Abstract
Colorectal cancer (CRC) is one of most common malignancies worldwide and its incidence is still growing. In spite of recent advances in targeted therapies, their clinical efficacy has been limited, non-curative and unaffordable. A growing body of literature indicates that CRC is a multi-modal disease, where a variety of factors within the tumor microenvironment play a significant role in its pathogenesis. For instance, imbalance in gut microbial profiles and impaired intestinal barrier function contribute to the overall intestinal inflammation and initiation of CRC. Moreover, persistent chronic inflammation favors a tumor microenvironment for the growth of cancer. In addition, autophagy or ‘self-eating’ is a surveillance mechanism involved in the degradation of cellular constituents that are generated under stressful conditions. Cancer stem cells (CSCs), on the other hand, engage in the onset of CRC and are able to endow cancer cells with chemo-resistance. Furthermore, the aberrant epigenetic alterations promote CRC. These evidences highlight the need for multi-targeted approaches that are not only safe and inexpensive but offer a more effective alternative to current generation of targeted drugs. Curcumin, derived from the plant Curcuma longa, represents one such option that has a long history of its use for a variety of chronic disease including cancer, in Indian ayurvedic and traditional Chinese medicine. Scientific evidence over the past few decades have overwhelmingly shown that curcumin exhibits a multitude of anti-cancer activities orchestrated through key signaling pathways associated with cancer. In this article, we will present a current update and perspective on this natural medicine – incorporating the basic cellular mechanisms it effects and the current state of clinical evidence, challenges and promise for its use as a cancer preventative and potential adjunct together with modern therapies for CRC patients.
Keywords: curcumin, colorectal cancer, chemoprevention, chemotherapy, gut microbiota, intestinal barrier, inflammation, autophagy, cancer stem cells, epigenetics
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer diagnosed in males and second most common malignancy in females, with over 1.8 million new cases and approximate 861,000 deaths reported in 2018 [1]. The prognosis of CRC varies according to cancer stage, with 5-year survival rate of about 90% for stage I disease and a dismal ~10% for stage IV patients. Although 60% of patients present with a resectable disease at the time of diagnosis, nearly half of these patients that undergo curative surgery alone and another 20–25% who receive post-surgical adjuvant chemotherapy, experience cancer relapse, metastatic disease and eventual death [2–4]; highlighting the inadequacy of current state of modern treatment choices for this fatal malignancy at the current time. Not surprisingly, there is a growing awareness that cancer prevention, in the long run, has likely a significant potential for saving more human lives compared to the classic chemotherapies and targeted drugs that are currently available for the management of this disease. Given the toxicity profile and exorbitant expense associated with modern therapies, there is a growing interest in identifying possible natural product, that are safe and more affordable for the prevention of CRC and possible adjunctive treatments together with conventional treatments currently offered to such patients.
It is well established that environmental and genetic factors increase the likelihood for developing CRC, such as older age, chronic inflammation for prolonged periods of time, a personal or family history of colorectal polyps and unhealthy diets and lifestyles, obesity and lack of exercise. Moreover, in the recent years, numerous studies have indicated a potential dual role of the gut microbiota in preserving host’s health or exacerbation of disease due to the overabundance of specific microbes that promote tumorigenesis in the colon. In this context, intestinal dysbiosis has been shown to tightly correlate with colorectal inflammation and tumorigenesis [5–7]. Under these environmental or genetic stresses, diverse cellular pathways coordinate to manifest phenotypic alterations that occur within the colonic epithelial cells, triggering a cascade of events that transform a healthy normal cell to an intermediate adenoma (or polyp) and eventually an advanced adenocarcinoma. While this biological process is well understood, this offers an opportunity for a timely intervention or regression (prevention) of disease, because normal-adenoma-cancer progression happens over a decade or longer, and various factors that promote such epithelial transformation including dietary and lifestyle choices, chronic inflammation, and benign pre-malignant lesions, can be potentially modified; hence, making cancer prevention or delayed onset a realistic possibility for a disease such as CRC which manifests over decades.
While the anecdotal evidence has existed for centuries in certain for some of the natural remedies cultures (e.g. Ayurvedic medicine from India and Chinese traditional medicine), a select number of such anti-cancer compounds derived from various natural medicines have been well-studied for their ability to prevent or even reverse early-stage cancer progression by virtue of their potent anti-inflammatory and anti-oxidant activities. Curcumin (CUR), derived from the roots of the Curcuma longa, is one such compound that has been very likely one of the leading and most-studied natural medicine for its role in cancer prevention. Several thousand studies to date have been published highlighting the biological impact of curcumin on multiple cellular functions, many of them that play a central role predominantly against cancer [8–20]. In this review, we aim to elucidate the underlying mechanisms underlying the interaction between curcumin and key biological processes or molecular pathways associated with CRC development. We further summarize various preclinical studies and clinical trials involving curcumin and its anti-cancer activity in CRC; hence highlighting its potential role for exploitation in cancer prevention or even its use as an adjunctive treatment together with classic drugs for improving therapeutic outcomes in CRC patients.
1. Curcumin – a potent bioactive natural medicine for various human diseases
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione), also referred to as diferuloylmethane, constitutes one of the major curcuminoids present in the root of plant Curcuma longa (turmeric), which has been used for centuries as a dietary spice, as well as a traditional medicine in India and China [21]. Well known for its healing property, the earliest mention of curcumin was reported by Albert Oppenheimer in 1937 for the treatment of cholecystitis [22]. Subsequently, curcumin has been virtually studied for most known diseases in humans, and has been shown to be highly effective in various medical ailments, such as cancer, arthritis, major depression, liver disease, dyslipidemia, chronic obstructive pulmonary disease, to name a few [8, 21, 23–27].
Structurally, curcumin is a symmetric molecule with two similar looking aromatic rings. Due to the presence of conjugated double bonds, curcumin serves as an effective electron donor to counteract formation of reactive oxygen species in many redox reactions [9]; hence, acting as a potent anti-oxidant. In addition to its anti-oxidative potential, a plethora of other biological functions have been attributed to curcumin, including its role as an anti-inflammatory, anti-tumor, anti-microbial, lipid-modifier and an anti-analgesic [25, 28, 29]. Accumulating evidence indicates that curcumin acts in a multi-targeted fashion and has the ability to interfere with various cellular signaling pathways by directly targeting bioactive proteins or epigenetically regulating gene expression, in key disease-associated pathways [30–32].It is noteworthy to mention that curcumin has hitherto been found to be effective in suppressing various phases of CRC development, and its therapeutic potency against CRC has been validated in several preclinical and clinical investigations [10, 33]. Due to its active anti-tumor activity, it is reasonable to conclude that curcumin has the potential for its use as a natural product for CRC prevention, and possible even for the treatment of this disease singularly or in conjunction with other treatments.
2. Curcumin and its multifaceted activity in colorectal cancer prevention and treatment
2.1. Curcumin as a modulator of gut microbial environment
Without a question, the role of gut microbiota in modulating various diseases, especially conditions of the gastrointestinal tract, has received increased attention over the last decades. In essence, gut microbiome represents a diverse microbial community that primarily comprises of various classes of bacteria, fungi, viruses and a few other rare species [34]. We now recognize that gut microbial species plays important roles in host physiology, perform numerous fundamental metabolic, structural and protective functions for the host health. Accordingly, any perturbations in gut microbiome profile or dysbiosis, can have important consequences for humans to be at-risk for developing various diseases, including CRC [35, 36]. A surge of interesting studies has revealed that microbiome alterations are most likely to be involved during the initial origin of the disease and possibly continue to play some role during the development of CRC.
Different hypotheses have been put forward to uncover the role of microbiome alterations in the tumorigenesis of CRC. In general, the collective activities of gut microbes and their metabolites can affect immune responses and therefore lead to chronic inflammation. Butyrate, for instance, is a short chain fatty acid produced by gut microbial players. As a histone deacetylase (HDAC) inhibitor, butyrate has anti-tumor effects in several key oncogenic signaling pathways including JAK2-STAT3 and VEGF pathways. In addition, butyrate reduces gut inflammation by promoting T-regulatory cell differentiation through the suppression of master transcription factor NF-κB and its downstream mediator STAT3 pathway [37]. In contrast to the beneficial metabolic effects of butyrate, growing evidence indicates that it can also lead to elimination of several bacterial species which can result in direct damage of gut cells or produce toxins to promote inflammatory responses. For example, Bacteroides fragilis toxin (BFT), produced by Bacteroides fragilis has the potential to initiate CRC through the induction of a pro-carcinogenic inflammatory cascade [38]. In contrast, Fusobacterium nucleatum, a well-known bacterium linked to CRC pathogenesis, can directly invade colonic epithelial cells through its surface protein[39–41]. Such an interaction between its surface protein FadA and E-cadherin subsequently triggers activation of Wnt signaling, which promotes transformation of normal colonic epithelial cells [42, 43]; highlighting how the modulation of gut microbiota is a promising intervention for improving the overall health within the gastrointestinal tract and potentially preventing CRC.
Interestingly, curcumin is capable of exerting direct influences on gut microbiome (Figure 1). The administration of curcumin has been shown to remarkably shift the ratio between beneficial and pathogenic microbes in several animal and human studies. As a matter of fact, curcumin may reduce intestinal inflammation, at least in part, through regulation of gut microbiota. In an experimental DSS-colitis model, curcumin was shown to ameliorate intestinal inflammation by reducing NF-κB activation in colonic epithelial cells and by increasing expansion of CD4+ Foxp3+ regulatory T cells in the colonic mucosa[44].Interestingly, the abundance of butyrate-producing bacteria, Clostridium cluster IV and XIVa, were observed to be significantly increased by curcumin, which was accompanied by elevation of fecal butyrate levels [44]. This is of significance because studies have demonstrated that these two clusters of Clostridium can induce mucosal Treg cells by producing butyrate [45, 46], suggesting that curcumin in such situations may ameliorate Treg-associated inflammation by promoting abundance of these commensal bacteria. A pilot study investigated the gut microbiome profiles in human subjects who used curcumin as a dietary supplement, and reported that curcumin treatment showed increased species such as Clostridium, Enterobacter, while reduced the relative abundance of several Blautia and Ruminococcus species[47]. It is worth noting that the increased population of Ruminococcus species has been been previously linked to patients with CRC [48–50]. Although the exact pathogenic role of Ruminococcus in the CRC development remains unclear, the fact that curcumin suppresses their level indicates yet one of the potential ways it might exert its anti-cancer activity for CRC prevention.
Figure 1:
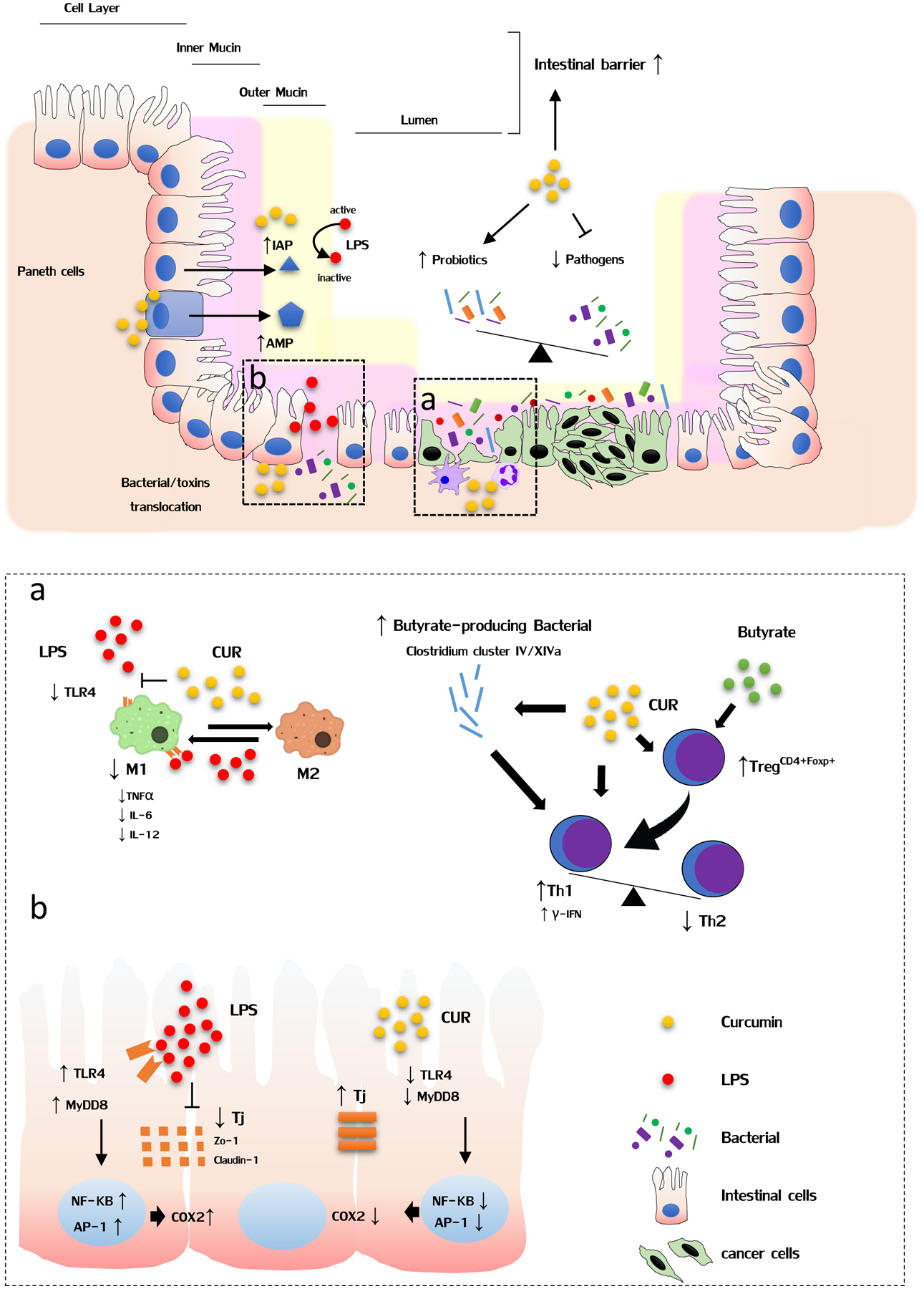
Curcumin modulates gut microbiome, enhances intestinal barrier function and inhibits inflammation in the gut. The administration of curcumin could remarkably result in the shift of ratio between beneficial and pathogenic microbes, leading to the reduction in toxins such as LPS. Furthermore, the enhanced intestinal barrier function by curcumin can prevent translocation of bacterial or their derived-products. Moreover, curcumin can promote paneth cells mediated secretion of antimicrobial peptide (AMP) to prevent entry of pathogenic bacteria or colonic epithelial cells to release intestinal alkaline phosphatase (IAP) to detoxify LPS. Finally, curcumin exerts its anti-inflammatory effects through regulation of several inflammatory pathways in immune cells (a) and colon cells (b).
