Abstract
Background
Medications licensed for the treatment of dementia have limited efficacy against cognitive impairment or against the distressed behaviours (behavioural and psychological symptoms, or behaviour that challenges) which are also often the most distressing aspect of the disorder for caregivers. Complementary therapies, including aromatherapy, are attractive to patients, practitioners and families, because they are perceived as being unlikely to cause adverse effects. Therefore there is interest in whether aromatherapy might offer a safe means of alleviating distressed behaviours in dementia.
Objectives
To assess the efficacy and safety of aromatherapy for people with dementia.
Search methods
We searched ALOIS, the Cochrane Dementia and Cognitive Improvement Group Specialized Register, on 5 May 2020 using the terms: aromatherapy, lemon, lavender, rose, aroma, alternative therapies, complementary therapies, essential oils. In addition, we searched MEDLINE, Embase, PsycINFO (all via Ovid SP), Web of Science Core Collection (via Thompson Web of Science), LILACS (via BIREME), CENTRAL (via the Cochrane Library), ClinicalTrials.gov and the World Health Organization (WHO) trials portal (ICTRP) on 5 May 2020.
Selection criteria
We included randomised controlled trials which compared fragrance from plants in an intervention defined as aromatherapy for people with dementia with placebo aromatherapy or with treatment as usual. All doses, frequencies and fragrances of aromatherapy were considered. Participants in the included studies had a diagnosis of dementia of any subtype and severity.
Data collection and analysis
Two reviewers independently selected studies for inclusion, extracted data and assessed risk of bias in included studies, involving other authors to reach consensus decisions where necessary. We did not perform any meta‐analyses because of heterogeneity between studies, but presented a narrative synthesis of results from the included trials. Because of the heterogeneity of analysis methods and inadequate or absent reporting of data from some trials, we used statistical significance (P ≤ or > 0.5) as a summary metric when synthesising results across studies. As far as possible, we used GRADE methods to assess our confidence in the results of the trials, downgrading for risk of bias and imprecision.
Main results
We included 13 studies with 708 participants. All participants had dementia and in the 12 trials which described the setting, all were resident in institutional care facilities. Nine trials recruited participants because they had significant agitation or other behavioural and psychological symptoms in dementia (BPSD) at baseline. The fragrances used were lavender (eight studies); lemon balm (four studies); lavender and lemon balm, lavender and orange, and cedar extracts (one study each). For six trials, assessment of risk of bias and extraction of results was hampered by poor reporting. Four of the other seven trials were at low risk of bias in all domains, but all were small (range 18 to 186 participants; median 66), reducing our confidence in the results. Our primary outcomes were agitation, overall behavioural and psychological symptoms, and adverse effects. Ten trials assessed agitation using various scales. Among the five trials for which our confidence in the results was moderate or low, four trials reported no significant effect on agitation and one trial reported a significant benefit of aromatherapy. The other five trials either reported no useable data or our confidence in the results was very low. Eight trials assessed overall BPSD using the Neuropsychiatric Inventory and we had moderate or low confidence in the results of five of them. Of these, four reported significant benefit from aromatherapy and one reported no significant effect. Adverse events were poorly reported or not reported at all in most trials. No more than two trials assessed each of our secondary outcomes of quality of life, mood, sleep, activities of daily living, caregiver burden. We did not find evidence of benefit on these outcomes. Three trials assessed cognition: one did not report any data and the other two trials reported no significant effect of aromatherapy on cognition. Our confidence in the results of these studies was low.
Authors' conclusions
We have not found any convincing evidence that aromatherapy (or exposure to fragrant plant oils) is beneficial for people with dementia although there are many limitations to the data. Conduct or reporting problems in half of the included studies meant that they could not contribute to the conclusions. Results from the other studies were inconsistent. Harms were very poorly reported in the included studies. In order for clear conclusions to be drawn, better design and reporting and consistency of outcome measurement in future trials would be needed.
Plain language summary
Aromatherapy for dementia
Background to the review Medication prescribed for the treatment of dementia is not always effective at relieving symptoms of the condition such as problems with thinking, behaviour, mood, and sleep. Natural therapies, including aromatherapy (the use of fragrant essential oils from plants), are attractive options for treating these distressing symptoms of dementia as they are often thought to have a low risk of side effects.
Review question Is aromatherapy safe and effective at relieving symptoms of dementia?
What we did We searched the medical literature up to 5 May 2020, looking for studies which compared aromatherapy for people with dementia to a control treatment, which could be either usual care or ‘dummy’ aromatherapy involving a non‐fragrant oil. To make the comparison fair, the studies had to assign people randomly to aromatherapy or to the control treatment. We looked at the effect on agitation, behavioural and mental health issues, and other important symptoms of dementia. We also looked for reports of side effects. Because the studies were so different from each other, we were not able to combine results statistically so we described the results of individual studies and assessed how confident we could be in them.
Study characteristics We found 13 studies to include in the review. There were 708 participants in total. All had dementia and were living in care homes. The most commonly used aromatherapy fragrance was lavender. Studies also used lemon balm, orange and cedar extracts.
Main findings Ten studies assessed agitation, but five did not report data we could use or our confidence in their results was very low. We had moderate or low confidence in the results of the other five: four reported no significant effect of aromatherapy and one reported a significant benefit. Eight studies assessed behavioural and mental health issues, but three did not report any usable data, or our confidence in the results was very low. Of the other five, for which our confidence was moderate or low, four reported a significant benefit from aromatherapy and one reported no significant effect. Side effects of treatment were either poorly reported or not reported at all. No more than three studies reported our secondary outcomes which were quality of life, cognition (thinking), mood, sleep, activities of daily living, and caregiver burden. We found no evidence that aromatherapy was helpful for any of these outcomes.
Quality of the evidence Overall the quality of the evidence was poor. Many of the studies were poorly reported and some did not report any data we could use. Most studies were very small so that there was a lot of uncertainty about their results. Results of different studies did not agree with one another.
Conclusions We have found no convincing evidence that aromatherapy is beneficial for people with dementia although there are many limitations to the data reported by the studies so conclusions cannot be drawn with confidence. In order to determine whether aromatherapy is safe and effective at relieving symptoms of dementia, larger, well‐designed studies with clearer reporting are needed.
Summary of findings
Summary of findings 1. Aromatherapy versus control (placebo aromatherapy / no intervention) for dementia.
| Aromatherapy versus control (placebo aromatherapy / no intervention) for dementia | |||
| Patient or population: Dementia Setting: Care facilities or hospital wards Intervention: Aromatherapy Comparison: Control (placebo aromatherapy / no intervention) | |||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE) |
| Agitation assessed with: CMAI, PAS, individual study assessment tools follow up: range 1 to 12 weeks | 5 trials provided either no usable data or data in which our confidence was very low. Of the remaining 5 trials, 4 reported no statistically significant effect on agitation and 1 reported a significant benefit. | 593 (10 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 4 |
| Overall behavioural and psychological symptoms assessed with: NPI follow up: range 2 to 12 weeks | 3 trials provided either no useable data or data in which our confidence was very low. Of the remaining 5 trials, 4 trials reported a significant reduction in overall behavioural and psychological symptoms and 1 trial did not find a significant effect of aromatherapy. | 346 (8 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 4 5 |
| Adverse effects follow up: range 1 to 12 weeks | Adverse effects were reported in only 4 of 12 trials. None reported any adverse effects. | 206 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 4 |
| Quality of life assessed with: Blau Quality of Life, Dementia Care Mapping follow up: range 4 to 12 weeks | 1 trial reported a significant beneficial effect of aromatherapy on quality of life. The other trial did not find any significant effect of aromatherapy on quality of life. | 134 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 4 6 7 |
| Mood assessed with: CSDD‐C, PGCARS follow up: range 1 to 9 weeks | 1 trial reported no significant effect of aromatherapy on mood. The other trial reported a statistically significant beneficial effect of aromatherapy on depressive symptoms. | 120 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 4 8 |
| Sleep | 1 trial provided no useable data. | 21 (1 RCT) |
‐ |
| Activities of daily living assessed with: Barthel Index for Activities of Daily Living, follow up: 12 weeks | 1 trial provided no useable data. 1 trial found no significant effect of aromatherapy on activities of daily living. | 91 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 4 10 |
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | |||
1 Inconsistency: downgraded by 1 level due to inconsistent findings.
2 Risk of bias: downgraded by 1 level due to study limitations. Random sequence generation (selection bias): low risk of bias in 6 studies, unclear risk of bias in 4 studies. Allocation concealment (selection bias): low risk of bias in 6 studies, unclear risk of bias in 4 studies. Blinding of participants and personnel (performance bias): low risk of bias in 6 studies, unclear risk of bias in 3 studies, high risk of bias in 1 study. Blinding of outcome assessment (detection bias): low risk of bias in 8 studies, unclear risk of bias in 1 study, high risk of bias in 1 study. Incomplete outcome data: low risk of bias in 8 studies, unclear risk of bias in 1 study, high risk of bias in 1 study. Selective reporting (reporting bias): low risk of bias in 7 studies, high risk of bias in 3 studies. Other bias: low risk of bias in 9 studies, unclear risk of bias in 1 study.
3 Imprecision: downgraded by 2 levels due to small sample size in all studies.
4 Publication bias: downgraded by 1 level because included studies did not publish usable data on outcomes they measured.
5 Risk of bias: downgraded by 1 level due to study limitations. Random sequence generation (selection bias): low risk of bias in 4 studies, unclear risk of bias in 4 studies. Allocation concealment (selection bias): low risk of bias in 4 studies, unclear risk of bias in 4 studies. Blinding of participants and personnel (performance bias): low risk of bias in 5 studies, unclear risk of bias in 1 study, high risk of bias in 2 studies. Blinding of outcome assessment (detection bias): low risk of bias in 5 studies, unclear risk of bias in 2 studies, high risk of bias in 1 study. Incomplete outcome data (attrition bias): low risk of bias in 4 studies, unclear risk of bias in 2 studies, high risk of bias in 2 studies. Selective reporting (reporting bias): low risk of bias in 7 studies, high risk of bias in 1 study. Other bias: low risk of bias in 7 studies, high risk of bias in 1 study.
6 Risk of bias: Ballard 2002 and Burns 2011 were at low risk of bias in all domains.
7 Indirectness: downgraded by 1 level due to Ballard 2002 using Dementia Care Mapping to assess quality of life, which we consider to be an indirect measure.
8 Risk of bias: downgraded by 1 level due to study limitations. Random sequence generation (selection bias): low risk of bias in 1 study, unclear risk of bias in 1 study. Allocation concealment (selection bias): low risk of bias in 1 study, unclear risk of bias in 1 study. Blinding of participants and personnel (performance bias): low risk of bias in 1 study, unclear risk of bias in 1 study. Blinding of outcome assessment (detection bias): low risk of bias in both studies. Incomplete outcome data (attrition bias): low risk of bias in both studies. Selective reporting (reporting bias): low risk of bias in 1 study, high risk of bias in 1 study. Other bias: low risk of bias in both studies.
9 Risk of bias: downgraded by 1 level due to study limitations. Random sequence generation (selection bias): unclear risk of bias. Allocation concealment (selection bias): unclear risk of bias. Blinding of participants and personnel (performance bias): low risk of bias. Blinding of outcome assessment (detection bias): low risk of bias. Incomplete outcome data (attrition bias): high risk of bias. Selective reporting (reporting bias): low risk of bias. Other bias: high risk of bias.
10 Risk of bias: downgraded by 1 level due to study limitations. Random sequence generation (selection bias): low risk of bias in 1 study, unclear risk of bias in 1 study. Allocation concealment (selection bias): low risk of bias in 1 study, unclear risk of bias in 1 study. Blinding of participants and personnel (performance bias): low risk of bias in 1 study, high risk of bias in 1 study. Blinding of outcome assessment (detection bias): low risk of bias in both studies. Incomplete outcome data (attrition bias): low risk of bias in both studies. Selective reporting (reporting bias): low risk of bias in both studies. Other bias: low risk of bias in both studies.
Background
Description of the condition
Dementia is a condition in which acquired cognitive impairment is severe enough to affect a person's ability to manage everyday activities. Usually it occurs in later life and is caused by progressive, neurodegenerative conditions, of which the most common are Alzheimer's disease and cerebrovascular disease. The cognitive deficits are often accompanied by psychiatric and behavioural symptoms, such as apathy, mood changes and, especially in the later stages of the illness, agitated behaviours (such as restlessness, shouting or physical aggression to carers) which may be expressions of distress (Kales 2015). Dementia is devastating both to the person directly affected and to families, who undertake most of the care for people with dementia and who experience high levels of distress and burden (Cheng 2017). In high‐income countries it is estimated that 34% of patients with severe dementia are cared for in residential or nursing facilities (Prince 2015).
Dementia is a major healthcare challenge with an estimated 50 million people worldwide suffering from the condition and nearly 10 million new cases every year (WHO 2019). This creates an enormous challenge for informal and professional care systems. Currently, there are no medical treatments which can prevent or alter the course of any form of dementia. The licensed medications which are available have limited efficacy against cognitive symptoms and little or no effect on the distressed behaviours (behavioural and psychological symptoms, or behaviour that challenges) which often present the greatest burden to caregivers (Battle 2019; Birks 2006; Birks 2015; Birks 2018; McShane 2019). Other medications which are used for behavioural and psychological symptoms in dementia (BPSD) also have limited efficacy and have been associated with significant adverse effects (Reus 2016). National Institute for Health and Care Excellence (NICE) guidelines recommend non‐pharmacological strategies should be considered (NICE 2018). Many people with dementia and their carers use complementary and alternative therapies, but there is a lack of high‐quality research to guide their use (Alzheimer's Society 2014).
In the context of aromatherapy, it is pertinent to note that people with dementia have, as a population, a greater prevalence of olfactory impairment (impaired sense of smell) and that this may be a very early sign of some of the neurodegenerative diseases associated with cognitive decline (Bathini 2019).
Description of the intervention
Complementary (or alternative) therapies are popular approaches to a wide range of health problems. There is evidence to show that complementary medicine use is a substantial and growing part of healthcare behaviour in Europe, Australia and North America (Harris 2012). Aromatherapy is one of the main complementary therapies practised by nurses and other healthcare professionals in hospital, hospice, and community settings (Buckle 2003).
Aromatherapy is a part of the discipline of phytotherapy (the use of whole plants or parts of plants for medicinal purposes) and uses pure essential oils from fragrant plants (such as lavender (Lavandula angustifolia), lemon balm (Melissa officinalis), peppermint, sweet marjoram, and rose) to help relieve health problems and improve quality of life in general (OnHealth 2020). Essential oils have been defined as "highly fragrant essences extracted from plants by distillation, which evaporate readily" (Tisserand 1988). They may be applied directly to the skin or vaporised and administered through inhalation only.
Essential oils are many and varied, with presumed different potential effects. These are claimed to include promotion of relaxation and sleep, relief of pain, reduction of agitation and depressive symptoms (for example Spirit Scents 2020). Aromatherapy might be of particular use as an intervention for people who are confused, have little or no preserved language function, or for whom verbal interaction is difficult, and for whom conventional medicine is seen as being of only marginal benefit. Aromatherapy has, therefore, been used to address behavioural and psychological symptoms in dementia, aiming for example to reduce disturbed behaviour (Brooker 1997; Lin 2007; Nguyen 2008), promote sleep ( Hwang 2015; Wolfe 1996), and stimulate motivated behaviour (MacMahon 1998).
Essential oils selected for aromatherapy have been reported to have very low toxicity profiles and, if administered by qualified practitioners, have been presented as safer than conventional pharmacological medications (Perry 2006). However, common assumptions about the safety of aromatherapy have been questioned. A review of published case reports and case series found that aromatherapy has potential to cause adverse effects, some serious, and commented that the frequency of such effects is unknown (Posadzki 2012).
While pharmacological medications are highly standardised, extraction techniques for essential oils are variable across manufacturers (Barnes 2003). Other factors, such as agricultural, storage and processing factors, can also influence the content and concentration of constituents (Barnes 2003). There are, however, established systems of quality control (Shinde 2009; Turek 2013); and some manufacturers produce standardised extracts to achieve within‐manufacturer consistency, similar to pharmaceutical quality (Barnes 2003). The 'dose' delivered to each person also depends on the mode of delivery, the volume of oil, temperature, room size and air flow. Complete standardisation of treatments is therefore hard to achieve.
How the intervention might work
The essential oils used in aromatherapy are most commonly delivered through electric diffusers and vaporizers or massaged into the skin: thus the oil evaporates and the aroma stimulates the olfactory sense (Kong 2009). The aromas used are generally experienced as pleasant and so the immediate effect may be a positive emotional response. It has also been suggested that olfactory sensations may be effective means of stimulating implicit memories (Degel 2001). Although deterioration of explicit memory is a prominent symptom of dementia, there is evidence to suggest that implicit memory can remain intact in patients with the disease (Fleischman 2005). The implicit memory may include an emotional response based on the person's past experience (Holmes 2004). Some authors have also suggested pharmacological actions of essential oils, relating to for example inhibition of acetylcholinesterase (Arruda 2012).
Why it is important to do this review
Current guidelines issued by NICE in the UK recommend that aromatherapy may be considered to promote well‐being in people with dementia (NICE 2018). Despite such recommendations and an increase in popularity, the rationale for aromatherapy is based on limited scientific research, with the majority of evidence coming from studies at high risk of bias (case series, uncontrolled studies, etc.). Additionally, despite the implementation of regulatory processes such as the European Directive on Traditional Herbal Medicinal Products (Directive 2004/24/EC), the absence of a regulatory body to approve the manufacturing practice of unlicensed products such as essential oils makes it impossible to identify those that reach acceptable standards. Hence uncertainties about both efficacy and safety remain. This review aims to address these uncertainties by identifying and synthesising the best available evidence.
Objectives
To assess the efficacy and safety of aromatherapy for people with dementia.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) only.
Types of participants
Participants in the included studies had a diagnosis of dementia of any type and severity. We accepted formal diagnoses based on criteria such as the International Statistical Classification of Diseases and Related Health Problems (ICD‐10) (WHO 1993) and the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) (APA 1994), or clinical diagnoses, or cognitive test scores consistent with dementia on well‐validated assessment scales for cognitive function, such as the Mini‐Mental State Examination (MMSE) (Folstein 1975) and the Alzheimer's Disease Assessment Scale‐cognitive subscale (ADAS‐Cog) (Rosen 1994).
Types of interventions
We included trials which used fragrance from plants in an intervention defined as aromatherapy for people with dementia. There were no restrictions on fragrance, dose, frequency or duration of treatment.
The comparator group was placebo aromatherapy or treatment as usual/no treatment.
Types of outcome measures
Primary outcomes
Agitation
Overall behavioural and psychological symptoms (BPSD)
Adverse effects
Secondary outcomes
Quality of life
Mood
Sleep
Cognition
Activities of daily living
Caregiver burden or distress, or both
'Summary of findings' table
We used the GRADE approach to assess the overall quality of evidence behind each result (Schünemann 2008); and we used the GRADE profiler to import data from Review Manager 5 (RevMan 5) to create 'Summary of findings' tables (Review Manager 2014). These tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the intervention examined, and the sum of available data on the outcomes that we rated as most important to patient care. We selected the following outcomes for inclusion in the 'Summary of findings' tables.
Agitation
Overall behavioural and psychological symptoms
Adverse effects
Quality of life
Activities of daily living
Mood
Sleep
Search methods for identification of studies
Electronic searches
We searched ALOIS, the Cochrane Dementia and Cognitive Improvement Group Specialised Register, on 5 May 2020. The search terms we used were: aromatherapy, lemon, lavender, rose, aroma, alternative therapies, complementary therapies, essential oils.
ALOIS is maintained by the Information Specialists of the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia prevention, dementia treatment, and cognitive enhancement in healthy individuals. The studies are identified from:
quarterly search of the Central Register of Controlled Trials (CENTRAL) in the Cochrane Library;
monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL, PsycINFO, and LILACS;
monthly searches of a number of trial registers: International Standard Randomised Controlled Trial Number (ISRCTN); the World Health Organization (WHO) portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials; and the Netherlands National Trials Register; plus others);
six‐monthly searches of a number of grey literature sources: ISI Web of Science Conference Proceedings.
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL and conference proceedings can be viewed in the ‘Methods used in reviews’ section within the editorial information about the Dementia and Cognitive Improvement Group.
We performed additional searches in many of the sources listed above to ensure that the search for the review was as up to date and as comprehensive as possible. The search strategies we used can be seen in Appendix 1.
Electronic searches carried out in the previous versions of the review can be viewed in Appendix 2 and Appendix 3.
Searching other resources
We searched reference lists of included trials for additional studies.
Data collection and analysis
Selection of studies
For the original review, LTF and AS independently screened the titles and abstracts extracted by the searches for their eligibility for potential inclusion in the review based on the above criteria. They discussed this with MO.
For the 2008 update, FEH and TPHB assessed the new study found by the March 2008 search using the same criteria as previously used.
For the 2014 update, NM and KSW independently screened 28 studies, again using the same criteria as previously used.
For the 2020 update, BO‐B, AG and ELB independently screened titles and abstracts. BO‐B, AG, ELB, SDS, JH and JMcC all contributed to full‐text screening. At least two authors independently assessed the papers and we resolved disagreements by discussion with the full author team.
Data extraction and management
We extracted the data from the published reports. At least two authors independently extracted the data and we resolved disagreements by discussion with the full author team.
Assessment of risk of bias in included studies
For the original review, NM undertook assessment of the risk of bias of all the included trials according to the methods in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and KSW checked these. For the 2020 update, this was reviewed by ELB, BO‐B, JH, SDS and JMcC and the authors added the risk of bias for the newly included studies.
The 'Risk of bias' tool examines five key domains for bias: selection bias, performance bias, attrition bias, detection bias, and reporting bias. We assessed and classified each domain as either a low or a high risk of bias, or where insufficient detail was reported in a study to assess the risk we reported it as 'unclear'. In addition, we reported any other risk of bias noted in the study.
We used the Cochrane 'Risk of bias' tool in RevMan 5 (Review Manager 2014).
Measures of treatment effect
All outcomes in the review are continuous measures. We used the mean difference (MD) between groups and its 95% confidence interval (CI) as the measure of treatment effect.
Unit of analysis issues
Where studies used a cross‐over method, we intended to extract paired data. These were not available in any of the reports of the included cross‐over trials so we reported results based on analyses of unpaired data, recognising that this reduces the power of the study to detect an effect.
Where studies reported outcomes at more than one time point, we used the outcome data from the end of the intervention period where possible. A few studies reported data which summarised effects throughout the intervention period and we also included these.
Dealing with missing data
We reported the amount of missing outcome data in each trial. When reporting trialists' own analyses, we favoured intention‐to‐treat analyses and reported any imputation methods.
Assessment of heterogeneity
We assessed clinical heterogeneity between studies, considering the participants, characteristics of the intervention, and the outcomes reported.
Assessment of reporting biases
There was insufficient data for formal assessment of reporting biases.
