Abstract
Neurological disorders are commonly reported among veterans who returned from the Gulf war. Veterans who suffer from Gulf War illness (GWI) complain of continued symptom persistence that includes neurological disorders, muscle weakness, headaches, and memory loss, that developed during or shortly after the war. Our recent research showed that chemical exposure associated microbial dysbiosis accompanied by a leaky gut connected the pathologies in the intestine, liver, and brain. However, the mechanisms that caused the symptoms to persist even 30 years after the war remained elusive to investigators. In this study, we used a rodent model of GWI to investigate the persistence of microbiome alterations, resultant chronic inflammation, and its effect on neurotrophic and synaptic plasticity marker BDNF. The results showed that exposure to GW chemicals (the pesticide permethrin and prophylactic drug pyridostigmine bromide) resulted in persistent pathology characterized by the low relative abundance of the probiotic bacteria Akkermansia muciniphila in the gut, which correlated with high circulatory HMGB1 levels, blood-brain barrier dysfunction, neuroinflammation and lowered neurotrophin BDNF levels. Mechanistically, we used mice lacking the NLRP3 gene to investigate this inflammasome’s role in observed pathology. These mice had significantly decreased inflammation and a subsequent increase in BDNF in the frontal cortex. This suggests that a persistently low species abundance of Akkermansia muciniphila and associated chronic inflammation due to inflammasome activation might be playing a significant role in contributing to chronic neurological problems in GWI. A therapeutic approach with various small molecules that can target both the restoration of a healthy microbiome and decreasing inflammasome activation might have better outcomes in treating GWI symptom persistence.
Keywords: Dysbiosis, Akkermansia muciniphila, BDNF, RAGE, 3-nitrotyrosine, peroxynitrite, inflammasomes, NLRP3
Introduction
Neurological disorders are commonly reported among veterans who returned from the Gulf war (GW) of 1990-1991.1-3 Afflicted veterans complain of problems including neuralgias, migraine headaches, muscle weakness and coordination, and memory problems.2,4,5 These issues occur in combination with other Gulf War illness (GWI) symptoms, and their pathology is not very well understood. Most veterans who suffer from GWI developed their symptoms during or shortly after the war, and these symptoms persist 30 years later. Although the causes of these symptoms are difficult to pinpoint, epidemiological studies have established a compelling link between these symptoms in different GW veteran cohorts and environmental exposures which occurred during the war, or chemicals that were applied to the warriors before or shortly after the war.6-8 Such exposures include dust from desert storms, depleted uranium, combustion byproducts from oil wells, possible chemical weapons, pesticides, vaccines, and prophylactic medicines such as pyridostigmine bromide (PB).9-12
In recent years, research has focused on studying symptoms as well as elucidating mechanisms of these disorders, using GW veteran cohorts as well as animals and in vitro studies. For example, Van Riper et al13 reported widespread disruption in white matter microstructure distribution across brain regions involved in the processing and modulating chronic pain. James et al found that there was a significant positive correlation between C-reactive protein (CRP), pain, and neurocognitive mood in GW veterans. Another study by Abou-Donia et al14 reported elevated autoantibodies to neurons and other brain cells, eg, tau proteins, glial acidic fibrillary protein (GFAP), and myosin basic protein (MBP) which indicate neuronal injury or gliosis in GW veterans. In other recent animal studies, Zakirova et al15 found cognitive deficits in mice several weeks after treatment with GW chemicals, and these deficits were associated with increased astrogliosis and a reduction in synaptophysin in mouse hippocampi and cerebral cortex. Furthermore, Madhu et al16 found that cognitive impairments which persisted 10 months post-exposure to GW chemicals were associated with increased density of activated microglia and astrocytes in rats and inflammation with elevated levels of HMGB1 in the cerebral cortex.
These studies provide evidence that exposure to GW chemicals plays a significant role in the persistence of neurological dysfunction. This may generally be through the disruption of neuronal networks, reactive glia, which fuel inflammation or weak neuronal growth and neuroplasticity. Many neurological disorders such as Parkinson’s disease (PD), Alzheimer’s disease (AD), bipolar disorders, and neuropathic pain17-21 are associated with decreased levels of neurotrophins or impairments in their signaling pathways.22,23 These disorders also commonly present with chronic neuroinflammation.24-26 Brain-derived neurotropic factor (BDNF) is the most prevalent neurotrophins in the brain and has been very widely studied in several diseases and brain functions.23,27 It is produced by neurons, and it plays crucial functions in neuroplasticity, growth, and survival of neurons.28
To date, our previous research has focused mainly on the possible role of an altered microbiome (bacteriome and virome) in contributing to a persistent inflammatory phenotype, in acute models of GWI.29-32 We proposed that exposure to GW chemicals alters the microbiome in rodents, which then drives inflammation through the production of immunostimulatory particles, ie, damage-associated molecular pattern (DAMPS) and pathogen-associated molecular patterns (PAMPS). These DAMPS may then continuously trigger inflammation in different organ systems. Although our results largely supported our hypothesis, we were still limited in knowing whether the observed changes and mechanisms in the microbiome and associated chronic inflammation due to an altered microbiome indeed persisted.
In this present study, we used a persistence rodent model of GWI in which mice were exposed to GW chemicals for 2 weeks (representing the war phase) after which no further GW chemicals were applied for the next 20 weeks (to represent 20 years after the war). We then investigated the persistence of microbiome alterations, chronic inflammation, and its effect on neuronal health (BDNF levels). We further used an NLRP3 KO mouse to study its potential role of this inflammasome as a primary contributor to the observed neuroinflammation.
Materials and Methods
Materials
We purchased PB and permethrin from Sigma-Aldrich (St. Louis, MO). Anti-RAGE, anti-Claudin 5, anti-HMGB1, anti-IL-1β, and anti-ASC-2 were purchased from Santacruz Biotechnology (Dallas, TX), Anti-BDNF from cell signaling technology (Danvers, MA), while anti-NLRP3, anti-3-nitrotyrosine, anti-IL-6, anti-IL-18, anti-TMEM 119 primary antibodies were purchased from Abcam (Cambridge, MA). Species specific biotinylated conjugated secondary antibodies and Streptavidin-HRP (Vectastain Elite ABC kit) were purchased from Vector Laboratories (Burlingame, CA). Fluorescence conjugated (Alexa Fluor) secondary antibodies, ProLong Diamond antifade mounting media with DAPI and Pierce LAL chromogenic endotoxin quantitation kit were bought from Thermo Fisher Scientific (Waltham, MA) while enzyme-linked immunosorbent assay (ELISA) kits were purchased from ProteinTech (Rosemond, IL). Unless otherwise specified, all other chemicals used were purchased from Sigma. Paraffin-embedding of tissue and sectioning were done by AML laboratories (Baltimore, MD) and at the Instrument Resources Facility, University of South Carolina School of medicine (Columbia, SC). Microbiome analysis was done by Cosmos ID (Rockville, MD).
Animal experiments
Adult (10 weeks old) wild type male (C57BL/6J mice) and NLRP3 deficient adult (10 weeks) male (B6N.129-Nlrp3tm3Hhf/J) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Mice experiments were implemented in accordance with National Institutes of Health (NIH) guidelines for humane care and use of laboratory animals and local Institutional Animal Care and Use Committee (IACUC) standards. All procedures were approved by the University of South Carolina at Columbia, SC. Mice were housed individually and fed on a chow diet at 22°C to 24°C with a 12 h light/12 h dark cycle. All mice were sacrificed after animal experiments had been completed. Right after anesthesia, blood from the mice was drawn using cardiac puncture, to preserve serum for further experimentation. Their brains were removed immediately, and the frontal cortex dissected out and was fixed using Bouin’s fixative solution. We also collected the fecal pellets and luminal contents for microbiome analysis.
