Abstract
The COVID-19 pandemic with its severe respiratory disease has caused overflow to hospitals and intensive care units. Elevated troponins and natriuretic peptides are related to cardiac injury and poor prognosis. We present a young woman with COVID-19 infection with haemodynamic instability caused by acute perimyocarditis and cardiac tamponade. Troponin T was modestly elevated. Focused cardiac ultrasound made the diagnosis. Echocardiography revealed transient thickening of the myocardial walls. After pericardial drainage and supportive care, she improved significantly within 1 week without targeted therapy. The case illustrates the importance of cardiac diagnostic imaging in patients with COVID-19 and elevated cardiac biomarkers.
Keywords: pericardial disease, infectious diseases
Background
The COVID-19 pandemic is a challenge to healthcare systems and societies around the world. The virus has in some countries already caused overflow to hospitals and intensive care units (ICUs). Most deaths are related to severe respiratory failure.1 However, data from the Wuhan region in China show that cardiac injury assessed by elevation of troponin I and N-terminal pro brain natriuretic peptide (NT-proBNP) was present in more than half of those who died.2 3 It has been argued that cardiac imaging may not be needed in patients with COVID-19 with moderate elevation of troponins.4 The Middle East respiratory syndrome coronavirus (MERS-CoV) is another coronavirus confirmed in approximately 2500 individuals since 2012 (https://www.who.int/emergencies/mers-cov/en). MERS-CoV was linked to a case of acute myocarditis in the anterolateral and apical left ventricular myocardial wall.5 A couple of COVID-19 associated acute myocarditis cases are published, though not confirmed by biopsy.6 7 In addition, a case of life-threatening cardiac tamponade caused by acute perimyocarditis was recently presented.8 Data regarding the cause of elevated levels troponins and NT-proBNP in patients with COVID-19 are scarce, and it is not well described whether the myocardial injury is secondary to respiratory distress or primarily caused by acute cardiac disease.9 It has furthermore been hypothesised that the COVID-19 may not directly affect the heart.10
We present a case of acute perimyocarditis and cardiac tamponade in a previously healthy middle-aged woman with COVID-19 infection without respiratory disease. The patient was treated with pericardial drainage and supportive care at the ICU. The report highlights elements of potential importance for both diagnostics and treatment during the ongoing COVID-19 pandemic with emphasis of diagnostics.
Case presentation
A previously healthy 55-year-old woman with confirmed COVID-19 infection was hospitalised due to fatigue and near-syncope. Nine days prior to admission, her daughter returned from another European country and 3 days thereafter the daughter developed upper airways symptoms. The next day (day 0), the patient developed fatigue and unspecific body discomfort, with no airway symptoms. COVID-19 was confirmed in both the next days. Her medical condition improved temporarily until hospitalisation on day 7 due to several episodes of near-syncope and increased body and chest discomfort.
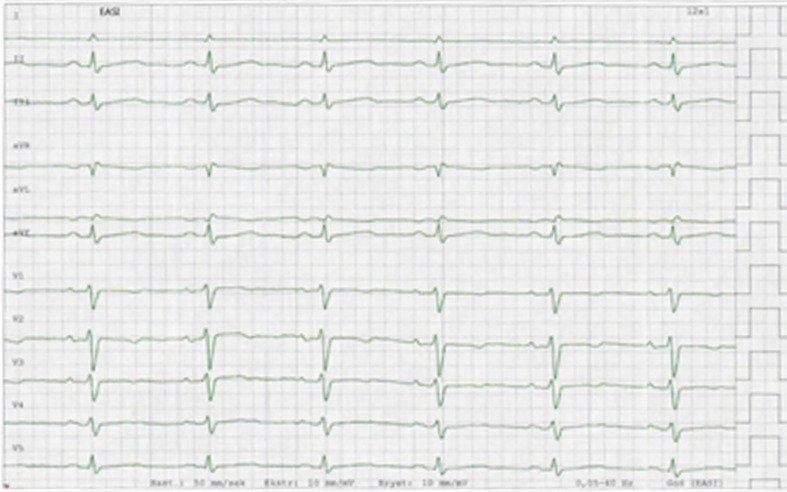
