Abstract
Social interaction is impaired in schizophrenia, including the use of hand gestures, which is linked to poor social perception and outcome. Brain imaging suggests reduced neural activity in a left-lateralized frontoparietal network during gesture preparation; therefore, gesturing might be improved through facilitation of left hemispheric brain areas or via disruption of interhemispheric inhibition from the right homolog. This study tested whether repetitive transcranial magnetic stimulation (rTMS) protocols would improve gesture performance in schizophrenia. This randomized, placebo-controlled, double-blind, crossover trial applied 3 different protocols of rTMS separated by 48 h. Twenty right-handed schizophrenia patients and 20 matched healthy controls received facilitatory intermittent theta burst stimulation (iTBS) over the left inferior frontal gyrus (IFG), inhibitory continuous theta burst stimulation (cTBS) over right inferior parietal lobe (IPL), and placebo over left IPL in randomized order. Primary outcome was change in the test of upper limb apraxia (TULIA), rated from video recordings of hand gesture performance. Secondary outcome was change in manual dexterity using the coin rotation task. Participants improved on both tasks following rTMS compared with baseline. Only patients improved gesture performance following right IPL cTBS compared with placebo (P = .013). The results of the coin rotation parallel those of the TULIA, with improvements following right IPL cTBS in patients (P = .001). Single sessions of cTBS on the right IPL substantially improved both gesture performance accuracy and manual dexterity. The findings point toward an inhibition of interhemispheric rivalry as a potential mechanism of action.
Keywords: gesture, praxis network, dexterity, nonverbal skills, motor domain, RCT
Introduction
Impaired social interaction is one of the most important features of schizophrenia, strongly related to poor functional outcome.1–3 Social interaction includes processes of nonverbal communication, such as the use and integration of posture, gestures, facial expressions, or dyadic synchrony of prosocial behaviors.4,5 One critical component of nonverbal communication is the use of hand gestures to substitute or accompany speech.6 Performance and perception of hand gestures is impaired at multiple levels in schizophrenia. For example, patients with psychosis show spatial, temporal, or content errors when performing gestures,7–9 which correlates with poor nonverbal social perception and poor social functioning.7,10 Gesture deficits are also tied to poor frontal lobe function and motor abnormalities in schizophrenia, including both basic motor impairment such as parkinsonism or dyskinesia, but also impaired motor control such as compromised manual dexterity.7–9,11 In addition, patients use gestures less frequently than healthy subjects12,13 or with incorrect content.14 Finally, perception and interpretation of hand gestures is severely impaired, when patients misperceive incidental movements as gestures, neutral gestures as threatening, or fail to detect the mismatch between spoken language and co-speech gestures.15–17 Thus, interventions targeting poor nonverbal behavior would be most needed to aid social interaction. First evidence suggests that the interpretation of metaphoric gestures can be improved in schizophrenia in single sessions of transcranial direct current stimulation on the left frontal cortex.18
Correct performance of hand gesture requires proper function of a left-lateralized frontoparietal network, called the praxis network. In schizophrenia, this network is less active when planning hand gestures, particularly the left inferior frontal gyrus (IFG), supplementary motor area, left superior parietal lobe (SPL) and inferior parietal lobe (IPL).19 Likewise, another functional magnetic resonance imaging (fMRI) study found left IPL to be less active in schizophrenia patients during a finger imitation task.20 Given that gesture deficits in schizophrenia are linked to poor activation of the praxis network and that a large proportion of the praxis network is located underneath the skull, it is a promising target for brain stimulation.
Repetitive transcranial magnetic stimulation (rTMS) is currently applied in a variety of neuropsychiatric disorders to improve symptoms either by stimulating hypoactive brain areas or by using inhibitory stimulation to suppress surrounding noise or to antagonize inhibitory processes. Noninvasive brain stimulation such as rTMS has different effects on brain function depending on the frequency and type of stimulation, eg, intermittent theta burst stimulation (iTBS) has facilitatory effects, whereas continuous theta burst stimulation (cTBS) has inhibitory effects.21 rTMS may also be used to interfere with normal processes such as interhemispheric rivalry, in which the active brain region is inhibiting concurrent activity in the contralateral homolog through transcallosal fibers.22,23 In hemispatial neglect, inhibition of this interhemispheric rivalry has yielded beneficial results via inhibitory stimulation of the contralateral side.24,25
We may expect that increasing neural activity in the left IFG or IPL would improve hand gesture performance in schizophrenia. In fact, inhibitory stimulation of the left IFG perturbed gesture performance in healthy subjects.26 Thus, facilitatory stimulation may improve impaired gesturing in schizophrenia. Likewise, inhibitory cTBS on the left IPL disrupted gesture performance in healthy subjects,27 suggesting that in contrast increased neural activity in the left IPL would be beneficial, which may be achieved by disturbing the interhemispheric inhibition from the right IPL. Finally, modulation of the praxis network by rTMS may also improve manual dexterity.
