Abstract
Prolonged Grief Disorder (PGD) is a debilitating condition affecting between 7% and 10% of bereaved individuals. Past imaging and psychological studies have proposed links between PGD’s characteristic symptoms - in particular, profound yearning - and the neural reward system. We conducted a systematic review to investigate this connection. On December 19, 2019, we searched six bibliographic databases for data on the neurobiology of grief and disordered grief. We excluded studies of the hypothalamic-pituitary-adrenal (HPA) axis, animal studies, and reviews. After abstract and full-text screening, twenty-four studies were included in the final review. We found diverse evidence for the activation of several reward-related regions of the brain in PGD. The data reviewed suggest that compared to normative grief, PGD involves a differential pattern of activity in the amygdala and orbitofrontal cortex (OFC); likely differential activity in the posterior cingulate cortex (PCC), rostral or subgenual anterior cingulate cortex (ACC), and basal ganglia overall, including the nucleus accumbens (NAc); and possible differential activity in the insula. It also appears that oxytocin signaling is altered in PGD, though the exact mechanism is unclear. Our findings appear to be consistent with, though not confirmative of, conceptualizing PGD as a disorder of reward, and identify directions for future research.
Keywords: bereavement, Prolonged Grief Disorder (PGD), Complicated Grief (CG), neuroimaging, neurobiology
1. Introduction
Grief, the sorrow caused by the loss of a loved one, develops into an all-consuming, persistent, and debilitating condition for roughly 7–10% of bereaved survivors (Prigerson et al. 2009, Lundorff et al. 2017). This syndrome, referred to as Prolonged Grief Disorder (PGD), has been validated in numerous studies (Prigerson et al. 1997, Latham and Prigerson 2004, Prigerson et al. 2009), included in the International Classification of Diseases (ICD) −11 (ICD-11 for Mortality and Statistics, 2020), and approved by the Diagnostic and Statistical Manual (DSM) Steering Committee as a new mental disorder for inclusion in DSM-5-TR.1 PGD is characterized by persistent yearning for the deceased and disabling symptoms, which can include emotional numbness, a sense of disbelief about the death, identity disruption, and an inability to move forward in life following the death of the significant other (Prigerson et al. 2009). Those who develop PGD are at an increased risk of suicide (Latham and Prigerson 2004; Maciejewski et al. 2016; Prigerson et al. 1999), acute and chronic medical conditions such as heart disease, myocardial infarction, and cancer (Prigerson et al. 1997; Prigerson et al. 2009; Tofler et al. 2019), substance misuse (Prigerson et al. 1997, Parisi et al. 2019), and impaired quality of life (Silverman et al. 2000; Boelen and Prigerson 2007, Maciejewski et al. 2016, MacCallum and Bryant 2020). There is a need to develop the evidence base of mental health interventions for vulnerable bereaved individuals. Research on the neural mechanisms of PGD can inform the development of interventions targeting this syndrome; it may be especially helpful for pharmacological treatments, as medication trials have had disparate and mixed results (Bui et al. 2012).
Studies of neurobiological correlates of PGD have hypothesized that the onset and maintenance of symptoms involve neural reward system activity associated with thoughts of the deceased (O’Connor 2012). The reward system (illustrated in Figure 1) facilitates the seeking of both intrinsic and extrinsic (learned) rewards through pathways that link dopaminergic neurons in the midbrain with limbic and cortical areas, which evaluate the motivational salience of a stimulus. Evidence suggests that reward system activation is linked to the mitigation of pain sensations, putatively by generating endorphins, a class of endogenous opioids (Younger 2010). Reward signaling integrates not only dopamine but also oxytocin (OT) – a neuropeptide hormone involved in social bonding and attachment – and the endogenous opioids. Among other areas, dopamine, oxytocin, and opioid receptors converge in the nucleus accumbens (NAc), a primary node of the reward system (Trezza et al. 2011, facilitating interplay between extrinsic and intrinsic sources of reward and the formation of social bonds (Johnson et al. 2015). Reward signaling is not the only function of the NAc, which is also active during motion, avoidance, and complex behavioral decisions (Floresco 2015). Similarly, the anterior cingulate cortex (ACC), insula, and orbitofrontal cortex (OFC) activate during a wide variety of psychological processes, and are not exclusively indicative of reward signaling. We present their activity in the context of reward because the symptom profile of PGD may accord with a reward disorder and because animal microinjection studies (Bosch et al. 2016) have shown reward-related signaling cascades in the NAc to be integral to attachment and its disruption.
Figure 1:
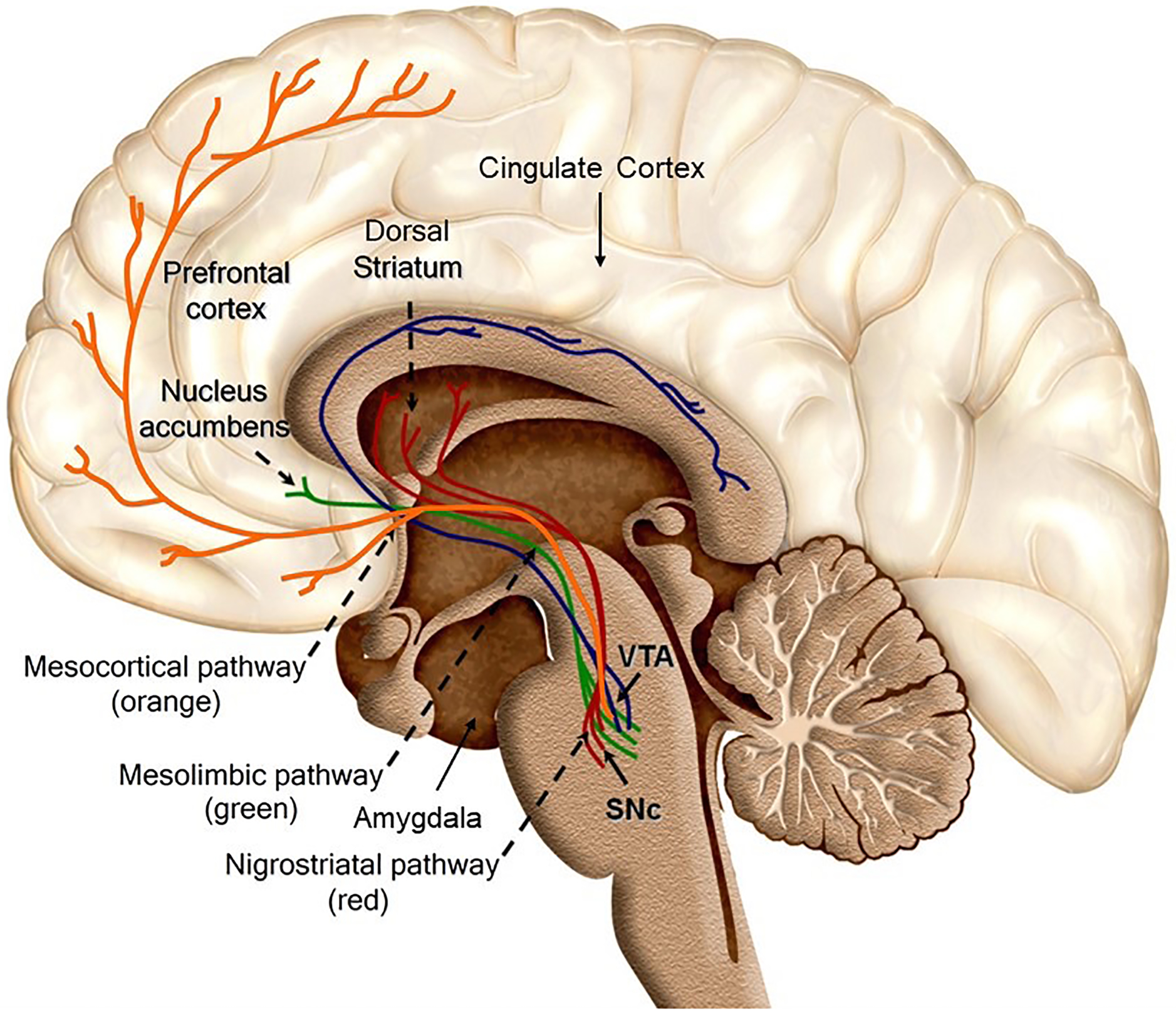
Key areas and pathways involved in neural reward signaling. (Image: JG, adapted from Wikimedia Commons. [Arias-Carrión O., Stamelou M., Murillo-Rodríguez E., Menéndez-González M., Pöppel E., 2010. Dopaminergic reward system: a short integrative review International Archives of Medicine 3, 24. doi:10.1186/1755-7682-3-24.])
Love and loss are known to affect the reward system. Attachment figures play a psychological and physiological regulatory role, providing emotional stability during times of distress and acting as safe buoys in times of uncertainty (Coan et al. 2006, Fogel et al. 2007). Neurobiologically speaking, attachment offers not only security, but also sustained reward, facilitated by OT and mediated largely by endogenous opioids (Inagaki 2018), the withdrawal of which can send an individual into a state of psychological and physiological “disorganization” when facing a significant loss (Sbarra and Hazan 2008). Site-specific agonist studies in prairie voles (Bosch et al. 2009, Bosch et al. 2016) have found that a drop in OT levels in the NAc, mediated by corticotropin-releasing factor (CRF), induces a depressive-like response in male voles separated from their partners.
PGD, then, may be associated with a persistent disruption in reward signaling. An elegant 2019 review by LeRoy et al. (2019) proposed that in order for an individual’s attachment hierarchy to be adaptively reorganized, and for the individual to reach homeostasis after a loss, the individual must pass from disorganization to a “protest” stage, in which they are first disoriented and then agitated and angered over deprivation of the deceased, to a “despair” stage, characterized by depression and lack of motivation or withdrawal. LeRoy et al. (2019) suggested that dysregulated or prolonged grief develops when bereaved individuals continue to rely on the deceased as a primary attachment figure, with motivational activity in the reward system causing them to yearn for the deceased – a core symptom of PGD. The bereaved individual experiences despair presumably because the deceased attachment figure is unavailable to fulfill the same interpersonal functions that they had previously.
