Abstract
Early life is a decisive stage for the development of physiological and psychological characteristics of an individual. Any stress or disruption of healthy development at this stage has serious long-lasting consequences for the remaining life. Unfortunately, early life stress is a common occurrence in humans and other animals. In this context, we investigated if the provision of environmental enrichment during the pre-weaning phase of rat pups and dams could alter the consequences of early-life maternal-separation stress. Pre-weaning enrichment rescued the effects of maternal separation on the excess secretion of adrenal stress hormones and anxiety-like behavior during adulthood. Enrichment also reduced the effect of stress on the spine density of basolateral amygdala neurons, a brain region critical for stress-induced facilitation of emotional behaviors. Pre-weaning enrichment, provided during early-life, blunted the effects of maternal separation stress on decreased intra-nuclear translocation of glucocorticoid receptors within the amygdala neurons when tested later in adulthood. Early-life, pre-weaning environmental enrichment also increased the amount of brain-derived neurotrophic factor within adult basolateral amygdala. Our observations showed that environmental manipulation during early formative years could be utilized to build lifelong resilience to stress. Complex naturalistic housing and sensory enrichment is, thus, an useful buffer against an impoverished and stressful childhood.
Subject terms: Emotion, Stress and resilience
Introduction
Early life is an important period for brain development. Experiences during this period often result in a long-lasting realignment of the stress-response for rest of life. Exposure to stress in early life causes a sustained vulnerability to stress-responsive psychiatric disorders, including anxiety, depression, and post-traumatic stress disorder1,2. In order to potentially blunt the harmful effects of stress, it is crucial to understand the mechanistic basis of the relationship between early-life stress and later development of emotional disorders3.
Maternal separation is a particularly significant and established model of early life stress4. Interestingly, the emergence of aversive stress response in pups is moderated by maternal presence through a reduction in adrenal glucocorticoid release and through the reduction in activity of the amygdala within brain5. Thus, maternal presence is an important regulator for stress response in rodent offspring; and maternal absence or separation results in long-lasting stress hyper-responsivity.
A perceived threat or an adverse environment leads to the adrenal release of glucocorticoids through the hypothalamic–pituitary–adrenal axis (HPA axis)6,7. Regulation of the HPA axis plays a crucial role in restoring homeostasis after exposure to acute stressors8–10. HPA axis remains subdued during early life of rat pups between post-natal days 3 through 14, a phase known as stress hypo-responsive period. Early life stress disrupts the HPA quiescence during this period through increasing glucocorticoid secretion, leading to ontogenic disruption of stress-related behaviors and brain regions. The emergence of adult-like fear response after the stress hypo-responsive period is mediated by greater ability of the adrenals to secrete glucocorticoids and the action of adrenal glucocorticoids within the basolateral amygdala11. This suggests that synchrony between glucocorticoids and amygdala is critical in healthy normative development of the stress response.
Early life is also a suitable window to institute stress resilience since it is the phase when the environment can create long-term changes in brain development. Environmental enrichment, is known to exert beneficial effects on stress physiology, synaptic plasticity, molecular changes, and behavioral manifestation of stress12–15. For example, post-weaning environmental enrichment rescues the effects of early life stress on anxiety-like behavior and HPA axis13. Effects of environmental enrichment during pre-weaning stages, a stage when maternal presence is an expectant event, remains underexplored. This opens a possibility that short-term environmental enrichment provided to the mother–pup dyad early in life may counter the damaging effects of maternal separation on emotional reactivity and commensurate plasticity in the brain during adulthood.
Anxiety is a common maladaptive behavioral outcome of stress. In this context, earlier work demonstrated the central role of structural changes within the basolateral amygdala in stress-induced anxiogenesis12,13. Additionally, elevated secretion of adrenal glucocorticoids is a reliable indicator of stress hyper-sensitivity. Glucocorticoids are also known to interact with the basolateral amygdala neurons16, specifically through structural plasticity in the dendrites. These interactions are essential for fear and anxiety. The importance of glucocorticoid-mediated signaling is evident through the observation that blocking of glucocorticoid binding to its receptors within the basolateral amygdala reduces stress-induced anxiogenesis17,18. Similarly, the provision of glucocorticoids within amygdala enhances fear and anxiety. Apart from glucocorticoids, neurotrophins like brain-derived neurotrophic factor (BDNF) are essential regulators of stress-induced neuronal plasticity. GR and BDNF are known to interact with each other, with BDNF facilitating GR signaling and consequent gene transcriptional response to stress through glucocorticoid response element (GRE)19–21.
In this backdrop, we investigated if maternal separation causes coordinated long-term changes in stress response, anxiety-like behavior, basolateral amygdala structural plasticity, regulation of GR activation and regulation of BDNF within the basolateral amygdala. We also investigated the ameliorative effects of a short-term sensory environmental enrichment on all of these parameters.
Materials and methods
Animals
Rats of Wistar strain were procured from Charles River, USA. Animals were maintained two per cage in a 12:12 light–dark cycle (lights on at 0700 h). Food and water were available ad libitum. All procedures were approved by the Nanyang Technological University Institutional Animal Care and Use Committee (IACUC). All animal experiments were congruent with and were performed in accordance with the guidelines of the IACUC.
At the start of the experiments, breeding pairs were set by co-housing one male and one female rat. Both mating partners were sexually inexperienced. Male was removed after confirmation of pregnancy at approximately two weeks after the start of the co-housing. Day of birth was designated as PND0 (post-natal day 0). Pups were weaned on PND21.
Experimental treatments
All experimental treatments were conducted between PND2 and PND21 (post-natal day 2 and 21). During this time, experimental subjects were not weaned from the dams. Pups remained unhandled during the course of the experiment, except during the maternal separation procedures. Experimental animals were weaned at PND21. All behavioral, physiological, and histological measurements were conducted when these weaned animals reached adulthood (PND60 through PND70). Experimenters were blind to the treatment groups during the measurement of endpoints. Only male animals were used for the quantification, although litters remained unmanipulated and consisted of pups of both sexes.
Pups assigned to receive maternal separation were separated from the dams once a day for three hours from PND2 through PND14 (post-natal day 2 through 14). The dam was first gently removed and placed in a new cage with fresh bedding. The pups were then removed and placed into a new cage with fresh bedding as a group, brought into a neighboring room, and placed on a heating pad for three hours. Both pups and dams were returned to the home-cage after three hours. Soiled bedding was changed on PND2, PND9, and PND14, by returning pups and mother to a new cage with fresh bedding, which had been previously sprinkled with a handful of soiled bedding and nesting material from the previous cage. Adult animals that received pre-weaning maternal separation were designated as 'MS', and those without maternal separation were designated as 'Control’ in this manuscript (for example, Fig. 1).
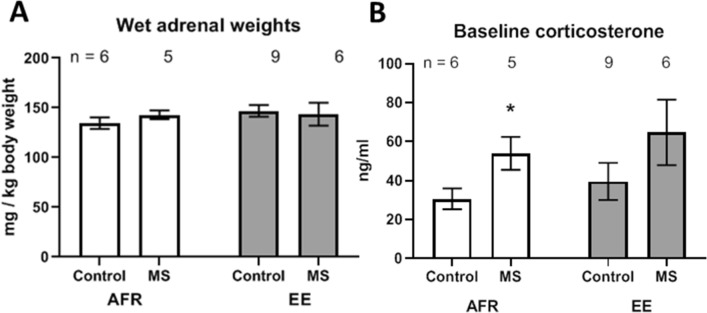
Figure 1.
