Abstract
Background:
Anxiety and stress reactivity are risk factors for the development of affective disorders. However, the behavioral and neurocircuit mechanisms that potentiate maladaptive emotion regulation are poorly understood. Neuroimaging studies have implicated the amygdala and dorsolateral prefrontal cortex (DLPFC) in emotion regulation, but how anxiety and stress alter their context-specific causal circuit interactions are not known. Here we use computational intervention.
Methods:
Forty-five children (ages 10–11; 25 male) reappraised aversive stimuli during fMRI scanning. Clinical measures of anxiety and stress were acquired for each child. Drift-diffusion modeling of behavioral data and causal circuit analysis of fMRI data, with an NIMH Research Domain Criteria approach, were used to characterize latent behavioral and neurocircuit decision-making dynamics driving emotion regulation.
Results:
Children successfully reappraised negative responses to aversive stimuli. Drift-diffusion modeling revealed that, emotion regulation was characterized by increased initial bias towards positive reactivity during viewing of aversive stimuli, and increased drift rate which captured evidence accumulation during emotion evaluation. Crucially, anxiety and stress reactivity impaired latent behavioral dynamics associated with reappraisal and decision-making. Anxiety and stress increased dynamic casual influences from the right amygdala to DLPFC, but not the reverse. In contrast, DLPFC, but not amygdala, reactivity was correlated with evidence accumulation and decision-making during emotion reappraisal.
Conclusions:
Our findings provide new insights into how anxiety and stress in children impact decision-making and amygdala-DLPFC signaling during emotion regulation, and uncover latent behavioral and neurocircuit mechanisms of early risk for psychopathology.
Keywords: emotion regulation, anxiety, stress, decision-making dynamics, causal amygdala-DLPFC circuits, children
Introduction
Childhood is a vulnerable period for the development of symptoms and syndromes of anxiety, ranging from typical developmental experiences to pathological experiences (1). Nearly all affective disorders have an onset in childhood, and affective disorders are the most frequent mental disorders in children and adolescents (1,2). Cognitive and neural models of anxiety have implicated impaired emotion regulation in the etiology and maintenance of anxiety disorders (3–5). Few studies have investigated the cognitive and neural dynamics of emotion regulation in children, and how these processes are altered by anxiety and stress reactivity, which is important for the development of clinically useful biomarkers for early diagnosis and treatment implementation. Here, we investigate how anxiety and stress reactivity affect latent behavioral emotion regulation processes and dynamic causal neural circuits in a developmentally homogenous group of children.
Reappraisal relies on a frontoparietal network linking the amygdala with the prefrontal cortex (PFC) regions involved in cognitive control, including the dorsolateral PFC (DLPFC), ventrolateral PFC (VLPFC), and medial PFC (7–11). The amygdala detects and encodes emotionally salient stimuli and mediates threat learning and vigilance (12). The DLPFC, along with coordinated interactions with other PFC regions, regulates adaptive responses to emotionally negative stimuli, thereby attenuating or heightening affective response (13–17). The DLPFC is of particular interest because it plays a critical role in supporting mental representations of affective states and their manipulation in working memory, processes that are essential for emotion regulation (8,18). Brain imaging studies of emotion regulation have also identified the DLPFC as a locus of deficits in several psychiatric disorders (19). Lastly, the DLPFC has an outsized role as an intervention target in brain stimulation studies that are designed to alleviate treatment-resistant anxiety and mood disorders in adults (20–23).
Attentional Control Theory posits that excessive anxiety biases bottom-up signals from the amygdala, disrupting cognitive functions associated with the DLPFC, limiting attentional resources necessary for emotion regulation (24). This theory has not been empirically tested within a causal circuit analysis framework. As anxiety is associated with a bias towards negative interpretations, anxious individuals may not have the prerequisite abilities to alter distorted perceptions (25). However, extant behavioral studies suggest that anxious individuals perform similarly to non-anxious individuals on emotion reappraisal tasks (26, 27). Thus, observed behavioral measures (reaction time, accuracy) may not be adequate to uncover dynamic processes driving reappraisal and its modulation by anxiety and stress (28). Computational modeling approaches are needed to uncover latent behavioral and neuronal processes and their links to emotional reappraisal and reactivity in children (6, 29–31).
Here, we use computational modeling to determine how individual differences in anxiety and stress reactivity influence latent behavioral dynamics and causal bottom-up and top-down neural signaling between the amygdala and DLPFC during emotion regulation. A developmentally homogenous (ages 10–11) group of children performed an emotion reappraisal task during which they downregulated their emotional experience of aversive visual images (6, 31). Trait-like measures of anxiety (worry) and stress reactivity (temperament), which are associated with appraising situations as more stressful and threatening and are risk factors for developing anxiety disorders (32, 33) were assessed in each child. Drift-diffusion modeling (DDM) (34) was used to dissociate observed behavior into latent dynamic processes that represent distinct cognitive-affective components, including initial bias during viewing of aversive stimuli and a subsequent decision-making process during evaluation of emotional reaction. We tested the hypothesis that anxiety and stress reactivity negatively impact both these latent emotion regulation processes. As worry is cognitively demanding, we anticipated that anxiety would have broad effects on latent emotion regulation decision-making processes.
To investigate the role of causal amygdala-DLPFC circuits in emotion regulation, we used a state-space multivariate dynamical systems (MDS) model (35) to compute directional (bottom-up and top-down) causal interactions between amygdala and DLPFC in (latent) quasi-neuronal space, unconfounded by regional variations in hemodynamic response. We hypothesized that anxiety and stress in children would be associated with greater bottom-up causal interactions from the amygdala, reflecting maladaptive influence on DLPFC cognitive control mechanisms. Alternatively, greater top-down causal interactions from the DLPFC might reflect an adaptive role in ameliorating the effects of anxiety and stress. Finally, we hypothesized that the DLPFC would be sensitive to decision-making and evaluation of reactivity to aversive stimuli during emotion regulation.
