Abstract
Aims
There is mixed evidence for an association between depression and/or anxiety (DA) and carotid intima-media thickness (IMT), and limited information on the related role of dyslipidaemia. Here we report associations between DA and IMT in the Whitehall II cohort, considering the moderating effects of sex and dyslipidaemia.
Methods
2,822 males and 1,112 females (61±6 years) were studied during phase 7 (2002–4) of the Whitehall II study. IMT and lipid levels were assessed, and questionnaires (General Health Questionnaire [GHQ] and Centre for Epidemiologic Studies Depression Scale [CES-D]) completed. Linear regression was used to explore relationships between DA and IMT and the moderating effects of sex and dyslipidaemia.
Results
1,461 participants were categorised with DA. The association between DA and IMT differed between men and women so analyses were undertaken separately by sex. In men, IMT was significantly associated with dyslipidaemia (p=0.002) but not DA (p=0.29). In women, both dyslipidaemia and DA were independently associated with IMT (p=0.028 & p=0.031). The greatest IMT was in women with both DA and dyslipidaemia. These results were replicated when GHQ score was substituted for DA and non-HDL cholesterol for dyslipidaemia.
Conclusions
DA is associated with increased IMT in women but not in men. Dyslipidaemia is associated with IMT in both men and women. Women with both DA and dyslipidaemia are potentially at the greatest risk of CVD.
Keywords: Depression, Anxiety, Intima-media thickness, lipids
Introduction
Depression and anxiety, which have a high level of comorbidity, are associated with increased risk of cardiovascular disease within the general population and risk of recurrent coronary events.1–4 However, whether these chronic stressors trigger cardiovascular events in those with underlying disease and/or contribute to the development of atherosclerotic disease is unclear.1–3, 5
Depression and/or anxiety (DA) disorders are more prevalent in women, whereas cardiovascular disease (CVD) is traditionally more prevalent in men,6, 7 with conflicting associations between depression and CVD in men and women.8–11
Increased intima-media thickness (IMT) reflects sub-clinical arterial disease and predicts clinical cardiovascular events.12 Both depression and anxiety have been associated with increased IMT in some studies but others found no association, or differing results by sex.13–18
Dyslipidaemia is a key determinant of atherosclerosis and is associated with increased IMT.19 However, the relationship between DA and dyslipidaemia is less clear.20, 21 Regardless of how DA influences CVD risk factors, the effects of DA on atherogenesis may be greater in those with risk factors (e.g. dyslipidaemia), but have not been investigated specifically.
To investigate this issue, we hypothesised that DA would be associated with carotid IMT, that this relationship differed by sex and would be stronger among participants with dyslipidaemia.
Patients and methods
Participants
The Whitehall II cohort comprised 10,308 civil servants recruited between 1985 and 1988, undergoing regular follow-up. In 2002–4 (Phase 7), 4,097 participants completed questionnaires including questions regarding depression and anxiety symptoms and underwent assessment of cardiovascular disease risk factors, disease status, body habitus and carotid artery IMT (Supplementary Figure 1). The University College London Medical School committee on the ethics of human research provided ethical approval.
Carotid intima-media thickness
The IMT of the far wall of both common carotid arteries was measured. The mean of the two arteries was taken (supplement).
Depression and/or anxiety
Participants completed the General Health Questionnaire (GHQ) and the Centre for Epidemiologic Studies Depression Scale (CES-D) (supplement) and were categorised thereby into two groups as having DA or neither. Depression was defined if meeting ≥1 of the following criteria: score ≥4 on GHQ depression subscale; score ≥16 on the CES-D scale; prior diagnosis of depression; prescribed antidepressant medications. Anxiety was defined if meeting ≥1 of the following criteria: score ≥5 on GHQ anxiety subscale and/or use of anxiolytic medication.
Dyslipidaemia
Fasting blood was collected for total cholesterol (TC), high density lipoprotein (HDL-C) and triglycerides (Trigs); low density lipoprotein (LDL-C) was calculated using the Friedewald formula; non-HDL-C was calculated from TC and HDL-C22. Participants were categorized with dyslipidaemia if meeting ≥1 of the following: TC >6.0, Trigs>1.7, HDL-C<1.0 and LDL-C>4.0mmol/L or receiving lipid lowering therapy. Glucose, C-reactive protein (CRP) and interleukin-6 (IL-6) were also measured (supplement).