The aim of this randomized, double-blind, placebo-controlled, crossover trial was to determine whether single sessions of 2 different rTMS protocols would improve gesture performance in schizophrenia. We hypothesized that facilitatory iTBS over the left IFG or inhibitory cTBS over the right IPL would be superior to baseline and placebo stimulation. Furthermore, we predicted that patients would benefit more from brain stimulation than controls due to dysfunctions in the praxis network. Finally, we tested whether manual dexterity would improve with active TMS protocols in patients.
Methods and Materials
Participants
Twenty patients were recruited from the in- and outpatient departments of the University Hospital of Psychiatry, Bern, Switzerland. In addition, 20 matched healthy controls were recruited from the community using flyers, the department internet website, and word of mouth. All subjects received 100 Swiss francs compensation for participation. Inclusion criteria were schizophrenia spectrum disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria, right-handedness, and growing up in Switzerland due to cultural influences on gesture behavior. Exclusion criteria were any other psychiatric disorder, epilepsy, pregnancy, neurological, or medical impairment affecting gesture, such as the history of stroke or multiple sclerosis, or rTMS treatment within the past 3 months. In controls, further exclusion criterion was any first-degree relative with schizophrenia spectrum disorders. Diagnoses were given by trained psychiatrists using the complete case files as well as the Mini-International Neuropsychiatric Interview.28 The study protocol adheres to the Declaration of Helsinki and had been approved by the local ethics committee. All participants provided written informed consent. The trial was registered at www.clinicaltrials.gov NCT03483909. Study recruitment lasted from January 2018 to March 2019.
Procedures
This was a double-blind, randomized, placebo-controlled, crossover trial conducted at one site. Each participant had a baseline assessment and 3 visits including rTMS in a randomized order with at least 48 h between visits to avoid carryover effects. Each visit included a gesture performance task, a manual dexterity test, and screen for side effects. Blinding was secured in 3 ways: participants could neither see the protocol selected nor see the site being stimulated. The person administering rTMS was different from the person assessing the outcome variables (hand gesture performance and manual dexterity), which were recorded on video. Moreover, a third member of the team evaluated outcomes blind to group and intervention received. Each stimulation protocol was assigned a number and the randomization of the order of the 3 numbers was performed. A randomization document listed treatment order according to the order of recruitment. This list was created before the study start and was kept separately from all study material. Allocation of stimulation order was placed in sealed envelopes and was only disclosed to the investigator performing rTMS. Participants were informed that the study included 3 different stimulations, one at each visit. After the stimulation participants would immediately go to a different room meeting the assessor, who also inquired about their impression of which stimulation they had received. Assessors and raters were trained by the principal investigator (S.W.) to ensure reliability. Symptom severity was rated using the Positive And Negative Syndrome Scale.29 Working memory performance was assessed with the digit span backward. Frontal lobe function was rated according to the frontal assessment battery.30
Repetitive TMS
Repetitive TMS was delivered using MagPro R30 with theta burst option (MagVenture, Inc.) using a standard figure of eight coil. The application of stimulation followed the TMS guidelines.31,32 Before each session, the resting motor threshold was determined to identify the individual intensity of stimulation. The positions for the coil placement according to the EEG 10–20 system were left IFG at the center between F5/F7/FC5/FT7 for iTBS, right IPL at CP4/6 for cTBS, and left IPL at CP3/5 for placebo (supplementary figure S1). iTBS included 2 s trains of TBS repeated every 10 s for a total of 190 s, resulting in a total of 600 pulses at 80% individual resting motor threshold.33 cTBS had 801 pulses in 267 bursts, with each burst of 3 pulses at 30 Hz with an interburst interval of 100 ms for a total duration of 44 s at 100% resting motor threshold.24 Finally, the placebo protocol was identical to iTBS but administered with a placebo coil that looks identical to the real coil, emits similar sounds, but produces no magnetic pulses. The neural effects of single sessions of iTBS or cTBS on the motor cortex last approximately 20–30 mins.33
Measures
Hand gesture performance was assessed with the test of upper limb apraxia (TULIA),34 which has been validated in schizophrenia.8,9 The TULIA tests the accuracy of performance of 48 hand gestures, 24 of which are performed on demonstration of the experimenter (imitation), whereas 24 are performed following verbal instructions (pantomime). Gestures span the following semantic categories: meaningless (eg, put your hand flat on your head), intransitive (symbolic, eg, salute like a soldier), or transitive (tool related, eg, demonstrate the use of a screwdriver). The order of assessment between domains (imitation vs pantomime) or semantic categories was randomized across visits and participants. Gesture performance is videotaped and later rated according to the manual from the video recordings, taking into account spatial, temporal or content errors. The TULIA total score ranges 0–240 with higher values indicating superior performance.
