Abstract
Background:
Hepatic resection of colorectal liver metastases (CRLM) is associated with long-term survival. This study analyzes actual 10-year survivors following resection of CRLM, reports the observed rate of cure, and identifies factors that preclude cure.
Methods:
A single-institution, prospectively-maintained database was queried for all initial resections for CRLM 1992–2004. Observed cure was defined as actual 10-year survival with either no recurrence or resected recurrence with at least 3 years of disease-free follow-up. Clinical risk score (CRS) was dichotomized into low (0–2) and high (3–5). Semiparametric proportional hazards mixture cure model was utilized to estimate probability of cure.
Results:
1211 patients were included with a median follow-up for survivors of 11 years. Median DSS was 4.9 years (95% CI: 4.4–5.3). 295 patients (24.4%) were actual 10-year survivors. The observed cure rate was 20.6% (n=250). Among 250 cured patients, 192 (76.8%) had no recurrence and 58 (23.2%) had a resected recurrence with at least 3 years of disease-free follow-up. Extrahepatic disease (EHD, n=88), CEA >200 (n=119), positive margin (n=109), and >10 tumors (n=31) had observed cure rates less than 10%. In cure model analysis, patients with both EHD and high CRS (n=31) had an estimated probability of cure of 3.5%.
Conclusion:
Actual 10-year survival after resection of CRLM is 24% with an observed 20% cure rate. Patients with both high CRS and EHD have an estimated probability of cure less than 5%. When such factors are identified, strong consideration may be given to preoperative strategies, such as neoadjuvant chemotherapy, to help select patients for surgical therapy.
TOC Statement- 20171017
Actual 10-year survival after resection of CRLM is 24% with an observed 20% cure. The significance of this report is that no single factor precludes cure, although patients with both a high CRS and EHD had an estimated probability of cure of 3.5%.
Introduction
Resection of colorectal liver metastases (CRLM) in selected patients has become standard treatment over the last 3 decades. As this operation became more prevalent, surgical series reported 10-year survival rates between 16% and 23%.1–3 10-year follow-up ensured that late recurrences were taken into account and delineated a portion of patients apparently cured of disease. These published results, in which all patients had a minimum of 10 years potential follow-up, demonstrated the true curative potential for resection in a time when chemotherapy options were limited.
In the interval time, management of patients undergoing hepatic resection of CRLM has evolved. Although randomized trials have demonstrated that perioperative adjuvant systemic chemotherapy does not increase overall survival, exposure to modern chemotherapy throughout patients’ entire clinical courses in select studies has significantly increased and improved outcomes.4–7 Furthermore, salvage resection and/or ablation for limited recurrence can effectively control disease and is being successfully utilized.8, 9 The profile of selected patients has also changed over time with liver resections being performed on patients with worse clinical and pathologic characteristics including limited and resectable extrahepatic disease, extensive liver disease and close margins.10–12 For contemporary physicians, an updated assessment of the outcomes and cure rates of hepatic resection for CRLM are needed in the current clinical context.
Perioperative risk scores derived from clinicopathologic factors are used for prognostication, but few if any factors (individually or as conglomerate scores) precluded long-term survival and cure in previous analyses.2, 13–16 In our initial publication of patients that underwent hepatic resection from 1985–1994, one patient died of disease beyond 10 years and only a positive hepatic margin correlated with the absence of 10-year survival.2 Therefore, patient selection remained challenging, and further improvements in prognostication are necessary to identify patients unlikely to benefit from resection.
The aim of this study was to analyze the characteristics of actual 10-year survivors following resection of CRLM in a modern cohort and, analyze the observed cure rate, and identify any factors that preclude cure.
Methods
Study Design and Patients
All patients evaluated by a hepatopancreatobiliary surgeon at Memorial Sloan Kettering Cancer Center (New York, NY) are entered into a prospectively-maintained database. With approval of the Institutional Review Board and a waiver of informed consent, all patients undergoing initial hepatic resection for CRLM without residual macroscopic (R2) disease were obtained from the database. Between 1992 and 2004, 1316 patients met these criteria and were reviewed. All patients within this chosen interval had the potential for 10 years of follow-up. From this group, the following exclusion criteria were applied: 90 day postoperative death (n=35) or no follow-up beyond 90 days from operation (n=70). The remaining patients formed the study population. General guidelines for resectability have been described previously.13 Most patients had disease confined to the liver or in highly selected cases had limited and resectable extrahepatic metastases.11 Hepatic artery infusion pump (HAIP) placement was performed selectively as previously described.17–19 Resection and intraoperative ablation were used in the same setting for some patients, but patients treated with ablation only were not included in the initial query.
Clinical risk score (CRS) has been previously defined and is comprised of five factors: >1 tumor, >5cm tumor, CEA >200 ng/mL, lymph node positive primary, and DFI <12 months.13 CRS was dichotomized into low (0–2) and high (3–5) risk groups. Positive margin was defined as malignant cells extending to the inked surface of the transected liver.12 Extrahepatic disease represented those patients with metastatic lesions known at the time of the hepatic resection and either resected at the same time or within 6 months of hepatectomy.11 Perioperative chemotherapy represented any chemotherapy administered within 3 months of surgery.
