Abstract
Background:
Shared decision making (SDM) facilitates delivery of medical therapies that are in alignment with patients’ goals and values. Medicare national coverage decision for several interventions now includes SDM mandates, but few have been evaluated in nationwide studies. Based upon a detailed needs assessment with diverse stakeholders, we developed pamphlet and video patient decision aids (PtDA) for implantable cardioverter defibrillator (ICD) implantation, ICD replacement, and cardiac resynchronization therapy with defibrillation to help patients contemplate, forecast, and deliberate their options. These PtDA are the foundation of the Multicenter Trial of a Shared Decision Support Intervention for Patients Offered Implantable Cardioverter-Defibrillators (DECIDE-ICD), a multicenter, randomized trial sponsored by NHLBI aimed at understanding the effectiveness and implementation of a SDM support intervention for patients considering ICDs. Finalization of a Medicare coverage decision mandating the inclusion of SDM for new ICD implantation occurred shortly after trial initiation, raising novel practical and statistical considerations for evaluating study endpoints.
Methods/Design:
A stepped-wedge randomized controlled trial was designed, guided by the RE-AIM planning and evaluation framework using an effectiveness-implementation hybrid type II design. Six electrophysiology programs from across the United States will participate. The primary effectiveness outcome is decision quality (defined by knowledge and values-treatment concordance). Patients with heart failure who are clinically eligible for an ICD are eligible for the study. Target enrollment is 900 participants.
Discussion:
Study findings will provide a foundation for implementing decision support interventions, including PtDAs, with patients who have chronic progressive illness and are facing decisions involving invasive, preference-sensitive therapy options. RCT# NCT03374891
Keywords: decision aids, implantable cardioverter defibrillator, cardiac resynchronization therapy, patient-centered care, shared decision-making, implementation, RE-AIM
INTRODUCTION
Over 200,000 implantable cardioverter defibrillators (ICD) are implanted annually in the US1 to prevent sudden cardiac death (SCD), including both new devices and replacement procedures at the end of routine battery life. In appropriately selected patients, ICDs reduce mortality from SCD resulting in roughly a 5% absolute increase in survival in the 5 years after implant.2-5 However, a number of potential clinical and quality of life (QOL) threats exist. ICDs require surgical implantation and regular follow up. Patients have described an ICD shock as “getting kicked in the chest by a mule,”6 leading some to have their ICDs removed for fear of repeated shocks.7 Inappropriate shocks can occur.8 Some studies suggest that patients with ICDs have more heart failure admissions,9 a lower QOL - particularly if shocked by their devices10,11 - and an increased incidence of anxiety, depression, and post-traumatic stress disorder.12 Furthermore, if not properly deactivated, ICDs can cause unnecessary suffering at the end of life.13-16
Research also suggests problems with current ICD decision making. An integrative review of patient perspectives highlighted a paternalistic approach to decision making.6,17 Patients with ICDs frequently report never having had a conversation about periprocedural risks, expected benefits, or potential QOL problems.6 With historical approaches to communication, patients tend to overestimate the benefits of ICDs, underestimate the risks, and are underinformed about device deactivation.18 Recent policy changes, including a mandate in October 2018 by the Centers for Medicare and Medicaid Services requiring that “a formal shared decision making encounter must occur between the patient and an independent physician…using an evidence-based decision tool on ICDs prior to initial ICD implantation”19 reflect the importance of this need and help address concerns regarding good shared decision making (SDM).
Patient decision aids (PtDA) have strong efficacy data. A Cochrane review of 105 randomized trials demonstrated that PtDAs improve knowledge, satisfaction, patient/provider communication, increase patient involvement in decision making, and reduce patient decisional conflict and regret.20 Despite their established efficacy, PtDAs are not often implemented outside the research setting. A recent systematic review of PtDA implementation identified a host of logistical barriers, including clinicians’ perception of time necessary to use PtDAs, lack of reimbursement, and perceived bias inherent in the PtDAs themselves.21
Guided by the International Patient Decision Aid Standards (s) and following the Ottawa Decision Support Framework,22 we developed a decision making support intervention consisting of (a) video and pamphlet PtDA23 and (b) a clinician-directed decision support training for ICD decision making. The “Multicenter Trial of a Shared Decision Support Intervention for Patients Offered Implantable Cardioverter-Defibrillators” (DECIDE-ICD) hypothesizes that this decision support intervention will improve patient decision quality. Additionally, guided by the RE-AIM framework24, DECIDE-ICD will explore ways to optimize implementation and increase understanding of implementation processes. This article describes the methods for this multicenter effectiveness-implementation hybrid trial.
METHODS/DESIGN
The DECIDE-ICD study will test the effectiveness and implementation of SDM support intervention for ICDs in the contexts of initial implantation, reimplantation, and CRT-D. With the goal of more rapid translation to clinical practice, this study was designed as an effectiveness-implementation hybrid type II design.25 This allows the study team to conduct effectiveness testing traditionally done with decision-support interventions, while simultaneously assessing a real-world implementation strategy of the intervention. The dual focus will help ensure that should the intervention be effective, it can also feasibly be adopted outside of the study environment. This trial has been registered on www.clinicaltrials.gov (#NCT03374891).
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01HL136403. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents. The content does not necessarily represent the official views of the National Institutes of Health.
Lessons Learned from Previous Trials
ICD Decision Aid Pilot Trial
From April 2012 to March 2013, 21 participants were enrolled across 3 settings in the Denver metropolitan area to assess the acceptability and feasibility of the ICD PtDA. Participants included adult patients (>18 years of age) with systolic heart failure who were being presented with a decision to get an ICD for primary prevention. The study tested desirability of 4 different PtDA types: option grids, infographics, a video, and a website. In addition, 3 different recruitment methods were tested. Out of 3 recruitment methods, using a medical team member was the most efficient way of identifying appropriate patients. PtDA were found to be highly acceptable: 67% of those included in the study found the PtDA to be unbiased, 89% found them helpful, and 100% would recommend them to others. Patients preferred infographic and video types of decision aids compared to option grids or a website.
Decision Quality was measured as a secondary outcome in the pilot trial via the domains of knowledge and value concordance. The Pilot trial showed that knowledge remained the same between intervention and control, whereas value concordance significantly increased in the 1 month follow-up but not the 3 month follow-up. However, this trial was underpowered to make strong conclusions about these outcomes.
Some design choices in the DECIDE-ICD trial based on the results of the pilot study include: (1) having electrophysiologists as site-PIs to ensure multi-level buy-in; (2) encouraging sites to include schedulers, nurses, and others to be involved in discussions and implementation to create multidisciplinary engagement; (3) changing the timing of the baseline survey to after a discussion with a medical team member as identifying patients pre-discussion makes meeting recruitment targets unfeasible; (4) PtDA should be comprised of videos and paper pamphlets; and (5) have enough power with 900 subjects to show significance in Decision Quality. Additionally, our team has also learned from a similarly designed trial for patients making decisions with LVADs.23,26
Stepped-Wedge, Randomized, Multicenter Trial Design
A quasi-experimental multicenter trial randomized at the hospital level was chosen for a phased roll-out of the SDM support intervention.27 This variant of the cluster trial design is termed the stepped-wedge trial (Table I): a 1-way crossover cluster trial where all programs will receive the intervention, but the time when they receive this is randomly ordered. In a stepped-wedge randomized design, each site begins in the control phase, where usual care consists of the program’s current education, decision making, and informed consent process. When sites reach their randomly assigned time to transition to the intervention, the intervention is formally integrated into the existing process.
