containing compounds as hydrogen sulfide donors and broad‐spectrum antiviral agents. Washington: Washington State University. [Google Scholar]
- Palamara, A. T. , Perno, C. F. , Ciriolo, M. R. , Dini, L. , Balestra, E. , D'agostini, C. , … Garaci, E. (1995). Evidence for antiviral activity of glutathione: in vitro inhibition of herpes simplex virus type 1 replication. Antiviral Research, 27, 237–253. 10.1016/0166-3542(95)00008-a [DOI] [PubMed] [Google Scholar]
- Pan, L. L. , Liu, X. H. , Gong, Q. H. , Yang, H. B. , & Zhu, Y. Z. (2012). Role of cystathionine gamma‐lyase/hydrogen sulfide pathway in cardiovascular disease: A novel therapeutic strategy? Antioxidants & Redox Signaling, 17, 106–118. 10.1089/ars.2011.4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, K. H. , & Park, W. J. (2015). Endothelial dysfunction: Clinical implications in cardiovascular disease and therapeutic approaches. Journal of Korean Medical Science, 30, 1213–1225. 10.3346/jkms.2015.30.9.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti, M. , & D'acquisto, F. (2009). Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nature Reviews. Immunology, 9, 62–70. 10.1038/nri2470 [DOI] [PubMed] [Google Scholar]
- Polhemus, D. J. , Li, Z. , Pattillo, C. B. , Gojon, G. Sr. , Gojon, G. Jr. , Giordano, T. , & Krum, H. (2015). A novel hydrogen sulfide prodrug, SG1002, promotes hydrogen sulfide and nitric oxide bioavailability in heart failure patients. Cardiovascular Therapeutics, 33, 216–226. 10.1111/1755-5922.12128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouokam, E. , & Althaus, M. (2016). Epithelial electrolyte transport physiology and the gasotransmitter hydrogen sulfide. Oxidative Medicine and Cellular Longevity, 2016, 4723416–4723413. 10.1155/2016/4723416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prawan, A. , Saw, C. L. , Khor, T. O. , Keum, Y. S. , Yu, S. , Hu, L. , & Kong, A. N. (2009). Anti‐NF‐κB and anti‐inflammatory activities of synthetic isothiocyanates: Effect of chemical structures and cellular signaling. Chemico‐Biological Interactions, 179, 202–211. 10.1016/j.cbi.2008.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renieris, G. , Katrini, K. , Damoulari, C. , Akinosoglou, K. , Psarrakis, C. , Kyriakopoulou, M. , … Giamarellos‐Bourboulis, E. J. (2020). Serum hydrogen sulfide and outcome association in pneumonia by the SARS‐CoV‐2 corona virus. Shock, Publish Ahead of Print 10.1097/SHK.0000000000001562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios, E. C. , Szczesny, B. , Soriano, F. G. , Olah, G. , & Szabo, C. (2015). Hydrogen sulfide attenuates cytokine production through the modulation of chromatin remodeling. International Journal of Molecular Medicine, 35, 1741–1746. 10.3892/ijmm.2015.2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, B. , Moss, C. , George, G. , Santaolalla, A. , Cope, A. , Papa, S. , & Van Hemelrijck, M. (2020). Associations between immune‐suppressive and stimulating drugs and novel COVID‐19—A systematic review of current evidence. Ecancer Medical Science, 14, 1022 10.3332/ecancer.2020.1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardu, C. , Gambardella, J. , Morelli, M. B. , Wang, X. , Marfella, R. , & Santulli, G. (2020). Hypertension, thrombosis, kidney failure, and diabetes: Is COVID‐19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. Journal of Clinical Medicine, 9 10.3390/jcm9051417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schett, G. , Sticherling, M. , & Neurath, M. F. (2020). COVID‐19: Risk for cytokine targeting in chronic inflammatory diseases? Nature Reviews. Immunology, 20, 271–272. 10.1038/s41577-020-0312-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestito, S. , Daniele, S. , Pietrobono, D. , Citi, V. , Bellusci, L. , Chiellini, G. , … Rapposelli, S. (2019). Memantine prodrug as a new agent for Alzheimer's disease. Scientific Reports, 9, 4612 10.1038/s41598-019-40925-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestito, S. , Pruccoli, L. , Runfola, M. , Citi, V. , Martelli, A. , Saccomanni, G. , … Rapposelli, S. (2019). Design and synthesis of H2S‐donor hybrids: A new treatment for Alzheimer's disease? European Journal of Medicinal Chemistry, 184, 111745 10.1016/j.ejmech.2019.111745 [DOI] [PubMed] [Google Scholar]
- Shibuya, N. , Tanaka, M. , Yoshida, M. , Ogasawara, Y. , Togawa, T. , Ishii, K. , & Kimura, H. (2009). 3‐Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxidants and Redox Signaling, 11(4), 703–714. 10.1089/ars.2008.2253 [DOI] [PubMed] [Google Scholar]
- Szabo, C. , & Papapetropoulos, A. (2017). International Union of Basic and Clinical Pharmacology. CII: Pharmacological modulation of H2S levels: H2S donors and H2S biosynthesis inhibitors. Pharmacological Reviews, 69, 497–564. 10.1124/pr.117.014050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama, K. , Hirose, A. , Suda, I. , Miyazaki, A. , Oguchi, M. , Onotogi, M. , & Fotopoulos, G. (2011). Comparison of the anti‐inflammatory and analgesic effects in rats of diclofenac‐sodium, felbinac and indomethacin patches. International Journal of Biomedical Sciences, 7, 222–229. [PMC free article] [PubMed] [Google Scholar]
