Abstract
Breast cancer is the second leading cause of mortality among women worldwide. Despite the available therapeutic regimes, variable treatment response is reported among different breast cancer subtypes. Recently, the effects of the tumor microenvironment on tumor progression as well as treatment responses have been widely recognized. Hypoxia and hypoxia inducible factors in the tumor microenvironment have long been known as major players in tumor progression and survival. However, the majority of our understanding of hypoxia biology has been derived from two dimensional (2D) models. Although many hypoxia-targeted therapies have elicited promising results in vitro and in vivo, these results have not been successfully translated into clinical trials. These limitations of 2D models underscore the need to develop and integrate three dimensional (3D) models that recapitulate the complex tumor-stroma interactions in vivo. This review summarizes role of hypoxia in various hallmarks of cancer progression. We then compare traditional 2D experimental systems with novel 3D tissue-engineered models giving accounts of different bioengineering platforms available to develop 3D models and how these 3D models are being exploited to understand the role of hypoxia in breast cancer progression.
Keywords: Three-dimensional (3D) models, Microtumor models, Breast cancer, Hypoxia, Cancer hallmarks
1. Introduction
Breast cancer is the second leading cause of mortality among women worldwide.[1] According to the recent GLOBOCAN 2018, cancer incidence and mortality statistics, female breast cancer accounted for almost one in four cases among women with a mortality rate of 11.6% worldwide. [2] Though current conventional chemotherapies and immunotherapies have been successful in management of 70–80% of early stage breast cancer patients, treatment of metastatic breast cancer still remains a challenge. [3] The variable treatment responses are further aggravated by breast cancer heterogeneity at the molecular and cellular level. Breast cancer also exhibits various molecular subtypes namely, luminal A, luminal B, human epidermal growth factor receptor 2 (HER2) positive and triple negative breast cancer (TNBC). [4–6] It is widely accepted that the surrounding tumor microenvironment (TME) that consists of non-cellular factors (oxygen, nutrient, pH gradients, etc.), extracellular matrix (ECM) and cellular factors (tumor cells, stromal cells) plays important role in tumor progression and treatment response.[7–11] Of note, hypoxic changes in surrounding TME are being increasingly highlighted to guide the crosstalk between the hypoxic tumor and stromal cells.[12, 13] The role of hypoxia inducible factors 1 alpha and beta (HIF-1α and HIF-1β) in the TME are being extensively studied to understand how hypoxia regulates the hallmarks of tumor progression in breast cancer.[9, 14]
Hypoxia, characterized by low oxygen levels, has been illustrated to activate hypoxia-inducible signaling via HIF-1α and HIF-1β, which mediates adaptive changes in tumor cells followed by rapid proliferation of tumor cells and remodeling of tumor vasculature.[15, 16] These hypoxic transformations sequentially guide tumor survival and progression in breast cancer cells via metabolic adaptations, angiogenesis, stromal cell recruitment, migration, tissue invasion, ECM remodeling, extravasation to metastatic sites and drug resistance.[17, 18] The clinical significance of hypoxia in tumor progression has also been studied in breast cancer patients.[19–21] Elevated HIF-1α levels correlated with poor prognosis and tamoxifen resistance in breast cancer patients.[22] Over-expression of HIF-1 pathway genes (carbonic anhydrase IX (CAIX), vascular endothelial growth factor (VEGF), BCL2 interacting protein 3 (BNIP-3) and ascorbate) correlated with advanced tumor stage and grade, increased necrosis, invasion and poor disease-free survival rates.[23] Similar results were also reported from another study where increased HIF-1α and CAIX overexpression predicted poor outcome in early-stage triple negative breast cancer patients.[24]
In view of the clinical relevance of hypoxia, different hypoxia inhibitors (Tirapazamine, PR104, TH-302 and Banoxanthane) have been tested in clinical trials; however, hypoxia-targeted therapies combined with current standard-of-care therapies (e.g., chemotherapy or radiotherapy) have exhibited varied outcomes in several clinical trials.[25–28] The failures warrant further investigation into related mechanisms along with the role of TME in influencing therapeutic response. It should be noted that traditional two-dimensional (2D) cell monolayers used to test efficacy of hypoxia-targeted therapies are limited in their translational efficacy as they do not completely emulate the complex in vivo tumor cell-cell and cell-ECM interactions of solid tumors. To address the limitations of 2D models, three-dimensional (3D) models have been recently engineered to effectively recapitulate complex tumor-TME interactions.
In view of these developments, this review first summarizes current knowledge of how complex biological processes and cancer hallmarks are modulated by hypoxia followed by the recent progress in the development of novel 3D models to study cancer biology and finally, how these 3D models have contributed to our understanding of hypoxia biology with particular focus on breast cancer progression.
2. Hypoxia Biology
Hypoxia-inducible factors (HIF-1α and HIF-1β) play a major role in tumor survival and progression in breast cancer cells through various cellular processes including metabolic adaptations, promoting tumor vascularization (angiogenesis), stromal cell recruitment, migration, tissue invasion, extracellular matrix modelling, extravasation to metastatic sites and drug resistance.[18, 29] The recent pre-clinical studies in breast cancer patients have also highlighted the promising results of co-administration of HIF inhibitors with chemotherapeutic drugs.[26, 30–35] In the following sub-sections, we summarize the role of hypoxia in promoting various hallmarks of breast cancer progression, which is also comprehensively illustrated in Figure 1.
Figure 1: A schematic representation of hypoxia signaling cascades modulated by hypoxia inducible factors (HIFs) in tumor cells.
HIFs promote metabolic adaptations, oxidative strees, angiogenesis, stromal cell recruitment, extracellular matrix modelling, extravasation, metastasis, migration and drug resistance through different signaling pathways. ATP-binding cassette subfamily G member 2 (ABCG2), AKT serine/threonine protein kinase B (AKT), Angiopoietin-1 (Ang-1), Angiopoietin-2 (Ang-2), B-cell lymphoma 2 (Bcl-2), Carbonic anhydrase IX (CAIX), Cyclooxygenase-2 (COX-2), C-X-C motif chemokine 4 (CXCR4), E-cadherin (E-cad), Forkhead box O3 (FOXO3A), Fibroblast growth factor (FGF), Glucose transporter 1 (GLUT-1), Lysyl oxidase (LOX), Inhibitor of apoptosis (IAP), Interleukin-6 (IL-6), Mitogen activated protein kinase (MAPK), Phospholipase C-gamma (PLC-γ), Monocarboxylate transporter 4 (MCT-4), Macrophage inhibitory cytokine-1 (MIC-1), Matrix metalloproteinases (MMPs), Mammalian target of rapamycin (mTOR), Nuclear factor kappa light chain enhancer of activated B cells (NF-κB), Platelet derived growth factor (PDGF), Phosphoglycerate kinase 1 (PGK1), Phosphatidylinositol 3-kinase (PI3K), Pyruvate kinase (PKM), Receptor tyrosine kinase (RTK), Stromal cell-derived factor 1 (SDF-1), Vascular endothelial growth factor (VEGF), Vascular endothelial growth factor receptor 1 (VEGFR1), Vascular endothelial growth factor receptor 2 (VEGFR2), von Hippel-Lindau tumor suppressor (VHL).
2.1. Metabolic Stress and Lipolysis
Metabolic reprogramming is rapidly emerging as a major hallmark of cancer; however, the precise metabolic markers still need extensive validation.[36] Breast cancer cells differentially rewire the metabolic adaptations in response to hypoxia in different subtypes of breast cancer. [37] HIF-1α is reported to mediate coupling of redox regulation and glucose metabolism in breast cancer cells, which modulates stem cell phenotype in breast cancer cells.[38] HIF-1α also mediates the metabolic responses to hypoxia via a number of mechanisms; increased flux through the glycolytic pathway thereby regulating mitochondrial reactive oxygen species (ROS) production and through changes in the serine synthesis pathway and mitochondrial folate cycle leading to increased production of glutathione and nicotinamide adenine dinucleotide phosphate (NADPH). [17, 38] A recent study reported that the metabolic coupling involving oxidative changes and the Warburg effect in the glycolytic stromal cells promoted mitochondrial metabolism in breast cancer cells. [39] This was driven by the HIF-1α-mediated activation of nuclear factor kappa B subunit 1 (NF-κB) and the c-Jun N-terminal kinase (JNK) pathway, which was modulated through ROS production leading to a catabolic phenotype. The expression of caveolin-1 (CAV-1) and monocarboxylate transporter 4 (MCT4) by this catabolic phenotype served as novel stromal cell markers indicative of the Warburg effect in breast cancer cells.[39] Additionally, dysregulation of adipocytes along with that of metabolic substrates (cholesterol, lipids, triglycerides, free fatty acids, hormones, leptin, adiponectin) in the breast TME have also been shown to mediate tumor progression via the induction of hypoxia (recently reviewed in detail [40]). Walsh et al. accentuated the critical metabolic plasticity seen in breast cancer stem cells, which allows tumor initiating cells to switch between oxidative phosphorylation and glycolysis guided through metabolic enzymes, energy and stress sensors, epigenetic chromatin modifications, transcription factors, non-coding RNAs and transcriptional repressors.[41] These mechanisms collectively indicate metabolic adaptations driven by ROS, which further induce catabolic phenotypes (mitophagy, autophagy, and glycolysis) in breast cancer cells and emphasize the importance of deciphering metabolic synergy along with epithelial-stromal interactions in breast cancer cells.
2.2. Apoptosis
Hypoxia mainly activates anti-apoptotic pathways that mediate tumor growth, progression, and survival.[42, 43] In contrast, a few studies have shown activation of growth-inhibitory genes and pro-apoptotic pathways under hypoxic conditions. [44] Hypoxia has been shown to induce cell cycle arrest and apoptosis via different pathways, which are discussed hereafter. HIF-1α has been shown to interact with p53 in MCF-7 cells transfected with HIF-1α, which leads to stabilization of p53 and the induction of apoptosis.[45] Synchronous results were reported from another study in which induction of p53 target genes i.e. pleckstrin homology like domain family A member 3 (PHLDA3) and inositol polyphosphate-5-phosphatase (INPP5D) by hypoxia led to apoptosis due to AKT serine/threonine kinase (AKT) inhibition. [46] The interactions and interplay between p53 family members and HIFs have previously been reviewed by Amelio and Melino [47] and Petrova [48]. In another study, expression of BNIP3 (a cell death factor) was upregulated in presence HIF-1α that was attributed to activation of the BNIP3 promoter containing HIF-1 response elements (HRE). [49] Similar findings were reported from another study where HIF-1 led to induction of BNIP3 and its homologue Nip3 like protein X (NIX) in T47D, MCF-7 and MDA-MB-231 breast tumors cells [50]. Hypoxia has also been shown to induce the TNF-related apoptosis inducing ligand (TRAIL) and the nuclear localization of breast cancer susceptibility gene 1 (BRCA1) in MCF-7 and MDA-MB-468 breast cancer cells, which further enhanced TRAIL-induced apoptosis in breast cancer cells.[51] Hypoxia-induced apoptosis in human breast cancer cells has also been reported to be mediated via signal transducer and activator of transcription 5B (STAT5b) and a caspase-3 dependent pathway.[52]
2.3. Angiogenesis and Vascular Mimicry
Abnormal angiogenesis is a well-established hallmark of tumor progression and survival and is triggered by hypoxic microenvironments. These hypoxic conditions in turn initiates an imbalance between pro- and anti-angiogenic factors. The hypoxic response has been coupled to increased vascularization or angiogenesis via angiogenic sprouting and elevated expression of matrix metalloproteinases (MMPs) in breast cancer cells.[53–55] Additionally, the hypoxic response has also been shown to modulate activation of pro-angiogenic factors i.e. VEGF [56–59], fibroblast growth factor (FGF) [15, 60], platelet-derived growth factor-β (PDGF-β) [61, 62], stromal-derived factor 1α (SDF-1α) [63, 64], angiopoietin-1 (Ang-1) [65, 66], angiopoietin-2 (Ang-2) [65, 67] and transforming growth factor beta 1 (TGF-β) [68–70] in breast cancer cells. In totality, elevated expression of these hypoxia-induced pro-angiogenic factors has been reported to promote neo-angiogenesis in breast tumors. In a recent report, elevated high-mobility group box 1 (HMGB1) was reported to increase HIF-1α expression, which in turn elevated VEGF expression in MCF-7 breast cancer cells via AKT phosphorylation and the activation of phosphatidylinositol 3-kinase (PI3K) pathway.[71] Further, shRNA mediated knockdown of HMGB1 downregulated HIF-1α and VEGF, which inhibited vessel formation in MCF-7 cells. [71] Hypoxia induction was also shown to drive expression of ADAM metallopeptidase domain 17 (ADAM17), which resulted in shedding of ectodomain of nectin-4 in the TME, which interacts with integrin-β4 on endothelial cells. [72] This interaction led to activation of PI3K/AKT, proto-oncogene tyrosine-protein kinase Src (Src) and inducible nitric oxide synthase (iNOS) pathway, which in turn promoted angiogenesis in MCF-10A breast tumor cells. [72]
HIF-1 and hypoxia regulated genes are also being targeted via different drugs to test if they can rewire normal vascularization in breast tumor cells. In one of the recent studies, administration of metformin was shown to inhibit progression in breast cancer cells through decreased hypoxia and induction of vessel normalization via downregulation of PDGF-β. [73] Centchroman (a novel estrogen receptor modulator) was reported to regulate breast cancer angiogenesis through inhibition of HIF-1α and its downstream target VEGF (i.e. HIF-1 α/VEGFR2 signaling axis) via decreased phosphorylation of extracellular signal regulated kinase (ERK)/AKT in MCF-10A cells. [74] In yet another interesting study, a bromodomain and extra-terminal (BET) inhibitor JQ1 was shown to downregulate HIF regulated genes, carbonic anhydrase (CA9) and vascular endothelial growth factor A (VEGF-A), which inhibited binding of HIF to hypoxia response element in CA9 promoter site in MDA-MB-231 triple negative breast cancer cells. This inhibition of CA9 and VEGF-A in turn inhibited xenograft vascularization in MDA-MB-231 cells. [75]
Besides angiogenesis and neo-vascularization, an interesting concept of vascular mimicry (VM) was highlighted in uveal melanomas in the last decade, which has gained impetus over the years in cancer biology. [76] The concept of VM refers to de novo formation of microvascular channels by the malignant cell population and has been extensively studied in breast cancer cells.[77–83] This concept of VM has also been linked to hypoxia in breast cancer cells and has been reviewed by Li and colleagues. [84] Though presently we have few studies delineating the mechanism underlying hypoxia induced VM, research is ongoing to explore this dimension of cancer biology. In one of the initial studies, combretastatin A4 phosphate (CA4P), a vascular targeting agent was reported to induce intra-tumoral hypoxia (elevated HIF-1α) along with a VM network in mice injected with W256 breast cancer cells via activation of the HIF-1α/ erythropoietin producing hepatoma receptor A2 (EphA2)/PI3K/MMP signaling pathway. [85] Also, treatment of MDA-MB-231 and Hs578T tumor bearing mice with sunitinib, a receptor tyrosine kinase (RTK) inhibitor, was shown to induce VM in TNBC along with upregulation of expression of HIF-1α, vascular endothelial cadherin (VE-cadherin), matrix metallopeptidase 2 (MMP2) and Twist1, which was not observed in mice injected with non-TNBC cells (MCF-7 and BT474).[86]
Further, VM targeting with 6,6’-bis(2,3-dimethoxybenzoyl)-a,a-D-trehalose (DMBT), an anti-invasive agent, was shown to inhibit hypoxia-induced VM in MDA-MB-231 and MCF-7 breast cancer cells. DMBT treatment decreased expression of HIF-1α, matrix metallopeptidase 9 (MMP-9), cell division cycle 42 (Cdc42), VE-cadherin, p-AKT, epidermal growth factor receptor (EGFR) and phosphorylated mammalian target of rapamycin (mTOR) via downregulating HIF-1α/VE-cadherin/MMP signaling pathways.[87] Tumor protein p53-inducible nuclear protein 1 (TP52INP1) has also been shown to inhibit hypoxia-induced VM by downregulating the ROS/ glycogen synthase kinase 3 beta (GSK-3β) /Snail signaling axis leading to decreased HIF-1α, Snail and VE-cadherin expression. [88] In an interesting study, ectopic restoration of miR-204 was illustrated to inhibit hypoxia-induced VM in MDA-MB-231 cells via cooperative downregulation of 13 target proteins involved in VEGF, PI3K/AKT, focal adhesion kinase (FAK)/Src and Raf-1 proto-oncogene, serine/threonine kinase (RAF1)/ mitogen activated protein kinase (MAPK) signaling pathways. [89] In conclusion, the existing scientific literature suggests that HIF-1α plays essential roles in inducing tumor angiogenesis and VM in breast cancer cells via different signaling cascades.
2.4. Pro-inflammatory Pathways
Tumor hypoxia is known to promote inflammation-mediated metastasis via activation of pro-inflammatory signaling molecules in malignant cells and the adjacent TME. Mechanisms of how hypoxia mediates inflammatory signaling have been discussed in detail in a recent article [90]. The crosstalk between hypoxia and inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6) and leptin is suggested to deregulate Notch signaling in breast cancer.[91] This deregulated Notch signaling, a critical downstream target of IL-6 induces tumor angiogenesis in breast cancer. It has also been reported that hypoxia-mediated downregulation of estrogen signaling, particularly CCAAT/enhancer binding protein-α (C/EBPα) promoted breast cancer growth via cytokines, IL-1β/IL-6 and tumor necrosis factor–alpha (TNF-α) in the microenvironment. [12] HIF-1α upregulates genes of the purinergic pathway, namely, CD23, CD73, A2A adenosine receptor (A2AAR) and A2B adenosine receptor (A2BAR) of which CD73 plays a major role in tumor progression via downregulating PI3K/AKT and Wnt/β-catenin pathways. CD73 in the TME has been shown to negatively regulate interferon-gamma (IFN-γ) and TNF-α expression via binding of CD73-derived adenosine to natural killer (NK) cells and macrophages (increased interleukin-4 (IL-4) and interleukin-10 (IL-10) production). Targeting A2 adenosine receptors (A2AR) has been shown to decrease metastasis via elevated expression of granzyme B and perforin.[92] The role of inflammatory mediators namely IL-6, interleukin-8 (IL-8), TGF-β, TNF-α, connective tissue growth factor (CTGF), VEGF, cyclooxygenase-2 (COX-2) and C-C motif chemokine ligand 2 (CCL2) in regulation of hypoxia in different malignancies and their function in tumor progression is discussed.[93] Additionally, the mechanistic inhibition of hypoxia signaling pathways and hypoxia-activated pro-drugs have been shown to modulate the anti-inflammatory response.[29, 94] As hypoxia has been reported to induce these inflammatory mediators, drugs targeting these inflammatory molecules have also been studied to regulate progression in malignancies. [95–99] LY2157299 (Galunisertib, a TGF-β receptor I kinase inhibitor) has been shown to inhibit tumor progression in triple negative breast cancer in vitro and has also been shown to alleviate tumor recurrence post paclitaxel treatment in mouse xenograft models. [95] Anti-IL6 treatment has also been shown to inhibit bone metastasis in breast cancer murine model. [99] Vorinostat (8-(hydroxyamino)-8-oxo-N-phenyl-octanamide), a histone deacetylase (HDAC) inhibitor, known to inhibit HIF-1α translation has been shown to decrease inflammatory mediators (IL-6, interleukin-1 beta (IL-1β) and TNF-α) in a dose-dependent manner in a mouse model. [98] Human peripheral blood mononuclear cells were reported to secrete decreased IL-1β, interleukin-12 (IL-12), TNF-α and IFN-γ upon administration of vorinostat. [96, 97] These studies elicit the role of HDAC inhibitors in modulating HIF-1α levels along with suppression of inflammatory molecules. Over the recent years, pro-inflammatory molecules modulated via hypoxia have emerged as potential candidates for targeted therapy. However, further studies still need to elucidate the potential of these small molecule inhibitors as specific targeted therapies.
