ABSTRACT
The intestinal tract is the largest barrier between a person and the environment. In this role, the intestinal tract is responsible not only for absorbing essential dietary nutrients, but also for protecting the host from a variety of ingested toxins and microbes. The intestinal barrier system is composed of a mucus layer, intestinal epithelial cells (IECs), tight junctions (TJs), immune cells, and a gut microbiota, which are all susceptible to external factors such as dietary fats. When components of this barrier system are disrupted, intestinal permeability to luminal contents increases, which is implicated in intestinal pathologies such as inflammatory bowel disease, necrotizing enterocolitis, and celiac disease. Currently, there is mounting evidence that consumption of excess dietary fats can enhance intestinal permeability differentially. For example, dietary fat modulates the expression and distribution of TJs, stimulates a shift to barrier-disrupting hydrophobic bile acids, and even induces IEC oxidative stress and apoptosis. In addition, a high-fat diet (HFD) enhances intestinal permeability directly by stimulating proinflammatory signaling cascades and indirectly via increasing barrier-disrupting cytokines [TNFα, interleukin (IL) 1B, IL6, and interferon γ (IFNγ)] and decreasing barrier-forming cytokines (IL10, IL17, and IL22). Finally, an HFD negatively modulates the intestinal mucus composition and enriches the gut microflora with barrier-disrupting species. Although further research is necessary to understand the precise role HFDs play in intestinal permeability, current data suggest a stronger link between diet and intestinal disease than was first thought to exist. Therefore, this review seeks to highlight the various ways an HFD disrupts the gut barrier system and its many implications in human health.
Keywords: high-fat diet, intestinal permeability, gut barrier, tight junction, inflammatory bowel disease, inflammation, bile acids, superficial unstirred mucus layer, shedding-proliferation axis, gut microbiota
Introduction
The intestinal tract comprises the largest boundary between the host and its environment. In this role, the intestinal tract retains a robust barrier system in order to protect the host from external toxins, dietary antigens, bacteria, and other potentially hazardous substances. This barrier is multifaceted and comprises various protective mechanisms, such as a mucus layer, a constantly renewing epithelial boundary, tight junctions (TJs), and even the gut microbiome, to preserve intestinal health. Throughout the host's lifetime, these systems provide rigid protection yet remain malleable to allow normal physiological remodeling with minimal alteration to barrier integrity. However, stimuli such as dietary substances, especially fats, compromise the barrier and enhance permeation of luminal contents into the mucosal and submucosal layers in proximity to resident immune cells. These changes promote inflammatory responses that damage intestinal superstructures and predispose the host to various enteropathies like inflammatory bowel disease (IBD), celiac disease, and irritable bowel syndrome.
It is well known that dietary components substantially alter intestinal physiology and specifically modulate intestinal barrier integrity. For example, in celiac disease, permeability of hypersensitized intestines to gluten leads to profound mucosal inflammation, villous atrophy, and malabsorption diarrhea. Remarkably, simple removal of dietary gluten tends to ameliorate most intra- and extra-intestinal pathologies and symptoms (1). Like celiac disease, dietary components may play a larger role in the pathophysiology of other intestinal diseases than was initially thought. Differences in diet among various regions and cultures may explain the divergent incidence rates of intestinal diseases. For example, celiac disease, IBD, and even colorectal cancer tend to localize to Westernized countries, such as the United States, Canada, and Europe, where a diet high in fats and refined carbohydrates, and low in fiber, predominates (2). Underlying these diseases is gut barrier degeneration due to as yet unidentified reasons. Diet seems to play a major role in these observations and thus the need to understand how it modulates intestinal barrier integrity is of great significance.
Dietary fat has been a focus of research into gut barrier regulation due to its ubiquitous nature, particularly in Western countries. For example, high-fat diet (HFD) consumption promotes and exacerbates experimental colitis in dietary and genetic mouse models of IBD (3, 4). In humans, consuming a Western-style diet is associated with an increased risk of developing IBD, a condition characterized by intestinal hyperpermeability (5–7). In addition, high dietary fat consumption enhances the number and intensity of IBD flares, presumably due to enhanced mucosal permeation of gut microbes or dietary antigens (3). Specifically, there is a striking positive correlation between IBD incidence rates and the industrialization status of a country, as diets in these societies tend to be richer in processed fats and sugars (8). Similarly, migrants traveling from countries with low IBD incidence rates to countries with high incidence rates adopt the risk of developing IBD with the latter (9). Taken together, dietary fats play an integral role in intestinal disease pathogenesis, prompting a need to understand the molecular mechanisms underlying dietary fat–mediated intestinal permeability. Indeed, knowledge of diet-induced intestinal changes may prove invaluable for successful treatment of intestinal disease, such as IBD. Diet alteration is not only a simpler choice for therapeutic targeting, it is relatively inexpensive, carries less risk of adverse effects, and can be fine-tuned by either the physician or patient. Moreover, a regimen consisting of dietary changes enhances treatment adherence as patients are more involved and have greater control of their treatment compared to conventional pharmacologic regimens (10). Therefore, this review explores the various mechanisms underlying the effects of dietary fat on intestinal permeability.
The Western-Style Diet
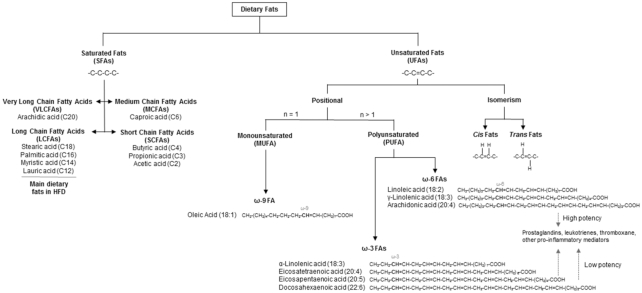
Dietary fats come in many forms and are classified based on the number and position of double bonds within the hydrocarbon chain (Figure 1). For example, SFAs have no double bonds whereas MUFAs contain one and PUFAs contain more than one double bond. Additionally, unsaturated fatty acids (UFAs) can be configured in the cis- or trans-orientation with respect to their double bonds, where the former is natural, and the latter is processed. Diets rich in UFAs, such as in a Mediterranean diet, are associated with anti-inflammatory properties (11, 12). In contrast, diets rich in SFAs, such as with a Western-style diet, are associated with negative health impacts including obesity, diabetes, cardiovascular disease, and even IBD (8, 13–15).
FIGURE 1.
Classification of dietary fats. Dietary fats come in a variety of forms and are classified based on the presence of double bonds in the hydrocarbon chain. SFAs have no double bonds while unsaturated fatty acids (UFAs) contain double bonds. UFAs are subcategorized based on the orientation, number, and position of the double bonds. Cis fats contain cis-oriented double bonds and are natural, while trans fats contain trans-oriented double bonds and are usually synthetic or processed. Fatty acids with one double bond are MUFAs, while PUFAs contain more than one double bond. Last, UFAs can be further classified depending on the position of the chain-terminal double bond. For example, ω-3 PUFAs contain a double bond 3-carbons away from the terminal carbon on the fatty acid tail. PUFAs are usually regarded as safe and beneficial; but that depends on potency, concentration, and oxidizability. HFD, high-fat diet.
The “Western-style diet,” as it is known colloquially, began around the time of the Industrial Revolution and developed throughout the 20th century as meats rich in SFAs became available year-round, refined vegetable oils and dairy products became more affordable, and foods containing sugars and grains were heavily processed (16). Today, 35–45% of calories derived from a Western-style diet is from fat, to which 11.1% is from SFAs alone (17). As of 2015, the American Heart Association recommends reducing SFA intake as a percentage of daily calories to 5–6% (13.1 g in a typical 2000-kcal/d diet) and to replace SFAs for PUFAs in the diet at a 1:1 ratio where possible in order to reduce cardiovascular disease risk (18). Unfortunately, these fats are mainly derived from dairy and lard-based foodstuffs, which are ubiquitous and culturally ingrained in Westernized countries.
In order to study how dietary fats affect biochemical processes and impact human health, researchers feed mice various diets with different fat contents designated as a percentage of total daily energy intake in kilocalories. Typically, control low-fat diets (LFDs) derive approximately 8–15% kcals from fats while HFDs derive either 40–50% kcals (models a Western-style diet) or 60–80% kcals (models diet-induced obesity) from fats (19). For simplicity, in this article “LFD” is used only to refer to diets deriving ≤15% kcal from fat, and “HFD” is used to refer to deriving ≥35% kcals from fat unless noted otherwise.
Dietary Fat Disrupts Tight Junctions in Intestinal Epithelium
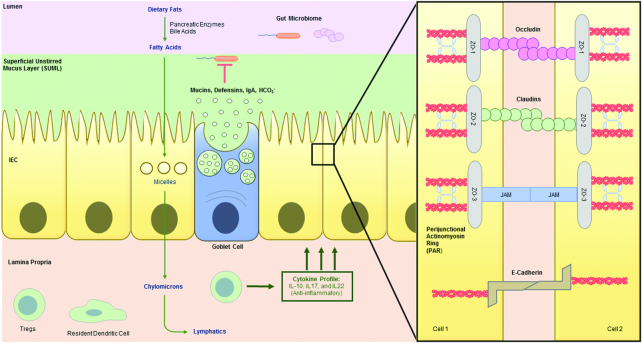
One mechanism by which dietary fat modulates intestinal permeability is through altering the distribution and expression of intestinal tight junctions (TJs), directly or indirectly. TJs and adherens junctions (AJs) comprise the apical junctional complex (AJC), one of the best understood components of the intestinal barrier system (for an extensive review on TJs see references 20 and 21). TJ and AJ components exist as multimeric complexes consisting of transmembrane proteins such as E-cadherin, occludin (OCLN), claudin (CLDN), and junctional adherens molecule (JAM). The structure of these proteins includes extracellular and intracellular domains (Figure 2). The extracellular domain binds the lateral-apical epithelial surface with adjacent intestinal epithelial cells (IECs) harboring TJ proteins of the same type, while the intracellular domains bind “bridging” proteins such as catenins, zona occludens (ZO, TJP1) −1, −2, and −3, that anchor the TJs directly to F-actin (22). Thus, the AJC connects adjacent IECs to form a lumen-tight seal that selectively allows nutrients to permeate paracellularly. Indeed, this barrier inhibits unwarranted permeation of environmental toxins, luminal antigens, and bacteria, which prevents potential focal enteropathy or systemic disease (Figure 3). Moreover, IEC permeability can be dynamically altered through rearrangement of cellular cytoskeletal elements, expression of AJC proteins, or posttranslational modifications. Therefore, disruption of AJC complexes results in intestinal hyperpermeability.
