The bacterium Bacillus subtilis has long been an important subject for basic studies. However, this organism has also had industrial applications due to its easy genetic manipulation, favorable culturing characteristics for large‐scale fermentation, superior capacity for protein secretion, and generally recognized as safe (GRAS) status. In addition, as the metabolically dormant form of B. subtilis, its spores have attracted great interest due to their extreme resistance to many environmental stresses, which makes spores a novel platform for a variety of applications.
KEYWORDS: Bacillus subtilis, biotechnology, endospores, material sciences
ABSTRACT
The bacterium Bacillus subtilis has long been an important subject for basic studies. However, this organism has also had industrial applications due to its easy genetic manipulation, favorable culturing characteristics for large‐scale fermentation, superior capacity for protein secretion, and generally recognized as safe (GRAS) status. In addition, as the metabolically dormant form of B. subtilis, its spores have attracted great interest due to their extreme resistance to many environmental stresses, which makes spores a novel platform for a variety of applications. In this review, we summarize both conventional and emerging applications of B. subtilis spores, with a focus on how their unique characteristics have led to innovative applications in many areas of technology, including generation of stable and recyclable enzymes, synthetic biology, drug delivery, and material sciences. Ultimately, this review hopes to inspire the scientific community to leverage interdisciplinary approaches using spores to address global concerns about food shortages, environmental protection, and health care.
INTRODUCTION
Bacillus subtilis is a Gram-positive bacterium that can survive in adverse environments through the formation of spores via sporulation, a process which is typically triggered by a shortage of nutrients (1–3). The B. subtilis spores are relatively easily purified and are ellipsoidal in shape and 0.8 to 1.2 μm in length (4) (Fig. 1). The spore structure is very different than that of a growing cell, with an outer coat that has multiple layers and then two peptidoglycan layers, the large outer cortex and the smaller germ cell wall, with the inner spore membrane under the germ cell wall and the outer membrane outside the cortex and inside the spore coat (Fig. 1) (5–7). The central core contains the spore DNA, ribosomes, and metabolic and biosynthetic enzymes but has a very low water content, ∼35% of wet weight, and contains a number of spore-specific components (8). As a consequence of their novel structure and composition, dormant spores are metabolically inactive, exhibit no gene expression, and are extremely resistant to all manner of environmental extremes, including high heat or radiation fluences, desiccation, toxic chemicals, and pH extremes (8). All of these properties allow spores to survive for many years in the absence of nutrients (9). However, if appropriate nutrients are provided, most usually l-alanine, B. subtilis spores can rapidly break their dormancy in the process of germination using specific germination proteins present in spores. Given sufficient nutrients, the germinated spores can then return to vegetative growth in the process of outgrowth. The molecular mechanisms underlying B. subtilis sporulation, spore resistance, and spore germination have been extensively studied for many decades, and much is now known about them (3, 4). Importantly, B. subtilis is also nonpathogenic and generally recognized as safe, which has enabled its wide use in a number of applications (10, 11). This review will focus on recent advances in using B. subtilis spores in biotechnological applications that integrate other disciplines, including food science, synthetic biology, immunology, drug delivery, and material science (Table 1).
FIG 1.
Structure of B. subtilis spores. (Left) Scanning electron microscopy (SEM) image of purified B. subtilis spores. Bar, 5 μm. (Right) Schematic of a B. subtilis spore and location of anchor proteins used for display of heterologous proteins (spore layers are not drawn to scale) (5–7).
TABLE 1.
A list of applications using B. subtilis spores
| Application | B. subtilis derivative or relativea | Anchor protein | Target moleculeb | Specific function (reference[s]) |
|---|---|---|---|---|
| Improving enzyme thermostability | DB403 | CotB | Lipase Tm1350 (extremophile) | Food processing and biodiesel, etc. (25) |
| WB800 | CotZ | DPEase (extremophile) | Food processing (26) | |
| DB104 | CotG | Haloalkane dehalogenase | Bioremediation (30) | |
| DB104 | CotE | Tyrosinase | Bioremediation, biosensor, biomedicine, and biocatalysis (29, 94) | |
| PY79 | CotG | Chitinase | Biopesticide (38) | |
| PY79 | CotG, OxdD | Phytase | Feed enzyme for monogastric animals (31) (16) | |
| WB800N | CotC | Trehalose synthase | Food processing, pharmaceuticals, cosmetics, and agricultural products (32) | |
| PY79 | CotZ, OxdD | β-Galactosidase | Food processing (33) (16) | |
| Recombinant vaccines | PY79 | CotB | TTFC | Against tetanus toxin (13) |
| WB600 | CotC | CsPmy | Against Clonorchis sinensis (95) | |
| PY79 | CotC | OmpC | Against Salmonella (50) | |
| PY79 | COtB | VP28 | Against white spot syndrome virus (51) | |
| GC5 | CotC | Sip | Against Streptococcus agalactiae (52) | |
| Nonrecombinant vaccines | PY79 | TTFC | Against tetanus toxin (54) | |
| PY79 | PA | Against Bacillus anthracis (54) | ||
| PY79 | GST-Cpa | Against Clostridium perfringens (54) | ||
| HU58 and DS127 | MPT64 and Acr-Ag85B | Against tuberculosis (96) | ||
| 1012 | DNA vaccine | Against human papillomavirus and type 1 human herpes virus (97) | ||
| Adjuvant | PY79 | TTFC | Adjuvant for tetanus vaccine (98) | |
| PY79 | Inactivated H5N1 virus | Adjuvant for H5N1 influenza (99) | ||
| Nanobody production | WB800N | Caffeine, methotrexate, CTLA-4, PD-L1 | Small molecule capturing or antigen detection (60) | |
| Drug delivery | B. coagulans | Curcumin and folate | Colon cancer (63) | |
| PY79 | CotB | Antibody and paclitaxel | Colon cancer (65) | |
| 3D printing | PY79 | Living materials (71) | ||
| Self-healing concrete | B. sphaericus | Precipitation of CaCO3 | Fixing cracks in concrete (79) | |
| NCTC 8236 | Precipitation of CaCO3 | Fixing cracks in concrete (100) | ||
| MTCC 441 | Formation of Ca2Al2SiO7 | Fixing cracks in concrete (80) | ||
| Biosorption | PY79 and RH201 | CotB | Toxic metal ions (e.g., Ni2+) via an 18-histidine amino acid display | Bioremediation (91) |
| Various derivatives | Rare earth ions | Biosorption (87) |
Unless specified with a species names, organisms are B. subtilis derivatives.
Unless specified as from an extremophile, enzymes are derived from mesophiles.
APPLICATIONS OF B. SUBTILIS SPORES
B. subtilis spores as enzyme expression and delivery platforms.
Early uses of B. subtilis spores in biotechnology applications focused on expressing and anchoring heterologous proteins on the spore, referred to as spore display, in attempts to enhance the functionality and stability of recombinant proteins (12). Over the past 2 decades since the first report that displayed a heterologous protein on the spore in 2001 by Isticato et al. (13), this technology has continued to advance (14, 15). Spore display typically starts with genetic modifications in growing cells of B. subtilis by fusing the gene for a target protein with a gene for a spore coat protein. This is followed by sporulation to generate recombinant spores that express the fusion protein on the spore. Of note, researchers have attempted to fuse and anchor various recombinant proteins with myriad coat proteins on the spore, which include, but are not limited to, CotA, CotB, CotC, CotD, CotE, CotF, CotG, CotW, CotX, CotY, and CotZ. Expression of these fusion proteins almost always has minimal if any effects on sporulation, spore resistance, or germination (5, 16–19). Figure 1 shows the localization of these coat proteins in the B. subtilis spore. Immobilization of recombinant proteins on the spore bypasses the need for protein purification and subsequent immobilization on synthetic matrices, which simplifies downstream processes such as biocatalysis, as well as reuse of the immobilized proteins, as discussed below.
