Abstract
Bovine colostrum has been advocated as a source of immunity against external environmental microorganisms. Recurrent upper respiratory tract infections present a critical public health concern in the developing countries. The present case report was performed to assess the influence of bovine colostrum in preventing recurrent upper respiratory tract infections caused by respiratory viruses in an adult in Jordan in addition to its effect on respiratory microbiome. In conclusion, bovine colostrum was successful in the prevention of upper respiratory tract infections and significantly affected the nasal swab microbiome. This is the first case report investigating the influence of bovine colostrum on the nasal swab microbiome.
Keywords: Colostrum, Microbiome, Upper respiratory tract infection
1. Introduction
Mammalian mothers’ lactate to feed their young, and the milk produced immediately after giving birth is called colostrum, which continues for 2–3 days. In cows, this milk is called bovine colostrum (BC), and it is supplemented by high levels of immunoglobulins since BC is the only way to provide the newborn calf immunity against external environmental microorganisms [[1], [2], [3]]. Although bovine and human colostrum is homologous, BC has a much higher concentration of immunoglobulins [1,2,4]. Immunoglobulin levels in BC are reportedly almost a hundred-fold more than that in regular bovine milk, and it also contains numerous factors responsible for the acquired and innate immune systems. Furthermore, BC contains a series of antimicrobial fractions [3,5]. What sets this hyperimmune BC apart from conventional antimicrobials is that the rich concentration of specifically targeted IgG ensures that the integrity of the gut microflora in animals is not disturbed, nor does it pose a possible threat of emergence of new, antibiotic-resistant microorganisms [[1], [2], [3]]. The potential use of BC as a passive immunotherapeutic agent to combat a variety of pathogens has been researched [[1], [2], [3]].
Recurrent respiratory infections present a critical public health concern in the developing countries [3,6]. Upper respiratory tract infection (URTI) is one such concern, and similar to other inner lining and tract infections, it is mostly caused by respiratory viruses, and bacteria [3,[6], [8]].
So far, however, there has been little clinical studies about the influence of BC in the case of recurrent infections. Therefore, the present research was performed to assess the effectiveness and safety of BC in preventing recurrent URTI in addition to its effect on respiratory microbiome. We report a case of recurrent URTI caused by respiratory viruses in an adult in Jordan. This is the first case report investigating the influence of bovine colostrum on the nasal swab microbiome in the Kingdom of Jordan.
2. Materials and methods
2.1. Clinical data
The patient was 27 years male Jordanian patient with generally healthy diet and moderate exercise activities. No previous surgery, hospitalization, and Flu vaccine were reported. The patient was non-smoker and non-alcoholic. A history of recurrent viral URTI; 5 times during the year past the beginning of BC regimen was reported. After obtaining informed written consent from the patient, the plan included in Table 1 was performed. The novel BC regimen consisted of 3 periods; A, B, and C. Period A, the beginning of BC regimen, represented the daily supplementation of 1 g (2 capsules each 500 mg) of BC at the Dawn time for 4 weeks. Period B was 2 weeks after the end of period A and represented supplementation of 1 g of BC at the Dawn time for 3 continuous days. Period C was 2 weeks after the end of period B with supplementation of 1 g of BC at the Dawn time two times weekly (Monday and Thursday) for 4 weeks. Then cycles of period B then C and so forth were implemented until completing the year from the beginning of the regimen (Table 1).
Table 1.
Schedule for obtaining nasal swab (NS) samples and BC regimen.
