Abstract
Treating persistent neuropathic pain remains a major clinical challenge. Current conventional treatment approaches carry a substantial risk of toxicity and provide only transient pain relief. In this work, we show that the activity and expression of the inflammatory mediator secretory phospholipase-A2 (sPLA2) enzyme increases in the spinal cord after painful nerve root compression. We then develop phospholipid micelle-based nanoparticles that release their payload in response to sPLA2 activity. Using a rodent model of neuropathic pain, phospholipid micelles loaded with the sPLA2 inhibitor, thioetheramide-PC (TEA-PC), are administered either locally or intravenously at the time of painful injury or 1–2 days afterward. Local micelle administration immediately after compression prevents pain for up to 7 days. Delayed intravenous administration of the micelles attenuates existing pain. These findings suggest that sPLA2 inhibitor-loaded micelles can be a promising anti-inflammatory nanotherapeutic for neuropathic pain treatment.
Keywords: phospholipase, inhibitor, phospholipid micelles, inflammation, neuropathic pain
GRAPHICAl ABSTRACT:
Neuropathic pain, which develops from direct or secondary neural tissue injury or damage, occurs in approximately 10% of the U.S. population1 and often persists for several years or even a lifetime after an initial injury.2 Despite its prevalence, neuropathic pain remains largely resistant to treatment,3,4 leading to chronic disability,5 with staggering societal and economic costs.6,7 Current neuropathic pain treatments include surgical intervention and pharmacologic approaches, but are largely ineffective, providing only transient pain relief.7–10 Although treatment of neuropathic pain through opioid11 and nonopioid analgesics12 has been extensively pursued, these approaches are often accompanied by undesired side effects including addiction and increased pharmacological tolerance.13 Accordingly, there is a tremendous need to develop new interventional platforms for greater effectiveness to treat chronic neuropathic pain.
In addition to the primary injury to neural tissue that occurs with nerve root trauma and spinal cord injury,14 painful neuropathic injuries are accompanied by a robust secondary neuroinflammatory response, both at the site of injury and remote to it in the spinal cord.15–17 Many different animal models of neuropathic pain have defined a host of inflammatory cascades both at the site of injury and in the central nervous system (CNS),18–23 contributing to central sensitization that is responsible for persistent pain.24–27 During central sensitization there is increased neuronal hyperexcitability and spontaneous activity in spinal neurons, which can result from and be exacerbated by inflammatory mediators that directly act on neurons.28 Among the potent inflammatory mediators involved in spinal neuroimmune regulation of pain is the family of phospholipase-A2 (PLA2) enzymes. These enzymes recognize and catalytically hydrolyze the sn-2 ester bond of glycerophospholipids, releasing free fatty acids, such as arachidonic acid (AA) and lysophospholipids, that are involved in tissue damage and neuronal injury, as well as a host of inflammatory and neurological disorders.29,30 Many studies have reported expression of the secretory PLA2 (sPLA2), an isoform of the PLA2 enzyme, to be associated with a number of painful pathologies characterized by inflammation, such as disc herniation, disc degeneration, discogenic pain, and neuropathic pain.31–36 sPLA2 is normally present in the mammalian spinal cord and brain.37,38 Yet, under pathological conditions sPLA2 can be induced by multiple cascades and effector molecules including inflammatory cytokines,39 free radicals,40 and excitatory amino acids.41 Elevated sPLA2 expression after neuropathic injury is observed both centrally in the spinal cord and in the peripheral dorsal root ganglion (DRG), both anatomic sites that contribute to the potentiation and amplification of chronic pain after trauma.42,43 As such, if sPLA2 is a sensitive marker for spinal neuroinflammation and painful neuropathic injury, it can be exploited to also act as a therapeutic target for pain treatment. However, no study has harnessed the specific sPLA2 response for the treatment of neuropathic pain.
Clinical management of neuropathic pain is further complicated by lack of effective drug delivery. Systemic delivery of small-molecule drugs provides only a small portion of medication at the site of injury, and even less drug is delivered to the spinal cord, where pain modulation occurs.8,12 Yet, delivery of drugs directly to the spinal cord via intrathecal injection is invasive, imposes toxicity to uninjured spinal levels, and is difficult to perform in nonhospital settings.10,44,45 Recently, nanoparticle-based drug delivery has been extensively explored in the treatment of cancer.46 Such drug delivery systems not only increase the concentration of drug that can be delivered to target tissues, lowering the overall dose needed, but also reduce off-target toxicity and side-effects.10,45 Imaging agents can also be incorporated into these systems as theranostic agents,47 which may be promising for painful neuropathy treatment, which is often hindered by heterogeneous diagnostic criteria.48 A delivery platform that enables tunable drug release based on the pathological conditions that are induced by the pain state itself offers a long-term clinical option to treat patients with neuropathic pain. Therefore, in this study we first defined if spinal sPLA2 expression after a nerve root compression injury is specific to a painful state, enabling its possible utility as a potential therapeutic target for pain modulation. On the basis of those positive findings, we fabricated sPLA2 inhibitor-loaded micelle nanoparticles (Figure 1A). The effectiveness of these sPLA2 inhibitor-loaded micelles was tested in both preventing and abolishing pain and spinal inflammation using both local and systemic administration paradigms in a well-established rodent model of persistent neuropathic pain (Figure 1B).
Figure 1.
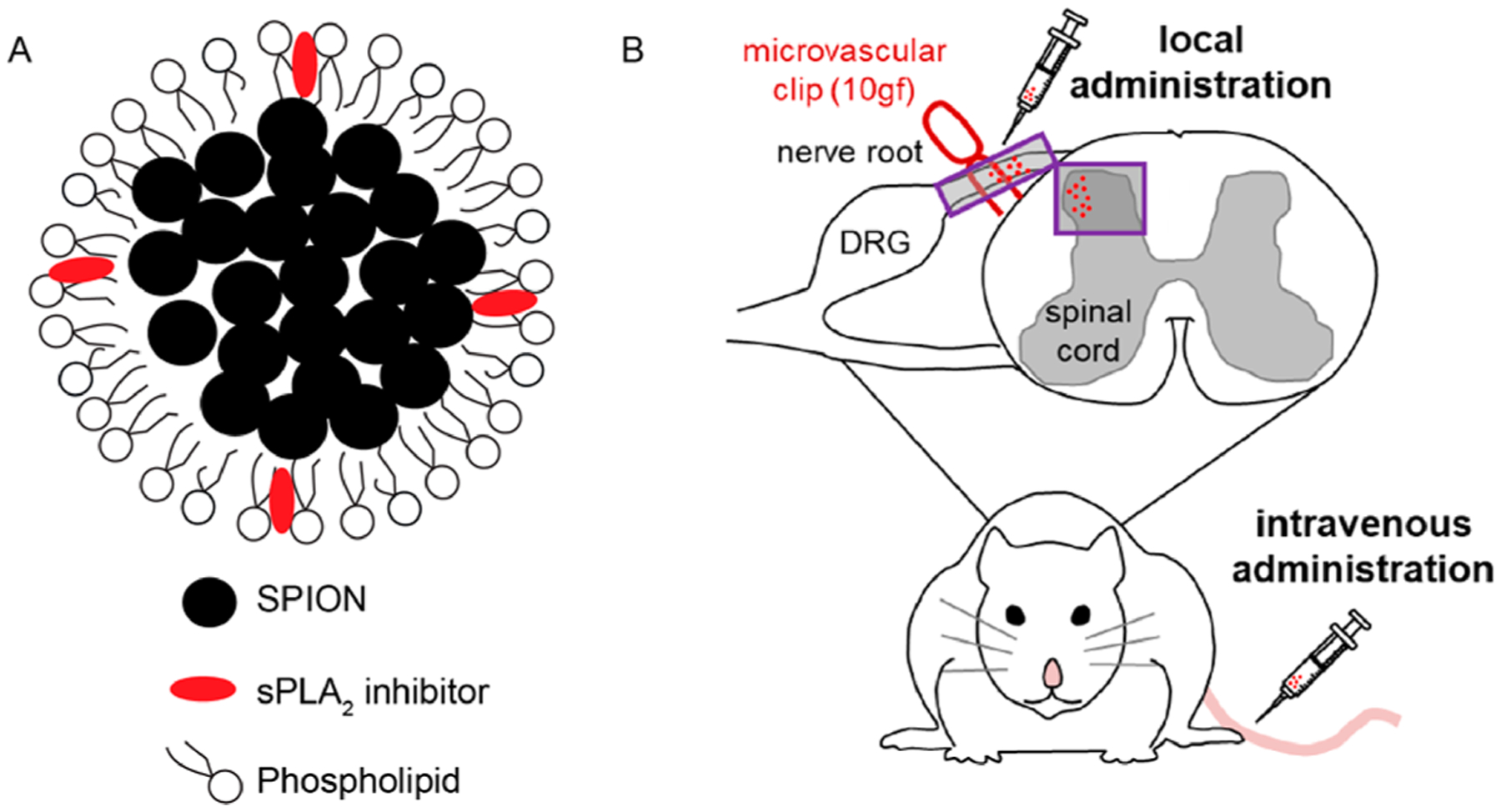
Schematic diagrams of sPLA2 inhibitor-loaded micelles and their administration following a painful nerve root compression. (A) sPLA2 inhibitor-loaded micelles were formed through the coassembly of the phospholipid and the small hydrophobic SPIO nanoparticles. sPLA2 inhibitor was incorporated into the phospholipid membrane during the micelle preparation. (B) Nerve root compression injury was performed using a microvascular clip (10 gf for 15 min) applied to the right C7 dorsal nerve root. sPLA2 inhibitor-loaded micelles or control unloaded micelles were injected directly onto the nerve root ipsilateral to injury or given by intravenous injection at the tail.
RESULTS AND DISCUSSION
Spinal sPLA2 Expression after Painful Neuropathic Injury to the Nerve Root.
Spinal sPLA2 is elevated only after a painful nerve root injury. In fact, early (at day 1) after nerve root compression (Figure 2A), spinal sPLA2 increases in the spinal cord only after a painful compression, with the extent of spinal sPLA2 1.85 ± 1.20-fold greater than levels at that time after nonpainful root compression (p = 0.048) and 1.97 ± 0.82 times the levels after a nonpainful sham surgery (p = 0.044) (Figure 2B). Further, at day 7, the increase in spinal sPLA2 expression is even more robust, with further increases (p = 0.043) by 48.7 ± 9.4% over levels at day 1 (Figure 2). Although sPLA2 levels in both the nonpainful and sham control groups increase at day 7 from levels at day 1, those increases are not significant (Figure 2B). At day 1, the majority of spinal sPLA2 is expressed in microglia, with the lowest extent of sPLA2 expression observed in astrocytes (Figure 2C). Similar to total spinal sPLA2 levels, both microglial and neuronal sPLA2 expression at day 1 are significantly higher (p < 0.006 and p < 0.038, respectively) after painful injury compared to nonpainful and sham surgeries (Figure 2D). There are no differences among groups observed for astrocytic sPLA2 levels at day 1. Together, these data support that increased spinal sPLA2 expression appears to be sensitive to a painful injury and may be an appropriate molecular target for neuropathic pain treatment.
Figure 2.
