Abstract
We analyzed 44 patients with newly diagnosed systemic light-chain amyloidosis (AL) and del 17p, a rare finding in AL. Predictors of overall and progression-free survival were cardiac involvement at diagnosis and hematologic response to therapy, respectively. Median survivals of patients with > 50% and ≤ 50% del 17p plasma cells were 28 and 52 months (P = .08).
Introduction:
Deletion 17p (del 17p) portends a poor prognosis in myeloma, but its significance in light-chain amyloidosis is unknown.
Patients and Methods:
We identified patients with light-chain amyloidosis and del 17p at diagnosis, and analyzed presenting characteristics, treatments, and clinical outcomes. All had baseline biopsy results showing amyloid and serologic and marrow studies, including standard fluorescence in-situ hybridization determinations of del 17p using commercial probes. Consensus criteria for hematologic and organ involvement, progression, and response were used. Kaplan-Meier (log rank) analyses and Cox regression analysis of baseline variables were used to identify predictors of overall and progression-free survival (PFS). Six-month landmark analyses were performed to assess the impact of treatment-related variables.
Results:
We identified 44 patients from 7 countries with median marrow and del 17p plasma cells of 22% (range, 3%–100%) and 30% (2%–93%). Ninety-five percent had cardiac involvement, including 44% stage III. Two-thirds of the patients initially received bortezomib-based therapy. Forty-nine percent of patients experienced complete response or very good partial response, with median time to best response of 4 months (range, 1–28 months). Median overall survival and PFS were 49 and 32 months. Cardiac stage and hematologic response were the key predictors of outcomes. Patients with > 50% and ≤ 50% del 17p in clonal plasma cells had median survivals of 28 and 52 months, respectively (P = .08). In landmark analyses, only hematologic response predicted both overall survival and PFS.
Conclusion:
Cardiac stage, hematologic response, and del 17p percentage impact outcomes in these cases. Emphasis should be placed on optimizing supportive care and achieving a deep hematologic response.
Keywords: AL amyloidosis, Deletion 17p, FISH cytogenetics, Plasma cells, Prognosis
Introduction
Light-chain amyloidosis (AL) is a rare clonal plasma-cell neoplasm that causes morbidity and mortality through toxic effects of misfolded light chains and deposition of amyloid fibrils affecting key organs such as the heart. In multiple myeloma, age, renal failure, and chromosomal abnormalities are powerful predictors of overall survival (OS), and deletion 17p (del 17p) is considered a high-risk abnormality because it has been associated with shorter survival.1 However, depth of response to treatment can mitigate this association and enable those high-risk patients who experience complete responses (CRs) and negative minimal residual disease status to live significantly longer.2
Bone marrow cytogenetic and fluorescence in-situ hybridization (FISH) studies are part of the standard staging evaluation in multiple myeloma, but not in other plasma-cell disorders such as monoclonal gammopathy of undetermined significance. Our understanding of the role for such studies in AL is evolving.3,4 Several retrospective studies have characterized the significance of cytogenetic abnormalities in AL.5–8 Abnormal FISH was identified as a risk factor for death,5 and gain 1q216 and translocation (11;14)7,8 have both been shown to be associated with adverse outcomes. Del 17p is rarely found in AL patients, occurring at a frequency of 2% to 6%,5–7 and its significance has not been well defined.
We performed a retrospective analysis of 44 AL patients with del 17p at diagnosis and analyzed the factors affecting outcomes and survival.
Patients and Methods
This study retrospectively analyzed patients with systemic AL amyloidosis with del 17p from medical centers in Germany, France, United Kingdom, Greece, Spain, Italy, and the United States. All patients had detection of del 17p by FISH due to a plasma-cell dyscrasia. Patients with concurrent monoclonal gammopathy of undetermined significance, or smoldering or symptomatic myeloma (defined as the presence of lytic lesions) and AL at diagnosis were included. AL patients with del 17p detected at relapse and symptomatic myeloma patients who developed AL at relapse were excluded. All patient data were deidentified and were obtained from each center’s institutionally approved database.