That the reward system is vulnerable to such disruptions is evident from its role in addiction, which develops as overall dopamine levels in the mesolimbic reward system drop, prompting the compulsive reward-seeking typical of addiction in order to achieve equilibrium (Edwards and Koob 2010). Functional neuroimaging studies show that brain regions associated with reward and substance addiction also activate upon viewing stimuli related to an attachment figure, such as a romantic partner (Fisher et al. 2010, Younger et al. 2010). Anecdotally, Dr. Prigerson is aware of three patients with PGD who received naltrexone, a competitive opioid antagonist commonly used to treat addiction, and who all rapidly (within two days of initiation) exhibited dramatic alterations in their behavior and reduction of their PGD symptoms. Within days of starting naltrexone, each of these three bereaved family members who had met criteria for PGD became able to put away mementos and venture outside of their home in contrast to former behavior in which they had been home-bound and spent the day viewing pictures or videos of the deceased (personal communication). Taken together, this led us to hypothesize that naltrexone would make the rewarding stimulus (e.g., images of the deceased) and behaviors (e.g., ruminations of times together with the deceased) markedly less pleasurable, thereby freeing them from the hold they had and permitting an openness to explore other activities and develop relationships outside the home. Admittedly scant, these examples suggested to us that the reward system might be implicated in PGD and prompted us to review the neurobiological research in support of this scientific premise.
Behaviorally, PGD manifests in some behaviors that resemble disorders of reward. People with PGD seek out connection with the deceased (approach); this is complicated by the tendency for them to simultaneously avoid painful reminders that the person they lost is gone (avoidance). The conflict between approach and avoidance characterizes substance addiction (Breiner et al. 1999). Research by MacCallum and colleagues (2015) found that individuals with PGD show an approach bias in an approach-avoidance task, responding more quickly to grief-related stimuli than to neutral stimuli. Those with PGD also showed attentional bias to grief-related cues over neutral cues during an emotional counting Stroop (ecStroop) task study (MacCallum and Bryant 2010). MacCallum et al. (2015) suggested that negative affect in PGD is maintained by consistent attentional bias toward the loss, a process that is also observed in substance addiction (Field et al. 2009).
The notion that the neurological reward system underlies craving for the deceased gained empirical support from a 2008 fMRI study by O’Connor and colleagues (2008), who found that participants with PGD had heightened activation of the NAc when viewing grief-related stimuli. It is important to note that the seminal O’Connor et al. (2008) study used a cluster correction of 10 voxels to account for multiple comparisons across the brain. On its own, this approach has been shown to have unacceptably high false positive rates (see Eklund et al. 2016). However, subsequent neuroimaging studies observed activity in other areas involved in reward signaling, such as the dorsal anterior cingulate cortex (dACC), in those with PGD (O’Connor 2012). More recent work has suggested differences in OT signaling in PGD (Bui et al. 2019), further implicating the reward system. While the role of this system in PGD is of increasing interest, a comprehensive examination of the research in this area has been lacking. The purpose of this systematic review is to summarize the state of the science and review the literature to evaluate the extent to which neurological reward pathways are associated with PGD.
2. Methods
All procedures were conducted according to guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria (Moher et al. 2015). On December 19, 2019, a comprehensive electronic literature search was conducted using the following six databases: Medline/PubMed (legacy), Embase.com, Scopus, Web of Science, PsycInfo (Ovid), and the Cochrane Central Register of Controlled Trials. The search strategy consisted of two components, related to bereavement/grief and relevant neurological/biological phenomena, respectively. The search terms used were subject headings (MeSH, Emtree, APA’s Thesaurus of Psychological Index Terms) and/or keywords in the title, abstract and author keywords fields. Boolean Operators OR and AND were used to combine the search terms and the search strategy components. The searches in all databases except for Cochrane CENTRAL were limited to the English language. Results were not restricted by date, in order to capture all existing literature. Search results were compiled using the citation management tool EndNote and duplicates were removed following the Bramer method (2017). Covidence was utilized to facilitate article screening and selection. For a complete overview of the search strategy, see the accompanying PubMed search displayed in Appendix A.
Studies were deemed eligible for inclusion if they described neurological pathways, structures or components and their association with grief, prolonged grief, or complicated grief. Studies describing other biological associations with grief (e.g., HPA axis, cortisol activation, genetic factors, neurocognitive effects, cardiac effects) were excluded from this review. Studies that did not clearly indicate that they were focused on grief, in contrast to other outcomes such as depression or anxiety, were excluded.
After duplicates were removed, seven members of the study team independently reviewed a random selection of titles/abstracts for initial eligibility. In addition to the above inclusion and exclusion criteria, non-human animal studies were excluded, as were reviews and chapters. Each article was reviewed by two coders and any discrepancies were resolved in a consensus meeting with the entire coding team. The full text of the remaining articles was reviewed by two independent coders each to determine if each article met inclusion criteria. Discrepancies were again discussed in a consensus meeting with the entire coding team. Finally, the first author completed a spreadsheet to extract prespecified information about the sample, instruments used, methods, and results from the included articles.
Two articles were added from outside the original search. One (Bryant et al. 2020) was found when the authors conducted an informal search on March 20, 2020, using the original search terms, to ensure the review included the most up-to-date material. The second (Fernández-Alcántara et al. 2020) was identified on publication because it cited work by two of the authors. (See Figure 2 for the PRISMA flow diagram.)
Figure 2:
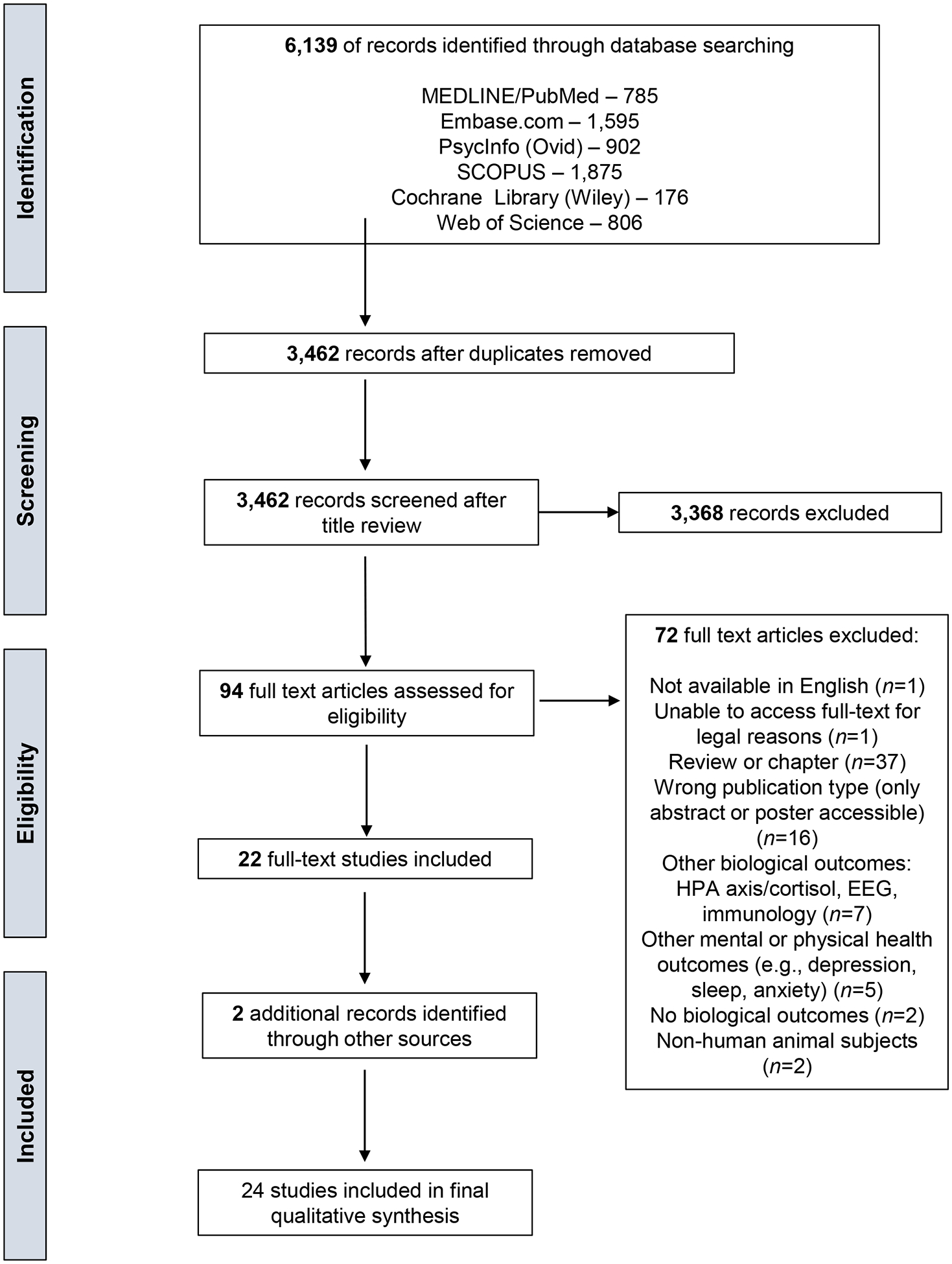
PRISMA flow diagram outlining the systematic review process.
3. Results
3.1. Overview of Utilized Assessment Tools
The 24 included studies varied in their use of the terms “prolonged grief” and “complicated grief,” as well as in their choice of instruments to assess grief and other constructs of interest. In this review, we use the term Prolonged Grief Disorder (PGD) for consistency and clarity. In all, 8 of the 24 included studies used neuroimaging to investigate PGD specifically. Eight of these studies assessed PGD caseness using the Inventory of Complicated Grief (ICG), with one (Bui et al. 2019) including ICG-diagnosed patients as well as those with “probable” PGD as assessed by the Structured Clinical Interview for Complicated Grief in analyses. All studies using the ICG applied a cutoff score of 30 for PGD diagnosis, except for two: one by Saavedra-Perez and colleagues that conducted separate analyses using cutoffs of 22 and 30 (Saavedra Perez et al. 2015); and a study by Fernández-Alcántara and colleagues (2020) that used a cutoff of score of 25 but confirmed diagnoses by a clinical interview. A 2008 neuroimaging study by O’Connor et al. (2008) diagnosed PGD using an early structured clinical interview (SCI), The Traumatic Grief Evaluation of Response to Loss (Prigerson and Jacobs 2001), for what was then termed “traumatic grief,” rather than the ICG. Bryant et al. (2020) used the Prolonged Grief-13 scale to assess PGD.
Of the non-neuroimaging studies we reviewed, three investigated the role of OT in PGD, offering indirect support for the activity of the reward system in the disorder. One study measured the effect of intranasal OT on approach and avoidance in PGD using a joystick push-pull task (Arizmendi 2018). Another study measured peripheral (bloodstream) OT levels in PGD as scored by the ICG (Bui et al. 2019). A separate study examined genomics: participants were genotyped for a polymorphism possibly affecting oxytocin binding in PGD, which was assessed using the ICG (Schiele et al. 2018).