Other covariates
Anthropometric measures including height, weight, hip and waist measurements were taken and body mass index (BMI) was calculated. Seated brachial blood pressure was assessed using an automated Omron 907 device.
Statistical analysis
In brief, right-skewed distributions were log transformed and cardiovascular risk factors compared between those included and excluded from the analytical sample using standard tests. The relationship between DA and IMT was explored using multiple regression and adjusted for cardiovascular risk factors. Interaction terms between DA and sex showed that the effects of DA on IMT differed between men and women. Subsequent analyses, therefore, were conducted separately in men and women. (Full details in supplement).
Results
The cohort consisted of 3,934 participants with complete data on IMT, GHQ score and all covariates (supplementary Figure 1). These participants did not differ in age from the 2,521 who were not included in this sample, but had a more favourable cardiovascular risk profile (Supplementary Table 1). Study population characteristics are shown by sex in Table 1 (combined in Supplementary Table 1).
Table 1:
Participant characteristics of the whole population and divided by sex.
| Mean ± Standard deviation / Percentage (N) | ||
|---|---|---|
| Men (n=2822) | Women (n=1112) | |
| Age (yrs) | 61.1 ± 5.8 | 60.9 ± 5.9 |
| Waist circumference (cm) | 93.7 ± 10.4 | 82.8 ± 12.4 |
| Body mass index (kg/m2) | 26.4 ± 3.7 | 26.7 ± 5.1 |
| Low employment grade | 3.5% (99) | 26.5% (295) |
| Current smokers | 6.2% (174) | 8.4% (92) |
| Antidepressants | 2.5% (69) | 4.4% (49) |
| Anxiolytics | 0.3% (8) | 0.4% (4) |
| Lipid lowering therapy | 12.0% (337) | 9.1% (101) |
| Antihypertensive therapy | 22.6% (637) | 23.7% (263) |
| Systolic blood pressure (mmHg) | 127.4 ± 15.4 | 125.2 ± 173 |
| Diastolic blood pressure (mmHg) | 74.2 ± 10.2 | 72.5 ± 10.4 |
| Heart rate (bpm) | 66.6 ± 11.6 | 67.8 ± 9.7 |
| Fasting glucosea (mmol/L) | 5.43 ± 0.16 | 5.20 ± 0.15 |
| Total cholesterol (mmol/L) | 5.63 ± 0.99 | 5.92 ± 1.01 |
| HDL-cholesterol (mmol/L) | 1.48 ± 0.39 | 1.82 ± 0.49 |
| Triglycerides (mmol/L) | 1.41 ± 0.90 | 1.21 ± 0.68 |
| LDL-cholesterol (mmol/L) | 3.51 ± 0.91 | 3.54 ± 0.96 |
| NonHDL-cholesterol (mmol/L) | 4.15 ± 1.01 | 4.09 ± 1.06 |
| Dyslipidaemia | 60% (1692) | 58% (647) |
| C-reactive proteina (mg/L) | 1.21 ± 1.06 | 1.43 ± 1.13 |
| Interleukin 6a (pg/ml) | 1.88 ± 0.57 | 1.75 ± 0.56 |
| Depression and/or anxiety | 33% (937) | 47% (524) |
| General health questionnaire scoreb | 2.59 ± 5.13 | 3.34 ± 5.80 |
| Intima-media thickness (mm) | 0.80 ± 0.16 | 0.77 ± 0.14 |
HDL = high density lipoprotein, LDL = low density lipoprotein
Estimates shown for fasting glucose, C-reactive protein and interleukin 6 are the geometric mean ± standard deviation of the logged values.
Estimates shown are for untransformed GHQ. Log(GHQ+1.0) transformation used in all other analyses.
Relationship between depression and/or anxiety and IMT
1,461(37%) participants (937 [33%] men and 524 [47%] women) were categorised with DA.
IMT was correlated with all risk factors, whereas DA was negatively correlated with age, systolic blood pressure and IMT (Supplementary Table 2).