Methods and Materials
Participants
A total of 76 children were recruited from a suburban public school district in northern California as part of a larger study examining health and wellness in a historically low socioeconomic status and high-adversity community. Participants were excluded from the present study if they demonstrated excessive head motion during functional magnetic resonance imaging (fMRI) acquisition (n = 12), if they failed to engage in the task or if their behavioral data could not be acquired owing to equipment failure (n = 8), or if they did not complete clinical measures (n = 11). Our final sample size included 45 participants (Figure 1A; Supplementary Materials). Participant demographics are summarized in Table S1.
Figure 1.
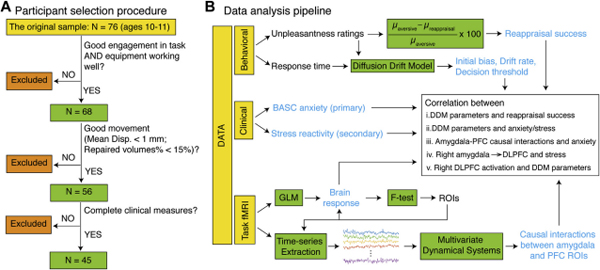
Schematic view of participant selection procedure and data analysis pipeline. (A) Children (10–11 years of age) were excluded if they failed to engage in the task or if their behavioral data could not be acquired owing to equipment failure (n = 8), demonstrated excessive motion during functional magnetic resonance imaging (fMRI) acquisition (n = 12), or did not complete dnical measures (n = 11), yielding a final sample size of 45 participants. (B) Reappraisal success and latent behavioral dynamics were computed using behavioral data. The Behavior Assessment System for Children. Second Edition (BASC) anxiety and Response to Stress Questionnaire stress reactivity subscales were the clinical measures of interest. Brain responses to task conditions were estimated using a general linear model (GLM) and an omnibus F test to identify amygdala and prefrontal cortex (PFC) regions of interest (ROIs). Time series were extracted from each ROI and were used to estimate causal interactions between amygdala and PFC ROIs using a multivariate dynamical state-space model. Finally, the relations among latent behavioral dynamics, anxiety/stress, and casual brain circuit measures were examined. DDM, drift-drffusion modeling, DLPFC, dorsolateral PFC.
Clinical measures of anxiety and stress reactivity
The anxiety subscale from the Behavioral Assessment System for Children, Second Edition (BASC) (36) was used to evaluate predominately worry-related anxiety symptoms. The involuntary response to stress subscale from the Response to Stress Questionnaire (RSQ) (37) was used to assess physiological and/or temperamental reactions to stressors (stress reactivity).
fMRI experimental design and emotion regulation task
Consistent with well-validated procedures (31), participants were trained on the experimental paradigm prior to scanning. Participants were told that they would see an instructional cue followed by an image. For ‘LOOK’ cues, participants were asked to notice their feelings towards the picture. For ‘LESS’ cues, participants were asked to reappraise aversive images by telling themselves a story to make the pictures seem less negative, or more positive (Supplementary Materials).
During fMRI acquisition, participants completed two scanning runs, each consisting of 30 experimental trials. Each trial began with a 2-second instructional cue word (‘LOOK’ or ‘LESS’), followed by an aversive or neutral image appearing for 7.5-seconds, followed by a rating scale appearing for 2-seconds (Figure 2A). Participants rated their emotional state for the following conditions: looking at neutral images and responding naturally (‘LOOK’; Neutral Condition), looking at aversive images and responding naturally (‘LOOK’; Aversive Condition), and reappraising aversive images (‘LESS’; Reappraisal Condition). There were 20 trials in each of the three task conditions: Neutral, Aversive, and Reappraisal. The rating scale consisted of numbers 1 “Okay” through 4 “Very Bad”. Participant ratings served as a behavioral index of reappraisal effectiveness. Reappraisal success was computed using the following equation: ((μaversive−μreappraisal)/μaversive)*100, with higher scores indicating better reappraisal ability.
Figure 2.
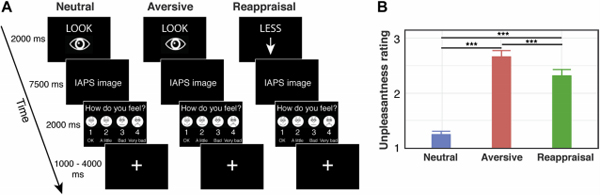
Functional magnetic resonance imaging (fMRI) experimental design, behavioral performance, and emotion regulation abilities. (A) Children 10–11 years of age were presented with cues (LOOK or LESS) followed by neutral or aversive images. They were asked to notice their feelings toward the picture when LOOK was presented, and to reappraise aversive images by telling themselves a story to make the pictures seem less negative or more positive when LESS was presented. The neutral condition consisted of viewing a neutral picture, the aversive condition consisted of viewing an aversrve picture, and the reappraisal condition consisted of reframing an aversrve picture as less negative. Following the presentation of a neutral or aversrve image, a rating scale consisting of numbers from 1 (okay) to 4 (very bad) was shown for children to indicate their emotional evaluation. (B) Children reported neutral stimuli to be significantly less unpleasant than the aversrve images, regardless of the instructional cue, and reported the aversive stimuli to be significantly less unpleasant during the reappraisal condition. ***p < .001. IAPS, International Affective Picture System.
fMRI data acquisition and pre-processing
Images were pre-processed using a standard SPM12 pipeline. For each participant, contrast images corresponding to Aversive vs. Neutral, Reappraisal vs. Neutral, and Reappraisal vs. Aversive task conditions were generated using a GLM. An omnibus F-test was used to identify brain regions showing significant group-level responses to Reappraisal vs Neutral or Aversive vs Neutral task conditions, with a height threshold p < 0.005 and FWE corrections for multiple comparisons at p < 0.01 (minimum cluster size = 87 voxels or 696 mm3). Activation peaks in bilateral amygdala, DLPFC and other PFC regions were identified and used to construct 6mm sphere ROIs for subsequent dynamic causal and latent brain-behavior analyses (Figures 1B, Supplemental Figure S2; Supplementary Materials).