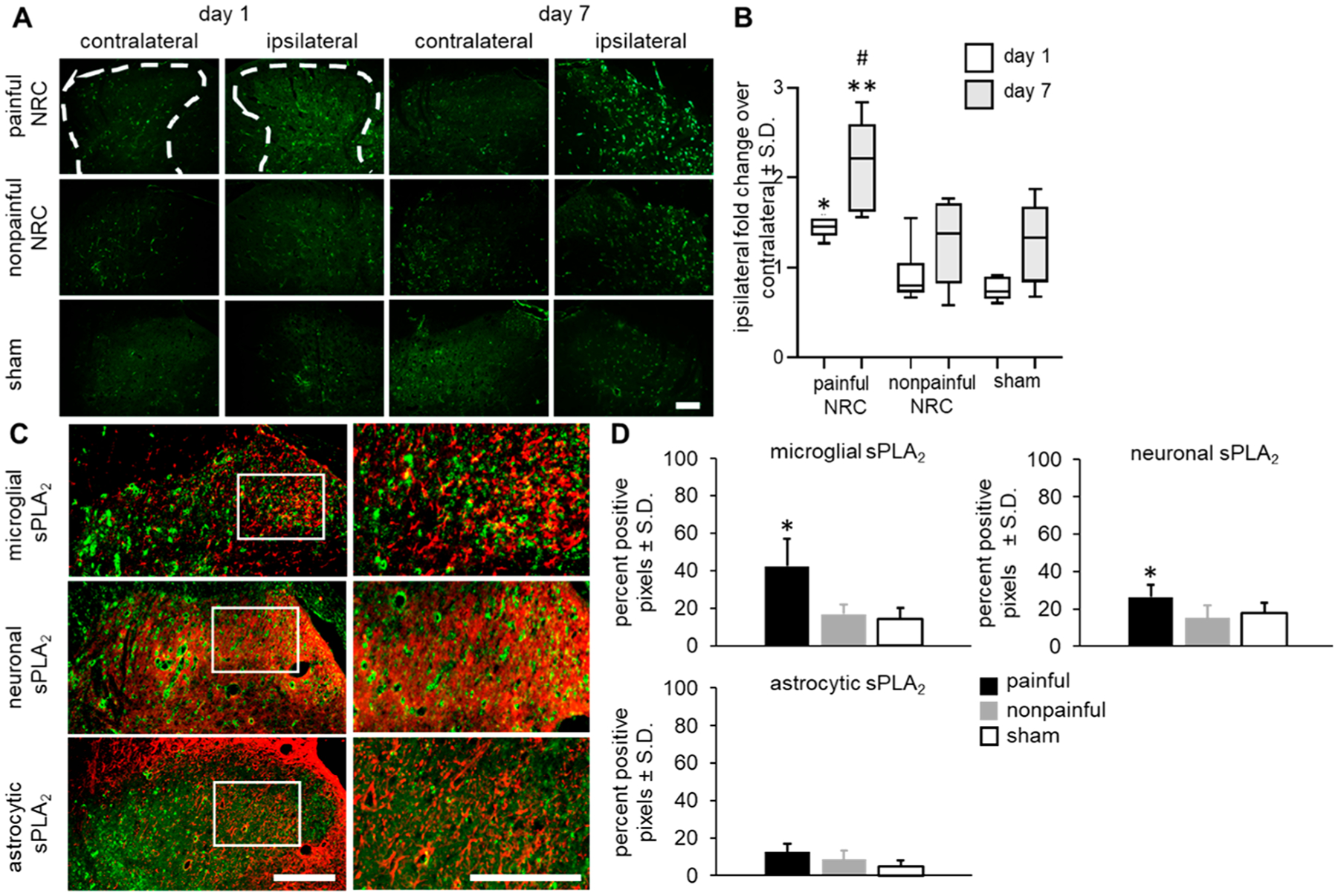
Spinal sPLA2 expression increases after a painful compression. (A) Representative images of the bilateral spinal dorsal horns show increased sPLA2 immunoreactivity only in the ipsilateral spinal cord in all groups at day 7 compared to expression at day 1, with greatest expression in the painful group at each day. The scale bar is 200 μm and applies to all panels. (B) Quantification of sPLA2 expression shows the painful group is significantly greater than expression after both a nonpainful compression and sham surgery on both days 1 (*p < 0.048) and 7 (**p < 0.009). Expression in the painful group at day 7 is significantly higher than levels at day 1 (#p < 0.043). (C) Representative images show the greatest extent of sPLA2 immunoreactivity with microglia. The scale bar is 100 μm for lower magnification images and 50 μm for higher magnification panels (white box). (D) At day 1 after painful injury, both microglial (p < 0.006) and neuronal (p < 0.038) sPLA2 expression is significantly higher than the nonpainful and sham groups. There were no significant differences in astrocytic sPLA2 expression between groups.
Synthesis and Characterization of sPLA2 Inhibitor-Loaded Phospholipid Micelles.
Based on the findings that spinal sPLA2 expression increases after painful neuropathic injury to the nerve root, therapeutic nanoparticles were prepared by incorporating sPLA2 inhibitor thioetheramide-PC (TEA-PC) into small hydrophobic superparamagnetic iron oxide nanoparticle (SPION, 7 nm, Figure S1)-loaded phospholipid micelles. Due to the amphiphilic nature of phospholipid molecules, these SPIO-loaded micelles were highly soluble in aqueous solutions. Dynamic light scattering (DLS) revealed that the sPLA2 inhibitor-loaded phospholipid micelles had a mean diameter of 60 nm with a polydispersity index (PDI) of 0.20 in water (Figure 3A). However, a large aggregation for the micelles was found when the amount of incorporated sPLA2 inhibitor increased from 1 mg to 2 mg in the preparation of the current formulation. Given the drug-loading efficiency and appropriate size, we therefore chose to prepare a micelle formulation with 1 mg sPLA2 inhibitor loading for further in vitro and in vivo testing. The morphologies of these micelles were further confirmed by transmission electron microscopy (TEM) (Figure 3B), in which the SPIONs were found to be tightly packed into a spherical core. The SPIO-loaded micelles exhibited superparamagnetic properties with an R2 value of 464 mM−1 s−1 (Figure S2). Micelle stability was also evaluated by incubating sPLA2 inhibitor-loaded micelles in PBS buffer for up to 7 days. No changes were observed in the hydrodynamic diameter of the micelles at days 1 or 7, suggesting there was no aggregation or precipitation of micelles during the incubation period (Figure S3).
Figure 3.
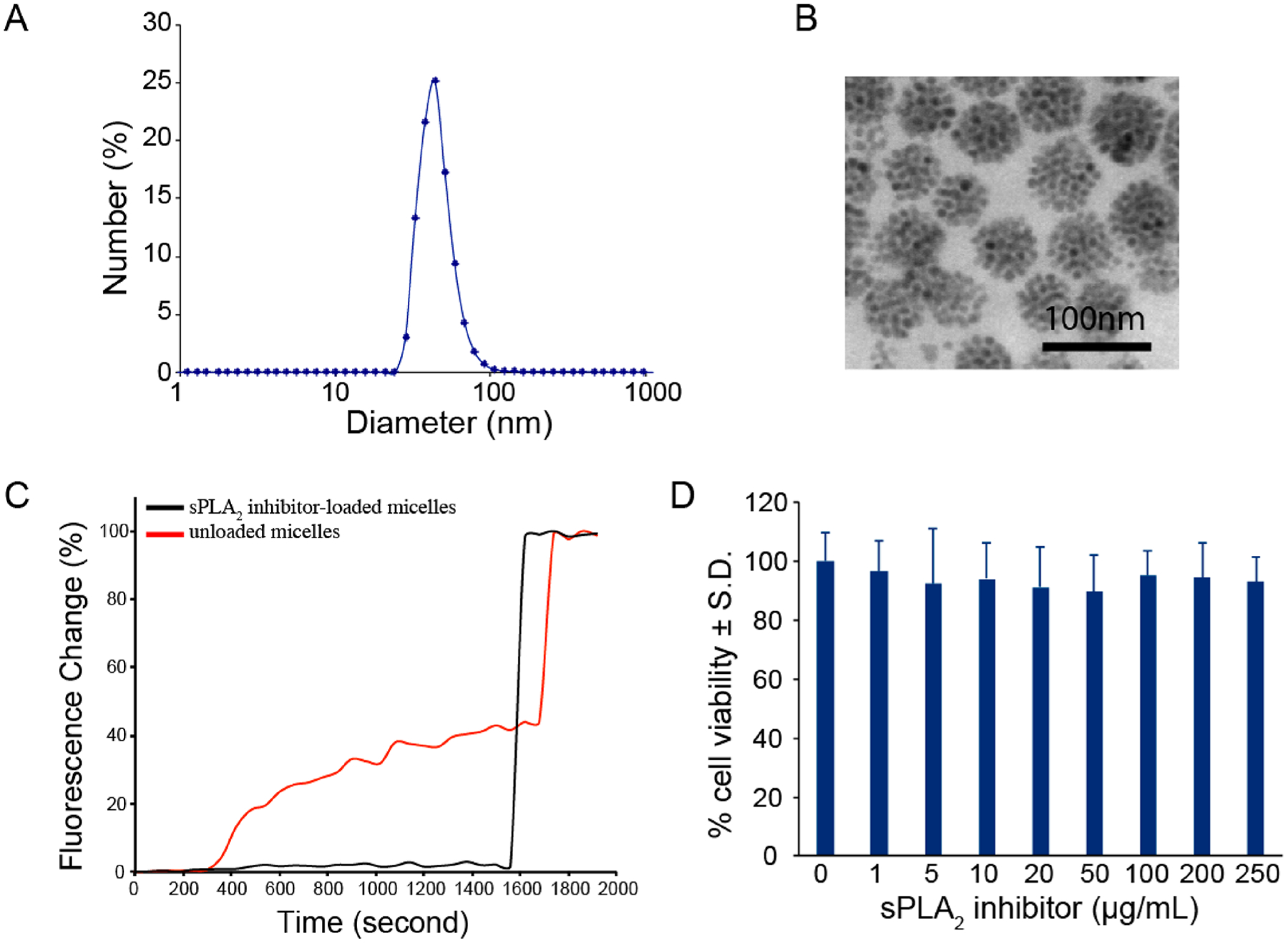
Characterization of sPLA2 inhibitor and SPIO-loaded phospholipid micelles. (A) Number-weighted size distribution of sPLA2 inhibitor-loaded micelles by dynamic light scattering (DLS). The average hydrodynamic diameter is 60 nm. (B) Transmission electron microscopy (TEM) image of sPLA2 inhibitor-loaded micelles. The electron micrograph shows tightly packed SPIONs within the hydrophobic core (scale bar: 100 nm). (C) In vitro response of sPLA2 inhibitor-loaded micelles. sPLA2 inhibitor-loaded micelles significantly inhibit PLA2 activity. (D) Incubation of the sPLA2 inhibitor-loaded micelles with rat dorsal root ganglion cells does not decrease cell viability from control (0 μg/mL) for any loaded concentration of sPLA2 inhibitor.
To study the ability of sPLA2 inhibitor-loaded micelles to inhibit sPLA2 enzyme, phospholipid hydrogenated soy phosphatidylcholine (HSPC) liposomes doped with the fluorescent lipid 1-palmitoyl-2-{6-[(7-nitro-2–1,3-benzoxadia-zol-4-yl)amino]hexanoyl}-sn-glycero-3-phosphocholine (NBD-PC) were prepared as a fluorescent sensor for sPLA2 activity. The fluorophore NBD was attached to the sn-2 position of the phospholipid with a spacer (C6) between NBD and the lipid backbone since native sPLA2 enzymes specifically recognize and catalytically hydrolyze the sn-2 acyl bond of phospholipids.49 When 20 mol % NBD-PC was incorporated into the HSPC liposomal membrane, the NBD was highly quenched within the phospholipid membrane.49 Little to no change in fluorescence was observed over a 24 h time period when these NBD-incorporated liposomes were incubated in buffer (10 mM HEPES, pH 7.4). However, a significant increase in the fluorescence intensity was observed immediately upon the addition of the preincubation mixture of control unloaded micelles (i.e., no sPLA2 inhibitor) and sPLA2 enzyme (Figure 3C). The sPLA2-induced change in fluorescence was due to the release of NBD into the surrounding bulk solution upon the hydrolysis of the phospholipid liposomal membrane by the sPLA2 enzyme. The released NBD led to fluorescence dequenching, resulting in a higher fluorescence intensity. For comparison, sPLA2 inhibitor-loaded micelles were also tested under similar conditions. No change in fluorescence intensity was observed when the preincubation mixture of sPLA2 inhibitor-loaded micelles and sPLA2 enzyme was added into the NBD-incorporated liposomal suspension, revealing that the release of NBD was significantly inhibited. Furthermore, to evaluate if sPLA2 inhibitor-loaded micelles reduced sPLA2 activity in a dose-dependent manner, inhibitor micelles of three separate concentrations of sPLA2 inhibitor (0–4.83 μg/mL) were incubated under a constant amount of sPLA2 enzyme. Indeed, the greater reductions in the extent of fluorescence activation were observed with increasing inhibitor concentrations (Figure S4), suggesting that sPLA2 inhibitor-loaded micelles inhibit sPLA2 activity in a concentration-dependent manner.