To further investigate the effect of curcumin-supplemented diets in CRC tumorigenesis and its relationship with gut microbial profiles, a mice model evaluated the effect of a well-established mutagenic agent azoxymethane (AOM). This study revealed that AOM treated mice displayed decreased abundance of Lactobacillus. Nevertheless, dietary curcumin restored the Lactobacillus numbers to control levels, which have previously been demonstrated to exhibit anti-tumor function through mediation of cell cycle arrest and induction of apoptosis in colon cancer cell lines [51]. In other words, studies like this point towards one of the many ways dietary curcumin may potentially lead to enrichment of unique microbial species within the digestive tract to promote cancer inhibitory activity in humans.
2.2. Curcumin protects intestinal barrier function
Intestinal barrier primarily consists of four layers. The first layer is comprised of intestinal alkaline phosphatase, which is secreted by colonic epithelial cells to detoxify bacterial endotoxin lipopolysaccharide (LPS). The mucous layer forms the second layer and prevents entry of pathogenic bacteria. The third layer is composed of tight junctions between intestinal epithelial cells that restrict transcellular or paracellular transport of bacteria or their products into the systemic circulation. The final layer consists of antibacterial proteins, which are produced by paneth cells, and block bacteria that have penetrated the overlying layer in this intestinal barrier [52]. Not surprisingly, any defects in intestinal barrier integrity can result in invasion of bacteria into normal colonic tissue, eliciting local inflammation, and thereby generation of genotoxicity against intestinal epithelial cells for stimulating their oncogenic transformation [53, 54]. Thus, any intervention that allows maintenance of intestinal barrier integrity and allow reduced translocation of bacterial derived-products, represents a promising strategy for CRC prevention.
In an elegant study on this topic, Wang and colleagues discovered that LPS could increase secretion of cytokine IL-1β from intestinal epithelial cells (Caco-2 and HT-29 cells) and PMA-differentiated human THP1 macrophages[55]. IL-1β subsequently induced activation of p38/MAPK signaling pathway to up-regulate the expression of myosin light chain kinase, which phosphorylates junction proteins and causes disruption of normal arrangement. Interestingly, pretreatment with curcumin in intestinal epithelial cells and macrophages significantly decreased LPS-induced IL-1β levels and restored intestinal barrier dysfunction, highlighting the protective role of curcumin in gut barrier dysfunction [55]. Moreover, curcumin was found to predominately attenuate H2O2-induced disruption of paracellular permeability in Caco-2 enterocytic monolayers, which ties into the notion that the protective effect of curcumin is dependent on the heme oxygenase-1 (HO-1) pathway [56]. From a human cancer perspective, an interesting study demonstrated that western diets lead to the alteration of gut microbial profiles in manner that are more conducive to promoting release of gut bacteria-derived products into systemic circulation; hence supporting the increased burden of cancer incidence in the western populations. To provide a mechanistic support for this paradigm, the mice were fed western diets for 16 weeks, and then either received antibiotics (Neomycin and polymyxin for selective gut decontamination) in drinking water, or gavaged daily with curcumin. Consequently, selective gut decontamination and supplementation with curcumin effectively decreased plasma LPS levels by western diet, and dramatically enhanced intestinal alkaline phosphatase activity and up-regulated expression of tight junction proteins, ZO-1 and Claudin-1 [57]. Taken together, curcumin mediated improvement of intestinal barrier function providing a direct evidence for its potential role in CRC prevention (Figure 1).
2.3. Curcumin promotes anti-inflammatory activity in the gastrointestinal tract
Chronic inflammation is believed to be one of the primary underlying reasons for the initiation of CRC. In addition, immune cells often infiltrate tumors, referred to as ‘tumor-elicited inflammation’, and promote tumor development by favoring the growth of cancer cells. In the past decades, overwhelming body of literature has convincingly proven that curcumin is perhaps one of the most potent natural-remedies for impeding inflammation through its activity on multitude of signaling pathways; thus supporting its role in the prevention and treatment of CRC.
Numerous studies have demonstrated that the hyperactivity of toll-like receptor 4 (TLR4) tightly associates with the development of CRC [58, 59]. In this context, the activation of TLR4 receptor triggers innate immune response and subsequent inflammation. Furthermore, TLR4 is well-known for recognizing LPS, as well as other endogenous proteins including low-density lipoprotein and heat shock proteins. Upon the recognition of LPS, the signaling effects are passed on to the intracellular adaptor molecules such as MyD88, which are finally continued through either NF-κB or AP-1, both of which are frequently up-regulated in various inflammatory diseases as well as caner [60]. Recent studies have shown that curcumin is capable of attenuating LPS-induced inflammation by inhibiting activation of TLR4/MyD88/NF-κB signaling axis [61, 62]. Moreover, curcumin can prevent NF-κB subunit movement to the nucleus and mitigate the expression of other pro-inflammatory genes, which are otherwise over-activated in cancer [63]. In addition, curcumin was found to reduce IκB kinase activity, inhibiting degradation of IκBα and leading to the block of nuclear translocation of the NF-κB subunit [63]. Curcumin has also been shown to suppress TNFα or IL-1β-induced p38 and JNK activation, causing the eventual suppression of IκBα degradation in HT29 cells [64]. From an epigenetic viewpoint, while the overexpression of oncogenic miR-21associates with ulcerative colitis and colitis-associated CRC [65–67], curcumin treatment has been reported to significantly down-regulate miR-21 expression through competitive inhibition of its binding activity to AP-1. Since miR-21 can directly target several tumor suppressor genes including Pdcd4 (programmed cell death protein 4), curcumin-treated cells exhibited G2/M phase arrest and suppressed tumor growth and invasion [68].
In addition to targeting TLR4 pathway, curcumin directly regulates several other important inflammation-related molecules. Cyclo-oxygenase 2 (COX2) is an inducible isoform of prostaglandin H synthase, and functions primarily for prostaglandin synthesis during inflammation. A number of studies have confirmed that COX2 plays an important role in CRC [69–72], and that curcumin inhibits COX2 induction by suppression of NF-κB activity in colon cancer cells [72, 73]. Such inhibitory effects of curcumin in COX2 inhibition in CRC were also validated in a dextran sulfate sodium (DSS)-mice model [74], as well as in an animal model with azoxymethane (AOM)-treatment [75].
In addition to its direct effects on colonic epithelia cells, curcumin also modulates a number of immune cells that are actively engaged in the progression of intestinal inflammation. As illustrated in Figure-1, the roles of tumor microenvironment, encompassing tumor macrophages and their role in promoting colorectal inflammation and cancer have been appreciated in the past decade[76, 77]. Now we know that the M1 type macrophage could produce several pro-inflammatory cytokines or chemokines to trigger and promote inflammation. Several studies have demonstrated that curcumin down-regulated TLR4 pathway in macrophages and inhibited the production of TNF-α, IL-6, IL-12, and other inflammatory cytokines[78–80]. Moreover, curcumin-treated macrophages possess an enhanced ability to capture antigens and endocytose via the mannose receptor. Furthermore, curcumin can significantly polarize or repolarize macrophages toward the M2 phenotype, leading to a potential reduction in mucosal inflammation [78–80].
It is also known that regulatory T cells (Treg) play an important role in tumor immune tolerance and evasion. Therefore, effective targeting of Treg cells holds a clinical promise for prevention or even treatment of CRC. In clinical studies, patients with curcumin treatment exhibited decreased number of peripheral Treg cells and increased Th1 cells[81–83]. Further experiments revealed that inhibition of Foxp3 expression and enhanced IFN-γ secretion in Treg cells by curcumin treatment caused conversion of Treg cells to Th1 cells. Since Th1 cells possess antitumor properties, it may be possible to use curcumin as an immunotherapy approach in CRC patients, by itself or in combination with other conventional drugs [81–83]. Collectively, the above studies clearly reveal and emphasize the pharmacological roles of curcumin in immunomodulation, and making an argument favoring its potential as a natural medicine of choice for CRC prevention.
2.4. Curcumin modulates autophagy in cancer cells
A growing body of literature supports the role of autophagy in CRC, particularly in the initial stages of its development. Autophagy is primarily perceived as a surveillance mechanism, whereby failure to remove damaged or toxic substances by autophagy can result in the accumulation of DNA mutations or other malignant entities that favor cancer progression. In contrast, autophagy supports tumor growth by dealing with increased stress tolerance or shortage of nutrients. The clearance of unnecessary proteins or damaged organelles, in turn, fuels cancer cell survival [84]. Therefore, modulation of autophagy in different scenarios provides encouraging pharmacological targets for CRC prevention.
The autophagy is initiated by the activation of Unc-51 like kinase 1 (ULK1) complex, and subsequently class III phosphatidylinositol 3-kinase (PI3K) complex I triggers the formation of phagophores. A number of factors are recruited along the way for the elongation of the phagophore membrane and mediation of the fusion between autophagosome and lysosome (as illustrated in Figure-2). Consequently, the cargo proteins are degraded by the lysosomal enzymes. Accumulating studies have shown that the molecular targets of rapamycin complex 1 (mTORC1) are an upstream inhibitor of autophagy, and different pathways, including PI3K/AKT pathway, RAS/MEK/ERK signaling, or JNK pathway, can regulate mTORC1 activity or direct targets of autophagy associated factors [85–87]. In this regard, it was found that curcumin significantly suppresses PI3K/Akt/mTOR signaling pathways to induce protective autophagy within cancer cells. Moreover, mTOR inhibitors or PI3K/Akt inhibitor LY294002 could enhance curcumin-induced apoptosis, highlighting its anti-cancer role in autophagy [88, 89]. In human colon cancer cells, curcumin was found to substantially induce autophagy, through conversion of microtubule-associated protein 1 light chain 3 (LC3)-I to LC3-II or up-regulation of Beclin-1 [88, 89].
Figure 2:
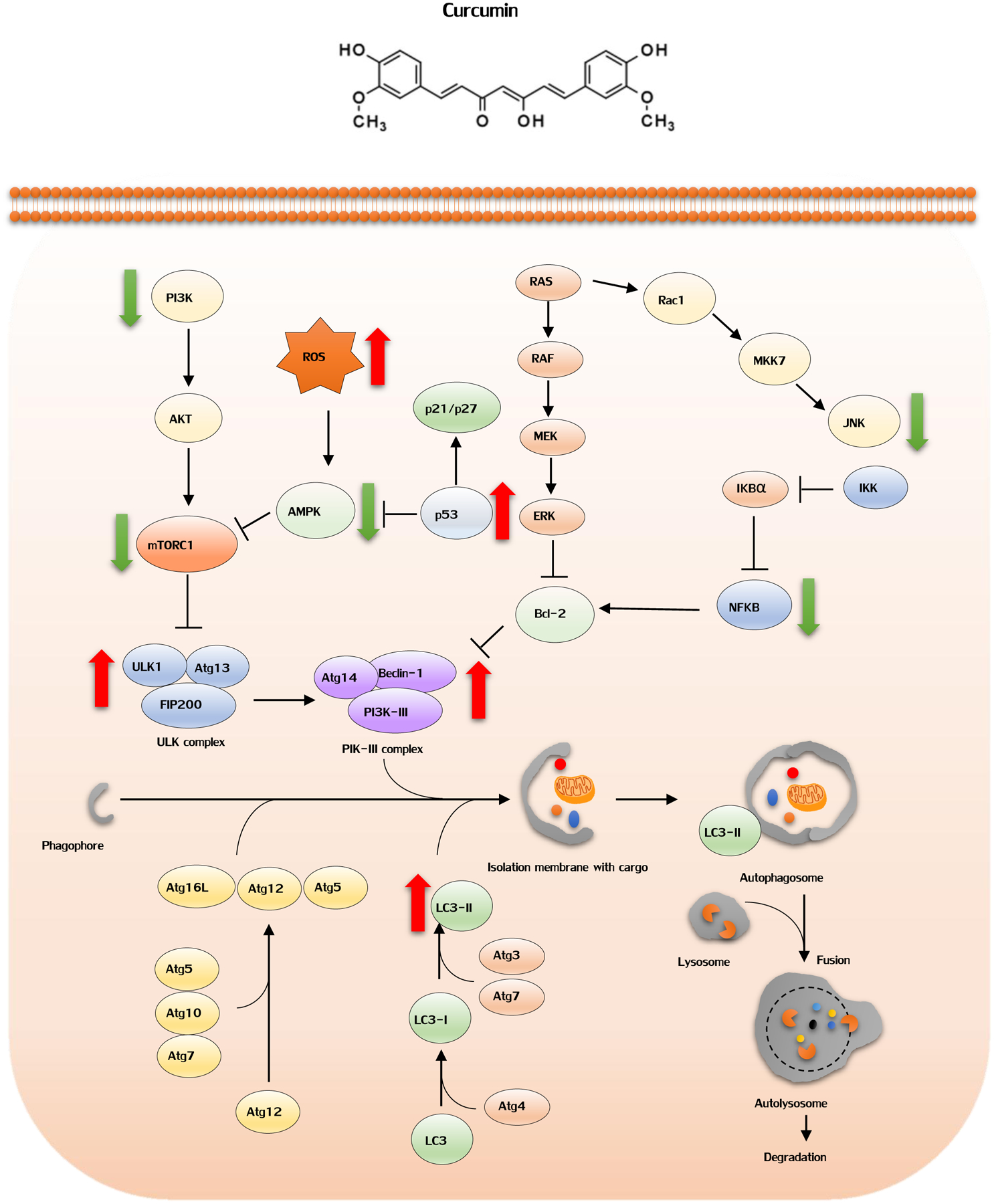
The effects of curcumin in autophagy pathway. Red arrows: stimulatory interaction; Green arrow: inhibitory interaction. The autophagy is initiated by the activation of Unc-51 like kinase 1 (ULK1) complex, and subsequently class III phosphatidylinositol 3-kinase (PI3K) complex I triggers the formation of phagophores. A number of factors are recruited along the way for the elongation of the phagophore membrane and mediation of the fusion between autophagosome and lysosome. Consequently, the cargo proteins are degraded by the lysosomal enzymes. Molecular targets of rapamycin complex 1 (mTORC1) are an upstream inhibitor of autophagy, and different pathways, including PI3K/AKT pathway, RAS/MEK/ERK signaling can regulate mTORC1 activity or direct targets of autophagy associated factors. Curcumin suppresses PI3K/Akt/mTOR signaling pathways to induce protective autophagy within cancer cells. p53 activation could lead to autophagy, indicating autophagy as one of the protective functions of p53. Curcumin increased p53 expression and may therefore trigger induction of autophagy. AMP-activated protein kinase (AMPK) activation enables induction of autophagy in colorectal cancer, and its expression can be regulated by ROS or p53. Curcumin can activate AMPK to induce autophagy.