A number of specific enzymes are crucial for biocatalyses and biotransformations in many industrial processes because of these enzymes’ high catalytic efficiency and specificity (20). Given enzymes’ potential costs, it would be advantageous to recycle them, but it can be very challenging to separate and recycle enzymes from their products in a bulk reaction. However, an effective approach for increasing enzyme recyclability is through their immobilization on solid substrates such as spores (21). This is typically accomplished by genetically fusing the gene encoding the enzyme of interest to the N- or C-terminal coding regions of a gene encoding of a spore coat protein (22). Important factors that determine the choice of the coat protein for enzyme fusion are both the levels of the coat protein and its location on the spore, which may affect the fused enzyme’s access to its substrates (23). Additionally, the spore surface appears to be covered by polysaccharides, and their influence on spore surface properties is not fully understood (24). Because spores are inherently more stable at higher temperatures than most enzymes, enzymes immobilized on spores, including on both live and inactivated spores, could in theory confer greater thermostability to the immobilized enzymes compared to that of the free enzyme. Importantly, stabilization of the surface-displayed enzymes has generally been seen with enzymes from both extremophiles and mesophiles (25, 26).
One example of a spore-displayed enzyme is lipase, which is a subclass of esterases involved in hydrolysis of dietary lipids (e.g., triglycerides, fats, and acylglycerols) (27). Traditionally, applications of lipases in industry are limited by these enzymes’ low thermostability, high production cost, and low reusability. To address these issues, Chen et al. successfully expressed and displayed lipase Tm1350 from a hyperthermophile on the B. subtilis spore anchored to CotB (25). Notably, the anchored enzyme was more stable than the free enzyme in high-temperature and alkaline environments, and this increased thermostability has generally been seen for proteins from mesophiles as well (Table 1) (28). Importantly, recycling experiments showed that the spore-displayed lipase could be reused for three reaction cycles without a significant decrease in its catalytic efficiency. In a second example, given the high interest in utilizing tyrosinases for bioremediation of phenol-polluted environments and for production of levodopa (l-DOPA) from l-tyrosine as a drug for the treatment of Parkinson's disease, Hosseini-Abari et al. successfully displayed tyrosinase on B. subtilis spores using CotE as the anchor protein (29). The anchored tyrosinase exhibited many advantages over the free enzyme, including ease of enzymatic assays, enhanced stability, and high recyclability, with ∼62% of enzyme activity retained after six usages. Another example is d-psicose 3-epimerase (DPEase) from Clostridium scindens ATCC 35704, which can produce d-allulose from d-fructose by biocatalytic synthesis (26). The fusion enzyme CotZ-DPEase, displayed on B. subtilis spores, exhibited high thermostability and reusability, with an optimal reaction temperature of 55°C and retention of 60% of the initial activity after five cycles of utilization. As a result, spore display of DPEase has proven to be an alternative to the current platform using Escherichia coli for d-allulose production. Other enzymes that have been displayed on the B. subtilis spore for potential industrial applications include, but are not limited to, (i) trehalose synthase producing trehalose as a food additive, (ii) E. coli periplasmic acid phosphatase, phytase, or AppA, a dietary supplement for animal digestion that generates phosphate from phytin, (iii) β-galactosidase, which is widely used in the food industry to improve sweetness, solubility, and digestibility of dairy products, and (iv) haloalkane dehalogenase DhaA, which rapidly degrades sulfur mustard (a warfare agent) in an environmentally friendly manner (30–34).
In addition to producing valuable industrial products as noted above, spore-displayed enzymes have also found utility in agriculture. For instance, chitin is the second most abundant polysaccharide in the world after cellulose (35). While cellulose is the major ingredient in plant cell walls, chitin constitutes a vital structural component for cell walls of plant-associated fungi and arthropods, but not for plants (36). This difference has been utilized to kill fungal infections in plants through the use of chitinase to break down chitin (37). Notably, repurposing chitinase to inhibit or kill pests can avoid potential environmental pollution in soil. For example, Rostami et al. fused chitinase with the B. subtilis spore coat protein CotG and displayed it on the spore as a potential biopesticide (29, 38). The recombinant spores demonstrated inhibitory effects on growth of several plant fungi, specifically Rhizoctonia solani and Trichoderma harzianum, which suggests that these spores could have potential as a new ecofriendly biopesticide. In addition to utilizing enzymes as biopesticides, it was also reported that one B. subtilis strain, CPA-8, could produce lipopeptides that exhibit antifungal activities against Monilinia laxa and Monilinia fructicola (39). While this review primarily focuses on B. subtilis, it is important to note that another spore-forming Bacillus species, Bacillus thuringiensis (Bt) can produce toxins with potent and specific insecticidal activity in the mother cell cytoplasm during spore formation, which is released, generally in crystal form, along with the spore upon mother cell lysis (40, 41). Mechanistically, these toxins kill insects by binding to and creating pores in midgut membranes of insects. As a result, Bt spores and their associated toxins have been widely used as topical pesticides to protect crops.
Expression and delivery of mucosal vaccines.
Mucosal vaccination via oral or intranasal administration can generate effective immune responses against infections associated with mucosal barriers such as the gastrointestinal tract (GIT), reproductive tract, and oral cavity (42). In this context, B. subtilis spores have been explored for mucosal immunization due to several attractive features, which include a safety record of human and animal use, resistance to acidic pH (for oral administration), and ease of genetic manipulation (43). Importantly, B. subtilis spores can interact with immune cells and trigger production of the body’s protective immune response as they transit the GIT (44). To use spores as carriers, two main approaches have been developed to present antigens on the spore (45). One approach is through the use of conventional spore display technology, fusing an antigen of interest with spore coat proteins, including but not limited to CotB, CotC, CotG, and CotZ (13, 46, 47); the other is a nonrecombinant approach, in which antigen proteins are adsorbed to the spore by hydrophobic and electrostatic forces.
The first antigen that was displayed on spores was the tetanus toxin fragment C (TTFC), which was genetically fused with the coat protein CotB (13). While this seminal study did not examine the immune responses of the displayed TTFC in animal studies, a follow-up study showed that the CotB-TTFC fusion protein markedly enhanced the recognition of the antigen by the mucosa-associated lymphoid tissue afferent sites, leading to the induction of mucosal (immunoglobulin A [IgA]) and systemic (immunoglobulin G [IgG]) antigen-specific antibody responses (48). In addition to antibody responses, further studies showed that recombinant spores elicited robust cellular immunity, which was associated with both systemic (spleen) and local (mesenteric lymph nodes) T-lymphocyte responses (49). Besides targeting pathogenic infections in humans, spore-based vaccines have also been explored in poultry. For example, Salmonella enterica subsp. enterica serovar Pullorum can cause an acute septicemic disease called Pullorum disease in poultry, which has resulted in significant worldwide economic losses. To address this problem, Dai et al. generated a chimera between OmpC, an antigen of Salmonella serovar Pullorum, and the coat protein CotC. Following oral immunization of mice with the genetically modified spores, there was a significant increase in OmpC-specific IgG and IgA antibodies that were produced to fight against Salmonella infections (50). The same recombinant spores were found to demonstrate cross protection in mice that received a lethal challenge with Salmonella enterica subsp. enterica serovar Typhimurium. However, this study did not examine possible side effects associated with administration of recombinant spores. In addition to potential utility in poultry, B. subtilis surface-displayed antigens on spores have also been used to vaccinate shrimp with protein VP28, an antigen from white spot syndrome virus, as well as tilapia with surface immunogenic protein (Sip) to protect against Streptococcus agalactiae infection (51, 52).