Computational modeling of latent behavioral dynamics during emotion regulation
The emotion evaluation process was modeled as a drift diffusion process, in which evidence accumulates over time resulting in a decision when a decision threshold is reached. The evaluations were coded as positive (ratings of 1 or 2) or negative (ratings of 3 or 4) (Figure 3A). The initial bias represented the starting point for the drift diffusion process, and captures the initial reaction during image viewing, prior to the decision window. The drift rate parameter (δ) characterizes evidence accumulation, with higher values indicating a greater proportion of positive responses, and higher absolute values of the drift rate characterizing faster responses. For this task, drift rate indexes not only evidence accumulation, but also the decision to make an evaluative response (“Ok” to “Very Bad”) when presented with an image. The decision threshold parameter (α) captures response caution, or the degree of confidence required to conclusively evaluate the emotion, with higher values characterizing slower and more consistent responses. The decision threshold for an individual was allowed to vary by instruction – viewing (Look) versus reappraisal (Less). The drift rate and initial bias could vary by instruction (Look, Less) and stimulus type (Neutral, Aversive, and Reappraisal). The non-decision time, reflecting perceptual processes prior to evidence accumulation, for each individual was fixed across instructions and stimulus types. The initial bias for aversive reappraisal condition was constrained to lie between viewing neutral and aversive conditions. The DDM was implemented within a Bayesian inference framework using JAGS (38). Model fit was validated by comparing the posterior predictive model emotion evaluations and response times under the three different conditions to the actual values (Supplementary Materials).
Figure 3.
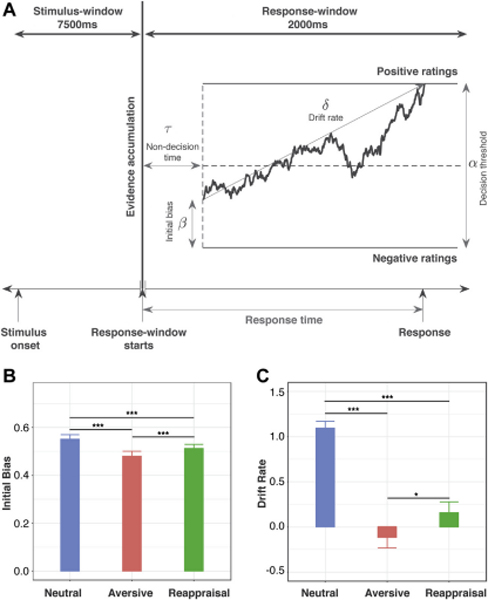
Drift-diffusion model of latent behavioral dynamics. (A) Illustration of a single trial of the drift-diffusion process, in which the random walk represents noisy evidence accumulation over time for a positive versus negative evaluation of the stimulus. When the evidence accumulation process hits either decision boundary (separated by the decision threshold), a response is made. The initial bias captures the bias toward positivity or negativity that is built up over the 7500-ms stimulus window and acts as a starting point for the random walk. The drift rate captures the rate of evidence accumulation during the 2000-ms response window. (B) Children showed significantly greater initial bias under the reappraisal than the aversive condition. Initial bias was highest in the neutral condition. (C) Children showed significantly higher drift rates under the reappraisal than the aversive condition. The drift rate was highest in the neutral condition, *p < .05; **p < .001
Computational modeling of dynamic causal interactions between the amygdala and DLPFC
We used multivariate dynamical systems (MDS), a state-space model for estimating context-dependent causal interactions between multiple brain regions while accounting for regional variation in hemodynamic responses (35). MDS has been validated using extensive simulations (35, 39, 40). See Supplementary Materials for details of the computational model and Variational Bayes solution used to infer model parameters.
Results
Behavioral performance and emotion regulation abilities
Children rated their emotional reaction to negative stimuli during Reappraisal and Aversive task conditions, and to stimuli in a Neutral task condition. Stimuli were rated as less unpleasant during the Reappraisal condition than during the Aversive condition (t(44) = −3.57, p = 0.001) (Figure 2B). Stimuli were rated as more unpleasant during the Aversive (t(44) = 13.66, p < 0.001) and Reappraisal (t(44) = 8.55, p < 0.001) conditions than the Neutral condition. Results demonstrate that children are able to modulate their negative affective ratings of aversive stimuli, with more positive evaluations reflecting a higher degree of reappraisal success.
Latent behavioral dynamics during emotion regulation
A novel implementation of DDM was used to determine initial bias, which captures the initial reaction during viewing of aversive images, prior to the decision window and the drift rate, which captures the ability to regulate emotion evaluation during the response (decision) window, and a decision threshold, which measures response caution (Figure 3A). Children showed a greater initial bias during the Reappraisal condition (0.51 ± 0.11) than during the Aversive condition (0.48 ± 0.14) (t(44) = 3.62, p < 0.001) (Figure 3B). Children also showed higher drift rates during the Reappraisal condition (0.22 ± 0.88) than during the Aversive condition (−0.12 ± 0.79) (t(44) = 2.67, p = 0.011) (Figure 3C). Children did not show a significant difference in the decision threshold parameter during the Reappraisal condition (2.83 ± 1.1) than during the Aversive condition (2.83 ± 1.08) or during the Neutral condition (2.83 ± 1.08). Results show that emotion regulation is characterized by increased positivity bias while viewing images under the reappraisal condition and higher drift rate during the decision period when evaluating their emotional reaction.
