Abstract
This paper is part of the celebration of the 50th anniversary of founding of the journal Hormones and Behavior, the official journal of the Society for Behavioral Neuroendocrinology. All sex differences in phenotypic development stem from the sexual imbalance in X and Y chromosomes, which are the only known differences in XX and XY zygotes. The sex chromosome genes act within cells to cause differences in phenotypes of XX and XY cells throughout the body. In the gonad, they determine the type of gonad, leading to differences in secretion of testicular vs. ovarian hormones, which cause further sex differences in tissue function. These current ideas of sexual differentiation are briefly contrasted with a hormones-only view of sexual differentiation of the last century. The multiple, independent action of diverse sex-biasing agents means that sex-biased factors can be synergistic, increasing sex differences, or compensatory, making the two sexes more equal. Several animal models have been fruitful in demonstrating sex chromosome effects, and interactions with gonadal hormones. MRI studies of human brains demonstrate variation in brain structure associated with both differences in gonadal hormones, and in the number of X and Y chromosomes. Five unanswered questions are posed as a challenge to future investigators to improve understanding of sexual differentiation throughout the body.
In the 50 years since the founding of the journal Hormones and Behavior in 1969, concepts of sexual differentiation have changed dramatically. In this highly selective review, I briefly discuss the current status of mammalian sexual differentiation theory, and contrast it with the 20th Century view that it replaced. I allude to studies in animals and humans that have clarified some aspects of sexual differentiation, particularly the effects of sex chromosome genes. I then pose five questions that are currently unanswered, as a challenge for future investigators who are building the next levels of understanding of sexual differentiation in diverse tissues.
Current and previous sexual differentiation theories
The current theory of mammalian sexual differentiation provides a change in emphasis relative to older theories (Figure 1)(Arnold, 2017a; Arnold, 2019b). It states that the primary sex-biasing factors are encoded by the sex chromosomes, which are the only molecules that are known to differ in male and female zygotes. Thus, all sex differences in gene expression and tissue phenotype must be downstream of the X and Y genes and chromatin that are unequally represented in males and females. The transcriptome of XX and XY embryos is sexually differentiated essentially as soon as it is activated, in the 2-8 cell stage (Bramble et al., 2016; Lowe et al., 2015; Werner et al., 2017). Therefore the sex of the embryonic cells can be assessed based on measurement of the cellular phenotype (gene expression) at the very earliest stages. Some X genes that escape X-inactivation are expressed at higher levels in XX than XY embryos. And, a few Y genes are expressed only in XY embryos. A major event in sexual differentiation is the pre-implantation expression of Xist only in XX embryos (Cloutier et al., 2018; Sado and Ferguson-Smith, 2005), which causes transcriptional silencing of one X chromosome in XX but not XY cells. This inactivation is part of the process that brings the dose of most X genes in female cells to be within the male range, relative to autosomal genes with which they interact extensively in most gene networks (Birchler et al., 2006; Disteche, 2016; Nguyen and Disteche, 2006). The expression of Xist thus has pervasive effects on molecular pathways underlying many emergent phenotypes. Thus, one of the earliest sex-specific events in development has the effect of offsetting an inherent sexual inequality in number of X chromosomes, illustrating the paradox that sexual differentiation and inequality in expression of one gene can operate to make the two sexes more equal in phenotypes. A dominant theme is that sexual balance and sexual imbalance are both byproducts of sexually differentiated processes (Arnold, 2017b).
Figure 1.
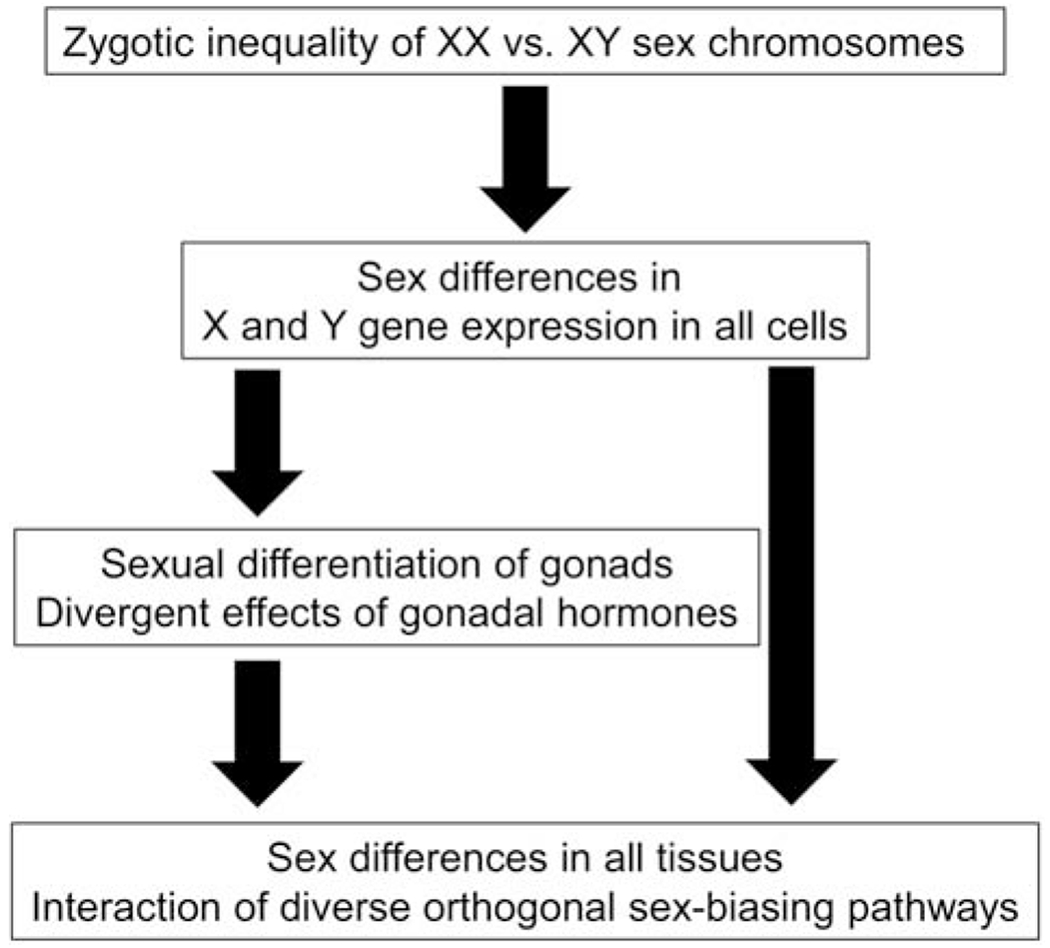
A schematic diagram illustrating sex-biasing influences of sex chromosomes and gonadal hormones, which act independently and interdependently on cells in diverse tissues. Because different sex-biasing factors can work together or in opposition, their effect can be to make or reduce sex differences in emergent phenotypes. From (Arnold, 2019b), reprinted with permission of Elsevier.
Another major milestone of placental and marsupial mammalian sexual differentiation is the expression of Sry from the Y chromosome in XY embryos, again because of its widespread downstream sex-specific effects. Sry, the testis-determining gene, is expressed in the undifferentiated gonad and commits that tissue to a testicular fate (Capel, 2017). Sry is inherently sex biased because it is Y-linked, and makes males different from females. In the absence of Sry, genes shared by both sexes (autosomal and X genes) are activated only in XX embryos to commit the gonad to an ovarian fate. Both differentiation programs, testicular and ovarian, activate sex-specific gene networks to cause sex-typical gonadal development, but also inhibit the alternative pathway of development, thus normally ensuring that the production of testes or ovaries is dichotomous with relatively few intermediates. The differentiation of the gonads is extremely important for sexual differentiation of most non-gonadal tissues of the body, because the testes and ovaries secrete different levels of hormones (predominantly estrogens, androgens, progestins, and anti-Müllerian hormone) that cause many sex differences in tissue function throughout the body, including the brain.
