Abstract
Whether kidney transplant recipients are capable of mounting an effective anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) adaptive immune response despite chronic immunosuppression is unknown and has important implications for therapy. Herein, we analyzed peripheral blood cell surface and intracellular cytokine phenotyping by flow cytometry along with serum antibody testing in 18 kidney transplant recipients with active coronavirus disease 2019 (COVID-19) infection and 36 matched, transplanted controls without COVID-19. We observed significantly fewer total lymphocytes and fewer circulating memory CD4+ and CD8+ T cells in the COVID-19 subjects. We also showed fewer anergic and senescent CD8+ T cells in COVID-19 individuals, but no differences in exhausted CD8+ T cells, nor in any of these CD4+ T cell subsets between groups. We also observed greater frequencies of activated B cells in the COVID-19 patients. Sixteen of 18 COVID-19 subjects tested for anti-SARS-CoV-2 serum antibodies showed positive immunoglobulin M or immunoglobulin G titers. Additional analyses showed no significant correlation among immune phenotypes and degrees of COVID-19 disease severity. Our findings indicate that immunosuppressed kidney transplant recipients admitted to the hospital with acute COVID-19 infection can mount SARS-CoV-2-reactive adaptive immune responses. The findings raise the possibility that empiric reductions in immunosuppressive therapy for all kidney transplant recipients with active COVID-19 may not be required.
KEYWORDS: immune regulation, immunobiology, immunosuppressant – other, immunosuppression/immune modulation, infection and infectious agents – viral, kidney transplantation/nephrology, translational research/science
Abbreviations: AKI, acute kidney injury; ALT, alanine aminotransferase; ARBs, angiotensin II receptor blockers; AST, aspartate aminotransferase; BREG, regulatory B cell; CCR-, C-C chemokine receptor-; COVID-19, coronavirus disease 2019; CPK, creatine phosphokinase; CRP, C-reactive protein; CXCR-, C-X-C chemokine receptor-; CyTOF, time-of-flight mass cytometry; ELISA, enzyme-linked immunosorbent assay; H1N, influenza A virus subtype H1N1; ICU, intensive care unit; IFN-γ, interferon γ; IgG, immunoglobulin G; IgM, immunoglobulin M; IL-, interleukin-; LAG, lymphocyte-activation gene; LDH, lactate dehydrogenase; MMF, mycophenolate mofetil; mTOR, mammalian target of rapamycin; NK cell, natural killer cell; PBMC, peripheral blood mononuclear cell; PD-1, programmed cell death-1; RAAS-blockers, renin-angiotensin-aldosterone system blockers; RT-PCR, real-time polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TFH, follicular helper T cell; TIM, T cell membrane protein; TNFα, tumor necrosis factor α; TREG, regulatory T cell
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of the clinical illness coronavirus disease 2019 (COVID-19), was first described in humans in December 2019 in Wuhan, China and has led to a global pandemic.1 Among the risk factors for poor outcome are advanced age and presence of comorbidities such as hypertension, cardiovascular disease, diabetes, lung disease, and obesity. Chronic kidney disease, tumor, and dysfunction of the immune system seem to be additional risk factors for developing complications during COVID-19.2
Immunosuppressed kidney transplant recipients are potentially at risk for poor outcome following COVID-19 infection, but relatively little has been reported in this population. Mortality across kidney transplant recipients with COVID-19 is still unclear. Although some US studies reported high mortality rates (about 20%-30%),3 , 4 a European study showed a reduced mortality in transplanted patients with COVID-19 compared to the general population (9.5% vs 12.5%, respectively).5 Because of the possibility that immunosuppression may inhibit development of protective anti-COVID-19 immunity, many centers empirically reduce antirejection immunosuppression upon hospital admission for COVID-19 infection. As immunosuppression reduction increases the risk of graft rejection, understanding whether transplant recipients can mount immune responses to COVID-19 has important implications for devising best practice strategies in these patients. In an effort to address this unmet medical need, herein we set out to analyze peripheral blood immune cell phenotypes and serum antibodies in kidney transplant recipients hospitalized with acute COVID-19 infection and compare them to those of stable controls without COVID-19.
2. METHODS
2.1. Study population
We included 18 kidney transplant recipients admitted at Mount Sinai Hospital due to active COVID-19 from March 24 to May 10, 2020. The positivity for SARS-CoV-2 was diagnosed through real-time polymerase chain reaction (RT-PCR) of nasopharyngeal swab samples. We obtained serial samples from 3 subjects collected at least 23 days apart during admission. We recorded epidemiological, clinical, and laboratory data.
As controls, we used previously biobanked samples from COVID-19 negative stable kidney transplant outpatients collected at the University Hospital in Verona, Italy (n = 8) or peripheral blood mononuclear cells from subjects (n = 28) enrolled in the ongoing multicenter Clinical Trials in Organ Transplantation (CTOT)-19 trial (NCT02495077). In CTOT-19, recipients of deceased donor kidneys with Kidney Donor Profile Index scores 20%-90% were treated with antithymocyte globulin induction and randomized to receive a single intraoperative infusion of anti-tumor necrosis factor alpha (TNFα) monoclonal antibody infliximab (Remicade, Janssen, Raritan, NJ) or saline. Maintenance immunosuppression consisted of tacrolimus, mycophenolate mofetil, and prednisone. As the 2-year follow-up is not completed for all enrollees, the study remains blinded to the perioperative intervention. However, because infliximab half-life is <2 weeks6 and samples included in this study were collected >4 months after induction, it is unlikely that this treatment affected immune phenotype of enrollees. We chose COVID-19 negative controls by matching for sex, age (±10 years), and time after transplant (<6; 6-12; >12 months) with the COVID-19 positive cases.
We collected data using an ad hoc database with the appropriate approval of the Ethics and Scientific Committees of the participating centers (institutional review board [IRB] titles/numbers: IRB-20-03454, Mount Sinai Hospital; EudraCT: 2013-004538-14, University of Verona; CTOT-19 participating sites obtained approval by IRBs at the participating sites). We obtained serum levels of interleukin-6 (IL-6), C-reactive protein (CRP), and ferritin on the day of blood collection from the clinical laboratory data at each participating center.
As additional controls, we used COVID-19 negative (n = 14) and positive (n = 16) individuals from the general population admitted at Mount Sinai Hospital, New York. Whole blood samples from these individuals were analyzed by time-of-flight mass cytometry (CyTOF).7 Table S1 includes a list of the markers we used for these analyses.
Flow cytometry analyses and anti-SARS-CoV-2 antibody measurements are detailed in Data S1.
2.2. Statistical analyses
We expressed results as mean and standard deviation or standard error unless stated otherwise. Comparison of continuous variables between groups was performed by unpaired t test and categorical variables by 2-sided chi-square or 2-sided Fisher’s exact test, where applicable. A value of P < .05 was considered as statistically significant. No correction was made for multiple testing. Statistical analysis was performed using GraphPadPrism® version 8.4.2 software package (GraphPad Software Inc, La Jolla, CA).
