Abstract
Glioma is one of the most common types of tumor of the central nervous system. Due to the aggressiveness and invasiveness of high-level gliomas, the survival time of patients with these tumors is short, at ~15 months, even after combined treatment with surgery, radiotherapy and/or chemotherapy. Recently, a number of studies have demonstrated that long non-coding RNA (lncRNAs) serve crucial roles in the multistep development of human gliomas. Gliomas acquire numerous biological abilities during multistep development that collectively constitute the hallmarks of glioma. Thus, in this review, the roles of lncRNAs associated with glioma hallmarks and the current and future prospects for their development are summarized.
Keywords: glioma, long non-coding RNA, hallmark, glioma stem cells, glioma endothelial cell
1. Introduction
Hallmarks of glioma
The eight hallmarks of cancer are also hallmarks of gliomas (1) and are as follows: i) Maintenance of growth signals; ii) resistance to growth inhibition signals; iii) resistance to apoptosis; iv) ability to replicate indefinitely; v) angiogenesis; vi) invasion; vii) reprogramming of energy metabolism; and viii) evading immune cell attack (1,2). In addition to these eight hallmarks, the tumor microenvironment (TME) is another feature of gliomas (1–3). The composition and role of the TME have been extensively studied, and it has been demonstrated that the TME serves an important role when tumors acquire the aforementioned hallmarks during tumor progression (1–3). The blood-brain barrier (BBB) provides the brain with immune resistance through physical protection (3,4). Although gliomas usually change the basal lamina of their internal blood vessels and destroy the endothelial barrier, which increases endothelial permeability through the high expression of vascular endothelial growth factor (VEGF) (5–8), the BBB serves a specific role in immune resistance in the early stage of glioma (5–8); thus, the TME in gliomas is different from that in other parts of the body.
Functions of lncRNAs in cancer
Only ~2% of the genes in the human genome encode proteins, whereas the remainder constitute non-coding RNAs (ncRNAs) (9,10). ncRNAs can be divided into two types: Long ncRNAs (lncRNAs, >200 nt) and short nc RNAs (<200 nt) (11). lncRNAs account for ~80% of all ncRNAs (12) and can be divided into three categories according to their function: i) Functional lncRNAs, which function through their own sequences; ii) non-functional lncRNAs; and iii) lncRNAs that affect transcription independently of their sequence (13,14). The human genome encodes ~28,000 distinct lncRNAs, a number of which remain to be explored (15). lncRNAs have been demonstrated to serve important roles in the development of cancer, as the expression of a number of lncRNAs is abnormal in various cancer types (16–18). lncRNAs can be involved in cell cycle changes, escape from apoptosis and invasion, and can cause various types of cancer, such as glioma, and lung, liver, breast, colorectal, ovarian and prostate cancer (12,19–22). In addition, maternally expressed 3 (MEG3), a class of tumor suppressor lncRNA, was the first lncRNA group discovered to possess tumor suppressor functions (22). In conclusion, the roles of lncRNAs in cancer are complex and diverse.
2. Roles of lncRNAs in the hallmarks of glioma
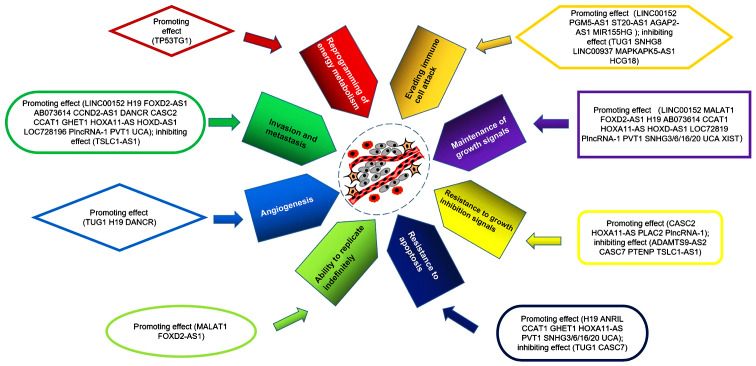
Increasing numbers of studies have demonstrated that lncRNAs serve important roles in the hallmarks of glioma (23–25). lncRNAs promote or inhibit the development of glioma through a variety of pathways; lncRNAs serve a number of roles, and multiple lncRNAs are involved in the hallmarks of glioma (Table I; Fig. 1), which are described in this section.
Table I.
Roles of different lncRNAs in glioma hallmarks.
| lncRNA | Expression in glioma | Maintenance of growth signals | Resistance to growth inhibition signals | Resistance to apoptosis | Ability to replicate indefinitely | Angiogenesis | Invasion | Reprogramming of energy metabolism | Evading immune cell attack | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|
| H19 | ↑ | + | + | + | + | (21,52,69, 72,73,91) | ||||
| XIST | ↑ | + | (23,24) | |||||||
| HOXD-AS1 | ↑ | + | + | (25) | ||||||
| CCAT1 | ↑ | + | + | + | (26) | |||||
| LOC728196 | ↑ | + | + | (27) | ||||||
| PLAC2 | ↓ | – | (31) | |||||||
| PTENP1 | ↓ | – | (32) | |||||||
| HOXA11-AS | ↑ | + | + | + | + | (33,76) | ||||
| Plncrna-1 | ↑ | + | + | + | (34–37) | |||||
| ADAMTS9-AS2 | ↓ | – | (38) | |||||||
| CASC2 | ↑ | + | + | (39) | ||||||
| ANRIL | ↑ | + | (41) | |||||||
| SNHG3/6/16/2O | ↑ | + | + | (47–50) | ||||||
| TUG1 | ↓ | – | – | (51,82) | ||||||
| CASC7 | ↓ | – | – | (53) | ||||||
| GHET1 | ↑ | + | + | (54) | ||||||
| MALAT1 | ↑ | + | + | (56,57) | ||||||
| FOXD2-AS1 | ↑ | + | + | + | (59–61) | |||||
| TUG1 | ↑ | + | (62) | |||||||
| PVT1 | ↑ | + | + | + | (64–66,93) | |||||
| AB073614 | ↑ | + | + | (67,68) | ||||||
| TSLC1-AS1 | ↓ | – | – | (69) | ||||||
| DANCR | ↑ | + | + | (71,75) | ||||||
| CCND2-AS1 | ↑ | + | (72) | |||||||
| UCA | ↑ | + | + | + | (78) | |||||
| TP53TG1 | ↑ | + | (79) | |||||||
| LINC00152 | ↑ | + | + | + | (82,89) | |||||
| PGM5-AS1 | ↑ | + | (83) | |||||||
| ST20-AS1 | ↑ | + | (83) | |||||||
| AGAP2-AS1 | ↑ | + | (83) | |||||||
| MIR155HG | ↑ | + | (83) | |||||||
| SNHG8 | ↓ | – | (83) | |||||||
| LINC00937 | ↓ | – | (83) | |||||||
| MAPKAPK5-AS1 | ↓ | – | (83) | |||||||
| HCG18 | ↓ | – | (83) |
↑, increased expression; ↓, decreased expression; +, promoting effect; −, inhibitory effect; lncRNA, long non-coding RNA.
Figure 1.
lncRNAs with different glioma hallmarks. lncRNAs with different glioma hallmarks have been reported. A number of these RNAs have been reported to have multiple glioma hallmarks, such as H19 and CCAT1. XIST and PLAC2 have been reported only one hallmark function. Others have been reported in other tumors and may serve a role in gliomas, such as MALAT1 and FOXD2-AS1.