To assess cytotoxicity of the sPLA2 inhibitor-loaded micelles prior to in vivo administration, micelles were incubated for 24 h with dorsal root ganglia neurons. Incubation of the sPLA2 inhibitor-loaded micelles did not reduce neuronal viability at any concentration tested compared to untreated control cultures (Figure 3D). Additionally, incubation of the sPLA2 inhibitor-loaded micelles in neuronal cultures did not significantly increase cell lysis compared to control cultures, with the greatest concentration of micelles inducing 4.42 ± 1.77% lysis for the total culture (Figure S5). These data suggest that the sPLA2 inhibitor-loaded micelles are safe for in vivo administration.
Therapeutic Efficacy Following Local Administration of sPLA2 Inhibitor-Loaded Micelles.
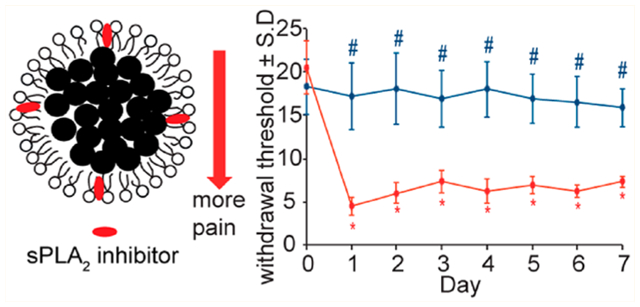
Local administration of sPLA2 inhibitor-loaded micelles immediately after a painful root compression prevents the onset of pain as early as 1 day after injury, lasting for up to 7 days (Figure 4A). Withdrawal thresholds in the ipsilateral forepaw after treatment with sPLA2 inhibitor-loaded micelles are significantly higher (p < 0.016) than those thresholds after injury treated with unloaded micelles on all days of testing. Treatment with the control unloaded micelles does not prevent pain development, with ipsilateral paw withdrawal thresholds on all days (days 1–7) remaining significantly lower (p < 0.0001) than the preinjury response. Additionally, there are no significant differences between thresholds in the contralateral paws for both groups for any post-treatment day (Figure 4B).
Figure 4.
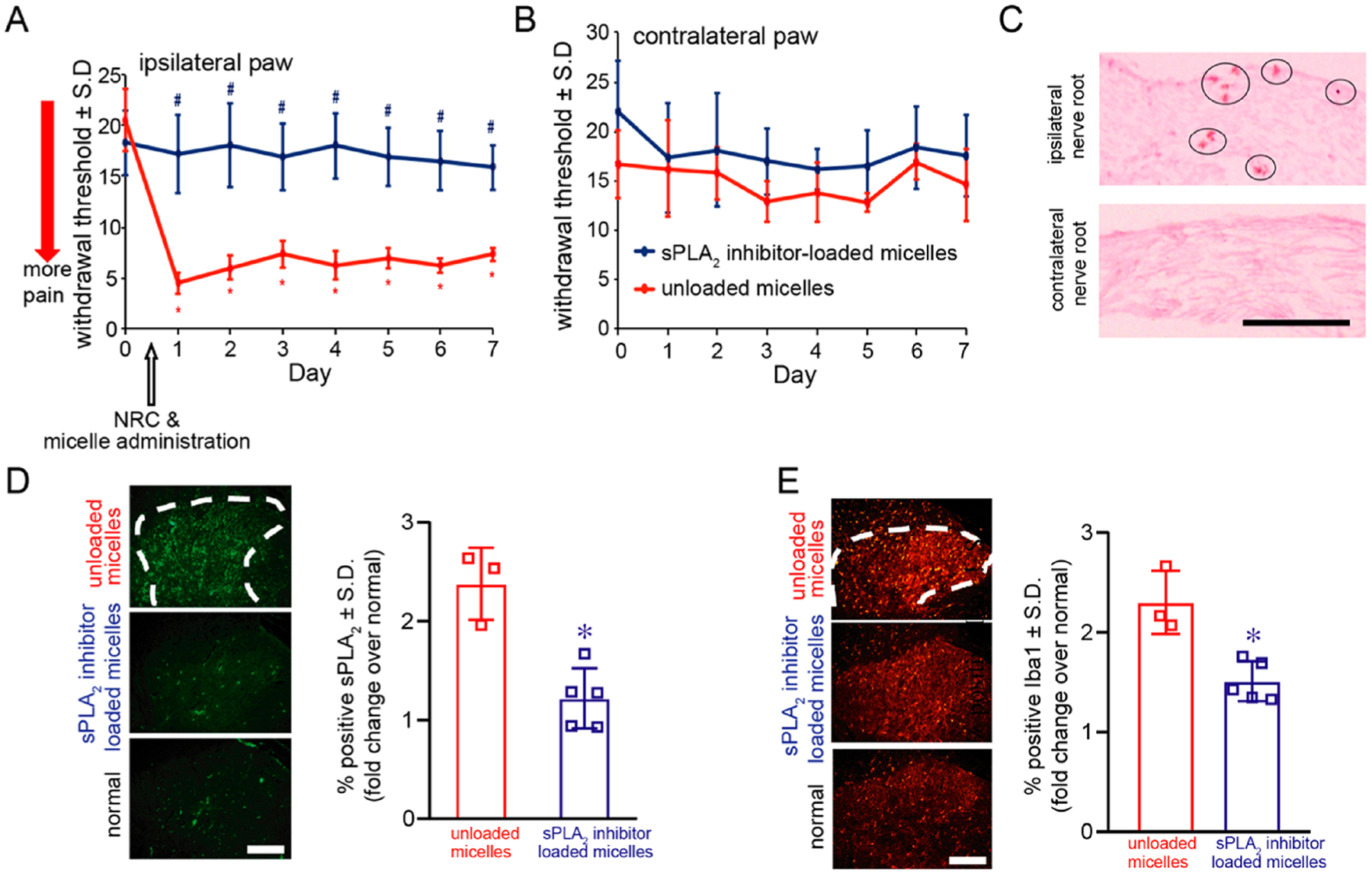
Local administration of sPLA2 inhibitor-loaded micelles immediately after a painful neuropathic nerve root compression injury prevents the development of pain and reduces the spinal sPLA2 and Iba1 expression. (A) Ipsilateral paw withdrawal thresholds following treatment with sPLA2 inhibitor-loaded micelles are significantly higher (#p < 0.016) than thresholds following treatments with micelles alone and are maintained at preinjury (day 0) levels. Administration of control micelles does not prevent pain, with withdrawal thresholds significantly lower than preinjury levels, lasting for 7 days (*p < 0.0001). (B) The withdrawal threshold in the contralateral paw is not different between groups on any day tested. (C) sPLA2-inhibitor- and SPIO-loaded micelles are found to localize at day 7 to the injured nerve root after local administration at the time of injury (day 0). Iron is detected (circles) only in the ipsilateral C7 dorsal nerve root after a nerve root compression. There is no evidence of iron in the contralateral C7 nerve root. The scale bar is 100 μm and applies to all panels. (D) sPLA2 expression is significantly decreased (*p < 0.0001) for treatment with sPLA2 inhibitor-loaded micelles compared to levels treated with control unloaded micelles and is comparable to normal levels. (E) Spinal Iba1 expression is also decreased (*p = 0.0002) at day 7 after treatment with sPLA2 inhibitor-loaded micelles compared to treatment with unloaded micelles alone. The scale bars are 100 μm in all panels.
Prussian Blue staining was performed to identify if iron oxide particles are present in the injured C7 dorsal nerve roots, after treatment with both sPLA2 inhibitor-loaded micelles and control unloaded micelles. Following local administration of the sPLA2 inhibitor-loaded micelles and unloaded control micelles, iron oxide particles are evident only in the injured (compressed) nerve root (Figure 4C and Figure S6), confirming accumulation of micellar treatment at the injury site. There are no iron oxide particles evident in any contralateral uninjured nerve root after either micelle treatment.
Local administration of sPLA2 inhibitor-loaded micelles immediately after injury not only prevents pain but also prevents the increase in spinal sPLA2 that is observed after a painful nerve root injury (Figure 4D). Spinal sPLA2 expression at day 7 is significantly lower (*p < 0.0001) after treatment compared to sPLA2 levels in the spinal cord of an untreated injury condition (Figure 4D). In addition, microglial activation, a common hallmark of spinal inflammation, was evaluated using labeling for Iba1 and is lowered in the superficial dorsal horn at day 7 after treatment with sPLA2 inhibitor-loaded micelles (Figure 4E). Similar to spinal sPLA2 expression, Iba1 levels are significantly reduced (p = 0.0002) following treatment with sPLA2 inhibitor-loaded micelles, while Iba1 levels in the untreated spinal cord remain 2.32 ± 1.25-fold over normal levels (Figure 4E).
Nanoparticle Structural Integrity during Intravenous Administration.
The sPLA2 activity in the blood and C7 spinal cord tissue was measured to assess the relative levels of sPLA2. sPLA2 activity in ipsilateral injured spinal tissue was significantly higher, 6.07 ± 1.16-fold (p = 0.0004), over circulating sPLA2 activity in serum samples (Figure S7A). To assess nanoparticle integrity in the presence of circulating sPLA2 enzymes, fluorescence-quenched HSPC liposomes were incubated with serum that was collected 1 day after a painful injury. No significant change in fluorescence was observed, either immediately upon mixing or 24 h later (Figure S7B). Increased fluorescence, a proxy for disrupted liposomes, was observed only following the addition of Triton-X, indicating that activity from circulating sPLA2 enzymes does not affect the sPLA2 inhibitor-loaded phospholipid micelles.
Therapeutic Efficacy Following Delayed Intravenous Administration of sPLA2 Inhibitor-Loaded Micelles.
Since local administration of sPLA2 inhibitor-loaded micelles prevents the onset of pain after nerve root compression (Figure 4A) and seems to modulate spinal inflammation even at later times (Figure 4E), we sought to test if giving these sPLA2 inhibitor-loaded micelles via systemic routes after pain has been established would be effective as a treatment to abolish or reduce pain. A pilot study was first performed by administering sPLA2 inhibitor-loaded micelles on day 1 after painful nerve root injury, at the same dose shown to reduce pain when administered locally to the nerve root after compression. Following treatment only on day 1, withdrawal thresholds were significantly lower (p < 0.0007) on days 3–7 compared to preinjury (day 0) thresholds (Figure S8), suggesting that a single dose of micelles is not sufficient to relieve existing pain. As such, the sPLA2 inhibitor-loaded micelles were given repeatedly via the tail vein on days 1 and 2 postinjury, at the same dose that prevents pain via the local administration after nerve root compression. As expected, the two groups, which received sPLA2 inhibitor-loaded and unloaded micelles, subjected to painful nerve root compression exhibited a significant reduction (p < 0.0053) in withdrawal thresholds compared with preinjury thresholds as early as day 1 postinjury (Figure 5A). Since those behavioral assessments are made prior to any micelle treatment, withdrawal thresholds in the unloaded and inhibitor-loaded micelle groups are also significantly lower (p < 0.001) compared to the sham surgical control group on day 1.