The proximate hormonal effects that induce sexual differentiation have classically been divided into two functional classes: organizational and activational effects. This classification was first articulated by Phoenix et al. (Phoenix et al., 1959), ten years before the founding of Hormones and Behavior, who studied sexual differentiation of guinea pig sexual behavior. They thought that secretions of androgens by the fetal testes laid down a permanent male substrate, probably a morphological change in the brain, upon which later gonadal hormones acted to activate sexual behaviors and other functions (Arnold, 2009). The permanent hormonal structural masculinization of the brain was documented starting 12 years later (Arnold and Gorski, 1984). The early organizational effects are permanent, and are discriminated from later activational effects that are reversible. Since 1959 the concept of organizational effects has been broadened to include any long-lasting effect of gonadal hormones, such as effects of androgens acting during puberty in hamsters (Sisk and Zehr, 2005), or effects of estrogens acting postnatally to help program the capacity for female reproductive behaviors in mice (Bakker and Brock, 2010). Previously, organizational effects were considered part of the process of sexual differentiation (producing “true” and permanent sex differences), whereas activational effects were sometimes excluded from sexual differentiation because they could come and go. That attitude probably stemmed from the expectation that tissue differentiation is a process of commitment to an irreversible fate. Today, I would consider any hormonal (or non-hormonal) effect as part of the sexual differentiation process as long as it produces sex differences in phenotype.
Thus, in studies of animals that have unequal sex chromosomes, three major classes of proximate sex-biasing factors are currently recognized: sex chromosome effects (the differential action of X and Y genes or chromatin that are out of balance in XX and XY genomes), and organizational and activational effects of gonadal hormones (Arnold, 2014). The roles of the three different classes are operationally defined and tested using specific experimental designs. Sex chromosome effects are measured by comparing mice that differ in number and types of sex chromosomes, but have similar levels of gonadal hormones. The most common models include the Four Core Genotypes and XY* mouse models, discussed below (see also (Case and Teuscher, 2015)). To assess organizational effects of hormones, animals are compared that differ in the level of gonadal hormones early in life, but their phenotype is measured later in life when gonadal hormone levels are similar across groups (e.g., after gonadectomy with or without hormone replacement). Activational effects are those that change dynamically as hormone levels wax and wane. Classically, to activate full masculine sexual behavior, as demonstrated by Phoenix et al. (1959), androgens must exert both organizational effects during pre- and postnatal development, and activational effects in adulthood. The dichotomy between organizational and activational effects, developed in the study of behavior and the brain, is now in wide use in studies of sex differences in many different tissues (e.g., (Norheim et al., 2019).
The current concept of sexual differentiation is more complete than earlier theories of sexual development. The classic theory, which emerged during the first half of the 20th Century, proposed two phases, sex determination followed by sexual differentiation (Achermann and Jameson, 2017). Sex is defined by type of gonad and gametes produced. Sex determination, then, is the process controlling gonadal type, the primary and first event of sexual development. The model suggests that the two stages occur in succession, the second (differentiation) phase dependent on the first (determination), and that different agents operate in the two stages to produce male and female phenotypes. Sex determination is genetic (presence or absence of Sry), whereas sexual differentiation of non-gonadal tissues is controlled by gonadal hormones. The testes secrete two hormones that cause masculine phenotypic development. Testosterone causes masculine differentiation of the penis, scrotum and brain, and Müllerian Inhibiting Hormone prevents formation of the uterus and oviducts. Ovarian estrogens and progestins, while not required for the decision to develop female internal and external genitalia, are important for the control of female reproduction and the cycle of ovulation.
I have criticized the classic model because it leaves out important sex differences and mechanisms of sexual differentiation (Arnold, 2011; Arnold, 2019b). Phenotypic sex differences occur throughout the body, in the germline (Chuva de Sousa Lopes et al., 2008; Sangrithi et al., 2017) and in non-gonadal tissues (Bermejo-Alvarez et al., 2010; Burgoyne et al., 1995; Dewing et al., 2003; Lowe et al., 2015), prior to differentiation of the gonads, and thus cannot be downstream of Sry or gonadal differentiation. Thus, it does not make sense to envision a process of sex determination that excludes some kinds of sex differences. Moreover, sex differences in nearly all cell types are caused by sex chromosome effects that occur before and after gonadal differentiation (Arnold, 2019b). Thus, I question the need to discriminate between sex determination and sexual differentiation, and prefer to embrace a unitary process of sexual differentiation in which sex differences are primarily caused by sex chromosome genes and their downstream pathways including dramatic effects of gonadal hormones.
Interaction of multiple independent sex-biasing factors
The current theory indicates that multiple, independent factors contribute to sex differences in tissue phenotypes, replacing a small set of gonadal hormones as the only proximate factors that initiate sex-biased development of non-gonadal tissues (Arnold, 2011). The independence of hormonal and sex chromosome effects has the important ramification that the two sex-biasing factors can have “opposite” rather than synergistic effects (Arnold, 2019b; De Vries, 2004; McCarthy and Arnold, 2011). For example, previously Y chromosome genes were always thought to cause males to be phenotypically different than females. Instead, we can now imagine that a Y chromosome gene can act to make males more like females, canceling out a sex difference caused by another factor such as testosterone, or different effects of one vs. two X chromosomes (Arnold, 2017b). We see evidence for such compensatory actions in the following examples: (1) Female-specific expression of Xist compensates for the presence of a second X chromosome in females, and reduces the sexual disparity in expression of X genes that would otherwise occur. (2) Not infrequently, both sex chromosomes and gonadal hormones contribute to sex differences, for example in mouse models of autoimmune disease, metabolism and adiposity, numerous cardiovascular diseases, cancer, and stroke (Arnold et al., 2017; Chen et al., 2012; Du et al., 2014; Kaneko and Li, 2018; McCullough et al., 2016; Smith-Bouvier et al., 2008). In these cases, the effect of sex chromosomes is often “opposite” in direction to the sex-biasing effects of gonadal hormones. For example, genes on the X chromosome increase body weight and adiposity in mice when they are present in two copies, as in females, relative to one copy, as in males (Chen et al., 2012). Having ovaries or estradiol, however, reduces body weight and fat (Chen et al., 2012; Litwak et al., 2014). The simultaneous action of the two female-typical factors produces a more sexually equivalent phenotype than if either factor operated alone. Although two sex-biased factors might have opposite effects with regard to an emergent phenotype such as body weight, they may or may not interact with each other mechanistically to achieve the end result. (3) In Turner’s syndrome (X,45), women have a single X chromosome. Having a single X chromosome, in the absence of another sex chromosome, is thought to be lethal for the vast majority of human conceptuses (Cockwell et al., 1991; Hook and Warburton, 2014). Moreover, those individuals who survive past birth have a complex of syndromic features including the inability to reproduce (Gravholt et al., 2019). Having a second sex chromosome of either type prevents the syndromic effects of X monosomy. Thus, the Y chromosome, found only in males, has a similar Turner’s-preventing effect as a second X chromosome, producing greater sexual equality in survival than if the Y chromosome is absent (Arnold, 2019a). Similarly, in some strains of mice, a second sex chromosome of either type reverses the lower body weight found in XO mice (Chen et al., 2013).
Animal models have allowed discovery of many diverse sex chromosome effects on sexual differentiation.
