Abstract
According to the Gestalt theorists, restructuring is an essential component of insight problem-solving, contributes to the “Aha!” experience, and is similar to the perceptual switch experienced when reinterpreting ambiguous figures. Previous research has demonstrated that pupil diameter increases during the perceptual switch of ambiguous figures, and indexes norepeinephrine functioning mediated by the locus coeruleus. In this study, we investigated if pupil diameter similarly predicts the switch into awareness people experience when solving a problem via insight. Additionally, we explored eye movement dynamics during the same task to investigate if the problem-solving strategies used are linked to specific oculomotor behaviors. In 38 participants, pupil diameter increased about 500 msec prior to solution only in trials for which subjects report having an insight. In contrast, participants increased their microsaccade rate only prior to non-insight solutions. Pupil dilation and microsaccades were not reliably related, but both appear to be robust markers of how people solve problems (with or without insight). The pupil size change seen when people have an “Aha!” moment represents an indicator of the switch into awareness of unconscious processes humans depend upon for insight, and suggests important involvement of norepinephrine, via the locus coeruleus, in sudden insight.
Keywords: Insight, Problem-solving, Eye movements, Attention
Insight problem-solving was first introduced by the Gestalt psychologists who compared it to the perceptual switch people have when looking at ambiguous figures (Kohler, 1925). According to the Gestalt theorists, both cases are accompanied by an unpredictable restructuring of the figure/problem elements that allows a solution (or percept) to reach awareness, which is phenomenologically indexed by the exclamation “Aha!”.
When exposed to ambiguous figures people experience what is called “perceptual rivalry”, where their visual experience switches between the different possible alternatives rather than staying fixed on one interpretation (e.g., Necker’s cube effect). A recent study demonstrated that participants who better identified two alternative perspectives in ambiguous images were also better in solving insight problems; and showed that insight problem-solving ability improved when participants were first presented with a Necker’s cube in a conflict version (i.e., promoting perceptual rivalry) (Laukkonen and Tangen, 2017). The authors speculated that the relationship between insight problems and images presenting perceptual rivalry, specifically using the Necker cube, hinges on the same cognitive process of representational change (or shifting perspectives) when people have an insight.
The study of insight problem-solving has moved several steps forward since the Gestalt school first introduced it. We are now able to study its neural bases (e.g., for a review see Kounios and Beeman, 2014) and its association with behavioral responses such as ocular movements and involuntary reflexes indicating possible brain regions and functions underpinning insight problem-solving (e.g., Salvi et al., 2015; Shen et al., 2018). Based on Laukkonen and Tangen’s results, in this research, we assumed that these two switches could be associated with “corollary” behavioral responses and that if the Gestalt hypothesis is valid, they should mediate similar behavioral markers. In other words, we hypothesize that because perceptual and conceptual “representational changes” (Ohlsson, 1992) rely on similar processes, they should present similar behavioral responses. One candidate behavioral marker is pupil dilation. Differences in pupil diameter have been registered both during perceptual switch (specifically with the Necker’s cube - Einhäuser et al., 2008) and during problem-solving (Hess and Polt, 1964). Variation of pupil diameter is a marker of attentional shifts and surprise (Preuschoff et al., 2011; Konishi et al., 2017), which are also a core component of the “Aha!” phenomenology (Danek and Wiley, 2017; Litchfield and Ball, 2011; Salvi et al., 2015; Thomas and Lleras, 2009). Nevertheless, studies that directly investigated if pupil diameter changes when people experience a sudden insight are missing.
1. Visual perception and pupil dilation
Prior studies demonstrated that the average pupil diameter deviates from the baseline before perceptual reports, preceding the conscious recognition of ambiguous stimuli (Einhäuser et al., 2008; Kietzmann et al., 2011). Studies in this field showed that participants’ pupil diameter increased just before the perceptual switch of Necker’s cube and the relative amount of dilation before the switch was a predictor of the following duration of perceptual stability. The switch coincided, specifically, with the strongest slope of pupil dilation, and pupil dilation was largest at the time of the change in perception (Einhäuser et al., 2008). Again, in two different studies, the time of pupil dilation indicates the timing of the decision (Einhäuser et al., 2010) and surprise (Preuschoff et al., 2011). Pupillary dilation is inversely, and monotonically, related to outcome probability conditions of uncertainty, where rare stimuli trigger a larger pupil response indexing a strong relationship between pupil dilation and the feeling of surprise (Friedman et al., 1973). Also, newer research suggests that pupil dilation is directly related to decision uncertainty (Urai et al., 2017).
While the link between pupil dilation and arousal has been known for a long time (Bradshow, 1968; Hess and Polt, 1960; Simpson and Hale, 1969), only recent evidence indicates that the pupillary response may provide an index to the switch to awareness of processes that occur below the threshold of awareness (Laeng and Teodorescu, 2002). Another study, investigating visual patterns preceding the conscious recognition of ambiguous stimuli, demonstrated that eye-movements recorded prior to conscious recognition predict the later perceptual outcome, suggesting that different eye movement patterns, and therefore the underlying neuronal mechanisms, occur prior to awareness of an ambiguous object and problem solution (Kietzmann et al., 2011). A recent study presented participants with ambiguous transforming images, and while looking at these images they had to report when they recognized the object and the corresponding confidence level. Pupil dilation increased along with the recognition state of the ambiguous stimulus, and it was associated with awareness of object recognition, regardless of meta-cognitive confidence levels (Suzuki et al., 2018).
Finally, pupil dilation indirectly indexes the activity of norepinephrine-containing neurons in the brainstem nucleus locus coeruleus (LC-NE). Direct evidence that pupil dilation is associated with LC activation is demonstrated by concomitant event-driven changes in monkeys’ LC spiking activity and pupil diameter (Aston-Jones and Cohen, 2005). Further, pupil diameter covaries with neuronal activity in the cortex, reflecting modulation by the LC-NE system (Ebitz and Platt, 2015; Eldar et al., 2013; Reimer et al., 2014; Vinck et al., 2015), where LC activation precedes changes in pupil dilation (Joshi et al., 2016). Pupil diameter is also simultaneously associated with BOLD signal changes as well as LC activation patterns (Elman et al., 2017). The LC innervates brain areas involved in selective attention (e.g., parietal cortex, pulvinar nucleus, superior colliculus; Foote and Morrison, 1987). The NE system (including the LC) modulates cognitive flexibility in problem-solving, and it mediates the functional integration of the whole attentional brain system (Beversdorf et al., 1999; Campbell et al., 2008; Corbetta et al., 2008; Coull et al., 1999; Sara, 2009). Although attention and consciousness depend on different cerebral structures, and have different functions, they appear highly related(Koch and Tsuchiya, 2007). The role given to subcortical structures, as the LC and the amygdala, is that of apprising and alerting the frontal cortical areas to switch the course of the ongoing processing so as to give relevance to new stimuli or concepts (Duncan and Barrett, 2007; Gompf et al., 2010; Laeng et al., 2012; Sterpenich et al., 2006). Thus, pupil diameter change, as a marker of LC-NE activity, could index the switch into awareness that characterizes idea generation when people have an “Aha!” moment.
2. Problem-solving and pupil dilation
Hess and Polt (1964) were among the first showing that the pupil dilates, reaching the maximum diameter, immediately before a problem is solved, and then it reverts to its’ baseline size. Another seminal study found a peak in pupillary dilation at the moment of a verbalized solution on single-solution anagram problems, followed by a rapid constriction (Bradshow, 1968). Two years later, another study reported that overall pupil diameter increases especially at the later phase of the response period when participants were approaching the solution. Greater increase in dilation was associated with greater accuracy (Boersma et al., 1970). The researchers argued that changes in pupil size during problem-solving can be used as a direct measure of mental activity, and it covaries along with problem difficulty. These findings have been replicated by a large body of studies involving mathematical problems (Ahern and Beatty, 1979; Boersma et al., 1970; Bradshow, 1968; Klingner et al., 2011; Payne et al., 1968; Schaefer et al., 1968). A more recent study found that pupil dilation increases in an analogy task where participants are asked to compare figures with similar geometric structures, whereas it is not significantly associated with pupil dilation when participants solved algebra tasks (Bornemann et al., 2010). Further, differences in pupil diameter have been found when participants learned how to solve the game named “the beauty contest” gradually vs. all-at-once (Chen and Krajbich, 2017). Again, a significant increase in pupil diameter has been found during idea generation vs. normal reading and in correlation with internally directed cognition (vs. externally directed cognition) when participants worked on anagram and sentence generation tasks (Benedek et al., 2017; Walcher et al., 2017).