Treatments and rodent model of Gulf War illness
Mice were exposed to GW chemicals (PB and permethrin) based on established rodent models of GWI with some modifications.31,33-35 The treated mice group (GWP) and NLRP3KO (GWP-NLRP3KO) mice group were dosed tri-weekly for 2 weeks with PB (2 mg/kg) and permethrin (200 mg/kg) by oral gavage, after which no further treatments with GW chemicals were applied, and mice were fed on a normal chow diet for 20 weeks. The control group (CONT) of mice received vehicle (0.6% dimethyl sulfoxide in phosphate-buffered saline [PBS]) by oral gavage as in other experiments above. Each group had a starting sample size of n = 6 until the end of the experiments. All experimental mice had an average starting weight of 26.5 grams and a final weight of 33.5 grams at the end of the study. There was no signifcant difference of weight difference between controls and Gulf War chemical treated groups over the 20 week period.
Microbiome analysis
Microbiome analysis was done by CosmosID (Rockville, MD, USA) from fecal pellets and luminal contents, which were collected from the animals of each group after sacrifice. DNA isolation, sequencing, and analysis of gut microbiome were performed according to vendor optimized protocol. Briefly, DNA was isolated from fecal samples using the ZymoBIOMICS Miniprep kit, following the manufacturer’s instructions. 16S sequencing was carried out on the V3V4 (341 nt–805 nt) region of the 16S ribosomal RNA (rRNA) gene with a 2-step polymerase chain reaction (PCR) strategy. First, PCR was performed using 16S-optimized primer set to amplify the V3–V4 regions of 16S ribosomal DNA (rDNA) within the metagenomic DNA. Then the PCR products from the previous steps were mixed at equal proportions and used as templates in the second step to produce Illumina dual-index libraries for sequencing, with both adapters containing an 8bp index allowing for multiplexing. The dual-indexed library amplification products are purified using Ampure beads (Beckman Coulter). Library quantification was performed using Qubit dsDNA HS assay (Thermo Fisher) and qualified on a 2100 Bioanalyzer instrument (Agilent) to show a distribution with a peak in the expected range. A final qualitative PCR (qPCR) quantification was performed before loading onto a MiSeq (Illumina) sequencer for PE250 (v2 chemistry). The sequences for each sample were then run on the 16S pipeline of the CosmosID GENIUS software, and results were analyzed.
Laboratory Methods
Immunohistochemistry
The fixed brain tissues were embedded in paraffin and sliced into 5 μM thick sections. These sections were deparaffinized following optimized standard protocols. Epitope retrieval solution and steamer (IHC-Word, Woodstock, MD) were used for epitope retrieval for deparaffinized sections. About 3% H2O2 was used for the recommended time to block the endogenous peroxidase. After serum blocking, the primary antibodies were applied at recommended and optimized concentrations. Species-specific biotinylated conjugated secondary antibodies and streptavidin conjugated with HRP were used to implement antigen-specific immunohistochemistry. 3,3'-Diaminobenzidine (DAB) (Sigma Aldrich, St Louis, MD) was used as a chromogenic substrate. Mayer’s hematoxylin solution (Sigma Aldrich) was used as a counterstain. Sections were washed between steps using PBS 1×. Finally, stained sections were mounted in Simpo-mount (GBI Laboratories, Mukilteo, WA). Tissue sections were observed using Olympus BX63 microscope (Olympus, America). Cellsens software from Olympus America (Center Valley, PA) was used for morphometric analysis of images.
Immunofluorescence staining
Paraffin-embedded sections were deparaffinized using the standard protocol. Epitope retrieval solution and steamer were used for epitope retrieval of sections. Primary antibodies were used at recommended dilutions. Species-specific secondary antibodies conjugated with Alexa Fluor (633-red and 488-green) were used at advised dilution. In the end, the stained sections were mounted using prolong diamond antifade reagent with DAPI. Sections were observed under Olympus fluorescence microscope BX63 using 20X, 40X, 60X objective lens.
Real-time quantitative PCR
Total RNA was isolated from frontal cortex tissue homogenization in TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions and purified with the use of RNeasy mini kit columns (Qiagen, Valencia, CA, USA). Complementary DNA (cDNA) was synthesized from purified RNA (1 µg) using iScript cDNA synthesis kit (Bio-rad, Hercules, CA, USA) following the manufacturer’s standard protocol. Real-time qPCR (qRTPCR) was performed with the gene-specific primers using Sso Advanced SYBR Green Supermix and CFX96 thermal cycler (Bio-rad, Hercules, CA, USA). Threshold cycle (Ct) values for the selected genes were normalized against respective samples internal control 18S. Each reaction was carried out in triplicates for each gene and for each sample. The relative fold change was calculated by the 2−∆∆Ct method. The sequences for the primers used for real-time PCR are provided in Table 1.
Table 1.
Primer sequences.
| Primer | Sequence |
|---|---|
| mm-Claudin 5 |
Sense: TTCGCCAACATTGTCGTCC Antisense: TCTTCTTGTCGTAGTCGCCG |
| mm-18 S |
Sense: TTCGAACGAACGTCTGCCCTATCAA Antisense: ATGGTAGGCACGGCGATA |
Endotoxin level detection by Litmus Amebocyte Lysate assay
Serum bacterial endotoxin levels (EU/mL) were detected using the Pierce LAL Chromogenic Endotoxin Quantification Kit (Waltham, MA) according to the manufacturer’s instructions. Briefly, serum samples were obtained from mice and diluted 1:80 with endotoxin-free water. The endotoxins were then quantified.
Western blot analysis
About 30 mg of tissue from each brain tissue sample was immediately homogenized in 300 μL of RIPA buffer with protease and phosphatase inhibitors cocktail (Pierce, Rockford, IL) using slow speed mechanical homogenizer. The homogenate was centrifuged, and the supernatant was collected and saved for experimental use. About 30 μg of denatured protein from each sample was loaded per well of Novex 4% to 12% bis-tris gradient gel (Life Technologies, Carlsbad, CA) and subjected for standard sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Separated protein bands were transferred to nitrocellulose membrane using precut nitrocellulose/filter paper sandwiches (Bio-Rad, Hercules, CA) and Trans-Blot Turbo transfer system (Bio-Rad) using 30-minute transfer protocol. Furthermore, blots were blocked with 3% bovine serum albumin solution prepared in Tris-buffered saline with 0.05% tween-20 (TBS-T). Primary antibodies were used at recommended dilutions in 1.5% blocking buffer and incubated overnight at 4°C. Species-specific anti-IgG secondary antibody conjugated with HRP were used at recommended dilutions in 1% blocking buffer and incubated for 2 h at room temperature. Pierce ECL Western Blotting substrate (Thermo Fisher Scientific Inc, Rockford, IL) was used in dark to develop the blot. Finally, the blot was imaged using G:Box Chemi XX6 (Syngene imaging systems) and subjected to densitometry analysis using Image J software.
ELISA
Serum IL-1β, IL-6, and TNF-α were estimated using ELISA kits from ProteinTech (Rosemont, IL) according to manufacturer protocol.
Statistical analysis
We conducted calculations for each experimental condition prior to initiation of the study. Preliminary data confirmed that the sample size was enough to achieve a minimum statistical power of 0.80 at an alpha of 0.05. One-way analysis of variance (ANOVA) was used with post hoc comparisons among different exposure conditions or treatments (eg, least significant differences [LSD] and Bonferroni correction) to compare means among multiple groups. Student t tests was used to compare means between two groups at the termination of treatment. Correlative associations were tested using Pearson’s correlation coefficient analysis with Graph pad prism software (GraphPad Software Inc, La Jolla, CA). A p value of less than p=0.05 was considered statistically significant.