FIGURE 2.
Intestinal barrier system under normal dietary conditions. In steady state, the intestinal tract utilizes various strategies to prevent foreign material translocating from the lumen into the lamina propria (LP). The goblet cell–derived mucus prevents bacterial breach of the enterocyte monolayer by forming a physical barrier, enhancing degradation via defensins, and stimulating regulated immune responses through secreted IgA. In addition, the LP is maintained under an anti-inflammatory state due to the presence of anti-inflammatory dendritic cells and associated cytokines. Last, tight junctions (TJs) formed between enterocytes are numerous and selectively allow permeation of molecules while simultaneously walling the LP from the lumen. There are many proteins involved in TJ maintenance. including occludin, claudins, cadherins, etc. and these proteins are integral in gut barrier maintenance. IEC, intestinal epithelial cell.
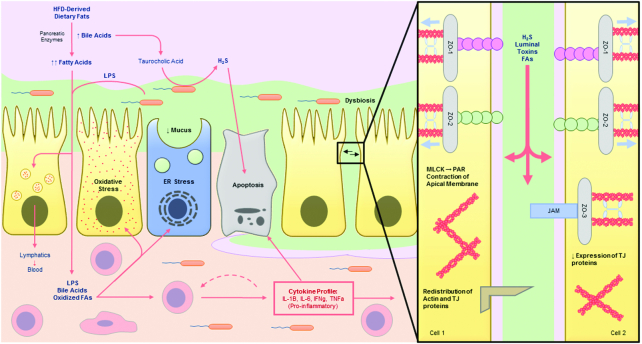
FIGURE 3.
Intestinal barrier system under a chronic high-fat diet (HFD). Chronically consuming an HFD has many detrimental effects on intestinal barrier integrity. An HFD reduces tight-junction (TJ) tightness by downregulating expression of TJ proteins or stimulating aberrant perijunctional-actinomyosin ring (PAR) contraction, permitting influx of luminal components to the lamina propria (LP). Translocated BAs and fatty acids induce enterocyte oxidative stress and apoptosis, further reducing the lumen tight seal. Additionally, fatty acid–induced unfolded protein response (UPR) stress in goblet cells inhibits the secretion of mucus, which in addition to elevated hydrophobic BA load, negatively impacts both the quality and quantity of mucus, permitting gut microbiota invasion. Changes to the bacterial flora by HFD increases the abundance of pathogenic strains, such as Desulfovibrio spp., which deconjugate TCA-producing genotoxic H2S gas. All these factors promote an inflammatory response which furthers the cycle of intestinal barrier degeneration, predisposing the host to gastrointestinal pathologies. BA, bile acid; ER, endoplasmic reticulum; MLCK, myosin-light chain kinase; PAR, perijunctional actinomyosin ring; TCA, taurocholic acid.
Dietary fat directly modulates TJ distribution and expression levels
Recent studies have shown that dietary fats directly modulate AJC integrity, and thus intestinal permeability. For example, a long-term HFD reduces expression of gut TJ proteins and correlates with intestinal hyperpermeability in mice (23, 24). In corroboration, continuous feeding of C57BL/6J mice with an unsaturated, but not saturated, fatty acid–enriched diet significantly reduced TJ expression and increased flux of fluorescein isothiocyanate–conjugated 4-kDa dextran, a measurable fluorescent marker of paracellular permeability (25). Similarly, Long-Evans Tokushima Otsuka and Otsuka Long-Evans Tokushima Fatty rats, which develop obesity as a result of enhanced neuropeptide Y–mediated hyperphagia, fed an HFD exhibited reduced expression of Ocln, Jam2, Cldn1, andCldn3, which originated with the dietary fat itself and not the metabolic consequences of the diet (26).
Dietary fats may also modulate intestinal permeability through stimulation of intracellular signaling pathways. Notably, a typical Western-style diet contains an abundance of γ-linolenic (18:3, n–6) and docosahexaenoic acid (22:6, n–3), both of which are known potent signaling molecules (Figure 1). Direct supplementation of these fatty acids to differentiated human colon adenocarcinoma cell line (Caco2) monolayers, a well-established in vitro enterocyte model, enhanced intestinal permeability through protein kinase C (PKC)-mediated redistribution of actin and TJ proteins, an effect ameliorated with PKC inhibitors (27, 28). Additionally, Caco2 cells can convert eicosapentaenoic acid (20:5, n–3), another Western-style diet PUFA, into bioactive metabolites that enhance monolayer permeability through stimulation of endogenous cyclooxygenase and lipooxygenase activity (29). Taken together, these results confirm that dietary fats directly alter intestinal barrier integrity by interacting with intestinal epithelial cells.
Dietary fat–induced bile acid production damages the gut mucosal barrier
It has long been known that dietary fat stimulates both bile acid (BA) synthesis and bile expulsion by the gallbladder. The BAs emulsify luminal fat and increase the total surface area for lipase-mediated digestion of micelles for absorption by intestinal epithelial cells. Multiple studies have shown a direct correlation between fat intake and BA secretion/synthesis (30–32), and this phenomenon can be explained briefly. First, digested chyme containing fat stimulates cholecystokinin (CCK) secretion by duodenal I-cells. Next, CCK stimulates gallbladder contraction and expulsion of stored bile. Following that, luminal BAs return to the liver via the entero-hepatic circulation, which reabsorbs and recycles 95% of the intraluminal bile at the ileum. Finally, recycled bile is reabsorbed by hepatocytes and secreted back into bile canaliculi [for a more thorough review on BA metabolism and signaling, see (33)]. Under normal physiologic conditions, IECs are usually resistant to the solubilizing effects of BAs. However, chronically high intraluminal and fecal BA levels, especially those seen in HFDs, may induce intestinal hyperpermeability through alteration of TJ dynamics (26, 34–36).
Stenman et al. (36) was one of the first to investigate the detrimental effects of BAs on intestinal permeability in vivo. This group showed that long-term HFD, but not LFD, increased BA synthesis 10-fold and enriched bile composition in hydrophobic BAs such as deoxycholic acid (DCA), lithocholic acid (LCA), and chenodeoxycholic acid (CDCA) (36). In particular, hydrophobic BAs induced intestinal hyperpermeability when administered at concentrations observed in HFD, but not LFD (36). One possible mechanism for DCA- and CDCA-induced intestinal hyperpermeability is by epidermal growth factor receptor (EGFR) stimulation (35). In Caco2 cells, treatment with supra-physiologic concentrations of DCA and CDCA led to rapid stimulation of EGFR-mediated signaling, resulting in 1) SRC kinase activation; 2) downstream serine-threonine dephosphorylation of the OCLN cytoplasmic tail; 3) OCLN-ZO1 complex dissociation; and 4) enhanced intestinal permeability (35). However, changes in intestinal permeability were reversed upon administration of synthetic EGFR inhibitors or sustained epidermal growth factor (35). In an associated mechanism, treatment of Caco2 cells with supra-physiologic, but not physiologic, hydrophobic BA concentrations enhanced IEC generation of reactive oxygen species (ROS) resulting in oxidative stress (OS)-mediated phosphoinositide 3-kinase (PI3K) stimulation (Figure 3) (34). In this setting, PI3K activation promotes intestinal permeability through increased intercellular translocation of the regulatory p85 subunit, enabling PI3K-mediated tyrosine phosphorylation of ZO1 and β catenin, inhibiting structural interactions with OCLN and E-cadherin isomers, respectively (37, 38). Last, the oxidation product of a linoleic acid (18:2, n–6), 13-hydroxyoctadecadienoic acid, the most abundant PUFA in a Western-style diet, is at its highest concentration in the intestinal lumen after a fat-rich meal and may promote intestinal permeability similarly to LCA as a result of close structural identity (39).
In contrast, recent evidence suggests that supplementation with hydrophilic BAs, such as ursodeoxycholic acid, which are thought to act in an opposite manner to hydrophobic BAs, improves the intestinal epithelium barrier integrity in murine models through a variety of mechanisms, including reducing OS (40–43). These results indicate that dietary fat–induced shifts in BA quantity and quality negatively impact intestinal permeability and may be a promising therapeutic route in treating intestinal diseases characterized by enhanced colonic bile output, such as bile acid malabsorption diarrhea, commonly associated with Crohn's ileitis (44).
Dietary Fat–Associated Intestinal Inflammation Enhances Intestinal Permeability
It has long been known that the integrity of the intestinal barrier is exquisitely sensitive to the inflammatory state of the gut. For example, the intestinal hyperpermeability observed in Crohn's disease (CD) is a direct result of pathologic changes in IEC signaling, viability, and cohesiveness induced by proinflammatory mediators such as IL1B, IL6, IFNγ, and TNFα, to name a few. Part of the IBD spectrum, CD is an autoimmune condition characterized by intestinal transmural inflammation, noncaseating granulomas, abdominal pain, and remitting diarrhea. CD may occur anywhere along the gastrointestinal (GI) tract, but primarily localizes to the terminal ileum. Specifically, the CD-associated T1H inflammatory response is directly responsible for the pathologic degeneration of the intestinal barrier through cytokine signaling to immune cells and IECs (Table 1) (45–74). Moreover, these cytokines have been prime targets for therapy as inflammation, in addition to intestinal permeability parameters, return to normal when pharmacologically inhibited (75–77).
TABLE 1.