In men and women combined, unadjusted analysis showed IMT was negatively associated with DA (Cohen’s D=−0.09, p=0.006). However, adjustment for cardiovascular risk factors removed this association (p=0.96). Inclusion of the interaction variable “DA by sex” demonstrated that the association of DA with IMT differed between men and women (sex*DA interaction, p=0.016). Therefore, further analyses were conducted separately for men and women. Adjustment for the presence of dyslipidaemia confirmed the sex differences in the relationship between DA and IMT (Table 2).
Table 2.
Association between IMT and, (A) depression and/or anxiety and dyslipidaemia, and (B) depression and/or anxiety and non-HDL, separately in men and women
| Men | Women | |||
|---|---|---|---|---|
| N | Mean IMTa (95%CI) | N | Mean IMTa (95%CI) | |
| Analysis A | ||||
| Depression and/or anxiety | ||||
| No | 1885 | 0.798 (0.790, 0.805) | 588 | 0.766 (0.755, 0.776) |
| Yes | 937 | 0.791 (0.781, 0.801) | 524 | 0.782 (0.771, 0.793) |
| p-value | 0.27 | 0.031 | ||
| Dyslipidaemia | ||||
| No | 1130 | 0.785 (0.775, 0.794) | 465 | 0.765 (0.753, 0.777) |
| Yes | 1692 | 0.803 (0.796, 0.811) | 647 | 0.783 (0.773, 0.793) |
| p-value | 0.002 | 0.028 | ||
| Analysis B | ||||
| Depression and/or anxiety | ||||
| No | 1671 | 0.794 (0.786, 0.801) | 536 | 0.764 (0.753, 0.775) |
| Yes | 814 | 0.787 (0.777, 0.797) | 475 | 0.782 (0.771, 0.794) |
| p-value | 0.32 | 0.025 | ||
| Non-HDL | ||||
| Lowest third (≤ 3.6) | 642 | 0.776 (0.764, 0.788) | 340 | 0.761 (0.748, 0.775) |
| Middle third (3.7 – 4.5) | 945 | 0.788 (0.778, 0.797) | 337 | 0.771 (0.757, 0.785) |
| Highest third (> 4.5) | 898 | 0.808 (0.798, 0.818) | 334 | 0.786 (0.772, 0.801) |
| p-value (trend) | <0.001 | 0.016 | ||
Associations of depression and/or anxiety and dyslipidaemia (Analysis A) and of depression and/or anxiety and non-HDL (Analysis B), are mutually adjusted. All analyses are also adjusted for age, systolic blood pressure, waist circumference, body mass index, fasting glucose, c-reactive protein, interleukin-6, smoking status and socio-economic status.
In men, there was an association between dyslipidaemia and IMT (Cohen’s D=0.12, p=0.002), but no association between DA and IMT (p=0.27) plus no interaction between dyslipidaemia and DA on IMT (p=0.50).
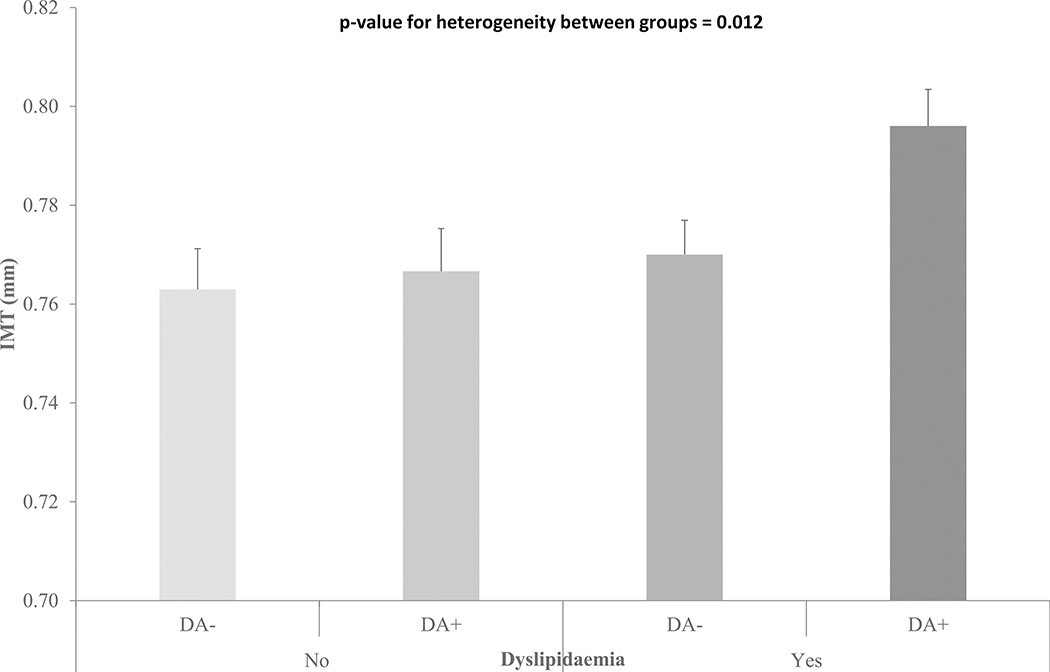
However, in women there were independent associations between IMT and both dyslipidaemia (Cohen’s D=0.14, p=0.028) and DA (Cohen’s D=0.13, p=0.031), but no evidence of an interaction between dyslipidaemia and DA on IMT (p=0.15). Thus, women with both DA and dyslipidaemia have greater IMT than those in other categories (Figure 1). Further adjustment for use of antihypertensive medication did not affect these findings (data not shown). The associations between DA and IMT were unaffected following sensitivity analysis using a more extreme cut-off for anxiety (Supplementary Table 