(A) Effects of maternal separation (MS) and environmental enrichment (EE) on wet weight of adrenal glands and (B) baseline circulating corticosterone. Graphs depict Mean ± SEM. *p < 0.05; ***p < 0.001. Both MS and EE were provided pre-weaning, while endpoints were quantified during adulthood. n refers to number of animals.
Pups assigned to receive environmental enrichment (EE) were placed in enriched housing between PND2 through PND21. One dam and her litter were placed in one enriched housing cage that was much bigger in dimension (72 × 51 × 110 cm3 compared to standard of 37 × 22 × 18 cm3) and provisioned with a highly sensory environment. A sensorially rich environment was created within the cages by the provision of nesting materials, wooden climbing planks, metal climbing chains, bells, burrowing tubes of various sizes, enrichment toys, and fruit-flavored chow. The EE cages also contained wire meshes on the walls for rats to climb. The location of the objects within EE cages was changed every fourth day to maintain novelty. Water and regular rat chow were provided ad libitum. Apart from the regular food, flavored cereals and sunflower seeds were sprinkled every fourth day. The light and dark cycle was similar to that of the standard cages. This sensory enrichment paradigm did not include the provision of a running wheel. Adult animals that received pre-weaning environmental enrichment are designated as 'EE,' and those without maternal separation are designated as 'AFR' (animal-facility reared) in this manuscript (Fig. 1).
Animals in eleven litters were randomly assigned to one of the four experimental groups: with/without maternal separation (MS or Control), raised in either standard housing (AFR) or enriched housing (EE). After weaning, all animals were housed in individually ventilated standard cages with two animals/cage (37 × 22 × 18 cm3).
Corticosterone measurement
50–200 µl of blood was collected through lateral tail vein incisions for baseline corticosterone measurement at P5622. Blood was centrifuged (4,700 rpm, 15 min; Thermo Scientific Sorvall Legend XTR, Germany) and the separated serum was stored at – 80 °C until further analysis. The concentration of serum corticosterone was quantified using an enzyme-linked immunoassay based commercial kit (Enzo Life Sciences, NY, USA). Of note, adrenal and endocrine measures reported here reflect the tone of the stress endocrine response and do not provide information about sensitivity to novel stressors. Adrenal glands were collected bilaterally during sacrifice, and their weight were taken, reported as normalized to body weight at sacrifice.
Open-field test
An open field test was conducted in a circular Perspex arena (radius = 1 m) between 0900 and 1200 h. Animals were placed at the periphery of the dimly illuminated open field for a trial duration of 300 s. Apparatus was cleaned with 70% ethanol between trials. Time spent in the center of the arena was quantified from recorded videos. The center was defined as a concentric circular region of interest with a radius of 0.33 m. In a subset of animals, total time spent by animals being mobile in the arena was also quantified as an index of locomotion.
Quantification of dendritic spines
The staining methods used in this study were similar to those previously described12. In brief, freshly harvested brains were obtained by sacrifice through decapitation sans anesthesia. Blocks of brain tissue containing BLA (Bregma − 2.04 to − 3.36) were processed for Golgi-Cox stain using a commercial kit (FD Neurotechologies, Columbia, USA). After processing, 100 µm thick coronal sections were mounted on glass slides, counter-stained with cresyl violet (Sigma-Aldrich, Singapore) dehydrated using an ascending series of alcohol and xylene, and coverslipped in non-aqueous medium.
Analysis of spine density was performed using methods similar to those previously described23. Spine density on primary and secondary dendrites of spiny principal basolateral amygdala neurons was calculated at 1000× optical magnification using an oil-immersion objective lens (Olympus BX43). Dendrites directly originating from cell soma were classified as primary dendrites, and those arising from primary dendrites were classified as secondary dendrites. Starting from the origin of the branch, and continuing away from cell soma, spines were counted along a 60 µm stretch of primary and 60 µm of secondary dendrite. Density was expressed as number of spines per µm, averaged across primary and secondary dendrites.
Tissue preparation for immunohistochemistry
Anesthetized rats (gaseous isoflurane, 5%) were sacrificed through transcardial perfusion of phosphate-buffered saline followed by 4% paraformaldehyde dissolved in buffered saline. Harvested brains were equilibrated with 30% sucrose in buffered saline. These brains were cryosectioned in coronal planes at 40 µm thickness and collected in a 24-well plate with anti-freeze media and stored at − 20 °C until further use. Three randomly chosen sections between Bregma − 2.76 to − 2.92 mm were used for immunostaining for glucocorticoid receptors. Another set of three sections were used for immunostaining of brain-derived neurotrophic factor.
Quantification of intra-nuclear glucocorticoid receptors (GR)
Sections were washed in phosphate-buffered saline (3×) and then incubated with 5% bovine serum albumin for one hour. Primary antibody (rabbit anti-GR; Santa Cruz, USA) was diluted (1:500) in a solution containing 0.1% Triton × 100 and 3% bovine serum albumin in buffered saline. Brain sections were incubated with the primary antibody for 48 h at 4 °C with gentle shaking. Sections were thoroughly washed in the buffer after incubation. Localization of primary antibody was visualized using anti-rabbit antibody raised in goat and conjugated with DyLight-549 (1:1,000, Vector Laboratories, USA; 2 h at room temperature). Nuclear boundaries were subsequently stained after washing unbound antibodies and incubating sections with 4′, 6-diamidino-2-phenylindole (DAPI) for 1 min. Sections were washed and mounted in aqueous medium on superfrost slides and stored at − 20 °C until imaging.
Four randomly selected regions of interest (ROI: BLA, Bregma − 2.04 to − 3.36) for each animal was imaged at 400× magnification using the confocal laser scanning microscope LSM 710 (Carl Zeiss, Singapore). 35–40 confocal slices were collected per region of interest at 1 µm interval. The number of DAPI-containing nuclei with or without GR co-localization was manually counted. GR nuclear localization was expressed as number of nuclei showing colocalization relative to the sum of nuclei with the presence or absence of co-localization24,25.
Quantification of brain-derived neurotrophic factor (BDNF)
Methods similar to GR immunohistochemistry were used for BDNF visualization. The primary antibody consisted of mouse anti-BDNF antibody (1:300, Developmental Studies Hybridoma Bank, University of Iowa). The secondary antibody was DyLight-488 horse anti-mouse IgG (1:1,000, Abcam).
35–40 confocal slices were collected per region of interest at 1 µm interval. Three slices (#6, #12, and #18) were chosen for the subsequent analysis. The fluorescent signal derived from BDNF was converted to an 8-bit image such that the intensity of the signal varied between 0 (no signal) to 255 (maximum). The number of pixels at each intensity was then measured. Out of all pixels with non-zero intensities, 99.2 ± 0.2% exhibited intensities lower than 150, demonstrating that the confocal images were not saturated. The normative histogram for each experimental group is depicted in Supplementary Fig. 2. The relationship between intensity and the corresponding cumulative number of pixels was modeled using an exponential plateau function, Y = Ymax (1 − e(−k×X)). Ymax in this equation estimates asymptote for the number of pixels with positive staining values. On the other hand, k, or rise constant, estimates relative preponderance of pixels with lower intensity values in the histogram.