Compilation of studies showing which cytokines are changed during HFD feeding and how they mechanistically alter the integrity of the gut barrier system1
| Cytokine | Effects of HFD (reference2) | Permeability effects | Mechanism of action(s) on barrier integrity | Reference(s) |
|---|---|---|---|---|
| IL1B | ↑ (45) | ↑ | Reduction of OCLN expression | (46) |
| MAPKKK-mediated NFκB activation → MLCK-induced PAR contraction | (47–49) | |||
| IL4/IL13 | ↑ (50) | ↑ | PI3K-mediated actin reorganization | (51) |
| STAT6–unknown | (52) | |||
| IL6 | ↑ (53) | ↑ | Reduction of TJP1 expression | (54) |
| Claudin switching—↑ pore-forming claudins3 | (55) | |||
| IL10 | ↓ (56) | ↓ | Reduction in proinflammatory cytokine signaling—specifically IFNγ | (57) |
| p38 MAPK–mediated increase in cadherin levels | (58) | |||
| IL12 | ↑ (59) | ↑ | Unknown | |
| IL17/IL23 | ↓ then ↑ (60) | ↓ | Act1-mediated OCLN association with F-actin | (61) |
| IL18 | ↑ (45) | ↑ | Unknown | |
| IL22 | ↓ then ↑ (62) | ↓ | Increased IEC proliferation and migration | (63) |
| Reduction in enterocyte ER stress | ||||
| Replenishment of mucus layer by GCs | (64) | |||
| IFNγ | ↑ (65) | ↑ | Internalization of TJ proteins via macropinocytosis | (66) |
| PI3K-mediated NFκB activation → MLCK-induced PAR contraction | (67) | |||
| TNFα | ↑ (68) | ↑ | ↑ IEC apoptosis | (69) |
| Internalization of TJ components | (67) | |||
| MLCK-dependent contraction of PAR | (70, 71) | |||
| Claudin switching—↑ pore-forming claudins | (72) | |||
| Perforin | ↑ (73) | ↑ | Unknown | |
| Granzyme B | ↑ (73) | ↑ | Cleave TJ and AJ components | (74) |
AJ, adherens junction; ER, endoplasmic reticulum; GC, goblet cell; HFD, high-fat diet; IEC, intestinal epithelial cell; MLCK, myosin light chain kinase; OCLN, occludin; PAR, peri-actinomyosin ring; TJ, tight junction; TJP1, zona-occludens 1 (ZO1) gene.
These references address research into how HFD alters permeability generally. Right column references address research into specific mechanisms.
Interestingly, the same cytokine state encountered in the intestines of CD patients is also present during HFD feeding, an idea supported by studies linking increased dietary fat intake with enhanced risk of CD development and increased flare-up number and severity. (For a review on how dietary fats regulate the immune system and how cytokines influence gut barrier integrity, please see references 78 and 79). In concordance with enhanced immune-cell infiltration of the lamina propria (LP) in CD, direct exposure of rat duodenum to olive oil activates leukocyte homing via stimulation of adhesion protein expression, primarily intercellular adhesion molecule 1, on postcapillary venules of Peyer's patches (80). Likewise, dietary fat enhances the expression of endothelial mucosal vascular addressin cell adhesion molecule 1 and vascular cell adhesion molecule 1, which enhances LP-homing of circulating immune cells during feeding (80–82). Mechanistically, Kawano et al. (45) showed that the enhanced infiltration of C-X-C motif chemokine receptor 3 (CXCR3+) macrophages of the murine LP observed during HFD feeding was due to stimulation of chemokine (C-C motif) ligand 2, the cognate chemokine of CXCR3, expression and secretion by IECs. This finding corroborates previous studies which show enhanced macrophage infiltration and exacerbation of pre-existing inflammation and tissue damage by HFD feeding in both chemically-induced (dextran-sulfate sodium and 2,4,6-trinitrobenzenesulfonic acid) and genetic (Mdr1a−/− and Muc2−/−-TnfΔARE) murine models of IBD (3, 4, 83, 84).
The prevailing theory behind how an HFD promotes intestinal inflammation is as follows: HFD induces preliminary intestinal hyperpermeability, transient gut microbiota dysbiosis (explained later), and enhanced recruitment of proinflammatory leukocytes to the LP. These changes may ultimately increase the risk of developing intestinal inflammation and exacerbates pre-existing inflammation such as in IBD. When inflammation is present, local cytokine signaling facilitates greater recruitment of proinflammatory leukocytes and induces strong pathologic degeneration of the intestinal barrier, furthering the cycle. In addition to what has been mentioned, dietary free fatty acids also participate in the inflammatory response by directly stimulating inflammatory signaling, which further potentiates the intestinal hyperpermeability. The different mechanisms in which dietary fats modulate the immune response in the context of modulating permeability are explored below.
HFD promotes LPS-induced gut barrier dysfunction
One of the first studies to link dietary fats with intestinal immunity was from Miura et al. (85), who demonstrated that feeding mice a lard-based diet resulted in mesenteric lymph node leukocyte proliferation, presumably due to enhanced lymphatic chylomicronemia. Although the exact mechanism was unknown at the time, it is now thought that this response is due to increased intestinal permeation of LPS, which is directly incorporated into chylomicrons and delivered to the circulatory system (86). A major component of the outer cell membranes of gram-negative bacteria, LPS consists of an O-antigen, core polysaccharide, and immunogenic lipid A. Specifically, lipid A is composed of branched chain SFAs and is the primary component recognized by the proinflammatory Toll-like receptor 4 (TLR4)–CD14 system in immune cells and intestinal epithelial cells.
In the gut, LPS is derived from the regular turnover of microflora-derived gram-negative bacteria and, depending on the intestinal permeability and dietary fat content, becomes incorporated into chylomicrons via its lipid A tail (86). Intestinal permeability facilitates passive translocation of luminal LPS or LPS-containing bacteria into the LP, promoting greater translocation of LPS into blood (termed endotoxemia) independent of diet. This relationship may partially explain the elevated serum LPS observed in patients with IBD and other intestinal pathologies such as necrotizing enterocolitis (87, 88). In fact, serum LPS is now an accepted surrogate marker for assessing in vivo intestinal permeability (89).
Similarly, dietary fat can enhance serum LPS levels independent of permeability status, mainly through luminal micellar incorporation and stimulation of lipid raft-mediated endocytosis (90). Cani et al. (91) was the first to demonstrate that serum LPS levels varied in the fast compared with the fed state and that an HFD was responsible for 3-fold greater serum LPS in mice. In addition, Cani et al. showed that HFD feeding significantly enriched the gut microflora with LPS-containing bacteria. Concerning the components of an HFD, the elevation of serum LPS was solely dependent on the dietary content of SFAs, but not PUFAs, presumably due to lipid rafts (90). These results may explain the original findings that humans consuming an LFD have serum LPS levels ranging from 0 to 0.2 ng/mL while those consuming diets high in fat may have levels up to 2 ng/mL (92). This mechanism also confirms findings that consuming diets high in calorie content (fats) is associated with elevated serum LPS in healthy men (93). Interestingly, a popular theory explaining insulin resistance observed in metabolic syndrome is that it may be a result of low-grade inflammation due to metabolic endotoxemia in the obese state, which could be a combinatorial effect of resting intestinal hyperpermeability and diet-induced LPS translocation (94–96).
Indeed, LPS is best known for stimulating a TLR4-CD14–dependent proinflammatory response that directly alters the intestinal barrier. In addition, LPS has been found to directly modulate TJ organization and enhance permeability of Caco2 (as measured by decreased transepithelial electrical resistance) monolayers via TLR4-CD14–mediated activation of NFκB (97). Furthermore, LPS directly induces rapid IEC shedding without compensatory TJ-resealing (98). Last, LPS may promote IEC OS, mitophagy, and overall mitochondrial failure resulting in enhanced intestinal permeability (99). These results provide an indirect mechanism by which an HFD promotes intestinal hyperpermeability; HFD-feeding enhances local and serum LPS levels which 1) promote intestinal inflammation, 2) modulate TJ organization through signaling cascades, and 3) induce IEC dysfunction, ultimately resulting in hyperpermeability.
SFAs directly promote an LPS-like inflammatory response
It has been proposed that the structural similarity between dietary SFAs and the lipid A component of LPS may permit SFA-TLR4 interactions, which would provide a direct link between HFD consumption and intestinal inflammation (100). Indeed, it was initially found that SFAs, specifically lauric acid, but not PUFAs, activated NFκB-signaling in a TLR4-dependent manner, which was later attributed to TLR4-homodimerization via lipid-raft incorporation (101, 102). Interestingly though, it was also discovered that SFAs can promote TLR4-independent inflammation through stimulation of TLR2 heterodimerization with TLR1 or TLR6 (an activating signal) via lipid-raft incorporation (103, 104). However, although only a limited number of SFAs have been found to promote TLR activation, there is still active research on other mechanisms by which dietary fats promote inflammation, and therefore, intestinal permeability.
Dietary fats inhibit probarrier signaling between immune cells and IECs
There is increasing evidence that the gut immune system coordinates with IECs to promote gut barrier maintenance (Table 1), and that this cross-talk is dysregulated during acute HFD-feeding (105). For example, intestines of mice fed an HFD showed markedly reduced IL17, a cytokine that promotes gut barrier TJ organization via Act1-mediated association of OCLN with F-actin (61). Similarly, rats fed an HFD showed drastic reduction of intestinal IL10 as soon as within 1 wk of starting the diet (57, 58). IL10 is the principle anti-inflammatory cytokine that helps maintain the intestinal barrier through inhibiting proinflammatory cytokine signaling (57, 58). Importantly, HFD feeding depletes the LP of T-regulatory cells, the primary source of IL10, through a yet unknown mechanism and may be a contributing factor behind the unfavorable cytokine environment and intestinal permeability status observed with this diet (65). Lastly, mice fed an HFD demonstrated reduced IL22 (source yet to be identified), another gut barrier–promoting cytokine, in the acute period, followed by dramatic elevation in the chronic phase due to compensatory forces (62). Mechanistically, IL22 is thought to enhance gut barrier function through PI3K-mediated IEC proliferation, enhancing the wound-healing response, and reduce fatty acid–induced ER stress (63, 64).