| Date | URTIs yearly frequency | URTI no. | Sample and purpose | Intervention | Comments |
|---|---|---|---|---|---|
| May-2017 | 5 URTIs | URTI no. 1 | – | All 4 NS were subjected to qPCR viral test and 1/3 (before BC) was subjected to microbiome analysis | |
| URTI no. 2 | - | ||||
| URTI no. 3 | 1 NS (qPCR) | - | |||
| URTI no. 4 | 1 NS (qPCR) | - | |||
| URTI no. 5 |
2 NS (1 NS for qPCR and microbiome analysis; 1 NS for qPCR) |
– |
The third and fourth NS samples are 24-h apart; the third NS was taken then a single BC dose was given and after 24 hours another NS was taken. The first NS sample was used for microbiome analysis (one week before starting BC regimen) |
||
| May-2018 | – | - | - | A | Period A (repeated yearly): The beginning of BC regimen. Daily supplementation of 1 g of BC at the Dawn time for 4 weeks. |
| - | - | B1 | Period B: 2 weeks after the end of period A: supplementation of 1 g of BC at the Dawn time for 3 continuous days | ||
| - | - | C1 | Period C: 2 weeks after the end of period B: supplementation of 1 g of BC at the Dawn time two times weekly (Monday and Thursday) for 4 weeks | ||
| - | 1 NS (microbiome analysis) | B2 | Then cycles of period B then C and so forth until completing the year from the beginning of the regimen. | ||
| - | - | C2 | |||
| - | - | B3 | |||
| - | - | C3 | |||
| - | - | B4 | |||
| - | - | C4 | |||
| - | - | B5 | |||
| - | - | C5 | |||
| - | - | 1-month rest |
2.2. Clinical samples’ collection
A nasal swab (gentle brushing) was taken from each nostril and placed in 1-ml phosphate buffered saline solution. The nasal swab performed for this study did not enter the nostril as deeply as the pharyngeal-nasal swabs that are done for hospital admissions and other studies. Samples were obtained and processed by research nurse and stored in −80 °C freezer for retrospective analysis at the completion of the study. Frozen samples were transferral to the Laboratory on dry ice.
2.3. Nucleic acid extraction
Nasal swab samples were thawed in a water-bath on 37 °C before nucleic acid extraction. Samples were then placed in 2-ml empty tubes cryosafe-labelled with laboratory ID and date. The MagNA Pure 96 extraction platform (Roche Diagnostics Ltd., Burgess Hill, UK) and the QIAamp nucleic acid Kit (Qiagen, UK) were used for total nucleic acid extraction from 200-μl samples. After extraction, the eluted nucleic acid samples (100- μl) were transferred to 1.5-ml conical tubes and frozen on −80 °C until ready for testing. Nucleic acids’ concentration for the extracts of the nasal swab's samples were measured via FLUOstar Omega (BMG Labtech, Offenburg, Germany).
2.4. Respiratory viral detection
Presence of a cold or respiratory viral infection was confirmed by positive virology test results. Patient had to have two symptom-free weeks between apparent respiratory infections for them to be considered as separate events.
The nasal swab samples were subjected to real-time qPCR testing (amplification mix preparation) in a respiratory viral panel, including the following:
-
•
Adenovirus (ADV), detection of all serotypes;
-
•
Bocavirus (BOV), detection of all serotypes;
-
•
Coronavirus (COV), detection of OC43, 229E, NL63, and HKU1 types;
-
•
Generic influenza (FLU), detection of all varieties of H1, H3, and H5;
-
•
Human rhinovirus (HRV 1 and 2 assays), detection of all serotypes;
-
•
Metapneumovirus (MPV), detection of A and B types;
-
•
Parainfluenza (PF), detection of 1, 2, and 3 types;
-
•
Respiratory syncytial virus (RSV), detection of A and B types.
To evaluate the quality of the samples and to recognize PCR inhibition, all samples were tested for ribonuclease P (RNP), which is a gene responsible for the maintenance of basic cellular function. All qPCR assays were TaqMan assays (reporter-quencher hydrolysis probe) (Appendix). 8-μl aliquots of the amplification mix were transferred into 384 white LightCycler- 480 multi-well plates (Roche Diagnostics Ltd., UK). The eluted nucleic acid from each sample was transferred in 2- μl volumes into the wells of the plates containing the amplification mix. LightCycler 480 sealing foil was then used to seal the plates before centrifugation at 4000 rpm (2576×g). The plates were then placed in the LightCycler-480 (Roche Diagnostics, West Sussex, UK) for amplification according to the following: [50 °C for 15-min, 95 °C for 5-min, 45 cycles of 95 °C for 10s then 60 °C for 1-min, and finally 40 °C for 15s].
2.5. Library preparation and the Illumina MiSeq sequencing
Library preparation from samples was applied for sequencing of the 16S rRNA gene V4 region (Appendix), which consists of four main PCR steps. The ends of every read were intersected to create full-length reads of the V4 region with a high-quality in a one 65-h run using the MiSeq v3 reagents and the paired 300-bp reads.
2.6. Outcome evaluation
One of the outcome measures was decrease in URTI episodes’ number over one-year following BC regimen which was evaluated every 2–4 weeks. We also followed-up the adherence to BC regimen. The patient assisted as his own control. The parent was monitored every 2–4 weeks for any side effects during the study period.
