Abstract
With an aging population that continues to enlarge in recent years, the need for the development of therapeutic approaches for treatment of Neurodegenerative disorders (ND) has increased. ND, which are characterized by the progressive loss of structure or function of neurons, often associate with neuronal death. In spite of screening numerous drugs, currently there is no specific treatment that can cure these diseases or slow down their progression. Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), frontotemporal dementia, Huntington’s disease, and prion disease belong to ND which affect enormous numbers of people globally. There are some main possible reasons for failure in the treatment of neurodegenerative diseases such as limitations introduced by the blood–brain barrier (BBB), the Blood-Cerebrospinal Fluid Barrier (BCFB) and P-glycoproteins. Current advances in nanotechnology present opportunities to overcome mentioned limitations by using nanotechnology and designing nanomaterial improving delivering active drug candidates. Some of the basic and developing strategies to overcome drug delivery impediments are the local delivery of drugs, receptor-mediated transcytosis, physicochemical disruption of BBB, cell-penetrating peptides and magnetic disruption. Recently, the application of nanoparticles has been developed to improve the efficiency of drug delivery. Nanoengineered particles as nanodrugs possess the capacity to cross the BBB and also show decreased invasiveness. Example include inorganic, magnetic, polymeric and carbonic nanoparticles that have been developed to improve drug delivery efficiency. Despite numerous papers published in this filed, there are some unsolved issues that need to be addressed for successfully treatment of neurodegenerative diseases. These are discussed herein.
Keywords: Nanotechnology, Drug Delivery, Nanomaterial, Neurodegenerative Disorders
Graphical Abstract
The recent advances in application of nanotechnology including inorganic,polymeric,magnetic and carbon nanomaterials in drug delivery for treatment of neurodegenerative diseases

1. Introduction
In recent years, the increase in the proportion of the ageing population worldwide has escalated the incidence of Neurodegenerative disorders (ND) 1 ND, characterized by the progressive loss of structure or function of neurons, is often associated with neuronal death and morbidity. In spite of many studies and some progress, successful early diagnosis and treatment strategies of these diseases are still limited. Furthermore, the majority of current available treatments are symptomatic and unable to reform the quality of life and delay or ameliorate damage. Based on the global statistics, stroke is considered as the third main cause of death 2 and the second most common disease after Alzheimer’s in the United States, which leads to dramatic damage and finally the death of the brain cells 3. The risk of being affected by a neurodegenerative disease correlates dramatically with advancing age. Most research predicts the that a large section of the population will be affected by neurodegenerative diseases in the coming decades, which drives the need to investigate their basic causes and develop new approaches for their prevention, early detection and treatment 4–6. Nevertheless, the mechanism by which neurodegenerative diseases originate and progress is still an unsolved question, and we still lack clinical therapeutics for their treatment 7, 8. The leading obstacles for this remain insufficient targeting of the brain. This is due mainly to the presence of the Blood–Brain Barrier (BBB), the Blood-Cerebrospinal Fluid Barrier (BCFB) and P-glycoproteins which work as defense systems and prevents the penetration of the majority of drugs and medicines, causing peripheral (side) effects 9. Moreover, the diagnostic markers used are not specific and work only on probable or possible diagnosis 10–13. In recent years, nanomaterials as nanomedicines have played an indispensable role in diagnosis and treatment of numerous neurological disorders globally. The application of nanomaterials at commercial and industrial scales has remarkably increased. Furthermore, they have been broadly used in disease diagnostics, therapeutic medicines and medical approaches 14, 15. Administration of nanotechnology in drug delivery systems has improved the bioavailability and kinetic profile of drugs and medicines in biological systems.
Thus, nanomedicine is an emerging field where engineered nanomaterials (NMs) are utilized for the clinical objectives in multiple diseases including neurological disorders. They are considered as one of the most promising and multifunctional drug delivery systems into unreachable areas like the brain, being able to provide protection to therapeutic compounds while efficiently delivering them into the damaged regions 15, 16. Advances in nanotechnology has provided impactful developments in novel technological inventions in the form of nanoparticles (NPs), nanotubes, nanomedicines, nanocarriers, microparticles (MPs), and polymeric NMs, which aid in management of neurodegenerative diseases and accurately deliver drugs to specific molecular targets and sites of action 15. The continuous release capability of nano-drug delivery systems improves the controlled and safe release of loaded drugs, thereby minimizing the dosage-regimen 17. What renders engineered NMs attractive, mainly in the biomedical field, is their versatility. In particular, their physical properties can be exploited for diagnosis or therapy and also for tissue engineering and regeneration. In addition, chemical functionalization may confer them targeting specificity 9. In spite of the numerous published research and established findings, currently the reliable treatments available for neurodegenerative diseases remain limited and more investigation is needed to elucidate the mechanisms involved in neurological disorders 18, 19. However, the worldwide growth of aging population implies an urgent need to accelerate the diagnostic and therapeutic approaches for treatment of neurodegenerative disease. Based on these facts, this review we will have an overview on neurodegenerative diseases and the current obstacles and available strategies to overcome these impediments. We will also discuss about the current status of nanomaterials and their application in drug delivery system of neurological disorders.
2. Neurodegenerative Diseases
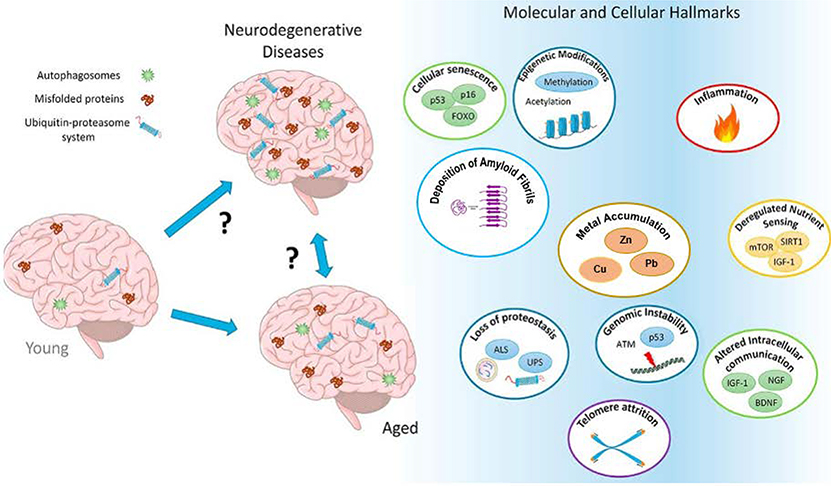
Neurodegenerative diseases occur when nerve cells in the brain lose their functionality and/or undergo untimely death 9, 20, 21. Neurodegenerative diseases such as Alzheimer’s, Huntington’s and Parkinson’s diseases have been considered to be multifactorial diseases which are affected by different factors contributing to their progression. Their complexity, as well as their rapid progress, has prompted researchers to design multitarget-directed ligands to address the complementary pathways involved in these diseases 22. Genetics can be considered as a key factor in the pathogenesis of neurodegenerative diseases. Moreover, accumulation of amyloid fibrils, chronic inflammation, oxidative stress, cellular senescence and genome instability, proteostasis dysregulation and excessive metal (Cu, Zn, Pb, Fe) accumulation in the human brain and defects in mitochondrial function seem to play a crucial role in the pathophysiology of neurodegenerative diseases and the resulting neuronal damage (Figure 1) 22, 23. Although there are pharmaceutical cares associated with neurodegenerative diseases which can lessen their substantial side-effects, currently there is no specific treatment that can cure the disease or slow down its progression 12, 16, 24. Herein, the most common symptoms and characteristics of these diseases will be discussed.
Figure 1.
The multifactorial and complex nature of neurodegenerative diseases. Adapted with permission from Daniele et al. 23. with minor corrections. Copyright 2018 ELSEVIER.
2.1. Alzheimer’s Disease
Alzheimer’s disease is associated with prominent cognitive deficits 1, 25. The intracellular hyperphosphorylated neurofibrillary tangles and amyloid plaques (extracellular deposits of amyloid-beta (Aβ) peptide) in the brain play important roles in the early-onset of AD (Figure 2) 15. Numerous evidences also suggested that the activation of the glutamatergic system plays a critical role in AD pathology 1, 26, 27. The symptoms will start with limited forgetfulness and trouble in memory imprinting, which leads to short-term memory loss and finally to long-term memory problems 10, 12, 28. Eventually, the plaques and tangles spread all over the brain area and the patient becomes definitely dependent on others 6. Current treatments in AD patients require the long-term administration of high doses of antibodies against Aβ which can pass through the BBB and remove Aβ plaques from the brain. Considering that only up to 0.1% of anti-Aβ antibodies administered peripherally can reach the brain, large portion of antibodies remain in the bloodstream which is the basic problem in AD treatment 29. Overall, the treatment process of AD is very complex and needs further efforts and more specific research 30, 31.
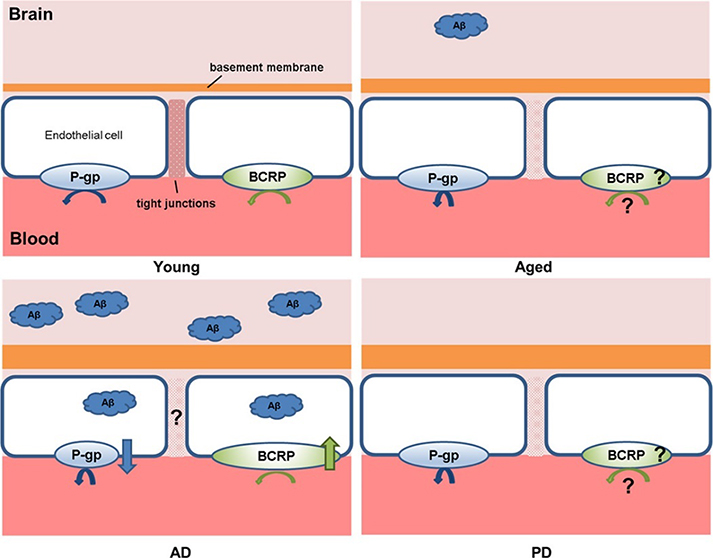
Figure 2.
Pathological alterations to the BBB in aging, AD and PD, relative to the BBB in the young adult, including a thickened basement membrane, tight junctions breakdown, and modified expression and/or function of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP). Smaller shorter arrows underneath each transporter represents reduced function, whereas the arrow pointing up or down indicates upregulation and downregulation, respectively. Given the conflicting studies or lack of data, the status of the paracellular route in AD and the expression/function of BCRP have been depicted with a “?”Adapted with permission from Pan et al.31. Copyright 2018 ELSEVIER.
2.2. Parkinson’s Disease
Parkinson’s disease currently ranks second after Alzheimer’s disease with more than 6.3 million people suffering from this chronic disease worldwide 32, 33. It is characterized by progressive deterioration of motor functions due to loss of dopamine-releasing neurons in the substantia nigra of the brain, though the existence of nonmotor symptoms correspondingly supports the neuronal loss in nondopaminergic areas (Figure 3) 34, 35. As the serious motor impairments are the core causes of this disease the diagnosis of this disease is largely delayed, resulting in more difficulty in its management. Among the available treatment approaches used for Parkinson’s disease, use of protein like human glial cell line-derived neurotrophic factor (hGDNF) was found to be promising. A quick and simple method has been demonstrated to produce a high amount of purified hGDNF using a mammalian cell derived system 6, 36.
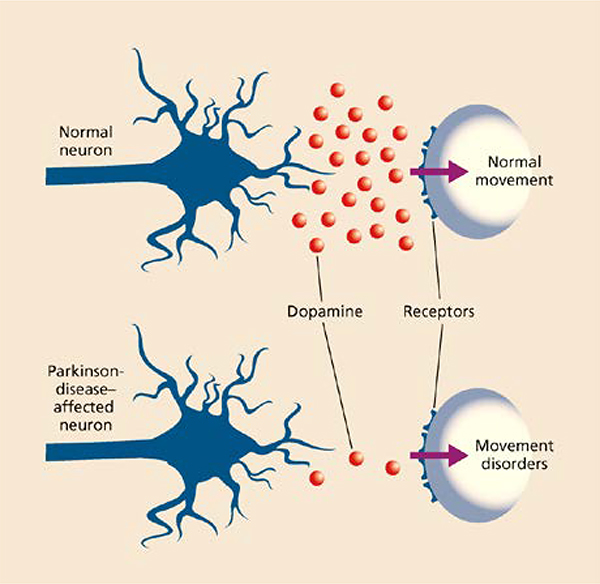
Figure 3.