3. RESULTS
3.1. Patient population
Clinical characteristics of the 18 hospitalized COVID-19 positive kidney transplant recipients and 36 matched outpatient kidney transplant recipients without COVID-19 are shown in Table 1. Overall the subjects were mostly males, with a mean age of 53 years, and generally at over 1 year after transplant. Approximately half of the patients were African American (Table 1).
TABLE 1.
Patients’ characteristics
| Overall (n = 54) | Controls (n = 36) | COVID-19 (n = 18) | P | |
|---|---|---|---|---|
| Age (y) | 53.0 ± 11.0 | 51.8 ± 9.2 | 55.2 ± 14.0 | .3 |
| Sex; n (%) | 1 | |||
| Female | 24 (44.4) | 16 (44.4) | 8 (44.4) | |
| Male | 30 (55.6) | 20 (55.6) | 10 (55.6) | |
| Time after transplant; n (%) | 1 | |||
| <6 mo | 9 (16.7) | 6 (16.7) | 3 (16.7) | |
| 6-12 mo | 3 (5.6) | 2 (5.6) | 1 (5.6) | |
| >12 mo | 42 (77.7) | 28 (77.7) | 14 (77.7) | |
| Race; n (%) | .04 | |||
| White | 17 (31.5) | 14 (38.9) | 3 (16.7) | |
| Black | 24 (44.4) | 17 (47.2) | 7 (38.9) | |
| Unknown/Other | 13 (24.1) | 5 (13.9) | 8 (44.4) | |
| Ethnicity; n (%) | .04 | |||
| Hispanic or Latino | 12 (22.2) | 5 (13.9) | 7 (38.9) | |
| Not Hispanic or Latino | 42 (77.8) | 31 (86.1) | 11 (61.1) | |
| Chronic immunosuppression; n (%) | .9 | |||
| Tacrolimus | 49 (90.7) | 32 (88.9) | 17 (94.4) | |
| Prednisone | 45 (83.3) | 30 (83.3) | 15 (83.3) | |
| MMF/MPA | 42 (77.8) | 27 (75.0) | 15 (83.3) | |
| Azathioprine | 1 (1.9) | 1 (2.8) | 0 (0) | |
| Immunosuppression withdrawal or tapering; n (%) | ||||
| Tacrolimus | 1 (1.9) | na | 1 (5.6) | |
| Prednisone | 1 (1.9) | na | 1 (5.6) | |
| MMF/MPA | 5 (9.3) | na | 5 (27.8) | |
| Add-on prednisone; n (%) | 2 (3.7) | na | 2 (11.1) | |
| Days between symptoms onset and sample collection | 16.1 ± 10.1 | na | 16.1 ± 10.1 | |
| Days between admission and sample collection | 9.8 ± 6.4 | na | 9.8 ± 6.4 | |
| Laboratory at the time of blood collection | ||||
| White blood cell (×103/µL) | 7.7 ± 4.3 | 6.8 ± 3.2 | 9.5 ± 5.5 | .03 |
| Lymphocytes (%) | 14.4 ± 10.6 | 18.7 ± 10.9 | 8.4 ± 6.5 | .0009 |
| Hemoglobin (g/dL) | 11.9 ± 2.3 | 12.9 ± 1.8 | 10.1 ± 2.1 | <.0001 |
| Platelet (×103/µL) | 216.2 ± 101.1 | 216.7 ± 80.7 | 215.4 ± 134.2 | .9 |
| Serum creatinine (mg/dL) | 2.0 ± 2.2 | 1.9 ± 2.5 | 2.3 ± 1.7 | .5 |
Note: Data are average ± SD or n (%).
Abbreviations: COVID-19, coronavirus disease 2019; MMF, mycophenolate mofetil; MPA, mycophenolic acid.
Immunosuppression included triple therapy with mycophenolate mofetil (MMF) or mycophenolic acid (MPA), tacrolimus, and steroids in most of the patients (Table 1). At the time of blood collection (day 16.1 ± 10.1 after onset of symptoms) for the COVID-19 subjects, the practicing physicians had withdrawn MMF/MPA in 5 cases (1 patient withdrew all immunosuppressive agents), and in the remaining 13 MMF/MPA was continued at reduced doses. Two patients not receiving steroids as part of their antirejection therapy had introduced steroids (Table 1). Graft function was slightly impaired in both groups (Table 1). CRP, D-dimer, and ferritin levels were all above normal ranges in COVID-19 patients ( Table 2). Analysis of serum IL-6 levels (a proinflammatory mediator) in the COVID-19 positive subjects revealed median values of 237.5 pg/mL (95% confidence interval [CI]: 55.3-422.7) (Table 2) that are remarkably higher than those reported by others in stable, noninfected, kidney transplant recipients (<5 pg/mL).8
TABLE 2.