Western blots
The brain from a subset of animals was harvested after rapid decapitation, frozen in liquid nitrogen, and stored in – 80 °C. Harvested brains were sectioned in coronal planes at 100 µm thickness and mounted on glass slides. The basolateral amygdala was collected from the sections using bilateral micro-punches (Bregma − 2.04 to − 3.36 mm). Collected tissue was lyzed in the presence of protease inhibitors, homogenized and centrifuged at 4 °C, and then used to estimate the amount of GR and BDNF amount relative to protein product of an internal housekeeping gene (β-actin). Equivalent amounts of post-lysis samples were loaded on pre-cast sodium dodecyl sulfate polyacrylamide gel and allowed to electrophorese for 45 min at 145 V. Gels were then transferred onto a membrane (Trans-Blot Turbo Cassette; BioRad), cut in accordance to the expected size of the protein products, and blocked in 5% bovine serum albumin. Membrane fragments were incubated with primary antibodies for 14 h, washed, and incubated with secondary antibodies for 1 h. All antibody solutions were diluted with 5% bovine serum albumin. Secondary antibodies were visualized. The relative intensity of the protein bands was quantified using NIH ImageJ (version 1.50b; NIH)26.
For GR protein (90–95 kDa), anti-GR rabbit IgG was used as a primary antibody (1:500, Santa Cruz) and goat anti-rabbit IgG as secondary antibody (1:2,000, Santa Cruz). For BDNF (14–15 kDa), anti-BDNF mouse IgG was used as a primary antibody (1:300, University of Iowa) and donkey anti-mouse IgG as secondary antibody (1:2,000, Santa Cruz). For β-actin (37 kDa), anti-β-actin mouse IgG was used as a primary antibody (1:1,000, Santa Cruz) and donkey anti-mouse IgG as secondary antibody (1:2,000, Santa Cruz). The relative intensity of the protein bands was quantified using ImageJ software26.
Quantification of DNA methylation
Basolateral amygdala tissue was isolated using microdissection, as described in the previous section. DNA was isolated using a commercial kit (AllPrep DNA/RNA kit, Qiagen), and shipped to a commercial vendor for methylation quantification (QIAGEN GmbH). We assessed methylation status using direct bisulfite DNA sequencing. Bisulfite-treated samples were amplified through polymerase chain reaction using primers targeted at CpG-rich regions within GR. Amplified products were purified using a gel extraction kit and sequenced using the reverse primer. Peak values of A and G were quantified on the electopherogram. For each CpG site within the target region methylation status was quantified using ratio between G relative to the sum of A and G. Universally methylated and unmetlylated standards were run concurrently. Three CpG sites on GR promotor were quantified, and average methylation across three sites was used as an endpoint. The amplicon and four sites were as follows (CpG sites underlined):
GTTCTGCGGCACGCCCACTTCTAGCAGATAAGGCCGGGCGGGCGA.
Statistics and analysis
Outliers were identified and removed using the ROUT method with a maximum false discovery rate set at 1%27. The experimental design included two treatments, namely, stress exposure and housing conditions. The main effects of these experimental conditions were estimated using a two-way analysis of variance. Omnibus post-hoc comparisons were not conducted in order to avoid multiple pair-wise contrasts28.
Experiments were designed to determine if the effects of stress were dependent on housing conditions. In keeping with this a priori interest, two orthogonal planned comparisons were constructed using independent sample t-test28. These were (1) between non-stressed controls and stressed animals under animal-facility rearing (AFR versus MS); and (2) between non-stressed controls and stressed animals reared in enriched housing (EE versus MSEE). Information about the number of samples (n) is included in the figures and figure legends.
Results
Bodyweight
Both maternal separation and exposure to enriched housing were terminated before weaning of experimental subjects at 3 weeks of the age. We started by analyzing the weight of animals at weaning and gain in weight between weaning and sacrifice at 9–10 weeks of age.
Maternal separation did not cause significant difference in weaning weight in standard animal facility rearing conditions (Supplementary Fig. 1A; Δx̅ = − 2.1 ± 3.0 g; t14 = 0.69, p = 0.499). These two groups also did not exhibit a significant difference in weight gain during adulthood (Supplementary Fig. 1B; Δx̅ = 14.9 ± 11.6 g; t14 = 1.29, p = 0.218). In contrast, stressed animals had significantly lower weight at weaning compared to corresponding controls when animals were raised in the enriched housing (Δx̅ = − 7.0 ± 2.1 g; t14 = 3.30, p = 0.005). Stressed animals in enriched housing also gained lesser weight in adulthood compared to corresponding unstressed controls (Δx̅ = − 22.00 ± 10.06 g; t14 = 2.19, p = 0.046).
Analysis of variance showed significant main effect of environmental enrichment on body weight at weaning (Δx̅ = − 8.8 ± 1.8 g; p < 0.001) and for weight gain during adulthood (Δx̅ = − 23.7 ± 7.7 g; p < 0.004). Maternal stress presented with a significant main effect on body weight at weaning (Δx̅ = − 4.5 ± 1.8 g; p = 0.019). However, this effect became statistically non-significant for body weight gain during adulthood (Δx̅ = − 3.5 ± 7.7 g; p = 0.648).
Glucocorticoids
Glucocorticoid tone was measured through the wet weight of adrenal glands during sacrifice and baseline corticosterone levels during adulthood (Fig. 1). Adrenal weights were normalized to body weight of the respective animals (mg/kg body weight). Maternal separation did not cause statistically significant differences in normalized adrenal weights, in absence (Δx̅ = 8.21 ± 7.47 mg/kg; t9 = 1.1, p = 0.300) or presence (Δx̅ = − 3.34 ± 11.7 mg/kg; t13 = 0.29, p = 0.780) of enriched housing. When reared in standard housing environment, maternal separation significantly increased baseline corticosterone levels (Δx̅ = 23.4 ± 9.6 ng/ml; t9 = 2.43, p = 0.038). In contrast, effects of stress on basal corticosterone were not statistically significant when animals were reared in enriched housing before weaning (Δx̅ = 25.2 ± 17.9 ng/ml; t13 = 1.41, p = 0.183).
Analysis of variance revealed statistically insignificant effects of pre-weaning stress and enrichment on normalized adrenal weight during adulthood (p = 0.750 for maternal separation and p = 0.394 for environmental enrichment). Stress increased the amount of circulating basal corticosterone (Δx̅ = 24.3 ± 11.3 ng/ml; p = 0.042), while enrichment did not cause any statistically significant change (Δx̅ = 9.9 ± 11.3 ng/ml; p = 0.390).
Anxiety-like behavior
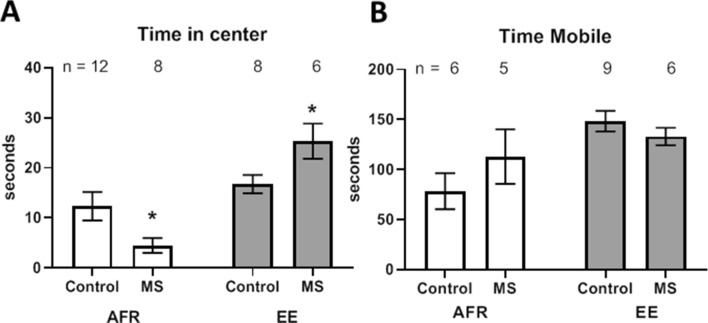
The effect of pre-weaning stress and enrichment on adult anxiety-like behavior was measured using the time spent in the anxiogenic center of an open field (Fig. 2A). Stress and enrichment caused contrasting effects on anxiety-like behavior. Maternal separation reduced exploration on anxiogenic center (Δx̅ = − 7.8 ± 3.7 s; t18 = 2.1, p = 0.050), while environmental enrichment increased such exploration (Δx̅ = 8.583 ± 3.692 s; t12 = 2.32, p = 0.038).