Cardiac staging was assessed by the Mayo system.9 Cardiac, hematologic, and renal responses were evaluated by previously published criteria.10–12 All other organs were evaluated by consensus guidelines.11 Cytogenetics and FISH studies used standard methods and probes used for analysis of multiple myeloma chromosomal abnormalities. Estimates of del 17p percentages were made on the basis of the percentages of clonal plasma cells in marrow aspirates and the percentages of cells deemed del 17p positive by cytopathologists at the individual centers.
Epidemiologic and clinical characteristics included age, gender, cardiac and renal staging, organs involved, echocardiographic estimates of wall thickness, marrow studies, features of monoclonal gammopathies, serial paraprotein measurements, specific therapies, hematologic responses, and survival. Results are reported by intention to treat except as otherwise noted. OS was calculated from the date of diagnosis to the date of last contact or death; one patient was censored at the time of heart transplantation. Progression-free survival (PFS) was defined as date of death, of hematologic progression, of unsatisfactory response (ie, no response), or of second-line therapy. We used Kaplan-Meier (log rank) analyses and Cox regression analysis of baseline variables to identify predictors of OS and PFS. Six-month landmark analyses were performed to assess the impact of treatment-related variables. MedCalc Statistical Software version 17.9.6 (MedCalc Software, Ostend, Belgium; http://www.medcalc.org; 2017) was used for all statistical analyses.
Results
Patients
Fifty-one cases of AL with del 17p detected in marrow plasma cells were collected from Germany (n = 17), France (n = 7), the United Kingdom (n = 2), Spain (n = 1), Greece (n = 1), Italy (n = 1), and the United States [Mayo Rochester (n = 9), Memorial Sloan Kettering Cancer Center (n = 7), and Tufts Boston (n = 6)]. Two cases with incomplete survival data and 5 cases of del 17p detected at relapse were excluded from further analyses. Characteristics of the 44 patients included are shown in Table 1. Patients were diagnosed between January 19, 2007, and May 30, 2016. Median follow-up of survivors was 31 months (range, 16–61 months).
Table 1.
Characteristics of 44 Patients
| Characteristic | Value |
|---|---|
| Age at diagnosis (years) | 65 (38–81) |
| Male | 27 (61%) |
| Concurrent MGUS or myeloma | 5 (11%) |
| Marrow PCs | 22% (3%−100%) |
| Patients with PC > 10% | 37 (84%) |
| Cytogenetics and FISH | 100% |
| del 17p | 44 (100%) |
| del 17p % (n = 36) | 30 (2–93) |
| t(11;14) | 14 (32%) |
| t(11;14) % (n = 11) | 76 (12–98) |
| del 13 | 19 (43%) |
| del 13 % (n = 11) | 67 (3–96) |
| Gain 1q21 | 7 (16%) |
| With other complex deletions or gains | 6 (14%) |
| Trisomies | 4 (9%) |
| t(4;14), t(14;16) | 4 (9%) |
| Other IgH rearrangements | 2 (5%) |
| Clonal λ light-chain isotype | 39 (89%) |
| Involved FLC (mg/L) | 295 (10.1–11,300) |
| dFLC (mg/L) | 284 (10–11,292) |
| Organs Involved | |
| Number | 2 (1–5) |
| Cardiac | 42 (95%) |
| Renal | 31 (70%) |
| Liver/GI | 20 (45%) |
| Soft tissue | 16 (36%) |
| PNS | 8 (18%) |
| AL Cardiac Stage I/II/IIIa/IIIb | 3/18/15/6 |
| NT-proBNP (pg/mL) | 4076 (59–27,547) |
| Troponin I or T greater than threshold | 16 (39%) |
| hs-cTnT (ng/L) (n = 14) | 38 (7–567) |
| hs-cTnT > 14 ng/L | 11 (27%) |
| Echocardiogram IVSd (mm) | 14 (9–22) |
| Renal Stage I/II/III | 15/12/4 |
| Proteinuria, g/24 hours | 1.9 (0–14.4) |
| Serum creatinine (mg/dL) | 1 (0.4–8.0) |
| Alkaline phosphatase (mg/dL) | 94 (43–1089) |
Data are presented as median (range) or n (%) unless otherwise indicated.
Abbreviations: dFLC = difference between involved and uninvolved free light chain; FISH = fluorescence in-situ hybridization; FLC = free light chain; hs-cTnT = highly sensitive cardiac troponin T; MGUS = monoclonal gammopathy of undetermined significance; NT-proBNP = N-terminal pro b-type natriuretic peptide; PC = plasma cell.