As an alternative to genetic modifications for spore display of antigens, a nonrecombinant approach was explored by adsorbing protein antigens onto the surface of spores via hydrophobic and electrostatic interactions that exploited the adjuvanticity potential of spores (44, 53). This method can obviate concerns regarding the use of genetically modified microorganisms. In this setting, a variety of antigens have been adsorbed on the surface of B. subtilis spores to immunize animal models through the nasal or oral route. For example, antigens such as TTFC of Clostridium tetani, the protective antigen of Bacillus anthracis, glutathione S-transferase (GST) from Schistosoma japonica, and a GST fusion to the carboxy-terminal domain of Clostridium perfringens alpha toxin were efficiently adsorbed on the spore, and all were shown to induce protective immune responses in nasally immunized mice (54). In addition, this study also found that heat-inactivated spores were as effective as live spores in adsorbing and delivering various antigens intranasally, suggesting that the germination of spores in the body may not be required for efficacy in the setting of intranasal immunization. Indeed, it may be argued that use of inactivated spores will be preferred over live ones from the perspective of biosafety, despite the fact that B. subtilis is generally regarded as safe. Finally, inspired by use of spore coat proteins, spore-sized silica beads were developed recently with a lipid bilayer, termed a synthetic spore husk-encased lipid bilayer (SSHEL), presenting two structural proteins (SpoVM and SpoIVA). These artificial spores were able to deliver various proteins and small-molecule drugs of interest by covalently linking to SpoIVA. In one application, they were designed to display vaccine on their surface with enhanced protection against Staphylococcus aureus infection (55).
A versatile and stable platform for production of nanobodies.
Nanobodies (Nbs), single-domain antibodies, are a novel type of antibody characterized by small size, high solubility, and great thermal stability (56). Because of these properties, Nbs have been explored as diagnostic and therapeutic reagents in the areas of oncology and infectious diseases, as well as research tools for fundamental biology (57, 58). While the majority of Nbs are being produced in E. coli, this expression system is associated with (i) potential contamination with endotoxin, (ii) a requirement for bacterial lysis to release intracellular Nbs, and (iii) E. coli’s lack of resistance to environmental extremes (59). To address these limitations, an expression platform using B. subtilis was recently developed as a stable and efficient microbial factory to produce Nbs on demand (60). In this study, Nbs were genetically fused to a cellulose-binding protein to allow for immobilization on low-cost cellulose matrices for antigen capturing, or were modified to contain appropriate amino acid sequences for antigen detection. As expected, the spores of the engineered B. subtilis strains displayed high resistance to wet heat, acidic pH, and desiccation. Even after exposure to these conditions, the spores were able to germinate, outgrow, and secrete Nbs without any decrease in yield and quality. Notably, as a Gram-positive bacterium, B. subtilis does not have endotoxin, and the ability to secrete Nbs directly into the culture supernatant can ease the downstream purification process. These attributes may make using spores advantageous in the development of diagnostic devices with high portability and long shelf lives.
Delivery vehicles for cancer drugs.
Over the last few decades, many synthetic drug carriers have been developed to release drugs in a controlled manner, which helps to decrease drug side effects and frequency of drug administration (61). While synthetic carriers utilize physical diffusion or hydrolytic decomposition (e.g., enteric coating) to release drugs (62), spores can be loaded with hydrophobic cancer drugs on the surface and will release drugs after germination in the GIT. Regarding the mechanism of germination in the GIT, based on an in vitro study that used simulated intestinal and gastric fluids (63), as well as a mouse study that examined the germination of B. subtilis spores after oral gavage (64), it was found that spores can germinate under conditions such as those in the intestine but not under gastric conditions, which can inhibit spore germination. Additionally, by genetically displaying targeting moieties, such as antibodies, on the surface of drug-loaded spores, modified spores can simultaneously target and kill cancer cells. For example, Nguyen et al. engineered B. subtilis spores to display streptavidin, which can bind biotinylated cetuximab, an FDA-approved monoclonal antibody drug specific for epidermal growth factor receptor overexpressed by certain cancer cells (65). By combining cetuximab and paclitaxel, the latter a cancer chemotherapeutic, modified spores were shown to exhibit enhanced anticancer effects in a human colon cancer cell line, HT29, compared to those of free paclitaxel. While this review focuses on B. subtilis spores, in another example, Yin et al. developed a colon cancer-targeted drug carrier (termed SPORE-CUR-FA) based on spores of Bacillus coagulans, a close relative of B. subtilis, by covalently conjugating curcumin (CUR), a drug with putative anticancer effects, and folate (FA) with the outer coat proteins on spores (63). In this setting, curcumin serves as the therapeutic payload, while folate acts as the targeting moiety for the folate receptor, which is highly upregulated on the surface of colon cancer cells compared to normal cells. Pharmacokinetic studies confirmed that SPORE-CUR-FA indeed efficiently improved the oral bioavailability of curcumin. Moreover, SPORE-CUR-FA exhibited remarkable antitumor effects in colon cancer cell lines, as well as in animal studies. To investigate the mechanism underlying drug release, researchers subjected SPORE-CUR-FA to simulated gastric fluid (pH 1.3) and intestinal fluid (pH 7.6), respectively, and it was found that significant curcumin and FA were released as nanomicelles in the simulated intestinal fluid but not in gastric fluids. Based on this evidence, the authors speculated that spores would germinate in the intestine of mice. However, this work warrants further animal studies on whether spores germinated in the GIT and thereby released the drugs.
B. subtilis spores as building blocks for functional materials.
Three-dimensional (3D) printing of materials represents a new area for tissue engineering and regenerative medicine (66). 3D printing can precisely incorporate natural or recombinant live organisms into tunable scaffolding materials, referred to as living materials, and equip them with the intelligent attributes of live cells to sense and respond to multiple environmental stimuli, thus performing various functions such as sensing, chemical production, self-healing, self-powering, self-cleaning, and self-adhering (67, 68). However, a key challenge for 3D printing of living materials is to maintain the viability of cells in the harsh printing conditions of heat, toxic chemicals, and shear pressure (69). While more advanced 3D printing techniques are under development to improve biocompatibility (70), González et al. incorporated B. subtilis spores into a hydrogel made of agarose to develop a resilient 3D living material (71). The construction of 3D printed materials that contain spores enables living functions to be used for applications that require long-term storage or exposure to environmental stresses. In addition to demonstrating improved resistance to harsh printing conditions, these workers genetically engineered spores with synthetic gene circuits that can sense and respond to environmental cues. In this study, these cues were represented by nutrients, which were introduced exogenously to induce germination of spores in the hydrogel. Subsequently, programmed genetic circuits were activated in the growing vegetative bacteria to produce fluorescent proteins or antimicrobial compounds. In addition to 3D printing, the Sahin group generated high-density energy inspired by the mechanical response of Bacillus spores to water gradients, which was 2 orders of magnitude higher than synthetic water-responsive materials. As a proof of concept, the Sahin group used spores to build an energy-harvesting device that can remotely generate electrical power from an evaporating body of water. They also identified mutations that could confer greater energy density than that of the wild-type spores (72).
B. subtilis spores for self-healing of concrete.
Concrete plays a pivotal role in architecture due to its rigidity and fire resistance (73). However, concrete suffers from low tensile strength that makes it prone to cracking. The formation of cracks allows the ingress of water and harmful chemicals, which can damage the protective film covering the concrete structures over time. Ultimately, these initial cracks reduce the load-carrying capacity and life expectancy of the concrete (74). Therefore, fixing these cracks at an early stage is of paramount importance for reinforcing concrete structures. While various human-made repairs have been employed, these methods are limited to macrocracks and are associated with high operational cost and frequent assessment followed by periodic maintenance. To address these issues, the development of an automated approach that does not require any external interventions would be beneficial (75). One intriguing system is using bacteria to naturally precipitate calcium carbonate (CaCO3) as a means of self-healing within the concrete (76). The principle underlying bacterial crack healing is that in the presence of appropriate nutrients, certain bacteria can precipitate insoluble inorganic calcite crystals that help seal the cracks (77). Notably, to enable prolonged survival of bacteria inside the concrete, bacteria must be protected from elements of the harsh concrete environment, such as high alkalinity and mechanical pressure. While a variety of bacteria have been investigated for this purpose, spore-forming Bacillus species represent an ideal candidate due to formation of spores that are intrinsically resistant to environmental extremes.