By admittance to hospital, the patient was isolated due to the COVID-19 infection. Blood pressure was 102/72 mm Hg, heart rate was 100 beats/min, respiratory rate was 17/min and rectal temperature was 37.2°C. Clinical examination revealed normal organ findings including pulmonary and cardiac examination. The ECG showed sinus tachycardia, insignificant ST-elevation in inferior leads and T-wave inversion in precordial leads (figure 1). Furthermore, there was low-voltage ECG with peak-to-peak QRS amplitude less than 5 mm in the standard leads and 10 mm in the precordial leads (V5 and V6). All ECG findings were stationary during the course. Laboratory findings showed glucose 7.2 mmol/L, normal electrolytes, mild elevation of C-reactive protein (CRP) 11 and significantly elevated leucocyte count 13.2×109/L, troponin T 108 ng/L, NT-proBNP 1025 ng/L. Comprehensive description of laboratory tests and therapy is shown in online supplementary tables 1 and 2. Bedside cardiac ultrasound examination performed by the on-call cardiac resident using a hand-held ultrasound device revealed moderate concentric left ventricular hypertrophy, reduced left ventricular EF, dilated inferior caval vein with reduced respiratory variation and pericardial effusion at a maximum of 18 mm, a small right ventricle and a slight impression of the right atrium (video 1). She was subsequently transferred to the ICU and treated with fluid support and norepinephrine. Day to day fluid and pressor treatment are highlighted in online supplementary table 2. The results of the point-of-care ultrasound examination were confirmed by echocardiography at the ICU. The left ventricle presented with a moderate concentric hypertrophy with a small cavity with end-diastolic volume less than 70 mL. Furthermore, there was a striking hyperechogenic pattern of the endocardium and epicardium (figure 2 and video 2). She deteriorated the following day with decreasing blood pressure and diuresis, with only a modest response on supportive treatment with fluid and pressor (norepinephrine (NA) and dobutamine). Fluid support was provided due to extensive peripheral capillary leakage. The extremities were cold and wet, but cognition was normal and beside international normalization ratio (INR) 1.2–1.3 there was no other sign of organ failure. Pericardiocentesis was performed at day 9 due to subacute deterioration and echocardiographic signs of a tamponade including compression of the right atrium, dilated inferior vena cava without inspiratory diameter reduction and respiratory variation in volumes and flow velocities (figure 3 and video 3). After initial drainage of 300 mL of serosal pericardial fluid, there was an immediate reduction of the respiratory variation of inflow patterns. The systolic blood pressure increased from 60 mm Hg to 95 mm Hg followed by increased diuresis. Size and function of the cardiac chambers together with mitral inflow pattern and filling of the left ventricle normalised gradually over the following days (figures 2 and 4, videos 2 and 4). The left ventricular myocardial thickness and striking echogenicity were reduced in the same period. Cardiac output and blood pressure improved correspondingly. The patient needed supportive fluid therapy due to capillary leakage until day 15 when she was transferred to the ward.
Figure 1.
EASI ECG representative for the course of disease. The ECG was recorded on day 14. Lead V6 is lacking in the stored version. There is insignificant ST-elevation in inferior leads and T-wave inversion in precordial leads. Furthermore, low voltage findings are present with peak-to-peak QRS amplitude less than 5 mm in the standard leads and 10 mm in the precordial leads (V5 and V6). Similar findings were seen in the printed 12-lead ECGs recorded at admittance to hospital and during the hospital stay.
Video 1.
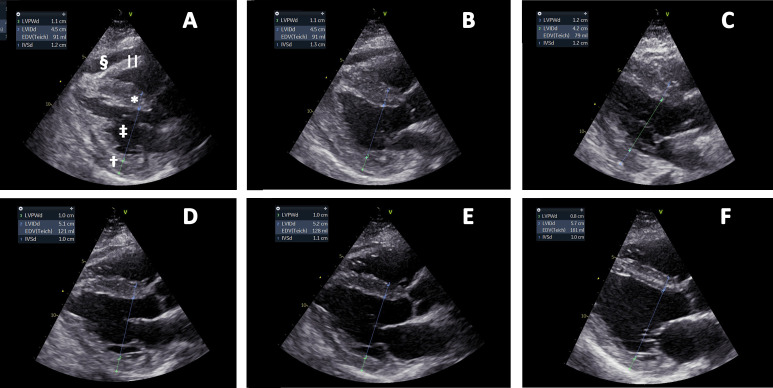
Figure 2.