2.5. Migration and metastasis
Epithelial to mesenchymal transition (EMT) and metastasis is intricately regulated by remodeling of the cytoskeleton in breast cancer cells that plays important roles in migration and invasion of breast cancer cells into surrounding TME, intravasation into blood vessels and extravasation and colonization at secondary sites. [100–103] The hypoxic microenvironment is known to be a critical factor in EMT, tumor cell migration and metastasis and was recently reviewed by Gao and colleagues [7], Gilkes [8], Schito and Ray [10] and the Liu group [9]. In addition to activation of well-known hypoxia-mediated signaling pathways including the hepatocyte growth factor (HGF)-c-Met axis, Ras homolog family member A (RhoA)/ Rho associated coiled-coil containing protein kinase 1 (ROCK1) transduction pathway, NOTCH-integrin-linked kinase (ILK) signaling pathways, platelet-derived growth factor B (PDGF) pathway and MAPK/ERK pathway that have been implicated in tumor cell migration, other groups have identified roles for less studied signaling molecules in inducing migration in hypoxic cells. In one of the recent studies, SRY-box-2 (SOX2) was shown to promote hypoxia-induced migration in MDA-MB-231 and MDA-MB-468 breast cancer cells through induction of NEDD9 (neural precursor cell expressed, developmentally down-regulated 9) expression and activation of Rac family small GTPase 1 (Rac1)/HIF-1α signaling pathway. [104] Also, nuclear factor erythroid-2-related factor 2 (Nrf2) expression was reported to inhibit kelch-like-ECH-associated protein 1 (Keap1) and induce activation of HIF-1α, glucose-6-phosphate dehydrogenase (G6PD) and Notch 1 (G6PD/HIF-1α/Notch 1 axis) in MCF-7 and MDA-MB-231 cells. [105] Activation of the Notch signaling pathway modulated downstream Hes family BHLH transcription factor 1 (HES-1) expression, which led to EMT and Nrf-2 driven metastasis in breast cancer cells. Hypoxia was also reported to induce migration in MDA-MB-231 cells via activation of cadherin-22 (a cell-cell adhesion molecule), which is in turn regulated by overexpression of eukaryotic translation initiation factor 4E type 2 (elF4E2).[106] In another study, epigenetic regulation of zinc finger MYND-type containing 8 (ZMYND8) leading to ZMYND8 acetylation at lysine 1007 and 1034 activated HIF-1 mediated metastasis with recruitment of bromodomain containing 4 (BRD4) and release of RNA polymerase II. [107] These studies highlighted the plausible role of epigenetic mechanism in HIF activation along with HIF-mediated breast cancer migration and metastasis.
2.6. Drug Resistance
It is well-established that hypoxia leads to drug resistance against conventional anti-cancer agents in breast cancer. [108] Different research groups have postulated different mechanisms of inherent or acquired drug resistance, which is a major player in poor treatment response rates and can also predispose patients to early relapse or refractory disease. A recent hypothesis emphasizes the role of cancer stem cells (CSCs), which are regulated by HIF-2α, which in turn, modulates the stemness in breast cancer cells while the role of HIF-1α has been implicated in tumor cell survival. Various mechanisms and signaling cascades responsible for drug resistance involve prominent ABC drug transporters such as P-gylcoprotein (P-gp) including breast cancer resistance protein (BCRP), multidrug resistance gene 1 (MDR1), multidrug resistance associated protein (MRP1 and MRP2) responsible for efflux of chemotherapeutic drugs, thereby conferring drug resistance.[109, 110] The regulation of MRP1 and MRP2 in hypoxic microenvironments involves different signaling pathways such as NF-κB, peroxisome proliferators-activated receptor alpha (PPARα), constitutive androstane receptor (CAR), pregnane X receptor (PXR), farnesoid X receptor (FXR) and microRNAs (miRNAs) during chemoresistance. [13] The PI3K/AKT signaling pathway has also emerged as a major pathway upregulated in drug resistance wherein hypoxic conditions are known to activate PI3K/AKT coupled to mTOR along with accumulation of HIF-1α and HIF-2α. Besides the PI3K/AKT pathway, drug resistance in breast cancer cells is also modulated through MAPK pathway, ERK, MEK (MAPK/ERK), and phospholipase C-gamma (PLC-γ). Drug resistance is also reported to be regulated by the TME including contributions by stromal cells (vascular endothelial cells, immune-inflammatory cells and fibroblasts), extracellular matrix and soluble factors in addition to intrinsic tumor specific pathways involving Ang-2, FGF/FGF receptor (FGFR) and MET proto-oncogene (MET)/HGF pathway. [111, 112] Cancer-associated fibroblasts (CAFs) have been reported to induce CSC phenotypes, which are chemoresistant due to secretion of CCL2.[113] Also, stromal cell-derived factor (SDF-1)/C-X-C motif chemokine 12 (CXCL12) have also been reported to protect small cell lung cancer (SCLC) cells from chemotherapy induced apoptosis.[114] Tumor associated macrophages (TAMs) have also been shown to enhance chemoresistance to paclitaxel in MDA-MB-231 breast cancer cells via cathepsin proteases.[115] Further details into the mechanism of chemoresistance mediated via TME has been discussed by Velaei’s group.[112] The detailed mechanisms of drug resistance induced by hypoxia (HIF-1α and HIF-2α) in breast cancer cells has also been reviewed by Schoning et al. and Mahdi et al. [116, 117]
Although the field of hypoxia biology has evolved greatly with deep understanding of downstream signaling pathways, it should be noted that most of these mechanisms were discovered/studied using 2D cultures, which do not recapitulate the in vivo TME observed in solid tumors. While 2D cultures can be easily adapted to high throughput assays and drug screening approaches, 2D in vitro models have some major limitations for the study of cancer biology. Hence, there are a multitude of efforts to generate physiologically relevant 3D experimental models to mimic the in vivo TME of solid tumors. In the following sections, we first assess the limitations of current experimental models followed by general advances in various technologies to generate 3D experimental models recapitulating different aspects of TME, followed by hypoxia-related studies that highlight differences in 2D and 3D cultures. We then discuss 3D experimental models used to investigate the role of hypoxia in promoting various hallmarks discussed above with a focus on breast cancer progression.
3. General Overview of current experimental systems used in cancer biology
3.1. Limitations of 2D and in vivo models in studying hypoxia biology
Solid tumor microenvironment is composed of tumor and stromal cells (vascular, immune and fibroblast cells) and ECM components where cell-cell interactions, cell-ECM interactions and local gradients of nutrients, growth factors, secreted factors and oxygen regulate cancer cell function and behavior. Traditionally, 2D cell-based in vitro tumor models have been extensively used to study hypoxia biology due to the relative ease of their implementation and capacity to provide for high-throughput screening. Traditional 2D cell culture tumor models refer to flat monolayer cell culture on plastic dishes or glass wherein cells are exposed to hypoxia using hypoxic chambers that maintain hypoxic conditions over a defined concentration of pO2. Cell culture in hypoxic chambers cannot reproduce the spatial oxygen gradients and spatial heterogeneity observed in solid tumors in vivo, where only an inner hypoxic core exists. This is because in hypoxic chambers, all cells in 2D are equally exposed to hypoxia. They also do not capture the constantly evolving dynamic TME in vivo, and hence, they are considered poor surrogates for evaluating tumor progression as well as drug efficacy (summarized in Table I).
Table I:
Two-dimensional (2D) vs. three-dimensional (3D) cell culture models.
| Culture Techniques | Two-dimensional (2D) | Three dimensional (3D) |
|---|---|---|
| In vivo like | Do not mimic in vivo tissue and interactions | Mimics in vivo tissues and interactions |
| Culture formation | Few minutes to hours | Few hours to few days |
| Culture Quality |
|
|
| Architecture | Cells interact partially |
|
| Assess to essential compounds | Equally exposure to nutrients, oxygen, metabolites and signaling molecules in growth media | Variable access- Nutrients, Oxygen, Metabolites and signaling molecules access is modulated on surface and core |
| Cell Proliferation | High proliferation rate in monolayer | Replicate in vivo cell proliferation |
| Cell Polarity | Partial polarization | Accurately replicate cell polarization |
| Cell cycle stage | Cells in same stage due to equal exposure to media | Heterogeneous cells in different cell cycle stages in spheroids (proliferating, hypoxic, quiescent, necrotic) |
| Cell Morphology | Flat, sheet-like monolayer cells | Aggregate/spheroid structures |
| Stiffness | High stiffness (~3*109 Pa) | Low stiffness (>4000Pa) |
| Cell interactions |
|
|
| Drug response | Lack of correlation between human tumors undergoing drug screening in comparison to 2D cells | 3D tumor spheroids show similar drug resistance pattern as in patients |
| Maintenance Cost |
|
|
In vivo xenograft models have also been used to study hypoxia biology via gene knock-in/knock-out experiments [118–121]. Xenograft models mainly rely on the manipulation of molecular targets and signaling pathways regulated by hypoxia. Although the xenograft mouse models and patient-derived xenografts (PDXs) better resemble solid tumors than 2D cultures, they do not completely represent the etiology and pathophysiology of tumor development in human.[122, 123] Preclinical animal models rely heavily on mouse tumor models that are expensive and time consuming, thereby limiting the numbers of agents that can be tested and are also limited by ethical considerations. The differences in (for syngeneic immunocompetent models) and/or lack of immune component (immunocompromised mouse xenograft models) and lack of human-specific stromal interactions further limit the relevance of rodent models to human solid tumors.[124, 125] This contributes to poor predictions of drug efficacy and patient responses in clinical trials.
In view of these limitations of 2D and xenograft models, a major advantage of biomimetic tissue-engineered in vitro models is that they can be designed to better mimic the cell–cell interactions, cell–matrix interactions, and the heterogeneous TME of human solid tumors observed in vivo. Biomimetic in vitro 3D models made of human cancer cell lines or human tissues can be exploited to elucidate the role of non-cellular microenvironmental factors (e.g. hypoxia, ECM molecules, etc.) as well as cellular factors (epithelial-stromal cell interactions) in human disease progression within the context of a defined and controlled microenvironment. [126–130] Compared to 2D monolayer cultures, tumor cells cultured in 3D microenvironments experience different cellular cues that modify their responses to various stimuli. For example, tumor cells growing in 3D cell cultures are exposed to dramatically different adhesive, topographical and mechanical forces than cells growing in 2D on treated surfaces.[131, 132] It has been well documented that the 3D microenvironment alters oxygen/nutrient diffusion gradients leading to hypoxia, changes in pH, etc., which further affect numerous cellular and functional activities including cell morphology, viability, proliferation, differentiation, and migration through altered signal transduction, histone acetylation, gene expression, and protein expression. [132, 133] Additionally, the cell-cell and cell-ECM interactions of cells in solid tumors and multi-layer tumor spheroids constitute a permeability barrier through which therapeutic agents must penetrate leading to altered drug penetration efficacy, drug metabolism, and drug sensitivity. [134, 135] Technological advances in the field of tissue engineering, microfluidics and bioprinting has enabled fabrication of variety of 3D tumor models recapitulating various aspects of the TME. [136]
3.2. Overview of technologies to generate 3D tumor models
Multicellular Tumor Spheroids
The most widely used 3D models to study cancer biology and drug response are multicellular tumor spheroids (MCTS, also called as microtumors, tumoroids, or tumor aggregates) (summarized in Table II). Tumor spheroids can be homotypic (made of single tumor cell types) or heterotypic (aggregates of different tumor cell types or mixture of tumor and stromal cells) and have been widely used to study cell–cell interactions, microenvironmental cues important for tumor growth, and mechanistic investigations of various hallmarks of cancer such as angiogenesis, metastasis, metabolic adaptations, and drug resistance. [137–139] Tumor spheroids are generated using different technologies including spinner flasks, rotatory system, hanging drop, microcarrier beads, bioreactors (Figure 2 A–F), etc. [140–143] However, these techniques are both time-consuming and labor intensive and most importantly, they generate aggregates with a wide range of irregular sizes and shapes, which presents a critical challenge to recreate reproducible physio-chemical microenvironments and makes assay standardization difficult. Indeed, variations in size and shape can play a critical role in the establishment of differential gradients such as hypoxia, pH, nutrients, growth factors, cytokines, waste products, and metabolic stress within the TME, which in turn can affect cancer biology, cell behaviors and cellular responses to the drug treatments.
Table II:
Advantages and challenges of three-dimensional (3D) tumor models.
| Techniques | Graphical Representation | Advantages | Challenges | Research Stage | Ref |
|---|---|---|---|---|---|
| Liquid Overlay Culture and Hanging Drop |  |
|
|
|
[210, 220, 221] |
| Hydrogel |  |
|
|
|
[222–226] |
| Bioreactors |  |
|
|
|
[161–163, 227–233] |
| Scaffolds |  |
|
|
|
[157, 234–237] |
| Microtitre plate |  |
|
|
|
[132] |
| Microfluidics |  |
|
|
|
[238–240] |
| 3D Bioprinting |  |
|
|
|
[165, 168, 169, 241] |
Extracellular matrix (ECM), High-throughput setting (HTS), High content screening (HCS).
Figure 2: A schematic representation of three-dimensional (3D) models. (A) Non-adhesive culture;
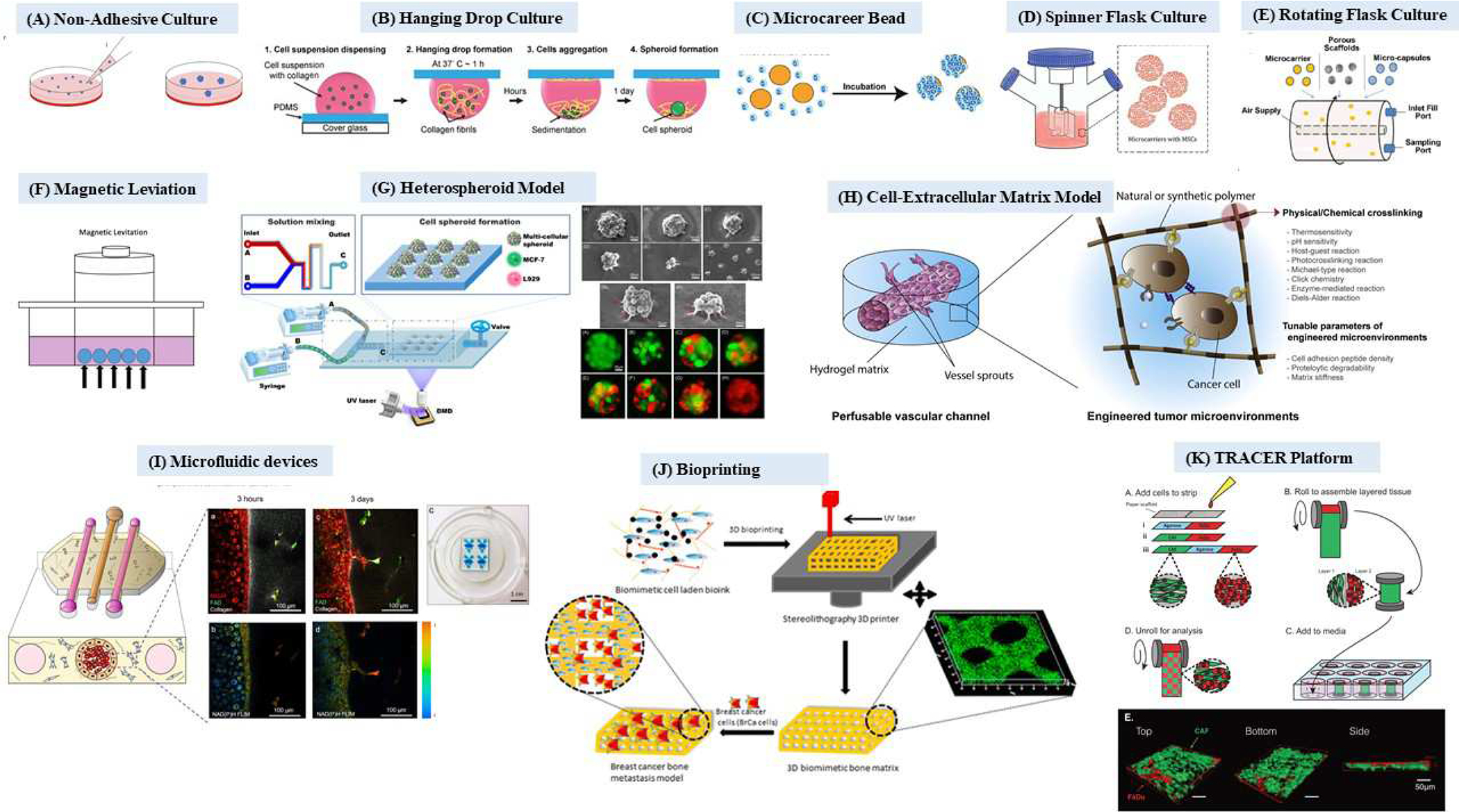
Adapted with permission [213], (B) Hanging drop culture; Adapted with permission [214], (C) Microcarrier beads; Adapted with permission [215], (D) Spinner flask culture; Adapted with permission [216], (E) Rotating flask culture; Adapted with permission [217] (F) Magnetic levitation, (G) Heterospheroid model; Adapted with permission [146], (H) Cell-extracellular matrix model; Adapted with permission [218], (I) Microfluidic device; Adapted with permission [137], (J) Bioprinting; Adapted with permission [219], (K) TRACER platform; Adapted with permission [147].
While tumor spheroids enable studying role of non-cellular microenvironmental factors such as pH, oxygen, and nutrient gradients as well as cell-cell interactions on tumor cell behaviors, they do not recapitulate tumor-stromal cell interactions. This limitation can be overcome by generation of heterotypic spheroids (Figure 2 G–K), which have been used to recreate intra-tumoral heterogeneity, for example by mixing cancer cells of different subtypes. [144, 145] Heterotypic spheroids have also been used to recreate tumor-stromal cell interactions where stromal cells such as immune cells, fibroblasts, endothelial cells, etc. are mixed with tumor cells. [146] [147] For example, McGuigan and colleagues established (TRACER) platform (Tissue Roll for the Analysis of Cellular Environment and Response) to investigate tumor-stroma interactions using co-culture of patient-derived CAFs and squamous carcinoma cells of the hypopharynx (Figure 2L).[147]
3D Scaffolds
3D spheroids can also be grown on prefabricated scaffolds in which cells attach and migrate along the scaffold fibers. Scaffolds are generally seeded with cell lines, sometimes along with growth factors for promoting their growth and providing tumor microenvironment to recapitulate molecular crosstalk and in vivo architecture. These scaffolds can be of natural (gum, gellan, hyaluronic acid, silk, etc.) or synthetic origin (polylactide/glycolide, polycaprolactone or polystyrene) and provide mechanical support to the seeded cells. [148] They can be engineered to emulate the extracellular matrix (ECM) since the ECM is known to promote key pathways that modulate angiogenesis, metastasis, migration, proliferation and cellular organization. [149–152] With the advancements in the field of biomaterial fabrication, scaffolds are being tailored to mimic biophysical and biochemical properties of ECM. The porous poly(lactide-co-glycolide) (PLG) fabricated scaffolds were used to establish in vivo microenvironmental cues in a 3D human oral cancer model. In this model, oral squamous cell carcinoma cells (OSCC-3) cultured in PLG scaffolds displayed enhanced malignant potential with increased secretion of VEGF, bFGF and IL-8. [151] Similar results were reported from another study where hypoxic MCF-7 cells seeded on PLG scaffolds showed elevated secretion of angiogenic factors IL-8 and VEGF. [153] Similarly, an open-faced 3D collagen scaffold has been shown to create microenvironment suitable for promoting signaling pathways essential for spheroid formation, differentiation and organization. [154] The major advantages of working with scaffold-based 3D tumor models is the ability to fine tune physico-chemical properties of the scaffold such as scaffold porosity providing more surface area to cells, scaffold surface functionalization to alter tumor cell adhesion properties, and mechanical properties to study role of mechanotransduction in tumor progression. The disadvantages of scaffold-based approaches include scaffold to scaffold variations, non-transparency and thickness creating challenges for imaging, and difficulty in removing cells for protein analysis. [155, 156] One could overcome these shortcomings by improving 3D scaffold material compositions and combining biofabrication techniques (Table II). In addition to the natural and synthetic scaffolds, decellularization of native healthy and diseased tissues is being explored to preserve native ECM structure and composition. [150] Such decellularized matrices are actively being used as bioactive scaffolds for studying tumor progression, EMT as well as a platform for drug testing.[157] The limitations in using decellularized patient-derived matrix to fabricate scaffolds is potentially the availability of patient biopsies and more importantly, the ethical issues associated with obtaining informed content for the same.