Observed Cure and Follow-up Status
Observed cure was defined as 10-year survival with either no recurrence or resected recurrence with at least 3 years of disease-free follow-up. Previous studies from our institution have utilized 3 years of disease-free survival as effective salvage therapy after resection of recurrence due to the rare incidence of recurrence after this period of time.8, 20 Therefore, the same duration of disease-free follow-up was required for a 10-year survivor to be considered cured. At last clinical follow-up, patients were considered without disease (NED) if they were alive without documented recurrence or had all sites of recurrent disease resected. Patients that died of disease (DOD) had a date of recurrence and subsequent date of death. Patients were considered dead of unknown cause (DUC) if no identifiable cause of death was found in the medical record and the patient did not have a documented recurrence. Patients were considered dead of other cause (DOC) if a clearly attributable non-cancer reason for death was reported. Ten-year survivors that had died but were lost to follow-up were classified as either DOC or DUC; those with greater than 5 years of disease-free follow-up were considered DOC, while those that died with less than 5 years of disease-free follow-up were classified as DUC.
Data Analysis and Cure Model
Disease-specific survival (DSS) was calculated from the date of hepatic resection until cancer-related death. Patients who died of non-cancer related causes were censored at the date of last follow-up. DSS was estimated using Kaplan-Meier methods, and compared using log-rank test between CRS (0–2 vs 3–5).
A plateau in the DSS curve suggested that this sample consisted of a mixture of patients: a fraction that are long-term survivors and another fraction that experienced the event of interest. Standard Cox proportional hazard model presumes a homogeneous population and the assumption of proportional hazards can fail when survival curves have plateaus at the tails.21 Hence, a semiparametric proportional hazards mixture cure model was used to estimate the probability of cure and assess heterogeneity between patients that were long-term survivors and those that were not. In this model, the probability of being cured was modeled with logistic regression and the survival probability for patients who experienced the event of interest was estimated using a proportional hazards model.22–27 All analyses were done in SAS version 9.3 (SAS Institute Inc, Cary, NC) or R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria) using the “smcure” package. 25 All p-values were two-sided and values of <0.05 were considered statistically significant.
Results
Patient Characteristics and Follow-up Status
Between 1992 and 2004, 1211 patients met criteria for inclusion and formed the study population. Perioperative and postoperative characteristics are reported in Table 1. The majority of patients had a node-positive primary (n=727, 60.0%) and metachronous disease (n=720, 59.5%). Patients with extrahepatic disease comprised a small minority of the patients (n=88, 7.3%). Three hundred and forty-eight patients (28.7%) received perioperative HAIP chemotherapy.
Table 1.
Demographic characteristics of patients with resection of CRLM, 1992–2004.
| Characteristics | Total (N=1211) | |
|---|---|---|
| Age at surgery, years-Median (range) | 63 (23–88) | |
| Gender, Male- N (%) | 695 | (57.5) |
| Synchronous Disease- N (%) | 491 | (40.5) |
| Positive nodes in primary – N (%) | ||
| Yes | 727 | (60.0) |
| No | 473 | (39.1) |
| Unknown | 11 | (0.9) |
| CEA – N (%) | ||
| >200 | 119 | (9.8) |
| ≤200 | 936 | (77.3) |
| Unknown | 156 | (12.9) |
| DFI < 12 months– N (%) | 637 | (52.6) |
| No. of hepatic tumors | ||
| Median (range) | 2 (1–50) | |
| 1 | 588 | (48.6) |
| 2–4 | 477 | (39.4) |
| 5–10 | 115 | (9.5) |
| >10 | 31 | (2.6) |
| Size of hepatic tumor, cm- Median (range) | 4.0 (0–40) | |
| Clinical Risk Score – N (%) | ||
| Low (0,1,2) | 627 | (51.8) |
| High (3,4,5) | 420 | (34.7) |
| Unknown | 164 | (13.5) |
| Extrahepatic Disease- N (%) | 88 | (7.3) |
| Surgical margin – N (%) | ||
| Positive | 109 | (9.0) |
| Negative | 1102 | (91.0) |
| Perioperative chemotherapy – N (%) | ||
| Yes | 1120 | (92.5) |
| No | 91 | (7.5) |
| Perioperative HAIP- N (%) | ||
| Yes | 348 | (28.7) |
| No | 863 | (71.3) |
| Vital Status at last contact – N (%) | ||
| NED | 302 | (24.9) |
| AWD | 35 | (2.9) |
| DOD | 748 | (61.8) |
| DOC | 63 | (5.2) |
| DUC | 63 | (5.2) |
Abbreviations: CEA, carcinoembryonic antigen; DFI, disease-free interval; HAIP, hepatic artery infusion pump; NED, no evidence of disease; AWD, alive with disease; DOD, dead of disease; DOC, dead other cause; DUC, dead unknown cause.