Four studies did not explicitly investigate PGD caseness, but rather used the ICG to measure “grief severity” (Schneck et al. 2017, 2018, 2019a, 2019b) and the Impact of Events Scale (IES) to measure intrusive thoughts and avoidance – a measure of posttraumatic stress disorder (PTSD) symptoms. One study of neurobiological correlates of adult attachment style (AAS) in grief (Acosta et al. 2018) used the anxiety and avoidance subscales of the Relationship Scales Questionnaire (RSQ) to examine attachment style, and the List of Threatening Experiences Questionnaire (LTE-Q) to tally affective losses in the past five years. Three studies focused on the neurobiology of normative grief, measured by the Texas Revised Inventory of Grief (TRIG) (Huang et al. 2019); levels of inflammation (O’Connor et al. 2009); and subjective grief ratings on a scale of 1 to 10 (O’Connor et al. 2007), respectively. Another study examining individuals who had lost a pet (Freed et al. 2009) used the Texas Revised Inventory of Grief (TRIG) to measure grief intensity and the Impact of Event Scale-Revised (IES-R) to measure intrusive and avoidant thoughts as predictors of grief intensity (though it should be noted that the IES is a measure of PTSD, not PGD). Of two other papers, one examined anticipatory grief, measured by the Marwit and Meuser Caregiver Grief intensity scale (Jain et al. 2019), and the other examined grief following a romantic break-up, using the ICG (Najib et al. 2004).
Below, we summarize study findings from our review, organizing them according to the brain regions implicated. Although the wide variety of methods and samples in our studies preclude us from reaching specific conclusions about any one region, we found diverse evidence that neural structures associated with the reward system are active in PGD. The areas for which we found some evidence for differential activity in PGD are the basal ganglia, including the NAc; the amygdala; the insula; the cingulate cortex, with specific support for the subgenual anterior cingulate cortex (sgACC); and the orbitofrontal cortex (OFC). We also found evidence for differential OT signaling in PGD, some of which indicated a possible genetic component to the disorder. We recognize that the activation of these brain regions and the presence of differential OT signaling do not necessarily implicate neural reward signaling. We present these findings to evaluate the evidence in support of a connection to the reward system and to suggest potentially fruitful areas of future research. A summary of studies is found in Table 1.
Table 1.
Description of design and outcomes of studies reviewed.
| Author (Year) | Sample | Design/Method | Measures | Results | Grief Associated Regions and Mechanisms |
|---|---|---|---|---|---|
| Acosta et al. (2018) |
|
|
|
|
|
| Arizmendi et al. (2016)* |
|
|
|
|
|
| Arizmendi et al. (2018)* |
|
|
|
|
|
| Bryant et al. (2020)* |
|
|
|
|
|
| Bui et al. (2019)* |
|
|
|
|
|
| Fernández-Alcántara et al. (2020) |
|
|
|
|
|
| Freed et al. (2009) |
|
|
|
|
|
| Gundel et al. (2003) |
|
|
|
|
|
| Huang et al. (2018) |
|
|
|
|
|
| Jain et al. (2019) |
|
|
|
|
|
| McConnell et al. (2018) |
|
|
|
|
|
| Najib et al. (2004) |
|
|
|
|
|
| O’Connor et al. (2018)*⌘ |
|
|
|
|
|
| O’Connor et al. (2015)*⌘ |
|
|
|
|
|
| O’Connor et al. (2007) |
|
|
|
|
|
| O’Connor et al. (2009) |
|
|
|
|
|
| O’Connor et al. (2013) |
|
|
|
|
|
| O’Connor et al. (2008)* |
|
|
|
|
|
| Saavedra Perez et al. (2015)* |
|
|
|
|
|
| Schiele et al. (2018) |
|
|
|
|
|
| Schneck et al. (2017) |
|
|
|
|
|
| Schneck et al. (2019) |
|
|
|
|
|
| Schneck et al. (2019) |
|
|
|
|
|
| Schneck et al. (2018) |
|
|
|
|
|
includes mechanisms implicated in PGD,
abstract only
3.2. The Basal Ganglia
The basal ganglia are a multifarious group of nuclei, including the NAc, that bridge the midbrain and forebrain. They play a significant role in movement: for example, degeneration of dopaminergic neurons in the basal ganglia is responsible for disordered movements like those observed in Parkinson’s disease (Blandini et al. 2000). Most relevant to this review, however, the basal ganglia form the chief part of the mesolimbic reward pathway. Regions of the basal ganglia such as the subthalamic nucleus and the NAc process and integrate reward signals. Specifically, the basal ganglia integrate impulses from the midbrain and cortical regions to determine incentive salience (Lanciego et al. 2012, Berridge and Kringelbach 2015). In addiction, they allow an addictive substance to gain incentive salience, and diminish dopamine and opioid signaling in its absence (Koob and Volkow 2016).
In bereaved individuals, the basal ganglia may be activated when thinking of the deceased. Two included studies by Schneck and colleagues (2017, 2018) applied neural network-based pattern identification to fMRI data from bereaved participants completing sustained-attention tasks and ecStroop tasks (which measure participants’ response latency when counting words that have either emotional or neutral valence). The authors identified neural patterns of activation that were associated with mental representation of the deceased (d-MR) and the suppression of deceased-related thoughts (deceased-related selective attention, or d-SA). Schneck et al. (2017) identified an activation pattern for d-MR that involved the basal ganglia and orbitofrontal cortex. This pattern was associated with experiential avoidance, as measured by scores on the Avoidance subscale of the IES, and frequency of thoughts about the deceased during a sustained attention task, both predictors of poorer grief outcomes. Because the activation pattern predicted deceased-related thinking independent of total ICG score, Schneck et al. (2018) proposed that the pattern may be indicative of a subtype of PGD.
A second study by the same group (Schneck et al. 2019b) identified two separate patterns characteristic of avoidant grievers, as indicated by the IES: a frontotemporoparietal network for deceased-related selective attention (d-SA) and the aforementioned basal ganglia-orbitofrontal network for mental representation of the deceased (d-MR). Schneck et al. suggested that these two networks are responsible for the avoid-approach conflict characteristic of avoidant grief, with the d-SA network monitoring for cues related to the deceased in order to prevent the d-MR network activating. Paradoxically, the study found that in the absence of a cue, participants with avoidant grief, as indicated by the IES, had more frequent intrusive thoughts about the deceased than those with normative grief, suggesting that heightened d-SA primes the brain for deceased-related thoughts (Schneck et al. 2019b).
The NAc, a basal ganglia nucleus, has received considerable attention in grief research because of its role in the reward pathway. A striatal area receiving dopaminergic input from the Ventral Tegmental Area (VTA) and OT and opioid signals from other areas, the NAc modulates signaling from the midbrain to the limbic system, facilitating motivated behavior (Salamone et al. 2007). Animal studies in the prairie vole, a monogamous mammal, implicate the NAc in the systemic response to partner loss. Vole research has found that increased levels of corticotropin-releasing factor (CRF) following partner separation mediate depressive behavior, akin to Bowlby’s “despair” phase (Bowlby 1973), by causing OT levels in the NAc to drop precipitously (Bosch and Young 2017).
Human research has painted a murkier picture. An early influential study by O’Connor et al. (2008) implicated the NAc in the maintenance of PGD. The authors compared fMRI data from 11 subjects with PGD to 12 with normative grief and observed higher activity in the NAc among participants with PGD than without when viewing a picture of the deceased. The NAc co-activated with areas associated with pain, such as the insula and periaqueductal grey, prompting O’Connor et al. (2008) to suggest that “craving” for the deceased was experienced as an affliction, as in addiction. A subsequent study attempted to replicate this finding with an older sample, but found no significant difference in NAc activation between PGD and non-PGD groups (McConnell et al. 2018). The authors suggested this null finding may have been due to a small sample size or to the age of the subjects (the average age was 71 years, as opposed to 44 years in their 2008 paper), citing non-human animal research that shows a decline in certain NAc activity with advancing age (Ruegsegger et al. 2017). However, it is unknown whether the animal findings extend to humans, or whether they would be detectable in an fMRI. Furthermore, the high false positive rate associated with the statistical method used by O’Connor et al. (2008) may cast doubt upon that paper’s findings. Thus, the role of the NAc in PGD remains unclear.
3.3. Amygdala
The amygdala, a key node of the limbic system, contributes to emotional learning and the formation of desire and aversion (Clark 1995). Importantly, the amygdala sends glutamatergic projections to the NAc, which are active in determining emotional salience (Nieh et al. 2013). Because of its dual roles in avoiding unpleasant stimuli and seeking pleasure (Fernando et al. 2013), one would expect to see activation in the amygdala in response to grief stimuli in PGD.
Functional neuroimaging studies show simultaneous activation of the amygdala with reward-oriented areas of the brain in PGD. Arizmendi et al. (2016) found heightened activation in the amygdala as well as in the orbitofrontal cortex (OFC), a reward-related region discussed in greater detail below, when individuals with PGD were exposed to a deceased-related cue. In their study of pet loss, Freed et al. (2009) found that functional connectivity between the amygdala and prefrontal cortex (PFC) was indeed inversely correlated with intrusive thoughts and avoidance as measured by the IES-R. The authors proposed that altered regulation of the amygdala could lead to pathological grief symptoms, although their data showed that amygdala activity alone predicted sadness, not yearning (the latter being more uniquely characteristic of PGD). Bryant et al. (2020) found heightened activity in the amygdala in those with PGD viewing sad faces, but this activity was also observed in participants with Post-Traumatic Stress Disorder (PTSD), leading the authors to suggest that amygdala activation reflects a separate network active in multiple disorders. This finding is supported to some extent by a study conducted by Fernandéz-Alcántara et al. (2020) in which the authors found higher activity in the amygdala of participants with PGD while viewing death-valenced images. However, the latter study (Fernández-Alcántara 2020) is limited by an entirely nonbereaved control group; it is therefore unclear whether its finding of heightened amygdala activity is distinct in PGD or merely reflective of normative grief. In addition, the general role of the amygdala in strong emotion (Clark 1995) precludes interpretation specific to PGD.
Heightened amygdala activation is consistent with findings from two non-fMRI papers we reviewed. The first tested circulating catecholamine levels in participants with PGD in a trial comparing Interpersonal Therapy to Complicated Grief Treatment (O’Connor et al. 2013). The study found that higher levels of peripheral epinephrine predicted higher PGD symptomatology after therapy, regardless of intervention assignment. Circulating catecholamines are a measure of stress (O’Neill 2019), which the amygdala integrates into memory consolidation and recall through its connections with the hippocampal complex and prefrontal cortex (Roozendaal et al. 2009). The second paper, by Saavedra Pérez et al. (2015), reported cross-sectional analyses of psychometric tests and volumetric analyses of MRI data across an aging cohort of over 5,000 individuals enrolled in the Rotterdam Study (Hofman et al. 1991). Saavedra Pérez et al. (2015) tentatively suggest that aging-related synaptic loss in the amygdala (among other regions) may predispose older adult patients to PGD by reducing their ability to regulate the stress of grieving and mourning.