Data synthesis
We considered the studies unsuitable for meta‐analysis because of clinical diversity, the heterogeneity of analysis methods and inadequate or absent reporting of outcome data from some trials. For these reasons, we present a narrative review of the results, using statistical significance (P ≤ or > 0.5) as a summary metric when synthesising results across studies.
Subgroup analysis and investigation of heterogeneity
We did not undertake any subgroup analyses.
Sensitivity analysis
We did not undertake any sensitivity analyses.
Summary of findings and assessment of the certainty of the evidence
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies for details of the studies considered for this review.
Results of the search
Searches up to and including May 2020 identified 3649 records. One record was identified through forward citation and one record through other sources. After we had removed duplicates, 2358 records remained. CDCIG information specialists were able to identify 1818 as irrelevant. Review authors screened 540 titles or abstracts and selected 71 to be assessed in full text. We excluded 43 records (see Characteristics of excluded studies); we identified two ongoing trials from trial registry entries (see Characteristics of ongoing studies); six further trials, also described in trial registry entries, and one published study are awaiting classification while we seek additional information about eligibility from trial authors (see Characteristics of studies awaiting classification). We included 13 trials, described in 19 records, in the current update (see Characteristics of included studies); seven of these trials were included in the last version of the review in 2014. The process of study selection is summarised in Figure 1.
1.
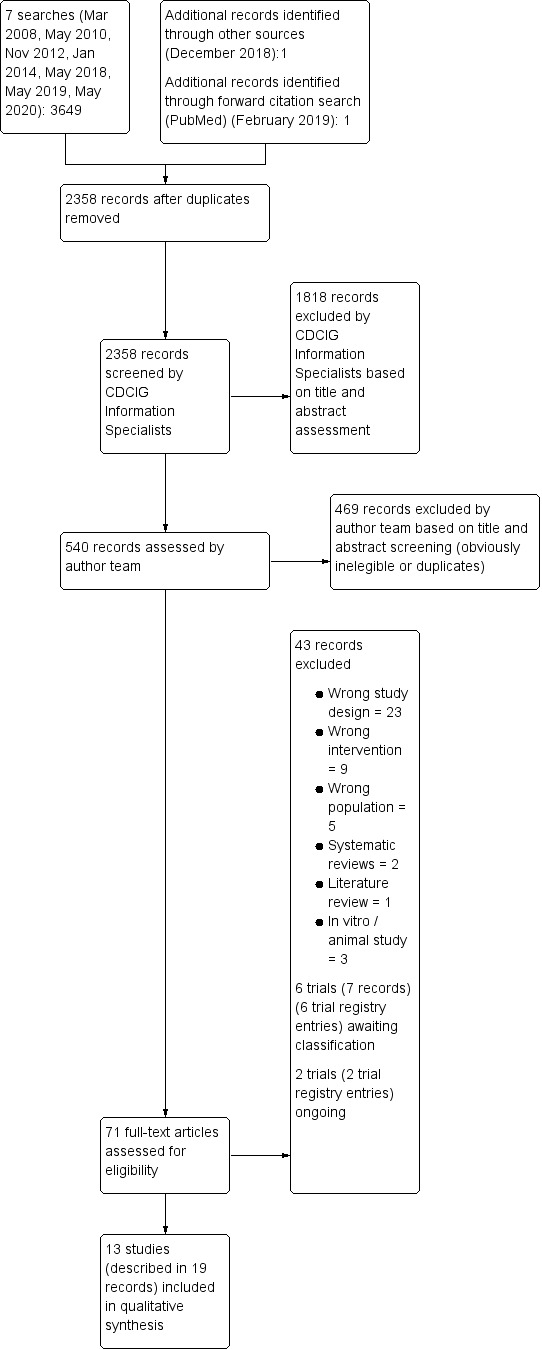
Study flow diagram.
Included studies
We included 13 studies with 708 participants (Ballard 2002; Burns 2011; Cameron 2011; Fu 2013; Fujii 2008; Hanson 2013; Lin 2007; O'Connor 2013; Smallwood 2001; Takahashi 2020; Watson 2019; Yang 2015; Yang 2016). One study was described only in a conference poster (Hanson 2013). For the 2014 version of this review, additional unpublished data was provided by the authors of Ballard 2002.
1. Study design
All trials were RCTs. Eleven trials randomised individuals and two were cluster‐RCTs (Ballard 2002 and Yang 2015) with residential care facilities as the unit of randomisation. In Ballard 2002 eight nursing homes were matched in pairs and within each pair homes were allocated randomly to active treatment or control. Similarly, Yang 2015 included three retirement homes in each of two categories: veterans' homes and other long‐term care facilities. When a veterans' home was randomly assigned to the aroma‐acupressure, aromatherapy, or control condition, a long‐term care facility was also assigned to this condition. Five trials used a cross‐over design (Cameron 2011, Hanson 2013, Lin 2007, Watson 2019 and O'Connor 2013). Hanson 2013 had no washout period between treatments. The other cross‐over trials used washout periods ranging from four days to two weeks in length.
2. Setting
Lin 2007 was conducted in Hong Kong; Yang 2015 and Yang 2016 were both based in Taiwan; Fujii 2008 and Takahashi 2020 were conducted in Japan. Fu 2013, Watson 2019 and O'Connor 2013 were based in Australia; Hanson 2013 was conducted in Minnesota, USA; and the remaining four studies were based in the UK.
Participants in 12 trials were resident in institutions, which were described in different ways. Ballard 2002 and Burns 2011 included residents in specialist nursing homes; Fu 2013 and Yang 2016 included participants from long‐term care facilities; Yang 2015 included participants from three long‐term care facilities and three retirement homes for veterans; Hanson 2013 recruited participants from memory care units in an assisted living facility; Lin 2007 was conducted in a 'care and attention home'; O'Connor 2013 recruited participants from eight specialist psychogeriatric nursing homes and three private nursing homes; Smallwood 2001 included inpatients in a district general hospital ward; Fujii 2008 included patients in long‐term care in a hospital; Cameron 2011 included inpatients but did not report the setting; and Watson 2019 included patients from six residential aged care facilities. One trial did not report the setting (Takahashi 2020).
3. Participants
In 11 studies, all participants were identified as having dementia. Nine of these trials also specified that participants should be exhibiting agitation or other BPSD at baseline. Hanson 2013 provided no information about participant diagnoses but recruited from memory care units in an assisted living facility. Watson 2019 included participants with and without dementia, but in this review we consider only the 56 participants with dementia. The mean age of participants included in the studies ranged from 66.8 years (Smallwood 2001) to 85.7 years (Hanson 2013) (no data from Cameron 2011). The mean age of all participants in Watson 2019 was 89.3 years, but demographic data was not provided separately for participants with dementia. The percentage of female participants was approximately 59% (no data from Cameron 2011 or from Watson 2019 for participants with dementia).
Ballard 2002 included 72 people with severe dementia, diagnosed with the Clinical Dementia Rating scale (Hughes 1982), and clinically significant agitation.
Burns 2011 included 114 participants with a National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA) diagnosis of probable or possible Alzheimer’s disease and agitation. Sixty‐three participants were randomised to the two groups included in this review.
In Cameron 2011 the 18 participants had moderate to severe dementia and 'behavioural and psychological symptoms in dementia' (BPSD); they did not report the diagnostic criteria.
Fu 2013 included 67 participants with cognitive functional impairment indicative of a dementia condition and features of Alzheimer’s disease according to the American Psychiatric Association DSM‐IV‐TR, with a documented history of agitation or aggression.
Fujii 2008 included 28 participants with dementia diagnosed according to DSM‐IV criteria.
Hanson 2013 included 22 participants but did not specify the participant inclusion criteria in the poster or abstract.
In Lin 2007, the participants were 70 Chinese older persons with dementia diagnosed according to the DSM‐IV and with clinically significant agitation (Chinese CMAI). The causes of dementia were reported as Alzheimer's disease, vascular and other unstated dementias.
O'Connor 2013 included 66 participants with at least mild dementia on the Clinical Dementia Rating scale and physically agitated behaviour not due primarily to pain, physical illness, depression, or psychosis.
Smallwood 2001 included 21 patients with a clinical diagnosis of severe dementia made by a psychiatrist.
Takahashi 2020 included 36 patients with a clinical diagnosis of Alzheimer's disease according to NINCDS/ADRDA.
Watson 2019 included 75 subjects with a ‘cognitive level of moderate or higher’ defined by an MMSE score greater than 10. Included in this review were the 56 participants (75%) who had a clinical diagnosis of dementia.
Yang 2015 included 186 participants who were diagnosed with dementia according to DSM‐IV criteria and scored 35 or above on the CMAI. One hundred and thirty participants were randomised to the two groups included in this review.
Yang 2016 included 59 people with mild to severe dementia who displayed symptoms of agitation or depression in the two weeks prior to the study. The diagnosis of dementia was based on the Short Portable Mental Status Question (SPMSQ) (scoring ≤ 8) or the Mini‐Mental State Examination (MMSE) (scoring ≤ 17 if the participant had a high school education or ≤ 23 if the participant had a high school education or higher. Agitation and depressive symptoms were reported by caregivers using the Chinese version of the CMAI (long version) and Cornell Scale for Depression in Dementia‐Chinese Version (CSDD‐C); cut‐offs for inclusion were not reported.
4. Interventions
Lavender was the most commonly used fragrance, administered in nine studies. In one study (Yang 2016), lavender oil was combined with orange oil. One study included both lavender and lemon balm aromatherapy groups (Watson 2019). Three studies used lemon balm aromatherapy only (Ballard 2002; Burns 2011; Cameron 2011). One study used aroma from cedar extracts (Takahashi 2020). Six studies administered aromatherapy using touch or massage (Ballard 2002; Burns 2011; Cameron 2011; Hanson 2013; O'Connor 2013; Yang 2016). Four studies administered aromatherapy by exposure to fragrance only (Fujii 2008; Lin 2007; Takahashi 2020; Watson 2019). Two studies used more than one application technique. In one of these studies (Fu 2013), aromatherapy was administered via a mist and accompanied with a hand massage, or just administered via the mist. Smallwood 2001 applied aromatherapy either through massage or via a diffuser, which was accompanied with conversation. One study administered aromatherapy by applying aromatherapy oil to acupressure points without any pressure (Yang 2015)
Ballard 2002 used 10% lemon balm and base oil applied topically to the arms and face for one to two minutes. The control condition was sunflower oil applied in the same way. The oil was applied twice daily for four weeks.
Burns 2011 used 10% lemon balm oil which was gently massaged on the hands and upper arms for one to two minutes. The control condition was sunflower oil applied in the same way. The oil was applied twice daily for 12 weeks. Both the aromatherapy and placebo aromatherapy groups received placebo medication. The study also included a third group which involved placebo aromatherapy and Donepezil medication; we have not, however, included this group in this review.
Cameron 2011 used less than 2% lemon balm oil aromatherapy which was applied by gently rubbing the patient's forearm for one minute twice a day. The control condition was 1% geranium and 0.5% lemon oil applied in the same way. There were two treatment phases of three weeks and a one‐week washout period between phases; and the trial was repeated after one year.
Fu 2013 had two aromatherapy intervention groups: both used 3% lavender mist aromatherapy, one group with and another without hand massage. Three sprays of the lavender mist were applied to the participants' upper chest. The control condition was water mist applied in the same way. The interventions were given twice a day for six weeks.
Fujii 2008 used lavender oil as the aromatherapy intervention. Two drops of lavender oil were applied to the collar of the patients' underwear, approximately one hour after meals, three times a day for four weeks. The control group did not receive any active treatment.
Hanson 2013 used lavender oil applied to the spine, back and neck at bedtime, followed by a 20‐minute diffuser containing lavender oil. The control condition was almond oil administered in the same way. During acclimation (week 1), while dressing for participants' bedtime, resident assistants applied lotion. Participants then received either lavender or placebo (almond) oil in week 2, and switched to the other oil during week 3. A diffuser containing the corresponding treatment was also turned on for 20 minutes.
Lin 2007 used 100% lavender essential oil which was dropped onto cosmetic cotton and placed into two diffusers at each side of the participant's pillow. The diffusers were used for at least one hour whilst the participants slept at night. The control condition was sunflower oil administered in the same way. One of the interventions was administered for the first three weeks of the study, followed by a two‐week washout period, and then the second intervention was administered for three weeks.
O'Connor 2013 used 30% lavender in jojoba oil which was massaged into both forearms for one minute each. The control condition was jojoba oil administered in the same way. The intervention was administered three times during the first week of the study when nursing staff reported that agitated behaviour was likely to be displayed. There was a four‐day washout period followed by the second intervention being administered three times in the last week of the study.
Smallwood 2001 used two aromatherapy groups: lavender applied topically through massage and lavender in a diffuser accompanied by conversation. The control condition was massage only using plain oil. The interventions were administered twice a week for four weeks.
Takahashi 2020 used cedar extracts in a distilled ethanol solution. Each day, 2.3 mL of the distilled solution with cedar extracts was placed in the residents' living room and bedroom and the solution was diffused using rattan sticks. A few times a day, the distilled solution with cedar was also sprayed as a mist on the patients' clothing and bedding. The control condition was the distilled ethanol solution without the cedar extracts, administered in the same way. The interventions were performed for eight weeks.
Watson 2019 used three interventions: lavender; lemon balm; and a placebo. Two drops of oils were applied to a cotton patch which was attached to the participant's collar for two hours for 14 consecutive days. This was followed by a washout period before commencing the next intervention.
Yang 2015 used 2.5% lavender oil which was applied at five acupoints, followed by a warm‐up exercise carried out for five minutes. The duration of each protocol was no longer than 15 minutes, and each protocol was conducted once per day for five days per week for four weeks total. The control condition was normal daily care routine continued as usual without interventions. The study also included a third group, the aroma‐acupressure group (five acupoints were pressed for two minutes with 2.5% lavender oil followed by a warm‐up exercise carried out for five minutes), but we are not including this group in this review.
Yang 2016 used three drops of both lavender and orange oil in 5 ml of “essential oil” applied topically around neck, shoulders and arms for 30 minutes once per week for eight weeks (weeks 2 to 9 of the study) as their intervention. The control condition was usual care with participation in regular activities (e.g. group singing, watching movies) in the long‐term care facilities.
5. Outcomes
Most trials applied validated outcome scales at baseline and at the end of the intervention period, or – in the case of the cross‐over trials – at the beginning and end of each treatment period. Less than half of the trials assessed outcomes at intermediate time points, but we did not include these data. Two trials (O'Connor 2013 and Smallwood 2001) used intensive observation to collect outcome data before and after each treatment application and synthesised these data to provide an outcome score. Similarly Hanson 2013, which was the only trial to assess sleep, used actigraph data collected throughout the whole intervention period to derive their sleep outcome. Only two trials (Fu 2013 and Yang 2015) looked for persistent effects by re‐assessing outcomes six and three weeks respectively after the end of the intervention period.
Outcome assessment tools
1. Agitation
i) Cohen‐Mansfield Agitation Inventory (CMAI) (Cohen‐Mansfield 1999): in Ballard 2002, Cameron 2011, Watson 2019 and Fu 2013, which used the short version; and Lin 2007, Yang 2015 and Yang 2016, which used the Chinese version of this scale. This is a seven‐point rating scale that assesses the frequency of agitated behaviour. A higher score indicates more agitation.
ii) Pittsburgh Agitation Scale (PAS) (Rosen 1994): in Burns 2011 and Cameron 2011. This scale measures agitation using four behaviour groups of aberrant vocalisation, motor agitation, aggressiveness, and resisting care. A higher score indicates more agitation.
iii) O'Connor 2013 measured agitation by recording whether the behaviour was absent or present over three 30‐minute observation periods.
iv) Smallwood 2001 used video records to assess agitated behaviour at baseline and immediately after treatment. Smallwood 2001 used a video camera to record behaviour for 15‐minute periods over a day in a specified sequence and frequency. The video records were sampled and coded into six behaviour categories developed by two blinded raters.
2. Overall behavioural and psychological symptoms
i) Neuropsychiatric Inventory (NPI) (Cummings 1994): in Ballard 2002, Burns 2011, Cameron 2011, Fujii 2008, Hanson 2013, Takahashi 2020, and Watson 2019; and Lin 2007, using the Chinese version of this scale. This scale assesses either 10 or 12 behavioural disturbances common in dementia: delusions, hallucinations, dysphoria, anxiety, agitation or aggression, euphoria, disinhibition, irritability or lability, apathy, and aberrant motor activity. A higher score indicates greater severity of these behaviours.
3. Adverse effects
i) Adverse effects were measured in Burns 2011, Cameron 2011, Fu 2013 and O'Connor 2013.
4. Quality of life
i) Blau Quality of Life (Blau 1977): in Burns 2011. This scale measures subjective quality of life in a mental health setting using 10 items. A higher score indicates better quality of life.
ii) Dementia Care Mapping (Kitwood 1992): in Ballard 2002. Dementia Care Mapping is an observational method to evaluate quality of care and life in people with dementia.
5. Mood
i) Cornell Scale for Depression in Dementia‐Chinese Version (CSDD‐C) (Lin 2008): in Yang 2016, using the Chinese version of this scale. This scale uses a comprehensive interviewing approach that derives information from the patient and the informant. The interviews focus on depressive symptoms and signs occurring during the week preceding the interview. The final ratings of the CSDD items represent the rater's clinical impression rather than the responses of the informant or the patient.
ii) Philadelphia Geriatric Center Affect Rating Scale (PGCARS) (Lawton 1996): in O'Connor 2013. This scale assesses affect including pleasure, anger, sadness, contentment, interest and anxiety/fear.
6. Sleep
i) Hanson 2013 assessed sleep by measuring time spent sleeping across four epochs of actigraph data and a sleep log completed by staff.
7. Cognition
i) Mini Mental State Examination (MMSE) (Folstein 1975): in Fu 2013 and Fujii 2008. This scale measures cognitive impairment. A higher score indicates less cognitive impairment.
ii) Alzheimer's Disease Assessment Scale‐cognitive subscale (ASDAS‐cog) (Rosen 1984): in Takahashi 2020. This scale measures cognitive dysfunction in Alzheimer's disease.
8. Activities of daily living
i) Barthel Scale of Activities of Daily Living (Mahoney 1965): in Burns 2011 and Fujii 2008. This scale measures performance in activities of daily living. A higher score indicates better functioning.
9. Caregiver burden or distress, or both
i) Japanese version of the Zarit Caregiver Burden interview (J‐ZBI) (Arai 1997): in Takahashi 2020. This tool measures caregiver burden.
6. Additional data obtained from study authors
For the previous version of this review (Forrester 2014), Professor Ballard provided access to the individual patient data from his cluster‐randomised study (Ballard 2002). Reviewers performed analyses additional to those that had been published using the PROC MIXED procedure in SAS® 1999. The nursing homes were the units of randomisation. For each outcome, the mean change from baseline of all residents within a home was the outcome value for the home. The treatment effect for an outcome was the difference between the overall means of the four homes on treatment and the four homes on placebo (Table 2). The contribution from each home was weighted and this weight depended on the precision of the mean value for each home. Analysis of covariance was used for all outcomes, with the nursing home being treated as a random effect. There were several participant‐level covariates that could be included in the model, such as age, sex, baseline outcomes, and the medication being taken (Table 3). When tested in the model for each outcome, the only medication variable that had a significant effect was whether the patient was taking atypical antipsychotics. Sex and the baseline value of the outcome measure also had significant effects. Therefore, the estimate of the treatment effect was adjusted for sex, baseline measure of the outcome, and use of atypical antipsychotic medication.
1. Effect of aromatherapy compared with placebo (Ballard 2002).
| OUTCOME | Effect (S.E.) | T value | P value | 95% confidence interval | Favours |
| CMAI total (change from baseline at 4 weeks) | −11.08 (3.62) | −3.06 | 0.022 | −19.95 to −2.21 | Aromatherapy |
| CMAI physical aggression (change from baseline at 4 weeks) | −3.27 (1.78) | −1.84 | 0.115 | −7.62 to 1.80 | ‐ |
| CMAI physical non‐aggressive (change from baseline at 4 weeks) | −5.36 (1.42) | −3.77 | 0.009 | −8.84 to −1.88 | Aromatherapy |
| CMAI verbal aggression (change from baseline at 4 weeks) | −0.39 (0.49) | −0.80 | 0.456 | −1.58 to 0.81 | ‐ |
| CMAI verbal non‐aggressive (change from baseline at 4 weeks) | −2.92 (0.91) | −3.22 | 0.018 | −5.14 to −0.70 | Aromatherapy |
| NPI total (change from baseline at 4 weeks) | −15.80 (3.50) | −4.51 | 0.004 | −24.37 to −7.22 | Aromatherapy |
| NPI agitation (change from baseline at 4 weeks) | −2.31 (0.89) | −2.59 | 0.041 | −4.50 to −0.12 | Aromatherapy |
| NPI aberrant motor behaviour (change from baseline at 4 weeks) | −3.01 (1.23) | −2.45 | 0.050 | −6.02 to 0.00 | Aromatherapy |
CMAI ‐ Cohen Mansfield Agitation Inventory NPI ‐ Neuropsychiatric Inventory
2. Baseline characteristics for each group (Ballard 2002).
| VARIABLE | CONTROL | TREATMENT |
| Age | 79.7 (8.5) | 77.2 (7.6) |
| CMAITOT | 60.6 (16.6) | 68.3 (15.0) |
| NPITOT | 34.9 (15.0) | 37.6 (17.6) |
| Number taking atypical neuroleptic medication | 12/36 | 16/36 |
| Number taking benzodiazepine | 19/36 | 16/36 |
| Number taking antidepressant medication | 7/36 | 19/36 |
| Number taking neuroleptic medication | 18/36 | 23/36 |
| Number taking other psychotropic medication | 12/36 | 14/36 |
| Number taking any psychotropic medication | 33/36 | 33/36 |
| Number taking cognitive enhancer | 0/36 | 1/36 |
CMAITOT ‐ Cohen Mansfield Agitation Inventory Total score NPITOT ‐ Neuropsychiatric Inventory Total score
Excluded studies
We excluded 43 studies: two were systematic reviews and one was a literature review; two studies were in vitro studies and one was an animal study; 21 were not randomised; two did not have a control condition; nine studies did not have aromatherapy as the intervention; and in five studies the participants did not have dementia.
Ongoing studies
There were two ongoing studies ACTRN12617001159347 and ChiCTR‐INR‐17013281. Our attempts to contact the authors for further information were unsuccessful. See Ongoing studies for details.
Risk of bias in included studies
See also Characteristics of included studies, Figure 2, and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We considered six studies to be at low risk of bias for sequence generation and seven studies to be at unclear risk. For allocation concealment, we considered the risk of bias to be low in six trials and unclear in all remaining trials.
Blinding
Seven trials had a low risk of bias for blinding of participants and personnel, four were unclear, and two trials were high risk. Ten were low risk for blinding of outcome assessors, two trials were unclear, and one trial was high risk. Authors went to considerable lengths to try to blind personnel, including use of nose pegs and masking oils applied to the upper lip of personnel administering the treatments. Active and placebo oils were identified by letters and provided in identical containers. In some studies, personnel were not informed of the study hypothesis. We did not consider lack of blinding of participants to present a major risk of bias because of their degree of cognitive impairment.