Parasternal long-axis views day 8–day 16. (A) Day 8, (B) day 9, (C) day 10, (D) day 11, (E) day 12 and (F) day 16, respectively. Interventricular septum* and left ventricular posterior wall† thicknesses (IVSd and LVPWd) are reduced from (A) to (F), accompanied by increased end-diastolic left ventricular internal dimension‡ (LVIDd). Pericardial effusion§ is shown in (A) and (B) and increased right ventricular wall||thickness is best visualised in (A). Annotations are shown in (A) only.
Video 2.
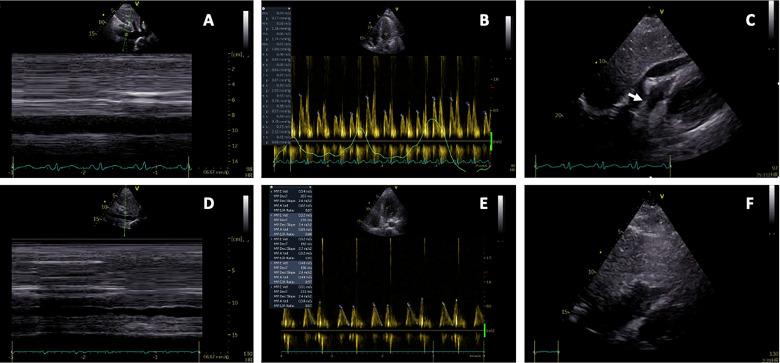
Figure 3.
Echocardiography immediately before and after pericardial drainages due to tamponade. (A–C) These show pericardial tamponade: (A) subcostal view of a dilated inferior caval vein, (B) mitral inflow with exaggerated respiratory variability (>25%) and (C) subcostal view with pericardial fluid, and a compressed right ventricle and right atrium (arrow). (D–F) These show postdrainage: (D) subcostal view of inferior caval vein with normal respiratory variability, (E) mitral inflow with normal respiratory variability and (F) subcostal view without pericardial fluid and no compression of right ventricle and right atrium.
Video 3.
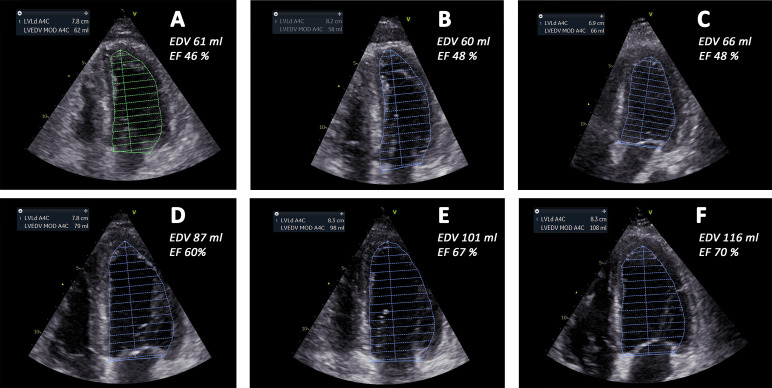
Figure 4.
End-diastolic four-chamber views from day 8 to day 16. (A) Day 8, (B) day 9, (C) day 10, (D) day 11, (E) day 12 and (F) day 16, respectively. (A–F) These show left ventricular end-diastolic volume (LVEDV) traces from four-chamber views. In the top left boxes, the volume estimate is shown. End-diastolic volumes increased from day 8 to day 16, accompanied by increased ejection fraction (EF) and increased stroke volume. End-diastolic volume (EDV) and EF of the left ventricle were calculated by Simpson’s equation in four-chamber and two-chamber views and the appropriate numbers are indicated in the panels. A4C, four-chamber; LVLd, left ventricular length; MOD, method of disc summation.