Figure 5.
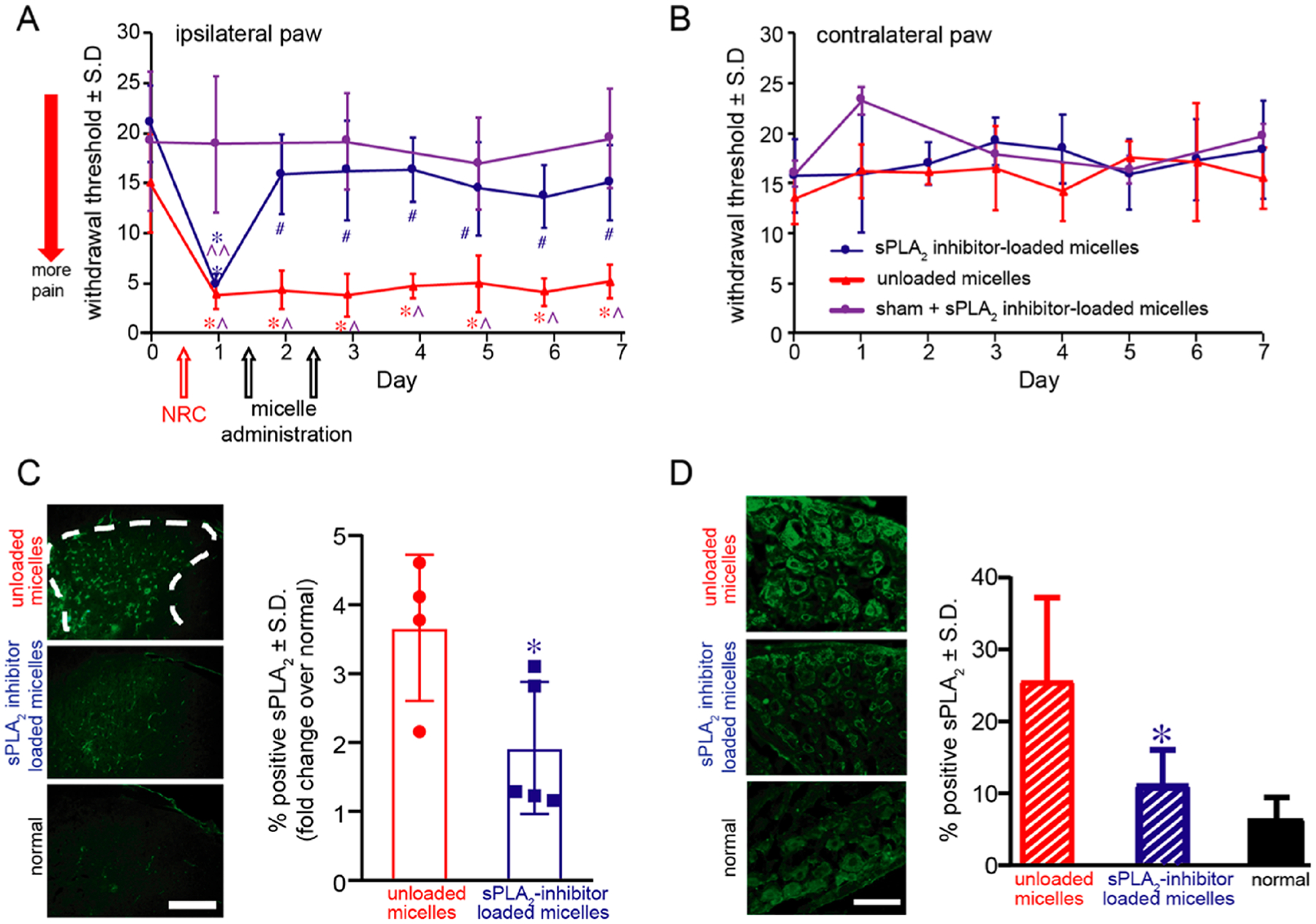
Repeated intravenous (i.v.) administration of sPLA2 inhibitor-loaded micelles on days 1 and 2 after painful nerve root compression (NRC) abolishes pain until day 7 and decreases sPLA2 expression in the spinal cord and dorsal root ganglia (DRG) at day 7. (A) A painful NRC neuropathic injury significantly reduces withdrawal thresholds in the ipsilateral forepaw from preinjury (day 0) by day 1 (^p = 0.0053) and compared to thresholds after a sham surgery (*p = 0.001). Withdrawal thresholds remain significantly decreased from sham withdrawal thresholds (^p < 0.002) after repeated administration on days 1 and 2 of control unloaded micelles (without PLA2 inhibitor) for up to 7 days. Similarly, prior to treatment with sPLA2 inhibitor-loaded micelles, thresholds on day 1 are significantly lower than those of the preinjury response on day 0 (#p = 0.0001) and sham responses on day 1 (**p = 0.0004). Within 1 day of the i.v. micellar inhibitor treatment on day 1, withdrawal thresholds are significantly increased back to preinjury and sham response levels by day 2 and remain significantly elevated (*p < 0.0024) through day 7. (B) There are no differences in the contralateral withdrawal thresholds for any of the groups. (C) Spinal sPLA2 expression is significantly decreased (*p = 0.038) in the superficial dorsal horn after treatment of micelles with inhibitor compared to treatment with unloaded micelles alone. Representative images are shown with a scale bar (100 μm) that applies to all panels. (D) Peripheral sPLA2 expression in the DRG is also significantly (*p = 0.002) reduced after treatment with sPLA2 inhibitor-loaded micelles and is comparable to normal levels.
However, after behavioral testing on day 1, treatment with sPLA2 inhibitor-loaded micelles was given and the withdrawal threshold increases on day 2 to sham and preinjury levels, indicating less pain (Figure 5A). After the second micellar treatment dose on day 2, withdrawal thresholds are still elevated on day 3 and remain the same as preinjury through day 7 (Figure 5A). After sPLA2 inhibitor-loaded micelle treatment, the responses are significantly increased (*p < 0.0024) through day 7 over thresholds in the group receiving a painful nerve root compression and unloaded micelles (Figure 5A). In fact, there are no differences between the micelle treatment group and sham group on any post-treatment days. However, repeated intravenous administration of control micelles without the inhibitor does not alter the pain response, and thresholds remain significantly lower than baseline (p < 0.0001) and sham surgical control responses (p < 0.002) (Figure 5A). Similar to local administration of sPLA2 inhibitor-loaded micelles (Figure 4B), the contralateral withdrawal thresholds are not different between the two treatment groups or sham group at any day postinjury (Figure 5B). In addition, as with the local administration of sPLA2 inhibitor-loaded micelles (Figure 4C), iron oxide particles localize only to the injured C7 dorsal nerve root after intravenous delivery of the sPLA2 inhibitor-loaded micelles (Figure S9A), with no evidence of accumulation in the treated sham and naïve C7 nerve roots. Iron oxide particles are also observed in the white and gray matter of the ipsilateral but not the contralateral C7 spinal sections after painful injury, but not in naïve spinal tissues (Figure S9B). These studies validate sufficient selective accumulation of sPLA2 inhibitor-loaded micelles to the injury C7 spinal level with systemic delivery.
Systemic treatment with sPLA2 inhibitor-loaded micelles also significantly reduces (p = 0.038) sPLA2 expression in the spinal dorsal horn compared to treatment with control unloaded micelles at day 7, almost to levels of a normal naïve spinal cord (Figure 5C). Similarly, sPLA2 expression in the DRG is also significantly (p = 0.002) reduced by 2.29 ± 0.39-fold to normal levels, after treatment with sPLA2 inhibitor-loaded micelles (Figure 5D). Similar to local administration, systemic treatment with sPLA2 inhibitor-loaded micelles also significantly reduces (p < 0.0002) spinal Iba1 expression to normal levels compared to treatment with unloaded micelles alone (Figure S10A). Systemic treatment with inhibitor micelles also significantly decreased (p = 0.0002) microglial sPLA2 expression, to normal levels, compared to treatment with unloaded micelles (Figure S10B and C). At day 7, spinal Iba1 expression after treatment with unloaded micelles is significantly higher (p < 0.0002) than treatment with sPLA2 inhibitor-loaded micelles and normal (uninjured) levels. Consistent with the histological evidence of micelle accumulation at the site of injury (nerve root) and spinal cord (Figure S9), decreased sPLA2 expression in the spinal cord and DRG after systemic micellar treatment further suggests that the sPLA2 inhibition occurs at the injured C7 spinal level.
Pharmacokinetics, In Vivo Toxicity, and Micelle Accumulation Following Delayed Intravenous Administration of sPLA2 Inhibitor-Loaded Micelles.
Following intravenous injection of rhodamine-labeled sPLA2 inhibitor-loaded micelles 1 day after painful nerve root compression, micelle blood circulation half-life is calculated to be approximately 45 min (Figure S11A). In vivo toxicity was also assessed 7 days after intravenous administration of sPLA2 inhibitor-loaded micelles in major organs using histological staining, and no major abnormalities are observed in the heart, liver, spleen, lung, kidney, and brain following micelle administration compared to saline-injected controls (Figure S11B).
In this work, we show that sPLA2 expression after painful injury is significantly increased (Figure 2). This finding suggests that sPLA2 can be a signature of painful tissue and may act as a therapeutic target for neuropathic pain treatment. However, traditional approaches for delivery of anti-inflammatory and neuromodulatory drugs are not very effective for attenuating or abolishing the pain. Drug delivery systems utilizing nanoparticles are increasingly being used to improve therapeutic delivery owing to their ability to increase the effective drug concentration at sites of inflammation while reducing off-target toxicity.45 We have recently reported the nanoparticle based on SPIO-loaded phospholipid micelles.49 In this study, we incorporated the sPLA2 inhibitor TEA-PC into this nanoformulation. These micelle nanoparticles can release their payload based on the pathological conditions by the pain state itself, i.e., different sPLA2 activity and expression. It was found that leveraging the robust neuroinflammation that occurs with painful neuropathy effectively directs the accumulation and release of encapsulated drugs from micelles and provides long-lasting pain relief (Figures 4A and 5A). Capitalizing on increased expression of sPLA2 in the dorsal root ganglia50 ensures localized release of the inhibitor drug where it can have the most impact on phospholipase activity. Administration of these sPLA2 inhibitor-loaded micelles immediately after compression and directly to the nerve root fully prevents the pain that typically develops after a painful root injury (Figure 4A). However, these studies were only carried out to postoperative day 7, and it is unknown if pain develops in the following weeks. More clinically relevant is the finding that its intravenous delivery after painful neuropathy is already established abolishes existing pain (Figure 5A). The intravenous dose of the sPLA2 inhibitor (0.25 mg/mL) used in these micelles not only was comparable to previous intrathecal doses of PLA2 inhibitors shown to provide pain relief51,52 but was also approximately 30 times lower than the oral PLA2 inhibitor dose and 1000 times lower than the COX-2 inhibitor dose53,54 needed to attenuate neuropathic pain.55 Further, unlike many pharmacologic neuropathic pain treatments that require continuous and invasive intrathecal delivery or repeated daily dosing,53,54 the sPLA2 inhibitor-loaded micelles were effective in fully preventing pain with only a single low dose or only two separate doses to attenuate existing pain. These studies suggest that these targeted drug delivery methods could provide a much-needed solution to the limited efficacy of current neuropathic pain therapeutics. As a platform, sPLA2 inhibitor-loaded micelles could not only remove the need for invasive treatments but increase the therapeutic index of many pain therapeutics that are currently not used in the clinic due to substantial side-effects.