Latent behavioral dynamics during decision-making are correlated with reappraisal scores
We determined whether DDM-derived latent cognitive parameters are related to reappraisal success. Individual reappraisal scores were correlated with change in drift rate between the Reappraisal and Aversive conditions (t(42) = 12.96, r = 0.89, p < 0.001) (Figure S1). Hierarchical linear regressions included reappraisal success as the dependent variable and changes in initial bias, drift rate, and decision threshold under reappraisal versus aversive conditions as the independent variables, and revealed an excellent model fit (adjusted R2 = 0.78, F(3, 41) = 53.64, p < 0.001). Change in drift rate from the Aversive to Reappraisal conditions was the only independent variable that contributed unique variance and thus emerged as the dominant predictor (t(41) = 11.36, β = 0.99, p < 0.001; See Supplementary Results). Results suggest that success in emotion regulation is characterized by decision-making during the post-viewing, response period and not initial bias during reappraisal.
Anxiety and stress impair latent behavioral dynamics during viewing and evaluation
Anxiety scores were negatively correlated with initial bias (t(43) = −2.18, r = −0.32, p = 0.035), drift rate (t(43) = −2.36, r = −0.34, p = 0.023), and decision threshold (t(43) = −2.28, r = −0.33, p = 0.028) during Reappraisal (Figure 4A-C). Stress reactivity was negatively correlated with the decision threshold during Reappraisal (t(43) = −2.34, r = −0.34, p = 0.024; Figure 4D). Anxiety and stress reactivity were not correlated with reappraisal success (ps > 0.4). Results demonstrate that anxiety and stress impair latent behavioral dynamics of emotion regulation and that DDM captures their influence on behavior in ways that traditional response selection and reaction time measures by themselves do not.
Figure 4.
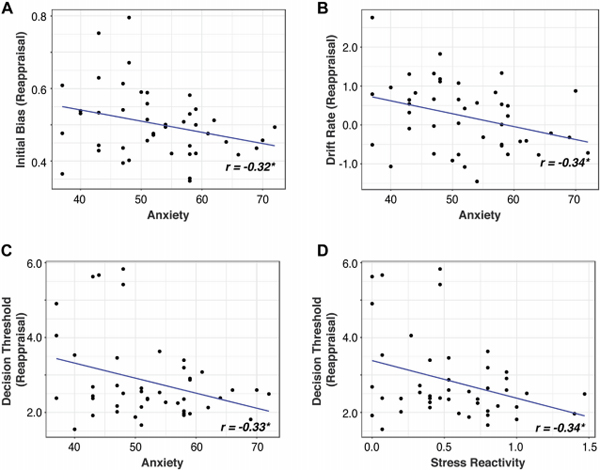
Relationship between latent behavioral dynamics and anxiety and stress. The Behavior Assessment System for Children, Second Edition anxiety scores were negatively correlated with (A) initial bias, (B) drift rate, and (C) decision threshold during reappraisal. (D) Stress reactivity was negatively correlated with the decision threshold during reappraisal.
Brain areas activated during Reappraisal and Aversive emotion processing
An omnibus F-test contrasting Reappraisal vs. Neutral or Aversive vs. Neutral conditions revealed significant activation in bilateral amygdala, DLPFC, DMPFC, VLPFC, posterior parietal cortex, posterior cingulate cortex, and occipital cortex (Figures 5, S2; Tables S2-S5), consistent with previous reports (7–10, 41) (Supplementary Results).
Figure 5.
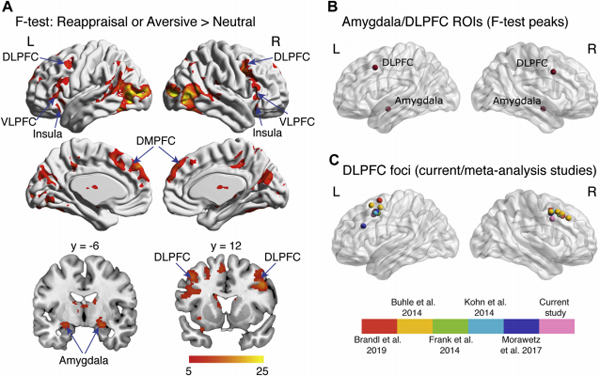
Region-of-interest (ROI) identification. (A) An omnibus F test was conducted to identify brain regions showing greater responses in either aversrve and reappraisal vs. neutral conditions. Significant activation (p < .005; minimum cluster size = 87 voxels or 696 mm3) was detected in the bilateral amygdala, bilateral dorsolateral prefrontal cortex (DLPFC). bilateral insula, bilateral caudate, bilateral dorsomedial PFC (DMPFC). left ventrolateral PFC (VLPFC). right supramarginal gyrus, bilateral precuneus, and bilateral occipital cortices. (B) ROIs were centered (6-mm radii) around activation peaks in the amygdala (Montreal Neurological Institute coordinates: [−24, −6, −14] and [22, −6, −14]) and DLPFC (Montreal Neurological Institute coordinates: [−42, 12, 44] and [40, 8, 38]). (C) Companson of DLPFC ROIs from the present study with those identified in previous meta-analysis studies of emotion regulation (7–10,41). Our DLPFC ROIs were localized to the middle frontal gyrus/inferior frontal function (55). L, left: R, right.
Anxiety increases causal interactions between the amygdala and DLPFC during emotion regulation
To identify amygdala and DLPFC regions of interest (ROIs) for causal circuit and latent brain-behavior analyses, we used task-related activation identified by the F-test, as described above, thereby avoiding biases associated with selection of regions specific to either task condition. We also conducted additional control analyses using multiple PFC regions (DLPFC, VLPFC, DMPFC, and anterior insula) identified by the F-test (Table S5), with FDR-corrections for multiple comparisons.
We then used MDS to compute task condition-specific causal circuit interactions between amygdala and DLPFC ROIs in each hemisphere. A contrast of the strength of causal interactions between the Reappraisal and Aversive conditions was used to probe how anxiety influences causal circuit interactions during emotion regulation. Both forward (amygdala → DLPFC) and backward (DLPFC → amygdala) links in both hemispheres were tested, with FDR corrections (p < 0.05) for multiple comparisons.