Microfluidic devices
Tumor-on-a-chip or microfluidic devices have miniaturized and revolutionized cell culture platforms. Microfluidic devices provide a controlled cellular environment, adaptive to different cellular and metabolic changes and are easy to handle with high reproducibility. [158, 159] A major advantage of microfluidic devices is the feasibility of measuring dynamic cellular changes over time on the same chip rather than a single endpoint analysis. Recently, the application of microfluidic approaches has been extended to investigate cells from liquid biopsies because of their ability to successfully mimic the interactions and flow of different types of cells. Other advantages include feasibility to develop co-culture and cell localization studies involving use of chemical gradients (Table II). However, one of the limitations of tumor-on-a-chip models is its limited scalability for high throughput screening (HTS); we still need to precisely streamline automated loading of micro-physiological components on the model, which is still done manually. This necessitates future developments in tumor-on-a-chip models as both automation and miniaturization need to be well-balanced for microfluidic devices to better respond to HTS requisites of pharmaceutical companies.
Bioreactors
Versatile bioreactors often used in pharmaceutical processes have also been employed to culture tumor cells as spheroids [160] or microcarriers [161]. They help in easy culturing coupled with high throughput production of spheroids (Table II) These bioreactors precisely monitor metabolites, temperature, pH and nutrients under strict control. Perfusion culture systems were later developed, which allowed for monitoring dynamic 3D tumor growth under reduced media conditions to bridge the gap between microfluidic chips and bioreactors. Examples include 3DKube™ [162] and IVtech™ [163]. Perfused bioreactor systems offer the possibility of dynamic visualization due to their transparency and the potential for advancing cancer research by making it feasible to investigate interactions in patient derived 3D tumor cells cultured in a cancer niche. However, bioreactors have their own share of disadvantages like requirement of specialized equipment, less uniformity in cell control and size and unwanted shear force exposure on cells (Table II).
3D Bioprinting
3D bioprinting employs a layer-by-layer deposition technique ideally to capture complexity and spatial heterogeneity of a tissue-like structure by combining tumor/stromal cells and ECM-like biomaterials. [164] The advantage of using these bio-ink hydrogels is the maintenance of cell viability and 3D architecture because they provide suitable mechanical and physicochemical interactions imitating the tumor tissue. An added advantage of bioprinting is minimal manipulation of 3D cell cultures, which enhances its biological replication, consistency and reliability in drug screening assay read-outs. 3D bioprinted models can be used to study the cell–ECM and cell-cell interactions between tumor cells and different stromal cell populations (macrophages, fibroblasts) along with ECM components. [165–167] [168] [169] Although bioprinting is a promising and versatile technique, a major challenge with 3D bioprinting is to simultaneously print material (bio-ink) and the tumor cells without replacing the chemical solvents and maintaining cell viability. Further, standardization of bioprinting protocols will help promote the use of these 3D tumor models in pre-clinical settings.[165]
3.3. Comparative studies between 2D and 3D models
It has been shown that expression of genes and proteins can be differentially regulated in 2D vs. 3D cultures. For instance, hypoxia was reported to differentially regulate estrogen receptor alpha (ERα) expression in 2D and 3D T47D breast cancer cells. In this study, the 3D model consisted of paper-based scaffolds-cells containing a single zone surrounded by a wax-patterned border suspended in collagen I matrix. Surprisingly, ERα mRNA levels were insensitive to hypoxia (1% O2 via hypoxic chamber) in 3D whereas in 2D, a decrease in ERα transcription activity was reported. [170] In another study, the exposure of MCF-7 breast cancer spheroids and MCF-7 monolayer cells to estradiol showed similar expression profile for estrogen-regulated transcription targets (progesterone receptor (PGR); amphiregulin (AREG) and PDZ containing domain 1 (PDZK1)); however, they showed differentially activated cell-cell interactions pathways [171], which was attributed to differences in cellular differentiation in 2D and 3D cultures. In our own study [172], size-controlled 3D microtumors generated using hydrogel microwells of defined diameter (150 and 600 μm) showed differential microtumor size-dependent ERα expression. While small non-hypoxic 150 μm microtumors maintained ERα expression, large hypoxic 600 μm microtumors displayed loss of ERα expression both at mRNA and protein levels.
When cancer drug responses are compared in 2D and 3D tumor models, differential drug sensitivity has been reported to be manifested as either greater resistance or enhanced sensitivity. [132, 138] The drug response to cisplatin and cetuximab was evaluated in head and neck squamous cell carcinoma (HNSCC) cells (LK0858B, LK0902 and LK1122) cultured as monolayers in 2D and as spheroids in ultra-low attachment plates. Counter-intuitively, LK0902 cells were shown to be more sensitive to cetuximab treatment in 3D tumor spheroids in comparison to 2D culture. [173] In another study conducted in colorectal cancer cells, cells cultured in type-I collagen were reported to be sensitive to cetuximab treatment while the 2D cells were non-responsive. [174] In another study, AU565, HCC1569, SKBR3, and BT549 breast cancer cells cultured in matrigel were more sensitive to HER2-targeting agents (lapatinib, pertuzumab and trastuzumab) in 3D compared to cells cultured in 2D.[175] MCF-7 cells cultured in 3D laminin-rich ECM also showed elevated sensitivity to doxorubicin in comparison to 2D monolayer cultures. This was attributed to prevention of activation of p53-DRAM-autophagy.[176]
Based on this evidence, a broad consensus has emerged that in vitro 3D tumor models serve better as physiologically relevant preclinical models to evaluate the efficacy of cancer drug leads, and that the application of these models have the potential to improve the success rate of drug candidates that advance into mouse tumor models and clinical trials (Table II). The next section highlights application of 3D models to investigate role of hypoxia in specific hallmarks in breast cancer progression.
4. Three Dimensional Models Used to Study Hypoxia Biology in Breast Cancer
4.1. Metabolic Stress and Lipolysis
Altered cancer cell metabolism under hypoxic conditions exert a selection pressure, which modulates different cellular processes coupled with downregulation of oxidative phosphorylation and inhibition of fatty acids. Altered metabolic adaptations and lipid profile of tumor cells is a well-established hallmark of cancer progression. Currently, lipid profiling and mapping in breast cancer in vitro models has been extensively studied using 2D cell culture to investigate the association between lipid production and tumor progression. In view of the technological advancements, 3D models are being used to understand the underlying metabolic changes, which facilitate tumor cell survival and proliferation. Presently, there is only one published study investigating hypoxia-initiated modulation of breast cancer metabolic adaptations. [137] In this study, starvation-induced spatial-temporal metabolic adaptations were investigated in an organotypic microfluidic MCF-10A breast cancer model. To recapitulate the ductal carcinoma in situ (DCIS) microenvironment, the authors developed a microfluidic model, in which MCF-10A cells were grown inside a luminal mammary duct model, embedded in a 3D hydrogel with mammary fibroblasts. [137] A hypoxia sensing dye was added to the collagen hydrogel before polymerization to detect oxygen levels. It was shown that metabolic signaling pathways specific to glycolysis and hypoxia were altered. The study also identified gradients of different metabolic phenotypes across the mammary duct model. Also, administration of the hypoxia-activated prodrug; Tirapazamine selectively destroyed hypoxic DCIS cells. [137] The results from this study highlights the metabolic adaptations of tumor cells to endure hypoxia.
In addition to the metabolic changes inherent in breast cancer cells undergoing tumor progression and survival, modulation of lipid metabolism is another major source of energy to support tumor cell proliferation. In 2D monolayer models of breast cancer metabolism, aberrant accumulation of lipids along with altered lipid profiles are reported to be associated with malignant progression. [177] This altered lipid metabolism is well-known hallmark of cancer progression. It is also well known that malignant cells store surplus lipids in the form of intracellular droplets or in organelles involved in transport and storage of lipids, which provides the essential energy for tumor proliferation. The recent high-throughput technological advancements in Mass spectrometry (MS) and Raman microscopy has enabled investigations of the lipid profile (lipidomics) and special distribution of lipids in cancer tissues. In one of comprehensive study design, Vidavsky et al. [178] mapped and profiled lipid distribution in a 3D breast cancer model of tumor progression. They used liquid chromatography coupled with MS to extensively illustrate the difference in the lipid composition of the MCF-10A breast cancer progression when cultured as 2D monolayers vs. 3D spheroids. Further, lipid staining, and Raman chemical imaging was used to study the spatial distribution of lipids. While the amount of lipids decreased significantly, the ratio of acylglycerols to the membrane lipids increased in 3D cultured cells, suggesting formation of large lipid droplets, which were absent in 2D cultures. In malignant invasive 3D MCF-10A spheroids, lipid accumulation was observed in the hypoxic core of the tumor spheroids with declining gradient towards the periphery. Similarly, large lipid droplets were present in the hypoxic core, which was attributed to activation of the de novo sphingolipid synthesis signaling cascade. [178] In another study, Jiang and colleagues investigated the heterogeneous spatial response of tumors to hypoxia using MALDI-MS imaging (MSI) in 3D MDA-MB-231-HRE-tdTomato (hypoxia response element with control of red fluorescent tdTomato protein construct) breast tumor xenografts.[179] The findings from the study highlighted co-localization of lipid species in the hypoxic regions of tumors using principal component analysis-linear discriminant analysis (PCA-LDA) with identification of a total of 34 hypoxia regulated lipid species for which strong positive correlations were observed for phosphatidylcholine (PC) (16:0/18:0; 16:0/18:1; 18:0/18:1 and 18:1/18:1) and sphingomyelin (SM) (d18:1/24:0) suggestive of their high expression in the hypoxic cores. [179]
Tumor metabolic pathways and lipid metabolism have been extensively studied in 2D cultures and still lacks extensive studies using appropriate 3D culture systems, which better recapitulate the in vivo malignant tumor progression. In the view of limited scientific literature on the evolution of metabolic stress and lipolysis in hypoxic conditions using 3D models, more well-designed experimental studies are needed to reconnoiter lipid metabolism for therapeutic or targeted modulation.
4.2. Apoptosis
The studies reporting activation of the pro-apoptotic pathway in hypoxic 3D breast cancer models are sparse. In breast cancer cells, the mechanism of action of hypoxia in modulating pro-apoptotic pathways has not been very well explored in 3D tumor models and presently there has been only one study publishes. In this study, a 3D scaffold-based MDA-MB-231 breast cancer model recreating a hypoxic environment was used to study its effects on phenotypic features and growth dynamics. [180] A decrease in the percentage of live cells was seen in 3D scaffolds, which was directly proportional to increased number of apoptotic events. Also, the hypoxic niche was shown to induce migration on collagen fibrils eventually leading to cellular senescence in MDA-MB-231 breast cancer cells illustrated by overexpression of senescence associated β-galactosidase marker. MDA-MB-231 breast cancer cells cultured in these scaffolds expressed increased expression of Bcl-2 associated X protein (Bax), caspase 3 and caspase 9 in comparison to monolayer cultures. [180]
4.3. Angiogenesis and Vascular Mimicry
Hypoxia is an established key regulator of tumor angiogenesis and vascular mimicry, which modulates angiogenic events in malignant cells via expression of HIFs. Angiogenesis as a whole is has been extensively studied and is shown to have a major correlation with hypoxia. However, along with hypoxia one must take into consideration other potential targets, which might engage in crosstalk with other immune cells and promote tumor angiogenesis.
An interesting biomimetic 3D model was created using macroporous type I collagen scaffolds (average porosity: 85 ± 6.3%) to study how hypoxic microenvironment affects the growth dynamics and phenotypic features in MCF-7 and MDA-MB-231 breast cancer cells. [180] These scaffolds induced hypoxic microenvironment in 3D MCF-7 and MDA-MB-231 breast cancer cells with elevated HIF-1α protein expression and activation of the glycolytic pathway indicated by high Glut-1 expression. Hypoxia also induced angiogenesis with elevated VEGF levels in scaffold media of MCF7 (p=0.006) and MDA-MB-231 cells (p=0.008) on day 7 in comparison to monolayer culture cells.[180] In one study [181], microfabricated alginate hydrogels (4% w/v) were used for 3D culture in an oxygen controlled (1% O2) environment to study how 3D culture influenced hypoxic responses and angiogenesis in MDA-MB-231 breast cancer cells. The findings supported a positive correlation between culture dimensionality and hypoxia response, which further regulated tumor angiogenesis via VEGF. This VEGF-mediated sprouting angiogenesis was reported to be enhanced via the action of IL-8, a pro-inflammatory cytokine.[181] Further, the effect of cyclic hypoxia (exposure to cycles of hypoxia) on angiogenesis and tumor vascularization was also studied in MCF-7 CSCs. [182] MCF-7 cell mammospheres were generated using ultra-low attachment plates and were exposed to hypoxic conditions continuously or intermittently. The secreted media from intermittently hypoxic CSCs showed elevated VEGF levels (1159±90 pg/ml) compared to continuously hypoxic stem cells (934±28 pg/ml) and control normoxic conditioned media (225±24 pg/ml). [182] Similar upregulation of VEGF-A and HIF-1α expression were reported in another study, which explored the angiogenic potential of MDA-MB-231 bioengineered tumors cultured in collagen I hydrogels exposed to closed vasculature of 150–200μm diameter induced hypoxia.[183]
In addition to the role of hypoxia in stimulating angiogenesis in the TME of malignant cells, intra-tumoral hypoxia has also been shown to induce formation of vessel-like tubes from tumor cells (VM).[84] In the context of breast cancer cells, presently there are few published studies, which have explored VM in 3D matrigel and collagen matrix. In the first study, Walker 256 (W256) rat breast carcinoma cells were cultured in cold matrigel under 2% O2 in a humidified tri-gas incubator and treated with CA4P, a vascular targeting agent responsible for depolymerizing microtubule. [85] The CA4P treatment elevated HIF-1α and induced tube formation indicative of VM network in W256 cells. Further, they also established a W256 breast tumor bearing mouse model, which when exposed to CA4P, induced intra-tumoral hypoxia and increased VM network formation in vivo. It was proposed that the induction of hypoxia and VM networks by CA4P was due to activation of HIF-1α/EphA2/PI3K/MMP signaling pathways. [85] In another study, MDA-MB-231 cells cultured in matrigel and transfected with pre-miR-2014 (30nM) were incubated in hypoxic conditions (1% O2) were observed to induce 3D tunnel formation (matrix-associated VM) in MDA-MB-231 cells. [89] Ectopic restoration of miR-204 was shown to inhibit VM and reduced patterned 3D channels. This miR-204 was reported to impair VM via downregulation of PI3K/AKT, VEGF, FAK/Src and RAF1/MAPK signaling pathways, thereby emphasizing the importance of PI3K/AKT/FAK pathways in VM formation.
Another study explored the influence of topographical organization of high-density collagen matrix in vivo on tumor progression, migration and metastasis. [184] The high-density collagen matrix triggered transcriptional response (upregulation of integrin subunit beta 1 (ITGB1), jagged canonical Notch ligand 1 (JAG1) and fibroblast growth factor receptor 1 (FGFR1) leading to motility switch in MDA-MB-231 breast cancer cells independent of matrix stiffness, density or hypoxia in comparison to low-density collagen matrix, which did not show migration. This high-density collagen matrix induced migration post one-week culture in vitro along with formation of interconnected network resembling endothelial tubulogenesis/VM. This migration phenotype was identified as collagen-induced network phenotype (CINP), which was shown to be driven by driven by β1-integrin upregulation and correlated with poor patient survival. [184]
4.4. Pro-Inflammatory Pathways
Recent developments in the field of microfluidic devices have made it possible to recapitulate 3D tumor microenvironments in vitro to study the effect of hypoxia on pro-inflammatory pathways. In an initial study, MDA-MB-231 breast cancer cells cultured in collagen I and exposed to 5% O2 levels were reported to have decreased JNKs, cortactin expression and activated transforming growth factor beta-1 (TGFβ1)/Smad signaling cascade. [14] This dysregulated JNK expression, activated Smad 2/4 and Snail1 further inhibited formation of invadopodia and expression of matrix metalloproteinase 14 (MMP14). [14] Following the initial report, a hypoxic 3D microenvironment was shown to regulate the hypoxic response and angiogenesis via pro-inflammatory pathways. The authors used an alginate-based hypoxic (oxygen-controlled) 3D model and reported elevated IL-8-mediated angiogenic sprouting in a 3D endothelial invasion assay. They further highlighted role of pro-inflammatory molecules and signaling cascade as a major modulators of hypoxic tumor responses in the TME. [181] Similarly, Yoshino and Funamoto demonstrated oxygen-dependent (normoxic: 21% O2 and hypoxic: 1% O2) contraction and degradation of ECM when human MDA-MB-231 breast cancer cells were co-cultured with HUVECs in a collagen gel. This was attributed to tumor-endothelial cell interactions coupled with downregulation of matrix metalloproteinase-7 (MMP-7). [185] Recently, 3D-heterotypic spheroid models (composed of RAW 264.7 macrophages and MDA-MB 231 breast cancer cells) were used to evaluate the role of TAMs in the TME. [186] The effect of hypoxia on this tumor/macrophage heterotypic 3D spheroid model exhibited elevated IL-10 secretion leading to activation of M2 TAMs differentiation pathway in the TME. [186] Further, an in-silico agent-based model was used to study hypoxia, chemokine receptor C-C chemokine receptor type 5 (CCR5) and cancer stem cell interactions in MDA-MB-231 cells. The group reported hypoxia-induced tumor progression in MDA-MB-231 cells, further showing that treatment with Maraviroc (a CCR5 inhibitor) decreased tumor cell growth. [187]
Although development of 3D microfluidic platforms made it possible to better replicate the TME in vitro, only a limited number of scientific studies have investigated hypoxic microenvironments. Further studies are warranted to understand the precise mechanism of which hypoxia modulates ECM dynamics and the pro-inflammatory pathways in the TME since these may become promising therapeutic targets.
