Abstract
Background:
Non-muscle-invasive bladder cancer (NMIBC) disproportionately affects older adults who often have co-existing chronic conditions such as metabolic syndrome (MetS). Although prior research suggests that MetS is a risk factor for NMIBC, limited data exist on whether MetS is associated with NMIBC recurrence. Our objective was to evaluate the association between MetS and recurrence in older adults treated for NMIBC.
Methods:
We identified 1485 older (age ≥60 years) NMIBC patients (AJCC Stage ≤1) from two community-based health systems. Using data from the health systems’ electronic medical record, MetS was defined as the presence of three of the following: diagnosis codes indicating hypertension, hyperlipidemia, diabetes, or body mass index >30. Follow up time was determined by date of the last follow up in the tumor registry and censored at 10 years. Cox proportional hazards regression of time to recurrence that accounts for the competing risk of death included adjustment for age, sex, smoking status, health system, NMIBC stage/grade, tumor size, and number of specimens with cancer.
Results:
Overall, 341 patients (23%) met MetS criteria. Median follow up was 5.9 years and 582 patients (39.2%) died. Patients with MetS were more frequently male (84.2%), and mostly current/former smokers (82.6%). By 10 years, 34.1% of the cohort had experienced a recurrence. After accounting for the competing risk of death, there was no association between MetS and time to recurrence (adjusted hazard ratio, 0.88, 95% confidence interval 0.70–1.11, p=0.28). Patients without MetS had more 0a/low grade recurrences (49.1% versus 41.4%), though differences were not significant.
Conclusion:
We found no association between MetS and risk of NMIBC recurrence in this large, multi-site cohort of older adults with NMIBC. In order to design personalized care for older NMIBC patients, future research is needed to evaluate associations between common chronic conditions and a variety of oncologic outcomes.
Keywords: bladder cancer, geriatric oncology, outcomes, metabolic syndrome, multiple chronic conditions
1. INTRODUCTION
Two-thirds of new cancers are diagnosed in older adults over the age of 65 years.1 Older cancer patients often have two or more coexisting chronic conditions, also known as multiple chronic conditions (MCC), which may impact cancer diagnosis and management. For example, cancer treatments may exacerbate chronic conditions, just as those conditions and corresponding medications may positively or negatively impact cancer biology, may increase treatment toxicity, and may shorten life expectancy.2, 3
The longitudinal interplay among aging, MCC, and cancer is of particular importance in bladder cancer, the sixth most common cancer in the United States with the highest median age at diagnosis of all cancer sites (73 years).4, 5 Over three-quarters of bladder cancers are diagnosed in the early stages as non-muscle-invasive bladder cancer (NMIBC). NMIBC has a low risk of cancer-specific death and a high rate of recurrence (30–70%).6 Advanced age and smoking are major risk factors for bladder cancer, and also for metabolic syndrome (MetS) and related chronic conditions such as hypertension, hyperlipidemia, diabetes, and obesity.7
Similar to bladder cancer, MetS also disproportionately affects older adults with the highest prevalence in those aged 60 years and older.8 Over 40 million American adults have the syndrome. The five components of MetS are abdominal obesity, elevated blood pressure, impaired blood sugar, elevated triglycerides, and low high-density lipoprotein (HDL).8, 9 This constellation of traits is closely associated with an increased risk of cardiovascular disease.
Prior studies suggest a link between MetS and the development of bladder cancer; however limited data exist on whether MetS is associated with recurrence of NMIBC.10, 11 Given the high recurrence rate of NMIBC, understanding the association between MetS and NMIBC recurrence may reduce the need for frequent surgical treatments in this growing population of older, medically complex patients by informing risk-based interventions in the future. We conducted a retrospective cohort study using existing data from two large community-based health systems with the objective of determining the association between MetS and NMIBC recurrence in older adults. In order to design personalized care that incorporates an individual’s profile of medical complexity, it is important to elucidate the relationships between MCC and cancer in older adults.
2. PATIENTS AND METHODS
2.1. Data/Study Population
We previously described the study cohort from two community-based health systems, Geisinger and Kaiser Permanente Northwest (KPNW).12 After Institutional Review Board approval, we identified 1835 older adults (age ≥60 years at diagnosis) who were diagnosed with NMIBC (AJCC stage ≤ 1) between 2003 to 2015 from the tumor registry. We excluded 12 patients with non-urothelial histology and 23 patients with missing follow-up data. As the current study focuses on older adults who received treatment for NMIBC, we excluded 315 patients who did not have Current Procedural Terminology (CPT) procedure codes for transurethral resection of bladder tumor (52204, 52224, 52235, and 52240) and/or intravesical instillation (90586 and 51720) within 6 months of NMIBC diagnosis. The final cohort for analysis included 1485 patients: 695 from Geisinger and 790 from KPNW.