Roles of lncRNAs in the maintenance of growth signals
The expression of the lncRNA X-inactive specific transcript (XIST) is increased in glioma compared with that in normal controls, and XIST can directly bind microRNA (miR)-29c to inhibit its expression (23,24). In glioma, the XIST/miR-29c axis induces an increase in the protein levels of MutS Homolog 6, transcription factor specificity protein 1 and O -methylguanine-DNA methyltransferase through mutations in the mismatch repair pathway, which promotes cell proliferation and reduces the temozolomide (TMZ)-induced inhibition of cell proliferation (23). XIST also promotes angiogenesis in glioma by targeting miR-137 (24).
The lncRNA HOXD cluster antisense RNA 1 (HOXD-AS1) acts as a miR-204 sponge to promote the proliferation, migration and invasion of glioma cells, and to confer cisplatin resistance (25). miR-204 directly inhibits the expression of its target oncogenes high-mobility group box-1 and RAB22A, a member of the Ras oncogene family, in various malignancies, such as glioma, cervical cancer and breast cancer (25).
In glioma, the expression of the lncRNA colon cancer-associated transcript-1 (CCAT1) is increased, whereas the expression of miR-181b is decreased compared with that in normal brain tissues from patients with cerebral trauma (26). Additionally, studies have demonstrated that the expression of these two RNAs is negatively correlated in glioma, and that miR-181b inhibits the expression of fibroblast growth factor receptor 3 (FGFR3) and platelet-derived growth factor receptor (PDGFR) by directly binding to the 3′-untranslated regions (UTRs) of these genes (26). Thus, CCAT1 may be a competitive endogenous RNA (ceRNA) of miR-181b, which increases the expression of FGFR3 and PDGFR by regulating miR-181b, thus increasing cell proliferation (26).
In glioma, the expression of the lncRNA LOC728196 is increased, whereas the expression of miR-513c is decreased compared with adjacent normal tissues, demonstrating a negative correlation, and high expression of LOC728196 increases the proliferation of glioma cells (27). A previous study has also demonstrated that LOC728196 reduces the targeted inhibition of transcription factor 7 (TCF7) by binding to miR-513c (27). TCF7 is involved in the Wnt/β-catenin signaling pathway to leads to excessive activation of the pathway, which leads to the development of malignant tumors (28,29).
Another study demonstrated that cell proliferation is inhibited in glioma U87 cells following small nucleolar RNA host gene (SNHG)20-knockdown; in addition, a decrease in the levels of cyclin A1 and an increase in the levels of p21 were observed in U87 cells of SNHG20 siRNA group compared with the NC siRNA group (30). Overall, XIST, HOXD-AS1, CCAT1, LOC728196 and SNHG20 increased expression in glioma compared with normal brain tissue or brain tissue adjacent to the tumor and promoted the maintenance of growth signals.
Roles of lncRNAs in resistance to growth inhibition signals
The lncRNA placenta-specific protein 2 (PLAC2) is expressed at lower levels in glioma compared with those in normal brain tissue (31). The study also has demonstrated that PLAC2 binds to STAT1 and inhibits the expression of the ribosomal protein L36 by binding to its promoter in the nucleus (31). In addition, cytoplasmic PLAC2 inhibits the nuclear translocation of STAT1 and inhibits cell proliferation (31).
The lncRNA PTEN pseudogene 1 (PTENP1) is a tumor suppressor, the expression of which is decreased in glioma; its overexpression inhibits cell proliferation and invasion, which may be achieved by promoting the expression of p21 and inhibiting the p38 mitogen-activated protein kinase (MAPK) pathway (32).
The expression of the lncRNA homeobox A11 antisense (HOXA11-AS) in glioma tissues and cells, which has been demonstrated to be significantly increased compared with normal brain tissue (33), promotes proliferation by upregulating cyclin-dependent kinase 2/4, cyclin D1 and cyclin E, and promotes the downregulation of p16, p21, p27 and retinoblastoma tumor suppressor protein (33).
Prostate cancer-upregulated lncRNA 1 (PlncRNA-1) promotes cancer progression by regulating androgen receptors and activating transforming growth factor-β1 (34–36). Recent a study reported that PlncRNA-1 is significantly upregulated in glioma and indicates a poor survival prognosis (37). PlncRNA-1 has also been demonstrated to promote the proliferation and invasiveness of glioma, which is achieved by upregulating the notch pathway-related proteins Notch-1, Jagged-1 and Hes family bhlh transcription factor 1 (37).
The lncRNA ADAMTS9-antisense RNA 2 (ADAMTS9-AS2) is a tumor suppressor that inhibits the migration of glioma cells and is negatively regulated by DNA methyltransferase-1, which results in its decreased expression in glioma (38).
The lncRNA cancer susceptibility 2 (CASC2) exerts tumor inhibitory effects and is significantly decreased in glioma compared with normal brain tissue (39). CASC2 can directly bind miR-181a to upregulate PTEN, inhibit tumor proliferation and increase the sensitivity of glioma cells to TMZ (39).
In summary, PLAC2, PTENP1, ADAMTS9-AS2 and CASC2 inhibits resistance to growth inhibition signals and are decreased in glioma. Increased HOXA11-AS and PlncRNA-1 expression in glioma and promotes resistance to growth inhibition signals.
Roles of lncRNAs in the resistance to apoptosis
In glioma, the expression of lncRNA antisense non-coding RNA in the INK4 locus (ANRIL) is negatively associated with the expression of the anticancer gene miR-203a (40). Studies have demonstrated that ANRIL promotes carcinogenesis by affecting the expression of miR-203a, and that knockdown of ANRIL inhibits twist-related protein 1 and c-jun transcription by inhibiting the phosphorylation of the AKT signaling pathway components, which promotes apoptosis (40–42). c-myc is an oncogene that inhibits the expression of p21 (40); p21 is a tumor suppressor gene that can promote stagnation of the tumor cell cycle and inhibit the expression of bcl-2 (41,42), an anti-apoptotic gene that inhibits apoptosis by inhibiting the activity of apoptosis-related caspase proteins (43). A number of studies have reported that ANRIL can inhibit the expression of c-myc, thereby promoting cell cycle arrest in tumor cells and increasing caspase-3/8/9 activity, which promotes apoptosis (40–44).
The lncRNAs SNHG3/6/16/20 are primarily expressed in the nucleus and are increased in glioma compared with normal brain tissue (45–50). SNHG3 recruits enhancer of zeste homologue 2 (EZH2) to the promoter region of Kruppel-like factor 2 (KLF2) and p21 through sponging EZH2 (45). EZH2 binds to the KLF2 and p21 promoters to inhibit their expression (45,46), which inhibits apoptosis and increases cell proliferation (47). In transfected normal astrocytes with an SNHG6 overexpression vector, the development of malignant phenotypes is observed, which indicates that SNHG6 induces the transformation of cells with a normal phenotype to those with a malignant phenotype. In addition, knockdown of SNHG6 promotes cell proliferation by enhancing the mRNA and protein levels of p21 in glioma cells compared with negative control-transfected glioma cells (48). Additionally, in SNHG6-knockdown glioma cells, the apoptosis-related proteins caspase-3 and caspase-9 are activated, promoting apoptosis (48). SNHG16 upregulates the expression of protein arginine methyltransferase 5 (PRMT5) through sponging miR-4518 to promote the relevant functions of PRMT5 in glioma (49). In addition, SNHG16 inhibits apoptosis by regulating the expression of p21, bcl-2 and the components of the PI3K/AKT pathway (49). In SNHG20-knockout glioma cells, inhibition of the PTEN/PI3K/AKT signaling pathway and increased apoptosis were observed compared with negative control-transfected glioma cells, suggesting that SNHG20 may regulate apoptosis through the PTEN/PI3K/AKT signaling pathway (50).