The strength of causal influence from right amygdala to right DLPFC (Figure 6A) during emotion regulation (Reappraisal vs. Aversive conditions) was positively correlated with BASC anxiety (t(42) = 3.28, r = 0.45, p FDR-corrected = 0.008) (Figure 6B). No such effects were observed in the reverse connectivity pattern (DLPFC → amygdala). Post-hoc analysis of left amygdala → DLPFC link with anxiety showed a marginally significant effect (t(42) = 1.84, r = 0.27, uncorrected p = 0.074).
Figure 6.
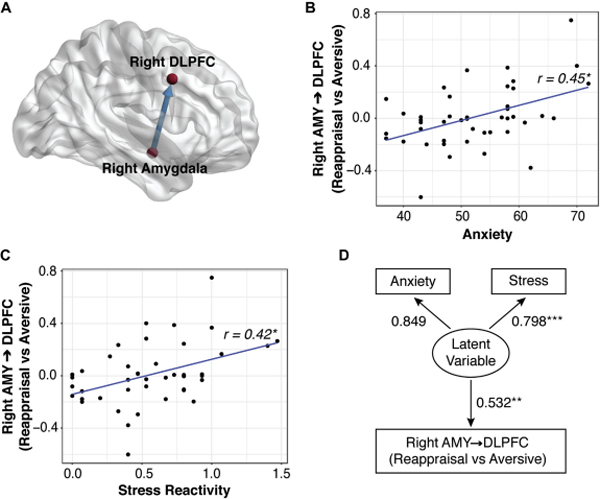
Anxiety and stress increase causal interactions between the amygdala (AMY) and dorsolateral pretrontal cortex (DLPFC) during emotion regulation. (A) The strength of causal influence from the right amygdala to the right DLPFC during emotion regulation was positively correlated with (B) Behavior Assessment System For Chidren, Second Edition anxiety and (C) Response to Stress Questionnaire stress reactivity scores. (D) Structurai equation modeling revealed that shared variance between anxiety and stress drives right amygdala → DLPFC interactions during emotion regulation.
We conducted an additional analysis using bilateral VLPFC, DMPFC, and anterior insula regions that also showed significant activation associated with emotion processing (Table S6). Again, only the right amygdala → DLPFC link was significantly correlated with anxiety (p FDR-corrected = 0.033).
Stress reactivity increases causal interactions between amygdala and DLPFC during emotion regulation
Next, we examined whether the strength of causal influence from the right amygdala → DLPFC was also correlated with stress reactivity. We found that right amygdala → DLPFC was positively correlated with stress reactivity (t(42) = 3.04, r = 0.42, p FDR-corrected = 0.016) (Figure 6C). No such effects were observed for the reverse direction (DLPFC → amygdala).
Amygdala-DLPFC causal circuit is a common pathway for anxiety and stress during emotion regulation
To disentangle the roles of anxiety and stress in their relation to amygdala → DLPFC causal interaction during emotion regulation, we conducted additional analyses using residualized anxiety, derived by regressing stress out from anxiety, and residualized stress, derived by regressing anxiety out from stress. The strength of causal influence from the right amygdala to right DLPFC was not correlated with residualized anxiety (t(42) = 1.54, r = 0.23, p = 0.13) or residualized stress (t(42) = 1.06, r = 0.16, p = 0.30). Formal structural equation modelling revealed a significant relationship between the strength of right amygdala → DLPFC causal interactions and a latent factor underlying anxiety and stress (Figure 6D). These results suggest that shared variance between anxiety and stress reactivity drives bottom-up amygdala → DLPFC signaling during emotion regulation (Supplementary Results).
Right DLPFC reactivity is correlated with latent behavioral dynamics
Finally, we investigated the role of the DLPFC in decision-making during emotion regulation. Activation in right DLPFC was correlated with difference in drift rate between Reappraisal vs Aversive conditions (t(42) = 2.48, r = 0.36, p = 0.017) (Figure 7). No such relation was observed with amygdala response or causal interactions between amygdala and right DLPFC (ps > 0.05). Results demonstrate that right DLPFC, rather than amygdala, reactivity underlies evidence accumulation and decision-making during emotion regulation.
Figure 7.
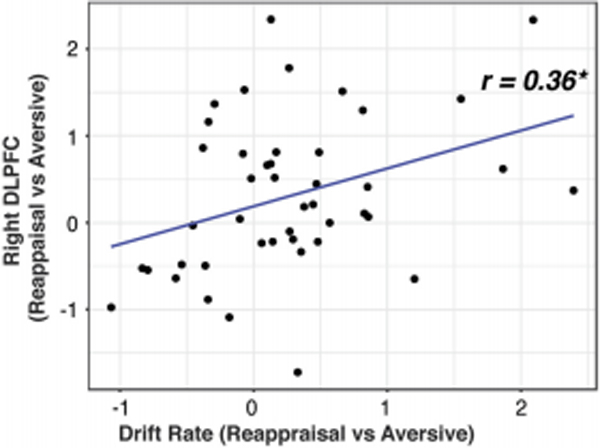
Right dorsolateral prefrontai cortex (DLPFC) reactiviy increases writh evidence accumiiation during emotion regulation. Increased activation in right DLPFC (reappraisal vs. aversive conditions) was correlated with evidence accumulation during evaluation of emotional reaction, as assessed by change in drift rate between the conditions.
Discussion
Although anxiety and stress are known risk factors for the development of affective disorders, the behavioral and neurocircuit mechanisms that potentiate maladaptive emotion regulation behaviors are poorly understood. We used computational tools to investigate how anxiety and stress impact latent decision-making processes and dynamic causal amygdala-PFC interactions during emotion regulation in children. An NIMH Research Domain Criteria (RDoC) approach (42, 43) allowed us to capture dimensional and shared representations of childhood anxiety and stress reactivity. We found that emotion regulation in children is characterized by increased initial bias during viewing of aversive stimuli and a more positive evaluation of their emotional reaction to negative stimuli during reappraisal. Anxiety impaired multiple latent behavioral dynamic measures, including initial bias and decision-making during evaluation, and stress reactivity resulted in less confident, more impulsive decision-making. State-space circuit modeling revealed that directed causal influences from amygdala to DLPFC, but not the reverse, were exacerbated by anxiety and stress reactivity during reappraisal. Furthermore, DLPFC, but not amygdala, reactivity was correlated with weak evidence accumulation during emotion reappraisal. Control analyses confirmed the specificity of our findings with respect to the amygdala, DLPFC, and their functional circuit interactions. Our findings reveal latent dynamic behavioral and neurocircuit mechanisms of early pathophysiology during childhood.