Cardiac involvement was present in 95% of patients, and 48% had stage III disease. The median number of marrow plasma cells was 22% (range, 3%–100%), and 84% had ≥ 10%. All had del 17p identified by FISH in marrow plasma cells; percentages of plasma cells containing del 17p were available for 36 patients, and the median was 30% (range, 2%–93%). Concomitant t(11;14), del 13, or gain 1q21 was observed in 32%, 45%, and 16% of patients. Chromosomal aberrations involving t(4;14) or t(14;16) were seen in 9% of patients.
Treatments and Response
For first-line therapy, patients received a bortezomib-based regimen (n = 28), dexamethasone and cyclophosphamide or melphalan (n = 9), and dexamethasone and lenalidomide (n = 4), or underwent stem-cell transplantation (SCT, n = 3). Nine patients underwent SCT after induction; therefore, 12 patients received SCT. Sixteen patients received second-line therapy; a variety of regimens were used including bortezomib based (n = 10, 5 of whom were naive to bortezomib), thalidomide (n = 2), cyclophosphamide (n = 1), bendamustine (n = 2), and melphalan (n = 1), all with dexamethasone.
Hematologic responses to first-line therapy were scored in 39 patients [CR/very good partial response (VGPR) 19, partial response 14, no response 6]. Median time to best response was 4 months (range, 1–28 months). Of 25 cases evaluable for cardiac responses, 28% (n = 7) experienced them, and of 23 cases evaluable for renal responses, 26% (n = 6) experienced them. Median OS and PFS were 49 months (range, 0.5–69 months) and 32 months (range, 0.5–67 months), respectively (Figure 1A and B). Two-year OS was 67%. Del 17p most commonly occurred with other cytogenetic aberrations such as del 13, t(11;14), and gain 1q21. There was no correlation between the copresence of these abnormalities and the percentage of plasma cells. However, patients with