2.2. Definition of Metabolic Syndrome (MetS)
We separated the cohort into two groups: individuals who had MetS and those who did not. We defined MetS as the presence of three of the following: coded diagnoses of hypertension, hyperlipidemia, diabetes, or body mass index (BMI) ≥30 kg/m2. To calculate BMI, we selected height and weight measurements closest to and within 2 years of NMIBC diagnosis date. If height and/or weight data was missing, individuals with International Classification of Diseases, Ninth Edition (ICD-9) codes for obesity (278.00, 278.01, 278.02) attached to three or more outpatient encounters or one inpatient encounter were considered obese. If both height/weight and ICD-9 codes were missing, individuals were categorized as non-obese. To determine baseline hypertension, hyperlipidemia, and diabetes, we used a combination of Agency for Healthcare Research and Quality’s Chronic Condition Indicator and Clinical Classifications Software tools applied to all ICD-9 codes attached to clinical encounters prior to NMIBC diagnosis using methods described in our previous work (Supplemental Table 1).7
2.3. Other Covariates
In multivariable analyses, we controlled for age at diagnosis, sex, health system (Geisinger or KPNW), smoking status (current/former or never), initial tumor size (<3cm or ≥3cm), number of specimens positive for cancer at initial diagnosis (1 or ≥2), and NMIBC stage and grade. For tumor size, we reported the aggregate size if multiple tumors were present. As NMIBC management and prognosis are determined by cancer stage and histologic grade, we created a categorical variable combining American Joint Committee on Cancer (AJCC) stage and grade as follows: 0a/low grade, 0a/high grade, 0is/high grade, and 1/high grade.
2.4. Outcomes
The primary outcome variable was time to NMIBC recurrence within 10 years of diagnosis (recurrence-free survival). Two urologic oncologists (TG and MEN) reviewed all pathology reports from Geisinger and KPNW, respectively, to assess initial stage/grade, tumor size, and number of tumors, as well as to confirm NMIBC recurrence and recurrence stage/grade.
Recurrence-free survival time was calculated as the time from date of initial NMIBC diagnosis to recurrence of NMIBC, death, or last tumor registry follow-up date. Individuals were censored at the time of recurrence, and those without recurrence were censored at last follow up date as recorded in the tumor registry or at the end of the 10 year observation period. Individuals without recurrences who died during the observation period were censored at death, but not counted as a recurrence based on competing risks analysis using the method of Fine and Gray.13
2.4. Statistical Analysis
Baseline characteristics were compared by MetS status. Continuous variables were compared using the Student t test, and categorical variables were compared using the chi-squared test. Reverse Kaplan-Meier methods censoring for death were used to determine median follow-up time for the cohort. Follow-up time was defined by date of initial NMIBC diagnosis to date of last follow-up or death listed in the tumor registry. Due to the older age of the cohort and high proportion of co-existing chronic conditions in the NMIBC population12, we modeled the association between MetS status and recurrence-free survival using multivariable Cox proportional hazards models adjusting for competing risks.13 We first modeled the association between MetS and recurrence-free survival adjusting for covariates that were significant on univariate analysis: sex and smoking status. The final model was adjusted for age, sex, health system, smoking status, NMIBC stage/grade, initial tumor size, and number of specimens with cancer. The covariates in the final model were selected a priori due to known or potential associations with the outcome. The multivariable analysis excluded 135 patients with missing data. Analyses were conducted in SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
3.0. RESULTS
Of the total cohort, 341 (23%) met criteria for MetS. Mean age was 73.5 years, 78.8% were male, and the majority were white (97.3%) which is consistent with national demographic trends for bladder cancer (Table 1).5 Patients with MetS were more frequently male (84.2%), and were mostly current/former smokers (82.6%). Figure 1 displays the distribution of MetS conditions stratified by MetS group. Consistent with national trends among older adults with cancer, hypertension and hyperlipidemia were the most frequent MetS components in both groups.14 There were no significant differences in age, race/ethnicity, health system, initial tumor size, number of specimens with cancer, and stage/grade distribution.