Dietary Fat Disrupts the Intestinal Epithelial Shedding–Proliferation Axis
The intestinal tract bears the tremendous responsibility of absorbing different nutrients from the diet while protecting the body from ingested toxins, bacteria, and other foreign substances. For this reason, IECs are constantly shed and replaced by new enterocytes every 2–6 d, guided by an active proliferating layer at the base of the crypts of Lieberkühn guided by Wnt signaling (106, 107). It has been estimated that approximately 1011 IECs are lost daily and that this number requires a regenerative supply, making the intestines one of the sites of the fastest fixed-cell turnover rate in the human body (108). The balance between proliferation and shedding is controlled by a complex process of superficial enterocyte apoptosis, extrusion with rapid resealing of AJCs, and basal cell proliferation with upward movement and differentiation. This process is highly coordinated and tightly regulated and thus is susceptible to a myriad of stimuli, one of which is diet-induced OS [for an extensive review on this subject see (108)].
Effects of oxidative stress on the enterocyte shedding–proliferation axis
It has been proposed that the pathogenesis of multiple HFD-associated diseases, such as obesity, diabetes, and metabolic syndrome, involves self-propagating inflammation secondary to OS (109). OS involves an intracellular redox balance shift from a reducing environment to an oxidizing one and is caused by dysfunction or depletion of antioxidant molecules such as vitamin E, ascorbic acid, and glutathione by pro-oxidant electrophiles generated from cellular respiration or exogenous toxins. HFDs are rich in PUFAs, which contain double bonds that are susceptible to oxidation via exogenous or endogenous sources (110). Previous studies have shown that oxidation of PUFAs may be caused by different premeal preparation techniques, such as thermophilic-oxidative conversion or frying, or endogenous oxidation via transit through oxidative environments of the GI tract, such as the stomach (111–114). These PUFAs are delivered to the intestines, where they are prepared for absorption by IECs via interaction between bile salts, pancreatic lipases, and brush border enzymes. It has been proposed that their oxidized counterparts passively diffuse across the apical membrane and induce intracellular oxidative stress. Additionally, it is suggested that oxidized PUFAs peroxidize plasma membrane phospholipid components from the luminal compartment. Both mechanisms result in free-radical initiation, propagation, and OS (115). Studies have shown that OS predisposes the intestines to chronic diseases such as IBD primarily through disruption of the gut barrier function via alteration in the enterocyte shedding-proliferation axis, disruption of the OCLN-ZO1 pathway, and induction of inflammation (116).
The importance of maintaining the proliferation-shedding axis is exemplified by studies which show that conditions favoring enterocyte apoptosis, such as endotoxemia or systemic inflammation, induce basement membrane damage and the formation of numerous mucosal “gaps,” thereby increasing intestinal paracellular permeability (117, 118). By the same measure, other studies have linked chronic enterocyte OS with dysregulation of the enterocyte shedding-proliferation axis, either through depressed proliferation, disruption of AJC resealing, or enhanced apoptosis, particularly through HFD-derived oxidized PUFAs (119). For example, treatment of Caco2 cells with lipid peroxides (LOOHs), which can be found at detectable concentrations in a typical Western-style diet, not only induced pronounced oxidative stress (decreased reduced glutathione:oxidized glutathione ratio), but also depressed G0/G1 cell transition and cyclin D/cyclin-dependent kinase 4 expression, while enhancing DNA oxidation in a concentration-dependent manner (120, 121). In addition, LOOH-induced apoptosis of Caco2 cells was uninhibited by redox homeostasis restoration, suggesting that chronic HFD-induced oxidative stress results in permanent IEC damage (115). Moreover, intestinal absorption of 4-hydroxy-2-hexenal, a natural decomposition product of oxidized PUFAs, not only elicited site specific (duodenal) OS and inflammation, but also markedly enhanced IEC apoptosis both in vitro and in vivo (110). However, although none of these groups directly studied the effect of enterocyte turnover on intestinal permeability, some have speculated that HFD-related intestinal oxidative stress may be the initiating factor underlying the gut barrier dysfunction and endotoxemia observed in type 2 diabetes and various other intestinal and extraintestinal diseases (89–91). More studies are needed to solidify the connection between dietary fats and intestinal OS, and how these factors influence intestinal permeability and risk of enteric pathology.
Dietary Fats Alter Intestinal Mucus Properties
In addition to the numerous TJs that inhibit permeation of luminal contents, the intestinal barrier consists of a superficial unstirred mucus layer (SUML) which coats IECs with a protective milieu of bicarbonate, antimicrobials, IgA, glycoproteins, and lubricant. Research into the SUML has revealed its importance in maintaining intestinal homeostasis mainly by filtering out bacteria, luminal toxins, and insoluble material, thereby preventing damage to bathed enterocytes (122, 123). Indeed, the SUML was shown to be integral to gut health as mice deficient in mucin 2 (MUC2), a glycoprotein which forms the bulk of the mucus, spontaneously developed colitis, with a presentation similar to dextran sulfate-sodium–induced colitis (124). Recent evidence points to the ability of dietary fats to modulate the composition and function of the SUML, which is primarily produced by goblet cells (GCs) and becomes more numerous moving distally through the intestinal system. For example, mice fed an HFD stimulated the GC unfolded protein response (UPR) pathway via localized generation of OS, resulting in enhanced misfolding of MUC2 and a degeneration of the SUML morphology (Figure 3) (64). In this setting, the destruction of the mucus layer correlated directly to gut barrier dysfunction, assessed by enhanced endotoxemia, either as a result of diminished TJ protein expression, increased TJ protein misfolding, or inflammation (64, 125). Moreover, HFD feeding repressed the expression of the cystic fibrosis transmembrane receptor (Cftr) gene in mouse ileal enterocytes via activation of peroxisome-proliferating factor gamma. The CFTR enhances chloride secretion, which hydrates the SUML and promotes its fluidity and robustness. In contrast, reduced CFTR levels decrease the viscosity and density of the SUML, promoting the loss of intestinal barrier integrity (126).
Other studies have subsequently parsed out the role other contents of the SUML play on maintaining intestinal barrier integrity. For example, the SUML contains a reservoir of antioxidants which actively protect the intestinal mucosa from luminal pro-oxidative molecules like oxidized PUFAs (127). Indeed, one study showed that mucus derived from inflamed pancreases, which is rich in oxidative compounds (modified via the inflammatory environment), depletes the antioxidant capacity of the SUML (as measured by enhanced nitrosylation and carbonylation of mucus proteins) and increases intestinal permeability (127). Likewise, studies have linked HFD with increased OS, and it is possible that oxidized FAs may deplete the antioxidant capacity of the mucus and increase intestinal permeability (64, 128).
In another possible connection between dietary fats and intestinal mucus–associated changes, GC fatty acid synthase (FAS), is responsible for the palmitoylation of the N-terminus, and therefore proper secretion, of MUC2 and therefore may play a role in intestinal barrier functionality (129). For example, knockout of FAS in GCs resulted in significantly diminished mucus MUC2 content and increased intestinal permeability. As FAS is a metabolic keystone enzyme, it is subjected to heavy regulation. HFD feeding diminishes FAS activity in hepatocytes, thus leaving open the possibility that a similar regulatory process may occur in GCs or even IECs, although no studies to date exist (130, 131). Interestingly, because FAS is an insulin-sensitive enzyme, FAS downregulation in the setting of HFD-induced insulin resistance (diabetes or metabolic syndrome) may provide additional insight into the pathophysiology of enhanced intestinal permeability and associated endotoxemia seen in these patients (95, 132, 133). Although research into the dietary modulation of the intestinal mucus layer is still in its infancy, the effects of HFD on intestinal permeability may be explained partly by disruption of the SUML. However, more research is needed to fully describe these interactions.
Dietary Fat–Associated Gut Dysbiosis Alters Intestinal Permeability
It is now well established that diet modulates the gut microbial composition, maintaining intestinal homeostasis in the normal individual. It is estimated that trillions of microbes colonize the gut (∼1011–1014 bacteria per g of colon weight) and have a multitude of functions which include, but are not limited to, metabolism, inflammation, and maintenance of gut barrier integrity (91, 134–138). These microbes colonize the gut early at birth and differentially develop up until adulthood with a composition primarily reflecting the dominant diet (Figure 2) (139). This is one of the primary reasons why colonic bacterial composition is similar within a geographical region and drastically different between regions (140, 141). However, although established at an early age, the microbiome diversity is both dynamic and malleable as sustained, but not short-term, changes in diet can directly alter microbiome richness and composition (142). In addition, 16s ribosomal RNA “fingerprinting” of colonic bacteria has revealed dominant bacterial species are associated with certain diets such as Prevotella (carbohydrates and fiber) and Bacteroides (meat and fats) (143, 144). Gut microbial diet–associated patterns can be explained by the fact that colonic microbes inhabit the same luminal space and directly compete for undigested nutrients supplied by the diet. Thus, microbiome balance is in part dependent on nutritional balance, and thus dietary imbalances in quantity or quality are reflected as microbial imbalances, or dysbiosis (Figure 3). Moreover, gut dysbiosis has been implicated in numerous disease processes, most notably diet-induced obesity, and has direct effects on gut permeability.
Obesity-mediated dysbiosis has been directly linked to dietary excess of fats and manifests in a reduced overall microbial load, a shift in species abundance, and an overall increase in intestinal permeability (136, 145). This concept was exemplified in a landmark study by Bäckhed et al. (146), in which gut microbiota transplanted from HFD-induced obese mice into sterilized mice colons resulted in the development of a metabolic syndrome phenotype with epithelial barrier dysfunction independent of recipient diet. In addition, as previously mentioned, several studies have shown that obesity, as well as high-fat intake, was associated with enhanced gut-derived endotoxemia as a direct result of barrier dysfunction and enhanced paracellular permeation of luminal LPS in both mice and humans (91, 135, 147–149). Some have argued that this endotoxemia is partly responsible for the low-grade inflammatory state seen in obesity. For example, one group showed that germ-free mice fed an HFD did not develop systemic inflammatory markers associated with obesity (150). This same group also revealed that transplantation of bacteria from HFD mice to germ-free mice resulted in elevated intestinal Nfkb1 expression, indicating that the presence of diet-induced dysbiosis alone is sufficient for inflammation (150). Furthermore, the dominant bacterial species distributed in an HFD gut support the general notion that there exists a link between dietary fats, colonic microflora composition, and inflammation, which greatly influences gut permeability.