Figure 2.
(A) Effects of MS and EE on time spent in the anxiogenic center of an open field. *p < 0.05. n refers to number of animals. (B) Effects of MS and EE on total locomotion, measured as time sent being mobile in the arena.
Analysis of variance showed a significant main effect of enrichment, demonstrating that enrichment increased time spent in center of the open field (Δx̅ = 12.6 ± 2.7 s; p < 0.001. Main effect of stress did not reach statistical significance (Δx̅ = 0.4 ± 2.7 s; p = 0.891).
Maternal separation did not significantly affect locomotion, as measured through time spent being mobile in the arena, both in absence (Fig. 2B, Δx̅ = 3.7 ± 29.0 s; t9 = 0.1, p = 0.902) and presence of environment enrichment (Δx̅ = − 15.3 ± 14.5 s; t13 = 1.1, p = 0.400).
Spine density of principal basolateral amygdala neurons
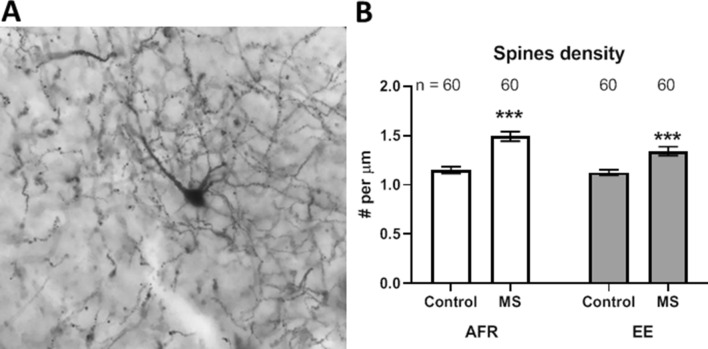
The density of dendritic spines on principal basolateral amygdala neurons was measured in Golgi-stained preparations (Fig. 3A). Spine density was quantified in adulthood as a function of pre-weaning stress and enrichment (Fig. 3B). Maternal separation caused a significant increase in spine density, both in absence of enrichment (Δx̅ = 0.34 ± 0.06/µm; t118 = 5.81, p < 0.001) and in presence of enrichment (Δx̅ = 0.22 ± 0.05/µm; t118 = 4.07, p < 0.001).
Figure 3.
(A) A representative image depicting a Golgi stained neuron at 400× optical magnification. (B) Effects of MS and EE on the spine density of principal neurons in basolateral amygdala. ***p < 0.001. n refers to number of neurons.
Analysis of variance revealed contrasting main effects of stress and enrichment on the spine density. While maternal separation caused an increase in spine density (Δx̅ = 0.28 ± 0.04/µm; p < 0.001), environmental enrichment reduced it (Δx̅ = − 0.09 ± 0.04/µm; p = 0.027).
Intra-nuclear glucocorticoid receptors (GR)
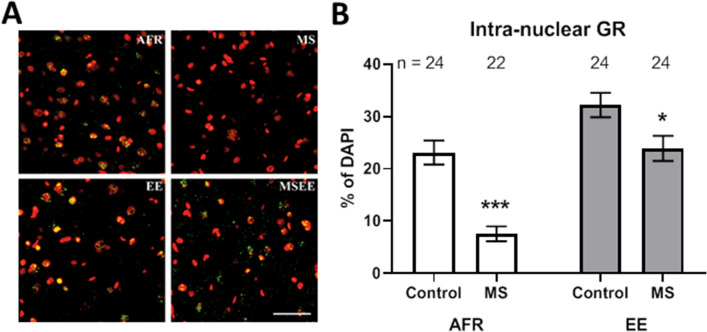
The extent of intra-nuclear localization of GR was quantified as percentage of DAPI-positive total nuclei (Fig. 4A,B). Maternal separation before weaning resulted in statistically significant reduction in intra-nuclear GR during adulthood, both in absence (t44 = 5.62, p < 0.001) and presence (t46 = 2.46, p = 0.018) of pre-weaning environmental enrichment. Magnitude of the stress effect was more pronounced in absence of enrichment (Δx̅ = − 15.5 ± 2.8%) compared to that in presence of enrichment (Δx̅ = − 8.3 ± 3.4%).
Figure 4.
(A) Representative images depicting labelling for glucocorticoid receptors (green) and DAPI (psudocolored red). Scale bar = 50 µm. (B) Effects of MS and EE on the percentage of basolateral amygdala cells exhibiting intra-nuclear localization of glucocorticoid receptors (GR). *p < 0.05; ***p < 0.001. n refers to number of sections.
Analysis of variance showed opposing main effects of stress and enrichment on intra-nuclear GR localization. Maternal separation reduced movement of GR within nuclei (Δx̅ = 11.9 ± 2.2%; p < 0.001). In contrast, environmental enrichment increased intra-nuclear localization (Δx̅ = − 12.7 ± 2.2%; p < 0.001).
Western blot analysis of total GR content within basolateral amygdala tissue did not reveal significant differences between stressed and non-stressed groups (Fig. 5; t10 = 0.97, p = 0.355 in the absence of enrichment and t10 = 0.40, p = 0.670 in the presence of enrichment). Similarly, main effects of stress and enrichment did not exhibit statistical significance (p = 0.339 and 0.995, respectively). Genomic DNA from basolateral amygdala of a subset of animals was also probed for DNA methylation status on five sites on the GR promoter (Fig. 6). This analysis showed very weak DNA methylation of GR promoter in the basolateral amygdala across all groups (x̅ = 1.9 ± 0.1%; range from 1.2 to 2.8%). Planned comparisons did not reveal significant effects of stress on absence or presence of enrichment (t8 = 1.60, p = 0.149 and t8 = 1.76, 0.116, respectively; Δx̅ < 0.5%).
Figure 5.
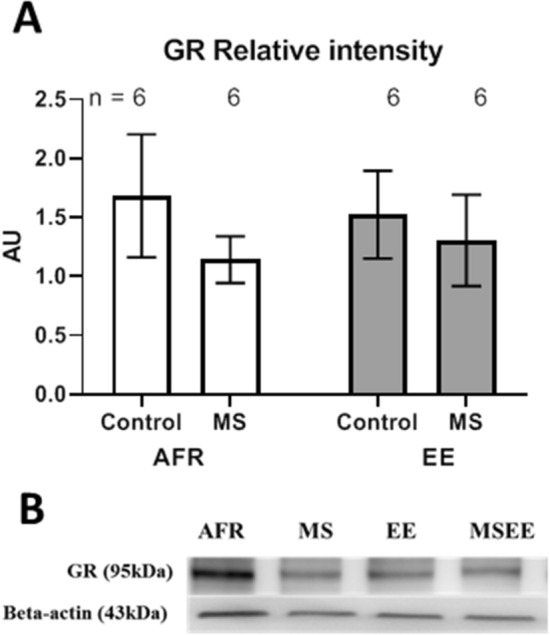
(A) Effects of maternal separation (MS) and environmental enrichment (EE) on amount of total GR protein. (B) Representative Western blot images for GR and an internal control (Beta-actin). n refers to number of animals.
Figure 6.