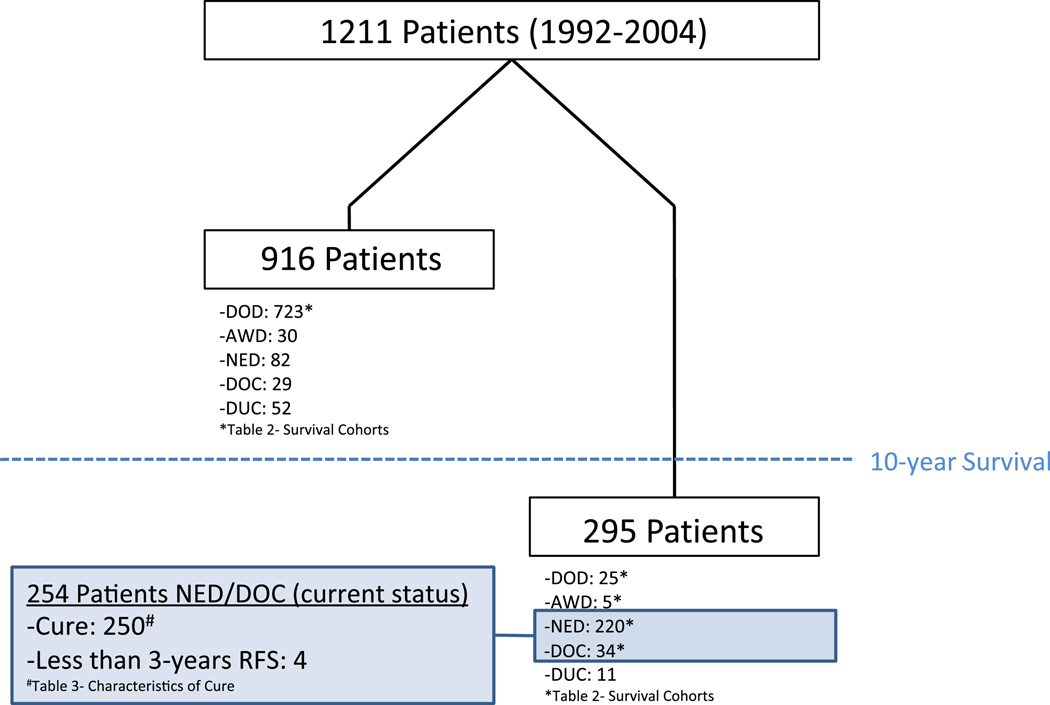
Disease status at last follow-up included 748 patients (61.8%) that were DOD and 35 (2.9%) that were alive with disease (AWD). Sixty-three patients (5.2%) were DOC and another 63 (5.2%) were DUC. Three hundred and two patients (24.9%) were NED, however 82 (6.8%) of these patients were lost to follow-up prior to 10 years. There was wide variation regarding when these 82 patients were censored with a median follow-up time of 3.7 years (range 0.25–9.99 years). Figure 1 illustrates a flowchart of the current status and outcomes of patients.
Figure 1.
Flowchart representing current status and observed cure in study population
Disease-Specific Survival
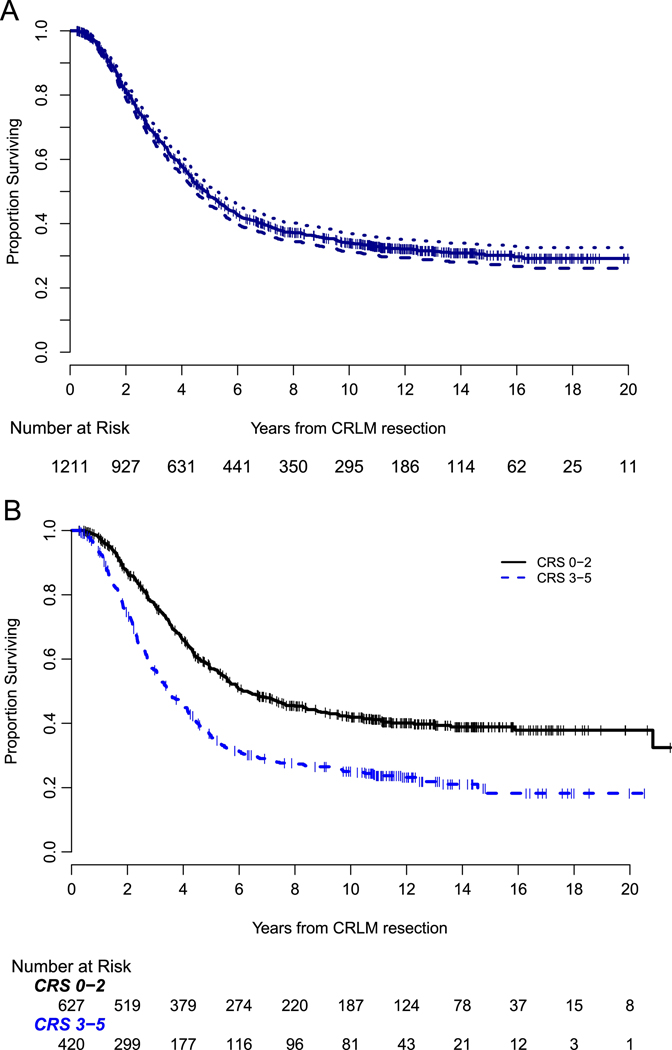
Figure 2a demonstrates the DSS for the entire cohort (n=1211). Median follow-up was 11 years for survivors, and median DSS was 4.9 years (95% CI: 4.4–5.3). The estimated 10-year DSS was 34% (95% CI: 31–37%), with 193 patients censored prior to 10 years. Stratified by CRS, patients with a low CRS had a 10-year DSS of 42% (95% CI: 37–46%) compared to 25% (95% CI: 21–29%) for those with a high CRS (p<0.001), Figure 2b.