4.5. Migration
Several 3D breast tumor models are being developed to understand the underlying mechanisms of tumor migration. The initial attempt to decipher the migration profile of breast cancer cells was carried out by Funamoto and colleagues [188] who designed a novel microfluidic platform to study the effect of controlled hypoxic microenvironment on MDAMB-231 cells. The microfluidic platform designed by the group consisted of a central 3D gel which acted as an extracellular matrix. This central region was flanked by media channels and peripheral gas channels that maintained the controlled hypoxic microenvironment (0% O2).[188] This microfluidic platform was used for imaging the migratory phenotypes of MDA-MB-231 cells every 10 min for >8h and enhanced migration was reported under hypoxia via activation of hypoxia associated signaling pathways in comparison to normoxic conditions. A rotatory culture method was used to generate 3D SUM159 and SUM149 breast spheroids, which were exposed to 1% O2 in a modular incubator chamber and evaluated for selectivity of hypoxia to induce integrin receptor expression, migration, and metastasis. The results highlighted the role of integrin alpha 5 (ITGA5) in inducing migration and metastasis to lymph nodes and lungs under hypoxic conditions (induced via elevated HIF-1α expression).[189] An innovative 3D breast tumor platform was developed consisting of photo-crosslinked hydrogel encapsulated with 21PT and 21MT-2 breast cancer cells derived from patient samples, which were exposed to 5% O2 levels in a tri-gas incubator.[190] This study showed decrease in tumor size and density, increase in EMT transition and migration, that was coupled to upregulation of lysyl oxidase (LOX). The model was also used to generate a breast cancer and lung cells contact model by stacking 3D hydrogel constructs with breast cancer cells onto lung mesenchymal cell (LMC) laden hydrogels. This unique model showed migration of breast cancer cells towards LMC cells under hypoxic conditions. In an interesting study carried out on MCF-7 and T47D spheroids cultured using matrigel and exposed to variable oxygen gradients in a hypoxic chamber (0.2–1% oxygen), hypoxia was shown to induce miR-191 in a time-dependent manner, which promoted migration in breast spheroids via upregulation of TGF-β2 in the hypoxic TME along with upregulation of its downstream target genes VEGFA, CTGF, SMAD family member 3 (SMAD3) and bone morphogenetic protein 4 (BMP4). [191]
3D models have also been exploited to determine the efficacy of inhibitors targeting hypoxia signaling pathway. Interestingly, the efficacy of five novel ureido-substituted CAIX inhibitors (FC11409B, FC9398A, S4, FC9403, and FC9396A) were assessed under normoxic (21%) and hypoxic (0.5% O2) in 3D breast spheroids cultured in spinner flasks, an ex-vivo explant model, and a MDA-MB-231 xenograft model. [192] Post-CAIX treatment, 3D breast cancer cells were shown to have decreased cell migration, extravasation and invasion potential. S4, FC9403A and FC9398A inhibited invasion into collagen while FC9403A was also reported to reverse established invasion. FC9398A was shown to reduce xenograft tumor growth. [192] Similar results were reported from another study where CAIX inhibitor (CAI017) inhibited migration in a dose-dependent manner in MDA-MB-231 cells cultured in matrigel and hypoxic (1% O2) conditions and orthotopic mice bearing breast tumors.[193] CAI017 inhibited migratory phenotype via modulation of downstream regulation of the mTORC1 axis. [193] In addition to CAIX inhibitors, LOX inhibitors have also been shown to inhibit migration and EMT. [190]
Currently, there are no preclinical in vitro models that show tumor progression in real time in the same tumor model using the same cell line without any genetic manipulations or any artificial culture conditions. Our lab has previously engineered an array of uniform size hydrogel microwells using microfabricated PDMS micro-posts of defined diameter and height (Figure 3A)[172, 194–197] The hydrogel microwells are made of polyethylene glycol dimethacrylate (PEGDMA), a non-adhesive polymer that does not allow cell-hydrogel interaction, and thus, maximizes cell-cell interaction forming hundreds of uniform size cell aggregates (referred henceforth as ‘microtumors’). The size of microtumor is determined by the diameter of the microwells on the array, the compaction ability of the cells used and the cell seeding density.[194, 195] For example, T47D breast cancer cells seeded on a device containing 150 μm diameter hydrogel microwells result in microtumors of average diameter of 128±16 μm on day 6 (referred as ‘small’ microtumors) while that containing 600 μm diameter hydrogel microwells result in microtumors of average diameter of 550 ± 57 μm (referred as ‘large’ microtumors) by day 6.[195] Each 1×1 cm2 device of 150 μm size microwells generates 350–450 small microtumors while each 1×1 cm2 device of 600 μm size microwells generates 60–80 large microtumors.[195] Thus, the hydrogel microwell platform can generate hundreds of defined yet uniform size microtumors from the same parent cells. We have shown that precise control over microtumor size naturally creates spatial oxygen/nutrient diffusion gradients leading to controlled yet reproducible hypoxic environments.[195] Hypoxia, ROS, and propidium iodide (PI)+ cells were seen in the large microtumor cores, along with increased HIF-1α and metabolic stress-induced Glut-1 expression, but not in non-hypoxic small microtumors (Figure 3B). Interestingly, without any additional stimulus, large T47D microtumors exhibit a migratory phenotype as early as day 3–4 while small microtumors generated from the same parent cells never migrate (Figure 3C).[172, 195, 196] That is, the migratory phenotype emerged spontaneously based on differential microtumor size only. These results are consistent with the clinical findings that critical genetic mutations pre-exist prior to the onset of tumor migration/invasion, and that microenvironmental factors, like tumor size-induced hypoxia and metabolic stress, drive transition from a non-migratory to a migratory phenotype.[141, 198–202] In addition, we observed the emergence of heterogeneous cellular populations inside each large microtumor (‘intra-tumoral’ heterogeneity) based on the expression of the epithelial marker, E-Cadherin (E-Cad) and mesenchymal marker, Vimentin (VIM) along with tumor-to-tumor variation in the migration kinetics (‘inter-tumoral’ variation).[195, 196] Thus, by manipulating microtumor size alone and without any genetic manipulations or exogenous stimuli, we reproducibly generated two distinct phenotypes from the same noninvasive parent breast cancer cells: small non-hypoxic microtumors represent a non-migratory phenotype whereas large hypoxic microtumors exhibit a migratory phenotype. These microtumor models recapitulate three key hallmarks of pre-invasive to invasive disease progression that is observed in vivo: 1) Increasing tumor size drives hypoxia and metabolic stress; 2) Heterogeneous tumor cells spontaneously emerge; and 3) Cells begin to migrate from the parent tumor.
Figure 3: Size controlled 3D uniform micro-tumors recapitulate tumor microenvironment.
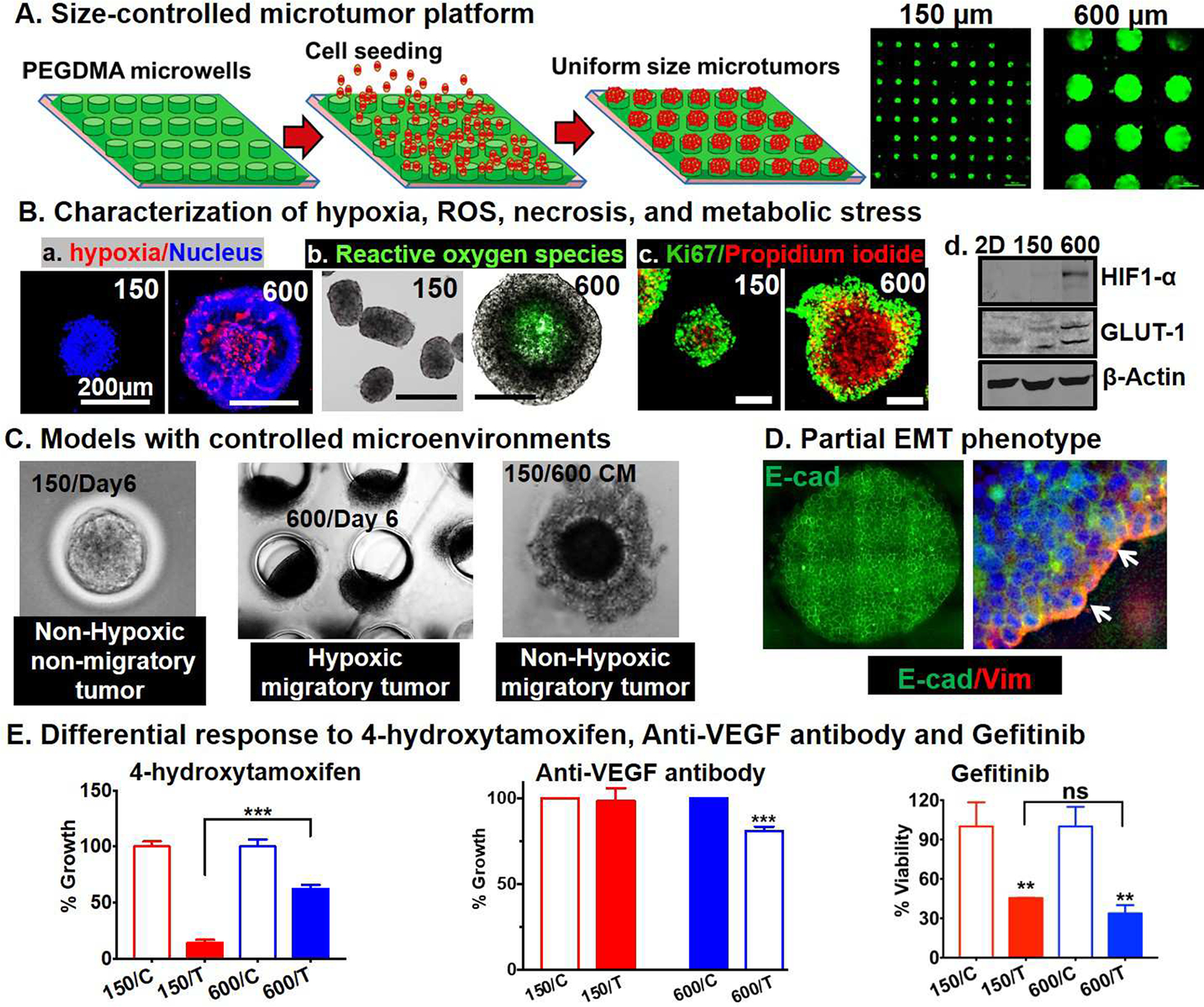
(A) Fabrication of 150μm and 600 μm micro-well devices on polyethylene glycol dimethacrylate 1000 (PEGDMA) hydrogel microarrays to develop 3D micro-tumors. (B) (a-c) Large microtumors, 600μm show high hypoxia, reactive oxygen species (ROS) in the core of tumor spheroids and high Ki67 staining in the periphery. (d) 600μm microtumors express high hypoxia inducible factor 1 (HIF-1) and glucose transporter 1 (GLUT-1) (C) Microscopic images showing non-migratory and non-hypoxic - 150μm micro-tumors, hypoxic and migratory - 600μm micro-tumors and non-hypoxic, migratory 150μm micro-tumors incubated with 600μm media. (D) Migratory front in 600μm micro-tumors show tumor heterogeneity and both E-cadherin (E-cad) and Vimentin (VIM) expression representative of the partial- epithelial to mesenchymal transition (EMT) phenotype. (E) Differential response of 150μm and 600μm micro-tumors treated with 4-hydroxytamoxifen (4-OHT), anti-vascular endothelial growth factor (VEGF) and gefitinib.
Another interesting feature of our microtumor model is that the large microtumors exhibit partial or hybrid EMT phenotype (Figure 3D) without loss of E-cad along with upregulation of mesenchymal markers, Snail, Slug and Vim. [195] Our large T47D microtumors recapitulate the collective migration observed in clinical samples of carcinoma.[203, 204] Additionally, the large microtumors were shown to retain their migratory phenotype even after removal of hypoxia suggesting that the migratory phenotype is irreversible.[195] To further delineate the mechanisms underlying this irreversible migratory phenotype, we generated non-hypoxic, yet migratory microtumor models by exposure of small non-hypoxic microtumors to the conditioned media of large microtumors. [196] This study showed that the hypoxic secretome, specifically, soluble E-cad, MMP9 and fibronectin in the conditioned media of large migratory microtumors induced collective migration in the non-hypoxic, non-migratory small microtumors. We proposed a novel two-stage tumor progression mechanism, where hypoxia is important in the early ‘initiation stage’ of tumor progression, and the positive feedback loop among the secretome factors works synergistically to maintain the migratory phenotype (‘maintenance stage’). Computational and experimental studies in 3D microtumor models (using cell lines and patient derived cancer cells) showed that inhibition of tumor-secreted factors effectively halts microtumor migration despite tumor-to-tumor variation in migration kinetics, while inhibition of hypoxia is effective only within a time window and is compromised by tumor-to-tumor variation, supporting our notion that hypoxia initiates migratory phenotypes but does not sustain it.
In summary, 3D models have advanced our understanding of tumor-intrinsic, cell-cell and cell-ECM mediated mechanisms related to hypoxia, EMT and migration.
4.6. Drug Resistance
Hypoxia is widely known to promote drug resistance to conventional chemotherapeutic drugs and radiation therapy. However, the precise mechanism as to how hypoxic cells acquire drug resistance still remains unknown. It is postulated that reduced metabolism, genetic instability, loss of p53-mediated sensitivity to apoptosis or activation of genes regulating multiple drug resistance (e.g. MDR1 encoding P-gp) may be key modulators in acquisition of drug resistance. Recently, numerous studies have also highlighted the role of CSCs with attributes of self-renewal, drug resistance and tumor relapse along with regulation of miRNAs as major contributors for failure of cancer treatments. In view of the limitations of 2D experimental systems, 3D models have become the choice of experimental models due to their ability to replicate tumor biology in vivo and investigate responses to chemotherapeutic agents. 3D spheroids or scaffold-based models are also important for the study of tumor drug resistance. Using the conventional ultralow attachment plate for spheroid formation, different groups have tried to explore the drug resistance biology in breast cancer cells. In one of the initial studies, 3% O2 in a humidified hypoxic chamber was identified as sufficient to induce HIF-1α activation in MCF-7 spheroids.[205] In this study, MCF-7 spheroids were resistant to doxorubicin (3μmol/L), which was associated with elevated P-gp expression via activation of HIF-1α.[205] In another study, [206] MCF-10A cells exposed to humidified hypoxic chamber (1% O2) induced HIF-1α activation, which in turn, induced lapatinib resistance via activation of the downstream Erb-B2 receptor tyrosine kinase 2 (ERBB2) signaling cascade, ERK/AKT. The study also elucidated that administration of trametinib (a MEK inhibitor) or over-expression of dual-specificity phosphatase 2 (DUSP2) reversed hypoxia-induced lapatinib resistance.[206] Recent technology advancements, have provided new 3D models to study the effect of hypoxia on drug resistance, which are summarized below.
When MDA-MB-231 spheroids encapsulated in collagen matrix were placed in a hypoxic chamber (1% O2), it was found that hypoxia stimulated proliferation of spheroids in cells treated with paclitaxel (IC50 532.8±74.4) and doxorubicin (IC50 21.0±28.5 nM) vs. the untreated tumor spheroids emphasizing the role of hypoxia induced chemoresistance.[207] Similarly, hypoxic MDA-MB-231 spheroids cultured in 0.6% agar showed activated cleaved Notch 1 levels and AMP-activated protein kinase (AMPK). [208]I another study, a semisolid culture system was established for MCF-7 spheroids and exposed to hypoxic chamber (0.1% pO2 and 5% CO2) to study effect of hypoxia on stemness.[209] The group reported that the hypoxic MCF-7 cells were resistant to paclitaxel, gemcitabine and adriamycin and overexpressed miR-210 in breast cancer stem cells, which suppressed E-cad expression by targeting open reading frame the (ORF) of E-cad. [209] In another study, the hanging drop method was used to generate BT-474 spheroids, which were then exposed to hypoxia using two different methods i.e. 1% O2 in hypoxic chamber and CoCl2 method.[210] An increase in breast cancer stem cell population was observed in hypoxic HER2+ BT-474 spheroids (CD44+/CD24low) with acquired trastuzumab resistance. [210] In a study carried out in MDA-MB-157 triple negative breast cancer cells, tumor spheroids were generated using polymeric aqueous two-phase system (ATPS) technology, which generated uniform yet controlled size spheroids (100μm and 200μm) depending on cell densities (1.5*104 cells for 100μm and 1.0*105 for 200 μm spheroids). [211] The larger MDA-MB-157 spheroids treated with doxorubicin (100*10−9M) showed ten-fold higher drug resistance (IC50 of 481*10−9M) than smaller spheroids. Further, the group co-treated larger spheroids with doxorubicin (100*10−9M) and TH-302 (a hypoxia activated prodrug, 10*10−6M) and the combined treatment was seen to decrease cell viability by 30% and had a combination index (CI) of 0.02 (measure of high synergistic effect of two drugs). The larger MDA-MB-157 spheroids were also seen to express the cancer stem cell markers, namely, CD24, CD133 and Nanog. These results were supported by the hypothesis of hypoxia leading to transcriptional activation of genes encoding stem cells. [211] In a recent study from another group [212], hypoxia induced a switch via SNAT2/Solute carrier family 38 member 2 (SLC38A2) signaling regulation, which was responsible for generation of endocrine resistance in hypoxic (0.1% O2) MCF-7 spheroids. Additionally, the 3D-heteotypic spheroid model (tumor cell (MDA-MB 231) and macrophage (RAW 264.7)) discussed above has also been used to evaluate the effect of paclitaxel treatment. [186] The first heterotypic spheroid model, mimicked the TAMs infiltrated into tumor mass and showed resistance to paclitaxel while in the second model, macrophages diffusely seeded around the spheroids showed the same metabolic profiles irrespective of the presence of tumor spheroids.[186] The findings from this study highlight the synergistic growth pattern of TAMs and tumor cells in a heterospheroid model mimicked in vivo conditions which play a role in paclitaxel drug resistance. In yet another interesting study, Alhawarat and colleagues illustrated higher chemoresistance in prolonged hypoxia treated CSC spheroids.[182] The 3D-MCF-7 CD44+/CD24− CSCs under normoxic conditions exhibited high drug resistance to doxorubicin (4.08 fold) in comparison to parent 2D cultures, which increased further (3.5 fold) when hypoxic MCF-7 CSCs were exposed to repetitive long-term continuous/intermittent cycles of hypoxia for four months.[182]
We have shown differential drug response in our non-migratory small (150 μm) vs. migratory large microtumor models (600 μm) highlighting a significant role for the TME in dictating molecular mechanisms involved in tumor progression and consequently, tumor response to molecular therapies.[172] Although small and large microtumors were generated from the same ER+ parental T47D cell line, size-dependent microenvironmental changes such as hypoxia, ROS, and pro-angiogenic factors induced downstream signaling such as loss of ER-α and upregulation of VEGF in large microtumors. Such differential molecular changes between 150 and 600 μm microtumors further led to differential response to ER-targeted anti-estrogen, and anti-VEGF antibodies without much difference in EGFR-targeted tyrosine kinase inhibitor (TKI), gefitinib response (Figure 3E). Non-migratory small microtumors retaining ERα expression were more sensitive to anti-estrogen 4-hydroxytamoxifen similar to early stage breast cancer patients while migratory large microtumors with loss of ERα showed endocrine resistance; however, upregulated VEGF levels rendered them more sensitive to anti-VEGF therapy similar to the advanced stage breast cancer patients. With similar expression levels of signaling molecules involved in the EGFR pathway, both small and large microtumors responded in a size-independent manner to EGFR-targeted TKI gefitinib. We confirmed that differential drug response observed in 150 and 600 μm microtumors was not due to microtumor size-mediated drug diffusion limitations but indeed due to the differential microenvironment-mediated signaling changes. This study underscores the importance of TME in regulation of molecular mechanisms involved in tumor progression and hence, it warrants development of physiologically relevant 3D models that recapitulate underlying molecular mechanisms of disease progression. These efforts will be instrumental for effective screening of targeted therapies. These studies collectively provide the scientific evidence of hypoxia induced chemoresistance (Table III). This also leads the way for therapeutic evaluation of key pathway molecules modulating hypoxia induced chemo-resistance and their inhibitors, which is important to develop better treatment modalities.