Characteristics of COVID-19 positive patients stratified by disease severity
| COVID-19 disease severity grade | P | |||
|---|---|---|---|---|
| 3 - 4 (n = 7) | 5 (n = 6) | 6 (n = 5) | ||
| Age (y) | 49.7 ± 18.3 | 53.0 ± 10.4 | 65.6 ± 2.9 | .1 |
| Sex; n (%) | .5 | |||
| Female | 2 (28.6) | 3 (50) | 3 (60) | |
| Male | 5 (71.4) | 3 (50) | 2 (40) | |
| Medical history, n (%) | ||||
| Heart disease | 1 (14.3) | 1 (16.7) | 1 (20) | .9 |
| Hypertension | 7 (100) | 5 (83.3) | 5 (100) | .3 |
| Cancer | 0 (0) | 2 (33.3) | 2 (40) | .2 |
| Lung disease | 2 (28.6) | 1 (16.7) | 0 (0) | .4 |
| HIV/AIDS | 0 (0) | 2 (33.3) | 1 (20) | .3 |
| Obesity | 4 (57.1) | 2 (33.3) | 0 (0) | .1 |
| Symptoms, n (%) | ||||
| Fever | 5 (71.4) | 6 (100) | 4 (80) | .4 |
| Dyspnea | 5 (71.4) | 4 (66.7) | 5 (100) | .4 |
| Diarrhea | 2 (28.6) | 5 (83.3) | 0 (0) | .01 |
| Myalgia | 5 (71.4) | 5 (83.3) | 3 (60) | .7 |
| Signs, n (%) | ||||
| Respiratory rate ≥24/min | 0 (0) | 5 (83.3) | 5 (100) | .0007 |
| Heart rate >100 beats/min | 2 (28.6) | 3 (50) | 2 (40) | .7 |
| Complications, n (%) | ||||
| Acute kidney injury | 5 (71.4) | 6 (100) | 5 (100) | .2 |
| Admission to ICU | 1 (14.3) | 5 (83.3) | 5 (100) | .004 |
| Laboratory | ||||
| White blood cell (×103/µL) | 5.1 ± 2.0 | 11.4 ± 5.6 | 13.3 ± 5.2 | .01 |
| Neutrophils (%) | 76.9 ± 8.8 | 76.9 ± 27.4 | 88.8 ± 7.9 | .4 |
| Lymphocytes (%) | 12.7 ± 6.0 | 6.6 ± 6.2 | 4.6 ± 4.7 | .06 |
| Neutrophils/lymphocytes | 8.3 ± 6.9 | 33.5 ± 31.7 | 45.2 ± 34.3 | .06 |
| Hemoglobin (g/dL) | 11.0 ± 2.3 | 9.8 ± 2.4 | 9.3 ± 0.7 | .4 |
| Platelet (×103/µL) | 245.1 ± 150.1 | 237.2 ± 144.6 | 147.6 ± 92.6 | .4 |
| C-reactive protein (mg/L) | 83.8 ± 60.5 | 137.7 ± 109.0 | 138.4 ± 99.9 | .5 |
| Procalcitonin (ng/mL) | 0.2 ± 0.2 | 2.8 ± 5.3 | 0.5 ± 0.2 | .3 |
| Ferritin (ng/mL) | 2263.4 ± 1982.4 | 3087.8 ± 2785.1 | 2015.2 ± 2706.9 | .7 |
| D-dimer (µg/mL) | 1.8 ± 1.0 | 9.9 ± 8.5 | 6.3 ± 6.6 | .08 |
| IL-6 (pg/mL) | 34.7 ± 25.1 | 94.3 ± 51.1 | 652.6 ± 943.6 | .1 |
| Creatine phosphokinase (U/L) | 120.0 ± 133.2 | 185.7 ± 214.4 | 62.5 ± 26.4 | .5 |
| AST (U/L) | 36.9 ± 19.5 | 36.2 ± 27.3 | 30.8 ± 17.8 | .9 |
| ALT (U/L) | 35.0 ± 30.2 | 33.3 ± 42.0 | 37.4 ± 35.6 | .9 |
| LDH (U/L) | 311.7 ± 100.0 | 602.2 ± 280.8 | 426.2 ± 160.0 | .05 |
Note: According to a meta-analysis on 1035 renal transplant recipients, mean IL-6 value of in kidney transplant recipients is 3.25 pg/mL (95% CI: 2.17, 4.32).14 Acute kidney injury is defined by Acute Kidney Injury Network (AKIN) criteria. Disease severity score: (1) not hospitalized with resumption of normal activities; (2) not hospitalized but unable to resume normal activities; (3) hospitalized not requiring supplemental oxygen; (4) hospitalized, requiring supplemental oxygen; (5) hospitalized, requiring nasal high flow oxygen therapy, noninvasive mechanical ventilation or both; (6) hospitalized, requiring extracorporeal membrane oxygenation (ECMO), invasive mechanical ventilation or both; (7) death.12
Abbreviations: AIDS, acquired immune deficiency syndrome; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; COVID-19, coronavirus disease 2019; HIV, human immunodeficiency virus; ICU, intensive care unit; IL-6, interleukin-6; LDH, lactate dehydrogenase. Ref range: C reactive protein 0.0-5.0 mg/L; D-dimer 0.00-0.50 µg/mL; ferritin 30-400 ng/mL.
Over a median (interquartile range [IQR]) follow-up of 86.5 days (IQR: 81.5-97.8) since admission, 7 COVID-19 patients died and 11 were discharged. No patients developed biopsy-proven acute rejection during this period, but 16 patients showed acute renal graft injury (Table 2).
3.2. Naïve, effector, memory, dysfunctional, and regulatory CD4+ T cell subsets
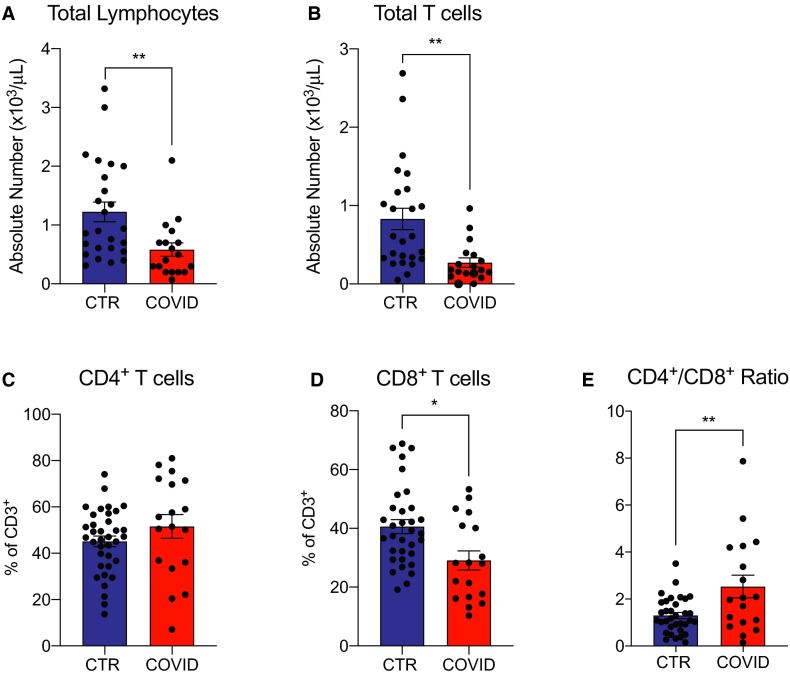
Flow cytometric analysis showed significantly fewer lymphocytes and total T cells in the COVID-19 kidney transplant recipients vs controls (Table 1; Figure 1A,B), similar to what has been reported by others in COVID-19 positive patients in the general population.1 , 9 Within the T cell population, we observed lower percentages of CD8+ T cells (Figure 1D), and higher CD4+/CD8+ T cell ratio (Figure 1E) in the COVID-19 group, whereas percentages of CD4 + T cells did not differ between the 2 groups (Figure 1C; Figure S1A).
FIGURE 1.