Dopamine levels in a normal and Parkinson disease-affected neuron. Adapted with permission from Tiwari et al. 37. Copyright 2017 AICH.
2.3. Huntington’s Disease
Huntington’s disease (HD) is a genetic neurodegenerative disorder caused by the abnormal repetition of CAG nucleotides in the Huntingtin (HTT) gene. The expanded CAG repeat of HTT gene results in the pathological expansion of polyglutamine (polyQ) and aggregation of the mutated HTT protein in the brain, more specifically in the striatum 38. Similar to other neurodegenerative disease, HD is also associated with protein aggregations including mutant HTT, polyQ-expanded ataxins and α-synuclein in the brain cells 39, 40.
Despite the differences in amino acid sequences, amyloid proteins share common structural features allowing conformation-dependent, oligomer-specific antibodies to recognize diverse amyloid deposits. An amyloid oligomer-specific scFv antibody (W20) conjugated to PEGylated superparamagnetic iron oxide nanoparticles crossed the BBB and specifically bound to the oligomer area. It shows the potential of early-stage diagnostic of HD and a promising strategy for crossing the BBB 41. However, Currently, there is no proven medical therapy to alleviate the onset or progression of HD 6, 41, 42.
2.4. Amyotrophic Lateral Sclerosis (ALS)
Motor neuron disease, interchangeably known as amyotrophic lateral sclerosis (ALS), is a neurodegenerative disorder characterized by a progressive muscular paralysis reflecting degeneration of motor neurons in the primary motor cortex, brain stem, and spinal cord. The phenotypic expression of ALS is highly heterogeneous and determined by four factors: (1) body region of onset, (2) relative mix of upper motor neuron (UMN) and lower motor neuron (LMN) involvement, (3) rate of progression, and (4) cognitive impairment. The initial hallmarks of ALS may appear so mild that they are usually disregarded: like muscle twitching, cramping, stiffness, weakness, involuntary jerking movements, tremors, inability to control the bowels or the bladder, or inability to move or open the eyes completely. Up to now the diagnosis of ALS is still a clinical one and electromyography (EMG) is the most important technical test to support the diagnosis. Laboratory tests and imaging, including MRI, do not yield diagnostically positive results, but are necessary to exclude other potentially better treatable diseases 43.
2.5. Prion Disease
Prion disease, also known as transmissible spongiform encephalopathies (TSEs), have been observed in both humans and animals. Prion diseases are a group of rapidly progressive disorders characterized by a defined spectrum of clinical abnormalities. Recently, the number of human and animal diseases recognized as TSEs showed an increasing trend. They all share similar symptoms such as the spongiform degeneration of the brain and variable amyloid plaque formation (PrPSc). In fact, PrPSc is the disease-associated isoform of the endogenously expressed prion protein (PrPC), which may be present as amyloid deposits 44.
3. Obstacles of Drug Delivery to Brain Targeting
Despite screening numerous drug candidates, only limited candidates, such as galantamine (AChE inhibitors), donepezil, galantamine, tacrine, Curcumin, rivastigmine, and memantine (NMDA inhibitor) 45 are currently utilized in the aid of clinical therapy of neurodegenerative disorders 1. The central nervous system (CNS) is the most complicated and sensitive system in the human body. It is tightly sealed by the BBB and the BCFB 21, 46. A key component of the system for regulating the CNS internal milieu is the presence of several transporter systems, which are located at the BBB and are able to transport substances across the BBB. One of the most important transporters at the BBB is the multidrug resistance protein P-glycoprotein (Pgp), belonging to the family of ATP-binding cassette transporters 47 (Figure 4).
Figure 4.
The schematic representation of the main barriers for drug delivery to the brain targeting
Due to the complex nature of the CNS, there are several limitations in targeted drug delivery from the blood brain to the CNS. These include a lack of information about the drug’s function, pharmacokinetics (half-life) or their bioavailability for the brain cells, their side-effect and the unpredictable interaction of off-targets-drugs with unspecific receptors and enzymes. This is due to the complex pharmacology of various drugs, the long latency period of neurodegenerative disorders and inefficiency of drugs after progression of diseases, inaccurate dosage of drugs, genotype of patient population and different response to drugs, and the volatility (oxidation, hydrolysis) index of evaluated drugs. Furthermore, with regards to the brain, the problems will become even more complicated 10, 11, 13. Here, the three major barriers preventing drug delivery into the brain will be discussed.
3.1. Blood Brain Barrier
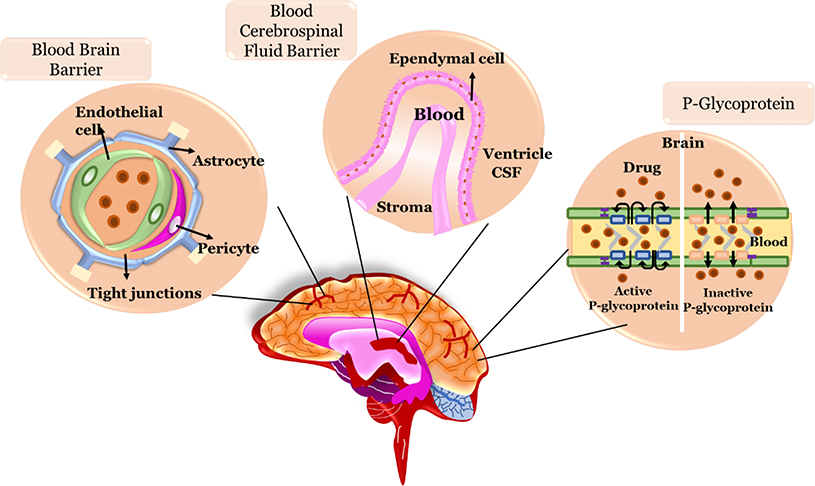
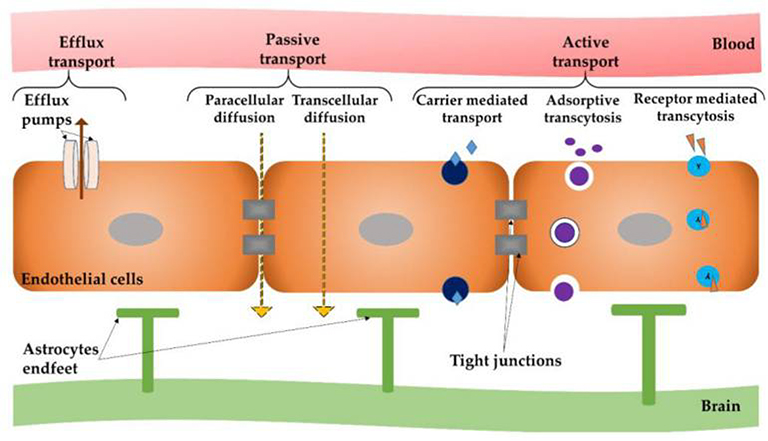
Neuropharmaceutics is the largest potential growth sector of the pharmaceutical industry. However, this growth is blocked by the need to circumvent the BBB. Up to now successful therapeutic strategies have been failed to achieve due to the complexity of CNS and an inhospitable environment in and around the lesion site for cell transplantation. The next limitation is the low diffusion of drugs/biologics across the BBB which further restricts the application of well-known delivery methods, such as oral and intravenous 48. The tight junctions in the BBB prevent significant passive transfer of small hydrophilic molecules from the blood to the brain but several transport systems (influx systems), able to trigger the BBB mediate the entry of essential substances with high or low molecular weights, hydrophilic and lipophilic, such as glucose, amino acids, purine bases and nucleosides, choline, monocarboxylic acids, amines, thyroid hormones . The influx systems could also trigger the passage of macromolecules by exploiting endocytosis or transcytosis mediated by specific receptor interactions. Along with these influx systems, at the BBB level, efflux systems are present, protecting the brain from damaging agents and xenobiotic exposure, thus defending the brain from toxic insults (Figure 5).
Figure 5.
Different modes of transport across the blood-brain barrier (BBB). Adapted with permission from Teleanu el al. 53. Copyright 2019 MDPI AG.
The BBB is primarily composed of brain endothelial cells which are lined with micro-vessels and capillaries in the brain and are knitted very tightly with junctions leaving no gaps between the cells and possess few fenestrae and few endocytic vesicles as compared to capillaries of other organs 21, 49. Brain capillary endothelial cells are surrounded by extracellular matrix, astrocytes, pericytes and microglial cells. The brain base endothelial cells are enclosed by basal lamina, which consist of fibronectin, type IV collagen, laminin, and heparin sulfate 50. Unfortunately, it is estimated that approximately 98% of drugs active against brain pathologies do not cross the BBB., which is insufficient for the treatment of these highly complex disorders 51, 52.
3.2. Blood-Cerebrospinal Fluid Barrier
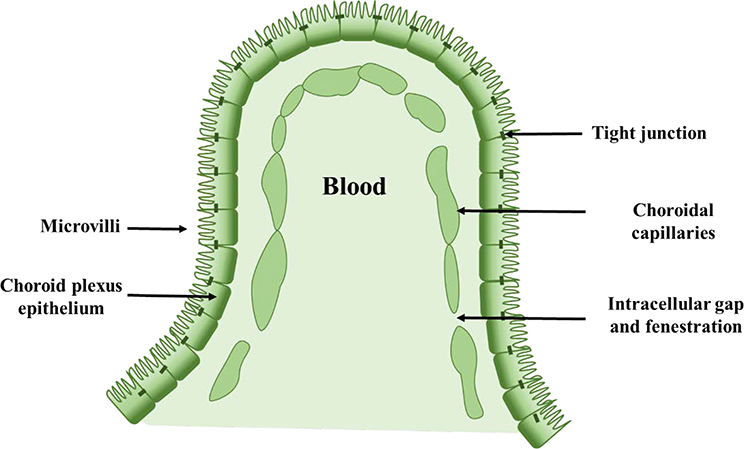
The Blood-Cerebrospinal Fluid Barrier (BCFB) is the second barrier (after BBB) that a systemically administered drug molecule encounters before entering the brain 54. This is built by the epithelial cells of the choroid plexus (CP). The choroid plexus epithelium (CPE) is a secretory epithelium, par excellence, with unique cellular transport mechanisms (Figure 6). The cells of the CPE are among the most efficient tissues in terms of secretory rate 55. Similar to the BBB, this epithelial barrier system separates the blood from the CSF and is also sealed by tight junctions between adjacent cells which are located at the CSF-facing surface. The barrier-forming cells that separate the blood from the CNS are joined by a continuous belt of tight junction proteins that seal the paracellular cleft. It constitutes a machinery based on multi-specific efflux transport proteins and detoxifying enzymes that collectively prevent the entry of potentially toxic compounds into the CNS 56. Various drug delivery strategies have been developed to improve drug penetration into the brain. Most strategies target the microvascular endothelium forming the BBB proper. Targeting the BCFB formed by the epithelium of the choroid plexuses in addition to the BBB may offer added value for the treatment of CNS diseases 56.
Figure 6.
The schematic representation of Blood-Cerebrospinal Fluid Barrier
3.3. Multidrug Resistance Proteins
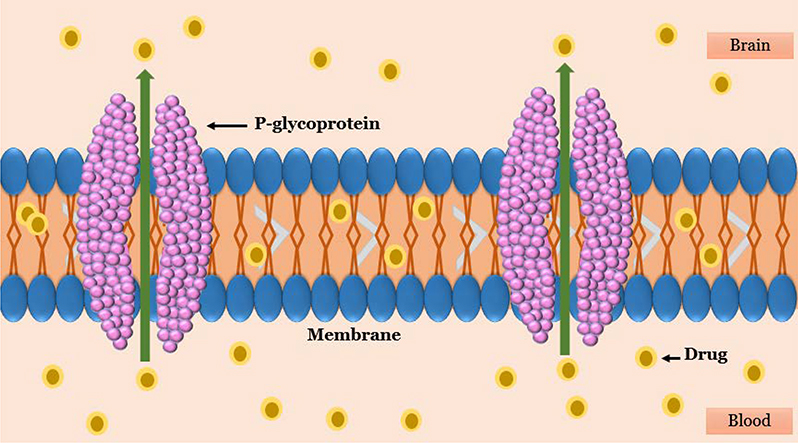
Until recently, it was believed that the BBB acts as a static lipid membrane barrier. Physical features of the cerebral endothelial cells including tight junctions, deficiency of vesicles or caveolae, and high electrical resistance were considered as the primary factors which provide the membrane selectivity of the BBB to a wide range of circulating compounds from the periphery (Figure 7). However, findings from molecular biology, immunocytochemistry, biochemistry, and transport studies illustrated that the cerebral endothelial cells possess a variety of metabolic enzymes (i.e., alkaline phosphatase, glutathione transferases, cytochrome P450 enzymes) and energy-dependent efflux transport proteins (i.e., P-Glycoprotein (P-gp) and multidrug-resistance proteins) that have been arranged asymmetrically and serve in a barrier capacity 47. P-gp, a 170–180 kDa plasma membrane-associated protein, a product of the multidrug resistance protein (MDR1) gene , is one of the most important ATP-dependent efflux transporters at the BBB 57. It restricts a variety of drugs from reaching their specific therapeutic targets. In contrast, when the expression and the activity of the P-gp are prevented, the amount of drugs entering the CNS is increased. Xu et al. supposed that P-gp may prevent brucine across the BBB and consequently, its entry into the CNS. As such, P-gp inhibitors and inducers may have the potential to affect the pharmacokinetics and so the pharmacodynamics of brucine 57.