- Teman, N. R. , Thomas, J. , Bryner, B. S. , Haas, C. F. , Haft, J. W. , Park, P. K. , … Napolitano, L. M. (2015). Inhaled nitric oxide to improve oxygenation for safe critical care transport of adults with severe hypoxemia. American Journal of Critical Care, 24, 110–117. 10.4037/ajcc2015570 [DOI] [PubMed] [Google Scholar]
- Testai, L. , Marino, A. , Piano, I. , Brancaleone, V. , Tomita, K. , Di Cesare, M. L. , … Calderone, V. (2016). The novel H2S‐donor 4‐carboxyphenyl isothiocyanate promotes cardioprotective effects against ischemia/reperfusion injury through activation of mitoKATP channels and reduction of oxidative stress. Pharmacological Research, 113, 290–299. 10.1016/j.phrs.2016.09.006 [DOI] [PubMed] [Google Scholar]
- Tian, M. , Wang, Y. , Lu, Y. Q. , Yan, M. , Jiang, Y. H. , & Zhao, D. Y. (2012). Correlation between serum H2S and pulmonary function in children with bronchial asthma. Molecular Medicine Reports, 6, 335–338. 10.3892/mmr.2012.904 [DOI] [PubMed] [Google Scholar]
- Tisoncik, J. R. , Korth, M. J. , Simmons, C. P. , Farrar, J. , Martin, T. R. , & Katze, M. G. (2012). Into the eye of the cytokine storm. Microbiology and Molecular Biology Reviews, 76, 16–32. 10.1128/MMBR.05015-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toohey, J. I. (2011). Sulfur signaling: Is the agent sulfide or sulfane? Analytical Biochemistry, 413, 1–7. 10.1016/j.ab.2011.01.044 [DOI] [PubMed] [Google Scholar]
- Toohey, J. I. (2012). The conversion of H2S to sulfane sulfur. Nature Reviews. Molecular Cell Biology, 13, 803author reply p 803. 10.1038/nrm3391-c2 [DOI] [PubMed] [Google Scholar]
- Wang, W. , Ye, L. , Ye, L. , Li, B. , Gao, B. , Zeng, Y. , … She, Y. (2007). Up‐regulation of IL‐6 and TNF‐alpha induced by SARS‐coronavirus spike protein in murine macrophages via NF‐kappaB pathway. Virus Research, 128, 1–8. 10.1016/j.virusres.2007.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, D. , & Yang, X. O. (2020). TH17 responses in cytokine storm of COVID‐19: An emerging target of JAK2 inhibitor fedratinib. Journal of Microbiology, Immunology, and Infection, 53, 368–370. 10.1016/j.jmii.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, W. , Zheng, S. , Dweik, R. A. , & Erzurum, S. C. (2006). Role of epithelial nitric oxide in airway viral infection. Free Radical Biology & Medicine, 41, 19–28. 10.1016/j.freeradbiomed.2006.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, G. (2020). H2S as a potential defence against COVID‐19? American Journal of Physiology. Cell Physiology, 319, C244–C249. 10.1152/ajpcell.00187.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. , Minkler, P. , Grove, D. , Wang, R. , Willard, B. , Dweik, R. , & Hine, C. (2019). Non‐enzymatic hydrogen sulfide production from cysteine in blood is catalyzed by iron and vitamin B6. Communications Biology, 2, 194 10.1038/s42003-019-0431-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, X. , Feng, F. , Xiang, Z. , & Ge, L. (2005). The effects of allitridin on the expression of transcription factors T‐bet and GATA‐3 in mice infected by murine cytomegalovirus. Journal of Medicinal Food, 8, 332–336. 10.1089/jmf.2005.8.332 [DOI] [PubMed] [Google Scholar]
- Zanardo, R. C. , Brancaleone, V. , Distrutti, E. , Fiorucci, S. , Cirino, G. , & Wallace, J. L. (2006). Hydrogen sulfide is an endogenous modulator of leukocyte‐mediated inflammation. The FASEB Journal, 20, 2118–2120. 10.1096/fj.06-6270fje [DOI] [PubMed] [Google Scholar]
- Zhang, D. , Wang, X. , Chen, S. , Chen, S. , Yu, W. , Liu, X. , … Jin, H. (2019). Endogenous hydrogen sulfide sulfhydrates IKKβ at cysteine 179 to control pulmonary artery endothelial cell inflammation. Clinical Science (London, England), 133, 2045–2059. 10.1042/CS20190514 [DOI] [PubMed] [Google Scholar]
- Zhansheng, L. , Liu, Y. , Fang, Z. , Yang, L. , Zhuang, M. , Zhang, Y. , & Lv, H. (2019). Natural sulforaphane from broccoli seeds against influenza a virus replication in MDCK cells (Vol. 14). SAGE. in Press [Google Scholar]
- Zhao, Y. , Bhushan, S. , Yang, C. , Otsuka, H. , Stein, J. D. , Pacheco, A. , … Xian, M. (2013). Controllable hydrogen sulfide donors and their activity against myocardial ischemia‐reperfusion injury. ACS Chemical Biology, 8, 1283–1290. 10.1021/cb400090d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen, H. , Fang, F. , Ye, D. Y. , Shu, S. N. , Zhou, Y. F. , Dong, Y. S. , … Li, G. (2006). Experimental study on the action of allitridin against human cytomegalovirus in vitro: Inhibitory effects on immediate‐early genes. Antiviral Research, 72, 68–74. 10.1016/j.antiviral.2006.03.017 [DOI] [PubMed] [Google Scholar]
- Zheng, Y. , Yu, B. , De La Cruz, L. K. , Roy Choudhury, M. , Anifowose, A. , & Wang, B. (2018). Toward hydrogen sulfide based therapeutics: Critical drug delivery and developability issues. Medicinal Research Reviews, 38, 57–100. 10.1002/med.21433 [DOI] [PubMed] [Google Scholar]