The lncRNA taurine upregulated 1 (TUG1) is a tumor-inhibiting lncRNA, the expression of which is decreased in glioma. TUG1 activates caspase-3/9 and inhibits the bcl-2-mediated anti-apoptosis pathway to promote apoptosis in glioma cells (51). Notably, another study has reported that TUG1 is upregulated in glioblastoma, that it induces the expression of vascular endothelial growth factor A (VEGFA) and that it promotes tumor angiogenesis through sponging miR-299 (52).
In glioma, the expression of lncRNA CASC7 is significantly decreased compared with normal brain tissues (53). A previous study demonstrated that CASC7 inhibits glioma progression, promotes apoptosis and inhibits cell proliferation by inactivating the Wnt/β-catenin signaling pathway (53). Additionally, increased caspase-3 activity was also observed in glioma cell lines that overexpressed CASC7 (53).
The expression of the lncRNA gastric carcinoma proliferation enhancing transcript 1 (GHET1) is increased in glioma compared with normal brain tissues, and may inhibit apoptosis by downregulating the Numb protein, which in turn downregulates p53 and upregulates matrix metalloproteinase 2/9 (54).
The expression of the lncRNA urothelial cancer-associated 1 (UCA1) is increased in glioma compared with normal brain tissues, where it inhibits miR-182 expression (55). Notably, apoptosis-stimulating protein inhibitor of p53 (iASPP) and its 3′-UTR are inhibited by binding miR-182; thus, UCA1 may increase the expression of iASPP and inhibit apoptosis (55). In summary, ANRIL, SNHG3/6/16/20 and GHET1 increased expression in glioma compared with normal brain tissue and promoted the resistance to apoptosis. TUG1 and CASC7 decreased expression in glioma and promoted apoptosis.
Roles of lncRNAs in indefinite replication
Telomerase reverse transcriptase (TERT) is a catalytic subunit of telomerase that serves an important role in the replication of telomeres (56). A previous study has demonstrated that in BRL-3A cells, metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) regulates telomerase activity by regulating TERT (57). In glioma, MALAT1 expression is increased and promotes glioma cell proliferation compared with normal brain tissues (58); thus, MALAT1 is likely to have a positive effect on telomerase levels in glioma cells, although no relevant studies have been published to date.
A previous study has demonstrated that FOXD2 adjacent opposite strand RNA 1 (FOXD2-AS1) upregulates TERT expression in thyroid cancer (59). FOXD2-AS1 is also expressed in glioma, and its expression level is higher compared with that in normal brain tissue. Additionally, FOXD2-AS1 promotes glioma cell proliferation and migration, and inhibit apoptosis (60,61). In summary, MALAT1 and FOXD2-AS1 increased expression in glioma compared with normal brain tissue or brain tissue adjacent to the tumor and promotes indefinite replication.
Roles of lncRNAs in angiogenesis
In glioma cells, TUG1 expression is increased compared with normal astrocytes, and knockdown of TUG1 reduces the expression of VEGFA (52). The study also demonstrated that TUG1 can be used as a ceRNA of miR-299 (52). Notably, miR-299 inhibits VEGFA expression by targeting its 3′-UTR; thus, TUG1 may promote angiogenesis by inhibiting miR-299 and increasing VEGF expression in glioma cells (52). Another study has demonstrated that overexpression of H19 in glioma cells also has an angiogenic effect similar to that of TUG1 (62).
Roles of lncRNA in invasion
The lncRNA plasmacytoma variant translocation 1 (PVT1) is highly expressed in glioma and can sponge miR-128-3p to inhibit its activity. miR-128-3p inhibits gremlin 1 (GREM1) protein expression by binding to the 3′-UTR of GREM1 mRNA (63). Studies have demonstrated that PVT1 regulates the bone morphogenetic protein (BMP) signaling pathway through the miR-128-3p/GREM1 axis, and can upregulate BMP2 and BMP4 (63), promote cell proliferation and invasion, and inhibit apoptosis in glioma (64). In addition, knockdown of PVT1 negatively regulates miR-424 to inhibit cell activity and the invasiveness of glioma (65). PVT1 also promotes connective tissue growth factor and angiopoietin 2 expression by sponging and degrading miR-26b (66).
The lncRNA AB073614 is highly expressed in glioma and is used as an independent indicator of poor prognosis for patients with glioma (67). AB073614 promotes the epithelial-mesenchymal transition (EMT), proliferation and migration of glioma cells (68).
The lncRNA TSLC1-AS1 exerts an antitumor effect, and its expression is decreased in glioma compared with normal brain tissues (69). TSLC1-AS1 is the antisense transcript of the tumor suppressor TSLC1, and their expression levels are positively correlated in glioma (69). TSLC1-AS1 overexpression inhibits the proliferation, migration and invasiveness of glioma U87 cells (69). In contrast, knockdown of TSLC1-AS1 exerts the opposite effect; for example, in knockdown experiments, double-stranded TSLC1-AS1 small interfering (si)RNA significantly reduces the expression of TSLC1-AS1 and TSLC1 and promotes the proliferation, migration and invasiveness of glioma U87 cells compared with negative control-transfected glioma cells (69). Additionally, in a study where the sense and antisense strand of TSLC1-AS1 siRNA were transfected into glioma cells, it was demonstrated that the expression levels of TSLC1-AS1 and TSLC1 were decreased in glioma cells transfected with the sense strand, but not the antisense strand (69). The aforementioned study also demonstrated that increased TSLC1-AS1 expression upregulated the tumor suppressors neurofibromin 1, von Hippel-Lindau and phosphoinositide-3-kinase regulatory subunit 1, and inhibited the expression of the oncogene B-Raf proto-oncogene (69).
CCND2-antisense RNA 1 (CCND2-AS1), H19 and differentiation antagonizing non-protein coding RNA (DANCR) can activate the Wnt/β-catenin signaling pathway to promote EMT, further increase the resistance of glioma cells to TMZ, and promote cell proliferation and migration (70–72). The activation of H19 may be mediated by miR-675 (73). In addition, H19 reduces the inhibition of miR-140 on iASPP through sponging miR-140, upregulating iASPP expression, and promoting tumor proliferation and invasion (74). Additionally, previous studies have demonstrated that in osteosarcoma and hepatocellular carcinoma, DANCR binds miR-33a-5p and miR-15b-5p to promote tumor growth (75,76).
HOXA11-AS promotes the invasiveness of glioma by binding miR-130a-5p to reduce the inhibition of miR-130a-5p on high-mobility group protein B2 (77).
Another study has demonstrated that UCA1 reduces the inhibitory effect of miR-204-5p on zinc finger E-box binding homeobox 1 (ZEB1) through sponging miR-204-5p, which upregulates ZEB1 and further increases the invasiveness of glioma and EMT (78).
In summary, increased expression of PVT1, AB073614, CCND2-AS1, H19, DANCR, HOXA11-AS and UCA1 in glioma compared with normal brain tissue promotes invasion. TSLC1-AS1 decreased expression in glioma and inhibits invasion.