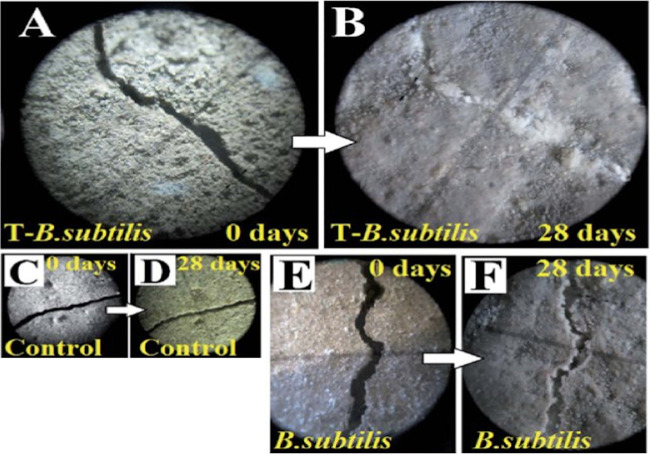
In one study that utilized Bacillus pseudofirmus as the healing bacteria, spores were prepared and incorporated into the cement (78). In this setting, B. pseudofirmus spores were found to survive in the cement paste for up to 93 days. After artificial microcracks were generated and Na+ ions, alanine, and inosine were exogenously introduced to induce spore germination and subsequent growth, vegetative cells were able to seal microcracks (∼1 mm in length) by precipitation of CaCO3. While this experiment provides a proof of concept, how to trigger germination and subsequent cell growth in a practical setting remains unclear. Pungrasmi et al. provided a different practical solution to crack filling recently by using another spore-forming Bacillus species, Bacillus sphaericus (79). The spores of this organism were first encapsulated along with nutrients in a dry state in the concrete. After artificial microcracks were made, the researchers introduced water to simulate a condition in which the internal moisture rose after rain. The spores then germinated and outgrew, and the vegetative cells were able to repair cracks in a self-healing manner (79). The results of this study indicated that (i) the healing ratio (defined as the healing percentage of the initial and final crack areas) was higher (48 to 80%) than that without bacteria (18 to 50%), (ii) the maximum width healed by bacteria was 970 μm, ∼4 times that without bacteria (maximum, 250 μm), and (iii) the overall water permeability of the concrete with bacteria was ∼10 times lower than that of concrete without bacteria. In contrast to using wild-type B. subtilis, Sarkar et al. genetically modified B. subtilis vegetative cells to express a gene from a thermophilic anaerobic bacterium that can induce biosilicification. As a proof of concept, spores prepared from the recombinant B. subtilis were incorporated in mortar, and then artificial cracks were induced mechanically. To assess the self-healing potential of spore-loaded concrete, they incubated cracked mortars with a rich bacterial growth medium, which induced spore germination, outgrowth, and transgene expression in vegetative cells. As shown in Fig. 2, after 28 days, the expression of the biosilicification gene resulted in formation of a novel compound called gehlenite (Ca2Al2SiO7), which improved concrete’s self-healing ability in comparison to that created by wild-type B. subtilis, which only formed CaCO3 (80).
FIG 2.
Images of cracked and healed mortars treated with wild-type and modified bacteria. Spores of B. subtilis with the biosilicification gene were incorporated into a cracked mortar on day 0, and the cracked mortar was incubated with a rich bacterial growth medium for 28 days. (A and B) T-B. subtilis, B. subtilis carrying the biosilicification gene; (C and D) control, no bacterial treatment; (E and F) B. subtilis, wild type. (All images are reprinted from reference 80 with permission of the publisher [copyright 2015, Royal Society of Chemistry].)
Spores for capturing metal ions.
Currently, the majority of global rare earth elements (REEs) are obtained by large-scale mining and refining activities (81). Because of the low abundance of REEs, the conventional approach is to use strong acids and organic solvents to extract REEs from rocks (82), procedures which have inevitably resulted in environmental pollution due to the release of toxic chemicals in mining areas (83). In contrast, a variety of microorganisms have been explored to extract REEs by a low-cost and environmentally friendly strategy referred to as biosorption (84, 85). While most previous studies utilized growing bacteria to absorb REEs, a few reports have examined REE absorption by bacterial spores (86). Notably, Dong et al. recently reported that B. subtilis spores could be a candidate for the adsorption of the REEs Tb3+ and Dy3+ (87). They found that Tb3+ and Dy3+ are incorporated into spores’ outer layers (Fig. 3) within a few minutes of incubation in a neutral-pH environment up to 2 to 3% of spore dry weight, and the uptake of these ions had minimal effects on spore wet heat resistance or germination. In addition to spore adsorption of these ions, their desorption is also necessary, and the ions were completely released upon spore germination. The release of adsorbed Tb3+/Dy3+ was also rapidly achieved by adding exogenous dipicolinic acid, a chemical compound comprising ∼10% of the dry weight of spores that binds REE ions very tightly. Questions not answered in this study are as follows: (i) while Tb3+/Dy3+ was found to accumulate in the spore’s outer layers as evidenced by transmission electron microscopy (Fig. 3), the functional group(s) responsible for REE absorption are unknown; if these were known, it might be possible to engineer spores to adsorb even more REEs; and (ii) whether inactivated spores that cannot germinate will adsorb REEs, although this seems likely. If the latter is true, then the spores could be reused many times over. Altogether, this seminal work suggests the possibility for a unique system for biosorption of REEs, especially in contrast to the extreme harsh conditions associated with traditional mining.
FIG 3.
TEM micrographs of B. subtilis spores incubated with or without Tb3+. Spores incubated without (A and C) or with (B and D) Tb3+ were examined by TEM. Panels C and D show higher magnifications than panels A and B (87). TEM, transmission electron microscopy; Ct, spore coat; Sm, spore matrix. The composition of Sm has not yet been well defined, but it is hypothesized to contain both proteins and carbohydrates (87). White arrows indicate the spore coat, and black arrows denote electron-dense areas where Tb3+ is adsorbed. (All images are reprinted from reference 87 with permission.)
In addition to biosorption of REEs, use of B. subtilis spores has also been explored in bioremediation of heavy metal-contaminated environments (88, 89). Heavy metal contamination is a serious environmental pollution problem due to the nonbiodegradability and subsequent accumulation of heavy metals in living organisms to toxic levels (88). Various physical and chemical strategies have been investigated to clear toxic metals in polluted environments (90), but most of these strategies are costly or not environmentally friendly. Bioremediation using a microbial biomass as a biosorbent to bind and accumulate heavy metals has drawn broad attention, but the biosorption capacity of such biomass is limited. To address this problem, Hinc et al. reported an improved nickel bioremediation method by genetically fusing an 18-histidine-residue array with the C terminus of B. subtilis spore coat protein CotB, in which the histidine residues serve as metal ion chelators (91). This work found that spores carrying the chelators exhibited higher nickel binding efficiency than wild-type spores, and the metal binding capacity correlated with the quantities of spores but was relatively independent of pH and temperature. While it is potentially attractive to consider use of spores to extract heavy metals from contaminated areas, given the high abundance of heavy metals in such areas, it may be challenging to scale up the system from a practical perspective. In contrast, the scarcity of REEs and their high economic value may make spores a practical tool to extract REEs from natural sources.