3.4. Insula
A wide range of functions are credited to the insula, which is known to be active in sensing social and physical pain as well as in interoception, salience, and reward anticipation (Tsurumi et al. 2014, Uddin et al. 2017). Studies linking damage to the insula with the cessation of addictive craving make it highly relevant to research on dysregulated reward signaling in other disorders (Naqvi and Bechara 2009, Droutman et al. 2015). However, perhaps due to the heterogeneity in its functions, evidence for the insula’s involvement in PGD is mixed. In a recent fMRI study comparing PGD, PTSD, and Major Depressive Disorder (MDD; the latter two disorders in nonbereaved participants), Bryant et al. (2020) found heightened insula activity across all three disorders during subliminal processing of happy faces, which were displayed in rapid succession. In their examination of grief, the two neural pattern recognition studies introduced above (Schneck et al. 2017, 2018) identified increased activity in the insula as part of the basal ganglia-orbitofrontal d-MR pattern. The pattern predicted both experiential avoidance and thoughts about the deceased (which, when intense and frequent, are symptoms of PGD), even when controlling for total ICG score (Schneck et al. 2018). On the other hand, in their 2008 study demonstrating increased activity in the NAc among those with PGD, O’Connor et al. (2008) found that participants with PGD had lower insula activity during grief elicitation than those without PGD. These studies used similar methods to elicit grief, but with distinct samples. Both neural pattern identification studies (Schneck et al. 2017, 2018) were performed with a cohort with a high proportion of suicide-bereaved participants and a high average score on the ICG (26.5; PGD caseness was not assessed), while the O’Connor et al. (2008) study included women who had lost a mother or a sister to breast cancer, half of whom met criteria for PGD based on a structured clinical interview.
3.5. Cingulate Cortex
The anterior cingulate cortex (ACC) integrates stimulus evaluation with emotional and physiological affect. It interacts directly with the reward system through a projection to the NAc, as well as with autonomic areas, including the amygdala, hypothalamus, and brainstem (Stevens et al. 2011). The evidence for differential activation of the ACC in PGD is mixed and reflects the likelihood - according to basic neurobiological studies - that the several subregions comprising the ACC perform different roles (Jin et al. 2018, Tang et al. 2019).
Two included studies revealed no evidence for differential activation of the ACC on the basis of PGD diagnosis. O’Connor et al. (2008) found no significant difference in ACC activation between subjects with PGD and without during grief elicitation, which used picture-word composites. An fMRI study by the same group (O’Connor et al. 2007) examined neural correlates of baseline parasympathetic arousal (inversely measured by respiratory sinus arrhythmia) in bereaved women and found that ACC activation was not associated with parasympathetic activity. The authors note that this decreased parasympathetic activity is a common feature of disorders such as MDD and PTSD, though participants with Axis I disorders diagnosable by a structured clinical interview were excluded in this study and participants were not screened for PGD.
The evidence concerning subregions of the ACC is more nuanced. One study testing Mentalizing Imagery Therapy (MIT), a mindfulness intervention, for anticipatory grief in dementia caregivers (Jain et al. 2019), found activation of the dorsal ACC (dACC) during grief elicitation at baseline; the authors suggested that this region is activated in social pain. However, the level of activation of the broader cingulate gyrus during grief elicitation after completing MIT predicted lower grief intensity. This may mean that both regions of the cingulate cortex are associated with anticipatory grief. An fMRI study of 8 bereaved women exhibiting normative grief (Gundel et al. 2003) found that the dACC was activated by viewing pictures of the deceased loved one. The authors suggested that dACC activation is associated with intense feelings of grief, because the dACC is known to be active in awareness of emotion (Stevens et al. 2011) and the engagement of attention (Gundel et al. 2003). Arizmendi et al. (2016) observed significantly lower recruitment of the rostral ACC (rACC) among participants with PGD during an ecStroop task. The authors suggested that those with PGD process grief stimuli via different pathways than those without PGD. As the task progressed through repeated rounds of words, they saw greater activation in the dACC in participants with PGD and in nonbereaved controls, but not in those with normative grief, which the authors proposed means those with PGD lack an adaptation common to normative grief. The authors suggested that the dACC’s role in self-awareness facilitates monitoring of error, and that while the rACC is recruited in normative grief, the dACC “turns on” in PGD to compensate for the failure of the rACC to activate (Arizmendi et al. 2016).
Subregions of the rACC may also be differentially implicated. A recent fMRI study (Bryant et al. 2020) found that compared to bereaved controls, participants with PGD had higher functional connectivity between the pregenual ACC (pgACC) and right pallidum, a region that inhibits reward processing, during subliminal processing of happy faces. However, pgACC activity in PGD did not differ from that in nonbereaved participants with PTSD or MDD. The difference in functional connectivity could therefore be indicative of reward inhibition patterns in several psychological disorders (Price and Drevets 2010). The subgenual ACC (sgACC), which is the foremost area of the rostral section, may also play a role in symptomatic yearning in PGD. sgACC activation during grief elicitation was associated with circulating levels of inflammatory markers in a study of 18 women who had lost a mother or sister to breast cancer (O’Connor et al. 2009). The authors proposed that sgACC activation is associated with a stressful grief experience, though PGD was not assessed. A later study by McConnell et al. (2018) observed no significant differences in sgACC activation between those with PGD and those without. However, in a symptom-specific analysis of all bereaved participants, the authors found that yearning (as assessed by the yearning item in the ICG) was associated with activation in the sgACC, independent of other ICG items or total score (O’Connor et al. 2018). This result is consistent with other studies that have found the sgACC to be active in ruminative thought, both in depression (Cooney et al. 2010) and in grief (Eisma et al. 2015).
The role of the posterior cingulate cortex (PCC) in autobiographical memory and pain (Nielsen et al. 2005, Leech and Sharp 2014) makes it a candidate for grief-related activity. Though we found no evidence that its activation predicts PGD, differential reward-related PCC activation may be associated with the disorder. Four functional neuroimaging studies we reviewed found heightened PCC activity during grief elicitation in normatively grieving participants (Gundel et al. 2003, O’Connor 2007, Schneck et al. 2018, Jain et al. 2019). Gündel et al. (2003) and O’Connor et al. (2007) found that grief-related words significantly induced PCC activation; similarly, in Jain et al.’s (2019) study of anticipatory grief in dementia caregivers, pictures of a terminally ill loved one induced PCC activation. Schneck et al.’s (2017) machine learning study of neural activation patterns in grief identified the PCC as part of a pattern of mental representation of the deceased. Another study observed reduced PCC activity in grieving individuals completing an ecStroop task after they had taken a mindfulness course; the authors suggested that the PCC has a role in emotional distress and painful recall in acute normative grief (Huang et al. 2019).
The PCC’s connectivity to the orbitofrontal cortex (OFC), which conducts reward processing (see next section), could implicate it in reward-related activity in grief, though a direct link to PGD is not evident. O’Connor et al. (2007) found that bereaved individuals with greater baseline arousal, as measured by respiratory sinus arrhythmia (RSA), had PCC activation when presented with cues reminding them of the deceased. The PCC activation was associated with activity in the OFC. Schneck et al. (2017) found PCC activity to be part of a larger network indicating mental representation of the deceased, which also included the OFC. This machine learning-generated pattern predicted participants’ self-reported repetitive thinking about the loss and experiential avoidance. It is possible, therefore, that the PCC is recruited in patterns of repetitive thought that contribute to the clinical presentation of PGD.
3.6. Orbitofrontal Cortex
The OFC, the dysfunction of which is implicated in MDD, performs multiple functions in reward-oriented behavior. Lesion studies in macaques suggest that the lateral OFC links representations of reward to stimulus choice, while the ventromedial OFC facilitates repeated choice based on the likelihood of reward (Noonan et al. 2012). Enhanced activity in the OFC has been observed in individuals with a drug addiction when exposed to a drug-related stimulus (Schoenbaum and Shahan 2008). As noted above in the review of evidence for activity in the PCC, the OFC has been implicated in repetitive thinking about the deceased (O’Connor et al. 2007, Schneck et al. 2017). Two additional studies have demonstrated dysregulated OFC activity in PGD. In O’Connor et al.’s (2009) aforementioned study of inflammation in grief, participants with higher levels of inflammatory markers also had higher activation in the OFC upon recalling grief-related words. More recently, Arizmendi et al. (2016) found that a PGD diagnosis was associated with heightened activation in the OFC during a grief-eliciting Stroop task. In comparing participants with PGD to those with PTSD and MDD, Bryant et al. (2020) also found higher medial OFC activity in those with PGD while viewing sad faces, suggestive of reward processing. These data suggest heightened OFC activity in individuals with PGD thinking of their deceased loved one.
3.7. Oxytocin Signaling
Studies of OT signaling were included in this review because it is the neuropeptide hormone most directly responsible for emotional disequilibrium following partner loss in animals (Hurlemann and Scheele 2016), and because variations in OT levels and binding efficiency within the brain play a primary role in attachment (King et al. 2016). Variations in OT signaling appear to impact bereaved individuals’ responses to their loss; however, the studies reviewed differed in their findings.
One study administered intranasal OT to participants with and without PGD before they engaged in an approach-avoidance task (Arizmendi 2018). Those with PGD, but not those without, were significantly slowed in their execution of the task with administration of OT, regardless of the image they were shown (a deceased loved one, a living loved one, or a stranger). The difference in reaction times suggests that OT signaling may uniquely influence the approach-avoidance calculus in PGD. An abstract included in our review reported similar results, with OT slowing participants with PGD in their responses to grief-related and neutral images (O’Connor 2018).
Attempts to measure overall levels of OT associated with PGD symptoms have been inconclusive. One study that examined circulating OT levels in bereaved participants with normative grief, PGD, and MDD (Bui et al. 2019) initially found that those with MDD had significantly lower OT levels than those without. In contrast, participants with ICG scores greater than 30, which has been used as the clinical cutoff for PGD in most studies, did not differ significantly from those with normative grief. However, a secondary analysis adding those with “probable” PGD as determined by the Structured Clinical Interview for Complicated Grief (SCI-CG) found that all participants with PGD, even those with comorbid MDD, had higher OT levels than those without. The authors proposed that these differences in levels of OT distinguish PGD, which was associated with higher levels, from MDD, which was associated with lower levels (Bui et al. 2019). However, this result must be interpreted with caution, as other research has found that peripheral OT levels may not accurately reflect central OT levels (Valstad et al. 2017).