either or both t(11;14) or gain 1q21 exhibited a trend toward shorter survival; median survivals with either or neither of these abnormalities were 28 and 53 months, respectively (Figure 1C). Patients with > 50% del 17p marrow plasma cells, although small in number (n = 12), also exhibited a trend toward shorter survival compared to those with ≤ 50%; medians were 28 and 52 months, respectively (Figure 1D). One case in our series was notable because of clonal evolution into a leukemic phase with progressive amyloidosis and is described in Supplemental Data S1 in the online version.
Figure 1.
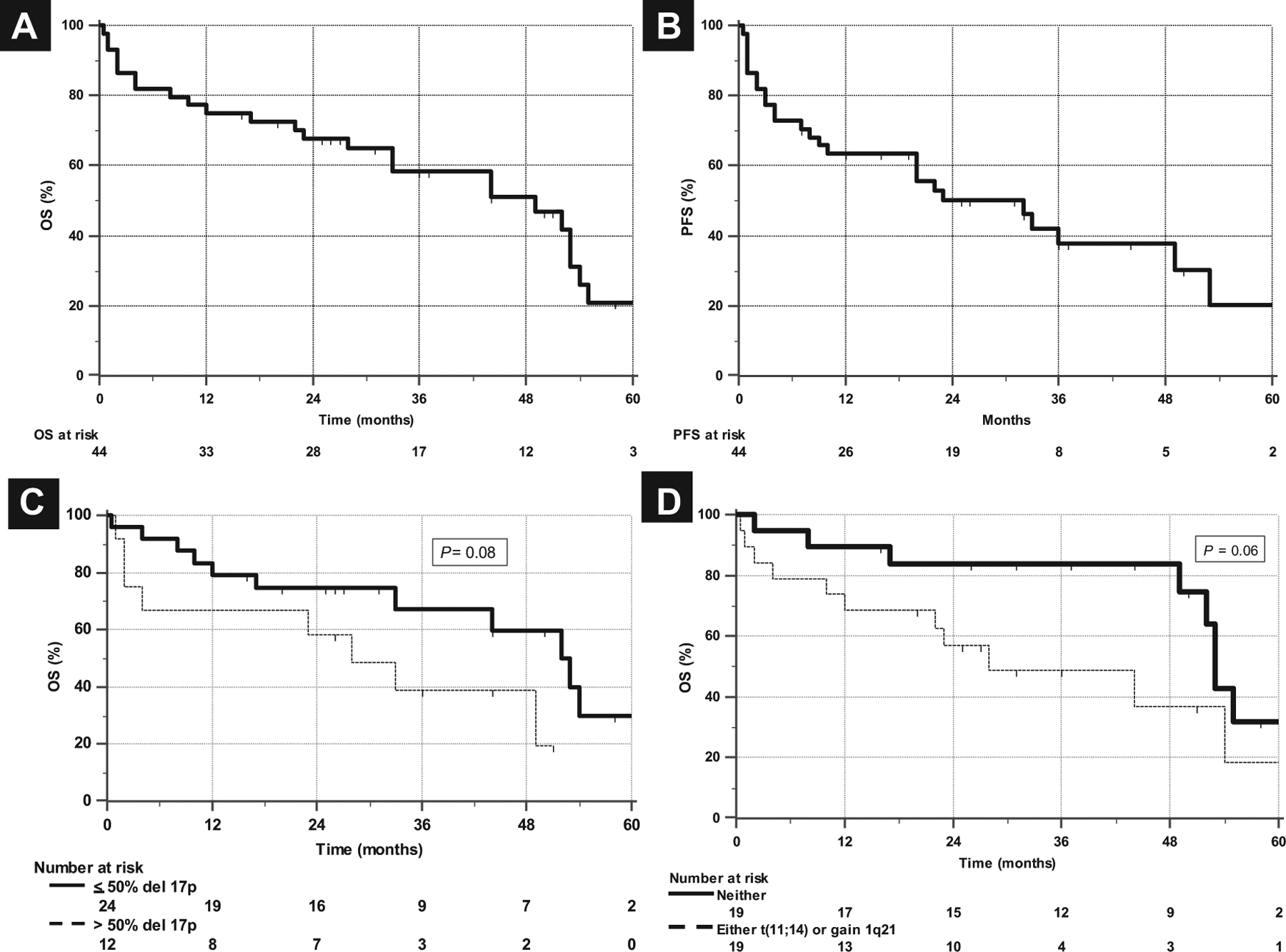
Median OS and PFS. (A) OS and (B) PFS of 44 Patients With AL and del 17p at Diagnosis, Showing Median OS of 49 Months and PFS of 32 Months. (C) Patients With > 50% del 17p Marrow Plasma Cells, Although Small in Number (n = 12), Exhibited Trend Toward Shorter Survival (Median, 28 and 52 Months). (D) Survival Curve of Patients With Either t(11;14) or Gain 1q21 Exhibited Trend Toward Shorter Survival (Median, 28 and 53 Months)
Abbreviations: AL = light-chain amyloidosis; OS = overall survival; PFS = progression-free survival.
Predictors of PFS and OS
Patients who received bortezomib-based initial treatment had a similar OS compared to those who received alternative first-line therapies (Figure 2A), while those receiving SCT had significantly better OS than those who were not treated with SCT (Figure 2B); however, patients who underwent SCT were younger and had less advanced cardiac involvement. SCT patients were a median of 60 years old (range, 38–65 years) and were from Germany (n = 6), United States (n = 5), and the United Kingdom (n = 1). Seven were cardiac stage II and three were stage IIIa. Hematologic responses in these 12 patients were CR/VGPR (n = 7), partial response (n = 4), and no response (n = 1).
Figure 2.