Table III:
In vitro studies of drug resistance in 3D-breast cancer tumor models
| Cell line model | Drugs Tested | Conclusion | Ref |
|---|---|---|---|
| MCF-7 | Doxorubicin (3μM,18 h) Verapamil (Pgp inhibitor, 100μM) YC-1 (5μM, 24h) |
MCF-7 3D-spheroids resistant to doxorubicin associated with elevated Pgp expression via activation of HIF-1α | [205] |
| MDA-MB-231 | Paclitaxel (1–10nM) Doxorubicin (0–10nM) |
Hypoxic spheroids resistant to paclitaxel and doxorubicin show progression | [207] |
| BT-549 | Paclitaxel Doxorubicin |
Dense spheroid formation simulates hypoxia, anti-apoptotic features and drug resistance | [138] |
| BT-474 | |||
| T-47D | |||
| MCF-7 | |||
| DA-MB-231 | |||
| HCC-1954 | |||
|
MCF10A-ERBB2 MTEC-Neu SK-BR3 |
Lapatinib (1μM) Trametinib (MEK inhibitor) |
HIF-1 blocks lapatinib mediated effects in ERBB2 cells and maintain downstream ERK signaling via inhibition of DUSP2. Trametinib treatment reverses hypoxia- mediated lapatinib resistance | [206] |
| MDA-MB-157 3D | Doxorubicin (100*10−9M) 5-Fluorouracil TH-302 (Hypoxia-activated prodrug) |
Combination treatment (TH-302 and doxorubicin) targets drug resistant spheroids | [211] |
|
MDA-MB-231 RAW 264.7 |
Paclitaxel (10ng/mL) Tyrphostin AG 1478(EGFR inhibitor) |
Macrophages in heterotypic spheroids increase resistance to paclitaxel | [186] |
| BT-474 | HER2+ BT-474 spheroids resistant to trastuzumab with increased breast cancer cells | [210] | |
| MCF-7 | Doxorubicin | Hypoxic cancer stem cells illicit high drug resistance to doxorubicin compared to normoxic cancer stem cells | [182] |
|
MCF-7 MDA-MB-231 |
Paclitaxel Gemcitabine Adriamycin |
Survival rates of breast cancer stem cells high in MCF-7 spheroid cells with drug treatment | [209] |
| MDA-MB-231 | Doxycycline (5μg/μL) Doxorubicin (1μM) | AMPK mediates hypoxia induced drug resistance in MDA-MB-231 cells via Notch1 signaling | [208] |
| MCF-7 | Tamoxifen Fulvestrant |
Hypoxia induces switch in SNAT2/SLC38A2 regulation which leads to resistance to anti-hormone therapy | [212] |
P-glycoprotein (Pgp), Hypoxia inducible factor 1 alpha (HIF-1α), Erb-B2 receptor tyrosine kinase 2 (ERBB2), Extracellular signal regulated kinase (ERK), Dual specificity phosphatase 2 (DUSP2), Mitogen activated protein kinase (MEK), Epidermal growth factor receptor (EGFR), Human epidermal growth factor receptor 2 (HER2), 5’-Adenosine monophosphate activated protein kinase (AMPK), Sodium-coupled neutral amino acid transporter 2 (SNAT2), Solute carrier family 38 member 2 (SLC38A2).
5. Future Prospects and Conclusion
This review highlights the role of hypoxia in modulating cellular signaling pathways in directing oncogenic transformations breast cancer progression at different phases of tumor progression. The hypoxic transformations in the tumor cells mediated via expression of hypoxia-inducible factors promote tumor survival and progression in breast cancer cells via metabolic adaptations, angiogenesis, stromal cell recruitment, migration, tissue invasion, extracellular matrix modelling, extravasation to metastatic sites and drug resistance. It is important to note that the current understanding of molecular mechanisms in cancer biology in general and hypoxia biology specifically is heavily derived from 2D cultures that do not recapitulate important components of TME: tumor cells, stromal cells, and non-cellular factors (hypoxia, pH, metabolic stress, ECM, etc.). Hence, there is significant need to validate the currently known mechanisms using physiologically relevant 3D models. We have highlighted several studies that have used 3D in vitro culture systems to mimic invasive behavior of cancer cells, epithelial and mesenchymal transition, and cell-cell/cell-ECM interactions. We have also highlighted studies with effort to understand the effect of therapeutic agents on signaling pathways in a 3D microenvironment. 3D tumor spheroids still remain the most widely used model for studying tumor biology. Although the field is recognizing the importance of 3D models in cancer biology and is moving in the right direction, most studies discussed here have utilized hypoxic chambers to create hypoxic microenvironments in 3D.
Recent technological advances in tissue engineering and materials science have launched newer methods for generating 3D models like bioreactors, bioprinters and advanced complex microfluidics. These methods are being fine-tuned to offer greater control over culture complexity in terms of incorporating multiple components of TME as well as culture conditions such as oxygen, nutrient supply etc. Technologies are also evolving to monitor/image dynamic changes in cellular behaviors in 3D cultures over a period of time. Similarly, there are efforts to characterize and validate the changes in 3D cultures using genomic and transcriptomic profiles. With the advantages of 3D models, these insights will provide us the understanding of the evolution of the TME in 3D and its relevance to clinical samples.
Although there are significant technological advancements in generating variety of 3D tumor models and a huge repertoire of available models to choose from, translation of these models to study hypoxia biology in particular is a long way ahead. This is supported by the limited studies, which have exploited the strengths of 3D models to understand the fundamental role of hypoxia biology in regulating breast cancer progression. It should also be borne in mind that each 3D model may not be able to recapitulate all aspects of in vivo TME and disease pathology and that the context, the hypothesis will play significant role in selecting the appropriate model. Hence, it is important to determine the essential components of the 3D models to successfully test the hypothesis. It is equally important to consider validation process for the 3D models. The validation steps could be related to the validation of the known molecular mechanisms or the clinical response to the known drugs. The validation could also include use of patient-specific samples to support the findings observed in these in vitro 3D models. In order to advance our understanding of genetic and epigenetic mechanisms involved in hypoxia mediated tumor progression and to unravel novel therapeutic targets for benefit of the patients, it is instrumental that we promote crosstalk among multiple disciplines including cancer biology, pharmacology, materials science, bioengineering and bioinformatics.
Highlights.
Role of hypoxia in directing oncogenic transformations i.e. metabolic adaptations, angiogenesis, stromal cell recruitment, migration, tissue invasion, extracellular matrix modeling, extravasation to metastatic sites and drug resistance via modulation of cellular signaling pathways.
Techniques to develop 3D in vitro models replicating tumor microenvironments and hallmarks of cancer progression such as metabolic stress, angiogenesis, epithelial and mesenchymal transition, migration, invasion, and drug resistance.
Opportunities and challenges for the translation of 3D models to understand the fundamental role of hypoxia biology in regulating breast cancer progression.
7. Funding
This work is supported by the National Institute of Health (NIH) [R37CA232209] to SS.
Abbreviations
- 2D
Two-dimensional
- 3-D
Three-dimensional
- TME
Tumor microenvironment
- ECM
Extracellular matrix
- TNBC
Triple negative breast cancer
- HIF-1
Hypoxia inducible factor 1
- HIF-1α
Hypoxia inducible factor 1 alpha
- HIF-1β
Hypoxia inducible factor 1 beta
- SNAT2
Sodium-coupled neutral amino acid transporter 2
- CAIX
Carbonic anhydrase IX
- VEGF
Vascular endothelial growth factor
- BNIP-3
BCL2 interacting protein 3
- ROS
Reactive oxygen species
- NADPH
Nicotinamide adenine dinucleotide phosphate
- JNK
c-Jun N-terminal kinase
- NF-κB
Nuclear factor kappa B subunit
- CAV-1
Caveolin-1
- MCT4
Monocarboxylate transporter 4
- PHLDA3
Pleckstrin homology like domain family A member 3
- INPP5D
Inositol polyphosphate-5-phosphatase
- HRE
HIF-1 response element
- NIX
Nip3 like protein X
- TRAIL
TNF-related apoptosis inducing ligand
- BRCA1
Breast cancer susceptibility gene 1
- STAT5b
Signal transducer and activator of transcription 5B
- MMPs
Matrix metalloproteinases
- FGF
Fibroblast growth factor
- PDGF-β
Platelet-derived growth factor-β
- SDF-1α
Stromal-derived factor 1α
- Ang-1
Angiopoietin-1
- Ang-2
Angiopoietin-2
- TGF-β
Transforming growth factor beta 1
- PPARα
Peroxisome proliferators-activated receptors
- mTOR
Mammalian target of rapamycin
- MAPK
Mitogen-activated protein kinase
- ERK
Extracellular signal-regulated kinase
- MAPK/ERK
MEK
- TAMs
Tumor associated macrophages
- P-gp
P-glycoprotein
- HMGB1
High-mobility group box 1
- AKT
AKT serine/threonine kinase
- PI3K
Phosphatidylinositol-3-kinase
- iNOS
Inducible nitric oxide synthase
- Src
proto-oncogene tyrosine-protein kinase Src
- ADAM17
ADAM metallopeptidase domain 17
- BET
Bromodomain and extra-terminal
- CA9
Carbonic anhydrase
- VEGF-A
Vascular endothelial growth factor A
- VM
Vascular mimicry
- CA4P
Combretastatin A4 phosphate
- RTK
Receptor tyrosine kinase
- VE-cadherin
Vascular endothelial cadherin
- DMBT
6,6’-bis(2,3-dimethoxybenzoyl)-a,a-D-trehalose
- EGFR
Epidermal growth factor receptor
- EphA2
Erythropoietin producing hepatoma receptor A2
- MMP2
Matrix metallopeptidase 2
- MMP9
Matrix metallopeptidase 9
- Cdc42
Cell division cycle 42
- TP52INP1
Tumor protein p53-inducible nuclear protein 1
- GSK-3β
Glycogen synthase kinase 3 beta
- FAK
Focal adhesion kinase
- RAF1
Raf-1 proto-oncogene, serine/threonine kinase
- IL-1
Interleukin-1
- IL-6
Interleukin-6
- C/EBPα
CCAAT/enhancer binding protein-α
- TNF-α
Tumor necrosis factor–alpha
- A2AAR
A2A adenosine receptor
- A2BAR
A2B adenosine receptor
- NK
Natural killer
- IL-4
Interleukin-4
- IL-10
Interleukin-10
- A2AR
A2 adenosine receptors
- CTGF
Connective tissue growth factor
- CCL2
C-C motif chemokine ligand 2
- COX-2
Cyclooxygenase-2
- HDAC
Histone deacetylase
- IL-1β
Interlekuin-1 beta
- IL-12
Interleukin-12
- EMT
Epithelial to mesenchymal transition
- IL-8
Interleukin-8
- HGF
Hepatocyte growth factor
- RhoA
Ras homolog family member A
- ROCK1
Rho associated coiled-coil containing protein kinase 1
- ILK
Integrin-linked kinase
- PDGF
Platelet-derived growth factor B
- SOX2
SRY-box-2
- NEDD9
Neural precursor cell expressed, developmentally down-regulated 9
- Nrf2
Nuclear factor erythroid-2-related factor 2
- Keap1
Kelch-like-ECH-associated protein 1
- G6PD
Glucose-6-phosphate dehydrogenase
- HES-1
Hes family BHLH transcription factor 1
- elF4E2
Eukaryotic translation initiation factor 4E type 2
- ZMYND8
Zinc finger MYND-type containing 8
- BRD4
Bromodomain containing 4
- CSCs
Cancer stem cells
- BCRP
Breast cancer resistance protein
- MDR1
Multidrug resistance gene 1
- MRP1/2
Multidrug resistance associated protein 1/2
- PPARα
Peroxisome proliferators-activated receptor alpha
- CAR
Constitutive androstane receptor
- PXR
pregnane X receptor
- FXR
Farnesoid X receptor
- FGF
Fibroblast growth factor
- FGFR
FGF receptor
- CAFs
Cancer-associated fibroblasts
- SDF-1
Stromal cell-derived factor
- CXCL12
C-X-C motif chemokine 12
- SCLC
Small cell lung cancer
- PDXs
Patient-derived xenografts
- MCTS
Multicellular tumor spheroids
- DMD
Digital micromirror device
- TRACER
Tissue roll for the analysis of cellular environment and response
- PDMS
Polydimethylsiloxane
- hBM_MSCs
Human bone marrow-derived mesenchymal stem cells
- HUVECs
Human umbilical vein endothelial cells
- OD
Osteo-differentiated
- HTS
High-throughput screening
- GEM
Global Eukaryotic Microcarriers
- 3D-PREDICT
3D-prediction of patient-specific response
- ERα
Estrogen receptor alpha
- PGR
Progesterone receptor
- AREG
Amphiregulin
- PDZK1
PDZ containing domain 1
- HNSCC
Head and neck squamous cell carcinoma
- DCIS
Ductal carcinoma in situ
- MS
Mass spectrometry
- MSI
MALDI-MS imaging
- PCA-LDA
Principal component analysis-linear discriminant analysis
- PC
Phosphatidylcholine
- SM
Sphingomyelin
- Bax
Bcl-2 associated X protein
- MMP14
Matrix metalloproteinase 14
- MMP-7
Matrix metalloproteinase-7
- CCR5
C-C chemokine receptor type 5
- ITGA5
Integrin alpha 5
- LOX
Lysyl oxidase
- LMC
Lung mesenchymal cells
- SMAD3
SMAD family member 3
- PEGDMA
Polyethylene glycol dimethacrylate
- E-cad
E-Cadherin
- VIM
Vimentin
- miRNAs
MicroRNAs
- DUSP2
Dual-Specificity Phosphatase 2
- ERBB2
Erb-B2 receptor tyrosine kinase 2
- AMPK
AMP-activated protein kinase
- ORF
Open reading frame
- ATPS
Aqueous two-phase system
- CI
Combination index
- SLC38A2
Solute carrier family 38 member 2
- FGFR1
Fibroblast growth factor receptor 1
- JAG1
Jagged canonical Notch ligand 1
- ITGB1
Integrin subunit beta 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no conflict of interest.
References
- [1].Bray F, McCarron P, Parkin DM, The changing global patterns of female breast cancer incidence and mortality, Breast Cancer Res, 6 (2004) 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA Cancer J Clin, 68 (2018) 394–424. [DOI] [PubMed] [Google Scholar]
- [3].Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J, Cardoso F, Breast cancer, Nat Rev Dis Primers, 5 (2019) 66. [DOI] [PubMed] [Google Scholar]
- [4].Provenzano E, Ulaner GA, Chin SF, Molecular Classification of Breast Cancer, PET Clin, 13 (2018) 325–338. [DOI] [PubMed] [Google Scholar]
- [5].Vuong D, Simpson PT, Green B, Cummings MC, Lakhani SR, Molecular classification of breast cancer, Virchows Arch, 465 (2014) 1–14. [DOI] [PubMed] [Google Scholar]
- [6].Aggarwal V, Priyanka K, Tuli HS, Emergence of Circulating MicroRNAs in Breast Cancer as Diagnostic and Therapeutic Efficacy Biomarkers, Mol Diagn Ther, (2020). [DOI] [PubMed] [Google Scholar]
- [7].Gao T, Li JZ, Lu Y, Zhang CY, Li Q, Mao J, Li LH, The mechanism between epithelial mesenchymal transition in breast cancer and hypoxia microenvironment, Biomed Pharmacother, 80 (2016) 393–405. [DOI] [PubMed] [Google Scholar]
- [8].Gilkes DM, Implications of Hypoxia in Breast Cancer Metastasis to Bone, Int J Mol Sci, 17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu ZJ, Semenza GL, Zhang HF, Hypoxia-inducible factor 1 and breast cancer metastasis, J Zhejiang Univ Sci B, 16 (2015) 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schito L, Rey S, Hypoxic pathobiology of breast cancer metastasis, Biochim Biophys Acta Rev Cancer, 1868 (2017) 239–245. [DOI] [PubMed] [Google Scholar]
- [11].Aggarwal V, Das A, Bal A, Srinivasan R, Das R, Prakash G, Malhotra P, Varma S, MYD88, CARD11, and CD79B Oncogenic Mutations are Rare Events in the Indian Cohort of De Novo Nodal Diffuse Large B-Cell Lymphoma, Appl Immunohistochem Mol Morphol, 27 (2019) 311–318. [DOI] [PubMed] [Google Scholar]
- [12].Khan S, Shukla S, Sinha S, Meeran SM, Role of adipokines and cytokines in obesity-associated breast cancer: therapeutic targets, Cytokine Growth Factor Rev, 24 (2013) 503–513. [DOI] [PubMed] [Google Scholar]
- [13].Luo B, Li W, Wang R, Lu H, Yang T, Jia Z, [Effect of hypoxia on expression of multidrug resistance protein 2 and its regulation mechanism], Zhong Nan Da Xue Xue Bao Yi Xue Ban, 42 (2017) 98–107. [DOI] [PubMed] [Google Scholar]
- [14].Lee MS, Kim S, Kim BG, Won C, Nam SH, Kang S, Kim HJ, Kang M, Ryu J, Song HE, Lee D, Ye SK, Jeon NL, Kim TY, Cho NH, Lee JW, Snail1 induced in breast cancer cells in 3D collagen I gel environment suppresses cortactin and impairs effective invadopodia formation, Biochim Biophys Acta, 1843 (2014) 2037–2054. [DOI] [PubMed] [Google Scholar]
- [15].Harris AL, Hypoxia--a key regulatory factor in tumour growth, Nat Rev Cancer, 2 (2002) 38–47. [DOI] [PubMed] [Google Scholar]
- [16].Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG, Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis, Neuro Oncol, 7 (2005) 134–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Aggarwal V, Tuli HS, Varol A, Thakral F, Yerer MB, Sak K, Varol M, Jain A, Khan MA, Sethi G, Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements, Biomolecules, 9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Semenza GL, The hypoxic tumor microenvironment: A driving force for breast cancer progression, Biochim Biophys Acta, 1863 (2016) 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G, Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer, Cancer Res, 60 (2000) 4693–4696. [PubMed] [Google Scholar]
- [20].Dhani N, Fyles A, Hedley D, Milosevic M, The clinical significance of hypoxia in human cancers, Semin Nucl Med, 45 (2015) 110–121. [DOI] [PubMed] [Google Scholar]
- [21].Vaupel P, Mayer A, Hypoxia in cancer: significance and impact on clinical outcome, Cancer Metastasis Rev, 26 (2007) 225–239. [DOI] [PubMed] [Google Scholar]
- [22].Jogi A, Ehinger A, Hartman L, Alkner S, Expression of HIF-1alpha is related to a poor prognosis and tamoxifen resistance in contralateral breast cancer, PLoS One, 14 (2019) e0226150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Campbell EJ, Dachs GU, Morrin HR, Davey VC, Robinson BA, Vissers MCM, Activation of the hypoxia pathway in breast cancer tissue and patient survival are inversely associated with tumor ascorbate levels, BMC Cancer, 19 (2019) 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jin MS, Lee H, Park IA, Chung YR, Im SA, Lee KH, Moon HG, Han W, Kim K, Kim TY, Noh DY, Ryu HS, Overexpression of HIF1alpha and CAXI predicts poor outcome in early-stage triple negative breast cancer, Virchows Arch, 469 (2016) 183–190. [DOI] [PubMed] [Google Scholar]
- [25].Mack PC, Redman MW, Chansky K, Williamson SK, Farneth NC, Lara PN Jr., Franklin WA, Le QT, Crowley JJ, Gandara DR, Swog, Lower osteopontin plasma levels are associated with superior outcomes in advanced non-small-cell lung cancer patients receiving platinum-based chemotherapy: SWOG Study S0003, J Clin Oncol, 26 (2008) 4771–4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Patel A, Sant S, Hypoxic tumor microenvironment: Opportunities to develop targeted therapies, Biotechnol Adv, 34 (2016) 803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].von Pawel J, von Roemeling R, Gatzemeier U, Boyer M, Elisson LO, Clark P, Talbot D, Rey A, Butler TW, Hirsh V, Olver I, Bergman B, Ayoub J, Richardson G, Dunlop D, Arcenas A, Vescio R, Viallet J, Treat J, Tirapazamine plus cisplatin versus cisplatin in advanced non-small-cell lung cancer: A report of the international CATAPULT I study group. Cisplatin and Tirapazamine in Subjects with Advanced Previously Untreated Non-Small-Cell Lung Tumors, J Clin Oncol, 18 (2000) 1351–1359. [DOI] [PubMed] [Google Scholar]