Table 1:
Baseline Characteristics Stratified by Metabolic Syndrome Status
| All (n = 1485) | MetS: No (n = 1144) | MetS: Yes (n = 341) | p value* | |
|---|---|---|---|---|
| Age (mean, SD) | 73.5 (8.17) | 73.6 (8.37) | 73.2 (7.44) | 0.33 |
| Sex, Male, N (%) | 1170 (78.8%) | 883 (77.2%) | 271 (84.2%) | 0.01 |
| Race/Ethnicity, White, N (%) | 1445 (97.3%) | 1113 (97.3%) | 332 (97.4%) | 1.00 |
| Health System, N (%) | ||||
| Geisinger | 695 (46.8%) | 538 (47.0%) | 157 (46.0%) | 0.76 |
| KPNW | 790 (53.2%) | 606 (53.0%) | 184 (54.0%) | |
| Smoking Status, N (%) | ||||
| Never | 364 (24.8%) | 305 (27.1%) | 59 (17.4%) | <0.01 |
| Current/Former | 1103 (75.2%) | 822 (72.9%) | 281 (82.6%) | |
| Unknown | 18 | 17 | 1 | |
| Diabetes, N (%) | 357 (24.0%) | 116 (10.1%) | 241 (70.7%) | |
| Hyperlipidemia, N (%) | 633 (42.6%) | 337 (29.5%) | 296 (86.8%) | |
| Hypertension, N (%) | 798 (53.7%) | 475 (41.5%) | 323 (94.7%) | |
| Obesity, N (%) | 518 (34.9%) | 261 (22.8%) | 257 (75.4%) | |
| Stage/Grade, N (%) | ||||
| 0A Low Grade | 705 (47.5%) | 554 (49.1%) | 151 (44.6%) | 0.39 |
| 0A High Grade | 296 (19.9%) | 219 (19.4%) | 77 (22.7%) | |
| 0is High Grade | 76 (5.1%) | 60 (5.3%) | 16 (4.7%) | |
| 1 High Grade | 391 (26.3%) | 296 (26.2%) | 95 (28.0%) | |
| Unknown | 17 | 15 | 2 | |
| Tumor Size, N (%) | ||||
| <3cm | 989 (71.5%) | 748 (70.6%) | 241 (74.6%) | 0.18 |
| ≥3cm | 394 (28.5%) | 312 (29.4%) | 82 (25.4%) | |
| Unknown | 102 | 84 | 18 | |
| Number of Specimens Positive for Cancer, N (%) | ||||
| 1 | 1104 (78.0%) | 844 (77.6%) | 260 (79.0%) | 0.65 |
| ≥2 | 312 (22.0%) | 243 (22.4%) | 69 (21.0%) | |
| Unknown | 69 | 57 | 12 | |
| Recurrence, N (%) | 507 (34.1%) | 403 (35.2%) | 104 (30.5%) | 0.12 |
Comparing MetS No versus MetS Yes
Abbreviations: MetS, metabolic syndrome; SD, standard deviation; KPNW, Kaiser Permanente Northwest
Figure 1:
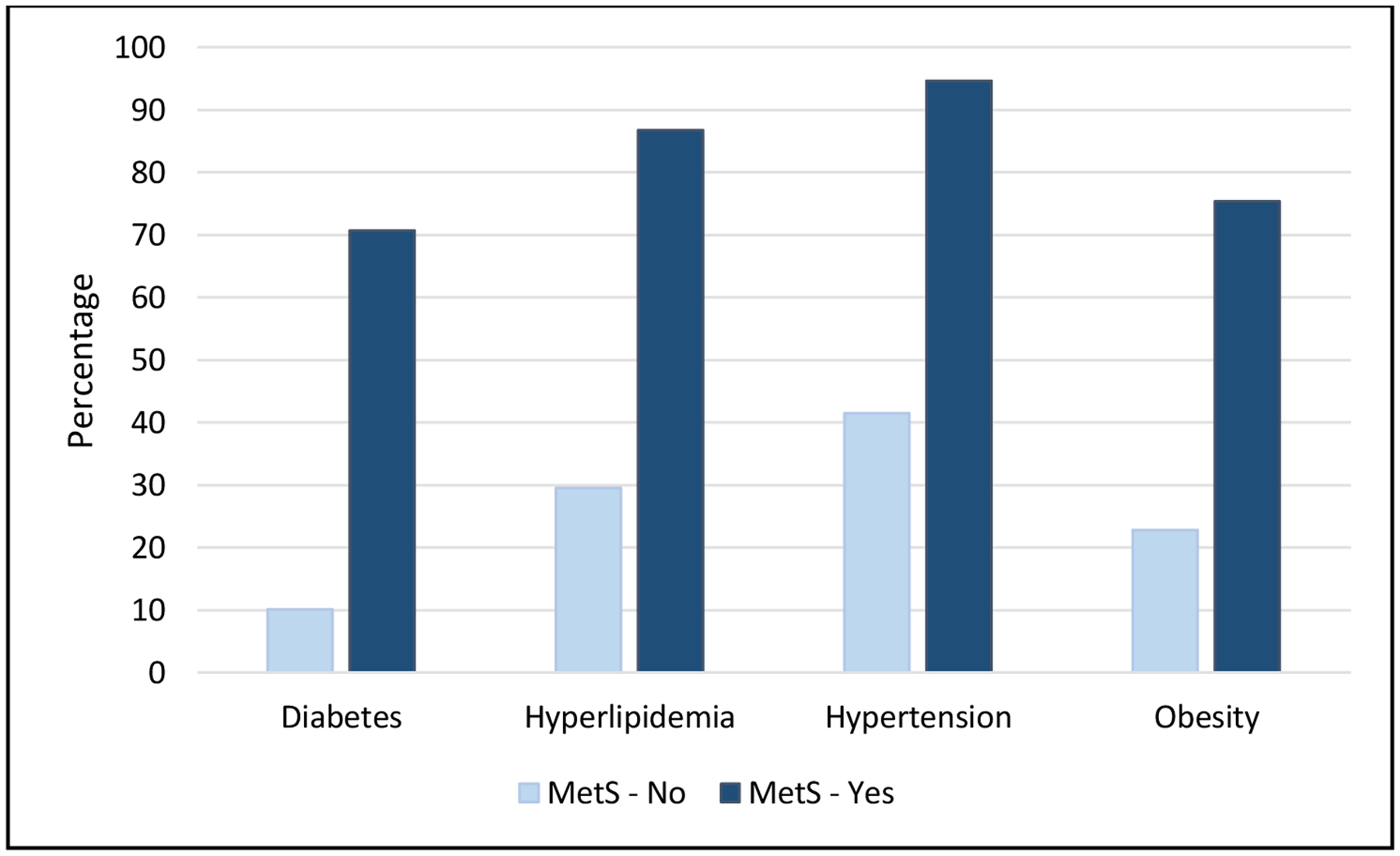
Frequencies of Individual Metabolic Syndrome (MetS) Traits Stratified by MetS Status
Based on reverse Kaplan-Meier methods, median follow-up time for the whole cohort was 5.9 years (95% confidence interval [CI] 5.5–6.3 years) during which 582 patients (39.2%) died. Median follow-up time was 4.8 years for those with MetS (95% CI 3.7–5.4) versus 6.3 years for those without MetS (95% CI 5.9–6.9). Similar proportions of patients died in the MetS group (39%) as compared to those without MetS (39.3%) during the follow-up time.
