Abstract
Study design:
Fixed-order crossover design with a standardized out-of-laboratory activity protocol.
Background:
Previous studies investigating limb volume change with elevated vacuum (EV) have shown inconsistent results, and have been limited by out-of-socket volume measurements and short, single-activity protocols.
Objectives:
To evaluate the effectiveness of EV for managing limb fluid volume compared to suction suspension (SS) with an in-socket measurement modality during many hours of activity.
Methods:
Transtibial electronic EV users participated in two sessions. EV was used during the first session, and suction suspension in the second. Participants completed a 5.5-hour protocol consisting of multiple intervals of activity. In-socket residual limb fluid volume was continuously measured using a custom portable bioimpedance analyzer.
Results:
12 individuals participated. Overall rate of fluid volume change was not significantly different, though the rate of posterior fluid volume change during Cycle 3 was significantly lower with EV. Though individual results varied, 11 participants experienced lower overall rates of fluid volume loss in at least one limb region using EV.
Conclusions:
EV may be more effective as a volume management strategy after accumulation of activity. Individual variation suggests the potential to optimize the limb fluid volume benefits of EV by reducing socket vacuum pressure for some users.
Keywords: Elevated vacuum, prosthetic socket, artificial limbs, residual limb volume, volume accommodation, bioimpedance, suction, lower limb
BACKGROUND
Daily limb volume loss is a challenge for many individuals with transtibial amputation.1 Residual limb volume loss greatly impacts the fit of the prosthetic socket.2 As the residual limb reduces in volume, the socket may become loose, adversely affecting interface pressure and shear stress distributions.3 Poor socket fit may affect limb health and may also lead to skin breakdown, unstable gait, and injurious falls.1,4
Advances in prosthetic socket technology have served to increase the number of volume accommodation strategies available to individuals with lower limb amputation. However, they do not all perform the same. Strategies such as prosthetic socks, pads, and adjustable paneled sockets are only temporarily effective and often lead to additional volume loss since the size of the interior socket is reduced, compressing the limb.5,6 Other strategies, such as socket release,7–9 may be effective but are not always convenient. Elevated vacuum (EV), a suspension method that applies continuous negative pressure between the liner and socket to pull the soft tissue outward to the socket wall,10–14 has been suggested as a strategy for preventing or slowing daily residual limb volume change without reducing the interior socket volume.11 EV may influence fluid volume by reducing residual limb pressures during stance phase loading and increasing negative pressure during swing phase.11,15 The reduced pressure between the liner and the socket lowers interstitial fluid pressure, potentially increasing fluid volume transport to the interstitial space. This reduces residual limb volume loss and better maintains socket fit over time.16
Research assessing the effectiveness of EV in managing residual limb volume has shown inconsistent results when compared to other suspension methods such as locking pin and suction.11,12,16–18 Suction suspension (SS) uses a one-way valve and sealing sleeve; the user’s body weight expels air through the valve to create negative pressure during swing phase.19 Use of EV resulted in volume gains11,17 and less volume fluctuation18 in several studies but did not improve volume management in others.12,16 With the exception of Sanders et al.,16 each of these previous studies reported out-of-socket limb volume measurements such as alginate casting with water displacement11,17 and optical scanning12,18 which require the socket to be doffed. Residual limb volume changes rapidly with the removal of the socket and these changes vary by individual.20 Bioimpedance analysis does not require the socket to be doffed thus is more appropriate for evaluation of the effectiveness of volume accommodation strategies such as EV. Another methodological limitation of prior EV studies is that volume change was primarily evaluated during a single activity (i.e. walking) over a short time period ranging from 3 minutes16 to 30 minutes;12 therefore, results may not be indicative of volume changes that occur when performing various activities over an entire day.
The goal of this study is to evaluate the effectiveness of EV for managing residual limb fluid volume across many hours of activity compared to a control condition, SS.
METHODS
Volunteers were recruited from prosthetics clinics in Seattle, WA and Edmond, OK, and from individuals who had previously participated in studies in our laboratory. Inclusion criteria for participation in this study were a transtibial amputation of at least 18 months and classification as a limited community ambulator with a Medicare Functional Classification Level (MFCL) of K-2 or higher. Participants were required to report using a properly fitting EV socket with an electronic vacuum pump for at least 6 hours per day. Electronic pumps were required because they provide a more controlled and continuous level of vacuum compared to mechanical pumps.21 Exclusion criteria included actively undergoing socket revisions and any presence of skin breakdown. A University of Washington Institutional Review Board approved the study procedures, and informed consent was obtained from each participant before beginning study procedures.
The study used a fixed-order crossover design consisting of two visits spaced approximately one week apart. For the first test session, participants used their regular EV system at the maximum vacuum setting within the allowable range established by each participant’s prosthetist. For the second test session, the pump was deactivated (i.e. standby mode) before beginning the activity protocol, preventing vacuum regulation and simulating SS. The EV system was returned to each participant’s regular settings following the second test session. SS was deemed an appropriate control due to its popularity as a standard suspension method.19 Using SS also ensured that the same socket and components were used for each test condition. A fixed-order design was selected for safety to ensure participants were comfortable completing the protocol with their standard prosthesis before using the modified suspension, SS. Test sessions were conducted remotely at TGG Prosthetics & Orthotics in Edmond, OK, as well as locally at the University of Washington located in Seattle, WA. During each visit, an approximately 5.5-hour standardized protocol was conducted outside of the clinic/lab environment along indoor hallways. A portable bioimpedance analyzer was used to assess residual limb fluid volume throughout the protocol. A wireless monitor to detect within-socket vacuum pressure (LimbLogic Communicator LimbLogic EV, WillowWood, Mt. Sterling, OH, USA) was available if a loss of vacuum suspension was suspected and verification of vacuum was needed.