CONCLUSION
In summary, this review has highlighted key advances in the application of B. subtilis spores in different areas of biotechnology, from traditional recombinant protein expression to emerging biomaterials and applications such as engineered living materials, self-healing concrete, and biosorption. In particular, the resistance of spores to various environmental stresses may confer greater stability of enzymes and antigens, and it may provide the advantages of facile purification and the recycling of immobilized enzymes. In certain applications, it will also be imperative to prevent spores from germinating. For example, if spores were to be used for enzyme display and the spores recycled, spontaneous spore germination must be blocked, or enzymes will be lost from the spore after germination and the germinated spores will also likely be lost as well. Molecules released from germinating spores can also trigger germination of nearby spores as well (92). Notably, using spores inactivated by heat may not be applicable to enzyme display, since most enzymes cannot tolerate the temperatures required to inactivate spores. In contrast, when using spores to administer vaccines, through either genetic fusion or passive absorption, germination may even be essential, depending on the specific route of inoculation. For example, by comparing two studies from the Cutting group, it was found that germination is required for immunization efficacy in oral delivery but not in intranasal delivery (54, 93).
In the case of using spore-forming bacteria to repair cracks in the concrete, most, if not all studies have incorporated spores into the concrete to prolong its viability in harsh conditions. Thus, it is essential to keep spores from germinating before the occurrence of cracking. However, once repair is needed, nutrients must be provided to allow spores to form vegetative cells, which subsequently promote calcium carbonate precipitation in the cracks. In this sense, induction of germination is necessary for self-healing of cracks. On the other hand, the concept of using bacteria to heal cracks in concrete is a scientifically intriguing process and could pave the way for sustainable buildings by applying appropriate self-healing and ecofriendly bacteria in appropriate composite materials. However, there are many challenges to overcome in this area, including the filling of large cracks and safety considerations associated with maintaining concrete’s required compressive strength. For biosorption of metal ions such as REEs, it may also be necessary to block germination when spores are used in harsh conditions. Additionally, further engineering efforts to improve the selectivity and capacity of spores for specific ions could greatly increase the capacity and selectivity of spores’ REE adsorption.
Finally, merging interdisciplinary sciences such as synthetic biology and engineering materials could make spores viable options for the development of multifunctional living materials equipped with diverse functionalities. Overall, ongoing progress in the field of different engineering disciplines seems likely to further the use of spores as a highly versatile platform for a variety of technological applications.
ACKNOWLEDGMENTS
Our work was supported by Northeastern University Faculty startup funding and by the Peer Reviewed Medical Research Program from the Department of Defense’s Congressionally Directed Medical Research Programs.
We are grateful to Yanpu He at MIT, who assisted in taking the scanning electron microscopy (SEM) image of spores.
REFERENCES
- 1.Cai D, Rao Y, Zhan Y, Wang Q, Chen S. 2019. Engineering Bacillus for efficient production of heterologous protein: current progress, challenge and prospect. J Appl Microbiol 126:1632–1642. doi: 10.1111/jam.14192. [DOI] [PubMed] [Google Scholar]
- 2.Elshaghabee FMF, Rokana N, Gulhane RD, Sharma C, Panwar H. 2017. Bacillus as potential probiotics: status, concerns, and future perspectives. Front Microbiol 8:1490. doi: 10.3389/fmicb.2017.01490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Setlow P, Wang S, Li Y-Q. 2017. Germination of spores of the orders Bacillales and Clostridiales. Annu Rev Microbiol 71:459–477. doi: 10.1146/annurev-micro-090816-093558. [DOI] [PubMed] [Google Scholar]
- 4.Setlow P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol 101:514–525. doi: 10.1111/j.1365-2672.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- 5.Driks A, Eichenberger P. 2016. The spore coat. Microbiol Spectr 4:TBS-0023-2016. doi: 10.1128/microbiolspec.TBS-0023-2016. [DOI] [PubMed] [Google Scholar]
- 6.Shuster B, Khemmani M, Abe K, Huang X, Nakaya Y, Maryn N, Buttar S, Gonzalez AN, Driks A, Sato T, Eichenberger P. 2019. Contributions of crust proteins to spore surface properties in Bacillus subtilis. Mol Microbiol 111:825–843. doi: 10.1111/mmi.14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKenney PT, Driks A, Eskandarian HA, Grabowski P, Guberman J, Wang KH, Gitai Z, Eichenberger P. 2010. A distance-weighted interaction map reveals a previously uncharacterized layer of the Bacillus subtilis spore coat. Curr Biol 20:934–938. doi: 10.1016/j.cub.2010.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Setlow P. 2016. Spore resistance properties In Driks A, Eichenberger P (ed), The bacterial spore: from molecules to systems. ASM Press, Washington, DC. [Google Scholar]
- 9.Setlow P, Johnson EA. 2019. Spores and their significance, p 23–64. In Doyle MP, Buchanan R (ed), Food microbiology, fundamentals and frontiers, 4th ed ASM Press, Washington, DC. [Google Scholar]
- 10.Hosoi T, Hirose R, Saegusa S, Ametani A, Kiuchi K, Kaminogawa S. 2003. Cytokine responses of human intestinal epithelial-like Caco-2 cells to the nonpathogenic bacterium Bacillus subtilis (natto). Int J Food Microbiol 82:255–264. doi: 10.1016/s0168-1605(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 11.Wenzel M, Muller A, Siemann-Herzberg M, Altenbuchner J. 2011. Self-inducible Bacillus subtilis expression system for reliable and inexpensive protein production by high-cell-density fermentation. Appl Environ Microbiol 77:6419–6425. doi: 10.1128/AEM.05219-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin P, Yuan H, Du J, Liu K, Liu H, Wang T. 2020. Progress in research and application development of surface display technology using Bacillus subtilis spores. Appl Microbiol Biotechnol 104:2319–2331. doi: 10.1007/s00253-020-10348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isticato R, Cangiano G, Tran HT, Ciabattini A, Medaglini D, Oggioni MR, De Felice M, Pozzi G, Ricca E. 2001. Surface display of recombinant proteins on Bacillus subtilis spores. J Bacteriol 183:6294–6301. doi: 10.1128/JB.183.21.6294-6301.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J, Schumann W. 2009. Display of proteins on Bacillus subtilis endospores. Cell Mol Life Sci 66:3127–3136. doi: 10.1007/s00018-009-0067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isticato R, Ricca E. 2014. Spore surface display. Microbiol Spectr 2:TBS-0011-2012. doi: 10.1128/microbiolspec.TBS-0011-2012. [DOI] [PubMed] [Google Scholar]