None of these studies directly measured insight problem-solving by asking participants if they experienced an insight, by measuring how sudden the solution came to mind, or by looking at any temporal over-lapping between pupil dilation and having an “Aha!” moment. However, they led to the hypothesis that pupil dilation may represent a physiological signature of solving a problem via insight, and index the switch into awareness that corresponds with having an Aha! moment.
3. LC/NA and attention
A complementary hypothesis driving this research regards the attention system. Former studies suggested that solutions via insight, and creative ideation in general, are characterized by internal attention allocation (for a review see Benedek et al., 2017; Kounios and Beeman 2014; Salvi and Bowden, 2016; Salvi et al., 2015). Research on the neural correlates of insight problem-solving revealed a distinctive neural activation that, among other areas, involves the vision system and led scientists to conclude that different attention allocations are involved when people solve problems via insight vs. analysis (i.e., step-by-step). For one thing, a sudden surge in alpha-frequency activity has been registered over right occipito-parietal cortex, compared to analytic solutions (Jung-Beeman et al., 2004). Given that the alpha-frequency activity over occipital cortex indexes an active suppression of input (Haegens et al., 2011; Händel et al., 2011), this burst prior to insight has been interpreted as an attention shift, away from the visual stimulus and toward internal processing, whereas solving by analysis apparently involves greater attention to external inputs. These results were corroborated by oculomotor behavior, which indicates input suppression. Specifically, higher blink duration and pattern of eye fixations oriented away from the problem were found prior to insightful (but not analytical) solutions. This putatively reflects the suppression of distracting visual inputs, allowing participants to retrieve weak internal associations that lead to solutions (Salvi et al., 2015). By contrast, stronger activity in the occipital cortex has been found during the preparatory period preceding the presentation of problems solved by analysis (Kounios et al., 2006).
A large body of results in rodents, primates, as well as in humans, demonstrated that the noradrenergic system has a determinant role in attentional shifting and behavioral flexibility (Devauges and Sara, 1990; Aston-Jones and Cohen, 2005; Bouret and Sara, 2005; Yu and Dayan, 2005; McGaughy et al., 2008). Specifically, LC-NA activation has been associated with cortical control of attention by Corbetta et al. (2008). They described two distinct functional anatomical networks underlying attention: the ventral frontoparietal network that interrupts and resets ongoing activity and is oriented to detect salient or behaviorally relevant stimuli; and the dorsal frontoparietal network that directs attention to expected stimuli connecting them to appropriate responses (Corbetta and Shulman, 2002). According to this theory, the ventral network is actively suppressed and responds only to behaviorally relevant, or unexpected, stimuli. During the reorienting of attention, outputs from the ventral attention network send “circuit-breaker” signals to the dorsal region, interrupting ongoing selective attention and shifting attention toward the novel stimulus (Corbetta et al., 2008; Corbetta and Shulman, 2002). This scenario for cortical control of attention is mediated by LC-NA system activation (Sara and Bouret, 2012). Bouret and Sara (2005) explained how the activation of the LC-NA system has a specific role in interrupting ongoing functional networks, and causes the emergence of new ones, through a “reset” in the target structures. According to the authors, NA would act as a signal from LC, leading the ventral network to “reset” the dorsal region and promote the shift in attention. Thus, LC neurons should be activated together with several elements of an arousal response and NA release in target structures, allowing the redirection of the network and switch of attention. As we mentioned above, a similar redirection of attention happens when people have an insight. An “Aha!” momentalso entails a pervasive break in a train of thought, redirecting attention toward the insightful idea. It has been seen that the noradrenergic system plays a role in problem-solving when it requires an attentional shift, or a shift in responding, from familiar to novel stimuli (Devauges and Sara, 1990). Several studies have proven the involvement of LC-NA on cognitive flexibility showing that attentional set-shifting certainly requires the noradrenergic system through its action in the medial prefrontal cortex (Lapiz and Morilak, 2006; Tait et al., 2007; McGaughy et al., 2008), and that it helps mediate the functional integration of attention systems in the brain (Beversdorf et al., 1999; Campbell et al., 2008; Sara, 2009). Experiments with mice showed how increased firing rate of LC neurons and release of NA, facilitates the ability of rats to switch between problem-solving strategies. Specifically, this was seen when the rats were required to shift attention to a different strategy and modify their behavior. Although it is not possible to measure “Aha!” moments in mice yet, we know from Kohler’s studies (1925) that when animals attempt to solve a problem they show an abrupt behavioral switch that leads to the correct solution. We know that such a switch is mediated by the LC-NA system. Considering pupil indirectly indexes the activity of LC-NA system, we expect to find a significant difference in pupil dilation at the insight moment.
Additionally, the fact that the LC plays a key role in both focusing attention and disengaging ongoing action/thought is, in itself, a good neurophysiological reason for using the pupil as a window to changes in states of consciousness (Bouret and Sara, 2005) in a discontinuous off-on problem-solving. A recent vein of research on creativity, mind wandering, imagination, etc., supports a broader idea of internal processing being associated with an “off-line mode” characterized by an internal attention allocation. (e.g., Benedek, 2018; Benedek et al., 2017; Benedek et al., 2017; Walcher et al., 2017, Konishi et al., 2017; Schooler et al., 2011; Smallwood et al., 2011; Smallwood et al., 2007; Palmiero et al., 2016). This “mode” requires a decoupling of attention from perception in order to isolate competing streams of internal and external information. Research on ocular markers associated with this off-line state revealed its association with differences in pupil diameter, microsaccades, and a peculiar eye behavior oriented towards “looking at nothing” (i.e., in an empty portion of our visual field such as a white wall) (Franklin et al., 2013; Konishi et al., 2017; Salvi and Bowden, 2016; Smallwood et al., 2011; Walcher et al., 2017). This state of visual disengagement is thought to help isolate the internal thoughts from external interfering distractions (Smallwood et al., 2007). In addition to pupil size and eye blink differences, two recent studies report this state of internally-directed cognition, and specifically that idea generation is associated with several eye-related markers such as longer eye blinks and fewer microsaccades (Benedek et al., 2017; Walcher et al., 2017).
Considering these findings, our study sought to investigate whether different problem-solving styles would be reflected in pupil size and microsaccades, thus we decided to run a second set of analysis on the data previously collected and published in Salvi et al. (2015).
In sum, we hypothesized that we would find differences in pupil dilation when people solve a problem via insight as an index of the switch into awareness. This hypothesis is based on previous studies comparing the perceptual rivalry switch recorded when looking at ambiguous figures, and the conceptual reframing switch that allows having an insight; on several studies in the problem-solving field showing that pupil dilates immediately before a problem is solved, and as an index of a switch toward internal attention mediated by LC-NE activity.
Our previous results on eye blink and fixation demonstrated that solutions via insight are associated with an off-line state of internally-directed attention, whereas solutions via analysis are paired with external attention allocation, coinciding with more fixations outside of the problem area. Since 2015, research in the creativity field confirmed our former results and found new markers of the internal/external attention distinction, such as microsaccades. Thus, we decided to further analyze our data specifically to examine pupil diameter and microsaccades (c.f., Benedek et al., 2017; Walcher et al., 2017).
4. Materials and methods
4.1. Participants
Thirty-eight students from Northwestern University were recruited (20.12; ±3.04 years old; 22 females and 16 males). Participants had normal or corrected-to-normal vision, were skilled readers, right-handed, and native speakers of American English.
In the current paper, we analyze new facets of the data collected for Salvi et al. (2015): Analyses of eye blink and fixation rates were previously published, whereas here we analyze pupil size and microsaccades.