Total lymphocytes and T cells in coronavirus disease 2019 (COVID-19) transplant patients and in COVID-19 negative controls. A,B, Total lymphocytes and CD3+ percentage of acquired cells. C, D, CD4+ and CD8+ cells percentage of CD3+ and E, calculated CD4+/CD8+ ratio. For 20 control patients, lymphocyte and total T cell absolute numbers were not available. Data are represented as mean and standard error of the mean (SEM). Each dot represents an individual value. *P < .05; **P < .01; ***P < .001. CTR, controls [Color figure can be viewed at wileyonlinelibrary.com]
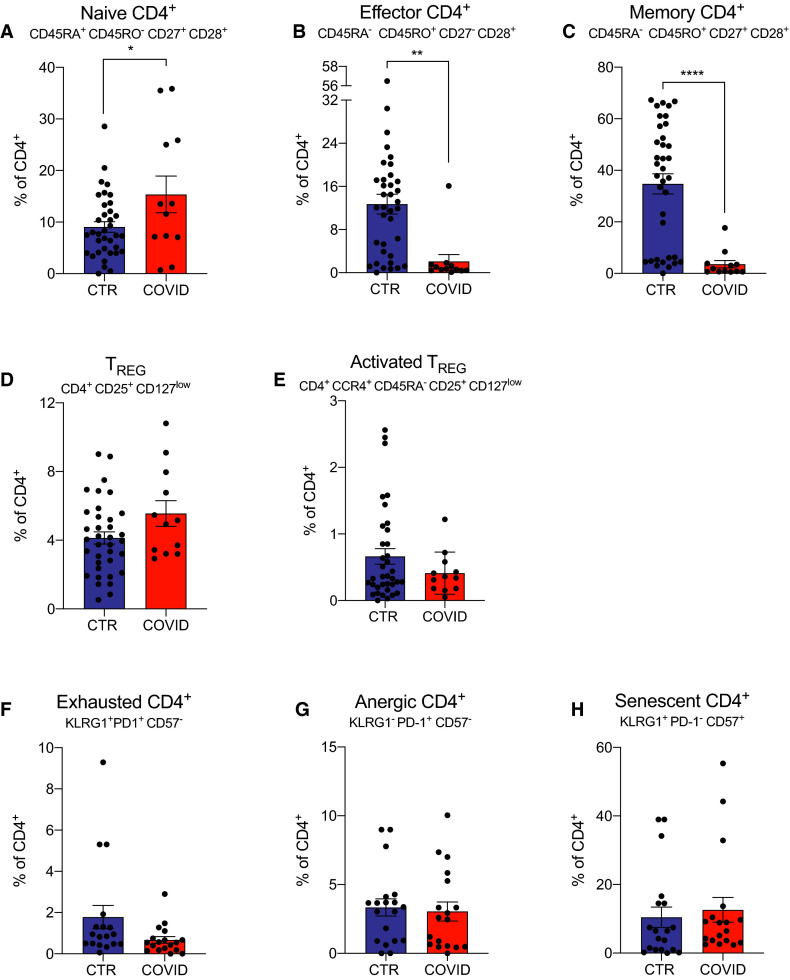
To assess effects on CD4+ T cell subsets we quantified naïve (CD45RA+CD45RO−CD27+CD28+), effector (CD45RA−CD45RO+CD27−CD28+), and memory (CD45RA−CD45RO+CD27+CD28+) CD4+ T cells in each COVID-19 positive and control subject by flow cytometry (gating strategy Figure S1B). These analyses revealed significantly more naïve and fewer effector and memory CD4+ T cells in COVID-19 patients compared to controls ( Figure 2A-C).
FIGURE 2.
CD4+ T cells and cytokines in coronavirus disease 2019 (COVID-19) transplant patients and in COVID-19 negative controls. A-C, Naïve, effector, and memory CD4+ cells percentage of total CD4+ T cells. D,E, Regulatory T cells (TREG) and activated TREG percentage of CD4+. F-H, Dysfunctional CD4+ T cell percentages of total CD4+ T cells. We did not have enough cells to perform naïve, effector, memory, and TREG staining for 6 COVID-19 patients. For 17 control patients, staining was not done for exhaustion, anergic, or senescent cells. Data are represented as mean and standard error of the mean (SEM). Each dot represents an individual value. *P < .05; ** P < .01; ***P < .001. CTR, controls; TREG, regulatory T cells [Color figure can be viewed at wileyonlinelibrary.com]
We did not detect differences in frequencies of CD4+CD25+CD127low regulatory T cells (TREG) or CD4+CCR4+CD45RA−CD25+CD127low activated TREG 10 between COVID-19 patients and controls (Figure 2D,E; Figure S1C).
Previous work by others studying nontransplanted COVID-19 patients with severe disease showed the emergence of dysfunctional (exhausted, anergic, or senescent) T cells that could negatively affect outcomes.11 When we quantified CD4+ T cells with phenotypic characteristics of exhaustion (KLRG1+PD-1+CD57−), anergy (KLRG1−PD-1+CD57−), or senescence (KLRG1+PD-1−CD57+) we did not detect differences between groups (Figure 2F-H; Figure S1D).
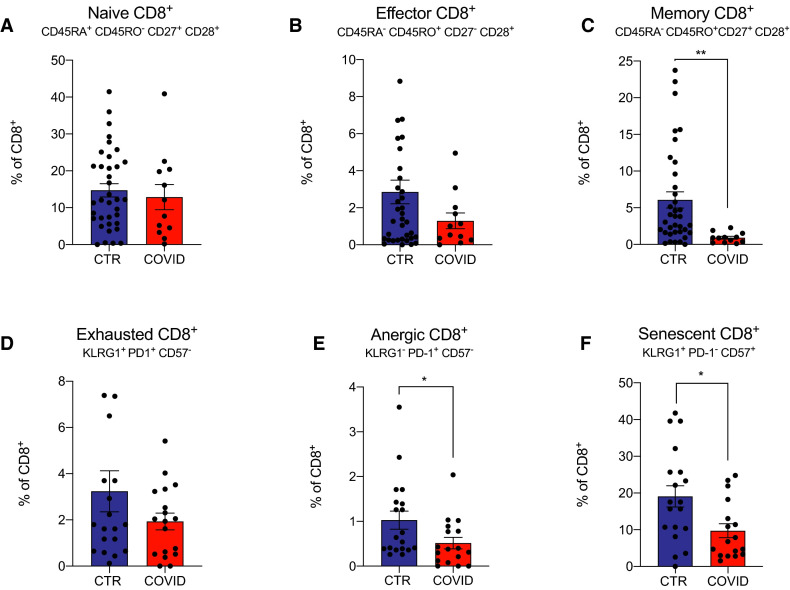
3.3. Naïve, effector, memory, and dysfunctional CD8+ T cells
Analysis of CD8+ T cell subsets similarly revealed no differences in naïve or effector subsets between groups ( Figure 3A,B), although we observed fewer circulating memory CD8+ T cells in the COVID-19 subjects (Figure 3C). Although we did not observe differences in phenotypically exhausted CD8+ T cells between COVID-19 and control subjects (Figure 3D), our analyses interestingly showed fewer anergic and senescent CD8+ T cells in COVID-19 enrollee (Figure 3E,F).
FIGURE 3.