It is believed that p53 activation could lead to autophagy, indicating autophagy as one of the protective functions of p53 [90]. The administration of curcumin in patients with CRC promoted apoptosis of tumor cells, and increased p53 expression in tumor tissues [91], suggesting that curcumin can induce p53 expression and may therefore trigger induction of autophagy. Accumulating studies have demonstrated that AMP-activated protein kinase (AMPK) activation enables induction of autophagy in colorectal cancer ([92–94], and its expression can be regulated by ROS or p53 [92, 95]. Since curcumin can cause accumulation of ROS and increased p53 expression, it can indirectly activate AMPK to induce autophagy. In addition, current studies have shown that curcumin acts as a novel AMPK agonist, which directly enhances phosphorylation level of AMPK for promoting autophagy in cancer cells [96]. Collectively, the favorable contribution of stimulating an autophagic cascade by curcumin can be considered as yet another potential mechanism for the cancer preventative potential of curcumin.
2.5. Curcumin targets cancer stem cells and acts as a chemosensitizer in cancer
In the recent years, there has been a renewed interest that cancer stem cells (CSC), also referred to as tumor initiating cells, are the primary causes for the initiation of tumorigenesis, as well as an Achilles heel for cancer treatment [97, 98]. As its name implies, CSCs are a small subpopulation of tumor cells that have an enhanced potency and ability to self-renew, differentiate, and give rise to a new cancer cells [99]. In the colon, since adenomas serve as the precursor for adenocarcinoma, an interesting study on familial adenomatous polyposis (FAP) patients revealed that mutations within the adenomatous polyposis coli (APC) gene resulted in overpopulation of multipotent cell expansion at the intestinal epithelial crypt base during adenoma development, suggesting that mutant crypt stem cells may be the origin for CRC [100]. Therefore, targeting CSCs in adenomas or early-stage cancers is deemed to be a potential approach for CRC prevention.
Recently, a study reported that curcumin by itself, or together with an anticancer drug dasatinib, was able to suppress >90% of the spontaneous intestinal adenomas in APCMin+/− mice, which was accompanied by reduced expression of various CSC markers (e.g. ALDH, CD133, CD44 and CD166) in tumor remnants from APCMin+/− mice [101]. This study also confirmed that a combination of dasatinib and curcumin significantly inhibited transformation of CSCs in colon cancer cell lines; thus providing a rationale for utilizing curcumin to target CSCs for cancer prevention [101].
Another recent study showed that tissue glutamine levels are gradually decreased during adenomatous polyp to CRC development in humans. Curcumin may bind to CD44 at the cell membrane to block transport of glutamine into colorectal CSCs, resulting in low levels of glutamine, as well as induction of apoptosis in CSCs [102]. Intriguingly, high density tumor microenvironment co-cultures with MRC-5 fibroblasts synergistically favored HCT116-derived CSC survival characterized by increased levels of CD133, CD44 and ALDH1 [103]. Curcumin, as expected, can significantly suppress such synergistic crosstalk between CSCs and tumor microenvironment, and inhibit CSCs survival.
CSCs not only play a critical role in the initiation of cancer, but also contribute towards imparting resistance to conventional chemotherapies and targeted drugs, as well as are an important cause of cancer recurrence in cancer patients. As shown in Table 1, several studies have demonstrated that curcumin enhanced sensitivity of resistant CRC cells to chemotherapeutic drug 5-fluorouracil or oxaliplatin, due to the reduction of CSCs. Our group have shown that high density tumor microenvironment co-cultures favored CSC survival, and significantly contributes to 5-FU resistance [103]. Such synergistic crosstalk can be remarkably broken by the presence of curcumin, accordingly sensitizing CSCs to 5-FU treatment. In consistence, we also found that curcumin potentiates and chemosensitizes resistant HCT116 cells to 5-FU [17]. To further support this, we have demonstrated the mechanistic role of curcumin in 5-FU chemoresistance. Our group showed that curcumin can modulate several key epigenetic events, including DNA methylation[14] and miRNA[17, 20], to sensitize colon cancer cells to 5-FU treatment. An insight into the molecular mechanism(s) has revealed that curcumin has substantial inhibitory activity in multiple CSC-related pathways, suggesting that curcumin, with its promising anti-CSC potential, can be developed as a promising adjunctive treatment to enhance the efficacy and reduce the adverse toxicity profile associated with existing treatment modalities [104].
Table1:
Molecular targets of curcumin in colorectal cancer
| Experimental model | Biological response | invovled genes/pathway | Reference | |
|---|---|---|---|---|
| Cell lines | 5-FU resistant HCT8 cells | ↑ sensitivity to 5-FU | ↑ Nrf2 | [137] |
| Cell lines | HCT116, SW620 cells | ↓ proliferation | ↓ YAP | [138] |
| ↑ autophagy | ||||
| Cell lines | Irinotecan resistant LoVo cells | ↑ apoptosis of cancer stem cells | unknown | [139] |
| Cell lines | HCT116, DLD1, HCT15 cells | ↓ Xenograft tumor growth | ↓ SIRT1 | [140] |
| Animal model | HCT116-xenograft | |||
| Cell lines | LoVo, HT29 cells | ↑ sensitivity to irinotecan | ↑ ROS generation, ER stress | [141] |
| Cell lines | SW480, LoVo cells | ↓ adhesion and proliferation | ↑ AMPK | [142] |
| ↓ NF-κ B, uPA and MMP9 | ||||
| Cell lines | DLD1 cells | ↑ apoptosis | ↓ BMI1 | [143] |
| Cell lines | HT29, LoVo, DLD1 and their corresponding oxaliplatin resistant cells. | ↑ sensitivity to oxaliplatin | ↓ NF-κ B | [144] |
| Cell lines | SW620 cells | ↓ EMT progression | ↓ NKD2- Wnt- CXCR4 | [145] |
| Animal model | patient-derived colorectal liver metastases xenografts | ↓ cancer stem cell phenotypes | ↓ number of ALDHhigh/CD133-cells | [123] |
| ↑ anti-proliferative and pro-apoptotic effects by FOLFOX treatment | ||||
| Cell lines | HCT116 and corresponding 5-FU resistant HCT116 cells | ↑ sensitivity to 5-FU | ↓ NF-κ B | [17] |
| Cell lines | HCT116, HCT116p53−/−, and SW480 cell lines | ↓ cellular proliferation | ↑ miR-34a | [19] |
| ↑ induced apoptosis and cell-cycle arrest | ↓ miR-27a | |||
| Cell lines | HCT116, SW480 cells | ↑ sensitivity to 5-FU | ↑ miR-200c | [20] |
| Cell lines | HT29 cells | ↓ anchorage-independent growth | ↑ DLEC1 | [107] |
| Animal model | LoVo-xenograft | ↑ sensitivity to oxaliplatin, apoptosis | ↑ Bax, caspase-3, and PARP | [146] |
| Cell lines | HCT116 and HT29 cells | ↓ aerobic glycolysis | ↓ HKII | [147] |
| ↑ apoptosis | ||||
| Cell lines | LoVo cells | ↓ invasion | ↓ PI3K/Akt | [148] |
| ↑ apoptosis | ||||
| Cell lines | HCT116, HCT116+ch3, and corresponding 5- FU resistant HCT116 and HCT116+ch3 cells | ↑ sensitivity to 5-fluorouracil in resistant MMR- deficient CRC cells | unknown | [16] |
| ↓ cancer stem cells | ||||
| Cell lines | HCT116, HT29, HCT15, HCC2998, Colo205, Km12, and SW620 cells | ↓ migration, invasion, and colony formation in vitro cells | ↓ Sp-1, FAK | [149] |
| ↑ E-Cadherin | ||||
| ↓ tumor growth and liver metastasis in mice model. | ||||
| Cell lines | HCT15 cells | ↓ proliferation | ↓ p53 and Prp4B | [150] |
| ↑ apoptosis | ||||
| Cell lines | HCT116, HT29 and RKO cells | ↓ Cell viability and proliferation | ↑ DNA Methylation | [14] |
| Cell lines | HCT116, HCT116+ch3 cells | ↑ sensitivity to 5-fluorouracil | ↓ NF-κ B/PI3K/Src | [16] |
| Cell lines | RKO and SW480 cells | ↑ ROS, apoptosis | ↓ SP1,SP3 and SP4 | [117] |
| ↓ cell growth | ||||
| Cell lines | HCT116 and Caco-2 cells | ↑ G(2)/M stage arrest | ↑ DNA damage | [151] |
| ↑ mitotic spindle abnormalities and defects in chromosomal congression | ||||
| Cell lines | HCT116 cells | ↑ S and G2/M phase arrest | ↑ DNA damage | [152] |
| Animal model | DSS-induced tumor mice | ↓ disease activity index, ↓ neoplasic lesions | ↓ ß-catenin,COX2,iNOS | [74] |
| Animal model | oxaliplatin-resistant HCT116-xenograft | ↑ sensitivity to oxaliplatin | ↓ Ki-67 and Notch-1 | [134] |
| Cell lines | RKO and HCT116 cells | ↓ tumour growth, invasion and in vivo metastasis | ↓ miR-21 | [68] |
| Cell lines | HCT116 (p53+/+ and p53−/−),HT29 | ↑ apoptosis | ↑ oxidative stress | [153] |
| Cell lines | HCT116 and HT29 cells | ↑ sensitivity to FOLFOX | ↓ EGFR, IGF-1R | [154] |
| Cell lines | HCT116, HT29, and SW620 cells | ↑ radiosensitivity | ↓ NF-κ B | [155] |
| Cell lines | HCT116 cells | ↓ proliferation | ↓ mTOR | [156] |
| Animal model | orthotopically implanted CRC tumors (HCT116) | ↓ growth and metastasis | ↓ NF-κ B | [157] |
| ↑ sensitivity to capecitabine | ||||
| Cell lines | HCT-116 and SW480 cell | ↓ proliferation | ↓ proteasome | [158] |
| ↑ apoptosis | ||||
| Animal model | HCT116-xenograft | ↑ radiosensitivity | ↓ NF-κ B | [159] |
| Cell lines | HCT116 cells | ↑ apoptosis, sensitivity to oxaliplatin | ↓ TGF-β/Smads | [160] |
2.6. Curcumin as an epigenetic modulator in cancer cells
Aberrant epigenetic regulation of cancer-associated genes is a frequent and important event in cancer initiation, progression and metastasis, and plays a central role for the gain of function through inadvertent activation of oncogenes and inactivation of tumor suppressor genes in cancer cells [105, 106]. Intriguingly, a large number of studies have recently reported that that curcumin can biologically regulate all major epigenetic alterations-DNA methylation, histone modification and the expression of non-coding RNAs including microRNAs (miRNA) in CRC cells, making it a promising candidate for CRC prevention (Figure 3).