Table I:
Overview of the Stepped-Wedge Design
| Site | Baseline (5 months) |
Intervention rollout sequentially across all sites (3 years) |
|||||
|---|---|---|---|---|---|---|---|
| Random Site 1 | |||||||
| Random Site 2 | |||||||
| Random Site 3 | |||||||
| Random Site 4 | |||||||
| Random Site 5 | |||||||
| Random Site 6 | |||||||
Setting and Participants
Participants are enrolled from 6 centers across the United States, including a Veteran’s Affairs hospital, 2 academic medical centers, 2 private medical centers, and one combined county safety-net hospital/academic medical center, drawing both urban and rural patients from diverse settings across the country (Appendix I). Any initial differences in approach to treatment between these distinct sites will further highlight the efficacy of a more standardized approach to defibrillator education via the decision aids and may serve as a model for future implementation across cultures and countries.
Patients
A planned total of 900 patients will be enrolled across 6 sites during a 36-month enrollment period, beginning April 2018 and ending March 2021. Participants will be adult (age > 18 years) patients who have had a discussion about an ICD, ICD replacement, or CRT-D for primary prevention of heart failure. This excludes patients with subcutaneous ICDs. These discussions will take place between a patient and a member of the clinical team, which can be a cardiologist, nurse, electrophysiologist, or anyone else partaking in their care. Discussions may include risk and benefit, next steps, and other items, but can vary in length of time, content, and specificity between clinics and patients. As this is an implementation trial, there is not a specific protocol for how this discussion will be done with each patient. However, at the beginning of each implementation roll-out at each site, three talks will be given by the study team to staff who will be initiating these discussions. These meetings will include the importance and intent of SDM, teaching techniques for EPs to use SDM, and a meeting with the clinic staff where they decide the best way for their clinic to have these discussions and dissemination. The study staff will record this implementation strategy, and periodically ask and record any changes to each clinic’s implementation strategy. Given that the PtDAs and study assessments are currently in English, recruitment is limited to English speakers. Study staff will identify eligible patients through clinical referrals or by screening patient charts in electronic medical records. They will look at referrals, notes, or problem lists to ascertain indications for inclusion.
Effectiveness of the Intervention
The effectiveness of the PtDA intervention will be evaluated.23 Using a step-wedge design, after a 5-month control period for all sites, a new site will begin the intervention every 5 months and continue through the end of the trial. Paper and video PtDA will be handed out in combination to eligible patients before a discussion about a defibrillator. Both the pamphlet and the video contain information about what an ICD/CRT is, risks and benefits, and important questions about the device. The videos are 8-20 minutes long and contain patient narratives about their decision-making processes regarding getting or not getting a device (see Appendix II). These PtDA have been shown to be helpful, largely unbiased, and highly acceptable to patients.28
We plan for the PtDA to be adapted as new evidence arises, consistent with IPDAS.29 Evidence will be reviewed throughout the study and changes to the PtDA made as necessary. An evidence document will be maintained and updated every 6-12 months, reviewing feedback and documenting any changes to the PtDA.
Implementation Strategy
At the time of intervention implementation, each site will participate in (1) a grand rounds-style presentation given by the principal investigator and targeting cardiologists and electrophysiologists, which explains the background to the project, pilot work conducted for the intervention, and the objectives of implementation science; (2) a demonstration of the PtDA materials and a 30 to 60 minute coaching session for staff directly or indirectly involved in defibrillator patient education and care, which includes important aspects of SDM such as discussing approaches to tough questions faced with previous patients; and (3) a 30-60 minute coaching and strategizing session for staff directly involved in implementing defibrillator patient education and care. Along with tenets learned from the decision coaching sessions, the PtDA materials will be integrated into existing education and decision making processes at the site level for all patients undergoing defibrillator evaluation.
Data Collection
Eligible patients are identified by the study team at each site during the time that a discussion around defibrillators is initiated for the patient. These discussions will be identified through chart review notes or through clinician referrals to study staff. Patients will be recruited within 1 week after the defibrillator discussion. Baseline, 1-month, and 6-month surveys will be completed. Medical record data (e.g.: outcomes, adverse events, treatment decisions) for patients will also be collected at baseline and 6 months. Study coordinators, with the help of the clinical staff, will complete the Study Coordinator Checklist for each patient. The primary physician or staff who had the defibrillator discussion with the enrolled patient will complete the Clinician’s Perceptions of Patient Appropriateness for SDM survey, reporting his/her opinion on the best therapy for the patient and an estimate of risk of unfavorable outcome. Data collection is the same during control and intervention periods.
The RE-AIM framework will be used for evaluation.30 The RE-AIM framework assesses an intervention’s potential for dissemination and public health impact using five criteria: Reach, Effectiveness, Adoption, Implementation, and Maintenance. The RE-AIM framework has been used to translate research into practice and to help plan programs and improve their chances of working in “real-world” settings.30. Highlighted below are the primary outcomes of the trial, and Table III provides details of all 5 criteria of the RE-AIM framework30-33.
Table III:
RE-AIM Framework
| Conceptual Area | Definition | Measurement in DECIDE-ICD |
|---|---|---|
| Reach | Proportion and representativeness of the target population who participate in the intervention. | The number of patients who receive and remember the PtDA; over the number of patients eligible for ICD, replacement, or CRT-D. Comparison to those not participating. |
| Effectiveness | The impact of an intervention on important outcomes and any unanticipated outcomes. | Domains of decision quality: 1) knowledge and 2) value-choice concordance; will be measured by participant questionnaires. |
| Adoption | Proportion and representativeness of staff who are willing to initiate a program. | Clinician attitudes toward SDM and the intervention will be measured in pre/post interviews and a 6 question per-patient survey for each patient enrolled. Comparison to those not participating. |
| Implementation | How closely staff members follow the program that the developers provide, and adaptations made. | Interviews with clinicians pre-, during, and post-intervention will measure implementation of SDM with PtDA. Additionally, a study coordinator checklist for patients will measure implementation and adaptation. |
| Maintenance | The extent to which a program or policy becomes part of the routine organizational practices and policies. | This will be assessed primarily by seeing whether each of the 6 sites decides at the conclusion of the study to maintain, modify, or discontinue the PtDAs. |
Reach
Reach is defined as the proportion and representativeness of the target population who participate in the intervention. While Reach is a relatively straightforward measure, defining the denominator of eligible patients can be difficult. We will assess the percentage of patients that both receive and remember reviewing the PtDAs. Representativeness will be assessed by comparing participants to those who opt out on a range of available demographics and clinical indicators (e.g., age, gender, comorbidities) from chart review. As we are only enrolling a subset of patients into the trial at each site, we will ask the staff delivering the PtDA to maintain a log of intervention recipients.
Effectiveness
A variety of secondary outcomes and covariates will be measured at baseline and follow-up. A number of effectiveness measures will be collected (Table IV). Appendix III provides more detail on the effectiveness measures.
Table IV:
Effectiveness Measures
| Baseline Pre Decision |
1 Month Post Decision |
6 Months Post Decision |
|
|---|---|---|---|
| PRIMARY OUTCOMES | |||
| - Decision Quality – Knowledge34,35 | X | X | X |
| - Decision Quality – Value-choice concordance34,35 | X | X | X |
| SECONDARY OUTCOMES | |||
| - Process – 6 IPDAS-endorsed process measures29 | X | X | X |
| - Decision choice36,37 | X | X | |
| - Decision conflict20,29,38 | X | X | X |
| - Decision regret20,29,39 | X | X | |
| - HADS-A Anxiety Measure40 | X | X | |
| - ICD Experiences (+/− shock; +/− complications etc.) | X | X | |
| COVARIATES | |||
| - Demographics (combined survey and short chart review) | X | ||
| - Medications, Comorbidities, Heart Failure Type/Severity | X | ||
| - Literacy (REALM-R 7 item)41 and Subjective Numeracy42 | X |
Primary outcome: The primary effectiveness outcome will be decision quality. Decision quality is an essential element of the Ottawa Decision Support Framework, defined as “the extent to which the implemented decision reflects the considered preferences of a well-informed patient.”34 Decision quality measures consist of two domains: knowledge and value-choice concordance.
