ABSTRACT
The gut microbiota plays a relevant role in determining an individual's health status, and the diet is a major factor in modulating the composition and function of gut microbiota. Gluten constitutes an essential dietary component in Western societies and is the environmental trigger of celiac disease. The presence/absence of gluten in the diet can change the diversity and proportions of the microbial communities constituting the gut microbiota. There is an intimate relation between gluten metabolism and celiac disease pathophysiology and gut microbiota; their interrelation defines intestinal health and homeostasis. Environmental factors modify the intestinal microbiota and, in turn, its changes modulate the mucosal and immune responses. Current evidence from studies of young and adult patients with celiac disease increasingly supports that dysbiosis (i.e., compositional and functional alterations of the gut microbiome) is present in celiac disease, but to what extent this is a cause or consequence of the disease and whether the different intestinal diseases (celiac disease, ulcerative colitis, Crohn disease) have specific change patterns is not yet clear. The use of bacterial-origin enzymes that help completion of gluten digestion is of interest because of the potential application as coadjuvant in the current treatment of celiac disease. In this narrative review, we address the current knowledge on the complex interaction between gluten digestion and metabolism, celiac disease, and the intestinal microbiota.
Keywords: gluten, celiac disease, microbiota, gluten-free diet, gut
Introduction
The way human diseases develop has considerably changed over the last century. In the new global scenario immunological conditions have increased, mediated either by allergic or autoimmune mechanisms (1–3). Because genetics cannot explain these changes, new factors have been added into the discussion, and the influence of environmental factors on the human microbiota has become a relevant issue. It is well known that individuals who develop celiac disease carry specific risk genes, but their presence alone is not enough to develop the disease; environmental factors are also required to trigger the disease. The intestinal microbiota is one such factor (4). Microbiota can be referred to as the group of micro-organisms inhabiting the human body in the oral cavity, nasal passages, skin, intestine, and urogenital tract (5). The gastrointestinal microbiota plays a relevant role in determining the health status of an individual throughout life, especially those individuals suffering from diseases with treatment consisting mainly of restrictive diets (6, 7).
The diet greatly influences the gut microbiota composition and function, and, in turn, the intestinal microbiota modulates immunological homeostasis and metabolic regulation (8–10). Microbiota alterations lead to dysbiosis, characterized by modifications of the constituting communities (11), mainly affecting the diversity and proportions among its various constituents. Recent studies have consistently shown that altered microbiota (i.e., dysbiosis) associates with some chronic bowel diseases, all of which are related to immune homeostasis disruption and autoimmune manifestations. For example, in the gastrointestinal tract (GIT), changes in microbiota are reported in celiac disease, ulcerative colitis, and Crohn disease, with the changes mainly being seen in diversity and proportion of the main components Firmicutes, Bacteroidetes, and Actinobacteria (see next paragraphs). However, the evidence available to date is unclear and fails to discriminate between these diseases (4, 12).
Celiac disease is an autoimmune pathology, triggered by gluten, affecting the small intestine in genetically susceptible individuals, with broad clinical manifestations derived from multiple contributing factors (13). At present, dietary gluten is described as the main environmental factor involved in celiac disease pathogenesis. The high proline content of the gluten protein hampers its digestion by human gastro-intestinal enzymes, which lack prolyl endopeptidase activity (14). Incomplete gluten digestion leads to the presence of immunogenic peptides that interact with the intestinal epithelium and mucosa (14). Current research points to a dysbiotic pattern in patients with celiac disease, but results are still somewhat controversial. Several studies show an imbalance in the microbiota composition when patients with celiac disease are compared to healthy individuals (15–17), but other reports show no such differences (18, 19). One should keep in mind that most of these studies have analyzed the colon microbiota, whereas only few have focused on the small intestine, which is where relevant events for this disease take place (18, 20, 21). Gluten is an essential component of diets in Western societies (22) and is intimately involved in celiac disease pathophysiology. Currently available data show that gluten is able to modify the intestinal microbiota, and it has become relevant to understand the relation and interaction between gut microbiota and gluten. In this narrative review, we will review the existing knowledge on the complex interactions between gluten digestion and metabolism, the intestinal microbiota and celiac disease, excluding a detailed description of the immune system involvement in these issues.
Current Status of Knowledge
Gluten
Gluten in the world
Cereals are the most important crops worldwide, representing a total of ∼2000 million tons of grain per year (23); 70% of the cereals consumed by the world population are wheat, corn, and rice (23). Wheat is one of the most widespread crops; its harvest accounts for ∼600 million tons over often large geographical areas (24). Because wheat is a hexaploid species and some of the genes coding for gluten proteins originate from duplicate loci, 1 variety of wheat may contain several hundred different gluten proteins (25).
Wheat farming dates back to the beginning of human agriculture ca. 10,000 years ago, in the great belt of Southeast Asia (Turkey, Palestine, Lebanon, and northern Iraq), where a wide variety of wild cereals, including the currently used wheat and barley (26), spontaneously appeared. Today, gluten consumption is estimated to be 10–20 g per person per day in Western societies (24); such high consumption facilitates identification of individuals who cannot tolerate it.
Gluten proteins
Core wheat grain contains 8–15% protein, of which 10–15% is albumin/globulin and 85–90% gluten (24, 27). Gluten is a complex mixture of hundreds of different proteins, mainly gliadin and glutenin, collectively called prolamins. They are water-insoluble proteins, extractable in aqueous ethanol, and characterized by high glutamine (38%) and proline residues (20%) (27) grouped in polyglutamine sequences or glutamine/proline sequences. The polyglutamine sequences are located in the C-terminal domain of gliadin, while the repetitive glutamine/proline regions are in the protein central area (28). Gluten proteins can be classified into subgroups according to their sulfur content and molecular weight, but their different primary structures also classify them into α-, β-, γ-, and ω-gliadins (29). Gluten proteins are connected by strong covalent and noncovalent unions, which, together with the structure and interactions of the different proteins, provide the unique properties of gluten (30). One of its most relevant characteristics is its ability to retain air in the protein matrix (31), which facilitates and improves bread production.
Gluten digestion
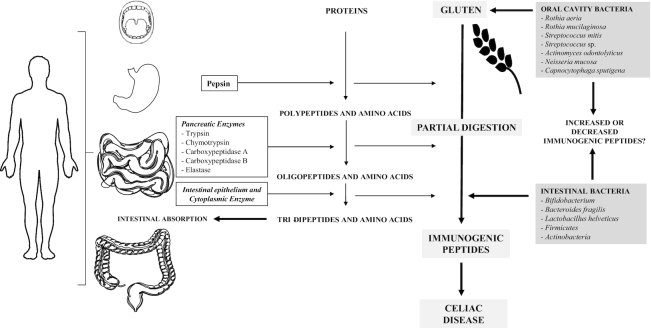
Digestion of gluten proteins begins in the oral cavity where proteases of bacterial origin are described to hydrolyze tripeptides, which frequently appear among the gluten prolamins resistant to human enzymes (32). Some studies describe microbial enzymes isolated from the dental plaque, which are highly active and can potentially neutralize gluten peptide epitopes. The substantial fraction of glutamine and proline residues (27) has a fundamental role in protecting peptides against proteolytic degradation, where these proline-glutamine-rich epitopes are especially resistant to enzymatic processing (14). The main bacterial strains described as related to this gluten metabolism are Rothia, Staphylococcus epidermidis, Streptococcus pneumoniae, Streptococcus mitis, and Bifidobacterium (33, 34) (Figure 1).
FIGURE 1.
Digestion of gluten and food proteins. The digestion of food proteins, a fundamental substrate for human nutrition, is a metabolic process that takes place initially at the level of the stomach, where pepsin (a digestive endopeptidase) acts by promoting degradation of the original protein in polypeptides and amino acids. Then, these components follow further digestion at the level of the small intestine, where the digestion of food proteins is particularly important. It is here that enzymes from the pancreas, the striated border, and also some cytoplasmic enzymes, continue to digest dietary proteins up to tripeptides, dipeptides, and amino acids, and to optimize the absorption of proteins. The gluten protein shows peculiarities in its amino acid structure. Its amino acid content is composed of an important fraction of glutamine and proline residues, which have a fundamental role in protection of peptides against human proteolytic degradation. Proline-glutamine-rich epitopes are exceptionally resistant to enzymatic processing. Therefore, incompletely digested gluten protein and resulting peptides accumulate in the gastrointestinal tract. These protein fractions have antigenic capacity and can trigger autoimmune processes as characteristic of celiac disease. They can also become a substrate for bacteria that are part of the commensal or opportunistic microbiota at the oral or intestinal level, an interaction that can promote or reduce the antigenicity of these peptides. This figure was produced using “Servier Medical Art” from https://smart.servier.com.