Figure 7.
The schematic representation of P-glycoprotein structure of cerebral endothelial cells
Thus, currently one of the major challenges of pharmaceutical research is to discover devices enabling an effective and efficacious delivery of drugs into the CNS 43.
4. Application of Nanotechnology Tools for Neurodegenerative Disease Therapy
Nanoparticles are materials containing at least one dimension less than 100 nm. Based on their structure, they can be classified into zero dimensional, which includes nanoparticles and quantum dots; one dimensional, which involves nanofibers, nanotubes, and nanowires; and two dimensional, consisting of graphene 6, 51.
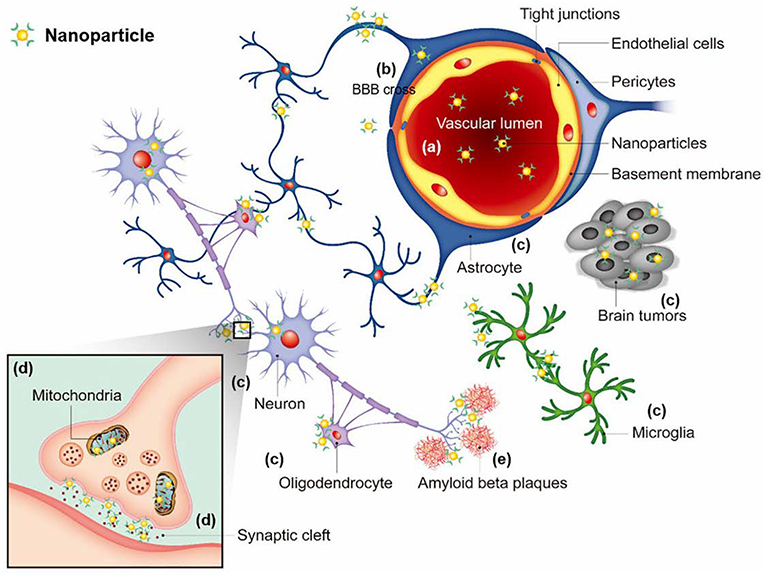
In recent years, nanomaterials include nanotubes, nanofibers, quantum dots, and nanoparticles have broad applications in several biomedical fields such as drug delivery, biosensors, bioimaging, etc. 6, 58–60. Neuronanomedicine refers to the engineered nanomaterials designed as drugs for neurodegenerative disorders 61. Nanotechnology designs engineered nanomaterials to produce nanoscale devices which can interact with biological systems at a molecular level 62. These nanotechnological devices can trigger, respond, and interact with target cells and tissues to bring about the preferred physiological response while lowering harmful side effects 6, 63. Some of nanoparticles are designed to enhance the penetration of BBB and to target specific domains within cells. Their efforts focus on localization of intracellular or to reach extracellular molecules, such as amyloid beta plaques in Alzheimer’s disease (Fig 8).
Figure 8.
Nanoparticles with targeting ability used for the brain diseases. Nanoparticles circulating in a blood stream (a) need to cross the BBB (b), and then localize to target cells (e.g. neurons, astrocytes, oligodendrocytes, microglia, tumor cells) (c), and sometimes target cellular organelles (e.g. mitochondria, synaptic cleft) (d) or extracellular molecules (e.g. amyloid beta plaques in Alzheimer’s disease) (e). Adapted with permission from Jin et al.64 Copyright © 2020 by SAGE Publications Ltd
Surface modification of NPs to make highly positive particles would enable them to cross BBB through adsorptive and receptor/transporter-mediated transcytosis. Recognition of apolipoprotein E (Apo E) by specific receptors located on BBB facilitates transportation of lipoproteins (LPs) to the brain. Functionalization of NPs with LPs, like conjugation of Apo E to albumin-NPs or liposomes would enable them to be recognized and transferred by specific receptors on BBB 64.
Most importantly, nanotechnology represents mechanisms to adjust complex biological systems with higher selectivity and specificity. In fact, achieving nanocarriers that are able to remain stable in the bloodstream, protect the drug from metabolism, and promote the long-lasting release of the drug is still a pivotal prerequisite for nanomedicine, but it is now deemed insufficient. Active targeting of specific pathological cells is now the challenge for pharmaceutical nanotechnologists, who are faced with difficulties in colloidal chemistry and most of all in the characterization of the engineered nanocarriers from a technological and physiological point of view 43. Nanoengineered particles as nanodrugs possess the ability to cross the BBB and also show decreased invasiveness 65. Liposomes, polymeric NPs, and solid-lipid NPs (SLN) are the most studied NPs in terms of noninvasive brain drug-delivery materials with specific characteristics like biocompatibility, stability, low antigenicity, and high biodegradability. They have emerged as one the most effective smart platforms for controlled discharge of their cargo in target sites. Therefore, the most important application of nanoparticles in the biomedical field can be for drug delivery systems (Figure 9). Herein, we will discuss some applications of these mentioned nanomaterials for drug delivery system in treatment of neurodegenerative diseases (Table 1):
Figure 9.
Graphical overview of the process toward nanomaterial-mediated treatment of neurological diseases. Adapted with permission from Furtado et al. 21. Copyright 2018 John Wiley & Sons.
Table 1.
Different type of nanoparticles/nanomaterials and their application in drug delivery
| Types of NPs and or NMs | Size | Advantage or Application | Key References | |
|---|---|---|---|---|
| Inorganic NPs | Gold NPs | 1–150 nm | Inhibit formation of amyloid fibrils, Neural survival by increasing the expression of brain-derived neurotrophic factor | 69–73 |
| Silver NPs | 1–100 nm | Alter gene and protein expressions of Aβ deposition | 74, 77 | |
| Magnetic NMs | 1 nm to 0.5 μm | Regulate the metal homeostasis in the brain, carry a large dose of drug to achieve high local concentration and avoid toxicity, Target and detect amyloid plaques in AD | 106–109 | |
| Polymeric NMs | 1–1000 nm | Cross the BBB and enhance the viability of brain cell, Improved oral bioavailability, Increased brain uptake, Enhanced the bioactivity of drugs | 82, 89, 110 | |
| Carbonic NMs | Graphene | 1–100 nm | Neuronal cell survival, assist in neuronal regeneration | 93–96 |
| Fullerene | 0.4–1.6 nm 111 | Antioxidant activity against cytotoxicity of oxidative stress, induce neural stem cell (NSC) proliferation and rescue the function of injured CNS | 97, 98 | |
| Carbon Nanotubes | 1–100 nm | Promoting functional recovery of neurons after brain damage, Promote the neuronal activity, Network communication and Synaptic formation | 103, 112 | |
4.1. Inorganic Nanoparticles
4.1.1. Gold nanoparticles
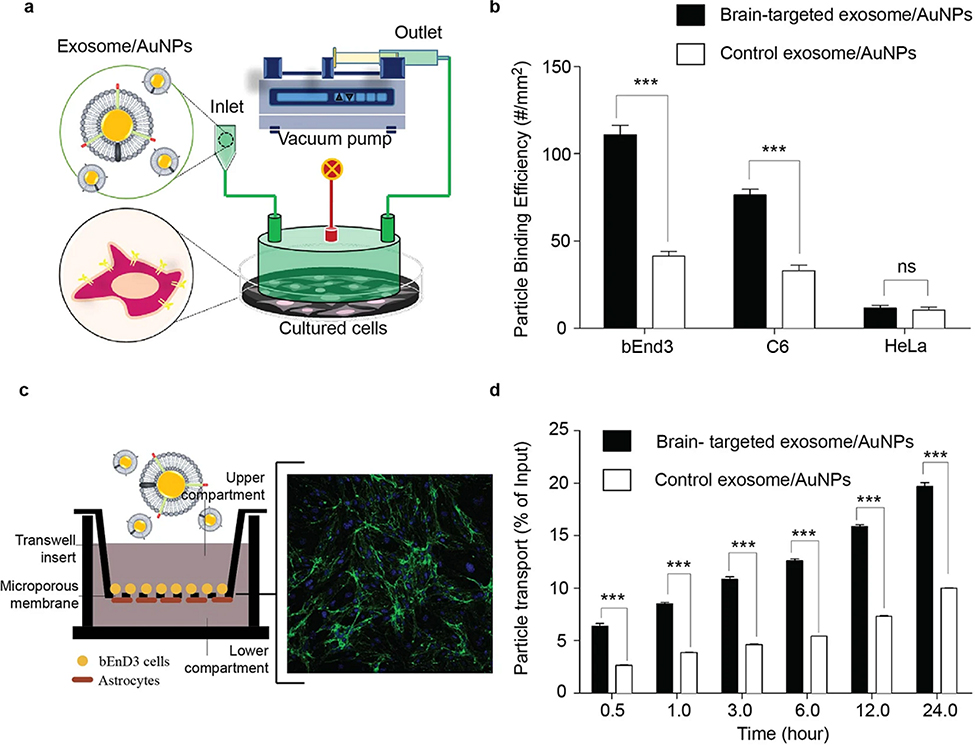
Gold nanoparticles (AuNPs) have been extensively used as nanomaterials for theranostic applications due to their multifunctional characteristics in therapeutics, imaging, and surface modification 66. It has been proven that exosome-derived membranes in combination with synthetic AuNPs have unique functionalities for targeted delivery to brain. Also, the bioluminescence imaging revealed that after intravenous injection, targeted-exosome coated AuNPs could accumulate in the mouse brain. The surface modification of synthetic AuNPs with the brain-targeted exosome can be a highly novel and effective strategy to provide efficient brain targeting (Figure 10) 67.
Figure 10.
The brain-targeting property of AuNPs after fabrication with neuron-targeted exosome. (a) Schematic diagram showing the arrangement of the flow chamber perfusion system. The instrument required brain cells cultured on a tissue culture plate. A flow chamber was then assembled over the cultured cells and targeted exosome-coated AuNPs were flowed over the cultured cells. (b) Adhesion of nanoparticles to brain cells under flow conditions. Brain cells were exposed to targeted exosome-coated AuNPs or control AuNPs under flow condition. Hela cells were used as negative controls. As nanoparticles had been fluorescently labelled, the binding of nanoparticles to cells was visualized and analyzed using fluorescence microscopy. (c) An in vitro blood-brain barrier (BBB) model being composed of co-culture with endothelial (bEnd.3) and astrocyte-like (ALT) cells was established to evaluate the transcytosis of AuNPs coated with neuron-specific rabies viral glycoprotein (RVG)- or unmodified exosomes. The expression of tight junction protein claudin-5 and ZO-1 in BBB model. Green: claudin-5 proteins; Blue: cell nucleus. (d) The percentage of exosome-coating AuNPs transported across the BBB over 20 hr. targeted exosome-coated AuNPs or control AuNPs were added to the apical chamber and incubated at 37 °C up to 24 hr. Signals of fluorescently labelled nanoparticles in the basal chamber were measured in 0.5 ml aliquots at different time points. Adapted with permission from Khongkow et al. 68. Copyright 2019 Springer Nature Limited.
In amyloidosis disorders, which are associated with misfolding of normally soluble, functional peptides and proteins and causing amyloid fibrils, generation of toxic intermediates in the process of self-assembly cause amyloid diseases such as Alzheimer, Parkinson, etc. Therefore, a considerable number of therapies for Alzheimer’s disease rely on inhibition/delay/ dissociation of Aβ oligomers and fibrils. In this case, nanoparticles demonstrated substantial effects on the Aβ fibrillation process; however, their effects on progressive cognitive decline and memory have been poorly investigated in vivo. In an effort to evaluate the effect of AuNPs on the amyloid formation, α-lactalbumin protein was used as a good sample for study the amyloid formation due to the formation of molten globule state. They found that AuNPs inhibit formation of amyloid fibrils in reduced α-lactalbumin. This protective effect can be due to increase protein adsorption to the surface of the nanoparticles and therefore prevents their structural changes. Nanoparticle by binding to the monomer prevented them from joining together and elongating the core of amyloid fibrils. It can thus be used as effective therapeutic agent for prevention of formation amyloid and treatment of amyloid disease 69.