5). When the DA group was separated into D only, A only and D+A, there was no overall difference in IMT for either men or women (supplementary Table 3). However, women with D+A had greater IMT than those with neither (Cohen’s D=0.17 p=0.047). This was replicated in the sensitivity analysis using a greater threshold for anxiety and resulted in greater separation in IMT between those with D+A versus neither (Cohen’s D=0.22 p=0.025) (Supplementary Table 5).
Figure 1:
IMT in women with and without DA and dyslipidaemia. IMT is adjusted for age, systolic blood pressure, waist circumference, body mass index, fasting glucose, c-reactive protein, interleukin-6, smoking status and socio-economic status (Bars show standard errors of the means).
We also explored the relationship between non-HDL-C, DA and sex (excluding those on lipid lowering therapy). The association between DA and IMT still differed by sex (DA by sex interaction, Cohen’s D=0.19, p=0.013), therefore, the association between DA and non-HDL-C was examined separately in men and women.
Furthermore, consideration of non-HDL-C confirmed the differences in the effect of DA on IMT between the sexes (Table 2). In men, there was a strong positive association between non-HDL-C and IMT (Cohen’s D=0.22, p<0.001), but no association between DA and IMT (p=0.32). There was no evidence for an interaction effect between non-HDL-C and DA on IMT (p=0.93). In women, both non-HDL-C (Cohen’s D=0.20, p=0.016) and DA (Cohen’s D=0.14, p=0.025) were independently associated with IMT with no interaction (p=0.70). Thus, women with both DA and high non-HDL-C have greater IMT than those in other categories (as for women with DA and dyslipidaemia).
Relationship between GHQ score and IMT
GHQ score was higher in women than men (Cohen’s D=0.17, p<0.001), positively correlated with BMI, and negatively correlated with age, systolic and diastolic blood pressure and IMT (Supplementary Table 4). However, following adjustment for major cardiovascular risk factors, this association between GHQ score and IMT disappeared (p=0.33). The addition of an interaction term between sex and GHQ score again showed that the association of GHQ score with IMT differed between men and women (Cohen’s D=0.07, p=0.05). The results mirrored those with DA such that, in women, both dyslipidaemia (Cohen’s D=0.14, p=0.024) and GHQ score (Cohen’s D=0.08, p=0.010) were independently associated with IMT, but without interaction (p=0.82). In men, dyslipidaemia (Cohen’s D=0.13, p=0.002), but not GHQ score (p=0.93), was associated with IMT and there was no interaction (p=0.31).
Discussion
Our principal findings are finding that women with both DA and dyslipidaemia have greater IMT than women within the other categories. Sex had a moderating effect on the relationship between DA and IMT: women with DA were more likely to have greater IMT than those without DA, but no such relationship is seen in men.