Incomplete outcome data
We considered the risk of bias due to incomplete outcome data to be low in nine trials; unclear in two trials due to lack of information (Cameron 2011; Takahashi 2020); and high in two trials (Hanson 2013 where outcome data were reported for only 50% of participants; Watson 2019 where there was no reporting of attrition by group).
Selective reporting
Three studies had a high risk of bias for selective reporting; the other studies had a low risk of bias.
Other potential sources of bias
We rated Yang 2015 as unclear bias because there was possible recruitment bias before or after cluster allocation. There was also possible analysis bias as the study does not specify whether clustering was taken into account in the statistical analysis. We rated Hanson 2013 as high risk of bias as limited information about the methods was presented on the conference poster and abstract. We rated the remaining studies as low risk of bias.
Effects of interventions
See: Table 1
The diversity of the data and inadequate reporting in many studies meant that no pooling of data was possible. We present here the results from each included study. For each study, we also make an assessment of our confidence in the result, taking into account concerns about risk of bias, analysis method, imprecision and indirectness.
Unless otherwise stated, all the results reported are from the end‐of‐treatment time points.
We report only total CMAI and total NPI scores and not domain‐specific subscores.
Aromatherapy versus placebo
Primary outcomes
1. Agitation
Ten studies measured the effects of aromatherapy on agitated behaviour.
Ballard 2002 (71 participants) applied lemon balm essential oil or placebo oil twice daily for four weeks. Agitation was assessed using the CMAI. The authors of a previous version of this review conducted analyses on individual patient data provided by the study authors (see point 6 in Included studies above). The analysis was adjusted for clustering and for several participant‐level covariates. The mean difference between aromatherapy and placebo groups in change from baseline in the mean total CMAI score after four weeks of treatment was −11.10 favouring the aromatherapy group (95% CI −20.00 to −2.20; 1 study, 71 participants). The study was at low risk of bias in all domains and we were moderately confident in this result (downgraded one level due to imprecision because of the small sample size).
Burns 2011 (63 participants) applied lemon balm essential oil or placebo oil twice daily for 12 weeks. Agitation was assessed using the PAS at baseline and after four and 12 weeks of treatment. PAS data were reported as medians because of a non‐normal distribution and treatment groups were compared using a Kruskal‐Wallis test. The authors reported no significant difference between placebo and aromatherapy groups on the PAS at week 4 or week 12. The study was at low risk of bias in all domains and we were moderately confident in this result (downgraded one level due to imprecision).
Cameron 2011 (18 participants) applied lemon balm oil or placebo oil twice a day for three weeks. This was a cross‐over study. Agitation was assessed using the PAS and CMAI. No numerical data were reported. The authors reported no significant difference between treatment groups, but no details were given of the analysis method. We had very low confidence in this result due to lack of information on participant attrition, outcome data and analysis methods, and the very small sample size.
Fu 2013 (61 participants) applied 3% lavender mist aromatherapy twice a day for six weeks. Water mist was used in the control condition. Agitation was assessed using the CMAI‐SF. They did not report any numerical data for total CMAI‐SF scores, nor any analysis of between‐group differences.
Lin 2007 (70 participants) administered lavender essential oil or a placebo oil in a diffuser for one hour per night for three weeks. Agitation was assessed using the Chinese version of the CMAI (CCMAI). This was a cross‐over study. Mean (SD) scores on the CCMAI were reported for aromatherapy and placebo groups at baseline and after three weeks of treatment. Paired data were not reported. First‐period‐only data were not reported. We used the final scores reported for each treatment group to calculate a mean difference between aromatherapy and placebo groups after three weeks of treatment. Negative scores favour the aromatherapy group. The MD was −5.13 (95% CI −13.21 to 2.95; 1 study, 70 participants). We had low confidence in this result due to serious concerns about risk of bias (especially lack of blinding of outcome assessment), our inability to conduct or report a paired analysis suitable to the cross‐over design, and imprecision.
O'Connor 2013 (64 participants) administered lavender essential oil or control oil three times over the course of one week. Physically agitated behaviours were measured by observing and counting target behaviours for 30 minutes before the intervention and 60 minutes after the intervention and then calculating a mean behaviour count for each of one 30‐minute pre‐exposure period and two 30‐minute post‐exposure periods. This was a cross‐over study. Paired data were not reported. First‐phase‐only data were not reported. Data were analysed using binomial regression. The study reports that behaviour counts reduced significantly following both aromatherapy and placebo interventions, but that there was no statistically significant treatment effect (no significant 'treatment × time' interaction). The risk of bias was low in all domains. Our confidence in the study was moderate, reduced due to imprecision.
Smallwood 2001 (21 participants) administered lavender oil or a control oil either via a diffuser or via massage, twice a week for four weeks. Agitation was assessed by measuring motor behaviour from 15‐minute video recordings of participants. No numerical data were reported. The authors compared the two aromatherapy conditions and the placebo group using a one‐way ANOVA and reported no significant between‐group differences (P > 0.1 for all comparisons). We had very low confidence in this result due to imprecision, indirectness (motor behaviour as a proxy for agitated behaviour), lack of data presented in the paper, and risk of bias.
Watson 2019 (39 participants) administered lavender, lemon balm or placebo oil once a day for two weeks. Agitation was assessed using the CMAI, but the study did not report any data on CMAI scores in the separate treatment groups and did not report any relevant analysis of between‐group differences.
Yang 2015 (130 participants) administered lavender oil once per day, five days a week for four weeks. The comparator was no intervention. Agitation was assessed using the CCMAI. This was a cluster‐randomised trial. There was no indication that analyses were adjusted for clustering. The authors reported that the CMAI score was significantly higher in the aromatherapy group than the control (no intervention) group before treatment. From the data given, there was no change between baseline and end of treatment in the CMAI score in the aromatherapy group; the CMAI score in the control group increased over the treatment period to a score very similar to that in the aromatherapy group, suggesting that the authors’ conclusion of a positive effect of aromatherapy may have been attributable largely to the baseline imbalance. Our confidence in the results of this study was very low due to serious concerns about risk of bias, incorrect analysis and imprecision.
Yang 2016 (56 participants) administered lavender and orange oil once per week for eight weeks. The comparator was no intervention. Agitation was assessed using modified scoring of the 24‐hour CCMAI (no information on validation given). Measurements were made at baseline, and 24 hours after massage halfway through the 8‐week intervention and at the end of the intervention period. The authors used a general linear model repeated measurement analysis and they report that “no significant difference was noted between groups regarding overall agitation” (P = 0.316). Our confidence in this result was low due to serious concerns about risk of bias and imprecision.
In summary, although agitation was an outcome in 10 trials, two of them provided no relevant data or analyses and our confidence in the results of three more was very low. We had low confidence in the results of two trials, in both of which aromatherapy had no significant effect on agitation. We were moderately confident in the results of the three remaining trials, which were of a similar size and used similar interventions. One of these trials found a statistically significant effect of aromatherapy on agitation while the other two did not. The balance of the evidence is against a positive effect of aromatherapy on agitation, but inconsistency, serious study limitations, imprecise results and publication bias make this a tentative conclusion.
2. Overall behavioural and psychological symptoms
Eight studies measured the effects of aromatherapy on behavioural and psychological symptoms.
Ballard 2002 (71 participants) assessed overall behavioural and psychological symptoms using the NPI. The authors of a previous version of this review conducted analyses on individual patient data provided by the study authors (see point 6 in Included studies above). The analysis was adjusted for clustering and for several participant‐level covariates. The mean difference between aromatherapy and placebo groups in change from baseline in the mean total NPI score after four weeks of treatment was −15.80, favouring the aromatherapy group (95% CI −24.40 to −7.20; 1 study, 71 participants). The study was at low risk of bias in all domains and we were moderately confident in this result (downgraded one level due to imprecision because of the small sample size).
Burns 2011 (63 participants) assessed overall behavioural and psychological symptoms using the NPI and found no significant difference in behavioural symptoms between those treated with aromatherapy and those treated with placebo after 12 weeks (MD 2.80, 95% CI −5.84 to 11.44; 63 participants). The study was at low risk of bias in all domains and we were moderately confident in this result (downgraded one level due to imprecision).
Cameron 2011 (18 participants) assessed overall behavioural and psychological symptoms using the NPI. This study was a cross‐over study. No numerical data were presented. The authors report no significant difference between treatment groups, but no details were given of the analysis method. We had very low confidence in this result due to lack of information on participant attrition, outcome data and analysis methods, and the very small sample size.
Fujii 2008 (28 participants) administered lavender oil three times a day for four weeks. The comparator was no intervention. Overall behavioural and psychological symptoms were assessed using the NPI. We used the final scores reported for each treatment group to calculate a mean difference between aromatherapy and placebo groups at four weeks. The mean difference was −9.00 favouring the aromatherapy group (95% CI −17.89 to −0.11; 1 study, 28 participants). We had low confidence in this result due to imprecision and risk of bias.
Hanson 2013 (21 participants) administered lavender oil or placebo oil via touch and via a diffuser. Overall behavioural and psychological symptoms were assessed using the NPI, but the study reported no useable data. This was a cross‐over study.
Lin 2007 (70 participants) assessed overall behavioural and psychological symptoms using the NPI. This was a cross‐over study. Paired data were not reported. First‐period‐only data were not reported. We used the final scores reported for each treatment group to calculate a mean difference between aromatherapy and placebo groups after three weeks of treatment. Negative scores favour the aromatherapy group. The MD was −6.64 (95% CI −10.85 to −2.43; 1 study, 70 participants). We had low confidence in this result due to serious concerns about risk of bias (especially lack of blinding of outcome assessment), our inability to conduct or report a paired analysis suitable to the cross‐over design, and imprecision.
Takahashi 2020 (36 participants) used a distilled cedar and ethanol solution diffused as a room fragrance as well as a mist spray, sprayed on patients' clothes a few times a day. Takahashi 2020 assessed overall behavioural and psychological symptoms using the NPI and report that the NPI score of the aromatherapy group significantly decreased in comparison to the control group. We used the final scores reported for each group to calculate a mean difference between aromatherapy and placebo groups at eight weeks. The mean difference was −4.26 favouring the aromatherapy group (95% CI −9.46 to 0.94; 1 study, 36 participants). We had low confidence in this result due to imprecision and concerns about risk of bias.
Watson 2019 (39 participants) assessed overall behavioural and psychological symptoms using the NPI but did not report any data on NPI scores in the separate treatment groups and did not report any relevant analysis of between‐group differences.
In summary, eight studies assessed overall behavioural and psychological symptoms using the total NPI score. Two studies provided no useable data and we have very low confidence in the result of one more study. We had low confidence in the results of three trials, all of which showed a reduction in overall behavioural and psychological symptoms following aromatherapy. We were moderately confident in the results of the two remaining trials, which were of a similar size and used similar interventions. One of these trials found a statistically significant effect of aromatherapy on overall behavioural and psychological symptoms; the other did not.
3. Adverse effects
Four studies mentioned adverse events occurring during the trial.
Burns 2011 (63 participants) reported that two participants in the aromatherapy group and two participants in the control group suffered serious adverse events. The authors also report 27 adverse events but do not specify which treatment group suffered the adverse events. The study was at low risk of bias in all domains and we were moderately confident in this result (downgraded one level due to imprecision because of the small sample size).
Cameron 2011 (18 participants) reported three deaths but stated these deaths were not as a result of the aromatherapy treatment. There was no information on systematic assessment of adverse events.
Fu 2013 (61 participants) reported that there were no adverse events in the control group or treatment group.
O'Connor 2013 (64 participants) reported that there were no adverse events in the control group or treatment group.
In summary, adverse events were poorly reported or not reported at all in most trials. What data there were did not raise concern about adverse effects of aromatherapy in this patient population.
Secondary outcomes
1. Quality of life (QoL)
One study assessed the effect of aromatherapy on a quality of life scale (Burns 2011). Ballard 2002 included Dementia Care Mapping (DCM) and reported the effect of aromatherapy on the percentage of time participants spent socially withdrawn or participating in constructive activities. We decided to report this here under the QoL outcome, but to consider it an indirect measure of QoL.
Ballard 2002 (71 participants) reported DCM data as medians. Compared to the control group, the aromatherapy group spent a significantly lower percentage of time socially withdrawn (P = 0.05) and a significantly higher percentage of time engaged in constructive activities (P = 0.01). The study was at low risk of bias in all domains but we considered it indirect in relation to quality of life, hence our confidence in this as a QoL result was low (downgraded due to imprecision because of the small sample size and due to indirectness).
Burns 2011 (63 participants) assessed QoL using the Blau QoL Scale at baseline and after four and 12 weeks of treatment. The authors reported no significant difference between placebo and aromatherapy groups on the Blau QoL Scale at week 4 or at week 12 (MD = 19.00, 95% CI −23.12 to 61.12; 1 study, 63 participants). The study was at low risk of bias in all domains and we were moderately confident in this result (downgraded one level due to imprecision).
In summary, two trials assessed QoL, or aspects of QoL, using different assessment tools. We were moderately confident in one trial which did not find any significant effect of aromatherapy on QoL. Because of the indirectness of the measure used in the other study, we had low confidence that the beneficial effect of aromatherapy in this trial reflected an effect on overall QoL.
2. Mood
Two studies measured the effect of aromatherapy on mood.
O'Connor 2013 (64 participants) assessed mood using the PGCARS, recording the main type of affect displayed every minute for 30 minutes before the intervention and 60 minutes after the intervention, and then calculating mean scores for positive and negative affects for each of one 30‐minute pre‐exposure period and two 30‐minute post‐exposure periods. This was a cross‐over study. Paired data were not reported. First‐phase‐only data were not reported. Data were analysed using binomial regression. The study reports no effect of treatment on positive or negative affects (no 'treatment × time' interactions). The risk of bias was low in all domains. Our confidence in the study was moderate, reduced due to imprecision (small sample size).
Yang 2016 (56 participants) assessed mood by using the CSDD‐C. Measurements were made at baseline, and 24 hours after massage halfway through the 8‐week intervention and at the end of the intervention period. There appears to be a baseline imbalance with higher depressive symptom scores in the aromatherapy group at baseline. The authors used a general linear model repeated measurement analysis and report that “Depressive symptoms decreased significantly over time for the intervention group compared to the control group” (P < 0.001). Our confidence in this result was low due to serious concerns about risk of bias and imprecision (small sample size).
In summary, mood or affect was an outcome in two trials. We were moderately confident in the results of one trial which found no significant effect of aromatherapy on affect. Another trial did report a statistically significant benefit of aromatherapy on depressive symptoms, but we had low confidence in this result.
3. Sleep
One study measured the effect of aromatherapy on sleep.
Hanson 2013 (21 participants) assessed sleep but reported no useable data. This was a cross‐over study.
4. Cognition
Three studies measured the effect of aromatherapy on cognition.
Fu 2013 (61 participants) stated that they assessed cognition using the MMSE at baseline and at the end of the trial but did not report this outcome.
Fujii 2008 (28 participants) assessed cognition using the MMSE. We used the final scores reported for each treatment group to calculate a mean difference between aromatherapy and placebo groups at four weeks. The mean difference was 1 MMSE point (95% CI −4.19 to 6.19; 1 study, 28 participants). We had low confidence in this result due to concerns about risk of bias and imprecision.
Takahashi 2020 (36 participants) assessed cognition using ADAS‐cog and reported no difference between the aromatherapy and control groups. We used the final scores reported for each treatment group to calculate a mean difference between aromatherapy and placebo groups at eight weeks. The mean difference was −0.36 (95% CI −6.60 to 5.88; 1 study, 36 participants). We had low confidence in this result due to concerns about risk of bias and imprecision.
In summary, three trials apparently assessed cognitive outcomes. One did not report any data. The two other trials found no significant effect of aromatherapy on cognition; our confidence in the results of these studies was low.
5. Activities of daily living
Two studies measured the effect of aromatherapy on activities of daily living.
Burns 2011 (63 participants) assessed activities of daily living using the Barthel Index for Activities of Daily Living. They found no significant difference in activities of daily living between those treated with aromatherapy and those treated with placebo after 12 weeks, mean difference −0.50 (95% CI −1.79 to 0.79; 1 study, 63 participants). The study was at low risk of bias in all domains and we were moderately confident in this result (downgraded one level due to imprecision).
Fujii 2008 (28 participants) assessed activities of daily living using the Barthel Index for Activities of Daily Living. The study measured this but did not include quantitative data and did not conduct between‐group analysis.
In summary, two trials assessed activities of daily living. One did not report any useable data. The other found no significant effect of aromatherapy on activities of daily living; we were moderately confident in this result.
6. Caregiver burden or distress, or both
One study measured the effect of aromatherapy on caregiver burden.
Takahashi 2020 (36 participants) assessed caregiver burden using the Japanese version of the Zarit Caregiver Burden interview. The study reported that the caregiver burden score was significantly lower in the aromatherapy group than in the placebo group. We used the final scores reported for each treatment group to calculate a mean difference between aromatherapy and placebo groups at eight weeks. The mean difference was −6.27 (95% CI −12.29 to −0.25; 1 study, 36 participants). We had low confidence in this result due to concerns about risk of bias and imprecision.
Discussion
Summary of main results
See Table 1
For all efficacy outcomes there was inconsistency between trials largely because one study ‒ Ballard 2002 ‒ reported beneficial effects of aromatherapy. We had varying levels of confidence in the trials. We found no convincing benefit of aromatherapy on agitation and overall behavioural and psychological symptoms. Reporting of harms was very poor, with only four trials making any mention of adverse events. Two trials assessed quality of life and reported inconsistent results. Two trials assessed mood and reported inconsistent results. One study in our review assessed sleep but reported no useable data. Cognition was reported in three trials: two trials reported no significant effect of aromatherapy on cognition; the other trial reported no useable data. Activities of daily living were reported in two trials: one did not report any useable data; the other trial found no significant effect of aromatherapy on activities of daily living. One study measured caregiver burden and reported a reduction following the aromatherapy intervention.
Overall completeness and applicability of evidence
All of the participants in the trials had dementia and were recruited from care facilities or hospital wards (one trial did not specify where the participants were recruited from); therefore findings from these trials cannot be applied to community settings. As discussed above, the range of reported outcomes was limited and there was very little systematic reporting of adverse events. Aromatherapy involves exposure to plant‐based aromas. One study used cedar extracts, the remaining twelve studies used essential oils. Complementary medicine practitioners may consider this to be exposure to essential oil fragrances rather than aromatherapy.
Quality of the evidence
We could not perform a meta‐analysis as the trials were heterogeneous and many did not report any useable numerical data. We reported numerical data from individual studies where possible, but were only able to compare results of studies using a P value either ≤ or > 0.5 in our narrative synthesis, which we recognise is a poor metric.
We used GRADE to assess the quality of the evidence for each outcome; this ranged from very low to moderate. The major problem was imprecision due to small sample sizes. Among higher‐quality studies there was inconsistency in their results.
Key methodological issues include the quality of the blinding, the placebo effect, poor reporting of the concentration of constituents in the aromatherapy substances used, and the comparability of different interventions. Many of the aromatherapy interventions involve an increase in interaction with other people, which could help to relieve the symptoms of dementia irrespective of the aromatherapy treatment. Three of the included studies compared an aromatherapy intervention to usual care and therefore do not control for an increase in attention from others. No studies assessed whether the aromatherapy substances were present systemically, providing no insight into the pharmacokinetics of aromatherapy.
Potential biases in the review process
We are unable to exclude publication bias.
Agreements and disagreements with other studies or reviews
Kim 2019 is a systematic review of the effect of aromatherapy on agitation in people with dementia. Kim 2019 reported that aromatherapy is beneficial at improving agitation in individuals with dementia. The review included 12 aromatherapy trials, eight of which are included in the current review (Ballard 2002, Burns 2011, Fu 2013, Lin 2007, O'Connor 2013, Yang 2015, two studies were referenced in relation to Yang 2016). The four other trials ‒ Akhondzadeh 2003, Holmes 2002, Snow 2004 and Yoshiyama 2015 ‒ were not included in the current review because Akhondzadeh 2003 administered aromatherapy orally; Holmes 2002 and Snow 2004 were not randomised controlled trials; and Yoshiyama 2015 is currently awaiting classification ‒ we have contacted the lead author as no useable data were presented in this small pilot study.
Leng 2019 is a systematic review on the use of non‐pharmacological interventions for agitation in people with dementia. Leng 2019 reported that aromatherapy did not have a beneficial effect on agitation in individuals with dementia. The review included six aromatherapy trials, four of which are included in the current review (Burns 2011, Lin 2007, Yang 2015 and Yang 2016). The other two trials (Dimitriou 2018 and Kaymaz 2017) were not included in the current review because Kaymaz 2017 was not a randomised controlled trial and Dimitriou 2018 did not include a control group.
Oliveira 2015 is a systematic review on the use of non‐pharmacological interventions to reduce behavioural and psychological symptoms of dementia. Oliveira 2015 reports that aromatherapy may be beneficial at reducing agitation in patients with dementia. The review includes only three aromatherapy trials (Burns 2011, Lin 2007 and Yang 2015), all of which are included in the current review.
Livingston 2014 is a systematic review on the use of non‐pharmacological interventions for agitation in dementia in randomised control trials. Livingston 2014 reports that aromatherapy appears to be effective when the intervention is non‐blinded; but when raters are blinded, aromatherapy does not appear to be effective in reducing agitation. The review includes only two aromatherapy trials (Ballard 2002 and Burns 2011), both of which are included in the current review.
Fung 2012 is a systematic review on the use of aromatherapy in treating behavioural problems in dementia. Fung 2012 reports that there is some evidence that aromatherapy has a positive effect on cognitive functioning and reducing BPSDs. However, although the review authors stated that they included only RCTs, six of the 11 included studies were not randomised and one was not testing aromatherapy, and so we have not included these studies in our review. This accounts for the differences in our results.
Authors' conclusions
Implications for practice.
The use of aromatherapy for people with dementia in long‐term care facilities and hospital wards is feasible. From the available evidence, it is not possible to be certain whether or not patients with dementia and agitation or other signs of distress will benefit from aromatherapy. Reporting of adverse events in the trials was very poor. Although the four trials which mentioned them did not detect adverse effects, it is not possible to assume that aromatherapy is without risk of harm.
Implications for research.
A promising start has been made in systematically investigating the effect of aromatherapy for dementia; well‐designed, larger RCTs that fully report the data are needed, however, before conclusions can be drawn as to its effectiveness. Many methodological issues need to be addressed such as the quality of the blinding, the comparability of different interventions, and the placebo effect. Control conditions should account for any increase in social interaction that occurs during the aromatherapy intervention. Treatment effects at different severities of dementia should be investigated. Future research should involve pragmatic randomised controlled trials of the most widely used aromatherapy fragrances, using patient‐important outcome measures, preferably from a well‐derived core outcome set and assessing systematically for any harms. The concentration of constituents in the aromatherapy substances should be reported.
Feedback
Abstract, March 2008
Summary
Please can you edit the abstract of the review?
In the abstract, it is not clear what outcomes you looked for, how many studies you found, how many studies are included and what the results are. The Plain Language Summary provides more information than the abstract.
Reply
We have edited the abstract.