Designing multifunctional Aβ inhibitor using AuNPs such as AuNPs@POMD-pep (POMD: polyoxometalate with Wells–Dawson structure, pep: peptide) can improve synergistic effects in inhibiting Aβ aggregation, dissociating Aβ fibrils, decreasing Aβ-mediated peroxidase activity and Aβ-induced cytotoxicity 70. Some other studies also reported that using AuNPs conjugated to the specific peptides can be an effective way to destroy the toxic aggregates of Aβ. AuNPs conjugated to the specific modified β-sheet breaker peptide called CLPFFD have the ability of destroy the toxic aggregates of Aβ. To modify the capacity of drug delivery of this compound, the peptide sequence of THRPPMWSPVWP is introduced into the gold nanoparticle CLPFFD conjugate. Interaction of this peptide sequence with the transferrin receptor present in the microvascular endothelial cells of the BBB caused an increase in permeability of the conjugate in brain 71. AuNPs also have inhibitory effect on the fibrillogenesis process of insulin fibrils. Co-incubation of AuNPs with insulin lead to a one-week delay in the structural transformation of insulin fibrils into amyloid-like fibrils. Furthermore, the formed fibrils revealed altered structure, shape and dynamics which further reduced the growth rate of fibril, as well as the stability of available amyloid-like fibrils with cross-β structure for aggregation. The AuNPs have the capacity to disrupt insulin amyloid fibrillation and further prevent fibril types that are shorter and more compact. Therefore, this property of AuNPs may serve as a useful solution in solving diagnostic strategies problems for amyloid-related disorders 72. AuNPs can improve the acquisition and retention of spatial learning and memory in Aβ treated rats. Also the use of AuNPs improved the neural survival by increasing the expression of brain-derived neurotrophic factor, BDNF, cAMP response element binding protein, CREB, and stromal interaction molecules, e.g., STIM1 and STIM2 67. Electromagnetized gold nanoparticles in the presence of specific Electromagnetic fields (EMF) conditions facilitate an efficient direct lineage reprogramming to induced dopamine neurons. The dopaminergic neuron reprogramming by EMF stimulation of AuNPs efficiently and non-invasively can alleviate of symptoms in Parkinson’s disease models 73. According to these recent findings, it can be concluded that AuNPs can be highly relevant for the therapeutic applications for molecular surgery in the treatment of ND.
4.1.2. Silver Nanoparticles
Silver nanoparticles (SNPs) translocate to the brain through the blood stream after they are implanted in vivo. Distribution of SNPs crossing through the BBB in an in vitro model of rat brain micro-vessel vascular endothelial cells (BMVECs) revealed that in a medium containing 100 μg/mL of either SNPs or silver microparticles (SMPs), after 4 hours of culture, SNPs can cross the BBB and accumulate inside BMVECs, while the SMPs cannot. Passing the SNPs through the BBB could be mainly by transcytosis of capillary endothelial cells 74. Similar results about the ability of SNPs to pass across the BBB revealed that after subcutaneously injection of SNPs and SMPs (62.8 mg/kg) into brain of the rats, only SNPs can traverse the BBB and move into the brain in form of the particle. SNPs injected into the brain can enter into neurons, accumulate in the brain and induce toxicity effects, resulting in the loss of neuron function. Decomposition of neural cell membranes can lead to the release of SNPs, affect other adjacent neurons and eventually, induce neural cell apoptosis and pathological transformations. The possible mechanisms of entry of SNPs into the brain through disruption of BBB are specifically vascular endothelial cell transcytosis and BBB disruption by weakening of the tight junctions or by dissolving the membrane of the endothelial cells. They can induce further neuronal degeneration and necrosis by accumulating in the brain over a long period of time. The risk of long-term retention of some nanomaterials in the brain can be of great concern, particularly for solid core NPs that would not degrade 75. Newly engineered nanoplatforms are designed as impactful delivery devices with unique nano-properties for the manipulation of cell metabolism and cell-cell interaction. However, the oxidative modification of these novel materials by the immune system may lead to unexpected reactions and adverse effects on cell functions. Although effective timely degradation of these drug carriers is an essential factor in their design, it needs to be optimized based on nano-carrier vs drug-payload degradation 76. It is important to consider the side-effects of some of these nanoparticles; i.e., the cause of their possible unknown negative effects on metabolic pathways. It has been reported that after AgNPs treatment of neural cells, the gene expression of amyloid precursor protein (APP) was induced. Also, two main factors in suppression of Alzheimer’s disease progression, neprilysin, a major Aβ-degrading enzyme in brain, and low-density lipoprotein receptor, which is responsible for enhancing Aβ uptake and degradation in the brain, were both reduced in neural cells as well as their protein level. Thus, it is necessary to take notice of AgNPs distribution in the environment 77.
4.2. Magnetic Nanomaterials
One of the great of interest fields for researchers are magnetic nanoparticles from a wide range of disciplines, including magnetic fluids, catalysis, biotechnology/ biomedicine, magnetic resonance imaging, data storage, and environmental remediation. The use of magnetic nanoparticles (MNPs) has become an area of increasing interest in biomedicine. MNPs have unique features, such as their reaction to a magnetic force, that can be utilized in drug targeting and cell sorting. The interaction of iron oxide nanoparticles (IONPs) with astrocytes has been extensively investigated 78.
The concept of magnetic targeting is to inject magnetic nanoparticles to which drug molecules are attached, to guide these particles to a chosen site under the localized magnetic field gradients, hold them there until the therapy is complete, and then to remove them. The magnetic drug carriers have the potential to carry a large dose of drug to achieve high local concentration, and avoid toxicity and other adverse side effects arising from high drug doses in other parts of the organism.
However, optimizing the size of these NPs is a critical factor in their design and development for biomaterial-based therapies. Until recently, most studies have been focused on a relatively small size range of IONPs. The novel micro/nanomotor technologies using methods based on thin-film deposition, glancing angle deposition, direct laser writing, membrane template-assisted electrodeposition, and spiral water-conduction have been developed for fabrication of magnetic micro/nanomotors. Drugs loaded on these micro/nanomotors increase targeting efficiency, control particles direction and also improve drug concentration at the target location. Furthermore, increasing the nanoparticle diameter and magnetic field strength (MFS) will improve the magnetophoretic force and the particle capture efficiency. Investigation on IONPs diameters distributed over a much wider size range (more than 300 nm) compared to the regular range of NPs (lower than 100 nm) showed greater retention and accumulation in tumors than did small nanoparticles. The possible explanation for remaining of larger NPs in tumors is that smaller particles can move faster in the blood vessels, compare to the larger ones and thus there is greater possibility to exit the tumor 79. Clinical trials in human patients affected with prostate and brain tumors are conducted using local magnetic hyperthermia in combination with radiotherapy. Superparamagnetic particles exposed to an alternating magnetic field can be used for heat induction. The BBB activity can be reduced for 60 min when temperatures reach 42.5–43 °C, promising an improvement in combined chemotherapies of brain tumors. Superparamagnetic nanoparticles based on a core consisting of iron oxides (SPION) that can be targeted through external magnets are very promising nanoparticles. SPION can be coated with biocompatible materials and to be functionalized with drugs, proteins or plasmids. Using an external magnetic field, SPION functionalized with reversibly bound drugs could be delivered to specific locations and localized in place 80.
4.3. Polymeric Nanomaterials
Polymeric nanoparticles refer to particles formed by polymers with a size of 1–1000 nm generally 81. In particular degradable nanoparticles have become the main type of neurodegenerative drug carriers, due to their low toxicity, adjustable degradation rates and high drug loading capacity and their ability to pass through BBB and target CNS 82.
The therapeutic benefits of various water-soluble/insoluble drugs and bioactive agents, such as solubility, bioavailability, and retention time, are promoted by the frequent use of biodegradable polymer-based NPs 17. For the successful application of these polymeric systems in medicine and pharmaceuticals, the most important properties to consider are the biodegradability, biocompatibility, nontoxic, nonimmunogenic, and noncarcinogenic characteristics of these materials 83. Polylactic acid (PLA), polyglycolic acid (PGA), and poly lactic-glycolic acid (PLGA) have been widely used in medicine and pharmaceuticals 84.
PLGA NPs can be designed with various formulation protocols, e.g. bottom-up and top-down techniques. Although representing a significant potential to carry drugs for CNS therapies, unmodified PLGA NPs have some downsides such as negative charge, hydrophobic structure, and non-targeting of the BBB. Recently, some of these drawbacks have been resolved by engineering techniques. These include polymersome creation, core-shell type hybridization, cell-PLGA hybridization, surface derivatization, receptor-specific ligand-PLGA conjugation. Surface modification of PLGA NPs improved their ability to cross the BBB, tissue targeting for encapsulated nucleic acids (siRNA) and some specific drugs. This strategy also facilitates the slowly controlled release of drugs in their target sites 85. Surface-coated PLGA-NPs with polysorbate 80 and poloaxmer 188 had shown an improved CNS penetration 86. (531) Poly (lactide-co-glycolide) (PLGA), a biodegradable polymer, can be decomposed into lactic and glycolic acids and converted further into carbon dioxide through the tricarboxylic acid cycle 87. PLGA could form uniform nanoparticles (NPs) in microemulsion. A study on delivering pharmaceuticals against human immunodeficiency virus residing in the brain showed that antiretroviral nevirapine-entrapped PLGA NPs could permeate human brain-microvascular endothelial cells (HBMECs), the key cellular component of the BBB. Also, polyacrylamide (PAAM) is a synthetic cationic biopolymer with good biocompatibility 88. Kou and Tsai developed polyacrylamide (PAAM)-cardiolipin (CL)-poly(lactide-co-glycolide) (PLGA) NPs grafted with surface 83–14 monoclonal antibody (MAb) to carry rosmarinic acid and curcumin. This drug delivery system was used to cross BBB and enhance the viability of SK-N-MC cells (from human neuroblastoma) insulted with β-amyloid (Aβ) deposits. Experimental evidence revealed that an increase in the concentration of 83–14 MAb enhanced the permeability coefficient of rosmarinic acid and curcumin using the nanocarriers 89. In order to offer controlled release of paclitaxel, an optimal paclitaxel microemulsion prepared by self-micro-emulsifying drug delivery system (SMEDDS) was developed which included a mixture of paclitaxel, tetraglycol, Cremophor ELP, and Labrafil 1944 and a paclitaxel microemulsion containing poly (d,l-lactide-co-glycolide) (PLGA). The release behavior of paclitaxel from microemulsion containing PLGA having various molecular weights (8K, 33K, and 90K) exhibited a biphasic pattern characterized by a fast initial release during the first 48 h, followed by a slower and continuous release for 144 h, in contrast that the release of paclitaxel from microemulsion without PLGA was finished during 24 h . Solanum tuberosum lectin (STL) conjugated poly (DL-lactic-co- glycolic acid) (PLGA) nanoparticle (STL-NP) was constructed as a novel biodegradable nose-to-brain drug delivery system. STL-NP demonstrated 1.89–2.45 times higher brain targeting efficiency in different brain tissues than unmodified NP 90. There is evidence that siRNA-chitosan nanoparticles are able to efficiently silence the P-glycoprotein (P-gp) gene in a BBB model. The knockdown resulted in a considerable reduction in P-gp substrate efflux and improved delivery and efficacy of doxorubicin, which can be used as a model drug.
PLA-NPs pre-loaded with a flavonoid breviscapine were able to penetrate the BBB in a size-dependent manner, with larger particles (~300 nm) delivering more drug concentrations to the brain than smaller ones (~200 nm). In another case, trans-activating transcriptor (TAT) peptide, associated with the surface of PLA-NPs, promoted an increase in transport of the same NPs through the BBB via the bypass of efflux transporters. These findings suggest that a nanoparticle mediated delivery of anti-P-gp siRNA could be a promising approach to improve the treatment of various diseases in the CNS where drug delivery is currently limited by the BBB 91.