At 10 years of follow-up, 34.1% of the total cohort had a recurrence. A smaller proportion of patients in the MetS group had a recurrence compared to the non-MetS group (30.5% versus 35.2%). Median time to recurrence was slightly longer for those without MetS (3.8 years versus 3 years). The unadjusted cumulative incidence function estimate for recurrence-free survival was slightly higher at each time point in the group without MetS (Supplemental Table 2); however, when compared for each group, there was no significant difference in recurrence-free survival over 10 years (Wald chi-square p value=0.31, Figure 2).
Figure 2:
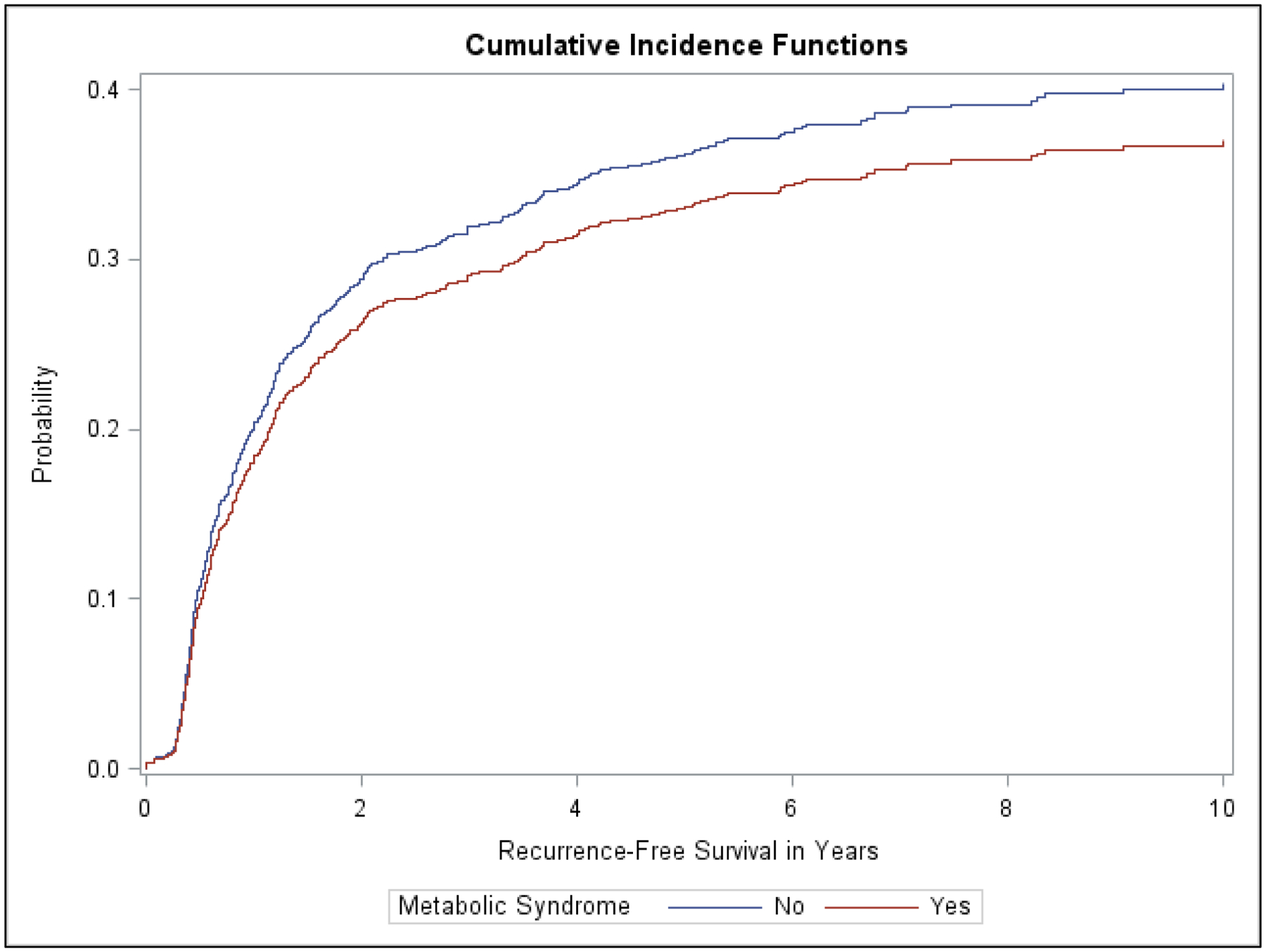
Cumulative Incidence Function Curves for Recurrence Free Survival Stratified by Metabolic Syndrome Status
In the preliminary Cox proportional hazards model accounting for competing risks, we adjusted for sex and smoking status which were significant on univariate analysis. There was no association between MetS and recurrence free survival (adjusted hazard ratio [HR] 0.87, 95% CI 0.70–1.08, p=0.21, Supplemental Table 3). In the final Cox proportional hazards model accounting for competing risks, there was no association between MetS and NMIBC recurrence within ten years (adjusted HR 0.88, 95% CI 0.70–1.11, p=0.28, Table 2). Initial tumor size (adjusted HR 1.46, 95% CI 1.19–1.78) and number of specimens with cancer (adjusted HR 1.47, 95% CI 1.19–1.80) were highly associated with time to recurrence.
Table 2:
Multivariable Cox Proportional Hazards Model Adjusting for Competing Risks for the Association Between MetS and Time to Recurrence*
| Hazard Ratio | p value | |
|---|---|---|
| Age | 0.99 (0.98–1.002) | 0.12 |
| Sex (ref: Female) | 1.12 (0.89–1.41) | 0.34 |