Results
Gulf War chemical exposure results in a decreased relative abundance of Akkermansia muciniphila, which negatively correlates with increased circulatory HMGB1 levels
Our previous studies have strongly suggested that exposure to GW chemicals alters the microbiome and these alterations may contribute to the persistence of GWI symptoms through the release of DAMPs and PAMPs.30-32 In this study, we analyzed the microbiome for alterations in specific bacterial species that have a notable role in inflammation persistence in chronic diseases of the gut, metabolic reprogramming, and neuronal deficiencies. We analyzed 10 distinct bacterial species that had a fold change difference in abundance and has been found to contribute to inflammation and metabolic responses (Figure 1A) We found that mice treated with GW chemicals (GWP) had a significantly lower abundance of A muciniphila (significant, Figure 1B, *P = .008; n = 5), Bacteroides thetaiotomicron, and Dorea Sp (not significant) when compared with mice treated with only vehicle control (Figure 1A). Notably, A muciniphila has been associated with several health benefits.36-38 Furthermore, we found that mice which were treated with GW chemicals (GWP) had significantly higher HMGB1 levels in their serum compared with mice treated with vehicle control only (CONT) *P = .034; n = 6 (Figure 1C and D). We then carried out statistical analyses to determine whether the increased levels of HMGB1 were related to the observed decreased relative abundance of A muciniphila. In Figure 1E, we found that there was a negative correlation between A muciniphila abundance and circulatory HMGB1 levels (Pearson’s r = −0.50; R2 COD = 0.255).
Figure 1.
Exposure to GW chemicals results in decreased relative abundance of Akkermansia muciniphila and chronic high levels of circulatory HMGB1. (A) Percentage abundance of gut bacteria species. Percentage abundance of 10 most abundant species in the gut bacteriome are represented comparing GW chemical treated groups (GWP) to vehicle control treated group groups (CONT). Data are represented as the mean of 6 mice per group. (B) Percentage relative abundance of A muciniphila. Percentage relative abundance was determined from duplicate fecal samples of 5 mice per group treated with GW chemicals (GWP) compared with mice treated with vehicle control only (CONT). Data are represented as mean ± SEM (*P < .05; n = 5). (C) Serum HMGB1 levels. Western blot of HMGB1 levels in serum for mice treated with GW chemicals (GWP) compared with mice treated with vehicle control (CONT) only. Data are represented as mean ± SEM. (D) Densitometry of HMGB1 immunoblots, normalized against Ponceau red (*P < .05; n = 6). (E) Relationship between A muciniphila and circulatory HMGB1 levels. A correlative analysis was carried out to determine how A muciniphila is related to serum HMGB1 levels. We found a negative correlation between A muciniphila and serum HMGB1 levels (Pearson’s r = −0.50; R2 COD = 0.255 shaded area represents 95% confidence bands). GW indicates Gulf War; SEM, standard error of the mean; COD, coefficient of dipersion.
Exposure to GW chemicals is associated with blood-brain barrier tight junction protein dysregulation, and the changes persist five months after exposure
The study by Abou-Donia et al14 suggests the presence of a leaky blood-brain barrier (BBB) among veterans who returned from the GW, and this may be a portal for immunostimulatory particles such as DAMPS and PAMPS to continuously fuel neuroinflammation. We studied the messenger RNA (mRNA) and protein expression levels of Claudin 5, the major tight junction protein in the complex that makes up the BBB. We found that GWP mice also exhibited significantly lower Claudin 5 mRNA and protein levels compared with vehicle control treated mice (CONT) P = .042 and P = .03; n = 6 (Figure 2A to C). Furthermore, we studied the levels of Claudin 5 in the BBB by observing colocalizations between Claudin 5 and CD31, a marker for endothelial cells that make up the lining of the blood vessels (Figure 2D and E). We found that there was a significant decrease in the number of colocalizations (yellow spots) constituting Claudin 5 and CD31 in GWP mice compared with controls (CONT) (P = .04; n = 6). This result points to the fact that at least one major component of BBB integrity is repressed at the protein level, paving the way for possible dysfunctional BBB, and this may lead to the passage of DAMPS such as HMGB1 leaking into the brain and triggering several immune responses.
Figure 2.
Exposure to GW chemicals is associated with altered Claudin 5 levels in the frontal cortex. (A) Claudin 5 mRNA levels in the frontal cortex: mRNA levels of Claudin 5 as studied by RTqPCR show that mice exposed to GW chemicals (GWP) had significantly decreased Claudin 5 mRNA levels compared with mice treated with vehicle control only (CONT). (B) Claudin 5 protein levels in frontal cortex. Western blot analysis of Claudin 5 protein levels in CONT and GWP treated mice. (C) Morphometry analysis of Claudin 5 immunoblots, normalized against β-actin (*P < .05; n = 6). Data are represented as mean ± SEM. (D) Representative immunofluorescence micrographs of the blood-brain barrier showing colocalization of tight junction protein Claudin 5 (labeled in red) and endothelial cell marker CD31 (labeled in green) as yellow spots around a BBB (magnification 60× and scale bar 10 µm) and DAPI stained nucleus (labeled in blue). See Supplemental Figure S2 for images of separate channels. Inset (magnification 40× and scale bar 20 µm) shows the whole micrograph field, from which the main image was obtained. (E) Quantitative morphometry analysis of colocalizations for every 100 cells represented as % ROI (*P < .05; n = 6). Data are represented as mean ± SEM. BBB indicates blood-brain barrier; GW, Gulf War; SEM, standard error of the mean; ROI, region of interest.
GW chemical exposure is associated with persistent activation of microglia via the HMGB1-RAGE pathway resulting in increased reactive oxygen species and triggering of the NLRP3 inflammasome
Chronic neurological disorders such as AD and PD are characterized by activation of immune cells such as microglia, the resident macrophages of the brain.39 These cells may be activated by the presence of pathogens or DAMPS, such as HMGB1. We studied the protein expression levels of activated microglia marker TMEM119. The results showed that there was a significant increase in activated microglia in the frontal cortex of mice treated with GW chemicals (GWP) compared with controls (CONT) (*P = .02; n = 6) (Figure 3A and B) even after 3 months of exposure. Furthermore, we found that there was evidence of activated HMGB1-RAGE signaling, as indicated by colocalization events. Figure 3C and D shows a significantly high expression of RAGE protein levels (*P = .04; n = 6) and subsequent increased RAGE-HMGB1 colocalizations (*P = .001; n = 6) in GWP mice compared with controls (Figure 3E and F). RAGE is a receptor, which binds several ligands including HMGB1. Interaction of HMGB1-RAGE can lead to increasing reactive oxygen species (ROS) generation, eg, peroxynitrite, which reacts with tyrosine in proteins to form the stable adduct 3 nitrotyrosine. In Figure 4A and B, we detected significantly higher levels 3-nitrotyrosine (3NT) in GW chemical treated mice (GWP) compared with vehicle control treated mice (CONT); (*P = .01; n = 6). We also found that mice exposed to GW chemicals had significantly higher inflammasome activation compared with vehicle control treated mice (CONT) Figure 4C and D (*P < .001; n = 6). This activation was detected as yellow dots indicating a colocalization between the NLRP3 protein complex (labeled with red antibody) and the adapter protein ASC2 (marked with green), which facilitate the processing of pro-inflammatory cytokines from their basal inactive to more active form via protein cleavage.