3. Results
There was no URTI episodes over the one-year following initiation of BC regimen compared to 5 episodes past BC regimen over the previous year (p < 0.05). Of those 3 nasal swabs taken before BC regimen, 2 (the first and third) specimens showed HRV detection and one specimen (the second one) showed dual viruses (HRV and COV). Real-time qPCR showed there was a decrease in the nasal swab viral load (indicated by a higher Ct value; from 19 to 25 cycles) 24 hours after the single BC regimen. BC was well tolerated with no stated allergy or adverse effects in the patient of this case report.
We compared the bacterial community structure in the nasal swab sample at the baseline and 3 months after BC regimen (3MABCR) to gain insight into whether BC is associated with significant changes in the respiratory microbiome. The number of genera detected was significantly higher in the nasal swab collected 3MABCR compared with that collected at the baseline (p-values = <0.005). The numbers of detected genera represent the numbers of any genera with a relative percentage abundance greater than 0%.
Both aerobic and anaerobic bacteria were detected in the baseline and sample collected 3MABCR. Aerobic genera detected in the nasal swab collected 3MABCR included Streptococcus; Rothia; Haemophillus; and Pseudomonas, whereas, Streptococcus and Rothia, were among the genera which were detected in the samples collected at the baseline. Anaerobes genera including Prevotella, Veillonella, and Actinomyces were also detected in the sample collected 3MABCR, in addition to the baseline sample. Also, Fusobacterium was detected in the baseline sample.
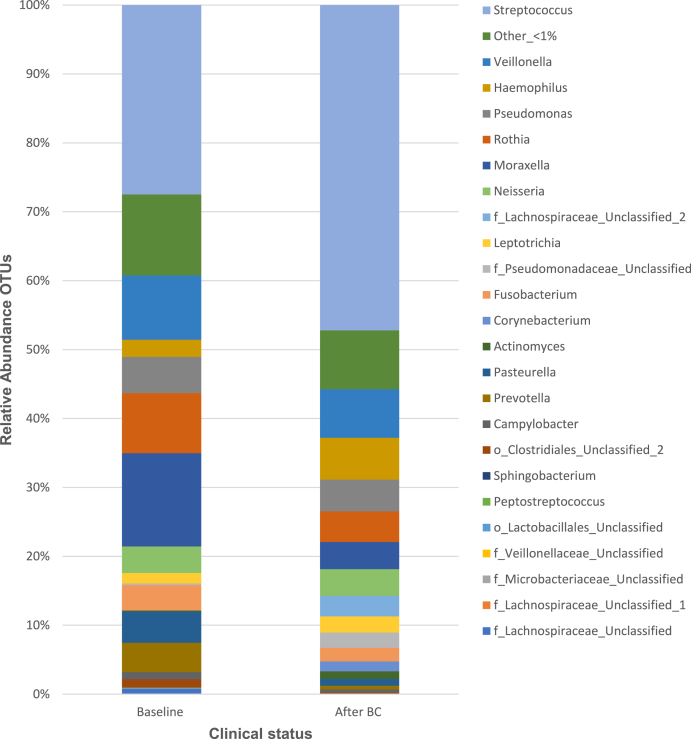
Communities were most frequently dominated by Streptococcus in both types of samples. A significantly higher relative abundance of Prevotella genus in the baseline than the sample collected 3MABCR (p-value = 0.003, the related samples Wilcoxon Signed-Rank test). Whereas, the relative abundances of Actinomyces, Corynebacterium, Haemophillus, and Streptococcus genera, in addition to Lachnospiraceae and Pseudomonadaceae families were significantly higher in the sample collected 3MABCR than the baseline sample (p-values = 0.005, 0.005, 0.044, 0.005, 0.005, 0.003, respectively, Wilcoxon Signed-Rank test) (Fig. 1).
Fig. 1.
Comparison of the relative abundance of the main recognised genera in the nasal swab specimens (at baseline versus 3 months following BC regimen).
Taxa name with “unclassified” suffix might not be assigned to the level of genus and are shown at the lowest known taxon. BC: Bovine Colostrum; OTUs: operational taxonomic units.
No significant difference was observed (p = 0.639, Wilcoxon Signed-Rank test) in the bacterial community diversity (Shannon-Wiener diversity index). However, a significant increase in the median/mean taxonomic richness (an indication for the number of species existing in the specimen) and a decrease in the median/mean evenness (an indication for the relative abundance of diverse species that form the richness in that area) of the sample obtained 3MABCR compared with the baseline specimen (p < 0.005; Wilcoxon Signed-Rank test).