Figure 2.
a. Kaplan-Meier plot of DSS for 1211 patients undergoing resection of CRLM from 1992–2004, 95% confidence interval represented by dotted lines.
b. Kaplan-Meier DSS curve stratified by clinical risk score (CRS, 0–2 vs 3–5). Low CRS (0–2) had a 10-year DSS of 42% (95% CI: 37–46%) compared to 25% (95% CI: 21–29%) for those with a high CRS (3–5) (p < 0.001).
Actual 10-Year Survivors
Table 2 reports the characteristics of patients in specific survival cohorts with patients grouped into less than 2 years, 2–5 years, 5–10 years, and greater than 10-year survival. Patients that were either DUC, DOC, or lost to follow-up prior to 10 years were excluded from the respective survival cohorts. The 10-year survivor cohort included all patients except those DUC (n=11). Overall, 295 patients (24.4%) survived 10 years. Excluding the 11 DUC patients, there were 284 patients in the 10-year survivor cohort. Among the 10 year survivors (n=284), the majority were NED (n=220, 77%). Thirty-four (2.8 %) patients died a non-cancer related death, 5 were AWD, and 25 ultimately died of disease greater than 10 years after their initial hepatectomy. Only one patient developed a first recurrence beyond 10 years.
Table 2.
Prognostic factors across survival cohorts.
| <2yr Survival Ɨ | 2–5yr Survival Ɨ | 5–10yr Survival Ɨ | >10yr Survival * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Number | % | Number | % | Number | % | Number | % | |
| 1007 | 217 | 21.5% | 361 | 35.8% | 145 | 14.4% | 284 | 28.2% | |
| Preoperative Factors | |||||||||
| Synchronous | 426 | 93 | 21.8% | 170 | 39.9% | 53 | 12.4% | 110 | 25.8% |
| Node-Positive | 616 | 153 | 24.8% | 237 | 38.5% | 86 | 14.0% | 140 | 22.7% |
| N1 | 389 | 85 | 21.9% | 148 | 38.0% | 63 | 16.2% | 93 | 23.9% |
| N2 | 227 | 68 | 30.0% | 89 | 39.2% | 23 | 10.1% | 47 | 20.7% |
| Preoperative CEA >200 | 107 | 37 | 34.6% | 43 | 40.2% | 11 | 10.3% | 16 | 15.0% |
| DFI <12 months | 543 | 131 | 24.1% | 205 | 37.8% | 64 | 11.8% | 143 | 26.3% |
| No. of hepatic tumors | |||||||||
| Median | 2 | 2 | 1 | 1 | |||||
| 1 | 468 | 77 | 16.5% | 159 | 34.0% | 82 | 17.5% | 150 | 32.1% |
| 2 to 4 | 412 | 92 | 22.3% | 156 | 37.9% | 49 | 11.9% | 115 | 27.9% |
| 5 to 10 | 101 | 33 | 32.7% | 38 | 37.6% | 12 | 11.9% | 18 | 17.8% |
| >10 | 26 | 15 | 57.7% | 8 | 30.8% | 2 | 7.7% | 1 | 3.8% |
| Largest Hepatic Tumor >5cm | 340 | 95 | 27.9% | 129 | 37.9% | 48 | 14.1% | 68 | 20.0% |
| Largest tumor, median cm | 5cm | 4.0cm | 3.65cm | 3.20cm | |||||
| Extrahepatic Disease | 77 | 29 | 37.7% | 29 | 37.7% | 10 | 13.0% | 9 | 11.7% |
| Clinical Risk Score | 884 | 182 | 20.6% | 324 | 36.7% | 119 | 13.5% | 259 | 29.3% |
| <2 | 511 | 78 | 15.3% | 174 | 34.1% | 78 | 15.3% | 181 | 35.4% |
| ≥3 | 373 | 104 | 27.9% | 150 | 40.2% | 41 | 11.0% | 78 | 20.9% |
| Postoperative | |||||||||
| Margin positive | 103 | 31 | 30.1% | 45 | 43.7% | 17 | 16.5% | 10 | 9.7% |
| Resection >3 segments | 655 | 165 | 25.2% | 243 | 37.1% | 83 | 12.7% | 164 | 25.0% |
| Perioperative HAIP | 314 | 43 | 13.7% | 97 | 30.9% | 55 | 17.5% | 119 | 37.9% |
| Perioperative Chemo | 937 | 199 | 21.2% | 335 | 35.8% | 136 | 14.5% | 267 | 28.5% |
Note:
Patients DUC, DOC, or lost to follow-up with less than 10 years survival were excluded.
Among 10-year survivors, cohort includes all patients except dead of unknown cause (DUC, n=11).