4.2. Procedure
Stimuli were displayed binocularly to participants on a 19-inch Viewsonic E90FB CRT monitor driven at 75 Hz with a 1,024×768 pixel resolution, subtending 33.6 pixels per degree using the Eyelink Builder software. Words were shown in 28-point Times New Roman black font on a white background, with each character subtending 0.61° vertically. Three problem words were presented in a horizontal orientation, one above each other (standard) in the center of the monitor, separated by 2.04° of empty space. Each session lasted approximately 1 h.
Participants had their heads placed on a forehead-height eye-tracking apparatus 56 cm from the screen. The chin rest was removed so they could speak freely, but the forehead rest and sidebars remained in place as to keep their head still. The participants were asked to solve 120 compound remote associates (CRA) word problems (Bowden and Jung-Beeman, 2003). Each CRA problem consisted of three stimulus words (e.g., Dog, Catcher, and Fast) presented simultaneously. The participants were tasked with generating an additional word (e.g., Food) that could form a common compound word or a familiar two-word phrase within 15 s. Problems were presented in four blocks balanced for difficulty on the basis of prior data (Bowden and Jung-Beeman, 2003). Blocks order was randomized. Trials began with a prompt response, and when participants were ready a central fixation cross lasting 1000 msec was followed by the three problem words that were presented simultaneously on the screen. If participants found the problem solution within the time limit (15 s), they had to press a button and immediately report the problem solution to the experimenter. Afterward, the problem words were erased and participants had to report, via button press, whether they had solved the problem via insight or analysis. Self-reports for insight and analytic solving have been established in numerous behavioral and neuroimaging studies (e.g., Cristofori et al., 2018; Jung-Beeman et al., 2004; Kounios et al., 2006; Laukkonen and Tangen, 2018; Salvi et al., 2018; Salvi et al., 2016a,b; Santarnecchi et al., 2019; Salvi et al., 2020; Salvi and Bowden, 2019). No feedback was given to participants regarding whether the solution they had provided was correct.
Participants were given three practice CRA problems before the main battery of problems. During the practice they were instructed on how to distinguish between insight and analytic problem-solving. Insight problem-solving was described as: “The answer suddenly comes to mind even though you are unable to articulate how you achieved the solution. Sometimes this is called the Aha! moment.” Analytical problem-solving was described as: “You deliberately and consciously tested out different words until you found the solution, and you are able to report the steps that you used to reach the solution.” Participants were informed that neither solution type was superior to the other.
4.3. Eye-movement recording and data processing
Eye movements were recorded using an EyeLink 1000 Desktop Mount the EyeLink 1000 Tower Mount (SR Research, Ontario, Canada). The video eye tracker was used to record the right eye at 1 kHz. Signals of eye position were calibrated at the beginning of each experimental session, and after every 40 trials in order to reduce possible eye position measurement errors due to repositioning by the participants. The average calibration error was 0.28° (median 0.24°, standard deviation 0.18°).
Data analysis was performed with Matlab R2015b. We analyzed the oculomotor dynamics during 1000 msec fixation windows (baseline) preceding the problem presentation when participants fixated the central fixation cross that was followed by the three problem words. To match this time we analyzed the pupil dilation during the 1000 msec window preceding subjects’ responses (Figs. 1 and 2). We compared the oculomotor dynamics (eye movement characteristics and pupil size, across time), across these two windows. The data was recorded in a separate room with no windows, at a constant and controlled ambient light (levels below 0.1 cd/m2). About 20% of trials were discarded due to eye blinks.
Fig. 1.
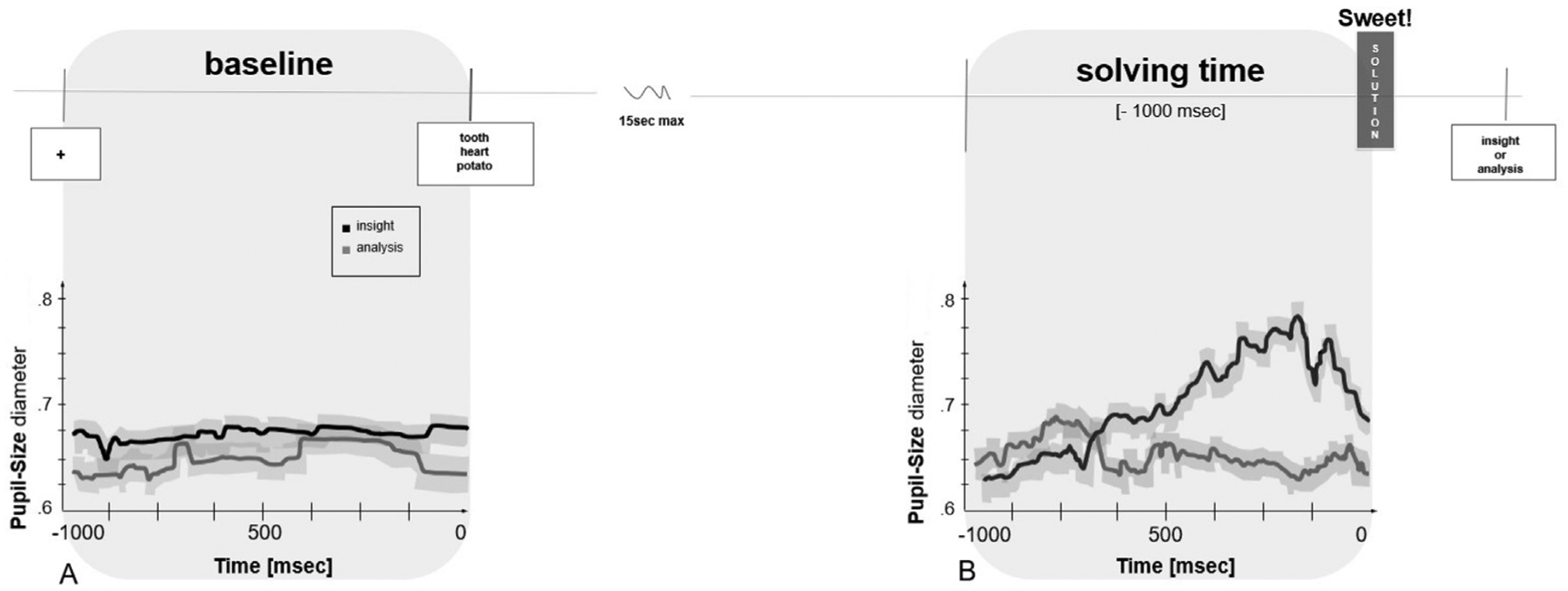
(A) Average fluctuation of pupil dilation/constriction across problems following solved via insight versus via analysis (black line and gray line respectively), within a 1-sec temporal window at fixation, preceding the appearance of the problem. (B) Average fluctuation of pupil dilation/constriction preceding the solution time (1000 msec before the button press) across problems solved via insight and via analysis (black line and gray line respectively). Thick lines denote means over participants, gray shadows represent the standard errors. Data include all the attempted problems (correct/incorrect, insight/analysis).
Fig. 2.