4. Discussion
Although bovine and human colostrum is homologous, BC has a much higher concentration of immunoglobulins [1,2,4]. Comparisons between normal bovine milk and BC have shown that the concentration of immunoglobulins is higher in BC by a factor of almost a hundred. BC also contains factors responsible for activation of the acquired and innate immune systems, and also some antimicrobial fractions [3,5]. BC, rich in targeted IgG, is different from the conventional antimicrobials as it does not disturb the integrity of the gut microbiota, nor will it potentially lead to the emergence of new antibiotic-resistant organisms microorganisms [1,5]. The potential use of BC as an immunotherapeutic agent to combat different pathogens has been researched [[1], [2], [3]].
In this case report, there was no URTI episodes over the one-year following initiation of BC regimen compared to 5 episodes past BC regimen over the previous year (p < 0.05). The results of this case report indicate that BC was effective in preventing URTIs. These results match those observed in earlier studies [9,10]. In contrast to earlier findings which found that significantly less participants taking BC stated symptoms of URTI within only 7 weeks following termination of BC compared with those taking placebo. In a previous study, BC had no impact on symptoms once they had developed [9], however, our finding suggest a possible reduction in the viral load and symptoms. These findings cannot be extrapolated to all patients unless a larger study confirms.
Patel and Rana performed a non-comparative study of the effects of a daily 3g dose of BC in 551 children after recurrent episodes of acute URTI observed over the preceding six months. The number of reported cases of URTI following supplementation was reduced by 73%, 83%, and 91% at 4, 8, and 12 weeks, respectively, compared to the number of cases in the six months preceding BC supplementation [3]. However, other authors have showed the various limitations that was noted in this research [11]. Therefore, cautious interpretation of the results of this study is required [11]. Cesarone et al. [12] stated that BC decreased flu cases, and advised that administration of BC might be more efficient than influenza virus vaccines.
These data illustrate that BC has a prophylactic influence against URTI. However, these studies were not individually significant and were varied with respect to BC dosage, participants, the quality of methods, and findings. Therefore, the impact of BC must be confirmed by performing randomized, controlled, longitudinal studies.
BC administration was well tolerated with no stated allergy or adverse effects in the patient of this case report. Similarly, according to a systematic review, there were no cases of severe adverse effects in 51 studies, comprising 2326 individuals. Reported adverse effects included complaints of an unpleasant taste, nausea, flatulence, diarrhea, skin rash, and unspecified abdominal discomfort, all of which were deemed mild. Nine studies definitely stated an absence of side effects. In general, BC is considered to be safe and well-tolerated in humans [5].
One of the aims of this case report has been to define and comparatively examine the respiratory bacterial microbiome at the baseline, as well as 3MABCR. To facilitate this, the NGS Illumina MiSeq was employed, thereby comprehensively illuminating the respiratory microbiome composition, and facilitating the detection of less prevalent pathogens that are associated with culture difficulties. Molecular methods for the characterisation of the microbiota are innovative, and so the present research, which depends on the NGS Illumina MiSeq, brings with it new data pertaining to the microbiota of nasal swab in patient with recurrent URTI at the baseline and 3MABCR. It is particularly noteworthy that significant disparities were observed between the two types of samples in our research, although it is challenge to determine the role of BC on the respiratory microbiome. High-throughput Illumina MiSeq uncovered substantially high bacterial diversity in nasal swabs.
In the α-diversity analyses, when the two groups (at the baseline and 3MABCR) were compared at the genus level, no significant difference were seen in biodiversity in the term of Shannon index. However, we observed a significant increase in the taxonomic richness 3 months after BC regimen compared with the nasal swab sample collected at the baseline (p < 0.005) in addition to the significant decrease in the evenness (p < 0.005). This indicate that implemented BC regimen decrease the dominance of certain bacteria. We hypothesise that recurrent URTIs could be caused by alterations in respiratory bacterial community composition, the arising of novel species, or the spread of infection by one species to different areas of the respiratory tract. This shift in the respiratory bacterial community structure reflects that replacement of much of the respiratory microbiome by the key pathogens, might be a factor in the decreasing microbial richness observed in this case report.