- 16.Potot S, Serra CR, Henriques AO, Schyns G. 2010. Display of recombinant proteins on Bacillus subtilis spores, using a coat-associated enzyme as the carrier. Appl Environ Microbiol 76:5926–5933. doi: 10.1128/AEM.01103-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartels J, Lopez Castellanos S, Radeck J, Mascher T. 2018. Sporobeads: the utilization of the Bacillus subtilis endospore crust as a protein display platform. ACS Synth Biol 7:452–461. doi: 10.1021/acssynbio.7b00285. [DOI] [PubMed] [Google Scholar]
- 18.Imamura D, Kuwana R, Takamatsu H, Watabe K. 2011. Proteins involved in formation of the outermost layer of Bacillus subtilis spores. J Bacteriol 193:4075–4080. doi: 10.1128/JB.05310-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abhyankar W, Beek AT, Dekker H, Kort R, Brul S, de Koster CG. 2011. Gel-free proteomic identification of the Bacillus subtilis insoluble spore coat protein fraction. Proteomics 11:4541–4550. doi: 10.1002/pmic.201100003. [DOI] [PubMed] [Google Scholar]
- 20.Choi JM, Han SS, Kim HS. 2015. Industrial applications of enzyme biocatalysis: current status and future aspects. Biotechnol Adv 33:1443–1454. doi: 10.1016/j.biotechadv.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Sheldon RA. 2007. Enzyme immobilization: the quest for optimum performance. Adv Synth Catal 349:1289–1307. doi: 10.1002/adsc.200700082. [DOI] [Google Scholar]
- 22.Schuurmann J, Quehl P, Festel G, Jose J. 2014. Bacterial whole-cell biocatalysts by surface display of enzymes: toward industrial application. Appl Microbiol Biotechnol 98:8031–8046. doi: 10.1007/s00253-014-5897-y. [DOI] [PubMed] [Google Scholar]
- 23.Nicks TB, Nair NU. 2019. Anchoring proteins for spore-display of enzymes are best selected using surface availability of the anchoring terminus as opposed to loading density of the anchor protein. Cell Free Syst Conf, Orlando, FL, 10 to 15 November 2019. [Google Scholar]
- 24.Shuster B, Khemmani M, Nakaya Y, Holland G, Iwamoto K, Abe K, Imamura D, Maryn N, Driks A, Sato T, Eichenberger P. 2019. Expansion of the spore surface polysaccharide layer in Bacillus subtilis by deletion of genes encoding glycosyltransferases and glucose modification enzymes. J Bacteriol 201:e00321-19. doi: 10.1128/JB.00321-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen H, Tian R, Ni Z, Zhang Q, Zhang T, Chen Z, Chen K, Yang S. 2015. Surface display of the thermophilic lipase Tm1350 on the spore of Bacillus subtilis by the CotB anchor protein. Extremophiles 19:799–808. doi: 10.1007/s00792-015-0755-0. [DOI] [PubMed] [Google Scholar]
- 26.He W, Jiang B, Mu W, Zhang T. 2016. Production of d-allulose with d-psicose 3-epimerase expressed and displayed on the surface of Bacillus subtilis spores. J Agric Food Chem 64:7201–7207. doi: 10.1021/acs.jafc.6b03347. [DOI] [PubMed] [Google Scholar]
- 27.Vanleeuw E, Winderickx S, Thevissen K, Lagrain B, Dusselier M, Cammue BPA, Sels BF. 2019. Substrate-specificity of Candida rugosa lipase and its industrial application. ACS Sustainable Chem Eng 7:15828–15844. doi: 10.1021/acssuschemeng.9b03257. [DOI] [Google Scholar]
- 28.Kang SJ, Park EA, Lee DH, Hong KW. 2019. Comparison of the stability of eGFP displayed on the Bacillus subtilis spore surface using CotB and C-terminally truncated CotB proteins as an anchoring motif under extreme conditions. Appl Biol Chem 62:41. doi: 10.1186/s13765-019-0448-y. [DOI] [Google Scholar]
- 29.Hosseini-Abari A, Kim BG, Lee SH, Emtiazi G, Kim W, Kim JH. 2016. Surface display of bacterial tyrosinase on spores of Bacillus subtilis using CotE as an anchor protein. J Basic Microbiol 56:1331–1337. doi: 10.1002/jobm.201600203. [DOI] [PubMed] [Google Scholar]
- 30.Wang F, Song T, Jiang H, Pei C, Huang Q, Xi H. 2019. Bacillus subtilis spore surface display of haloalkane dehalogenase DhaA. Curr Microbiol 76:1161–1167. doi: 10.1007/s00284-019-01723-7. [DOI] [PubMed] [Google Scholar]
- 31.Mingmongkolchai S, Panbangred W. 2019. Display of Escherichia coli phytase on the surface of Bacillus subtilis spore using CotG as an anchor protein. Appl Biochem Biotechnol 187:838–855. doi: 10.1007/s12010-018-2855-7. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Yang S, Wang X, Wang T. 2019. Production of trehalose with trehalose synthase expressed and displayed on the surface of Bacillus subtilis spores. Microb Cell Fact 18:100. doi: 10.1186/s12934-019-1152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Yang R, Hua X, Zhao W, Zhang W. 2015. Functional display of active β-galactosidase on Bacillus subtilis spores using crust proteins as carriers. Food Sci Biotechnol 24:1755–1759. doi: 10.1007/s10068-015-0228-3. [DOI] [Google Scholar]
- 34.Kwon SJ, Jung HC, Pan JG. 2007. Transgalactosylation in a water-solvent biphasic reaction system with beta-galactosidase displayed on the surfaces of Bacillus subtilis spores. Appl Environ Microbiol 73:2251–2256. doi: 10.1128/AEM.01489-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doucet D, Retnakaran A. 2012. Insect chitin, p 437–511. In Dhadialla TS. (ed), Insect growth disruptors. Academic Press, Oxford, UK. [Google Scholar]
- 36.Swiontek Brzezinska M, Jankiewicz U, Burkowska A, Walczak M. 2014. Chitinolytic microorganisms and their possible application in environmental protection. Curr Microbiol 68:71–81. doi: 10.1007/s00284-013-0440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Awad HM, El-Enshasy HA, Hanapi SZ, Hamed ER, Rosidi B. 2014. A new chitinase-producer strain Streptomyces glauciniger WICC-A03: isolation and identification as a biocontrol agent for plants phytopathogenic fungi. Nat Prod Res 28:2273–2277. doi: 10.1080/14786419.2014.939083. [DOI] [PubMed] [Google Scholar]
- 38.Rostami A, Hinc K, Goshadrou F, Shali A, Bayat M, Hassanzadeh M, Amanlou M, Eslahi N, Ahmadian G. 2017. Display of B. pumilus chitinase on the surface of B. subtilis spore as a potential biopesticide. Pestic Biochem Physiol 140:17–23. doi: 10.1016/j.pestbp.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Yánez-Mendizábal V, Zeriouh H, Viñas I, Torres R, Usall J, de Vicente A, Pérez-García A, Teixidó N. 2012. Biological control of peach brown rot (Monilinia spp.) by Bacillus subtilis CPA-8 is based on production of fengycin-like lipopeptides. Eur J Plant Pathol 132:609–619. doi: 10.1007/s10658-011-9905-0. [DOI] [Google Scholar]
- 40.Liu YB, Tabashnik BE, Moar WJ, Smith RA. 1998. Synergism between Bacillus thuringiensis spores and toxins against resistant and susceptible diamondback moths (Plutella xylostella). Appl Environ Microbiol 64:1385–1389. doi: 10.1128/AEM.64.4.1385-1389.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanahuja G, Banakar R, Twyman RM, Capell T, Christou P. 2011. Bacillus thuringiensis: a century of research, development and commercial applications. Plant Biotechnol J 9:283–300. doi: 10.1111/j.1467-7652.2011.00595.x. [DOI] [PubMed] [Google Scholar]
- 42.Shakya AK, Chowdhury MYE, Tao W, Gill HS. 2016. Mucosal vaccine delivery: current state and a pediatric perspective. J Control Release 240:394–413. doi: 10.1016/j.jconrel.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheng S, Jia H, Topiol S, Farinas ET. 2017. Engineering CotA laccase for acidic pH stability using Bacillus subtilis spore display. J Microbiol Biotechnol 27:507–513. doi: 10.4014/jmb.1608.08026. [DOI] [PubMed] [Google Scholar]