Figure 3:
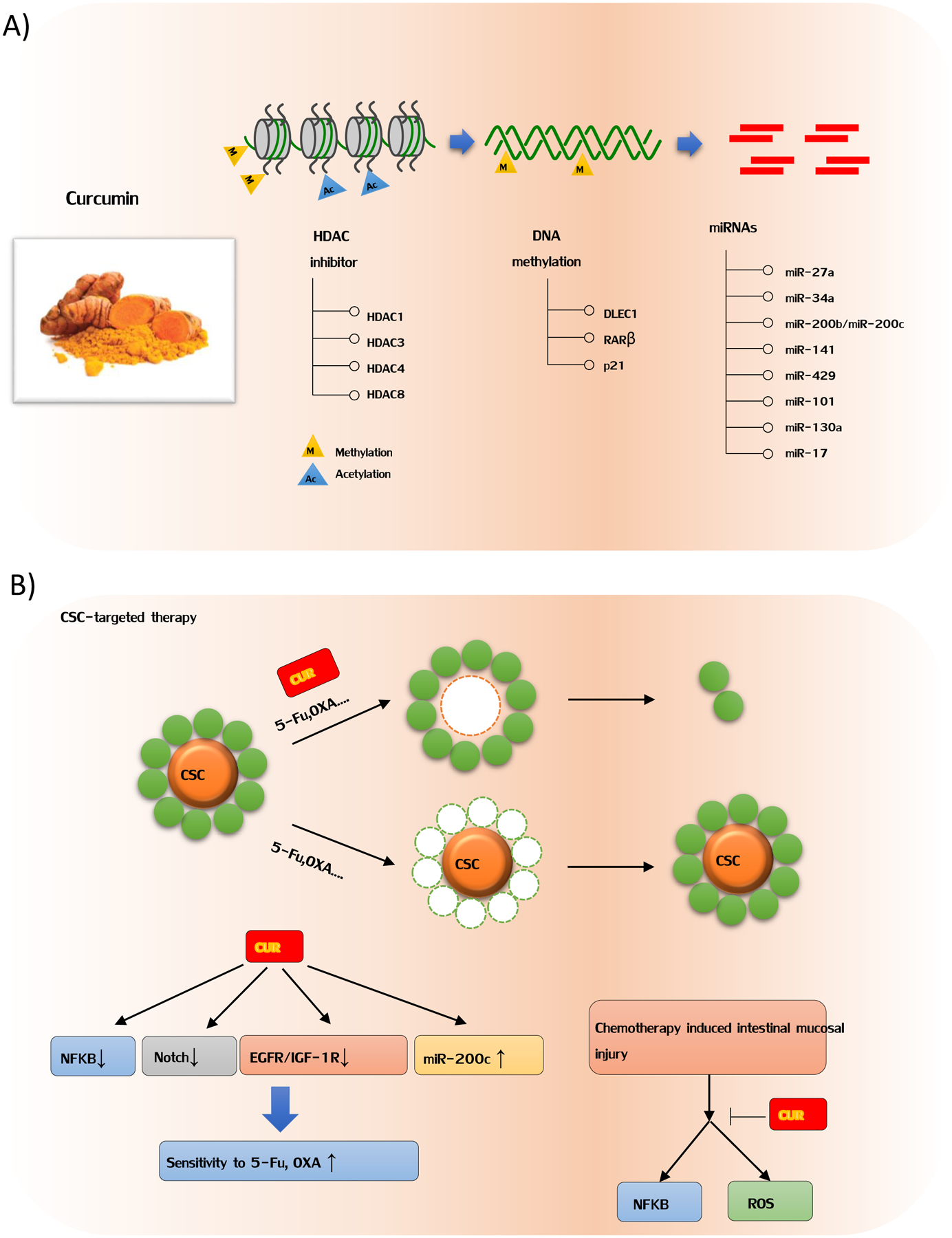
Curcumin as an epigenetic modulator and suppressor of cancer stem cell in colorectal cancer. A) Curcumin can biologically restore expression of several tumor suppressors by DNA de-methylation. Furthermore, curcumin exerts its antitumor effects by serving as histone deacetylation inhibitor and miRNA regulator. B) CSCs are a small subpopulation of tumor cells that have an enhanced potency and ability to self-renew, differentiate, and give rise to a new cancer cells. Several studies have demonstrated that curcumin enhanced sensitivity of resistant CRC cells to chemotherapeutic drug 5-fluorouracil or oxaliplatin, due to the reduction of CSCs. As shown in figure 3B, curcumin has substantially inhibitory effect on multiple CSC-related pathways, suggesting that curcumin, with its promising anti-CSC potential, can be developed to enhance the efficacy of existing treatment modalities.
In this text, our group was among the first to evaluate the effect of curcumin on DNA methylation in a panel of colon cancer cell lines including HCT116, HT29 and RKO [14]. Compared to non-specific global hypomethylation induced by 5-aza-CdR, curcumin treatment led to demethylation of specific CpG Loci, providing new mechanistic insights into the potent chemopreventative effect of this dietary nutraceutical [14]. In fact, a few other studies since then have also reported similar observations that curcumin can epigenetically restore expression of specific tumor suppressor genes, such as RARβ, DLEC1 and p21 [107–109]. Furthermore, in an azoxymethane-dextran sulfate sodium (AOM-DSS) mice model to investigate potential epigenetic mechanisms of curcumin in the initiation of CRC, it was observed that unique differentially methylated genes and associated pathways were modulated by curcumin, suggesting that curcumin may be clinically used as epigenetic modulator in inflammation-associated colon cancer [110].
Histone modifications are believed to be distinct epigenetic alterations that control expression of specific genes. Histone modifications comprise of two major modifications: histone acetylation and deacetylation. Histone deacetylases (HDACs) are chief enzymes that remove acetyl groups from the histone proteins, leading to their deacetylation, resulting in epigenetic silencing of the corresponding gene(s). The imbalance of acetylation and deacetylation levels within histone complexes is often associated with onset of cancer [111]. Indeed, numerous in vitro studies and clinical trials have shown HDAC inhibitors can be used as potential agents for cancer prevention, by virtue of their substantial inhibitory effects on cancer, including CRC [112]. With regard to curcumin, it has been confirmed to be a potent compound for the effective inhibition of HDAC activity, compared to other naturally-occurring HDAC inhibitors including sodium butyrate [113]. It has been reported that curcumin primarily inhibits HDAC isoforms 1, 3, 4 and 8, leading to an overall reduced HDAC activity, and resulting reactivation of various tumor suppressors and concomitant inhibition of cancer cell growth [113–116].
To date, among various classes of non-coding RNAs, microRNAs (miRNAs) have been most intensively studied and recognized as key players in CRC pathogenesis due to their ability to inhibit the expression of multiple downstream genes, many of which play a central role in carcinogenesis. It is of great interest to understand the underlying mechanisms by which natural dietary compounds, such as curcumin, affect the expression of such short non-coding RNAs in CRC. We have previously reported that treatment of curcumin in CRC cells induced up-regulation of miR-27a and miR-34a, which are well known for their tumor suppressive activity in CRC [19]. Furthermore, we also noted that curcumin sensitized CRC cells that were otherwise resistant to 5-fluorouracil, a process that was mediated through up-regulation of EMT-suppressive miRNAs, such as miR-200b, miR-200c, miR-141, miR-429 and miR-101 [20]. Furthermore, it has been shown that curcumin can suppress miR-21 expression, leading to inhibition of invasion and metastasis in CRC [68], as well as suppression of miR-130a preventing activation of Wnt/β-Catenin signaling pathway. Noteworthy of mentioning, a recent study revealed that curcumin treatment of CRC cells inhibited expression of miR-27a, miR-20a and miR-17–5p, resulting in activation of ZBTB10 and ZBTB4, which are suppressors of specificity protein (Sp) transcription factors [117]. The up-regulation of ZBTB10 and ZBTB4 expression significantly inhibited SP transcription factors, and subsequently the expression of various downstream target genes such as EGFR, c-MET, cyclin D1 and NFκB, leading to suppression of cancer cell growth and induction of apoptosis [117]. These findings highlight the epigenetic modulatory effects of curcumin in CRC, and consequently provide a mechanistic evidence for this botanical to serve as multi-targeted chemopreventive agent in cancer.
2.7. Additional molecular targets of curcumin
A large body of evidence has demonstrated various cell signaling pathways modulated by curcumin. As described in Table 1, curcumin results in impacting several biological functions in CRC cells, such as suppression of cell proliferation, cell cycle arrest, cell invasion inhibition, induction of apoptosis and even sensitization of chemo-resistant cells. In other words, in addition to well-established mechanisms, curcumin has additional potential activities that can restore tumor suppressor gene expression or inhibit oncogene activation. Thus, curcumin may prevent the risk of CRC, and even exert a synergistic effect when employed along with standard chemotherapeutic agents, in mitigating the overall risk of cancer.
3. Clinical evidence for curcumin in the prevention and treatment of CRC
3.1. Clinical trials with curcumin for CRC prevention
The recent years have witnessed an increase in the number of clinical trials involving curcumin for a variety of chronic inflammatory diseases including cancer. In the context of CRC, one of the first trials was a phase IIA clinical trial, aimed at evaluating whether curcumin treatment can prevent formation of aberrant crypt foci (ACF), reduce pro-carcinogenic eicosanoids prostaglandin E2 (PGE2) and 5-hydroxyeicosatetraenoic acid (5-HETE) and inhibit proliferation (Ki67 expression) in normal mucosa [118]. Through colonoscopic screening, 41 subjects with 8 or more ACF were recruited and completed this trial. Although curcumin did not affect PGE2 or 5-HETE levels, nor Ki-67 expression in ACF or normal mucosa tissues, a remarkable reduction of 40% in the number of ACF was observed with a dose of 4g. Moreover, the 4g group with ACF reduction showed 5-fold increase in post-treatment plasma curcumin/conjugate levels compared to pre-treatment level [118]. In contrast, another clinical trial was performed to evaluate the inhibitory effect of curcumin on size and numbers of polyps from 44 patients with familial adenomatous polyposis (FAP) with either curcumin or placebo treatment [119]. In this study the patients received a lower dose of 3g/day curcumin.
Unfortunately, this study did not report any significant differences in either the number of polyps (22.6; 95% CI, 12.1–33.1 vs. 18.6; 95% CI, 9.3–27.8) or the polyp size (2.1 mm; 95% CI, 1.5–2.7 vs. 2.3 mm; 95% CI, 1.8–2.8) in the curcumin vs. placebo groups [119]. These results suggested that higher dosage or long-term treatment of curcumin might be needed for treating patients with FAP. Interestingly, in another study, a combination of curcumin with other inhibitors was seen to have synergistic efficacy in the colon. To this end, Cruz-Correa and colleagues enrolled 5 patients with FAP, and gave them 480mg curcumin and 20 mg quercetin, orally 3 times a day [120]. Surprisingly, all patients exhibited a significantly reduced polyp number (60.4% compared to baseline number) and size (50.9% compared to baseline size) after 6 months of treatment with curcumin and quercetin; highlighting the chemopreventive efficacy of curcumin in pre-cancerous polyp formation in the colon.
3.2. Clinical trials using curcumin as an adjunct to chemotherapy in cancer
Majority of advanced CRC patients require a palliative treatment. However, either 5FU-based or Oxaliplatin-based chemotherapy is often prescribed, which often accompanies with increased adverse effects and toxicity including peripheral neuropathy. In view of this, there is an urgent need for identification of additional safe and inexpensive natural compounds with low-toxicity, that can serve as adjuncts to currently available treatment options. Curcumin offers such promise based upon a series of studies supporting such a role for its patients with advanced CRC. Sharma and colleagues conducted a phase I trial, and noted that 450mg-3600mg of curcumin treatment for up to 4 months was safe in patients (n=15) with advanced CRC [121, 122]. In further support of this study, another clinical trial demonstrated that curcumin was safe and well-tolerated as an adjunct to FOLFOX chemotherapy in 12 patients with colorectal liver metastasis when given 2g of curcumin daily [123]. This study also established patient-derived colorectal liver metastases stem cell models, and noted that a combination of curcumin with 5-FU or oxaliplatin led to synergistic anti-tumor effects in patient-derived explants, accompanied by reduced number of CSCs [123]. Besides these, as described in Table 2, several other clinical trials have to date studied curcumin in CRC and have shown varying degree of efficacy of curcumin in these human studies.