Currently, the best animal models for discovering sex chromosome effects involve comparisons of mice with different sex chromosomes but in which gonadal hormone levels are similar or are unlikely to cause the group differences. In the Four Core Genotypes (FCG) model, XX and XY mice are produced that have the same type of gonad (Burgoyne and Arnold, 2016; De Vries et al., 2002). This comparison is possible because the Sry gene is removed from the Y chromosome and inserted as a transgene onto chromosome 3. Thus, XY mice can have Sry and testes, or lack Sry and possess ovaries (Figure 2A). Similarly, XX mice can have either type of gonad. Comparison of XX and XY mice, with the same type of gonad, has uncovered sex chromosome effects on a wide variety of phenotypes, including behaviors (pain, juvenile play, reproductive, parental, feeding, addictive, learning) (Barker et al., 2010; Bonthuis et al., 2012; Chen et al., 2015; Cox et al., 2014; Cox et al., 2015; Gatewood et al., 2006; Gioiosa et al., 2008; Lewejohann et al., 2009; Quinn et al., 2007; Seu et al., 2014), autoimmune disease (Du et al., 2014; Itoh et al., 2019; Smith-Bouvier et al., 2008), adiposity and fat metabolism (Chen et al., 2012; Link and Reue, 2017)(Figure 2A), blood pressure (Caeiro et al., 2011; Dadam et al., 2017; Ji et al., 2010), stroke (McCullough et al., 2016), ischemia/reperfusion injury (Li et al., 2014), pulmonary hypertension (Umar et al., 2018), atheroschlerosis (AlSiraj et al., 2019), aortic aneurysms (Alsiraj et al., 2016; Arnold et al., 2017), aging and Alzheimer’s (Broestl et al., 2015; Davis et al., 2019), neural tube closure defects (Chen et al., 2008), bladder cancer (Kaneko and Li, 2018), volumes of brain regions (Corre et al., 2016; Raznahan et al., 2015; Vousden et al., 2018), and ability to synthesize estradiol in the fetal brain (Cisternas et al., 2015). A role for the Y chromosome in sexual differentiation is implied by the finding that male mice with different types of Y chromosome show variation in phenotypes (e.g., autoimmune disease and immune response to viral infection) (Arnold, 2017b; Case and Teuscher, 2015; Krementsov et al., 2017; Spach et al., 2009).
Figure 2.
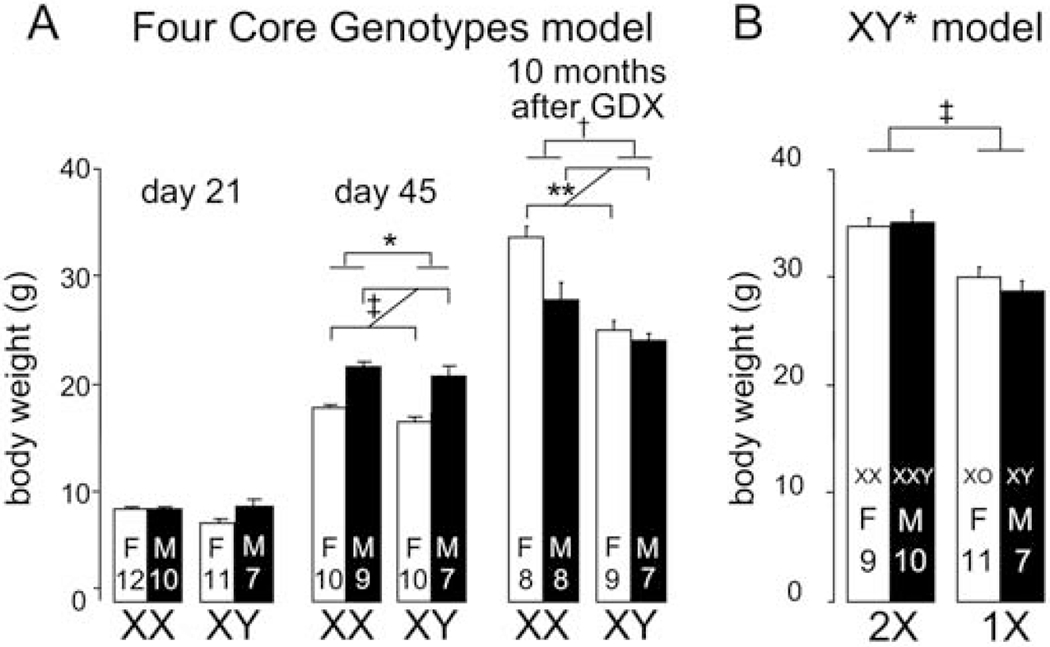
An illustration of a sex chromosome effect on body weight in mice, which is thought to be caused in part by sex chromosome effects on feeding behavior (Chen et al., 2012; Chen et al., 2015). A. In Four Core Genotypes (FCG) mice, the four different groups do not differ in body weight at weaning (day 21). After puberty, at day 45, gonadal male (M) mice weigh more than gonadal female (F) mice (‡ p<0.000001), and XX mice weigh more than XY mice (* p<0.05). Ten months after mice are gonadectomized at 75 days of age, XX mice weigh more than XY († p<0.0001), and gonadal females weigh more than males (** p<0.01), an effect that is more prominent in gonadal females than males (interaction * p<0.05). B. In mice from the XY* model, nine months after gonadectomy at 75 days, mice with two X chromosome weigh more than those with one X chromosome (‡ p<0.000001), irrespective of gonadal sex. Modified from (Chen et al., 2012).
When a sex chromosome effect is discovered in FCG mice, the next step is to discover the X or Y genes that are responsible for the effect, and their mechanisms of action. To narrow down the choice of genes, it is helpful to determine if the effect is X- or Y-linked. A convenient model for that purpose is the XY* mouse model in which the male has an altered Y* chromosome that pairs abnormally with the X chromosome during spermatogenesis, to produce progeny with genotypes similar to XO, XX, XY, and XXY (Arnold, 2014; Burgoyne and Arnold, 2016; Cox et al., 2014). Thus, mice with one vs. two X chromosomes can be compared (XO vs. XX, XY vs. XXY) to discover X effects (Figure 2BC), and mice with or without a Y chromosome can be compared (XO vs. XY, XX vs. XXY) to discover Y chromosome effects. In numerous cases, the difference between XX and XY mice with the same type of gonad is found to be caused by differences in the number of X chromosomes ((Chen et al., 2012; Chen et al., 2008; Cox et al., 2014; Itoh et al., 2019; Kaneko and Li, 2018; Li et al., 2014)(Figure 2), although effects of the Y chromosome have also been discovered (Umar et al., 2018). Numerous reviews discuss the use of these and other mouse models (Arnold, 2017b; Burgoyne and Arnold, 2016; Case et al., 2013; Cox et al., 2014; Krementsov et al., 2017; Lue et al., 2010; Spach et al., 2009; Wistuba, 2010).
The easy availability of the FCG and XY* mouse models has meant that virtually all of the research on sex chromosome effects in mammals has been done in mice. In other mammalian species, models are not yet available that allow comparison of individuals with different sex chromosome but with the same type of gonad, or lacking a gonad. The concentration of research in one species is a severe limitation, because broad comparative investigation is required for adequate tests of principles of biology. The effects of sex chromosomes in mice, for example, may or may not be generalizable. Although the sex chromosome of mice share many attributes and genes with sex chromosomes of other mammalian species, there are significant differences. For example, X-linked sex chromosome effects might be more important in humans than in mice, because a greater percentage of X genes escape inactivation and are constitutively expressed at higher levels in XX females than in XY males. In humans, about 25% of X genes show higher expression in females, across many tissues, and many pseudoautosomal X genes are expressed higher in females than males (Carrel et al., 1999; Tukiainen et al., 2017). In mice, the percent of genes escaping X inactivation is thought not to exceed about 8% (Berletch et al., 2015; Berletch et al., 2010). The higher number of sex-biased X genes in humans means that more of them are available as drivers of sexual differentiation, to be favored by natural selection when such sex differences increase fitness. Given the current resources for investigating sex chromosome effects in animal models, the most likely avenue for translation of animal research to humans is that individual X or Y genes can be discovered unequivocally to contribute to sex differences in mice, which then leads to tests of the role of the same gene in humans. Hopefully more non-mouse sex chromosome models will be developed in the near future.
Sex chromosome and androgen effects on human brain sexual differentiation is supported by magnetic resonance imaging of individuals with diverse genotypes.