We observed the Prevotella genus were significantly less abundant in the sample collected 3MABCR compared with the baseline sample. It is important to recognize that the results may be restricted by upper airway contamination [13], and it is also the case that clinical trials have yet to be conducted to investigate the degree to which these pathogens can be treated in an efficacious way. Ultimately, the degree to which these pathogens are clinically significant is not yet understood.
Furthermore, the respiratory microbiome can be integrated with other data sets to enhance precision medicine therapy and could have beneficial clinical impact. Precision medicine that combines genomics, bioinformatics analysis, and clinical interpretation is the future of healthcare and has the potential to provide substantial benefits to patients and predict more accurately which prevention and treatment strategies for a particular disease will benefit which groups of patients.
Some limitations can be noticed for this case report. First of all, due to financial restriction, we could not investigate immune functions in our patient using any assay of markers. Further well-designed, prospective, clinical controlled studies with a large sample size are encouraged to confirm our results and we will investigate the effect of BC on the coronavirus infectious disease-19 (COVID-19).
5. Conclusions
BC was effective in the prevention of URTI and we suggest that BC could be recommended as a treatment option for individuals with recurrent URTI. BC significantly affected the nasal swab microbiome. Moreover, this knowledge is necessary to implement the precision medicine.
Declaration of competing interest
All Authors declare no conflict of interest related to this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2020.101189.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Steele J., Sponseller J., Schmidt D., Cohen O., Tzipori S. Hyperimmune bovine colostrum for treatment of GI infections: a review and update on Clostridium difficile. Hum. Vaccines Immunother. 2013;9(7):1565–1568. doi: 10.4161/hv.24078. [DOI] [PubMed] [Google Scholar]
- 2.Patıroğlu T., Kondolot M. The effect of bovine colostrum on viral upper respiratory tract infections in children with immunoglobulin A deficiency. Clin. Respir. J. 2013;7(1):21–26. doi: 10.1111/j.1752-699X.2011.00268.x. [DOI] [PubMed] [Google Scholar]
- 3.Patel K., Rana R. Pedimune in recurrent respiratory infection and diarrhoea—the Indian experience—the pride study. Indian J. Pediatr. 2006;73(7):585–591. doi: 10.1007/BF02759923. [DOI] [PubMed] [Google Scholar]
- 4.Ballard O., Morrow A.L. Human milk composition: nutrients and bioactive factors. Pediatric Clin. 2013;60(1):49–74. doi: 10.1016/j.pcl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rathe M., Müller K., Sangild P.T., Husby S. Clinical applications of bovine colostrum therapy: a systematic review. Nutr. Rev. 2014;72(4):237–254. doi: 10.1111/nure.12089. [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez L., Cervantes E., Ortiz R. Malnutrition and gastrointestinal and respiratory infections in children: a public health problem. Int. J. Environ. Res. Publ. Health. 2011;8(4):1174–1205. doi: 10.3390/ijerph8041174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saad K., Mohamed S.A., Metwalley K.A. Recurrent/persistent pneumonia among children in Upper Egypt. Mediterr. J. Hematol. Infect. Dis. 2013;5(1) doi: 10.4084/MJHID.2013.028. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Brinkworth G.D., Buckley J.D. Concentrated bovine colostrum protein supplementation reduces the incidence of self-reported symptoms of upper respiratory tract infection in adult males. Eur. J. Nutr. 2003;42(4):228–232. doi: 10.1007/s00394-003-0410-x. [DOI] [PubMed] [Google Scholar]
- 10.Crooks C., Cross M.L., Wall C., Ali A. Effect of bovine colostrum supplementation on respiratory tract mucosal defenses in swimmers. Int. J. Sport Nutr. Exerc. Metabol. 2010;20(3):224–235. doi: 10.1123/ijsnem.20.3.224. [DOI] [PubMed] [Google Scholar]
- 11.Ramesh Menon P., Lodha R., Kabra S. Bovine colostrum in pediatric respiratory diseases: a systematic review. Indian J. Pediatr. 2010;77(1):108–109. doi: 10.1007/s12098-009-0257-0. [DOI] [PubMed] [Google Scholar]
- 12.Liberati A., Altman D., Tetzlaff J., Mulrow C., G0tzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Goddard A.F., Staudinger B.J., Dowd S.E., Joshi-Datar A., Wolcott R.D., Aitken M.L., Fligner C.L., Singh P.K. Direct sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiota. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109(34):13769–13774. doi: 10.1073/pnas.1107435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.