Observed Cure
The observed cure rate was 20.6 % (n=250). One hundred and ninety-two patients (15.6%) were 10-year survivors without recurrence, while 58 (4.8%) had a recurrence and subsequent resection before meeting the criteria for cure. Among the cured patients with a documented recurrence, isolated lung recurrence (n=29) was more frequent than other recurrence patterns (liver only, n=18; liver/lung, n=1; other, n=10). Among the 10 patients with extrahepatic and non-pulmonary recurrence, there was a variety of recurrence patterns and subsequent treatment: 5 recurred in the peritoneum and had subsequent surgery, 2 patients had anastomotic recurrence in the colon years after their initial colectomy, and 3 patients had a chest wall or needle tract recurrence with subsequent resection. In all situations, the patients survived at least 10 years and had 3 years of DFS following resection of recurrence.
No analyzed individual prognostic factors precluded cure, although observed cure rates were 8%, 8.3%, and 9.2% for patients with EHD, margin positive resection, and CEA >200, respectively. One patient with more than 10 hepatic tumors met our definition of cure (3.2%), and final pathologic review for that patient revealed 11 tumors (Table 3).
Table 3.
Characteristics of patients with observed cure and probability of cure estimated from the semiparametric mixture cure model.
| All Patients | Observed Cure* | Probability of Cure Ɨ | ||
|---|---|---|---|---|
| Total | Number | % of Patients | % | |
| 1211 | 250 | 20.6% | - | |
| Synchronous | 491 | 96 | 19.6% | 22.5% |
| Metachronous | 720 | 154 | 21.4% | 27.8% |
| Node-Positive Primary | 727 | 121 | 16.6% | 21.4% |
| N1 | 466 | 82 | 17.6% | 21.1% |
| N2 | 261 | 39 | 14.9% | 20.3% |
| Node-Negative Primary | 473 | 128 | 27.1% | 30.0% |
| Preoperative CEA >200 | 119 | 11 | 9.2% | 11.4% |
| Preoperative CEA ≤200 | 936 | 217 | 23.2% | 25.7% |
| DFI <12 months | 637 | 121 | 19.0% | 23.4% |
| DFI ≥12 months | 574 | 129 | 22.5% | 24.9% |
| No. of hepatic tumors | ||||
| Median | 2 | 1 | ||
| 1 | 588 | 134 | 22.8% | 24.3% |
| 2 to 4 | 477 | 100 | 21.0% | 25.8% |
| 5 to 10 | 115 | 15 | 13.0% | 15.1% |
| >10 | 31 | 1 | 3.2% | 11.7% |
| Largest Hepatic Tumor >5cm | 399 | 58 | 14.5% | 18.6% |
| Largest Hepatic Tumor ≤5cm | 812 | 192 | 23.6% | 26.7% |
| Largest tumor, median cm | 4.0cm | 3.25cm | ||
| Extrahepatic Disease | 88 | 7 | 8.0% | 10.4% |
| No Extrahepatic Disease | 1123 | 243 | 21.6% | 25.2% |
| Fong Score | ||||
| <2 | 627 | 165 | 26.3% | 27.2% |
| ≥3 | 420 | 63 | 15.0% | 17.7% |
| Resection ≥3 segments | 771 | 145 | 18.8% | 25.2% |
| Perioperative HAIP | 348 | 105 | 30.2% | 29.5% |
| No Perioperative HAIP | 863 | 145 | 16.8% | 21.3% |
| Perioperative Chemo | 1120 | 234 | 20.9% | 24.6% |
| No Perioperative Chemo | 91 | 16 | 17.6% | 23.0% |
| Margin positive | 109 | 9 | 8.3% | 8.0% |
| Margin negative | 1102 | 241 | 21.9% | 27.2% |
Note:
Observed Cure defined as 10-year survival with either no recurrence or resected recurrence with at least 3 years of disease-free follow-up,
Probability of cure was estimated from semiparametric mixture cure models
Cure Model
A plateau in the DSS survival curve was observed at approximately 13 years after initial hepatectomy. Using semiparametric mixture cure model analysis, clinicopathologic factors were assessed for their association with the probability of being cured. In univariate analysis, the estimated probability of cure for patients with positive margin was 8.0% and for patients with EHD was 10.4% (Table 3). High CRS (CRS 3–5) and EHD were known preoperative clinical factors to have poor survival outcomes. Among the subset of patients with both features (n=31), the median DSS was 2.3 (95% CI: 1.3–3.4) years and the probability of cure was 3.5%.
Discussion
The demographics of 10-year survivors and patients cured of CRLM have not been well described in a modern cohort. This study reports the outcomes of the largest series at a single-institution focused on actual 10-year survival and cure following resection of CRLM. In this study, resection of CRLM had a 20% minimum observed rate of cure. Individual poor prognostic factors (CEA>200, extrahepatic disease, greater than 10 tumors, and positive margin) did not preclude cure but had observed cure rates less than 10%. In cure model analysis, a combination of poor preoperative factors (high CRS and EHD, n=31) reveals a subset of patients with an estimated probability of cure of 3.5%.