Anxiety and stress impair latent behavioral dynamics during emotion regulation
We devised a novel DDM to disentangle three latent components supporting emotion regulation: (i) initial reaction while viewing aversive stimuli, captured by changes in initial bias, (ii) decision-making during emotion evaluation, captured by changes in drift rate, and (iii) response caution, captured by changes in decision threshold (44) (Figure 3A).
DDM revealed that children with higher anxiety demonstrated lower positivity bias (initial reaction), lower drift rate (lower ability to regulate), and lower decision threshold (less consistent and controlled evaluation) during reappraisal. These results point to lower and less consistent positivity ratings under reappraisal for higher anxiety scores and highlight latent mechanisms by which anxiety impacts the reappraisal process (Figure 4). Stress reactivity effects were only observed in relation to decision threshold, indicating that reappraisal is associated with less cautious, and more impulsive decision-making in children with higher reactive stress responses. These effects were specific to latent behavioral dynamic measures as overt ratings of reappraisal were not correlated with clinical measures of anxiety or stress reactivity. Thus, DDM provided a more sensitive measure of anxiety and stress reactivity effects on behavior than traditional response times and accuracy measures (28). Our findings provide new insights into the mechanisms by which anxiety and stress impair emotion regulation in children, and suggest that anxiety and stress may not manifest overtly in behavioral performance measures of emotion regulation (45).
Anxiety and stress are correlated with causal right-hemisphere amygdala → DLPFC interactions during emotion regulation
We focused on dissociations between bottom-up and top-down causal interactions between the amygdala and DLPFC as this pathway is theorized to be critical for regulating reactivity to negative emotions (8, 18, 46). Our causal circuit analysis addressed an important gap in the literature as the effects of anxiety and stress on this core pathway have been poorly understood. Our analysis revealed that the strength of dynamic causal interaction from right amygdala to right DLPFC was enhanced by both anxiety and stress reactivity during emotion regulation. Crucially, top down influences from DLPFC to amygdala were not correlated with anxiety or stress, highlighting the specificity of bottom-up signaling from amygdala to DLPFC. These effects were also specific to the DLPFC, as amygdala interactions with insula, VMPFC, and DMPFC were not correlated with anxiety and specific to right hemisphere amygdala-DLPFC interactions. We also found that variance shared between anxiety and stress reactivity drives right amygdala to DLPFC signaling, suggesting that these interactions reflect a transdiagnostic circuit in childhood. Furthermore, asymmetric involvement of right amygdala-DLPFC circuits is consistent with right-hemispheric dominance for anxiety and anxiety-related processes observed in adults (47–51).
Our findings that both anxiety and stress are associated with bottom-up, context-dependent functional signaling from the amygdala are consistent with Attentional Control Theory which posits that excessive anxiety biases bottom-up signals from the amygdala (24). Our findings add important developmental dimensions to emerging neurobiological models of anxiety and stress close to the age at which these symptoms manifest, suggesting that early adverse experiences are associated with modulation of causal dynamics in amygdala-DLPFC circuitry rather than amygdala reactivity itself.
Right DLPFC reactivity drives evidence accumulation and decision-making during emotion regulation
We next investigated DLPFC involvement in decision-making during emotion regulation in children, given its central role in cognitive and affective control (8). Right DLPFC activity was modulated by drift rate, reflecting the efficiency of evidence accumulation and decision-making process during emotion regulation. These effects were specific to DLPFC reactivity as drift rate did not modulate amygdala activation or causal interactions between the amygdala and DLPFC. Furthermore, reaction time and response selection were not associated with DLPFC activity demonstrating that latent behavioral dynamic measures provide new insights into the role of the DLPFC that cannot be obtained from overt behavioral measures. While DDM has been widely used to investigate perceptual decision-making, to our knowledge, no previous studies have examined decision-making processes associated with emotion regulation. It is noteworthy that aversive stimuli remained perceptually unchanged while the child reappraised its negative content, revealing a novel aspect of DLPFC function based on internally generated cognitive control processes during reappraisal. These findings further highlight the role of the DLPFC in children’s decision-making during emotion regulation.
Integrative view of findings
Increased right amygdala → DLPFC connectivity with anxiety and stress raises the question of whether such signaling reflects adaptive or maladaptive function. Our findings suggest that enhanced signaling in this pathway represents “hijacking” a key cortical circuit involved in cognitive control. If it were primarily an adaptive function that signaled the need for more top-down control, we would expect that causal DLPFC → amygdala connectivity would be negatively correlated with anxiety and stress, which we did not find evidence for. More likely, this signaling is not effectual in increasing top-down control. Moreover, based on latent behavioral findings that anxiety and stress reactivity impair emotion regulation decision-making dynamics, enhanced anxiety- and stress-related causal amygdala-DLPFC signaling points to ineffective engagement of cognitive control.
Clinical implications
Anxiety disorders are generally chronic and persist into adulthood, and childhood is a critical period for their onset. Our findings may represent a promising target for understanding early pathophysiology and are in line with the recent focus on identifying early psychological and biological dimensional factors that cut across diagnoses to explain mental illness (52). Bottom-up causal amygdala-DLPFC signaling may represent a critical transdiagnostic circuit as trait-like aspects of negative emotional reactivity, including cognitive (worry) and temperamental (stress reactivity), are present in some form across all anxiety disorders and related psychopathologies (53, 54). Our characterization of trait-like anxiety and stress reactivity effects on a circumscribed functional circuit in the developing brain may provide a fruitful approach for understanding the emergence and course of early pathological anxiety and related disorders.