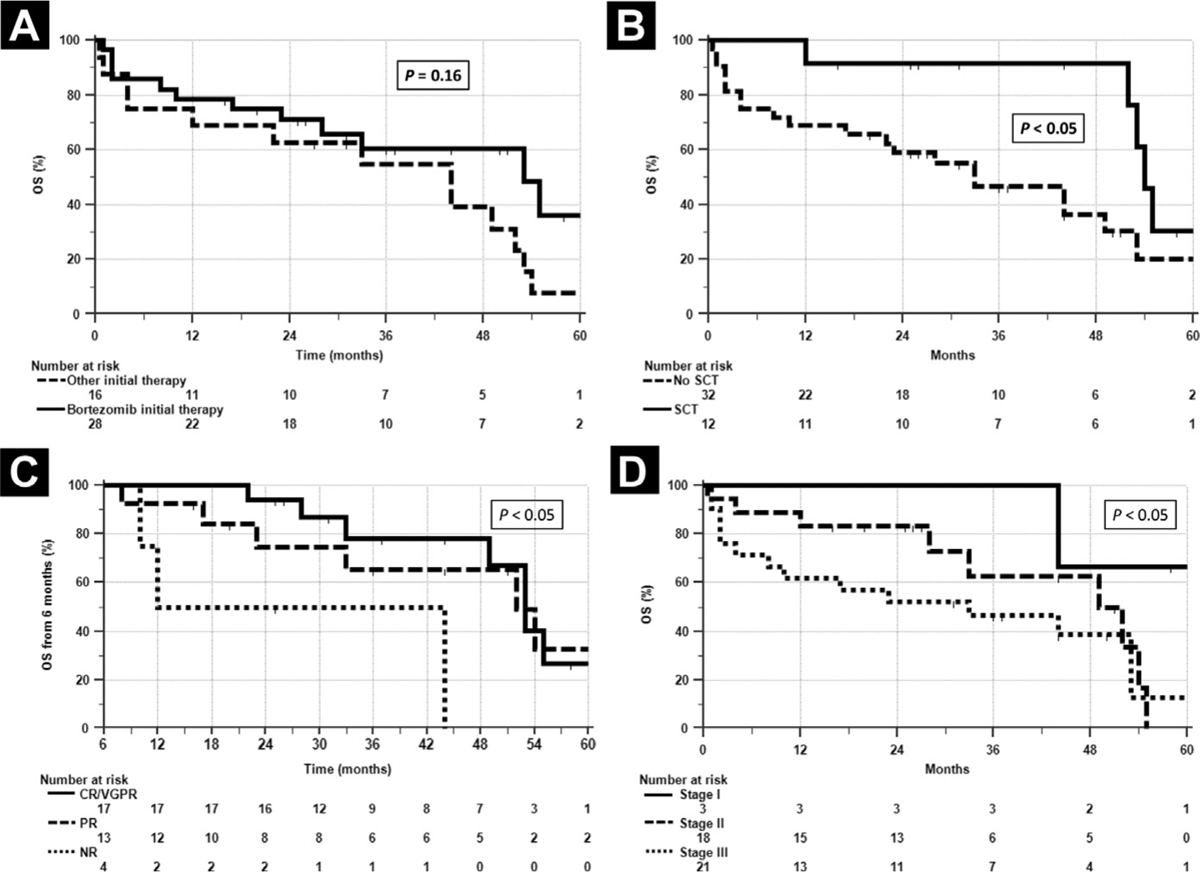
Survival According to Treatment With Bortezomib or Alternative First-Line Therapy. (A) Survival curves of Patients Initially Treated With Either Bortezomib or Other Therapies Overlap, Indicating That Initial Therapy With Bortezomib did Not Provide Significant Survival Advantage to Patients in This Small Cohort. (B) OS of Patients Who Underwent SCT compared to Those Who did Not showed Favorable Outcomes for SCT Patients (P < .05); However, Patients Who Underwent SCT Were Younger and Had Less Advanced Cardiac Involvement. (C) Six-Month landmark Analysis of OS Based on Hematologic Response, Indicating that Patients With Responsive Disease (CR/VGPR, PR) Had Better Survival Than Those With Nonresponsive (Not Reported, NR) Disease (P < .05). (D) OS As Function of Cardiac Stage, Predictor of OS by Cox Regression Analysis (P < .05)
Abbreviations: CR = complete response; OS = overall survival; PR = partial response; SCT = stem cell transplantation; VGPR = very good partial response.
We performed a Cox regression analysis with baseline variables including age at diagnosis, gender, percentage of marrow plasma cells, free light chains, cardiac stage, renal stage, liver involvement, and autonomic and peripheral nervous system involvement in order to identify predictors of OS. Cardiac stage was the only significant baseline predictor of OS (P < .05) (Figure 2C). We also performed 6-month landmark Cox regression analyses that included hematologic response to initial therapy, seeking predictors of PFS and OS. Hematologic response was the only significant predictor of duration of PFS and of OS in the landmark analyses (Figure 2D).