Manual dexterity was tested with the coin rotation (CR) task.35,36 In this task, participants have to rotate a 0.5 Swiss franc coin of 1-cm diameter as fast as possible between thumb, index, and middle finger. The coin has the same size as a US dime. Participants performed the task with their right hand while sitting at a table. At each session, 3 trials were conducted of 10 s each and videotaped. The first trial was discarded. The second and third trials were rated on video by an expert blind to group and session. The score of the second and third trial was averaged and used as CR score for further analyses. The CR scores were calculated according to the formula: CR score = half turns – [(coin drops × 0.1) × half turns].
Side effects were assessed after each stimulation using a questionnaire of side effect severity with 4 steps (none, mild, moderate, and severe) for nausea, headaches, neck pain, fatigue, and other. Furthermore, the participants indicated whether they had received placebo or real TMS.
Statistical Analyses
Descriptive statistics include chi-squared or t tests. Normal distribution was tested with Kolmogorov-Smirnov tests. To test the effects of rTMS on gesture performance or dexterity, we entered the TULIA total score or the CR score of the right hand into a repeated measures ANCOVA with the order of stimulation as covariate. ANCOVAs had a within-subject factor stimulation (baseline, IFG, IPL, placebo) and a between-subject factor group (patients, controls). We applied Greenhouse-Geisser correction when sphericity was violated. Effects were followed up within groups with repeated measures ANCOVAs correcting for stimulation order. In patients, we also performed a repeated measures ANCOVA testing for the TULIA domains (imitation, pantomime) and stimulation. In addition, we calculated the proportional change from baseline for each TMS protocol and corrected for the order of stimulation. Finally, we correlated proportional change with baseline scores and clinical variables. Two-sided P -values < .05 were considered significant. All tests were performed with SPSS, version 25.
Results
Table 1 provides clinical and demographic information. Patients were less educated and had working memory impairments.
Table 1.
Demographic and Clinical Data
| Variables | Patients (20) | Controls (20) | Statistics | ||
|---|---|---|---|---|---|
| Tests | df | P | |||
| Sex (m/f) | (12/8) | (13/7) | X 2 = 0.1 | 2 | .744 |
| Age (y) | 34.3 (12.6) | 30.5 (11.5) | T = –1.0 | 38 | .324 |
| Education (y) | 12.2 (2.6) | 14.8 (2.1) | T = 3.6 | 38 | .001 |
| DSB | 9.2 (5.7) | 15.1 (5.6) | T = 3.3 | 38 | .002 |
| FAB | 15.2 (1.7) | 15.5 (1.2) | T = 0.6 | 38 | .536 |
| CPZ (mg) | 478.9 (638.1) | ||||
| DOI (y) | 11.6 (8.9) | ||||
| PANSS positive | 21.7 (10.5) | ||||
| PANSS negative | 28.4 (7.4) | ||||
| PANSS total | 96.6 (24.2) |
Note: CPZ, chlorpromazine equivalents; DOI, duration of illness; PANSS, Positive and Negative Syndrome Scale; DSB, digit span backward; FAB, frontal assessment battery. Significant values highlighted in bold.
rTMS Effects on Gesture Performance
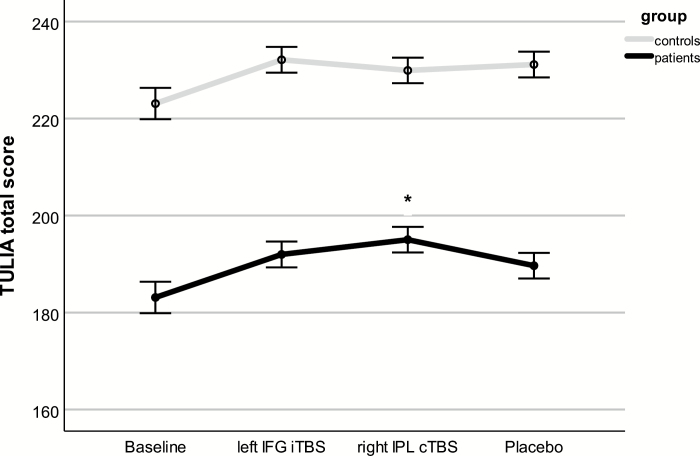
We detected a main effect for stimulation (F = 4.56, df = 3, P = .008, η 2 = .28), a main effect of group (F = 122.3, df = 1, P < .001, η 2 = .77), and a stimulation × group interaction (F = 3.36, df = 3, P = .030, η 2 = .22; figure 1). Post hoc tests indicated that every rTMS stimulation improved TULIA scores compared with baseline (all Ps < .001), along with a trend toward higher TULIA scores following right IPL cTBS compared with placebo (P = .07).