Knowledge: Consistent with methods developed by Sepucha et al.,34,35 we have developed a knowledge measure. We will use this measure to test knowledge at baseline, 1 month, and 6 months.
Value-choice concordance: We will calculate concordance between patients’ values and the treatment they choose according to the validated methods of Sepucha et al.34,35 We will measure this in two ways: 1) we will measure the values-clarity sub-scale of the decision conflict measure (test-retest reliability and Cronbach’s alpha > 0.78, correlated with knowledge, regret, and treatment discontinuance) and 2) we will explore the prevailing value trade-off between “living longer even if it means getting an invasive therapy” versus “not living as long and avoiding an invasive therapy” as this item was able to discriminate between groups.
Secondary outcomes: Additionally, we will collect the following secondary outcomes:
IPDAS process measures: We will use six questions based on key domains of decision process as outlined in the IPDAS background document. In prior work, these questions had significant reliability (Cronbach’s alpha of 0.78).29
Decision choice: We will use single item measures of decision predisposition, choice, and enactment. These questions have test-retest reliability of 0.9 and correlates with values.36,37
The Decision Conflict Scale (DCS): DCS is a 10-item instrument that measures decision quality and determines decisional uncertainty.38 DCS reliability measures include test-retest correlation and Cronbach’s alpha coefficients exceeding 0.78-0.90.20,29 The DCS discriminates between groups who make and delay decisions.20.
The Decision Regret Scale: Decision regret is a 5-item scale which measures regret with the decision making. It is a commonly used measure in decision aid trials and has good reliability (Cronbach’s alpha of 0.82-0.91).20,29 It correlates with satisfaction, decision conflict, and QOL.39
Hospital Anxiety & Depression Scale (HADS): The HADS is a 14-item measure assessing symptoms of anxiety and depression among general medical patient populations.40.
ICD experiences: These are questions developed and used previously to describe ICD-specific issues such as whether the participant has experienced a shock or complication and whether they have considered discussing their ICD in an advance directive or with a surrogate decision maker.
Literacy: The REALM-R is an 11-item test used to identify people with low health literacy.41
Subjective Numeracy: The subjective numeracy test identifies how comfortable people are with numbers, and determine subjective comfort with numerical preferences.42
Adoption
RE-AIM defines adoption as the absolute number, proportion, and representativeness of settings and intervention agents (people who deliver the program) who are willing to initiate a program. For this trial, we approached 7 sites and 6 agreed to participate giving an adoption rate of 86% at the site level since all 6 programs have agreed to participate. We will also assess adoption at the clinician level, where each clinician can adopt SDM each time they see a patient. We hypothesize that physicians will be more open to SDM as patients become older and sicker. We will explore clinicians’ adoption of SDM in two ways:
Clinician Attitudes Toward SDM: A quantitative survey will measure attitudes towards SDM at baseline and year 5.
Clinician’s Perceptions of Patient Appropriateness for SDM: We have developed and piloted a 6 question survey, which will be asked of clinicians in regard to each patient with whom they have a defibrillator discussion. (Table V) By exploring this preference on a per-patient basis, we will begin to understand how patient characteristics influence adoption of SDM and PtDAs.
Table V:
Clinician’s Perceptions of Patient Appropriateness for SDM (9 point Likert scale)
| Low | Middle | High | |
|---|---|---|---|
| 1) Regarding ____<<patient>>____, whom you have cared for recently, what was your recommendation regarding defibrillation? | Strongly recommended | Neither recommended for or against device | Strongly recommended against |
| 2) How was the decision about the device made? | The majority of the final decision was made by the patient. | The patient and I decided together. | The majority of the final decision was made by me. |
| 3) How easy did you feel the discussion(s) with this patient were? | Very easy | Very difficult | |
| 4) How well did the patient understand the device? | Not well | Very well | |
| 5) Based on this patient’s clinical situation, I would say: | The downsides of the device outweigh the benefits. | The downsides and benefits of the device are about equal. | The benefits of the device outweigh the downsides. |
| 6) Based on this patient’s values & opinions, I would say: | The downsides of the device outweigh the benefits. | The downsides and benefits of the device are about equal. | The benefits of the device outweigh the downsides. |
Implementation
The RE-AIM framework defines implementation as the extent to which the intervention is implemented as intended, adaptations made, and costs to deliver the program.30 Consistency of PtDA delivery will be assessed across hospitals, providers, patient subgroups, and time through the Study Coordinator Checklist and key informant interviews. The Study Coordinator Checklist will be used to assess how the intervention was implemented for each patient, with questions regarding the types of materials, the way in which they were provided, timeline, and use. In addition, factors associated with variation in implementation, such as who provides educational materials and when, will be identified. Key informant interviews will be conducted with various clinical staff at each site at baseline, after implementation of the intervention, and after study completion. The goals of these interviews are to (1) identify issues, facilitators, or barriers in the effectiveness of the intervention from various staff perspectives; (2) identify strategies that may be useful in the refinement of subsequent roll-out phases and future dissemination; and (3) ensure regular, uniform use of the educational materials. Adaptations will be assessed from the intervention checklists to assess deviations from the protocol and reasons. In addition, we will conduct periodic interviews to assess frequency, timing, source, type and reasons for adaptations using interview guides from Rabin et al.43
Maintenance
The RE-AIM framework defines maintenance as the continued use of a practice after a study has ended. This will be assessed primarily by seeing whether each of the 6 sites decides at the conclusion of the study to maintain, modify, or discontinue the PtDAs.
Pragmatism of Trial
As this is designed as a pragmatic trial, the internal team scored this study via the PRagmatic Explanatory Continuum Indicator Summary (PRECIS-2)44 (Figure I). Most domains showed similar assessments by the team. For the domains of Organization and Primary Outcome, there were larger ranges between raters. Organization variation (range 2-5) resulted in a discussion between low pragmatism due to the use of a formal investigator-based kick-off at the beginning of intervention for each site verses the high pragmatism of each site being able to carry out the study in a user-specific way. Primary Outcome variation (range 2-5) resulted in a rich discussion contrasting the effectiveness and implementation arms of the study. Raters scored low pragmatism due to hospital procedures and detailed research questionnaires as the measures of success, but others raters scored high pragmatism based on those processes ultimately being relevant to patients. The overall high pragmatism of the trial is promising for high generalizability of the implementation methods of this trial.
Figure I:
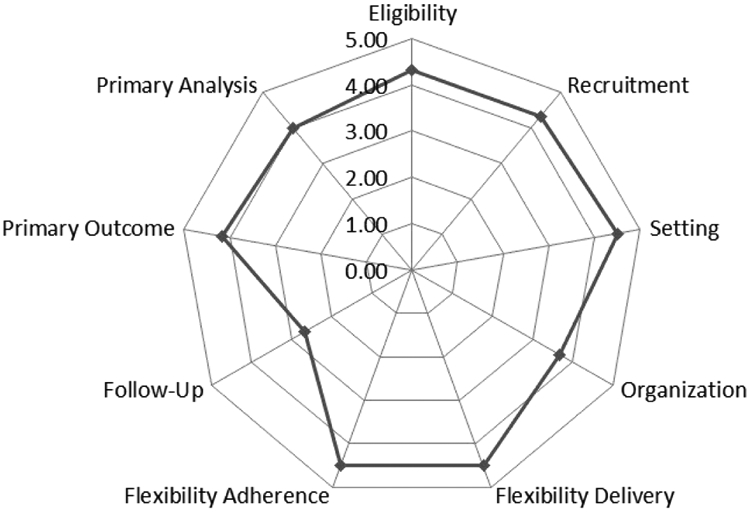
PRECIS-2 Diagram outlining pragmatism of proposed trial in 9 domains, compared to usual clinical care.