Most protein digestion begins in the stomach, where pepsin acts by generating large peptides, leaving gluten proteins incompletely digested (14, 35). This partial hydrolysis of gluten proteins means that high-molecular-weight gluten polypeptides reach the duodenum (36, 37). As a result, quite large peptides with immunogenic sequences (such as the 57–89 peptide and 33-mer peptide in α-gliadin) (38) spend a long time in the intestinal lumen, increasing the opportunity of contact with the gut epithelium. Overall, the 33-mer peptide in α-gliadin contains 6 copies of 3 different epitopes (PYPQPQLPY, PQPQLYPQ, PFPPQPQLPY) to which most patients with celiac disease react (36, 39) with the activation of the immune cascade leading to the inflammatory response (35, 36).
The main protein digestion occurs in the upper part of the small intestine through the pancreatic enzymes (trypsin, chymotrypsin, carboxypeptidase A, carboxypeptidase B, and elastase). The resulting digestion products then get in contact with the brush border enzymes, generating dipeptides, tripeptides, and amino acids that are easily absorbed by the enterocytes (40). A proportion of gluten prolamins are resistant to pancreatic and brush border enzymes in vitro (37). However, in the intestinal epithelium, some of the enzymes (e.g., dipeptidyl peptidase IV, dipeptidyl carboxypeptidase 1, and aminopeptidase N) can partially hydrolyze peptides with proline residues. Some of these relatively large peptides rich in proline and glutamine will cause the toxic effect that contributes to the inflammatory processes in susceptible (celiac) individuals (14, 36).
Gluten and celiac disease
The mechanism through which gluten triggers the onset of celiac disease is not fully understood. The appearance of mucosal lesions in the small intestine typically found in celiac disease requires the involvement of both innate and adaptive immune responses. Although such a relation is yet to be completely clarified, the presence of specific risk alleles is a necessary condition for the disease to appear (41). The gluten-derived proline-rich gliadin peptides pass through the intestinal epithelium and reach the lamina propria, where tissue transglutaminase 2 deamidates the peptide and exposes new epitopes; this conformational change dramatically increases the affinity for the human leukocyte antigen (HLA)-DQ2 and HLA-DQ8 of the human antigen presenting cells (42), amplifying the T cell response. In turn, this leads to T cell activation (instead of regulatory T lymphocyte appearance) (43) and proinflammatory interleukins such as IFN-γ, IL-15, and others are released, contributing to enhance the inflammatory cascade and to the further activation of cytotoxic intraepithelial lymphocytes (44, 45). The mechanisms through which antibodies are made against tissue transglutaminase 2 and gliadin are still unclear. It is also unclear how the specific antibody isotype (mainly IgA and IgG) is decided. The passage of these antibodies (or antigens) to the bloodstream provides a possible explanation for the appearance of extraintestinal manifestations in patients with celiac disease and gluten-sensitive individuals, including the central nervous system, such as ataxia, peripheral neuropathy, and migraine among others (46).
Damage by gluten
There is no definitive evidence that proves what amount of gluten harms patients with celiac disease. The international consensus applies 3, 5, 10, or 20 mg/kg gluten as cutoff values for processed “gluten-free” foods. Many countries accept that products containing <10 mg gluten are unlikely to cause significant histological abnormalities (47). Although unproven, in daily life most patients accept that a total daily intake <50 mg gluten is safe (48). However, the values reported for a tolerable daily gluten intake greatly vary: while some patients tolerate an average of 34–36 mg gluten/d, others who consumed ca. 10 mg/d develop mucosal abnormalities (47). In 2011, the FDA conducted a Health Hazard Assessment for Gluten Exposure. After analyzing all available evidence (49), they concluded that the tolerable daily gluten intake was 0.4 mg and 0.015 mg gluten/d for adverse morphological and clinical effects, respectively. Recently, Leffler et al. (50) showed that a gluten challenge with 3 g or 7.5 g gluten ingested over a 14-d period was sufficient to induce detectable histological and serological changes in patients with celiac disease. Patients may react to different immunogenic peptides (51) and gluten content may differ among foods, which could explain at least part of the variable results reported.
Gut microbiota
The collective genome of the microbiota in humans is called the microbiome. It includes bacteria, viruses, archaea, eukaryotes, and protozoa that live in several sites of the human body (52). From birth, the gastrointestinal microbiota contributes to development of normal bowel functions. The microbiota participates in harvesting energy (53) and promoting maturation of the immune system (54, 55), strengthening gut integrity, and shaping the intestinal epithelium (56).
The GIT is one of the largest interfaces (250–400 m2) between the human host, the environment, and body antigens (57). Throughout human life, a total of nearly 60 tons of food passes through the GIT, with an enormous number of environmental micro-organisms thriving in the gut: this poses a great challenge for the maintenance of intestinal integrity (58). Historically, the number of micro-organisms inhabiting the GIT was estimated at 1014, which represents 10 times more bacterial cells than the human cell content and 100 times more genomic content (microbiome) than the human genome (59, 60). However, a recent review has changed this view by estimating the proportion of human cells to bacteria at a ratio of 1:1 (61). Some authors have proposed that the whole host cells together with those of the micro-organisms that inhabit the gut should be referred to as a “super-organism” (60, 62).
Under physiological conditions, a symbiotic relation between the microbiota and the host has been widely described (63, 64). The immunological and metabolically active microbial organisms maintain a symbiotic tolerance relation with their host, remaining relatively constant throughout life (65). The gut microbiota is composed mainly of bacteria from 3 major phyla: Firmicutes, Bacteroidetes, and Actinobacteria (66). This diverse and complex microbiome functionally collaborates with the host genome, contributing to regulate its physiology (67). The host-bacterial symbiotic interaction is fundamental to human health (68). The co-metabolism between the host's systems and microbiota, with the same bacteria controlling the digestive system and the brain, is a recently cradled concept (64), which helps to understand the gut–brain axis (69). Disruption in the microbiota equilibrium (e.g., because of inflammatory conditions or use of antibiotics) alters the host's general homeostasis (70), leads to dysbiosis, and potentially to some intestinal and extraintestinal diseases (71–73).
Gluten and microbiota
Gluten effects on the intestinal mucosa and microbiota
The effects of gluten on the diet of mammals were investigated in gluten-sensitive juvenile macaques (74): when dietary gluten was introduced, the animals developed chronic diarrhea, had elevated IgA and IgG plasma antigliadin, and marked villous blunting, crypt hyperplasia, and increased intraepithelial lymphocytosis in duodenal biopsies. Clinical, histological, and serological changes returned to basal figures after feeding a gluten-free diet (GFD); after re-introduction of gluten, they altered again (74). In a second study and using the same gluten-sensitive juvenile macaque model, when dietary gluten was consumed by gluten-sensitive animals, a reduction of the gut microbial α-diversity was observed, mainly in the Firmicutes phylum (P = 0.02) (75). After re-introduction of the customary GFD, evaluation at days 14, 28, 42, and 70 showed the restoration of the gut microbiome composition to that observed in normal healthy control individuals. Interestingly, the messenger RNA and claudin-1 protein expression, which is a validated tight junction protein, was significantly downregulated in the jejunal epithelium of gluten-sensitive macaques (75).
Gluten and duodenal and colonic microbiota
In humans, the duodenal microbiota and gluten metabolism are associated (76) (Figure 1). Specific patterns of gliadin degradation in duodenal biopsies of patients with celiac disease have been described, suggesting that particular microbes may be responsible for some of the proteolytic activity observed (77). Despite lower microbial diversity in the small intestine, the high luminal concentration of gluten proteins promotes proteolytic bacteria proliferation (78). The currently high intake of gluten in Western societies allows the presence of abundant gluten peptides in the small intestine, and those peptides become a substrate to different duodenal bacteria that contribute to dysbiosis in patients with celiac disease (77).
Once in the large intestine, partially digested gluten peptides get in contact with an extensive and diverse microbiota, in which different proteolytic bacteria are found. The genetic diversity of the microbiota of the large intestine produces different biochemical pathways, which differ from those found in the host, allowing digestion of gluten peptides resistant to human digestive enzymes (79). For example, Bifidobacterium and Bacteroides fragilis, which can hydrolyze gluten peptides, have been described in patients with celiac disease and also evaluated in animal models (80–82). Nevertheless, the partially digested gluten found in fecal samples from patients with celiac disease and healthy subjects shows that at least part of the dietary gluten passes through the digestive tract and is excreted in feces (83).
The main bacteria involved in the gluten metabolism belong to the Firmicutes phylum, mainly the Lactobacillus genus followed by Streptococcus, Staphylococcus, and Clostridium (84). The large variety of bacteria that are able to hydrolyze gluten proteins and peptides, present in the human intestine, raises a promising opportunity for searching alternative new treatments for celiac disease (84). Herrán et al. (76) characterized the microbes possibly involved in gluten hydrolysis in the small intestine of healthy volunteers and patients with celiac disease, evaluating in duodenal mucosa-associated microbiota: 114 bacterial strains were isolated belonging to 32 species, 85 of such strains growing in a gluten-containing medium as the sole source of nitrogen, 31 having extracellular proteolytic activity digesting gluten proteins, and 27 having peptidolytic activity against the 33-mer peptide (76). The study of these bacteria and their enzymes is relevant because 1) it would help to understand their involvement in celiac disease, revealing whether intestinal dysbiosis occurs before the onset of the disease or whether the gluten load promotes altered colonization leading to the appearance of the disease in susceptible individuals; 2) it may establish such bacteria and enzymes as a potential coadjuvant of treatment by consumption of a GFD, as collaborating in digestion of gluten when involuntarily consumed.