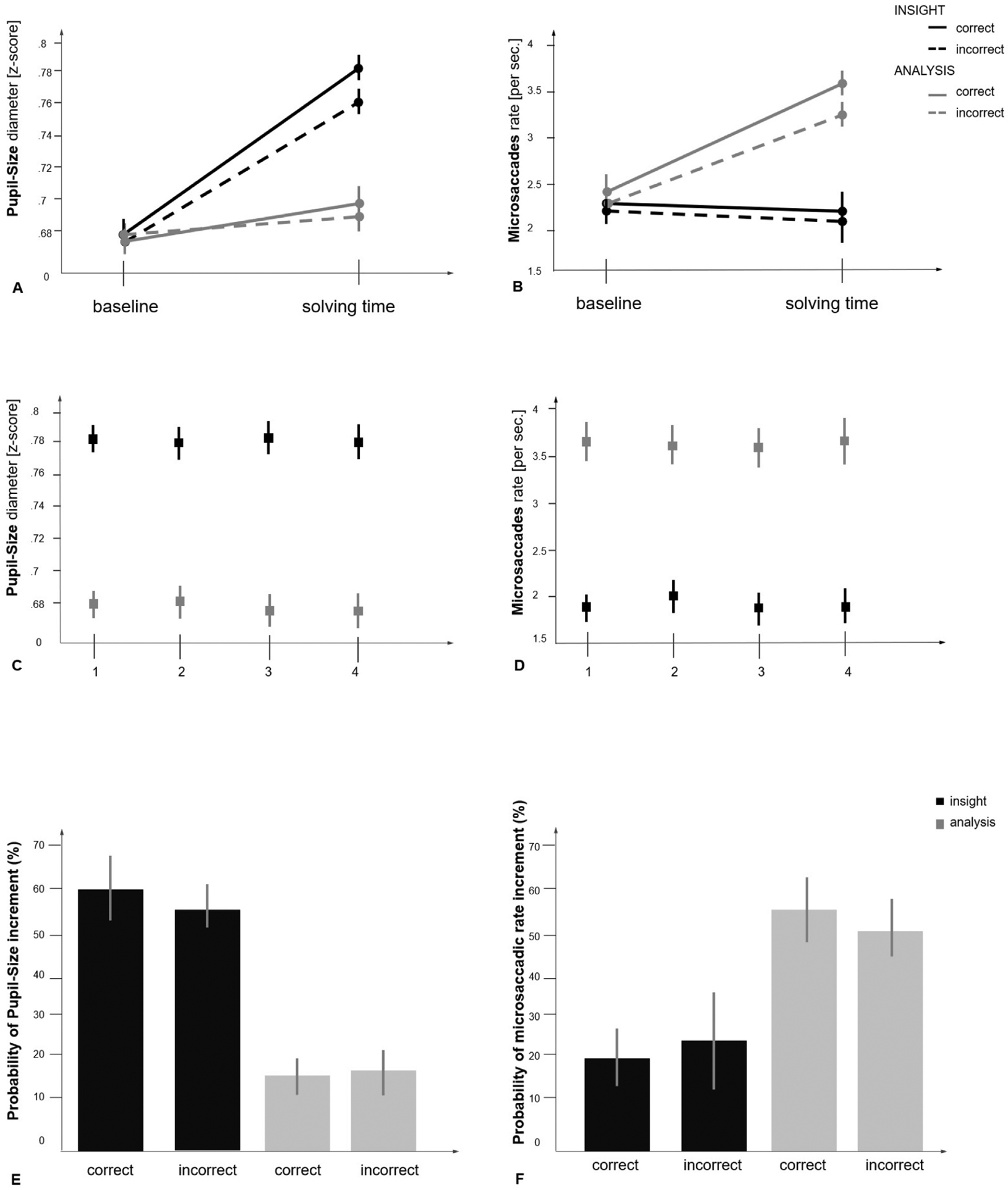
Graph A shows the average and standard error of pupil diameter at the baseline and at the solving time (1000 msec before problem-solving), within problems solved correctly and incorrectly, via insight and via analysis (respectively in black and gray). Graph B shows the average and standard error of microsaccadic rate at the baseline and at the solving time (1000 msec before problem-solving) within problems solved correctly and incorrectly via insight and via analysis (respectively in black and gray). Graph C shows the average and standard error of pupil diameter for problems solved via insight and via analysis across the four experimental blocks. No significant difference across the blocks was found (respectively in black and gray). Graph D shows the average and standard error of microsaccadic rate for problems solved via insight and via analysis across the four experimental blocks. No significant difference across the blocks was found (respectively in black and gray). Graph E shows the probability of pupil size increment across problems solved correctly and incorrectly via insight and via analysis (respectively in black and gray). Graph F shows probability of microsaccadic increment across problems solved correctly and incorrectly via insight and via analysis (respectively in black and gray).
4.4. Microsaccades detection and pupil size analysis
A microsaccade is a ballistic involuntary eye movement with the same characteristics as a saccade (Ko et al., 2010) but with a smaller amplitude. Here we define microsaccades as eye movements characterized by an amplitude between 0.08 and 1.2° and with speed higher than 3°/sec (Collewijn and Kowler, 2008; Engbert and Mergenthaler, 2006a; Ko et al., 2010). Microsaccades were detected by using a Microsaccade Toolbox for Matlab with a velocity-based algorithm (Engbert and Kliegl, 2003). The time series of eye positions were computed separately for the baseline period (1000 msec) and the solving time period (1000 msec before the subject’s answer) and transformed into 2D velocity space (see Fig. 1 for details). Separate thresholds were computed for horizontal and vertical velocities.
Pupil size was recorded using the Eyelink system that computes the true diameter of the pupil on the camera. We normalized the pupil diameters to z scores to compare data across observers. In the two temporal windows (baseline and solving time), we used the average peak of the pupil size and the corresponding standard errors (SE). To make sure that our data were not biased by blinks, we excluded all the blinks periods: from 100 ms before blink to 100 ms after each blink ended.
5. Results
Out of all the problems administered, participants offered solutions to 47.7% (mean n of responses, M = 60.2; SD = 16.2) of the problems (i.e., correct and incorrect). Within these solution attempts, 63.6% (SE 11.6) were by insight and the rest (36.4%; SE = 11.6) were solved with analysis. Among the problems solved via insight 93.7% were correct (mean n of responses, M = 29.3, SD = 11.4) and among all responses labeled as analytic, an average of 78.3% were correct (mean n of responses, M = 19.1, SD = 11.2). These results were reported also in (Salvi et al., 2015) and are in line with recent findings on the “accuracy effect” of insight problem-solving (Danek et al., 2014; Danek and Salvi, 2018; Hedne et al., 2016; Laukkonen et al., 2018; Laukkonen et al., 2018; Salvi et al., 2016a, b; Webb et al., 2016).
For the pupil analysis, we discarded all problems solved in less than 2 s, since these are thought to be considered immediate recognition and do not reflect problem-solving processing (e.g., Cranford and Moss, 2012). On average, for solutions longer than 2 s, participants solved 41.2% (mean n of responses, M = 48.1; SD = 14.3) of problems (i.e., correct and incorrect). Within these solution attempts, 65.7% (SE 11.2) were by insight and the rest (34.3%; SE = 11.2) were solved with analysis. Among the problems solved via insight 96.1% were correct (mean n of responses, M = 24.2, SD = 11.5) and among all responses labeled as analytic, an average of 80.1% were correct (mean n of responses, M = 16.3, SD = 11.7). The average response times were, respectively, 5.5 s (SD = 1.8 s) for insight and 7.1 s (SD = 2.9 s) for analysis.
We focused on time windows of 1000 msec each: A) the time immediately preceding the problem presentation while the fixation cross was stable on the screen considered as the baseline; B) the time immediately before participants button press considered as the solving time i.e., when participants might have the Aha! moment (see Fig. 1 for the time course of pupil response during these temporal windows).
As Fig. 1 shows, during the solving time period, starting ~600 msec before the participants responded (mean 550.7 msec; SE 55.3), the pupil size of problems solved via insight increased [2 (baseline vs. solving time) x 2 (insight vs. analysis) comparison F (1,37) = 28.57, p < .001, η2 = 0.81] with a peak of increment around ~200 msec before the participants responded (mean 215.7; msec SE 73.8). The result was stable for both correct p < .001, η2 = 0.39 and incorrect attempts p < .001, η2 = 0.42. Within the solution time period, pupil size is significantly higher F (1,37) = 26.41, p < .001, η2 = 0.78 for trials solved via insight compared to analysis, for correct attempts (p < .001, η2 = 0.45) and incorrect attempts (p < .001, η2 = 0.43). During the baseline time period pupil size remained stable for problems solved via insight and via analysis. Within the baseline time period, pupil size did not change for trials solved via insight compared to analysis, for correct attempts p > .05. (See Fig. 2A for the average pupil size increment and the standard error at baseline and solving time).