| Smoking (ref: Never) | 1.09 (0.88–1.35) | 0.44 |
| Health System (ref: Geisinger) | 1.13 (0.93–1.36) | 0.23 |
| Stage/Grade (ref: 0A Low Grade) | ||
| 0A High Grade | 1.23 (0.97–1.57) | 0.09 |
| 0is High Grade | 1.286 (0.87–1.89) | 0.20 |
| 1 High Grade | 1.070 (0.85–1.35) | 0.57 |
| Tumor Size ≥3cm (ref: <3cm) | 1.46 (1.19–1.78) | 0.0003 |
| Number of Specimens Positive for Cancer (ref: 1) | 1.47 (1.19–1.8) | 0.0003 |
| Metabolic Syndrome (ref: No) | 0.88 (0.70–1.11) | 0.28 |
The analysis excluded 135 patients with missing data.
Abbreviations: MetS, metabolic syndrome; ref, reference
The histologic grade of recurrence varied by MetS status. Proportionally, there were more 0a/low grade recurrences in patients without MetS (49.1% versus 41.4%, Figure 3). A total of 34 recurrences were muscle-invasive disease (Stage II), which occurred more frequently in patients without MetS (7.6% versus 3.9%, Figure 3). Overall, there were no significant differences between stage/grade of recurrence by MetS status.
Figure 3:
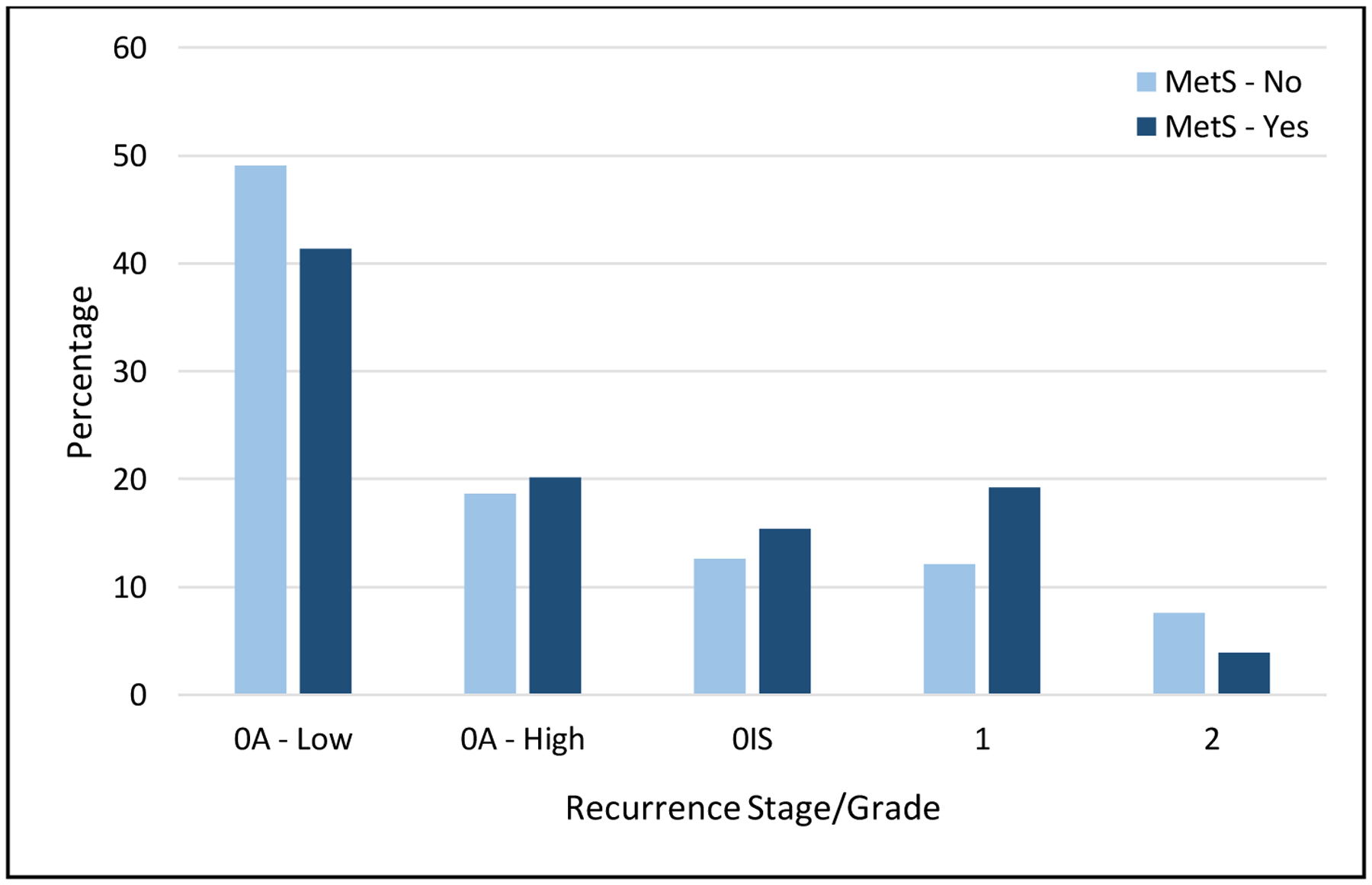
Proportions of Combined Stage/Grade at Recurrence Stratified by Metabolic Syndrome Status
4.0. DISCUSSION
In this study of 1485 older NMIBC patients from two community-based health systems, our objective was to determine whether MetS is associated with time to recurrence. Nearly one-quarter of patients in this NMIBC cohort met our criteria for MetS; however, we found that there was no association between MetS and recurrence-free survival. Prior studies were limited to examining associations between individual components of MetS and oncologic outcomes15, or have evaluated the role of MetS or individual MetS traits on the risk of developing bladder cancer.10 Limited data from small cohorts have examined the effect of combined MetS traits on oncologic outcomes in NMIBC, and, to our knowledge, no prior work has specifically studied older adults.