Analysis
Effectiveness
The analyses of effectiveness will use a repeated-measures mixed model. This strategy allows for partially incomplete data and relaxes the missing data assumptions to missing at random conditional on observed data. Before these analyses, the participants in the 2 phases of the study will be contrasted, identifying any participant or site characteristics that are unbalanced. If more than 3 to 5 variables are identified, a propensity score for the likelihood of being in the intervention phase will be developed. Each analysis model will include an indicator variable for the intervention phase, indicators for each of the sites, and the variables identified above. We have three pre-specified subgroups that we plan to evaluate as these groups may have important differences in terms of the primary outcome: 1) Age: >=70 vs. <70; 2) Ischemic vs. Non-ischemic cardiomyopathy; 3) Type of procedure: CRT-D, reimplant, or initial ICD. Important covariates that will be explored include health literacy, education, and subjective numeracy.
Power Estimation (knowledge)
The primary outcome is decision quality. The power estimation is based on the primary subdomain knowledge. With a sample size of 900 patients and a standard deviation of 18%, the study would have a power of 0.98 to detect an improvement in knowledge by 10% (assuming intraclass correlation 0.01) and 90 for a difference of 6% (in the 2011 Cochran review knowledge improve an average of 13.8%, in our pilot 12%). Even with variance inflation for correlation within sites (assuming intraclass correlation 0.10), the power for the 6% difference remains acceptable at 0.86. This level of power will afford the ability to assess some heterogeneity of effect across vulnerable patient groups, and this size of a study will also allow for adequate assessment of secondary effectiveness measures and implementation evaluation.
Reach, adoption, implementation and maintenance will be assessed by descriptive data (percentages, means, and indices of dispersion) and univariate and bivariate analyses (e.g., regression) of the effect of patient, staff and setting factors on implementation outcomes.
Qualitative Exploration (implementation)
The process and impact of implementing PtDA into the clinical workflow will be characterized using qualitative interviews of key informant ICD-involved clinicians at each study site. Analysis of these interviews will be guided by a grounded theory approach to develop a framework for understanding the process of implementing PtDA at each site.45 In accordance with this process, analysis will occur as a continuous, iterative process throughout data collection and final analysis. Transcripts will be read repeatedly by multiple analysts to achieve immersion, followed by coding using an emergent approach, emphasizing interviewee perspectives rather than speculation from the research team.46 As these data and emerging thematic maps are described, they will be contextualized according to provider type, site characteristics, and other demographic indicators to highlight qualitative comparisons between groups. We will also analyze interview data on adaptations using categories from Rabin et al.43 All analyses will be conducted using Dedoose analytic software (V. 8.0.35, 2018. Los Angeles, CA: SocioCultural Research Consultants, LLC. www.dedoose.com) to facilitate data storage, team-based analysis, code organization, and visualization.
Discussion
In February of 2018, 2 months before enrollment was set to begin, The Centers for Medicare and Medicaid Services (CMS) updated their national coverage determination decision memo to require that “a formal shared decision making encounter must occur between the patient and an independent physician…using an evidence-based decision tool on ICDs prior to initial ICD implantation,”19 The mandate referenced PtDAs created by the principal investigator, which were intended for use in the intervention phase of DECIDE-ICD. The mandate took effect October 1, 2018. While this was a major positive endorsement of the intervention, this mandate created a scientific problem for DECIDE-ICD by potentially contaminating the ICD part of the control phases of this trial. The ICD replacement and CRT-D parts of the trial were unaffected.
The study team requested a temporary exemption from this mandate for participating research sites from CMS in April 2018. This request was declined; however, representatives from CMS decided from this complication that future mandates would include exceptions for research studies. After discussions with the trial’s DSMB, project officer at the NHLBI, and other site-PIs, it was determined that sites wishing to meet the CMS mandate during control phases of DECIDE-ICD could use the paper tools developed by Dr. Matlock’s lab or other tools, but during intervention phases will use both paper and video tools developed by Dr. Matlock’s lab. As a consequence of this mandate, the trial may have some contamination in the control phase making achieving effectiveness more challenging. However, the advantage of the mandate is there is now more pressure to implement the intervention successfully.
Conclusion
Patients making decisions about whether to pursue invasive technologies in the setting of chronic progressive illness face arguably some of the most complicated decisions in medicine. Learning how to help patients, their loved ones, and their clinicians better make high-quality decisions is an area of need. PtDAs are increasingly available; however, they have not been widely adopted because of the challenging nature of integrating them into clinical practice. With the recent CMS mandate for SDM prior to ICD implantation, understanding the effectiveness and implementation of PtDA into practice is more important than ever. The goal of the DECIDE-ICD trial is to address this by evaluating and implementing a SDM support intervention for patients pursuing defibrillators based on previous evidence. The DECIDE-ICD study is a platform to evaluate introduction, implementation, and dissemination of PtDA with patients facing end-of-life decisions. Our findings should inform both research and practice in SDM, implementation science, and clinical care.
Table II:
Intervention activities during implementation intervention
| Implementation Action |
Target Group | Components |
|---|---|---|
| Grand Rounds | Hospital wide – cardiology and electrophysiology |
|
| Focus Group | Electrophysiology Team |
|
| Nuts and Bolts Group | Team who will be involved with implementation |
|
Acknowledgements:
SOURCES OF FUNDING: This work was supported through a National Heart, Lung and Blood Institute (NHLBI) Program Award (R01HL136403), Bethesda, MD. All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the NHLBI, its Board of Governors or Methodology Committee.
Appendix I:
In an effort to increase the diversity of our study population, a 7th site of Denver Health was added to improve the diversity of our sample. Denver Health is also a safety net hospital. Below is the expected demographics chart for our trial:
TABLE:
Anticipated Gender, Ethnicity, and Racial Distribution for Participants in DECIDE-ICD
| DHMC/U. of CO |
BIDMC | Provi dence |
Baptist | DVAMC | MAHI | Total | ||
|---|---|---|---|---|---|---|---|---|
| N=75 % |
75 % |
150 % |
150 % |
150 % |
150 % |
150 % |
900 % |
|
| GENDER | ||||||||
| Female | 52% | 34% | 27% | 36% | 53% | 5% | 40% | 34% |
| Male | 48% | 66% | 73% | 64% | 47% | 95% | 60% | 66% |
| ETHNICITY | ||||||||
| Hispanic | 28% | 3% | 1% | 9% | 5% | 3% | 4% | 6% |
| Non-Hispanic | 72% | 97% | 99% | 91% | 95% | 97% | 96% | 94% |
| RACE | ||||||||
| American Indian/Alaskan Native | 1% | 2% | 0% | 1% | 0% | 2% | 1% | 1% |
| Asian | 3% | 2% | 1% | 7% | 2% | 2% | 1% | 3% |
| Black or African American | 13% | 10% | 6% | 6% | 17% | 10% | 12% | 10% |
| More than one race | 2% | 4% | 5% | 2% | 2% | 3% | 3% | |
| Native Hawaiian/other Pacific Islander | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1% |
| Unknown or not reported | 20% | 3% | 4% | 4% | 2% | 3% | 1% | 4% |
| White | 64% | 81% | 88% | 76% | 74% | 81% | 82% | 79% |
U of CO – University of Colorado; BIDMC – Beth Israel Deaconess Medical Center; DVAMC – Denver Veterans Affairs Medical Center; MAHI – Mid America Heart Institute
Appendix II:
All current versions of our patient decision aids can be found on our website at https://patientdecisionaid.org/.
Below are the current versions at the time of this submission.