It is challenging to separate sex chromosome from hormonal effects on sex differences in the brains of humans. One issue is that the investigator is unable to manipulate the relevant independent variables separately, holding others constant. A second complication is that differences in sex-biased biological variables (hormones, sex chromosome genes) are strongly correlated with gendered differences in the individual’s environment, making it difficult to determine if sex differences in the brain are caused by sex-biased biological variables, or by the different environments experienced by each sex. Nevertheless, progress has been made by analyzing specific genetic variations that are associated with changes in effectiveness of hormones or dose of sex chromosome genes (reviewed by (Bakker, 2019). For example, structural MRI of brains has been applied to a population of humans with variations in effectiveness of the androgen receptor (AR), caused by different lengths of CAG repeats encoding polyglutamine tracts in the AR gene (Raznahan et al., 2010). In males, shorter CAG repeats (causing more effective function of the AR) were found to be associated with more masculine adolescent developmental changes in cortical thickness of the inferior parietal lobule in males, suggesting that some sex differences in the cortex are caused by greater action of androgens in males. Moreover, in females more effective variants of the AR were associated with more masculine patterns of adolescent change in the inferior frontal gyrus, suggesting that androgen action in females may account for some phenotypic variations among females. Another informative genetic group are humans with a mutation of the androgen receptor, leading to complete androgen insensitivity syndrome (CAIS) and external female body phenotype in XY CAIS women. CAIS women are reported to have regional brain volumes that are similar to control XX females in some cases, but similar to control XY males in other cases (Savic et al., 2017). These results imply that androgens may be responsible for some sex differences, but sex chromosome effects might contribute to other sex differences in brain structure (Bakker, 2019). Because of the unequivocal proof of androgenic masculinization of brain development in many other vertebrate species, the evidence in humans for similar effects is convincing. Of course, in all of these conditions there exists the possibility of environmental effects that are difficult to separate from biological effects.
Other structural MRI studies focus on humans with diverse sets of sex chromosomes, including XO, XX, XXX, XY, XXY, XYY, and XXYY. In a study of cerebral cortex structure in individuals between ages 5 and 25 (Raznahan et al., 2016), increases in the number of X chromosome were associated with a decrease in total brain volume, cortical volume, and cortical surface area, without an effect on cortical thickness. These X effects occurred in both gonadal males and females, and did not interact with gonadal sex. In contrast, all four of these variables were increased with increasing number of Y chromosomes (Figure 3). However, when the effects of sex chromosome aneuploidy were measured within smaller regions of the cortex, some characteristics (for example, surface area of the occipitoparietal surface area and absolute rostrofrontal cortical thickness) were influenced in the same direction by increasing X or Y chromosome dose. The authors suggest that brain regions showing similar effects of X and Y dose are predominantly involved in “biological motion perception, autobiographical memory, interoception, emotion and reward processing, and language” (Raznahan et al., 2016). These opposite or synergistic patterns of X vs. Y chromosome dose may potentially be explained by effects of different types of sex chromosome genes. For example, many genes escaping X inactivation are expressed at inherently higher levels in females than males, without any known compensatory effect of a gene on the Y chromosome (Disteche, 2016; Tukiainen et al., 2017). And, Y genes have only male-specific effects, some of which are unlike the effects of X genes (Hughes and Page, 2015). However, some X and Y genes have similar effects, and might account for the instances of convergent effects of X and Y dose on specific brain parameters. These genes reside in the pseudoautosomal region found on both chromosomes, or are X-Y gene pairs with overlapping functions (Bellott et al., 2014; Cortez et al., 2014; Mankiw et al., 2017; Raznahan et al., 2018; Shpargel et al., 2012).
Figure 3.
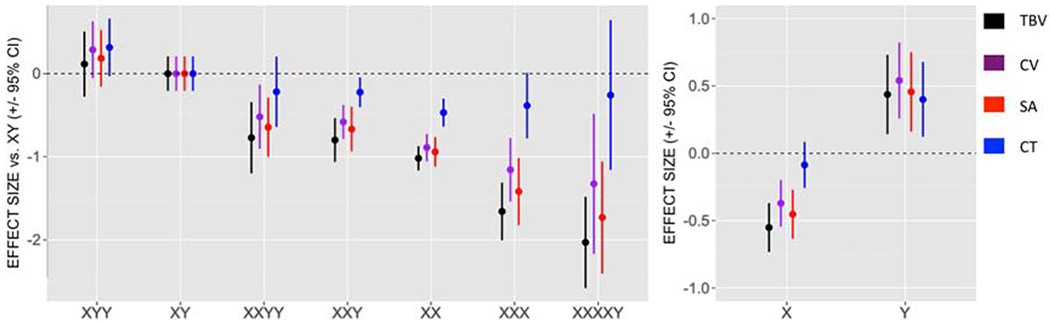
In an MRI study of brains of 5-25 year old humans, groups differed in the number and type of sex chromosomes. The left graph represents effect-size deviations of brain volumes relative to XY males. An increase in number of X chromosomes was associated with a reduction in total brain volume (TBV), cortical volume (CV), and surface area (SA), but not cortical thickness (CT). An increase in number of Y chromosomes was associated with an increase in all four variables. The right graph shows standardized effect-size estimates (± 95% confidence interval) for addition of one X or one Y chromosome on each anatomic dependent variable. From (Raznahan et al., 2016), by permission of Oxford University Press.
The study of sex chromosome aneuploidy supports the role of sex chromosome dose as a determinant of brain structure. However, it is not clear how much these dosage effects contribute to sex differences. It is not easy to unravel these effects from the effects of gonadal hormones in comparisons of euploid humans (XX females vs. XY males). One issue is that the sex chromosome effects are confounded with hormone levels in cross-sex comparisons. Another issue is that gonadal hormones and sex chromosome effects can be synergistic or in opposition (compensatory), as discussed above, so that regional variations in the direction of effects of sex chromosomes could reflect variation in effects of hormones that interact with sex chromosome effects (Raznahan et al., 2016). Further light will be shed by measuring the effects of specific X and Y genes that vary in dose in aneuploid populations of humans, and in the comparison of XX females vs. XY males.
Five unanswered questions about sexual differentiation
The considerations above lead to five areas requiring more investigation to improve our understanding of sexual differentiation of mammals including humans.
1. How important are sex chromosome effects?
A vast number of sex differences in animals have been shown to be caused or strongly influenced by gonadal hormones. In contrast, the number of sex chromosome effects is small. Indeed, the potent and pervasive effects of gonadal hormones were the experimental basis for the hormone-only theory of sexual differentiation, and help explain its persistence despite evidence for sex chromosome effects. Today, many biologists appear not yet to be aware that sex chromosome complement influences sexual differentiation via non-hormonal mechanisms. Nevertheless, there may be reasons for some skepticism about the conclusion that sex chromosome effects are minor. Experiments testing the role of gonadal hormones are much easier to perform compared to experiments testing sex chromosome effects. Manipulations of hormone levels are relatively easy in many species, including humans. In contrast, tractable animal models for studying sex chromosome effects have been developed relatively recently, and mostly in mice. Most investigators interested in sex differences are well-trained in endocrinology, but have little familiarity with studies of the sex chromosome evolution and function, or with models (FCG, XY*, etc.) to test for sex chromosome effects. Is the dominance of hormones really accurate, or in part a reflection of the paucity of studies of sex chromosome effects?
Relatively few studies have manipulated both gonadal hormones and sex chromosomes, to judge their relative influence on sex differences. In an early microarray study of sex differences in gene expression in mouse liver, 2597 genes were found to be expressed at different levels in gonad-intact XY males vs. XX females (n=5 per group) (Van Nas et al., 2009). The number of genes that reach significance in such studies depends on group size and statistical power. Other better-powered studies find many more genes that are expressed differently at that mRNA level (Yang et al., 2006). But, keeping group size at n=5, the sex differences in expression of liver genes were virtually abolished after gonadectomy of adults, with only 12 genes retaining sex differences. That result implies that most sex differences in gene expression in liver are caused by activational effects of gonadal hormones that were removed by gonadectomy. One might surmise that the remaining 12 genes were sexually differentiated by organizational or sex chromosome effects. However, other genes might show organizational or sex chromosome effects when gonadal hormones are present at the time of measurement of gene expression. A more complete investigation would also compare groups of mice that differ only in the levels of testicular hormones around the time of birth, to estimate the role of organizational effects more directly. In a comparably powered study of FCG mice that were GDX as adults, only 10 genes were expressed differently in liver between XX and XY mice, suggesting again a much smaller role for sex chromosome complement, compared to activational effects of hormones (Van Nas et al., 2009).