Table2:
Clinical trials with curcumin in colorectal cancer
| NCT Number | Condition | Title | Intervention/treatment | Enrollment | Phase | Primary Purpose | Reference |
|---|---|---|---|---|---|---|---|
| NCT02439385 | Unresectable | First Line Avastin/FOLFIRI in Combination with Curcumin- containing Supplement in Colorectal Cancer Patients with Unresectable Metastasis | Drug. ‘vastin/i-OLFIRI | 50 | 2 | Treatment | NA |
| Metastasis | Dietary Supplement: Curcumin | ||||||
| NCT00973869 | CRC patients | A Pilot Study of Administration of Curcumin to Determine Colonic Curcumin Tissue Levels in Patients Awaitir, Col.ectal Endoscopy or Patients with Colorectal Cancer Awaiting Resection | Dietary Supplement: curcumin | 30 | 1 | Prevention | NA |
| Procedure: diagnostic endoscopic | |||||||
| procedure | |||||||
| Procedure: therapeutic | |||||||
| conventional surgery | |||||||
| NCT01859858 | Advanced | A Prospective Evaluation of the Effect of Curcumin on Dose- limiting Toxicity and Pharmacokinetics of Irinotecan in Colorectal Cancer Patients | Dietary Supplement: curcumin | 23 | 1 | Basic Science | NA |
| Colorectal Cancer | Drug: Irinotecan | ||||||
| NCT01333917 | CRC patients | Curcumin Chemoprev ‘ntki o’ Colorectal Neoplasia | Drug: Curcumin C3 tablet | 40 | 1 | Curcumin | NA |
| Biomarkers | |||||||
| NCT02724202 | 5’Fu-Resistant | A Pilot, Feasibility Study of Curcumin in Combination With 5FU | Drug: Curcumin | 13 | 1 | Treatment | NA |
| Metastatic patients | for Patients With 5FU-Resistant Metastatic Colon Cancer | Drug: 5-flurorouracil | |||||
| NCT01490996 | Metastatic patients | A Phase I/IIa Study Combining Curcumin (Curcumin C3- Complex, Sabinsa) With Standard Care FOLFOX Chemotherapy in Patients With Inoperable Colorectal Cancer | Drug: Oral complex C3 curcumin + chemotherapy | 51 | 1,2 | Treatment | [161] |
| Drug: Chemotherapy only | |||||||
| NCT01294072 | CRC patients | Phase I Clinical Trial Investigating the Ability of Plant Exosomes co Deliver Curcumin to Normal and Malignant Colon Tissue | Dietary Supplement: curcumin | 7 | 1 | Treatment | [162] |
| Dietary Supplement: Curcumin conjugated with plant exosomes | |||||||
| NCT00118989 | AP | Phase II Double Blind Placebo-Controlled Trial of Curcuminoids’ Effect on Cellular Proliferation, Apoptosis and COX-2 Expression in the Colorectal Mucosa of Subjects With Recently Resected Sporadic Adenomatous Polyps | Dietary Supplement: Curcuminoids | 56 | NA | Prevention | NA |
| NCT00641147 | FAP | Curcumin for Treatment of Intestinal Adenomas in Familial Adenomatous Polyposis (FAP) | Drug: Curcumin | 44 | 2 | Treatment | [119] |
| NCT00927485 | FAP | Use of Curcumin for Treatment of Intestinal Adenomas in Familial Adenomatous Polyposis (FAP) | Drug: Calcumin (Curcumin) | 44 | NA | Treatment | NA |
| NCT00745134 | FAP | A Randomized Double Blinded Study of Curcumin with Pre-operative Capecitabine and Radiation Therapy Followed by Surgery for Rectal Cancer | Drug: Capecitabine | ||||
| Dietary Supplement: Curcumin | 45 | 2 | Treatment | NA |
FAP: Familial Adenomatous Polyposis; AP: Adenomatous Polyps; CRC: colorectal cancer; NA: Not applicable Data from ClinicalTrials.gov (https://clinicaltrials.gov)
3.3. Curcumin as a potential nature booster for development of effective immunotherapy against cancer
The PD-1/PD-L1 blockade is currently considered as a promising immune intervention for cancer therapy. Recent study has shown that curcumin can augment anti-tumor activity of the PD-1/PD-L1 blockade[124]. The expression of PD-L1 was significantly reduced in curcumin treated cancer cells. Furthermore, curcumin treatment can obviously improve anti-tumor immune response by increasing CD8 positive T cells and inhibiting T regulatory cells (Tregs) and Myeloid-derived suppressor cells (MDSCs).
It is believed that tumor cells can develop several strategies to escape from the immune surveillance. One of these strategies is to increase the population of Treg cells, which impaired the anti-tumor activity of immune effector cells, such as CD8+ T cells, natural killer cells. Therefore, inhibition of Treg cells is a promising approach for effective immunotherapy[125].In a clinical study, 40 colon cancer patients and 30 healthy subjects were recruited and treated with curcumin. The data showed the number of peripheral Tregs was significantly decreased, while Th1 cells increased. Furthermore, they demonstrated that curcumin treatment promotes conversion of Tregs to Th1 cells, as well as enhancing their IFN-γ production, highlighting the antitumor role of curcumin in colorectal cancer[81]. In line with above results, a recent clinical investigation in lung cancer patients (n=30) and control healthy patients (n=6) validate that curcumin treatment is capable of converting peripheral Tregs to Th1 cells[126]. Owing to the role of Tregs in cancer progression, curcumin may serve as a potential adjuvant immunotherapy for patients with malignant diseases.
4. The concerns with poor bioavailability of curcumin in cancer prevention and treatment
Although curcumin’s potential in cancer prevention and treatment is supported by plenty of preclinical studies or even clinical studies, there is some concern regarding the bioavailability of curcumin following oral ingestion. While this is a highly debated issue, poor bioavailability of curcumin is often attributed to poor absorption, rapid metabolism, and rapid systemic elimination, hampering its application as therapeutic agent [127, 128]. To overcome this practical limitation for the bioavailability of curcumin, numerous approaches have been adopted to enhance its systemic absorption [129]. As a result, several creative formulations of curcumin have been developed over the years to enhance its systemic bioavailability, including the use of liposomal curcumin, curcumin nanoparticles, curcumin phospholipid complexes and other structural analogues of curcumin [128]. For example, Chaurasia and colleagues developed polymeric nanoparticles of curcumin, called CENPs, which exhibited a significantly improved bioavailability vs. standard curcumin in a wistar rat model [130, 131]. Furthermore, oral administration of CENPs exhibited increased antitumor activity, as observed by reduced tumor growth and increased survival rate of animals with xenograft CRC [130, 131]. Likewise, another group identified a new structural analogue of curcumin, Dehydrozingerone. In-vitro studies showed that Dehydrozingerone led to the accumulation of intracellular ROS, induced cell-cycle arrest at the G2/M phase and up-regulated p21 expression in HT-29 cells, suggesting an anticancer potential of innovative curcumin analogues as potential chemotherapeutic agents in CRC [132]. On similar lines, a curcumin-phosphatidylcholine complex, exhibited increased absorption and high curcuminoid levels in plasma [133]. In an elegant study, while comparing the efficacy of this form of curcumin alone or together with oxaliplatin in oxaliplatin-resistant tumor mice revealed that the combination therapy had the most significant tumor suppressive effects [134]. These data suggest that such curcumin-phosphatidylcholine complexes may add to the clinical benefit of current chemotherapy regimens in CRC patients [134]. Table 3 summarizes various studies involving modified curcumin formulations with improved efficacy in colorectal cancer.
Table 3.
Curcumin formulations and their clinical or experimental outcome in colorectal cancer
| Formulations | Clinical/in vivo | outcome | Reference |
|---|---|---|---|
| Meriva (patented and commercialized); a complex of curcumin with phosphatidylcholine | tumor-bearing mice | enhanced oxaliplatin efficacy | [134] |
| CENPs (curcumin-loaded Eudragit E 100 nanoparticles); curcumin nanoparticles | tumor-bearing mice | improved bioavailability and anti-cancer efficacy of curcumin. | [131] |
| Curcumin C3 (standardized patented curcumin extract); a complex of curcumin, desmethoxycurcumin and bis- | MAC16 tumor-bearing mice | oral curcumin c3 results in the prevention and reversal of weight loss, implying curcumin c3 may be an effective adjuvant therapy against cachexia. | [163] |
| Desmethoxycurcumin; analogue of curcumin | HT-29 cells | accumulation of intracellular ROS, induced cell-cycle arrest at the G2/M phase and up-regulated p21 expression | [132] |
| Theracurmin (patented and commercialized); curcumin dispersed with colloidal submicron-particles | Apc-mutant mice | inhibited intestinal polyp development and suppressed MCP-1 and IL-6 mRNA expression levels in the parts of the intestine with polyps. | [164] |
| Lipocurc (patented and commercialized); liposomal curcumin | Clinical trial (32 patients | Phase I study. 300 mg/m2 liposomal curcumin over 6 h was the maximum tolerated dose, and recommended starting dose for anti-cancer trials. | [165] |
| Liposomal curcumin | tumor-bearing mice | significant tumor growth inhibition in Colo205 and LoVo xenografts; showed antiangiogenic effect, including reduction in CD31, VEGF and IL-8 | [166] |
| Phytosomal curcumin | CT-28 cells | anti-proliferative, anti-migratory and apoptotic activity in-vitro, and reduce tumor growth and enhance 5-fluorouracil (5-FU) anti-tumor effect in-vivo. | [167] |
| Curcumin loaded PEG nanoparticles | Caco-2 cells | antiproliferative effect and growth arrest, and significantly inhibited hTERT gene expression. | [168] |
As mentioned above, several phase I clinical studies have clearly showed the safety of oral curcumin. However, there are a couple of studies that have indicated potential caution with curcumin’s use in the clinic. It was found that high concentrations of curcumin could potentially provoke glutathione depletion and caspase-3 activation, and possibly result in hepatocytotoxicity [135]. In another study, it was reported that curcumin can interfere systemic iron metabolism by repressing hepcidin synthesis, indicating limited application of curcumin in cancer patients with marginal iron stores, chronic disease or anemia [136]. Nonetheless, overwhelming body of literature supports the safety of curcumin in clinical studies, as novel preparations of curcumin allow superior absorption and consequently improved efficacy in cancer.
CONCLUSIONS
In the past decades, curcumin has received global attention for its multi-targeted pharmacological activities. In this review article, we discussed various mechanisms of curcumin’s anti-cancer activity including its ability to modulate gut microbiome, improving intestinal barrier function and alleviating intestinal inflammation. Moreover, curcumin is considered as a potent modulator of autophagy, which can prevent transformation of normal colonic epithelium in the healthy colon. Furthermore, curcumin has the ability to target directly or indirectly multitude of pathways involved in the self-renewal of CSCs, suggesting its potential application in chemoprevention or as an adjunctive treatment to cytotoxic chemotherapy. Finally, we also described that curcumin is capable of regulating epigenetic machinery. Several studies have shown that cancer suppressive effects of curcumin in CRC depend on regulation of DNA methylation, histone modification or miRNA. To date, data from several clinical trials haven shown that curcumin may prevent CRC by reducing polyp number and size. Moreover, curcumin has shown tremendous synergistic effects on the efficacy of current anticancer drugs. To address potential concerns surrounding its bioavailability, several new curcumin formulations have been developed, which heighten the promise for this natural medicine to garner further attention as a safe and inexpensive option available to patients with chronic diseases including cancer, particularly for the prevention and treatment of colorectal cancer.
Sources of funding
The present work was supported by the grants R01 CA72851, CA181572, CA184792, CA202797, and U01 CA187956 from the National Cancer Institute, National Institutes of Health, pilot grants from the Baylor Sammons Cancer Center and Foundation, funds from the Baylor Scott & White Research Institute and financial support from the Beckman Research Institute of City of Hope, to Ajay Goel. This work was also supported by grants No.81672826, No. 81874179 from National Natural Science Foundation of China, No.2017YQ044 from Municipal Human Resources Development Program for Outstanding Young Talents in Medical and Health Sciences in Shanghai, 18PJD047 from Shanghai Pujiang Talent Plan to W Weng.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No Conflicts to disclose
Disclosures: The views expressed in the submitted article are the authors’ own and not an official position of the institutions or funders.