In addition to preventing and abolishing pain, both administration paradigms of sPLA2 inhibitor-loaded micelles also normalize the spinal sPLA2 expression at the C7 spinal level (Figure 4D and Figure 5C), confirming that the active drug is released as observed with the in vitro experiments (Figure 3C). This is further corroborated by the >6-fold difference in sPLA2 activity in serum and in the injured spinal cord (Figure S7A), suggesting that greater inhibitor release from micelles occurs at the injured nerve root and spinal cord rather than during circulation. Further, the extent of the decreases in spinal sPLA2 is similar for both administration paradigms (Figure 4D and Figure 5C), suggesting that the systemic administration achieved similar inhibitory activity at the injured spinal level to that of locally administered injections. However, since neither the sPLA2 inhibitor TEA-PC nor the encapsulated TEA-PC readily cross the blood spinal cord barrier (BSCB),56 it may have trafficked there when the barrier was disrupted, as evidenced by the spinal accumulation of iron oxide particles after painful nerve root compression at day 7 (Figure S9). BSCB breakdown occurs as early as 1 day after spinal cord and radicular injuries but is restored by day 7. Although both treatments may leverage the BSCB being open to meditate sPLA2 in the spinal cord, it is unknown if the same effects would be observed with a later delivery time. Despite this uncertainty, current neuropathic pain treatments such as morphine and cytokine antagonists, even given as early as day 1 and to the injury site or directly to the spinal cord, fail to completely abolish pain.57,58 Whether the encapsulated drug relieves pain by acting exclusively in the periphery, spinally, or through a combination of both, the use of sPLA2-responsive phospholipid micelles as a delivery platform ensures the drug’s highest possible therapeutic value.
Despite sPLA2’s established role in neuroinflammation,51,59 few studies have utilized it for pain treatment. Although direct application of sPLA2 to the naïve spinal cord induces pain,60 most interventions focus on its downstream inflammatory effects, including blocking the COX enzymes, which utilize arachidonic acid for the synthesis of prostaglandins.60,61 Yet, COX inhibitors and the larger class of nonsteroidal anti-inflammatory drugs (NSAIDs) have limited utility for neuropathic pain since they have substantial renal and cardiovascular toxicities and limited efficacy without daily repeated administration.62 Further, although other phospholipase A2 inhibitors reduce pain after neuropathy, analgesia is only transient.52 Intrathecal administration of the cytosolic phospholipase A2 inhibitor (cPLA2) AACOCF3 reduces behavioral sensitivity when given 1 day after chronic constriction of the sciatic nerve, but only if given under daily repeated dosing.45 The differences in dosing between sPLA2 and cPLA2 inhibition may depend on differences between arachidonic acid generation from these two phospholipase A2 isoforms. In vitro activity studies suggest that cPLA2 may have more of a regulatory role in cellular membrane maintenance, whereas sPLA2 may be more responsible for arachidonic acid production and exacerbating downstream inflammatory cascades.63 Inhibiting sPLA2 decreased the activation of spinal microglia and microglial sPLA2 expression at day 7 (Figure 4E and Figure S10). Since the increased neuroinflammation evident days after injury is responsible for the progression toward persistent neuropathic pain, it is likely that inhibiting sPLA2 in this early window interferes with the establishment of persistent pain altogether.64 In addition to inflammation, sPLA2 has also been shown to modulate neuronal excitatory transmission65,66 and may contribute to the heightened neuronal activity that maintains neuropathic pain.67 In pilot studies with glutamate-stimulated cortical neurons,66 treatment with TEA-PC reduces neuronal calcium events compared to media vehicle controls by 62.8 ± 13.3%, suggesting that sPLA2 inhibition may reduce the heightened neuronal activity that maintains nerve root-mediated pain.64 These studies suggest that, mechanistically, sPLA2 may contribute to both the inflammation and increased neuronal activity attributed to persistent neuropathic pain, and probing these mechanisms further would provide better insights into the timing and dosing of these inhibitor micelles for patients.
Although here the repeated dose was needed to attenuate pain (Figure 5A and Figure S8), whether a greater administration dose of sPLA2 inhibitor-loaded micelles on day 1 would provide long-lasting pain relief in a single dose is unknown. Since the systemic dose used to inhibit sPLA2 on days 1 and 2 is sufficient to reduce sPLA2 expression in the spinal cord and DRG at day 7 (Figure 5C and D), early sPLA2 inhibition in both regions may be necessary to attenuate pain. Therefore, further studies are also needed to identify the individual contributions of peripheral and spinal sPLA2 to the progression of persistent pain. Nevertheless, these data together suggest that systemic sPLA2 inhibition may be a potent alternative to current anti-inflammatory strategies used to manage persistent neuropathic pain.
In this study, the incorporation of the SPIO core into sPLA2 inhibitor-loaded micelles was used to identify accumulation of the micelle in vivo. The use of the SPIO core can also be used to detect sites of neuroinflammation68 as well as localize sites of macrophage infiltration after painful nerve root compression using MR imaging.17 Therefore, future work seeks to combine drug (sPLA2 inhibitor) and MR imaging agents (SPIOs) in a single nanoformulation. Noninvasive MR imaging could be used for in vivo real-time tracking of theranostic micelle accumulation and assessing local pathological states. By combining therapeutics and diagnostic information into a single platform, sPLA2 inhibitor and SPIO-loaded micelles could provide important insights into heterogeneities between pain symptoms and pathologies at the individual patient level, which may be crucial in developing effective treatments for painful neuropathies.48
CONCLUSIONS
This study demonstrates that sPLA2-responsive phospholipid micelles provide localized delivery of encapsulated drugs and can even be more efficacious than traditional intrathecal delivery methods for treating chronic pain. Leveraging sPLA2 activity in painful syndromes helps exploit the release of the inhibitor and possibly other drugs locally to the areas that are directly involved in pain, facilitating both pain treatment and possibly image tracking of the active disease state. Given the role of sPLA2 in many different inflammatory and neuropathic pain syndromes,59,69 sPLA2-responsive phospholipid micelles have a potentially broad reach. Further, this sPLA2 micelle platform can be expanded to encapsulate other drugs including neuromodulatory drugs, which despite success in animal models, have not been adopted into the clinic since systemic administration requires high doses, which induce substantial off-target side-effects. sPLA2 inhibitor-loaded phospholipid micelles may potentially address the clinical challenge of delivering pain therapeutics to achieve long lasting pain relief, without the debilitating off-target toxicities.
METHODS
Materials.
TEA-PC was purchased from Cayman Chemical (Ann Arbor, MI, USA). HSPC, 1,2-distearoyl-sn-glycero-3-phosphoethanol-amine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000), and NBD-PC were purchased from Avanti Polar Lipids, Inc. Secreted phospholipase A2 enzyme from Naja mossambica was purchased from Sigma-Aldrich Co. 3′,3-Diaminobenzidine (DAB) was purchased from Vector Inc. Goat polyclonal antibody to sPLA2-IIA and rabbit polyclonal antibody to Iba1 were purchased from Santa Cruz Biotechnology Inc. and Wako, respectively. All other chemicals were used as received. All of the buffer solutions were prepared with deionized water.
Synthesis of Hydrophobic 7 nm Superparamagnetic Iron Oxide Nanoparticles.
Oleic acid-coated SPIONs were prepared by thermal decomposition as previously described.49 After allowing the reaction to cool to room temperature, acetone was added and the sample was centrifuged to precipitate the nanoparticles. The particles were further washed in hexane and precipitated again using acetone followed by centrifugation. This washing procedure was repeated until the supernatant was clear. The particles were then allowed to air-dry and dissolved in toluene at ~40 mg/mL.
Synthesis of PLA2 Inhibitor and SPION-Loaded Micelles.
sPLA2 inhibitor-loaded micelles were prepared using an oil-in-water emulsion method.49 A mixture (230 μL) containing HSPC (1 mg in 20 μL of chloroform), PLA2 inhibitor TEA-PC (1 mg in 100 μL of ethanol), DSPE-PEG2000 (0.25 mg in 10 μL of chloroform), and SPIO NPs (2 mg in 100 μL of toluene) was injected into a glass vial containing 4 mL of water, and the sample was sonicated (Branson Ultrasonics, Danbury, CT, USA) until a homogeneous mixture was achieved. The toluene and chloroform were then allowed to evaporate overnight. sPLA2 inhibitor- and SPION-loaded phospholipid micelle samples were then centrifuged (Eppendorf microcentrifuge 5418) at 1000 rpm for 30 min to remove large aggregates. In order to remove empty micelles, the resulting supernatant was then centrifuged at 10 000 rpm for half an hour, and the pellet was resuspended in water. The stock solution of sPLA2 inhibitor- and SPION-loaded phospholipids micelles was stored in the dark at 4 °C. The unloaded micelles, i.e., without PLA2 inhibitor, were prepared using similar procedures to the sPLA2 inhibitor- and SPION-loaded phospholipid micelles. Synthesized sPLA2 inhibitor-loaded micelles were incubated in 0.1 M PBS (pH 7.4) for the stability study. DLS was used to monitor the particle size over a total of 1 week.
In Vitro PLA2 Response Study.
To study the efficiency of cargo release from sPLA2 inhibitor-loaded micelles, the dequenching of NBD-incorporated liposomes incubated with sPLA2 inhibitor-loaded micelles in the presence of sPLA2 enzyme was directly measured. To develop the NBD-incorporated liposomes, a 80 mol % HSPC/20 mol % NBD-PC mixture was prepared in chloroform in a glass vial using a total 1 mg amount of HSPC. The chloroform solvent was removed using a direct stream of nitrogen, prior to vacuum desiccation for a minimum of 4 h. After vacuum desiccation and the formation of a dried lipid film, liposomal samples were then incubated with 0.2 mL in a 50 °C water bath for 0.5 h followed by another 0.5 h of direct sonication at the same temperature to yield the NBD-incorporated liposomes. The stock solution of NBD-incorporated liposomes was stored in the dark at 4 °C.
Dequenching measurements were performed by first preincubating a mixture of either sPLA2 inhibitor-loaded or unloaded micelles with the sPLA2 enzyme (10 μL of sPLA2 inhibitor-loaded [TEA-PC = 0.125 and 0.25 mg/mL] or unloaded micelles + 6.67 μL of sPLA2 enzyme [PLA2 = 7.5 U/mL]) for 20 min. Fluorescent measurements of the NBD-incorporated liposomes in buffer (20 μL of NBD-incorporated liposomal suspension ([HSPC] = 1 mg/mL) + 0.48 mL of 0.01 M HEPES (pH 7.4) buffer solution containing 2 mM CaCl2) were taken for 5 min prior to the addition of the incubation mixture of the sPLA2 inhibitor-loaded or unloaded micelles. The fluorescence intensity at 520 nm was measured using an excitation at 460 nm. The amount of NBD dequenched (% NBD dequenched) was calculated as ([Ix− I0]/[It − I0]) × 100, where I0 is the fluorescence intensity of the liposomal suspension containing NBD at the initial time, Ix is the fluorescence intensity at any given time, and It is the fluorescence intensity after addition of 20 μL of Triton X-100 (50 mM) to the suspension at the end of experiment.
In Vitro Cytotoxicity.