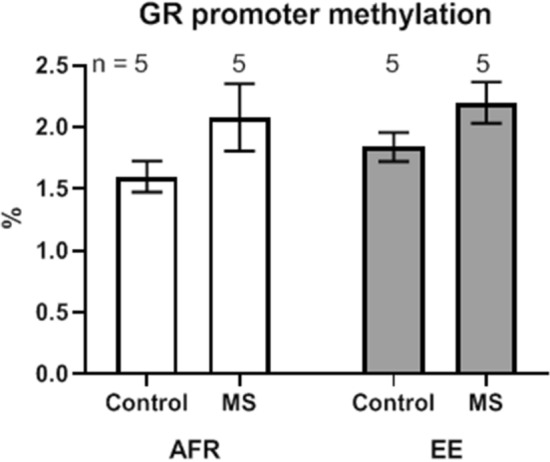
Effects of maternal separation (MS) and environmental enrichment (EE) on percentage DNA methylation at the GR promoter site. n refers to number of animals.
Brain-derived neurotrophic factor (BDNF)
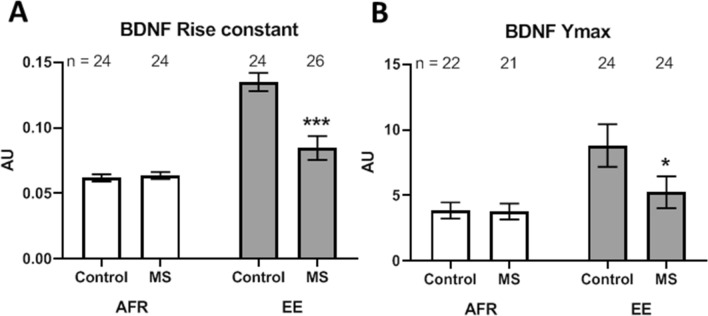
The amount of BDNF across experimental groups was quantified as a function of staining intensity (Fig. 7). The percentage number of pixels at each fluorescent intensity from 1 to 255 was quantified. These values were fitted with an exponential plateau function to calculate the rise constant and Ymax for each image. Inter-group variation in these endpoints was then analyzed.
Figure 7.
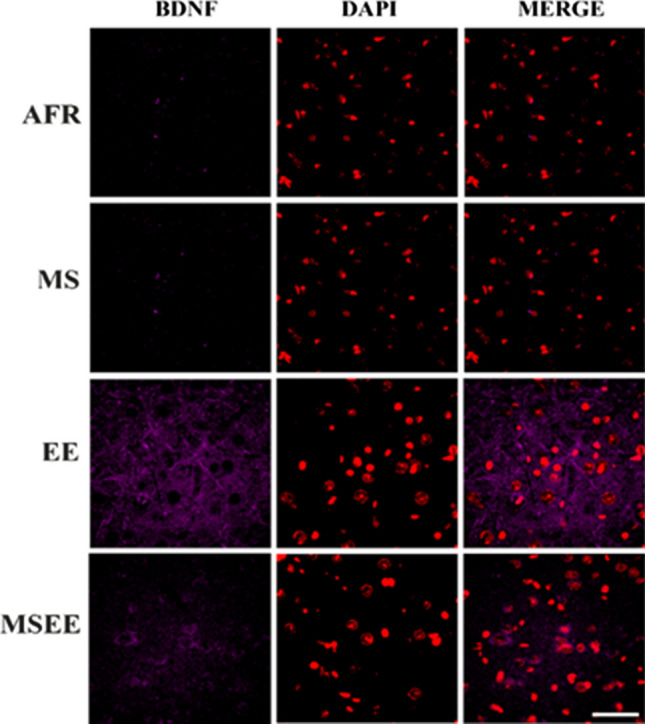
Representative images depicting labelling for BDNF (psudocolored magenta) and DAPI (psudocolored red). Scale bar = 50 µm.
Preponderance of signal with lower intensity within the total amount of fluorescence was estimated using rise constant of the exponential plateau function. Pre-weaning maternal separation did not cause significant change in rise constant of BDNF (Fig. 8A; Δx̅ = 0.002 ± 0.004; t46 = 0.49, p = 0.623). Rise constant of BDNF in non-stressed controls was significantly higher than stressed groups when animals were provided with pre-weaning enrichment (Δx̅ = − 0.050 ± 0.012; t48 = 4.31, p < 0.001). Main effects during analysis of variance showed that environmental enrichment increased rise constant (Δx̅ = 0.47 ± 0.006; p < 0.001). In contrast, maternal separation decreased rise constant (Δx̅ = 0.024 ± 0.006; p < 0.001).
Figure 8.
(A) Effects of MS and EE on rise constant of brain-derived neurotrophic factor signal within the basolateral amygdala and its asymptote (B, Ymax). *p < 0.05; ***p < 0.001. n refers to number of sections.
Total extent of the fluorescence staining was estimated using Ymax, or the asymptote, of the exponential plateau function. Maternal separation did not cause a significant change in Ymax of BDNF in absence (Fig. 8B; Δx̅ = − 0.076 ± 0.867; t41 = 0.09, p = 0.931) of environmental enrichment. Ymax in non-stressed controls was significantly higher than stressed groups when animals were provided with pre-weaning enrichment (Δx̅ = − 4.491 ± 1.813; t46 = 2.48, p = 0.017). Analysis of variance revealed significant increase Ymax due to environmental enrichment (Δx̅ = 3.211 ± 1.151; p = 0.006). Main effect of maternal separation was not statistically significant (Δx̅ = − 1.818 ± 1.151; p = 0.188).
Congruent with Ymax, relative intensity of BDNF protein during Western blot analysis showed lack of significant difference due to maternal stress in absence of enrichment (Fig. 9; Δx̅ = 0.23 ± 0.26; t10 = 0.87, p = 0.404) and a significant decrease in the stressed group in presence of enrichment (Δx̅ = − 0.66 ± 0.20; t10 = 3.28, p = 0.008). Analysis of variance for relative amount of BDNF did not have significant main effects of either enrichment (Δx̅ = − 0.041 ± 0.166; p = 0.809) or stress (Δx̅ = − 0.215 ± 0.166; p = 0.210).
Figure 9.
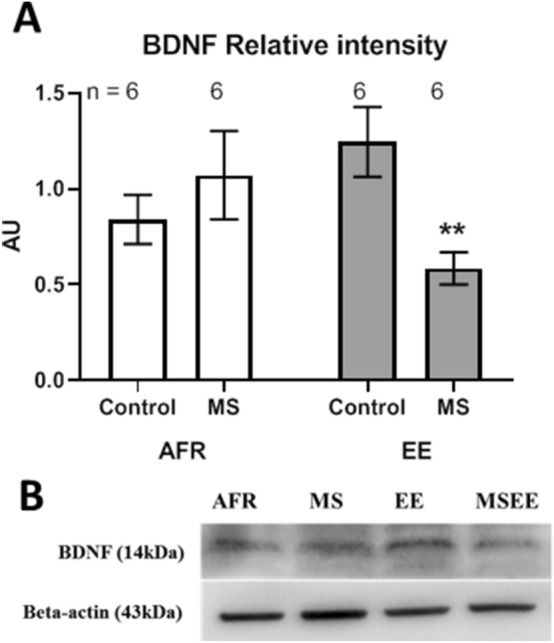
(A) Effects of maternal separation (MS) and environmental enrichment (EE) on amount of total BDNF protein. (B) Representative Western blot images for BDNF and an internal control (Beta-actin). n refers to number of animals.