Figure 3.
Activation of macrophages and associated HMGB1/RAGE complex formation. (A) TMEM119 immunoreactivity in frontal cortex. Representative immunohistochemistry micrographs of TMEM 119 reactivity in control (CONT) and GW chemical treated (GWP) mice (magnification 40× and scale bar 50 µm). (B) Morphometric analysis (represented as % ROI) obtained from 10 to 15 images from different microscopy fields from each mouse sample. Data are represented as mean ± SEM (*P < .05; n = 6). (C) Western blots of RAGE protein levels in the frontal cortex of GWP and CONT treated mice. (D) Morphometry analysis of all immunoblots normalized against β-actin (n = 5) (*P < .05; n = 6). Data are represented as mean ± SEM. (E) RAGE/HMGB1 complex formation. HMGB1 (labeled in red) and RAGE (labeled in green) and DAPI stained nucleus (labeled in blue) in GWP and CONT mice. Colocalizations are shown as yellow dots and marked with arrows in the micrographs (magnification 40× and scale bar 20 µm). See Supplemental Figure S3 for images of separate channels. (F) Morphometric analysis (represented as % ROI) obtained from 6 to 8 images from different microscopy fields from each mouse sample. Data are represented as mean ± SEM. (*P < .05; n = 6). GW indicates Gulf War; SEM, standard error of the mean; ROI, region of interest.
Figure 4.
Increased ROS is associated with NLRP3 inflammasome activation. (A) 3-nitrotyrosine immunoreactivity in frontal cortex. Representative immunohistochemistry micrographs of 3-nitrotyrosine reactivity in control (CONT) and GW chemical treated (GWP) mice (magnification 40× and scale bar 50 µm). (B) Morphometric analysis (represented as % ROI) obtained from 10 to 15 images from different microscopy fields from each mouse sample. Data are represented as Mean ± SEM (*P < .05; n = 6). (C) Immunofluorescence micrographs showing activation of NLRP3 inflammasome protein. NLRP3 (labeled red) ASC2 (labeled in green) and DAPI stained nucleus (labeled in blue) in GWP and CONT mice. Colocalizations are shown as yellow dots and marked with arrows in the micrographs. See Supplemental Figure S4 for images of separate channels. Magnification 60× and scale bar 20 µm. (D) Morphometric analysis (represented as % ROI) obtained from 6 to 8 images from different microscopy fields from each mouse sample. Data are represented as mean ± SEM (*P < .05; n = 6). GW indicates Gulf War; ROS, reactive oxygen species; SEM, standard error of the mean; ROI, region of interest.
Exposure to GW chemicals, decreased abundance of A muciniphila is associated with a persistently increased neuroinflammation and low levels of BDNF
Inflammasomes are large immune complexes found in several cell types and are responsible for processing the inflammatory cytokines IL-1β and IL-18 by cleaving them from their precursors.40 Uncontrolled activation of these complexes can lead to chronic inflammation, as has been found in cancer, diabetes, and neurodegenerative disease.41-44 In our study, we found that NLRP3 inflammasome activation was also significantly associated with increased IL-1β (Figure 5A and B; P = .001, n = 6), IL-18 (Figure 5C and D; P = .021, n = 6), and IL-6 (Figure 5E and F; P = .01, n = 6) protein levels in GWP mouse frontal cortex when compared with mice treated with only vehicle control (CONT) (Figure 5A and F). Neurodegenerative diseases are often characterized by neuroinflammation, accompanied by decreased levels of neurotrophins.18,45 Similarly, in this study, we observed that mice which were treated with GW chemicals had significantly lower levels of the neurotrophin BDNF when compared with mice treated with only vehicle control (CONT) (Figure 5G and H, P = .018 and Figure 5I and J, P = .001 n = 6).
Figure 5.
GW chemical exposure is associated with chronic neuroinflammation and decreased brain-derived neurotrophic factor (BDNF) levels in frontal cortex.
(A, C, E) Immunohistochemistry micrographs of frontal cortex tissues of GWP and CONT treated mice showing immunoreactivity of IL-1β, IL-18, and IL-6 (magnification 20× and scale bar 50 µm). (B, D, F) Morphometric analysis (as % ROI) obtained from 10 to 15 images from different microscopic fields from each mouse sample. (*P < .05; n = 6). Data are represented as mean ± SEM. (G) Western blots of BDNF protein levels in the frontal cortex of GWP and CONT treated mice. (H) Morphometry analysis of immunoblots normalized against β-actin (n = 5) (*P < .05; n = 5). Data are represented as mean ± SEM. (I) BDNF immunoreactivity. Representative immunohistochemistry micrographs showing BDNF in frontal cortex tissues of GWP and CONT treated mice (magnification 40× and scale bar 50 µm). (J) Morphometric analysis (represented as % ROI) obtained from 10 to 15 images from different microscopy fields from each mouse sample (*P < .05; n = 6). Data are represented as mean ± SEM. BDNF indicates brain-derived neurotrophic factor; GW, Gulf War; SEM, standard error of the mean; ROI, region of interest.
A muciniphila relative abundance correlates with BDNF levels and persistent neuroinflammation
A high abundance of A muciniphila has been linked to decreased inflammation in chronic diseases.38,46,47 To study whether the host bacteria’s abundance played a role in affecting chronic inflammation and sustained BDNF levels in our model of GWI, we carried out correlative analyses to determine whether there were statistically significant relationships between the bacterial abundance, inflammation, and BDNF levels. The results showed that there was a significant positive correlation between BDNF levels and abundance of A muciniphila (Pearson’s r = 0.83, R2 COD = 0.73; P = .0024), and a significant negative correlation with IL-1β protein levels (Pearson’s r = −0.684; R2 COD = 0.46; P = .02) (Figure 6A and B). Shaded area represents 95% confidence bands.
Figure 6.
The decreased relative abundance of A muciniphila correlates with IL-1β and BDNF levels in the frontal cortex. (A) Correlation between A muciniphila and IL-1β levels in GW chemical (GWP) and vehicle control (CONT) treated mice. We carried out a linear regression analysis to determine the relationship between IL-1β and A muciniphila in GWP and CONT mice. There was a negative correlation between A muciniphila and IL-1β in the FC (Pearson’s r = −0.68; R2 COD = 0.46 and P = .02). (B) Correlation between A muciniphila and BDNF levels in GW chemical (GWP) and vehicle control (CONT) treated mice. We carried out a linear regression analysis to determine the relationship between BDNF and A muciniphila in GWP and CONT mice. There was a positive correlation between A muciniphila relative abundance and BDNF levels (Pearson’s r = 0.83, R2 COD = 0.7 and P = .0024). BDNF indicates brain-derived neurotrophic factor; GW, Gulf War; COD, coefficient of dispersion.