To assess in vitro cytotoxicity to neural cells, dorsal root ganglia were isolated from embryonic day 18 Sprague–Dawley rats and stored in Hibernate-E medium (Thermo-Scientific, Waltham, MA, USA) supplemented with 2% B-27 and 0.5 mM GlutaMAX (Thermo-Scientific) for 2 days until dissociation. To perform DRG dissociation, Hibernate-E medium was aspirated and DRGs were incubated with warm 0.25% trypsin in EDTA for 1 h at 37 °C. After that, feeding medium (Neurobasal supplemented with 2% B-27, 0.5 mM GlutaMAX, 1% fetal bovine serum (FBS), 0.02 μg/mL nerve growth factor (NGF), 2.5 mg/mL glucose, and 40 μM M2 (5-fluoro-2′-deoxyridine 10 mM, uridine 10 mM) with an additional 5% FBS was added to inhibit trypsin activity. DRGs were then manually titurated with a pestle, centrifuged at 1000 rpm for 5 min at room temperature, isolated from their supernatant, and then reconstituted with fresh feeding medium. Dissociated DRGs were then plated (3.97 × 106 cells/mL) onto a 24-well plastic tissue culture plate treated with poly-D-lysine (PDL) and laminin. Feeding medium was changed at day in vitro (DIV) 1 and DIV 3. On DIV 4, cultures were separately incubated for 24 h with micelles at different PLA2 inhibitor concentrations: 0, 1, 5, 10, 20, 50, 100, 200, and 250 μg/mL. Cell viability was tested in triplicate for each concentration using a CellTiter 96 AQ One Solution cell proliferation assay (Promega, Madison, WI, USA). Cytotoxicity measurements from treated groups were normalized to untreated controls as previously described.70,71 Average percent cell viability was compared using a one-way ANOVA (group) with Tukey’s test.
In Vivo Injury and sPLA2 Inhibitor-Loaded Micelles Administration.
All studies were approved by our Institutional Animal Care and Use Committee and carried out under the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain. First, spinal sPLA2 expression was characterized after a painful root injury. As such, rats underwent either a nerve root compression that was painful (15 min; n = 11) or a compression that served as a control since it was nonpainful (3 min; n = 11); sham injuries (n = 10) were also included as surgical controls. Under isoflurane anesthesia (4% induction, 2% maintenance), male Holtzman rats underwent a painful nerve root compression in which the right C7 nerve root was surgically exposed and compressed for 15 min with a 10g force microvascular clip as previously described.64
To test the effectiveness of sPLA2 inhibitor-loaded micelles in preventing the pain that develops from a neuropathic injury, sPLA2 inhibitor-loaded micelles were administered in vivo after a painful nerve root compression.64 Immediately after tissue compression, two separate groups of rats received either 100 μL (TEA-PC concentration: 0.25 mg/mL) of sPLA2 inhibitor-loaded micelles in saline (sPLA2 inhibitor-loaded micelles; n = 5) or a comparable dose of control micelles without any sPLA2 inhibitor loaded in saline (unloaded micelles; n = 3) administered directly to the nerve root. After each surgery, incisions were closed using 3–0 polyester sutures and surgical staples, and the rats were monitored during recovery in room air.
To assess if the same dose of inhibitor micelles that were effective in preventing pain with local administration could be as effective when administered systemically, in a pilot study (sPLA2 inhibitor-loaded micelles; n = 4), rats received 100 μL of sPLA2 inhibitor-loaded micelles (TEA-PC concentration: 0.25 mg/mL) diluted in PBS that were administered via tail vein only on day 1 after a painful nerve root compression. In a second study, separate groups of rats received either 100 μL of sPLA2 inhibitor-loaded micelles (TEA-PC concentration: 0.25 mg/mL), sPLA2 (inhibitor-loaded micelles, n = 5) or a comparable dose of unloaded control micelles (unloaded micelles; n = 4) diluted in 400 μL of PBS administered intravenously through the tail vein after the nerve root compression on both day 1 and day 2.17 Additional groups of rats were included as sham-surgical controls (sham + sPLA2 inhibitor-loaded micelles; n = 4) in which rats received identical surgical procedures in which the nerve root was exposed but not compressed.
Behavior Testing for Pain Sensitivity.
Behavioral sensitivity following either micelle administration paradigm (local: sPLA2 inhibitor-loaded micelles; n = 5; unloaded micelles; n = 3; and intravenous: sPLA2 inhibitor-loaded micelles; n = 5; unloaded micelles; n = 4) was assessed by measuring the bilateral forepaw withdrawal threshold to mechanical stimulation, using a modified Chaplan’s up–down method.64 Testing was performed at baseline before injury (day 0) and daily for 7 days after and was quantified as the paw withdrawal threshold to an applied mechanical stimulus. Rats were randomly placed into different test chambers and allowed to acclimate for 20 min prior to testing. The thresholds for eliciting a withdrawal response were quantified by stimulating each forepaw using a series of von Frey filaments (Stoelting; Wood Dale, IL, USA) with increasing strengths (1.4, 2, 4, 6, 8, 15, and 26 gf). An observer, blinded to the group, applied each filament five times before advancing to the next highest strength, and if two consecutive filaments both induced a withdrawal response, the lower strength filament was recorded as the threshold. The withdrawal threshold was taken as the lowest von Frey filament to elicit a response and was confirmed by the next filament also provoking a response.72 Testing sessions consisted of 3 rounds each separated by 10 min of rest. The withdrawal thresholds were separately averaged across rounds for each day, and differences in thresholds between groups were compared separately for the ipsilateral and contralateral forepaw using a repeated-measures ANOVA with posthoc Tukey’s test. No rats were excluded from behavioral testing or analysis.
Tissue Harvest, Prussian Blue Staining, and Immunohis-tochemistry.
For the studies characterizing sPLA2 expression in the bilateral spinal cord, tissue at the C7 level was separately harvested on days 1 (painful n = 6; nonpainful n = 5; sham n = 5) and day 7 (painful n = 5; nonpainful n = 6; sham n = 5). For all micelle treatment groups, on postoperative day 7 after behavior testing, the C7 nerve roots, DRG, and spinal cord were harvested for histology and immunolabeling. Rats were deeply anesthetized with sodium pentobarbital (65 mg/kg) and transcardially perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. Sub-sequently, all neural tissues were postfixed in 4% paraformaldehyde overnight, cryoprotected in 30% sucrose, and embedded in OCT medium (VWR, Bridgeport, NJ, USA). All nerve root, DRG, and spinal cord tissues were cryosectioned along the long axis at 14 μm and thaw-mounted onto slides.
For spinal sPLA2 characterization studies, both ipsilateral and contralateral C7 spinal sections were blocked using 10% normal donkey serum (Vector, Burlingame, CA, USA) with 0.3% Triton-X 100 and incubated in goat anti-sPLA2 IIA (1:500; Santa Cruz, Dallas TX, USA). In a separate run, spinal tissue sections were colabeled separately with sPLA2 IIA and mouse-anti MAP2 (1:250; Covance; Cumberland, VA, USA), rabbit anti-Iba1 (1:1000, Wako, Osaka), and rabbit anti-GFAP (1:500; Millipore; Billerca, MA, USA) to label for neuronal, microglial, and astrocytic sPLA2, respectively Tissue sections were then rinsed with PBS and incubated for 2 h at room temperature in either donkey anti-goat 488, donkey anti-mouse 555, or donkey anti-rabbit 546 (1:500, Invitrogen, Carlsbad CA, USA) secondary antibodies. For each rat, 3–6 spinal cord sections were captured using a Leica DM6000 (Wetzlar, Germany) with deconvolution at 20×, and both ipsilateral and contralateral images were cropped to 750 × 200 pixels2 to contain the superficial (I and II) laminae of the spinal cord dorsal horn. A custom densitometry MATLAB script (Mathworks, Natick, MA, USA) was used to quantify the extent of positive spinal sPLA2, and spinal cord sections from naïve rats (n = 2) were acquired and used to set the pixel intensity thresholds used to quantify positive labeling for sPLA2. The ipsilateral expression of total spinal sPLA2 was presented as a fold change over contralateral expression. To quantify colocalization of spinal sPLA2 in neurons, microglia, and astrocytes, the total number of pixels positive for sPLA2 and either MAP2, Iba1, or GFAP is divided by the total number of pixels positive for sPLA2 for each image in the ipsilateral dorsal horn. Spinal neuronal, microglia, and astrocytic sPLA2 and total spinal sPLA2 were compared across groups using a one-way ANOVA with Tukey’s posthoc test.
To determine the expression of sPLA2 and the extent of Iba1 positive microglia in the spinal cord and sPLA2 expression in the DRG after micelle treatment, sections were similarly blocked and incubated in goat anti-sPLA2 IIA (1:500; Santa Cruz) and rabbit anti-Iba1 (1:1000, Wako, Osaka Japan) primary antibodies as well as donkey anti-goat 488 and donkey anti-rabbit 546 (1:500, Invitrogen) secondary antibodies. Spinal sections were similarly imaged using a Leica DM6000 and cropped to only include the superficial dorsal horn for analysis. Images of the DRG were cropped (450 × 450 pixels) to include 10–20 random neurons per image for intensity analysis. A custom densitometry MATLAB script (Mathworks) was used to quantify the extent of positive spinal sPLA2 and Iba1 labeling and sPLA2 labeling in the DRG, as previously described.73,74 DRG and spinal cord sections from naïve rats (n = 2) were acquired and used to set the pixel intensity thresholds used to quantify positive labeling for sPLA2 and Iba1 separately. Spinal microglial sPLA2 expression after micelle treatment was also evaluated through quantifying the colocalization of sPLA2 and Iba1 labeling as performed in earlier spinal sPLA2 characterization studies. The number of pixels positive for either sPLA2 or Iba1 was calculated as a percent of the total dorsal horn area for each label. Similarly, the positive pixels for sPLA2 were evaluated over the area of selected DRG neurons and compared across groups using a one-way ANOVA with Tukey’s posthoc test. Total levels of each of sPLA2, Iba1, or microglial sPLA2 labeling were then separately compared to the normal levels and expressed as a fold-change over normal levels for each rat. Differences in normalized intensity between the sPLA2 inhibitor-loaded micelles and the unloaded micelles were compared using separate Student’s t tests for each label in the spinal cord.
To assess micelle localization, Prussian Blue staining was used to detect SPIONs via iron oxide staining.17 An additional group of naive rats that received no surgery, but the same intravenous dose of sPLA2 inhibitor-loaded micelles (normal + sPLA2 inhibitor-loaded micelles; n = 2), was included as histological controls. Nerve root and spinal cord sections were incubated with 0.3% hydrogen peroxide in PBS and a 1:1 mixture of 2% potassium ferrocyanide and 2% hydrochloric acid for 30 min. Visualization of iron in the tissue was enhanced by DAB (Vector) for an additional 15 min. Tissue sections were then counterstained with eosin (Sigma-Aldrich, St. Louis, MO, USA), cleared with xylene (Sigma-Aldrich), and coverslipped using DPX mounting media (Sigma-Aldrich). Spinal sections were imaged using an Olympus 5XB1 microscope (Olympus, Tokyo, Japan).
Pharmacokinetic and In Vivo Toxicity Experiments.
Blood circulation of micelles was tested in a separate group of rats (n = 3) that received a painful nerve root compression followed by injection of dye-incorporated sPLA2 inhibitor-loaded micelles 1 day after injury via tail vein injections. Dye-loaded sPLA2 inhibitor-loaded micelles were fabricated by incorporating 10 mol % rhodamine-PE during the micelle preparation. One day after painful nerve root injury, rats received 500 μL of the dye-loaded sPLA2 inhibitor-loaded micelles (TEA-PC; 0.5 mg/mL) diluted in saline. Whole blood samples were collected in heparinized tubes at baseline prior to injection (0 h) and then 0.5, 1, 2, 4, 8, 12, and 24 h after injection. Fluorescence spectra of rhodamine from blood samples were collected by a FluoroMax-3 spectrofluorimeter (Horiba Jobin Yvon, Edison, NJ, USA). For in vivo toxicity studies, tissue from the same group of rats was harvested with fresh PBS at day 7, and major organs were postfixed in 4% paraformaldehyde for 48 h. A second group of rats (n = 2) that did not receive surgery but did receive an intravenous injection of 500 μL of saline was included as histological controls. Organ tissue was then prepared for paraffin embedding and sectioned serially at a 6 μm thickness across the entire organ. Tissue sections were then stained with hematoxylin and eosin (H&E) to assess cellular and tissue architecture as previously described.75
sPLA2 Activity Assay and Circulating sPLA2 Experiment.