CD8+ T cells in coronavirus disease 2019 (COVID-19) transplant patients and in COVID-19 negative controls. A-C, Naïve, effector, and memory CD8+ cells percentage of total CD8+ T cells. We did not have enough cells to perform staining for 6 COVID-19 patients. D-F, Dysfunctional CD8+ cells percentage of total CD8+ T cells. For 17 control patients, staining was not done for exhaustion, anergic, or senescent cells. Data are represented as mean and standard error of the mean (SEM). Each dot represents an individual value. *P < .05; ** P < .01; ***P < .001. CTR, controls [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Evidence of enhance humoral immune activation in COVID-19-infected transplant recipients
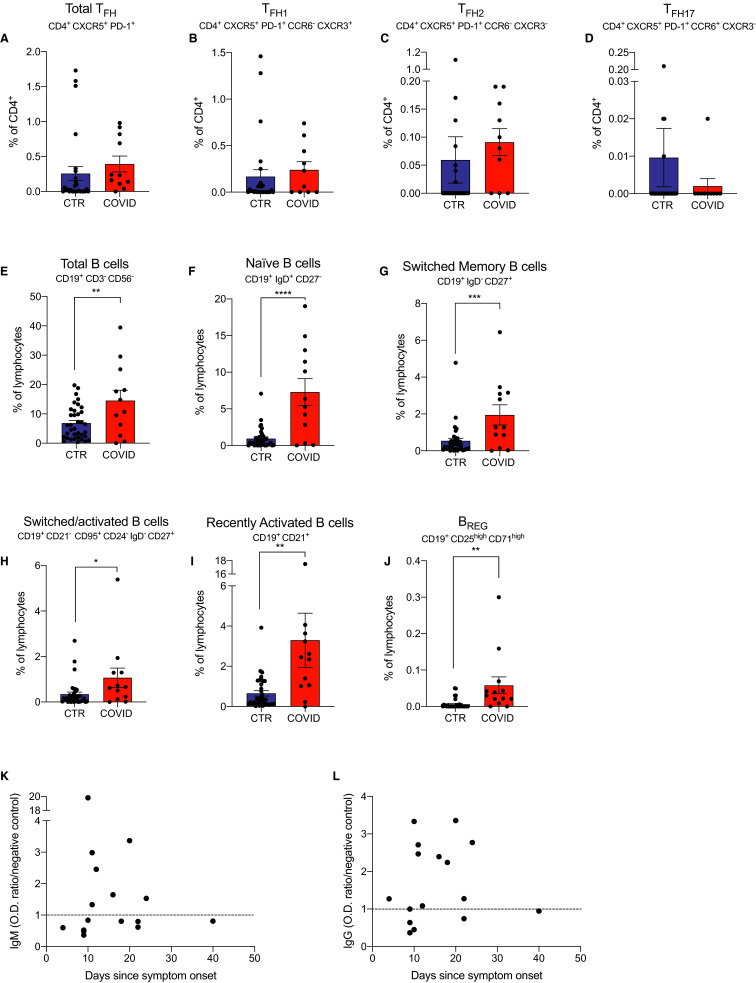
Follicular helper T cells (TFH) promote class switch recombination and affinity maturation in germinal centers,12 are essential for rapid immunoglobulin G (IgG) antibody production in response to Ebola virus13 , 14 and influenza virus14 and are likely needed for development of effective anti-COVID-19 antibody responses.
When we quantified peripheral blood CD4+CXCR5+PD-1+ TFH by flow cytometry (gating strategy Figure S3), we did not observe significant differences between the 2 groups ( Figure 4A). We also did not observe significant differences in the frequencies of CD4+CXCR5+PD-1+CCR6−CXCR3+ TFH1 cells (implicated as crucial in antiviral antibody responses15), CD4+CXCR5+PD-1+CCR6−CXCR3− TFH2 cells (important for maturation of B cells into IgG4-secreting cells16), nor in the CD4+CXCR5+PD-1+CCR6+CXCR3− TFH17 cells17 between groups (Figure 4B-D).
FIGURE 4.
TFH and B cells subsets and anti-SARS-CoV-2 IgG and IgM antibody levels in coronavirus disease 2019 (COVID-19) transplant patients and in COVID-19 negative controls. A-D, Total TFH and TFH subsets percentage of CD4+; E-J, B cell subsets that differ significantly between groups. We did not have enough cells to perform TFH staining for 6 COVID-19 patients. For 2 control patients, staining was not done for Total TFH and TFH subsets. Data are represented as mean and standard error of the mean (SEM). Each dot represents an individual value. *P < .05; **P < .01; ***P < .001. K, Anti-SARS-CoV-2 IgM and L, IgG at various time-points after symptoms onset. Each dot represents an individual value. Dotted line represents optical density (OD) ratio to negative control. *P < .05. CTR, controls; IgG, immunoglobulin G; IgM, immunoglobulin M; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TFH, follicular helper T cell [Color figure can be viewed at wileyonlinelibrary.com]
Analysis of peripheral blood B cells (gating strategy in Figure S4) showed higher frequencies of total B cells and all B cell subsets (including transitional [IgD+CD27−CD19+], switched memory [IgD−CD27+CD19+], switched/activated [CD21−CD95+CD24−IgD−CD27+CD19+], recently activated [CD19+CD21+], and regulatory B cells [BREG] [CD25highCD71highCD19+]) in COVID-19 subjects compared to controls (Figure 4E-J). Analysis of percentages of peripheral blood plasma cells (CD19+CD38highCD27highCD138+) and plasmablasts (CD19+CD38highCD27highCD138−) showed no differences between groups (data not shown).
3.5. Anti-SARS-CoV-2 antibodies
To assess whether these immunosuppressed kidney transplant recipients could mount an antibody response to SARS-CoV-2, we measured specific immunoglobulin M (IgM) and IgG antibodies by ELISA in 16 patients with available sera. When we plotted anti-SARS-CoV-2 IgM and IgG over time after symptoms, we noticed that most patients developed antivirus antibodies starting 10 days after the symptom onset (Figure 4K-L).
We did not observe significant differences in the incidence of anti-SARS-CoV-2 (60.0% vs 36.4%, respectively; P: .6) or IgG (60.0% vs 63.6%; P > .9) in subjects who underwent MMF/MPA withdrawal vs those who maintained MMF/MPA.