4.4. Carbon Nanomaterials
Of all the types of nanomaterials, carbon-based nanomaterials with hydrophobic surfaces, including zero-dimensional fullerene (C60), one-dimensional carbon nanotubes (CNTs) and two-dimensional graphene have aroused considerable interest in nanomedicine due to their unique combinations of chemical and physical properties (i.e., thermal and electrical conductivity, high mechanical strength, and optical properties) 92. Here some application of these nanomaterials will be discussed.
4.4.1. Graphene
Graphene and graphene oxide (GO), with their advantageous properties, have recently emerged as new and competitive drug delivery systems with the potential to be applied in systemic, targeted, and local drug delivery systems 93. Graphene-based materials generally aggregate in aqueous medium containing salts, proteins, or other ions, and require chemical modification or functionalization to have the desired properties. Such functionalization enables researchers to change the basic electrical and optical properties of graphene. These modifications also allow conjugation of contrast agents, antibodies, peptides, ligands, drugs, and genes to the surface of graphene nanoparticle 94.
Graphene-heparin/poly-L-lysine polyelectrolytes were assembled via layer-by-layer (LbL) deposition onto 2D surfaces and 3D electrospun nanofibers. Cell culture experiments showed that both 2D and 3D graphene−PEMs supported neuron cell adhesion and neurite outgrowth, with no appreciable cell death. This electroactive scaffold modification may therefore assist in neuronal regeneration, for creating functional and biocompatible polymer scaffolds for electrical entrainment or biosensing applications 95.
Electroactive materials have been investigated as next-generation neuronal tissue engineering scaffolds to enhance neuronal regeneration and functional recovery after brain injury. Graphene, an emerging neuronal scaffold material with charge transfer properties, has shown promising results for neuronal cell survival and differentiation in vitro 96.
4.4.2. Fullerenes
The protective function of fullerenes is via two main ways: radical sponge and hydrophobic surface. The special structure of fullerene is exemplified by a “radical sponge,” with capability to entrap several radicals in a single molecule sphere that culminates in an effective antioxidant activity against cytotoxicity induced by intracellular oxidative stress 97. The protective effects of the polyhydroxylated fullerene derivative C60(OH)24 have been examined in a 1-methyl-4-phenylpyridinium (MPP1)- induced acute cellular Parkinson’s disease model in human neuroblastoma cells. Results suggested that Polyhydroxylated Fullerene Derivative C60(OH)24 is an effective antioxidant with powerful mitochondrial protective antioxidant with direct radical scavenging activity and indirect antioxidant inducing activity and is a potential agent for preventing mitochondrial dysfunction and oxidative damage in an MPP1-cells.
In another study, water-soluble C60 fullerene derivatives with different types of linkages between the fullerene cage and the solubilizing addend were synthesized (compounds 1−3: C−C bonds, compounds 4−5: C−S bonds, compound 6: C−P bonds, and compounds 7−9: C−N bonds). Fullerene derivatives 1−6 were observed to induce neural stem cell (NSC) proliferation in vitro and rescue the function of injured CNS in zebrafish. Fullerene derivatives 7−9 were found to inhibit glioblastoma cell proliferation in vitro and reduce glioblastoma formation in zebrafish. These effects were correlated with the cell metabolic changes. Particularly, compound 3 bearing residues of phenylbutyric acids significantly promoted NSC proliferation and neural repair without causing tumor growth. Meanwhile, compound 7 with phenylalanine appendages significantly inhibited glioblastoma growth without retarding neural repair. It can be concluded that the surface functional group determines the properties as well as the interactions of C60 with NSCs and glioma cells, producing either a neuroprotective or antitumor effect for possible treatment of CNS-related diseases, induced cellular model of Parkinson’s disease 98.
4.4.3. Carbon Nano Tubes
Carbon nano tubes are allotropes of carbon with a cylindrical nanostructure. Nanotubes are part of the fullerene family, members of which have a long hollow structure made of sheets one atom thick. They include one or several layers of carbon categorized as single-wall and multiwall CNTs 99, 100. These carbon-based NPs are valuable in medicine 101. The CNTs have special chemical, mechanical, and electrical properties 30, 102. The pure and modified (by a variety of polymers) forms of CNTs have been evaluated. The formation of nanotube-neural hybrid networks can promote the neuronal activity, network communication, and synaptic formation 3. In the future, CNT-based technologies are likely to be particularly useful for promoting functional recovery of neurons after brain damage due to the outstanding physical properties of these nanomaterials together with their recently documented ability to interface with neuronal circuits, synapses, and membranes.
Zhang et al. have demonstrated the electrical conductive capacity and strong mechanical properties of CNTs, and shown the morphological characteristics of CNTs to be similar to those of neurons 19. Using single-cell electrophysiology techniques, electron microscopy analysis and theoretical modelling, Cellote et al. revealed that nanotubes improve the responsiveness of neurons by forming tight contacts with the cell membranes that might favor electrical shortcuts between the proximal and distal compartments of the neuron 103. Multiwalled carbon nanotubes (CNTs) coated with neurotrophin were used to regulate the differentiation and survival of neurons. By enzyme-linked immunosorbent assay (ELISA), it has been demonstrated that neurotrophin-coated CNTs carry neurotrophin. These findings suggest that neurotrophin-coated CNTs have biological activity and stimulate the neurite outgrowths of neurons 104. Maintenance of the interaction between CNTs and stem cells introduces a new vision for the application of these carbon-based NPs in the design and fabrication of nervous tissue by cellular simulation 3, 105 (Figure 11).
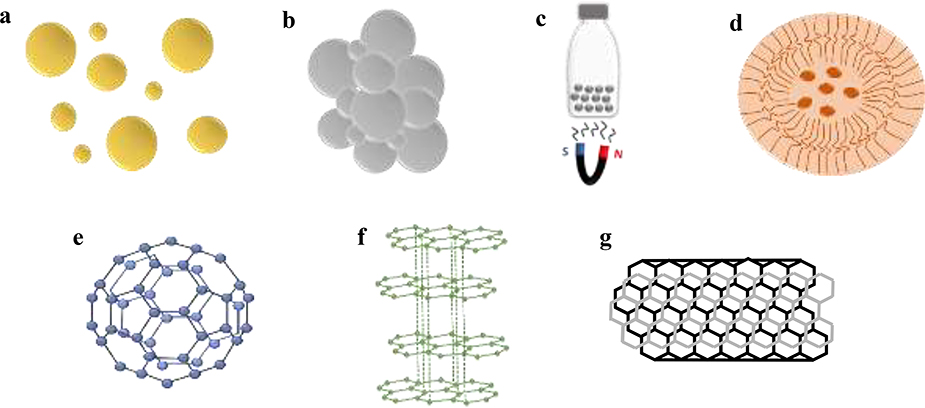
Figure 11.
Schematic representation of a) Gold Nanoparticles, b) Silver Nanoparticles, c) Magnetic Nanomaterials, d) Polymeric Nanomaterials, e) Fullerene, f) Graphene g) Carbon Nanotube
5. Strategies to Overcome Drug Delivery Impediments
Numerous clinical data suggested that Alzheimer’s Disease patients exhibit severe impairment of cholinergic–neurotransmitter systems, possibly due to the suppression of acetylcholine (responsible for neural synapse) by Acetylcholinesterase (AChE) activity 1, 113. Subsequently, accumulating evidence also suggested that the activation of the glutamatergic system plays a significant role in Alzheimer’s Disease pathology 26, 27. Based on these two scientific leads, researchers have developed few approved drug candidates for treatment of neurodegenerative diseases, such as tacrine, donepezil, rivastigmine, galantamine (AChE inhibitors), and memantine (NMDA inhibitor) 1, 45, 114.
However, as implied in the previous section, there are some major barriers for delivering these drugs to target brain cells. Current advances in nanotechnology present opportunities to overcome mentioned limitations by using nanomedicines and designing nanomaterials improving delivering active drug candidates. Modification of properties associated with surface modulation has resulted in NPs as excellent candidates to cross the blood brain barrier (BBB) and improve drug delivery to the brain1. Teleanu et al. recently discussed an emerging role for neuronanomedicine as a new field that constitutes a link between neurological science and nanotechnology 115. It is a promising field for the diagnosis and treatment of CNS disorders 6. The first reported drug delivery using nanoparticles to transport across the BBB was dalargin, a hexapeptide, which was transported into the brain using poly (butylcyanoacrylate) nanoparticles coated with polysorbate 80 43.
Recently, noninvasive techniques based on colloidal carriers could represent a huge potential, since nanocarriers can protect drugs (or gene material) and deliver them across the BBB to target specific populations of brain cells 13. Some of these approaches will be discussed here.
5.1. Local Delivery
Numerous studies have been focused on the local delivery system of drugs into the CNS and many therapeutic strategies have been developed to improve local delivery of various nanodrugs into their target which mainly is located in CNS 116. Applying polymer pellets can deliver extremely high doses of drug to brain for a sustained period and its efficiency depends on the local rates of transportation and elimination of drug from the brain tissues. Although an enhanced dose of drug could be delivered to the tumor resection, the limited penetration of the drug released from the implants over the tumor margin greatly limited the therapeutic efficacy 116. So, neurologists searched for various chemotherapy agents which because of their physical properties can penetrate further into the brain cells and tissues near the polymer carrier. Nanoparticles such as liposomal ones have recently attracted the attention of researchers as good candidates for local delivery of drug into CNS 117, 118[139,140]. Previously, the main problem in the local delivery system was the application of huge microparticles such as polymer microspheres of poly (lactic-co-glycolic acid) PLGA, poly (methylidene malonate) (PMM), poly (epsilon-caprolactone), and chitosan 119. These polymers have great potential for treating many brain diseases, but their transport is impeded by their size and surface properties. Recently, the intracranial infusion or convection enhanced delivery (CED) approach has been overcome and by using this system, proteins and several kinds of particles, including liposomes and polymeric nanoparticles less than 100 nm in size can be used for drug delivery instead of larger microparticles. Design of microfluidic probes can increase the effective pore size of the brain extracellular matrix (ECM). To enhance the transport of the infused nanoparticles, two methods were used; the dilation of ECM and enzymatic digestion of specific constituents of ECM. The findings demonstrated that using this probe potentially enhance nanoparticle transport through the brain parenchyma. Their findings revealed that both enzymatic treatment and dilation of the extracellular space significantly enhance the transport of polymeric nanoparticles 120. The CED can be an effective approach in gene therapy applications and delivering large molecules and particles to the CNS or transduced large areas of cortex by infusing the bioorganism in different parts of the brain 121, 122. The CED has been shown more advantages compared to conventional diffusion-based injections to deliver more homogeneous vector densities across the expressing regions 121, 123. This approach provides relatively large volumes of drugs which are infused into the parenchyma of target tissue, to create pressure that drives bulk fluid flow (convection). The much faster delivery rate in conventional techniques enables infusion of large volumes of vector (up to 200 μl for thalamic delivery) via a single infusion site over 45 min. The small number of injections and faster infusion rates make it practical to plan and infuse the viral vector under the guidance of MRI, which enables precise targeting and real time monitoring 123.
5.2. Receptor-Mediated Transcytosis
Delivery of many endogenous macromolecules into the brain depends on the receptor-mediated transcytosis pathway. The receptor mediated transcytosis mechanism is based on the reciprocal action of nanoparticles surface ligand and a specific receptor in the BBB 53, relying on the presence of receptors that are highly expressed by BBB cells, to selectively drive functionalized nanomaterial across the BBB endothelium 9. Most nanoparticles cannot freely pass through the BBB and need receptor-mediated transcytosis 6, 9, 124. If these nanocarriers are not engineered on their surface in order to take advantage of BBB transport mechanisms, they are unable to satisfactorily target and reach the brain only to a poor extent (up to 0.1–1% of the injected dose). The ligand-based approach, one of the different approaches included in NP engineering, became an interesting choice in order to achieve a more specific and selective drug delivery to the CNS 43. In this method, nanoparticles are functionalized with different types of ligands, such as insulin, transferring, lactoferrin, or surfactants like polysorbate 80 16, 51, 124. The interaction between nanoparticles bounded with ligands and the brain endothelial cells leads to plasma membrane retraction followed promote the formation of corpuscles via endocytosis pathway. Next, the formed corpuscles release nanoparticles from ligands and enter the brain parenchyma in CNS and cross the BBB via exocytosis to exercise their function, without damaging the BBB 6, 61, 125, 126. Therefore, receptor-mediated BBB-crossing and improving the knowledge about the upregulation of different receptors can be considered as a promising technological tool for delivery of therapeutic agents into the CNS. There are several receptor-ligand approaches of which the most common examples are insulin receptors, transferrin receptors, interleukin 13 receptors, lipoprotein receptors, lactoferrin receptors 127.