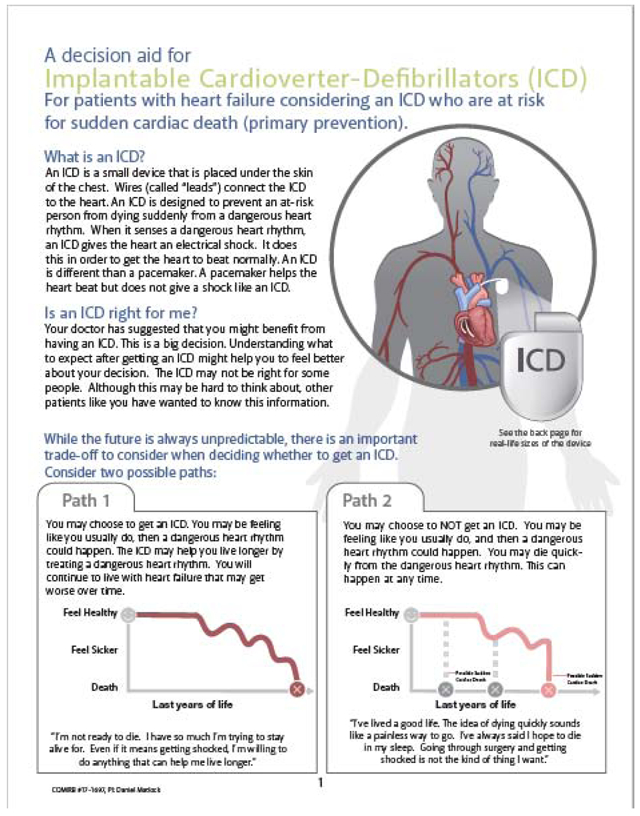

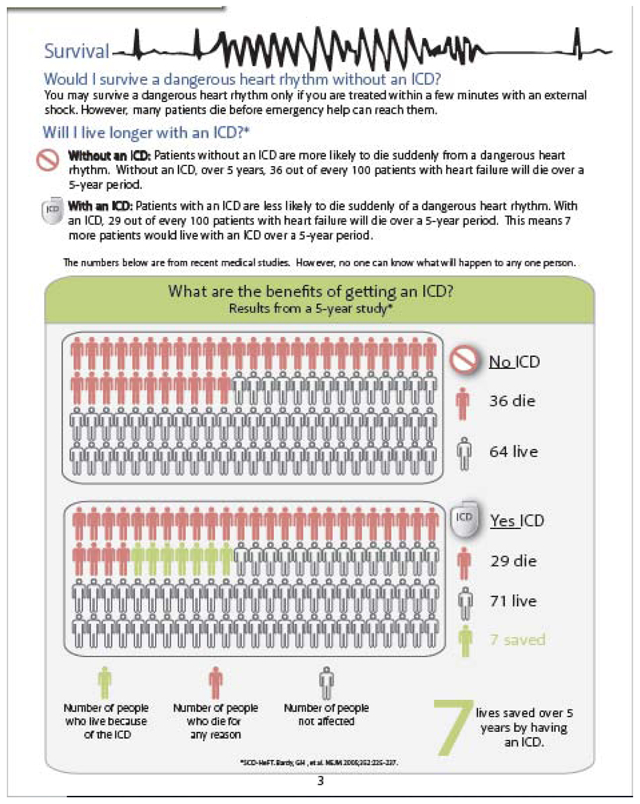

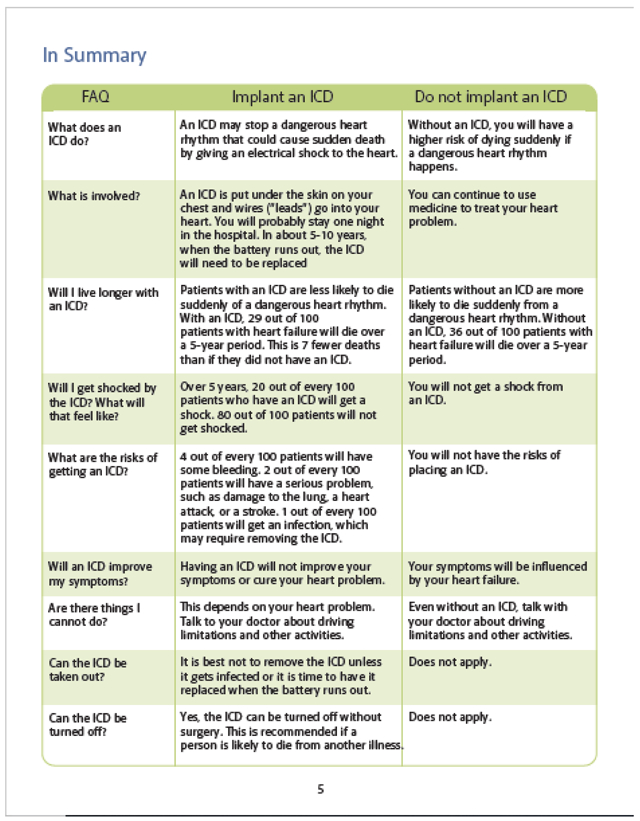




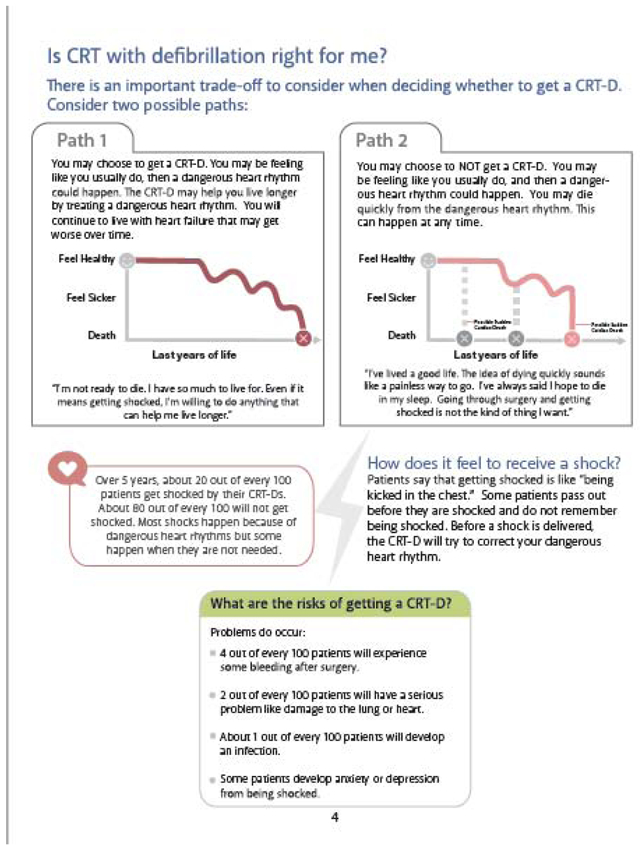

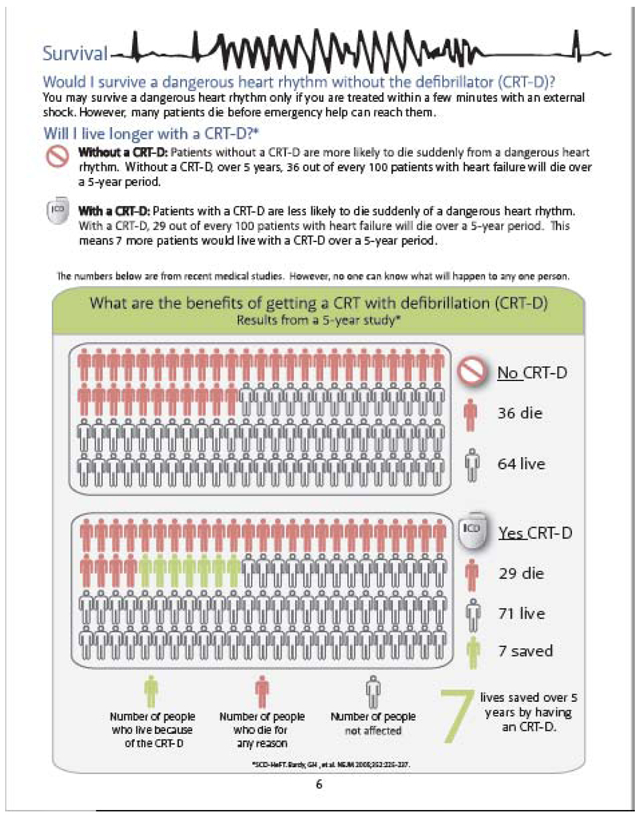
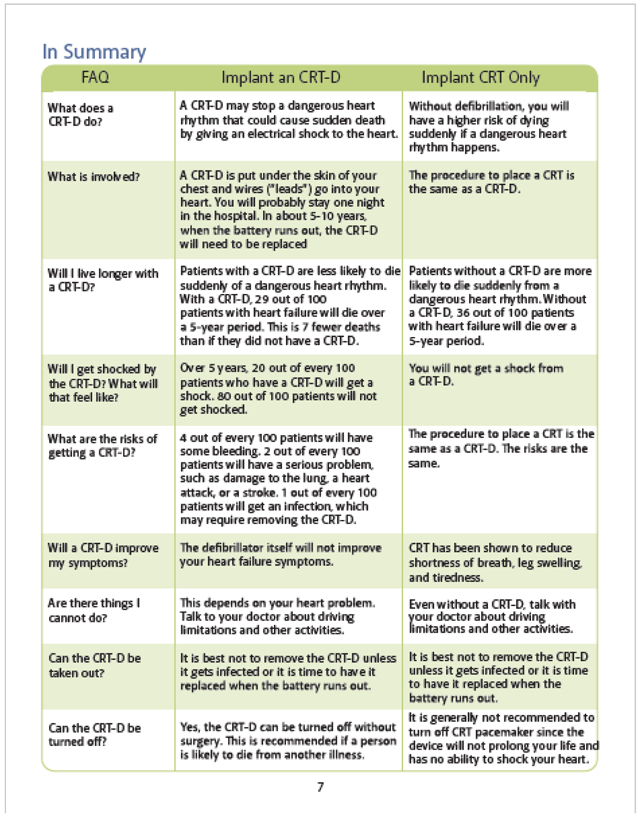


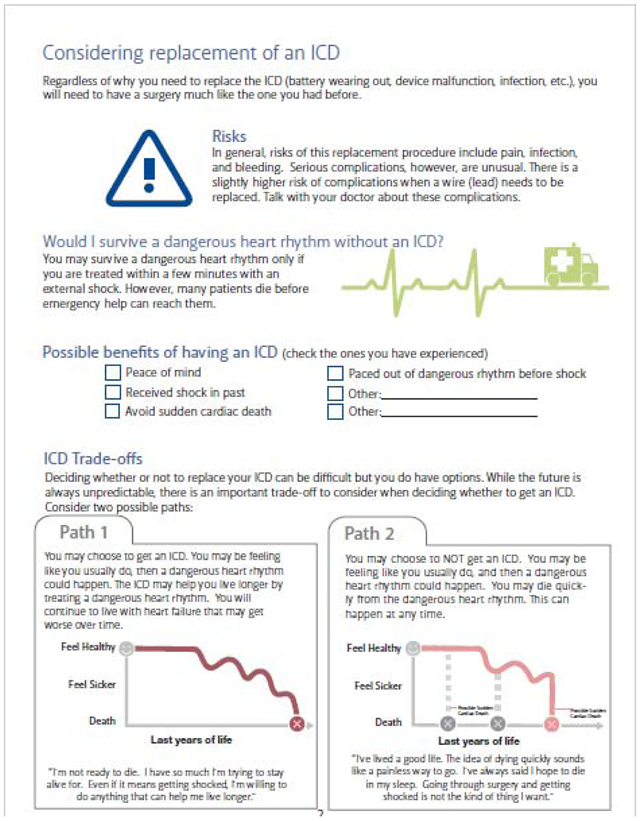


Appendix III:
RE-AIM is an important framework for understanding implementation science. The DECIDE-ICD trial was designed around RE-AIM, as described below:
Reach
Reach is defined as the proportion and representativeness of the target population who participate in the intervention. While Reach is a relatively straightforward measure, defining the denominator of eligible patients can be difficult. We will assess the percentage of patients that both receive and remember reviewing the PtDAs. Representativeness will be assessed by comparing participants to those who opt out on a range of available demographics and clinical indicators (e.g., age, gender, comorbidities) from chart review. As we are only enrolling a subset of patients into the trial at each site, we will ask the staff delivering the PtDA to maintain a log of intervention recipients.
Effectiveness
A variety of secondary outcomes and covariates will be measured at baseline and follow-up. A number of effectiveness measures will be collected. (Table IV)
Table IV:
Effectiveness Measures
| Effectiveness Measures | Baseline Pre Decision |
1 Month Post Decision |
6 Months Post Decision |
|---|---|---|---|
| EFFECTIVENESS OUTCOMES (see descriptions below) | |||
| - *Decision Quality – Knowledge | X | X | X |
| -*Decision Quality – Value-choice concordance | X | X | X |
| - Process – 6 IPDAS-endorsed process measures | X | X | X |
| - Decision choice | X | X | |
| - Decision conflict | X | X | X |
| - Decision regret | X | X | |
| - HADS-A Anxiety Measure | X | X | |
| - ICD Experiences (+/− shock; +/− complications etc.) | X | X | |
| COVARIATES | |||
| - Demographics (combined survey and short chart review) | X | ||
| - Medications, Comorbidities, Heart Failure Type/Severity | X | ||
| - Literacy (REALM-R 7 item)41 and Subjective Numeracy42 | X |
Primary outcome measures