We also investigated variables related to internal vs. external attention allocation and visual exploration during the baseline and the solution time. Our results show an increment of microsaccades in trials solved (correctly and incorrectly) via analysis, specifically in the solution time period compared to baseline [2 (baseline vs. solving time) x 2 (insight vs. analysis) comparison F (1,37) = 35.2, p < .001, η2 = 0.83. The result was stable for both correct p < .001, η2 = 0.44 and incorrect attempts p < .001, η2 = 0.47] (Fig. 2B). Within the problem solving time period the microsaccadic rate increased significantly in trials solved via analysis compared to insight F (1,37) = 37.51, p < .001, η2 = 0.86, for both correct p < .001, η2 = 0.49 and incorrect attempts p < .001, η2 = 0.45. During the baseline time period microsaccadic rate remained stable for problems solved via insight and via analysis.
Our results are constant across the different blocks, did not change as a function of learning or habituation, and did not depend upon the difficulty of the problems presented (Fig. 2C–D).
For trials solved via insight pupil size incremented with a probability of 60.5% SE 5.2 for correct responses, and 57.5%, SE 3.4 for incorrect responses; while for trials solved via analysis pupil size incremented with a probability of 12.2%, SE 3.3 for correct responses and 14.1%, SE 4.5 for incorrect responses. (Fig. 2E). For trials solved via insight the microsaccadic rate incremented with a probability of 19.2%, SE 4.3 for correct responses, and 21.2% SE 8.3 for incorrect responses; while for trials solved via analysis the microsaccadic rate incremented with a probability of 58.7%, SE 2.3 for correct responses and 54.4%, SE 3.1 for incorrect responses. (Fig. 2F). As in much of the literature, we treated insight and analysis as independent variables although they were not directly manipulated in the design.
6. Discussion
When exposed to ambiguities we tend to search for a recognizable structure from our perceptual or imaginative representations, analogous to “connecting the dots” puzzles. Having an insight involves identifying this structure below awareness, quickly re-organize the stimulus set that suddenly engages awareness accompanied by a feeling of surprise and which is characterized by the exclamation “Aha!”. Our results allow us to speculate that pupil size is a marker of this switch into awareness.
In this study, we demonstrate that Gestalt psychologists’ conceptualization of insight problem-solving, like the structural re-organization of visual ambiguous figures, is associated with an increase of pupil diameter. The change in pupil dilation was observed regardless of insight accuracy, corroborating the idea that false insights have the same phenomenology of accurate insights (Danek and Wiley, 2017; Laukkonen et al., 2020). Separately, solutions arising from analytic processes are related to increased frequencies of microsaccades, but are not to pupil diameter.
This data completes a series of results on the eye behaviors associated with insight problem-solving. In the prior analysis, indeed, we found that insight problem-solving is associated with higher blink frequency and duration and an eye-fixations away from the problem words (vs. more fixations on the problems’ words before solutions via analysis). This sensory gating demonstrated decreasing attention to external inputs (i.e., the problem words), likely to avoid prepotent, but distracting, associations. These results led us to conclude that suppressing external inputs facilitated retrieving the non-prepotent associations that yield insights. The new results we report here allow us to provide further details to the neural and cognitive processes involved in insight problem-solving: here we speculate on a possible role played by the NE-LC system.
Because changes in pupil dilation link to NE activity in the LC, our results suggest that the LC-NE system plays a role in insight problem-solving. Together with prior studies demonstrating that the average pupil diameter deviates from the baseline before the conscious recognition of ambiguous stimuli (Einhäuser et al., 2008; Kietzmann et al., 2011), our data suggest that pupil diameter variation could be a valid index for switch into awareness of unconscious processes in insight problem-solving. This conclusion is based upon prior evidence indicating that the pupillary response is a marker of unconscious-conscious transition (Laeng and Teodorescu, 2002). LC has been demonstrated to induce, and regulate, cortical arousal increasing prior to the transition from sleep to wakefulness (Berridge, 2008 for a comprehensive review), which resembles the off-on discontinuous switch into awareness that characterizes having an “Aha!” moment. Studies using optogenetics to manipulate LC activity prove its crucial role in the sleep-wakefulness cycle as well as in behavioral and cortical arousal (Carter et al., 2010). Among other data, our previous results (Salvi et al., 2015) showed that solutions via analysis are associated with decision aspects of problem-solving, such as looking at the three target words (probably checking if the candidate solution word would fit those on the screen) whereas solutions via insight are preceded by looking outside of the problem area indexing internal attention orientation. This state of external disengagement combined with pupil dilation preceding an insight, allows us to speculate that the pupil dilation signals the switch into awareness of an idea. The current data does not allow us to determine if the feeling of insight precedes or follow pupil dilation. Yet, Chapman, Oka, Bradshaw, Jacobson, and Donaldson (1999) explicitly suggested that the cognitive aspects of the pupillary response are pre-awareness. In other words, the pupil would index processing that takes place before conscious perception and that may be needed for phenomenal awareness (cf. Block, 2005). We believe future research, using EEG/imaging coupled with eye-taking might be able to determine the sequence of physiological events paired with such a feeling.
Non-luminance-mediated pupil dilation/constriction has been used as a biomarker of arousal and cognitive effort for a long time, yet only recently it has been associated with the feeling of surprise, salience, decision biases, problem-solving, explore-exploit trade-off, and other aspects of information processing (Alnaes et al., 2014; Beatty and Kahneman, 1966; de Gee et al., 2014; Einhäuser et al., 2010, 2008; Eldar et al., 2013; Gilzenrat et al., 2010; Granholm and Steinhauer, 2004; Krugman, 1964; Lavín et al., 2014; Nassar et al., 2012; Richer and Beatty, 1987; Takeuchi et al., 2011; Wang et al., 2014). When cognitive processes, such as focused attention, learning, memory, and perception are impacted by a new state, activity of NE neuromodulatory neurons, covaries with new psychological and physiological factors, mediating the ongoing behavioral state in the central nervous system (Sara and Bouret, 2012). A wealth of experiments indeed demonstrates the crucial role played by the LC input to frontal cortex in regulating complex cognitive processes (e.g., Devauges and Sara, 1991; Birrell and Brown, 2000; Lapiz and Morilak, 2006; Tait et al., 2007; McGaughy et al., 2008). Our data enrich this literature by extending it also to insight problem-solving and corroborates prior evidence on that this switch is mediated by LC-NA activation.
Together with prior results, we demonstrate that insight is associated with a shift of attention from external to internal processes in an off-on manner (Kounios et al., 2006; Jung-Beeman et al., 2004; Salvi et al., 2015). Based on prior evidence, our data let us speculate that the LC-NA involvement in insight is implicated during the interruption of ongoing functional networks at the emergence of a new idea, causing a ‘“reset” in the target structures. NA release should be activated in target structures allowing the redirection of the network and switch of attention toward novel ideas together with several elements of an arousal response causing the classic “Aha!” feeling.
Our findings also corroborate research showing that problem-solving, creativity and idea generation are associated with a specific eye movement pattern of attention oriented to avoid visual distractors (“looking at nothing behavior”) and by an increased eye blink rate (Akbari Chermahini and Hommel, 2010; Benedek et al., 2017; Salvi et al., 2015; Walcher et al., 2017). Other eye-related biomarkers indicate that when people are deeply absorbed in thinking, there is a shift from external to internal attention and thinking by reducing cognitive load and enhancing attention to internally evolving activation (Salvi and Bowden, 2016). Pupil dilation has been found to be an index of “load on attentional capacity” (Kahneman, 1973) and many studies have clearly demonstrated a relationship between pupillary dilation and executive load or working memory load (e.g., Ahern and Beatty, 1979; Hyona et al., 1995; Kahneman and Peavler, 1969) as well as interference or competition between stimuli and/or responses [e.g. Laeng et al., 2011; Moresi et al., 2008; Siegle et al., 2008]. Similarly, studies have found that mind wandering (i.e., the involuntary slipping away of attention from an external task to an unrelated internal train of thought), is associated with characteristic changes in eye behavior such as eye blinks and spontaneous pupil activity (Franklin et al., 2013; Smallwood et al., 2011; Smilek et al., 2010) suggesting how variables related to the visual system (including pupil dilation) represent a reliable biomarker of an attentional switch from an external to internal focus such as during idea generation. External visual information may be irrelevant and even distracting to such internal activity. From these results we can conclude that pupil size and microsaccadic rate represent reliable predictors of the problem-solving strategy used in tasks such as ours.