By 2030, cancer incidence in older adults is expected to increase by 67%.16 Older cancer patients have high rates of co-existing chronic conditions which may influence tumor biology to impact downstream oncologic outcomes.2 Hypertension, hyperlipidemia, and diabetes are the most common chronic conditions among cancer survivors and older adults, and the early stages of these conditions comprise MetS.17
While several definitions of MetS exist, the most widely used is the National Cholesterol Education Program Adult Treatment Panel III (ATP III).9 ATP III criteria were selected due to each trait’s association with cardiovascular disease and include any three of the following: abdominal obesity, elevated triglycerides, low high-density lipoprotein (HDL), elevated blood pressure, and fasting plasma glucose ≥100mg/dL. It remains unknown whether MetS traits are unified by an underlying etiology, though obesity may be a common thread.
Obesity causes downstream metabolic changes such as inflammation, higher insulin levels, insulin-like growth factor production, and changes in sex hormones, all of which have been associated with cancer.18, 19 However, obesity and MetS are complex issues in older adults. BMI tends to be less accurate secondary to physical changes that coincide with aging such as decreased height, muscle loss, and increased fat in locations that confer greater insulin resistance (e.g., visceral, subcutaneous, intramuscular, and intrahepatic fat).20 The loss of muscle mass with aging, or sarcopenia, is particularly important as it leads to insulin resistance and MetS.