Sex differences in the associations of depression and/or anxiety with IMT
Our finding of a moderating effect of sex on the association between DA and IMT with higher IMT in women with DA agrees with previous work.17, 23 However, studies tended to consider depression or anxiety individually, despite co-morbidity between the two conditions. In a middle-aged Korean cohort, females with depressive symptoms had greater IMT, but there was no such difference in males.17 In contrast, in a Hispanic cohort, a moderating effect of sex on IMT in depression was only found in males.16 The reasons for these differences are unclear, but neurological influences may contribute. Women appear to have greater amygdala activity in response to negative emotion, linked to greater prevalence of depression.24 A small study found that greater amygdala activity, in response to behaviourally relevant stimuli, is associated with increased IMT.25 Loss of the protective effect of oestrogen in postmenopausal women may also contribute as IMT progression accelerates beyond the age of 50,26 as may methodological differences in the assessment of cIMT and DA symptoms.16, 17 Furthermore, depression develops earlier in women, potentially extending exposure promoting subclinical atherogenesis.27
Dyslipidaemia, depression and/or anxiety
The observation that women with DA who also have dyslipidaemia had greater IMT is particularly interesting. Although differences between the groups were small, the adjusted effect of DA on IMT in women is similar in magnitude to that of dyslipidaemia in both sexes (according to both absolute and Cohen’s D values). Thus, the cumulative combined effect on the arteries could have a clinically significant impact over time, as seen in both the healthy population and those with pre-existing coronary disease.1, 2, 20, 21 Our findings identify a group of older women with high lipid levels and DA symptoms, that are potentially at greater risk of future cardiovascular events. Analyses categorising the participants into the separate depression, anxiety or depression and anxiety indicated that both groups with depression had greater IMT, but it was only the combined group where this difference was significant when compared with those without either depression or anxiety. However, diminishing numbers in these subgroups limits analysis power.
Our study explored a prespecified hypothesis that the presence of dyslipidaemia, itself associated with subclinical vascular pathophysiology and worse cardiovascular outcome,19, 28, 29 would be associated with DA and arterial disease. Patients with depression or anxiety have been shown to have increased lipid levels and increased risk of cardiovascular disease1, 2, 21 and patients with more severe symptoms of DA are more prone to dyslipidaemia alongside obesity.20 Notably, we found the relationship between dyslipidaemia and IMT remained after adjustment for obesity measures. The combination of depression and dyslipidaemia resulting in the greatest IMT in these women may be due to a mixture of lifestyle factors, and biological mechanisms such as inflammation (although we found no evidence of a further interaction between DA and CRP or IL6 [data not shown]), as well as other possible psychophysiological influences on lipid metabolism.
Little previous work has explored the impact of specific cardiovascular risk factors on the relationship between DA and subclinical vascular pathophysiology. Violanti et al found an association between depressive symptoms and IMT but only in those without hypertension.30 Wagner et al found that type 2 diabetes had no impact on the association between lifetime history of depression and impaired endothelial function.31 Therefore, the finding that women with DA and dyslipidaemia have greater IMT, may have important clinical implications, suggesting that there may be a particular benefit in treating dyslipidaemia in women with DA. Our findings confirm the importance of dyslipidaemia in men but suggest no incremental impact of DA on atherogenesis. We hypothesised that dyslipidaemia would be associated with subclinical arterial disease (cIMT) in DA in our prespecified analysis plan, rather than undertaking a “hypothesis-generating” exploratory analysis of multiple primary risk factors of interest (e.g. smoking, hypertension, diabetes, inflammation), but fully adjusted for these parameters in our multivariable analyses.
Potential mechanisms
A number of potential mechanisms may mediate the risk of cardiovascular disease in depression/anxiety, including increased hypothalamic-pituitary-adrenal axis activity (HPA) and inflammation, which promote cardiovascular and platelet dysfunction.32–34
Lifestyle behaviours including cigarette smoking, lower physical activity and poor diet are all associated with common mental disorders and increased cardiovascular risk3, 35 through creation of an atherogenic vascular milieu. Although we adjusted for socioeconomic status, smoking and obesity, other less well-characterised “lifestyle”-related factors may have contributed.