To assess if circulating sPLA2 in blood affects inhibitor release from sPLA2 inhibitor-loaded micelles, both blood and spinal cord tissue were collected from rats 1 day after receiving a painful nerve root compression and assayed using an sPLA2 activity kit from Cayman Chemical (Ann Arbor, MI, USA). In a subset of rats (n = 3) that received only a painful nerve root compression, serum samples were collected from whole blood samples as previously described.65 Blood was collected from the tail vein before surgery and 1 day after surgery and centrifuged to separate the serum from other whole-blood components. At the same time point, ipsilateral C7 spinal cord also was collected, flash frozen, and stored at −70 °C until assayed. Spinal cords at the C7 level from naïve uninjured rats (n = 2) were also harvested as controls. Both serum and spinal cord samples were assayed for sPLA2 activity as per the kit instructions. Activity measurement from injured serum was normalized to each rat’s baseline (prior to compression injury), and spinal cord activity was normalized to naïve spinal cord measurements. Both serum and spinal cord sPLA2 activity were compared to each other using Student’s t test.
Serum samples were also used to test liposomal integrity in the presence of circulating sPLA2 enzymes in vitro. In separate studies, 30 μL of an NBD-incorporated liposomal suspension in 10 mM HEPES (2 mM CaCl2, pH 7.4) was first mixed with 30 μL of serum from healthy or injured rats. Either immediately after mixing or after 24 h of incubation, 20 μL of the mixture was added to 480 μL of HEPES buffer and fluorescent measurements were taken. Fluorescent intensity measurements were acquired using a single excitation at 460 nm. The fluorescent emission was collected from 490 to 580 nm. At the end of the experiment, total NBD fluorescence was determined by the addition of Triton X-100. The fluorescence intensity was then normalized relative to the intensity of the Triton-treated sample at 540 nm.
Instrumentation.
Fluorescence spectra measurements were made on a SPEX FluoroMax-3 spectrofluorometer (Horiba Jobin Yvon). DLS measurements were performed on a Zetasizer Nano (Malvern Instruments). The scattering angle was held constant at 90°. Transmission electron microscopy (JEM-1010) was used to characterize the size and shape of the small hydrophobic SPION and SPION-loaded phospholipid micelles. T2 relaxation times were determined using a Bruker mq60 MR relaxometer operating at 1.41 T (60 MHz). The Fe concentration in samples was determined by ICP-OES analysis using a Genesis ICPOES (Spectro Analytical Instruments GMBH; Kleve, Germany).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the National Institutes of Health R01NS100892 (Z.C.), the Cervical Spine Research Society, Catherine Sharpe Foundation, and the PENN ITMAT-CT3N Pilot Project.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsnano.0c00999.
Additional information (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).DiBonaventura MD; Sadosky A; Concialdi K; Hopps M; Kudel I; Parsons B; Cappelleri JC; Hlavacek P; Alexander AH; Stacey BR; Markman JD; Farrar JT The Prevalence of Probable Neuropathic Pain in the Us: Results from a Multimodal General-Population Health Survey. J. Pain Res 2017, 10, 2525–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Scholz J; Woolf CJ The Neuropathic Pain Triad: Neurons. Nat. Neurosci 2007, 10, 1361–1368. [DOI] [PubMed] [Google Scholar]
- (3).Moalem G; Tracey DJ Immune and Inflammatory Mechanisms in Neuropathic Pain. Brain Res. Rev 2006, 51, 240–264. [DOI] [PubMed] [Google Scholar]
- (4).Harden N; Cohen M Unmet Needs in the Management of Neuropathic Pain. J. Pain Symptom Manage 2003, 25, S12–17. [DOI] [PubMed] [Google Scholar]
- (5).Radhakrishnan K; Litchy WJ; O’Fallon WM; Kurland LT Epidemiology of Cervical Radiculopathy. A Population-Based Study from Rochester, Minnesota, 1976 through 1990. Brain 1994, 117, 325–335. [DOI] [PubMed] [Google Scholar]
- (6).Waters TR National Efforts to Identify Research Issues Related to Prevention of Work-Related Musculoskeletal Disorders. J. Electromyogr. Kinesiol 2004, 14, 7–12. [DOI] [PubMed] [Google Scholar]
- (7).Cote P; Cassidy JD; Carroll LJ; Kristman V The Annual Incidence and Course of Neck Pain in the General Population: A Population-Based Cohort Study. Pain 2004, 112, 267–273. [DOI] [PubMed] [Google Scholar]
- (8).Gilron I; Baron R; Jensen T Neuropathic Pain: Principles of Diagnosis and Treatment. Mayo Clin. Proc 2015, 90, 532–545. [DOI] [PubMed] [Google Scholar]
- (9).Henschke N; Kamper SJ; Maher CG The Epidemiology and Economic Consequences of Pain. Mayo Clin. Proc 2015, 90, 139–147. [DOI] [PubMed] [Google Scholar]
- (10).Chizh BA; Headley PM Nmda Antagonists and Neuropathic Pain - Multiple Drug Targets and Multiple Uses. Curr. Pharm. Des 2005, 11, 2977–2994. [DOI] [PubMed] [Google Scholar]
- (11).McNicol ED; Midbari A; Eisenberg E Opioids for Neuropathic Pain. Cochrane Database Syst. Rev 2013, 8, Cd006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Moore RA; Chi CC; Wiffen PJ; Derry S; Rice AS Oral Nonsteroidal Anti-Inflammatory Drugs for Neuropathic Pain. Cochrane Database Syst. Rev 2015, 10, Cd010902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Dworkin RH; O’Connor AB; Audette J; Baron R; Gourlay GK; Haanpaa ML; Kent JL; Krane EJ; Lebel AA; Levy RM; Mackey SC; Mayer J; Miaskowski C; Raja SN; Rice AS; Schmader KE; Stacey B; Stanos S; Treede RD; Turk DC; et al. Recommendations for the Pharmacological Management of Neuropathic Pain: An Overview and Literature Update. Mayo Clin. Proc 2010, 85, S3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Trbovich M; Yang H Capsaicin 8% Patch for Central and Peripheral Neuropathic Pain of Persons with Incomplete Spinal Cord Injury: Two Case Reports. Am. J. Phys. Med. Rehabil 2015, 94, e66–72. [DOI] [PubMed] [Google Scholar]
- (15).Rothman SM; Winkelstein BA Chemical and Mechanical Nerve Root Insults Induce Differential Behavioral Sensitivity and Glial Activation That Are Enhanced in Combination. Brain Res. 2007, 1181, 30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Kelly J. D. t.; Aliquo D; Sitler MR; Odgers C; Moyer RA Association of Burners with Cervical Canal and Foraminal Stenosis. Am. J. Sports Med 2000, 28, 214–217. [DOI] [PubMed] [Google Scholar]
- (17).Thorek DL; Weisshaar CL; Czupryna JC; Winkelstein BA; Tsourkas A Superparamagnetic Iron Oxide-Enhanced Magnetic Resonance Imaging of Neuroinflammation in a Rat Model of Radicular Pain. Mol. Imaging 2011, 10, 206–214. [PubMed] [Google Scholar]
- (18).Znaor L; Lovric S; Hogan Q; Sapunar D Association of Neural Inflammation with Hyperalgesia Following Spinal Nerve Ligation. Croat. Med. J 2007, 48, 35–42. [PMC free article] [PubMed] [Google Scholar]
- (19).Hashizume H; DeLeo JA; Colburn RW; Weinstein JN Spinal Glial Activation and Cytokine Expression after Lumbar Root Injury in the Rat. Spine (Philadelphia) 2000, 25, 1206–1217. [DOI] [PubMed] [Google Scholar]
- (20).Winkelstein BA; DeLeo JA Nerve Root Injury Severity Differentially Modulates Spinal Glial Activation in a Rat Lumbar Radiculopathy Model: Considerations for Persistent Pain. Brain Res. 2002, 956, 294–301. [DOI] [PubMed] [Google Scholar]
- (21).DeLeo JA; Yezierski RP The Role of Neuroinflammation and Neuroimmune Activation in Persistent Pain. Pain 2001, 90, 1–6. [DOI] [PubMed] [Google Scholar]
- (22).Sweitzer SM; Hickey WF; Rutkowski MD; Pahl JL; DeLeo JA Focal Peripheral Nerve Injury Induces Leukocyte Trafficking into the Central Nervous System: Potential Relationship to Neuropathic Pain. Pain 2002, 100, 163–170. [DOI] [PubMed] [Google Scholar]
- (23).Watkins LR; Milligan ED; Maier SF Spinal Cord Glia: New Players in Pain. Pain 2001, 93, 201–205. [DOI] [PubMed] [Google Scholar]
- (24).Rutkowski MD; Pahl JL; Sweitzer S; van Rooijen N; DeLeo JA Limited Role of Macrophages in Generation of Nerve Injury-Induced Mechanical Allodynia. Physiol. Behav 2000, 71, 225–235. [DOI] [PubMed] [Google Scholar]
- (25).Rutkowski MD; Winkelstein BA; Hickey WF; Pahl JL; DeLeo JA Lumbar Nerve Root Injury Induces Central Nervous System Neuroimmune Activation and Neuroinflammation in the Rat: Relationship to Painful Radiculopathy. Spine 2002, 27, 1604–1613. [DOI] [PubMed] [Google Scholar]
- (26).Winkelstein BA; Rutkowski MD; Sweitzer SM; Pahl JL; DeLeo JA Nerve Injury Proximal or Distal to the Drg Induces Similar Spinal Glial Activation and Selective Cytokine Expression but Differential Behavioral Responses to Pharmacologic Treatment. J. Comp. Neurol 2001, 439, 127–139. [PubMed] [Google Scholar]
- (27).Watkins LR; Wiertelak EP; Goehler LE; Smith KP; Martin D; Maier SF Characterization of Cytokine-Induced Hyperalgesia. Brain Res. 1994, 654, 15–26. [DOI] [PubMed] [Google Scholar]
- (28).Latremoliere A; Woolf CJ Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J. Pain 2009, 10, 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Zhao Z; Liu N; Huang J; Lu PH; Xu XM Inhibition of Cpla2 Activation by Ginkgo Biloba Extract Protects Spinal Cord Neurons from Glutamate Excitotoxicity and Oxidative Stress-Induced Cell Death. J. Neurochem 2011, 116, 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Toborek M; Malecki A; Garrido R; Mattson MP; Hennig B; Young B Arachidonic Acid-Induced Oxidative Injury to Cultured Spinal Cord Neurons. J. Neurochem 1999, 73, 684–692. [DOI] [PubMed] [Google Scholar]
- (31).Miyamoto H; Saura R; Harada T; Doita M; Mizuno K The Role of Cyclooxygenase-2 and Inflammatory Cytokines in Pain Induction of Herniated Lumbar Intervertebral Disc. Kobe J. Med. Sci 2000, 46, 13–28. [PubMed] [Google Scholar]
- (32).Carabaza A; Cabre F; Garcia AM; Gomez M; Sanchez J; Mauleon D; Carganico G Inhibition of Phospholipase A2 Purified from Human Herniated Disc. Biochem. Pharmacol 1993, 45, 783–786. [DOI] [PubMed] [Google Scholar]