Our current finding on microsaccades is consistent with this literature. Previous studies demonstrated that microsaccades are an index of attention to spatial location (Engbert and Mergenthaler, 2006b; Engbert and Kliegl, 2003; Galfano et al., 2004; Hafed and Clark, 2002; Rolfs et al., 2008). Thus, we speculate that increased microsaccade rate in solutions via analysis is due to external attention allocation. This conclusion is supported by findings that microsaccade rate increased in conditions that involved complex/meaningful sources of stimuli such as natural vs. blank scene, or faces vs. non-faces images, and when cognitive/attentional demands increased, such as in tasks as “Where’s Waldo” vs. free visual exploration. In accordance they see that exploration of a blank scene, was conducive to longer fixation periods, yet low microsaccade rates (Otero-millan et al., 2008).
Acknowledgements
This research was supported by a grant from the Smart Family Foundation of New York to JG. CS was supported in part by NIH training grant T32 NS047987, and in part by the United States Air Force Research Laboratory FA8650-15-2-5518 to MB. We thank Steve Franconeri and his lab for the equipment, lab material, and advice provided to run this study.
References
- Ahern S, Beatty J, 1979. Pupillary responses during information processing vary with scholastic aptitude test scores. Science. 10.1126/science.472746. [DOI] [PubMed] [Google Scholar]
- Akbari Chermahini S, Hommel B, 2010. The (b)link between creativity and dopamine: spontaneous eye blink rates predict and dissociate divergent and convergent thinking. Cognition 115 (3), 458–465. 10.1016/j.cognition.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Alnaes D, Sneve MH, Espeseth T, Endestad T, van de Pavert SHP, Laeng B, 2014. Pupil size signals mental effort deployed during multiple object tracking and predicts brain activity in the dorsal attention network and the locus coeruleus. J. Vis 10.1167/14.4.1. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD, 2005. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci 28, 403–450. 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Beatty J, Kahneman D, 1966. Pupillary changes in two memory tasks. Psychonomic Sci. 10.3758/BF03328444. [DOI] [Google Scholar]
- Benedek M, 2018. Internally directed attention in creative cognition In: Jung R, Vartanian O (Eds.), The Cambridge Handbook of the Neuroscience of Creativity. Cambridge University Press. [Google Scholar]
- Benedek M, Stoiser R, Benedek M, Stoiser R, Walcher S, Körner C, 2017. Eye behavior associated with internally versus externally directed cognition. Front. Psychol (July) 10.3389/fpsyg.2017.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beversdorf DQ, Hughes JD, Steinberg BA, Lewis LD, Heilman KM, 1999. Noradrenergic modulation of cognitive flexibility in problem-solving. In: Neuroreport, vol. 10 Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/10511436. [DOI] [PubMed] [Google Scholar]
- Boersma F, Wilton K, Barham R, Miur W, 1970. Effects of arithmetic problem difficulty on pupillary dilation in normals and educable on retardates. J. Exp. Child Psychol 9, 142–155. [DOI] [PubMed] [Google Scholar]
- Bornemann B, Foth M, Horn J, 2010. Mathematical Cognition: Individual Differences in Resource Allocation, pp. 555–567. 10.1007/s11858-010-0253-x. [DOI] [Google Scholar]
- Bouret S, Sara SJ, 2005. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 28, 574–582. [DOI] [PubMed] [Google Scholar]
- Bowden EM, Jung-Beeman M, 2003. Normative data for 144 compound remote associate problems. Behav. Res. Methods Instrum. Comput.: J. Psychon. Soc. Inc 35 (4), 634–639. [DOI] [PubMed] [Google Scholar]
- Bradshow J, 1968. Pupillary changes and reaction time with varied stimulus uncertainty. Psychonomic Sci. 13 (2), 69–70. [Google Scholar]
- Campbell HL, Tivarus ME, Hillier A, Beversdorf DQ, 2008. Increased task difficulty results in greater impact of noradrenergic modulation of cognitive flexibility. Pharmacol. Biochem. Behav 88 (3), 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H, Kowler E, 2008. The significance of microsaccades for vision and oculomotor control. J. Vis 10.1167/8.14.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, 2002. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci 3 (1), 201–215. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL, 2008. The reorienting system of the human brain: from environment to theory of mind. Neuron. 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Büchel C, Friston KJ, Frith CD, 1999. Noradrenergically mediated plasticity in a human attentional neuronal network. Neuroimage 10 (6), 705–715. 10.1006/nimg.1999.0513. [DOI] [PubMed] [Google Scholar]
- Cristofori I, Salvi C, Beeman M, Grafman J, 2018. The effects of expected reward on creative problem solving. Cognit. Affect Behav. Neurosci 5 (18), 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danek AH, Fraps T, von Müller A, Grothe B, Öllinger M, 2014. Working wonders? Investigating insight with magic tricks. Cognition 130 (2), 174–185. 10.1016/j.cognition.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Danek H, Salvi C, 2018. Moment of truth: why aha! Experiences are correct. J. Creativ. Behav 10.1002/jocb.380, 0, 1–3. [DOI] [Google Scholar]
- Danek Amory H., Wiley J, 2017. What about False Insights? Deconstructing the Aha! Experience along its Multiple Dimensions for Correct and Incorrect Solutions Separately, vol. 7, pp. 1–14. 10.3389/fpsyg.2016.02077. January. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danek AH, Wiley J, 2017. What about False Insights ? Deconstructing the Aha ! Experience along Its Multiple Dimensions for Correct and Incorrect Solutions Separately. Front. Psychol (January), 1–14. 10.3389/fpsyg.2016.02077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gee JW, Knapen T, Donner TH, 2014. Decision-related pupil dilation reflects upcoming choice and individual bias. In: Proceedings of the National Academy of Sciences. 10.1073/pnas.1317557111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devauges V, Sara SJ, 1990. Activation of the noradrenergic system facilitates an attentional shift in the rat. Behav. Brain Res 39, 19–28. [DOI] [PubMed] [Google Scholar]
- Duncan S, Barrett LF, 2007. The role of the amygdala in visual awareness. In: Trends in Cognitive Sciences. 10.1016/j.tics.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebitz RB, Platt ML, 2015. Neuronal activity in primate dorsal anterior cingulate cortex signals task conflict and predicts adjustments in pupil-linked arousal. Neuron. 10.1016/j.neuron.2014.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhäuser W, Koch C, Carter OL, 2010. Pupil dilation betrays the timing of decisions. Front. Hum. Neurosci 4 (February), 18 10.3389/fnhum.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhäuser W, Stout J, Koch C, Carter O, 2008. Pupil dilation reflects perceptual selection and predicts subsequent stability in perceptual rivalry. Proc. Natl. Acad. Sci. U. S. A 105 (5), 1704–1709. 10.1073/pnas.0707727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar E, Cohen JD, Niv Y, 2013. The effects of neural gain on attention and learning. Nat. Neurosci 10.1038/nn.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman JA, Panizzon MS, Hagler DJ, Eyler LT, Granholm EL, Gennema-Notestine C, Lyons MJ, McEvoy LK, Franz CE, Dale AM, Kremen WS, 2017. Task-evoked pupil dilation and BOLD variance as indicators of locus coeruleus dysfunction. Cortex 97, 60–69. 10.1016/j.cortex.2017.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbert R, Mergenthaler K, 2006. Microsaccades are triggered by low retinal image slip. Proc. Natl. Acad. Sci. Unit. States Am 103 (18), 7192–7197. 10.1073/pnas.0509557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbert Ralf, Kliegl R, 2003. Microsaccades uncover the orientation of covert attention. Vis. Res 43 (9), 1035–1045. 10.1016/S0042-6989(03)00084-1. [DOI] [PubMed] [Google Scholar]