Video 4.
bcr-2020-236218supp001.pdf (285.2KB, pdf)
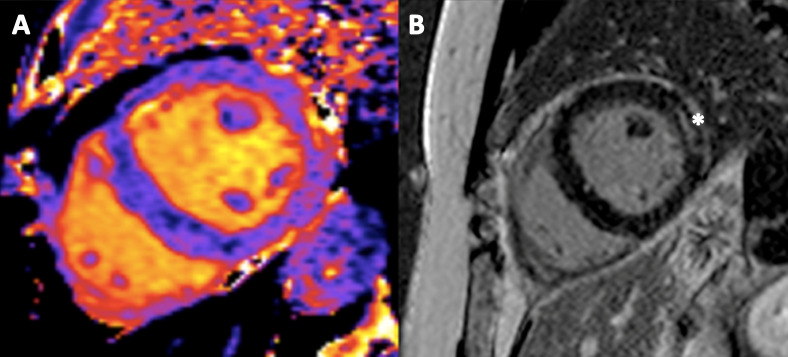
The pericardial fluid tested negative in PCR for COVID-19. Cardiac (CMRI) study performed at day 15 was consistent with the diagnosis of acute perimyocarditis. T1-mapping showed relaxation times of 1260–1270 ms in the anterolateral wall compared with 1090 ms in the septum. The corresponding T2-mapping relaxation times were 60–61 ms and 52–53 ms, respectively. Inversion recovery sequences showed a moderate epicardial late gadolinium enhancement in the anterolateral wall (figure 5). Myocardial biopsy was postponed due to the COVID-19 situation and rapid improvement.
Figure 5.
Cardiac MRI performed at day 15. (A) Shows a native short-axis T1-map and (B) shows late gadolinium enhancement* in the left ventricular anterolateral wall. The findings are consistent with a subacute perimyocarditis.
Supplementary findings and investigations
Chest X-ray at admission did not reveal any pulmonary involvement, which coincides with no respiratory symptoms (online supplementary figure 1). Thus, a CT scan was not performed. At the ICU, several chest X-rays were performed showing minor amounts of bilateral pleural effusion and basal atelectasis in the left lung (online supplementary figure 2).
bcr-2020-236218supp002.pdf (234.6KB, pdf)
Furthermore, consecutive laboratory findings revealed only a mild to moderate increase in CRP, a modest troponin T elevation with a small variation (online supplementary table 1). Moreover, extensive examination of the pericardial fluid and blood/serum did not reveal any signs of other causes like infectious and systemic diseases or coexisting rheumatologic disorder.
Outcome and follow-up
The patient was discharged on day 17 still without respiratory symptoms. She was called on day 39 and presented full recovering. She is planned for echocardiographic follow-up within 2 months.
Discussion
We present a case with acute perimyocarditis and cardiac tamponade as the main finding in a former healthy woman with COVID-19 infection. The clinical picture was followed by extensive capillary leakage, oedema formation and hypotension. The patient needed extensive fluid support during the ICU stay and vasopressor was administered. Improvement in cardiac function was seen ahead of clinical improvement and reduction of capillary leakage. Important elements include the findings of (1) a life-threatening perimyocardial complication of COVID-19 infection without significant respiratory symptoms and findings, (2) early diagnosis with point-of-care cardiac ultrasound, (3) detailed information of the cardiac and pericardial injury and (4) the development and pathway of the clinical picture.
Pericardial tamponade and acute perimyocarditis compose potentially lethal conditions.11 Early and adequate diagnostics and treatment are mandatory and lifesaving. During the COVID-19 pandemic, much emphasis has been on the potential lethal acute respiratory distress syndrome and the poor prognosis of those with cardiac injury (elevated cardiac biomarkers).1–3 9 12 The possibility of unrevealed but treatable cardiac or pericardial pathology among deceased COVID-19 victims should not be excluded. The presented case was confirmed COVID-19 positive ahead of admission. The cardiac biomarkers were modestly elevated, and by the early hand-held cardiac ultrasound examination, the correct diagnosis was made. This allowed for appropriate treatment. There are recent case presentations of patients with COVID-19 demonstrating non-respiratory symptoms.6 8 13 Thus, we believe that early echocardiography or focused cardiac ultrasound is warranted to reveal important information in patients with COVID-19 with cardiac injury. In line with the recommendations, emergency echocardiography and/or hand-held cardiac ultrasound echocardiography is indicated in patients with symptoms suspicious cardiac disease or in haemodynamic instability.14 15 Careful examination of the patients is needed even in case of modest elevations of troponins.