Over the last 3 decades, hepatic resection of CRLM in selected patients has been established as an effective treatment with potential for long-term survival. Early studies demonstrated five-year survival between 20 and 30%.28–30 However, as surgical series matured, 10-year survival was reported to describe the actual outcomes and frequency of late recurrences. In these early analyses, the majority of 10-year survivors were disease-free and died non-cancer related deaths.1–3 Disease-specific survival curves plateaued at the 10-year time period, and patients that lived to 10 years appeared cured of disease. Our group has previously analyzed survival trends over different time periods, with an increase in DSS but not RFS seen in more recent patients.10 As such, it was necessary to update the demographics of actual 10-year survivors, report of the rates of cure, and reassess factors that preclude cure in the current clinical context.
In our initial publication reporting long-term outcomes of hepatic resections performed between 1985 and 1994, median survival for the entire cohort was less than 4 years (44 months).2 In this updated analysis (1992–2004), median survival approaches 5 years and 10-year survival was no longer restricted to patients cured of disease. In the original publication, only one patient died of disease beyond 10 years. However, in this update, 25 patients had a disease-related death greater than 10 years after initial hepatic resection and five current 10-year survivors are AWD. Our data suggests that patient selection, salvage resection, and modern chemotherapy may be associated with a prolongation of survival in patients who are not cured following resection of CRLM.8 Modern chemotherapy, that may prolong survival when used to treat a recurrence, has not been associated with improved OS in a recent randomized trial of resectable CRLM.4 Therefore, perioperative chemotherapy likely should not impact the overall rate of cure in a sample with hepatic resection of CRLM from 1992–2004. This data, although it represents hepatic resections performed over 10 years ago, provides necessary clinical follow-up and describes the long-term survival outcomes of patients with resection of CRLM.
Furthermore, recurrence after hepatic resection does not preclude long-term survival and cure. Our group utilized a definition of cure which required 10-year survival and either no recurrence or a minimum of 3 years disease-free interval from the time of resection of recurrence. This conservative definition restricts cure to a small group with strict criteria. 250 patients (20.6%) were classified as being cured. Seventy-seven percent of cured patients (192/250) underwent a single liver resection. This percentage is lower than our original report, but notably higher than Japanese series with increased utilization of parenchymal-sparing surgery.9, 31 However, 58 patients within this group had a repeat resection and greater than 3 years of disease-free survival to qualify for inclusion. Among these cured patients, isolated lung recurrence was more frequent than other recurrence patterns. These results are consistent with what has previously been described regarding recurrence patterns of CRLM and outcomes in which patients with resected isolated lung recurrence can have prolonged survival and cure.20 In our previous descriptive report, only 16 of 102 10-year survivors had a recurrence, and it was evenly split between the liver and lung. While an early recurrence has a particularly poor prognosis, patients with recurrence and subsequent resection are still able to achieve 10-year survival and cure.32
Contrary to our original report on long-term survival, microscopic positive resection margin does not preclude 10-year survival or cure. Our group has proposed that a microscopic positive margin may be an indicator of aggressive tumor biology as opposed to surgical technique.12 Furthermore, this factor is not easily predictable preoperatively and has limited utility in patient selection. Within our data, the observed cure rate for a positive margin was 8.3%. Although low, this number represents a real possibility of cure in a group with aggressive tumor biology. In the same way, CEA >200 and extrahepatic disease had particularly low observed rates of cure below 10%; however, only patients with greater than 10 tumors had an observed cure rate less than 5% for a single prognostic variable.
Actual 10-year survivors were used to define the observed rates of cure, but it does not take into account the patients that are lost to follow-up or discharged from clinic prior to 10 years. The semiparametric proportional hazards mixture cure model accounts for censored patients and models the shape and plateau of the DSS curve for cured and uncured patients separately. This has the advantage over the conventional methods to determine the association between clinical factors and long-term effect. In a study sample that consists of a mixture of long-term survivors and those that die of recurrent disease, the proportional hazards mixture cure model provides better insight into the pattern of long-term survival than conventional Cox models.22–24, 26, 27 Conventional Cox models assume that survival approaches zero, or that every patient eventually will be an event. As evidence by the observed rates of cure following hepatectomy for CRLM, this assumption is not valid in this dataset with extensive follow-up, and a conventional Cox model does not adequately describe the relationship between specific clinical factors and long-term survival. Thus, the cure model most appropriately addresses the clinical question of identifying factors that preclude cure. The estimated probabilities, in general, are slightly higher than observed cure rates, but not substantially different. However, this is an expected finding given the strict definition of observed cure utilized for this analysis. Therefore, by combining preoperative variables with a poor prognosis (both high CRS and EHD) in the model, we identify a subset of patients with a probability of cure at 3.5%.
The outcome of the small subset of patients with both high CRS and extrahepatic disease (n=31), demonstrates that patients with this particular presentation of adverse prognostic factors can rarely if ever be cured. The median survival for this group of patients was just over 2 years. With only 31 patients in the time interval of this study, it highlights the fact that the majority of patients with these poor disease characteristics never undergo resection. In such high-risk patients with known extrahepatic disease, hepatic resection may have limited impact on the outcome beyond the best modern systemic chemotherapy.33 These factors provide prognostic information about the potential for cure and require careful consideration during patient selection.