- [28].Williamson SK, Crowley JJ, Lara PN Jr., McCoy J, Lau DH, Tucker RW, Mills GM, Gandara DR, Southwest S Oncology Group Trial, Phase III trial of paclitaxel plus carboplatin with or without tirapazamine in advanced non-small-cell lung cancer: Southwest Oncology Group Trial S0003, J Clin Oncol, 23 (2005) 9097–9104. [DOI] [PubMed] [Google Scholar]
- [29].Wigerup C, Pahlman S, Bexell D, Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer, Pharmacol Ther, 164 (2016) 152–169. [DOI] [PubMed] [Google Scholar]
- [30].Borad MJ, Reddy SG, Bahary N, Uronis HE, Sigal D, Cohn AL, Schelman WR, Stephenson J Jr., Chiorean EG, Rosen PJ, Ulrich B, Dragovich T, Del Prete SA, Rarick M, Eng C, Kroll S, Ryan DP, Randomized Phase II Trial of Gemcitabine Plus TH-302 Versus Gemcitabine in Patients With Advanced Pancreatic Cancer, J Clin Oncol, 33 (2015) 1475–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chawla SP, Cranmer LD, Van Tine BA, Reed DR, Okuno SH, Butrynski JE, Adkins DR, Hendifar AE, Kroll S, Ganjoo KN, Phase II study of the safety and antitumor activity of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma, J Clin Oncol, 32 (2014) 3299–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].DiSilvestro PA, Ali S, Craighead PS, Lucci JA, Lee YC, Cohn DE, Spirtos NM, Tewari KS, Muller C, Gajewski WH, Steinhoff MM, Monk BJ, Phase III randomized trial of weekly cisplatin and irradiation versus cisplatin and tirapazamine and irradiation in stages IB2, IIA, IIB, IIIB, and IVA cervical carcinoma limited to the pelvis: a Gynecologic Oncology Group study, J Clin Oncol, 32 (2014) 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hicks KO, Siim BG, Jaiswal JK, Pruijn FB, Fraser AM, Patel R, Hogg A, Liyanage HD, Dorie MJ, Brown JM, Denny WA, Hay MP, Wilson WR, Pharmacokinetic/pharmacodynamic modeling identifies SN30000 and SN29751 as tirapazamine analogues with improved tissue penetration and hypoxic cell killing in tumors, Clin Cancer Res, 16 (2010) 4946–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hunter FW, Young RJ, Shalev Z, Vellanki RN, Wang J, Gu Y, Joshi N, Sreebhavan S, Weinreb I, Goldstein DP, Moffat J, Ketela T, Brown KR, Koritzinsky M, Solomon B, Rischin D, Wilson WR, Wouters BG, Identification of P450 Oxidoreductase as a Major Determinant of Sensitivity to Hypoxia-Activated Prodrugs, Cancer Res, 75 (2015) 4211–4223. [DOI] [PubMed] [Google Scholar]
- [35].Rischin D, Peters LJ, O’Sullivan B, Giralt J, Fisher R, Yuen K, Trotti A, Bernier J, Bourhis J, Ringash J, Henke M, Kenny L, Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group, J Clin Oncol, 28 (2010) 2989–2995. [DOI] [PubMed] [Google Scholar]
- [36].Hsu CC, Tseng LM, Lee HC, Role of mitochondrial dysfunction in cancer progression, Exp Biol Med (Maywood), 241 (2016) 1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ogrodzinski MP, Bernard JJ, Lunt SY, Deciphering metabolic rewiring in breast cancer subtypes, Transl Res, 189 (2017) 105–122. [DOI] [PubMed] [Google Scholar]
- [38].Semenza GL, Hypoxia-inducible factors: coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype, EMBO J, 36 (2017) 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Martinez-Outschoorn U, Sotgia F, Lisanti MP, Tumor microenvironment and metabolic synergy in breast cancers: critical importance of mitochondrial fuels and function, Semin Oncol, 41 (2014) 195–216. [DOI] [PubMed] [Google Scholar]
- [40].Chu DT, Phuong TNT, Tien NLB, Tran DK, Nguyen TT, Thanh VV, Quang TL, Minh LB, Pham VH, Ngoc VTN, Kushekhar K, Chu-Dinh T, The Effects of Adipocytes on the Regulation of Breast Cancer in the Tumor Microenvironment: An Update, Cells, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Walsh HR, Cruickshank BM, Brown JM, Marcato P, The Flick of a Switch: Conferring Survival Advantage to Breast Cancer Stem Cells Through Metabolic Plasticity, Front Oncol, 9 (2019) 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jing X, Yang F, Shao C, Wei K, Xie M, Shen H, Shu Y, Role of hypoxia in cancer therapy by regulating the tumor microenvironment, Mol Cancer, 18 (2019) 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Koumenis C, ER stress, hypoxia tolerance and tumor progression, Curr Mol Med, 6 (2006) 55–69. [DOI] [PubMed] [Google Scholar]
- [44].Wykoff CC, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ, Identification of novel hypoxia dependent and independent target genes of the von Hippel-Lindau (VHL) tumour suppressor by mRNA differential expression profiling, Oncogene, 19 (2000) 6297–6305. [DOI] [PubMed] [Google Scholar]
- [45].An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM, Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha, Nature, 392 (1998) 405–408. [DOI] [PubMed] [Google Scholar]
- [46].Leszczynska KB, Foskolou IP, Abraham AG, Anbalagan S, Tellier C, Haider S, Span PN, O’Neill EE, Buffa FM, Hammond EM, Hypoxia-induced p53 modulates both apoptosis and radiosensitivity via AKT, J Clin Invest, 125 (2015) 2385–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Amelio I, Melino G, The p53 family and the hypoxia-inducible factors (HIFs): determinants of cancer progression, Trends Biochem Sci, 40 (2015) 425–434. [DOI] [PubMed] [Google Scholar]
- [48].Petrova VS, Barlev NA, TUMOR MICROENVIRONMENT REGULATION BY HYPOXIA-INDICIBLE FACTORS (HIFs), AND p53 FAMILY PROTEINS, Tsitologiia, 59 (2017) 259–270. [PubMed] [Google Scholar]
- [49].Bruick RK, Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia, Proc Natl Acad Sci U S A, 97 (2000) 9082–9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL, HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors, Cancer Res, 61 (2001) 6669–6673. [PubMed] [Google Scholar]
- [51].Fitzgerald LD, Bailey CK, Brandt SJ, Thompson ME, BRCA1 accumulates in the nucleus in response to hypoxia and TRAIL and enhances TRAIL-induced apoptosis in breast cancer cells, FEBS J, 274 (2007) 5137–5146. [DOI] [PubMed] [Google Scholar]
- [52].Joung YH, Lim EJ, Kim MS, Lim SD, Yoon SY, Lim YC, Yoo YB, Ye SK, Park T, Chung IM, Bae KY, Yang YM, Enhancement of hypoxia-induced apoptosis of human breast cancer cells via STAT5b by momilactone B, Int J Oncol, 33 (2008) 477–484. [PubMed] [Google Scholar]
- [53].Choi JY, Jang YS, Min SY, Song JY, Overexpression of MMP-9 and HIF-1alpha in Breast Cancer Cells under Hypoxic Conditions, J Breast Cancer, 14 (2011) 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Stetler-Stevenson WG, Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention, J Clin Invest, 103 (1999) 1237–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].van Hinsbergh VW, Koolwijk P, Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead, Cardiovasc Res, 78 (2008) 203–212. [DOI] [PubMed] [Google Scholar]
- [56].Blancher C, Moore JW, Talks KL, Houlbrook S, Harris AL, Relationship of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression to vascular endothelial growth factor induction and hypoxia survival in human breast cancer cell lines, Cancer Res, 60 (2000) 7106–7113. [PubMed] [Google Scholar]
- [57].Ciccone V, Terzuoli E, Donnini S, Giachetti A, Morbidelli L, Ziche M, Stemness marker ALDH1A1 promotes tumor angiogenesis via retinoic acid/HIF-1alpha/VEGF signalling in MCF-7 breast cancer cells, J Exp Clin Cancer Res, 37 (2018) 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Liang H, Xiao J, Zhou Z, Wu J, Ge F, Li Z, Zhang H, Sun J, Li F, Liu R, Chen C, Hypoxia induces miR-153 through the IRE1alpha-XBP1 pathway to fine tune the HIF1alpha/VEGFA axis in breast cancer angiogenesis, Oncogene, 37 (2018) 1961–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ruohola JK, Valve EM, Karkkainen MJ, Joukov V, Alitalo K, Harkonen PL, Vascular endothelial growth factors are differentially regulated by steroid hormones and antiestrogens in breast cancer cells, Mol Cell Endocrinol, 149 (1999) 29–40. [DOI] [PubMed] [Google Scholar]
- [60].Shi YH, Bingle L, Gong LH, Wang YX, Corke KP, Fang WG, Basic FGF augments hypoxia induced HIF-1-alpha expression and VEGF release in T47D breast cancer cells, Pathology, 39 (2007) 396–400. [DOI] [PubMed] [Google Scholar]
- [61].Bos R, van Diest PJ, de Jong JS, van der Groep P, van der Valk P, van der Wall E, Hypoxia-inducible factor-1alpha is associated with angiogenesis, and expression of bFGF, PDGF-BB, and EGFR in invasive breast cancer, Histopathology, 46 (2005) 31–36. [DOI] [PubMed] [Google Scholar]
- [62].Schito L, Rey S, Tafani M, Zhang H, Wong CC, Russo A, Russo MA, Semenza GL, Hypoxia-inducible factor 1-dependent expression of platelet-derived growth factor B promotes lymphatic metastasis of hypoxic breast cancer cells, Proc Natl Acad Sci U S A, 109 (2012) E2707–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Jin F, Brockmeier U, Otterbach F, Metzen E, New insight into the SDF-1/CXCR4 axis in a breast carcinoma model: hypoxia-induced endothelial SDF-1 and tumor cell CXCR4 are required for tumor cell intravasation, Mol Cancer Res, 10 (2012) 1021–1031. [DOI] [PubMed] [Google Scholar]
- [64].Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC, Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1, Nat Med, 10 (2004) 858–864. [DOI] [PubMed] [Google Scholar]
- [65].Herr D, Rodewald M, Fraser HM, Hack G, Konrad R, Kreienberg R, Wulff C, Potential role of Renin-Angiotensin-system for tumor angiogenesis in receptor negative breast cancer, Gynecol Oncol, 109 (2008) 418–425. [DOI] [PubMed] [Google Scholar]
- [66].Liang H, Ge F, Xu Y, Xiao J, Zhou Z, Liu R, Chen C, miR-153 inhibits the migration and the tube formation of endothelial cells by blocking the paracrine of angiopoietin 1 in breast cancer cells, Angiogenesis, 21 (2018) 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lam SW, Nota NM, Jager A, Bos MM, van den Bosch J, van der Velden AM, Portielje JE, Honkoop AH, van Tinteren H, Boven E, Team ATXT, Angiogenesis- and Hypoxia-Associated Proteins as Early Indicators of the Outcome in Patients with Metastatic Breast Cancer Given First-Line Bevacizumab-Based Therapy, Clin Cancer Res, 22 (2016) 1611–1620. [DOI] [PubMed] [Google Scholar]
- [68].Krock BL, Skuli N, Simon MC, Hypoxia-induced angiogenesis: good and evil, Genes Cancer, 2 (2011) 1117–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hung SP, Yang MH, Tseng KF, Lee OK, Hypoxia-induced secretion of TGF-beta1 in mesenchymal stem cell promotes breast cancer cell progression, Cell Transplant, 22 (2013) 1869–1882. [DOI] [PubMed] [Google Scholar]
- [70].Varadan V, Kamalakaran S, Gilmore H, Banerjee N, Janevski A, Miskimen KL, Williams N, Basavanhalli A, Madabhushi A, Lezon-Geyda K, Bossuyt V, Lannin DR, Abu-Khalaf M, Sikov W, Dimitrova N, Harris LN, Brief-exposure to preoperative bevacizumab reveals a TGF-beta signature predictive of response in HER2-negative breast cancers, Int J Cancer, 138 (2016) 747–757. [DOI] [PubMed] [Google Scholar]
- [71].He H, Wang X, Chen J, Sun L, Sun H, Xie K, High-Mobility Group Box 1 (HMGB1) Promotes Angiogenesis and Tumor Migration by Regulating Hypoxia-Inducible Factor 1 (HIF-1alpha) Expression via the Phosphatidylinositol 3-Kinase (PI3K)/AKT Signaling Pathway in Breast Cancer Cells, Med Sci Monit, 25 (2019) 2352–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Siddharth S, Nayak A, Das S, Nayak D, Panda J, Wyatt MD, Kundu CN, The soluble nectin-4 ecto-domain promotes breast cancer induced angiogenesis via endothelial Integrin-beta4, Int J Biochem Cell Biol, 102 (2018) 151–160. [DOI] [PubMed] [Google Scholar]
- [73].Wang JC, Li GY, Wang B, Han SX, Sun X, Jiang YN, Shen YW, Zhou C, Feng J, Lu SY, Liu JL, Wang MD, Liu PJ, Metformin inhibits metastatic breast cancer progression and improves chemosensitivity by inducing vessel normalization via PDGF-B downregulation, J Exp Clin Cancer Res, 38 (2019) 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dewangan J, Kaushik S, Rath SK, Balapure AK, Centchroman regulates breast cancer angiogenesis via inhibition of HIF-1alpha/VEGFR2 signalling axis, Life Sci, 193 (2018) 9–19. [DOI] [PubMed] [Google Scholar]
- [75].da Motta LL, Ledaki I, Purshouse K, Haider S, De Bastiani MA, Baban D, Morotti M, Steers G, Wigfield S, Bridges E, Li JL, Knapp S, Ebner D, Klamt F, Harris AL, McIntyre A, The BET inhibitor JQ1 selectively impairs tumour response to hypoxia and downregulates CA9 and angiogenesis in triple negative breast cancer, Oncogene, 36 (2017) 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ, Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry, Am J Pathol, 155 (1999) 739–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Izawa Y, Kashii-Magaribuchi K, Yoshida K, Nosaka M, Tsuji N, Yamamoto A, Kuroyanagi K, Tono K, Tanihata M, Imanishi M, Onishi M, Sakiyama M, Inoue S, Takahashi R, Stem-like Human Breast Cancer Cells Initiate Vasculogenic Mimicry on Matrigel, Acta Histochem Cytochem, 51 (2018) 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Jafarian AH, Kooshkiforooshani M, Rasoliostadi A, Mohamadian Roshan N, Vascular Mimicry Expression in Invasive Ductal Carcinoma; A New Technique for Prospect of Aggressiveness, Iran J Pathol, 14 (2019) 232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Shen Y, Quan J, Wang M, Li S, Yang J, Lv M, Chen Z, Zhang L, Zhao X, Yang J, Tumor vasculogenic mimicry formation as an unfavorable prognostic indicator in patients with breast cancer, Oncotarget, 8 (2017) 56408–56416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wagenblast E, Soto M, Gutierrez-Angel S, Hartl CA, Gable AL, Maceli AR, Erard N, Williams AM, Kim SY, Dickopf S, Harrell JC, Smith AD, Perou CM, Wilkinson JE, Hannon GJ, Knott SR, A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis, Nature, 520 (2015) 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Delgado-Bellido D, Serrano-Saenz S, Fernandez-Cortes M, Oliver FJ, Vasculogenic mimicry signaling revisited: focus on non-vascular VE-cadherin, Mol Cancer, 16 (2017) 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Fernandez-Cortes M, Delgado-Bellido D, Oliver FJ, Vasculogenic Mimicry: Become an Endothelial Cell “But Not So Much”, Front Oncol, 9 (2019) 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ge H, Luo H, Overview of advances in vasculogenic mimicry - a potential target for tumor therapy, Cancer Manag Res, 10 (2018) 2429–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Li S, Meng W, Guan Z, Guo Y, Han X, The hypoxia-related signaling pathways of vasculogenic mimicry in tumor treatment, Biomed Pharmacother, 80 (2016) 127–135. [DOI] [PubMed] [Google Scholar]
- [85].Yao N, Ren K, Jiang C, Gao M, Huang D, Lu X, Lou B, Peng F, Yang A, Wang X, Ni Y, Zhang J, Combretastatin A4 phosphate treatment induces vasculogenic mimicry formation of W256 breast carcinoma tumor in vitro and in vivo, Tumour Biol, 36 (2015) 8499–8510. [DOI] [PubMed] [Google Scholar]
- [86].Sun H, Zhang D, Yao Z, Lin X, Liu J, Gu Q, Dong X, Liu F, Wang Y, Yao N, Cheng S, Li L, Sun S, Anti-angiogenic treatment promotes triple-negative breast cancer invasion via vasculogenic mimicry, Cancer Biol Ther, 18 (2017) 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Li S, Zhang Q, Zhou L, Guan Y, Chen S, Zhang Y, Han X, Inhibitory effects of compound DMBT on hypoxia-induced vasculogenic mimicry in human breast cancer, Biomed Pharmacother, 96 (2017) 982–992. [DOI] [PubMed] [Google Scholar]
- [88].Wang Y, Sun H, Zhang D, Fan D, Zhang Y, Dong X, Liu S, Yang Z, Ni C, Li Y, Liu F, Zhao X, TP53INP1 inhibits hypoxia-induced vasculogenic mimicry formation via the ROS/snail signalling axis in breast cancer, J Cell Mol Med, 22 (2018) 3475–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Salinas-Vera YM, Marchat LA, Garcia-Vazquez R, Gonzalez de la Rosa CH, Castaneda-Saucedo E, Tito NN, Flores CP, Perez-Plasencia C, Cruz-Colin JL, Carlos-Reyes A, Lopez-Gonzalez JS, Alvarez-Sanchez ME, Lopez-Camarillo C, Cooperative multi-targeting of signaling networks by angiomiR-204 inhibits vasculogenic mimicry in breast cancer cells, Cancer Lett, 432 (2018) 17–27. [DOI] [PubMed] [Google Scholar]
- [90].DiGiacomo JW, Gilkes DM, Tumor Hypoxia As an Enhancer of Inflammation-Mediated Metastasis: Emerging Therapeutic Strategies, Target Oncol, 13 (2018) 157–173. [DOI] [PubMed] [Google Scholar]
- [91].Guo S, Liu M, Gonzalez-Perez RR, Role of Notch and its oncogenic signaling crosstalk in breast cancer, Biochim Biophys Acta, 1815 (2011) 197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Soleimani A, Taghizadeh E, Shahsavari S, Amini Y, Rashidpour H, Azadian E, Jafari A, Parizadeh MR, Mashayekhi K, Soukhtanloo M, Jaafari MR, CD73; a key ectonucleotidase in the development of breast cancer: Recent advances and perspectives, J Cell Physiol, (2019). [DOI] [PubMed] [Google Scholar]