Research into diet-induced colonic dysbiosis has provided insights into possible mechanisms explaining the inverse correlation between fat intake and gut barrier function. Numerous studies have probed dietary fat–induced alterations in gut microflora species and related them to changes in gut permeability (Table 2) (151–159). The results of these studies reveal that HFDs transiently reduce gut barrier–promoting microbes, such as Lactobacillus spp., Bifidobacterium spp., Bacteriodetes spp., Clostridiales spp., and Akkermansia muciniphilia, while augmenting microbes involved in decreasing barrier integrity, such as Oscillibacter spp., and Desulfovibrio spp. Although the exact mechanism of how these specific microbial species modulate intestinal permeability is still highly debated, it is speculated that they exert their effects either directly through secreted products, or indirectly through immune system provocation. For example, HFD increases levels of Desulfovibrio spp., specifically Bilophila wadsworthia, which produces genotoxic hydrogen sulfide (H2S) gas, causing IEC hypoplasia and hyperpermeability (156, 160–162). Specifically, B. wadsworthia uniquely utilizes taurocholic acid (TCA)-derived sulfur as a reducing equivalent in its electron transport chain system for survival and proliferation in the gut (157). The link between HFD, B. wadsworthia, and enhanced intestinal permeability is hypothesized to be a result of physiologic elevation of taurine-containing luminal bile salt levels in response to dietary fat, as discussed previously (Figure 3). TCA is then used as a reducing equivalent, thereby enhancing intracellular energy production, proliferative capacity, and H2S gas production, resulting in inhibition of butyrate oxidation, a step required for maintenance of both gut permeability and enterocyte energy balance (157). Indeed, increased colonic levels of TCA select for Bilophila spp., ultimately resulting in a nutritional advantage and increased representation in the gut flora. Moreover, HFD enhanced intestinal levels and the nutritional advantage of Oscillibacter spp., directly correlating with depressed IEC expression of Tjp1 and gut barrier dysfunction (163).
TABLE 2.
Compilation of data from different studies looking at the effects of high-fat feeding on gut microbiota composition and their effects on intestinal barrier integrity1
| GI microbe phylum | GI microbe subclass | Effects of HFD | Permeability effects | Potential function(s) | Reference(s) |
|---|---|---|---|---|---|
| Actinobacteria | Bifidobacterium spp. | ↓ | ↓ | Short-chain fatty acid production increases gut barrier integrity | (91, 142, 151) |
| Bacteriodetes | Bacteriodiodes spp. | ↑ | ↓ or ↑ | Decreases gut colonization of Enterobacteriaceae | (143, 144) |
| Prevotella spp. | ↓ | ↓ | Unknown | (152) | |
| Firmicutes | Clostridium spp. | ↑ | ↓ | Acetate, propionate, and toxin production | (153, 154) |
| Lactobacillus spp. | ↓ | ↓ | Increases expression of OCLN and TJP1 | (155) | |
| Oscillobacter spp. | ↑ | ↑ | Decreases expression of TJP1 | (156) | |
| Proteobacteria | Desulfovibio spp. | ↑ | ↑ | H2S production from TCA | (157) |
| Verrucomicrobia | Akkermansia spp. | ↓ | ↓ | Increases GC mucus and IEC TJ protein synthesis | (158, 159) |
GC, goblet cell; GI, gastrointestinal; HFD, high-fat diet; IEC, intestinal epithelial cell; OCLN, occludin gene; TCA, taurocholic acid; TJ, tight junction; TJP1, zona-occludens 1 (ZO1) gene.
In contrast, Bifidobacterium spp. and Lactobacillus spp., which are decreased in HFD, are associated with enhanced gut barrier function (163, 164). These microbial species act on the intestine through unknown mechanisms to stimulate enterocyte gene expression of the TJ proteins cingulin, OCLN, TJP1, and TJP2 (165–168). Similarly, Akkermansia muciniphila, which accounts for approximately 1–3% of total gut microflora, induces Tjp1 and Ocln gene expression in addition to counteracting HFD-induced thinning of the SUML, thus preventing increased permeation of luminal substrates and pathogens (158, 159). At this point, it is still unknown how the gut microbiome specifically modulates the zonulin pathway or leads to intestinal barrier dysfunction by other means, and thus more research is needed to elucidate mechanisms associated with gut–microbial cross-talk and its implications in diseases associated with enhanced intestinal permeability, such as IBD.
In addition to all that was described previously, there are many other ways by which HFD-associated changes in gut microbiota impact gut barrier functionality, and this field is still evolving. More research is continuously conducted on this topic and hopefully we will see microbe-targeted therapeutics for eliminating strains that negatively impact barrier function while preserving those species with positive impacts. This may be therapeutically relevant as it is possible that these treatments can be used in conjunction with what is already available to produce the best outcome in those with diseases of the gut.
Conclusions
The intestines serve as the first line of defense against toxins and antigens from ingested food. Although the intestinal tract employs a multilayered barrier system, consisting of a superficial mucus layer, an epithelial monolayer, the adherens-junctional complex system, an immune system, and gut microflora (Figure 2), these components may be disrupted by the diet, thus predisposing the host to various intestinal diseases. Here, we discuss how dietary fats disrupts every aspect of the intestinal barrier system and how this may manifest clinically (Figure 3).
Take-Home Message
As interest in the field of nutrition grows, there is heightened awareness in what we consume and how that affects intestinal health. Although the consequences of consuming a Western-style HFD are readily understood, such as obesity, diabetes, cardiovascular disease, and atherosclerosis, less is known about the effects of this diet on the GI tract. Bringing together data from a vast array of studies, it is clear that an HFD negatively impacts intestinal health by disrupting the intestinal barrier system through a variety of mechanisms. Thus, this review should bring greater awareness of the impacts of diet on intestinal physiology and how this may contribute to the etiology of diseases in addition to potentially providing an alternative/supplement to treating existing GI pathologies.
Acknowledgments
All authors have read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: MWR, CAN, TAR-R, and SP, no conflicts of interest.
Abbreviations used: AJ, adherens junction; AJC, apical junctional complex; BA, bile acid; CCK, cholecystokinin; CD, Crohn's disease; CDCA, chenodeoxycholic acid; CLDN, claudin; CTFR, cystic fibrosis transmembrane receptor; CXCR3, C-X-C motif chemokine receptor; DCA, deoxycholic acid; EGFR, epidermal growth factor receptor; ER, endoplasmic reticulum; FA, fatty acid; FAS, fatty acid synthase; GC, goblet cell; GI, gastrointestinal; HFD, high-fat diet; IBD, inflammatory bowel disease; IEC, intestinal epithelial cell; JAM, junctional adherens molecule; LCA, lithocholic acid; LFD, low-fat diet; LOOH, lipid peroxide; LP, lamina propria; MLC, myosin-light chain; MLCK, myosin-light chain kinase; MUC2, mucin 2; OCLN, occludin; OS, oxidative stress; PAR, perijunctional actinomyosin ring; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; ROS, reactive oxygen species; SUML, superficial unstirred mucus layer; TCA, taurocholic acid; TJ, tight junction; TJP1, zona-occludens 1 (ZO1) gene; TLR4, Toll-like receptor 4; UFA, unsaturated fatty acid; ZO, zona occludens.