REFERENCES
- [1].Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, Znaor A, Bray F, Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods, Int J Cancer 144(8) (2019) 1941–1953. [DOI] [PubMed] [Google Scholar]
- [2].Obrand DI, Gordon PH, Incidence and patterns of recurrence following curative resection for colorectal carcinoma, Dis Colon Rectum 40(1) (1997) 15–24. [DOI] [PubMed] [Google Scholar]
- [3].O’Connell MJ, Campbell ME, Goldberg RM, Grothey A, Seitz JF, Benedetti JK, Andre T, Haller DG, Sargent DJ, Survival following recurrence in stage II and III colon cancer: findings from the ACCENT data set, J Clin Oncol 26(14) (2008) 2336–41. [DOI] [PubMed] [Google Scholar]
- [4].Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A, Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial, J Clin Oncol 27(19) (2009) 3109–16. [DOI] [PubMed] [Google Scholar]
- [5].Allen J, Sears CL, Impact of the gut microbiome on the genome and epigenome of colon epithelial cells: contributions to colorectal cancer development, Genome Med 11(1) (2019) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Farhana L, Banerjee HN, Verma M, Majumdar APN, Role of Microbiome in Carcinogenesis Process and Epigenetic Regulation of Colorectal Cancer, Methods Mol Biol 1856 (2018) 35–55. [DOI] [PubMed] [Google Scholar]
- [7].Chen GY, The Role of the Gut Microbiome in Colorectal Cancer, Clin Colon Rectal Surg 31(3) (2018) 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bachmeier BE, Killian PH, Melchart D, The Role of Curcumin in Prevention and Management of Metastatic Disease, Int J Mol Sci 19(6) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chikara S, Nagaprashantha LD, Singhal J, Horne D, Awasthi S, Singhal SS, Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment, Cancer Lett 413 (2018) 122–134. [DOI] [PubMed] [Google Scholar]
- [10].Allegra A, Innao V, Russo S, Gerace D, Alonci A, Musolino C, Anticancer Activity of Curcumin and Its Analogues: Preclinical and Clinical Studies, Cancer Invest 35(1) (2017) 1–22. [DOI] [PubMed] [Google Scholar]
- [11].Goel A, Aggarwal BB, Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs, Nutr Cancer 62(7) (2010) 919–30. [DOI] [PubMed] [Google Scholar]
- [12].Goel A, Jhurani S, Aggarwal BB, Multi-targeted therapy by curcumin: how spicy is it?, Mol Nutr Food Res 52(9) (2008) 1010–30. [DOI] [PubMed] [Google Scholar]
- [13].Goel A, Kunnumakkara AB, Aggarwal BB, Curcumin as “Curecumin”: from kitchen to clinic, Biochem Pharmacol 75(4) (2008) 787–809. [DOI] [PubMed] [Google Scholar]
- [14].Link A, Balaguer F, Shen Y, Lozano JJ, Leung HC, Boland CR, Goel A, Curcumin modulates DNA methylation in colorectal cancer cells, PLoS One 8(2) (2013) e57709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ravindranathan P, Pasham D, Balaji U, Cardenas J, Gu J, Toden S, Goel A, A combination of curcumin and oligomeric proanthocyanidins offer superior anti-tumorigenic properties in colorectal cancer, Sci Rep 8(1) (2018) 13869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shakibaei M, Buhrmann C, Kraehe P, Shayan P, Lueders C, Goel A, Curcumin chemosensitizes 5-fluorouracil resistant MMR-deficient human colon cancer cells in high density cultures, PLoS One 9(1) (2014) e85397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shakibaei M, Kraehe P, Popper B, Shayan P, Goel A, Buhrmann C, Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer, BMC Cancer 15 (2015) 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shakibaei M, Mobasheri A, Lueders C, Busch F, Shayan P, Goel A, Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-kappaB and Src protein kinase signaling pathways, PLoS One 8(2) (2013) e57218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Toden S, Okugawa Y, Buhrmann C, Nattamai D, Anguiano E, Baldwin N, Shakibaei M, Boland CR, Goel A, Novel Evidence for Curcumin and Boswellic Acid-Induced Chemoprevention through Regulation of miR-34a and miR-27a in Colorectal Cancer, Cancer Prev Res (Phila) 8(5) (2015) 431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Toden S, Okugawa Y, Jascur T, Wodarz D, Komarova NL, Buhrmann C, Shakibaei M, Boland CR, Goel A, Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer, Carcinogenesis 36(3) (2015) 355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Willenbacher E, Khan SZ, Mujica SCA, Trapani D, Hussain S, Wolf D, Willenbacher W, Spizzo G, Seeber A, Curcumin: New Insights into an Ancient Ingredient against Cancer, Int J Mol Sci 20(8) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hu RW, Carey EJ, Lindor KD, Tabibian JH, Curcumin in Hepatobiliary Disease: Pharmacotherapeutic Properties and Emerging Potential Clinical Applications, Ann Hepatol 16(6) (2017) 835–841. [DOI] [PubMed] [Google Scholar]
- [23].Goncalves PB, Romeiro NC, Multi-target natural products as alternatives against oxidative stress in Chronic Obstructive Pulmonary Disease (COPD), Eur J Med Chem 163 (2019) 911–931. [DOI] [PubMed] [Google Scholar]
- [24].Khan H, Ullah H, Nabavi SM, Mechanistic insights of hepatoprotective effects of curcumin: Therapeutic updates and future prospects, Food Chem Toxicol 124 (2019) 182–191. [DOI] [PubMed] [Google Scholar]
- [25].Chen CY, Kao CL, Liu CM, The Cancer Prevention, Anti-Inflammatory and Anti-Oxidation of Bioactive Phytochemicals Targeting the TLR4 Signaling Pathway, Int J Mol Sci 19(9) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chandran B, Goel A, A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis, Phytother Res 26(11) (2012) 1719–25. [DOI] [PubMed] [Google Scholar]
- [27].Sanmukhani J, Satodia V, Trivedi J, Patel T, Tiwari D, Panchal B, Goel A, Tripathi CB, Efficacy and safety of curcumin in major depressive disorder: a randomized controlled trial, Phytother Res 28(4) (2014) 579–85. [DOI] [PubMed] [Google Scholar]
- [28].Eke-Okoro UJ, Raffa RB, Pergolizzi JV Jr., Breve F, Taylor R Jr., Group NR, Curcumin in turmeric: Basic and clinical evidence for a potential role in analgesia, J Clin Pharm Ther 43(4) (2018) 460–466. [DOI] [PubMed] [Google Scholar]
- [29].Naeini MB, Momtazi AA, Jaafari MR, Johnston TP, Barreto G, Banach M, Sahebkar A, Antitumor effects of curcumin: A lipid perspective, J Cell Physiol (2019). [DOI] [PubMed] [Google Scholar]
- [30].Mirzaei H, Masoudifar A, Sahebkar A, Zare N, Sadri Nahand J, Rashidi B, Mehrabian E, Mohammadi M, Mirzaei HR, Jaafari MR, MicroRNA: A novel target of curcumin in cancer therapy, J Cell Physiol 233(4) (2018) 3004–3015. [DOI] [PubMed] [Google Scholar]
- [31].Carlos-Reyes A, Lopez-Gonzalez JS, Meneses-Flores M, Gallardo-Rincon D, Ruiz-Garcia E, Marchat LA, Astudillo-de la Vega H, Hernandez de la Cruz ON, Lopez-Camarillo C, Dietary Compounds as Epigenetic Modulating Agents in Cancer, Front Genet 10 (2019) 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bahrami A, Amerizadeh F, ShahidSales S, Khazaei M, Ghayour-Mobarhan M, Sadeghnia HR, Maftouh M, Hassanian SM, Avan A, Therapeutic Potential of Targeting Wnt/beta-Catenin Pathway in Treatment of Colorectal Cancer: Rational and Progress, J Cell Biochem 118(8) (2017) 1979–1983. [DOI] [PubMed] [Google Scholar]
- [33].Salehi B, Stojanovic-Radic Z, Matejic J, Sharifi-Rad M, Anil Kumar NV, Martins N, Sharifi-Rad J, The therapeutic potential of curcumin: A review of clinical trials, Eur J Med Chem 163 (2019) 527–545. [DOI] [PubMed] [Google Scholar]
- [34].Cani PD, Human gut microbiome: hopes, threats and promises, Gut 67(9) (2018) 1716–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rajagopala SV, Vashee S, Oldfield LM, Suzuki Y, Venter JC, Telenti A, Nelson KE, The Human Microbiome and Cancer, Cancer Prev Res (Phila) 10(4) (2017) 226–234. [DOI] [PubMed] [Google Scholar]
- [36].Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA, The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy, Cancer Cell 33(4) (2018) 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chen J, Zhao KN, Vitetta L, Effects of Intestinal Microbial(−)Elaborated Butyrate on Oncogenic Signaling Pathways, Nutrients 11(5) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chung L, Thiele Orberg E, Geis AL, Chan JL, Fu K, DeStefano Shields CE, Dejea CM, Fathi P, Chen J, Finard BB, Tam AJ, McAllister F, Fan H, Wu X, Ganguly S, Lebid A, Metz P, Van Meerbeke SW, Huso DL, Wick EC, Pardoll DM, Wan F, Wu S, Sears CL, Housseau F, Bacteroides fragilis Toxin Coordinates a Pro-carcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells, Cell Host Microbe 23(2) (2018) 203–214 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang S, Cai S, Ma Y, Association between Fusobacterium nucleatum and colorectal cancer: Progress and future directions, J Cancer 9(9) (2018) 1652–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yang Y, Weng W, Peng J, Hong L, Yang L, Toiyama Y, Gao R, Liu M, Yin M, Pan C, Li H, Guo B, Zhu Q, Wei Q, Moyer MP, Wang P, Cai S, Goel A, Qin H, Ma Y, Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-kappaB, and Up-regulating Expression of MicroRNA-21, Gastroenterology 152(4) (2017) 851–866 e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Brennan CA, Garrett WS, Fusobacterium nucleatum - symbiont, opportunist and oncobacterium, Nat Rev Microbiol 17(3) (2019) 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gholizadeh P, Eslami H, Kafil HS, Carcinogenesis mechanisms of Fusobacterium nucleatum, Biomed Pharmacother 89 (2017) 918–925. [DOI] [PubMed] [Google Scholar]
- [43].Zhou Z, Chen J, Yao H, Hu H, Fusobacterium and Colorectal Cancer, Front Oncol 8 (2018) 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ohno M, Nishida A, Sugitani Y, Nishino K, Inatomi O, Sugimoto M, Kawahara M, Andoh A, Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells, PLoS One 12(10) (2017) e0185999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H, Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells, Nature 504(7480) (2013) 446–50. [DOI] [PubMed] [Google Scholar]
- [46].Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K, Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota, Nature 500(7461) (2013) 232–6. [DOI] [PubMed] [Google Scholar]
- [47].Peterson CT, Vaughn AR, Sharma V, Chopra D, Mills PJ, Peterson SN, Sivamani RK, Effects of Turmeric and Curcumin Dietary Supplementation on Human Gut Microbiota: A Double-Blind, Randomized, Placebo-Controlled Pilot Study, J Evid Based Integr Med 23 (2018) 2515690X18790725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mori G, Orena BS, Cultrera I, Barbieri G, Albertini AM, Ranzani GN, Carnevali I, Tibiletti MG, Pasca MR, Gut Microbiota Analysis in Postoperative Lynch Syndrome Patients, Front Microbiol 10 (2019) 1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Youssef O, Lahti L, Kokkola A, Karla T, Tikkanen M, Ehsan H, Carpelan-Holmstrom M, Koskensalo S, Bohling T, Rautelin H, Puolakkainen P, Knuutila S, Sarhadi V, Stool Microbiota Composition Differs in Patients with Stomach, Colon, and Rectal Neoplasms, Dig Dis Sci 63(11) (2018) 2950–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Li S, Fu C, Zhao Y, He J, Intervention with alpha-Ketoglutarate Ameliorates Colitis-Related Colorectal Carcinoma via Modulation of the Gut Microbiome, Biomed Res Int 2019 (2019) 8020785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].McFadden RM, Larmonier CB, Shehab KW, Midura-Kiela M, Ramalingam R, Harrison CA, Besselsen DG, Chase JH, Caporaso JG, Jobin C, Ghishan FK, Kiela PR, The Role of Curcumin in Modulating Colonic Microbiota During Colitis and Colon Cancer Prevention, Inflamm Bowel Dis 21(11) (2015) 2483–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Burge K, Gunasekaran A, Eckert J, Chaaban H, Curcumin and Intestinal Inflammatory Diseases: Molecular Mechanisms of Protection, Int J Mol Sci 20(8) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Takiishi T, Fenero CIM, Camara NOS, Intestinal barrier and gut microbiota: Shaping our immune responses throughout life, Tissue Barriers 5(4) (2017) e1373208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Salvo Romero E, Alonso Cotoner C, Pardo Camacho C, Casado Bedmar M, Vicario M, The intestinal barrier function and its involvement in digestive disease, Rev Esp Enferm Dig 107(11) (2015) 686–96. [DOI] [PubMed] [Google Scholar]
- [55].Wang J, Ghosh SS, Ghosh S, Curcumin improves intestinal barrier function: modulation of intracellular signaling, and organization of tight junctions, Am J Physiol Cell Physiol 312(4) (2017) C438–C445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wang N, Wang G, Hao J, Ma J, Wang Y, Jiang X, Jiang H, Curcumin ameliorates hydrogen peroxide-induced epithelial barrier disruption by upregulating heme oxygenase-1 expression in human intestinal epithelial cells, Dig Dis Sci 57(7) (2012) 1792–801. [DOI] [PubMed] [Google Scholar]
- [57].Ghosh SS, Bie J, Wang J, Ghosh S, Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR−/−mice--role of intestinal permeability and macrophage activation, PLoS One 9(9) (2014) e108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Li TT, Ogino S, Qian ZR, Toll-like receptor signaling in colorectal cancer: carcinogenesis to cancer therapy, World J Gastroenterol 20(47) (2014) 17699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Yesudhas D, Gosu V, Anwar MA, Choi S, Multiple roles of toll-like receptor 4 in colorectal cancer, Front Immunol 5 (2014) 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lu YC, Yeh WC, Ohashi PS, LPS/TLR4 signal transduction pathway, Cytokine 42(2) (2008) 145–151. [DOI] [PubMed] [Google Scholar]