The initial assessment revealed a combination of pericardial and myocardial disease judged as acute perimyocarditis. Echocardiography revealed transient left ventricular hypertrophy suspicious of myocardial oedema. The increased endocardial echogenicity seen initially may be an ultrasound artefact related to increased difference in the reflections from the myocardium and the fluid due to oedema. However, it may as well relate to myocardial inflammation. Similar features may be seen in eosinophilia and endomyocardial fibrosis.16 All pathological echocardiographic findings were resolved during the ICU stay. Importantly, the improvement took place without specific antiviral and anti-inflammatory therapy. However, she was given two doses of colchicine 0.5 mg but this was discontinued due to the uncertainty regarding the influence of anti-inflammatory therapy on COVID-19.17 For the same reason, she was not treated with corticosteroids or non-steroidal anti-inflammatory drugs.
The CMR study revealed myocardial oedema, increased pericardial thickness and apical and anterolateral myocardial affection consistent with acute perimyocarditis. Similar CMR findings were reported from previous cases of coronavirus-induced myocarditis presented during the MERS-CoV outbreak and in influenza-A virus.5 18 The moderate oedema and rise of T2 values may be explained by the delayed imaging performed. The echocardiographic examinations showed global affection of both left and right ventricles with initial reduction in systolic function. Chest ultrasound revealed both pleural effusion and comet-tail signs related to atelectasis. Both findings are unspecific in this case. Chest ultrasound may be of interest for future evaluation of patients with COVID-19 but its role in evaluation of such patients has to be established.19
The most likely differential diagnosis is acute pericarditis combined with a pronounced capillary leakage causing cardiac tamponade, oedema (in the posterior part of legs, torso and gluteal region), hypotension and need for fluid support. However, the CMR performed on day 15 was consistent with subacute perimyocarditis. The repetitive echocardiograms are in line with but not pathognomonic for perimyocarditis. However, an important limitation for the precise diagnosis is that no myocardial biopsy was performed. Due to the inhospital infection control preventive procedures, the biopsy was delayed and later discontinued due to the rapid improvement. The only modest elevated troponin T and the rapid improvement exclude fulminant myocarditis. We suggest that the pathogenetic mechanism for the transient biventricular hypertrophic appearance of the myocardium is a general capillary leak and not caused by a fulminant viral myocarditis.
The history, ECGs and repetitive echocardiograms and CMR exclude myocardial infarction. Except for two paroxysms of atrial fibrillation, no other arrhythmias were found, and her cardiac dysfunction and pathological ventricular morphology were resolved without specific therapy. Furthermore, extensive laboratory testing for other viruses and rheumatologic disorders was negative and cultures of pericardial effusion, blood and urine did not grow any microbes. Together with the medical history, this makes other viruses, microbes or rheumatic disorder unlikely as cause of the perimyocardial disorder. Others have suggested a cytokine storm syndrome caused by the virus as cause for multiorgan failure in COVID-19.20 The presented case had no significant fever, cytopenia, and ferritin was only 204 µg/L, and thus, the latter diagnosis is unlikely. Furthermore, we did not reveal findings suspicious of other causes for her disease. Thus, we conclude that an acute perimyocarditis with pericardial tamponade and modest myocardial involvement related to the COVID-19 infection is the most probable cause.
Learning points.
Elevated troponins and natriuretic peptides are common in patients infected with COVID-19 and indicate cardiac injury and poor prognosis. However, these findings may be caused by myopericardial involvement as perimyocarditis and cardiac tamponade.
Early focused cardiac ultrasound and echocardiography are indicated in patients with COVID-19 with haemodynamic instability or cardiac symptoms and may reveal reversible life-threatening causes of COVID-19-associated diseases.