Gluten metabolized by opportunistic pathogens and commensal duodenal bacteria has also been investigated in animal models (80, 85). In murine models, bacterial colonization resulted in different patterns of gluten degradation in the small intestine (85). Interestingly, Pseudomonas aeruginosa, an opportunistic pathogen commonly found in patients with celiac disease, showed elastase activity, which resulted in peptides with enhanced translocation through the murine intestinal barrier (86). Gluten peptides modified by Pseudomonas aeruginosa were able to activate the gluten-specific T cells of patients with celiac disease (86). Lactobacillusspp. obtained from the duodenum of healthy individuals without celiac disease (control individuals) collaborated in the degradation of gluten peptides, which could reduce their immunogenicity (86). Thus, the bacteria of the small intestine show different metabolic patterns to handle gluten in vivo, which can increase or decrease the immunogenicity of gluten peptides. Such microbial-gluten-host interactions can modulate the autoimmune risk in genetically susceptible individuals and may be at the basis of the reported dysbiosis found in celiac disease (86). Caminero et al. (87) have recently demonstrated that the duodenal biopsies of patients with celiac disease consuming a gluten-containing diet have greater proteolytic activity against gluten peptides than those from healthy control individuals, a finding that has been correlated with the abundance of Proteobacteria, including Pseudomonas. The same authors have also shown that in mice expressing risk genes for celiac disease, Pseudomonas aeruginosa elastase synergized with gluten to induce more severe inflammation, which was associated with a moderate reduction of villi height, thus suggesting that proteases expressed by opportunistic pathogens influence the host immune responses, which are relevant to the development of food sensitivities. In patients with celiac disease, these microbial changes potentially represent a trigger for the disease or consequences, as derived from intestinal damage or malabsorption (87).
Oral microbiome
Little consideration has been paid to the oral microbiome in terms of celiac disease until recently. However, it has been identified that the oral microbiome of patients with celiac disease or refractory celiac disease, this being a severe complication of the disease often associated with T cell lymphoma (88), differs from that of healthy subjects. Higher numbers of Lactobacilli may be responsible for the observed greater gluten degradation in patients with celiac disease (89). Oral microbe-derived enzyme activity was found to be elevated in patients with celiac disease, an interesting observation because it possibly influences gluten processing and the presentation of immunogenic gluten epitopes to the immune system once it reaches the small intestine (89). However, the mechanisms explaining the role of oral and gut microbiomes in celiac disease are unclear (Figure 1).
Microbiota and Dysbiosis in Celiac Disease
Several research groups have investigated the gut microbiota composition in celiac disease (3, 76–78). In general, changes in the number of bacterial species, their diversity, and proportions are described in celiac disease and other inflammatory bowel diseases. Decreased numbers of bacteria with anti-inflammatory capacity and increased bacteria with inflammatory capacity are reported in patients with inflammatory bowel diseases, when compared to healthy individuals (90, 91). The most consistent changes are a reduction in the diversity of gut microbiota and changes in the abundance of Firmicutes/Bacteroidetes. For brevity, our discussion does not include GIT diseases other than celiac disease. The results achieved in child and adult patients with celiac disease are summarized in Table 1.
TABLE 1.
Summary of studies evaluating the gut microbiota characteristics in patients with celiac disease1
| Study (publication year) (reference) | Patients | Age group | Sampling site/methods | Outcomes | Main findings |
|---|---|---|---|---|---|
| Forsberg et al. (2004) (92) | Patients with untreated celiac disease (n = 55), patients with treated celiac disease (n = 53), patients challenged for celiac disease (n = 42), and control individuals (n = 78) | Children with median (range) age of 5.4 (0.8–15.8), 5.9 (1.8–17.4), 6.8 (3–12.7), and 5.4 (0.8–17.9) y in the untreated, treated, challenged, and control group, respectively | Intestinal biopsies from proximal jejunum/scanning electron microscopy; qRT-PCR, antibody and lectin immunohistochemistry | Bacteria associated with intestinal epithelium, and epithelial innate immune status in jejunal biopsies | Bacteria commonly found in the intestinal mucosa of patients with celiac disease but not in that of control individualsRod-shaped bacteria frequently associated with the mucosa of patients with celiac disease, in both active and inactive diseaseRod-shaped bacteria frequently associated with the mucosa of patients with celiac disease, in both active and inactive diseaseUnique carbohydrate structures of the glycocalyx/mucous layer are features of patients with celiac diseaseIncreased production of mucin-2, α-defensins HD-5/HD-6, and lysozyme in patients with active celiac disease |
| Collado et al. (2007) (15) | Patients with celiac disease (n = 26) and age-matched control individuals (n = 23) | Infants, median (range) age of 26.3 (12–48) and 23.0 (11–45) mo in the celiac disease and control group, respectively | Fecal samples/FISH | Fecal microbiota composition | Higher amounts of Bacteroides, Clostridium, and Staphylococcus in patients with celiac diseaseBifidobacterium tended to be higher in healthy control individualsBifidobacteriumtended to be higher in healthy control individualsLevels of Bacteroides-Prevotella, Clostridium histolyticum, Eubacterium rectale-C. coccoides, Atopobium, and sulfate-reducing bacterial groups were also significantly higher in patients with celiac disease |
| Nadal et al. (2007) (20) | Patients with active celiac disease (n = 20), patients with inactive celiac disease consuming a GFD (n = 10), and control individuals (n = 8) | Children, mean (range) age 5.1 (1.6–12) y, 5.6 (2–7.8) y, 4.1 (1.9–9) y, in active and inactive disease, and control individuals, respectively | Duodenal biopsies/FISH and flow cytometry | Composition and duodenal microbiota | Higher gram-negative bacteria in patients with active celiac diseaseIncreased abundance of Bacteroides and Escherichia coli in patients with active celiac disease compared with control individualsLower Lactobacillus-Bifidobacterium to Bacteroides-Escherichia coli ratio in both groups of patients with celiac disease compared with control individuals |
| Collado et al. (2008) (93) | Patients with active celiac disease (n = 30), nonactive celiac disease (n = 18), and age-matched control individuals (n = 30)For duodenal biopsies: Patients with active celiac disease (n = 25), nonactive celiac disease (n = 8), and age-matched control individuals (n = 8) | Children, mean age 56.4 ± 38.5 mo, 65.2 ± 37.7 mo, and 45.0 ± 33.5 mo in active celiac disease, nonactive celiac disease, and control group, respectively | Fecal and duodenal biopsies/qRT-PCR analysis | Bifidobacterium species composition of duodenal biopsies and fecal samples | Lower numbers of total Bifidobacterium and B. longum species in feces and duodenal biopsies in patients with celiac disease than those of control individualsLower prevalence of B. catenulatumin duodenal biopsies of both patient groups with celiac disease. B. dentium higher in feces of patients with nonactive celiac disease compared with control individuals |
| Ou et al. (2009) (94) | Untreated patients with celiac disease (n = 33), patients with celiac disease consuming a GFD (n = 17) and challenger celiac disease (n = 3), and control individuals (n = 18) | Children, median (range) age 5.9 (1.2–16) y, 7.5 (2.4–17) y, 10.8 (5.2–18) y, 3.2 (1–18.5) y, in untreated, treated, challenged, and control groups, respectively | Intestinal biopsies (distal duodenum proximal jejunum/16S rDNA sequencing, culture-methods, and scanning electron microscopy) | Microbiota composition of the proximal small intestine | Quite similar proximal small intestine microbiota in patients with celiac disease compared with control individualsFrom celiac disease biopsies positive for rod-shaped bacteria at scanning electron microscopy, the microbiota was enriched in Clostridium, Prevotella, and Actinomyces |
| Sánchez et al. (2010) (81) | Patients with active celiac disease (n = 20), treated celiac disease (n = 12), and control individuals (n = 8) | Preschool children, mean age 51.1 ± 31.8 mo, 54.9 ± 25.6 mo, 50.1 ± 31.2 mo in active celiac disease, treated celiac disease, and control individuals, respectively | Duodenal biopsies/partial 16S rRNA gene sequencing and PCR-denaturing gradient gel electrophoresis | Bacteroides, Bifidobacterium, and lactic acid bacteria composition | Reduced Bacteroides strains in both groups of patients and higher Bifidobacterium diversity in patients with active celiac disease compared with control childrenHigher lactic acid bacteria diversity found in patients with treated celiac disease and control individuals than in patients with active celiac disease |
| De Palma et al. (2010) (17) | Patients with treated celiac disease (n = 18), patients with untreated celiac disease (n = 24), and healthy control individuals (n = 20) | Children, mean (range) age 5.5 (2.1–12) y, 5.5 (1–12.3) y, and 5.3 (1.8–10.8) y in untreated, treated, and healthy children | Fecal samples/FISH and flow cytometry | Fecal microbiota composition and percentage of immunoglobulin-coated bacteria | Reduced gram-positive to gram-negative bacteria ratio in both groups of patients with celiac diseaseLower abundance of Bifidobacterium, C. histolitycum, C. lituseburense, and Faecalibacterium prausnitzii in patients with untreated celiac disease |