3.6. Immune changes according to disease severity and time after symptom onset
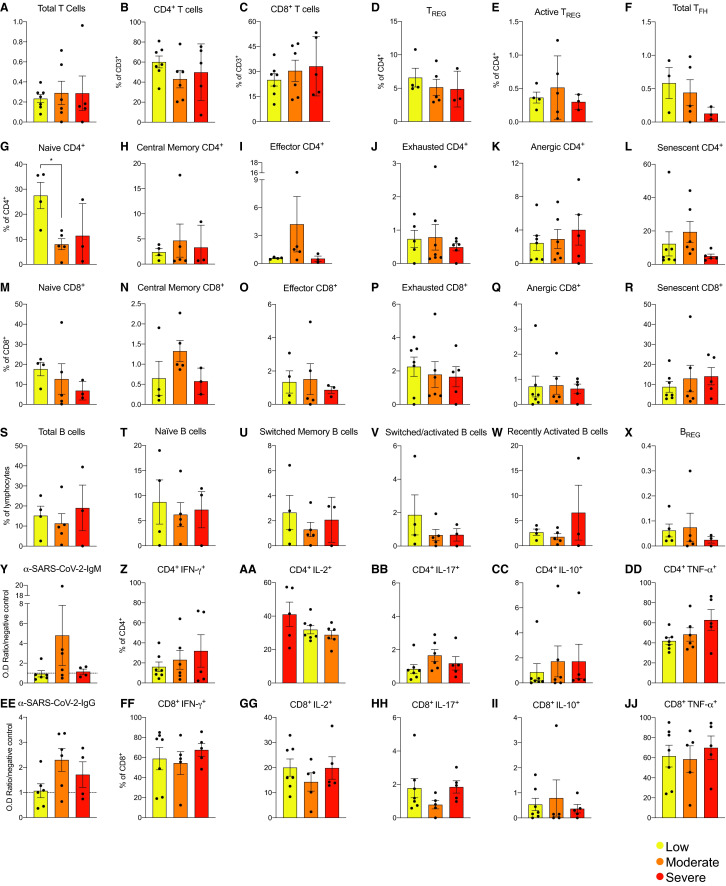
To test whether immune phenotypes associate with disease severity of disease (Data S1), we stratified patients into 3 groups, low (score 3-4), intermediate (score = 5), and high risk (score ≥6),18 and we compared the percentages of each T and B cell subset and anti-SARS-CoV-2 IgM and IgG across patients in the 3 categories (Table 2), noting that IL-6, a marker of inflammation, was highest in patients with most severe disease (Table 2).
We did not detect significant differences across the 3 groups for any of the analyzed T and B cell subsets ( Figure 5A-X), nor for anti-SARS-CoV-2 antibody levels (Figure 5Y,Z). Analysis of mitogen-induced cytokine production showed no significant differences in IL-2, IL-10, IL-17, interferon-gamma (IFN-γ), and TNFα in CD4+ (Figure 5AA-JJ) and CD8+ (Figure 5C) T cells.
FIGURE 5.
Cell subsets and anti-SARS-CoV-2 antibody levels according to coronavirus disease 2019 (COVID-19) severity. T and B cell subsets and anti-SARS-CoV-2 IgM and IgG levels in COVID-19 patients stratified in 3 groups according to disease severity at the time of sampling: 3 to 4, low (n = 7); 5, moderate (n = 6); and 6 to 7, severe (n = 5) . We defined COVID-19 severity using a scale from 1 (not hospitalized and resumed normal activities) to 7 (death).18 Data are represented as mean and standard error of the mean (SEM). Each dot represents an individual value. *P < .05. Average of control samples is shown by dotted horizontal line on bar charts. No subsets are significantly different between groups. IgG, immunoglobulin G; IgM, immunoglobulin M; IL, interleukin; IFN-γ, interferon γ; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNFα, tumor necrosis factor α [Color figure can be viewed at wileyonlinelibrary.com]
Notably, the PD1+ T cell subsets did not produce cytokines, verifying their dysfunctional status (Figure S2). Altogether, these data indicate that the immune changes in COVID-19 patients were independent of disease severity, at least in hospitalized patients.
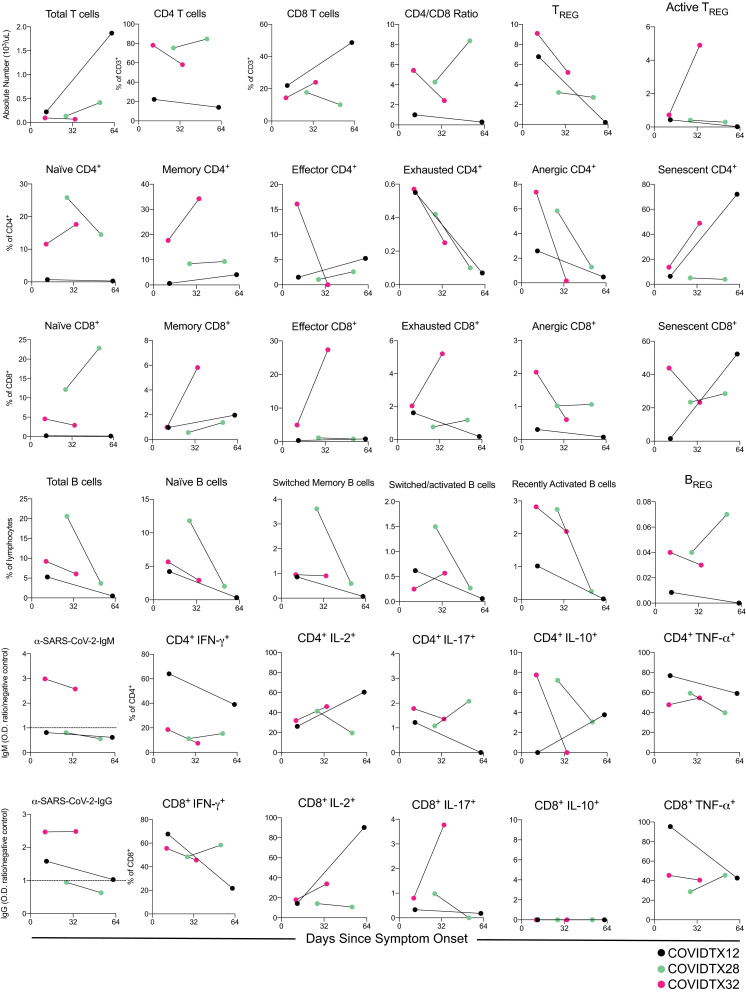
To assess the variations in COVID-19 immune response over time, we analyzed changes in the immune phenotype in serial samples from 3 COVID-19 kidney transplant recipients. Overall, data from these 3 patients suggest a trend toward lower percentages of circulating regulatory T cells (Tregs), exhausted T cells, B cell subsets, and anti-SARS-CoV-2 IgG levels. Interestingly, the absolute numbers of circulating T cells increased in the 2 patients who were successfully discharged, whereas they remained low in the patient who died (COVIDTX32) ( Figure 6).
FIGURE 6.