Limitations
IMT was only assessed during Phase 7 of the WHII study. Therefore, we were only able to explore cross-sectional but not prospective relationships between DA and IMT. The depression group is a composite of participants, including those with symptoms meeting depression ‘cut-offs’, those previously diagnosed as depressed and those taking anti-depressant medication. Thus, a wide range of symptom severity from mild- to severe clinical- depression, may have differing relationships with the vascular measures. Not all participants would have currently been undergoing a depressive episode, as participants previously diagnosed with depression were included in the depression group. Equally, both the GHQ and CES-D questionnaires only concern recent symptoms and so may miss those participants who have previously had a biologically relevant period of DA. This study also could not discriminate single or recurrent episodes of DA. These limitations also apply to some extent to those with anxiety.
DA was examined as a combined variable due to the considerable overlap in conditions. However, we did consider depression and anxiety conditions separately and combined but found no overall differences in IMT between the four symptom groups. Whether these tools for identifying DA symptoms are sensitive enough to fully disentangle A from D is uncertain. Furthermore, splitting the analysis by gender and DA category reduces the group size considerably and we cannot be sure that the study was sufficiently powered to assess the separate effect of D and A. A larger prospective cohort would be required. Despite this, most of the associations described in women have small effect sizes, below or close to 0.2 as indicated by Cohen’s D measure of the standardised effect (albeit similar in magnitude to the relationship between dsylipidaemia and IMT in men). Confirmatory findings from other studies are needed before these results could be generalised.
Only a small proportion of those classified with DA were taking psychotropic medication, similar to prior observations in EUROASPIRE cardiac patients.36 This may partly be due to our only assessing current, but not prior, symptoms and use of psychotropic medication; plus a large proportion of those with DA were likely to subclinical or mild symptomology. There may also have been a reluctance to declare psychotropic medication use due to perceived stigma.
As this was a relatively healthy population, the dyslipidaemia definition was quite broad, therefore participants meeting the criteria did not necessarily have severe dyslipidaemia. The lipid cut-offs were based on European Guidelines and associated with increased cardiovascular risk.37 Using more stringent lipid levels would reduce sample size, with consequent loss of power. However, similar relationships were observed when restricting the analysis to non-HDL-C.
Conclusion
In conclusion, we found that women with DA and dyslipidaemia have increased IMT. Regardless of the presence or absence of dyslipidaemia, DA did not have a significant effect on IMT in men.
Supplementary Material
Acknowledgements
We thank all of the participants of civil service departments; their welfare personnel and establishment officers; the British Occupational Health and Safety Agency; the British Council of Civil Service Unions; all civil servants who participated in the Whitehall II study; and all members of the Whitehall II study team.
Funding
This work was supported by the UK Medical Research Council [MR/R024227/1]; the US National Institute on Aging [NIA, R01AG056477], the British Heart Foundation [RG/16/11/32334]; and European Commission [LIFEPATH 633666]. MS is partly supported by the British Heart Foundation.
Footnotes
Conflict of interest: none declared
References
- 1.Nicholson A, Kuper H and Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. European Heart Journal 2006; 27: 2763–2774. 2006/11/04. DOI: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 2.Roest AM, Martens EJ, de Jonge P, et al. Anxiety and Risk of Incident Coronary Heart Disease: A Meta-Analysis. Journal of the American College of Cardiology 2010; 56: 38–46. DOI: 10.1016/j.jacc.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA 2008; 300: 2379–2388. DOI: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen SS, von Kanel R, Tully PJ, et al. Psychosocial perspectives in cardiovascular disease. Eur J Prev Cardiol 2017; 24: 108–115. 2017/06/18. DOI: 10.1177/2047487317703827. [DOI] [PubMed] [Google Scholar]
- 5.Brunner EJ, Shipley MJ, Britton AR, et al. Depressive disorder, coronary heart disease, and stroke: dose–response and reverse causation effects in the Whitehall II cohort study. European Journal of Preventive Cardiology 2014; 21: 340–346. DOI: 10.1177/2047487314520785. [DOI] [PubMed] [Google Scholar]
- 6.King M, Nazareth I, Levy G, et al. Prevalence of common mental disorders in general practice attendees across Europe. The British Journal of Psychiatry 2008; 192: 362–367. 10.1192/bjp.bp.107.039966. [DOI] [PubMed] [Google Scholar]
- 7.British Heart Foundation. Cardiovascular Disease Statistics 2017. (2017).