Roles of lncRNAs in the reprogramming of energy metabolism
The expression of TP53 target 1 (TP53TG1) is significantly higher in human glioma compared with that in normal brain tissue, and the expression of TP53TG1 is also increased in the conditions of glucose deprivation (79). Under low glucose conditions, the increased expression of TP53TG1 upregulates the expression of glucose-regulated protein 78 and isocitrate dehydrogenase (IDH)1, and downregulates the expression of pyruvate kinase 2 (PKM2) (79). Glucose deprivation promotes the production of reactive oxygen species (ROS) and ROS-mediated cell death. The pentose phosphate pathway (PPP) produces NADPH, which detoxifies ROS (80). IDH promotes NADPH production, whereas PKM2 inhibits the PPP (80,81). Therefore, TP53TG1 may increase the tolerance of glioma cells to glucose deprivation by changing the energy metabolism pathway of the tumor.
Roles of lncRNAs in evading immune cell attack
LINC00152 has been demonstrated to serve an immune-related role in glioma, although the specific mechanism has not yet been determined (82). Additionally, another study reported nine immunologically related lncRNAs in anaplastic glioma: Phosphoglucomutases 5-antisense RNA 1 (PGM5-AS1), ST20-antisense RNA 1 (ST20-AS1), ankyrin repeat and PH domain 2-antisense RNA 1 (AGAP2-AS1), MIR155 host gene (MIR155HG), SNHG8, LINC00937, TUG1, MAPK activated protein kinase 5-antisense RNA 1 (MAPKAPK5-AS1) and HLA complex group 18 (HCG18). The former four are risk-related genes, and the latter four are protective genes (83). Notably, a number of studies have demonstrated that TUG1, an lncRNA, is increased in glioma and that it promotes glioma-associated angiogenesis, although other studies have reported that its expression is decreased and that it regulates immune functions and reduces apoptosis (51,52,83). In addition, TUG1 has been demonstrated to be upregulated in certain types of tumors, such as oesophageal squamous cell carcinoma, but downregulated in others, such as non-small cell lung cancer (84,85).
3. Roles of lncRNAs in glioma subpopulations
Roles of lncRNAs in glioma stem cells (GSCs)
The expression of the lncRNA nuclear paraspeckle assembly transcript 1 (NEAT1) is increased in GSCs compared with astrocytes, and it has been demonstrated that NEAT1 exerts an effect of mutual inhibition with miR let-7e (86). let-7e is a tumor suppressor that can target and downregulate neuroblastoma ras (NRAS) to inhibit the malignant behavior of GSCs; thus, NEAT1 upregulates NRAS to promote the proliferation, invasion and apoptosis inhibition of GSCs by sponging let-7e (86). Additionally, another study has demonstrated that NEAT1 enhances the effects of GSCs on glioma through the miR-107/CDK6 pathway (87).
The expression of the lncRNA hypoxia-inducible factor 1-α-antisense RNA 2 (HIF1A-AS2) is upregulated in GSCs and has been demonstrated to be beneficial to the resistance of GSCs to hypoxia (88). HIF1A-AS2 interacts with insulin-like growth factor 2 mRNA-binding protein 2 and ATP-dependent RNA helicase A to enhance the expression of these two proteins and to promote the adaptation of GSCs to a hypoxic environment (88).
The expression of lncRNA linc00152 is upregulated in GSCs compared with astrocytes and can negatively regulate the levels of miR-103a-3p by sponging it (89). miR-103a-3p targets and upregulates FEZ family zinc finger protein 1, promoting the expression of cell division cycle 25A, which is an oncogene that promotes malignant behavior of GSCs by activating the PI3K/AKT pathway (89).
lncRNA growth arrest-specific 5 (GAS5) is decreased in GSCs, where it exerts an antitumor effect (90). GAS5 promotes the expression of the transcription factor forkhead box O1 (FOXO1) through sponging miR-196a-5p; FOXO1 subsequently inhibits the malignant biological behavior of GSCs by upregulating phosphotyrosine interaction domain containing 1 and migration and invasion inhibitory protein (91).
Roles of lncRNAs in glioma endothelial cells (GECs)
The expression of lncRNA H19 is significantly increased in GECs compared with astrocytes, where it upregulates vasohibin 2 to promote GEC proliferation, migration and tubule formation by sponging miR-29a (92).
The expression of the lncRNA PVT1 is significantly increased in GECs compared with astrocytes, where it interacts with miR-186 and reduces the expression of autophagy-related 7 (Atg7) and beclin1 by binding to their mRNAs (93); thus, PVT1 may enhance the expression of the autophagy-related proteins Atg7 and beclin1 through sponging miR-186. Atg7 and beclin1 induce protective autophagy; therefore, PVT1 may promote the proliferation, migration and angiogenesis of GECs by inducing protective autophagy (93).
Expression of the lncRNA SNHG15 is increased in GEC, where it sponges miR-153 to inhibit its expression (94). miR-153 inhibits the expression of VEGFA and CDC42 and in turn inhibits their ability to promote angiogenesis; thus, SNHG15 may upregulate VEGFA and CDC42 to promote GEC proliferation, migration and tubule formation (94).
For other cell subpopulations in glioma, such as immune cells, pericytes, fibroblasts and mesenchymal stem cells, no specific studies on lncRNAs have been published to date.
4. Conclusions and future perspectives
Numerous studies have demonstrated that lncRNAs serve an important role in human tumorigenesis. Overall, lncRNAs are involved in the acquisition of all eight markers of glioma. Some lncRNAs promote upregulation of hallmarker biomarkers in glioma, for example XIST, whereas others can inhibit biomarkers, for example PLAC2, leading to glioma gaining hallmark characteristics. Some lncRNAs can promote proliferation and migration in GSCs and GECs. GECs is a part of tumor microenvironment; however, there are no reports about the effect of lncRNA on other parts of tumor microenvironment except GECs, to the best of our knowledge.
The TME is composed of a variety of extracellular components, such as the extracellular matrix, various hormones, cytokines and growth factors, and a variety of cell types, including endothelial cells, stem cells (including mesenchymal stem cells), immune cells and fibroblasts (95). The TME serves an important role in the occurrence and development of tumors as it not only promotes the occurrence, progression and metastasis of tumors, and maintains the characterization of tumors in favor of various types of the cells and extracellular components contained in the tumor microenvironment, but also serves an important role in the formation of tumor resistance to chemotherapy (95–97). The TME promotes the formation of tumor drug resistance in a variety of ways, such as secreting soluble factors, cell adhesion and participating in the immune response (95). Therefore, TME intervention may serve an important role in the treatment of tumors. In recent years, increased attention has been paid to the role of the TME in glioma; it has been demonstrated that the TME serves an important role in glioma progression and treatment effects (3), but only a few studies on lncRNAs in GECs have been published, and no studies have been published on the function of lncRNAs in fibroblasts, immune cells and pericytes. Studies of glioma-associated mesenchymal stem cells (gbMSCs) are also receiving increasing attention; these cells may be divided into two subgroups (CD90 gbMSCs and CD90 gbMSCs), the proportion of which in glioma is associated with the survival rate of patients (98). Our previous studies demonstrated that CD90 gbMSCs can differentiate into pericytes, and that CD90 gbMSCs promote the growth of glioma cells, whereas CD90 gbMSCs promote angiogenesis (99–101). In addition, our previous study also identified specific lncRNAs in the two subgroups (99), but at present, no related studies on lncRNAs in gbMSCs have been reported. Therefore, based on the important role of the TME and gbMSCs in the development and treatment of glioma, future studies should focus on how lncRNAs affect these components of glioma.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- lncRNA
long non-coding RNA
- GSC
glioma stem cell
- GEC
glioma endothelial cell
- TME
tumor microenvironment
- ncRNA
non-coding RNA
- ceRNA
competitive endogenous RNA
Funding
This review was supported by the National Natural Science Foundation of China (grant no. 81572488).