- 44.Copland A, Diogo GR, Hart P, Harris S, Tran AC, Paul MJ, Singh M, Cutting SM, Reljic R. 2018. Mucosal delivery of fusion proteins with Bacillus subtilis spores enhances protection against tuberculosis by bacillus Calmette-Guérin. Front Immunol 9:346. doi: 10.3389/fimmu.2018.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Potocki W, Negri A, Peszyńska-Sularz G, Hinc K, Obuchowski M, Iwanicki A. 2017. The combination of recombinant and non-recombinant Bacillus subtilis spore display technology for presentation of antigen and adjuvant on single spore. Microb Cell Fact 16:151. doi: 10.1186/s12934-017-0765-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Negri A, Potocki W, Iwanicki A, Obuchowski M, Hinc K. 2013. Expression and display of Clostridium difficile protein FliD on the surface of Bacillus subtilis spores. J Med Microbiol 62:1379–1385. doi: 10.1099/jmm.0.057372-0. [DOI] [PubMed] [Google Scholar]
- 47.Mauriello EM, Duc Le H, Isticato R, Cangiano G, Hong HA, De Felice M, Ricca E, Cutting SM. 2004. Display of heterologous antigens on the Bacillus subtilis spore coat using CotC as a fusion partner. Vaccine 22:1177–1187. doi: 10.1016/j.vaccine.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 48.Guoyan Z, Yingfeng A, Zabed H, Qi G, Yang M, Jiao Y, Li W, Wenjing S, Xianghui Q. 2019. Bacillus subtilis spore surface display technology: a review of its development and applications. J Microbiol Biotechnol 29:179–190. doi: 10.4014/jmb.1807.06066. [DOI] [PubMed] [Google Scholar]
- 49.Mauriello EM, Cangiano G, Maurano F, Saggese V, De Felice M, Rossi M, Ricca E. 2007. Germination-independent induction of cellular immune response by Bacillus subtilis spores displaying the C fragment of the tetanus toxin. Vaccine 25:788–793. doi: 10.1016/j.vaccine.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 50.Dai X, Liu M, Pan K, Yang J. 2018. Surface display of OmpC of Salmonella serovar Pullorum on Bacillus subtilis spores. PLoS One 13:e0191627. doi: 10.1371/journal.pone.0191627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen AT, Pham CK, Pham HT, Pham HL, Nguyen AH, Dang LT, Huynh HA, Cutting SM, Phan TN. 2014. Bacillus subtilis spores expressing the VP28 antigen: a potential oral treatment to protect Litopenaeus vannamei against white spot syndrome. FEMS Microbiol Lett 358:202–208. doi: 10.1111/1574-6968.12546. [DOI] [PubMed] [Google Scholar]
- 52.Yao YY, Chen DD, Cui ZW, Zhang XY, Zhou YY, Guo X, Li AH, Zhang YA. 2019. Oral vaccination of tilapia against Streptococcus agalactiae using Bacillus subtilis spores expressing Sip. Fish Shellfish Immunol 86:999–1008. doi: 10.1016/j.fsi.2018.12.060. [DOI] [PubMed] [Google Scholar]
- 53.Farjadian F, Moghoofei M, Mirkiani S, Ghasemi A, Rabiee N, Hadifar S, Beyzavi A, Karimi M, Hamblin MR. 2018. Bacterial components as naturally inspired nano-carriers for drug/gene delivery and immunization: set the bugs to work? Biotechnol Adv 36:968–985. doi: 10.1016/j.biotechadv.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang JM, Hong HA, Van Tong H, Hoang TH, Brisson A, Cutting SM. 2010. Mucosal delivery of antigens using adsorption to bacterial spores. Vaccine 28:1021–1030. doi: 10.1016/j.vaccine.2009.10.127. [DOI] [PubMed] [Google Scholar]
- 55.Karauzum H, Updegrove TB, Kong M, Wu IL, Datta SK, Ramamurthi KS. 2018. Vaccine display on artificial bacterial spores enhances protective efficacy against Staphylococcus aureus infection. FEMS Microbiol Lett 365:fny190. doi: 10.1093/femsle/fny190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schumacher D, Helma J, Schneider AFL, Leonhardt H, Hackenberger C. 2018. Nanobodies: chemical functionalization strategies and intracellular applications. Angew Chem Int Ed Engl 57:2314–2333. doi: 10.1002/anie.201708459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steeland S, Vandenbroucke RE, Libert C. 2016. Nanobodies as therapeutics: big opportunities for small antibodies. Drug Discov Today 21:1076–1113. doi: 10.1016/j.drudis.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 58.Hoey RJ, Eom H, Horn JR. 2019. Structure and development of single domain antibodies as modules for therapeutics and diagnostics. Exp Biol Med (Maywood) 244:1568–1576. doi: 10.1177/1535370219881129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruano-Gallego D, Fraile S, Gutierrez C, Fernández LÁ. 2019. Screening and purification of nanobodies from E. coli culture supernatants using the hemolysin secretion system. Microb Cell Fact 18:47. doi: 10.1186/s12934-019-1094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang M, Zhu G, Korza G, Sun X, Setlow P, Li J. 2020. Engineering Bacillus subtilis as a versatile and stable platform for production of nanobodies. Appl Environ Microbiol 86:e02938-19. doi: 10.1128/AEM.02938-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Senapati S, Mahanta AK, Kumar S, Maiti P. 2018. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther 3:7. doi: 10.1038/s41392-017-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Makadia HK, Siegel SJ. 2011. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel)) 3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yin L, Meng Z, Zhang Y, Hu K, Chen W, Han K, Wu BY, You R, Li CH, Jin Y, Guan YQ. 2018. Bacillus spore-based oral carriers loading curcumin for the therapy of colon cancer. J Control Release 271:31–44. doi: 10.1016/j.jconrel.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 64.Casula G, Cutting SM. 2002. Bacillus probiotics: spore germination in the gastrointestinal tract. Appl Environ Microbiol 68:2344–2352. doi: 10.1128/aem.68.5.2344-2352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nguyen VA, Huynh HA, Hoang TV, Ninh NT, Pham AT, Nguyen HA, Phan TN, Cutting SM. 2013. Killed Bacillus subtilis spores expressing streptavidin: a novel carrier of drugs to target cancer cells. J Drug Target 21:528–541. doi: 10.3109/1061186X.2013.778262. [DOI] [PubMed] [Google Scholar]
- 66.Wang X, Jiang M, Zhou Z, Gou J, Hui D. 2017. 3D printing of polymer matrix composites: a review and prospective. Compos B Eng 110:442–458. doi: 10.1016/j.compositesb.2016.11.034. [DOI] [Google Scholar]
- 67.Nguyen PQ, Courchesne ND, Duraj-Thatte A, Praveschotinunt P, Joshi NS. 2018. Engineered living materials: prospects and challenges for using biological systems to direct the assembly of smart materials. Adv Mater 30:e1704847. doi: 10.1002/adma.201704847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang C, Huang J, Zhang J, Liu S, Cui M, An B, Wang X, Pu J, Zhao T, Fan C, Lu TK, Zhong C. 2019. Engineered Bacillus subtilis biofilms as living glues. Mater Today 28:40–48. doi: 10.1016/j.mattod.2018.12.039. [DOI] [Google Scholar]
- 69.Park JH, Jang J, Lee JS, Cho DW. 2017. Three-dimensional printing of tissue/organ analogues containing living cells. Ann Biomed Eng 45:180–194. doi: 10.1007/s10439-016-1611-9. [DOI] [PubMed] [Google Scholar]
- 70.Yao L, Ou J, Wang G, Cheng C-Y, Wang W, Steiner H, Ishii H. 2015. bioPrint: a liquid deposition printing system for natural actuators. 3D Print Addit Manuf 2:168–179. doi: 10.1089/3dp.2015.0033. [DOI] [Google Scholar]