Further studies examining genetic correlates of PGD may inform associations between OT signaling and PGD. One study in our review that used genomic sequencing in bereaved participants (Schiele et al. 2018) found that those bearing a particular single-nucleotide polymorphism (SNP) in the oxytocin receptor gene (OXTR), rs2254298, had higher ICG scores as a function of their levels of behavioral inhibition and separation anxiety. The region of OXTR affected is involved in epigenetic modification, putatively affecting GATA4 binding. The authors suggested a gene-by-environment effect, consistent with the Social Salience Hypothesis of Oxytocin: those with the SNP in question could be predisposed to develop PGD in the presence of certain environmental, experiential, or psychological factors (Schiele et al. 2018).
4. Discussion
4.1. Summary of Findings
The purpose of this review was to synthesize empirical investigations of neurobiological correlates of PGD, with a focus on the reward system in light of the growing body of research implicating its role in PGD in humans. The limited evidence synthesized in this review provides varied, though not abundant, support for the hypothesis that the reward system plays a role in the onset and maintenance of PGD symptoms. These findings suggested that reminders of the deceased are processed by the reward system, and that the protracted yearning characteristic of PGD may reflect a combination of craving (commonly observed in addiction disorders) and rumination (commonly observed in MDD and anxiety disorders).
A landmark 2008 finding by the O’Connor group of heightened NAc activity in subjects with PGD undergoing grief elicitation (O’Connor et al. 2008) spurred our interest in the role of the reward system in PGD. Notably, activation of the NAc did not emerge in other studies, including a recent attempt to replicate the O’Connor group’s 2008 study (McConnell et al. 2018). What did emerge, however, was evidence for PGD-associated activity in the amygdala and OFC; likely in the PCC, rACC, sgACC, and basal ganglia overall; and possibly in the insula. Taken together, these data suggest a subcortical-cingulate-orbitofrontal pattern of activity related to PGD. The areas identified align with neural reward processing: for example, a comprehensive review of neuroimaging studies of addiction (Suckling and Nestor 2017) reported heightened activity in the OFC and cue reactivity in the ACC, amygdala, and ventral striatum (which contains the NAc). Our review therefore offers moderate support for conceptualizing PGD as similar to other reward-oriented disorders, such as substance abuse. The results of the review also suggest that OT signaling is altered in PGD, which would be consistent with animal studies of separation (Bosch and Young 2017) and with disorders involving the reward system. However, it is unclear exactly how OT signaling is altered, or which brain regions are affected. OT signaling interacts with dopamine and endogenous opioid systems, and all three are key in the response to the loss of a loved one: OT in trust and bond formation; opioids in creating a positive experience of social contact, such as touch; and dopamine in motivating one to pursue social contact (O’Connor 2012).
Conceptualizing PGD as a reward-system-based syndrome would be consistent with observations that individuals with PGD struggle to process their loss because the deceased is a source of both pleasure and pain. The empirical support for involvement of the reward system in this review helps elucidate the “pleasure” side of this paradox, with yearning as an analogue to craving, which we would expect in a condition mediated by dysregulated reward signaling. Such dysregulation is further supported by evidence for the approach-avoidance problem central to PGD (Schneck et al. 2017, 2018): a bereaved person simultaneously yearns for the deceased while avoiding thoughts that trigger their painful grief.
4.2. Study Design Considerations, Caveats, and Future Directions
It is important to consider critically the studies we reviewed and what the nascent evidence base suggests about future directions for researchers. Studies generally had small samples [in 15 of the 18 fMRI studies included, N was less than 30, the minimum recommended for replicability by a recent fMRI study (Turner et al. 2018)], limiting statistical power. Samples were also quite heterogeneous, with different causes of death (e.g., suicide vs. cancer), relationships to the deceased, and length of time since the loss occurred, as well as variability in how PGD was assessed. Some studies also did not directly link findings to PGD symptoms, limiting the conclusions that can be drawn.
Such diversity in study design makes it difficult to interpret the variation in findings observed. It is also important to note that 5 of the 7 fMRI studies of populations with PGD involved members of one research group (O’Connor et al. 2008 Arizmendi 2018, Arizmendi 2016, McConnell 2018, O’Connor and Arizmendi 2015). This reflects the relatively primitive stage of the field and punctuates how important it is for researchers to attempt to replicate findings and pursue research in this area.
At the risk of repeating ourselves, we reiterate that these results cannot be taken as incontrovertible evidence that PGD is a disorder of reward. OT and lesion studies in voles explicitly manipulate neural mechanisms, giving strong evidence that CRF and OT cascades in the NAc dictate post-separation behavior. By contrast, causal inference from the diverse human fMRI data we present is not possible. The Schneck et al. (2017, 2018, 20_S1_Reference8919a, 2_S1_Reference90019b) studies demonstrate that a machine learning model can predict certain psychological processes (i.e., intrusive thinking, approach avoidance, etc.,) associated with PGD. Yet even here, we cannot conclude that the psychological function of these networks implicates reward processes specifically. Furthermore, the presence of reward signaling would not exclude other neural processes from important roles in PGD’s pathenogenesis or symptom maintenance. While reward is an alluring conclusion, it should not be taken to be the only one.
More research on the role of the reward system, and the NAc in particular, in the development and maintenance of PGD symptoms is needed. It would also be helpful to explore functional connectivity patterns associated with PGD, as addiction researchers have shown, for example, that differences in functional connectivity between the NAc, insula, precuneus, and prefrontal cortex (PFC) reduce the ability of the PFC to regulate craving (Chen et al. 2016). Additionally, to our knowledge, no studies have examined baseline OFC activation in PGD, which may be of interest given its disproportionately low activation during drug withdrawal and its heightened activation in addicted individuals exposed to a drug-related stimulus (Volkow and Fowler 2000).
4.3. Clinical Implications and Future Directions
The studies included in this review have implications for both pharmacological and psychosocial interventions. The existing evidence suggests that PGD’s core symptoms – yearning for and preoccupation with the deceased – may be linked to reward signals that reinforce the deceased as a source of pleasure. It is possible, therefore, that psychopharmacological interventions targeting reward signaling, such as those used to address behavioral addictions and substance use disorders (e.g., naltrexone), may be helpful for individuals with PGD. A review of neurobiological correlates of addiction (Goldstein and Volkow 2011) found that dysregulated prefrontal activity – especially in the OFC – may be due in part to changes in dopamine receptor availability elsewhere in the brain. Endogenous opioid signaling also appeared to be involved: in those addicted to cocaine and alcohol, higher mu opiate binding in the ACC and PFC persists during abstinence and may predict treatment outcome (Goldstein and Volkow 2011). Naltrexone, a competitive opioid antagonist, has been a successful treatment for alcoholism (Helstrom et al. 2016) and for broadly defined behavioral addictions, such as gambling (Mouaffak et al. 2017). Guanfacine, an alternative treatment that may work by normalizing dysregulated prefrontal signaling, may also be of interest (Fredriksson et al. 2015), as could memantine, an NMDA receptor agonist that reduced cue-induced alcohol craving in a small trial (Krupitsky et al. 2007).
However, treatment of PGD with medication is controversial for a variety of reasons. First, concerns about medicalizing PGD have been prominent in the literature; providers, researchers, theorists, and bereaved family members worry that a neurobiological conceptualization of PGD and its treatment may contribute to over-pathologizing and overmedicating the bereaved. Second, while the present review suggests a link between the neurobiological reward system and PGD, much about the etiology, onset, and maintenance of PGD remains unknown. Finally, despite the potential utility, little research has been done on potential psychopharmacological interventions for PGD. The findings from this review may serve to encourage efforts to explore pharmacological interventions, cautiously and conservatively.
Involvement of the reward system in PGD also has implications for psychosocial interventions. Psychotherapeutic approaches might include those shown to be effective in addiction research to address cravings and encourage adaptive behaviors (often in conjunction with pharmacological treatment). These include motivational interviewing (Nyamathi et al. 2011) and cognitive-behavioral strategies such as self-monitoring, goal setting, coping skill rehearsal, behavioral contracts, and positive reinforcement (Chawarski et al. 2011, Moore et al. 2013, Dugosh et al. 2016).
Although the literature examining neurobiological pathways associated with PGD is developing, adequate symptom assessment and identification of treatment moderators (e.g., symptom thresholds, type of loss, and duration and course of symptoms) remain central to the development of appropriate and effective treatments for PGD. While this review suggests there may be value in pursuing research on medications to treat the symptoms of PGD, in parallel, it is critical to be able to identify those who are most likely to benefit from a psychopharmacological treatment.
4.4. Conclusions
Studies in this systematic review suggest that activation of the neural reward system is associated with PGD. Reward signaling can be powerful, resulting in persistent and preoccupying yearning for the comforting, soothing connection to the deceased and hindering integration of the loss and adaptation. Conceptualizing PGD through a neurobiological lens can be validating for those struggling with debilitating grief reactions: it helps explain why they cannot simply “get over” their loss. However, this review also highlights the necessity for additional research in this area. Research using larger and more homogeneous samples, and consistent, psychometrically validated measures of PGD, is needed to replicate existing findings and further elucidate the role of the reward system in the neurobiology of PGD. In addition, researchers should explore adapting existing interventions that target the reward system, such as those used to treat addiction, to help those suffering from PGD.
Highlights.
Prolonged Grief Disorder (PGD) likely involves the neural reward system
Evidence associates the mesolimbic dopamine system and cortical regions with PGD.
Oxytocin function may also be altered in PGD.
Further research on disordered reward processing in PGD is needed
Acknowledgements
We thank Dr. Mary-Frances O’Connor for her clarification of details regarding her work.
Funding
This work was supported by grants from the National Cancer Institute [CA106370 (Prigerson), CA197730 (Prigerson), CA218313 (Prigerson/Lichtenthal), CA009461 (Ostroff), P30CA008748 (Thompson)]; the National Institute of Minority Health Disparities [MD007652 (Maciejewski/Prigerson)]; the National Institute of Nursing Research [NR018693 (Prigerson/Epstein)]; the National Institute of Mental Health grant [MH095378 (Lichtenthal/Prigerson)]; the National Institute on Aging [AG049666 (Reid/Prigerson)]; and the National Center for Advancing Translation Sciences [UL1 TR002384 (Imperato-McGinley)]. The funding sources did not play a role in the conception or conduct of this research or the writing of this paper.