Discussion
Exposure to early life stress causes potentiation of the stress response and related behaviors in adulthood29–34. In congruence with these earlier observations, results in this report show an increase in anxiety-like behavior and basal tone of hypothalamus–pituitary–adrenal (HPA) axis. Adult animals that were exposed to maternal separation before weaning exhibited more glucocorticoids circulating in the bloodstream at baseline and reduced spatial exploration of an anxiogenic environment. These detrimental effects occurred in parallel to an increase in the density of dendritic spines in the basolateral amygdala (BLA), a brain region known to be involved in stress-induced anxiogenesis35. These changes reflect a long-lasting footprint of early life stress through sustained neuroendocrine and neuroplastic reconfiguration into adulthood.
Interestingly, the effects of early life stress are dependent upon the housing environment of the mother–pup dyad during stress exposure36–38. Maternal separation stress during early life caused an increase in anxiety-like behavior in adulthood, yet such stress-induced anxiogenesis was prevented by simultaneous exposure to an enriched environment. While maternal separation still caused an increase in the dendritic spine density of BLA neurons, the magnitude of this effect was much reduced from a Cohen's d of 1.1 in the non-enriched cohort to 0.7 in the enriched cohort. The general theme of opposing effects of stress and enrichment was also reflected in contrasting the main effects of these treatments during the analysis of variance. The maternal separation had the main effect of more considerable anxiety-like behavior and denser BLA dendritic spines. In contrast, environmental enrichment had the main effect of reduced anxiety-like behavior and lowered spine density of the BLA neurons. These results suggest that the relationship between early-life stress and adult stress response as well as anxiety-like behavior is dependent on the immediate environment of early life. Individuals exposed to stress in early life take vastly different neuroendocrine trajectories, neuroplasticity and receptor regulation depending on the environmental context of the early housing of mother and offspring.
The modifying role of environment on the effects of stress is a recurring theme in a large body of scientific work14,39–41. For example, animals exposed to chronic stress in adulthood develop anxiety if they live in standard laboratory housing but not in environmentally enriched housing12. Similarly, enhanced anxiety-like behavior in adulthood brought about by early maternal separation can be effectively rescued by peripubertal environmental enrichment or by a relatively short period of enrichment later in adult life13. The effects of naturally occurring variations in maternal care also demonstrate such a contingency of effects on adult behavior and stress response38,42,43. Pups raised by low maternal care mothers show increased anxiety-like behavior, and hyper-responsive HPA system32,44, and this effect can be effectively reversed by providing environmental enrichment to the offspring after weaning14. In contrast to these earlier studies, stress and enrichment were concomitant during early life in the current study. Maternal separation occurred whilst the mother and the pups were living in either improvised laboratory housing or in enriched housing. This is an important distinction because dyadic interactions between the mother and the child play a crucial role in the development of behavior in many species45–47. In rats, maternal presence modulates stress response and shapes fear behavior in the pups5,38. In humans, lower quality of maternal care results in increased anxiety and disturbed social interactions during adulthood, leading to reduced lifespan and increased disease burden through allostatic load48–50. Results presented here suggest that modification of the environment where maternal care occurs can successfully preclude the harmful effects of the loss of maternal care.
Maternal separation caused a significant reduction in the number of BLA neurons manifesting intra-nuclear localization of glucocorticoid receptors (GR). This stress-induced suppression of GR nuclear localization, became blunted when animals were raised in the enriched housing. The mean difference between unstressed and stressed animals halved in the presence of enrichment, with a corresponding reduction in standardized effect size measured through Cohen’s d from 1.7 to 0.7. Glucocorticoids secreted by adrenal glands can readily cross blood–brain barrier and plasma membranes of neurons. These steroid molecules then act as ligands to cytosolic GR, initiating their traversal to the inside of the nucleus where GR acts as transcription factor by binding to GRE and eventually change the molecular milieu of the neurons51,52. Our results suggest that maternal separation reduces the ability of glucocorticoids to initiate downstream molecular changes in the BLA neurons despite the greater concentration of the circulating ligand. Simultaneous exposure to environmental enrichment partially reversed this phenomenon. The absence of statistically significant or numerically robust differences in GR total protein suggests that inter-group differences were not driven by expression of the GR but were mainly affected by nuclear localization of the GRs. Congruently, neonatal isolation for a week or brief maternal separation spanning two weeks does not alter GR expression within BLA53, in contrast to the known effects of early life stressors on hippocampal GR expression. Stressors applied during adulthood are known to decrease total GR expression within BLA54. In contrast, GR expression increases after chronic unpredictable stress and treatment with glucocorticoid analogs. Thus, it is likely that stress exposure during developmentally labile early life causes persistent rerouting of the biochemical steps involved between binding of GR to its ligand and its eventual transfer inside the nucleus to initiate transcriptional regulation. Apart from binding to glucocorticoids, GRs can also influence the secretion of their own ligands from the adrenals55. A genetic knockout of forebrain glucocorticoid receptors, for example, increases activity of hypothalamus–pituitary–adrenal axis56. Thus, it is plausible that reduced nuclear localization of GRs after maternal separation is responsible for hypercortisolism in the stressed group. Moreover, successful recruitment of GRs within BLA is known to be required from memory consolidation and facilitation of spatial memory during stressful events57. Thus, our results suggest that prior exposure to maternal separation might compromise the substrates that are later used for the mnemonic functions.
The effects of maternal separation and environmental enrichment on the brain-derived neurotrophic factor (BDNF) within BLA have not yet been studied. In other brain regions, BDNF is generally conceived to have a permissive effect of spinogenesis and structural plasticity of the neurons58–62. Previous work has established that stressors, including maternal separation, cause a long-lasting increase in dendritic material within BLA35,63. This is congruent with current observation that maternal separation increases spine density of BLA neurons. We have earlier shown that environmental enrichment during adulthood prevents dendritic growth in BLA brought about maternal separation13. These observations, together with neurotrophic effects of BDNF signaling, would suggest suppression of BDNF within BLA during environmental enrichment. In contrast, pre-weaning environmental enrichment increased the amount of BDNF within adult BLA, as inferred by a greater amount of the integrated signal. Previous studies have shown that binding of BDNF to its TrkB receptors is required for the effects of GRs on memory performance64. Thus, BDNF-TrkB signaling and its downstream effects on the extracellular signal-regulated kinase pathway might be a critical conduit for the effects of occupied GRs on molecular plasticity. Consistent with this, we observe that environmental enrichment leads to a diffused BDNF distribution within the BLA as would be expected by cleavage of BDNF to its active form ready to interact with its cognate receptor. This is indicated by larger rise constant for BDNF intensity in the enriched group. Thus, the developmental window at which animals experience enrichment might have important implications for long-term biochemical alterations in BDNF and its partner proteins. The period of maternal separation used in this study encompasses the critical temporal window for the development of the amygdala and its responsiveness to glucocorticoids.
Coincident presence of BDNF and glucocorticoids is required for efficient nuclear translocation of the GRs20,65,66. The presence of BDNF alone does not initiate GR nuclear localization. Yet, BDNF enhances the potency of GR mediated genomic response by increasing its nuclear transport when bound with glucocorticoids. It is then likely that a high BDNF environment within BLA, provided by enriched environment creates an enabling molecular backdrop for efficient GR signaling. In the non-enriched animals, lack of robust BDNF diminishes the ability of stress-induced glucocorticoids to drive genomic response and ensuing plasticity through GR, as seen in feeble nuclear localization of GRs in maternally separated cohort.