Deletion of NLRP3 is protective against persistent systemic and neuroinflammation and is associated with an increase in BDNF levels
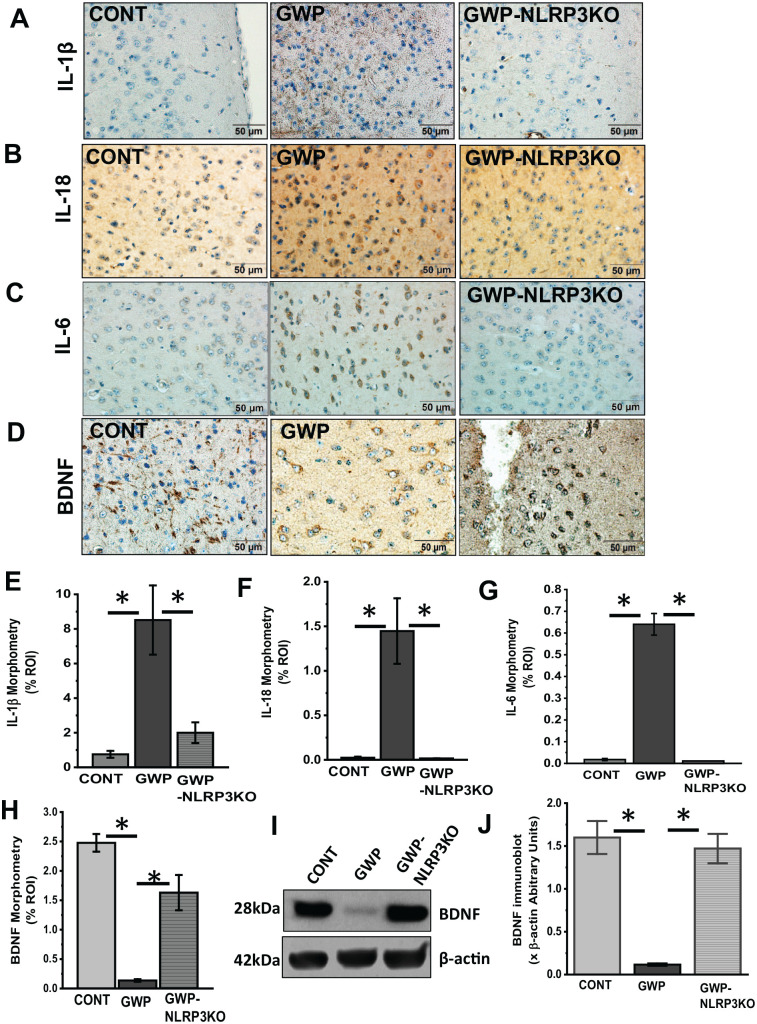
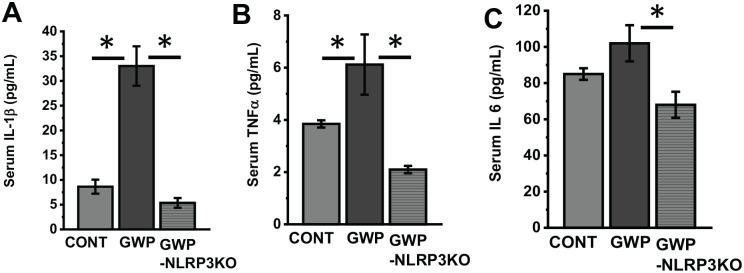
To study the role of NLRP3 in driving inflammation and lowering BDNF levels, we treated mice lacking NLRP3 with GW chemicals and then subjected them to our experimental conditions for 20 weeks or 5 months. We then studied the protein levels of IL-1β, IL-18, IL-6, and BDNF by western blot analysis and immunohistochemistry. Our results show that there were significantly lower levels of IL-1β (Figure 7A and E; P = .006, n = 6, IL-18 (Figure 7B and F; P = .01, n = 6), IL-6 (Figure 7C and G; P = .015, n = 6), and increased BDNF (Figure 7D and H; P = .007, and Figure 7I and J; P = .017, n = 6) levels in the frontal cortex of NLRP3 KO mice treated with GW chemicals (GWP-NLRP3KO), when compared with wild type mice treated with GW chemicals (GWP). Furthermore, we studied systemic inflammation levels in the three groups of mice by analyzing serum IL-1β (Figure 8A; P = .046, n = 6), TNF-α (Figure 8B; P = .032, n = 6) and IL-6 (Figure 8C; P = .042, n = 6) levels using an ELISA. Our results show that there is significantly lower inflammation in NLRP3KO mice treated with GW chemicals (GWP-NLRP3KO) compared with mice treated with GW chemicals (GWP).
Figure 7.
Deletion of NLRP3 is associated with decreased neuroinflammation and lower BDNF levels. (A to D) IL-1β, IL-18, IL-6, and BDNF immunoreactivity in the frontal cortex. Representative immunohistochemistry micrographs showing IL-1β, IL-18, IL-6, and BDNF, respectively, in frontal cortex tissues of GW chemical treated (GWP), GW chemical treated NLRP3KO (GWP-NLR3KO), and vehicle control (CONT) treated mice (magnification 40× and scale bar 50 µm). (E to H) Morphometric analysis (represented as % ROI) obtained from 10 to 15 images from different microscopy fields from each mouse sample. Data are represented as mean ± SEM. (*P < .05, n = 6). (I) Western blots of BDNF protein levels in the frontal cortex of GWP and CONT treated mice. (J) Morphometry analysis of all immunoblots normalized against β-actin. Data are represented as mean ± SEM (*P = .05, n = 5). BDNF indicates brain-derived neurotrophic factor; GW, Gulf War; SEM, standard error of the mean.
Figure 8.
Deletion of NLRP3 is associated with decreased serum cytokine levels. (A to C) Serum cytokine levels. IL-1β, TNF-α, and IL-6 levels, respectively, determined by ELISA in the serum of vehicle control (CONT), GW chemical treated (GWP) and GW chemical treated NLRP3KO mice (GWP-NLRP3KO). Data are represented as mean ± SEM (*P < .05, n = 6). GW indicates Gulf War; SEM, standard error of the mean.
Discussion
In our previous studies, we reported that there was a general alteration in microbiome accompanied by endotoxemia, a leaky gut, and inflammation in different organs such as the small intestine, brain, and liver.30-32 We found significant increases in phyla Firmicutes and Tenericutes over Bacteriodetes in GW chemical exposed mice when compared with controls.31 However, it was not clear whether these alterations persisted long after GW chemical exposure or would eventually resolve over time through the repopulation and reconstitution of the host microbiome. We also were eager to study the mechanisms that would connect the altered microbiome and the persistent inflammatory changes in the intestine and the neural-immune network. In this study, mice were exposed to the GW chemicals and allowed to ad libitum diet and water for 20 weeks. This was to simulate the period of exposure (during the GW) and the subsequent period following their return from the war. We found that exposure to GW chemicals (the pesticide permethrin and prophylactic drug PB) in mice resulted in persistent pathology characterized by the low abundance of A muciniphila, high circulatory HMGB1 levels, BBB dysfunction, neuroinflammation, and lowered neurotrophin BDNF levels. Our findings and the proposed mechanism are summarized as a schematic illustration in Figure 9.
Figure 9.

Schematic illustration of the proposed mechanism for persistent neuroinflammatory pathology in current study.