| Sanchez et al. (2012) (95) | Patients with active celiac disease (n = 20), and patients with inactive celiac disease (n = 18), healthy control individuals (n = 20) | Children, median (range) age of 3.9 (1.0–8.8) y, 6.2 (3.3–12.2) y, and 5.7 (2.5–10.8) y in patients with active celiac disease, inactive celiac disease, and healthy control individuals, respectively | Fecal samples/partial 16S rRNA gene sequencing | Diversity of Bacteroides spp. and its role in the generation of gliadin peptides with immunotoxic effect | Similar diversity distribution found among the 3 groups. Lower Renyi diversity in patients with active celiac disease compared with control individualsBacteroides fragilis more often found in patients with celiac disease compared with control individualsParabacteroides distasonis more often isolated from patients with active celiac disease. Bacteroides finegoldii more often isolated from control individuals compared with patients with active celiac disease, and Bacteroides ovatus more commonly found in control individuals than in patients |
| Cheng et al. (2013) (96) | Patients with celiac disease (n = 10), healthy control individuals (n = 9), adult patients with celiac disease consuming a GFD (n = 6) | Children, median (range) age 9.5 (3–14) y and 8.5 (4–16) y, in children with celiac disease and healthy control individuals, respectivelyAdult patients with celiac disease, mean age 46 (30–60) y | Duodenal biopsies/16S rRNA gene of intestinal phylotypes | To characterize duodenal microbiota and assess microbiota differences between groups | Similar microbiota composition and diversity, as well as similar microbe-associated molecular pattern of the microbiota, were found in patients and healthy control individuals8 bacterial profile groups differed in patients with celiac disease (Prevotella melaninogenica and Prevotella spp. were higher, while Prevotella oralis, Ruminococcus bromii, Papillibacter cinnamivorans, Proteus, and Clostridium stercorarium were decreased) |
| Wacklin et al. (2013) (97) | Patients with celiac disease with clinical symptoms (n = 33), asymptomatic patients with celiac disease (n = 8), and control subjects (n = 18) | Adults, mean (range) age 39 (18–67) y | Small bowel biopsy/PCR denaturing gradient gel electrophoresis and 16S rRNA gene sequencing | Duodenal microbiota composition and its relation with intestinal and extraintestinal symptoms | Patients with celiac disease with gastrointestinal symptoms or anemia exhibited lower microbial diversity compared with patients with dermatitis herpetiformisA higher abundance of Proteobacteria was found in patients with clinical symptomsSimilar microbiota composition between patients with dermatitis herpetiformis and control subjects (high abundance of Firmicutes) |
| Sanchez et al. (2013) (98) | Patients with active celiac disease (n = 32), patients with treated celiac disease (n = 17), and control individuals (n = 8) | Children, mean ± SD age 5.1 ± 3.2, 5.9 ± 1.2, and 6.9 ± 4.2 y in patients with active celiac disease, nonactive celiac disease, and control individuals, respectively | Duodenal biopsies/partial 16S rRNA gene sequencing | Composition and diversity of cultivable duodenal mucosa-associated bacteria | Higher amount of Proteobacteria with reduced abundance of Firmicutes in the active celiac disease group compared with nonactive celiac disease and control individualsIncreased abundance of Enterobacteriaceaeand Staphylococcaceae families, but a reduced abundance of members of the Streptococcaceae family in patients with active celiac disease compared with control individuals |
| Wacklin et al. (2014) (99) | Patients with celiac disease treated with a GFD, with (n = 18) and without (n = 18) persisting gastrointestinal symptoms | Middle-age adults with median (range) age of 54 (27–72) and 63 (42–75) y in the group with and without symptoms, respectively | Duodenal biopsies samples/16S rRNA gene pyrosequencing | Microbiota composition comparison between both groups of patients | Reduced microbiota richness with increased abundance of Proteobacteria and lower abundance of Bacteroidetes and Firmicutes in patients with celiac disease with persistent gastrointestinal symptoms |
| Francavilla et al. (2014) (100) | Patients with celiac disease (n = 13) consuming a GFD, and healthy control individuals (n = 13) | Children and early adolescents, mean ± SD age 9.7 ± 1.4 and 10.2 ± 1.4 in patients and control individuals, respectively | Saliva samples/culture-dependent techniques. Microbial diversity by pyrosequencing of the amplified V1-V3 region of 16S rRNA gene | Assessment of salivary microbiota and metabolomes | Reduced total number of anaerobes and lower diversity and substrate richness found in children with celiac diseaseIncreased Lachnospiraceae, Gemellaceae,and Streptococcus sanguinis, but reduced Streptococcus thermophilus in children with celiac diseaseAn increased level of Bacteroidetes with a reduced amount of Actinobacteria(Rothia mucilaginosa was the only Actinobacteria species found to be highest in children with celiac disease) |
| Caminero et al. (2015) (101) | Patients with celiac disease consuming a normal diet (n = 22), patients with celiac disease consuming a GFD (n = 20), healthy volunteers consuming a normal diet (n = 16), healthy volunteers consuming a GFD (n = 11) | Adult patients consuming a regular diet, mean (range) age 39.5 (15–60) y, and 30.6 (22–42) y in patients with celiac disease consuming a GFD; mean (range) age of 30.1 (25–45) y in healthy volunteers consuming a regular diet, and 32.2 (25–45) y in healthy volunteers consuming a GFD | Fecal samples/16S rDNA gene partial sequencing | Proteolytic activities, cultivable bacteria involved in gluten metabolism, SCFA, and the amount of gluten in fecal samples | Proteolytic activities and SCFA increased in patients with celiac diseaseAlterations in Clostridium, Lactobacillus, or Bacteroides were reported in patients with celiac diseaseCommensal microbial activity is important in the metabolism of gluten and is altered in patients with celiac disease |
| Lorenzo et al. (2015) (102) | Patients with celiac disease (n = 15) consuming a GFD and healthy control individuals (n = 15) | Children, mean (range) age 7.5 (3–14) y and 6.5 (2–11) y in patients with celiac disease and healthy control group, respectively | Fecal samples/culture-dependent techniques | Change in the composition of common bacteria from the intestinal microbiota | A lower number of Lactobacillus and the tendency to an increased Enterobacteria count in patients with celiac disease |
| Nistal et al. (2016) (19) | Patients with celiac disease (untreated, n = 9) and control individuals without celiac disease (n = 9) | N/A | Duodenal biopsies/pyrosequencing of 16S rRNA | Composition of the duodenal microbiota | A nonsignificant reduction in bacterial richness and diversity in patients with celiac diseaseMain bacterial communities belonged to Firmicutes and Proteobacteria phyla in both patients with celiac disease and control individuals without celiac disease, without differences in the duodenal bacterial composition between groups |
| Tian et al. (2017) (89) | Patients with celiac disease in remission (n = 21), and patients with refractory celiac disease (n = 8), healthy control individuals (n = 20) | Adults, mean ± SD age 36.2 ± 17, 54.1 ± 13.5, and 35.4 ± 15.7 y in patients with celiac disease in remission, refractory celiac disease, and healthy control individuals, respectively | Stimulated whole saliva samples/16S rRNA-based MiSeq analysis | Salivary enzymatic activities and oral microbial profiles | Higher levels of lactobacilli in both groups of patients with celiac diseaseLower microbial diversity in the celiac disease group compared with healthy control individualsHigher Bacteroidetes and Fusobacteria, but lower Actinobacteria in patients with celiac disease under remission compared with the celiac disease refractory groupSeveral species-level differences found when comparing healthy control individuals against both groups with celiac disease |
| Rintala et al. (2018) (103) | Children with high genetic risk of developing celiac disease, who developed (n = 9) or did not develop celiac disease at age 4 yHealthy control individuals (n = 18) | Median age at diagnosis 3.5 (2.6–4.2) y | Fecal samples at age 9 and 12 mo/16S rRNA gene sequencing | Fecal microbiota composition before the onset of the disease | Similar bacterial diversity index between groups at both ages 9 and 12 moNo between-group differences in bacterial composition regarding microbial abundances at phylum and genus levelsSimilar Bacteroides-Prevotella and Bacteroides-Bifidobacterium ratios between groups at both ages (9 and 12 mo) |
| Garcia-Mazcorro et al. (2018) (104) | Patients with celiac disease (n = 6), patients with NCGS (n = 12), and control subjects (n = 12) | Adults with median (range) age of 41.5 (25–73), 24.0 (21–59), and 25.5 (23–64) y in patients with celiac disease, NCGS patients, and control individuals, respectively | Small bowel mucosal biopsies (proximal duodenum) and fecal samples/16S rRNA gene sequencing | Gut microbiota composition | Novispirillum genus increased in duodenal biopsies on patients with celiac disease. Actinobacillus genus and Ruminococcaceae family were higher in duodenal and fecal samples of NCGS patients |