This is not the first study to apply a definition of cure and attempt to identify factors related to that outcome. An alternate statistical cure model by Cuchetti et al provides a separate definition that also has limitations.34 The probability of being cured was similar at 20%. Their statistical definition of cure was the time period when the mortality of patients following resection of CRLM returns to the same level as that of the general population. Disease-free survival is an important component, but the description of actual 10-year survivors in our data demonstrates that this is not the same as clinical cure. We have shown that cure is possible even in patients that develop a recurrence. Though informative, we believe that their statistical model fails to fully describe the outcomes of documented long-term survivors and patients cured of disease.
This study has several limitations. The generalizability of these results may be restricted to specialized centers with clinical experience and infrastructure to support high-volume hepatic resections. Approximately 30% of patients in our sample received perioperative hepatic artery infusion pump (HAIP) placement. This treatment strategy is not universally available, and may impact survival results that do not reflect hepatic resection for CRLM across all institutions. Our analysis of survival and cure was limited to patients that were evaluated beyond the perioperative encounter, so patients with postoperative death or no follow-up beyond 90 days were excluded. It is possible that our sample is biased towards patients with improved outcomes. However, if compared to the total number of hepatic resections during this time interval (n=1316) even before the study exclusions, the observed cure rate remains 19.0% (250/1316). In general, patients had surveillance imaging performed at least every 6 months following surgery. However, there was no standardized protocol for surveillance imaging in this entire surgical series; therefore the timing of recurrence is subject to some variation. This analysis also did not include molecular characteristics which are related to survival and now often collected on patients with metastatic colorectal cancer, such as BRAF and KRAS mutation status.35, 36 The years and scope of this project did not allow this correlation, but it will be an important prognostic factor to include in future analyses of cure. Nonetheless, this is the largest dataset documenting long-term survival and cure following resection of CRLM and serves as the best cohort to assess factors associated with these outcomes.
Conclusion
Resection of CRLM in selected patients is associated with a 20% observed cure rate. Poor prognostic factors such as positive margin, extrahepatic disease, CEA >200, and more than 10 hepatic tumors do not preclude cure but have rates less than 10%. Patients with both a high CRS and EHD have a predicted cure rate of 3.5%. When such factors are identified, strong consideration may be given to preoperative strategies, such as neoadjuvant chemotherapy, to help select patients for surgical therapy. Future correlation with molecular data, radiographic biomarkers, and other novel factors related to tumor biology are also needed to better select patients for surgical therapy.
Acknowledgments
Funding: This work was supported in part by NIH/NCI P30 CA008748 Cancer Center Support Grant.
Presentation: These results have not been previously presented.
Footnotes
Disclosures: No conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Pulitano C, Castillo F, Aldrighetti L, Bodingbauer M, Parks RW, Ferla G, et al. What defines ‘cure’ after liver resection for colorectal metastases? Results after 10 years of follow-up. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2010;12:244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–80. [DOI] [PubMed] [Google Scholar]
- 3.Vigano L, Ferrero A, Lo Tesoriere R, Capussotti L. Liver surgery for colorectal metastases: results after 10 years of follow-up. Long-term survivors, late recurrences, and prognostic role of morbidity. Annals of surgical oncology. 2008;15:2458–64. [DOI] [PubMed] [Google Scholar]
- 4.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. The Lancet Oncology. 2013;14:1208–15. [DOI] [PubMed] [Google Scholar]
- 5.Saltz LB, Kanowitz J, Kemeny NE, Schaaf L, Spriggs D, Staton BA, et al. Phase I clinical and pharmacokinetic study of irinotecan, fluorouracil, and leucovorin in patients with advanced solid tumors. J Clin Oncol. 1996;14:2959–67. [DOI] [PubMed] [Google Scholar]
- 6.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. The New England journal of medicine. 2000;343:905–14. [DOI] [PubMed] [Google Scholar]
- 7.Andre T, Bensmaine MA, Louvet C, Francois E, Lucas V, Desseigne F, et al. Multicenter phase II study of bimonthly high-dose leucovorin, fluorouracil infusion, and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and fluorouracil regimen. J Clin Oncol. 1999;17:3560–8. [DOI] [PubMed] [Google Scholar]
- 8.Butte JM, Gonen M, Allen PJ, Peter Kingham T, Sofocleous CT, DeMatteo RP, et al. Recurrence After Partial Hepatectomy for Metastatic Colorectal Cancer: Potentially Curative Role of Salvage Repeat Resection. Annals of surgical oncology. 2015;22:2761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi M, Hasegawa K, Oba M, Aoki T, Sakamoto Y, Sugawara Y, et al. Repeat resection leads to long-term survival: analysis of 10-year follow-up of patients with colorectal liver metastases. Am J Surg. 2015;210:904–10. [DOI] [PubMed] [Google Scholar]