- [93].Esquivel-Velazquez M, Ostoa-Saloma P, Palacios-Arreola MI, Nava-Castro KE, Castro JI, Morales-Montor J, The role of cytokines in breast cancer development and progression, J Interferon Cytokine Res, 35 (2015) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Phillips RM, Targeting the hypoxic fraction of tumours using hypoxia-activated prodrugs, Cancer Chemother Pharmacol, 77 (2016) 441–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Bhola NE, Balko JM, Dugger TC, Kuba MG, Sanchez V, Sanders M, Stanford J, Cook RS, Arteaga CL, TGF-beta inhibition enhances chemotherapy action against triple-negative breast cancer, J Clin Invest, 123 (2013) 1348–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Choi Y, Park SK, Kim HM, Kang JS, Yoon YD, Han SB, Han JW, Yang JS, Han G, Histone deacetylase inhibitor KBH-A42 inhibits cytokine production in RAW 264.7 macrophage cells and in vivo endotoxemia model, Exp Mol Med, 40 (2008) 574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Leoni F, Fossati G, Lewis EC, Lee JK, Porro G, Pagani P, Modena D, Moras ML, Pozzi P, Reznikov LL, Siegmund B, Fantuzzi G, Dinarello CA, Mascagni P, The histone deacetylase inhibitor ITF2357 reduces production of pro-inflammatory cytokines in vitro and systemic inflammation in vivo, Mol Med, 11 (2005) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Leoni F, Zaliani A, Bertolini G, Porro G, Pagani P, Pozzi P, Dona G, Fossati G, Sozzani S, Azam T, Bufler P, Fantuzzi G, Goncharov I, Kim SH, Pomerantz BJ, Reznikov LL, Siegmund B, Dinarello CA, Mascagni P, The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines, Proc Natl Acad Sci U S A, 99 (2002) 2995–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Luo X, Fu Y, Loza AJ, Murali B, Leahy KM, Ruhland MK, Gang M, Su X, Zamani A, Shi Y, Lavine KJ, Ornitz DM, Weilbaecher KN, Long F, Novack DV, Faccio R, Longmore GD, Stewart SA, Stromal-Initiated Changes in the Bone Promote Metastatic Niche Development, Cell Rep, 14 (2016) 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Medeiros B, Allan AL, Molecular Mechanisms of Breast Cancer Metastasis to the Lung: Clinical and Experimental Perspectives, Int J Mol Sci, 20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Tungsukruthai S, Petpiroon N, Chanvorachote P, Molecular Mechanisms of Breast Cancer Metastasis and Potential Anti-metastatic Compounds, Anticancer Res, 38 (2018) 2607–2618. [DOI] [PubMed] [Google Scholar]
- [102].Kozlowski J, Kozlowska A, Kocki J, Breast cancer metastasis - insight into selected molecular mechanisms of the phenomenon, Postepy Hig Med Dosw (Online), 69 (2015) 447–451. [DOI] [PubMed] [Google Scholar]
- [103].Wang D, Naydenov NG, Dozmorov MG, Koblinski JE, Ivanov AI, Anillin regulates breast cancer cell migration, growth, and metastasis by non-canonical mechanisms involving control of cell stemness and differentiation, Breast Cancer Res, 22 (2020) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wang Y, Bibi M, Min P, Deng W, Zhang Y, Du J, SOX2 promotes hypoxia-induced breast cancer cell migration by inducing NEDD9 expression and subsequent activation of Rac1/HIF-1alpha signaling, Cell Mol Biol Lett, 24 (2019) 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Zhang HS, Zhang ZG, Du GY, Sun HL, Liu HY, Zhou Z, Gou XM, Wu XH, Yu XY, Huang YH, Nrf2 promotes breast cancer cell migration via up-regulation of G6PD/HIF-1alpha/Notch1 axis, J Cell Mol Med, 23 (2019) 3451–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Kelly NJ, Varga JFA, Specker EJ, Romeo CM, Coomber BL, Uniacke J, Hypoxia activates cadherin-22 synthesis via eIF4E2 to drive cancer cell migration, invasion and adhesion, Oncogene, 37 (2018) 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Chen Y, Zhang B, Bao L, Jin L, Yang M, Peng Y, Kumar A, Wang JE, Wang C, Zou X, Xing C, Wang Y, Luo W, ZMYND8 acetylation mediates HIF-dependent breast cancer progression and metastasis, J Clin Invest, 128 (2018) 1937–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Karakashev SV, Reginato MJ, Progress toward overcoming hypoxia-induced resistance to solid tumor therapy, Cancer Manag Res, 7 (2015) 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Leslie EM, Deeley RG, Cole SP, Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense, Toxicol Appl Pharmacol, 204 (2005) 216–237. [DOI] [PubMed] [Google Scholar]
- [110].Sun YL, Patel A, Kumar P, Chen ZS, Role of ABC transporters in cancer chemotherapy, Chin J Cancer, 31 (2012) 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bueno MJ, Mouron S, Quintela-Fandino M, Personalising and targeting antiangiogenic resistance: a complex and multifactorial approach, Br J Cancer, 116 (2017) 1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Velaei K, Samadi N, Barazvan B, Soleimani Rad J, Tumor microenvironment-mediated chemoresistance in breast cancer, Breast, 30 (2016) 92–100. [DOI] [PubMed] [Google Scholar]
- [113].Tsuyada A, Chow A, Wu J, Somlo G, Chu P, Loera S, Luu T, Li AX, Wu X, Ye W, Chen S, Zhou W, Yu Y, Wang YZ, Ren X, Li H, Scherle P, Kuroki Y, Wang SE, CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells, Cancer Res, 72 (2012) 2768–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Hartmann TN, Burger JA, Glodek A, Fujii N, Burger M, CXCR4 chemokine receptor and integrin signaling co-operate in mediating adhesion and chemoresistance in small cell lung cancer (SCLC) cells, Oncogene, 24 (2005) 4462–4471. [DOI] [PubMed] [Google Scholar]
- [115].Shree T, Olson OC, Elie BT, Kester JC, Garfall AL, Simpson K, Bell-McGuinn KM, Zabor EC, Brogi E, Joyce JA, Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer, Genes Dev, 25 (2011) 2465–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Mahdi A, Darvishi B, Majidzadeh AK, Salehi M, Farahmand L, Challenges facing antiangiogenesis therapy: The significant role of hypoxia-inducible factor and MET in development of resistance to anti-vascular endothelial growth factor-targeted therapies, J Cell Physiol, 234 (2019) 5655–5663. [DOI] [PubMed] [Google Scholar]
- [117].Schoning JP, Monteiro M, Gu W, Drug resistance and cancer stem cells: the shared but distinct roles of hypoxia-inducible factors HIF1alpha and HIF2alpha, Clin Exp Pharmacol Physiol, 44 (2017) 153–161. [DOI] [PubMed] [Google Scholar]
- [118].Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, Richardson A, Violette S, Nikolskaya T, Nikolsky Y, Bauerlein EL, Hahn WC, Gelman RS, Allred C, Bissell MJ, Schnitt S, Polyak K, Regulation of in situ to invasive breast carcinoma transition, Cancer Cell, 13 (2008) 394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Lee S, Stewart S, Nagtegaal I, Luo J, Wu Y, Colditz G, Medina D, Allred DC, Differentially expressed genes regulating the progression of ductal carcinoma in situ to invasive breast cancer, Cancer Res, 72 (2012) 4574–4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Lyons TR, O’Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, Marusyk A, Tan AC, Schedin P, Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2, Nat Med, 17 (2011) 1109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Miller FR, Santner SJ, Tait L, Dawson PJ, MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ, J Natl Cancer Inst, 92 (2000) 1185–1186. [DOI] [PubMed] [Google Scholar]
- [122].Yoshida GJ, Applications of patient-derived tumor xenograft models and tumor organoids, J Hematol Oncol, 13 (2020) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Williams JA, Using PDX for Preclinical Cancer Drug Discovery: The Evolving Field, J Clin Med, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].de Jong M, Maina T, Of mice and humans: are they the same?--Implications in cancer translational research, J Nucl Med, 51 (2010) 501–504. [DOI] [PubMed] [Google Scholar]
- [125].Lampreht Tratar U, Horvat S, Cemazar M, Transgenic Mouse Models in Cancer Research, Front Oncol, 8 (2018) 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Hutmacher DW, Loessner D, Rizzi S, Kaplan DL, Mooney DJ, Clements JA, Can tissue engineering concepts advance tumor biology research?, Trends Biotechnol, 28 (2010) 125–133. [DOI] [PubMed] [Google Scholar]
- [127].Jedeszko C, Victor BC, Podgorski I, Sloane BF, Fibroblast hepatocyte growth factor promotes invasion of human mammary ductal carcinoma in situ, Cancer Res, 69 (2009) 9148–9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Simian M, Bissell MJ, Organoids: A historical perspective of thinking in three dimensions, J Cell Biol, 216 (2017) 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Sung KE, Yang N, Pehlke C, Keely PJ, Eliceiri KW, Friedl A, Beebe DJ, Transition to invasion in breast cancer: a microfluidic in vitro model enables examination of spatial and temporal effects, Integr Biol (Camb), 3 (2011) 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Marushima H, Shibata S, Asakura T, Matsuura T, Maehashi H, Ishii Y, Eda H, Aoki K, Iida Y, Morikawa T, Ohkawa K, Three-dimensional culture promotes reconstitution of the tumor-specific hypoxic microenvironment under TGFbeta stimulation, Int J Oncol, 39 (2011) 1327–1336. [DOI] [PubMed] [Google Scholar]
- [131].Antoni D, Burckel H, Josset E, Noel G, Three-dimensional cell culture: a breakthrough in vivo, Int J Mol Sci, 16 (2015) 5517–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Sant S, Johnston PA, The production of 3D tumor spheroids for cancer drug discovery, Drug Discov Today Technol, 23 (2017) 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Duval K, Grover H, Han LH, Mou Y, Pegoraro AF, Fredberg J, Chen Z, Modeling Physiological Events in 2D vs. 3D Cell Culture, Physiology (Bethesda), 32 (2017) 266–277. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- [134].Langhans SA, Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning, Front Pharmacol, 9 (2018) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Senthebane DA, Rowe A, Thomford NE, Shipanga H, Munro D, Mazeedi M, Almazyadi HAM, Kallmeyer K, Dandara C, Pepper MS, Parker MI, Dzobo K, The Role of Tumor Microenvironment in Chemoresistance: To Survive, Keep Your Enemies Closer, Int J Mol Sci, 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Brancato V, Oliveira JM, Correlo VM, Reis RL, Kundu SC, Could 3D models of cancer enhance drug screening?, Biomaterials, 232 (2020) 119744. [DOI] [PubMed] [Google Scholar]
- [137].Ayuso JM, Gillette A, Lugo-Cintron K, Acevedo-Acevedo S, Gomez I, Morgan M, Heaster T, Wisinski KB, Palecek SP, Skala MC, Beebe DJ, Organotypic microfluidic breast cancer model reveals starvation-induced spatial-temporal metabolic adaptations, EBioMedicine, 37 (2018) 144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Imamura Y, Mukohara T, Shimono Y, Funakoshi Y, Chayahara N, Toyoda M, Kiyota N, Takao S, Kono S, Nakatsura T, Minami H, Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer, Oncol Rep, 33 (2015) 1837–1843. [DOI] [PubMed] [Google Scholar]
- [139].Ismadi MZ, Gupta P, Fouras A, Verma P, Jadhav S, Bellare J, Hourigan K, Flow characterization of a spinner flask for induced pluripotent stem cell culture application, PLoS One, 9 (2014) e106493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Drost J, van Boxtel R, Blokzijl F, Mizutani T, Sasaki N, Sasselli V, de Ligt J, Behjati S, Grolleman JE, van Wezel T, Nik-Zainal S, Kuiper RP, Cuppen E, Clevers H, Use of CRISPR-modified human stem cell organoids to study the origin of mutational signatures in cancer, Science, 358 (2017) 234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Espina V, Mariani BD, Gallagher RI, Tran K, Banks S, Wiedemann J, Huryk H, Mueller C, Adamo L, Deng J, Petricoin EF, Pastore L, Zaman S, Menezes G, Mize J, Johal J, Edmiston K, Liotta LA, Malignant precursor cells pre-exist in human breast DCIS and require autophagy for survival, PLoS One, 5 (2010) e10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA, Multicellular tumor spheroids: an underestimated tool is catching up again, J Biotechnol, 148 (2010) 3–15. [DOI] [PubMed] [Google Scholar]
- [143].Lin RZ, Chang HY, Recent advances in three-dimensional multicellular spheroid culture for biomedical research, Biotechnol J, 3 (2008) 1172–1184. [DOI] [PubMed] [Google Scholar]
- [144].Gao Q, Yang Z, Xu S, Li X, Yang X, Jin P, Liu Y, Zhou X, Zhang T, Gong C, Wei X, Liu D, Sun C, Chen G, Hu J, Meng L, Zhou J, Sawada K, Fruscio R, Grunt TW, Wischhusen J, Vargas-Hernandez VM, Pothuri B, Coleman RL, Heterotypic CAF-tumor spheroids promote early peritoneal metastatis of ovarian cancer, J Exp Med, 216 (2019) 688–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Shang M, Soon RH, Lim CT, Khoo BL, Han J, Microfluidic modelling of the tumor microenvironment for anti-cancer drug development, Lab Chip, 19 (2019) 369–386. [DOI] [PubMed] [Google Scholar]
- [146].Yang W, Cai S, Yuan Z, Lai Y, Yu H, Wang Y, Liu L, Mask-free generation of multicellular 3D heterospheroids array for high-throughput combinatorial anti-cancer drug screening, Materials & Design, 183 (2019) 108182. [Google Scholar]
- [147].Young M, Rodenhizer D, Dean T, D’Arcangelo E, Xu B, Ailles L, McGuigan AP, A TRACER 3D Co-Culture tumour model for head and neck cancer, Biomaterials, 164 (2018) 54–69. [DOI] [PubMed] [Google Scholar]
- [148].Pradhan S, Hassani I, Clary JM, Lipke EA, Polymeric Biomaterials for In Vitro Cancer Tissue Engineering and Drug Testing Applications, Tissue Eng Part B Rev, 22 (2016) 470–484. [DOI] [PubMed] [Google Scholar]
- [149].Bersani F, Lee J, Yu M, Morris R, Desai R, Ramaswamy S, Toner M, Haber DA, Parekkadan B, Bioengineered implantable scaffolds as a tool to study stromal-derived factors in metastatic cancer models, Cancer Res, 74 (2014) 7229–7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Brancato V, Ventre M, Imparato G, Urciuolo F, Meo C, Netti PA, A straightforward method to produce decellularized dermis-based matrices for tumour cell cultures, J Tissue Eng Regen Med, 12 (2018) e71–e81. [DOI] [PubMed] [Google Scholar]
- [151].Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, Mooney DJ, Engineering tumors with 3D scaffolds, Nat Methods, 4 (2007) 855–860. [DOI] [PubMed] [Google Scholar]
- [152].Phan-Lai V, Florczyk SJ, Kievit FM, Wang K, Gad E, Disis ML, Zhang M, Three-dimensional scaffolds to evaluate tumor associated fibroblast-mediated suppression of breast tumor specific T cells, Biomacromolecules, 14 (2013) 1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Fischbach C, Kong HJ, Hsiong SX, Evangelista MB, Yuen W, Mooney DJ, Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement, Proc Natl Acad Sci U S A, 106 (2009) 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Chaicharoenaudomrung N, Kunhorm P, Noisa P, Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling, World J Stem Cells, 11 (2019) 1065–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Kundu B, Saha P, Datta K, Kundu SC, A silk fibroin based hepatocarcinoma model and the assessment of the drug response in hyaluronan-binding protein 1 overexpressed HepG2 cells, Biomaterials, 34 (2013) 9462–9474. [DOI] [PubMed] [Google Scholar]
- [156].Subia B, Dey T, Sharma S, Kundu SC, Target specific delivery of anticancer drug in silk fibroin based 3D distribution model of bone-breast cancer cells, ACS Appl Mater Interfaces, 7 (2015) 2269–2279. [DOI] [PubMed] [Google Scholar]
- [157].Jin Q, Liu G, Li S, Yuan H, Yun Z, Zhang W, Zhang S, Dai Y, Ma Y, Decellularized breast matrix as bioactive microenvironment for in vitro three-dimensional cancer culture, J Cell Physiol, 234 (2019) 3425–3435. [DOI] [PubMed] [Google Scholar]
- [158].Zhang YS, Zhang YN, Zhang W, Cancer-on-a-chip systems at the frontier of nanomedicine, Drug Discov Today, 22 (2017) 1392–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Caballero D, Kaushik S, Correlo VM, Oliveira JM, Reis RL, Kundu SC, Organ-on-chip models of cancer metastasis for future personalized medicine: From chip to the patient, Biomaterials, 149 (2017) 98–115. [DOI] [PubMed] [Google Scholar]
- [160].Estrada MF, Rebelo SP, Davies EJ, Pinto MT, Pereira H, Santo VE, Smalley MJ, Barry ST, Gualda EJ, Alves PM, Anderson E, Brito C, Modelling the tumour microenvironment in long-term microencapsulated 3D co-cultures recapitulates phenotypic features of disease progression, Biomaterials, 78 (2016) 50–61. [DOI] [PubMed] [Google Scholar]
- [161].Brancato V, Garziano A, Gioiella F, Urciuolo F, Imparato G, Panzetta V, Fusco S, Netti PA, 3D is not enough: Building up a cell instructive microenvironment for tumoral stroma microtissues, Acta Biomater, 47 (2017) 1–13. [DOI] [PubMed] [Google Scholar]
- [162].Benien P, Swami A, 3D tumor models: history, advances and future perspectives, Future Oncol, 10 (2014) 1311–1327. [DOI] [PubMed] [Google Scholar]
- [163].Cacopardo L, Costa J, Giusti S, Buoncompagni L, Meucci S, Corti A, Mattei G, Ahluwalia A, Real-time cellular impedance monitoring and imaging of biological barriers in a dual-flow membrane bioreactor, Biosens Bioelectron, 140 (2019) 111340. [DOI] [PubMed] [Google Scholar]
- [164].Charbe N, McCarron PA, Tambuwala MM, Three-dimensional bio-printing: A new frontier in oncology research, World J Clin Oncol, 8 (2017) 21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Albritton JL, Miller JS, 3D bioprinting: improving in vitro models of metastasis with heterogeneous tumor microenvironments, Dis Model Mech, 10 (2017) 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Belgodere JA, King CT, Bursavich JB, Burow ME, Martin EC, Jung JP, Engineering Breast Cancer Microenvironments and 3D Bioprinting, Front Bioeng Biotechnol, 6 (2018) 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Di Modugno F, Colosi C, Trono P, Antonacci G, Ruocco G, Nistico P, 3D models in the new era of immune oncology: focus on T cells, CAF and ECM, J Exp Clin Cancer Res, 38 (2019) 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Yi HG, Jeong YH, Kim Y, Choi YJ, Moon HE, Park SH, Kang KS, Bae M, Jang J, Youn H, Paek SH, Cho DW, A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy, Nat Biomed Eng, 3 (2019) 509–519. [DOI] [PubMed] [Google Scholar]
- [169].Mollica PA, Booth-Creech EN, Reid JA, Zamponi M, Sullivan SM, Palmer XL, Sachs PC, Bruno RD, 3D bioprinted mammary organoids and tumoroids in human mammary derived ECM hydrogels, Acta Biomater, 95 (2019) 201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Whitman NA, Lin ZW, Kenney RM, Albertini L, Lockett MR, Hypoxia differentially regulates estrogen receptor alpha in 2D and 3D culture formats, Arch Biochem Biophys, 671 (2019) 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Vantangoli MM, Madnick SJ, Wilson S, Boekelheide K, Estradiol Exposure Differentially Alters Monolayer versus Microtissue MCF-7 Human Breast Carcinoma Cultures, PLoS One, 11 (2016) e0157997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Singh M, Venkata Krishnan H, Ranganathan S, Kiesel B, Beumer JH, Sreekumar S, Sant S, Controlled Three-Dimensional Tumor Microenvironments Recapitulate Phenotypic Features and Differential Drug Response in Early vs Advanced Stage Breast Cancer, ACS Biomaterials Science & Engineering, 4 (2018) 421–431. [DOI] [PubMed] [Google Scholar]