We report that exposure to GW chemicals caused a decrease in A muciniphila or resulted in conditions that favor other bacteria populations repopulate over A muciniphila (Figure 1A). A muciniphila is a mucin degrading bacterium which exists as part of the normal human gut flora and is abundant in healthy individuals.47-50 In recent years, the herein reported bacterium is emerging as an important probiotic which can be consumed to improve health.51 This bacterium was found to improve ulcerative colitis in mice52 and restored colonic mucus layer thickness with decreased inflammation in aging mice.53 In another study, the abundance of this bacterium inversely correlated with inflammation and altered lipid metabolism in obese mice.51 Although the mechanism by which A muciniphila promotes these health benefits is not fully understood, studies report that the bacterium strengthens gut barrier integrity through its association with enterocytes and also produces high amounts of anti-inflammatory cytokine IL-8.48,54 It is possible that the low levels of this bacterium in the gut compromised gut barrier integrity, a condition that we have observed in our previous acute models of GWI.29,31 This condition of compromised gut barrier integrity has also been reported among veterans who suffer from gastrointestinal problems in GWI.55 Moreover, a recent study by Janulewicz et al56 showed that GWI afflicted veterans with gastrointestinal disturbances present gut dysbiosis among bacteria of the phylum Verrucomicrobia. Interestingly, A muciniphila belongs to the same phylum. Another study found that A muciniphila treatment normalized diet-induced metabolic endotoxemia, adiposity, and the adipose tissue marker CD11c in obese mice, which otherwise had increased inflammatory indicators in the intestine and aided in the metabolic disease development.46 Similarly, our studies of GWI mouse models have consistently found that altered levels of tight junction proteins in the gut was associated with increase in endotoxins and DAMPs such as HMGB1 and inflammation that was related to an alteration of gut microbiome abundance.29,32 Notably, mice in the present study (using a persistence model of GWI) that were exposed to similar chemicals showed a slight increase in serum endotoxin levels (low-level endotoxemia consistent with an obesity phenotype) in GW chemical treated mice when compared with vehicle control treated mice (Supplemental Figure S1). In addition, a significant increase in circulatory HMGB1 levels was observed in GW chemical exposed mice which negatively correlated with A muciniphila relative abundance (Figure 1C to E) suggesting that a sustained and consistent low inflammatory trigger was closely associated with a persistent change in the microbiome and decreased A Muciniphila abundance in these mice. Furthermore, the persistence of systemic inflammatory indicators such as pro-inflammatory cytokines, eg, IL-1β, IL-6, and TNF-α (Figure 8A to C), endotoxin levels and serum HMGB1 for such a long period that failed to ease even 20 weeks after exposure (equivalent to > 20 human years) and its connection with an altered microbiome triggered our interest to study their effects on neuronal structures and their networks.
Before we could study the neuroinflammatory indicators for persistence, we needed to assess the integrity of the BBB, a vital interface of neuronal physiology and pathology. Interestingly, our results showed that the expression of Claudin 5, a critical tight junction protein of the BBB in the brain, was decreased in the frontal cortex of mice treated with GW chemicals compared with controls (Figure 2A to E). This protein, together with others such as Claudin 1, zona occludens, and occludins make up tight junctions in the BBB. The BBB is a selective barrier found at the interface of blood vessels in the brain and brain tissue. It is made of a single cell layer of endothelial cells, astrocyte, and pericytes. Its unique properties allow it to tightly regulate the movement of particles between the circulation and brain tissue.57,58 Claudin 5 and other tight junction proteins are found between adjacent endothelial cells of blood vessels and help to anchor these cells to create a tightly regulated selective barrier that allows the passage of particles between the blood and the brain.59 Low levels of this protein have been found in neurodegenerative and neuroinflammatory diseases such as AD, PD, and schizophrenia.60,61 We found decreased mRNA and protein levels of this protein in GWP mice compared with controls (Figure 2A to E). This provides strong evidence that at least one key component of the BBB is dysregulated and this possibly compromised the barrier’s integrity, likely caused by the serum mediators endotoxins, HMGB1, and pro-inflammatory cytokines causing it to become leaky. However, we have no direct evidence of such an event in an in vitro experimental setup using BBB endothelial cells. We hypothesized that this leaky BBB allowed the passage of unwanted particles such as DAMPs and PAMPS, HMGB1, as one such example, which we found to be greatly increased in the serum. It is also worth noting that even though serum endotoxin levels are not significantly higher in GWP mice compared with controls, even low levels of endotoxins over a long time can be a toxic stimulus to the body and may contribute to observed pathology.62,63
HMGB1 is a DAMP known to trigger proinflammatory pathways through toll-like receptors (TLRs), eg, TLR4, and through the receptor for advanced glycation end products (RAGE).64,65 We found that there was increased activation of microglia in GWP mice compared with controls (Figure 3A and B) with an increased expression of RAGE receptors in the frontal cortex (Figure 3C and D). We also detected HMGB1/RAGE complex formation using immunofluorescence microscopy, which may indicate activation of RAGE signaling (Figure 3E and F). The activation of this pathway is known to result in the transcription of pro-inflammatory cytokines and the generation of ROS66 such as nitric oxide. High levels of nitric oxide in the presence of superoxide could result in the formation of peroxynitrite, a potent ROS which attacks tyrosine to form 3 nitrotyrosine denaturing them and rendering them dysfunctional. We found significantly higher levels of ROS in our GWP mice compared with controls (Figure 4A and B). High amounts of ROS are a known trigger of the NLRP3 inflammasome.67 Inflammasomes are large protein complexes that are assembled in response to infections, etc, and are involved in the processing of pro-inflammatory cytokines such as IL-1β and IL-18. We found that mice treated with GW chemicals had higher expression of NLRP3/ASC2 complex formation compared with controls (Figure 4C and D). Although inflammasomes are triggered as a defense mechanism, chronic activation of these complexes has been implicated in conditions characterized by chronic low-grade inflammation such as diabetes, PD, and ALS.68,69 This activation of the NLRP3 inflammasomes was followed by increased IL-1β and IL-18 levels in the brain (Figure 5A to D), which might contribute to the persistent neuroinflammation in GWI.
In neurological diseases, inflammation has been associated with poor neuronal health, with fewer neurons, decreased neuronal plasticity, and growth.70 This in part is due to low levels of neurotrophins such as BDNF. BDNF is a neurotrophin produced by neurons and is involved in neuronal growth, survival, and plasticity.23 Studies by Guan and Fang, and another by Lapchak, suggest that increased IL-1β levels interfered with BDNF synthesis,71,72 while Tong et al showed that increased IL-1β interfered with BDNF signaling through the PI3K/AKT pathway by preventing its activation of AKT. The above mechanisms resulted in decreased growth and survival of neurons.73 In this study, we report low levels of BDNF in the frontal cortex of mice, which were treated with GW chemicals compared with controls even 5 months post-exposure (Figure 5F and G). We also studied any correlations that BDNF, IL-1β with A muciniphila relative abundance may have to connect the intestinal, microbiome changes, and neuronal levels of these mediators referenced above. In Figure 6A and B, we show that A muciniphila relative abundance correlated negatively with IL-1β levels and positively with BDNF levels. The above result indicates that A muciniphila relative abundance might have played a role in modulating neuroinflammation and neurotrophin levels in GWI as has been the case in obesity and other diseases described previously.46 This could be through the bacterium’s production of anti-inflammatory compounds that counter inflammation or modulation of the intestinal barrier integrity. However, more studies need to be done to determine the exact mechanism. Finally, to study the role of NLRP3 inflammasome in contributing to neuroinflammation that persists for a prolonged period and its association with increased abundance of Akkermansia, we used a mouse model with the systemic knockout of NLRP3 gene (KO mouse). The results found that the deletion of this gene was associated with increased BDNF levels and protected the mice from neuroinflammation (Figure 7A to J). Deleting NLRP3 is protective through preventing inflammasome activation and subsequent processing of pro-inflammatory cytokines and distinctly proves that the persistent inflammation in GWI chemical exposed mice that had altered A muciniphila abundance is due to NLRP3-mediated inflammasome activation though there may be multiple molecular mediators for triggering such an activation. Our current study identifies some of these mediators such as gut-derived endotoxins, HMGB1, or peroxynitrite, to name a few. Still, the molecular mechanism of such a trigger remains speculative currently.
In conclusion, we report that persistence of GWI inflammatory symptoms is characterized by low relative abundance of A muciniphila and chronic high circulatory HMGB1 levels which trigger NLRP3 mediated neuroinflammation and decreased levels of neurotrophins through RAGE signaling as shown in Figure 9. These findings not only provide insight into the mechanism of persistent neurological disturbances in GWI but also provide possible therapeutic targets. First, the microbiome can be targeted through replacement or enhancing of A muciniphila gut bacterial populations and other significantly lowered probiotic bacteria, and second, through therapies that target RAGE signaling or NLRP3 inflammasomes to relieve persistent inflammation and improve quality of life of veterans who suffer from GWI veterans.