- (33).Biyani A; Andersson GB Low Back Pain: Pathophysiology and Management. J. Am. Acad. Orthop. Surg 2004, 12, 106–115. [DOI] [PubMed] [Google Scholar]
- (34).Franson RC; Saal JS; Saal JA Human Disc Phospholipase A2 Is Inflammatory. Spine (Philadelphia) 1992, 17, S129–132. [DOI] [PubMed] [Google Scholar]
- (35).Piperno M; Hellio le Graverand MP; Reboul P; Mathieu P; Tron AM; Perrin G; Peschard MJ; Richard M; Vignon E Phospholipase A2 Activity in Herniated Lumbar Discs. Clinical Correlations and Inhibition by Piroxicam. Spine (Philadelphia) 1997, 22, 2061–2065. [DOI] [PubMed] [Google Scholar]
- (36).Kawakami M; Tamaki T; Hayashi N; Hashizume H; Nishi H Possible Mechanism of Painful Radiculopathy in Lumbar Disc Herniation. Clin. Orthop. Relat. Res 1998, 351, 241–251. [PubMed] [Google Scholar]
- (37).Kishimoto K; Matsumura K; Kataoka Y; Morii H; Watanabe Y Localization of Cytosolic Phospholipase A2Messenger Rna Mainly in Neurons in the Rat Brain. Neuroscience 1999, 92, 1061–1077. [DOI] [PubMed] [Google Scholar]
- (38).Ong WY; Horrocks LA; Farooqui AA Immunocy-tochemical Localization of Cpla2 in Rat and Monkey Spinal Cord. J. Mol. Neurosci 1999, 12, 123–130. [DOI] [PubMed] [Google Scholar]
- (39).Murakami M; Nakatani Y; Atsumi G; Inoue K; Kudo I Regulatory Functions of Phospholipase A2. Crit. Rev. Immunol 1997, 17, 225–283. [DOI] [PubMed] [Google Scholar]
- (40).van Rossum GS; Drummen GP; Verkleij AJ; Post JA; Boonstra J Activation of Cytosolic Phospholipase A2 in Her14 Fibroblasts by Hydrogen Peroxide: A P42/44(Mapk)-Dependent and Phosphorylation-Independent Mechanism. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 2004, 1636, 183–195. [DOI] [PubMed] [Google Scholar]
- (41).Klein J Membrane Breakdown in Acute and Chronic Neurodegeneration: Focus on Choline-Containing Phospholipids. J. Neural Transm 2000, 107, 1027–1063. [DOI] [PubMed] [Google Scholar]
- (42).Sun GY; Xu JF; Jensen MD; Simonyi A Phospholipase a(2) in the Central Nervous System: Implications for Neuro-degenerative Diseases. J. Lipid Res 2004, 45, 205–213. [DOI] [PubMed] [Google Scholar]
- (43).Tang ZY; Shu B; Cui XJ; Zhou CJ; Shi Q; Holz J; Wang YJ Changes of Cervical Dorsal Root Ganglia Induced by Compression Injury and Decompression Procedure: A Novel Rat Model of Cervical Radiculoneuropathy. J. Neurotrauma 2009, 26, 289–295. [DOI] [PubMed] [Google Scholar]
- (44).Osikowicz M; Mika J; Przewlocka B The Glutamatergic System as a Target for Neuropathic Pain Relief. Exp. Physiol 2013, 98, 372–384. [DOI] [PubMed] [Google Scholar]
- (45).Wilczewska AZ; Niemirowicz K; Markiewicz KH; Car H Nanoparticles as Drug Delivery Systems. Pharmacol. Rep 2012, 64, 1020–1037. [DOI] [PubMed] [Google Scholar]
- (46).Xin Y; Huang M; Guo WW; Huang Q; Zhang LZ; Jiang G Nano-Based Delivery of RNAi in Cancer Therapy. Mol. Cancer 2017, 16, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Cheng Z; Al Zaki A; Hui JZ; Muzykantov VR; Tsourkas A Multifunctional Nanoparticles: Cost Versus Benefit of Adding Targeting and Imaging Capabilities. Science 2012, 338, 903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Finnerup NB; Attal N; Haroutounian S; McNicol E; Baron R; Dworkin RH; Gilron I; Haanpaa M; Hansson P; Jensen TS; Kamerman PR; Lund K; Moore A; Raja SN; Rice AS; Rowbotham M; Sena E; Siddall P; Smith BH; Wallace M Pharmacotherapy for Neuropathic Pain in Adults: A Systematic Review and Meta-Analysis. Lancet Neurol. 2015, 14, 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Gao Q; Yan L; Chiorazzo M; Delikatny EJ; Tsourkas A; Cheng Z Pla2-Responsive and Spio-Loaded Phospholipid Micelles. Chem. Commun. (Cambridge, U. K.) 2015, 51, 12313–12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Kartha S; Weisshaar CL; Philips BH; Winkelstein BA Pre-Treatment with Meloxicam Prevents the Spinal Inflammation and Oxidative Stress in Drg Neurons That Accompany Painful Cervical Radiculopathy. Neuroscience 2018, 388, 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Ma L; Uchida H; Nagai J; Inoue M; Aoki J; Ueda H Evidence for De Novo Synthesis of Lysophosphatidic Acid in the Spinal Cord through Phospholipase a(2) and Autotaxin in Nerve Injury-Induced Neuropathic Pain. J. Pharmacol. Exp. Ther 2010, 333, 540–546. [DOI] [PubMed] [Google Scholar]
- (52).Sung B; Wang S; Zhou B; Lim G; Yang L; Zeng Q; Lim JA; Wang JD; Kang JX; Mao J Altered Spinal Arachidonic Acid Turnover after Peripheral Nerve Injury Regulates Regional Glutamate Concentration and Neuropathic Pain Behaviors in Rats. Pain 2007, 131, 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Wang Y; Zhang XA; Guo QL; Zou WY; Huang CS; Yan JQ Cyclooxygenase Inhibitors Suppress the Expression of P2x(3) Receptors in the Drg and Attenuate Hyperalgesia Following Chronic Constriction Injury in Rats. Neurosci. Lett 2010, 478, 77–81. [DOI] [PubMed] [Google Scholar]
- (54).Schafers M; Marziniak M; Sorkin LS; Yaksh TL; Sommer C Cyclooxygenase Inhibition in Nerve-Injury- and Tnf-Induced Hyperalgesia in the Rat. Exp. Neurol 2004, 185, 160–168. [DOI] [PubMed] [Google Scholar]
- (55).Khan M; Shunmugavel A; Dhammu TS; Matsuda F; Singh AK; Singh I Oral Administration of Cytosolic Pla2 Inhibitor Arachidonyl Trifluoromethyl Ketone Ameliorates Cauda Equina Compression Injury in Rats. J. Neuroinflammation 2015, 12, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Pardridge WM Drug Transport across the Blood-Brain Barrier. J. Cereb. Blood Flow Metab 2012, 32, 1959–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Hook MA; Moreno G; Woller S; Puga D; Hoy K; Balden R; Grau JW Intrathecal Morphine Attenuates Recovery of Function after a Spinal Cord Injury. y 2009, 26, 741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Rothman SM; Winkelstein BA Cytokine Antagonism Reduces Pain and Modulates Spinal Astrocytic Reactivity after Cervical Nerve Root Compression. Ann. Biomed. Eng 2010, 38, 2563–2576. [DOI] [PubMed] [Google Scholar]
- (59).Titsworth WL; Cheng XX; Ke Y; Deng LX; Burckardt KA; Pendleton C; Liu NK; Shao H; Cao QL; Xu XM Differential Expression of Spla(2) Following Spinal Cord Injury and a Functional Role for Spla(2)-Iia in Mediating Oligodendrocyte Death. Glia 2009, 57, 1521–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Chacur M; Milligan ED; Sloan EM; Wieseler-Frank J; Barrientos RM; Martin D; Poole S; Lomonte B; Gutierrez JM; Maier SF; Cury Y; Watkins LR Snake Venom Phospholipase A2s (Asp49 and Lys49) Induce Mechanical Allodynia Upon Peri-Sciatic Administration: Involvement of Spinal Cord Glia, Proin-flammatory Cytokines and Nitric Oxide. Pain 2004, 108, 180–191. [DOI] [PubMed] [Google Scholar]
- (61).Ma WY; Du W; Eisenach JC Role for Both Spinal Cord Cox-1 and Cox-2 in Maintenance of Mechanical Hypersensitivity Following Peripheral Nerve Injury. Brain Res. 2002, 937, 94–99. [DOI] [PubMed] [Google Scholar]
- (62).Galluzzi KE Management of Neuropathic Pain. J. Am. Osteopath. Assoc 2005, 105, S12–19. [PubMed] [Google Scholar]
- (63).Han WK; Sapirstein A; Hung CC; Alessandrini A; Bonventre JV Cross-Talk between Cytosolic Phospholipase a(2)Alpha (Cpla(2 Alpha)) and Secretory Phospholipase a(2) (Spla(2)) in Hydrogen Peroxide-Induced Arachidonic Acid Release in Murine Mesangial Cells - Spla(2) Regulates Cpla(2)Alpha Activity That Is Responsible for Arachidonic Acid Release. J. Biol. Chem 2003, 278, 24153–24163. [DOI] [PubMed] [Google Scholar]
- (64).Smith JR; Galie PA; Slochower DR; Weisshaar CL; Janmey PA; Winkelstein BA Salmon-Derived Thrombin Inhibits Development of Chronic Pain through an Endothelial Barrier Protective Mechanism Dependent on Apc. Biomaterials 2016, 80, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Kolko M; de Turco EB; Diemer NH; Bazan NG Secretory Phospholipase A2-Mediated Neuronal Cell Death Involves Glutamate Ionotropic Receptors. NeuroReport 2002, 13, 1963–1966. [DOI] [PubMed] [Google Scholar]
- (66).DeCoster MA; Lambeau G; Lazdunski M; Bazan NG Secreted Phospholipase A2 Potentiates Glutamate-Induced Calcium Increase and Cell Death in Primary Neuronal Cultures. J. Neurosci. Res 2002, 67, 634–645. [DOI] [PubMed] [Google Scholar]
- (67).D’Mello R; Dickenson AH Spinal Cord Mechanisms of Pain. Br. J. Anaesth 2008, 101, 8–16. [DOI] [PubMed] [Google Scholar]
- (68).Stoll G; Bendszus M New Approaches to Neuroimaging of Central Nervous System Inflammation. Curr. Opin. Neurol 2010, 23, 282–286. [DOI] [PubMed] [Google Scholar]
- (69).Svensson CI; Lucas KK; Hua XY; Powell HC; Dennis EA; Yaksh TL Spinal Phospholipase a(2) in Inflammatory Hyperalgesia: Role of the Small, Secretory Phospholipase a(2). Neuroscience 2005, 133, 543–553. [DOI] [PubMed] [Google Scholar]
- (70).Chan FK; Moriwaki K; De Rosa MJ Detection of Necrosis by Release of Lactate Dehydrogenase Activity. Methods Mol. Biol 2013, 979, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Prabhu BM; Ali SF; Murdock RC; Hussain SM; Srivatsan M Copper Nanoparticles Exert Size and Concentration Dependent Toxicity on Somatosensory Neurons of Rat. Nano-toxicology 2010, 4, 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Chaplan SR; Bach FW; Pogrel JW; Chung JM; Yaksh TL Quantitative Assessment of Tactile Allodynia in the Rat Paw. J. Neurosci. Methods 1994, 53, 55–63. [DOI] [PubMed] [Google Scholar]
- (73).Nicholson KJ; Guarino BB; Winkelstein BA Transient Nerve Root Compression Load and Duration Differentially Mediate Behavioral Sensitivity and Associated Spinal Astrocyte Activation and Mglur5 Expression. Neuroscience 2012, 209, 187–195. [DOI] [PubMed] [Google Scholar]
- (74).Zeeman ME; Kartha S; Winkelstein BA Whole-Body Vibration Induces Pain and Lumbar Spinal Inflammation Responses in the Rat That Vary with the Vibration Profile. J. Orthop. Res 2016, 34, 1439–1446. [DOI] [PubMed] [Google Scholar]
- (75).Kartha S; Zeeman ME; Baig HA; Guarino BB; Winkelstein BA Upregulation of BDNF and NGF in Cervical Intervertebral Discs Exposed to Painful Whole-Body Vibration. Spine (Philadelphia) 2014, 39, 1542–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