Primary outcome: The primary effectiveness outcome will be decision quality. Decision quality is an essential element of the Ottawa Decision Support Framework, defined as “the extent to which the implemented decision reflects the considered preferences of a well-informed patient.”34 Decision quality measures consist of two domains: knowledge and value-choice concordance.
Knowledge: Consistent with methods developed by Sepucha et al.,34,35 we have developed a knowledge measure. We will use this measure to test knowledge at baseline, 1 month, and 6 months.
Value-choice concordance: We will calculate concordance between patients’ values and the treatment they choose according to the validated methods of Sepucha et al.34,35 We will measure this in two ways: 1) we will measure the values-clarity sub-scale of the decision conflict measure (test-retest reliability and Cronbach’s alpha > 0.78, correlated with knowledge, regret, and treatment discontinuance) and 2) we will explore the prevailing value trade-off between “living longer even if it means getting an invasive therapy” versus “not living as long and avoiding an invasive therapy” as this item was able to discriminate between groups.
Secondary outcomes: Additionally, we will collect the following secondary outcomes:
IPDAS process measures: We will use six questions based on key domains of decision process as outlined in the IPDAS background document. In prior work, these questions had significant reliability (Cronbach’s alpha of 0.78).29
Decision choice: We will use single item measures of decision predisposition, choice, and enactment. These questions have test-retest reliability of 0.9 and correlates with values.36,37
The Decision Conflict Scale (DCS): DCS is a 10-item instrument that measures decision quality and determines decisional uncertainty.38 DCS reliability measures include test-retest correlation and Cronbach’s alpha coefficients exceeding 0.78-0.90.20,29 The DCS discriminates between groups who make and delay decisions.20.
The Decision Regret Scale: Decision regret is a 5-item scale which measures regret with the decision making. It is a commonly used measure in decision aid trials and has good reliability (Cronbach’s alpha of 0.82-0.91).20,29 It correlates with satisfaction, decision conflict, and QOL.39
Hospital Anxiety & Depression Scale (HADS): The HADS is a 14-item measure assessing symptoms of anxiety and depression among general medical patient populations.40.