Contributors
Vasiliy Vlassov, Occupational Physician.
What's new
| Date | Event | Description |
|---|---|---|
| 5 May 2020 | New citation required but conclusions have not changed | 6 new studies were included and the content revised and updated. Conclusions unchanged. New author team. |
| 5 May 2020 | New search has been performed | An update search was performed on 5 May 2020 |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 15 May 2019 | New search has been performed | An update search was performed for this review on 15 May 2019. |
| 24 February 2014 | New citation required but conclusions have not changed | New citation; conclusions unchanged |
| 3 February 2014 | New search has been performed | A pre‐publication search was performed on 20 January 2014. Updated with two new studies |
| 10 March 2013 | New search has been performed | Updated with one new study |
| 26 November 2012 | New search has been performed | A pre‐publication search was performed for this review on 26 November 2012 |
| 17 January 2012 | New search has been performed | An update search was performed for this review on 17 January 2012. |
| 8 June 2010 | New search has been performed | An update search was performed for this review on 17 May 2010. The authors were left with 8 records to assess for possible relevance within the review |
| 10 November 2008 | New search has been performed | An update search was run in March 2008 that retrieved one study (Lin 2007) which has been included in the review. No data from this trial has been included as data from the first phase of this crossover trial was not reported in the study report and has not been forthcoming from the study author. This update has been conducted by Theo Birks and Francesca Holt and approved by Martin Orrell. |
| 8 July 2008 | Feedback has been incorporated | Feedback added |
| 3 April 2008 | Amended | Converted to new review format. |
| 8 May 2006 | New search has been performed | May 2006 Four new papers were identified in the search of April 2006. Three were of new trials, two were excluded and one is ongoing (Myers 2005). The fourth paper is a commentary on an existing included trial (Lee 2003 b attached to Smallwood 2001). This update was performed by the CDCIG editorial base and approved by Martin Orrell and the Contact Editor as the first author (Lene Thorgrimsen) could not be contacted. |
| 15 May 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Lene Forrester was the first author of the 2014 review; she kindly supported this update and wished to be acknowledged as a contributor.
Our thanks also to the following authors for their contributions to previous versions of this review: Theodore PH Birks; Louise D Buchan; Francesca Holt; Nicola Maayan; Martin Orrell; Karla Soares‐Weiser; Aimee E Spector
We would like to thank peer reviewers Daniel O'Connor, Elaine Perry and Nic Perry and consumer reviewer Cathie Hofstetter for their comments and feedback.
Appendices
Appendix 1. Update searches: January 2012, January 2014, May 2018, May 2019, May 2020
|
Source |
Search strategy | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) [Date of most recent search: 5 May 2020] |
Keyword search: aroma OR aromatherapy OR lemon OR rose OR lavender OR smell OR “essential oils” OR “essential oil” | Nov 2012: 48 (all dates) Jan 2014: May 2018: 3 May 2019: 16 5 May 2020: 8 |
| 2. MEDLINE In‐Process and other non‐indexed citations and MEDLINE 1950‐present (OvidSP) [Date of most recent search: 5 May 2020] |
1. exp Dementia/ 2. Delirium/ 3. Wernicke Encephalopathy/ 4. Delirium, Dementia, Amnestic, Cognitive Disorders/ 5. dement*.mp. 6. alzheimer*.mp. 7. (lewy* adj2 bod*).mp. 8. deliri*.mp. 9. (chronic adj2 cerebrovascular).mp. 10. ("organic brain disease" or "organic brain syndrome").mp. 11. ("normal pressure hydrocephalus" and "shunt*").mp. 12. "benign senescent forgetfulness".mp. 13. (cerebr* adj2 deteriorat*).mp. 14. (cerebral* adj2 insufficient*).mp. 15. (pick* adj2 disease).mp. 16. (creutzfeldt or jcd or cjd).mp. 17. huntington*.mp. 18. binswanger*.mp. 19. korsako*.mp. 20. ((cognit* or memory* or mental*) adj3 (declin* or impair* or los* or deteriorat*)).mp. 21. or/1‐20 22. "aroma therap*".mp. 23. exp *Aromatherapy/ 24. aromatherapy.mp. 25. "complementary therap*".mp. 26. exp Complementary Therapies/ 27. "alternative therap*".mp. 28. exp Complementary Therapies/ 29. "essential oil*".mp. 30. aroma*.ti,ab. 31. ("lemon balm" or "rose* oil*" or "lavender oil*").mp. 32. or/22‐31 33. 21 and 32 34. randomized controlled trial.pt. 35. controlled clinical trial.pt. 36. placebo.ab. 37. random*.ab. 38. trial.ab. 39. groups.ab. 40. or/34‐39 41. (animals not (humans and animals)).sh. 42. 40 not 41 43. 42 and 33 |
Nov 2012: 120 Jan 2014: May 2018: 384 May 2019: 145 5 May 2020: 157 |
| 3. EMBASE 1980‐2018 May 8 (OvidSP) [Date of most recent search: 5 May 2020] |
1. exp dementia/ 2. Lewy body/ 3. delirium/ 4. Wernicke encephalopathy/ 5. cognitive defect/ 6. dement*.mp. 7. alzheimer*.mp. 8. (lewy* adj2 bod*).mp. 9. deliri*.mp. 10. (chronic adj2 cerebrovascular).mp. 11. ("organic brain disease" or "organic brain syndrome").mp. 12. "supranuclear palsy".mp. 13. ("normal pressure hydrocephalus" and "shunt*").mp. 14. "benign senescent forgetfulness".mp. 15. (cerebr* adj2 deteriorat*).mp. 16. (cerebral* adj2 insufficient*).mp. 17. (pick* adj2 disease).mp. 18. (creutzfeldt or jcd or cjd).mp. 19. huntington*.mp. 20. binswanger*.mp. 21. korsako*.mp. 22. CADASIL.mp. 23. ((cognit* or memory* or mental*) adj3 (declin* or impair* or los* or deteriorat*)).mp. 24. or/1‐23 25. "aroma therap*".mp. 26. exp aromatherapy/ 27. aromatherapy.mp. 28. "complementary therap*".mp. 29. exp alternative medicine/ 30. "alternative therap*".mp. 31. alternative medicine/ 32. "essential oil*".mp. 33. aroma*.ti,ab. 34. ("lemon balm" or "rose* oil*" or "lavender oil*").mp. 35. or/25‐34 36. 35 and 24 37. randomized controlled trial/ 38. controlled clinical trial/ 39. placebo.ab. 40. random*.ab. 41. trial.ab. 42. groups.ab. 43. or/37‐42 44. 36 and 43 |
Nov 2012: 55 Jan 2014: May 2018: 418 May 2019: 81 5 May 2020: 105 |
| 4. PsycINFO 1806‐May week 2 2019 (OvidSP) [Date of most recent search: 5 May 2020] |
1. exp Dementia/ 2. exp Delirium/ 3. exp Huntingtons Disease/ 4. exp Kluver Bucy Syndrome/ 5. exp Wernickes Syndrome/ 6. exp Cognitive Impairment/ 7. dement*.mp. 8. alzheimer*.mp. 9. (lewy* adj2 bod*).mp. 10. deliri*.mp. 11. (chronic adj2 cerebrovascular).mp. 12. ("organic brain disease" or "organic brain syndrome").mp. 13. "supranuclear palsy".mp. 14. ("normal pressure hydrocephalus" and "shunt*").mp. 15. "benign senescent forgetfulness".mp. 16. (cerebr* adj2 deteriorat*).mp. 17. (cerebral* adj2 insufficient*).mp. 18. (pick* adj2 disease).mp. 19. (creutzfeldt or jcd or cjd).mp. 20. huntington*.mp. 21. binswanger*.mp. 22. korsako*.mp. 23. ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 24. ((cognit* or memory* or mental*) adj3 (declin* or impair* or los* or deteriorat*)).mp. 25. or/1‐24 26. "aroma therap*".mp. 27. exp Aromatherapy/ 28. aromatherapy.mp. 29. "complementary therap*".mp. 30. exp Alternative Medicine/ 31. "alternative therap*".mp. 32. "essential oil*".mp. 33. aroma*.ti,ab. 34. ("lemon balm" or "rose* oil*" or "lavender oil*").mp. 35. or/26‐34 36. exp Clinical Trials/ 37. random*.ti,ab. 38. placebo.mp. 39. trial*.ti,ab. 40. groups.ab. 41. or/36‐40 42. 25 and 35 and 41 |
Nov 2012:14 Jan 2014: May 2018: 34 May 2019: 10 5 May 2020: 23 |
| 5. CINAHL (EBSCOhost) [Date of most recent search: 5 May 2020] |
S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 TX "aroma therap*" S21 ("Aromatherapy") or (MH "Aromatherapy") S22 TX "complementary therap*" S23 (MH "Alternative Therapies") S24 TX "alternative therap*" S25 TX "essential oil*" S26 AB aroma* S27 AB "lemon balm" OR "rose* oil*" OR "lavender oil*" S28 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 S29 S19 and S28 S30 AB random* S31 AB placebo S32 (MH "Clinical Trials+") S33 AB groups S34 TX trial* S35 S30 or S31 or S32 or S33 or S34 S36 S29 and S35 |
Nov 2012: 24 Jan 2014: May 2018: 35 May 2019: 18 5 May 2020: 19 |
| 6. Web of Knowledge – all databases [Date of most recent search: 5 May 2020] |
Topic=("lemon balm" OR "rose* oil*" OR "lavender oil*" OR aroma* OR aromatherapy OR "essential oil*") AND Topic=(dementia* OR alzheimer* OR BPSD OR lewy OR "cognit* impair*" OR MCI OR VCI OR AD) AND Topic=(randomly OR placebo OR groups OR trial OR RCT OR randomized OR randomised OR "double‐blind*" OR "single‐blind*" OR CCT OR "cross‐over" OR crossover) |
Nov 2012: 77 Jan 2014: May 2018: 459 May 2019: 57 5 May 2020: 63 |
| 7. LILACS (BIREME) [Date of most recent search: 5 May 2020] |
aroma OR aromatherapy OR lemon OR limão OR limón OR lavender OR lavanda OR alfazema OR "essential oil$" [Words] and dementia OR alzheimer OR demência OR demencia OR cognición OR cognição OR cognition OR cognitive OR MCI [Words] | Nov 2012: 1 Jan 2014: May 2018: 0 May 2019: 0 5 May 2020: |
| 8. CENTRAL (The Cochrane Library) (Issue 5 of 12, 2018) [Date of most recent search: 5 May 2020] |
#1 MeSH descriptor Dementia explode all trees #2 MeSH descriptor Delirium, this term only #3 MeSH descriptor Wernicke Encephalopathy, this term only #4 MeSH descriptor Delirium, Dementia, Amnestic, Cognitive Disorders, this term only #5 dement* #6 alzheimer* #7 "lewy* bod*" #8 deliri* #9 "chronic cerebrovascular" #10 "organic brain disease" or "organic brain syndrome" #11 "normal pressure hydrocephalus" and "shunt*" #12 "benign senescent forgetfulness" #13 "cerebr* deteriorat*" #14 "cerebral* insufficient*" #15 "pick* disease" #16 creutzfeldt or jcd or cjd #17 huntington* #18 binswanger* #19 korsako* #20 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19) #21 "aroma therap*" #22 aromatherapy #23 "alternative therap*" #24 "essential oil*" #25 aroma* #26 "lemon balm" or "rose* oil*" or "lavender oil*" #27 MeSH descriptor Aromatherapy explode all trees #28 MeSH descriptor Complementary Therapies explode all trees #29 (#21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28) #30 (#29 AND #20) |
Nov 2012: 34 Jan 2014: May 2018: 123 May 2019: 83 5 May 2020: 0 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) [Date of most recent search: 5 May 2020] |
Interventional Studies | dementia OR alzheimers OR AD OR alzheimer's OR alzheimer OR lewy OR FTLD OR FLD OR MCI OR cognitive OR cognition | aroma OR aromatherapy OR lavender OR lemon OR rose OR essential oils | Nov 2012: 2 Jan 2014: May 2018: 35 May 2019: 7 5 May 2020: 8 |
| 10. ICTRP Search Portal (http://apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] [Date of most recent search: 15 May 2019] Databse not available 5 May 2020 |
(Interventional Studies | dementia OR alzheimers OR AD OR alzheimer's OR alzheimer OR lewy OR FTLD OR FLD OR MCI OR cognitive OR cognition | aroma OR aromatherapy OR lavender OR lemon OR rose OR essential oils | Adult, Senior | Nov 2012: 27 Jan 2014: May 2018: 22 May 2019: 7 |
| TOTAL before de‐duplication | Nov 2012: 402 Jan 2014: 326 May 2018: 1513 May 2019: 424 5 May 2020: 463 |
|
| TOTAL after de‐duplication and first assessment by CDCIG information specialists | Nov 2012: 7 Jan 2014: 4 May 2018: 105 May 2019: 62 5 May 2020: 384 |
|
Appendix 2. Update search: May 2010
| Source | Search strategy | Hits |
| MEDLINE In‐Process and other non‐indexed citations and MEDLINE 1950‐present (OvidSP) | 1. exp Dementia/ 2. Delirium/ 3. Wernicke Encephalopathy/ 4. Delirium, Dementia, Amnestic, Cognitive Disorders/ 5. dement*.mp. 6. alzheimer*.mp. 7. (lewy* adj2 bod*).mp. 8. deliri*.mp. 9. (chronic adj2 cerebrovascular).mp. 10. ("organic brain disease" or "organic brain syndrome").mp. 11. ("normal pressure hydrocephalus" and "shunt*").mp. 12. "benign senescent forgetfulness".mp. 13. (cerebr* adj2 deteriorat*).mp. 14. (cerebral* adj2 insufficient*).mp. 15. (pick* adj2 disease).mp. 16. (creutzfeldt or jcd or cjd).mp. 17. huntington*.mp. 18. binswanger*.mp. 19. korsako*.mp. 20. ((cognit* or memory* or mental*) adj3 (declin* or impair* or los* or deteriorat*)).mp. 21. or/1‐20 22. "aroma therap*".mp. 23. exp *Aromatherapy/ 24. aromatherapy.mp. 25. "complementary therap*".mp. 26. exp Complementary Therapies/ 27. "alternative therap*".mp. 28. exp Complementary Therapies/ 29. "essential oil*".mp. 30. aroma*.ti,ab. 31. ("lemon balm" or "rose* oil*" or "lavender oil*").mp. 32. or/22‐31 33. 21 and 32 34. randomized controlled trial.pt. 35. controlled clinical trial.pt. 36. placebo.ab. 37. random*.ab. 38. trial.ab. 39. groups.ab. 40. or/34‐39 41. (animals not (humans and animals)).sh. 42. 40 not 41 43. 42 and 33 44. (2008* or 2009* or 2010*).ed. 45. 43 and 44 |
134 |
| Embase 1980‐2010 week 19 (OvidSP) |
1. exp dementia/ 2. Lewy body/ 3. delirium/ 4. Wernicke encephalopathy/ 5. cognitive defect/ 6. dement*.mp. 7. alzheimer*.mp. 8. (lewy* adj2 bod*).mp. 9. deliri*.mp. 10. (chronic adj2 cerebrovascular).mp. 11. ("organic brain disease" or "organic brain syndrome").mp. 12. "supranuclear palsy".mp. 13. ("normal pressure hydrocephalus" and "shunt*").mp. 14. "benign senescent forgetfulness".mp. 15. (cerebr* adj2 deteriorat*).mp. 16. (cerebral* adj2 insufficient*).mp. 17. (pick* adj2 disease).mp. 18. (creutzfeldt or jcd or cjd).mp. 19. huntington*.mp. 20. binswanger*.mp. 21. korsako*.mp. 22. CADASIL.mp. 23. ((cognit* or memory* or mental*) adj3 (declin* or impair* or los* or deteriorat*)).mp. 24. or/1‐23 25. "aroma therap*".mp. 26. exp aromatherapy/ 27. aromatherapy.mp. 28. "complementary therap*".mp. 29. exp alternative medicine/ 30. "alternative therap*".mp. 31. alternative medicine/ 32. "essential oil*".mp. 33. aroma*.ti,ab. 34. ("lemon balm" or "rose* oil*" or "lavender oil*").mp. 35. or/25‐34 36. 35 and 24 37. randomized controlled trial/ 38. controlled clinical trial/ 39. placebo.ab. 40. random*.ab. 41. trial.ab. 42. groups.ab. 43. or/37‐42 44. 36 and 43 45. (2008* or 2009* or 2010*).em. 46. 44 and 45 |
39 |
| PsycINFO 1806‐May week 2 2010 (OvidSP) |
1. exp Dementia/ 2. exp Delirium/ 3. exp HuntingtonsDisease/ 4. exp Kluver Bucy Syndrome/ 5. exp Wernickes Syndrome/ 6. exp Cognitive Impairment/ 7. dement*.mp. 8. alzheimer*.mp. 9. (lewy* adj2 bod*).mp. 10. deliri*.mp. 11. (chronic adj2 cerebrovascular).mp. 12. ("organic brain disease" or "organic brain syndrome").mp. 13. "supranuclear palsy".mp. 14. ("normal pressure hydrocephalus" and "shunt*").mp. 15. "benign senescent forgetfulness".mp. 16. (cerebr* adj2 deteriorat*).mp. 17. (cerebral* adj2 insufficient*).mp. 18. (pick* adj2 disease).mp. 19. (creutzfeldt or jcd or cjd).mp. 20. huntington*.mp. 21. binswanger*.mp. 22. korsako*.mp. 23. ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 24. ((cognit* or memory* or mental*) adj3 (declin* or impair* or los* or deteriorat*)).mp. 25. or/1‐24 26. "aroma therap*".mp. 27. exp Aromatherapy/ 28. aromatherapy.mp. 29. "complementary therap*".mp. 30. exp Alternative Medicine/ 31. "alternative therap*".mp. 32. "essential oil*".mp. 33. aroma*.ti,ab. 34. ("lemon balm" or "rose* oil*" or "lavender oil*").mp. 35. or/26‐34 36. exp Clinical Trials/ 37. random*.ti,ab. 38. placebo.mp. 39. trial*.ti,ab. 40. groups.ab. 41. or/36‐40 42. 25 and 35 and 41 43. (2008* or 2009* or 2010*).up. 44. 42 and 43 |
13 |
| CINAHL (EBSCOhost) | S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 TX "aroma therap*" S21 ("Aromatherapy") or (MH "Aromatherapy") S22 (MH "Alternative Therapies") S23 (MH "Alternative Therapies") S24 TX "alternative therap*" S25 TX "essential oil*" S26 AB aroma* S27 AB "lemon balm" OR "rose* oil*" OR "lavender oil*" S28 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 S29 S19 and S28 S30 AB random* S31 AB placebo S32 (MH "Clinical Trials+") S33 AB groups S34 TX trial* S35 S30 or S31 or S32 or S33 or S34 S36 S29 and S35 S37 EM 2008 S38 EM 2009 S39 EM 2010 S40 S37 or S38 or S39 S41 S36 and S40 |
37 |
| Web of Science with Conference Proceedings (1945 to present) | Topic=(dement* OR alzheimer* OR lewy OR deliri* OR cerebro* OR creutzfeldt OR huntington* OR korsako* OR binswanger*) AND Topic=(aroma* OR "complementary therap*" OR "essential oil*" OR "lemon" OR "rose oil*" OR lavender) AND Topic=(trial OR random* OR placebo OR groups) Timespan=Latest 5 years |
112 |
| LILACS (South and Central American coverage) | aroma$ [Words] and demen$ OR alzheimer$ [Words] | 1 |
| ALOIS (for a list of what ALOIS covers: http://www.medicine.ox.ac.uk/alois/content/about‐alois) | aromatherapy OR lemon OR lavender OR rose OR aroma OR alternative therapies OR complementary therapies | 12 |
| Umin (Clinical Trial register of Japan) | aromatherapy OR lemon OR lavender OR rose OR aroma | 0 |
| CENTRAL (The Cochrane Library) | #1 MeSH descriptor Dementia explode all trees #2 MeSH descriptor Delirium, this term only #3 MeSH descriptor Wernicke Encephalopathy, this term only #4 MeSH descriptor Delirium, Dementia, Amnestic, Cognitive Disorders, this term only #5 dement* #6 alzheimer* #7 "lewy* bod*" #8 deliri* #9 "chronic cerebrovascular" #10 "organic brain disease" or "organic brain syndrome" #11 "normal pressure hydrocephalus" and "shunt*" #12 "benign senescent forgetfulness" #13 "cerebr* deteriorat*" #14 "cerebral* insufficient*" #15 "pick* disease" #16 creutzfeldt or jcd or cjd #17 huntington* #18 binswanger* #19 korsako* #20 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19) #21 "aroma therap*" #22 aromatherapy #23 "alternative therap*" #24 "essential oil*" #25 aroma* #26 "lemon balm" or "rose* oil*" or "lavender oil*" #27 MeSH descriptor Aromatherapy explode all trees #28 MeSH descriptor Complementary Therapies explode all trees #29 (#21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28) #30 (#29 AND #20), from 2008 to 2010 |
60 |
| ClinicalTrials.gov | Interventional Studies | aromatherapy OR lemon OR lavender OR rose OR aroma | received from 01/01/2008 to 05/17/2010 | 19 |
| ICTRP Search Portal which covers: ANZCTR; ClinicalTrials.gov; ISRCTN; Chinese ClinicalTrial Registry; India Clinical Trials Registry; German Clinical Trials Register and more. | ADVANCED SEARCH: (dementia OR alzheimers OR lewy OR cognitive OR cerebrovascular) AND (aromatherapy OR lemon OR lavender OR rose OR aroma) AND (Recruitment status: ALL) AND (date registered: 01/01/2008‐17/05/2010) | 4 |
| Total | 438 | |
| Total after first‐assess and de‐duplication by TSC | 8 | |
Appendix 3. Update search: March 2008
| Source | Search strategy | Hits |
| CDCIG SR | “aroma therap*” OR “complementary therap*” OR “alternative therap*” OR “essential oil*” | 1 |
| PubMed (June 2006‐March 2008) | “aroma therap*” OR “complementary therap*” OR “alternative therap*” OR “essential oil*” AND Phases 1‐3 of the Highly sensitive search strategies for identifying reports of randomized controlled trials in Medline (APPENDIX 5b, Cochrane Handbook, 2006), all terms searched as Title, abstract, keyword, Publication type. |
33 |
| Embase (2008 week 12) PsycINFO (March week 3 2008) CINHAL (March week 2 2008) CENTRAL Issue 1 2008 (all via OvidSP) |
“aroma therap*” OR “complementary therap*” OR “alternative therap*” OR “essential oil*” AND Phases 1‐3 of the Highly sensitive search strategies for identifying reports of randomized controlled trials in Medline (APPENDIX 5b, Cochrane Handbook, 2006), all terms searched as Title, abstract, keyword, Publication type. |
49 |
| LILACS (BIREME) | “aroma therap*” OR “complementary therap*” OR “alternative therap*” OR “essential oil*” AND LILACS terms for trials |
0 |
| Total | 83 | |
| Total after first‐assess and de‐duplication by TSC | 2 | |
Appendix 4. Previous version of the methods
Methods
Criteria for considering studies for this review
Types of studies
This review considered all relevant randomized controlled trials (RCTs). Owing to the nature of aroma therapy double‐blinding may not be possible when combined with informed consent. A minimum length of trial and requirements for a follow‐up were not inclusion criteria.