5.3. Physicochemical Disruption of BBB
Nanotechnology has beneficial applications for the manipulation and disruption of tight junctions of BBB with existing strategies such as Magnetic resonance imaging-ultrasound technique, cell penetrating peptides and magnetic disruption which can facilitate the entry of drugs into the brain116, 128.
5.3.1. Magnetic Resonance Imaging-Ultrasound
Magnetic Resonance Imaging (MRI) is one of the basic clinical imaging modalities used in diagnosis of brain diseases, with the ability of providing high spatial and temporal resolution.
Nanoplatforms have been designed for improving the delivery of contrast agents in this technique. This biodegradable nanoparticle-based BBB transport system have been developed to deliver BBB-impermeable molecular imaging probes into brain for targeted neuroimaging. Coating MRI contrast agents with nanoparticles increased the delivery of the agent to the brain several hundredfold. Active systems for drug delivery and imaging can respond to the input of external agents, e.g., targeted ultrasound, magnetic field and pH. Recently, a pH-sensitive nanoplatform has been designed as a systemic delivery system for tumor-targeted dual-mode MRI as well as chemotherapy without any toxic effects 129.
When applied in the presence of circulating microbubbles, focused ultrasound can safely and transiently open the BBB to facilitate the delivery of immunotherapeutic agents into the brain parenchyma. Another promising approach for stimulating therapeutic immune responses in the brain is the deposition of high-density acoustic energy via non-invasive focused ultrasound (FUS) 130. Typically performed under real-time image guidance using diagnostic ultrasound or magnetic resonance imaging (MRI), FUS can markedly enhance therapeutic drug and gene delivery and distribution, as well as potentiate immune responses in tissues 131, 132. The ultrasound based techniques depend on the energy of acoustically activated microbubbles to provide a transient and reversible permeabilization of vascular endothelium, promoting transportation of desired molecules into the CNS across the BBB 116, 132.
MRI-guided FUS (MRIgFUS) delivery of anti-Ab antibodies rapidly reduces plaque pathology. Researchers used intravenous injections of MRI and FUS contrast agents, as well as anti-Ab antibody into brain cells and within minutes, the MRI contrast agent entered the brain. This method provides the combined advantages of using a low dose of antibody and rapidly reducing plaque pathology 133. Using another mouse model of Alzheimer’s disease (B6C3-Tg), Raymond et al. suggested that anti-Ab antibodies given intravenously could enter FUS-targeted areas of the brain 134. MRIgFUS combined with microbubbles have the ability of local and noninvasive delivery of neurotrophic factors such as glial cell line-derived neurotrophic factor (GDNF) into the CNS. Without using this techniques, the application of GDNF to treat neurodegenerative disease effectively is restricted because of inhibitory nature of BBB which prevent local delivery of macromolecular therapeutic agents into the CNS (Figure 12) 135. This approach has been developed to enhance the brain delivery efficiency of small molecular compounds, large biomolecules, and even nanoparticles.
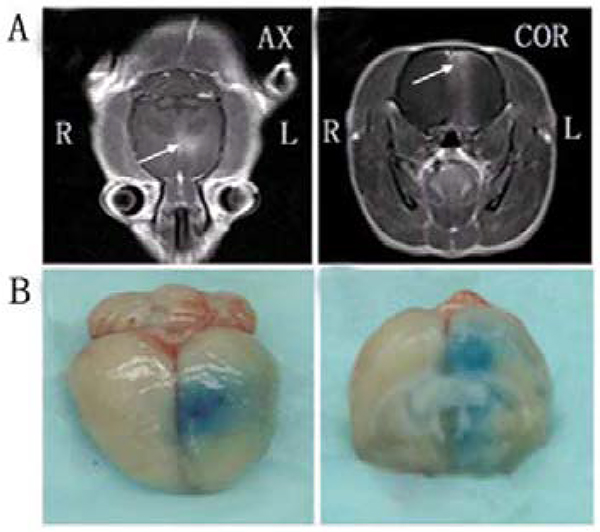
Figure 12.
MRI monitoring of BBB disruption and photographs of harvested brain showing BBB disruption induced by focused ultrasound. (A) BBB opening was monitored by leakage of the magnetic resonance (MR) contrast agent into the brain parenchyma on axial (AX) and coronal (COR) MR images (arrows). (B) The location of the BBB opening was confirmed by Evans Blue staining of the affected area. Adapted with permission from Wang et al. 135. Copyright 2012 PLOS.
5.3.2. Cell Penetrating Peptides
Cell penetrating peptides (CPP), are a class of diverse short (generally not exceeding 30 residues), water-soluble and partly hydrophobic, and/or polybasic peptides rich in basic amino acids such as arginine and lysine, widely used for siRNA, proteins and small molecular drugs delivery. They also have the ability to carry drugs to penetrate BBB efficiently 136–139. They help the conjugated therapeutic agents and have the capacity to ubiquitously cross biomaterials into the cell membranes 116, 138, 140, 141. Most common CPPs are positively charged peptides, though the presence of few anionic or hydrophobic CPPs was also demonstrated.
There are mainly three types of CPPs, peptides derived from proteins, chimeric peptides that are formed by the fusion of two natural sequences, and synthetic CPPs which are rationally designed sequences usually based on structure– activity studies 140.
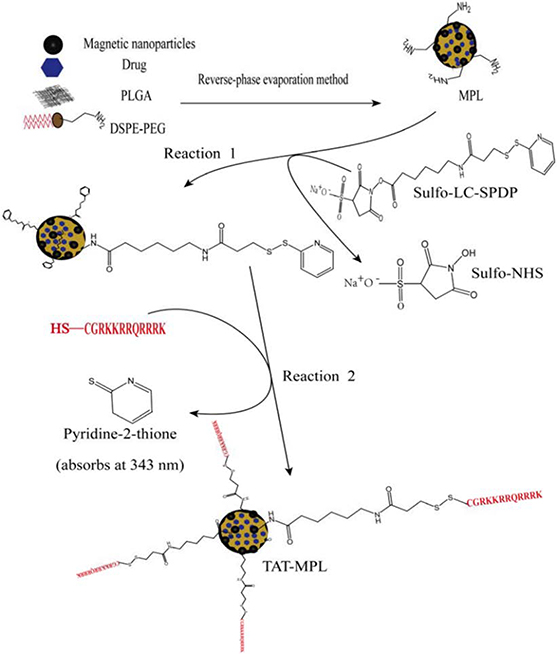
Although the cell membrane transportation mechanism of CPPs is still not clear, the binding of cationic charges in CPPs to the brain endothelial cell membrane surfaces via electrostatic interactions is helpful for the transportation of CPPs-modified nanomaterials across the BBB 116. Based on recent findings, the generally accepted mechanisms to facilitate membrane permeation of the CPPs include both endocytosis and direct translocation, in contrast to prior beliefs that only direct translocation was involved 142. However, due to their non-specific affinity to different cells, CPP-mediated brain delivery systems have high drug distribution in the whole brain, and this property would lead to unwanted toxicity to normal brain tissues 138. As a solution for this problem, a brain drug delivery system was designed for glioma chemotherapy based on transferrin and CCP dual-functioned liposome (Tf/TAT-lip), and their system efficiency was evaluated with doxorubicin (DOX) as a model drug. In vitro experiments demonstrated the Tf/TAT co-modified liposomes could deliver drugs across the BBB effectively. Moreover, the Ex Vivo biodistribution investigation revealed that the Tf/TAT-lip could deliver therapeutic agents across the BBB more effectively 138. Modulation of a second ligand to enhance cell penetration can be another effective strategy to improve transportation rate of nanoparticles into the brain using a Tf Receptor-mediated pathway. Using the bi-ligand technique in vivo, the concentration of liposomes reaching the brain increased, with in vitro experiments confirming the improvement of permeation into the parenchyma cells 143. Magnetic poly lipid nanoparticles (MPLs), compared to conjugated MPLs to trans-activating transcriptor (TAT), (TAT-MPLs) peptide, were designed to target the brain by magnetic guidance and TAT conjugation. The TAT-MPLs showed very strong fluorescence in the cytoplasm and cell nucleus, and delivered drugs to brain parenchyma cells efficiently. Therefore, it can potentially function as an effective drug delivery system that crosses the BBB (Figure 13) 144.
Figure 13.
Schematic of the preparation of stealth MPLs and the conjugation of TAT peptide to MPLs. Adapted with permission from Wen et al. 144. Copyright 2014 PLOS.
5.3.3. Magnetic Disruption
Magnetic gradients can improve the transportation rates of nanoparticle ferrofluids using nanoparticles which can become excited by an alternating magnetic field and producing heat which is called hyperthermia 116. Inside the brain microvasculature, such heat may thermally disrupt an intact BBB, thereby creating a transient entry for the therapeutic compounds to cross into the brain tissue. In fact, it has long been recognized that hyperthermia, otherwise known as elevation of body temperature, can lead to severe cellular stress and cause temporal disruption of the BBB 145. Among various methods of hyperthermia, whole body , microwave and radiofrequency hyperthermia 145, 146 are most frequently used to penetrate the BBB. In these techniques an entire area of the brain including neurons, astrocytes, vessel wall cells, and other glial cells are usually heated, which can result in many undesirable severe side effects during hyperthermic disruption of the BBB. Conversely, the induced hyperthermic disruption of the BBB by excitation of magnetic nanoparticles (MNPs) inside an alternating magnetic field, heat is exclusively dissipated to the ambient vessel wall cells by thermal conduction. Therefore, only the monolayer lining of the vessel walls and the endothelial cells are directly affected by the thermal stress 146. In addition, combination of magnetic disruption with the focused ultrasound can improve the BBB transportability of therapeutic magnetic nanoparticles 147. The effects of magnetic field enhanced convective diffusion (MFECD) were most apparent with the negatively charged N (trimethoxysilylpropyl) ethylenediaminetriacetate [EDT]- iron oxide nanoparticles (IONPs; EDT-IONPs). These results were consistent with enhanced bulk flow permeability of IONPs across transiently disrupted brain micro-vessel endothelial cells. The MFECD of EDT-IONPs provided higher delivery efficiency compared with either passive or active targeting of BBB vesicular transport processes. The use of MFECD in combination with methods for transient disruption of BBB permeability represents a potential method for enhancing drug delivery to the brain 148. However, despite these efforts and achievements, this technique has yet to develop into a workable clinical application. One of the reasons is the low payload capacity of existing MNPs 149, because payload (i.e. drugs) can only be attached on the surface or embedded in the double-layer coating around MNPs. To address this issue, one of the solutions is to utilize hollow MNPs, in which drugs could be loaded both inside their hollow core and on the surface 150.
6. Conclusion and Prospective
The critical role of BBB in maintaining the normal physiological function of CNS is definitely confirmed by numerous researches. However, there are also evidences that the BBB hinders the bioavailability of drugs, which is the most basic and critical issue in the treatment of neurodegenerative diseases. So, the design of brain-targeted drug carriers is particularly important. The rapid developments in nanotechnology, introduced many novel promising engineered nanoparticles and nanomaterials, represents the capability to overcome the barriers in the pathway of delivering drugs into targeting brain cells. Despite many studies focused on the application of nanoparticle on drug delivery systems, as a novel field, there are still many gaps and unsolved issues regarding to application of nanomaterials for biomedical purposes that remain to be discovered. Some of factors that need to be further considered include: 1) The side-effect of various nanoparticles on targeting cells, as well as on the other organs. Almost all the reported studies clearly demonstrate the potential for several nanomaterials to reach the CNS and induce toxic effects. One possible solution can be the coating of NPs with suitable biodegradable polymeric shells which are capable of improve the therapeutic efficacy of delivery systems, while reducing their undesired toxic effects. 2) Optimizing the amount of drug loaded on these particles by improving the structural design and maximizing their functionality. Currently, developed high-drug loading nanomedicines have a substantial problem concerning their degradation and excipient-excretion rates. As a solution, some types of carrier-free nanomedicines with no excipients have been developed. However, most current high drug-loading nanomedicine studies have focused on design and fabrication and lacked in vivo or clinical experiments. Therefore, much time and high costs are needed to improve these nanomedicines to get FDA approval. 3) Interaction of off-target drugs with non-specific receptors; in particular for receptor-mediated transcytosis methods. It has been proven that many nanomaterials may interfere with biological pathways such as oxidative stress, apoptosis and inflammation, which are common to most of the neurodegenerative disorders. 4) Research on novel nanoparticles which have not been investigated yet and addressing the uncovered gaps in drug delivery system for targeting the brain. Combination of nanotechnology with other advanced high-tech approaches such as designing implantable devices/ nanochips or multi reservoir drug delivery-chips can improve the efficiency of drug delivery by NPs. Development of engineered theranostic NPs with combined therapeutic and diagnostic applications can be also another practical technique to meet the challenges related to drug delivery systems.