- 71.Gonzalez LM, Mukhitov N, Voigt CA. 2020. Resilient living materials built by printing bacterial spores. Nat Chem Biol 16:126–133. doi: 10.1038/s41589-019-0412-5. [DOI] [PubMed] [Google Scholar]
- 72.Chen X, Mahadevan L, Driks A, Sahin O. 2014. Bacillus spores as building blocks for stimuli-responsive materials and nanogenerators. Nat Nanotechnol 9:137–141. doi: 10.1038/nnano.2013.290. [DOI] [PubMed] [Google Scholar]
- 73.Monteiro PJM, Miller SA, Horvath A. 2017. Towards sustainable concrete. Nat Mater 16:698–699. doi: 10.1038/nmat4930. [DOI] [PubMed] [Google Scholar]
- 74.Stanaszek-Tomal E. 2020. Bacterial concrete as a sustainable building material? Sustainability 12:696–708. doi: 10.3390/su12020696. [DOI] [Google Scholar]
- 75.Shahid S, Aslam MA, Ali S, Zameer M, Faisal M. 2020. Self‐healing of cracks in concrete using Bacillus strains encapsulated in sodium alginate beads. ChemistrySelect 5:312–323. doi: 10.1002/slct.201902206. [DOI] [Google Scholar]
- 76.Hammes F, Boon N, de Villiers J, Verstraete W, Siciliano SD. 2003. Strain-specific ureolytic microbial calcium carbonate precipitation. Appl Environ Microbiol 69:4901–4909. doi: 10.1128/aem.69.8.4901-4909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rohini I, Padmapriya RP. 2018. Effect of bateria subtilis on conrete substituted fully and partially with demolition wastes. Int J Civ Eng 9:230–243. [Google Scholar]
- 78.Sharma TK, Alazhari M, Heath A, Paine K, Cooper RM. 2017. Alkaliphilic Bacillus species show potential application in concrete crack repair by virtue of rapid spore production and germination then extracellular calcite formation. J Appl Microbiol 122:1233–1244. doi: 10.1111/jam.13421. [DOI] [PubMed] [Google Scholar]
- 79.Pungrasmi W, Intarasoontron J, Jongvivatsakul P, Likitlersuang S. 2019. Evaluation of microencapsulation techniques for MICP bacterial spores applied in self-healing concrete. Sci Rep 9:12484–12493. doi: 10.1038/s41598-019-49002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sarkar M, Adak D, Tamang A, Chattopadhyay B, Mandal S. 2015. Genetically-enriched microbe-facilitated self-healing concrete—a sustainable material for a new generation of construction technology. RSC Adv 5:105363–105371. doi: 10.1039/C5RA20858K. [DOI] [Google Scholar]
- 81.Jha MK, Kumari A, Panda R, Rajesh Kumar J, Yoo K, Lee JY. 2016. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 165:2–26. doi: 10.1016/j.hydromet.2016.01.035. [DOI] [Google Scholar]
- 82.Wu S, Wang L, Zhao L, Zhang P, El-Shall H, Moudgil B, Huang X, Zhang L. 2018. Recovery of rare earth elements from phosphate rock by hydrometallurgical processes - A critical review. Chem Eng J 335:774–800. doi: 10.1016/j.cej.2017.10.143. [DOI] [Google Scholar]
- 83.Haque N, Hughes A, Lim S, Vernon C. 2014. Rare earth elements: overview of mining, mineralogy, uses, sustainability and environmental impact. Resources 3:614–635. doi: 10.3390/resources3040614. [DOI] [Google Scholar]
- 84.Kucuker MA, Wieczorek N, Kuchta K, Copty NK. 2017. Biosorption of neodymium on Chlorella vulgaris in aqueous solution obtained from hard disk drive magnets. PLoS One 12:e0175255. doi: 10.1371/journal.pone.0175255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gupta NK, Gupta A, Ramteke P, Sahoo H, Sengupta A. 2019. Biosorption—a green method for the preconcentration of rare earth elements (REEs) from waste solutions: a review. J Mol Liq 274:148–164. doi: 10.1016/j.molliq.2018.10.134. [DOI] [Google Scholar]
- 86.Bonificio WD, Clarke DR. 2016. Rare-earth separation using bacteria. Environ Sci Technol Lett 3:180–184. doi: 10.1021/acs.estlett.6b00064. [DOI] [Google Scholar]
- 87.Dong W, Li S, Camilleri E, Korza G, Yankova M, King SM, Setlow P. 2019. Accumulation and release of rare earth ions by spores of Bacillus species and the location of these ions in spores. Appl Environ Microbiol 85:e00956-19. doi: 10.1128/AEM.00956-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Igiri BE, Okoduwa SIR, Idoko GO, Akabuogu EP, Adeyi AO, Ejiogu IK. 2018. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: a review. J Toxicol 2018:2568038. doi: 10.1155/2018/2568038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khulbe KC, Matsuura T. 2018. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl Water Sci 8:19–48. doi: 10.1007/s13201-018-0661-6. [DOI] [Google Scholar]
- 90.Imamura D, Kuwana R, Takamatsu H, Watabe K. 2010. Localization of proteins to different layers and regions of Bacillus subtilis spore coats. J Bacteriol 192:518–524. doi: 10.1128/JB.01103-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hinc K, Ghandili S, Karbalaee G, Shali A, Noghabi KA, Ricca E, Ahmadian G. 2010. Efficient binding of nickel ions to recombinant Bacillus subtilis spores. Res Microbiol 161:757–764. doi: 10.1016/j.resmic.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 92.Zhang J, Garner W, Setlow P, Yu J. 2011. Quantitative analysis of spatial-temporal correlations during germination of spores of Bacillus species. J Bacteriol 193:3765–3772. doi: 10.1128/JB.05154-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tam NK, Uyen NQ, Hong HA, Duc Le H, Hoa TT, Serra CR, Henriques AO, Cutting SM. 2006. The intestinal life cycle of Bacillus subtilis and close relatives. J Bacteriol 188:2692–2700. doi: 10.1128/JB.188.7.2692-2700.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fairhead M, Thony-Meyer L. 2012. Bacterial tyrosinases: old enzymes with new relevance to biotechnology. New Biotechnol 29:183–191. doi: 10.1016/j.nbt.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 95.Sun H, Lin Z, Zhao L, Chen T, Shang M, Jiang H, Tang Z, Zhou X, Shi M, Zhou L, Ren P, Qu H, Lin J, Li X, Xu J, Huang Y, Yu X. 2018. Bacillus subtilis spore with surface display of paramyosin from Clonorchis sinensis potentializes a promising oral vaccine candidate. Parasit Vectors 11:156. doi: 10.1186/s13071-018-2757-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reljic R, Sibley L, Huang JM, Pepponi I, Hoppe A, Hong HA, Cutting SM. 2013. Mucosal vaccination against tuberculosis using inert bioparticles. Infect Immun 81:4071–4080. doi: 10.1128/IAI.00786-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aps L, Tavares MB, Rozenfeld JHK, Lamy MT, Ferreira LCS, Diniz MO. 2016. Bacterial spores as particulate carriers for gene gun delivery of plasmid DNA. J Biotechnol 228:58–66. doi: 10.1016/j.jbiotec.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 98.Barnes AG, Cerovic V, Hobson PS, Klavinskis LS. 2007. Bacillus subtilis spores: a novel microparticle adjuvant which can instruct a balanced Th1 and Th2 immune response to specific antigen. Eur J Immunol 37:1538–1547. doi: 10.1002/eji.200636875. [DOI] [PubMed] [Google Scholar]
- 99.Song M, Hong HA, Huang JM, Colenutt C, Khang DD, Nguyen TV, Park SM, Shim BS, Song HH, Cheon IS, Jang JE, Choi JA, Choi YK, Stadler K, Cutting SM. 2012. Killed Bacillus subtilis spores as a mucosal adjuvant for an H5N1 vaccine. Vaccine 30:3266–3277. doi: 10.1016/j.vaccine.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 100.Khushnood RA, Qureshi ZA, Shaheen N, Ali S. 2020. Bio-mineralized self-healing recycled aggregate concrete for sustainable infrastructure. Sci Total Environ 703:135007. doi: 10.1016/j.scitotenv.2019.135007. [DOI] [PubMed] [Google Scholar]