Types of participants
Participants in included studies were to have a diagnosis of dementia of any type and severity, based on diagnostic criteria such as ICD‐10 (WHO 1993) and DSM‐IV (APA 1994), or well validated assessment scales for cognitive function, such as the MMSE (Folstein 1975) and ADAS‐Cog (Rosen 1994).
Types of interventions
This review considered trials using fragrance from plants, in an intervention defined as aroma therapy, for people with dementia. All doses, frequencies, and fragrances were considered.
Types of outcome measures
The outcomes considered in this review were: 1. cognitive function 2. functional performance 3. behaviour 4. quality of life 5. relaxation 6. wandering 7. sleep 8. mood
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois) ‐ the Cochrane Dementia and Cognitive Improvement Group’s Specialized Register on 26 November 2012. The search terms used were: aromatherapy, lemon, lavender, rose, aroma, alternative therapies, complementary therapies, essential oils.
ALOIS is maintained by the Trials Search Co‐ordinator of the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia prevention, dementia treatment and cognitive enhancement in healthy. The studies are identified from:
Monthly searches of a number of major healthcare databases: Medline, Embase, Cinahl, Psycinfo and Lilacs
Monthly searches of a number of trial registers: ISRCTN; UMIN (Japan's Trial Register); the WHO portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials and the Netherlands National Trials Register, plus others)
Quarterly search of The Cochrane Library’s Central Register of Controlled Trials (CENTRAL)
Six‐monthly searches of a number of grey literature sources: ISI Web of Knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS website.
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL and conference proceedings can be viewed in the ‘methods used in reviews’ section within the editorial information about the Dementia and Cognitive Improvement Group.
Additional searches were performed in many of the sources listed above to cover the timeframe from the last searches performed for ALOIS to ensure that the search for the review was as up‐to‐date and as comprehensive as possible. The search strategies used can be seen Appendix 1.
Electronic searches carried out in the previous version(s) of the review can be viewed in Appendix 2 and Appendix 3.
In addition the following online journals were searched: 'Complementary Therapies in Medicine', and 'Complementary Therapies in Nursing and Midwifery'.
Searching other resources
'Experts' in the field of complementary therapies were contacted to identify ongoing and unpublished research as well as the Aroma Therapy Organisations Council.
Selection of trials
LMT and AS independently screened the titles and abstracts extracted by the searches for their eligibility for potential inclusion in the review based on the above criteria, which were discussed with MO.
Update Sept. 2008: FEH and TPHB assessed the new study found by the March 2008 search using the same criteria as that previously used.
Data collection
Data were extracted from the published reports and unpublished company reports. The summary statistics required for each trial and each outcome for continuous data are the mean change from baseline, the standard error of the mean change, and the number of patients for each treatment group at each assessment. Where changes from baseline were not reported, the mean, standard deviation and the number of patients for each treatment group at each time point were extracted if available. For binary data the numbers in each treatment group and the numbers experiencing the outcome of interest were sought. The baseline assessment is defined as the latest available assessment prior to randomization, but no longer than two months prior. For each outcome measure, data were sought on every patient assessed. To allow an intention‐to‐treat analysis (ITT), the data were sought irrespective of compliance, whether or not the patient was subsequently deemed ineligible, or otherwise excluded from treatment or follow‐up. If ITT data were not available an analysis of patients who completed treatment was conducted. For continuous or ordinal variables which can be approximated to continuous variables, the main outcomes of interest were the assessment score at the time point being considered and the change from the baseline (i.e. pre‐randomization or at randomization) at this time point. For some binary and ordinal outcomes the endpoint category relative to baseline category was the outcome of interest. For other categorical outcomes, such as the Clinical Global Impression of Change (CIBIC‐Plus), the endpoint itself was of clinical relevance as all patients had begun, by definition, at the same baseline score. The baseline assessment score was the latest available score, no longer than two months prior to the randomization. Studies may have included a titration period prior to the randomization phase of the study. The data from these non‐randomized titration periods were not used to assess safety or efficacy since patients were not randomized, nor was treatment or dose allocation concealed. Data from any open follow‐on phase, after the randomized phase, were not used to assess safety or efficacy for the same reasons.
Quality assessment
A checklist for assessing the quality of all studies identified was developed, as presented below. Checklist for assessing methodological quality. Does the paper include: 1) A thorough review of the literature? 2) Hypothesis formulation/aims/power analysis? 3) Details of informed consent? 4) Description/justification of sampling procedure? 5) Description/justification of design (for non RCTs)? 6) Justification of lack of controls (for non RCTs)? 7) Details of randomization method(s) (selection bias)? 8) Number/details of drop‐outs (attrition bias)? 9) Description/justification/standardization of outcome measures? 10) Blind assessment/details of assessor(s) (detection bias)? 11) Clearly presented results (appropriate statistics)? 12) A description of limitations of study/design (for non RCTs)? 13) Intention to treat analysis? 14) Suggestions for future research?
Data analysis
Summary statistics (n, mean and standard deviation) were required for each rating scale at each assessment time for each treatment group in each trial for change from baseline.
When change from baseline results are not reported, the required summary statistics were calculated from the baseline and assessment time treatment group means and standard deviations. In this case a zero correlation between the measurements at baseline and assessment time was assumed. This method overestimates the standard deviation of the change from baseline, but this conservative approach is considered to be preferable in a meta‐analysis.
For binary outcomes, such as clinical improvement or no clinical improvement, the odds ratio was used to measure treatment effect.
For continuous or ordinal variables, such as psychometric test scores, clinical global impression scales, functional and quality of life scales, there are two possible approaches. If ordinal scale data appear to be approximately normally distributed or if the analysis that the investigators perform suggests parametric tests were appropriate, then the outcome measures were treated as continuous data. The second approach, which may not have excluded the first, was to concatenate into 2 categories which best represent the contrasting states of interest, and to treat the variable as binary. For binary outcomes such as institutionalization and death, the endpoint itself was of interest and the Peto method of the typical odds ratio was used.
A weighted estimate of the typical treatment effect across trials was calculated. Overall estimates of the treatment difference are presented. In all cases the overall estimate from a fixed‐effect model is presented and a test for heterogeneity using I2 statistic performed. Where there is evidence of heterogeneity of the treatment effect between trials then either only homogeneous results are pooled, or a random‐effects model is used (in which case the confidence intervals would be broader than those of a fixed‐effect model).
Data and analyses
Comparison 1. Aromatherapy versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Agitation, mean change (CMAI, high score=bad) | 1 | Other data | No numeric data | |
| 1.2 Behavioural symptoms, mean change (NPI, high score=bad) | 1 | Other data | No numeric data |
1.1. Analysis.
Comparison 1: Aromatherapy versus placebo, Outcome 1: Agitation, mean change (CMAI, high score=bad)
| Agitation, mean change (CMAI, high score=bad) | |||||
| Study | Mean difference | SE | Aromatherapy N | Placebo N | Mean difference (95%CI) |
| Ballard 2002 | ‐11.1 | 4.5409 | 35 | 36 | ‐11.10 [‐20.00, ‐2.20] |
1.2. Analysis.
Comparison 1: Aromatherapy versus placebo, Outcome 2: Behavioural symptoms, mean change (NPI, high score=bad)
| Behavioural symptoms, mean change (NPI, high score=bad) | |||||
| Study | Mean difference | SE | Aromatherapy N | Placebo N | Mean difference (95%CI) |
| Ballard 2002 | ‐15.8 | 4.3878 | 35 | 36 | ‐15.80 [‐24.40, ‐7.20] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ballard 2002.
| Study characteristics | ||
| Methods |
Treatment allocation: cluster‐randomised controlled trial
Study design: parallel group
Intervention: 4 weeks Assessments: conducted at baseline and following the 4‐week intervention |
|
| Participants |
Country: UK
Participants recruited from: 8 specialist nursing homes Number of participants randomised: 72 participants Number of participants included in analysis: 71 participants Mean age of participants: the mean age of participants who were randomised was 78.5 years, SD = 8.1 (active treatment = 77.2 years; placebo = 79.6 years) Sex of participants: 60% female (active treatment = 56% female; placebo = 64% female) Inclusion criteria: 1. Occupants of nursing homes were people with severe dementia (CDR = 3) and clinically significant agitation (defined as occurring on a daily basis and causing moderate to severe management problems) 2. Medication was allowed, but changes in psychotropic prescriptions were monitored and recorded Exclusion criteria: Not specified |
|
| Interventions |
Intervention groups included in this review: 1. 10% lemon balm essential oil and base oil (200 mg/day divided into 2 doses), applied topically to the face and both arms twice a day by a care assistant. N = 36 allocated to this intervention. 2. 10% sunflower oil and base oil (200 mg/day divided into 2 doses), applied topically to the face and both arms twice a day by a care assistant. N = 36 allocated to this intervention. |
|
| Outcomes |
Outcomes included in this review: 1. Agitation: CMAI 2. Behavioural symptoms: NPI 3. Quality of life: Dementia Care Mapping (% of time spent socially withdrawn, % of time engaged in constructive activities) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The facilities were matched in pairs (according to number of residents) and then assigned randomly (using the toss of a coin), to active treatment or placebo" |
| Allocation concealment (selection bias) | Low risk | "The facilities were matched in pairs (according to number of residents) and then assigned randomly (using the toss of a coin), to active treatment or placebo" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "In each facility only one of the aromatherapy substances was used, preventing comparisons between agents by staff. For the same reason staff were not informed of the nature of either the active treatment or placebo oils" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The assessments were repeated at weekly intervals for 4 weeks, by raters blind to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Seventy‐one (99%) participants completed the 4 week trial, one participant receiving active treatment died over the course of the study (unrelated to the study treatment)" |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | The published data were not adjusted for clustering; however, unpublished individual patient data were provided and we adjusted the data used in the analysis. |
Burns 2011.
| Study characteristics | ||
| Methods |
Treatment allocation: randomised controlled trial
Study design: parallel group
Intervention: 12 weeks Assessments: conducted at baseline, week 4 and week 12 |
|
| Participants |
Country: UK Participants recruited from: 3 clinical centres (Manchester, London and Southampton) Number of participants randomised: 114 participants, of whom 77 were randomised to the 2 groups included in this review Number of participants included in analysis: 63 participants (week 4), 55 (week 12) Mean age of participants: mean age of participants who completed the week 4 assessment was (active treatment = 85.6 years; placebo = 85.1 years). The mean age of participants who completed the week 12 assessment was not provided. Sex of participants: sex of participants who completed the week 4 assessment was (active treatment = 66% female; placebo = 48% female). The sex of participants who completed the week 12 assessment was not provided. Inclusion criteria: 1. Agitation for at least 4 weeks and score > 39 on the CMAI 2. NINCDS‐ADRDA diagnosis of probable or possible Alzheimer’s disease, clinical dementia rating of 3 3. Resident in a specialist nursing home or NHS continuing care facility 4. Age > 60 years 5. Free of psychotropic medication (antipsychotics and/or cholinesterase inhibitors) for at least 2 weeks Exclusion criteria: 1. Known sensitivity to cholinesterase drugs 2. Disability that may have prevented them from completing the study 3. Severe, unstable or poorly controlled medical conditions 4. History of stroke |
|
| Interventions |
Intervention groups included in this review: 1. Placebo medication and active aromatherapy (lemon balm oil). N = 38 allocated to this intervention. 2. Placebo medication and placebo aromatherapy. N = 39 allocated to this intervention. Additional intervention groups: 3. Active medication (donepezil) and placebo aromatherapy (sunflower oil). 5 mg of donepezil a day increasing to 10 mg after 1 month. N = 37 allocated to this intervention. The oil was administered twice a day by gently massage of the hands and upper arms for 1 to 2 minutes for 12 weeks. |
|
| Outcomes |
Outcomes included in this review: 1. Agitation: PAS 2. Behavioural symptoms: NPI 3. Adverse effects 4. Quality of life: Blau QOL scale 5. Activities of Daily Living: Barthel scale of Activities of Daily Living |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomised to 1 of the 3 groups by computer‐generated blocks of size 6 stratified by centre" |
| Allocation concealment (selection bias) | Low risk | "Participants were randomised to 1 of the 3 groups by computer‐generated blocks of size 6 stratified by centre" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Labelling of tablet bottles and oils was carried out by an external organization, and thus researchers and patients were blinded to the treatment allocation." The aromatherapy "was dispensed in opaque plastic dispensers" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Assessments were carried out at baseline, week 4 and week 12 by the research nurse who was blind to treatment group." "raters were required to wear nose clips to ensure that full blinding was maintained" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Eight subjects withdrew from the study at baseline, and an additional 12 subjects withdrew before the first follow‐up assessment at week 4. Of the remaining 94, 13 had no assessment data in week 12." Number of participants that withdrew at baseline: Donepezil = 2; lemon balm = 3; Placebo = 3 Number of participants that withdrew before week 4 follow up: Donepezil = 4; lemon balm = 3; Placebo = 5 Number of participants that withdrew before week 12 follow up: Donepezil = 5, lemon balm = 2; Placebo = 6 This review included the lemon balm and placebo groups in this review. Following attrition in the 2 groups, the below outlines the percentage of data that was available. Data available at baseline: 92% Data available at week 4 follow‐up: 82% Data available at week 12 follow‐up: 71% |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No additional biases |
Cameron 2011.
| Study characteristics | ||
| Methods |
Treatment allocation: randomised controlled trial
Study design: cross‐over
Intervention: Treatment 1: 3 weeks, washout 1 week, Treatment 2: 3 weeks. Assessments: assessments were conducted at baseline and during the weeks of oil application. During the weeks of oil application, PAS assessments were performed twice a day and NPI and CMAI assessments were conducted weekly. |
|
| Participants |
Country: UK Participants recruited from: inpatient ward Number of participants randomised: study states that 18 participants were included in the 'final study group' Number of participants included in analysis: The study states that 18 participants were included in the 'final study group' Mean age of participants: not reported Sex of participants: not reported Inclusion criteria: Not reported, "all inpatients were deemed eligible for inclusion in the study ... final study group consisted of 18 patients, all with moderate to severe dementia and significant BPSD" Exclusion criteria: "Patients were excluded if they refused to consent, withdrew consent at any time, or if their nearest relative refused to give assent" |
|
| Interventions |
Interventions included in this review: 1. Aromatherapy < 2% lemon balm oil 2. Placebo treatment 1% geranium and 0.5% lemon oil Protocol: The nursing staff gently rubbed the oil into 1 forearm of each patient for 1 minute, twice a day, for 3 weeks. This was followed by a 1‐week washout period before the alternative intervention was administered by rubbing the oil into 1 forearm of each patient for 1 minute, twice a day, for 3 weeks. |
|
| Outcomes |
Outcomes included in this review: 1. CMAI (no quantitative data reported) 2. PAS (no quantitative data reported) 3. NPI (no quantitative data reported) 4. Adverse effects (no quantitative data reported) |
|
| Notes | The trial was run twice, 1 year apart. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated to two groups by means of· an anonymous computer‐generated programme." |
| Allocation concealment (selection bias) | Low risk | "randomly allocated to two groups by means of· an anonymous computer‐generated programme." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind" "The two groups of oils appeared very similar in terms of smell, viscosity and texture. In a test prior to starting the trial, no member of the team was able to detect reliably which of the oils was either treatment or placebo." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double‐blind" "the PAS assessments were completed twice per day by nursing staff, and the NPI and CMAl assessments weekly by a consultant psychiatrist and a trained nurse." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported how many participants completed the trial |
| Selective reporting (reporting bias) | High risk | No data reported |
| Other bias | Low risk | No additional biases |
Fu 2013.
| Study characteristics | ||
| Methods |
Treatment allocation: randomised controlled trial
Study design: parallel group
Intervention: 6 weeks Assessments: cognitive assessment was measured at baseline and following the 6‐week intervention. "CMAI‐SF was administered five times in the study: 1. within the month prior to the intervention; 2. at the end of the second week of the intervention; 3. at the end of the fourth week of the intervention; 4. at the completion of the sixth week; and 5. six weeks after the completion of the intervention in week 12." |
|
| Participants |
Country: Australia
Participants recruited from: long‐term care facilities Number of participants randomised: 67 participants Number of participants included in analysis: 61 participants Mean age of participants: 84 years, SD = 6.36 (mean age of each intervention group was not specified) Sex of participants: 59% females (sex for each intervention group was not specified) Inclusion criteria: 1. Aged 60 or over 2. Living in a participating nursing home for at least 3 months 3. Cognitive functional impairment indicative of a dementia condition; MMSE score of 24 out of 30 or less; and features of Alzheimer’s disease according to American Psychiatric Association DSM‐IV‐TR 4. A documented history of a minimum of 2 weeks of agitation or aggression in total (consecutively or 14 single days), within the past 3 months 5. A documented history of physical and/or chemical restraint for agitation and aggression, including PRN (as required) medication 6. Consent for participation from resident's family or health‐attorney 7. No known allergic reaction to lavender oil 8. No recent skin tears, lacerations, bruises, or redness and swelling that might interfere with hand massage Exclusion criteria: 1. A diagnosis of schizophrenia or mental retardation to avoid the complication of dual diagnoses impacting on treatment effect 2. Expected to be transferred to another residential facility within the next 3 months |
|
| Interventions |
Intervention groups included in this review: 1. 3% lavender mist. N = 23 allocated to this intervention. 2. 3% lavender mist plus hand massage twice a day for 10 days; each hand massaged for 2.5 minutes. N = 22 allocated to this intervention. 3. Water mist. N = 22 allocated to this intervention. Protocol: The lavender mist consisted of 75 drops of pure 100% lavender oil mixed with 4 mL essential oil solubiliser and 125 cc purified water. 3 sprays of lavender/water mist applied to the participants’ chest within a 30 cm distance. All treatments were given twice a day, at 2 time periods ‒ 9 a.m. to 11 a.m. and 2 p.m. to 4 p.m. ‒ 7 days a week for 6 weeks. |
|
| Outcomes |
Outcomes included in this review: 1. Agitation: CMAI‐SF (quantitative data not reported separately for each treatment group) 2. Adverse events 3. MMSE (no data reported) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation assignments were given to participants following baseline testing; these were generated using a random number table" |
| Allocation concealment (selection bias) | Low risk | "A person not involved in the study randomised participants into three groups in each residential care facility." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were not blinded "Participants received treatments in a quiet and private environment, such as the participant’s room in an attempt to keep staff and family blind to the intervention type. If necessary, curtains and folding screens were used to screen participants from the view of the nursing staff." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All primary outcome measures were assessed by facility staff blind to treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "One male resident died in the first week of the study, and as no data were collected he was excluded. Five participants or their relatives withdrew consent for participation and data during the six weeks of the intervention stage of the study. Withdrawal of consent was related to family wanting reassurance their family member was in the intervention rather than control group and the team being unable to reassure the family. The data for these individuals was also excluded" |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | Low risk | No additional biases |
Fujii 2008.
| Study characteristics | ||
| Methods |
Treatment allocation: randomised controlled trial
Study design: parallel group
Intervention: 4 weeks Assessments: assessments were conducted at baseline and following the 4‐week intervention |
|
| Participants |
Country: Sendai, Japan Participants recruited from: long‐term care Sendai Tomizawa Hospital Number of participants randomised: 28 participants Number of participants included in analysis: 28 participants Mean age of participants: 78 years, SD = 10 (active treatment = 77 years, SD = 10; no treatment = 80 years, SD = 11) Sex of participants: 68% females (active treatment = 64% female; no treatment = 71% female) Inclusion criteria: 1. Dementia diagnosed according to DSM‐IV criteria 2. Physical condition stable for 3 months Exclusion criteria: 1. Major medical illness |
|
| Interventions |
Interventions groups included in this review: 1. 2 drops of lavender oil odorant (Farm Tomita, Hokkaido, Japan) was applied to the collar of the participants underwear. Aromotherapy was administered approximately 1 hour after meals, 3 times a day, for 4 weeks. N = 14 allocated to this intervention. 2. No active treatment. N = 14 allocated to this intervention. |
|
| Outcomes |
Outcomes included in this review: 1. Behavioural symptoms: NPI 2. Cognition: MMSE 3. Activities of daily living: Barthel Index tests (no quantitative data provided following the intervention) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "We randomly assigned patients with BPSD into two groups." No information provided about random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | "We randomly assigned patients with BPSD into two groups." No information provided about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Care staffs could smell lavender odour around the bedside of the patients for at least 2h." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "a trained nurse directly observed the patient and performed the NPI, MMSE and Barthel Index tests in a blinded manner to treatment status" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. Data was not provided for the Barthel Index tests; however, the study reports 'The Barthel Index did not significantly change in both groups'. |
| Other bias | Low risk | No additional biases. |
Hanson 2013.
| Study characteristics | ||
| Methods |
Treatment allocation: randomised controlled trial Study design: cross‐over Intervention: acclimation: 1 week; first intervention: 1 week; second intervention: 1 week. No washout period. Assessment: "Participants were monitored with actigraphs (Ambulatory Monitoring Inc,) for 3 weeks." "Weekday epochs of actigraph data (4 x 24 h, Mon noon to Fri noon)". An average of total sleep was calculated over the 4 nights. "Total minutes of sleep were calculated after sleep periods were manually defined using data from sleep logs." "Neuropsychiatric Inventory (NPI) measured every week." |
|
| Participants |
Country: USA (Minnesota) Participants recruited from: memory care units in the Deer Crest assisted living facility in Red Wing, Minnesota Number of participants randomised: 22 participants Number of participants included in analysis: 21 participants Mean age of participants: 85.7 years (unclear whether this was calculated based on the participants who were randomised or the participants who completed the study) Sex of participants: 71% female (unclear whether this was calculated based on the participants who were randomised or the participants who completed to study) Inclusion criteria: not specified Exclusion criteria: not specified |
|
| Interventions |
Interventions included in this review: 1. Lavender oil applied at bedtime to the spine, back and neck, followed by a diffuser containing lavender oil for 20 minutes. 2. Almond oil applied at bedtime to the spine, back and neck, followed by a diffuser containing almond oil for 20 minutes. |
|
| Outcomes |
Outcomes included in this review: 1. Sleep: total sleep time in minutes and percentage of sleep (time spent sleeping divided by the total sleep period) 2. Behavioural symptoms: NPI |
|
| Notes | Unable to find a published paper. Data extraction was conducted on poster and abstract data only. "This work was funded by a donation from a non‐profit private foundation." "Essential oils and diffusers were donated by Young Living, Inc." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Limited information given, "Double‐blinded, randomized‐controlled cross‐over trial" |
| Allocation concealment (selection bias) | Unclear risk | Limited information given, "Double‐blinded, randomized‐controlled cross‐over trial" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Limited information given, "Double‐blinded, randomized‐controlled cross‐over trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Limited information given, "Double‐blinded, randomized‐controlled cross‐over trial" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of participants enrolled = 22 Number of participants that completed the intervention = 21 Number of participants that adhered to intervention = 17 Number of participants with a complete dataset = 11 (50% of the data is available) |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported |
| Other bias | High risk | Poster and abstract data only, limited information regarding the methods |
Lin 2007.