Serial cell subsets and anti-SARS-CoV-2 antibody levels in 3 coronavirus disease 2019 (COVID-19) patients. T and B cell subsets and anti-SARS-CoV-2 IgM and IgG levels at 2 serial time points in 3 COVID-19 patients. Patients COVIDTX12 and COVIDTX28 were discharged at 47 and 45 d since symptom onset, respectively, while patient COVIDTX32 died on day 32. IgG, immunoglobulin G; IgM, immunoglobulin M; IL, interleukin; IFN-γ, interferon γ; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNFα, tumor necrosis factor α; TREG, regulatory T cells [Color figure can be viewed at wileyonlinelibrary.com]
3.7. Immune changes in COVID-19 negative and positive individuals from the general population
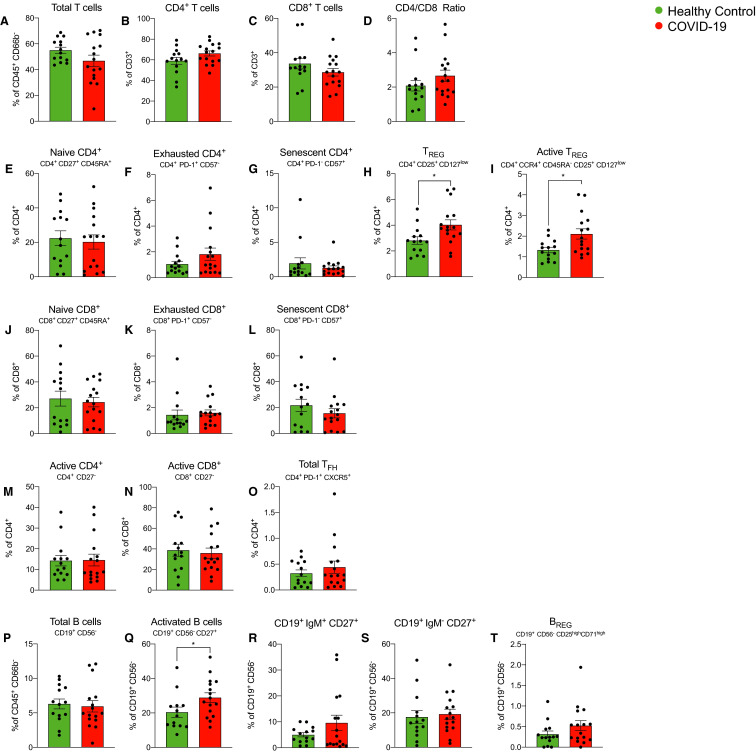
Finally, we tested whether the described immune changes associated with COVID-19 infection were unique to kidney transplant recipients or are similarly present in infected individuals in the general (nontransplanted) population. To address this issue, we analyzed samples from 16 individuals admitted for COVID-19 at Mount Sinai Hospital and 14 healthy controls (Table S3). These samples were analyzed by CyTOF (as part of a concerted multi-PI effort within the institution), a technology that generates data that correlates well with data obtained by flow cytometry.19
Similar to findings in kidney transplant recipients, we observed that COVID-19 individuals in the general population showed higher frequencies of active B cells (CD19+CD27+) compared to healthy controls ( Figure 7). At variance from transplant recipients, individuals from the general population with COVID-19 showed increased Tregs (Figure 7).
FIGURE 7.
Cell subsets in coronavirus disease 2019 (COVID-19) positive nontransplanted individual and in healthy controls. Time-of-flight mass cytometry (CyTOF) analyses of T and B cell subsets in COVID-19 patients stratified in positive nontransplanted individuals and in healthy controls. Data are represented as mean and standard error of the mean (SEM). Each dot represents an individual value. *P < .05. IgM, immunoglobulin M; TREG, regulatory T cells [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Our unique analysis of peripheral blood immune phenotypes in a cohort of COVID-19 infected kidney transplant recipients provides evidence that these individuals are capable of developing an adaptive immune response to SARS-CoV-2 despite immunosuppression to prevent graft rejection. We observed higher serum IL-6, broad activation of B cell subsets, and detectable serum anti-SARS-CoV-2 IgM and IgG as early as 10 days after the onset of clinical symptoms in the majority of COVID-19 kidney transplant recipients, findings similar to those reported by others in COVID-19-infected but nontransplanted subjects.20 We did not detect differences in circulating plasma cells or plasmablasts in our subjects. As the majority of antibody secreting cells are sequestered in the spleen or bone marrow,21 it is not surprising that similar frequencies of these cells are detected in the circulation of transplanted subjects with and without COVID-19 infection. In a separate cohort of nontransplant patients with COVID-19, we also found a significant increase in active B cells, indicating that immune changes observed in transplant recipients on chronic immunosuppression are similar to those observed in the general population.
Although MMF/MPA was withdrawn in 5/18 subjects at the time of hospitalization (Table 1), these changes did not appear to have an impact on the development of SARS-CoV-2-specific antibodies, but longer follow-up and additional analyses will be required to determine whether the subjects have truly developed protective, memory immunity.
The COVID-19 transplant patients in our study developed lymphopenia, similar to data reported by others in nontransplanted patients.1 Progressive lymphodepletion has been associated with clinical deterioration COVID-19 infection, whereas recovery of lymphocyte counts tended to directly precede clinical recovery.22 The cross-sectional nature of our study prevents to formally test whether the same trends occur also in kidney transplant recipients. However, the inverse relationship between disease severity and lymphocyte numbers (Table 2) suggests that a similar phenomenon occurs in transplant recipients. Intriguingly, in the 3 patients with serial samples analyzed in the present study, progressive lymphocyte decline was observed only in the patient with fatal outcome.
We found fewer memory CD8+ T cells in COVID-19 kidney transplant recipients compared to COVID-19 negative controls. Consistent with experimental data in mice infected with influenza,23 it is possible that antiviral CD8+ T cells are recruited into the lungs and other sites of SARS-CoV-2 infection. Our data indicate that kidney transplant recipients have fewer CD4+ and CD8+ IL-17+ T cells in the circulation. Similar to CD8+ memory T cells, it is possible that these cells are recruited in peripheral tissues. IL-17 has been implicated in the pathogenesis of COVID-19 also in nontransplanted patients.24 The fact that IFN-γ, IL-2, and TNF-α CD4+ and CD8+ T cells did not differ between COVID-19 positive and negative patients speaks against a skew in T cell polarization, but more studies are needed to better understand this mechanism.
We interestingly found no evidence that COVID-19 promotes T cell dysfunction in our study subjects. Exhaustion and other forms of T cell dysfunction are commonly observed in chronic viral infections and can be detrimental for viral clearance.25 Patients in our study developed disease ~2 weeks prior to blood collection, leaving a limited amount of time for the development of exhaustion. Quite surprisingly, others have found signs of T cell exhaustion in early phases of COVID-19 infection and suggested a causal link between T cell exhaustion and progression.26 Based on these findings,26 we stained CD4+ and CD8+ T cells for TIM3 and LAG3, additional markers of T cell exhaustion, but we did not detect any association between disease severity and CD4+ or CD8+ PD1+TIM3+ or PD1+LAG3+ T cells (not shown). It is tempting to speculate that immunosuppressive therapy, while allowing the formation of an adaptive immune response, prevents/delays the onset of T cell dysfunction, but more studies are needed to formally test this hypothesis.