Supplemental Material
Supplemental material, Supplimental_material for Host Akkermansia muciniphila Abundance Correlates With Gulf War Illness Symptom Persistence via NLRP3-Mediated Neuroinflammation and Decreased Brain-Derived Neurotrophic Factor by Diana Kimono, Dipro Bose, Ratanesh K Seth, Ayan Mondal, Punnag Saha, Patricia Janulewicz, Kimberly Sullivan, Stephen Lasley, Ronnie Horner, Nancy Klimas and Saurabh Chatterjee in Neuroscience Insights
Acknowledgments
We are grateful to the Instrument Resource Facility (University of South Carolina School of Medicine) and AML Labs (Baltimore MD) for providing excellent technical services. We are also thankful to Cosmos ID (Microbial Genomic Platform, Rockville, MD, USA) for their analysis of the bacteriome.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this project was provided by DoD-IIRFA grant: W81XWH1810374 and VA merit award I01 CX001923-01 to Saurabh Chatterjee; W81XWH-16-1-0556 to Dr Stephen Lasley; W81XWH-13-2-0072 to Dr Kim Sullivan. This material is based upon work supported (or supported in part) by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development vide grant no. CX001923-01 to Saurabh Chatterjee. Chatterjee is a 5/8th employee with the US Department of Veterans Affairs.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: SC conceived research, SC, DK and DB designed research. DK, DB, RS, AM, PS performed research. KS, PJ, SL, RH, NK and SC analyzed and interpreted data. DK, DB and SC drafted manuscript, SC wrote, edited and approved final version of the manuscript.
ORCID iD: Saurabh Chatterjee  https://orcid.org/0000-0003-1649-7149
https://orcid.org/0000-0003-1649-7149
Supplemental Material: Supplemental material for this article is available online.
References
- 1. White RF, Steele L, O’Callaghan JP, et al. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf war: effects of toxicant exposures during deployment. Cortex. 2016;74:449-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rose MR, Brix KA. Neurological disorders in Gulf War veterans. Philos Trans R Soc Lond B Biol Sci. 2006;361:605-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fukuda K, Nisenbaum R, Stewart G, et al. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA. 1998;280:981-988. [DOI] [PubMed] [Google Scholar]
- 4. Engdahl BE, James LM, Miller RD, et al. Brain function in Gulf War illness (GWI) and Associated Mental Health comorbidities. J Neurol Neuromedicine. 2018;3:24-34. [PMC free article] [PubMed] [Google Scholar]
- 5. Jeffrey MG, Krengel M, Kibler JL, et al. Neuropsychological findings in Gulf War illness: a review. Front Psychol. 2019;10:2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doebbeling BN, Clarke WR, Watson D, et al. Is there a Persian Gulf War syndrome? Evidence from a large population-based survey of veterans and nondeployed controls. Am J Med. 2000;108:695-704. [DOI] [PubMed] [Google Scholar]
- 7. Gray GC, Kaiser KS, Hawksworth AW, Hall FW, Barrett-Connor E. Increased postwar symptoms and psychological morbidity among U.S. Am J Trop Med Hyg. 1999;60:758-766. [DOI] [PubMed] [Google Scholar]
- 8. The Persian Gulf experience and health. NIH Technology Assessment Workshop Panel. JAMA. 1994;272:391-396. [PubMed] [Google Scholar]
- 9. Golomb BA. Acetylcholinesterase inhibitors and Gulf War illnesses. Proc Natl Acad Sci USA. 2008;105:4295-4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joshi U, Pearson A, Evans JE, et al. A permethrin metabolite is associated with adaptive immune responses in Gulf War illness. Brain Behav Immun. 2019;81:545-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brimfield AA. Chemicals of military deployments: revisiting Gulf War syndrome in light of new information. Prog Mol Biol Transl Sci. 2012;112:209-230. [DOI] [PubMed] [Google Scholar]
- 12. Mawson AR, Croft AM. Gulf War illness: unifying hypothesis for a continuing health problem. Int J Environ Res Public Health. 2019;16:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Riper SM, Alexander AL, Koltyn KF, et al. Cerebral white matter structure is disrupted in Gulf War veterans with chronic musculoskeletal pain. Pain. 2017;158:2364-2375. [DOI] [PubMed] [Google Scholar]
- 14. Abou-Donia MB, Conboy LA, Kokkotou E, et al. Screening for novel central nervous system biomarkers in veterans with Gulf War illness. Neurotoxicol Teratol. 2017;61:36-46. [DOI] [PubMed] [Google Scholar]
- 15. Zakirova Z, Tweed M, Crynen G, et al. Gulf War agent exposure causes impairment of long-term memory formation and neuropathological changes in a mouse model of Gulf War illness. PLoS ONE. 2015;10:e0119579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Madhu LN, Attaluri S, Kodali M, et al. Neuroinflammation in Gulf War illness is linked with HMGB1 and complement activation, which can be discerned from brain-derived extracellular vesicles in the blood. Brain Behav Immun. 2019;81:430-443. [DOI] [PubMed] [Google Scholar]
- 17. Khan N, Smith MT. Neurotrophins and neuropathic pain: role in pathobiology. Molecules. 2015;20:10657-10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiao SS, Shen LL, Zhu C, et al. Brain-derived neurotrophic factor protects against tau-related neurodegeneration of Alzheimer’s disease. Transl Psychiatry. 2016;6:e907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sampaio TB, Savall AS, Gutierrez MEZ, Pinton S. Neurotrophic factors in Alzheimer’s and Parkinson’s diseases: implications for pathogenesis and therapy. Neural Regen Res. 2017;12:549-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Allen SJ, Watson JJ, Dawbarn D. The neurotrophins and their role in Alzheimer’s disease. Curr Neuropharmacol. 2011;9:559-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grande I, Fries GR, Kunz M, Kapczinski F. The role of BDNF as a mediator of neuroplasticity in bipolar disorder. Psychiatry Investig. 2010;7:243-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tome D, Fonseca CP, Campos FL, Baltazar G. Role of neurotrophic factors in Parkinson’s disease. Curr Pharm Des. 2017;23:809-838. [DOI] [PubMed] [Google Scholar]
- 23. Lima Giacobbo B, Doorduin J, Klein HC, Dierckx RAJO, Bromberg E, de Vries EFJ. Brain-derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol Neurobiol. 2019;56:3295-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener. 2015;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee Y, Lee S, Chang SC, Lee J. Significant roles of neuroinflammation in Parkinson’s disease: therapeutic targets for PD prevention. Arch Pharm Res. 2019;42:416-425. [DOI] [PubMed] [Google Scholar]
- 27. Miranda M, Morici JF, Zanoni MB, Bekinschtein P. Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci. 2019;13:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci. 2015;11:1164-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kimono D, Sarkar S, Albadrani M, et al. Dysbiosis-associated enteric glial cell immune-activation and redox imbalance modulate tight junction protein expression in Gulf War illness pathology. Front Physiol. 2019;10:1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seth RK, Maqsood R, Mondal A, et al. Gut DNA virome diversity and its association with host bacteria regulate inflammatory phenotype and neuronal immunotoxicity in experimental Gulf War illness. Viruses. 2019;11:968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS ONE. 2017;12:e0172914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seth RK, Kimono D, Alhasson F, et al. Increased butyrate priming in the gut stalls microbiome associated-gastrointestinal inflammation and hepatic metabolic reprogramming in a mouse model of Gulf War illness. Toxicol Appl Pharmacol. 2018;350:64-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zakirova Z, Reed J, Crynen G, et al. Complementary proteomic approaches reveal mitochondrial dysfunction, immune and inflammatory dysregulation in a mouse model of Gulf War illness. Proteomics Clin Appl. 2017;11:1600190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Locker AR, Michalovicz LT, Kelly KA, Miller JV, Miller DB, O’Callaghan JP. Corticosterone primes the neuroinflammatory response to Gulf War Illness-relevant organophosphates independently of acetylcholinesterase inhibition. J Neurochem. 2017;142:444-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O’Callaghan JP, Kelly KA, Locker AR, Miller DB, Lasley SM. Corticosterone primes the neuroinflammatory response to DFP in mice: potential animal model of Gulf War illness. J Neurochem. 2015;133:708-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dao MC, Everard A, Aron-Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426-436. [DOI] [PubMed] [Google Scholar]