Availability of data and materials
Not applicable.
Authors' contributions
BZX and WX conceived the study, drafted and modified the manuscript. QZ, YHW, HFW, DYY, NXX, XBJ and HYZ equally contributed to data collection. PF revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Fouad YA, Aanei C. Revisiting the hallmarks of cancer. Am J Cancer Res. 2017;7:1016–1036. [PMC free article] [PubMed] [Google Scholar]
- 3.Quail DF, Joyce JA. The Microenvironmental landscape of brain tumors. Cancer Cell. 2017;31:326–341. doi: 10.1016/j.ccell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss N, Miller F, Cazaubon S, Couraud P. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys. 2009;1788:842–857. doi: 10.1016/j.bbamem.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Wolburg H, Wolburg-Buchholz K, Kraus J, Rascher-Eggstein G, Liebner S, Hamm S, Duffner F, Grote E, Risau W, Engelhardt B. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003;105:586–592. doi: 10.1007/s00401-003-0688-z. [DOI] [PubMed] [Google Scholar]
- 6.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 7.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 8.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 9.ENCODE Project Consortium, corp-author. Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang DQ, Fu P, Yao C, Zhu LS, Hou TY, Chen JG, Lu Y, Liu D, Zhu LQ. Long Non-coding RNAs, Novel culprits, or bodyguards in neurodegenerative diseases. Mol Ther Nucleic Acids. 2018;10:269–276. doi: 10.1016/j.omtn.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rynkeviciene R, Simiene J, Strainiene E, Stankevicius V, Usinskiene J, Miseikyte Kaubriene E, Meskinyte I, Cicenas J, Suziedelis K. Non-coding RNAs in Glioma. Cancers (Basel) 2018;11:17. doi: 10.3390/cancers11010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kornienko AE, Guenzl PM, Barlow DP, Pauler FM. Gene regulation by the act of long non-coding RNA transcription. BMC Biol. 2013;11:59. doi: 10.1186/1741-7007-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ulitsky I, Bartel DP. lincRNAs: Genomics, evolution, and mechanisms. Cell. 2013;154:26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tragante V, Moore JH, Asselbergs FW. The ENCODE project and perspectives on pathways. Genet Epidemiol. 2014;38:275–280. doi: 10.1002/gepi.21802. [DOI] [PubMed] [Google Scholar]
- 16.Bhan A, Mandal SS. Long noncoding RNAs: Emerging stars in gene regulation, epigenetics and human disease. ChemMedChem. 2014;9:1932–1956. doi: 10.1002/cmdc.201300534. [DOI] [PubMed] [Google Scholar]
- 17.Yuan J, Yue H, Zhang M, Luo J, Liu L, Wu W, Xiao T, Chen X, Chen X, Zhang D, et al. Transcriptional profiling analysis and functional prediction of long noncoding RNAs in cancer. Oncotarget. 2016;16:7. doi: 10.18632/oncotarget.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai M, Spitale RC, Chang HY. Long intergenic noncoding RNAs: New links in cancer progression. Cancer Res. 2011;71:3–7. doi: 10.1158/0008-5472.CAN-10-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie H, Ma H, Zhou D. Plasma HULC as a promising novel biomarker for the detection of hepatocellular carcinoma. Biomed Res Int. 2013;2013:136106. doi: 10.1155/2013/136106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parasramka MA, Maji S, Matsuda A, Yan IK, Patel T. Long non-coding RNAs as novel targets for therapy in hepatocellular carcinoma. Pharmacol Ther. 2016;161:67–78. doi: 10.1016/j.pharmthera.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Yang F, Yuan J, Yuan S, Zhou W, Huo X, Xu D, Bi H, Wang F, Sun S. Epigenetic activation of the MiR-200 family contributes to H19-mediated metastasis suppression in hepatocellular carcinoma. Carcinogenesis. 2013;34:577–586. doi: 10.1093/carcin/bgs381. [DOI] [PubMed] [Google Scholar]
- 22.Dai M, Li S, Qin X. Colorectal neoplasia differentially expressed: A long noncoding RNA with an imperative role in cancer. Onco Targets Ther. 2018;11:3755–3763. doi: 10.2147/OTT.S162754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du P, Zhao H, Peng R, Liu Q, Yuan J, Peng G, Liao Y. LncRNA-XIST interacts with miR-29c to modulate the chemoresistance of glioma cell to TMZ through DNA mismatch repair (MMR) pathway. Biosci Rep. 2017;37:BSR20170696. doi: 10.1042/BSR20170696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu H, Xue Y, Wang P, Liu X, Ma J, Zheng J, Li Z, Li Z, Cai H, Liu Y. Knockdown of long non-coding RNA XIST increases blood-tumor barrier permeability and inhibits glioma angiogenesis by targeting miR-137. Oncogenesis. 2017;6:e303. doi: 10.1038/oncsis.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou H, Ma Y, Zhong D, Yang L. Knockdown of lncRNA HOXD-AS1 suppresses proliferation, migration and invasion and enhances cisplatin sensitivity of glioma cells by sponging miR-204. Biomed Pharmacother. 2019;112:108633. doi: 10.1016/j.biopha.2019.108633. [DOI] [PubMed] [Google Scholar]
- 26.Cui B, Li B, Liu Q, Cui Y. lncRNA CCAT1 promotes glioma tumorigenesis by sponging miR-181b. J Cell Biochem. 2017;118:4548–4557. doi: 10.1002/jcb.26116. [DOI] [PubMed] [Google Scholar]
- 27.Wang O, Huang Y, Wu H, Zheng B, Lin J, Jin P. LncRNA LOC728196/miR-513c axis facilitates glioma carcinogenesis by targeting TCF7. Gene. 2018;679:119–125. doi: 10.1016/j.gene.2018.08.081. [DOI] [PubMed] [Google Scholar]
- 28.Wallmen B, Schrempp M, Hecht A. Intrinsic properties of Tcf1 and Tcf4 splice variants determine cell-type-specific Wnt/β-catenin target gene expression. Nucleic Acids Res. 2012;40:9455–9469. doi: 10.1093/nar/gks690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Xue L, Peng Q. Tunicamycin inhibits progression of glioma cells through downregulation of the MEG-3-regulated wnt/β-catenin signaling pathway. Oncol Lett. 2018;15:8470–8476. doi: 10.3892/ol.2018.8416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Shen F, Huang L, Hui L, Liu R, Ma Y, Jin B. lncRNA small nucleolar RNA host gene 20 predicts poor prognosis in glioma and promotes cell proliferation by silencing P21. Oncotargets Ther. 2019;12:805–814. doi: 10.2147/OTT.S192641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y, Kang C, Zhao J, Nie Y, Zheng L, Li H, Li X, Wang Q, Qiu Y. LncRNA PLAC2 down-regulates RPL36 expression and blocks cell cycle progression in glioma through a mechanism involving STAT1. J Cell Mol Med. 2018;22:497–510. doi: 10.1111/jcmm.