Another perspective is offered by the comparison of classic and more recent studies of sexual differentiation of brain structure. In the period between 1971 and 2000, numerous morphological sex differences in the brain were discovered using histological analysis in animal models. The most frequently studied sex differences were in areas of the brain involved in reproduction, including limbic and spinal nuclei controlling ovulation, sexual behavior, copulation, etc. (Arnold and Gorski, 1984; Cooke et al., 1998). Sex differences were also discovered in other brain areas not closely tied to reproduction, such as the cerebral cortex (Juraska, 1991). Because the sex differences were largest in limbic regions, these regions were most frequently studied to determine the underlying molecular and cellular mechanisms of sexual differentiation (McCarthy et al., 2017; McCarthy et al., 2015). The major tenets of brain sexual differentiation have come from studies of these limbic regions, and emphasize nearly exclusively the dominant role of gonadal hormones and their downstream pathways. That conclusion contrasts with the results of more recent MRI of sex differences across the entire brain (Corre et al., 2016; Spring et al., 2007; Vousden et al., 2018). MRI studies of FCG mouse brains confirm the sex differences in limbic brain regions, and confirm that these vary with the type of gonad rather than sex chromosome complement. However, an unexpectedly high proportion of brain sex differences were attributed to sex chromosome effects. Of 62 brain regions examined, 30 were significantly different among FCG groups, with 20 showing effects of gonadal hormones and 14 effects of sex chromosomes (Corre et al., 2016). Most of the sex chromosome effects discovered in gonad-intact mice were also found in adult mice gonadectomized before puberty (Vousden et al., 2018), indicating that are likely to be true sex chromosome effects. These studies raise the question whether traditional methods, which have uncovered the largest sex differences in areas of the brain controlling reproduction, might have led to an overestimate of the role of gonadal hormones relative to sex chromosomes in brain sexual differentiation. Much more work is needed to resolve this question, and to determine the factors contributing to sex differences in each brain region and function.
2. What are the downstream effects of primary sex-biasing factors?
As diverse hormonal and sex chromosome factors are implicated in causing sex differences in tissue function, a major effort will be devoted to establishing their mechanisms of action. A massive effort in the last 50 years has uncovered many mechanisms for gonadal hormone effects in diverse tissues. In the brain, for example, region-specific effects of gonadal hormones are mediated by a large diversity of molecular and cellular mechanisms (e.g., (McCarthy et al., 2017). Because sex chromosome effects have been implicated in sexual differentiation more recently, there is comparatively little knowledge of which X and Y genes have specific effects in specific tissues, and how those genes influence downstream molecular pathways. This situation will change rapidly in the next decades. One of the genes escaping X inactivation in mice, histone demethylase Kdm6a, expressed higher in XX than XY cells, has already been implicated in sex differences in autoimmune disease and bladder cancer (Itoh et al., 2019; Kaneko and Li, 2018). Other genes will soon be discovered, as will the downstream pathways that they regulate.
3. How do gonadal hormonal and sex chromosome factors interact to influence sex differences in the same phenotypes?
As discussed above, sex differences in phenotype are not infrequently influenced both by sex chromosome effects and by gonadal hormone effects. In mouse models of autoimmune disease, for example, sex differences are probably produced by a mix of effects of androgens, estrogens, X chromosome genes, and Y genes (Case and Teuscher, 2015; Gold and Voskuhl, 2016; Spence and Voskuhl, 2012; Voskuhl and Gold, 2012). In studies of body weight and adiposity in mice, estrogens, androgens, and X and Y chromosome genes all contribute to sex differences (Chen et al., 2012; Chen et al., 2013; Link and Reue, 2017; Palmer and Clegg, 2015; Palmisano et al., 2018). In studies of diverse cardiovascular diseases, including ischemia/reperfusion injury, atherosclerosis, and hypertension, both gonadal hormones and sex chromosomes influence sex differences in mouse models (AlSiraj et al., 2019; Arnold et al., 2017; Iorga et al., 2017; Li et al., 2014). In some cases, the hormones and sex chromosome genes appear to have “opposite” effects. For example, estradiol (higher in females) reduces body fat in mice, but having two X chromosome (as in females) increased body fat. Each effect therefore reduces the sex difference caused by the other, and constitutes a case of compensation (De Vries, 2004, 2005). Because disease processes can influence the level of an individual sex-biasing factor that is otherwise in balance with another sex-biasing factor, sex differences in disease may result from physiological changes in which two balanced sex-biasing factors move out of balance. A critical question is the mode of interaction of the different sex-biasing factors. Do sex chromosome and hormonal effects influence the same phenotype via different molecular pathways? Or, do they converge on a common molecular pathway to change the trait? We expect that the molecular basis of interaction will become clearer as more investigators choose to study both sex chromosome and hormonal effects in the same system.
4. What is the nature of permanence? What molecular pathways underlie effects of sex-biasing variables that are long-lasting?
The classic dichotomy between organizational and activational effects of gonadal hormones established that for a single phenotype a single hormone (e.g., testosterone, or estradiol) can have permanent effects at one time of life, and also transient effects at other times of life. Why are some effects permanent and others not? The basis of permanence was originally implied to be structural. Testosterone’s action to masculinize external genitalia was thought to be permanent because it created anatomical structures (penis, scrotum) that were stable and did not require further secretion of testosterone for maintenance of the structure for the rest of life. In describing organizational effects on the brain, Phoenix et al. imagined that organizational effects of testosterone involved organization of a male brain “substrate”, no doubt also structural, that was permanent because it also did not require androgens for maintenance. The discovery of permanent masculinization of brain structures by gonadal hormones (Arnold and Gorksi, 1983) confirmed this supposition of Phoenix et al.
One question is whether sex chromosome effects are organizational or activational, permanent or transient? The dichotomy may be less easy to test for sex chromosome effects. Hormones come and go, depending on stage of development (prenatal, pubertal, adult, aging), time of day, season, and reproductive cycle. It is possible that X or Y gene expression is less labile, making it more difficult to determine when it acts to influence sexual differentiation.
New ideas have recently arisen to account for the permanence of effects of hormonal or other sex-biasing factors. Because epigenetic marks in the genome can be long-lasting, and can even be carried across generations, it is possible that a cell is permanently changed in its gene expression, by epigenetic rather than by structural changes (Forger, 2016; Knoedler and Shah, 2018; McCarthy, 2019). Much further work is needed to understand what these epigenetic effects are and how long they last. Are the epigenetic effects reversible, and if so by what factors? Are epigenetic effects caused only by expression of specific genes, or are there direct effects of sex chromatin that do not require expression of specific X or Y genes, as has been documented in Drosophila (Silkaitis and Lemos, 2014; Wijchers and Festenstein, 2011)?
5. How can research on animals contribute to understanding the interaction of effects of sex-biasing biological factors and gendered environmental factors?
The terms “female” and “male” are used variably across and within cultures. Although the biological definitions of these terms have not changed substantially in recent years, social norms for males and females are rapidly changing. It is perhaps unfortunate that the terms have different biological and social meanings, because that leads to a great deal of confusion as people attempt to communicate with each other about the significance of sex and gender. As humans, males and females are different both because of innate biological differences, and because males and females have different social and physical environments. For many human traits, it is impossible to determine if an apparent difference between XX females and XY males is caused by biological or environmental variables, or what is the relative importance of the two sets of variables. The two sources of variation are completely intertwined and confounded with each other. For traits that are found only in humans, it is usually not possible to experimentally isolate of the effect of one variable from other. Biologists tend to emphasize the importance of biological variables, and sociologists tend to emphasize the role of gendered environments.