ICD experiences: These are questions developed and used previously to describe ICD-specific issues such as whether the participant has experienced a shock or complication and whether they have considered discussing their ICD in an advance directive or with a surrogate decision maker.
Literacy: The REALM-R is an 11-item test used to identify people with low health literacy.41
Subjective Numeracy: The subjective numeracy test identifies how comfortable people are with numbers, and determine subjective comfort with numerical preferences.42
Adoption
RE-AIM defines adoption as the absolute number, proportion, and representativeness of settings and intervention agents (people who deliver the program) who are willing to initiate a program. For this trial, we approached 7 sites and 6 agreed to participate giving an adoption rate of 86% at the site level since all 6 programs have agreed to participate. We will also assess adoption at the clinician level, where each clinician can adopt SDM each time they see a patient. The primary physician or staff who had the defibrillator discussion with the enrolled patient will complete the Clinician’s Perceptions of Patient Appropriateness for SDM survey (see Table V), reporting his/her opinion on the best therapy for the patient and an estimate of risk of unfavorable outcome. We hypothesize that physicians will be more open to SDM as patients become older and sicker. We will explore clinicians’ adoption of SDM in two ways:
-
3)
Clinician Attitudes Toward SDM: A quantitative survey will measure attitudes towards SDM at baseline and year 5, creating pre/post results for the intervention. We have developed a way of assessing clinician attitudes towards the importance of patient preferences relative to other factors such as guidelines and mortality data. These questions were used in the preliminary data project and demonstrated wide variability in the importance clinicians place on patient preferences.47 While this is not directly a measure of adoption, attitudes are an important predictor of adoption.
-
4)
Clinician’s Perceptions of Patient Appropriateness for SDM: We have developed and piloted a 6 question survey, which will be asked of clinicians in regard to each patient with whom they have a defibrillator discussion. The questions are related to: 1) the clinician’s assessment of the appropriateness of SDM for a particular patient; and 2) the clinician’s assessment of how well they think this particular patient will do with an ICD; both scored on a Likert scale (see Table V). While ICD is arguably a preference-sensitive decision, clinicians tend to think about preference sensitivity while integrating other aspects of patient clinical characteristics. We expect that this has been an important barrier to adoption of SDM in clinical practice. For example, a younger, patient with a higher risk of sudden death will find a clinician strongly encouraging an ICD while an older patient with multi-morbidity will find a clinician more comfortable engaging with SDM. This is why we organized this part of the evaluation under “adoption.” By exploring this preference on a per-patient basis, we will begin to understand how patient characteristics influence adoption of SDM and PtDAs.
Table V:
Clinician’s Perceptions of Patient Appropriateness for SDM (9 point Likert scale)
| LowL | MidMid | High | |
|---|---|---|---|
| 7) Regarding ____<<patient>>____, whom you have cared for recently, what was your recommendation regarding defibrillation? | Strongly recommended | Neither recommended for or against device | Strongly recommended against |
| 8) How was the decision about the device made? | The majority of the final decision was made by the patient. | The patient and I decided together. | The majority of the final decision was made by me. |
| 9) How easy did you feel the discussion(s) with this patient were? | Very easy | Very difficult | |
| 10) How well did the patient understand the device? | Not well | Very well | |
| 11) Based on this patient’s clinical situation, I would say: | The downsides of the device outweigh the benefits. | The downsides and benefits of the device are about equal. | The benefits of the device outweigh the downsides. |
| 12) Based on this patient’s values & opinions, I would say: | The downsides of the device outweigh the benefits. | The downsides and benefits of the device are about equal. | The benefits of the device outweigh the downsides. |
Implementation
The RE-AIM framework defines implementation as the extent to which the intervention is implemented as intended, adaptations made, and costs to deliver the program.30 Consistency of PtDA delivery will be assessed across hospitals, providers, patient subgroups, and time through the Study Coordinator Checklist and key informant interviews. The Study Coordinator Checklist will be used to assess how the intervention was implemented for each patient, with questions regarding the types of materials, the way in which they were provided, timeline, and use. In addition, factors associated with variation in implementation, such as who provides educational materials and when, will be identified. Key informant interviews will be conducted with various clinical staff at each site at baseline, after implementation of the intervention, and after study completion. Inclusion criteria include involvement in the defibrillator decision-making process or regulatory aspects of the defibrillator program. We anticipate a total of 24 to 30 participants will be interviewed. Semi-structured interview guides were developed based on the RE-AIM framework by a decision science expert, two implementation scientists, a social worker, and a health communication expert, with input from clinical staff. These interviews will provide an understanding of the current education and decision-making process in both the control phase and post intervention implementation. The goals of these interviews are to (1) identify issues, facilitators, or barriers in the effectiveness of the intervention from various staff perspectives; (2) identify strategies that may be useful in the refinement of subsequent roll-out phases and future dissemination; and (3) ensure regular, uniform use of the educational materials. Adaptations will be assessed from the intervention checklists to assess deviations from the protocol and reasons. In addition, we will conduct periodic interviews to assess frequency, timing, source, type and reasons for adaptations using interview guides from Rabin et al.43
The site-principal investigator and study coordinators will identify clinical staff for the key informant interviews. Formal e-mail invitations will be sent to each identified staff member to request an interview. Interviews will be audio recorded and summarized for analysis.
Maintenance
The RE-AIM framework defines maintenance as the continued use of a practice after a study has ended. This will be assessed primarily by seeing whether each of the 6 sites decides at the conclusion of the study to maintain, modify, or discontinue the PtDAs.
Footnotes
Disclosures/conflict of interest
Dr. Allen receives grant funding from American Heart Association, National Institutes of Health, and the Patient Centered Outcomes Research group; and consulting fees from ACI Clinical, Amgen/Cytokinetics, Boston Scientific, and Novartis.
Dr. Knoepke is supported by a career development award from the American Heart Association (18CDA34110026, Knoepke).
Dr. Tzou would like to disclose consulting, speaker honoraria, and grant support for Abbott, Boston Scientific, and Biosense Webster; consulting and speaker honoraria for Biotronik, consulting for BioSig, and speaker honoraria for Medtronic.
Dr. Gupta receives fellowship support and speaking honoraria from Boston Scientific and Medtronic. In addition, Dr. Gupta has research grants from Abbott/St. Jude Medical and Bristol-Meyers-Squibb.
Dr. Peterson receives grant funding from the National Institutes of Health and Astra Zeneca.
Dr. Matlock has received funding from the National Institutes of Health and the Patient Centered Outcomes Research Institute.