- [173].Melissaridou S, Wiechec E, Magan M, Jain MV, Chung MK, Farnebo L, Roberg K, The effect of 2D and 3D cell cultures on treatment response, EMT profile and stem cell features in head and neck cancer, Cancer Cell Int, 19 (2019) 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Li C, Singh B, Graves-Deal R, Ma H, Starchenko A, Fry WH, Lu Y, Wang Y, Bogatcheva G, Khan MP, Milne GL, Zhao S, Ayers GD, Li N, Hu H, Washington MK, Yeatman TJ, McDonald OG, Liu Q, Coffey RJ, Three-dimensional culture system identifies a new mode of cetuximab resistance and disease-relevant genes in colorectal cancer, Proc Natl Acad Sci U S A, 114 (2017) E2852–E2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Weigelt B, Lo AT, Park CC, Gray JW, Bissell MJ, HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment, Breast Cancer Res Treat, 122 (2010) 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Gomes LR, Vessoni AT, Menck CF, Three-dimensional microenvironment confers enhanced sensitivity to doxorubicin by reducing p53-dependent induction of autophagy, Oncogene, 34 (2015) 5329–5340. [DOI] [PubMed] [Google Scholar]
- [177].Baenke F, Peck B, Miess H, Schulze A, Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development, Dis Model Mech, 6 (2013) 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Vidavsky N, Kunitake J, Diaz-Rubio ME, Chiou AE, Loh HC, Zhang S, Masic A, Fischbach C, Estroff LA, Mapping and Profiling Lipid Distribution in a 3D Model of Breast Cancer Progression, ACS Cent Sci, 5 (2019) 768–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Jiang L, Chughtai K, Purvine SO, Bhujwalla ZM, Raman V, Pasa-Tolic L, Heeren RMA, Glunde K, MALDI-Mass Spectrometric Imaging Revealing Hypoxia-Driven Lipids and Proteins in a Breast Tumor Model, Anal Chem, 87 (2015) 5947–5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Liverani C, De Vita A, Minardi S, Kang Y, Mercatali L, Amadori D, Bongiovanni A, La Manna F, Ibrahim T, Tasciotti E, A biomimetic 3D model of hypoxia-driven cancer progression, Sci Rep, 9 (2019) 12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].DelNero P, Lane M, Verbridge SS, Kwee B, Kermani P, Hempstead B, Stroock A, Fischbach C, 3D culture broadly regulates tumor cell hypoxia response and angiogenesis via pro-inflammatory pathways, Biomaterials, 55 (2015) 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Alhawarat FM, Hammad HM, Hijjawi MS, Sharab AS, Abuarqoub DA, Al Shhab MA, Zihlif MA, The effect of cycling hypoxia on MCF-7 cancer stem cells and the impact of their microenvironment on angiogenesis using human umbilical vein endothelial cells (HUVECs) as a model, PeerJ, 7 (2019) e5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Szot CS, Buchanan CF, Freeman JW, Rylander MN, 3D in vitro bioengineered tumors based on collagen I hydrogels, Biomaterials, 32 (2011) 7905–7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Velez DO, Tsui B, Goshia T, Chute CL, Han A, Carter H, Fraley SI, 3D collagen architecture induces a conserved migratory and transcriptional response linked to vasculogenic mimicry, Nat Commun, 8 (2017) 1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Yoshino D, Funamoto K, Oxygen-dependent contraction and degradation of the extracellular matrix mediated by interaction between tumor and endothelial cells, AIP Advances, 9 (2019) 045215. [Google Scholar]
- [186].Tevis KM, Cecchi RJ, Colson YL, Grinstaff MW, Mimicking the tumor microenvironment to regulate macrophage phenotype and assessing chemotherapeutic efficacy in embedded cancer cell/macrophage spheroid models, Acta Biomater, 50 (2017) 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Norton KA, Wallace T, Pandey NB, Popel AS, An agent-based model of triple-negative breast cancer: the interplay between chemokine receptor CCR5 expression, cancer stem cells, and hypoxia, BMC Syst Biol, 11 (2017) 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Funamoto K, Zervantonakis IK, Liu Y, Ochs CJ, Kim C, Kamm RD, A novel microfluidic platform for high-resolution imaging of a three-dimensional cell culture under a controlled hypoxic environment, Lab Chip, 12 (2012) 4855–4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Ju JA, Godet I, Ye IC, Byun J, Jayatilaka H, Lee SJ, Xiang L, Samanta D, Lee MH, Wu PH, Wirtz D, Semenza GL, Gilkes DM, Hypoxia Selectively Enhances Integrin alpha5beta1 Receptor Expression in Breast Cancer to Promote Metastasis, Mol Cancer Res, 15 (2017) 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Wang Y, Mirza S, Wu S, Zeng J, Shi W, Band H, Band V, Duan B, 3D hydrogel breast cancer models for studying the effects of hypoxia on epithelial to mesenchymal transition, Oncotarget, 9 (2018) 32191–32203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Nagpal N, Ahmad HM, Chameettachal S, Sundar D, Ghosh S, Kulshreshtha R, HIF-inducible miR-191 promotes migration in breast cancer through complex regulation of TGFbeta-signaling in hypoxic microenvironment, Sci Rep, 5 (2015) 9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Ward C, Meehan J, Mullen P, Supuran C, Dixon JM, Thomas JS, Winum JY, Lambin P, Dubois L, Pavathaneni NK, Jarman EJ, Renshaw L, Um IH, Kay C, Harrison DJ, Kunkler IH, Langdon SP, Evaluation of carbonic anhydrase IX as a therapeutic target for inhibition of breast cancer invasion and metastasis using a series of in vitro breast cancer models, Oncotarget, 6 (2015) 24856–24870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Lock FE, McDonald PC, Lou Y, Serrano I, Chafe SC, Ostlund C, Aparicio S, Winum JY, Supuran CT, Dedhar S, Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche, Oncogene, 32 (2013) 5210–5219. [DOI] [PubMed] [Google Scholar]
- [194].Singh M, Close DA, Mukundan S, Johnston PA, Sant S, Production of Uniform 3D Microtumors in Hydrogel Microwell Arrays for Measurement of Viability, Morphology, and Signaling Pathway Activation, Assay Drug Dev Technol, 13 (2015) 570–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Singh M, Mukundan S, Jaramillo M, Oesterreich S, Sant S, Three-Dimensional Breast Cancer Models Mimic Hallmarks of Size-Induced Tumor Progression, Cancer Res, 76 (2016) 3732–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Singh M, Tian XJ, Donnenberg VS, Watson AM, Zhang J, Stabile LP, Watkins SC, Xing J, Sant S, Targeting the Temporal Dynamics of Hypoxia-Induced Tumor-Secreted Factors Halts Tumor Migration, Cancer Res, 79 (2019) 2962–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Singh M, Warita K, Warita T, Faeder JR, Lee REC, Sant S, Oltvai ZN, Shift from stochastic to spatially-ordered expression of serine-glycine synthesis enzymes in 3D microtumors, Sci Rep, 8 (2018) 9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Li H, Zhu R, Wang L, Zhu T, Li Q, Chen Q, Wang H, Zhu H, PIK3CA mutations mostly begin to develop in ductal carcinoma of the breast, Exp Mol Pathol, 88 (2010) 150–155. [DOI] [PubMed] [Google Scholar]
- [199].Ottesen GL, Carcinoma in situ of the female breast. A clinico-pathological, immunohistological, and DNA ploidy study, APMIS Suppl, (2003) 1–67. [PubMed] [Google Scholar]
- [200].Tamimi RM, Baer HJ, Marotti J, Galan M, Galaburda L, Fu Y, Deitz AC, Connolly JL, Schnitt SJ, Colditz GA, Collins LC, Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer, Breast Cancer Res, 10 (2008) R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Burkhardt L, Grob TJ, Hermann I, Burandt E, Choschzick M, Janicke F, Muller V, Bokemeyer C, Simon R, Sauter G, Wilczak W, Lebeau A, Gene amplification in ductal carcinoma in situ of the breast, Breast Cancer Res Treat, 123 (2010) 757–765. [DOI] [PubMed] [Google Scholar]
- [202].Castro NP, Osorio CA, Torres C, Bastos EP, Mourao-Neto M, Soares FA, Brentani HP, Carraro DM, Evidence that molecular changes in cells occur before morphological alterations during the progression of breast ductal carcinoma, Breast Cancer Res, 10 (2008) R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Christiansen JJ, Rajasekaran AK, Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis, Cancer Res, 66 (2006) 8319–8326. [DOI] [PubMed] [Google Scholar]
- [204].Khalil AA, Ilina O, Gritsenko PG, Bult P, Span PN, Friedl P, Collective invasion in ductal and lobular breast cancer associates with distant metastasis, Clin Exp Metastasis, 34 (2017) 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Doublier S, Belisario DC, Polimeni M, Annaratone L, Riganti C, Allia E, Ghigo D, Bosia A, Sapino A, HIF-1 activation induces doxorubicin resistance in MCF7 3-D spheroids via P-glycoprotein expression: a potential model of the chemo-resistance of invasive micropapillary carcinoma of the breast, BMC Cancer, 12 (2012) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Karakashev SV, Reginato MJ, Hypoxia/HIF1alpha induces lapatinib resistance in ERBB2-positive breast cancer cells via regulation of DUSP2, Oncotarget, 6 (2015) 1967–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].de Almeida Godet IS, Hypoxia in a 3D in vitro Spheroid Model: the Role on Tumor Progression and Response to Chemotherapy, (2015).
- [208].Lahiry M, Rangarajan A, AMPK promotes Notch1 stability to potentiate hypoxia-induced breast cancer stemness and drug resistance, bioRxiv, (2020) 458489. [Google Scholar]
- [209].Tang T, Yang Z, Zhu Q, Wu Y, Sun K, Alahdal M, Zhang Y, Xing Y, Shen Y, Xia T, Xi T, Pan Y, Jin L, Up-regulation of miR-210 induced by a hypoxic microenvironment promotes breast cancer stem cells metastasis, proliferation, and self-renewal by targeting E-cadherin, FASEB J, (2018) fj201801013R. [DOI] [PubMed] [Google Scholar]
- [210].Rodriguez CE, Berardi DE, Abrigo M, Todaro LB, Bal ED de Kier Joffe, G.L. Fiszman, Breast cancer stem cells are involved in Trastuzumab resistance through the HER2 modulation in 3D culture, J Cell Biochem, 119 (2018) 1381–1391. [DOI] [PubMed] [Google Scholar]
- [211].Ham SL, Joshi R, Luker GD, Tavana H, Engineered Breast Cancer Cell Spheroids Reproduce Biologic Properties of Solid Tumors, Adv Healthc Mater, 5 (2016) 2788–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Morotti M, Bridges E, Valli A, Choudhry H, Sheldon H, Wigfield S, Gray N, Zois CE, Grimm F, Jones D, Teoh EJ, Cheng WC, Lord S, Anastasiou D, Haider S, McIntyre A, Goberdhan DCI, Buffa F, Harris AL, Hypoxia-induced switch in SNAT2/SLC38A2 regulation generates endocrine resistance in breast cancer, Proc Natl Acad Sci U S A, 116 (2019) 12452–12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Gao W, Wu D, Wang Y, Wang Z, Zou C, Dai Y, Ng CF, Teoh JY, Chan FL, Development of a novel and economical agar-based non-adherent three-dimensional culture method for enrichment of cancer stem-like cells, Stem Cell Res Ther, 9 (2018) 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Kuo CT, Wang JY, Lin YF, Wo AM, Chen BPC, Lee H, Three-dimensional spheroid culture targeting versatile tissue bioassays using a PDMS-based hanging drop array, Sci Rep, 7 (2017) 4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Gaebler M, Silvestri A, Haybaeck J, Reichardt P, Lowery CD, Stancato LF, Zybarth G, Regenbrecht CRA, Three-Dimensional Patient-Derived In Vitro Sarcoma Models: Promising Tools for Improving Clinical Tumor Management, Front Oncol, 7 (2017) 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Phan J, Kumar P, Hao D, Gao K, Farmer D, Wang A, Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy, J Extracell Vesicles, 7 (2018) 1522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Tavassoli H, Alhosseini SN, Tay A, Chan PPY, Weng Oh SK, Warkiani ME, Large-scale production of stem cells utilizing microcarriers: A biomaterials engineering perspective from academic research to commercialized products, Biomaterials, 181 (2018) 333–346. [DOI] [PubMed] [Google Scholar]
- [218].Song HH, Park KM, Gerecht S, Hydrogels to model 3D in vitro microenvironment of tumor vascularization, Adv Drug Deliv Rev, 79–80 (2014) 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Zhou X, Zhu W, Nowicki M, Miao S, Cui H, Holmes B, Glazer RI, Zhang LG, 3D Bioprinting a Cell-Laden Bone Matrix for Breast Cancer Metastasis Study, ACS Appl Mater Interfaces, 8 (2016) 30017–30026. [DOI] [PubMed] [Google Scholar]
- [220].Costa EC, de Melo-Diogo D, Moreira AF, Carvalho MP, Correia IJ, Spheroids Formation on Non-Adhesive Surfaces by Liquid Overlay Technique: Considerations and Practical Approaches, Biotechnol J, 13 (2018). [DOI] [PubMed] [Google Scholar]
- [221].Costa EC, Gaspar VM, Coutinho P, Correia IJ, Optimization of liquid overlay technique to formulate heterogenic 3D co-cultures models, Biotechnol Bioeng, 111 (2014) 1672–1685. [DOI] [PubMed] [Google Scholar]
- [222].Fong EL, Martinez M, Yang J, Mikos AG, Navone NM, Harrington DA, Farach-Carson MC, Hydrogel-based 3D model of patient-derived prostate xenograft tumors suitable for drug screening, Mol Pharm, 11 (2014) 2040–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Grikscheit K, Frank T, Wang Y, Grosse R, Junctional actin assembly is mediated by Formin-like 2 downstream of Rac1, J Cell Biol, 209 (2015) 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Lal-Nag M, McGee L, Guha R, Lengyel E, Kenny HA, Ferrer M, A High-Throughput Screening Model of the Tumor Microenvironment for Ovarian Cancer Cell Growth, SLAS Discov, 22 (2017) 494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Liaw CY, Ji S, Guvendiren M, Engineering 3D Hydrogels for Personalized In Vitro Human Tissue Models, Adv Healthc Mater, 7 (2018). [DOI] [PubMed] [Google Scholar]
- [226].Yeung P, Sin HS, Chan S, Chan GC, Chan BP, Microencapsulation of Neuroblastoma Cells and Mesenchymal Stromal Cells in Collagen Microspheres: A 3D Model for Cancer Cell Niche Study, PLoS One, 10 (2015) e0144139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Brancato V, Comunanza V, Imparato G, Cora D, Urciuolo F, Noghero A, Bussolino F, Netti PA, Bioengineered tumoral microtissues recapitulate desmoplastic reaction of pancreatic cancer, Acta Biomater, 49 (2017) 152–166. [DOI] [PubMed] [Google Scholar]
- [228].Brancato V, Gioiella F, Profeta M, Imparato G, Guarnieri D, Urciuolo F, Melone P, Netti PA, 3D tumor microtissues as an in vitro testing platform for microenvironmentally-triggered drug delivery systems, Acta Biomater, 57 (2017) 47–58. [DOI] [PubMed] [Google Scholar]
- [229].Gioiella F, Urciuolo F, Imparato G, Brancato V, Netti PA, An Engineered Breast Cancer Model on a Chip to Replicate ECM-Activation In Vitro during Tumor Progression, Adv Healthc Mater, 5 (2016) 3074–3084. [DOI] [PubMed] [Google Scholar]
- [230].Li C, Zhao S, Zhao Y, Qian Y, Li J, Yin Y, Chemically crosslinked alginate porous microcarriers modified with bioactive molecule for expansion of human hepatocellular carcinoma cells, J Biomed Mater Res B Appl Biomater, 102 (2014) 1648–1658. [DOI] [PubMed] [Google Scholar]
- [231].Pradhan S, Clary JM, Seliktar D, Lipke EA, A three-dimensional spheroidal cancer model based on PEG-fibrinogen hydrogel microspheres, Biomaterials, 115 (2017) 141–154. [DOI] [PubMed] [Google Scholar]
- [232].Rebelo SP, Pinto C, Martins TR, Harrer N, Estrada MF, Loza-Alvarez P, Cabecadas J, Alves PM, Gualda EJ, Sommergruber W, Brito C, 3D-3-culture: A tool to unveil macrophage plasticity in the tumour microenvironment, Biomaterials, 163 (2018) 185–197. [DOI] [PubMed] [Google Scholar]
- [233].Zhuo S, Chen J, Xie S, Hong Z, Jiang X, Extracting diagnostic stromal organization features based on intrinsic two-photon excited fluorescence and second-harmonic generation signals, J Biomed Opt, 14 (2009) 020503. [DOI] [PubMed] [Google Scholar]
- [234].Godugu C, Singh M, AlgiMatrix-Based 3D Cell Culture System as an In Vitro Tumor Model: An Important Tool in Cancer Research, Methods Mol Biol, 1379 (2016) 117–128. [DOI] [PubMed] [Google Scholar]
- [235].Gomez-Roman N, Stevenson K, Gilmour L, Hamilton G, Chalmers AJ, A novel 3D human glioblastoma cell culture system for modeling drug and radiation responses, Neuro Oncol, 19 (2017) 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Lu WD, Sun RF, Hu YR, Lu JR, Gu L, Liu ZG, Lei GY, Qiang Z, Cai L, Photooxidatively crosslinked acellular tumor extracellular matrices as potential tumor engineering scaffolds, Acta Biomater, 71 (2018) 460–473. [DOI] [PubMed] [Google Scholar]
- [237].Sood D, Tang-Schomer M, Pouli D, Mizzoni C, Raia N, Tai A, Arkun K, Wu J, Black LD 3rd, Scheffler B, Georgakoudi I, Steindler DA, Kaplan DL, 3D extracellular matrix microenvironment in bioengineered tissue models of primary pediatric and adult brain tumors, Nat Commun, 10 (2019) 4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Edington CD, Chen WLK, Geishecker E, Kassis T, Soenksen LR, Bhushan BM, Freake D, Kirschner J, Maass C, Tsamandouras N, Valdez J, Cook CD, Parent T, Snyder S, Yu J, Suter E, Shockley M, Velazquez J, Velazquez JJ, Stockdale L, Papps JP, Lee I, Vann N, Gamboa M, LaBarge ME, Zhong Z, Wang X, Boyer LA, Lauffenburger DA, Carrier RL, Communal C, Tannenbaum SR, Stokes CL, Hughes DJ, Rohatgi G, Trumper DL, Cirit M, Griffith LG, Interconnected Microphysiological Systems for Quantitative Biology and Pharmacology Studies, Sci Rep, 8 (2018) 4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Lanz HL, Saleh A, Kramer B, Cairns J, Ng CP, Yu J, Trietsch SJ, Hankemeier T, Joore J, Vulto P, Weinshilboum R, Wang L, Therapy response testing of breast cancer in a 3D high-throughput perfused microfluidic platform, BMC Cancer, 17 (2017) 709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Lu S, Cuzzucoli F, Jiang J, Liang LG, Wang Y, Kong M, Zhao X, Cui W, Li J, Wang S, Development of a biomimetic liver tumor-on-a-chip model based on decellularized liver matrix for toxicity testing, Lab Chip, 18 (2018) 3379–3392. [DOI] [PubMed] [Google Scholar]
- [241].Lovitt CJ, Shelper TB, Avery VM, Cancer drug discovery: recent innovative approaches to tumor modeling, Expert Opin Drug Discov, 11 (2016) 885–894. [DOI] [PubMed] [Google Scholar]