In kidney transplant recipients, we found that, similar to the general population,1 COVID-19 was associated with a significant increase in inflammatory markers, including IL-6, CRP, ferritin, and D-dimer. Importantly the ratio between neutrophils and lymphocytes, a major risk factor for mortality in the general population,27 was also significantly higher in more severe cases of COVID-19 (Table 2). Altogether, these data indicate that COVID-19 is associated with a significant inflammatory response also in kidney transplant recipients, despite ongoing chronic immunosuppression.
Intriguingly, we found a significant increase in circulating Tregs in COVID-19 individuals from the general population that was not present in kidney transplant recipients. Others have shown a similar increase in Tregs in COVID-19 subjects from the general population.28 Lack of Treg expansion in kidney transplant recipients may be owing to the use of calcineurin inhibitors that prevent Treg induction,29 but more studies are needed to test this hypothesis. Others have shown that SARS-Cov-2-specific cells represent less than 1% of the total CD4+ and CD8+ T cells.30 Therefore, it is likely that the changes in frequencies of circulating lymphocytes that we report are in fact not driven mainly by SARS-CoV-2 specific cells but rather part of an overall activation of the adaptive immune response.
In summary, our findings indicate that stable kidney transplant recipients on maintenance calcineurin inhibitor-based immunosuppression are capable of developing immune responses to SARS-CoV-2 that at least early on are similar to those observed in the general population. Importantly, immunosuppression reduction/withdrawal was almost invariably associated with graft impairment, although no patients performed a biopsy to document the presence of acute rejection. Nonetheless, results of controlled prospective trials will be required to definitively address this clinically significant treatment decision.
ACKNOWLEDGMENTS
The authors sincerely thank the CTOT-19 site investigators and staff for their efforts, including D Brennan (Johns Hopkins, Baltimore, MD), J Bromberg (U Maryland, Baltimore, MD), S Bunnapradist (U of California at Los Angeles, Los Angeles, CA), Sindhu Chandra and Flavio Vincenti (U. California at San Francisco, San Francisco, CA), D Foley (U Wisconsin, Madison, WI), R Formica (Yale University, New Haven, CT), I Gibson, P Nickerson, D. Rush (U Manitoba, Winnipeg, Manitoba, Canada), D Hricik (University Hospitals Cleveland Medical Center, Cleveland, OH), R Mannon (U Alabama at Birmingham, Birmingham, AL), M C Menon (Mount Sinai, New York, NY), J Kim and K Tinckam (U Toronto, Toronto, Canada), K Newell (Emory U, Atlanta, GA), E Poggio (Cleveland Clinic, Cleveland, OH), M Samaniego and R Sung (U Michigan, Ann Arbor, MI).
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding information National Institute of Allergy and Infectious Diseases, Grant/Award Number: R01 AI132949-02
Footnotes
Susan Hartzell and Sofia Bin contributed equally to this manuscript.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W-J, Ni Z-Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cravedi P, Suraj SM, Azzi Y, et al. COVID-19 and kidney Transplantation: results from the TANGO International Transplant Consortium [published online ahead of print July 10, 2020]. Am J Transplant. 10.1111/ajt.16185 [DOI] [PMC free article] [PubMed]
- 5.Tschopp J, L’Huillier AG, Mombelli M, et al. First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study [published online ahead of print June 9, 2020]. Am J Transplant. 10.1111/ajt.16062 [DOI] [PMC free article] [PubMed]
- 6.Klotz U, Teml A, Schwab M. Clinical pharmacokinetics and use of infliximab. Clin Pharmacokinet. 2007;46(8):645–660. doi: 10.2165/00003088-200746080-00002. [DOI] [PubMed] [Google Scholar]
- 7.Fribourg M, Anderson L, Fischman C, et al. T-cell exhaustion correlates with improved outcomes in kidney transplant recipients. Kidney Int. 2019;96(2):436–449. doi: 10.1016/j.kint.2019.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omrani H, Jasemi SV, Sadeghi M, Golmohamadi S. Evaluation of serum interleukin-6 levels in the renal transplant recipients: a systematic review and meta-analysis of case-control studies. Open Access Maced J Med Sci. 2019;7(1):174–178. doi: 10.3889/oamjms.2018.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan LI, Wang QI, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood. 2012;119(19):4430–4440. doi: 10.1182/blood-2011-11-392324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29(1):621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 13.McElroy AK, Akondy RS, Davis CW, et al. Human Ebola virus infection results in substantial immune activation. Proc Natl Acad Sci USA. 2015;112(15):4719–4724. doi: 10.1073/pnas.1502619112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellebedy AH, Jackson KJL, Kissick HT, et al. Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat Immunol. 2016;17(10):1226–1234. doi: 10.1038/ni.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baiyegunhi O, Ndlovu B, Ogunshola F, et al. Frequencies of circulating Th1-biased T follicular helper cells in acute HIV-1 infection correlate with the development of HIV-specific antibody responses and lower set point viral load. J Virol. 2018;92(15) doi: 10.1128/JVI.00659-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akiyama M, Suzuki K, Yasuoka H, Kaneko Y, Yamaoka K, Takeuchi T. Follicular helper T cells in the pathogenesis of IgG4-related disease. Rheumatology. 2018;57(2):236–245. doi: 10.1093/rheumatology/kex171. [DOI] [PubMed] [Google Scholar]
- 17.Li X-Y, Wu Z-B, Ding J, et al. Role of the frequency of blood CD4+ CXCR5+ CCR6+ T cells in autoimmunity in patients with Sjögren’s syndrome. Biochem Biophys Res Commun. 2012;422(2):238–244. doi: 10.1016/j.bbrc.2012.04.133. [DOI] [PubMed] [Google Scholar]
- 18.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spitzer MH, Nolan GP. Mass cytometry: single cells, many features. Cell. 2016;165(4):780–791. doi: 10.1016/j.cell.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long Q-X, Liu B-Z, Deng H-J, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 21.Savage HP, Baumgarth N. Characteristics of natural antibody-secreting cells: Natural antibody secretion. Ann N Y Acad Sci. 2015;1362(1):132–142. doi: 10.1111/nyas.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Ling J, Mo P, et al. Restoration of leukomonocyte counts is associated with viral clearance in COVID-19 hospitalized patients [published online January 1, 2020]. medRxiv. 2020. 2020.03.03.20030437. 10.1101/2020.03.03.20030437 [DOI]
- 23.Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8(6):683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 24.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53(3):368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Du X, Chen J, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81(1):e6–e12. doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W, Su B, Pang L, et al. High-dimensional immune profiling by mass cytometry revealed immunosuppression and dysfunction of immunity in COVID-19 patients. Cell Mol Immunol. 2020;17(6):650–652. doi: 10.1038/s41423-020-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeiser R, Nguyen VH, Beilhack A, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108(1):390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501. doi: 10.1016/j.cell.2020.05.015. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.