References
- 1. Hollon J, Leonard Puppa E, Greenwald B, Goldberg E, Guerrerio A, Fasano A. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients. 2015;7:1565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL et al.. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–78. [DOI] [PubMed] [Google Scholar]
- 3. Paik J, Fierce Y, Treuting PM, Brabb T, Maggio-Price L. High-fat diet-induced obesity exacerbates inflammatory bowel disease in genetically susceptible Mdr1a-/- male mice. J Nutr. 2013;143:1240–7. [DOI] [PubMed] [Google Scholar]
- 4. Cheng L, Jin H, Qiang Y, Wu S, Yan C, Han M, Xiao T, Yan N, An H, Zhou X et al.. High fat diet exacerbates dextran sulfate sodium induced colitis through disturbing mucosal dendritic cell homeostasis. Int Immunopharmacol. 2016;40:1–10. [DOI] [PubMed] [Google Scholar]
- 5. Munkholm P, Langholz E, Hollander D, Thornberg K, Orholm M, Katz KD, Binder V. Intestinal permeability in patients with Crohn's disease and ulcerative colitis and their first degree relatives. Gut. 1994;35:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Michielan A, D'Incà R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm. 2015;2015:628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563. [DOI] [PubMed] [Google Scholar]
- 8. Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205–17. [DOI] [PubMed] [Google Scholar]
- 9. Barreiro-de Acosta M, Alvarez Castro A, Souto R, Iglesias M, Lorenzo A, Dominguez-Muñoz JE. Emigration to Western industrialized countries: a risk factor for developing inflammatory bowel disease. J Crohns Colitis. 2011;5:566–9. [DOI] [PubMed] [Google Scholar]
- 10. Martin LR, DiMatteo MR, Lepper HS. Facilitation of patient involvement in care: development and validation of a scale. Behav Med. 2001;27:111–20. [DOI] [PubMed] [Google Scholar]
- 11. Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505. [DOI] [PubMed] [Google Scholar]
- 12. Kris-Etherton PM, Harris WS, Appel LJ; American Heart Association Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57. [DOI] [PubMed] [Google Scholar]
- 13. Hellmann J, Zhang MJ, Tang Y, Rane M, Bhatnagar A, Spite M. Increased saturated fatty acids in obesity alter resolution of inflammation in part by stimulating prostaglandin production. J Immunol. 2013;191:1383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sears B, Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015;14:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Briggs MA, Petersen KS, Kris-Etherton PM. Saturated fatty acids and cardiovascular disease: replacements for saturated fat to reduce cardiovascular risk. Healthc. 2017;5:E29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O'Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81:341–54. [DOI] [PubMed] [Google Scholar]
- 17. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ et al.. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 18. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee I-M, Lichtenstein AH, Loria CM, Millen BE et al.. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–99. [DOI] [PubMed] [Google Scholar]
- 19. Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010;23:270–99. [DOI] [PubMed] [Google Scholar]
- 20. Günzel D, Yu ASL. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013;93:525–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Capaldo CT, Powell DN, Kalman D. Layered defense: how mucus and tight junctions seal the intestinal barrier. J Mol Med. 2017;95:927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res. 140:12–9. [DOI] [PubMed] [Google Scholar]
- 24. Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet–induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. [DOI] [PubMed] [Google Scholar]
- 25. Kirpich IA, Feng W, Wang Y, Liu Y, Barker DF, Barve SS, McClain CJ. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol Clin Exp Res. 2012;36:835–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Suzuki T, Hara H. Dietary fat and bile juice, but not obesity, are responsible for the increase in small intestinal permeability induced through the suppression of tight junction protein expression in LETO and OLETF rats. Nutr Metab. 2010;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Usami M, Komurasaki T, Hanada A, Kinoshita K, Ohata A. Effect of γ-linolenic acid or docosahexaenoic acid on tight junction permeability in intestinal monolayer cells and their mechanism by protein kinase C activation and/or eicosanoid formation. Nutr. 2003;19:150–6. [DOI] [PubMed] [Google Scholar]
- 28. Aspenström-Fagerlund B, Sundström B, Tallkvist J, Ilbäck N-G, Glynn AW. Fatty acids increase paracellular absorption of aluminium across Caco-2 cell monolayers. Chem Biol Interact. 2009;181:272–8. [DOI] [PubMed] [Google Scholar]
- 29. Usami M, Muraki K, Iwamoto M, Ohata A, Matsushita E, Miki A. Effect of eicosapentaenoic acid (EPA) on tight junction permeability in intestinal monolayer cells. Clin Nutr. 2001;20:351–9. [DOI] [PubMed] [Google Scholar]
- 30. Hill M. Bile, bacteria, and bowel cancer. Gut. 1983;871–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reddy BS, Mangat S, Sheinfil A, Weisburger JH, Wynder EL. Effect of type and amount of dietary fat and 1,2-dimethylhydrazineon billary bile acids, focal bile acids, and neutral sterols in rats. Cancer Res. 1977;37:2132–7. [PubMed] [Google Scholar]
- 32. Reddy BS. Diet and excretion of bile acids. Cancer Res. 1981;41:3766–8. [PubMed] [Google Scholar]
- 33. Chiang JYL. Bile acid metabolism and signaling. Compr Physiol. 2013;3:1191–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Araki Y, Katoh T, Ogawa A, Bamba S, Andoh A, Koyama S, Fujiyama Y, Bamba T. Bile acid modulates transepithelial permeability via the generation of reactive oxygen species in the Caco-2 cell line. Free Radic Biol Med. 2005;39:769–80. [DOI] [PubMed] [Google Scholar]
- 35. Raimondi F, Santoro P, Barone MV, Pappacoda S, Barretta ML, Nanayakkara M, Apicella C, Capasso L, Paludetto R. Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am J Physiol-Gastrointest Liver Physiol. 2008;294:G906–13. [DOI] [PubMed] [Google Scholar]
- 36. Stenman LK, Holma R, Korpela R. High-fat-induced intestinal permeability dysfunction associated with altered fecal bile acids. World J Gastroenterol. 2012;18:923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rao RK, Basuroy S, Rao VU, Karnaky Jr KJ, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368:471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sheth P, Seth A, Atkinson KJ, Gheyi T, Kale G, Giorgianni F, Desiderio DM, Li C, Naren A, Rao R. Acetaldehyde dissociates the PTP1B–E-cadherin–β-catenin complex in Caco-2 cell monolayers by a phosphorylation-dependent mechanism. Biochem J. 2007;402:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Penumetcha M, Khan-Merchant N, Parthasarathy S. Enhanced solubilization and intestinal absorption of cholesterol by oxidized linoleic acid. J Lipid Res. 2002;43:895–903. [PubMed] [Google Scholar]
- 40. Kullmann F, Arndt H, Gross V, Rüschoff J, Schölmerich J. Beneficial effect of ursodeoxycholic acid on mucosal damage in trinitrobenzene sulphonic acid-induced colitis. Eur J Gastroenterol Hepatol. 1997;9:1205–11. [PubMed] [Google Scholar]
- 41. Rodrigues CM, Fan G, Wong PY, Kren BT, Steer CJ. Ursodeoxycholic acid may inhibit deoxycholic acid-induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species production. Mol Med. 1998;4:165–78. [PMC free article] [PubMed] [Google Scholar]
- 42. Bernardes-Silva CF, Damião AOMC, Sipahi AM, Laurindo FRM, Iriya K, Lopasso FP, Buchpiguel CA, Lordello MLL, Agostinho CLO, Laudanna AA. Ursodeoxycholic acid ameliorates experimental ileitis counteracting intestinal barrier dysfunction and oxidative stress. Dig Dis Sci. 2004;49:1569–74. [DOI] [PubMed] [Google Scholar]
- 43. Perrone EE, Chen C, Longshore SW, Okezie O, Warner BW, Sun C-C, Alaish SM, Strauch ED. Dietary bile acid supplementation improves intestinal integrity and survival in a murine model. J Pediatr Surg. 2010;45:1256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lenicek M, Duricova D, Komarek V, Gabrysova B, Lukas M, Smerhovsky Z, Vitek L. Bile acid malabsorption in inflammatory bowel disease: assessment by serum markers. Inflamm Bowel Dis. 2011;17:1322–7. [DOI] [PubMed] [Google Scholar]
- 45. Kawano Y, Nakae J, Watanabe N, Kikuchi T, Tateya S, Tamori Y, Kaneko M, Abe T, Onodera M, Itoh H. Colonic pro-inflammatory macrophages cause insulin resistance in an intestinal Ccl2/Ccr2-dependent manner. Cell Metab. 2016;24:295–310. [DOI] [PubMed] [Google Scholar]
- 46. Al-Sadi RM, Ma TY. IL-1 causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Al-Sadi R, Ye D, Dokladny K, Ma TY. Mechanism of IL-1β-induced increase in intestinal epithelial tight junction permeability. J Immunol. 2008;180:5653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Al-Sadi R, Ye D, Said HM, Ma TY. IL-1β-induced increase in intestinal epithelial tight junction permeability is mediated by MEKK-1 activation of canonical NF-κB pathway. Am J Pathol. 2010;177:2310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Al-Sadi R, Guo S, Dokladny K, Smith MA, Ye D, Kaza A, Watterson DM, Ma TY. Mechanism of interleukin-1β induced-increase in mouse intestinal permeability in vivo. J Interferon Cytokine Res. 2012;32:474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Teixeira LG, Leonel AJ, Aguilar EC, Batista NV, Alves AC, Coimbra CC, Ferreira AV, de Faria AMC, Cara DC, Alvarez Leite JI. The combination of high-fat diet-induced obesity and chronic ulcerative colitis reciprocally exacerbates adipose tissue and colon inflammation. Lipids Health Dis. 2011;10:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ceponis PJM, Botelho F, Richards CD, McKay DM. Interleukins 4 and 13 increase intestinal epithelial permeability by a phosphatidylinositol 3-kinase pathway: lack of evidence for STAT6 involvement. J Biol Chem. 2000;275:29132–7. [DOI] [PubMed] [Google Scholar]
- 52. Madden KB, Whitman L, Sullivan C, Gause WC, Urban JF, Katona IM, Finkelman FD, Shea-Donohue T. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J Immunol. 2002;169:4417–22. [DOI] [PubMed] [Google Scholar]
- 53. Yoshida H, Miura S, Kishikawa H, Hirokawa M, Nakamizo H, Nakatsumi RC, Suzuki H, Saito H, Ishii H. Fatty acids enhance GRO/CINC-1 and interleukin-6 production in rat intestinal epithelial cells. J Nutr. 2001;131:2943–50. [DOI] [PubMed] [Google Scholar]
- 54. Yang R, Han X, Uchiyama T, Watkins SK, Yaguchi A, Delude RL, Fink MP. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol-Gastrointest Liver Physiol. 2003;285:G621–9. [DOI] [PubMed] [Google Scholar]