- 8.Haukkala A, Konttinen H, Uutela A, et al. Gender Differences in the Associations Between Depressive Symptoms, Cardiovascular Diseases, and All-Cause Mortality. Annals of Epidemiology 2009; 19: 623–629. DOI: 10.1016/j.annepidem.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Mendes de Leon CF, Krumholz HM, Seeman TS, et al. Depression and risk of coronary heart disease in elderly men and women: New Haven EPESE, 1982–1991. Established Populations for the Epidemiologic Studies of the Elderly. Arch Intern Med 1998; 158: 2341–2348. 1998/11/25. [DOI] [PubMed] [Google Scholar]
- 10.Penninx BWJH, Guralnik JM, de Leon CF Mendes, et al. Cardiovascular Events and Mortality in Newly and Chronically Depressed Persons >70 Years of Age. The American Journal of Cardiology 1998; 81: 988–994. DOI: 10.1016/S0002-9149(98)00077-0. [DOI] [PubMed] [Google Scholar]
- 11.Ferketich AK, Schwartzbaum JA, Frid DJ, et al. Depression as an antecedent to heart disease among women and men in the NHANES I study. National Health and Nutrition Examination Survey. Archives of Internal Medicine 2000; 160: 1261–1268. 2000/05/16. [DOI] [PubMed] [Google Scholar]
- 12.Lorenz MW, Markus HS, Bots ML, et al. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 2007; 115: 459–467. [DOI] [PubMed] [Google Scholar]
- 13.Faramawi MF, Gustat J, Wildman RP, et al. Relation Between Depressive Symptoms and Common Carotid Artery Atherosclerosis in American Persons ≥65 Years of Age†. The American Journal of Cardiology 2007; 99: 1610–1613. DOI: 10.1016/j.amjcard.2006.12.090. [DOI] [PubMed] [Google Scholar]
- 14.Elovainio M, Keltikangas-Jarvinen L, Kivimaki M, et al. Depressive Symptoms and Carotid Artery Intima-Media Thickness in Young Adults: The Cardiovascular Risk in Young Finns Study. Psychosomatic Medicine 2005; 67: 561–567. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Sun D, Wang B, et al. The relationship of depressive symptoms and functional and structural markers of subclinical atherosclerosis: A systematic review and meta-analysis. Eur J Prev Cardiol 2018; 25: 706–716. 2018/03/14. DOI: 10.1177/2047487318764158. [DOI] [PubMed] [Google Scholar]
- 16.Chirinos D, Medina-Lezama J, Salinas-Najarro B, et al. Depressive symptoms and carotid intima–media thickness in South American Hispanics: results from the PREVENCION study. Journal of Behavioral Medicine 2015; 38: 284–293. DOI: 10.1007/s10865-014-9599-9. [DOI] [PubMed] [Google Scholar]
- 17.Lee Y-H, Shin M-H, Choi J-S, et al. Gender Differences in the Association between Depressive Symptoms and Carotid Atherosclerosis among Middle-Aged and Older Koreans: The Namwon Study. Journal of Korean Medical Science 2014; 29: 1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rice SC, Zonderman AB, Metter EJ, et al. Absence of Relation Between Depressive Symptoms and Carotid Intimal Medial Thickness in the Baltimore Longitudinal Study of Aging. Psychosomatic Medicine 2009; 71: 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tonstad S, Joakimsen O, Stensland-Bugge E, et al. Carotid intima–media thickness and plaque in patients with familial hypercholesterolaemia mutations and control subjects. European Journal of Clinical Investigation 1998; 28: 971–979. DOI: 10.1046/j.1365-2362.1998.00399.x. [DOI] [PubMed] [Google Scholar]
- 20.Van Reedt Dortland AKB, Giltay EJ, Van Veen T, et al. Metabolic syndrome abnormalities are associated with severity of anxiety and depression and with tricyclic antidepressant use. Acta Psychiatrica Scandinavica 2010; 122: 30–39. DOI: 10.1111/j.1600-0447.2010.01565.x. [DOI] [PubMed] [Google Scholar]
- 21.Pizzi C, Manzoli L, Mancini S, et al. Analysis of potential predictors of depression among coronary heart disease risk factors including heart rate variability, markers of inflammation, and endothelial function. European Heart Journal 2008; 29: 1110–1117. 10.1093/eurheartj/ehn137. [DOI] [PubMed] [Google Scholar]