Abbreviations:
- AAP
Adult Attachment Projective Picture System
- AAS
Adult Attachment Scale
- ASA-27
Adult Separation Anxiety Scale
- ANOVA
Analysis of variance
- AAT
Approach Avoid Task
- BDI BDI II
Beck Depression Inventory
- BI
Behavioral Inhibition
- BC
Breast Cancer
- BSI
Brief Symptom Inventory
- CG
Complicated grief
- CRFR2
Corticotropin-releasing factor receptor 2
- d-SA
deceased-related selective attention
- DERS
Difficulties in Emotional Regulation Scale
- dACC
dorsal Anterior Cingulate Cortex
- DLPFC
Dorsolateral prefrontal cortex
- ECG
Electrocardiograph
- eCStroop
Emotional Counting Stroop
- FEW
Family-wise error correction
- FFMQ
Five Factor Mindfulness Questionnaire
- FST
Forced Swim Test
- fMRI
Functional Magnetic Resonance Imaging
- GAD-7
Generalized Anxiety Disorder-7
- IES-R
Impact of Event Scale-Revised
- IL-1β
Interleukin 1 beta
- IU
International Unit
- INT OT
Intranasal Oxytocin
- ICG
Inventory of Complicated Grief
- LDST
Letter-Digit Substitution Test
- LTE-Q
List of Threatening Experiences Questionnaire
- MIT
Mentalizing Imagery Therapy
- MBCT
Mindfulness-Based Cognitive Therapy
- MMSE
Mini Mental State Exam
- MAO-A
Monoamine Oxidase A
- MMQ
Multidimensional Mood Questionnaire
- non-CG
Non complicated grief
- NAcc
Nucleus Accumbens
- OFC
Orbitofrontal cortex
- OT
Oxytocin
- OXTR
Oxytocin Receptor
- PHG
Parahippocampal gyrus
- PVN
Paraventricular nucleus
- PHQ
Patient Health Questionnaire
- PAG
Periaqueductal gray
- PCC
Posterior cingulate cortex
- PMR
Progressive Muscle Relaxation
- QIDS
Quick Inventory of Depressive Symptomatology
- RSQ
Relationship Scales Questionnaire
- RSA
Respiratory Sinus Arrhythmia
- RSRI
Retrospective Self-Report of Inhibition
- rACC
rostral Anterior Cingulate Cortex
- SART
Selective Attention Related Thoughts
- SA
Seperation Anxiety
- K-22
Short Form of Social Support Questionnaire
- sTNFrII
Soluble tumor necrosis factor receptor type II
- STAI
State Trait Anxiety Index
- SCID
Structural Clinical Interview for DSM-IV
- SCI-CG
Structured Clinical Interview for Complicated Grief
- sACC
subgenual Anterior Cingulate Cortex
- SCL-90
Symptom Checklist-90
- TRIG
Texas Revised Inventory of Grief
Appendix A. MeSH Search Terms Used in Systematic Review
(“Bereavement”[Mesh] OR bereavement[tiab] OR bereaved[tiab] OR bereave[tiab] OR bereaving[tiab] OR grief[tiab] OR grieved[tiab] OR grieve[tiab] OR grieving[tiab]) AND (“Gene Expression”[MeSH] OR “Gene Expression Regulation”[MeSH] OR “Biomarkers”[MeSH] OR “Inflammation”[MeSH] OR “Cytokines”[Mesh] OR “Biogenic Amines”[Mesh] OR “Receptors, Biogenic Amine”[Mesh] OR “Catecholamines”[Mesh] OR “Receptors, Catecholamine”[Mesh] OR “Neuropeptides”[Mesh] OR “Oxytocin”[MeSH] OR “Hydrocortisone”[MeSH] OR “hydrocortisone receptor” [Supplementary Concept] OR “Blood Cells”[Mesh] OR “Cerebrovascular Circulation”[Mesh] OR “Synaptic Transmission”[Mesh] OR “Nerve Net”[Mesh] OR “Neural Pathways”[MeSH] OR “Neurons”[Mesh] OR “Neurotransmitter Agents”[Mesh] OR “Receptors, Neurotransmitter”[Mesh] OR “Brain”[Mesh] OR “Neurosciences”[Mesh] OR “Neuroimaging”[MeSH] OR “Electroencephalography”[Mesh] OR “Tomography”[Mesh] OR “Radionuclide Imaging”[Mesh] OR psychobiology[tiab] OR psychobiological[tiab] OR psychobiologic[tiab] OR neuroanatomy[tiab] OR neuroanatomical[tiab] OR neuroanatomic[tiab] OR neurobiology[tiab] OR neurobiological[tiab] OR neurobiologic[tiab] OR neurophysiology[tiab] OR neurophysiological[tiab] OR neurochemistry[tiab] OR neurochemical[tiab] OR neuroendocrine[tiab] OR neuroimmunomodulation[tiab] OR activation[tiab] OR psychoneuroimmunology[tiab] OR psychoneuroimmunological[tiab] OR psychoneuroimmunologic[tiab] OR biomarker[tiab] OR biomarkers[tiab] OR biological marker[tiab] OR biological markers[tiab] OR correlate[tiab] OR correlates[tiab] OR biogenic[tiab] OR biochemistry[tiab] OR biochemical[tiab] OR immunology[tiab] OR immunological[tiab] OR immunologic[tiab] OR immune[tiab] OR immunomodulation[tiab] OR inflammation[tiab] OR inflammatory[tiab] OR gene[tiab] OR genes[tiab] OR genotype[tiab] OR genotypes[tiab] OR phenotype[tiab] OR phenotypes[tiab] OR endocrine[tiab] OR endocrinology[tiab] OR endocrinological[tiab] OR endocrinologic[tiab] OR lipid[tiab] OR lipids[tiab] OR killer cell[tiab] OR killer cells[tiab] OR blood cell[tiab] OR blood cells[tiab] OR plasma[tiab] OR serum[tiab] OR platelet[tiab] OR platelets[tiab] OR erythrocyte[tiab] OR erythrocytes[tiab] OR leukocyte[tiab] OR leukocytes[tiab] OR lymphocyte[tiab] OR lymphocytes[tiab] OR neutrophil[tiab] OR neutrophils[tiab] OR serotonin[tiab] OR serotonergic[tiab] OR oxytocin[tiab] OR oxytocinergic[tiab] OR catecholamine[tiab] OR catecholamines[tiab] OR dopamine[tiab] OR acetylcholine[tiab] OR cortisol[tiab] OR cellular[tiab] OR intracellular[tiab] OR intercellular[tiab] OR protein[tiab] OR proteins[tiab] OR cytokine[tiab] OR cytokines[tiab] OR interleukin[tiab] OR interleukins[tiab] OR interferon[tiab] OR interferons[tiab] OR tumor necrosis factor[tiab] OR forebrain[tiab] OR brainstem[tiab] OR brain stem[tiab] OR nucleus accumbens[tiab] OR accumbens nucleus[tiab] OR hypothalamus[tiab] OR hypothalamic[tiab] OR thalamus[tiab] OR thalamic[tiab] OR cerebrum[tiab] OR cerebellum[tiab] OR hippocampus[tiab] OR hippocampal[tiab] OR parahippocampus[tiab] OR parahippocampal[tiab] OR cortex[tiab] OR cortical[tiab] OR subcortical[tiab] OR cingulum[tiab] OR cingulate[tiab] OR gyrus[tiab] OR gyri[tiab] OR lobe[tiab] OR lobes[tiab] OR frontal[tiab] OR fronto[tiab] OR prefrontal[tiab] OR gray matter[tiab] OR white matter[tiab] OR cerebral blood flow[tiab] OR cerebral circulation[tiab] OR hemisphere[tiab] OR hemispheres[tiab] OR caudate[tiab] OR amygdala[tiab] OR putamen[tiab] OR ganglion[tiab] OR ganglia[tiab] OR circuit[tiab] OR circuits[tiab] OR circuitry[tiab] OR pituitary[tiab] OR sympathetic[tiab] OR parasympathetic[tiab] OR neural[tiab] OR synapse[tiab] OR synapses[tiab] OR synaptic[tiab] OR neuronal[tiab] OR neurotransmitter[tiab] OR neurotransmitters[tiab] OR neurotransmission[tiab] OR neuropeptide[tiab] OR neuropeptides[tiab] OR neuroscience[tiab] OR tomography[tiab] OR tomogram[tiab] OR tomograms[tiab] OR magnetic resonance[tiab] OR mri[tiab] OR fmri[tiab] OR CT[tiab] OR SPECT[tiab] OR positron emission[tiab] OR neuroimaging[tiab] OR brain mapping[tiab] OR eeg[tiab] OR electroencephalography[tiab] OR electroencephalogram[tiab] OR electroencephalograms[tiab]) AND (English[lang])
Footnotes
Conflicts of Interest Statement
Declarations of interest: none.