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu S, Xu L, Li L, Luo D, Zhao H, Li D, Peng B. Overexpression of lncRNA PTENP1 suppresses glioma cell proliferation and metastasis in vitro. Onco Targets Ther. 2019;12:147–156. doi: 10.2147/OTT.S182537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Q, Zhang J, Liu Y, Zhang W, Zhou J, Duan R, Pu P, Kang C, Han L. A novel cell cycle-associated lncRNA, HOXA11-AS, is transcribed from the 5-prime end of the HOXA transcript and is a biomarker of progression in glioma. Cancer Lett. 2016;373:251–259. doi: 10.1016/j.canlet.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 34.Cui Z, Ren S, Lu J, Wang F, Xu W, Sun Y, Wei M, Chen J, Gao X, Xu C, et al. The prostate cancer-up-regulated long noncoding RNA PlncRNA-1 modulates apoptosis and proliferation through reciprocal regulation of androgen receptor. Urol Oncol. 2013;31:1117–1123. doi: 10.1016/j.urolonc.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 35.Fang Z, Xu C, Li Y, Cai X, Ren S, Liu H, Wang Y, Wang F, Chen R, Qu M, et al. A feed-forward regulatory loop between androgen receptor and PlncRNA-1 promotes prostate cancer progression. Cancer Lett. 2016;374:62–74. doi: 10.1016/j.canlet.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 36.Jin Y, Cui Z, Li X, Jin X, Peng J. Upregulation of long non-coding RNA PlncRNA-1 promotes proliferation and induces epithelial-mesenchymal transition in prostate cancer. Oncotarget. 2017;8:26090–26099. doi: 10.18632/oncotarget.15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Yan Y, Zhang C, Wei W, Ai X, Pang Y, Bian Y. Upregulation of lncRNA PlncRNA-1 indicates the poor prognosis and promotes glioma progression by activation of Notch signal pathway. Biomed Pharmacother. 2018;103:216–221. doi: 10.1016/j.biopha.2018.03.150. [DOI] [PubMed] [Google Scholar]
- 38.Yao J, Zhou B, Zhang J, Geng P, Liu K, Zhu Y, Zhu W. A new tumor suppressor LncRNA ADAMTS9-AS2 is regulated by DNMT1 and inhibits migration of glioma cells. Tumour Biol. 2014;35:7935–7944. doi: 10.1007/s13277-014-1949-2. [DOI] [PubMed] [Google Scholar]
- 39.Liao Y, Shen L, Zhao H, Liu Q, Fu J, Guo Y, Peng R, Cheng L. LncRNA CASC2 Interacts With miR-181a to modulate glioma growth and resistance to TMZ Through PTEN pathway. J Cell Biochem. 2017;118:1889–1899. doi: 10.1002/jcb.25910. [DOI] [PubMed] [Google Scholar]
- 40.Dorasamy MS, Choudhary B, Nellore K, Subramanya H, Wong P. Dihydroorotate dehydrogenase Inhibitors Target c-Myc and arrest melanoma, myeloma and lymphoma cells at S-phase. J Cancer. 2017;8:3086–3098. doi: 10.7150/jca.14835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang M, Xia P, Hou T, Qi Z, Liao S, Yang X. MicroRNA-190b inhibits tumor cell proliferation and induces apoptosis by regulating Bcl-2 in U2OS osteosarcoma cells. Pharmazie. 2017;72:279–282. doi: 10.1691/ph.2017.6921. [DOI] [PubMed] [Google Scholar]
- 42.Liu Z, Liu H, Yuan X, Wang Y, Li L, Wang G, Song J, Shao Z, Fu R. Downregulation of Pim-2 induces cell cycle arrest in the G0/G1 phase via the p53-non-dependent p21 signaling pathway. Oncol Lett. 2018;15:4079–4086. doi: 10.3892/ol.2018.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ju X, Yu H, Liang D, Jiang T, Liu Y, Chen L, Dong Q, Liu X. LDR reverses DDP resistance in ovarian cancer cells by affecting ERCC-1, Bcl-2, Survivin and Caspase-3 expressions. Biomed Pharmacother. 2018;102:549–554. doi: 10.1016/j.biopha.2018.03.092. [DOI] [PubMed] [Google Scholar]
- 44.Dai W, Tian C, Jin S. Effect of lncRNA ANRIL silencing on anoikis and cell cycle in human glioma via microRNA-203a. Onco Targets Ther. 2018;11:5103–5109. doi: 10.2147/OTT.S169809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seward S, Semaan A, Qazi AM, Gruzdyn OV, Chamala S, Bryant CC, Kumar S, Cameron D, Sethi S, Ali-Fehmi R, et al. EZH2 blockade by RNA interference inhibits growth of ovarian cancer by facilitating re-expression of p21(waf1/cip1) and by inhibiting mutant p53. Cancer Lett. 2013;336:53–60. doi: 10.1016/j.canlet.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 46.Taniguchi H, Jacinto FV, Villanueva A, Fernandez AF, Yamamoto H, Carmona FJ, Puertas S, Marquez VE, Shinomura Y, Imai K, Esteller M. Silencing of Kruppel-like factor 2 by the histone methyltransferase EZH2 in human cancer. Oncogene. 2012;31:1988–1994. doi: 10.1038/onc.2011.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fei F, He Y, He S, He Z, Wang Y, Wu G, Li M. LncRNA SNHG3 enhances the malignant progress of glioma through silencing KLF2 and p21. Biosci Rep. 2018;38:BSR20180420. doi: 10.1042/BSR20180420. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Cai G, Zhu Q, Yuan L, Lan Q. LncRNA SNHG6 acts as a prognostic factor to regulate cell proliferation in glioma through targeting p21. Biomed Pharmacother. 2018;102:452–457. doi: 10.1016/j.biopha.2018.03.083. [DOI] [PubMed] [Google Scholar]
- 49.Lu YF, Cai XL, Li ZZ, Lv J, Xiang YA, Chen JJ, Chen WJ, Sun WY, Liu XM, Chen JB. LncRNA SNHG16 Functions as an oncogene by sponging MiR-4518 and Up-regulating PRMT5 expression in glioma. Cell Physiol Biochem. 2018;45:1975–1985. doi: 10.1159/000487974. [DOI] [PubMed] [Google Scholar]
- 50.Guo LP, Zhang ZJ, Li RT, Li HY, Cui YQ. Influences of LncRNA SNHG20 on proliferation and apoptosis of glioma cells through regulating the PTEN/PI3K/AKT signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:253–261. doi: 10.26355/eurrev_201901_16771. [DOI] [PubMed] [Google Scholar]
- 51.Li J, Zhang M, An G, Ma Q. LncRNA TUG1 acts as a tumor suppressor in human glioma by promoting cell apoptosis. Exp Biol Med (Maywood) 2016;241:644–649. doi: 10.1177/1535370215622708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai H, Liu X, Zheng J, Xue Y, Ma J, Li Z, Xi Z, Li Z, Bao M, Liu Y. Long non-coding RNA taurine upregulated 1 enhances tumor-induced angiogenesis through inhibiting microRNA-299 in human glioblastoma. Oncogene. 2017;36:318–331. doi: 10.1038/onc.2016.212. [DOI] [PubMed] [Google Scholar]
- 53.Gong X, Liao X, Huang M. LncRNA CASC7 inhibits the progression of glioma via regulating Wnt/β-catenin signaling pathway. Pathol Res Pract. 2019;215:564–570. doi: 10.1016/j.prp.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 54.Ni W, Luo L, Zuo P, Li R, Xu X, Wen F, Hu D. lncRNA GHET1 down-regulation suppresses the cell activities of glioma. Cancer Biomark. 2018;23:9–22. doi: 10.3233/CBM-171002. [DOI] [PubMed] [Google Scholar]