- Foote SL, Morrison JH, 1987. Development of the noradrenergic, serotonergic, and dopaminergic innervation of neocortex. Curr. Top. Dev. Biol 10.1016/S0070-2153(08)60145-3. [DOI] [PubMed] [Google Scholar]
- Franklin MS, Broadway JM, Mrazek MD, Smallwood J, Schooler JW, 2013. Window to the wandering mind: pupillometry of spontaneous thought while reading. Q. J. Exp. Psychol 66 (12), 2289–2294. 10.1080/17470218.2013.858170,2006. [DOI] [PubMed] [Google Scholar]
- Friedman D, Hakerem G, Sutton S, Fleiss JL, 1973. Effect of stimulus uncertainty on the pupillary dilation response and the vertex evoked potential. Electroencephalogr. Clin. Neurophysiol 34 (5), 475–484. [DOI] [PubMed] [Google Scholar]
- Galfano G, Betta E, Turatto M, 2004. Inhibition of return in microsaccades. In: Experimental Brain Research 10.1007/s00221-004-2111-y. [DOI] [PubMed] [Google Scholar]
- Gilzenrat MS, Nieuwenhuis S, Jepma M, Cohen JD, 2010. Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cognit. Affect Behav. Neurosci 10 (2), 252–269. 10.3758/CABN.10.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompf HS, Mathai C, Fuller PM, Wood DA, Pedersen NP, Saper CB, Lu J, 2010. Locus ceruleus and anterior cingulate cortex sustain wakefulness in a novel environment. J. Neurosci 10.1523/JNEUROSCI.3037-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Steinhauer SR, 2004. Pupillometric measures of cognitive and emotional processes. Int. J. Psychophysiol 10.1016/j.ijpsycho.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Haegens S, Nacher V, Luna R, Romo R, Jensen O, 2011. α-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. In: Proceedings of the National Academy of Sciences of the United States of America, vol. 108, pp. 19377–19382, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Clark JJ, 2002. Microsaccades as an overt measure of covert attention shifts. Vis. Res 42 (22), 2533–2545. 10.1016/S0042-6989(02)00263-8. [DOI] [PubMed] [Google Scholar]
- Händel B, Haarmeier T, Jensen O, 2011. Alpha oscillations correlate with the successful inhibition of unattended stimuli. J. Cognit. Neurosci 23 (9), 2494–2502. [DOI] [PubMed] [Google Scholar]
- Hedne MR, Norman E, Metcalfe J, 2016. Intuitive feelings of warmth and confidence in insight and noninsight problem solving of magic tricks. Front. Psychol 7, 1314 10.3389/fpsyg.2016.01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess EH, Polt JM, 1964. Pupil size in relation to mental activity during simple problem-solving. Science 143 (3611), 1190–1192. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/17833905. [DOI] [PubMed] [Google Scholar]
- Hess Eckhard H., Polt JM, 1960. Pupil size as related to interest value of visual stimuli. Science 349–350. 10.1126/science.132.3423.349. [DOI] [PubMed] [Google Scholar]
- Hyona J, Tommola J, Alaja A, 1995. A pupil dilation as a measure of processing load in simultaneous interpretation and other language tasks pupil dilation as a measure of processing load in simultaneous interpretation and other langu. Q. J. Exp. Psychol. Sect A 598–612. 10.1080/14640749508401407. [DOI] [PubMed] [Google Scholar]
- Joshi S, Li Y, Kalwani RM, Gold JI, 2016. Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron 89 (1), 221–234. 10.1016/j.neuron.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung-Beeman M, Bowden EM, Haberman J, Frymiare JL, Arambel-Liu S, Greenblatt R, et al. , 2004. Neural activity when people solve verbal problems with insight. PLoS Biol. 2 (4), 500–510. 10.1371/journal.pbio.0020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D, 1973. Attention and effort. In: Cliffs E (Ed.), p. 1063 10.2307/1421603. [DOI] [Google Scholar]
- Kahneman D, Peavler WS, 1969. Incentive effects and pupillary changes in association learning. J. Exp. Psychol 79, 312–318 (2, Pt.1(Feb 1969). [DOI] [PubMed] [Google Scholar]
- Kietzmann TC, Geuter S, Ko P, 2011. Overt Visual Attention as a Causal Factor of Perceptual Awareness, 6 10.1371/Citation (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingner J, Tversky B, Hanrahan P, 2011. Effects of visual and verbal presentation on cognitive load in vigilance, memory, and arithmetic tasks. Psychophysiology. 10.1111/j.1469-8986.2010.01069.x. [DOI] [PubMed] [Google Scholar]
- Ko HK, Poletti M, Rucci M, 2010. Microsaccades precisely relocate gaze in a high visual acuity task. Nat. Neurosci 10.1038/nn.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C, Tsuchiya N, 2007. Attention and consciousness: two distinct brain processes. Trends Cognit. Sci 10.1016/j.tics.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Kohler W, 1925. The Mentality of Apes, second ed Harcourt Brace, New York. [Google Scholar]
- Konishi M, Brown K, Battaglini L, Smallwood J, 2017. When attention wanders: pupillometric signatures of fluctuations in external attention. Cognition 168, 16–26. 10.1016/j.cognition.2017.06.006, 2017. [DOI] [PubMed] [Google Scholar]
- Kounios J, Beeman M, 2014. The cognitive neuroscience of insight. Annu. Rev. Psychol 65 (1), 71–93. 10.1146/annurev-psych-010213-115154. [DOI] [PubMed] [Google Scholar]
- Kounios J, Frymiare JL, Bowden EM, Fleck JI, Subramaniam K, Parrish TB, Jung-beeman M, 2006. The prepared mind: neural activity prior to problem presentation predicts subsequent solution by sudden insight. Psychol. Sci 17 (10), 882–890. 10.1111/j.1467-9280.2006.01798.x. [DOI] [PubMed] [Google Scholar]
- Krugman HE, 1964. Some applications of pupil measurement. J. Market. Res 15–19. 10.2307/3150372. [DOI] [Google Scholar]
- Laeng B, Ørbo M, Holmlund T, Miozzo M, 2011. Pupillary stroop effects. Cognit. Process 10.1007/s10339-010-0370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeng B, Sirois S, Gredebäck G, 2012. Pupillometry: a window to the preconscious? Perspect. Psychol. Sci 7 (1), 18–27. 10.1177/1745691611427305. [DOI] [PubMed] [Google Scholar]
- Laeng B, Teodorescu D-S, 2002. Eye scanpath during visual imagery reenact those of perception of the same visual scene. Cognit. Sci 26, 207–231. [Google Scholar]
- Laukkonen RE, Schooler JW, Tangen JM, 2018. The Eureka Heuristic: Relying on Insight to Appraise the Quality of Ideas. (February), 1–44. 10.17605/OSF.IO/EZ3TN. [DOI] [Google Scholar]
- Laukkonen RE, Tangen JM, 2018. How to detect insight moments in problem solving experiments. Front. Psychol 9 (March), 1–5. 10.3389/fpsyg.2018.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukkonen R, Tangen JM, 2017. Can observing a Necker cube make you more insightful? Can observing a Necker cube make you more insightful? Conscious. Cognit 48 (January), 198–211. 10.1016/j.concog.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Morilak DA, 2006. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience 137, 1039–1049. [DOI] [PubMed] [Google Scholar]
- Laukkonen RE, Kaveladze BT, Tangen JM, Schooler JW, 2020. The dark side of Eureka: Artificially induced Aha moments make facts feel true. Cognition 196, 0–5 (January 2019). 10.1016/j.cognition.2019.104122. [DOI] [PubMed] [Google Scholar]
- Lavín C, San Martín R, Rosales Jubal E, 2014. Pupil dilation signals uncertainty and surprise in a learning gambling task. In: Frontiers in Behavioral Neuroscience. 10.3389/fnbeh.2013.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield D, Ball LJ, 2011. Using another’s gaze as an explicit aid to insight problem solving. Q. J. Exp. Psychol 64 (4), 649–656. 10.1080/17470218.2011.558628. 2006. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Ross RS, Eichenbaum H, 2008. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience 153, 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moresi S, Adam JJ, Rijcken J, Van Gerven PWM, Kuipers H, Jolles J, 2008. Pupil dilation in response preparation. Int. J. Psychophysiol 10.1016/j.ijpsycho.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Nassar MR, Rumsey KM, Wilson RC, Parikh K, Heasly B, Gold JI, 2012. Rational regulation of learning dynamics by pupil-linked arousal systems. Nat. Neurosci 10.1038/nn.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson S, 1992. Information-processing explanations of insight and related phenomena. Adv. Psychol. Thinking 1, 1–44. Retrieved from. http://ci.nii.ac.jp/naid/10013544833/. [Google Scholar]