Conversely, older adults are subject to the “Obesity Paradox” in which certain outcomes, such as survival, are better in obese patients as compared to normal weight or morbidly obese patients. Studies in other malignancies suggest that the Obesity Paradox is present, though data specific to older adults are limited.21–24 Several methodological issues may contribute to the Obesity Paradox in older adults including misclassification bias due to inaccuracies of BMI in older adults, detection bias due to cancer detection rates in patients with high healthcare utilization due to MCC, or reverse causality such that obese patients may have lost weight due to cancer treatment or lifestyle changes.25
Defining the complex relationships between chronic conditions such as MetS and cancer outcomes is increasingly relevant to bladder cancer for several reasons. Bladder cancer has the highest median age at diagnosis of all cancer sites (73 years).4 The association between smoking and bladder cancer coupled with older age at diagnosis leads to high rates of medical complexity with a median of eight co-existing chronic conditions.7 The incidence of bladder cancer among older adults is projected to increase by 54% by 2030, one of the largest increases of all cancer sites.16
Though NMIBC has a low risk of death; it has a high recurrence rate (30–50%).6 Understanding patient-related factors that may alter the likelihood of NMIBC recurrence is important in older adults because treatment of recurrences requires transurethral resection, a surgical procedure performed under general anesthesia. Though a seemingly minor procedure in terms of operative stress, complications of transurethral resection such as bleeding, bladder perforation, urinary incontinence, and hospitalization have long-lasting or irreversible effects on an older adult’s quality of life and functional status, and adversely affect overall survival in frail patients.26, 27 Therefore, understanding NMIBC prognosis within an individual’s chronic condition profile is a critical first step towards designing personalized care for older NMIBC patients.
To our knowledge, only one other study has evaluated the association between MetS and NMIBC recurrence; however, it was limited to a small sample of T1 high grade patients from a single institution. The authors identified 90 patients with T1 high grade NMIBC treated with transurethral resection and intravesical therapy of which 30% had MetS. In multivariable models, MetS was not associated with recurrence/progression as a combined outcome (adjusted HR 1.53, 95% CI 0.71–3.29).11 When evaluating individual MetS traits, BMI was significantly associated with time to recurrence or progression (adjusted HR 3.42, 95% CI 1.55–7.52). Similar to our study, MetS traits were defined using diagnosis codes from electronic health records.
Other studies have found that obesity may be associated with recurrence in patients with T1 high grade NMIBC. In a retrospective study of 892 patients, obesity was significantly associated with multiple outcomes including recurrence (adjusted HR 2.66, 95% CI 2.12–3.32) and progression (adjusted HR 1.49, 95% CI 1.00–2.21).28 Another study 726 NMIBC patients found no association between higher BMI and risk of recurrence (adjusted HR 1.33, 95% CI 0.94–1.89); however, current obese smokers were found to be at more than double the risk for recurrence as compared to normal weight smokers (adjusted HR 2.67, 95% CI 1.14–6.28).29
Our study has several important strengths. To our knowledge, this is the largest study examining the association between MetS and NMIBC recurrence. We had a large sample size from multiple institutions with long follow up. Our cohort was drawn from two community-based health systems which reflect real-world patients and practice patterns. We abstracted additional information on the stage and grade of recurrence which impacts the downstream management of NMIBC. In contrast to other studies, we incorporated competing risks in our time-to-event analysis. Accounting for the competing risk of death is an important consideration when studying outcomes in medically complex older adults because study subjects may die during the observation period before the end of the study or before an outcome would occur.
Our findings must be interpreted within the context of certain limitations. We utilized diagnosis codes rather than physical assessments to determine MetS. We were unable to control for medications such as metformin with suggested antitumor effects in NMIBC.30, 31 Conversely, some patients may have received thiazolidinediones such as pioglitazone for diabetes which have a suspected association with bladder cancer.32, 33 We were unable to address the role of intravesical therapy on NMIBC recurrence due to limitations of our dataset.
5.0. CONCLUSIONS
In this large, multi-institutional cohort of older NMIBC patients, we found no association between MetS and time to recurrence while accounting for the competing risk of death. Further research is needed to delineate the relationships between common chronic conditions such as MetS and oncologic outcomes to inform personalized care for older adults with NMIBC.
Supplementary Material
HIGHLIGHTS.
In 1485 older NMIBC patients from two health systems, 23% had metabolic syndrome.
Metabolic syndrome patients were mostly males and current or former smokers.
Over median follow up of 6 years, one-third of patients had a recurrence.
We found no association between metabolic syndrome and time to NMIBC recurrence.
FUNDING SOURCES:
This work was supported by the Health Care Systems Research Network (HCSRN)-Older Americans Independence Center (OAICs) AGING Initiative (R24AG045050), the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342), National Cancer Institute (1R21CA191610), National Institute on Aging (R03AG064382), and Pennsylvania Department of Health (SAP 4100070267). The funders had no involvement in study design, analysis and interpretation of the data, writing of the report, and the decision to submit this article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST:
Matthew E. Nielsen serves as a paid consultant to the American College of Physicians High Value Care Task Force and as a consultant/advisor to Grand Rounds for which he is paid via stock options. All other authors report No Conflict of Interest.