- 37. Ottman N, Geerlings SY, Aalvink S, de Vos WM, Belzer C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract Res Clin Gastroenterol. 2017;31:637-642. [DOI] [PubMed] [Google Scholar]
- 38. Remely M, Hippe B, Zanner J, Aumueller E, Brath H, Haslberger AG. Gut microbiota of obese, type 2 diabetic individuals is enriched in Faecalibacterium prausnitzii, Akkermansia muciniphila and Peptostreptococcus anaerobius after weight loss. Endocr Metab Immune Disord Drug Targets. 2016;16:99-106. [DOI] [PubMed] [Google Scholar]
- 39. Andreasson KI, Bachstetter AD, Colonna M, et al. Targeting innate immunity for neurodegenerative disorders of the central nervous system. J Neurochem. 2016;138:653-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Karan D. Inflammasomes: emerging central players in cancer immunology and immunotherapy. Front Immunol. 2018;9:3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lin C, Zhang J. Inflammasomes in inflammation-induced cancer. Front Immunol. 2017;8:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sepehri Z, Kiani Z, Afshari M, Kohan F, Dalvand A, Ghavami S. Inflammasomes and type 2 diabetes: an updated systematic review. Immunol Lett. 2017;192:97-103. [DOI] [PubMed] [Google Scholar]
- 44. Lang Y, Chu F, Shen D, et al. Role of inflammasomes in neuroimmune and neurodegenerative diseases: a systematic review. Mediators Inflamm. 2018;2018:1549549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ng TKS, Ho CSH, Tam WWS, Kua EH, Ho RC. Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients with Alzheimer’s disease (AD): a systematic review and meta-analysis. Int J Mol Sci. 2019;20:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110:9066-9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cani PD, de Vos WM. Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front Microbiol. 2017;8:1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Geerlings SY, Kostopoulos I, de Vos WM, Belzer C. Akkermansia muciniphila in the human gastrointestinal tract: when, where, and how? Microorganisms. 2018;6:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang T, Li Q, Cheng L, Buch H, Zhang F. Akkermansia muciniphila is a promising probiotic. Microb Biotechnol. 2019;12:1109-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lopez-Siles M, Enrich-Capo N, Aldeguer X, et al. Alterations in the abundance and co-occurrence of Akkermansia muciniphila and Faecalibacterium prausnitzii in the colonic mucosa of inflammatory bowel disease subjects. Front Cell Infect Microbiol. 2018;8:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schneeberger M, Everard A, Gomez-Valades AG, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bian X, Wu W, Yang L, et al. Administration of Akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front Microbiol. 2019;10:2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van der Lugt B, van Beek AA, Aalvink S, et al. Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging Ercc1 (-/Delta7) mice. Immun Ageing. 2019;16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reunanen J, Kainulainen V, Huuskonen L, et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol. 2015;81:3655-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang B, Verne ML, Fields JZ, Verne GN, Zhou Q. Intestinal hyperpermeability in Gulf War veterans with chronic gastrointestinal symptoms. J Clin Gastroenterol. 2019;53:e298-e302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Janulewicz PA, Seth RK, Carlson JM, et al. The Gut-microbiome in Gulf War veterans: a preliminary report. Int J Environ Res Public Health. 2019;16:3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Villabona-Rueda A, Erice C, Pardo CA, Stins MF. The evolving concept of the blood brain barrier (BBB): from a single static barrier to a heterogeneous and dynamic relay center. Front Cell Neurosci. 2019;13:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Greene C, Hanley N, Campbell M. Claudin-5: gatekeeper of neurological function. Fluids Barriers CNS. 2019;16:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Romanitan MO, Popescu BO, Spulber S, et al. Altered expression of claudin family proteins in Alzheimer’s disease and vascular dementia brains. J Cell Mol Med. 2010;14:1088-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nishiura K, Ichikawa-Tomikawa N, Sugimoto K, et al. PKA activation and endothelial claudin-5 breakdown in the schizophrenic prefrontal cortex. Oncotarget. 2017;8:93382-93391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Meessen ECE, Warmbrunn MV, Nieuwdorp M, Soeters MR. Human postprandial nutrient metabolism and low-grade inflammation: a narrative review. Nutrients. 2019;11:3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Netto Candido TL, Bressan J, Alfenas RCG. Dysbiosis and metabolic endotoxemia induced by high-fat diet. Nutr Hosp. 2018;35:1432-1440. [DOI] [PubMed] [Google Scholar]
- 64. Kokkola R, Andersson A, Mullins G, et al. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol. 2005;61:1-9. [DOI] [PubMed] [Google Scholar]
- 65. Chandrashekaran V, Seth RK, Dattaroy D, et al. HMGB1-RAGE pathway drives peroxynitrite signaling-induced IBD-like inflammation in murine nonalcoholic fatty liver disease. Redox Biol. 2017;13:8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Weber DJ, Allette YM, Wilkes DS, White FA. The HMGB1-RAGE inflammatory pathway: implications for brain injury-induced pulmonary dysfunction. Antioxid Redox Signal. 2015;23:1316-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Abais JM, Xia M, Zhang Y, Boini KM, Li PL. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22:1111-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ozaki E, Campbell M, Doyle SL. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflamm Res. 2015;8:15-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xie ZM, Wang XM, Xu N, et al. Alterations in the inflammatory cytokines and brain-derived neurotrophic factor contribute to depression-like phenotype after spared nerve injury: improvement by ketamine. Sci Rep. 2017;7:3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Guan Z, Fang J. Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav Immun. 2006;20:64-71. [DOI] [PubMed] [Google Scholar]
- 72. Lapchak PA, Araujo DM, Hefti F. Systemic interleukin-1 beta decreases brain-derived neurotrophic factor messenger RNA expression in the rat hippocampal formation. Neuroscience. 1993;53:297-301. [DOI] [PubMed] [Google Scholar]
- 73. Tong L, Balazs R, Soiampornkul R, Thangnipon W, Cotman CW. Interleukin-1 beta impairs brain derived neurotrophic factor-induced signal transduction. Neurobiol Aging. 2008;29:1380-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplimental_material for Host Akkermansia muciniphila Abundance Correlates With Gulf War Illness Symptom Persistence via NLRP3-Mediated Neuroinflammation and Decreased Brain-Derived Neurotrophic Factor by Diana Kimono, Dipro Bose, Ratanesh K Seth, Ayan Mondal, Punnag Saha, Patricia Janulewicz, Kimberly Sullivan, Stephen Lasley, Ronnie Horner, Nancy Klimas and Saurabh Chatterjee in Neuroscience Insights