Reference List
- Acosta H, Jansen A, Nuscheler B, Kircher T, 2018. A voxel-based morphometry study on adult attachment style and affective loss. Neuroscience 392, 219–229. doi: 10.1016/j.neuroscience.2018.06.045. [DOI] [PubMed] [Google Scholar]
- Arias-Carrión O, Stamelou M, Murillo-Rodríguez E, Menéndez-González M, Pöppel E, 2010. Dopaminergic reward system: a short integrative review International Archives of Medicine 3, 24. doi: 10.1186/1755-7682-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arizmendi B, 2018. The effect of intranasal oxytocin on neural functioning in widow(er)s. Dissertation Abstracts International: Section B: The Sciences and Engineering. [Google Scholar]
- Arizmendi B, Kaszniak AW, O’Connor MF, 2016. Disrupted prefrontal activity during emotion processing in complicated grief: An fMRI investigation. NeuroImage 124, 968–976. doi: 10.1016/j.neuroimage.2015.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML, 2015. Pleasure systems in the brain. Neuron 86, 646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandini F, Nappi G, Tassorelli C, Martignoni E, 2000. Functional changes of the basal ganglia circuitry in Parkinson’s disease. Prog Neurobio 62, 63–68. doi: 10.1016/s0301-0082(99)00067-2. [DOI] [PubMed] [Google Scholar]
- Boelen PA, Prigerson HG, 2007. The influence of symptoms of prolonged grief disorder, depression, and anxiety on quality of life among bereaved adults: a prospective study. Eur Arch Psychiatry Clin Neurosci. 257, 444–52. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Dabrowska J, Modi ME, Johnson ZV, Keebaugh AC, Barrett CE, Ahern TH, Guo J, Grinevich V, Rainnie DG, Neumann ID, Young LJ, 2016. Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology 64: 66–78. doi: 10.1016/j.psyneuen.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ, 2009. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology 34(6): 1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Young LJ, 2017. Oxytocin and Social Relationships: From Attachment to Bond Disruption In: Hurlemann R, Grinevich V (eds) Behavioral Pharmacology of Neuropeptides: Oxytocin. Current Topics in Behavioral Neurosciences, vol 35 Springer, Cham. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J, 1973. Attachment and Loss, volume II: Separation Anxiety and Anger. Basic Books. [Google Scholar]
- Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T, 2017. De-duplication of database search results for systematic reviews in EndNote [published correction appears in J Med Libr Assoc 105, 1]. J Med Libr Assoc 104, 240–243. doi: 10.3163/1536-5050.104.3.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, Andrew E, Korgaonkar MS, 2020. Distinct neural mechanisms of emotional processing in prolonged grief disorder. Psychological Medicine 2020, 1–9. doi: 10.1017/S0033291719003507. [DOI] [PubMed] [Google Scholar]
- Bui E, Nadal-Vicens M, Simon NM, 2012. Pharmacological approaches to the treatment of complicated grief: rationale and a brief review of the literature. Dialogues Clin Neurosci 14, 149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui E, Hellberg SN, Hoeppner SS, Rosencrans P, Young A, Ross RA et al. , 2019. Circulating levels of oxytocin may be elevated in complicated grief: A pilot study. European Journal of Psychotraumatology 10, 1646603. doi: 10.1080/20008198.2019.1646603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarski MC, Zhou W, Schottenfeld RS, 2011. Behavioral drug and HIV risk reduction counseling (BDRC) in MMT programs in Wuhan, China: A pilot randomized clinical trial. Drug Alcohol Depend 115, 237–239. doi: 10.1016/j.drugalcdep.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Yen JY, Wang PW, Liu GC, Yen CF, Ko CH, 2016. Altered Functional Connectivity of the Insula and Nucleus Accumbens in Internet Gaming Disorder: A Resting State fMRI Study. European Addiction Research 22, 192–200. doi: 10.1159/000440716. [DOI] [PubMed] [Google Scholar]
- Clark GA, 1995. Emotional learning: Fear and loathing in the amygdala. Current Biology 5, 246–248. doi: 10.1016/S0960-9822(95)00050-9. [DOI] [PubMed] [Google Scholar]
- Coan JA, Schaefer HS, Davidson RJ, 2006. Lending a Hand: Social Regulation of the Neural Response to Threat. Psychological Science 17, 1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Joormann J,, Eugène F, Dennis EL, Gotlib IH, 2010. Neural correlates of rumination in depression. Cogn Affect Behav Neurosci 10, 77–100. doi: 10.3758/CABN.10.4.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droutman V, Read SJ, Bechara A, 2015. Revisiting the role of the insula in addiction. Trends Cogn Sci 19, 414–420. doi: 10.1016/j.tics.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugosh K, Abraham A, Seymour B, McLoyd K, Chalk M, Festinger D, 2016. A Systematic Review on the Use of Psychosocial Interventions in Conjunction With Medications for the Treatment of Opioid Addiction. J Addict Med 10, 93–103. doi: 10.1097/ADM.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H 2016. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U.S.A 113, 7900–7905. 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Koob GF, 2010. Neurobiology of dysregulated motivational systems in drug addiction. Future Neurol. 5, 393–401. doi: 10.2217/fnl.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisma MC, Schut HAW, Stroebe MS, Boelen PA, van den Bout J, Stroebe W, 2015. Adaptive and maladaptive rumination after loss: A three‐wave longitudinal study. Br J Clin Psychol 54, 163–180. doi: 10.1111/bjc.12067 [DOI] [PubMed] [Google Scholar]
- Fernández-Alcántara M, Verdejo-Román J, Cruz-Quintana F, Pérez-García M, Catena-Martínez A, Fernández-Ávalos MI et al. , 2020. Increased amygdala activations during the emotional experience of death-related pictures in complicated grief: an fMRI study. Journal of Clinical Medicine 9, 851. doi: 10.3390/jcm9030851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando AB, Murray JE, Milton AL, 2013. The amygdala: securing pleasure and avoiding pain. Front Behav Neurosci 7, 190. doi: 10.3389/fnbeh.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Munafò MR, Franken IH, 2009. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychological bulletin 135, 589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher HE, Brown LL, Aron A, Strong G, Mashek D, 2010. Reward, Addiction, and Emotion Regulation Systems Associated With Rejection in Love. Journal of Neurophysiology 104, 51–60. doi: 10.1152/jn.00784.2009. [DOI] [PubMed] [Google Scholar]
- Floresco SB, 2015. The nucleus accumbens: an interface between cognition, emotion, and action. Annual Review of Psychology 66: 25–52. doi: 10.1146/annurev-psych-010213-115159 [DOI] [PubMed] [Google Scholar]
- Fogel A, Garvey A, 2007. Alive communication. Infant Behavior and Development 30, 251–257. doi: 10.1016/j.infbeh.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Fredriksson I, Jayaram-Lindström N, Wirf M et al. , 2015. Evaluation of guanfacine as a potential medication for alcohol use disorder in long-term drinking rats: behavioral and electrophysiological findings. Neuropsychopharmacology 40, 1130–1140. doi: 10.1038/npp.2014.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed PJ, Yanagihara TK, Hirsch J, Mann JJ, 2009. Neural mechanisms of grief regulation. Biological Psychiatry 66, 33–40. doi: 10.1016/j.biopsych.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2011. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12, 652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gündel H, O’Connor MF, Littrell L, Fort C, Lane RD, 2003. Functional neuroanatomy of grief: An FMRI study. American Journal of Psychiatry 160, 1946–1953. doi: 10.1176/appi.ajp.160.11.1946. [DOI] [PubMed] [Google Scholar]
- Helstrom AW, Blow FC, Slaymaker V, Kranzler HR, Leong S, Oslin D, 2016. Reductions in Alcohol Craving Following Naltrexone Treatment for Heavy Drinking. Alcohol and Alcoholism 51, 562–566. doi: 10.1093/alcalc/agw038. [DOI] [PubMed] [Google Scholar]
- Hofman A, Grobbee DE, de Jong PT, van den Ouweland FA, 1991. Determinants of disease and disability in the elderly: the Rotterdam Elderly Study. European Journal of Epidemiology 7, 403–422. doi: 10.1007/bf00145007. [DOI] [PubMed] [Google Scholar]
- Huang FY, Hsu AL, Hsu LM, Tsai JS, Huang CM, Chao YP et al. , 2019. Mindfulness improves emotion regulation and executive control on bereaved individuals: an fMRI study. Frontiers in Human Neuroscience 12, 541. doi: 10.3389/fnhum.2018.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R and Scheele D, 2016. Dissecting the Role of Oxytocin in the Formation and Loss of Social Relationships. Biological Psychiatry 79, 185–193. doi: 10.1016/j.biopsych.2015.05.013. [DOI] [PubMed] [Google Scholar]
- ICD-11 for Mortality and Statistics. 6B42 Prolonged Grief Disorder. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/1183832314. [Accessed March 23, 2020]
- Inagaki TK, 2018. Opioids and Social Connection. Current Directions in Psychological Science 27, 85–90. doi: 10.1177/0963721417735531. [DOI] [Google Scholar]
- Jain FA, Connolly CG, Christov-Moore L, Leuchter AF, Abrams M, Ben-Yelles RW et al. , 2019. Grief, mindfulness and neural predictors of improvement in family dementia caregivers. Frontiers in Human Neuroscience 13, 155. doi: 10.3389/fnhum.2019.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin F, Zheng P, Liu H, Guo H, Sun Z, 2018. Functional and anatomical connectivity-based parcellation of human cingulate cortex. Brain Behav 8, e01070. doi: 10.1002/brb3.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ, 2015. Neurobiological mechanisms of social attachment and pair bonding. Curr Opin Behav Sci. 3, 38–44. doi: 10.1016/j.cobeha.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LB, Walum H, Inoue K, Eyrich NW, Young LJ, 2016. Variation in the Oxytocin Receptor Gene Predicts Brain Region-Specific Expression and Social Attachment. Biol Psychiatry 80,160–169. doi: 10.1016/j.biopsych.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2016. Neurobiology of addiction: a neurocircuitry analysis. The Lancet: Psychiatry 3, 760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky EM, Neznanova O, Masalov D, Burakov AM, Didenko T, Romanova T, 2007. Effect of Memantine on Cue-Induced Alcohol Craving in Recovering Alcohol-Dependent Patients. American Journal of Psychiatry 164, 519–523. doi: 10.1176/ajp.2007.164.3.519 [DOI] [PubMed] [Google Scholar]
- Lanciego JL, Luquin N, Obeso JA, 2012. Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med 2, a009621. doi: 10.1101/cshperspect.a009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham AE, Prigerson HG, 2004. Suicidality and bereavement: complicated grief as psychiatric disorder presenting greatest risk for suicidality. Suicide Life Threat Behav. 34, 350–362. doi: 10.1521/suli.34.4.350.53737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ, 2014. The role of the posterior cingulate cortex in cognition and disease. Brain 137, 12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoy AS, Knee CR, Derrick JL, Fagundes CP, 2019. Implications for Reward Processing in Differential Responses to Loss: Impacts on Attachment Hierarchy Reorganization. Personality and Social Psychology Review 23, 391–405. doi: 10.1177/1088868319853895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundorff M, Holmgren H, Zachariae R, Farver-Vestergaard I, O’Connor M, 2017. Prevalence of prolonged grief disorder in adult bereavement: A systematic review and meta-analysis. J Affect Disord 212, 138–149. doi: 10.1016/j.jad.2017.01.030. [DOI] [PubMed] [Google Scholar]
- MacCallum F, Bryant RA, 2010. Attentional bias in complicated grief. J Affect Disord 125, 1–3. doi: 10.1016/j.jad.2010.01.070. [DOI] [PubMed] [Google Scholar]
- MacCallum F, Bryant RA, 2020. A Network Approach to Understanding Quality of Life Impairments in Prolonged Grief Disorder. J Trauma Stress 33, 106–115. [DOI] [PubMed] [Google Scholar]