| Caminero et al. (2019) (87) | Patients with celiac disease with clinical symptoms (n = 12), control individuals without celiac disease (n = 8) | Adults with mean (range) age of 42.3 (18–64), and 47 (30–72) y in celiac disease and control groups, respectively | Intestinal duodenal biopsies (second portion)/16S rRNA gene sequencing. Glutenasic activity by bioassay (gluten 1%) | Mucosa-associated microbiota | Proteobacteria and Firmicutes were the most commonly found bacterial types. No major between-group differences found regarding α or β diversityGlutenasic activity showed positive association with Proteobacteria and negative association with Firmicutesand Bacteroidetes relative abundanceIncreased glutenasic activity correlated with increased Pseudomonas and Janthinobacterium, and with reduced Lactobacillus and Clostridium |
| Bodkhe et al. (2019) (105) | Patients with celiac disease in gluten-containing diet (n = 23), healthy first-degree relatives of patients with celiac disease (n = 15), control individuals [patients with hepatitis B carriers or having functional dyspepsia (n = 24)] | Adults with mean ± SD age of 23.4 ± 9.5, 31.6 ± 10.8, 30.6 ± 12.3 y in patients with celiac disease, FDRs of patients, and control individuals, respectively | Duodenal biopsies and fecal samples/16S rRNA gene sequencing | Duodenal and fecal microbiota composition and changes in fecal microbiota between celiac disease, FDRs, and disease-control individuals | No significant differences in abundance of members of duodenal microbiota between celiac disease and FDRs or control. Similar duodenal and fecal α diversity between groupsIncreased duodenal Actinobacteria and Bacteroides phyla in FDRs compared to control individualsGreater abundance of the order Clostridiale in FDRs compared to control individuals, at duodenal and fecal samples. ASVs in duodenal samples was higher for Helicobacter and Megasphaera in patients with celiac diseaseSimilar abundance of phylum of fecal microbiota between groups. Reduced fecal abundance of ASVs classified as Akkermansia and Dorea in patients with celiac disease and FDRs compared to control individuals |
ASV, amplicon sequence variant; FDR, first-degree relative; FISH, fluorescence in situ hybridization; GFD, gluten-free diet; N/A, not available; NCGS, nonceliac gluten sensitivity.
In children with active celiac disease (patients consuming a GFD and a group of control children), Nadal et al. (20) showed in intestinal mucosal-associated microbiota that the proportions of total and gram-negative bacteria were significantly higher in the active celiac disease group and that the ratio of Lactobacillus-Bifidobacterium to Bacteroides-Escherichia coli was significantly reduced. Bacteroides and E. coli groups were more abundant in patients with active celiac disease than in control individuals, while such bacterial groups were not different between control individuals and symptom-free patients consuming a GFD (20). These authors speculated that the increased frequency of gram-negative and potentially proinflammatory bacteria in the duodenal microbiota of children with celiac disease might be related to the pathological process of the disease (20). Another study on children, assessing the gut Bifidobacteria composition reported that the total numbers of Bifidobacterium and B. longum species in feces and duodenal biopsies were lower in patients with active and nonactive celiac disease compared with control children (93). Bifidobacteria catenulatum also presented lower numbers in biopsies, whereas fecal B. dentium was higher in patients with nonactive celiac disease than in control individuals (93). The authors have suggested that the microbial indicators (i.e., the ratio between the fecal cell densities of lactic acid bacteria and the amounts of specific metabolites such as ethyl acetate, octyl-acetate, glutamine, and SCFA) could be a characteristic signature of celiac disease. Another report on children during and after the Swedish celiac disease outbreak showed that rod-shaped bacteria constituted a significant fraction of the proximal small-intestine mucosal microbiota (94). The presence of these bacterial groups conferred a 4-fold increased risk for celiac disease in children aged <2 y (94, 106).
In studies of children, other authors have reported that the Bacteroides diversity was smaller in duodenal biopsy samples of active and treated patients with celiac disease than in those from healthy control individuals, whereas Bifidobacterium diversity was higher in both groups of patients with celiac disease (81). Bacteroides dorei, Bifidobacterium adolescentis, and Bifidobacterium animalissubsp.lactis were more prevalent in patients with active celiac disease than in treated patients and control individuals (81). In turn, lactic acid bacteria were more prevalent in patients with treated celiac disease and control individuals than in patients with active celiac disease, suggesting that in patients with celiac disease the bacterial populations differ both in diversity and species composition (81).
Caminero et al. (101) analyzed the metabolic activity of intestinal bacteria associated with gluten intake in patients with celiac disease, their first-degree relatives (FDRs), and healthy individuals. The results were analyzed for fecal peptidase activity against the 33-mer peptide. The patients with celiac disease showed differences in fecal glutenase activity, fecal tryptic activity, SCFA, and fecal gluten content when compared to healthy volunteers. Alterations were identified in specific groups of bacteria that metabolize gluten, such as Clostridium and Lactobacillus. In FDRs, the indicators for SCFA and fecal tryptic activity and fecal glutenase activity were similar to those of patients compared to healthy volunteers (101). Other studies reported that Proteobacteria, and not Firmicutes, is the most abundant phylum in duodenal biopsy samples among patients with celiac disease, with the Neisseria genus being the most represented (107). Regardless of the exact extent to which intestinal dysbiosis is present in celiac disease, the available evidence shows that dysbiosis induced by dietary gluten in duodenal mucosa-associated bacteria is not easily restored after a GFD (98); in fact, a GFD itself can significantly alter the intestinal bacterial populations (6).
Wacklin et al. (99) evaluated the relation between the presence of an abnormal intestinal (duodenal biopsies) microbiota and the presence of persisting gastrointestinal symptoms in patients with celiac disease consuming a GFD. Treated patients with symptoms showed reduced microbial richness, and they were colonized by different duodenal microbiota, with a high relative abundance of Proteobacteria, but low abundance of Bacteroidetes and Firmicutes. This study suggested that dysbiosis is associated with the persistence of gastrointestinal symptoms in treated patients, even when they strictly adhere to a GFD (99). If this is further confirmed, restoring a normal microbiota appears to be another attractive possibility for complementing treatment for celiac disease.
It is well known that genetic factors confer susceptibility for the appearance of celiac disease, but they cannot fully explain the disease. Olivares et al. (108) investigated whether the HLA genotype is an independent factor influencing the early gut microbiota composition. In a cohort of healthy newborns, divided into high-risk HLA-DQ2 carriers or low-risk non-HLA-DQ2/8 carriers for celiac disease, the authors described in fecal samples significantly greater proportions of Firmicutes and Proteobacteria and lowered proportions of Actinobacteria in the high-risk group, independently of gluten ingestion. High-risk infants also showed a negative correlation in Bifidobacterium species and several genera of Proteobacteria (Escherichia/Shigella) and Firmicutes (Clostridium), raising the hypothesis that the specific genotype of the host may select the first intestinal colonizers, thus contributing to confer the risk of celiac disease (108). On evaluating the intestinal microbiota composition and functional profile in patients with celiac disease and nonceliac gluten sensitivity (NCGS), the genus Actinobacillus in duodenal biopsies and the Ruminococcaceae family in fecal samples were higher in patients with NCGS, while Novispirillum was higher in the duodenum of patients with celiac disease (104). An interesting finding was a difference in the increased abundance of duodenal Pseudomonas after 4 wk consuming a GFD in the NCGS group. The increase in abundance of Pseudomonas strains with gluten-degrading capability is relevant in NCGS and represents additional data on the relation between the gut microbiota and gluten-related disorders (104). A recent report (105) investigated the microbiome composition of both the small intestine and entire intestine in a subset of patients with celiac disease, FDRs, and control individuals, in duodenal biopsies and fecal samples. Duodenal microbiota of patients with celiac disease showed a higher abundance of amplicon sequence variant (ASV) for Megasphaera and Helicobacter compared to the FDRs, and the fecal microbiota from patients with celiac disease and FDRs showed reduced abundance of ASVs classified as Akkermansia and Dorea, when compared with that from control individuals. The predicted functional metagenome showed lower capacity for gluten degradation in fecal microbiota of patients with celiac disease compared to FDRs and control groups. The authors concluded that it is necessary to study the functional capacities of specific bacteria in healthy individuals, at-risk FDRs, and patients (105).