The role of animal models is that individual biological variables can be varied independently, to measure their effects in isolation from other variables. The results then give us good ideas about how the same variables might influence humans, and lead to investigations of the importance of these variables in humans. It is challenging, however, to model effects of human gendered environments in animals, because the environments are so uniquely human. Although some effects of environment in humans (e.g., stress, social dominance) can be modeled in lab animals, many others cannot.
Despite these challenges, further research on the interaction of biological sex and environment is critically important, in animal models. The cross-talk between animal and human research calls for extensive and creative hypothesis testing. What kinds of social and other environmental conditions can be translated to animals, to test their importance for sexual differentiation of diverse traits?
Conclusions
The study of sexual differentiation bears on many fundamental issues of biology and medicine. A basic question is the nature of femaleness and maleness, which influences our self-concept and perspective on the social and biological world. Sex-biased factors influence the incidence and progression of diseases, with the implication that sex-biasing variable can protect from disease or exacerbate disease. It is important to understand these variables, and how they act. With powerful methods that are already in hand, and with the development of better and more tractable animal models, it will be possible to make exceptionally exciting progress to answer the questions posed here, as well as numerous others.
Highlights.
Current theory suggests that sex chromosome genes and gonadal hormones are the primary proximate sex-biasing factors controlling sexual differentiation of tissues including the brain. The multiple, independent action of diverse sex-biasing agents means that sex-biased factors can be synergistic, increasing sex differences, or compensatory, making the two sexes more equal. Animal models and MRI studies of human brain support this model. Five unanswered questions are posed as a challenge for future study of sexual differentiation throughout the body.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Achermann JC, Jameson JL, 2017. Disorders of sex development, in: Jameson JL (Ed.), Harrison’s Endocrinology. McGraw Hill, New York, N.Y., pp. 146–158. [Google Scholar]
- AlSiraj Y, Chen X, Thatcher SE, Temel RE, Cai L, Blalock E, Katz W, Ali HM, Petriello M, Deng P, Morris AJ, Wang X, Lusis AJ, Arnold AP, Reue K, Thompson K, Tso P, Cassis LA, 2019. XX sex chromosome complement promotes atherosclerosis in mice. Nat Commun 10, 2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsiraj Y, Thatcher SE, Charnigo R, Kuey C, Blalock E, Daugherty A, Cassis LA, 2016. Female Mice with an XY Sex Chromosome Complement Develop Severe Angiotensin II-Induced Abdominal Aortic Aneurysms. Circulation 135, 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, 2009. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm. Behav 55, 570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, 2011. The end of gonad-centric sex determination in mammals. Trends Genet 28, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, 2014. Conceptual frameworks and mouse models for studying sex differences in physiology and disease: Why compensation changes the game. Exp. Neurol 259, 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, 2017a. A general theory of sexual differentiation. J. Neurosci. Res 95, 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, 2017b. Y chromosome’s roles in sex differences in disease. Proc. Natl. Acad. Sci. U. S. A 114, 3787–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, 2019a. The mouse as a model of fundamental concepts related to Turner syndrome. Am J Med Genet C Semin Med Genet 181, 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, 2019b. Rethinking sex determination of non-gonadal tissues. Curr Top Dev Biol 134, 289–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Cassis LA, Eghbali M, Reue K, Sandberg K, 2017. Sex Hormones and Sex Chromosomes Cause Sex Differences in the Development of Cardiovascular Diseases. Arterioscler. Thromb. Vasc. Biol 37, 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Gorski RA, 1984. Gonadal steroid induction of structural sex differences in the CNS. Annual Review of Neuroscience 7, 413–442. [DOI] [PubMed] [Google Scholar]
- Bakker J, 2019. The Sexual Differentiation of the Human Brain: Role of Sex Hormones Versus Sex Chromosomes. Curr Top Behav Neurosci. [DOI] [PubMed] [Google Scholar]
- Bakker J, Brock O, 2010. Early oestrogens in shaping reproductive networks: evidence for a potential organisational role of oestradiol in female brain development. J Neuroendocrinol 22, 728–735. [DOI] [PubMed] [Google Scholar]
- Barker JM, Torregrossa MM, Arnold AP, Taylor JR, 2010. Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. J. Neurosci 30, 9140–9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellott DW, Hughes JF, Skaletsky H, Brown LG, Pyntikova T, Cho TJ, Koutseva N, Zaghlul S, Graves T, Rock S, Kremitzki C, Fulton RS, Dugan S, Ding Y, Morton D, Khan Z, Lewis L, Buhay C, Wang Q, Watt J, Holder M, Lee S, Nazareth L, Rozen S, Muzny DM, Warren WC, Gibbs RA, Wilson RK, Page DC, 2014. Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature 508, 494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berletch JB, Ma W, Yang F, Shendure J, Noble WS, Disteche CM, Deng X, 2015. Escape from X inactivation varies in mouse tissues. PLoS. Genet 11, e1005079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berletch JB, Yang F, Disteche CM, 2010. Escape from X inactivation in mice and humans. Genome Biol 11, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Rizos D, Rath D, Lonergan P, Gutierrez-Adan A, 2010. Sex determines the expression level of one third of the actively expressed genes in bovine blastocysts. Proc. Natl. Acad. Sci. U. S. A 107, 3394–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler JA, Fernandez HR, Kavi HH, 2006. Commonalities in compensation. BioEssays 28, 565–568. [DOI] [PubMed] [Google Scholar]
- Bonthuis PJ, Cox KH, Rissman EF, 2012. X-chromosome dosage affects male sexual behavior. Horm. Behav 61, 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramble MS, Roach L, Lipson A, Vashist N, Eskin A, Ngun T, Gosschalk JE, Klein S, Barseghyan H, Arboleda VA, Vilain E, 2016. Sex-Specific Effects of Testosterone on the Sexually Dimorphic Transcriptome and Epigenome of Embryonic Neural Stem/Progenitor Cells. Sci. Rep 6, 36916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broestl L, Worden K, Wang D, Devidze N, Kim D, Chang K, Yu G-Q, Palop JJ, Miller BL, Arnold AP, Mucke L, Dubal DB, 2015. The X-chromosome decreases mortality and confers resilience against Alzheimer’s deficits. Annals of Neurology 78, S87. [Google Scholar]
- Burgoyne PS, Arnold AP, 2016. A primer on the use of mouse models for identifying direct sex chromosome effects that cause sex differences in non-gonadal tissues. Biol. Sex Differ 7, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne PS, Thornhill AR, Boudrean SK, Darling SM, Bishop CE, Evans EP, 1995. The genetic basis of XX-XY differences present before gonadal sex differentiation in the mouse. Philos. Trans. R. Soc. Lond B Biol. Sci 350, 253–260. [DOI] [PubMed] [Google Scholar]
- Caeiro XE, Mir FR, Vivas LM, Carrer HF, Cambiasso MJ, 2011. Sex chromosome complement contributes to sex differences in bradycardic baroreflex response. Hypertension 58, 505–511. [DOI] [PubMed] [Google Scholar]
- Capel B, 2017. Vertebrate sex determination: evolutionary plasticity of a fundamental switch. Nat. Rev Genet 18, 675–689. [DOI] [PubMed] [Google Scholar]