- 55. Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286:31263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hamilton MK, Boudry G, Lemay DG, Raybould HE. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am J Physiol-Gastrointest Liver Physiol. 2015;308:G840–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rajan S, Vyas D, Clark AT, Woolsey CA, Clark JA, Hotchkiss RS, Buchman TG, Coopersmith CM. Intestine-specific overexpression of IL-10 improves survival in polymicrobial sepsis. Shock. 2008;29:483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lorén V, Cabré E, Ojanguren I, Domènech E, Pedrosa E, García-Jaraquemada A, Mañosa M, Manyé J. Interleukin-10 enhances the intestinal epithelial barrier in the presence of corticosteroids through p38 MAPK activity in Caco-2 monolayers: a possible mechanism for steroid responsiveness in ulcerative colitis. PLoS One. 2015;10:e0130921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Suárez-Álvarez K, Solís-Lozano L, Leon-Cabrera S, González-Chávez A, Gómez-Hernández G, Quiñones-Álvarez MS, Serralde-Zúñiga AE, Hernández-Ruiz J, Ramírez-Velásquez J, Galindo-González FJ et al.. Serum IL-12 is increased in Mexican obese subjects and associated with low-grade inflammation and obesity-related parameters. Mediators Inflamm. 2013;2013:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Peluso I, Raguzzini A, Villano DV, Cesqui E, Toti E, Catasta G, Serafini M. High fat meal increase of IL-17 is prevented by ingestion of fruit juice drink in healthy overweight subjects. Curr Pharm Des. 2012;18:85–90. [DOI] [PubMed] [Google Scholar]
- 61. Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, Blumenschein WM, Judo M, Ayanoglu G, McClanahan TK et al.. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity. 2015;43:727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zou J, Chassaing B, Singh V, Pellizzon M, Ricci M, Fythe MD, Kumar MV, Gewirtz AT. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe. 2018;23:41–53. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte J-M, Diepolder H, Marquardt A, Jagla W, Popp A et al.. IL-22 is increased in active Crohn's disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827–838. [DOI] [PubMed] [Google Scholar]
- 64. Gulhane M, Murray L, Lourie R, Tong H, Sheng YH, Wang R, Kang A, Schreiber V, Wong KY, Magor G et al.. High fat diets induce colonic epithelial cell stress and inflammation that is reversed by IL-22. Sci Rep. 2016;6:28990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Luck H, Tsai S, Chung J, Clemente-Casares X, Ghazarian M, Revelo XS, Lei H, Luk CT, Shi SY, Surendra A et al.. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015;21:527–42. [DOI] [PubMed] [Google Scholar]
- 66. Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-γ-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16:5040–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–72. [DOI] [PubMed] [Google Scholar]
- 68. Fujiyama Y, Hokari R, Miura S, Watanabe C, Komoto S, Oyama T, Kurihara C, Nagata H, Hibi T. Butter feeding enhances TNF-α production from macrophages and lymphocyte adherence in murine small intestinal microvessels. J Gastroenterol Hepatol. 2007;22:1838–45. [DOI] [PubMed] [Google Scholar]
- 69. Piguet PF, Vesin C, Guo J, Donati Y, Barazzone C. TNF-induced enterocyte apoptosis in mice is mediated by the TNF receptor 1 and does not require p53. Eur J Immunol. 1998;28:3499–505. [DOI] [PubMed] [Google Scholar]
- 70. Su L, Nalle SC, Shen L, Turner ES, Singh G, Breskin LA, Khramtsova EA, Khramtsova G, Tsai P-Y, Fu Y-X et al.. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 2013;145:407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Suzuki M, Nagaishi T, Yamazaki M, Onizawa M, Watabe T, Sakamaki Y, Ichinose S, Totsuka M, Oshima S, Okamoto R et al.. Myosin light chain kinase expression induced via tumor necrosis factor receptor 2 signaling in the epithelial cells regulates the development of colitis-associated carcinogenesis. PloS One. 2014;9:e88369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ahmad R, Rah B, Bastola D, Dhawan P, Singh AB. Obesity-induces organ and tissue specific tight junction restructuring and barrier deregulation by claudin switching. Sci Rep. 2017;7(1):5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Robles EF, Vázquez VP, Emiliano JR, Amaro RG, Briones SL. High fat diet induces alterations to intraepithelial lymphocyte and cytokine mRNA in the small intestine of C57BL/6 mice. RSC Adv. 2017;7:5322–30. [Google Scholar]
- 74. Vergnolle N. Protease inhibition as new therapeutic strategy for GI diseases. Gut. 2016;65:1215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Noth R, Stüber E, Häsler R, Nikolaus S, Kühbacher T, Hampe J, Bewig B, Schreiber S, Arlt A. Anti-TNF-α antibodies improve intestinal barrier function in Crohn's disease. J Crohns Colitis. 2012;6:464–9. [DOI] [PubMed] [Google Scholar]
- 76. Cao M, Wang P, Sun C, He W, Wang F. Amelioration of IFN-γ and TNF-α-induced intestinal epithelial barrier dysfunction by berberine via suppression of MLCK-MLC phosphorylation signaling pathway. PloS One. 2013;8:e61944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zahs A, Bird MD, Ramirez L, Choudhry MA, Kovacs EJ. Anti-IL-6 antibody treatment but not IL-6 knockout improves intestinal barrier function and reduces inflammation after binge ethanol exposure and burn injury. Shock. 2013;39:373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. 2009;1788:864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Siracusa F, Schaltenberg N, Villablanca EJ, Huber S, Gagliani N. Dietary habits and intestinal immunity: from food intake to CD4+ TH cells. Front Immunol. 2019;9:3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tsuzuki Y, Miura S, Kurose I, Suematsu M, Higuchi H, Shigematsu T, Kimura H, Serizawa H, Hokari R, Akiba Y. Enhanced lymphocyte interaction in postcapillary venules of Peyer's patches during fat absorption in rats. Gastroenterology. 1997;112:813–25. [DOI] [PubMed] [Google Scholar]
- 81. Tsuzuki Y, Miyazaki J, Matsuzaki K, Okada Y, Hokari R, Kawaguchi A, Nagao S, Itoh K, Miura S. Differential modulation in the functions of intestinal dendritic cells by long- and medium-chain fatty acids. J Gastroenterol. 2006;41:209–16. [DOI] [PubMed] [Google Scholar]
- 82. Rolin J, Al-Jaderi Z, Maghazachi AA. Oxidized lipids and lysophosphatidylcholine induce the chemotaxis and intracellular calcium influx in natural killer cells. Immunobiology. 2013;218:875–83. [DOI] [PubMed] [Google Scholar]
- 83. Lu P, Bar-Yoseph F, Levi L, Lifshitz Y, Witte-Bouma J, de Bruijn ACJM, Korteland-van Male AM, van Goudoever JB, Renes IB. High beta-palmitate fat controls the intestinal inflammatory response and limits intestinal damage in mucin Muc2 deficient mice. PLoS One. 2013;8:e65878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gruber L, Kisling S, Lichti P, Martin F-P, May S, Klingenspor M, Lichtenegger M, Rychlik M, Haller D. High fat diet accelerates pathogenesis of murine Crohn's disease-like ileitis independently of obesity. PLoS One. 2013;8:e71661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Miura S, Imaeda H, Shiozaki H, Ohkubo N, Tashiro H, Serizawa H, Tsuchiya M, Tso P. Increased proliferative response of lymphocytes from intestinal lymph during long chain fatty acid absorption. Immunology. 1993;78:142–6. [PMC free article] [PubMed] [Google Scholar]
- 86. Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50:90–7. [DOI] [PubMed] [Google Scholar]
- 87. Guo Y, Zhou G, He C, Yang W, He Z, Liu Z. Serum levels of lipopolysaccharide and 1,3-β-D-glucan refer to the severity in patients with Crohn's disease. Mediators Inflamm. 2015;2015:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179:4808–20. [DOI] [PubMed] [Google Scholar]
- 89. Tabung FK, Birmann BM, Epstein MM, Martínez-Maza O, Breen EC, Wu K, Giovannucci EL. Influence of dietary patterns on plasma soluble CD14, a surrogate marker of gut barrier dysfunction. Curr Dev Nutr. 2017;1:e001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mani V, Hollis JH, Gabler NK. Dietary oil composition differentially modulates intestinal endotoxin transport and postprandial endotoxemia. Nutr Metab. 2013;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C et al.. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. [DOI] [PubMed] [Google Scholar]
- 92. Laugerette F, Vors C, Géloën A, Chauvin M-A, Soulage C, Lambert-Porcheron S, Peretti N, Alligier M, Burcelin R, Laville M et al.. Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. J Nutr Biochem. 2011;22:53–9. [DOI] [PubMed] [Google Scholar]
- 93. Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferriéres J. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87:1219–23. [DOI] [PubMed] [Google Scholar]
- 94. Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palù G, Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol-Gastrointest Liver Physiol. 2007;292:G518–25. [DOI] [PubMed] [Google Scholar]
- 95. Teixeira TFS, Collado MC, Ferreira CLLF, Bressan J, Peluzio M do CG. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr Res. 2012;32:637–47. [DOI] [PubMed] [Google Scholar]
- 96. Damms-Machado A, Louis S, Schnitzer A, Volynets V, Rings A, Basrai M, Bischoff SC. Gut permeability is related to body weight, fatty liver disease, and insulin resistance in obese individuals undergoing weight reduction. Am J Clin Nutr. 2017;105:127–35. [DOI] [PubMed] [Google Scholar]
- 97. Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol. 2013;182:375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Williams JM, Duckworth CA, Watson AJM, Frey MR, Miguel JC, Burkitt MD, Sutton R, Hughes KR, Hall LJ, Caamaño JH et al.. A mouse model of pathological small intestinal epithelial cell apoptosis and shedding induced by systemic administration of lipopolysaccharide. Dis Model Mech. 2013;6:1388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cao S, Zhang Q, Wang C, Wu H, Jiao L, Hong Q, Hu C. LPS challenge increased intestinal permeability, disrupted mitochondrial function and triggered mitophagy of piglets. Innate Immun. 2018;24:221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Schaeffler A, Gross P, Buettner R, Bollheimer C, Buechler C, Neumeier M, Kopp A, Schoelmerich J, Falk W. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immun. 2009;126:233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–51. [DOI] [PubMed] [Google Scholar]
- 102. Wong SW, Kwon M-J, Choi AMK, Kim H-P, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284:27384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Huang S, Rutkowsky JM, Snodgrass RG, Ono-Moore KD, Schneider DA, Newman JW, Adams SH, Hwang DH. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res. 2012;53:2002–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Snodgrass RG, Huang S, Choi I-W, Rutledge JC, Hwang DH. Inflammasome-mediated secretion of IL-1β in human monocytes through TLR2 activation; modulation by dietary fatty acids. J Immunol. 2013;191:4337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. McAlester AH, Kim M, Song R, Wu W, Diehl G. Acute high fat diet disrupts intestinal barrier repair. J Immunol. 2018;200:53.10. [Google Scholar]
- 106. Mayhew TM, Myklebust R, Whybrow A, Jenkins R. Epithelial integrity, cell death and cell loss in mammalian small intestine. Histol Histopathol. 1999;14:257–67. [DOI] [PubMed] [Google Scholar]
- 107. van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–60. [DOI] [PubMed] [Google Scholar]
- 108. Williams JM, Duckworth CA, Burkitt MD, Watson AJM, Campbell BJ, Pritchard DM. Epithelial cell shedding and barrier function: a matter of life and death at the small intestinal villus tip. Vet Pathol. 2015;52:445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jenkinson A, Franklin MF, Wahle K, Duthie GG. Dietary intakes of polyunsaturated fatty acids and indices of oxidative stress in human volunteers. Eur J Clin Nutr. 1999;53:523–8. [DOI] [PubMed] [Google Scholar]