Fig. 1.
Gesture performance following single sessions of rTMS. Depicted are estimated marginal means and standard errors of the mean, which are covaried for the order of stimulation. *Significant difference from placebo. Please note that the order of stimulation is randomized.
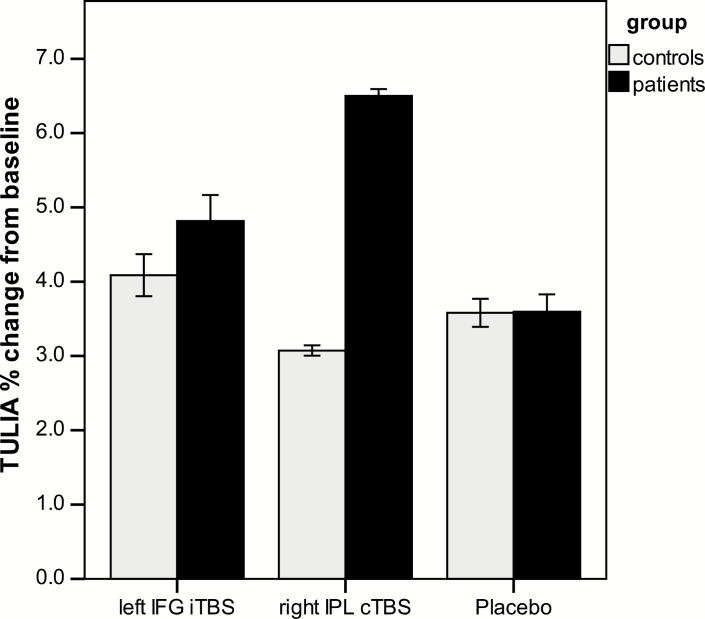
Within patients, repeated measures ANCOVA identified a significant effect of stimulation (F = 3.27, df =3, P = .028, η 2 = .15). Post hoc tests found increased TULIA scores for all stimulations compared with baseline (P < .006), and cTBS over right IPL increased TULIA more than placebo (mean difference: 5.3, 95% CI = 1.27–9.33, P = .013). The proportional change for each stimulation relative to baseline is depicted in figure 2. Within patients repeated measures ANCOVA including stimulation and TULIA domain detected a significant effect of domain with higher TULIA scores during imitation than pantomime (F = 44.70, df = 1, P < .001, η 2 = .71), but no stimulation × domain interaction (F = .39, df = 2.03, P = .66, η 2 = .02). The majority of patients benefits from both stimulations (supplementary figure S2). Finally, subjects with poor baseline performance seemed to have increased improvement during right IPL cTBS (r = –.48, P = .033), whereas clinical variables were unrelated to treatment effects (supplementary table S1).
Fig. 2.
Proportional changes in gesture performance from baseline. Depicted are proportional changes from baseline using the estimated marginal means and standard errors of the ANCOVA correcting for the order of stimulation.
Within controls, the repeated measures ANCOVA also detected a significant effect of stimulation (F = 4.04, df =2.1, P = .024, η 2 = .18), with all post hoc comparisons of stimulations vs baseline being significant (P < .005). Still, none of the stimulations produced gesture performance superior to placebo in controls.