Participation of the intestinal microbiota in celiac disease is a relevant point because it opens up opportunities for searching for new therapies. Improving dysbiosis and modulating the composition of the intestinal flora has potential as a probiotic-based therapy with bacteria producing specific protease inhibitors that interfere with the bacterial glutenasic activities. Therapeutic options based on enzymes of microbial origin that could completely digest gluten in the GIT should be investigated, as they could significantly improve management of celiac disease and patients’ quality of life.
Conclusions and Perspectives
Gluten is an essential dietary component in Western societies. Gluten consumption has greatly increased during the last century, coinciding with a considerable increase in prevalence of celiac disease. Gluten peptides exert specific effects on the intestinal mucosa, causing celiac disease in genetically susceptible individuals. Although our understanding of the proteolytic process and pathway followed by the gliadin-derived peptides that trigger the disease has significantly improved, many aspects of the pathways leading to the disease and its progression remain unclear. HLA-DQ genotype is a well-known factor that modulates the risk of developing celiac disease in susceptible individuals. Current evidence suggests that these genes participate in determining the intestinal microbiota composition in the host.
Gluten metabolism, pathophysiology of celiac disease, and gut microbiota are intimately related, with their interrelations defining intestinal health and homeostasis. Environmental factors modify the intestinal microbiota, and, in turn, changes in the microbiota count, diversity, and proportions of its constituents modulate mucosal and immune responses. Current evidence on patients with celiac disease increasingly supports that dysbiosis is present in celiac disease, but to what extent this is a cause or consequence of it and whether the different intestinal diseases have specific patterns of change is still unclear. Factors other than gluten may modulate the intestinal microbiota in celiac disease, but this requires clarification.
Knowledge has greatly improved but is still insufficient to define to what extent increased consumption of gluten, changes in the immune system (that increasingly respond towards allergy or autoimmunity instead of tolerance), and changes of the intestinal microbiota/dysbiosis (when diversity is owed to changes and/or dietary habits or is genetically determined) interact and trigger the appearance of celiac disease.
Acknowledgments
Figure 1 was produced using Servier Medical Art from https://smart.servier.com/. The authors’ responsibilities were as follows—KAB, MA, LE: conceptualized the review; KAB, MA: developed search strategies, conducted the research and decided on final studies to include; KAB, MA, LD, LR: interpreted studies and contributed to the revision of the manuscript; KAB, MA, LR, LE: wrote and critically reviewed the final manuscript; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: KAB, MA, LR, LD, and LE, no conflicts of interest.
Abbreviations used: ASV, amplicon sequence variant; FDR, first-degree relative; GFD, gluten-free diet; GIT, gastrointestinal tract; HLA, human leukocyte antigen; NCGS, nonceliac gluten sensitivity.
References
- 1. Sicherer SH, Sampson HA. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141:41–58. [DOI] [PubMed] [Google Scholar]
- 2. Bach J-F. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–20. [DOI] [PubMed] [Google Scholar]
- 3. Jacobson DL, Gange SJ, Rose NR, Graham NMH. Short analytical review. Clin Immunol Immunopathol. 1997;84:223–43. [DOI] [PubMed] [Google Scholar]
- 4. Cenit MC, Olivares M, Codoñer-Franch P, Sanz Y. Intestinal microbiota and celiac disease: Cause, consequence or co-evolution?. Nutrients. 2015;17:6900–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miniello VL, Colasanto A, Cristofori F, Diaferio L, Ficele L, Lieggi MS, Santoiemma V, Francavilla R. Gut microbiota biomodulators, when the stork comes by the scalpel. Clin Chim Acta. 2015;7:88–96. [DOI] [PubMed] [Google Scholar]
- 6. De Palma G, Nadal I, Collado MC, Sanz Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br J Nutr. 2009;102:1154–60. [DOI] [PubMed] [Google Scholar]
- 7. Albenberg LG, Wu GD. Diet and the intestinal microbiome: Associations, functions, and implications for health and disease. Gastroenterology. 2014;146:1564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li D, Wang P, Wang P, Hu X, Chen F. The gut microbiota: A treasure for human health. Biotechnol Adv. 2016;34:1210–24. [DOI] [PubMed] [Google Scholar]
- 9. Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy DN. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17:219–32. [DOI] [PubMed] [Google Scholar]
- 12. Sanz Y. Microbiome and Gluten. Ann Nutr Metab. 2015;67(Suppl 2):28–41. [DOI] [PubMed] [Google Scholar]
- 13. Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet. 2017;391:70–81. [DOI] [PubMed] [Google Scholar]
- 14. Hausch F, Shan L, Santiago NA, Gray GM, Khosla C. Intestinal digestive resistance of immunodominant gliadin peptides. Am J Physiol Gastrointest Liver Physiol. 2002;283(4):G996–1003. [DOI] [PubMed] [Google Scholar]
- 15. Collado MC, Calabuig M, Sanz Y. Differences between the fecal microbiota of coeliac infants and healthy controls. Curr Issues Intest Microbiol. 2007;8:9–14. [PubMed] [Google Scholar]
- 16. Sanz Y, Sánchez E, Marzotto M, Calabuig M, Torriani S, Dellaglio F. Differences in faecal bacterial communities in coeliac and healthy children as detected by PCR and denaturing gradient gel electrophoresis. FEMS Immunol Med Microbiol. 2007;51:562–8. [DOI] [PubMed] [Google Scholar]
- 17. De Palma G, Nadal I, Medina M, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol. 2010;10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nistal E, Caminero A, Herrán AR, Arias L, Vivas S, de Morales JMR, Calleja S, de Miera LES, Arroyo P, Casqueiro J. Differences of small intestinal bacteria populations in adults and children with/without celiac disease: Effect of age, gluten diet, and disease. Inflamm Bowel Dis. 2012;18:649–56. [DOI] [PubMed] [Google Scholar]
- 19. Nistal E, Caminero A, Herrán AR, Pérez-Andres J, Vivas S, Ruiz de Morales JM, Sáenz de Miera LE, Casqueiro J. Study of duodenal bacterial communities by 16S rRNA gene analysis in adults with active celiac disease vs non-celiac disease controls. J Appl Microbiol. 2016;120:1691–700. [DOI] [PubMed] [Google Scholar]
- 20. Nadal I, Donant E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalance in the composition of the duodenal microbiota of children with coeliac disease. J Med Microbiol. 2007;56(Pt 12):1669–74. [DOI] [PubMed] [Google Scholar]
- 21. Iebba V, Santangelo F, Totino V, Nicoletti M, Gagliardi A, De Biase RV, Cucchiara S, Nencioni L, Conte MP, Schippa S. Higher prevalence and abundance of Bdellovibrio bacteriovorus in the human gut of healthy subjects. PLoS One. 2013;8:e61608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanz Y, De Pama G, Laparra M. Unraveling the ties between celiac disease and intestinal microbiota. Int Rev Immunol. 2011;30:207–18. [DOI] [PubMed] [Google Scholar]
- 23. Shewry PR, Halford NG. Cereal seed storage proteins: structures, properties and role in grain utilization. J Exp Bot. 2002;53:947–58. [DOI] [PubMed] [Google Scholar]
- 24. Shewry PR. Wheat. J Exp Bot. 2009;60:1537–53. [DOI] [PubMed] [Google Scholar]
- 25. Feldman M. Origin of cultivated wheat. In: Alin PB, William JA, editors. The World Wheat Book: A History of Wheat Breeding. London: TCA VOC; 2001. p. 3–56. [Google Scholar]
- 26. Greco L. From the neolithic revolution to gluten intolerance: benefits and problems associated with the cultivation of wheat. J Pediatr Gastroenterol Nutr. 1997;24:S14–6. [DOI] [PubMed] [Google Scholar]
- 27. Wieser H. Chemistry of gluten proteins. Food Microbiol. 2007;24:115–9. [DOI] [PubMed] [Google Scholar]
- 28. Ang S, Kogulanathan J, Morris GA, Kök MS, Shewry PR, Tatham AS, Adams GG, Rowe AJ, Harding SE. Structure and heterogeneity of gliadin: A hydrodynamic evaluation. Eur Biophys J. 2010;39:255–61. [DOI] [PubMed] [Google Scholar]