Conclusions
Our study provides new insights into how anxiety and stress reactivity in children impact latent decision-making processes, dynamic causal interactions between the amygdala and DLPFC, and DLPFC reactivity during emotion regulation. Over time, it is likely these dynamics would impoverish cognitive control processes anchored in the right DLPFC, rendering it a node of vulnerability and a target for intervention. Our identification of a common circuit that impacts cognitive-emotional function as children enter adolescence may contribute to improved early treatment of anxiety disorders and related psychopathology.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Software; Algorithm | Matlab R2019a | Mathworks | ||
| Software; Algorithm | SPM12 | Wellcome Trust Department of Cognitive Neurology, Lodon, UK; https://www.fil.ion.ucl.ac.uk/spm/software/spm12/ | ||
| Software; Algorithm | R | R Core Team; https://www.R-project.org/ | ||
| Software; Algorithm | JAGS | Martyn Plummer |
Acknowledgements
This work was completed in partnership with the Ravenswood City, Alum Rock, and Orchard school districts and Pure Edge, Inc., and was supported by the Lucile Packard Foundation for Children’s Health (to VC); National Institutes of Health Grants Nos. EB022907, NS086085, and MH121069 (to VM); the Stanford Maternal Child Health Research Institute through the Transdisciplinary Initiatives Program (to VM); and a Stanford Institute for Computational & Mathematical Engineering GPU computing seed grant (to VM). We thank Dr. Lang Chen for valuable input on data analysis.
Footnotes
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beesdo K, Knappe S, Pine DS (2009): Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatric Clinics. 32:483–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, Ruscio AM, Shear K, Wittchen HU (2010): Epidemiology of anxiety disorders. Curr Top Behav Neurosci. 2:21–35. [PubMed] [Google Scholar]
- 3.Cisler JM, Olatunji BO, Feldner MT, Forsyth JP (2010): Emotion Regulation and the Anxiety Disorders: An Integrative Review. J Psychopathol Behav Assess. 32:68–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etkin A (2010): Functional neuroanatomy of anxiety: a neural circuit perspective. Curr Top Behav Neurosci. 2:251–277. [DOI] [PubMed] [Google Scholar]
- 5.Sheppes G, Suri G, Gross JJ (2015): Emotion regulation and psychopathology. Annu Rev Clin Psychol. 11:379–405. [DOI] [PubMed] [Google Scholar]
- 6.Ochsner KN, Silvers JA, Buhle JT (2012): Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 1251:E1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandl F, Corbi ZLH, Bratec SM, Sorg C (2019): Cognitive reward control recruits medial and lateral frontal cortices, which are also involved in cognitive emotion regulation: A coordinate-based meta-analysis of fMRI studies. NeuroImage. 200:659–673. [DOI] [PubMed] [Google Scholar]
- 8.Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. (2014): Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. 24:2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, et al. (2014): Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci Biobehav Rev. 45:202–211. [DOI] [PubMed] [Google Scholar]
- 10.Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U (2014): Neural network of cognitive emotion regulation--an ALE meta-analysis and MACM analysis. Neuroimage. 87:345355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin RE, Ochsner KN (2016): The neuroscience of emotion regulation development: implications for education. Current opinion in behavioral sciences. 10:142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF (2012): The brain basis of emotion: a meta-analytic review. Behav Brain Sci. 35:121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, et al. (2013): A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 33:4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, et al. (2012): The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc Cogn Affect Neurosci. 7:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Grundemann J, et al. (2014): Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 81:428–437. [DOI] [PubMed] [Google Scholar]
- 16.Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin RE, et al. (2017): vlPFC-vmPFC-Amygdala Interactions Underlie Age-Related Differences in Cognitive Regulation of Emotion. Cereb Cortex. 27:3502–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silvers JA, Shu J, Hubbard AD, Weber J, Ochsner KN (2015): Concurrent and lasting effects of emotion regulation on amygdala response in adolescence and young adulthood. Dev Sci. 18:771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etkin A, Buchel C, Gross JJ (2015): The neural bases of emotion regulation. Nat Rev Neurosci. 16:693–700. [DOI] [PubMed] [Google Scholar]
- 19.Blumberger DM, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, et al. (2018): Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 391:1683–1692. [DOI] [PubMed] [Google Scholar]
- 20.Bystritsky A, Kerwin LE, Feusner JD (2009): A preliminary study of fMRI-guided rTMS in the treatment of generalized anxiety disorder: 6-month follow-up. J Clin Psychiatry. 70:431432. [DOI] [PubMed] [Google Scholar]
- 21.Machado S, Paes F, Velasques B, Teixeira S, Piedade R, Ribeiro P, et al. (2012): Is rTMS an effective therapeutic strategy that can be used to treat anxiety disorders? Neuropharmacology. 62:125–134. [DOI] [PubMed] [Google Scholar]
- 22.Zilverstand A, Parvaz MA, Goldstein RZ (2017): Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. Neuroimage. 151:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diefenbach GJ, Bragdon LB, Zertuche L, Hyatt CJ, Hallion LS, Tolin DF, et al. (2016): Repetitive transcranial magnetic stimulation for generalised anxiety disorder: a pilot randomised, double-blind, sham-controlled trial. Br J Psychiatry. 209:222–228. [DOI] [PubMed] [Google Scholar]
- 24.Eysenck MW, Derakshan N, Santos R, Calvo MG (2007): Anxiety and cognitive performance: attentional control theory. Emotion. 7:336–353. [DOI] [PubMed] [Google Scholar]
- 25.Van Bockstaele B, Verschuere B, Tibboel H, De Houwer J, Crombez G, Koster EH (2014): A review of current evidence for the causal impact of attentional bias on fear and anxiety. Psychol Bull. 140:682–721. [DOI] [PubMed] [Google Scholar]