- 10.House MG, Ito H, Gonen M, Fong Y, Allen PJ, DeMatteo RP, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744–52, 52–5. [DOI] [PubMed] [Google Scholar]
- 11.Carpizo DR, Are C, Jarnagin W, DeMatteo R, Fong Y, Gonen M, et al. Liver Resection for Metastatic Colorectal Cancer in Patients with Concurrent Extrahepatic Disease: Results in 127 Patients Treated at a Single Center. Annals of surgical oncology. 2009;16:2138–46. [DOI] [PubMed] [Google Scholar]
- 12.Sadot E, Groot Koerkamp B, Leal JN, Shia J, Gonen M, Allen PJ, et al. Resection margin and survival in 2368 patients undergoing hepatic resection for metastatic colorectal cancer: surgical technique or biologic surrogate? Annals of surgery. 2015;262:476–85; discussion 83–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Annals of surgery. 1999;230:309–18; discussion 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zakaria S, Donohue JH, Que FG, Farnell MB, Schleck CD, Ilstrup DM, et al. Hepatic resection for colorectal metastases: value for risk scoring systems? Annals of surgery. 2007;246:183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–62. [PubMed] [Google Scholar]
- 16.Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daly JM, Kemeny N, Oderman P, Botet J. Long-term hepatic arterial infusion chemotherapy. Anatomic considerations, operative technique, and treatment morbidity. Archives of surgery. 1984;119:936–41. [DOI] [PubMed] [Google Scholar]
- 18.Allen PJ, Nissan A, Picon AI, Kemeny N, Dudrick P, Ben-Porat L, et al. Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: an institutional experience of 544 consecutive cases. J Am Coll Surg. 2005;201:57–65. [DOI] [PubMed] [Google Scholar]
- 19.Kemeny N, Huang Y, Cohen AM, Shi W, Conti JA, Brennan MF, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. The New England journal of medicine. 1999;341:2039–48. [DOI] [PubMed] [Google Scholar]
- 20.D’Angelica M, Kornprat P, Gonen M, DeMatteo RP, Fong Y, Blumgart LH, et al. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Annals of surgical oncology. 2011;18:1096–103. [DOI] [PubMed] [Google Scholar]
- 21.Othus M, Barlogie B, Leblanc ML, Crowley JJ. Cure models as a useful statistical tool for analyzing survival. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:3731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boag JW. Maximum Likelihood Estimates of the Proportion of Patients Cured by Cancer Therapy. J Roy Stat Soc B. 1949;11:15–53. [Google Scholar]
- 23.Yilmaz YE, Lawless JF, Andrulis IL, Bull SB. Insights from mixture cure modeling of molecular markers for prognosis in breast cancer. J Clin Oncol. 2013;31:2047–54. [DOI] [PubMed] [Google Scholar]
- 24.Berkson J, Gage RP. Survival Curve for Cancer Patients Following Treatment. J Am Stat Assoc. 1952;47:501–15. [Google Scholar]
- 25.Cai C, Zou Y, Peng Y, Zhang J. smcure: an R-package for estimating semiparametric mixture cure models. Computer methods and programs in biomedicine. 2012;108:1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sy JP, Taylor JM. Estimation in a Cox proportional hazards cure model. Biometrics. 2000;56:227–36. [DOI] [PubMed] [Google Scholar]
- 27.Taylor JM. Semi-parametric estimation in failure time mixture models. Biometrics. 1995;51:899–907. [PubMed] [Google Scholar]
- 28.D’Angelica M, Brennan MF, Fortner JG, Cohen AM, Blumgart LH, Fong Y. Ninety-six five-year survivors after liver resection for metastatic colorectal cancer. J Am Coll Surg. 1997;185:554–9. [DOI] [PubMed] [Google Scholar]
- 29.Jamison RL, Donohue JH, Nagorney DM, Rosen CB, Harmsen WS, Ilstrup DM. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Archives of surgery. 1997;132:505–10; discussion 11. [DOI] [PubMed] [Google Scholar]
- 30.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Annals of surgery. 2002;235:759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumura M, Mise Y, Saiura A, Inoue Y, Ishizawa T, Ichida H, et al. Parenchymal-Sparing Hepatectomy Does Not Increase Intrahepatic Recurrence in Patients with Advanced Colorectal Liver Metastases. Annals of surgical oncology. 2016;23:3718–26. [DOI] [PubMed] [Google Scholar]
- 32.Tan MC, Butte JM, Gonen M, Kemeny N, Fong Y, Allen PJ, et al. Prognostic significance of early recurrence: a conditional survival analysis in patients with resected colorectal liver metastasis. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2013;15:803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanoff HK, Sargent DJ, Campbell ME, Morton RF, Fuchs CS, Ramanathan RK, et al. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol. 2008;26:5721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cucchetti A, Ferrero A, Cescon M, Donadon M, Russolillo N, Ercolani G, et al. Cure model survival analysis after hepatic resection for colorectal liver metastases. Annals of surgical oncology. 2015;22:1908–14. [DOI] [PubMed] [Google Scholar]
- 35.Schirripa M, Bergamo F, Cremolini C, Casagrande M, Lonardi S, Aprile G, et al. BRAF and RAS mutations as prognostic factors in metastatic colorectal cancer patients undergoing liver resection. British journal of cancer. 2015;112:1921–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karagkounis G, Torbenson MS, Daniel HD, Azad NS, Diaz LA Jr., Donehower RC, et al. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer. 2013;119:4137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]