- MacCallum F, Sawday S, Rinck M, Bryant RA, 2015. The push and pull of grief: Approach and avoidance in bereavement. J Behav Ther Exp Psychiatry 48, 105–109. doi: 10.1016/j.jbtep.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Maciejewski PK, Maercker A, Boelen PA, Prigerson HG, 2016. “Prolonged grief disorder” and “persistent complex bereavement disorder”, but not “complicated grief”, are one and the same diagnostic entity: an analysis of data from the Yale Bereavement Study. World Psychiatry 15, 266–275. doi: 10.1002/wps.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell MH, Killgore WD, O’Connor MF, 2018. Yearning predicts subgenual anterior cingulate activity in bereaved individuals. Heliyon 4, e00852. doi: 10.1016/j.heliyon.2018.e00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M et al. , 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4, 1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Fazzino T, Barry DT et al. , 2013. The Recovery Line: A pilot trial of automated, telephone-based treatment for continued drug use in methadone maintenance. J Subst Abuse Treat 45, 63–69. doi: 10.1016/j.jsat.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouaffak F, Leite C, Hamzaoui S, Benyamina A, Laqueille X, Kebir O, 2017. Naltrexone in the treatment of broadly defined behavioral addictions: a review and meta-analysis of randomized controlled trials. European Addiction Research 23, 204–210. doi: 10.1159/000480539. [DOI] [PubMed] [Google Scholar]
- Najib A, Lorberbaum JP, Kose S, Bohning DE, George MS, 2004. Regional brain activity in women grieving a romantic relationship breakup. American Journal of Psychiatry 161, 12:2245–2256. doi: 10.1176/appi.ajp.161.12.2245. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A, 2009. The hidden island of addiction: the insula. Trends Neurosci 32, 56–57. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieh EH, Kim SY, Namburi P, Tye KM, 2013. Optogenetic dissection of neural circuits underlying emotional valence and motivated behaviors. Brain Res 1511, 73–92. doi: 10.1016/j.brainres.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen FA, Balslev D, Hansen LK, 2005. Mining the posterior cingulate: Segregation between memory and pain components. NeuroImage 27, 520–532. doi: 10.1016/j.neuroimage.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Noonan MP, Kolling N, Walton ME, Rushworth MF, 2012. Re-evaluating the role of the orbitofrontal cortex in reward and reinforcement. European Journal of Neuroscience 35, 997–1010. doi: 10.1111/j.1460-9568.2012.08023.x. [DOI] [PubMed] [Google Scholar]
- Nyamathi AM, Nandy K, Greengold B et al. , 2011. Effectiveness of intervention on improvement of drug use among methadone maintained adults. J Addict Dis 30, 6–16. doi: 10.1080/10550887.2010.531669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MF, 2012. Immunological and neuroimaging biomarkers of complicated grief. Dialogues Clin Neurosci. 14, 141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MF, 2018. Biobehavioral processes underlying complicated grief. Abstracts of the 76th Annual Scientific Meeting: Psychosomatic Medicine 80, A140. doi: 10.1097/PSY.0000000000000578. [DOI] [Google Scholar]
- O’Connor MF, Arizmendi B, 2015. Approach and avoidance in complicated grief: neuroscience results and clinical implications. Journal of Psychosomatic Research 6, 78. doi: 10.1016/j.jpsychores.2015.03.100. [DOI] [Google Scholar]
- O’Connor MF, Gündel H, McRae K, Lane RD, 2007. Baseline vagal tone predicts BOLD response during elicitation of grief. Neuropsychopharmacology 32, 2184–2189. doi: 10.1038/sj.npp.1301342. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Irwin MR, Wellisch DK, 2009. When grief heats up: Pro-inflammatory cytokines predict regional brain activation. Neuroimage 47, 891–896. doi: 10.1016/j.neuroimage.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MF, Shear MK, Fox R, Skritskaya N, Campbell B, Ghesquiere A et al. , 2013. Catecholamine predictors of complicated grief treatment outcomes. International Journal of Psychophysiology 88, 349–352. doi: 10.1016/j.ijpsycho.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MF, Wellisch DK, Stanton AL, Eisenberger NI, Irwin MR, Lieberman MD, 2008. Craving love? Enduring grief activates brain’s reward center. Neuroimage 42, 969–972. doi: 10.1016/j.neuroimage.2008.04.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill HA, 2019. A review on the involvement of catecholamines in animal behaviour. South African Journal of Animal Science 49, 29–39. doi: 10.4314/sajas.v49i1.1 [DOI] [Google Scholar]
- Parisi A, Sharma A, Howard MO, Blank Wilson A, 2019. The relationship between substance misuse and complicated grief: A systematic review. J Subst Abuse Treat 103, 43–57. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC, 2010. Neurocircuitry of mood disorders. Neuropsychopharmacology 35, 192–216. doi: 10.1038/npp.2009.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigerson HG, Bierhals AJ, Kasl SV, Reynolds CF, Shear MK, Day N, Beery LC, Newsom JT, Jacobs S, 1997. Traumatic grief as a risk factor for mental and physical morbidity. Am J Psychiatry 154, 616–23. [DOI] [PubMed] [Google Scholar]
- Prigerson HG, Horowitz MJ, Jacobs SC, Parkes CM, Aslan M, Goodkin K, Raphael B, Marwit SJ, Wortman C, Neimeyer RA, Bonanno GA, Block SD, Kissane D, Boelen P, Maercker A, Litz BT, Johnson JG, First MB, Maciejewski PK, 2009. Prolonged grief disorder: Psychometric validation of criteria proposed for DSM-V and ICD-11. PLoS Med. 2009, 6:e1000121. doi: 10.1371/journal.pmed.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigerson HG, Jacobs SC, 2001. Traumatic grief as a distinct disorder: a rationale, consensus criteria, and preliminary empirical test In: Stroebe MS, Hansson RO, Stroebe W, Schut H (Eds.), Handbook of Bereavement Research: Consequences, Coping and Care. American Psychological Association, Washington, D.C. pp. 613–645. [Google Scholar]
- Roozendaal B, McEwen B,, Chattarji S, 2009. Stress, memory, and the amygdala. Nat Rev Neurosci 10, 423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Prigerson HG, Bridge J, Maciejewski PK, Beery LC, Rosenheck RA, et al. 1999. Influence of traumatic grief on suicidal ideation among young adults. Am J Psychiatry 156: 1994–1995. [DOI] [PubMed] [Google Scholar]
- Ruegsegger GN, Toedebusch RG, Childs TE, Grigsby KB, Booth FW, 2017. Loss of Cdk5 function in the nucleus accumbens decreases wheel running and may mediate age-related declines in voluntary physical activity. J Physiol 595, 363–384. doi: 10.1113/JP272489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra Perez H, Ikram A, Direk N, Prigerson H, Freak-Poli R, Verhaaren B, et al. , 2015. Cognition, structural brain changes and complicated grief. A population-based study. Psychological Medicine 45, 1389–1399. doi: 10.1017/s0033291714002499. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A et al. , 2007. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology 191, 461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Sbarra DA, Hazan C, 2008. Coregulation, Dysregulation, Self-Regulation: An Integrative Analysis and Empirical Agenda for Understanding Adult Attachment, Separation, Loss, and Recovery. Personality and Social Psychology Review 12, 141–167. doi: 10.1177/1088868308315702. [DOI] [PubMed] [Google Scholar]
- Schiele MA, Costa B, Abelli M, Martini C, Baldwin DS, Domschke K, et al. , 2018. Oxytocin receptor gene variation, behavioural inhibition, and adult separation anxiety: Role in complicated grief. The World Journal of Biological Psychiatry 19, 471–479. doi: 10.1080/15622975.2018.1430374. [DOI] [PubMed] [Google Scholar]
- Schneck N, Haufe S, Tu T, Bonanno GA, Ochsner KN, Sajda P et al. , 2017. Tracking deceased-related thinking with neural pattern decoding of a cortical-basal ganglia circuit. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 2, 421–429. doi: 10.1016/j.bpsc.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneck N, Tu T, Michel CA, Bonanno GA, Sajda P, Mann JJ, 2018. Attentional bias to reminders of the deceased as compared with a living attachment in grieving. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 3, 107–115. doi: 10.1016/j.bpsc.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneck N, Tu T, Bonanno GA, Shear MK, Sajda P, Mann JJ, 2019a. Self-generated unconscious processing of loss linked to less severe grieving. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 4, 271–279. doi: 10.1016/j.bpsc.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneck N, Tu T, Haufe S, Bonanno GA, GalfaIvy H, Ochsner KN et al. , 2019b. Ongoing monitoring of mindwandering in avoidant grief through cortico-basal-ganglia interactions. Social Cognitive and Affective neuroscience 14, 163–172. doi: 10.1093/scan/nsy114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Shaham Y, 2008. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol Psychiatry 63, 256–262. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens FL, Hurley RA, Taber KH, 2011. Anterior cingulate cortex: unique role in cognition and emotion. Journal of Neuropsychiatry and Clinical Neuroscience 23, 121–125. doi: 10.1176/jnp.23.2.jnp121. [DOI] [PubMed] [Google Scholar]
- Silverman G Jacobs S, Kasl S, Shear M, Maciejewski P, Noaghuil S Prigerson H 2000. Quality of life impairments associated with diagnostic criteria for traumatic grief. Psychological Medicine, 30(4), 857–862. doi: 10.1017/S0033291799002524 [DOI] [PubMed] [Google Scholar]
- Suckling J, Nestor LJ, 2017. The neurobiology of addiction: the perspective from magnetic resonance imaging present and future. Addiction 112, 360–369. doi: 10.1111/add.13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Jbabdi S, Zhu Z et al. , 2019. A connectional hub in the rostral anterior cingulate cortex links areas of emotion and cognitive control. Elife 8, e43761. doi: 10.7554/eLife.43761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofler GH, Morel-Kopp MC, Spinaze M, Dent J, Ward C, McKinley S, et al. 2019. The effect of metoprolol and aspirin on cardiovascular risk in bereavement: A randomized controlled trial. Am Heart Journal 220:264–72. Epub 2020/01/11. doi: 10.1016/j.ahj.2019.11.003. [DOI] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Achterberg EJ,. Vanderschuren LJ, 2011. Nucleus accumbens μ-opioid receptors mediate social reward. J Neurosci. 31, 6362–70. doi: 10.1523/JNEUROSCI.5492-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurumi K, Kawada R, Yokoyama N et al. , 2014. Insular activation during reward anticipation reflects duration of illness in abstinent pathological gamblers. Front Psychol 5, 1013. doi: 10.3389/fpsyg.2014.01013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BO, Paul EJ, Miller MB et al. , 2018. Small sample sizes reduce the replicability of task-based fMRI studies. Commun Biol 1, 62. doi: 10.1038/s42003-018-0073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, Boucher O, 2017. Structure and Function of the Human Insula. J Clin Neurophysiol 34, 300–306. doi: 10.1097/WNP.0000000000000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valstad M, Alvares GA, Egknud M, Matziorinis AM, Andreassen OA, Westlye LT, Quintana DS, 2017. The correlation between central and peripheral oxytocin concentrations: A systematic review and meta-analysis. Neuroscience Biobehavioral Reviews 78, 117–124. doi: 10.1016/j.neubiorev.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler S, 2000. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral Cortex 10, 318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Younger J, Aron A, Parke S, Chatterjee N, Mackey S, 2010. Viewing Pictures of a Romantic Partner Reduces Experimental Pain: Involvement of Neural Reward Systems. PLOS ONE 5, e13309. doi: 10.1371/journal.pone.0013309 [DOI] [PMC free article] [PubMed] [Google Scholar]