Therefore, following up the recent updates in this promising filed can provide systematic knowledge to design future projects and to optimize the application of nanoparticles for treatment of neurodegenerative diseases.
Funding
MN and SMA would like to acknowledge NIH grant number 1SC3 GM111200 01A1.
Footnotes
Conflicts of interest
There are no conflicts to declare.
References
- 1.Karthivashan G, Ganesan P, Park S-Y, Kim J-S and Choi D-K, Drug delivery, 2018, 25, 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vijayan M and Reddy PH, Journal of Alzheimer’s Disease, 2016, 54, 427–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saeedi M, Eslamifar M, Khezri K and Dizaj SM, Biomedicine & Pharmacotherapy, 2019, 111, 666–675. [DOI] [PubMed] [Google Scholar]
- 4.Adams CF, Dickson AW, Kuiper J-H and Chari DM, Nanoscale, 2016, 8, 17869–17880. [DOI] [PubMed] [Google Scholar]
- 5.Dai X, Li Y and Zhong Y, Glob J Nanomed, 2018, 4, 001–004. [Google Scholar]
- 6.Rai M, Yadav A, Ingle AP, Reshetilov A, Blanco-Prieto MJ and Feitosa CM, in Nanobiotechnology in Neurodegenerative Diseases, Springer, 2019, pp. 1–398. [Google Scholar]
- 7.Spuch C, Saida O and Navarro C, Recent patents on drug delivery & formulation, 2012, 6, 2–18. [DOI] [PubMed] [Google Scholar]
- 8.Vieira DB and Gamarra LF, in Molecular Insight of Drug Design, IntechOpen, 2018, pp. 1–385. [Google Scholar]
- 9.Re F, Gregori M and Masserini M, Maturitas, 2012, 73, 45–51. [DOI] [PubMed] [Google Scholar]
- 10.Sharma U, Badyal PN and Gupta S, Int J Pharmacol, 2015, 2, 60–69. [Google Scholar]
- 11.Niu X, Chen J and Gao J, Asian Journal of Pharmaceutical Sciences, 2019, 14, 480–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De la Torre C and Ceña V, Pharmaceutics, 2018, 10, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serra A, Letunic I, Fortino V, Handy RD, Fadeel B, Tagliaferri R and Greco D, Scientific reports, 2019, 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddiqi KS and Husen A, Journal of Trace Elements in Medicine and Biology, 2017, 40, 10–23. [DOI] [PubMed] [Google Scholar]
- 15.Siddiqi KS, Husen A, Sohrab SS and Yassin MO, Nanoscale research letters, 2018, 13, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saraiva C, Praça C, Ferreira R, Santos T, Ferreira L and Bernardino L, Journal of Controlled Release, 2016, 235, 34–47. [DOI] [PubMed] [Google Scholar]
- 17.Kumari A, Yadav SK and Yadav SC, Colloids and surfaces B: biointerfaces, 2010, 75, 1–18. [DOI] [PubMed] [Google Scholar]
- 18.Kanwar JR, Sun X, Punj V, Sriramoju B, Mohan RR, Zhou S-F, Chauhan A and Kanwar RK, Nanomedicine: Nanotechnology, Biology and Medicine, 2012, 8, 399–414. [DOI] [PubMed] [Google Scholar]
- 19.John AA, Subramanian AP, Vellayappan MV, Balaji A, Mohandas H and Jaganathan SK, International journal of nanomedicine, 2015, 10, 4267–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alyautdin R, Khalin I, Nafeeza MI, Haron MH and Kuznetsov D, International journal of nanomedicine, 2014, 9, 795–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furtado D, Björnmalm M, Ayton S, Bush AI, Kempe K and Caruso F, Advanced Materials, 2018, 30, 1801362. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim MM and Gabr MT, Neural regeneration research, 2019, 14, 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniele S, Giacomelli C and Martini C, Biochemical pharmacology, 2018, 158, 207–216. [DOI] [PubMed] [Google Scholar]
- 24.Liu EY, Cali CP and Lee EB, Disease models & mechanisms, 2017, 10, 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Dam D and De Deyn PP, British journal of pharmacology, 2011, 164, 1285–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danysz W and Parsons CG, British journal of pharmacology, 2012, 167, 324–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Revett TJ, Baker GB, Jhamandas J and Kar S, Journal of psychiatry & neuroscience: JPN, 2013, 38, 6–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta M, Adem A and Sabbagh M, International Journal of Alzheimer’s disease, 2012, 2012, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordão JF, Ayala-Grosso CA, Markham K, Huang Y, Chopra R, McLaurin J, Hynynen K and Aubert I, PloS one, 2010, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modi G, Pillay V and Choonara YE, Annals of the New York Academy of Sciences, 2010, 1184, 154–172. [DOI] [PubMed] [Google Scholar]
- 31.Rahaman ST, World Journal of Pharmaceutical Sciences, 2018, 2321–3310. [Google Scholar]
- 32.Adams WR, PloS one, 2017, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adhikary RR, Sandbhor P and Banerjee R, ADMET and DMPK, 2015, 3, 155–181. [Google Scholar]
- 34.Maiti P, Manna J and Dunbar GL, Translational neurodegeneration, 2017, 6, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adam H, Gopinath SC, Arshad MM, Adam T and Hashim U, Process Biochemistry, 2019, 86, 32–39. [Google Scholar]
- 36.Ansorena E, Casales E, Aranda A, Tamayo E, Garbayo E, Smerdou C, Blanco-Prieto MJ and Aymerich MS, International journal of pharmaceutics, 2013, 440, 19–26. [DOI] [PubMed] [Google Scholar]
- 37.Tiwari PC and Pal R, Dialogues in clinical neuroscience, 2017, 19, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.André EM, Delcroix GJ, Kandalam S, Sindji L and Montero-Menei CN, Pharmaceutics, 2019, 11, 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernis ME, Babila JT, Breid S, Wüsten KA, Wüllner U and Tamgüney G, Acta neuropathologica communications, 2015, 3, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jansen AH, Batenburg KL, Pecho-Vrieseling E and Reits EA, Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 2017, 1863, 793–800. [DOI] [PubMed] [Google Scholar]
- 41.Liu X.-g., Lu S, Liu D.-q., Zhang L, Zhang L.-x., Yu X.-l.and Liu R.-t., Brain research, 2019, 1707, 141–153. [DOI] [PubMed] [Google Scholar]
- 42.Ramaswamy S and Kordower JH, Neurobiology of disease, 2012, 48, 243–254. [DOI] [PubMed] [Google Scholar]
- 43.Machtoub L and Kasugai Y, Amyotrophic Lateral Sclerosis: Advances and Perspectives of Neuronanomedicine, CRC Press, 2016. [Google Scholar]
- 44.Aulić S, Bolognesi ML and Legname G, International journal of cell biology, 2013, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birks JS and Evans JG, Cochrane Database of systematic reviews, 2015, CD001191. [DOI] [PubMed] [Google Scholar]
- 46.Erickson MA and Banks WA, Journal of Cerebral Blood Flow & Metabolism, 2013, 33, 1500–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Assema DM, Lubberink M, Boellaard R, Schuit RC, Windhorst AD, Scheltens P, Lammertsma AA and van Berckel BN, Molecular imaging and biology, 2012, 14, 771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tam RY, Fuehrmann T, Mitrousis N and Shoichet MS, Neuropsychopharmacology, 2014, 39, 169–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teleanu DM, Chircov C, Grumezescu AM, Volceanov A and Teleanu RI, Pharmaceutics, 2018, 10, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kasinathan N, Jagani HV, Alex AT, Volety SM and Rao JV, Drug delivery, 2015, 22, 243–257. [DOI] [PubMed] [Google Scholar]
- 51.Teleanu DM, Chircov C, Grumezescu AM, Volceanov A and Teleanu RI, Journal of clinical medicine, 2018, 7, 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mendes M, Sousa JJ, Pais A and Vitorino C, Pharmaceutics, 2018, 10, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teleanu DM, Negut I, Grumezescu V, Grumezescu AM and Teleanu RI, Nanomaterials, 2019, 9, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarkar A, Fatima I, Mohammad Sajid Jamal Q, Sayeed U, Khan KA, Akhtar S, Amjad Kamal M, Farooqui A and Haris Siddiqui M, Current drug metabolism, 2017, 18, 129–137. [DOI] [PubMed] [Google Scholar]
- 55.Damkier HH, Brown PD and Praetorius J, Physiological reviews, 2013, 93, 1847–1892. [DOI] [PubMed] [Google Scholar]
- 56.Strazielle N and Ghersi-Egea J-F, Current pharmaceutical design, 2016, 22, 5463–5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu D-H, Yan M, Fang P-F and Liu Y-W, European journal of pharmacology, 2012, 690, 68–76. [DOI] [PubMed] [Google Scholar]
- 58.Khatoon M, Shah KU, Din FU, Shah SU, Rehman AU, Dilawar N and Khan AN, Drug delivery, 2017, 24, 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lovisolo D, Dionisi M, Ruffinatti FA and Distasi C, AIMS Mol. Sci, 2018, 5, 1–13. [Google Scholar]
- 60.Zhang C, Xie B, Zou Y, Zhu D, Lei L, Zhao D and Nie H, Advanced drug delivery reviews, 2018, 132, 33–56. [DOI] [PubMed] [Google Scholar]
- 61.Tosi G, Vandelli MA, Forni F and Ruozi B, Expert Opinion on Drug Delivery, 2015, 12, 1041–1044. [DOI] [PubMed] [Google Scholar]
- 62.Ramanathan S, Archunan G, Sivakumar M, Selvan ST, Fred AL, Kumar S, Gulyás B and Padmanabhan P, International journal of nanomedicine, 2018, 13, 5561–5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaushik AC, Bharadwaj S, Kumar S and Wei D-Q, Scientific reports, 2018, 8, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jin G-Z, Chakraborty A, Lee J-H, Knowles JC and Kim H-W, Journal of Tissue Engineering, 2020, 11, 2041731419897460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sheikh S, Haque E and Mir SS, Journal of neurodegenerative diseases, 2013, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ajnai G, Chiu A, Kan T, Cheng C-C, Tsai T-H and Chang J, Journal of Experimental & Clinical Medicine, 2014, 6, 172–178. [Google Scholar]
- 67.Sanati M, Khodagholi F, Aminyavari S, Ghasemi F, Gholami M, Kebriaeezadeh A, Sabzevari O, Hajipour MJ, Imani M and Mahmoudi M, ACS chemical neuroscience, 2019, 10, 2299–2309. [DOI] [PubMed] [Google Scholar]
- 68.Khongkow M, Yata T, Boonrungsiman S, Ruktanonchai UR, Graham D and Namdee K, Scientific reports, 2019, 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Talebpour F and Ghahghaei A, International Journal of Peptide Research and Therapeutics, 2020, 1–10. [Google Scholar]
- 70.Gao N, Sun H, Dong K, Ren J and Qu X, Chemistry–A European Journal, 2015, 21, 829–835. [DOI] [PubMed] [Google Scholar]
- 71.Prades R, Guerrero S, Araya E, Molina C, Salas E, Zurita E, Selva J, Egea G, López-Iglesias C and Teixidó M, Biomaterials, 2012, 33, 7194–7205. [DOI] [PubMed] [Google Scholar]
- 72.Hsieh S, Chang C.-w. and Chou H.-h., Colloids and Surfaces B: Biointerfaces, 2013, 112, 525–529. [DOI] [PubMed] [Google Scholar]
- 73.Yoo J, Lee E, Kim HY, Youn D.-h., Jung J, Kim H, Chang Y, Lee W, Shin J and Baek S, Nature nanotechnology, 2017, 12, 1006–1014. [DOI] [PubMed] [Google Scholar]
- 74.Tang J, Xiong L, Zhou G, Wang S, Wang J, Liu L, Li J, Yuan F, Lu S and Wan Z, Journal of nanoscience and nanotechnology, 2010, 10, 6313–6317. [DOI] [PubMed] [Google Scholar]
- 75.Bony BA and Kievit FM, Pharmaceutics, 2019, 11, 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lombardo D, Kiselev MA and Caccamo MT, Journal of Nanomaterials, 2019, 2019. [Google Scholar]