- 22.Friedewald WT, Levy RI and Fredrickson DS. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clinical Chemistry 1972; 18: 499–502. [PubMed] [Google Scholar]
- 23.Santos IS, Goulart AC, Brunoni AR, et al. Anxiety and depressive symptoms are associated with higher carotid intima-media thickness. Cross-sectional analysis from ELSA-Brasil baseline data. Atherosclerosis 2015; 240: 529–534. DOI: 10.1016/j.atherosclerosis.2015.04.800. [DOI] [PubMed] [Google Scholar]
- 24.Stevens JS and Hamann S. Sex differences in brain activation to emotional stimuli: A meta-analysis of neuroimaging studies. Neuropsychologia 2012; 50: 1578–1593. DOI: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Gianaros PJ, Hariri AR, Sheu LK, et al. Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biol Psychiatry 2009; 65: 943–950. 2008/11/18. DOI: 10.1016/j.biopsych.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozàkovà M, Palombo C, Morizzo C, et al. Gender-specific differences in carotid intima-media thickness and its progression over three years: A multicenter European study. Nutrition, Metabolism and Cardiovascular Diseases 2013; 23: 151–158. DOI: 10.1016/j.numecd.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Salk RH, Hyde JS and Abramson LY. Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychological bulletin 2017; 143: 783–822. 2017/04/27. DOI: 10.1037/bul0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullen MJ, Kharbanda RK, Cross J, et al. Heterogenous Nature of Flow-Mediated Dilatation in Human Conduit Arteries In Vivo : Relevance to Endothelial Dysfunction in Hypercholesterolemia. Circulation Research 2001; 88: 145–151. [DOI] [PubMed] [Google Scholar]
- 29.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherocslerosis. Lancet 1992; 340: 1111–1115. [DOI] [PubMed] [Google Scholar]
- 30.Violanti J, Charles L, Gu J, et al. Depressive symptoms and carotid artery intima-media thickness in police officers. International Archives of Occupational and Environmental Health 2013; 86: 931–942. DOI: 10.1007/s00420-012-0829-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner J, Tennen H, Finan P, et al. Lifetime History of Depression, Type 2 Diabetes, and Endothelial Reactivity to Acute Stress in Postmenopausal Women. International Journal of Behavioral Medicine 2012; 19: 503–511. DOI: 10.1007/s12529-011-9190-5. [DOI] [PubMed] [Google Scholar]
- 32.Broadley AJM, Korszun A, Abdelaal E, et al. Metyrapone Improves Endothelial Dysfunction in Patients With Treated Depression. Journal of the American College of Cardiology 2006; 48: 170–175. [DOI] [PubMed] [Google Scholar]
- 33.Vogelzangs N, Beekman ATF, de Jonge P, et al. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry 2013; 3: e249 Original Article. DOI: 10.1038/tp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kivimäki M, Lawlor DA, Juonala M, et al. Lifecourse Socioeconomic Position, C-Reactive Protein, and Carotid Intima-Media Thickness in Young Adults: The Cardiovascular Risk in Young Finns Study. Arteriosclerosis, Thrombosis, and Vascular Biology 2005; 25: 2197–2202. DOI: 10.1161/01.ATV.0000183729.91449.6e. [DOI] [PubMed] [Google Scholar]
- 35.Bonnet F, Irving K, Terra J-L, et al. Anxiety and depression are associated with unhealthy lifestyle in patients at risk of cardiovascular disease. Atherosclerosis 2005; 178: 339–344. DOI: 10.1016/j.atherosclerosis.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 36.Pogosova N, Kotseva K, De Bacquer D, et al. Psychosocial risk factors in relation to other cardiovascular risk factors in coronary heart disease: Results from the EUROASPIRE IV survey. A registry from the European Society of Cardiology. Eur J Prev Cardiol 2017; 24: 1371–1380. 2017/05/23. DOI: 10.1177/2047487317711334. [DOI] [PubMed] [Google Scholar]
- 37.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol 2016; 23: Np1–np96. 2016/06/30. DOI: 10.1177/2047487316653709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.