rTMS Effects on Manual Dexterity
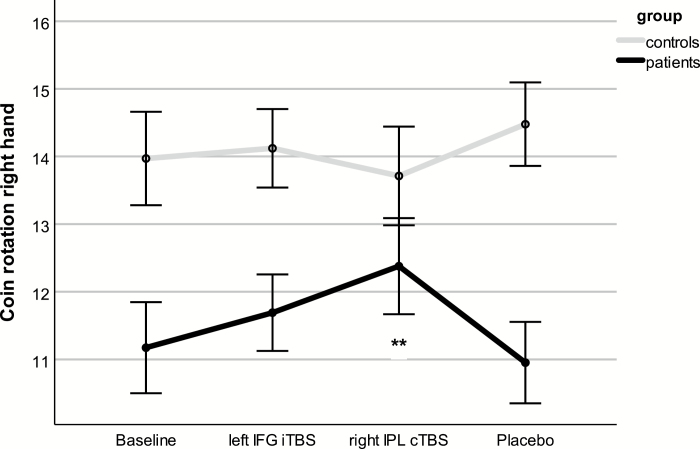
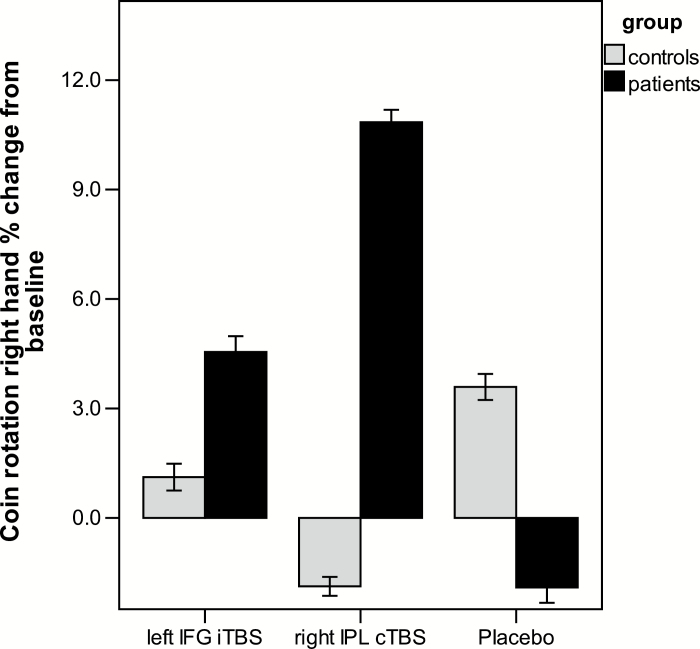
Repeated measures ANCOVA of the right-hand CR score detected no effect of stimulation (F = 1.03, df = 2.43, P = .37, η 2 = .03), but a group effect (F = 8.64, df = 1, P = .006), and a group × stimulation interaction (F = 5.85, df = 2.43, P = .002, η 2 = .14; figure 3). Within patients, there was a significant multivariate effect of stimulation (F = 3.44, df = 3, P = .042, η 2 = .39). Post hoc t tests indicated superior CR performance following right IPL cTBS compared with baseline (mean difference 1.21, 95% CI = 0.51–1.91, P = .002) and compared with placebo (mean difference 1.43, 95% CI = 0.68–2.17, P = .001). The proportional change for each stimulation relative to baseline is depicted in figure 4. Improvement of CR in patients with right IPL cTBS was correlated to baseline CR (r = –.48, P = .034), but not to concurrent TULIA improvement with right IPL cTBS (r = –.26, P = .276) or any clinical variable (supplementary table S1). There were no effects of rTMS on left hand CR (supplementary material).
Fig. 3.
Manual dexterity following single sessions of repetitive transcranial magnetic stimulation. Depicted are estimated marginal means and standard errors of the mean, which are covaried for the order of stimulation. **Significant difference from placebo. Please note that the order of stimulation is randomized.
Fig. 4.
Proportional changes in gesture performance from baseline. Depicted are proportional changes from baseline using the estimated marginal means and standard errors of the ANCOVA correcting for the order of stimulation.
Side Effects
Participants correctly identified placebo in 40%, iTBS in 59%, and cTBS in 63% with no significant differences between groups. Side effects occurred rarely with mild-to-moderate severity, with no significant differences between stimulation types and groups, except for more side effects of the category “other” in patients. Nausea was reported in 9%, headaches in 6%, neck pain in 5%, fatigue in 8%, and any other side effect in 25% of stimulations (for details see supplementary table S2). No serious adverse events occurred.
Discussion
This randomized, double-blind, placebo-controlled, crossover trial tested whether single sessions of different rTMS protocols would improve gesture performance in schizophrenia. In line with our hypotheses, inhibitory cTBS over the right IPL was superior over placebo enhancing both gesture performance and manual dexterity in patients. Importantly, single sessions of cTBS were able to improve gesture performance by 6.5% and manual dexterity by 10.8%. Facilitatory iTBS over the left IFG improved gesture performance by 5% but was not superior compared with placebo. In the majority of patients, we noted beneficial effects on gesture performance with either of the active stimulations. Single sessions of rTMS to modulate the praxis network were generally well tolerated and safe.
One of the tested protocols holds specific potential for treating gesture deficits in schizophrenia. Here, we report immediate offline effects of rTMS that are likely to decay within the next hour. However, repeated daily administration of iTBS or cTBS can intensify the neural and behavioral effects of single rTMS sessions. In fact, repeated administration of rTMS effectively treats psychiatric symptoms for weeks after the last stimulation and also with greater effects than single stimulation sessions, eg, in depression.37,38 This effect of repeated rTMS is achieved through modulation of brain network activity.39,40 Also, in schizophrenia patients with auditory verbal hallucinations receiving 10 or more daily sessions of inhibitory rTMS had lasting effects on hallucination severity and changed relevant aberrant brain activity.41–43 Given that the positive effects on gesture performance would be enduring and pronounced with repeated administration of cTBS, eg, in 10 sessions for 2 weeks, the stimulation may become a valuable treatment for deficits in gesture performance and impaired nonverbal communication in schizophrenia. Because of the association of gesture impairments with poor social functioning,7,10 we expect that repetitive cTBS application may exert downstream effects improving social skills and community functioning in schizophrenia. Currently, there are no other treatments available directly modulating gesture or nonverbal behaviors. Thus, if successful in further parallel arm designs, right IPL cTBS may in the near future become a mechanism-based add-on treatment for patients with gesture deficits.