Discussion
Chromosomal abnormalities as predictors of response and survival in multiple myeloma have been well described, and their significance continues to be refined. The Revised International Staging System has incorporated cytogenetic data into the risk stratification for myeloma at diagnosis.1 Although myeloma and AL are both plasma-cell malignancies, the role of such abnormalities cannot be extrapolated from one to the other because of the differences in the distribution of chromosomal abnormalities in these two diseases and because of the different ways these diseases affect organ function. Translocation (11;14), for example, is present at diagnosis in 20% of myeloma patients and in > 50% of AL patients. Del 17p in myeloma is present in about 10% of myeloma patients at diagnosis, and its prognostic impact depends in part on the percentage of clonal plasma cells with del 17p.13 Del 17p is rare in AL patients, hence the need for this collaborative effort. In both myeloma and AL, however, it is the case that the percentage of clonal cells with del 17p (≤ 50% or > 50%) and depth of hematologic response are key predictors of OS.2,13
Advanced cardiac involvement in AL patients at diagnosis remains a challenge, and despite improved outcomes over the past 15 years, it continues to cause early death in 25% of patients within 6 months of diagnosis.14 Recently a report on 1551 AL patients seen between 2000 and 2014 noted that among the 525 treated between 2010 and 2014 who had a median of 10% marrow plasma cells, 75% had cardiac involvement, 66% experienced CR or VGPR, and 38% of those receiving non-SCT chemotherapy survived at 4 years.14 In this series of 44 AL patients with del 17p at diagnosis who had a median of 22% marrow plasma cells, 93% had cardiac involvement, 49% experienced CR or VGPR (25% had SCT and 75% non-SCT therapy), and 50% survived at 49 months. Superficially, then, there does not appear to be a generic detriment with respect to OS associated with del 17p at diagnosis in AL patients.
In this series, the critical determinants of OS in AL remain cardiac involvement and hematologic response, although patients with > 50% del 17p plasma cells exhibit a trend toward shorter survival, as do those with the additional chromosomal abnormalities t(11;14) or gain 1q. We collaborated on this effort in order to identify the features of newly diagnosed systemic AL amyloidosis associated with del 17p, and although these findings require further evaluation in larger patient series and in clinical trials, they provide guidance to practitioners caring for patients with AL. The single case of progression to a leukemic phase with relapsed AL (Supplemental Data S1 in the online version) illustrates that rarely a more aggressive clone may emerge from an AL patient harboring del 17p at diagnosis. Interestingly, the only AL amyloidosis cell lines15 that have been generated to date (from a single patient) also harbor del 17p, which suggests that this chromosomal aberration in certain situations may endow AL plasma cells with stromal independence and proliferative capacity.
Conclusion
We characterize the outcomes of newly diagnosed AL amyloidosis patients with del 17p. OS in these patients is determined by cardiac involvement and hematologic response, with the latter also determining PFS. Patients with > 50% del 17p plasma cells and those with additional cytogenetic abnormalities such as t(11;14) or gain 1q may be at risk for shorter survival. Hematologic and organ responses, as well as OS and PFS, appear to be similar to previous reports.14,16 In rare instances, del 17p may be associated with the emergence of an aggressive plasma-cell clone.
Clinical Practice Points
Cytogenetic abnormalities are risk factors in multiple myeloma that affect hematologic response and survival.
In systemic AL, abnormalities such as translocation (11;14) and gain 1q may affect hematologic response.
In myeloma, del 17p is considered high risk and a marker of poor prognosis, but its impact, if any, in AL is unclear.
The finding of del 17p in a newly diagnosed AL patient may perplex the practitioner with respect to prognosis, particularly in patients with multiorgan involvement.
Unlike factors such as age and renal failure in myeloma and extent of cardiac damage and intractable hypotension in AL, the finding of del 17p in a newly diagnosed patient with AL can create uncertainty regarding the choice and duration of initial therapy and the use of melphalan in stem-cell transplantation.
Our analysis of this 44-patient case series indicates that cardiac involvement and hematologic response to therapy are the key predictors of OS and PFS, and that patients with > 50% clonal plasma cells with del 17p may be at risk for shorter survival.