- [61].Zhu HT, Bian C, Yuan JC, Chu WH, Xiang X, Chen F, Wang CS, Feng H, Lin JK, Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-kappaB signaling pathway in experimental traumatic brain injury, J Neuroinflammation 11 (2014) 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Fu Y, Gao R, Cao Y, Guo M, Wei Z, Zhou E, Li Y, Yao M, Yang Z, Zhang N, Curcumin attenuates inflammatory responses by suppressing TLR4-mediated NF-kappaB signaling pathway in lipopolysaccharide-induced mastitis in mice, Int Immunopharmacol 20(1) (2014) 54–8. [DOI] [PubMed] [Google Scholar]
- [63].Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, Sartor RB, Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity, J Immunol 163(6) (1999) 3474–83. [PubMed] [Google Scholar]
- [64].Moon DO, Jin CY, Lee JD, Choi YH, Ahn SC, Lee CM, Jeong SC, Park YM, Kim GY, Curcumin decreases binding of Shiga-like toxin-1B on human intestinal epithelial cell line HT29 stimulated with TNF-alpha and IL-1beta: suppression of p38, JNK and NF-kappaB p65 as potential targets, Biol Pharm Bull 29(7) (2006) 1470–5. [DOI] [PubMed] [Google Scholar]
- [65].Shi C, Yang Y, Xia Y, Okugawa Y, Yang J, Liang Y, Chen H, Zhang P, Wang F, Han H, Wu W, Gao R, Gasche C, Qin H, Ma Y, Goel A, Novel evidence for an oncogenic role of microRNA-21 in colitis-associated colorectal cancer, Gut 65(9) (2016) 1470–81. [DOI] [PubMed] [Google Scholar]
- [66].Schaefer JS, Attumi T, Opekun AR, Abraham B, Hou J, Shelby H, Graham DY, Streckfus C, Klein JR, MicroRNA signatures differentiate Crohn’s disease from ulcerative colitis, BMC Immunol 16 (2015) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Thorlacius-Ussing G, Schnack Nielsen B, Andersen V, Holmstrom K, Pedersen AE, Expression and Localization of miR-21 and miR-126 in Mucosal Tissue from Patients with Inflammatory Bowel Disease, Inflamm Bowel Dis 23(5) (2017) 739–752. [DOI] [PubMed] [Google Scholar]
- [68].Mudduluru G, George-William JN, Muppala S, Asangani IA, Kumarswamy R, Nelson LD, Allgayer H, Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer, Biosci Rep 31(3) (2011) 185–97. [DOI] [PubMed] [Google Scholar]
- [69].Cherukuri DP, Ishikawa TO, Chun P, Catapang A, Elashoff D, Grogan TR, Bugni J, Herschman HR, Targeted Cox2 gene deletion in intestinal epithelial cells decreases tumorigenesis in female, but not male, ApcMin/+ mice, Mol Oncol 8(2) (2014) 169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Xiang L, Wang S, Jin X, Duan W, Ding X, Zheng C, Expression of BMP2, TLR3, TLR4 and COX2 in colorectal polyps, adenoma and adenocarcinoma, Mol Med Rep 6(5) (2012) 973–6. [DOI] [PubMed] [Google Scholar]
- [71].Cox DG, Pontes C, Guino E, Navarro M, Osorio A, Canzian F, Moreno V, Bellvitge G Colorectal Cancer Study, Polymorphisms in prostaglandin synthase 2/cyclooxygenase 2 (PTGS2/COX2) and risk of colorectal cancer, Br J Cancer 91(2) (2004) 339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Goel A, Boland CR, Chauhan DP, Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells, Cancer Lett 172(2) (2001) 111–8. [DOI] [PubMed] [Google Scholar]
- [73].Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, Howells L, Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex, Oncogene 18(44) (1999) 6013–20. [DOI] [PubMed] [Google Scholar]
- [74].Villegas I, Sanchez-Fidalgo S, de la Lastra CA, Chemopreventive effect of dietary curcumin on inflammation-induced colorectal carcinogenesis in mice, Mol Nutr Food Res 55(2) (2011) 259–67. [DOI] [PubMed] [Google Scholar]
- [75].Kubota M, Shimizu M, Sakai H, Yasuda Y, Terakura D, Baba A, Ohno T, Tsurumi H, Tanaka T, Moriwaki H, Preventive effects of curcumin on the development of azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db obese mice, Nutr Cancer 64(1) (2012) 72–9. [DOI] [PubMed] [Google Scholar]
- [76].Zhong X, Chen B, Yang Z, The Role of Tumor-Associated Macrophages in Colorectal Carcinoma Progression, Cell Physiol Biochem 45(1) (2018) 356–365. [DOI] [PubMed] [Google Scholar]
- [77].Norton SE, Ward-Hartstonge KA, Taylor ES, Kemp RA, Immune cell interplay in colorectal cancer prognosis, World J Gastrointest Oncol 7(10) (2015) 221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Gao S, Zhou J, Liu N, Wang L, Gao Q, Wu Y, Zhao Q, Liu P, Wang S, Liu Y, Guo N, Shen Y, Wu Y, Yuan Z, Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13, J Mol Cell Cardiol 85 (2015) 131–9. [DOI] [PubMed] [Google Scholar]
- [79].Zhou Y, Zhang T, Wang X, Wei X, Chen Y, Guo L, Zhang J, Wang C, Curcumin Modulates Macrophage Polarization Through the Inhibition of the Toll-Like Receptor 4 Expression and its Signaling Pathways, Cell Physiol Biochem 36(2) (2015) 631–41. [DOI] [PubMed] [Google Scholar]
- [80].Mohammadi A, Blesso CN, Barreto GE, Banach M, Majeed M, Sahebkar A, Macrophage plasticity, polarization and function in response to curcumin, a diet-derived polyphenol, as an immunomodulatory agent, J Nutr Biochem 66 (2019) 1–16. [DOI] [PubMed] [Google Scholar]
- [81].Xu B, Yu L, Zhao LZ, Curcumin up regulates T helper 1 cells in patients with colon cancer, Am J Transl Res 9(4) (2017) 1866–1875. [PMC free article] [PubMed] [Google Scholar]
- [82].Rahimi K, Ahmadi A, Hassanzadeh K, Soleimani Z, Sathyapalan T, Mohammadi A, Sahebkar A, Targeting the balance of T helper cell responses by curcumin in inflammatory and autoimmune states, Autoimmun Rev 18(7) (2019) 738–748. [DOI] [PubMed] [Google Scholar]
- [83].Shafabakhsh R, Pourhanifeh MH, Mirzaei HR, Sahebkar A, Asemi Z, Mirzaei H, Targeting regulatory T cells by curcumin: A potential for cancer immunotherapy, Pharmacol Res 147 (2019) 104353. [DOI] [PubMed] [Google Scholar]
- [84].Burada F, Nicoli ER, Ciurea ME, Uscatu DC, Ioana M, Gheonea DI, Autophagy in colorectal cancer: An important switch from physiology to pathology, World J Gastrointest Oncol 7(11) (2015) 271–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Chen HT, Liu H, Mao MJ, Tan Y, Mo XQ, Meng XJ, Cao MT, Zhong CY, Liu Y, Shan H, Jiang GM, Crosstalk between autophagy and epithelial-mesenchymal transition and its application in cancer therapy, Mol Cancer 18(1) (2019) 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Udristioiu A, Nica-Badea D, Autophagy dysfunctions associated with cancer cells and their therapeutic implications, Biomed Pharmacother 115 (2019) 108892. [DOI] [PubMed] [Google Scholar]
- [87].Condello M, Pellegrini E, Caraglia M, Meschini S, Targeting Autophagy to Overcome Human Diseases, Int J Mol Sci 20(3) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Liu LD, Pang YX, Zhao XR, Li R, Jin CJ, Xue J, Dong RY, Liu PS, Curcumin induces apoptotic cell death and protective autophagy by inhibiting AKT/mTOR/p70S6K pathway in human ovarian cancer cells, Arch Gynecol Obstet 299(6) (2019) 1627–1639. [DOI] [PubMed] [Google Scholar]
- [89].Liu F, Gao S, Yang Y, Zhao X, Fan Y, Ma W, Yang D, Yang A, Yu Y, Antitumor activity of curcumin by modulation of apoptosis and autophagy in human lung cancer A549 cells through inhibiting PI3K/Akt/mTOR pathway, Oncol Rep 39(3) (2018) 1523–1531. [DOI] [PubMed] [Google Scholar]
- [90].White E, Autophagy and p53, Cold Spring Harb Perspect Med 6(4) (2016) a026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].He ZY, Shi CB, Wen H, Li FL, Wang BL, Wang J, Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin, Cancer Invest 29(3) (2011) 208–13. [DOI] [PubMed] [Google Scholar]
- [92].Sun J, Feng Y, Wang Y, Ji Q, Cai G, Shi L, Wang Y, Huang Y, Zhang J, Li Q, alpha-hederin induces autophagic cell death in colorectal cancer cells through reactive oxygen species dependent AMPK/mTOR signaling pathway activation, Int J Oncol 54(5) (2019) 1601–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Sun X, Zhu MJ, AMP-activated protein kinase: a therapeutic target in intestinal diseases, Open Biol 7(8) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Song X, Kim SY, Zhang L, Tang D, Bartlett DL, Kwon YT, Lee YJ, Role of AMP-activated protein kinase in cross-talk between apoptosis and autophagy in human colon cancer, Cell Death Dis 5 (2014) e1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Thent ZC, Zaidun NH, Azmi MF, Senin MI, Haslan H, Salehuddin R, Is Metformin a Therapeutic Paradigm for Colorectal Cancer: Insight into the Molecular Pathway?, Curr Drug Targets 18(6) (2017) 734–750. [DOI] [PubMed] [Google Scholar]
- [96].Liu Z, Cui C, Xu P, Dang R, Cai H, Liao D, Yang M, Feng Q, Yan X, Jiang P, Curcumin Activates AMPK Pathway and Regulates Lipid Metabolism in Rats Following Prolonged Clozapine Exposure, Front Neurosci 11 (2017) 558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Zhu P, Fan Z, Cancer stem cells and tumorigenesis, Biophys Rep 4(4) (2018) 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Munro MJ, Wickremesekera SK, Peng L, Tan ST, Itinteang T, Cancer stem cells in colorectal cancer: a review, J Clin Pathol 71(2) (2018) 110–116. [DOI] [PubMed] [Google Scholar]
- [99].Visvader JE, Lindeman GJ, Cancer stem cells: current status and evolving complexities, Cell Stem Cell 10(6) (2012) 717–728. [DOI] [PubMed] [Google Scholar]
- [100].Boman BM, Walters R, Fields JZ, Kovatich AJ, Zhang T, Isenberg GA, Goldstein SD, Palazzo JP, Colonic crypt changes during adenoma development in familial adenomatous polyposis: immunohistochemical evidence for expansion of the crypt base cell population, Am J Pathol 165(5) (2004) 1489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Nautiyal J, Kanwar SS, Yu Y, Majumdar AP, Combination of dasatinib and curcumin eliminates chemo-resistant colon cancer cells, J Mol Signal 6 (2011) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Huang YT, Lin YW, Chiu HM, Chiang BH, Curcumin Induces Apoptosis of Colorectal Cancer Stem Cells by Coupling with CD44 Marker, J Agric Food Chem 64(11) (2016) 2247–53. [DOI] [PubMed] [Google Scholar]
- [103].Buhrmann C, Kraehe P, Lueders C, Shayan P, Goel A, Shakibaei M, Curcumin suppresses crosstalk between colon cancer stem cells and stromal fibroblasts in the tumor microenvironment: potential role of EMT, PLoS One 9(9) (2014) e107514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ramasamy TS, Ayob AZ, Myint HH, Thiagarajah S, Amini F, Targeting colorectal cancer stem cells using curcumin and curcumin analogues: insights into the mechanism of the therapeutic efficacy, Cancer Cell Int 15 (2015) 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kagohara LT, Stein-O’Brien GL, Kelley D, Flam E, Wick HC, Danilova LV, Easwaran H, Favorov AV, Qian J, Gaykalova DA, Fertig EJ, Epigenetic regulation of gene expression in cancer: techniques, resources and analysis, Brief Funct Genomics 17(1) (2018) 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Esteller M, Epigenetics in cancer, N Engl J Med 358(11) (2008) 1148–59. [DOI] [PubMed] [Google Scholar]
- [107].Guo Y, Shu L, Zhang C, Su ZY, Kong AN, Curcumin inhibits anchorage-independent growth of HT29 human colon cancer cells by targeting epigenetic restoration of the tumor suppressor gene DLEC1, Biochem Pharmacol 94(2) (2015) 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Jiang A, Wang X, Shan X, Li Y, Wang P, Jiang P, Feng Q, Curcumin Reactivates Silenced Tumor Suppressor Gene RARbeta by Reducing DNA Methylation, Phytother Res 29(8) (2015) 1237–45. [DOI] [PubMed] [Google Scholar]
- [109].Chatterjee B, Ghosh K, Kanade SR, Curcumin-mediated demethylation of the proximal promoter CpG island enhances the KLF4 recruitment that leads to increased expression of p21Cip1 in vitro, J Cell Biochem 120(1) (2019) 809–820. [DOI] [PubMed] [Google Scholar]
- [110].Guo Y, Wu R, Gaspar JM, Sargsyan D, Su ZY, Zhang C, Gao L, Cheng D, Li W, Wang C, Yin R, Fang M, Verzi MP, Hart RP, Kong AN, DNA methylome and transcriptome alterations and cancer prevention by curcumin in colitis-accelerated colon cancer in mice, Carcinogenesis 39(5) (2018) 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Xu WS, Parmigiani RB, Marks PA, Histone deacetylase inhibitors: molecular mechanisms of action, Oncogene 26(37) (2007) 5541–52. [DOI] [PubMed] [Google Scholar]
- [112].Singh A, Patel P, Patel VK, Jain DK, Veerasamy R, Sharma PC, Rajak H, Histone Deacetylase Inhibitors for the Treatment of Colorectal Cancer: Recent Progress and Future Prospects, Curr Cancer Drug Targets 17(5) (2017) 456–466. [DOI] [PubMed] [Google Scholar]
- [113].Bora-Tatar G, Dayangac-Erden D, Demir AS, Dalkara S, Yelekci K, Erdem-Yurter H, Molecular modifications on carboxylic acid derivatives as potent histone deacetylase inhibitors: Activity and docking studies, Bioorg Med Chem 17(14) (2009) 5219–28. [DOI] [PubMed] [Google Scholar]
- [114].Chen CQ, Yu K, Yan QX, Xing CY, Chen Y, Yan Z, Shi YF, Zhao KW, Gao SM, Pure curcumin increases the expression of SOCS1 and SOCS3 in myeloproliferative neoplasms through suppressing class I histone deacetylases, Carcinogenesis 34(7) (2013) 1442–9. [DOI] [PubMed] [Google Scholar]
- [115].Chen Y, Shu W, Chen W, Wu Q, Liu H, Cui G, Curcumin, both histone deacetylase and p300/CBP-specific inhibitor, represses the activity of nuclear factor kappa B and Notch 1 in Raji cells, Basic Clin Pharmacol Toxicol 101(6) (2007) 427–33. [DOI] [PubMed] [Google Scholar]
- [116].Hu J, Wang Y, Chen Y, Curcumin-induced histone acetylation in malignant hematologic cells, J Huazhong Univ Sci Technolog Med Sci 29(1) (2009) 25–8. [DOI] [PubMed] [Google Scholar]