The exact mechanism of action for the effect of right IPL cTBS on gesture performance still requires elucidation. The left IPL is a key area of the praxis network,6,44 and inhibition of the left IPL in healthy subjects disrupts gesture imitation.27 Both fMRI studies on gesture performance in schizophrenia reported reduced left IPL activity during gesture planning in patients.19,20 In addition, both cortical thickness and gray matter volume of bilateral IPL are reduced in schizophrenia patients with severe gesture deficits.45,46 Inhibition of the interhemispheric rivalry has been suggested to exert beneficial effects in subjects with neglect.24,25 Likewise, we hypothesized that interhemispheric inhibition in schizophrenia would increase neural activity in the left IPL. Even though we have no neuroimaging evidence for this effect, behavioral results seem to support the notion. Our experiment demonstrated beneficial effects of right IPL cTBS on both gesture performance and manual dexterity in schizophrenia. In contrast, the stimulation had no effect on the CR performance of the left hand in either group. Therefore, the right IPL cTBS was specifically effective for dexterity in the right hand in patients.
Against our hypothesis, left IFG iTBS failed to improve gesture or dexterity more than placebo. The left IFG is also a critical component of the praxis network and inhibitory cTBS on the left IFG deteriorated gesture performance and gesture-speech integration in healthy subjects.26,47 Likewise, previous work suggests that left IFG is hypoactive in schizophrenia patients and has reduced gray matter in patients with severe gesture deficits.19,45,46 Left IFG iTBS still improved gesture performance by 5% and manual dexterity by 4% compared with baseline; however, this improvement was not significantly different from placebo. Still, with repeated administration of iTBS in schizophrenia, there might be a chance of significant improvement after all, particularly, for gestures with higher semantic content, which rely on left IFG function.18 Finally, the effects of IFG and IPL stimulation on gesture were correlated in patients; thus, we may consider testing combined stimulation.
Although we demonstrate beneficial effects of cTBS in patients, we fell short in detecting a similar effect in healthy controls. In line with our previous reports, we found a substantial group difference in gesture performance.7,9 Even though the TULIA is a demanding task designed to avoid ceiling effects in healthy subjects,34 controls still had very high scores on the baseline TULIA, leaving much less room for improvement than in patients. Nevertheless, controls improved gesture performance by 3%–4% with all stimulations compared with baseline. This might suggest a learning effect from first to second performance of the TULIA. Our protocol scheduled 48 h between assessments, which may have been too short to avoid learning the task. However, we randomized the order of stimulations and entered this variable as a covariate in the repeated measures ANCOVAs. Finally, a supplementary mixed linear model corroborated the specific effect of right IPL cTBS in patients, taking baseline TULIA and the timing of the randomized stimulations into account (supplementary material).
Some limitations of this study require discussion. First, we tested the effects of single sessions of theta burst stimulation, for which immediate offline effects may last up to 20–30 mins.33 Assessments of TULIA and bilateral CR were scheduled immediately after the stimulation and take approximately 15 mins. Thus, for some participants, the performance assessments were close to the decay of stimulation effects on brain function. However, this applies to all stimulation types. Second, we used prespecified locations targeting IPL and IFG to conduct a straightforward experiment. In contrast, neuronavigation may enhance rTMS precision with even stronger effects. Third, because gesture deficits are tightly linked to motor abnormalities in schizophrenia,7,8 improvements in gestures could have been driven by improvements in manual dexterity. However, the effect on manual dexterity and the effect on gesture were not correlated. Fourth, a proportion of the patients had acute psychotic episodes, which may interfere with gesture. However, in line with previous studies, we found no correlation between gesture performance and symptoms.7–9,12,14 Finally, it is unclear whether antipsychotic medication has also contributed to the strong rTMS effects in patients. CPZ dosage was unrelated to treatment effects. Still, mediation could exert effects beyond dosage.
Conclusions
In conclusion, our study demonstrates a beneficial effect of single sessions of inhibitory cTBS on the right IPL on both gesture performance and manual dexterity. Given the effects after single stimulation, we may hope that repeated sessions will have even stronger and enduring effects on gesture performance. Thus, future studies will have to determine whether this mechanism may improve nonverbal communication and social functioning in schizophrenia spectrum disorders.
Funding
None.