- 26.Carthy T, Horesh N, Apter A, Edge MD, Gross JJ (2010): Emotional reactivity and cognitive regulation in anxious children. Behaviour Research and Therapy. 48:384–393. [DOI] [PubMed] [Google Scholar]
- 27.Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ (2009): Neural Bases of Social Anxiety Disorder Emotional Reactivity and Cognitive Regulation During Social and Physical Threat. Arch Gen Psychiat. 66:170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White CN, Skokin K, Carlos B, Weaver A (2016): Using decision models to decompose anxiety-related bias in threat classification. Emotion. 16:196–207. [DOI] [PubMed] [Google Scholar]
- 29.Goldin PR, McRae K, Ramel W, Gross JJ (2008): The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 63:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN (2010): The neural bases of distraction and reappraisal. J Cogn Neurosci. 22:248–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. (2004): For better or for worse: neural systems supporting the cognitive down-and up-regulation of negative emotion. Neuroimage. 23:483–499. [DOI] [PubMed] [Google Scholar]
- 32.Newman MG, Llera SJ, Erickson TM, Przeworski A, Castonguay LG (2013): Worry and generalized anxiety disorder: a review and theoretical synthesis of evidence on nature, etiology, mechanisms, and treatment. Annu Rev Clin Psychol. 9:275–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLaughlin KA, Kubzansky LD, Dunn EC, Waldinger R, Vaillant G, Koenen KC (2010): Childhood social environment, emotional reactivity to stress, and mood and anxiety disorders across the life course. Depression and anxiety. 27:1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratcliff R, McKoon G (2008): The diffusion decision model: theory and data for twochoice decision tasks. Neural Comput. 20:873–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryali S, Supekar K, Chen T, Menon V (2011): Multivariate dynamical systems models for estimating causal interactions in fMRI. Neuroimage. 54:807–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds CR (2010): Behavior assessment system for children. The Corsini encyclopedia of psychology.1–2. [Google Scholar]
- 37.Connor-Smith JK, Compas BE, Wadsworth ME, Thomsen AH, Saltzman H (2000): Responses to stress in adolescence: measurement of coping and involuntary stress responses. Journal of consulting and clinical psychology. 68:976. [PubMed] [Google Scholar]
- 38.Plummer M (2003): JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. Proceedings of the 3rd international workshop on distributed statistical computing: Vienna, Austria, pp 10. [Google Scholar]
- 39.Ryali S, Chen TW, Supekar K, Tu T, Kochalka J, Cai WD, et al. (2016): Multivariate dynamical systems-based estimation of causal brain interactions in fMRI: Group-level validation using benchmark data, neurophysiological models and human connectome project data. J Neurosci Meth. 268:142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryali S, Shih YY, Chen T, Kochalka J, Albaugh D, Fang Z, et al. (2016): Combining optogenetic stimulation and fMRI to validate a multivariate dynamical systems model for estimating causal brain interactions. Neuroimage. 132:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morawetz C, Bode S, Derntl B, Heekeren HR (2017): The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: A meta-analysis of fMRI studies. Neurosci Biobehav Rev. 72:111–128. [DOI] [PubMed] [Google Scholar]
- 42.Cuthbert BN, Kozak MJ (2013): Constructing constructs for psychopathology: The NIMH research domain criteria. [DOI] [PubMed] [Google Scholar]
- 43.Kozak MJ, Cuthbert BN (2016): The NIMH research domain criteria initiative: background, issues, and pragmatics. Psychophysiology. 53:286–297. [DOI] [PubMed] [Google Scholar]
- 44.Forstmann BU, Ratcliff R, Wagenmakers EJ (2016): Sequential Sampling Models in Cognitive Neuroscience: Advantages, Applications, and Extensions. Annu Rev Psychol. 67:641666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berggren N, Derakshan N (2013): Attentional control deficits in trait anxiety: why you see them and why you don’t. Biol Psychol. 92:440–446. [DOI] [PubMed] [Google Scholar]
- 46.Ghashghaei HT, Barbas H (2002): Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 115:12611279. [DOI] [PubMed] [Google Scholar]
- 47.Davidson RJ (2002): Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiat. 51:68–80. [DOI] [PubMed] [Google Scholar]
- 48.Hilbert K, Lueken U, Beesdo-Baum K (2014): Neural structures, functioning and connectivity in Generalized Anxiety Disorder and interaction with neuroendocrine systems: a systematic review. J Affect Disord. 158:114–126. [DOI] [PubMed] [Google Scholar]
- 49.Nitschke JB, Heller W, Palmieri PA, Miller GA (1999): Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 36:628–637. [PubMed] [Google Scholar]
- 50.Pizzagalli DA, Nitschke JB, Oakes TR, Hendrick AM, Horras KA, Larson CL, et al. (2002): Brain electrical tomography in depression: the importance of symptom severity, anxiety, and melancholic features. Biol Psychiat. 52:73–85. [DOI] [PubMed] [Google Scholar]
- 51.Sharp PB, Miller GA, Heller W (2015): Transdiagnostic dimensions of anxiety: Neural mechanisms, executive functions, and new directions. Int J Psychophysiol. 98:365–377. [DOI] [PubMed] [Google Scholar]
- 52.Insel TR, Cuthbert BN (2015): Medicine. Brain disorders? Precisely. Science. 348:499–500. [DOI] [PubMed] [Google Scholar]
- 53.Ehring T, Watkins ER (2008): Repetitive negative thinking as a transdiagnostic process. International Journal of Cognitive Therapy. 1:192–205. [Google Scholar]
- 54.McEvoy PM, Hyett MP, Ehring T, Johnson SL, Samtani S, Anderson R, et al. (2018): Transdiagnostic assessment of repetitive negative thinking and responses to positive affect: Structure and predictive utility for depression, anxiety, and mania symptoms. J Affect Disord. 232:375–384. [DOI] [PubMed] [Google Scholar]
- 55.Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, et al. (2016): The human brainnetome atlas: a new brain atlas based on connectional architecture. Cerebral cortex. 26:3508–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