| Study characteristics | ||
| Methods |
Treatment allocation: randomised controlled trial
Study design: cross‐over
Intervention: treatment 1: 3 weeks; washout: 2 weeks; treatment 2: 3 weeks Assessments: assessments were conducted at baseline, following treatment 1, following the washout period and following treatment 2 |
|
| Participants |
Country: Hong Kong, China Participants recruited from: Care and attention homes in Hong Kong Number of participants randomised: 70 participants Number of participants included in analysis: 70 participants Mean age of participants: 78.29 years Sex of participants: 58.6% females Inclusion criteria: 1. Diagnosis of dementia according to DSM‐IV criteria 2. 'Clinically significant agitation' as determined by research team psychiatrist using Chinese version of CMAI 3. Concurrent psychotropic medication was allowed. 51.4% of subjects were receiving psychotropic medication. Their medication was not altered during the course of the trial. Exclusion criteria: |
|
| Interventions |
Interventions included in this review: 1. 100% lavender essential oil 2. Sunflower preparation essential oil 2 drops of the oil assigned to the patient were dropped onto cosmetic cotton. This was then placed into an aroma diffuser. 2 diffusers were then placed 1 on each side of the subject's pillow for at least 1 hour a night whilst they slept. |
|
| Outcomes |
Outcomes included in this review: 1. Agitation: Chinese CMAI 2. Agitation: Chinese NPI |
|
| Notes | Data for first period of cross‐over has not been received for re‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned to group A or B by blocked randomisation", no additional information provided |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "A staff in each C & A home was designated for implementing the interventions. The same staff member was then interviewed about the performance of study participants. It might lead to bias since they were not completely blinded to the treatments offered" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "A staff in each C & A home was designated for implementing the interventions. The same staff member was then interviewed about the performance of study participants. It might lead to bias since they were not completely blinded to the treatments offered" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "A total of 70 subjects were recruited and no dropout was reported" |
| Selective reporting (reporting bias) | Low risk | All outcome measures were reported |
| Other bias | Low risk | No additional biases |
O'Connor 2013.
| Study characteristics | ||
| Methods |
Treatment allocation: randomised controlled trial
Study design: cross‐over
Intervention: treatment 1: 1 week; washout: 4 days; treatment 2: 1 week Assessments: assessments were conducted 30 minutes before application, 30 minutes after application, 60 minutes after application |
|
| Participants |
Country: Australia Participants recruited from: 8 specialist psychogeriatric nursing homes and 3 private nursing homes Number of participants randomised: 66 Number of participants included in analysis: 64 Mean age of participants: 77.6 years, SD = 9.4 (unclear whether this was calculated based on the participants who were randomised or the participants who completed study) Sex of participants: 59% females (unclear whether this was calculated based on the participants who were randomised or the participants who completed study) Inclusion criteria: 1. At least mild dementia on the Clinical Dementia Rating scale 2. Physically agitated behaviour; behaviour was not due primarily to pain, physical illness, depression or psychosis 3. Residence in the facility for at least 3 months 4. Nursing and medical staff were asked not to alter participants’ psychotropic medications if possible 5. Consent to study participation by the next of kin or guardian Exclusion criteria: 1. An acute, life‐threatening illness 2. A variable psychotropic medication regime 3. A medical condition that precluded the use of topical oils |
|
| Interventions |
Interventions included in this review: 1. 30% lavender in jojoba oil 2. Jojoba oil Protocol: A nursing staff member massaged 1 mL of either the lavender or control oil into both forearms for 1 minute each, giving a total of 2 mL per session. The intervention was conducted 3 times during each of the treatment periods. Aromatherapy was administered at times when nursing staff reported that the selected physically agitated behaviour was most likely to be present. |
|
| Outcomes |
Outcomes included in this review: 1. Overall behavioural and psychological symptoms: observation of behaviour (data not reported for first phase of the trial) 2. Mood: Philadelphia Geriatric Center Affect Rating Scale (PGCARS) (data not reported for first phase of the trial) 3. Adverse effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were allocated randomly by the project manager using an Excel random number generator to either a lavender or control study condition with no pre‐set blocking" |
| Allocation concealment (selection bias) | Low risk | "Participants were allocated randomly by the project manager using an Excel random number generator to either a lavender or control study condition with no pre‐set blocking" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The lavender and control oils were stored in identical vials, marked as A or B."
"It was not considered practicable or desirable to attempt to blind participants, all of whom had marked cognitive impairment, to the treatment condition." "Only a single researcher, who had no other involvement in the study, was aware of the allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "To maintain observer blinding, nurses applying the oil wore a nose clip and research assistants, who completed the observations, applied a mixture of essential oils to their upper lip to disguise lavender’s fragrance." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants did not complete the trial, reasons not reported |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported |
| Other bias | Low risk | No additional biases |
Smallwood 2001.
| Study characteristics | ||
| Methods |
Treatment allocation: randomised controlled trial
Study design: parallel group
Intervention: 4 weeks (intervention administered twice weekly) Assessments: Assessments were conducted at baseline and following the intervention. |
|
| Participants |
Country: UK Participants recruited from: patients in a district general hospital ward Number of participants randomised: 21 participants Number of participants included in analysis: 21 participants Mean age of participants: 66.8 years (SD = 11.5) (unclear whether this was calculated based on the participants who were randomised or the participants who completed study). Mean age of intervention groups not reported. Sex of participants: 57% female (unclear whether this was calculated based on the participants who were randomised or the participants who completed study). Sex of intervention groups not reported. Inclusion criteria: 1. Diagnosis of severe dementia made by psychiatrist 2. Individual suitability for aromatherapy Exclusion criteria: Not specified |
|
| Interventions |
Intervention groups included in this review 1. Lavender in a diffuser with conversation twice a week. N = 7 allocated to this intervention. 2. Lavender applied via massage twice a week. N = 7 allocated to this intervention. 3. Plain oil applied via massage twice a week. N = 7 allocated to this intervention. Additional intervention groups None |
|
| Outcomes |
Outcomes included in this review: "Behaviour was recorded using a video camera for 15 minutes across four periods of the day (10‐11 am, 11‐12 noon, 2‐3 pm, and 3‐4 pm). A baseline measure of behaviour was recorded over a two‐week period preceding the study. Each patient's behaviour was recorded twice in each period of the day studied, giving a total of eight records or two hours of footage per individual. No two samples relating to any one individual were recorded on the same day." (Data was reported at baseline but not provided following the intervention. A reduction in motor behaviour after treatment was presented as a figure.) |
|
| Notes | Significant interaction with time. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Seven patients were randomly allocated to each of three conditions" "Random allocation of patients was made by two authors (EI and FC), neither of whom was involved in either data collection or data analysis" No additional information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The same aromatherapist administered each condition and was blind to condition but not hypothesis"; no blinding of staff to allocation was reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Video records were rated by two individuals, both blind to condition, and one who was blind to hypothesis" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "One subject was excluded following deterioration in health preceding the completion of the study period" |
| Selective reporting (reporting bias) | High risk | No useable data |
| Other bias | Low risk | No additional biases |
Takahashi 2020.
| Study characteristics | ||
| Methods |
Treatment allocation: randomised controlled trial
Study design: parallel group
Intervention: 8 weeks Assessments: assessments were conducted at baseline, following 4 weeks of the intervention, and following 8 weeks of the intervention |
|
| Participants |
Country: Japan Participants recruited from: not specified Number of participants randomised: not specified Number of participants included in analysis: 36 participants Mean age of participants: experimental group = 76.2 years, SD = 9.8 years. Control group = 75.8 years, SD = 7.8 years. Sex of participants: experimental group = 63% female, control group = 59% female Inclusion criteria: 1. Diagnosis of Alzheimer's disease according to NINCDS/ADRA. Patients with probable Alzheimer's disease were included. Exclusion criteria: 1. Patients with mild cognitive impairment 2. Patients with olfactory dysfunctions |
|
| Interventions |
Intervention groups included in this review: 1. Ethanol with cedar fragrance. N = 19 allocated to this intervention. 2. Ethanol without cedar fragrance. N = 17 allocated to this intervention. Cedar leaves were added to ethanol solution, the solution was then distilled. Each day, 2.3 mL of the distilled solution (with or without cedar) was used as a room fragrance (diffused using rattan sticks) in the residents' space (living room and bedroom). A few times a day, the distilled solution (with or without cedar) was also sprayed as a mist on the patients' clothing and bedding. |
|
| Outcomes |
Outcomes included in this review: 1. Behavioural symptoms: NPI 2. Cognition: Alzheimer's Disease Assessment Scale‐cognitive subscale 3. Cargiver burden: Japanese version of the Zarit Caregiver Burden interview |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'These patients were assigned randomly to the intervention group or control group.' |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | No additional biases. |
Watson 2019.
| Study characteristics | ||
| Methods |
Treatment allocation: randomised controlled trial
Study design: cross‐over
Intervention: 14‐day consecutive intervention treatment, followed by a 14‐day washout period, before commencing the next treatment. 3 treatments were administered to each participant. Assessments: NPI and CMAI was measured before and after each treatment cycle |
|
| Participants |
Country: Sydney, Australia Participants recruited from: 6 Residential Aged Care Facilities (RACF) Number of participants randomised: 56 participants with dementia, 19 participants without dementia Number of participants included in analysis: 39 participants with dementia, 10 participants without dementia. Mean age of participants: mean age of all participants included in the analysis is 89.31 years, SD = 6.30. The study does not provide the mean age for just the subjects with dementia. Sex of participants: percentage female of all of the participants included in the analysis is 75.5%. The study does not provide the sex for just the subjects with dementia. Inclusion criteria: 1. Age 65 years or older 2. Lived full time in the RACF for 3 months 3. A cognitive level of moderate or higher as demonstrated by a score above 10 on the MMSE 4. Agitated behaviours recorded on at least 1 of the Aged Care Funding Instruments (ACFI) behaviour domains 5. At least 1 agitated behaviour with a frequency of at least 6 occurrences observed by the nurse in the last 2 weeks, assessed on the NPI at baseline 6. An ability to detect scent as demonstrated in a scent test at screening 7. A valid signed resident or carer consent form 8. Participants stable on regular antipsychotic medication who exhibited observable agitation were included in this trial Exclusion criteria: 1. A diagnosis of psychosis or agitation resulting from brain damage 2. The presence of an acute life‐threatening condition as reported by staff or the medical officer 3. Any condition that was likely to confound the study such as schizophrenia, Parkinson's disease or another medical condition as determined by the researcher to interfere with interpretation of study results. |
|
| Interventions |
Interventions included in this review: Participants were randomly assigned a treatment sequence of lavender, lemon balm and sunflower oil. "The RA applied two drops of oil from the correctly assigned scent bottle to a 25 mm x 20 mm dark 100% cotton patch and attached the cloth to the participants collar area. The patch was dark in colour to obscure essential oil pigmentation. The RA did not engage in any non deliberate conversation with the participant and removed the patch after 2 hours." |
|
| Outcomes |
Outcomes included in this review: 1. Behavioural symptoms: NPI 2. Agitation: CMAI |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned a treatment sequence |
| Allocation concealment (selection bias) | Low risk | "The allocation sequence was concealed until data collection was completed and analysis finalised." "Assigned treatments were blinded in 6 bottles labeled with the letters A‐F. A second researcher accessed the computer‐generated assignment of each participant. Each participant was allocated a corresponding essential oil bottle letter for each treatment cycle. When the group assignment was confirmed the researcher informed the primary investigator of the assigned treatment to be implemented in each treatment period. Assigned treatments were blinded in 6 bottles and labeled with the letters A‐F" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study does not specify that the participants were blinded. "The RA was blinded to the allocation group and treatment by a nose peg and 3% Rosemary oil in Jojoba oil placed above the top lip." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No reporting of attrition by group. |
| Selective reporting (reporting bias) | Low risk | All outcome measures were reported. |
| Other bias | Low risk | |
Yang 2015.
| Study characteristics | ||
| Methods |
Treatment allocation: cluster‐randomised controlled trial Study design: parallel group Intervention: 4 weeks Assessments: CMAI was measured at baseline, following the 4‐week intervention and 3 weeks after the end of the intervention |
|
| Participants |
Country: Taiwan Participants recruited from: 6 institutions that specialise in the care of dementia patients (3 retirement homes for veterans and 3 long‐term care facilities) Number of participants randomised: 186 participants, of whom 130 were randomised to the 2 groups included in this review. Number of participants included in analysis: 130 participants were included in the analysis of the 2 groups included in this review Mean age of participants: active treatment = 83.67 years, SD = 4.96. Control group = 81.56 years, SD = 6.79. Sex of participants: 30% female (active treatment = 34% female; placebo = 24.6% female) Inclusion criteria: 1. Fulfil the DSM‐IV standard for dementia as diagnosed by psychiatrists or neurologist 2. Score 35 or above on the long form of the CMAI, defined as severe agitation 3. Expected to be present in the long‐term care facility every Monday to Friday during the period of the study 4. No broken skin or infection surrounding the acupoints Exclusion criteria: Not specified |
|
| Interventions |
Intervention groups included in this review: 1. 2.5% lavender oil was applied for 2 minutes at 5 acupoints. A warm‐up exercise was completed for 5 minutes. The protocol was conducted once per day, for 5 days per week, for a total of 4 weeks. N = 73 allocated to this intervention. 2. No intervention, daily care routine was conducted as usual. N = 57 allocated to this intervention. Additional intervention groups: 3. Each acupoint was pressed for 2 minutes with 2.5% lavender oil at 5 acupoints. A warm‐up exercise was completed for 5 minutes. The protocol was conducted once per day, for 5 days per week, for a total of 4 weeks. N = 56. |
|
| Outcomes |
Outcomes included in this review: 1. Agitation: CMAI‐Chinese version Additional outcomes: 1. Agitation: heart rate variability analyzer (Heart rate variability was considered in the paper to be a measure of agitation; however, this outcome has been excluded from this review as it is not an established or recognised measurement of agitation.) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each time, an institution in the veteran home was randomly assigned to the aroma‐ acupressure, aromatherapy, or control group and so was an institution in the long‐term care facility. The research assistant was blinded to the assignment procedure and allocation results. No further information given. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistant reported the outcome measures and was blinded to allocation group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear reporting of all attrition data. CMAI was analysed for all participants in the study. |
| Selective reporting (reporting bias) | Low risk | No protocol. All outcomes were appropriately discussed. |
| Other bias | Unclear risk | Possible recruitment bias before or after cluster allocation. Possible analysis bias as the study does not specify whether clustering was taken account in the statistical analysis. |
Yang 2016.
| Study characteristics | ||
| Methods |
Treatment allocation: randomised control trial
Study design: parallel group
Intervention: 8 weeks (intervention conducted between weeks 2 and 9 of study) Assessments: assessments conducted at week 1 (baseline) and 24 hours after massage in weeks 2, 5 and 9 |
|
| Participants |
Country: Taiwan
Participants recruited from: 5 long‐term care facilities
Number of participants randomised: 59 participants Number of participants included in analysis: 56 participants Mean age of participants: the mean age for the participants who were randomised is stated as "92 years, SD = 7 years" which is assumed to be a typographical error. (Experimental group = 83.34 years, SD = 6.41; control group = 80.67 years, SD = 7.44) Sex of participants: 61% female (experimental group = 65.5% female; control group = 56.7% female) Inclusion criteria: 1. Mild to severe dementia (SPMSQ score ≤ 8 or MMSE ≤ 17 if high school education or MMSE ≤ 23 if high school education or higher). 2. Demonstrated agitation or depressive symptoms in the past 2 weeks as reported by caregivers using the CCMAI and CSDD‐C (no cut‐off point was specified). Exclusion criteria: 1. Severity of behavioural problems prohibited interaction with researchers. |
|
| Interventions |
Intervention groups included in this review: 1. 3 drops of lavender oil and 3 drops of orange oil were added to 5 mL of essential oil. Aromatherapy oil was massaged around the neck, shoulders and arms for 30 minutes, once per week. The intervention was performed by trained research assistants. N = 29 allocated to this intervention. 2. No intervention, usual care was provided "participation in regular activities (e.g. group singing, watching movies) in the long‐term care facilities". N = 30 allocated to this intervention. |
|
| Outcomes | Outcomes included in this review: 1. Agitation: CCMAI (measured at baseline and within 24 hours of the massage in weeks 2, 5 and 9) 2. Mood: CSDD‐C (measured at baseline and within 24 hours of the massage in weeks 2, 5 and 9) | |
| Notes | Registry entry (NCT02126059)
Further information sought from authors on 4 November 2018 ‒ authors did not respond. Quality of life: WHO Quality of Life‐brief Taiwanese version (WHOQOL‐BREF) was an outcome mentioned in the trial registry but was not mentioned in the paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block technique not explained. Conducted solely by primary author. “Individuals with dementia were randomly assigned to the control or intervention groups through a randomized block technique performed by the researcher (i.e. intervener [J.J.‐W.].” |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information but probably not possible to blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Data collectors (i.e. caregivers) were blind to participant allocation.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants did not complete the study in the intervention group. Data available in the intervention group: 93% 1 participant did not complete the study in the control group. Data available in the control group: 97% |
| Selective reporting (reporting bias) | High risk | WHO‐QOL‐BREF not reported, although listed in trial registry entry |
| Other bias | Low risk | Part of a larger trial. No mention of how care facilities implementing aromatherapy were selected. |
BPSD ‐ behavioural and psychological symptoms in dementia CDR ‐ Clinical Dementia Rating Scale CMAI ‐ Cohen Mansfield Agitation Inventory CSDD‐C ‐ Cornell Scale for Depression in Dementia‐Chinese Version PAS ‐ Pittsburgh Agitation Scale NINCDS‐ADRDA ‐ National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association NPI ‐ Neuropsychiatric Inventory SD ‐ standard deviation QOL ‐ quality of life
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akhondzadeh 2003 | Wrong intervention (oral administration of lemon balm extract) |
| Bowles 2002 | Wrong study design (not RCT) |
| Brooker 1997 | Wrong study design (not RCT) |
| Burleigh 1997 | Wrong study design (not RCT) |
| Chiayana 2012 | In vitro study |
| Cohen‐Mansfield 2012a | Wrong intervention |
| Cohen‐Mansfield 2012b | Wrong intervention |
| Cooper 2012 | Systematic review |
| Dimitriou 2018 | Wrong study design (no control group) |
| Farokhnia 2014 | Wrong intervention |
| Fung 2012 | Systematic review |
| Fung 2018 | Wrong intervention |
| Gray 2002 | Wrong study design (not RCT) |
| Guendling 2010 | Wrong study design |
| Henry 1993 | Wrong study design |
| Holmes 2002 | Wrong study design |
| Jimbo 2009 | Wrong study design |
| Kaufmann 2011 | In vitro study |
| Kaymaz 2017 | Wrong study design (not RCT) |
| Kilstoff 1998 | Wrong study design (not RCT) |
| Kimura 2013 | Wrong study design (not RCT) |
| Klages 2011 | Wrong intervention |
| Korn 2012 | Wrong intervention |
| Li 2017 | Wrong study design (not RCT) |
| Lucian 2012 | Animal study |
| MacMahon 1998 | Wrong study design (not RCT) |
| Mitchell 1993 | Very limited methodological information. Very limited results. Probably wrong participants ("dementia‐related disorders"). |
| Moss 2003 | Wrong participants |
| NCT02518243 | Wrong study design (not RCT) |
| Ogun‐Semore 2019 | Wrong study design (no control group) |
| Opie 1999 | Literature review |
| Pengelly 2012 | Wrong intervention |
| Sakamoto 2012 | Wrong participants |
| Snow 2004 | Wrong study design (not RCT) |
| Tsolaki 2016 | Wrong participants |
| UMIN000019044 | Wrong study design (dose comparison) |
| UMIN000027692 | Wrong study design (dose comparison) |
| UMIN000027693 | Wrong study design (not RCT) |
| Watanabe 2010 | Wrong participants |
| West 1994 | Wrong study design (not RCT) |
| Wolfe 1996 | Wrong study design (not RCT) |
| Woods 1996 | Wrong intervention |
| Zalomonson 2019 | Wrong study design (not RCT) |
Characteristics of studies awaiting classification [ordered by study ID]
ISRCTN86563511.
| Methods |
Treatment allocation: randomised controlled trial Study design: not specified Intervention: 12 weeks Assessments: conducted at baseline and following 12 week intervention |
| Participants |
Country: Australia Participants recruited from: resident in nursing home Number of participants: not specified ‐ target number of participants was 100 to 130 people. Mean age of whole study population: not specified Sex of whole study population: not specified Inclusion criteria: 1. Living in the nursing home for more than 3 months 2. More than 65 years old 3. Already on an aromatherapy care plan; or deemed by the Director of Care or the care staff to be unlikely to be disturbed by the use of the aromatherapy lotion in place of their normal skin integrity lotion 4. English as their first language 5. Mini‐Mental State Examination (MMSE) score of 10 to 26 6. Diagnosis of dementia, short‐term memory loss or cognitive impairment that is not caused by any other diagnosis of mental illness 7. Residents with non‐acute concomitant diseases may participate if their disease is medically controlled. Exclusion criteria: 1. Myocardial infarction or stroke in previous 3 months 2. Epilepsy 3. Current treatment with anti‐cholinesterase or anti‐cholinergic drugs 4. Eczema, psoriasis or dermatitis around the neck and shoulders area 5. Known allergy to Eucalyptus, Cypress, Ginger, Lemongrass, Lime or Mandarin essential oils or aqueous cream 6. An adverse reaction to treatment patch‐tests given during screening process 7. Vision or hearing impairments that prevent them from undertaking the cognitive test |
| Interventions |
Intervention groups relevant to this review: 1. The 'active' treatment will contain 1 mL each of cypress (Cupressus sempervirens), lime (Citrus latifolia) and eucalyptus (Eucalyptus globulus) essential oils, diluted in a non‐fragranced aqueous cream lotion 2. The 'inactive' preparation will contain 1 mL each of ginger (Zingiber officinalis), lemon grass (Cymbopogon citratus) and mandarin (Citrus reticulata) essential oils, diluted in a non‐fragranced aqueous cream lotion 3. The placebo preparation will contain only non‐fragranced aqueous cream lotion and will be used during the washout periods. An important purpose of the placebo is to control for the possible effect of touch |
| Outcomes |
Outcomes relevant to this review: 1. Primary: MMSE 2. Secondary: NOSGER (Nurses' Observation Scale for Geriatric Patients) |
| Notes | Despite repeated attempts, CDCIG have been unable to get any reply from the study author Professor Stephen Myers: smyers@scu.edu.au. No data were available from this study. |
NCT03576170.