Supplementary Material
Acknowledgments
Dr Walther reports having received honoraria from Lundbeck, Janssen, and Sunovion, and research funding from the Swiss National Science Foundation (SNSF) and National Institute of Mental Health. Dr Stegmayer reports honoraria from Lundbeck and research funding from the SNSF and the Frutiger Foundation. Dr Bohlhalter reports research funding from the SNSF. All other authors reported no potential conflicts of interest.
References
- 1. Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nat Rev Neurosci. 2015;16(10):620–631. [DOI] [PubMed] [Google Scholar]
- 2. Schmidt SJ, Mueller DR, Roder V. Social cognition as a mediator variable between neurocognition and functional outcome in schizophrenia: empirical review and new results by structural equation modeling. Schizophr Bull. 2011;(suppl 37) 2:S41–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oliver LD, Haltigan JD, Gold JM, et al. Lower- and higher-level social cognitive factors across individuals with schizophrenia spectrum disorders and healthy controls: relationship with neurocognition and functional outcome. Schizophr Bull. 2018;10:629–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tschacher W, Giersch A, Friston K. Embodiment and schizophrenia: a review of implications and applications. Schizophr Bull. 2017;43(4):745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Knapp M, Hall JA, Horgan TG.. Nonverbal Communication in Human Interaction: Wadsworth, OH; 2013. [Google Scholar]
- 6. Walther S, Mittal VA. Why we should take a closer look at gestures. Schizophr Bull. 2016;42(2):259–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walther S, Stegmayer K, Sulzbacher J, et al. Nonverbal social communication and gesture control in schizophrenia. Schizophr Bull. 2015;41(2):338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walther S, Vanbellingen T, Müri R, Strik W, Bohlhalter S. Impaired pantomime in schizophrenia: association with frontal lobe function. Cortex. 2013;49(2):520–527. [DOI] [PubMed] [Google Scholar]
- 9. Walther S, Vanbellingen T, Müri R, Strik W, Bohlhalter S. Impaired gesture performance in schizophrenia: particular vulnerability of meaningless pantomimes. Neuropsychologia. 2013;51(13):2674–2678. [DOI] [PubMed] [Google Scholar]
- 10. Walther S, Eisenhardt S, Bohlhalter S, et al. Gesture performance in schizophrenia predicts functional outcome after 6 months. Schizophr Bull. 2016;42(6):1326–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Osborne KJ, Bernard JA, Gupta T, et al. Beat gestures and postural control in youth at ultrahigh risk for psychosis. Schizophr Res. 2017;185:197–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lavelle M, Healey PG, McCabe R. Is nonverbal communication disrupted in interactions involving patients with schizophrenia? Schizophr Bull. 2013;39(5):1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mittal VA, Tessner KD, McMillan AL, Delawalla Z, Trotman HD, Walker EF. Gesture behavior in unmedicated schizotypal adolescents. J Abnorm Psychol. 2006;115(2):351–358. [DOI] [PubMed] [Google Scholar]
- 14. Millman ZB, Goss J, Schiffman J, Mejias J, Gupta T, Mittal VA. Mismatch and lexical retrieval gestures are associated with visual information processing, verbal production, and symptomatology in youth at high risk for psychosis. Schizophr Res. 2014;158(1-3):64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. White TP, Borgan F, Ralley O, Shergill SS. You looking at me?: interpreting social cues in schizophrenia. Psychol Med. 2016;46(1):149–160. [DOI] [PubMed] [Google Scholar]
- 16. Bucci S, Startup M, Wynn P, Baker A, Lewin TJ. Referential delusions of communication and interpretations of gestures. Psychiatry Res. 2008;158(1):27–34. [DOI] [PubMed] [Google Scholar]
- 17. Nagels A, Kircher T, Grosvald M, Steines M, Straube B. Evidence for gesture-speech mismatch detection impairments in schizophrenia. Psychiatry Res. 2019;273:15–21. [DOI] [PubMed] [Google Scholar]
- 18. Schulke R, Straube B. Transcranial direct current stimulation improves semantic speech-gesture matching in patients with schizophrenia spectrum disorder. Schizophr Bull. 2018;45(3):522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stegmayer K, Bohlhalter S, Vanbellingen T, et al. Limbic interference during social action planning in schizophrenia. Schizophr Bull. 2018;44(2):359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thakkar KN, Peterman JS, Park S. Altered brain activation during action imitation and observation in schizophrenia: a translational approach to investigating social dysfunction in schizophrenia. Am J Psychiatry. 2014;171(5):539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55(2):187–199. [DOI] [PubMed] [Google Scholar]