- Carrel L, Cottle AA, Goglin KC, Willard HF, 1999. A first-generation X-inactivation profile of the human X chromosome. Proc. Natl. Acad. Sci. U. S. A 96, 14440–14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case LK, Teuscher C, 2015. Y genetic variation and phenotypic diversity in health and disease. Biol. Sex Differ 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case LK, Wall EH, Dragon JA, Saligrama N, Krementsov DN, Moussawi M, Zachary JF, Huber SA, Blankenhorn EP, Teuscher C, 2013. The Y chromosome as a regulatory element shaping immune cell transcriptomes and susceptibility to autoimmune disease. Genome Res 23, 1474–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, Reue K, 2012. The number of X chromosomes causes sex differences in adiposity in mice. PLoS Genet 8, e1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, McClusky R, Itoh Y, Reue K, Arnold AP, 2013. X and Y chromosome complement influence adiposity and metabolism in mice. Endocrinology 154, 1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang L, Loh D, Colwell C, Tache Y, Reue K, Arnold AP, 2015. Sex differences in diurnal rhythms of food intake in mice caused by gonadal hormones and complement of sex chromosomes. Horm. Behav 75, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Watkins R, Delot E, Reliene R, Schiestl RH, Burgoyne PS, Arnold AP, 2008. Sex difference in neural tube defects in p53-null mice is caused by differences in the complement of X not Y genes. Dev. Neurobiol 68, 265–273. [DOI] [PubMed] [Google Scholar]
- Chuva de Sousa Lopes S, Hayashi K, Shovlin TC, Mifsud W, Surani MA, McLaren A, 2008. X chromosome activity in mouse XX primordial germ cells. PLoS Genet 4, e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisternas CD, Tome K, Caeiro XE, Dadam FM, Garcia-Segura LM, Cambiasso MJ, 2015. Sex chromosome complement determines sex differences in aromatase expression and regulation in the stria terminalis and anterior amygdala of the developing mouse brain. Mol. Cell Endocrinol 414, 99–110. [DOI] [PubMed] [Google Scholar]
- Cloutier M, Harris C, Gayen S, Maclary E, Kalantry S, 2018. Experimental Analysis of Imprinted Mouse X-Chromosome Inactivation. Methods Mol Biol 1861, 177–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockwell A, MacKenzie M, Youings S, Jacobs P, 1991. A cytogenetic and molecular study of a series of 45,X fetuses and their parents. J. Med. Genet 28, 151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM, 1998. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol 19, 323–362. [DOI] [PubMed] [Google Scholar]
- Corre C, Friedel M, Vousden DA, Metcalf A, Spring S, Qiu LR, Lerch JP, Palmert MR, 2016. Separate effects of sex hormones and sex chromosomes on brain structure and function revealed by high-resolution magnetic resonance imaging and spatial navigation assessment of the Four Core Genotype mouse model. Brain Struct. Funct 221, 997–1016. [DOI] [PubMed] [Google Scholar]
- Cortez D, Marin R, Toledo-Flores D, Froidevaux L, Liechti A, Waters PD, Grutzner F, Kaessmann H, 2014. Origins and functional evolution of Y chromosomes across mammals. Nature 508, 488–493. [DOI] [PubMed] [Google Scholar]
- Cox KH, Bonthuis PJ, Rissman EF, 2014. Mouse model systems to study sex chromosome genes and behavior: Relevance to humans. Front Neuroendocrinol 35, 405–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KH, Quinnies KM, Eschendroeder A, Didrick PM, Eugster EA, Rissman EF, 2015. Number of X-chromosome genes influences social behavior and vasopressin gene expression in mice. Psychoneuroendocrinology 51, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadam FM, Cisternas CD, Macchione AF, Godino A, Antunes-Rodrigues J, Cambiasso MJ, Vivas LM, Caeiro XE, 2017. Sex chromosome complement involvement in angiotensin receptor sexual dimorphism. Mol. Cell Endocrinol 447, 98–105. [DOI] [PubMed] [Google Scholar]
- Davis EJ, Lobach I, Dubal DB, 2019. Female XX sex chromosomes increase survival and extend lifespan in aging mice. Aging Cell 18, e12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, 2004. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology 145, 1063–1068. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, 2005. Sex steroids and sex chromosomes at odds? Endocrinology 146, 3277–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP, 2002. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J. Neurosci 22, 9005–9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Shi T, Horvath S, Vilain E, 2003. Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Brain Res. Mol. Brain Res 118, 82–90. [DOI] [PubMed] [Google Scholar]
- Disteche CM, 2016. Dosage compensation of the sex chromosomes and autosomes. Semin. Cell Dev. Biol 56, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S, Itoh N, Askarinam S, Hill H, Arnold AP, Voskuhl RR, 2014. XY sex chromosome complement, compared with XX, in the CNS confers greater neurodegeneration during experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A 111, 2806–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, 2016. Epigenetic mechanisms in sexual differentiation of the brain and behaviour. Philos Trans R Soc Lond B Biol Sci 371, 20150114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF, 2006. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J. Neurosci 26, 2335–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioiosa L, Chen X, Watkins R, Klanfer N, Bryant CD, Evans CJ, Arnold AP, 2008. Sex chromosome complement affects nociception in tests of acute and chronic exposure to morphine in mice. Horm. Behav 53, 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SM, Voskuhl RR, 2016. Pregnancy and multiple sclerosis: from molecular mechanisms to clinical application. Semin Immunopathol 38, 709–718. [DOI] [PubMed] [Google Scholar]
- Gravholt CH, Viuff MH, Brun S, Stochholm K, Andersen NH, 2019. Turner syndrome: mechanisms and management. Nat Rev Endocrinol 15, 601–614. [DOI] [PubMed] [Google Scholar]
- Hook EB, Warburton D, 2014. Turner syndrome revisited: review of new data supports the hypothesis that all viable 45,X cases are cryptic mosaics with a rescue cell line, implying an origin by mitotic loss. Hum. Genet 133, 417–424. [DOI] [PubMed] [Google Scholar]
- Hughes JF, Page DC, 2015. The Biology and Evolution of Mammalian Y Chromosomes. Annu. Rev Genet 49, 507–527. [DOI] [PubMed] [Google Scholar]
- Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M, 2017. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ 8, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Golden LC, Itoh N, Matsukawa MA, Ren E, Tse V, Arnold AP, Voskuhl RR, 2019. The X-linked histone demethylase Kdm6a in CD4+ T lymphocytes modulates autoimmunity. J Clin Invest 130, 3852–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Zheng W, Wu X, Liu J, Ecelbarger CM, Watkins R, Arnold AP, Sandberg K, 2010. Sex chromosome effects unmasked in angiotensin II-induced hypertension. Hypertension 55, 1275–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraska JM, 1991. Sex differences in “cognitive” regions of the rat brain. Psychoneuroendocrinol 16, 105–119. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Li X, 2018. X chromosome protects against bladder cancer in females via a KDM6A-dependent epigenetic mechanism. Sci. Adv 4, eaar5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoedler JR, Shah NM, 2018. Molecular mechanisms underlying sexual differentiation of the nervous system. Curr Opin Neurobiol 53, 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krementsov DN, Case LK, Dienz O, Raza A, Fang Q, Ather JL, Poynter ME, Boyson JE, Bunn JY, Teuscher C, 2017. Genetic variation in chromosome Y regulates susceptibility to influenza A inflection. PNAS 114, 349–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewejohann L, Damm OS, Luetjens CM, Hamalainen T, Simoni M, Nieschlag E, Gromoll J, Wistuba J, 2009. Impaired recognition memory in male mice with a supernumerary X chromosome. Physiol Behav 96, 23–29. [DOI] [PubMed] [Google Scholar]
- Li J, Chen X, McClusky R, Ruiz-Sundstrom M, Itoh Y, Umar S, Arnold AP, Eghbali M, 2014. The number of X chromosomes influences protection from cardiac ischaemia/reperfusion injury in mice: one X is better than two. Cardiovasc. Res 102, 375–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link JC, Reue K, 2017. Genetic Basis for Sex Differences in Obesity and Lipid Metabolism. Annu Rev Nutr 37, 225–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwak SA, Wilson JL, Chen W, Garcia-Rudaz C, Khaksari M, Cowley MA, Enriori PJ, 2014. Estradiol prevents fat accumulation and overcomes leptin resistance in female high-fat diet mice. Endocrinology 155, 4447–4460. [DOI] [PubMed] [Google Scholar]