- 55.He Z, Wang Y, Huang G, Wang Q, Zhao D, Chen L. The lncRNA UCA1 interacts with miR-182 to modulate glioma proliferation and migration by targeting iASPP. Arch Biochem Biophys 623–624. 2017:1–8. doi: 10.1016/j.abb.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 56.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 57.Tan Y, Tang L, OuYang W, Jiang T, Zhang H, Li S. β-catenin-coordinated lncRNA MALAT1 up-regulation of ZEB-1 could enhance the telomerase activity in HGF-mediated differentiation of bone marrow mesenchymal stem cells into hepatocytes. Pathol Res Pract. 2019;215:546–554. doi: 10.1016/j.prp.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 58.Fu Z, Luo W, Wang J, Peng T, Sun G, Shi J, Li Z, Zhang B. Malat1 activates autophagy and promotes cell proliferation by sponging miR-101 and upregulating STMN1, RAB5A and ATG4D expression in glioma. Biochem Biophys Res Commun. 2017;492:480–486. doi: 10.1016/j.bbrc.2017.08.070. [DOI] [PubMed] [Google Scholar]
- 59.Liu X, Fu Q, Li S, Liang N, Li F, Li C, Sui C, Dionigi G, Sun H. LncRNA FOXD2-AS1 Functions as a Competing Endogenous RNA to Regulate TERT Expression by Sponging miR-7-5p in Thyroid Cancer. Front Endocrinol (Lausanne) 2019;10:207. doi: 10.3389/fendo.2019.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dong H, Cao W, Xue J. Long noncoding FOXD2-AS1 is activated by CREB1 and promotes cell proliferation and metastasis in glioma by sponging miR-185 through targeting AKT1. Biochem Biophys Res Commun. 2019;508:1074–1081. doi: 10.1016/j.bbrc.2018.12.050. [DOI] [PubMed] [Google Scholar]
- 61.Ni W, Xia Y, Bi Y, Wen F, Hu D, Luo L. FoxD2-AS1 promotes glioma progression by regulating miR-185-5P/HMGA2 axis and PI3K/AKT signaling pathway. Aging (Albany NY) 2019;11:1427–1439. doi: 10.18632/aging.101843. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Jiang X, Yan Y, Hu M, Chen X, Wang Y, Dai Y, Wu D, Wang Y, Zhuang Z, Xia H. Increased level of H19 long noncoding RNA promotes invasion, angiogenesis, and stemness of glioblastoma cells. J Neurosurg. 2016;2016:129–136. doi: 10.3171/2014.12.JNS1426. [DOI] [PubMed] [Google Scholar]
- 63.Yan K, Wu Q, Yan DH, Lee CH, Rahim N, Tritschler I, DeVecchio J, Kalady MF, Hjelmeland AB, Rich JN. Glioma cancer stem cells secrete Gremlin1 to promote their maintenance within the tumor hierarchy. Gene Dev. 2014;28:1085–1100. doi: 10.1101/gad.235515.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu C, Li D, Zhang X, Liu N, Chi G, Jin X. LncRNA PVT1 Facilitates Tumorigenesis and progression of glioma via regulation of MiR-128-3p/GREM1 Axis and BMP signaling pathway. Neurotherapeutics. 2018;15:1139–1157. doi: 10.1007/s13311-018-0649-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Han Y, Li X, Yan J, Ma C, Zheng X, Zhang J, Zhang D, Meng C, Zhang Z, Ji X, et al. Knockdown of LncRNA PVT1 inhibits glioma progression by regulating miR-424 expression. Oncol Res. 2019;27:681–690. doi: 10.3727/096504018X15424939990246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng J, Hu L, Cheng J, Xu J, Zhong Z, Yang Y, Yuan Z. lncRNA PVT1 promotes the angiogenesis of vascular endothelial cell by targeting miR26b to activate CTGF/ANGPT2. Int J Mol Med. 2018;42:489–496. doi: 10.3892/ijmm.2018.3595. [DOI] [PubMed] [Google Scholar]
- 67.Hu L, Lv Q, Chen S, Sun B, Qu Q, Cheng L, Guo Y, Zhou H, Fan L. Up-Regulation of long Non-coding RNA AB073614 predicts a poor prognosis in patients with glioma. Int J Environ Res Public Health. 2016;13:433. doi: 10.3390/ijerph13040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li J, Wang Y, Song Y. Knockdown of long noncoding RNA AB073614 inhibits glioma cell proliferation and migration via affecting epithelial-mesenchymal transition. Eur Rev Med Pharmaco. 2016;20:3997–4002. [PubMed] [Google Scholar]
- 69.Qin X, Yao J, Geng P, Fu X, Xue J, Zhang Z. LncRNA TSLC1-AS1 is a novel tumor suppressor in glioma. Int J Clin Exp Patho. 2014;7:3065–3072. [PMC free article] [PubMed] [Google Scholar]
- 70.Jia L, Tian Y, Chen Y, Zhang G. The silencing of LncRNA-H19 decreases chemoresistance of human glioma cells to temozolomide by suppressing epithelial-mesenchymal transition via the Wnt/β-catenin pathway. Onco Targets Ther. 2018;11:313–321. doi: 10.2147/OTT.S154339. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Li J, Zhou L. Overexpression of lncRNA DANCR positively affects progression of glioma via activating Wnt/β-catenin signaling. Biomed Pharmacother. 2018;102:602–607. doi: 10.1016/j.biopha.2018.03.116. [DOI] [PubMed] [Google Scholar]
- 72.Zhang H, Wei D, Wan L, Yan S, Sun Y. Highly expressed lncRNA CCND2-AS1 promotes glioma cell proliferation through Wnt/β-catenin signaling. Biochem Bioph Res Commun. 2017;482:1219–1225. doi: 10.1016/j.bbrc.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 73.Zhang T, Wang Y, Zeng F, Cao H, Zhou H, Wang Y. LncRNA H19 is overexpressed in glioma tissue, is negatively associated with patient survival, and promotes tumor growth through its derivative miR-675. Eur Rev Med Pharmaco. 2016;20:4891–4897. [PubMed] [Google Scholar]
- 74.Zhao H, Peng R, Liu Q, Liu D, Du P, Yuan J, Peng G, Liao Y. The lncRNA H19 interacts with miR-140 to modulate glioma growth by targeting iASPP. Arch Biochem Biophys. 2016;610:1–7. doi: 10.1016/j.abb.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 75.Jiang N, Wang X, Xie X, Liao Y, Liu N, Liu J, Miao N, Shen J, Peng T. lncRNA DANCR promotes tumor progression and cancer stemness features in osteosarcoma by upregulating AXL via miR-33a-5p inhibition. Cancer Lett. 2017;405:46–55. doi: 10.1016/j.canlet.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 76.Yang Y, Hou N, Wang X, Wang L, Chang S, He K, Zhao Z, Zhao X, Song T, Huang C. miR-15b-5p induces endoplasmic reticulum stress and apoptosis in human hepatocellular carcinoma, both in vitro and in vivo, by suppressing Rab1A. Oncotarget. 2015;6:16227–16238. doi: 10.18632/oncotarget.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu C, Xiao L, Liu Y, Chen L, Zheng S, Zeng E, Li D. The lncRNA HOXA11-AS promotes glioma cell growth and metastasis by targeting miR-130a-5p/HMGB2. Eur Rev Med Pharmaco. 2019;23:241–252. doi: 10.26355/eurrev_201901_16770. [DOI] [PubMed] [Google Scholar]