| Methods |
Treatment allocation: randomised controlled trial Study design: parallel group Intervention: not specified Assessments: "3 time points" |
| Participants |
Country: Hong Kong
Participants recruited from: not specified
Number of participants randomised: 112 participants Number of participants included in analysis: not specified Mean age of participants: not specified Sex of participants: not specified Inclusion criteria: 1. 60 years of age or older 2. Have a CMMSE score below 18 if illiterate, 19 if they have 1 to 2 years of education, and 20 if they had more than 2 years of education 3. Reported to have BPSD 4. Willing to participate in the research, with informed consent signed by their guardian or carer Exclusion criteria: 1. Allergic to essential oils 2. Refused to give consent 3. Over‐sensitive to tactile stimulation 4. Have a history of kidney or liver disease 5. Have ever had an epileptic seizure |
| Interventions |
Intervention groups relevant to this review: 1. Aroma‐scent 2. Aroma‐touch 3. Wait‐list |
| Outcomes |
Outcomes relevant to this review: 1. CCMAI 2. CNPI 3. CMMSE 4. Chinese version of the Barthel Index Additional outcomes 5. Chinese version of the index of social engagement |
| Notes | Registry entry. Study completion date 2018. No published paper identified. Contacted author to ask if data was available or if the trial had been published. The author did not respond. |
NCT03662360.
| Methods |
Treatment allocation: randomised controlled trial Study design: parallel groups Intervention: not specified Assessments: Baseline, day 4, day 7, day 13 |
| Participants |
Country: Switzerland
Participants recruited from: not specified "the acute geriatric ward"
Number of participants randomised: 32 participants Number of participants included in analysis: not specified Mean age of participants: not specified Sex of participants: not specified Inclusion criteria: 1. Patients admitted to the acute geriatric ward 2. Patients with a known diagnosis of dementia associated with BPSD or diagnosis performed during hospitalisation 3. 70 years or older Exclusion criteria: 1. Patients with alcohol‐based dementia 2. Patients with Mild Cognitive Impairment 3. Patients with language barrier 4. Patients already being treated with aromatherapy |
| Interventions |
Intervention groups relevant to this review: 1. Diffusion aromatherapy (lavender essential oil/wild orange essential oil) 2. No intervention |
| Outcomes |
Outcomes relevant to this review: 1. NPI‐Nursing Home Version |
| Notes | Registry entry. Study completion date 2018. No published paper identified. Contacted author to ask if data was available or if the trial had been published. The author did not respond. |
UMIN000020148.
| Methods |
Treatment allocation: randomised control trial Study design: cross‐over Intervention: 12 weeks (Group 1: 6‐week aroma hand massage followed by 6‐week live as usual; Group 2: 6‐week live as usual followed by 6‐week aroma hand massage) Assessments: assessments conducted prior to the first intervention/live as usual, following the first 6‐week intervention/live as usual and following the second 6‐week intervention/live as usual. |
| Participants |
Country: Japan Participants recruited from: not specified Number of participants randomised: not specified Mean age of whole study population: study planned to recruit participants aged between 20 and 80 years old. Exact ages are not specified. Sex of whole study population: study planned to recruit males and females. Percentages of males and females are not specified. Inclusion criteria: 1. Those who live around Matsuyama or Toon, Ehime Japan 2. Aged over 65 years old 3. Those who can participate in the trial in pairs of couples or friends 4. Those who can come to the examination for themselves 5. Those who are interested in preventing dementia or improving cognitive function 6. Following explanation of the trial, those who agree with participation and give written informed consent Exclusion criteria: 1. Severe heart disease 2. Severe hypertension (180/110 mmHg or more) 3. Severe arrhythmia 4. Pregnancy 5. Other severe diseases |
| Interventions |
Intervention groups relevant to this review: Group 1. First examination ‐ then aromatherapy hand massage for 10 minutes prior to sleeping at home (duration 6 weeks). Second examination ‐ followed by living as usual (duration not specified). Final examination. Group 2. First examination, followed by living as usual (duration not specified). Second examination ‐ then an aromatherapy hand massage for 10 minutes prior to sleeping at home (duration 6 weeks). Final examination. |
| Outcomes |
Outcomes relevant to this review: Cognitive function |
| Notes | Registry entry. Recruitment closed 2015. No published paper identified. Mixed population, including participants with dementia. Authors asked if trial competed and if data available for participants with dementia only. The author did not respond. |
UMIN000026366.
| Methods |
Treatment allocation: randomised control trial Study design: parallel group Intervention: 3 months Assessments: before and after the intervention |
| Participants |
Country: Japan Participants recruited from: not specified Number of participants randomised: not specified Mean age of whole study population: study planned to recruit participants aged between 60 and 85 years. Exact ages are not specified. Sex of whole study population: study planned to recruit males and females. Percentages of males and females are not specified. Inclusion criteria: 1. Males and females who live around Matsuyama city 2. Aged 60 to 85 years 3. Able to join in the initial and final examination 4. Can perform the intervention daily at home and are able to join the aroma foot massage class (6 sessions) on their own 5. Interested in the prevention of dementia or other disease, or promotion of health 6. Joined in the explanation session for participation in this study and gave written informed consent Exclusion criteria: 1. Participated in the cognitive function improvement effect of aromatherapy massage research study in 2015 and 2016 2. Severe cardiac disease 3. Severe hypertension (>180/110) 4. Severe arrhythmia 5. Other severe diseases 6. Allergy about the aroma oil (examined by patch test) For criteria 2 to 5, participation was permitted if symptoms were stable (due to medication) or a doctor provided consent. |
| Interventions |
Intervention groups relevant to this review: 1. Participants in the experimental group received 10 minutes of aroma foot massage daily for 3 months. Did not specify who administers the intervention. 2. The control group lived as usual |
| Outcomes |
Outcomes relevant to this review: Cognitive function: 1. The Montreal Cognitive Assessment (MoCA) 2. Digit Symbol Substitution Test (DSST) |
| Notes | Registry entry. No published paper identified. Mixed population, including participants with dementia. Authors have been contacted and asked if trial is completed and if data available for participants with dementia only ‒ no response. |
Yoshiyama 2015.
| Methods |
Treatment allocation: randomised controlled trial Study design: cross‐over Intervention: first oil administered for 4 weeks, followed by a 4‐week washout interval, followed by the second oil administered for 4 weeks Assessments: assessments were conducted before and after each trial and 4 weeks after the study |
| Participants |
Country: Nara, Japan Participants recruited from: a nursing home Number of participants randomised: 14 participants Mean age of whole study population: 82.8 years, SD = 9.503 years Sex of whole study population: 100% female Inclusion criteria: 1. Residence in the nursing home 2. Aged 65 years or older 3. Dementia diagnosed by the International Classification of Diseases 10th revision 4. Mild‐to‐moderate dementia (score of 10 to 26 on the MMSE) 5. Score of III on the Independence Degree of Daily Living for the Demented Elderly scale 6. Negative reaction on a patch test with jojoba oil and Delight & Harmony oil (D&H oil) 7. Consent for participation from patients and their families Exclusion criteria: 1. Any acute physical illness |
| Interventions |
Intervention groups relevant to this review: 1. D&H oil (1.02% essential oils and 0.2% lavender oil) (3 mL of oil was used for both hands) 2. Jojoba oil (3 mL of oil was used for both hands) Protocol: The first oil was administered 3 times per week for 4 weeks, with a 4‐week washout interval, followed by the second oil administered 3 times per week for 4 weeks. The oil was massaged gently on 1 hand and then the other in the following order: forearm, wrist, palm, fingers, and back of the hand. Treatment administered in the living room of the nursing home by a single researcher and aroma therapist, in the afternoon. |
| Outcomes |
Outcomes unable to use in the review (insufficient information provided): 1. Depression: CSDD 2. Agitation: CMAI (assess the frequency of agitated behavioural disturbance) 3. Degree of psychiatric symptoms and care burdens: NPI‐Q 4. Activities of daily living in dementia: Functional Independent Measure (FIM) (measuring the degree of disabilities and assistance required in ADLs). A single researcher evaluated the effect on BPSD and also ADLs. |
| Notes | Paper has been published in the Journal of Alternative and Complementary Medicine. The results provided in the published paper are unclear. Authors were asked to provide raw data for all outcomes ‒ no response. Aromatic wellness supplied the certified organic massage oil (Delight & Harmony oil). |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12617001159347.
| Study name | The effectiveness of topical essential oils for agitation in dementia: a cluster‐randomised, placebo‐controlled feasibility trial |
| Methods |
Treatment allocation: cluster‐randomised controlled trial Study design: parallel Intervention: 8 weeks Assessments: CMAI, PAS and QoL‐AD were measured in weeks 0, 4, 8 and 10. Frequency of adverse events were measured in weeks 1, 4 and 8. Study feasibility was measured in week 10. |
| Participants |
Country: Australia Participants recruited from: postcodes in Australia (5600 ‐ Whyalla, 5245 ‐ Hahndorf, 5245 ‐ Mannum, 5253 ‐ Murray Bridge) Number of participants randomised: accrual to date = 25 participants Mean age of whole study population: not specified Sex of whole study population: not specified Inclusion criteria: 1. Resident of the study site for a period of at least 4 weeks. 2. Diagnosis of dementia (as determined by Mini‐Mental State Examination (MMSE), DSM‐IV criteria or medical diagnosis). 3. Clinically significant agitation (as defined by a score of 39 or greater on the CMAI, or a score of 4 or greater on the Pittsburgh Agitation Scale [PAS]) 4. Informed consent, both directly (if appropriate) and via their next of kin. 5. Minimum age 40 Exclusion criteria: 1. Concurrent exposure to essential oils in any form 2. Concurrent exposure to other novel therapeutic interventions for agitation (e.g. Paro, Play up) 3. History of significant head trauma or brain lesions 4. Known allergy or sensitivity to any of the ingredients in the active or control interventions. |
| Interventions | 1. Intervention: participants in the intervention group will receive a bespoke blend of essential oils (4.5%) in a cream base, and a bespoke blend of essential oils (3%) in an oil base, at a dose of 20 mL 3 times daily (for the cream), and 10 mL 3 times daily, as required (for the oil), for eight consecutive weeks; the intervention will be administered topically (i.e. forearms/face/neck/shoulders for the cream [depending on participant preference], and lower legs for the oil) by trained nursing staff. Each blend will be personalised based on the participant’s odour preference, unique presentation of symptoms, and health history (including known sensitivities and contraindications to any oils or their chemical constituents). The interventions will be blended by a trained aromatherapist, who will select up to 5 appropriate essential oils from a list of 38 hypoallergenic oils. Fidelity will be assessed using a medication record, and by noting the remaining volume of cream in the intervention receptacle at weeks 4 (mid‐intervention) and 8 (post‐intervention). 2. Control: Participants in the control group will receive control cream (cream base only) and control oil (oil base only), at a dose of 20 mL 3 times daily (for the cream), and 10 mL 3 times daily, as required (for the oil), for eight consecutive weeks; the control treatment will be administered topically (i.e. forearms/face/neck/shoulders for the cream [depending on participant preference], and lower legs for the oil) by trained nursing staff. Fidelity will be assessed using a medication record, and by noting the remaining volume of cream in the control receptacle at weeks 4 (mid‐intervention) and 8 (post‐intervention). |
| Outcomes |
Primary Outcomes: 1. Mean Cohen Mansfield Agitation Inventory (CMAI) score 2. Mean Pittsburgh Agitation Scale (PAS) score Secondary Outcomes: 1. Mean Quality of Life – Alzheimer’s Disease scale (QoL‐AD) score 2. Mean frequency of use of PRN antipsychotic medication (as reported on the PAS) 3. Mean frequency of use of physical restraint (as reported on the PAS) 4. Frequency of adverse events (e.g. erythema, pruritus; measured using a standardised adverse event record) |
| Starting date | 2 January 2018 (date of first participant enrolment) |
| Contact information | Dr Matthew Leach Department of Rural Health University of South Australia 111 Nicolson Avenue Whyalla Norrie, SA, 5608 Australia matthew.leach@unisa.edu.au |
| Notes | Authors have been contacted to provide further information – no response. |
ChiCTR‐INR‐17013281.
| Study name | Comparison of the effects of aroma‐laser acupuncture and aromatherapy on depression in dementia patients |
| Methods |
Treatment allocation: randomised parallel controlled trial Study design: parallel Intervention: not specified Assessments: conducted at baseline and following the intervention |
| Participants |
Country: Taiwan, China Participants recruited from: long‐term care facilities: St. Joseph Home (Hsinchu County) & Suang Lien Elderly Center (New Taipei City) Number of participants randomised: not specified. Target is 41 in each of the 3 experimental groups. Mean age of whole study population: not specified Sex of whole study population: not specified Inclusion criteria: 1. Dementia case (MMSE less than 23) 2. Living in LTC facility during study period 3. The cases whose GDS‐SF15 > 5 points 4. The case's arms and legs have no infection or injury. 5. Aged 65 years or older Exclusion criteria: Not specified |
| Interventions | 1. Aroma‐laser acupuncture group: limonene is applied to acupoints then used the portable laser acupuncture device 2. Aromatherapy group: limonene is used with massage 3. Control group: base oil is used with massage |
| Outcomes | 1. Neuropsychiatric Inventory 2. GDS‐SF15 Outcomes were measured by the assistant. |
| Starting date |
Date of registration: 07 November 2017 Status: not yet recruiting |
| Contact information | Manhua Yang 13F, 368 Dayeh Road, Taipei, Taiwan, China mhyang@ym.edu.tw |
| Notes | Authors have been contacted to provide further information ‒ no response. |
CDCIG ‐ Cochrane Dementia and Cognitive Impairment Group CMAI ‐ Cohen Mansfield Agitation Inventory MMSE ‐ Mini‐Mental State Examination NOSGER ‐ Nurses' Observation Scale for Geriatric Patients
Differences between protocol and review
The Methods section has been updated to the current methods in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011); the previous version of the methods can be found in Appendix 4.
Primary outcome 'Behavioural symptoms' changed to 'Overall behavioural and psychological symptoms'.
Added that all outcomes had to be measured using validated scales.
Added that where studies used a cross‐over method, we used the final scores reported for each treatment group to calculate a mean difference between aromatherapy and control groups following the intervention.
Contributions of authors
ELB: selection of trials for inclusion and exclusion, data extraction, risk of bias assessment, interpretation of results, write‐up of review.
BO‐B: selection of trials for inclusion and exclusion, data extraction, risk of bias assessment, write‐up of review.
JH: selection of trials for inclusion and exclusion, data extraction.
SDS: selection of trials for inclusion and exclusion, data extraction.
AG: selection of trials for inclusion and exclusion.
JMcC: selection of trials for inclusion and exclusion, data extraction, risk of bias assessment, interpretation of results, write‐up of review.
Sources of support
Internal sources
Medical Research Council, University of Edinburgh and University of Glasgow (as part of the Precision Medicine Doctoral Training Programme), UK
External sources
NHS Research Scotland Ageing Speciality Group, UK
Health and Care Research Wales, UK
-
NIHR, UK
This update was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health
Declarations of interest
ELB ‐ none known
BO‐B ‐ none known
JH ‐ none known
SDS ‐ none known
AG ‐ none known
JMcC ‐ none known
These authors contributed equally to this work
These authors contributed equally to this work
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ballard 2002 {published data only}
- Ballard CG, O'Brien JT, Reichelt K, Perry EK. Aromatherapy as a safe and effective treatment for the management of agitation in severe dementia: the results of a double-blind placebo-controlled trial with Melissa. Journal of Clinical Psychiatry 2002;63(7):553-8. [DOI] [PubMed] [Google Scholar]
- Lee L, Reichelt K, Ballard C, Perry E. Melissa aromatherapy as safe and effective treatment. Nursing & Residential Care 2003;5(2):80-2. [Google Scholar]
Burns 2011 {published data only}
- Burns A, Perry E, Holmes C, Francis P, Morris J, Howes MJR, et al. A double-blind placebo-controlled randomized trial of Melissa officinalis oil and donepezil for the treatment of agitation in Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders 2011;31(2):158-64. [DOI] [PubMed] [Google Scholar]
Cameron 2011 {published data only}
- Cameron H, du Toit S, Richard G, Bearns L. Using lemon balm oil to reduce aggression and agitation in dementia: results of a pilot study. Journal of Dementia Care 2011;19(5):36-8.
Fu 2013 {published data only}
- Fu CY, Moyle W, Cooke M. A randomised controlled trial of the use of aromatherapy and hand massage to reduce disruptive behaviour in people with dementia. BMC Complementary and Alternative Medicine 2013;13:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fujii 2008 {published data only}
- Fujii M, Hatakeyama R, Fukuoka Y, Yamamoto T, Sasaki R, Moriya M, et al. Lavender aroma therapy for behavioral and psychological symptoms in dementia patients. Geriatrics & Gerontology International 2008;8(2):136-8. [DOI] [PubMed] [Google Scholar]
Hanson 2013 {published data only}
- Hanson L, Cagan A, Rinehimer K, Flottemesch T, Clairmont J, Sackett-Lundeen L, et al. Lavender essential oil improves sleep in residents of a memory care assisted living facility. In: Alzheimer's & Dementia. Vol. 9 (issue 4, supplement). 2013:P655-6.
- Hanson L, Cagan A, Rinehimer K, Stuck L, Flottemesch T, Clairmont J, et al. Lavender essential oil improved sleep in residents of a memory care assisted living facility (Poster). englishrosesuites.com/wp-content/uploads/2018/10/AAIC-2013-Lavender-Essential-Oil-Poster_LH-2.pdf.
Lin 2007 {published data only}
- Lin PW-K, Chan W-C, Ng BF-L, Lam LC-W. Efficacy of aromatherapy (Lavandula angustifolia) as an intervention for agitated behaviours in Chinese older persons with dementia: A cross-over randomized trial. International Journal of Geriatric Psychiatry 2007;22(5):405-10. [DOI] [PubMed] [Google Scholar]
O'Connor 2013 {published data only}
- O’Connor DW, Eppingstall B, Taffe J, Ploeg ES. A randomized, controlled cross-over trial of dermally-applied lavender (Lavandula angustifolia) oil as a treatment of agitated behaviour in dementia. BMC Complementary and Alternative Medicine 2013;13(1):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploeg ES, Eppingstall B, O'Connor DW. The study protocol of a blinded randomised-controlled cross-over trial of lavender oil as a treatment of behavioural symptoms in dementia. BMC Geriatrics 2010;10(1):49. [DOI] [PMC free article] [PubMed]
Smallwood 2001 {published data only}
- Smallwood J, Brown R, Coulter F, Irvine E, Copland C. Aromatherapy and behaviour disturbances in dementia: a randomized controlled trial. International Journal of Geriatric Psychiatry 2001;16(10):1010-3. [DOI] [PubMed] [Google Scholar]
Takahashi 2020 {published data only}
- Takahashi Y, Shindo S, Kanbayashi T, Takeshima M, Imanishi A, Mishima K. Examination of the influence of cedar fragrance on cognitive function and behavioural and psychological symptoms of dementia in Alzheimer type dementia. Neuropsychopharmacology Reports 2020;40(1):10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 2019 {published data only}
- Watson K, Hatcher D, Good A. A randomised controlled trial of lavender (lavandula angustifolia) and lemon balm (melissa officinalis) essential oils for the treatment of agitated behaviour in older people with and without dementia. Complementary Therapies in Medicine 2019;42:366-73. [DOI] [PubMed] [Google Scholar]
Yang 2015 {published data only}
- ChiCTR-TRC-14004810. Comparison of aroma-acupressure and aromatherapy in improving the problem behaviours of dementia. chictr.org.cn/historyversionpuben.aspx?regno=ChiCTR-TRC-14004810 (first received 01 May 2015).
- Yang MH, Lin LC, Wu SC, Chiu JH, Wang PN, Lin JG. Comparison of the efficacy of aroma-acupressure and aromatherapy for the treatment of dementia-associated agitation. BMC Complementary and Alternative Medicine 2015;15(1):93. [DOI: 10.1186/s12906-015-0612-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2016 {published data only}
- NCT02126059. Complementary interventions on patients with dementia: comparative and longitudinal research [The health effects of cognitive stimulation, reminiscence and aroma-massage complementary interventions on different behavior patterns of patients with dementia]. clinicaltrials.gov/ct2/show/NCT02126059 (first received 29 April 2014).
- Yang YP, Lee FP, Chao HC, Hsu FY, Wang JJ. Comparing the effects of cognitive stimulation, reminiscence and aroma-massage on agitation and depressive mood in people with dementia. Journal of the American Medical Directors Association 2016;17(8):719-24. [DOI] [PubMed] [Google Scholar]
- Yang YP, Wang CJ, Wang JJ. Effect of aromatherapy massage on agitation and depressive mood in individuals with dementia. Journal of Gerontological Nursing 2016;42(9):38-46. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akhondzadeh 2003 {published data only}
- Akhondzadeh S, Noroozian M, Mohammadi M, Ohadinia S, Jamshidi AH, Khani M. Melissa officinalis extract in the treatment of patients with mild to moderate Alzheimer's disease: a double blind, randomised, placebo controlled trial. Journal of Neurology, Neurosurgery, and Psychiatry 2003;74(7):863-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowles 2002 {published data only}
- Bowles EJ, Griffiths DM, Quirk L, Brownrigg A, Croot K. Effects of essential oils and touch on resistance to nursing care procedures and other dementia related behaviours in a residential care facility. International Journal of Aromatherapy 2002;12(1):22-9. [Google Scholar]
Brooker 1997 {published data only}
- Brooker DJR, Snape M, Johnson E, Ward E, Payne M. Single case evaluation of the effects of aromatherapy and massage on disturbed behaviour in severe dementia. British Journal of Clinical Psychology 1997;36(2):287-96. [DOI] [PubMed] [Google Scholar]
Burleigh 1997 {published data only}
- Burleigh S, Armstrong C. On the scent of a useful therapy. Journal of Dementia Care 1997;July/August:21-3. [Google Scholar]
Chiayana 2012 {published data only}
- Chaiyana W, Okonogi S. Inhibition of cholinesterase by essential oil from food plant. Phytomedicine 2012;19(8-9):836-9. [DOI] [PubMed] [Google Scholar]
Cohen‐Mansfield 2012a {published data only}
- Cohen-Mansfield J, Thein K, Marx MS, Dakheel-Ali M, Murad H, Freedman LS. The relationships of environment and personal characteristics to agitated behaviors in nursing home residents with dementia. Journal of Clinical Psychiatry 2012;73:392-9. [DOI] [PubMed] [Google Scholar]
Cohen‐Mansfield 2012b {published data only}
- Cohen-Mansfield J, Marx MS, Freedman LS, Murad H, Thein K, Dakheel-Ali M. What affects pleasure in persons with advanced stage dementia? Journal of Psychiatric Research 2012;46(3):402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2012 {published data only}
- Cooper C, Mukadam N, Katona C, Lyketsos CG, Ames D, Rabins P, et al. Systematic review of the effectiveness of non-pharmacological interventions to improve quality of life of people with dementia. International Psychogeriatrics 2012;24:856-70. [DOI] [PubMed] [Google Scholar]
Dimitriou 2018 {published data only}
- Dimitriou TD, Verykouki E, Papatriantafyllou J, Konsta A, Kazis D, Tsolaki M. Non-pharmacological interventions for agitation/aggressive behaviour in patients with dementia: a randomized controlled crossover trial. Functional neurology 2018;33(3):143-7. [PubMed] [Google Scholar]
Farokhnia 2014 {published data only}
- Farokhnia M, Shafiee Sabet M, Iranpour N, Gougol A, Yekehtaz H, Alimardani R, et al. Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer's disease: a double-blind randomized clinical trial. Human Psychopharmacology 2014;29(4):351-9. [DOI] [PubMed] [Google Scholar]
Fung 2012 {published data only}
- Fung KM, Tsang HW, Chung RC. A systematic review of the use of aromatherapy in treatment of behavioral problems in dementia. Geriatrics & Gerontology International 2012;12(3):372-82. [DOI] [PubMed] [Google Scholar]
Fung 2018 {published data only}
- Fung JKK-M, Tsang HW-H. Management of behavioural and psychological symptoms of dementia by an aroma-massage with acupressure treatment protocol: a randomised clinical trial. Journal of clinical nursing 2018;27(9-10):1812-25. [DOI] [PubMed] [Google Scholar]
Gray 2002 {published data only}
- Gray SG, Clair AA. Influence of aromatherapy on medication administration to residential-care residents with dementia and behavioural challenges. American Journal of Alzheimer's Disease & Other Dementias 2002;17(3):169-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guendling 2010 {published data only}
- Guendling A, Guendling PW. Aromatherapy in dementia. In: European Journal of Integrative Medicine. Vol. Conference: 3rd European Congress for Integrative Medicine, ECIM 2010 Berlin Germany. Conference Start: 20101203 Conference End: 20101204. Conference Publication. 2010:227.