Rat pups are born altricial, dependent on maternal care for protection and nourishment. Moreover, the developing brain of pups is highly responsive to variations in the maternal environment. For example, the presence of a mother can obliterate stress endocrine activation during physically painful stimuli and alter the course of subsequent conditioned responses5. In this study, environment enrichment was provided all through the pre-weaning stage, including early postnatal periods where eyes of the pups did not open and when the mother remained the main conduit for environmental effects on the pups. It is possible that enrichment induces changes in maternal interaction with her pups during the pre-weaning period. For example, pre-reproductive enrichment of females is known to increase pup-oriented behaviors to later offspring67. Hence, the effects reported in this manuscript could have risen from both direct sensory enrichment of pups as well as indirect enrichment effects of dams that secondarily reached the pups through maternal care. Our data does not delineate the relative influence of these sources. Nonetheless, the present data demonstrate the holistic value of early life intervention on long-lasting neural and behavioral changes.
Conclusions
Our paper shows for the first time that early environment can play a decisive role in projecting stress-vulnerability and stress-resilience in individuals during adult life. This contrasting outcome is driven at multiple levels, from behavior, anxiogenesis to physiological stress-response through amygdala neuroplasticity. Additionally, we show that the underlying cellular context of stress resilience and vulnerability, driven by early housing environment, involves corticosterone-receptor localization within the nucleus as well as cellular regulation of BDNF, a neurotrophic factor known to influence neuronal spine-plasticity.
Supplementary information
Acknowledgements
This work was supported by the Ministry of Education, Singapore (RG 144/17) to R.M. We thank Ajai Vyas for editing the manuscript.
Author contributions
A.H. and R.M. designed the experiments. A.H. and S.S. conducted experiments. A.H. and R.M. prepared figures. R.M. wrote the manuscript. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-70875-5.
References
- 1.Glaser D. Child abuse and neglect and the brain: A review. J. Child Psychol. Psychiatry. 2000;41:97–116. doi: 10.1017/S0021963099004990. [DOI] [PubMed] [Google Scholar]
- 2.Hill MN, Eiland L, Lee TTY, Hillard CJ, McEwen BS. Early life stress alters the developmental trajectory of corticolimbic endocannabinoid signaling in male rats. Neuropharmacology. 2019;146:154–162. doi: 10.1016/j.neuropharm.2018.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ. The association between prenatal stress and infant birth weight and gestational age at birth: A prospective investigation. Am. J. Obstet. Gynecol. 1993;169:858–865. doi: 10.1016/0002-9378(93)90016-c. [DOI] [PubMed] [Google Scholar]
- 4.Stanton ME, Levine S. Inhibition of infant glucocorticoid stress response: Specific role of maternal cues. Dev. Psychobiol. 1990;23:411–426. doi: 10.1002/dev.420230504. [DOI] [PubMed] [Google Scholar]
- 5.Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat. Neurosci. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pariante CM, Lightman SL. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Gunnar MR, Quevedo KM. Early care experiences and HPA axis regulation in children: A mechanism for later trauma vulnerability. Prog. Brain Res. 2008;167:137–149. doi: 10.1016/s0079-6123(07)67010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Entringer S, Kumsta R, Hellhammer DH, Wadhwa PD, Wust S. Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Horm. Behav. 2009;55:292–298. doi: 10.1016/j.yhbeh.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: Hypothalamo–pituitary–adrenocortical axis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Rostène W, et al. Steroid effects on brain functions: An example of the action of glucocorticoids on central dopaminergic and neurotensinergic systems. J. Psychiatry Neurosci. 1995;20:349–356. [PMC free article] [PubMed] [Google Scholar]
- 12.Ashokan A, Hegde A, Mitra R. Short-term environmental enrichment is sufficient to counter stress-induced anxiety and associated structural and molecular plasticity in basolateral amygdala. Psychoneuroendocrinology. 2016;69:189–196. doi: 10.1016/j.psyneuen.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Koe AS, Ashokan A, Mitra R. Short environmental enrichment in adulthood reverses anxiety and basolateral amygdala hypertrophy induced by maternal separation. Transl. Psychiatry. 2016;6:e729–e729. doi: 10.1038/tp.2015.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J. Neurosci. 2002;22:7840. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 16.Joels M, Fernandez G, Roozendaal B. Stress and emotional memory: A matter of timing. Trends Cogn. Sci. 2011;15:280–288. doi: 10.1016/j.tics.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Mitra R, Sapolsky RM. Expression of chimeric estrogen-glucocorticoid-receptor in the amygdala reduces anxiety. Brain Res. 2010;1342:33–38. doi: 10.1016/j.brainres.2010.03.092. [DOI] [PubMed] [Google Scholar]
- 18.Calvo N, Volosin M. Glucocorticoid and mineralocorticoid receptors are involved in the facilitation of anxiety-like response induced by restraint. Neuroendocrinology. 2001;73:261–271. doi: 10.1159/000054643. [DOI] [PubMed] [Google Scholar]
- 19.Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J. Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arango-Lievano M, et al. Neurotrophic-priming of glucocorticoid receptor signaling is essential for neuronal plasticity to stress and antidepressant treatment. Proc. Natl. Acad. Sci. U. S. A. 2015;112:15737–15742. doi: 10.1073/pnas.1509045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambert WM, et al. Brain-derived neurotrophic factor signaling rewrites the glucocorticoid transcriptome via glucocorticoid receptor phosphorylation. Mol. Cell Biol. 2013;33:3700–3714. doi: 10.1128/MCB.00150-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fluttert M, Dalm S, Oitzl MS. A refined method for sequential blood sampling by tail incision in rats. Lab. Anim. 2000;34:372–378. doi: 10.1258/002367700780387714. [DOI] [PubMed] [Google Scholar]
- 23.Ashokan A, Lim JWH, Hang N, Mitra R. Complex housing causes a robust increase in dendritic complexity and spine density of medial prefrontal cortical neurons. Sci. Rep. 2018;8:7308. doi: 10.1038/s41598-018-25399-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng H, Ruan Z, Long F, Simpson JH, Myers EW. V3D enables real-time 3D visualization and quantitative analysis of large-scale biological image data sets. Nat. Biotechnol. 2010;28:348–353. doi: 10.1038/nbt.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng H, Bria A, Zhou Z, Iannello G, Long F. Extensible visualization and analysis for multidimensional images using Vaa3D. Nat. Protoc. 2014;9:193–208. doi: 10.1038/nprot.2014.011. [DOI] [PubMed] [Google Scholar]
- 26.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression: A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 2006;7:123. doi: 10.1186/1471-2105-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruxton GD, Beauchamp G. Time for some a priori thinking about post hoc testing. Behav. Ecol. 2008;19:690–693. doi: 10.1093/beheco/arn020. [DOI] [Google Scholar]