- 29. Van Den Broeck HC, Van Herpen TWJM, Schuit C, Salentijn EMJ, Dekking L, Bosch D, Hamer RJ, Smulders MJM, Gilissen LJWJ, Van Der Meer IM. Removing celiac disease-related gluten proteins from bread wheat while retaining technological properties: A study with Chinese Spring deletion lines. BMC Plant Biol. 2009;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Biesiekierski JR. What is gluten?. J Gastroenterol Hepatol. 2017;32:78–81. [DOI] [PubMed] [Google Scholar]
- 31. Parada A, Araya M. [History of gluten and its effects on celiac disease]. Rev Med Chil. 2010;138:1319–25. [PubMed] [Google Scholar]
- 32. Helmerhorst EJ, Zamakhchari M, Schuppan D, Oppenheim FG. Discovery of a novel and rich source of gluten-degrading microbial enzymes in the oral cavity. PLoS One. 2010;5:e13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zamakhchari M, Wei G, Dewhirst F, Lee J, Schuppan D, Oppenheim FG, Helmerhorst EJ. Identification of rothia bacteria as gluten-degrading natural colonizers of the upper gastro-intestinal tract. PLoS One. 2011;6:e24455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fernandez-Feo M, Wei G, Blumenkranz G, Dewhirst FE, Schuppan D, Oppenheim FG, Helmerhorst EJ. The cultivable human oral gluten-degrading microbiome and its potential implications in coeliac disease and gluten sensitivity. Clin Microbiol Infect. 2013;19:E386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shan L, Qiao SW, Arentz-Hansen H, Molberg Ø, Gray GM, Sollid LM, Khosla C. Identification and analysis of multivalent proteolytically resistant peptides from gluten: Implications for Celiac Sprue. J Proteome Res. 2005;4:1732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shan L, Molberg Ø, Parrot I, Hausch F, Filiz F, Gray GM, Sollid LM, Khosla C. Structural basis for gluten intolerance in Celiac Sprue. Science. 2002;297:2275–9. [DOI] [PubMed] [Google Scholar]
- 37. Frazer AC, Fletcher RF, Shaw B, Ross CAC, Sammons HG, Schneider R. Gluten-induced enteropathy the effect of partially digested gluten. Lancet. 1959;2:252–5. [DOI] [PubMed] [Google Scholar]
- 38. Lammers KM, Herrera MG, Dodero VI. Translational chemistry meets gluten-related disorders. ChemistryOpen. 2018;7:217–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hardy MY, Girardin A, Pizzey C, Cameron DJ, Watson KA, Picascia S, Auricchio R, Greco L, Gianfrani C, La Gruta NL et al.. Consistency in polyclonal T-cell responses to gluten between children and adults with celiac disease. Gastroenterology. 2015;149:1541–52. e2. [DOI] [PubMed] [Google Scholar]
- 40. Ganapathy V. Chapter 59 - Protein Digestion and Absorption. In: Johnson LR, Ghishan FK, Kaunitz JD, Merchant JL, Said HM, Wood JDBT, editors. Physiology of the Gastrointestinal Tract (Fifth Edition). Boston: Academic Press; 2012. p. 1595–623. [Google Scholar]
- 41. Rosenbaum JT. Celiac disease and autoimmunity — the missing ingredient. N Engl J Med. 2017;377:1489–90. [DOI] [PubMed] [Google Scholar]
- 42. Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137:1912–33. [DOI] [PubMed] [Google Scholar]
- 43. Sollid LM, Khosla C. Future therapeutic options for celiac disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:140–7. [DOI] [PubMed] [Google Scholar]
- 44. Gianfrani C, Auricchio S, Troncone R. Adaptive and innate immune responses in celiac disease. Immunol Lett. 2005;99:141–5. [DOI] [PubMed] [Google Scholar]
- 45. Meresse B, Ripoche J, Heyman M, Cerf-Bensussan N. Celiac disease: from oral tolerance to intestinal inflammation, autoimmunity and lymphomagenesis. Mucosal Immunol. 2008;2:8. [DOI] [PubMed] [Google Scholar]
- 46. Sturgeon C, Lan J, Fasano A. Zonulin transgenic mice show altered gut permeability and increased morbidity/mortality in the DSS colitis model. Ann N Y Acad Sci. 2017;1397:130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Akobeng AK, Thomas AG. Systematic review: Tolerable amount of gluten for people with coeliac disease. Aliment Pharmacol Ther. 2008;27:1044–52. [DOI] [PubMed] [Google Scholar]
- 48. Catassi C, Fabiani E, Iacono G, D'Agate C, Francavilla R, Biagi F, Volta U, Accomando S, Picarelli A, De Vitis I et al.. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr. 2007;85:160–6. [DOI] [PubMed] [Google Scholar]
- 49. Food and Drug Administration. Health hazard assessment for gluten exposure in individuals with celiac disease: determination of tolerable daily intake levels and levels of concern for gluten. Office of Food Safety Center of Food Safety and Applied Nutrition Food and Drug Administration, U.S.A.; 2011. [Google Scholar]
- 50. Leffler D, Schuppan D, Pallav K, Najarian R, Goldsmith JD, Hansen J, Kabbani T, Dennis M, Kelly CP. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut. 2013;62:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cebolla Á, Moreno M de L, Coto L, Sousa C. Gluten immunogenic peptides as standard for the evaluation of potential harmful prolamin content in food and human specimen. Nutrients. 2018;10:1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baümler AJ, Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. 2016;535:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Natividad JMM, Verdu EF. Modulation of intestinal barrier by intestinal microbiota: Pathological and therapeutic implications. Pharmacol Res. 2013;69:42–51. [DOI] [PubMed] [Google Scholar]
- 57. Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bengmark S. Ecological control of the gastrointestinal tract. The role of probiotic flora. Gut. 1998;42:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. [DOI] [PubMed] [Google Scholar]
- 60. Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Luckey TD. Introduction to intestinal microecology. Am J Clin Nutr. 1972;25:1292–4. [DOI] [PubMed] [Google Scholar]
- 63. Obrenovich M, Sankar Chittoor Mana T, Rai H, Shola D, Sass C, McCloskey B, Levison BS. Recent findings within the microbiota–gut–brain–endocrine metabolic interactome. Pathol Lab Med Int. 2017;9:21–30. [Google Scholar]
- 64. Bauer KC, Huus KE, Finlay BB. Microbes and the mind: Emerging hallmarks of the gut microbiota-brain axis. Cell Microbiol. 2016;18:632–44. [DOI] [PubMed] [Google Scholar]
- 65. Kho ZY, Lal SK. The human gut microbiome – a potential controller of wellness and disease. Front Microbiol. 2018;9:1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tap J, Mondot S, Levenez F, Pelletier E, Caron C, Furet JP, Ugarte E, Muñoz-Tamayo R, Paslier DLE, Nalin R et al.. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol. 2009;11:2574–84. [DOI] [PubMed] [Google Scholar]
- 67. Hooper L V, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–8. [DOI] [PubMed] [Google Scholar]
- 68. Sjögren YM, Tomicic S, Lundberg A, Böttcher MF, Björkstén B, Sverremark-Ekström E, Jenmalm MC. Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses: gut microbiota and immune responses. Clin Exp Allergy. 2009;39:1842–51. [DOI] [PubMed] [Google Scholar]
- 69. Bienenstock J, Kunze W, Forsythe P. Microbiota and the gut-brain axis. Nutr Rev. 2015;73:28–31. [DOI] [PubMed] [Google Scholar]
- 70. Lange K, Buerger M, Stallmach A, Bruns T. Effects of antibiotics on gut microbiota. Dig Dis. 2016;34:260–8. [DOI] [PubMed] [Google Scholar]
- 71. Chang C, Lin H. Dysbiosis in gastrointestinal disorders. Best Pract Res Clin Gastroenterol. 2016;30:3–15. [DOI] [PubMed] [Google Scholar]
- 72. Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22:1079–89. [DOI] [PubMed] [Google Scholar]
- 73. Goulet O. Potential role of the intestinal microbiota in programming health and disease. Nutr Rev. 2015;73:32–40. [DOI] [PubMed] [Google Scholar]
- 74. Bethune MT, Ribka E, Khosla C, Sestak K. Transepithelial transport and enzymatic detoxification of gluten in gluten-sensitive rhesus macaques. PLoS One. 2008;3:e1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mohan M, Chow CT, Ryan CN, Chan LS, Dufour J, Aye PP, Blanchard J, Moehs CP, Sestak K. Dietary gluten-induced gut dysbiosis is accompanied by selective upregulation of microRNAs with intestinal tight junction and bacteria-binding motifs in a rhesus macaque model of celiac disease. Nutrients. 2016;8:E684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Herrán AR, Pérez-Andrés J, Caminero A, Nistal E, Vivas S, Ruiz de Morales JM, Casqueiro J. Gluten-degrading bacteria are present in the human small intestine of healthy volunteers and celiac patients. Res Microbiol. 2017;168:673–84. [DOI] [PubMed] [Google Scholar]