Henry 1993 {published data only}
- Henry J. Aroma groups improve the quality of life in Alzheimer's disease. The International Journal of Aromatherapy 1993;5(1):27-9. [Google Scholar]
Holmes 2002 {published data only}
- Holmes C, Hopkins V, Hensford C, MacLaughlin V, Wilkinson D, Rosenvinge H. Lavender oil as a treatment for agitated behaviour in severe dementia. International Journal of Geriatric Psychiatry 2002;17(4):305-8. [DOI] [PubMed] [Google Scholar]
Jimbo 2009 {published data only}
Kaufmann 2011 {published data only}
- Kaufmann D, Dogra AK, Wink M. Myrtenal inhibits acetylcholinesterase, a known Alzheimer target. Journal of Pharmacy and Pharmacology 2011;63(10):1368-71. [DOI] [PubMed] [Google Scholar]
Kaymaz 2017 {published data only}
- Kaymaz TT, Ozdemir L. Effects of aromatherapy on agitation and related caregiver burden in patients with moderate to severe dementia: A pilot study. Geriatric Nursing 2017;38(3):231-7. [DOI] [PubMed] [Google Scholar]
Kilstoff 1998 {published data only}
- Kilstoff K, Chenoweth L. New approaches to health and well-being for dementia day-care clients, family carers and day-care staff. International Journal of Nursing Practice 1998;4(2):70-83. [DOI] [PubMed] [Google Scholar]
Kimura 2013 {published data only}
- Kimura T, Takamatsu J. Pilot study of pharmacological treatment for frontotemporal lobar degeneration: Effect of lavender aroma therapy on behavioral and psychological symptoms. Geriatrics & Gerontology International 2013;13(2):516-7. [DOI: 10.1111/ggi.12025] [DOI] [PubMed] [Google Scholar]
Klages 2011 {published data only}
Korn 2012 {published data only}
- Korn L, Logsdon R, Polissar N, Gomez-Beloz A, Waters T, Ryser R. A randomized trial of polarity therapy for stress and pain reduction in American Indian and Alaska Native family dementia caregivers. In: BMC Complementary and Alternative Medicine. Vol. Conference: International Research Congress on Integrative Medicine and Health 2012 Portland, OR United States. Conference Start: 20120515 Conference End: 20120518. Conference Publication. 2012:1.
Li 2017 {published data only}
- Li RM, Gilbert B, Orman A, Aldridge P, Leger-Krall S, Anderson C, Castillo JH. Evaluating the effects of diffused lavender in an adult day care center for patients with dementia in an effort to decrease behavioral issues: a pilot study. Journal of Drug Assessment 2017;6(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lucian 2012 {published data only}
- Hritcu L, Cioanca O, Hancianu M. Effects of lavender oil inhalation on improving scopolamine-induced spatial memory impairment in laboratory rats. Phytomedicine 2012;19(6):529-34. [DOI] [PubMed] [Google Scholar]
MacMahon 1998 {published data only}
- MacMahon S, Kermode S. A clinical trial of the effects of aromatherapy on motivational behaviour in a dementia care setting using a single subject design. The Australian Journal of Holistic Nursing 1998;5(2):47-9. [PubMed] [Google Scholar]
Mitchell 1993 {published data only}
- Mitchell S. Aromatherapy's effectiveness in disorders associated with dementia. The International Journal of Aromatherapy 1993;5(2):20-3. [Google Scholar]
Moss 2003 {published data only}
- Moss M, Cook J, Wesnes K, Duckett P. Aromas of rosemary and lavender essential oils differentially affect cognition and mood in healthy adults. The International Journal of Neuroscience 2003;113(1):15-38. [DOI] [PubMed] [Google Scholar]
NCT02518243 {published data only}
- NCT02518243. Safe and easy for Alzheimer's disease and related pathologies. clinicaltrials.gov/ct2/show/NCT02518243 (first received 07 August 2015). [https://clinicaltrials.gov/ct2/show/NCT02518243]
Ogun‐Semore 2019 {published data only}
- Ogun-Semore OY. Lavender aromatherapy for agitation in elderly with dementia: A clinical scholarly project [Doctoral thesis]. ProQuest LLC, 2019. [Google Scholar]
Opie 1999 {published data only}
- Opie J, Rosewarne R, O'Connor DW. The efficacy of psychosocial approaches to behaviour disorders in dementia: a systematic literature review. Australian and New Zealand Journal of Psychiatry 1999;33(6):789-99. [DOI] [PubMed] [Google Scholar]
Pengelly 2012 {published data only}
- Pengelly A, Snow J, Mills SY, Scholey A, Wesnes K, Butler LR. Short-term study on the effects of rosemary on cognitive function in an elderly population. Journal of Medicinal Food 2012;15(1):10-7. [DOI] [PubMed] [Google Scholar]
Sakamoto 2012 {published data only}
- Sakamoto Y, Ebihara S, Ebihara T, Tomita N, Toba K, Freeman S, et al. Fall prevention using olfactory stimulation with lavender odor in elderly nursing home residents: a randomized controlled trial. Journal of the American Geriatrics Society 2012;60(6):1005-11. [DOI] [PubMed] [Google Scholar]
Snow 2004 {published data only}
- Snow AL, Hovanec L, Brandt J. A controlled trial of aromatherapy for agitation in nursing home patients with dementia. Journal of Alternative and Complementary Medicine 2004;10(3):431-7. [DOI] [PubMed] [Google Scholar]
Tsolaki 2016 {published data only}
- Tsolaki M, Karathanasi E, Lazarou I, Dovas K, Verykouki E, Karacostas A, Georgiadis K, Tsolaki A, Adam K, Kompatsiaris I, Sinakos Z. Efficacy and safety of Crocus sativus L. in patients with mild cognitive impairment: one year single-blind randomized, with parallel groups, clinical trial. Journal of Alzheimer's disease 2016;54(1):129-33. [DOI] [PubMed] [Google Scholar]
UMIN000019044 {published data only}
- UMIN000019044. The effectiveness of the bath powder which added aroma oil in Alzheimer's disease. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000022012 (first received 16 September 2015).
UMIN000027692 {published data only}
- UMIN000027692. Examination of the influence for cognitive function of aromatherapy using the essential oil which was extracted from Abies sachalinensis [Examination of the aromatherapy using the essential oil which was extracted from Abies sachalinensis]. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000031721 (first received 09 June 2017).
UMIN000027693 {published data only}
- UMIN000027693. Examination of the influence for cognitive function of aromatherapy using the essential oil which was extracted from natural green tea leaf [Examination of the aromatherapy using the essential oil which was extracted from natural green tea leaf]. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000031722 (first received 19 June 2017).
Watanabe 2010 {published data only}
- Watanabe S, Iino H, Miyajima M, Hara K, Ohta K, Matsuda A, et al. The effect of ylang-ylang aroma on auditory p300 event-related potentials in temporal lobe epilepsy. In: Epilepsy Currents. Vol. Conference: 64th Annual Meeting of the American Epilepsy Society, AES and 3rd Biennial North American Regional Epilepsy Congress San Antonio, TX United States. Conference Start: 20101203 Conference End: 20101207. Conference Publication. 2010:1.
West 1994 {published data only}
- West BJM, Brockman SJ. The calming power of aromatherapy. Journal of Dementia Care 1994;March/April(2):20-2. [Google Scholar]
Wolfe 1996 {published data only}
- Wolfe, N, Herzberg J. Can aromatherapy oils promote sleep in severely demented patients? International Journal of Geriatric Psychiatry 1996;11(10):926-7. [Google Scholar]
Woods 1996 {published data only}
- Woods DL, Craven R, Whitney J. The effects of therapeutic touch on disruptive behaviours of individuals with dementia of the Alzheimer's type. Alternative Therapies 1996;4(2):95-6. [Google Scholar]
Zalomonson 2019 {published data only}
- Zalomonson S, Freud T, Punchik B, Samson T, Lebedinsky S, Press Y. The results of a crossover placebo-controlled study of the effect of lavender oil on behavioral and psychological symptoms of dementia. Rejuvenation Research 2019;22(3):246-53. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ISRCTN86563511 {published data only}ISRCTN86563511
- ISRCTN86563511. Investigating cognitive effects of aromatherapy on people with dementia living in residential care facilities. www.isrctn.com/ISRCTN86563511 (first received 09 June 2005).
NCT03576170 {published data only}
- NCT03576170. Investigate the efficacy and effectiveness of aromatherapy for the management of behavioral and psychological symptoms of dementia. clinicaltrials.gov/ct2/show/NCT03576170 (first received 03 July 2018).
NCT03662360 {published data only}
- NCT03662360. Elderly demented patient: aromatherapy complementary to psychopharmacotherapy psychological disorders and BPSD behavior [Elderly patient with dementia in the department of acute geriatrics: pilot study, monocentric, randomized use of aromatherapy as a complementary treatment to psychopharmacotherapy in psychological disorders and BPSD behavior]. clinicaltrials.gov/ct2/show/NCT03662360 (first received 07 September 2018).
UMIN000020148 {published data only}
- UMIN000020148. Effect of Aroma hand massage on the improvement of cognitive function. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000020662 (first received 10 December 2012).
UMIN000026366 {published data only}
- UMIN000026366. Study on effect of aroma foot massage on cognitive function. rctportal.niph.go.jp/detail/um?trial_id=UMIN000026366 (first received 01 April 2017).
Yoshiyama 2015 {published data only}
- Yoshiyama K, Arita H, Suzuki J. The effect of aroma hand massage therapy for dementia patients. In: Integrative Medicine Research. Vol. 4. 2015:93-4. [DOI: 10.1016/j/imr.2015.04.151] [DOI] [PubMed]
- Yoshiyama K, Arita H, Suzuki J. The effect of aroma hand massage therapy for people with dementia. Journal of Alternative and Complementary Medicine 2015;21(12):759-65. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12617001159347 {published data only}
- ACTRN12617001159347. Essential oils for agitation in dementia (rELOAD) trial [The effectiveness of topical essential oils for agitation in dementia: a cluster-randomised, placebo-controlled feasibility trial]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373424 (first received 03 August 2017).
ChiCTR‐INR‐17013281 {published data only}
- ChiCTR-INR-17013281. Comparison of the effects of aroma-laser acupuncture and aromatherapy on depression in dementia patients. www.chictr.org.cn/showproj.aspx?proj=22768 (first received 07 November 2017).
Additional references
Alzheimer's Society 2014
- Alzheimer's Society. Alzheimer's Society's view on complementary medicine / alternative therapies. www.alzheimers.org.uk/about-us/policy-and-influencing/what-we-think/alternative-therapies (accessed 03 August 2020).
APA 1994
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edition (DSM-IV). Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
Arai 1997
- Arai Y, MD KK, Hosokawa T, Washio M, Miura H, Hisamichi S. Reliability and validity of the Japanese version of the Zarit Caregiver Burden Interview. Psychiatry and clinical neurosciences 1997;51(5):281-7. [DOI] [PubMed] [Google Scholar]
Arruda 2012
- Arruda M, Viana H, Rainha N, Neng NR, Rosa JS, Nogueira JMF, do Carmo Barreto M, et al. Anti-acetylcholinesterase and antioxidant activity of essential oils from Hedychium gardnerianum Sheppard ex Ker-Gawl. Molecules 2012;17(3):3082-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnes 2003
- Barnes J. Quality, efficacy and safety of complementary medicines: fashions, facts and the future. Part I. regulation and quality. Pharmacology Research and Perspectives 2003;55(3):226-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bathini 2019
- Bathini P, Brai E, Auber L. Olfactory dysfunction in the pathophysiological continuum of dementia. Ageing Research Reviews 2019;55:100956. [DOI] [PubMed] [Google Scholar]
Battle 2019
- Battle CE, Abdul-Rahim AH, Shenkin SD, Hewitt J, Quinn TJ. Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: a network meta-analysis. Cochrane Database of Systematic Reviews 2019, Issue 4. Art. No: CD013306. [DOI: 10.1002/14651858.CD013306] [DOI] [PMC free article] [PubMed] [Google Scholar]
Birks 2006
- Birks JS. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database of Systematic Reviews 2006, Issue 1. Art. No: CD005593. [DOI: 10.1002/14651858.CD005593] [DOI] [PMC free article] [PubMed] [Google Scholar]
Birks 2015
- Birks JS, Chong LY, Grimley Evans J. Rivastigmine for Alzheimer's disease. Cochrane Database of Systematic Reviews 215, Issue 9. Art. No: CD001191. [DOI: 10.1002/14651858.CD001191.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Birks 2018
- Birks JS, Harvey RJ. Donepezil for dementia due to Alzheimer's disease. Cochrane Database of Systematic Reviews 2018, Issue 6. Art. No: CD001190. [DOI: 10.1002/14651858.CD001190.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blau 1977
- Blau T. Quality of life, social indicators, and criteria of change. Professional Psychology 1977;8:464-73. [Google Scholar]
Buckle 2003
- Buckle J. Clinical Aromatherapy: Essential Oils in Practice. 2nd edition. Elsevier, 2003. [Google Scholar]
Cheng 2017
- Cheng S. Dementia caregiver burden: a research update and critical analysis. Current Psychiatry Report 2017;19(9):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen‐Mansfield 1999
- Cohen-Mansfield J. Measurement of inappropriate behaviour associated with dementia. Journal of Gerontological Nursing 1999;25(2):42-51. [DOI] [PubMed] [Google Scholar]
Cummings 1994
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44(12):2308-14. [DOI] [PubMed] [Google Scholar]
Degel 2001
- Degel J, Piper D, Koster EG. Implicit learning and implicit memory for odors: the influence of odor identification and retention time. Chemical Senses 2001;26(3):267-80. [DOI] [PubMed] [Google Scholar]
Directive 2004/24/EC
- Directive 2004/24/EC. Directive 2004/24/E of the European Parliament and Council of 31 March 2004, amending Directive 2001/83/EC on the Community code relating to medicinal products for human use. L 136/34- L 136/5. eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:136:0085:0090:EN:PDF (accessed 10 August 2020).
Fleischman 2005
- Fleischman DA, Wilson RS, Gabrieli JD, Schneider JA, Bienias JL, Bennett DA. Implicit memory and Alzheimer’s disease neuropathy. Brain 2005;128:2006-15. [DOI] [PubMed] [Google Scholar]
Folstein 1975
- Folstein NF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12(3):189-98. [DOI] [PubMed] [Google Scholar]
Harris 2012
- Harris PE, Cooper KL, Relton C, Thomas KJ. Prevalence of complementary and alternative medicine (CAM) use by the general population: a systematic review and update. International Journal of Clinical Practice 2012;66(10):924-939. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. [Google Scholar]
Holmes 2004
- Homes C, Ballard C. Aromatherapy in dementia. Advances In Psychiatric Treatment 2004;10(4):296-300. [Google Scholar]
Hughes 1982
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry 1982;140(6):556-72. [DOI] [PubMed] [Google Scholar]
Hwang 2015
- Hwang E, Shin S. The effects of aromatherapy on sleep improvement: a systematic literature review and meta-analysis. The Journal of Alternative and Complementary Medicine 2015;21(2):61-8. [DOI] [PubMed] [Google Scholar]
Kales 2015
- Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioural and psychological symptoms of dementia. BMJ 2015;350:h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2019
- Kim EK, Park H, Lee CH, Park E. Effects of aromatherapy on agitation in patients with dementia: a systematic literature review and meta-analysis. Journal of Korean Academy of Community Health Nursing 2019;30(2):183-194. [Google Scholar]
Kitwood 1992
- Kitwood T. A new approach to the evaluation of dementia care. J Adv Health Nurs Care 1992;1(5):41-60. [Google Scholar]
Kong 2009
- Kong E-H, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging and Mental Health 2009;13(4):512-20. [DOI] [PubMed] [Google Scholar]
Lawton 1996
- Lawton MP, Van Haitsma K, Klapper, J. Observed affect in nursing home residents with Alzheimer's disease. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences 1996;51(1):3-14. [DOI] [PubMed] [Google Scholar]
Leng 2019
- Leng M, Zhao Y, Wang Z. Comparative efficacy of non-pharmacological interventions on agitation in people with dementia: A systematic review and Bayesian network meta-analysis. International Journal of Nursing Studies 2019;102:103489. [DOI] [PubMed] [Google Scholar]
Lin 2008
- Lin JN, Wang JJ. Psychometric evaluation of the Chinese version of the Cornell Scale for depression in dementia. Journal of Nursing Research 2008;16(3):202-10. [DOI] [PubMed] [Google Scholar]
Livingston 2014
- Livingston G, Kelly L, Lewis-Holmes E, Baio G, Morris S, Patel N, Omar RZ, Katona C, Cooper C. Non-pharmacological interventions for agitation in dementia: systematic review of randomised controlled trials. The British Journal of Psychiatry 2014;205(6):436-442. [DOI] [PubMed] [Google Scholar]
Mahoney 1965
- Mahoney F, Barthel D. Functional evaluation: the Barthel Index: a simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Maryland State Medical Journal 1965;14:56-61. [PubMed] [Google Scholar]
McShane 2019
- McShane R, Westby MJ, Roberts E, Minakaran N, Schneider L, Farrimond LE, Maayan N, Ware J, Debarros J. Memantine for dementia. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD003154. [DOI: 10.1002/14651858.CD003154.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nguyen 2008
- Nguyen QA, Paton C. The use of aromatherapy to treat behavioural problems in dementia. International Journal of Geriatric Psychiatry 2008;23(4):337-46. [DOI] [PubMed] [Google Scholar]
NICE 2018
- NICE. Dementia: assessment, management and support for people living with dementia and their carers. www.nice.org.uk/guidance/ng97 (accessed 02 February 2019).
Oliveira 2015
- Oliveira AM, Radanovic M, Mello PC, Buchain PC, Vizzotto AD, Celestino DL, Stella F, Piersol CV, Forlenza OV. Nonpharmacological interventions to reduce behavioral and psychological symptoms of dementia: a systematic review. BioMed research international 2015:1-9. [DOI: 10.1155/2015/218980] [DOI] [PMC free article] [PubMed] [Google Scholar]
OnHealth 2020
- OnHealth with WebMD. Alternative Practices. web.archive.org/web/20010210192900/http://onhealth.webmd.com/alternative/resource/althealth/index.asp (accessed 10 August 2020).
Perry 2006
- Perry E. Aromatherapy for the treatment of Alzheimer's disease. The Journal of Quality Research In Dementia Issues 2006:3-6.
Posadzki 2012
- Posadzki P, Alotaibi A, Ernst E. Adverse effects of aromatherapy: A systematic review of case reports and case series. International Journal of Risk and Safety in Medicine 2012;24(3):147-61. [DOI] [PubMed] [Google Scholar]
Prince 2015
- Prince MJ, Wimo A, Guerchet MM, Ali GC, Wu YT, Prina M. The global impact of dementia: An analysis of prevalence, incidence, cost and trends. World Alzheimer Report. London: Alzheimer's Disease International 2015.
Reus 2016
- Reus VI, Fochtmann LJ, Eyler AE, Hilty DM, Horvitz-Lennon M, Jibson MD, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. American Journal of Psychiatry 2016;173(5):543-6. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosen 1984
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. The American journal of psychiatry 1984;141(11):1356–64. [DOI] [PubMed] [Google Scholar]
Rosen 1994
- Rosen J, Burgio L, Kollar M, Cain M, Allison M, Fogleman M, et al. The Pittsburg Agitation Scale: a user friendly instrument for rating agitation in dementia. American Journal of Geriatric Psychiatry 1994;2(1):52-9. [DOI] [PubMed] [Google Scholar]
SAS® 1999 [Computer program]
- SAS Institute Inc. SAS/STAT User's Guide, Version 8. Cary, NC: SAS Institute Inc., 1999.
Schünemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2008:359-83. [Google Scholar]
Shinde 2009
- Shinde VM, Dhalwal K, Potdar M, Mahadik KR. Application of quality control principles to herbal drugs. International Journal of Phytomedicine 2009;1(1):4-8. [Google Scholar]
Spirit Scents 2020
- Spirit Scents. Aromatherapy matrix of essential oils by therapeutic effect. www.spiritscents.com/matrix.html (accessed 10 August 2020).
Tisserand 1988
- Tisserand R. Aromatherapy for Everyone. London: Penguin, 1988. [Google Scholar]
Turek 2013
- Turek C, Stintzing FC. Stability of essential oils: a review. Comprehensive Reviews In Food Science and Food Safety 2013;12(1):40-53. [Google Scholar]
WHO 1993
- World Health Organization. The ICD-10 classification of mental and behavioural disorders: Diagnostic criteria for research. Geneva: World Health Organization, 1993. [Google Scholar]
WHO 2019
- WHO 2019. Dementia: Key Facts. www.who.int/news-room/fact-sheets/detail/dementia#:~:text=Worldwide%2C%20around%2050%20million%20people%20have%20dementia%2C%20with%20nearly%2060,is%20between%205%2D8%25. (accessed 03 August 2020).
References to other published versions of this review
Forrester 2014
- Forrester LT, Maayan N, Orrell M, Spector AE, Buchan LD, Soares-Weiser K. Aromatherapy for dementia. Cochrane Database of Systematic Reviews 2014, Issue 2. Art. No: CD003150. [DOI: 10.1002/14651858.CD003150.pub2] [DOI] [PubMed] [Google Scholar]
Thorgrimsen 2003
- Thorgrimsen LM, Spector AE, Wiles A, Orrell M. Aromatherapy for dementia. Cochrane Database of Systematic Reviews 2003, Issue 3. Art. No: CD003150. [DOI: 10.1002/14651858.CD003150] [DOI] [PubMed] [Google Scholar]