- 78.Liang C, Yang Y, Guan J, Lv T, Qu S, Fu Q, Zhao H. LncRNA UCA1 sponges miR-204-5p to promote migration, invasion and epithelial-mesenchymal transition of glioma cells via upregulation of ZEB1. Pathol Res Pract. 2018;214:1474–1481. doi: 10.1016/j.prp.2018.07.036. [DOI] [PubMed] [Google Scholar]
- 79.Chen X, Gao Y, Li D, Hao B, Cao Y. LncRNA-TP53TG1 participated in the stress response under glucose deprivation in glioma. J Cell Biochem. 2017;118:4897–4904. doi: 10.1002/jcb.26175. [DOI] [PubMed] [Google Scholar]
- 80.Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang J, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao S, Lin Y, Xu W, Jiang W, Zha Z, Wang P, Yu W, Li Z, Gong L, Peng Y, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324:261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang W, Wu F, Zhao Z, Wang K, Huang R, Wang H, Lan Q, Wang J, Zhao J. Long noncoding RNA LINC00152 is a potential prognostic biomarker in patients with high-grade glioma. CNS Neurosci Ther. 2018;24:957–966. doi: 10.1111/cns.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang W, Zhao Z, Yang F, Wang H, Wu F, Liang T, Yan X, Li J, Lan Q, Wang J, Zhao J. An immune-related lncRNA signature for patients with anaplastic gliomas. J Neurooncol. 2018;136:263–271. doi: 10.1007/s11060-017-2667-6. [DOI] [PubMed] [Google Scholar]
- 84.Zhang EB, Yin DD, Sun M, Kong R, Liu XH, You LH, Han L, Xia R, Wang KM, Yang JS, et al. P53-regulated long non-coding RNA TUG1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death Dis. 2014;5:e1243. doi: 10.1038/cddis.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu Y, Wang J, Qiu M, Xu L, Li M, Jiang F, Yin R, Xu L. Upregulation of the long noncoding RNA TUG1 promotes proliferation and migration of esophageal squamous cell carcinoma. Tumour Biol. 2015;36:1643–1651. doi: 10.1007/s13277-014-2763-6. [DOI] [PubMed] [Google Scholar]
- 86.Gong W, Zheng J, Liu X, Ma J, Liu Y, Xue Y. Knockdown of NEAT1 restrained the malignant progression of glioma stem cells by activating microRNA let-7e. Oncotarget. 2016;7:62208–62223. doi: 10.18632/oncotarget.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang X, Xiao Z, Du X, Huang L, Du G. Silencing of the long non-coding RNA NEAT1 suppresses glioma stem-like properties through modulation of the miR-107/CDK6 pathway. Oncol Rep. 2017;37:555–562. doi: 10.3892/or.2016.5266. [DOI] [PubMed] [Google Scholar]
- 88.Mineo M, Ricklefs F, Rooj AK, Lyons SM, Ivanov P, Ansari KI, Nakano I, Chiocca EA, Godlewski J, Bronisz A. The long Non-coding RNA HIF1A-AS2 facilitates the maintenance of mesenchymal glioblastoma Stem-like cells in hypoxic niches. Cell Rep. 2016;15:2500–2509. doi: 10.1016/j.celrep.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu M, Xue Y, Zheng J, Liu X, Yu H, Liu L, Li Z, Liu Y. Linc00152 promotes malignant progression of glioma stem cells by regulating miR-103a-3p/FEZF1/CDC25A pathway. Mol Cancer. 2017;16:110. doi: 10.1186/s12943-017-0677-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Coccia EM, Cicala C, Charlesworth A, Ciccarelli C, Rossi GB, Philipson L, Sorrentino V. Regulation and expression of a growth arrest-specific gene (gas5) during growth, differentiation, and development. Mol Cell Biol. 1992;12:3514–3521. doi: 10.1128/MCB.12.8.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao X, Liu Y, Zheng J, Liu X, Chen J, Liu L, Wang P, Xue Y. GAS5 suppresses malignancy of human glioma stem cells via a miR-196a-5p/FOXO1 feedback loop. Biochim Biophys Acta Mol Cell Res. 2017;1864:1605–1617. doi: 10.1016/j.bbamcr.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 92.Jia P, Cai H, Liu X, Chen J, Ma J, Wang P, Liu Y, Zheng J, Xue Y. Long non-coding RNA H19 regulates glioma angiogenesis and the biological behavior of glioma-associated endothelial cells by inhibiting microRNA-29a. Cancer Lett. 2016;381:359–369. doi: 10.1016/j.canlet.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 93.Ma Y, Wang P, Xue Y, Qu C, Zheng J, Liu X, Ma J, Liu Y. PVT1 affects growth of glioma microvascular endothelial cells by negatively regulating miR-186. Tumour Biol. 2017;39:1393395338. doi: 10.1177/1010428317694326. [DOI] [PubMed] [Google Scholar]
- 94.Ma Y, Xue Y, Liu X, Qu C, Cai H, Wang P, Li Z, Li Z, Liu Y. SNHG15 affects the growth of glioma microvascular endothelial cells by negatively regulating miR-153. Oncol Rep. 2017;38:3265–3277. doi: 10.3892/or.2017.5985. [DOI] [PubMed] [Google Scholar]
- 95.Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–68. doi: 10.1016/j.canlet.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 96.Wang Q, Hu B, Hu X, Kim H, Squatrito M, Scarpace L, deCarvalho AC, Lyu S, Li P, Li Y, et al. Tumor evolution of Glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell. 2017;32:42–56.e6. doi: 10.1016/j.ccell.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hanahan D, Coussens LM. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 98.Shahar T, Rozovski U, Hess KR, Hossain A, Gumin J, Gao F, Fuller GN, Goodman L, Sulman EP, Lang FF. Percentage of mesenchymal stem cells in high-grade glioma tumor samples correlates with patient survival. Neuro Oncol. 2017;19:660–668. doi: 10.1093/neuonc/now239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yi D, Xiang W, Zhang Q, Cen Y, Su Q, Zhang F, Lu Y, Zhao H, Fu P. Human Glioblastoma-derived mesenchymal stem cell to pericytes transition and angiogenic capacity in glioblastoma microenvironment. Cellular physiology and Biochemistry. 2018;46:279–290. doi: 10.1159/000488429. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Q, Yi D, Xue B, Wen WW, Lu YP, Abdelmaksou A, Sun MX, Yuan DT, Zhao HY, Xiong NX, et al. CD90 determined two subpopulations of glioma-associated mesenchymal stem cells with different roles in tumour progression. Cell Death Dis. 2018;9:1101. doi: 10.1038/s41419-018-1140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Q, Xiang W, Yi D, Xue B, Wen W, Abdelmaksoud A, Xiong N, Jiang X, Zhao H, Fu P. Current status and potential challenges of mesenchymal stem cell-based therapy for malignant gliomas. Stem Cell Res Ther. 2018;9:228. doi: 10.1186/s13287-018-0977-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.