- 77. Bernardo D, Garrote JA, Nadal I, León AJ, Calvo C, Fernández-Salazar L, Blanco-Quirós A, Sanz Y, Arranz E. Is it true that coeliacs do not digest gliadin? Degradation pattern of gliadin in coeliac disease small intestinal mucosa. Gut. 2009;58:886–7. [DOI] [PubMed] [Google Scholar]
- 78. Davila AM, Blachier F, Gotteland M, Andriamihaja M, Benetti PH, Sanz Y, Tomé D. Re-print of “intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host”. Pharmacol Res. 2013;69:114–26. [DOI] [PubMed] [Google Scholar]
- 79. Macfarlane GT, Allison C, Gibson SAW, Cummings JH. Contribution of the microflora to proteolysis in the human large intestine. J Appl Bacteriol. 1988;64:37–46. [DOI] [PubMed] [Google Scholar]
- 80. Laparra JM, Olivares M, Gallina O, Sanz Y. Bifidobacterium longum CECT 7347 modulates immune responses in a gliadin-induced enteropathy animal model. PLoS One. 2012;7:e30744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sánchez E, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Intestinal Bacteroides species associated with coeliac disease. J Clin Pathol. 2010;63:1105–11. [DOI] [PubMed] [Google Scholar]
- 82. Laparra JM, Sanz Y. Bifidobacteria inhibit the inflammatory response induced by gliadins in intestinal epithelial cells via modifications of toxic peptide generation during digestion. J Cell Biochem. 2010;109:801–7. [DOI] [PubMed] [Google Scholar]
- 83. Comino I, Real A, Vivas S, Síglez MÁ, Caminero A, Nistal E, Casqueiro J, Rodríguez-Herrera A, Cebolla Á, Sousa C. Monitoring of gluten-free diet compliance in celiac patients by assessment of gliadin 33-mer equivalent epitopes in feces. Am J Clin Nutr. 2012;95:670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Caminero A, Herrán AR, Nistal E, Pérez-Andrés J, Vaquero L, Vivas S, Ruiz de Morales JMG, Albillos SM, Casqueiro J. Diversity of the cultivable human gut microbiome involved in gluten metabolism: Isolation of microorganisms with potential interest for coeliac disease. FEMS Microbiol Ecol. 2014;88:309–19. [DOI] [PubMed] [Google Scholar]
- 85. D'Arienzo R, Maurano F, Lavermicocca P, Ricca E, Rossi M. Modulation of the immune response by probiotic strains in a mouse model of gluten sensitivity. Cytokine. 2009;48:254–9. [DOI] [PubMed] [Google Scholar]
- 86. Caminero A, Galipeau HJ, McCarville JL, Johnston CW, Bernier SP, Russell AK, Jury J, Herran AR, Casqueiro J, Tye-Din JA et al.. Duodenal bacteria from patients with celiac disease and healthy subjects distinctly affect gluten breakdown and immunogenicity. Gastroenterology. 2016;151:670–83. [DOI] [PubMed] [Google Scholar]
- 87. Caminero A, McCarville JL, Galipeau HJ, Deraison C, Bernier SP, Constante M, Rolland C, Meisel M, Murray JA, Yu XB et al.. Duodenal bacterial proteolytic activity determines sensitivity to dietary antigen through protease-activated receptor-2. Nat Commun. 2019;10:1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ritter J, Zimmermann K, Jöhrens K, Mende S, Seegebarth A, Siegmund B, Hennig S, Todorova K, Rosenwald A, Daum S et al.. T-cell repertoires in refractory coeliac disease. Gut. 2018;67:644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tian N, Faller L, Leffler DA, Kelly CP, Hansen J, Bosch JA, Wei G, Paster BJ, Schuppan D, Helmerhorst EJ. Salivary gluten degradation and oral microbial profiles in healthy individuals and celiac disease patients. Appl Environ Microbiol. 2017;83:e03330–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K et al.. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–6. [DOI] [PubMed] [Google Scholar]
- 91. Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Forsberg G, Fahlgren A, Hörstedt P, Hammarström S, Hernell O, Hammarstrôm ML. Presence of bacteria and innate immunity of intestinal epithelium in childhood celiac disease. Am J Gastroenterol. 2004;99:894–904. [DOI] [PubMed] [Google Scholar]
- 93. Collado MC, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalances in faecal and duodenal Bifidobacterium species composition in active and non-active coeliac disease. BMC Microbiol. 2008;8:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ou G, Hedberg M, Hörstedt P, Baranov V, Forsberg G, Drobni M, Sandström O, Wai SN, Johansson I, Hammarström ML et al.. Proximal small intestinal microbiota and identification of rod-shaped bacteria associated with childhood celiac disease. Am J Gastroenterol. 2009;104:3058–67. [DOI] [PubMed] [Google Scholar]
- 95. Sánchez E, Laparra JM, Sanz Y. Discerning the role of bacteroides fragilis in celiac disease pathogenesis. Appl Environ Microbiol. 2012;78:6507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cheng J, Kalliomäki M, Heilig HGHJ, Palva A, Lähteenoja H, de Vos WM, Salojärvi J, Satokari R. Duodenal microbiota composition and mucosal homeostasis in pediatric celiac disease. BMC Gastroenterol. 2013;13:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wacklin P, Kaukinen K, Tuovinen E, Collin P, Lindfors K, Partanen J, Mäki M, Mättö J. The duodenal microbiota composition of adult celiac disease patients is associated with the clinical manifestation of the disease. Inflamm Bowel Dis. 2013;19:934–41. [DOI] [PubMed] [Google Scholar]
- 98. Sánchez E, Donat E, Ribes-Koninckx C, Fernández-Murga ML, Sanz Y. Duodenal-mucosal bacteria associated with celiac disease in children. Appl Environ Microbiol. 2013;79:5472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wacklin P, Laurikka P, Lindfors K, Collin P, Salmi T, Lähdeaho ML, Saavalainen P, Mäki M, Mättö J, Kurppa K et al.. Altered duodenal microbiota composition in celiac disease patients suffering from persistent symptoms on a long-term gluten-free diet. Am J Gastroenterol. 2014;109:1933–41. [DOI] [PubMed] [Google Scholar]
- 100. Francavilla R, Ercolini D, Piccolo M, Vannini L, Siragusa S, De Filippis F, De Pasquale I, Di Cagno R, Di Toma M, Gozzi G et al.. Salivary microbiota and metabolome associated with celiac disease. Appl Environ Microbiol. 2014;80:3416–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Caminero A, Nistal E, Herrán AR, Pérez-Andrés J, Ferrero MA, Vaquero Ayala L, Vivas S, Ruiz De Morales JMG, Albillos SM, Casqueiro FJ. Differences in gluten metabolism among healthy volunteers, coeliac disease patients and first-degree relatives. Br J Nutr. 2015;114:1157–67. [DOI] [PubMed] [Google Scholar]
- 102. Lorenzo Pisarello MJ, Vintiñi EO, González SN, Pagani F, Medina MS. Decrease in lactobacilli in the intestinal microbiota of celiac children with a gluten-free diet, and selection of potentially probiotic strains. Can J Microbiol. 2015;61:32–7. [DOI] [PubMed] [Google Scholar]
- 103. Rintala A, Riikonen I, Toivonen A, Pietilä S, Munukka E, Pursiheimo JP, Elo LL, Arikoski P, Luopajärvi K, Schwab U et al.. Early fecal microbiota composition in children who later develop celiac disease and associated autoimmunity. Scand J Gastroenterol. 2018;53:403–9. [DOI] [PubMed] [Google Scholar]
- 104. Garcia-Mazcorro JF, Rivera-Gutierrez X, Cobos-Quevedo ODJ, Grube-Pagola P, Meixueiro-Daza A, Hernandez-Flores K, Cabrera-Jorge FJ, Vivanco-Cid H, Dowd SE, Remes-Troche JM. First insights into the gut microbiota of Mexican patients with celiac disease and non-celiac gluten sensitivity. Nutrients. 2018;10:1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bodkhe R, Shetty SA, Dhotre DP, Verma AK, Bhatia K, Mishra A, Kaur G, Pande P, Bangarusamy DK, Santosh BP et al.. Comparison of small gut and whole gut microbiota of first-degree relatives with adult celiac disease patients and controls. Front Microbiol. 2019;8:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ivarsson A, Persson L, Nyström L, Ascher H, Cavell B, Danielsson L, Dannaeus A, Lindberg T, Lindquist B, Stenhammar L et al.. Epidemic of coeliac disease in Swedish children. Acta Paediatr Int J Paediatr. 2000;89:165–71. [DOI] [PubMed] [Google Scholar]
- 107. D'Argenio V, Casaburi G, Precone V, Pagliuca C, Colicchio R, Sarnataro D, Discepolo V, Kim SM, Russo I, Del Vecchio Blanco G et al.. Metagenomics reveals dysbiosis and a potentially pathogenic N. flavescens strain in duodenum of adult celiac patients. Am J Gastroenterol. 2016;111:879–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Olivares M, Neef A, Castillejo G, De Palma G, Varea V, Capilla A, Palau F, Nova E, Marcos A, Polanco I et al.. The HLA-DQ2 genotype selects for early intestinal microbiota composition in infants at high risk of developing coeliac disease. Gut. 2015;64:406–17. [DOI] [PubMed] [Google Scholar]