- Otero-millan J, Troncoso XG, Macknik SL, Serrano-pedraza I, Martinez-conde S, 2008. Saccades and microsaccades during visual fi xation , exploration , and search: foundations for a common saccadic generator. J. Vis 8 (14), 1–18. 10.1167/8.14.21.Introduction. [DOI] [PubMed] [Google Scholar]
- Payne DT, Parry ME, Harasymiw SJ, 1968. Percentage of pupillary dilation as a measure of item difficulty. In: Perception & Psychophysics. 10.3758/BF03210453. [DOI] [Google Scholar]
- Palmiero M, Piccardi L, Nori R, Palermo L, Salvi C, Guariglia C, 2016. Creativity and mental imagery. Front. Psychol 7 (AUG) 10.3389/fpsyg.2016.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoff K, Marius B, Einhäuser W, 2011. Pupil dilation signals surprise: evidence for noradrenaline ‘ s role in decision making MATERIALS AND METHODS. Front. Neurosci 5 (September), 1–12. 10.3389/fnins.2011.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer J, Froudarakis E, Cadwell CR, Yatsenko D, Denfield GH, Tolias AS, 2014. Pupil fluctuations track Fast switching of cortical states during quiet wakefulness. Neuron 10.1016/j.neuron.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richer F, Beatty J, 1987. Contrasting effects of response uncertainty on the task-evoked pupillary response and reaction time. Psychophysiology. 10.1111/j.1469-8986.1987.tb00291.x. [DOI] [PubMed] [Google Scholar]
- Rolfs M, Kliegl R, Engbert R, 2008. Toward a model of microsaccade generation: the case of microsaccadic inhibition. J. Vis 8 (11) 10.1167/8.11.5, 5–5. [DOI] [PubMed] [Google Scholar]
- Salvi C, Beeman M, Bikson M, McKinley R, Grafman J, 2020. TDCS to the right anterior temporal lobe facilitates insight problem-solving. Sci. Rep 10 (1), 946 10.1038/s41598-020-57724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi C, Bricolo E, Franconeri SL, Kounios J, Beeman M, 2015. Sudden insight is associated with shutting out visual inputs. Psychon. Bull. Rev 22 (6), 1814–1819. 10.3758/s13423-015-0845-0. [DOI] [PubMed] [Google Scholar]
- Salvi C, Bricolo E, Kounios J, Bowden EM, Beeman M, 2016. Insight solutions are correct more often than analytic solutions. Think. Reas 22 (4), 1–18. 10.1080/13546783.2016.1141798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi C, Bowden E, 2019. The relation between state and trait risk taking and problem-solving. Psychol. Res 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi C, Bowden E, 2016. Looking for creativity: where do we look when we look for new ideas? Front. Psychol 10.3389/fpsyg.2016.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi C, Costantini G, Pace A, Palmiero M, 2018. Validation of the Italian remote associate test. J. Creativ. Behav 1–13. 10.1002/jocb.345, 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi C, Cristofori I, Grafman J, Beeman M, 2016. The politics of insight. Q. J. Exp. Psychol (February), 1–19. 10.1080/17470218.2015.1136338. 0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi E, Sprugnoli G, Bricolo E, Constantini G, Liew SL, Musaeus CS, Salvi C, Pascual-Leone A, Rossi A, Rossi S, 2019. Gamma tACS over the temporal lobe increases the occurrence of Eureka! moments. Sci. Rep 10.1038/s41598-019-42192-z (March), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ, 2009. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci 10 (3), 211–223. 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Schaefer T, Ferguson JB, Klein JA, Rawson EB, 1968. Pupillary responses during mental activities. Psychonomic Sci. 10.3758/BF03331236. [DOI] [Google Scholar]
- Schooler JW, Smallwood J, Christoff K, Handy TC, Reichle ED, Sayette M.a., 2011. Meta-awareness, perceptual decoupling and the wandering mind. Trends Cognit. Sci 15 (7), 319–326. 10.1016/j.tics.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Shen W, Tong Y, Yuan Y, Zhan H, Liu C, Luo J, 2018. Feeling the insight: uncovering somatic markers of the “ aha ” experience. Appl. Psychophysiol. Biofeedback 43 (1), 13–21. 10.1007/s10484-017-9381-1. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Ichikawa N, Steinhauer S, 2008. Blink before and after you think: blinks occur prior to and following cognitive load indexed by pupillary responses. Psychophysiology 45 (5), 679–687. 10.1111/j.1469-8986.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- Simpson M, Hale S, 1969. Pupillary changes during a decisionm making task. Percept. Mot. Skills 29 (2), 495–498. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Brown KS, Tipper C, Giesbrecht B, Franklin MS, Mrazek MD, et al. , 2011. Pupillometric evidence for the decoupling of attention from perceptual input during offline thought. PloS One 6 (3), e18298 10.1371/journal.pone.0018298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, McSpadden M, Schooler JW, 2007. The lights are on but no one’s home: meta-awareness and the decoupling of attention when the mind wanders. Psychon. Bull. Rev 14 (3), 527–533. 10.3758/BF03194102. [DOI] [PubMed] [Google Scholar]
- Smilek D, Carriere JSA, Cheyne JA, 2010. Out of mind, out of sight: eye blinking as indicator and embodiment of mind wandering. Psychol. Sci 21 (6), 786–789. 10.1177/0956797610368063. [DOI] [PubMed] [Google Scholar]
- Sterpenich V, D’Argembeau A, Desseilles M, Balteau E, Albouy G, Vandewalle G, et al. , 2006. The locus ceruleus is involved in the successful retrieval of emotional memories in humans. J. Neurosci 10.1523/JNEUROSCI.1001-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Minami T, Nakauchi S, 2018. Association between pupil dilation and implicit processing prior to object recognition via insight. Sci. Rep 1–10. 10.1038/s41598-018-25207-z (December 2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Puntous T, Tuladhar A, Yoshimoto S, Shirama A, 2011. Estimation of mental effort in learning visual search by measuring pupil response. PloS One. 10.1371/journal.pone.0021973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait DS, Brown VJ, Farovik A, Theobald DE, Dalley JW, Robbins TW, 2007. Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur. J. Neurosci 25, 3719–3724. [DOI] [PubMed] [Google Scholar]
- Thomas LE, Lleras A, 2009. Covert shifts of attention function as an implicit aid to insight. Cognition 111 (2), 168–174. 10.1016/j.cognition.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Urai A, Braun A, Donner T, 2017. Pupil-linked arousal is driven by decision uncertainty and alters serial choice bias. Nat. Commun 8, 14637 10.1038/ncomms14637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M, Batista-Brito R, Knoblich U, Cardin JA, 2015. Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 10.1016/j.neuron.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcher S, Körner C, Benedek M, 2017. Looking for ideas: eye behavior during goal-directed internally focused cognition ☆. Conscious. Cognit 53 (March), 165–175. 10.1016/j.concog.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-A, Boehnke SE, Itti L, Munoz DP, 2014. Transient pupil response is modulated by contrast-based saliency. J. Neurosci 10.1523/jneurosci.3550-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb ME, Little DR, Cropper SJ, 2016. Insight is not in the problem: investigating insight in problem solving across task types. Front. Psychol 7, 1424 10.3389/fpsyg.2016.01424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AJ, Dayan P, 2005. Uncertainty, neuromodulation, and attention. Neuron 46, 681–692. [DOI] [PubMed] [Google Scholar]