Key practice points are to optimize supportive care and seek an early and deep hematologic response.
The initial choice of therapy can be high-dose melphalan if the baseline clonal plasma-cell percentage is < 10%.
If a combination chemotherapy regimen is chosen as an initial therapy, and if there is no significant hematologic response, a change in therapy should be considered.
Supplementary Material
Acknowledgments
The authors thank the clinical research coordinators who contributed to this study. For their continued support, we also thank the Division of Hematology-Oncology and the Departments of Medicine and Pathology and Laboratory Medicine at Tufts, the Amyloidosis and Myeloma Research Fund at Tufts, the Cam Neely and John Davis Myeloma Research Fund, the John C. Davis Program for Myeloma and Amyloid at Tufts, the Sidewater Family Fund, the Lavonne Horowitz Trust, the Werner and Elaine Dannheiser Fund for Research on the Biology of Aging of the Lymphoma Foundation, David and Barbara Levine (in memoriam), and the Demarest Lloyd Jr Foundation.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
Supplemental Data
Supplemental data, including figures, accompanying this article can be found in the online version at https://doi.org/10.1016/j.clml.2018.07.292.
References
- 1.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol 2015; 33:2863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lahuerta JJ, Paiva B, Vidriales MB, et al. Depth of response in multiple myeloma: a pooled analysis of three PETHEMA/GEM clinical trials. J Clin Oncol 2017; 35: 2900–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergsagel PL, Mateos MV, Gutierrez NC, Rajkumar SV, San Miguel JF. Improving overall survival and overcoming adverse prognosis in the treatment of cytogenetically high-risk multiple myeloma. Blood 2013; 121:884–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berenson JR, Anderson KC, Audell RA, et al. Monoclonal gammopathy of undetermined significance: a consensus statement. Br J Haematol 2010; 150: 28–38. [DOI] [PubMed] [Google Scholar]
- 5.Warsame R, Kumar SK, Gertz MA, et al. Abnormal FISH in patients with immunoglobulin light chain amyloidosis is a risk factor for cardiac involvement and for death. Blood Cancer J 2015; 5:e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bochtler T, Hegenbart U, Kunz C, et al. Gain of chromosome 1q21 is an independent adverse prognostic factor in light chain amyloidosis patients treated with melphalan/dexamethasone. Amyloid 2014; 21:9–17. [DOI] [PubMed] [Google Scholar]
- 7.Bochtler T, Hegenbart U, Kunz C, et al. Translocation t(11;14) is associated with adverse outcome in patients with newly diagnosed AL amyloidosis when treated with bortezomib-based regimens. J Clin Oncol 2015; 33:1371–8. [DOI] [PubMed] [Google Scholar]
- 8.Bryce AH, Ketterling RP, Gertz MA, et al. Translocation t(11;14) and survival of patients with light chain (AL) amyloidosis. Haematologica 2009; 94:380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dispenzieri A, Gertz MA, Kyle RA, et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol 2004; 22:3751–7. [DOI] [PubMed] [Google Scholar]
- 10.Palladini G, Dispenzieri A, Gertz MA, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol 2012; 30:4541–9. [DOI] [PubMed] [Google Scholar]
- 11.Comenzo RL, Reece D, Palladini G, et al. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis. Leukemia 2012; 26:2317–25. [DOI] [PubMed] [Google Scholar]
- 12.Palladini G, Hegenbart U, Milani P, et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood 2014; 124:2325–32. [DOI] [PubMed] [Google Scholar]
- 13.An G, Li Z, Tai YT, et al. The impact of clone size on the prognostic value of chromosome aberrations by fluorescence in situ hybridization in multiple myeloma. Clin Cancer Res 2015; 21:2148–56. [DOI] [PubMed] [Google Scholar]
- 14.Muchtar E, Gertz MA, Kumar SK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood 2017; 129:2111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arendt BK, Ramirez-Alvarado M, Sikkink LA, et al. Biologic and genetic characterization of the novel amyloidogenic lambda light chain-secreting human cell lines, ALMC-1 and ALMC-2. Blood 2008; 112:1931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palladini G, Sachchithanantham S, Milani P. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood 2015; 126:612–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