Cardiac imaging with echocardiography and MRI indicates that COVID-19 may cause acute myopericardial life-threatening disease which may be underdiagnosed during the COVID-19 pandemic crisis.
In this case, an acute perimyocarditis with cardiac tamponade was treated with pericardial drainage and supportive care, and both cardiac function and the patient’s general condition were resolved within days without intensive immunosuppression.
There is no scientific evidence to support intensive immunosuppression or direct antiviral therapy in perimyocarditis due to COVID-19 infection, and as illustrated early diagnostics, pericardial drainage when indicated and supportive care may provide good results.
Footnotes
Contributors: HD, EH, AUG, J-AH, KHS, ATB, OCM, OR and RW discussed planning, conception and design of the case presentation, interpreted the data, revised and approved the manuscript. HD, EH, AUG, J-AH, KHS, ATB, OCM and OR acquired the data. HD, EH and RW drafted the manuscript. HD, EH, AUG, J-AH, KHS, ATB, OCM, OR and RW take full responsibility for the whole content of the manuscript and ensure that all questions regarding the accuracy and integrity will be investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Chen T, Wu D, Chen H, et al. . Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091. 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo T, Fan Y, Chen M, et al. . Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chapman AR, Bularga A, Mills NL. High-Sensitivity cardiac troponin can be an ally in the fight against COVID-19. Circulation 2020;141:1733–5. 10.1161/CIRCULATIONAHA.120.047008 [DOI] [PubMed] [Google Scholar]
- 5. Alhogbani T. Acute myocarditis associated with novel middle East respiratory syndrome coronavirus. Ann Saudi Med 2016;36:78–80. 10.5144/0256-4947.2016.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu H, Ma F, Wei X, et al. . Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J 2020. 10.1093/eurheartj/ehaa190. [Epub ahead of print: 16 Mar 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inciardi RM, Lupi L, Zaccone G, et al. . Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020. 10.1001/jamacardio.2020.1096. [Epub ahead of print: 27 Mar 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hua A, O'Gallagher K, Sado D, et al. . Life-Threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart J 2020;41:2130. 10.1093/eurheartj/ehaa253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shi S, Qin M, Shen B, et al. . Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020. 10.1001/jamacardio.2020.0950. [Epub ahead of print: 25 Mar 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu Z, Shi L, Wang Y, et al. . Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–2. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adler Y, Charron P, Imazio M, et al. . 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921–64. 10.1093/eurheartj/ehv318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis 2020. 10.1016/j.pcad.2020.03.001. [Epub ahead of print: 10 Mar 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pan L, Mu M, Yang P, et al. . Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol 2020;115:766–73. 10.14309/ajg.0000000000000620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Skulstad H, Cosyns B, Popescu BA, et al. . COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging 2020;21:592–8. 10.1093/ehjci/jeaa072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cardim N, Dalen H, Voigt J-U, et al. . The use of handheld ultrasound devices: a position statement of the European association of cardiovascular imaging (2018 update). Eur Heart J Cardiovasc Imaging 2019;20:245–52. 10.1093/ehjci/jey145 [DOI] [PubMed] [Google Scholar]
- 16. Grimaldi A, Mocumbi AO, Freers J, et al. . Tropical endomyocardial fibrosis: natural history, challenges, and perspectives. Circulation 2016;133:2503–15. 10.1161/CIRCULATIONAHA.115.021178 [DOI] [PubMed] [Google Scholar]
- 17. Ritchie AI, Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? Lancet 2020;395:1111. 10.1016/S0140-6736(20)30691-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weiss TW, Stensaeth KH, Eritsland J. Myocarditis in a juvenile patient with influenza A virus infection. Eur Heart J 2010;31:277. 10.1093/eurheartj/ehp566 [DOI] [PubMed] [Google Scholar]
- 19. Soldati G, Smargiassi A, Inchingolo R, et al. . Is there a role for lung ultrasound during the COVID-19 pandemic? J Ultrasound Med 2020;39:1459–62. 10.1002/jum.15284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mehta P, McAuley DF, Brown M, et al. . COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–4. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bcr-2020-236218supp001.pdf (285.2KB, pdf)
bcr-2020-236218supp002.pdf (234.6KB, pdf)