Mr. Wallace, Dr. Glasgow, Dr. Lewis, Dr. Fairclough, Ms. Helmkamp, Ms. Fitzgerald, Dr. Kramer, Dr. Varosy, Dr. Mandrola, and Dr. Brancato all have no significant disclosures or conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009--a World Society of Arrhythmia's project. Pacing and clinical electrophysiology : PACE 2011;34:1013–27. [DOI] [PubMed] [Google Scholar]
- 2.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. The New England journal of medicine 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- 3.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure.[see comment]. New England Journal of Medicine 350(21):2140–50, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. The New England journal of medicine 2002;346:877–83. [DOI] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 6.Lewis KB, Stacey D, Matlock DD. Making decisions about implantable cardioverter-defibrillators from implantation to end of life: an integrative review of patients' perspectives. The patient 2014;7:243–60. [DOI] [PubMed] [Google Scholar]
- 7.Kovacs AH, Feigofsky S, Goff JS, et al. Implantable cardioverter defibrillator implant-explant-implant case study: addressing the psychological adjustment to multiple shocks. Clinical cardiology 2006;29:274–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. The New England journal of medicine 2008;359:1009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldenberg I, Moss AJ, Hall WJ, et al. Causes and consequences of heart failure after prophylactic implantation of a defibrillator in the multicenter automatic defibrillator implantation trial II. Circulation 2006;113:2810–7. [DOI] [PubMed] [Google Scholar]
- 10.Dunbar SB, Dougherty CM, Sears SF, et al. Educational and psychological interventions to improve outcomes for recipients of implantable cardioverter defibrillators and their families: a scientific statement from the American Heart Association. Circulation 2012;126:2146–72. [DOI] [PubMed] [Google Scholar]
- 11.Mark DB, Anstrom KJ, Sun JL, et al. Quality of Life with Defibrillator Therapy or Amiodarone in Heart Failure. The New England Journal of Medicine 2008;359:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sears SF Jr., Conti JB. Psychological aspects of cardiac devices and recalls in patients with implantable cardioverter defibrillators. The American journal of cardiology 2006;98:565–7. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein N, Carlson M, Livote E, Kutner JS. Brief communication: Management of implantable cardioverter-defibrillators in hospice: A nationwide survey. Ann Intern Med 2010;152:296–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med 2004;141:835–8. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein NE, Mehta D, Siddiqui S, et al. "That's like an act of suicide" patients' attitudes toward deactivation of implantable defibrillators. Journal of general internal medicine 2008;23 Suppl 1:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein NE, Mehta D, Teitelbaum E, Bradley EH, Morrison RS. "It's like crossing a bridge" complexities preventing physicians from discussing deactivation of implantable defibrillators at the end of life. Journal of general internal medicine 2008;23 Suppl 1:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matlock DD, Nowels CT, Masoudi FA, et al. Patient and cardiologist perceptions on decision making for implantable cardioverter-defibrillators: a qualitative study. Pacing and Clinical Electrophysiology 2011;34:1634–44. [DOI] [PubMed] [Google Scholar]
- 18.Matlock DD, Nowels CT, Masoudi FA, et al. Patient and cardiologist perceptions on decision making for implantable cardioverter-defibrillators: a qualitative study. Pacing and clinical electrophysiology : PACE 2011;34:1634–44. [DOI] [PubMed] [Google Scholar]
- 19.Decision Memo for Implantable Cardioverter Defibrillators (CAG-00157R4). 2019. (Accessed 07/15/2019,
- 20.Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2014:Cd001431. [DOI] [PubMed] [Google Scholar]
- 21.Elwyn G, Scholl I, Tietbohl C, et al. "Many miles to go …": a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC medical informatics and decision making 2013;13 Suppl 2:S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ottawa decision support framework. (Accessed 06/21/2015, 2015, at http://decisionaid.Ohri.Ca/docs/develop/odsf.Pdf.)
- 23.Thompson JS, Matlock DD, McIlvennan CK, Jenkins AR, Allen LA. Development of a Decision Aid for Patients With Advanced Heart Failure Considering a Destination Therapy Left Ventricular Assist Device. JACC Heart Fail 2015;3:965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. American Journal of Public Health 1999;89:1322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Medical care 2012;50:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen LA, McIlvennan CK, Thompson JS, et al. Effectiveness of an Intervention Supporting Shared Decision Making for Destination Therapy Left Ventricular Assist Device: The DECIDE-LVAD Randomized Clinical Trial. JAMA Internal Medicine 2018;178:520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mdege ND MM, Taylor Nee Brown CA, Torgerson DJ. Systematic review of stepped wedge cluster randomized trials shows that design is particularly used to evaluate interventions during routine implementation. Clinical Epidemiology 2011;64:936–48. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins A, Jones J, Mellis BK, et al. Develop and pilot four implantable cardioverter-defibrillator (ICD) decision aids for primary prevention Society for Medical Decision Making. St. Louis: 2015. [Google Scholar]
- 29.Sepucha KR, Borkhoff CM, Lally J, et al. Establishing the effectiveness of patient decision aids: key constructs and measurement instruments. BMC medical informatics and decision making 2013;13 Suppl 2:S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glasgow RE, Klesges LM, Dzewaltowski DA, Estabrooks PA, Vogt TM. Evaluating the impact of health promotion programs: using the RE-AIM framework to form summary measures for decision making involving complex issues. Health education research 2006;21:688–94. [DOI] [PubMed] [Google Scholar]
- 31.Glasgow RE, Estabrooks PE. Pragmatic Applications of RE-AIM for Health Care Initiatives in Community and Clinical Settings. Preventing chronic disease 2018;15:E02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harden SM, Smith ML, Ory MG, Smith-Ray RL, Estabrooks PA, Glasgow RE. RE-AIM in Clinical, Community, and Corporate Settings: Perspectives, Strategies, and Recommendations to Enhance Public Health Impact. Frontiers in Public Health 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glasgow RE, Harden SM, Gaglio B, et al. RE-AIM Planning and Evaluation Framework: Adapting to New Science and Practice With a 20-Year Review. Frontiers in Public Health 2019;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sepucha KR, Levin CA, Uzogara EE, Barry MJ, O'Connor AM, Mulley AG. Developing instruments to measure the quality of decisions: early results for a set of symptom-driven decisions. Patient education and counseling 2008;73:504–10. [DOI] [PubMed] [Google Scholar]
- 35.Sepucha K, Ozanne E, Silvia K, Partridge A, Mulley AG Jr. An approach to measuring the quality of breast cancer decisions. Patient education and counseling 2007;65:261–9. [DOI] [PubMed] [Google Scholar]
- 36.O'Connor AM, Tugwell P, Wells GA, et al. Randomized trial of a portable, self-administered decision aid for postmenopausal women considering long-term preventive hormone therapy. Medical decision making : an international journal of the Society for Medical Decision Making 1998;18:295–303. [DOI] [PubMed] [Google Scholar]
- 37.O'Connor AM, Tugwell P, Wells GA, et al. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient education and counseling 1998;33:267–79. [DOI] [PubMed] [Google Scholar]
- 38.O'Connor AM. Validation of a decisional conflict scale. Medical decision making : an international journal of the Society for Medical Decision Making 1995;15:25–30. [DOI] [PubMed] [Google Scholar]
- 39.Brehaut JC, O'Connor AM, Wood TJ, et al. Validation of a decision regret scale. Medical decision making : an international journal of the Society for Medical Decision Making 2003;23:281–92. [DOI] [PubMed] [Google Scholar]
- 40.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of psychosomatic research 2002;52:69–77. [DOI] [PubMed] [Google Scholar]
- 41.Al Sayah F, Williams B, Johnson JA. Measuring health literacy in individuals with diabetes: a systematic review and evaluation of available measures. Health education & behavior : the official publication of the Society for Public Health Education 2013;40:42–55. [DOI] [PubMed] [Google Scholar]
- 42.Zikmund-Fisher BJ, Smith DM, Ubel PA, Fagerlin A. Validation of the Subjective Numeracy Scale: effects of low numeracy on comprehension of risk communications and utility elicitations. Medical decision making : an international journal of the Society for Medical Decision Making 2007;27:663–71. [DOI] [PubMed] [Google Scholar]
- 43.Rabin BA, McCreight M, Battaglia C, et al. Systematic, Multimethod Assessment of Adaptations Across Four Diverse Health Systems Interventions. Frontiers in Public Health 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson KE, Neta G, Dember LM, et al. Use of PRECIS ratings in the National Institutes of Health (NIH) Health Care Systems Research Collaboratory. Trials 2016;17:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Creswell J Qualitative Inquiry and Research Design: Choosing Among Five Approaches. Thousand Oaks, CA: Sage; 2013. [Google Scholar]
- 46.Thomas DR. A General Inductive Approach for Analyzing Qualitative Evaluation Data. American Journal of Evaluation 2006;27:237–46. [Google Scholar]
- 47.Caverly TJ, Al-Khatib SM, Kutner JS, Masoudi FA, Matlock DD. Patient preference in the decision to place implantable cardioverter-defibrillators. Archives of internal medicine 2012;172:1104–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