- 110. Awada M, Soulage CO, Meynier A, Debard C, Plaisancié P, Benoit B, Picard G, Loizon E, Chauvin M-A, Estienne M et al.. Dietary oxidized n-3 PUFA induce oxidative stress and inflammation: role of intestinal absorption of 4-HHE and reactivity in intestinal cells. J Lipid Res. 2012;53:2069–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Choe E, Min DB. Chemistry of deep-fat frying oils. J Food Sci. 2007;72:R77–86. [DOI] [PubMed] [Google Scholar]
- 112. Kanner J, Lapidot T. The stomach as a bioreactor: dietary lipid peroxidation in the gastric fluid and the effects of plant-derived antioxidants. Free Radic Biol Med. 2001;31:1388–95. [DOI] [PubMed] [Google Scholar]
- 113. Dobarganes C, Márquez-Ruiz G. Oxidized fats in foods. Curr Opin Clin Nutr Metab Care. 2003;6:157–63. [DOI] [PubMed] [Google Scholar]
- 114. Falade AO, Oboh G. Thermal oxidation induces lipid peroxidation and changes in the physicochemical properties and β-carotene content of Arachis Oil. Int J Food Sci. 2015;2015:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wang T, Gotoh Y, Jennings MH, Rhoads CA, Aw TY. Lipid hydroperoxide-induced apoptosis in human colonic CaCo-2 cells is associated with an early loss of cellular redox balance. FASEB J. 2000;14:1567–76. [DOI] [PubMed] [Google Scholar]
- 116. Kruidenier L, Verspaget HW. Oxidative stress as a pathogenic factor in inflammatory bowel disease—radicals or ridiculous?. Aliment Pharmacol Ther. 2002;16:1997–2015. [DOI] [PubMed] [Google Scholar]
- 117. Liu JJ, Wong K, Thiesen AL, Mah SJ, Dieleman LA, Claggett B, Saltzman JR, Fedorak RN. Increased epithelial gaps in the small intestines of patients with inflammatory bowel disease: density matters. Gastrointest Endosc. 2011;73:1174–80. [DOI] [PubMed] [Google Scholar]
- 118. Kiesslich R, Duckworth CA, Moussata D, Gloeckner A, Lim LG, Goetz M, Pritchard DM, Galle PR, Neurath MF, Watson AJM. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. 2012;61:1146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Noda N, Wakasugi H. Cancer and oxidative stress. Jpn Med Assoc J. 2001;44:535–9. [Google Scholar]
- 120. Gotoh Y, Noda T, Iwakiri R, Fujimoto K, Rhoads CA, Aw TY. Lipid peroxide-induced redox imbalance differentially mediates CaCo-2 cell proliferation and growth arrest. Cell Prolif. 2002;35:221–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tirosh O, Shpaizer A, Kanner J. Lipid peroxidation in a stomach medium is affected by dietary oils (olive/fish) and antioxidants: the Mediterranean versus Western diet. J Agric Food Chem. 2015;63(31):7016–23. [DOI] [PubMed] [Google Scholar]
- 122. Qin X, Caputo FJ, Xu D-Z, Deitch EA. Hydrophobicity of mucosal surface and its relationship to gut barrier function. Shock. 2008;29:372–6. [DOI] [PubMed] [Google Scholar]
- 123. Sharpe SM, Qin X, Lu Q, Feketeova E, Palange DC, Dong W, Sheth SU, Lee MA, Reino D, Xu D-Z et al.. Loss of the intestinal mucus layer in the normal rat causes gut injury, but not toxic mesenteric lymph nor lung injury. Shock. 2010;34:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Van der Sluis M, De Koning BAE, De Bruijn ACJM, Velcich A, Meijerink JPP, Van Goudoever JB, Büller HA, Dekker J, Van Seuningen I, Renes IB et al.. MUC2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–29. [DOI] [PubMed] [Google Scholar]
- 125. Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, Thornton DJ, Png CW, Crockford TL, Cornall RJ. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:1549–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Tomas J, Mulet C, Saffarian A, Cavin J-B, Ducroc R, Regnault B, Kun Tan C, Duszka K, Burcelin R, Wahli W et al.. High-fat diet modifies the PPAR-γ pathway leading to disruption of microbial and physiological ecosystem in murine small intestine. Proc Natl Acad Sci. 2016;113:E5934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Fishman JE, Levy G, Alli V, Zheng X, Mole DJ, Deitch EA. The intestinal mucus layer is a critical component of the gut barrier that is damaged during acute pancreatitis. Shock. 2014;42:264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Park M-Y, Kim MY, Seo YR, Kim J-S, Sung M-K. High-fat diet accelerates intestinal tumorigenesis through disrupting intestinal cell membrane integrity. J Cancer Prev. 2016;21:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wei X, Yang Z, Rey FE, Ridaura VK, Davidson NO, Gordon JI, Semenkovich CF. Fatty acid synthase modulates intestinal barrier function through palmitoylation of mucin 2. Cell Host Microbe. 2012;11:140–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Geelen SNJ, Blázquez C, Geelen MJH, Sloet van Oldruitenborgh-Oosterbaan MM, Beynen AC. High fat intake lowers hepatic fatty acid synthesis and raises fatty acid oxidation in aerobic muscle in Shetland ponies. Br J Nutr. 2001;86:31. [DOI] [PubMed] [Google Scholar]
- 131. Kim H, Choi S, Lee H-J, Lee J-H, Choi H. Suppression of fatty acid synthase by dietary polyunsaturated fatty acids is mediated by fat itself, not by peroxidative mechanism. J Biochem Mol Biol. 2003;36:258–64. [DOI] [PubMed] [Google Scholar]
- 132. Brun P, Castagliuolo I, Leo VD, Buda A, Pinzani M, Palu G, Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. AJP Gastrointest Liver Physiol. 2006;292:G518–25. [DOI] [PubMed] [Google Scholar]
- 133. König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, Whyte J, Troost F, Brummer R-J. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. 2016;7:e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Salyer A. Diet and the colonic environment: measuring the response of human colonic bacteria to changes in the host's diet. New York: Plenum Press; 1986;119–30. [Google Scholar]
- 135. Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. [DOI] [PubMed] [Google Scholar]
- 136. Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13:260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR et al.. Cultured gut microbiota from twins discordant for obesity modulate adiposity and metabolic phenotypes in mice. Science. 2013;341(6150). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP et al.. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Gupta VK, Paul S, Dutta C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front Microbiol. 2017;8:1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. ANR MicroObes consortium, ANR MicroObes consortium members, Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N et al.. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–8. [DOI] [PubMed] [Google Scholar]
- 143. Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2014;7:17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Singh RK, Chang H-W, Yan D, Lee KM, Ucmak D, Wong K, Abrouk M, Farahnik B, Nakamura M, Zhu TH et al.. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3. [DOI] [PubMed] [Google Scholar]
- 146. Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM, Dandona P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 2009;32:2281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Pendyala S, Neff LM, Suárez-Fariñas M, Holt PR. Diet-induced weight loss reduces colorectal inflammation: implications for colorectal carcinogenesis. Am J Clin Nutr. 2011;93:234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Caesar R, Reigstad CS, Bäckhed HK, Reinhardt C, Ketonen M, Östergren Lundén G, Cani PD, Bäckhed F. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut. 2012;61:1701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NMJ, Magness S, Jobin C, Lund PK. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5:e12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–83. [DOI] [PubMed] [Google Scholar]
- 152. Heisel T, Montassier E, Johnson A, Al-Ghalith G, Lin Y-W, Wei L-N, Knights D, Gale CA. High-fat diet changes fungal microbiomes and interkingdom relationships in the murine gut. mSphere. 2017;2(5):e00351–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lin H, An Y, Hao F, Wang Y, Tang H. Correlations of fecal metabonomic and microbiomic changes induced by high-fat diet in the pre-obesity state. Sci Rep. 2016;6:21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Jiao N, Baker SS, Nugent CA, Tsompana M, Guan L, Wang Y, Buck M, Genco RJ, Baker RD, Zhu R et al.. High-fat diet increases clostridium clusters XIVa in obese rodents: a meta-analysis. Gastroenterology. 2017;152:S1012. [Google Scholar]
- 155. Sun J, Qiao Y, Qi C, Jiang W, Xiao H, Shi Y, Le G-W. High-fat-diet-induced obesity is associated with decreased antiinflammatory Lactobacillus reuteri sensitive to oxidative stress in mouse Peyer's patches. Nutr. 2016;32:265–72. [DOI] [PubMed] [Google Scholar]
- 156. Lam YY, Ha CWY, Hoffmann JMA, Oscarsson J, Dinudom A, Mather TJ, Cook DI, Hunt NH, Caterson ID, Holmes AJ et al.. Effects of dietary fat profile on gut permeability and microbiota and their relationships with metabolic changes in mice. Obesity. 2015;23:1429–39. [DOI] [PubMed] [Google Scholar]
- 157. Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10 −/− mice. Nature. 2012;487:104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Derrien M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54:1469–76. [DOI] [PubMed] [Google Scholar]
- 159. Wu W, Lv L, Shi D, Ye J, Fang D, Guo F, Li Y, He X, Li L. Protective effect of Akkermansia muciniphila against immune-mediated liver injury in a mouse model. Front Microbiol. 2017;8:1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Zhang C, Zhang M, Pang X, Zhao Y, Wang L, Zhao L. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J. 2012;6:1848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Carbonero F, Benefiel AC, Alizadeh-Ghamsari AH, Gaskins HR. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front Physiol. 2012;3:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Peng L, Li Z-R, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139:1619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Lam YY, Ha CWY, Campbell CR, Mitchell AJ, Dinudom A, Oscarsson J, Cook DI, Hunt NH, Caterson ID, Holmes AJ et al.. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One. 2012;7:e34233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Ling X, Linglong P, Weixia D, Hong W. Protective effects of bifidobacterium on intestinal barrier function in LPS-induced enterocyte barrier injury of Caco-2 monolayers and in a rat NEC model. PLoS One. 2016;11:e0161635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Anderson RC, Cookson AL, McNabb WC, Park Z, McCann MJ, Kelly WJ, Roy NC. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010;10:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Miyauchi E, Morita H, Tanabe S. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J Dairy Sci. 2009;92:2400–8. [DOI] [PubMed] [Google Scholar]
- 167. Patel RM, Myers LS, Kurundkar AR, Maheshwari A, Nusrat A, Lin PW. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am J Pathol. 2012;180:626–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hsieh C-Y, Osaka T, Moriyama E, Date Y, Kikuchi J, Tsuneda S. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol Rep. 2015;3(3):e12327. [DOI] [PMC free article] [PubMed] [Google Scholar]