- 22. Fling BW, Benson BL, Seidler RD. Transcallosal sensorimotor fiber tract structure-function relationships. Hum Brain Mapp. 2013;34(2):384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jung P, Klein JC, Wibral M, et al. Spatiotemporal dynamics of bimanual integration in human somatosensory cortex and their relevance to bimanual object manipulation. J Neurosci. 2012;32(16):5667–5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cazzoli D, Müri RM, Schumacher R, et al. Theta burst stimulation reduces disability during the activities of daily living in spatial neglect. Brain. 2012;135(Pt 11):3426–3439. [DOI] [PubMed] [Google Scholar]
- 25. Nyffeler T, Vanbellingen T, Kaufmann BC, et al. Theta burst stimulation in neglect after stroke: functional outcome and response variability origins. Brain. 2019;142(4):992–1008. [DOI] [PubMed] [Google Scholar]
- 26. Bohlhalter S, Vanbellingen T, Bertschi M, et al. Interference with gesture production by theta burst stimulation over left inferior frontal cortex. Clin Neurophysiol. 2011;122(6):1197–1202. [DOI] [PubMed] [Google Scholar]
- 27. Vanbellingen T, Bertschi M, Nyffeler T, et al. Left posterior parietal theta burst stimulation affects gestural imitation regardless of semantic content. Clin Neurophysiol. 2014;125(3):457–462. [DOI] [PubMed] [Google Scholar]
- 28. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 (suppl 20):22–33;quiz 34–57. [PubMed] [Google Scholar]
- 29. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 30. Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55(11):1621–1626. [DOI] [PubMed] [Google Scholar]
- 31. Lefaucheur JP, André-Obadia N, Antal A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014;125(11):2150–2206. [DOI] [PubMed] [Google Scholar]
- 32. Rossi S, Hallett M, Rossini PM, Pascual-Leone A; Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. [DOI] [PubMed] [Google Scholar]
- 34. Vanbellingen T, Kersten B, Van Hemelrijk B, et al. Comprehensive assessment of gesture production: a new test of upper limb apraxia (TULIA). Eur J Neurol. 2010;17(1):59–66. [DOI] [PubMed] [Google Scholar]
- 35. Gebhardt A, Vanbellingen T, Baronti F, Kersten B, Bohlhalter S. Poor dopaminergic response of impaired dexterity in Parkinson’s disease: bradykinesia or limb kinetic apraxia? Mov Disord. 2008;23(12):1701–1706. [DOI] [PubMed] [Google Scholar]
- 36. Quencer K, Okun MS, Crucian G, Fernandez HH, Skidmore F, Heilman KM. Limb-kinetic apraxia in Parkinson disease. Neurology. 2007;68(2):150–151. [DOI] [PubMed] [Google Scholar]
- 37. Brunoni AR, Chaimani A, Moffa AH, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis. JAMA Psychiatry. 2017;74(2):143–152. [DOI] [PubMed] [Google Scholar]
- 38. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683–1692. [DOI] [PubMed] [Google Scholar]
- 39. Philip NS, Barredo J, van ‘t Wout-Frank M, Tyrka AR, Price LH, Carpenter LL. Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biol Psychiatry. 2018;83(3):263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weigand A, Horn A, Caballero R, et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol Psychiatry. 2018;84(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kindler J, Homan P, Flury R, Strik W, Dierks T, Hubl D. Theta burst transcranial magnetic stimulation for the treatment of auditory verbal hallucinations: results of a randomized controlled study. Psychiatry Res. 2013;209(1):114–117. [DOI] [PubMed] [Google Scholar]
- 42. Chen X, Ji GJ, Zhu C, et al. Neural correlates of auditory verbal hallucinations in schizophrenia and the therapeutic response to theta-burst transcranial magnetic stimulation. Schizophr Bull. 2019;45(2):474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kindler J, Homan P, Jann K, et al. Reduced neuronal activity in language-related regions after transcranial magnetic stimulation therapy for auditory verbal hallucinations. Biol Psychiatry. 2013;73(6):518–524. [DOI] [PubMed] [Google Scholar]
- 44. Goldenberg G. Apraxia and the parietal lobes. Neuropsychologia. 2009;47(6):1449–1459. [DOI] [PubMed] [Google Scholar]
- 45. Stegmayer K, Bohlhalter S, Vanbellingen T, et al. Structural brain correlates of defective gesture performance in schizophrenia. Cortex. 2016;78:125–137. [DOI] [PubMed] [Google Scholar]
- 46. Viher PV, Stegmayer K, Kubicki M, et al. The cortical signature of impaired gesturing: findings from schizophrenia. Neuroimage Clin. 2018;17:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao W, Riggs K, Schindler I, Holle H. Transcranial magnetic stimulation over left inferior frontal and posterior temporal cortex disrupts gesture-speech integration. J Neurosci. 2018;38(8):1891–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.