- 29.Anisman H, Zaharia MD, Meaney MJ, Merali Z. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int. J. Dev. Neurosci. 1998;16:149–164. doi: 10.1016/S0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- 30.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 31.Nusslock R, Miller GE. Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biol. Psychiatry. 2016;80:23–32. doi: 10.1016/j.biopsych.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol. Biochem. Behav. 2002;73:131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 33.Nugent NR, Tyrka AR, Carpenter LL, Price LH. Gene–environment interactions: Early life stress and risk for depressive and anxiety disorders. Psychopharmacology. 2011;214:175–196. doi: 10.1007/s00213-010-2151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McEwen BS. Early life influences on life-long patterns of behavior and health. Ment. Retard. Dev. Disabil. Res. Rev. 2003;9:149–154. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- 35.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hellstrom IC, Dhir SK, Diorio JC, Meaney MJ. Maternal licking regulates hippocampal glucocorticoid receptor transcription through a thyroid hormone-serotonin-NGFI-A signalling cascade. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2012;367:2495–2510. doi: 10.1098/rstb.2012.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fish EW, et al. Epigenetic programming of stress responses through variations in maternal care. Ann. N. Y. Acad. Sci. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- 38.Liu D, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science (New York, N.Y.) 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 39.Morley-Fletcher S, Rea M, Maccari S, Laviola G. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. Eur. J. Neurosci. 2003;18:3367–3374. doi: 10.1111/j.1460-9568.2003.03070.x. [DOI] [PubMed] [Google Scholar]
- 40.Moncek F, Duncko R, Johansson BB, Jezova D. Effect of environmental enrichment on stress related systems in rats. J. Neuroendocrinol. 2004;16:423–431. doi: 10.1111/j.1365-2826.2004.01173.x. [DOI] [PubMed] [Google Scholar]
- 41.Larsson F, Winblad B, Mohammed AH. Psychological stress and environmental adaptation in enriched vs. impoverished housed rats. Pharmacol. Biochem. Behav. 2002;73:193–207. doi: 10.1016/s0091-3057(02)00782-7. [DOI] [PubMed] [Google Scholar]
- 42.Wohr M, Schwarting RK. Maternal care, isolation-induced infant ultrasonic calling, and their relations to adult anxiety-related behavior in the rat. Behav. Neurosci. 2008;122:310–330. doi: 10.1037/0735-7044.122.2.310. [DOI] [PubMed] [Google Scholar]
- 43.Priebe K, et al. Maternal influences on adult stress and anxiety-like behavior in C57BL/6J and BALB/cJ mice: A cross-fostering study. Dev. Psychobiol. 2005;47:398–407. doi: 10.1002/dev.20098. [DOI] [PubMed] [Google Scholar]
- 44.Neumann ID, et al. Differential effects of periodic maternal separation on adult stress coping in a rat model of extremes in trait anxiety. Neuroscience. 2005;132:867–877. doi: 10.1016/j.neuroscience.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 45.Pratt M, et al. Mother–child adrenocortical synchrony; Moderation by dyadic relational behavior. Horm. Behav. 2017;89:167–175. doi: 10.1016/j.yhbeh.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Feng X, Shaw DS, Skuban EM, Lane T. Emotional exchange in mother-child dyads: Stability, mutual influence, and associations with maternal depression and child problem behavior. J. Fam. Psychol. JFP. 2007;21:714–725. doi: 10.1037/0893-3200.21.4.714. [DOI] [PubMed] [Google Scholar]
- 47.Smotherman WP. Mother–infant interaction and the modulation of pituitary–adrenal activity in rat pups after early stimulation. Dev. Psychobiol. 1983;16:169–176. doi: 10.1002/dev.420160303. [DOI] [PubMed] [Google Scholar]
- 48.Schulz LC. The Dutch Hunger Winter and the developmental origins of health and disease. Proc. Natl. Acad. Sci. U. S. A. 2010;107:16757–16758. doi: 10.1073/pnas.1012911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bateson P. Fetal experience and good adult design. Int. J. Epidemiol. 2001;30:928–934. doi: 10.1093/ije/30.5.928. [DOI] [PubMed] [Google Scholar]
- 50.Bowlby J. The nature of the child's tie to his mother. Int. Jo. Psycho-anal. 1958;39:350–373. [PubMed] [Google Scholar]
- 51.Meijer OC, Buurstede JC, Schaaf MJM. Corticosteroid receptors in the brain: Transcriptional mechanisms for specificity and context-dependent effects. Cell. Mol. Neurobiol. 2019;39:539–549. doi: 10.1007/s10571-018-0625-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scheschowitsch K, Leite JA, Assreuy J. New insights in glucocorticoid receptor signaling-more than just a ligand-binding receptor. Front. Endocrinol. 2017;8:16. doi: 10.3389/fendo.2017.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng W, Han F, Shi Y. Neonatal isolation modulates glucocorticoid-receptor function and synaptic plasticity of hippocampal and amygdala neurons in a rat model of single prolonged stress. J. Affect. Disord. 2019;246:682–694. doi: 10.1016/j.jad.2018.12.084. [DOI] [PubMed] [Google Scholar]
- 54.Han F, Ding J, Shi Y. Expression of amygdala mineralocorticoid receptor and glucocorticoid receptor in the single-prolonged stress rats. BMC Neurosci. 2015;15:77. doi: 10.1186/1471-2202-15-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions*. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 56.Solomon MB, et al. Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience. 2012;203:135–143. doi: 10.1016/j.neuroscience.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Finsterwald C, Alberini CM. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: From adaptive responses to psychopathologies. Neurobiol. Learn. Mem. 2014;112:17–29. doi: 10.1016/j.nlm.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohta KI, et al. Prolonged maternal separation attenuates BDNF-ERK signaling correlated with spine formation in the hippocampus during early brain development. J. Neurochem. 2017;141:179–194. doi: 10.1111/jnc.13977. [DOI] [PubMed] [Google Scholar]
- 59.Tyler WJ, Pozzo-Miller L. Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J. Physiol. 2003;553:497–509. doi: 10.1113/jphysiol.2003.052639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsutani S, Yamamoto N. Brain-derived neurotrophic factor induces rapid morphological changes in dendritic spines of olfactory bulb granule cells in cultured slices through the modulation of glutamatergic signaling. Neuroscience. 2004;123:695–702. doi: 10.1016/j.neuroscience.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 61.Alonso M, Medina JH, Pozzo-Miller L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn. Mem. (Cold Spring Harbor, N.Y.) 2004;11:172–178. doi: 10.1101/lm.67804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chapleau CA, Carlo ME, Larimore JL, Pozzo-Miller L. The actions of BDNF on dendritic spine density and morphology in organotypic slice cultures depend on the presence of serum in culture media. J. Neurosci. Methods. 2008;169:182–190. doi: 10.1016/j.jneumeth.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Padival MA, Blume SR, Rosenkranz JA. Repeated restraint stress exerts different impact on structure of neurons in the lateral and basal nuclei of the amygdala. Neuroscience. 2013;246:230–242. doi: 10.1016/j.neuroscience.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Revest JM, et al. BDNF-TrkB signaling through Erk1/2 MAPK phosphorylation mediates the enhancement of fear memory induced by glucocorticoids. Mol. Psychiatry. 2014;19:1001–1009. doi: 10.1038/mp.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daskalakis NP, De Kloet ER, Yehuda R, Malaspina D, Kranz TM. Early life stress effects on glucocorticoid-BDNF interplay in the hippocampus. Front. Mol. Neurosci. 2015;8:68. doi: 10.3389/fnmol.2015.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McEwen BS. Preserving neuroplasticity: Role of glucocorticoids and neurotrophins via phosphorylation. Proc. Natl. Acad. Sci. U. S. A. 2015;112:15544–15545. doi: 10.1073/pnas.1521416112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cutuli D, et al. Effects of pre-reproductive maternal enrichment on maternal care, offspring's play behavior and oxytocinergic neurons. Neuropharmacology. 2019;145:99–113. doi: 10.1016/j.neuropharm.2018.02.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.