REFERENCES
- 1.DeSantis CE, Lin CC, Mariotto AB, et al. : Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 64:252–271, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Bluethmann SM, Mariotto AB, Rowland JH: Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev 25:1029–1036, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. Older Adult Oncology (Version 1.2019) https://www.nccn.org/professionals/physician_gls/pdf/senior.pdf. Accessed December 31, 2019. [Google Scholar]
- 4.American Cancer Society: Cancer Facts & Figures 2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-ancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf. Accessed December 31, 2019. [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2019. CA Cancer J Clin 69:7–34, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Chang SS, Boorjian SA, Chou R, et al. : Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol 196:1021–1029, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Garg T, Young AJ, Kost KA, et al. : Burden of Multiple Chronic Conditions among Patients with Urological Cancer. J Urol 199:543–550, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Ford ES, Giles WH, Dietz WH: Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 287:356–359, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Alberti KGMM, Eckel RH Grundy SM, et al. : Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120:1640–1645, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Häggström C, Stocks T, Rapp K, et al. : Metabolic syndrome and risk of bladder cancer: prospective cohort study in the metabolic syndrome and cancer project (Me-Can). Int J Cancer 128:1890–1898, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Lenis AT, Asanad K, Blaibel M, et al. : Association between Metabolic Syndrome and Recurrence of Nonmuscle Invasive Bladder Cancer following bacillus Calmette-Guérin Treatment. Urology Practice 5:132–138, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg T, Young AJ, O’Keeffe-Rosetti M, et al. : Association between treatment of superficial bladder cancer and 10‐year mortality in older adults with multiple chronic conditions. Cancer 124:4477–4485, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine JP, Gray RJ: A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 94:496, 1999 [Google Scholar]
- 14.Buttorff Christine, Ruder Teague, and Bauman Melissa, Multiple Chronic Conditions in the United States. Santa Monica, CA: RAND Corporation, 2017. https://www.rand.org/pubs/tools/TL221.html. Accessed December 31, 2019 [Google Scholar]
- 15.Cantiello F, Cicione A, Salonia A, et al. : Association between metabolic syndrome, obesity, diabetes mellitus and oncological outcomes of bladder cancer: A systematic review. Int J Urol 22:22–32, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Smith BD, Smith GL, Hurria A, et al. : Future of Cancer Incidence in the United States: Burdens Upon an Aging, Changing Nation. Journal of Clinical Oncology 27:2758–2765, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Roy S, Vallepu S, Barrios C, et al. : Comparison of Comorbid Conditions Between Cancer Survivors and Age-Matched Patients Without Cancer. J Clin Med Res 10:911–919, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renehan AG, Zwahlen M, Egger M: Adiposity and cancer risk: new mechanistic insights from epidemiology. Nature Publishing Group 15:484–498, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Steele CB, Thomas CC, Henley SJ, et al. : Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity - United States, 2005–2014. MMWR Morb Mortal Wkly Rep 66:1052–1058, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cetin DC, Nasr G: Obesity in the elderly: More complicated than you think. Cleveland Clinic Journal of Medicine 81:51–61, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Hakimi AA, Furberg H, Zabor EC, et al. : An Epidemiologic and Genomic Investigation Into the Obesity Paradox in Renal Cell Carcinoma. J Natl Cancer Inst 105:1862–1870, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McQuade JL, Daniel CR, Hess KR, et al. : Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. The Lancet Oncology 19:310–322, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunner AM, Sadrzadeh H, Feng Y, et al. : Association between baseline body mass index and overall survival among patients over age 60 with acute myeloid leukemia. Am J Hematol 88:642–646, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pamoukdjian F, Aparicio T, Canoui-Poitrine F, et al. : Obesity survival paradox in cancer patients: Results from the Physical Frailty in older adult cancer patients (PF-EC) study. Clinical Nutrition 38:2806–2812, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Lennon H, Sperrin M, Badrick E, et al. : The Obesity Paradox in Cancer: a Review. Curr Oncol Rep 18:56, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suskind AM, Walter LC, Jin C, et al. : Impact of frailty on complications in patients undergoing common urological procedures: a study from the American College of Surgeons National Surgical Quality Improvement database. BJU Int 117:836–842, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinall MC Jr, Arya S, Youk A, et al. : Association of Preoperative Patient Frailty and Operative Stress With Postoperative Mortality. JAMA Surg e194620–9, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kluth LA, Xylinas E, Crivelli JJ, et al. : Obesity is Associated with Worse Outcomes in Patients with T1 High Grade Urothelial Carcinoma of the Bladder. JURO 190:480–486, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Wyszynski A, Tanyos SA, Rees JR, et al. : Body mass and smoking are modifiable risk factors for recurrent bladder cancer. Cancer 120:408–414, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richard PO, Ahmad AE, Bashir S, et al. : Impact of oral hypoglycemic agents on mortality among diabetic patients with non-muscle-invasive bladder cancer: A population-based analysis. CUAJ 12:1–8, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rieken M, Xylinas E, Kluth L, et al. : Association of diabetes mellitus and metformin use with oncological outcomes of patients with non-muscle-invasive bladder cancer. BJU Int 112:1105–1112, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Sun M, Wang F, et al. : Association between pioglitazone use and the risk of bladder cancer among subjects with diabetes mellitus: a dose-response meta-analysis. Int J Clin Pharmacol Ther 55:210–219, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Turner RM, Kwok CS, Chen-Turner C, et al. : Thiazolidinediones and associated risk of bladder cancer: a systematic review and meta-analysis. Br J Clin Pharmacol 78:258–273, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