- [117].Gandhy SU, Kim K, Larsen L, Rosengren RJ, Safe S, Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp) transcription factors by targeting microRNAs, BMC Cancer 12 (2012) 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, Kakarala M, Carpenter PM, McLaren C, Meyskens FL Jr., Brenner DE, Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia, Cancer Prev Res (Phila) 4(3) (2011) 354–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Cruz-Correa M, Hylind LM, Marrero JH, Zahurak ML, Murray-Stewart T, Casero RA Jr., Montgomery EA, Iacobuzio-Donahue C, Brosens LA, Offerhaus GJ, Umar A, Rodriguez LM, Giardiello FM, Efficacy and Safety of Curcumin in Treatment of Intestinal Adenomas in Patients With Familial Adenomatous Polyposis, Gastroenterology 155(3) (2018) 668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, Giardiello FM, Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis, Clin Gastroenterol Hepatol 4(8) (2006) 1035–8. [DOI] [PubMed] [Google Scholar]
- [121].Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, Pirmohamed M, Gescher AJ, Steward WP, Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance, Clin Cancer Res 10(20) (2004) 6847–54. [DOI] [PubMed] [Google Scholar]
- [122].Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, Pirmohamed M, Marnett LJ, Gescher AJ, Steward WP, Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer, Clin Cancer Res 7(7) (2001) 1894–900. [PubMed] [Google Scholar]
- [123].James MI, Iwuji C, Irving G, Karmokar A, Higgins JA, Griffin-Teal N, Thomas A, Greaves P, Cai H, Patel SR, Morgan B, Dennison A, Metcalfe M, Garcea G, Lloyd DM, Berry DP, Steward WP, Howells LM, Brown K, Curcumin inhibits cancer stem cell phenotypes in ex vivo models of colorectal liver metastases, and is clinically safe and tolerable in combination with FOLFOX chemotherapy, Cancer Lett 364(2) (2015) 135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Liao F, Liu L, Luo E, Hu J, Curcumin enhances anti-tumor immune response in tongue squamous cell carcinoma, Arch Oral Biol 92 (2018) 32–37. [DOI] [PubMed] [Google Scholar]
- [125].Tanaka A, Sakaguchi S, Regulatory T cells in cancer immunotherapy, Cell Res 27(1) (2017) 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Zou JY, Su CH, Luo HH, Lei YY, Zeng B, Zhu HS, Chen ZG, Curcumin converts Foxp3+ regulatory T cells to T helper 1 cells in patients with lung cancer, J Cell Biochem 119(2) (2018) 1420–1428. [DOI] [PubMed] [Google Scholar]
- [127].Liu W, Zhai Y, Heng X, Che FY, Chen W, Sun D, Zhai G, Oral bioavailability of curcumin: problems and advancements, J Drug Target 24(8) (2016) 694–702. [DOI] [PubMed] [Google Scholar]
- [128].Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB, Bioavailability of curcumin: problems and promises, Mol Pharm 4(6) (2007) 807–18. [DOI] [PubMed] [Google Scholar]
- [129].Toden S, Goel A, The Holy Grail of Curcumin and its Efficacy in Various Diseases: Is Bioavailability Truly a Big Concern?, J Restor Med 6(1) (2017) 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Jayaprakasha GK, Chidambara Murthy KN, Patil BS, Enhanced colon cancer chemoprevention of curcumin by nanoencapsulation with whey protein, Eur J Pharmacol 789 (2016) 291–300. [DOI] [PubMed] [Google Scholar]
- [131].Chaurasia S, Chaubey P, Patel RR, Kumar N, Mishra B, Curcumin-polymeric nanoparticles against colon-26 tumor-bearing mice: cytotoxicity, pharmacokinetic and anticancer efficacy studies, Drug Dev Ind Pharm 42(5) (2016) 694–700. [DOI] [PubMed] [Google Scholar]
- [132].Yogosawa S, Yamada Y, Yasuda S, Sun Q, Takizawa K, Sakai T, Dehydrozingerone, a structural analogue of curcumin, induces cell-cycle arrest at the G2/M phase and accumulates intracellular ROS in HT-29 human colon cancer cells, J Nat Prod 75(12) (2012) 2088–93. [DOI] [PubMed] [Google Scholar]
- [133].Cuomo J, Appendino G, Dern AS, Schneider E, McKinnon TP, Brown MJ, Togni S, Dixon BM, Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation, J Nat Prod 74(4) (2011) 664–9. [DOI] [PubMed] [Google Scholar]
- [134].Howells LM, Sale S, Sriramareddy SN, Irving GR, Jones DJ, Ottley CJ, Pearson DG, Mann CD, Manson MM, Berry DP, Gescher A, Steward WP, Brown K, Curcumin ameliorates oxaliplatin-induced chemoresistance in HCT116 colorectal cancer cells in vitro and in vivo, Int J Cancer 129(2) (2011) 476–86. [DOI] [PubMed] [Google Scholar]
- [135].Ghoneim AI, Effects of curcumin on ethanol-induced hepatocyte necrosis and apoptosis: implication of lipid peroxidation and cytochrome c, Naunyn Schmiedebergs Arch Pharmacol 379(1) (2009) 47–60. [DOI] [PubMed] [Google Scholar]
- [136].Jiao Y, Wilkinson J.t., Di X, Wang W, Hatcher H, Kock ND, D’Agostino R Jr., Knovich MA, Torti FM, Torti SV, Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator, Blood 113(2) (2009) 462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Zhang C, He LJ, Ye HZ, Liu DF, Zhu YB, Miao DD, Zhang SP, Chen YY, Jia YW, Shen J, Liu XP, Nrf2 is a key factor in the reversal effect of curcumin on multidrug resistance in the HCT8/5Fu human colorectal cancer cell line, Mol Med Rep 18(6) (2018) 5409–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Zhu J, Zhao B, Xiong P, Wang C, Zhang J, Tian X, Huang Y, Curcumin Induces Autophagy via Inhibition of Yes-Associated Protein (YAP) in Human Colon Cancer Cells, Med Sci Monit 24 (2018) 7035–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Su P, Yang Y, Wang G, Chen X, Ju Y, Curcumin attenuates resistance to irinotecan via induction of apoptosis of cancer stem cells in chemoresistant colon cancer cells, Int J Oncol 53(3) (2018) 1343–1353. [DOI] [PubMed] [Google Scholar]
- [140].Lee YH, Song NY, Suh J, Kim DH, Kim W, Ann J, Lee J, Baek JH, Na HK, Surh YJ, Curcumin suppresses oncogenicity of human colon cancer cells by covalently modifying the cysteine 67 residue of SIRT1, Cancer Lett 431 (2018) 219–229. [DOI] [PubMed] [Google Scholar]
- [141].Huang YF, Zhu DJ, Chen XW, Chen QK, Luo ZT, Liu CC, Wang GX, Zhang WJ, Liao NZ, Curcumin enhances the effects of irinotecan on colorectal cancer cells through the generation of reactive oxygen species and activation of the endoplasmic reticulum stress pathway, Oncotarget 8(25) (2017) 40264–40275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Tong W, Wang Q, Sun D, Suo J, Curcumin suppresses colon cancer cell invasion via AMPK-induced inhibition of NF-kappaB, uPA activator and MMP9, Oncol Lett 12(5) (2016) 4139–4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Adeyeni TA, Khatwani N, San K, Ezekiel UR, BMI1 is downregulated by the natural compound curcumin, but not by bisdemethoxycurcumin and dimethoxycurcumin, Physiol Rep 4(16) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Ruiz de Porras V, Bystrup S, Martinez-Cardus A, Pluvinet R, Sumoy L, Howells L, James MI, Iwuji C, Manzano JL, Layos L, Buges C, Abad A, Martinez-Balibrea E, Curcumin mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell lines through modulation of CXC-Chemokine/NF-kappaB signalling pathway, Sci Rep 6 (2016) 24675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Zhang Z, Chen H, Xu C, Song L, Huang L, Lai Y, Wang Y, Chen H, Gu D, Ren L, Yao Q, Curcumin inhibits tumor epithelialmesenchymal transition by downregulating the Wnt signaling pathway and upregulating NKD2 expression in colon cancer cells, Oncol Rep 35(5) (2016) 2615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Guo LD, Shen YQ, Zhao XH, Guo LJ, Yu ZJ, Wang D, Liu LM, Liu JZ, Curcumin combined with oxaliplatin effectively suppress colorectal carcinoma in vivo through inducing apoptosis, Phytother Res 29(3) (2015) 357–65. [DOI] [PubMed] [Google Scholar]
- [147].Wang K, Fan H, Chen Q, Ma G, Zhu M, Zhang X, Zhang Y, Yu J, Curcumin inhibits aerobic glycolysis and induces mitochondrial-mediated apoptosis through hexokinase II in human colorectal cancer cells in vitro, Anticancer Drugs 26(1) (2015) 15–24. [DOI] [PubMed] [Google Scholar]
- [148].Jiang QG, Li TY, Liu DN, Zhang HT, PI3K/Akt pathway involving into apoptosis and invasion in human colon cancer cells LoVo, Mol Biol Rep 41(5) (2014) 3359–67. [DOI] [PubMed] [Google Scholar]
- [149].Chen CC, Sureshbabul M, Chen HW, Lin YS, Lee JY, Hong QS, Yang YC, Yu SL, Curcumin Suppresses Metastasis via Sp-1, FAK Inhibition, and E-Cadherin Upregulation in Colorectal Cancer, Evid Based Complement Alternat Med 2013 (2013) 541695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Shehzad A, Lee J, Huh TL, Lee YS, Curcumin induces apoptosis in human colorectal carcinoma (HCT-15) cells by regulating expression of Prp4 and p53, Mol Cells 35(6) (2013) 526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Blakemore LM, Boes C, Cordell R, Manson MM, Curcumin-induced mitotic arrest is characterized by spindle abnormalities, defects in chromosomal congression and DNA damage, Carcinogenesis 34(2) (2013) 351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Lu JJ, Cai YJ, Ding J, Curcumin induces DNA damage and caffeine-insensitive cell cycle arrest in colorectal carcinoma HCT116 cells, Mol Cell Biochem 354(1–2) (2011) 247–52. [DOI] [PubMed] [Google Scholar]
- [153].Watson JL, Hill R, Yaffe PB, Greenshields A, Walsh M, Lee PW, Giacomantonio CA, Hoskin DW, Curcumin causes superoxide anion production and p53-independent apoptosis in human colon cancer cells, Cancer Lett 297(1) (2010) 1–8. [DOI] [PubMed] [Google Scholar]
- [154].Patel BB, Sengupta R, Qazi S, Vachhani H, Yu Y, Rishi AK, Majumdar AP, Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R, Int J Cancer 122(2) (2008) 267–73. [DOI] [PubMed] [Google Scholar]
- [155].Sandur SK, Deorukhkar A, Pandey MK, Pabon AM, Shentu S, Guha S, Aggarwal BB, Krishnan S, Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-kappaB activity, Int J Radiat Oncol Biol Phys 75(2) (2009) 534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Johnson SM, Gulhati P, Arrieta I, Wang X, Uchida T, Gao T, Evers BM, Curcumin inhibits proliferation of colorectal carcinoma by modulating Akt/mTOR signaling, Anticancer Res 29(8) (2009) 3185–90. [PMC free article] [PubMed] [Google Scholar]
- [157].Kunnumakkara AB, Diagaradjane P, Anand P, Harikumar KB, Deorukhkar A, Gelovani J, Guha S, Krishnan S, Aggarwal BB, Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model, Int J Cancer 125(9) (2009) 2187–97. [DOI] [PubMed] [Google Scholar]
- [158].Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumdar AP, Dou QP, Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo, Cancer Res 68(18) (2008) 7283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar A, Shentu S, Aggarwal BB, Krishnan S, Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products, Clin Cancer Res 14(7) (2008) 2128–36. [DOI] [PubMed] [Google Scholar]
- [160].Yin J, Wang L, Wang Y, Shen H, Wang X, Wu L, Curcumin reverses oxaliplatin resistance in human colorectal cancer via regulation of TGF-beta/Smad2/3 signaling pathway, Onco Targets Ther 12 (2019) 3893–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Irving GR, Iwuji CO, Morgan B, Berry DP, Steward WP, Thomas A, Brown K, Howells LM, Combining curcumin (C3-complex, Sabinsa) with standard care FOLFOX chemotherapy in patients with inoperable colorectal cancer (CUFOX): study protocol for a randomised control trial, Trials 16 (2015) 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Wu K, Xing F, Wu SY, Watabe K, Extracellular vesicles as emerging targets in cancer: Recent development from bench to bedside, Biochim Biophys Acta Rev Cancer 1868(2) (2017) 538–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Siddiqui RA, Hassan S, Harvey KA, Rasool T, Das T, Mukerji P, DeMichele S, Attenuation of proteolysis and muscle wasting by curcumin c3 complex in MAC16 colon tumour-bearing mice, Br J Nutr 102(7) (2009) 967–75. [DOI] [PubMed] [Google Scholar]
- [164].Adachi S, Hamoya T, Fujii G, Narita T, Komiya M, Miyamoto S, Kurokawa Y, Takahashi M, Takayama T, Ishikawa H, Tashiro K, Mutoh M, Theracurmin inhibits intestinal polyp development in Apc-mutant mice by inhibiting inflammation-related factors, Cancer Sci (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Greil R, Greil-Ressler S, Weiss L, Schonlieb C, Magnes T, Radl B, Bolger GT, Vcelar B, Sordillo PP, A phase 1 dose-escalation study on the safety, tolerability and activity of liposomal curcumin (Lipocurc()) in patients with locally advanced or metastatic cancer, Cancer Chemother Pharmacol 82(4) (2018) 695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Li L, Ahmed B, Mehta K, Kurzrock R, Liposomal curcumin with and without oxaliplatin: effects on cell growth, apoptosis, and angiogenesis in colorectal cancer, Mol Cancer Ther 6(4) (2007) 1276–82. [DOI] [PubMed] [Google Scholar]
- [167].Moradi-Marjaneh R, Hassanian SM, Rahmani F, Aghaee-Bakhtiari SH, Avan A, Khazaei M, Phytosomal Curcumin Elicits Anti-tumor Properties Through Suppression of Angiogenesis, Cell Proliferation and Induction of Oxidative Stress in Colorectal Cancer, Curr Pharm Des 24(39) (2018) 4626–4638. [DOI] [PubMed] [Google Scholar]
- [168].Lotfi-Attari J, Pilehvar-Soltanahmadi Y, Dadashpour M, Alipour S, Farajzadeh R, Javidfar S, Zarghami N, Co-Delivery of Curcumin and Chrysin by Polymeric Nanoparticles Inhibit Synergistically Growth and hTERT Gene Expression in Human Colorectal Cancer Cells, Nutr Cancer 69(8) (2017) 1290–1299. [DOI] [PubMed] [Google Scholar]