- Lowe R, Gemma C, Rakyan VK, Holland ML, 2015. Sexually dimorphic gene expression emerges with embryonic genome activation and is dynamic throughout development. BMC Genomics 16, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue YH, Wang C, Liu PY, Erkilla K, Swerdloff RS, 2010. Insights into the pathogenesis of XXY phenotype from comparison of the clinical syndrome with an experimental XXY mouse model. Pediatr. Endocrinol. Rev 8 Suppl 1, 140–144. [PubMed] [Google Scholar]
- Mankiw C, Park MTM, Reardon PK, Fish AM, Clasen LS, Greenstein D, Giedd JN, Blumenthal JD, Lerch JP, Chakravarty MM, Raznahan A, 2017. Allometric Analysis Detects Brain Size-Independent Effects of Sex and Sex Chromosome Complement on Human Cerebellar Organization. J. Neurosci 37, 5221–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, 2019. Is Sexual Differentiation of Brain and Behavior Epigenetic? Curr Opin Behav Sci 25, 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, 2011. Reframing sexual differentiation of the brain. Nat. Neurosci 14, 677–683. establishment of sex differences in the brain. Nat Rev Neurosci 18, 471-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Pickett LA, VanRyzin JW, Kight KE, 2015. Surprising origins of sex differences in the brain. Horm. Behav 76, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Mirza MA, Xu Y, Bentivegna K, Steffens EB, Ritzel R, Liu F, 2016. Stroke sensitivity in the aged: sex chromosome complement vs. gonadal hormones. Aging (Albany. NY.) 8, 1432–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DK, Disteche CM, 2006. Dosage compensation of the active X chromosome in mammals. Nat. Genet 38, 47–53. [DOI] [PubMed] [Google Scholar]
- Norheim F, Hasin-Brumshtein Y, Vergnes L, Chella Krishnan K, Pan C, Seldin MM, Hui ST, Mehrabian M, Zhou Z, Gupta S, Parks BW, Walch A, Reue K, Hofmann SM, Arnold AP, Lusis AJ, 2019. Gene-by-Sex Interactions in Mitochondrial Functions and Cardio-Metabolic Traits. Cell Metab 29, 932–949 e934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer BF, Clegg DJ, 2015. The sexual dimorphism of obesity. Mol. Cell Endocrinol 402, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmisano BT, Zhu L, Eckel RH, Stafford JM, 2018. Sex differences in lipid and lipoprotein metabolism. Mol Metab 15, 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC, 1959. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65, 369–382. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR, 2007. Sex chromosome complement regulates habit formation. Nat. Neurosci 10, 1398–1400. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Lee NR, Greenstein D, Wallace GL, Blumenthal JD, Clasen LS, Giedd JN, 2016. Globally Divergent but Locally Convergent X- and Y-Chromosome Influences on Cortical Development. Cereb. Cortex 26, 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, Clasen L, Addington A, Gogtay N, Rapoport JL, Giedd JN, 2010. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc. Natl. Acad. Sci. U. S. A 107, 16988–16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Lue Y, Probst F, Greenstein D, Giedd J, Wang C, Lerch J, Swerdloff R, 2015. Triangulating the sexually dimorphic brain through high-resolution neuroimaging of murine sex chromosome aneuploidies. Brain Struct. Funct 220, 3581–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Parikshak NN, Chandran V, Blumenthal JD, Clasen LS, Alexander-Bloch AF, Zinn AR, Wangsa D, Wise J, Murphy DGM, Bolton PF, Ried T, Ross J, Giedd JN, Geschwind DH, 2018. Sex-chromosome dosage effects on gene expression in humans. Proc. Natl. Acad. Sci. U. S. A 115, 7398–7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sado T, Ferguson-Smith AC, 2005. Imprinted X inactivation and reprogramming in the preimplantation mouse embryo. Hum Mol Genet 14 Spec No 1, R59–64. [DOI] [PubMed] [Google Scholar]
- Sangrithi MN, Royo H, Mahadevaiah SK, Ojarikre O, Bhaw L, Sesay A, Peters AH, Stadler M, Turner JM, 2017. Non-Canonical and Sexually Dimorphic X Dosage Compensation States in the Mouse and Human Germline. Dev. Cell 40, 289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic I, Frisen L, Manzouri A, Nordenstrom A, Linden Hirschberg A, 2017. Role of testosterone and Y chromosome genes for the masculinization of the human brain. Hum Brain Mapp 38, 1801–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seu E, Groman SM, Arnold AP, Jentsch JD, 2014. Sex chromosome complement influences operant responding for a palatable food in mice. Genes Brain Behav 13, 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpargel KB, Sengoku T, Yokoyama S, Magnuson T, 2012. UTX and UTY demonstrate histone demethylase-independent function in mouse embryonic development. PLoS Genet 8, e1002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silkaitis K, Lemos B, 2014. Sex-biased chromatin and regulatory cross-talk between sex chromosomes, autosomes, and mitochondria. Biol. Sex Differ 5, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL, 2005. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol 26, 163–174. [DOI] [PubMed] [Google Scholar]
- Smith-Bouvier DL, Divekar AA, Sasidhar M, Du S, Tiwari-Woodruff SK, King JK, Arnold AP, Singh RR, Voskuhl RR, 2008. A role for sex chromosome complement in the female bias in autoimmune disease. J. Exp. Med 205, 1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spach KM, Blake M, Bunn JY, McElvany B, Noubade R, Blankenhorn EP, Teuscher C, 2009. Cutting edge: the Y chromosome controls the age-dependent experimental allergic encephalomyelitis sexual dimorphism in SJL/J mice. J. Immunol 182, 1789–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence RD, Voskuhl RR, 2012. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front Neuroendocrinol 33, 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring S, Lerch JP, Henkelman RM, 2007. Sexual dimorphism revealed in the structure of the mouse brain using three-dimensional magnetic resonance imaging. Neuroimage 35, 1424–1433. [DOI] [PubMed] [Google Scholar]
- Tukiainen T, Villani AC, Yen A, Rivas MA, Marshall JL, Satija R, Aguirre M, Gauthier L, Fleharty M, Kirby A, Cummings BB, Castel SE, Karczewski KJ, Aguet F, Byrnes A, Lappalainen T, Regev A, Ardlie KG, Hacohen N, MacArthur DG, 2017. Landscape of X chromosome inactivation across human tissues. Nature 550, 244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umar S, Cunningham CM, Itoh Y, Moazeni S, Vaillancourt M, Sarji S, Centala A, Arnold AP, Eghbali M, 2018. The Y Chromosome Plays a Protective Role in Experimental Hypoxic Pulmonary Hypertension. Am. J. Respir. Crit. Care Med 197, 952–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nas A, GuhaThakurta D, Wang SS, Yehya N, Horvath S, Zhang B, Ingram-Drake L, Chaudhuri G, Schadt EE, Drake TA, Arnold AP, Lusis AJ, 2009. Elucidating the role of gonadal hormones in sexually dimorphic gene coexpression networks. Endocrinology 150, 1235–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl RR, Gold SM, 2012. Sex-related factors in multiple sclerosis susceptibility and progression. Nat. Rev. Neurol 8, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden DA, Corre C, Spring S, Qiu LR, Metcalf A, Cox E, Lerch JP, Palmert MR, 2018. Impact of X/Y genes and sex hormones on mouse neuroanatomy. Neuroimage 173, 551–563. [DOI] [PubMed] [Google Scholar]
- Werner RJ, Schultz BM, Huhn JM, Jelinek J, Madzo J, Engel N, 2017. Sex chromosomes drive gene expression and regulatory dimorphisms in mouse embryonic stem cells. Biol. Sex Differ 8, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijchers PJ, Festenstein RJ, 2011. Epigenetic regulation of autosomal gene expression by sex chromosomes. Trends Genet 27, 132–140. [DOI] [PubMed] [Google Scholar]
- Wistuba J, 2010. Animal models for Klinefelter’s syndrome and their relevance for the clinic. Mol. Hum. Reprod 16, 375–385. [DOI] [PubMed] [Google Scholar]
- Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, Drake TA, Lusis AJ, 2006. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res 16, 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]