- 77.Huang C-L, Hsiao I-L, Lin H-C, Wang C-F, Huang Y-J and Chuang C-Y, Environmental research, 2015, 136, 253–263. [DOI] [PubMed] [Google Scholar]
- 78.Migliore L, Uboldi C, Di Bucchianico S and Coppedè F, Environmental and molecular mutagenesis, 2015, 56, 149–170. [DOI] [PubMed] [Google Scholar]
- 79.Manshadi MK, Saadat M, Mohammadi M, Shamsi M, Dejam M, Kamali R and Sanati-Nezhad A, Drug delivery, 2018, 25, 1963–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Neuberger T, Schöpf B, Hofmann H, Hofmann M and Von Rechenberg B, Journal of Magnetism and Magnetic materials, 2005, 293, 483–496. [Google Scholar]
- 81.Hadavi D and Poot AA, Frontiers in bioengineering and biotechnology, 2016, 4, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ji W-H, Xiao Z-B, Liu G-Y and Zhang X, Chinese Chemical Letters, 2017, 28, 1829–1834. [Google Scholar]
- 83.Chiellini F, Piras AM, Errico C and Chiellini E, Nanomedicine, 2008, 3, 367–393. [DOI] [PubMed] [Google Scholar]
- 84.Shakeri S, Ashrafizadeh M, Zarrabi A, Roghanian R, Afshar EG, Pardakhty A, Mohammadinejad R, Kumar A and Thakur VK, Biomedicines, 2020, 8, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cai Q, Wang L, Deng G, Liu J, Chen Q and Chen Z, American journal of translational research, 2016, 8, 749–764. [PMC free article] [PubMed] [Google Scholar]
- 86.Calzoni E, Cesaretti A, Polchi A, Di Michele A, Tancini B and Emiliani C, Journal of functional biomaterials, 2019, 10, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A and Préat V, Journal of controlled release, 2012, 161, 505–522. [DOI] [PubMed] [Google Scholar]
- 88.Kuo Y-C, Lin P-I and Wang C-C, Nanomedicine, 2011, 6, 1011–1026. [DOI] [PubMed] [Google Scholar]
- 89.Kuo Y-C and Tsai H-C, Materials Science and Engineering: C, 2018, 91, 445–457. [DOI] [PubMed] [Google Scholar]
- 90.Chen J, Zhang C, Liu Q, Shao X, Feng C, Shen Y, Zhang Q and Jiang X, Journal of drug targeting, 2012, 20, 174–184. [DOI] [PubMed] [Google Scholar]
- 91.Malmo J, Sandvig A, Vårum KM and Strand SP, PLoS One, 2013, 8, e54182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cha C, Shin SR, Annabi N, Dokmeci MR and Khademhosseini A, ACS nano, 2013, 7, 2891–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu J, Cui L and Losic D, Acta biomaterialia, 2013, 9, 9243–9257. [DOI] [PubMed] [Google Scholar]
- 94.Biju V, Chemical Society Reviews, 2014, 43, 744–764. [DOI] [PubMed] [Google Scholar]
- 95.Zhou K, Thouas GA, Bernard CC, Nisbet DR, Finkelstein DI, Li D and Forsythe JS, ACS applied materials & interfaces, 2012, 4, 4524–4531. [DOI] [PubMed] [Google Scholar]
- 96.Zhou K, Motamed S, Thouas GA, Bernard CC, Li D, Parkington HC, Coleman HA, Finkelstein DI and Forsythe JS, PLoS One, 2016, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mohajeri M, Behnam B, Barreto GE and Sahebkar A, Pharmacological research, 2019. [DOI] [PubMed] [Google Scholar]
- 98.Hsieh F-Y, Zhilenkov A, Voronov I, Khakina E, Mischenko D, Troshin PA and Hsu S.-h., ACS applied materials & interfaces, 2017, 9, 11482–11492. [DOI] [PubMed] [Google Scholar]
- 99.Cellot G, Ballerini L, Prato M and Bianco A, Small, 2010, 6, 2630–2633. [DOI] [PubMed] [Google Scholar]
- 100.Singh B, Baburao C, Pispati V, Pathipati H, Muthy N, Prassana S and Rathode BG, International Journal of Research in Pharmacy and Chemistry, 2012, 2, 523–532. [Google Scholar]
- 101.Dizaj SM, Mennati A, Jafari S, Khezri K and Adibkia K, Advanced pharmaceutical bulletin, 2015, 5, 19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Komane PP, Choonara YE, du Toit LC, Kumar P, Kondiah PP, Modi G and Pillay V, Journal of Nanomaterials, 2016, 2016. [Google Scholar]
- 103.Cellot G, Cilia E, Cipollone S, Rancic V, Sucapane A, Giordani S, Gambazzi L, Markram H, Grandolfo M and Scaini D, Nature nanotechnology, 2009, 4, 126–133. [DOI] [PubMed] [Google Scholar]
- 104.Matsumoto K, Sato C, Naka Y, Kitazawa A, Whitby RL and Shimizu N, Journal of bioscience and bioengineering, 2007, 103, 216–220. [DOI] [PubMed] [Google Scholar]
- 105.Bokara KK, Kim JY, Lee YI, Yun K, Webster TJ and Lee JE, Anatomy & cell biology, 2013, 46, 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hu B, Dai F, Fan Z, Ma G, Tang Q and Zhang X, Advanced Materials, 2015, 27, 5499–5505. [DOI] [PubMed] [Google Scholar]
- 107.Cheng KK, Chan PS, Fan S, Kwan SM, Yeung KL, Wang Y-XJ, Chow AHL, Wu EX and Baum L, Biomaterials, 2015, 44, 155–172. [DOI] [PubMed] [Google Scholar]
- 108.Fernández T, Martínez-Serrano A, Cussó L, Desco M and Ramos-Gómez M, ACS chemical neuroscience, 2018, 9, 912–924. [DOI] [PubMed] [Google Scholar]
- 109.Lu AH, Salabas E. e. L. and Schüth F, Angewandte Chemie International Edition, 2007, 46, 1222–1244. [DOI] [PubMed] [Google Scholar]
- 110.Chen T, Li C, Li Y, Yi X, Wang R, Lee SM-Y and Zheng Y, ACS applied materials & interfaces, 2017, 9, 9516–9527. [DOI] [PubMed] [Google Scholar]
- 111.Goel A, Howard JB and Vander Sande JB, Carbon, 2004, 42, 1907–1915. [Google Scholar]
- 112.Silva GA, BMC neuroscience, 2008, 9, S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grothe M, Heinsen H and Teipel S, Neurobiology of aging, 2013, 34, 1210–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ferris SH and Farlow M, Clinical interventions in aging, 2013, 8, 1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Teleanu DM, Chircov C, Grumezescu AM and Teleanu RI, Pharmaceutics, 2019, 11, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xie J, Shen Z, Anraku Y, Kataoka K and Chen X, Biomaterials, 2019, 119491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Obermeyer JM, Tuladhar A, Payne SL, Ho E, Morshead CM and Shoichet MS, Tissue Engineering Part A, 2019, 25, 1175–1187. [DOI] [PubMed] [Google Scholar]
- 118.Chakroun RW, Zhang P, Lin R, Schiapparelli P, Quinones‐Hinojosa A and Cui H, Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 2018, 10, e1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Patel T, Zhou J, Piepmeier JM and Saltzman WM, Advanced drug delivery reviews, 2012, 64, 701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Neeves KB, Sawyer AJ, Foley CP, Saltzman WM and Olbricht WL, Brain research, 2007, 1180, 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kells AP, Hadaczek P, Yin D, Bringas J, Varenika V, Forsayeth J and Bankiewicz KS, Proceedings of the National Academy of Sciences, 2009, 106, 2407–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yazdan-Shahmorad A, Diaz-Botia C, Hanson TL, Kharazia V, Ledochowitsch P, Maharbiz MM and Sabes PN, Neuron, 2016, 89, 927–939. [DOI] [PubMed] [Google Scholar]
- 123.Yazdan-Shahmorad A, Tian N, Kharazia V, Samaranch L, Kells A, Bringas J, He J, Bankiewicz K and Sabes PN, Journal of neuroscience methods, 2018, 293, 347–358. [DOI] [PubMed] [Google Scholar]
- 124.Masserini M, ISRN biochemistry, 2013, 238428, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Silva Adaya D, Aguirre-Cruz L, Guevara J and Ortiz-Islas E, Journal of biomaterials applications, 2017, 31, 953–984. [DOI] [PubMed] [Google Scholar]
- 126.Rahaman ST, World Journal of Pharmaceutical Sciences, 2018, 6, 153–169. [Google Scholar]
- 127.Johnsen KB, Bak M, Kempen PJ, Melander F, Burkhart A, Thomsen MS, Nielsen MS, Moos T and Andresen TL, Theranostics, 2018, 8, 3416–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Toccaceli G, Barbagallo G and Peschillo S, Theranostics, 2019, 9, 537–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Singh AK, Yadav TP, Pandey B, Gupta V and Singh SP, in Applications of Targeted Nano Drugs and Delivery Systems, Elsevier, 2019, pp. 411–449. [Google Scholar]
- 130.Jagannathan J, Sanghvi NT, Crum LA, Yen C-P, Medel R, Dumont AS, Sheehan JP, Steiner L, Jolesz F and Kassell NF, Neurosurgery, 2009, 64, 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jang HJ, Lee J-Y, Lee D-H, Kim W-H and Hwang JH, Gut and liver, 2010, 4, S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Curley CT, Sheybani ND, Bullock TN and Price RJ, Theranostics, 2017, 7, 3608–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jordão JF, Ayala-Grosso CA, Markham K, Huang Y, Chopra R, McLaurin J, Hynynen K and Aubert I, PloS one, 2010, 5, e10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Raymond SB, Treat LH, Dewey JD, McDannold NJ, Hynynen K and Bacskai BJ, PloS one, 2008, 3, e2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang F, Shi Y, Lu L, Liu L, Cai Y, Zheng H, Liu X, Yan F, Zou C and Sun C, PLoS One, 2012, 7, e52925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Qin Y, Chen H, Zhang Q, Wang X, Yuan W, Kuai R, Tang J, Zhang L, Zhang Z and Zhang Q, International journal of pharmaceutics, 2011, 420, 304–312. [DOI] [PubMed] [Google Scholar]
- 137.Kuai R, Yuan W, Qin Y, Chen H, Tang J, Yuan M, Zhang Z and He Q, Molecular pharmaceutics, 2010, 7, 1816–1826. [DOI] [PubMed] [Google Scholar]
- 138.Zheng C, Ma C, Bai E, Yang K and Xu R, International journal of clinical and experimental medicine, 2015, 8, 1658–1668. [PMC free article] [PubMed] [Google Scholar]
- 139.Madani F, Lindberg S, Langel Ü, Futaki S and Gräslund A, Journal of biophysics, 2011, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bechara C and Sagan S, FEBS letters, 2013, 587, 1693–1702. [DOI] [PubMed] [Google Scholar]
- 141.Eugenin EA, Clements JE, Zink MC and Berman JW, Journal of Neuroscience, 2011, 31, 9456–9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhu X, Jin K, Huang Y and Pang Z, in Brain Targeted Drug Delivery System, Elsevier, 2019, pp. 159–183. [Google Scholar]
- 143.Nowak M, Helgeson ME and Mitragotri S, Advanced Therapeutics, 2020, 3, 1900073. [Google Scholar]
- 144.Wen X, Wang K, Zhao Z, Zhang Y, Sun T, Zhang F, Wu J, Fu Y, Du Y and Zhang L, PLoS One, 2014, 9, e106652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Masuda H, Hirata A, Kawai H, Wake K, Watanabe S, Arima T, Poulletier de Gannes F, Lagroye I and Veyret B, Journal of Applied Physiology, 2011, 110, 142–148. [DOI] [PubMed] [Google Scholar]
- 146.Tabatabaei SN, Duchemin S, Girouard H and Martel S, IEEE International Conference on Robotics and Automation, 2012, 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jones RM, Deng L, Leung K, McMahon D, O’Reilly MA and Hynynen K, Theranostics, 2018, 8, 2909–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sun Z, Worden M, Wroczynskyj Y, Yathindranath V, van Lierop J, Hegmann T and Miller DW, International journal of nanomedicine, 2014, 9, 3013–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kievit FM and Zhang M, Accounts of chemical research, 2011, 44, 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu C and Sun S, Advanced drug delivery reviews, 2013, 65, 732–743. [DOI] [PubMed] [Google Scholar]