Bioimpedance analysis monitors intracellular and extracellular resistance in limb segments and reflects primarily changes in muscle, skin, and blood fluid volume.22–24 Thin electrodes were custom produced using electrically conductive tape (ARCare 8881, Adhesives Research Incorporated, Glen Rock, PA, USA) and a thin layer of hydrogel (9880, 3M, Maplewood, MN, USA). A custom bioimpedance analyzer was used to inject bursts of electrical current (300 μA peak-to-peak) from the proximal thigh to the distal aspect of the residual limb. Each tone included 26 frequencies logarithmically spaced across a range of 3 kHz to 1 MHz.25 Pairs of voltage sensing electrodes were placed at the level of the patellar tendon distal to the fibular head and at the distal tibia, one pair positioned anterior laterally and the other pair along the posterior midline (Figure 1).26 Tissue through the limb cross-section between the proximal and distal electrode levels was monitored. Since current travels through the cross-section along the limb longitudinal axis, the interosseous membrane and the thick muscle fascia enveloping large muscle groups help to separate signal from the anterior and posterior regions, as demonstrated previously.27 De Lorenzo’s form of the Cole model is used to determine extracellular fluid resistance from demodulated bioimpedance data,28 and a geometric volume conduction model is applied to determine limb extracellular fluid volume.29,30 We use the term residual limb fluid volume to refer to the extracellular fluid volume calculated using this method. Extracellular fluid volume is investigated here because of its clinical relevance towards this application and its high-quality signal (error estimated as <0.1% fluid volume).25 Intracellular fluid volume changes are notoriously difficult to measure and bench tests demonstrated excessive error for this application.22,25
Figure 1.
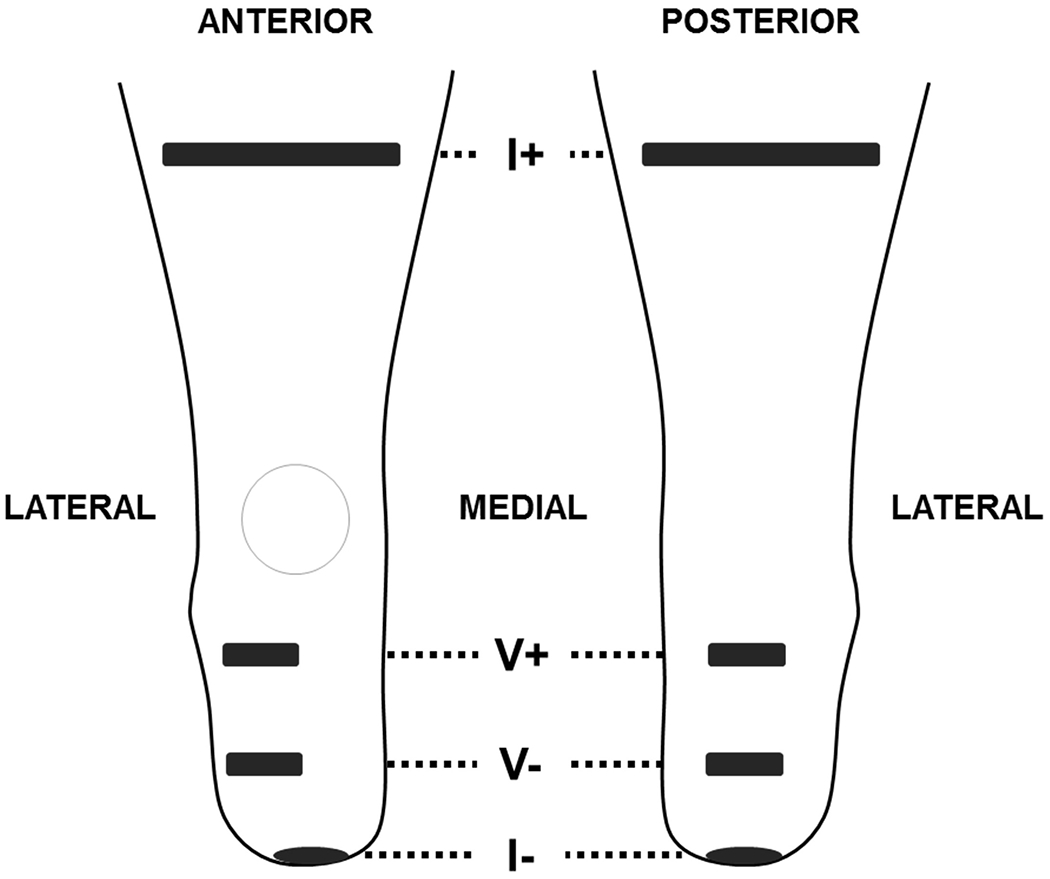
Electrode positioning for bioimpedance analysis. The I+ current electrodes are placed perpendicular to the limb axis on the posterior and anterior sides of the limb. The I- electrode is placed centrally on the distal end. The anterior voltage sensing electrodes are centered over the anterior lateral muscle compartment. V+ is at the proximal edge of the compartment, and V- is placed at the distal end of the tibia. The posterior voltage sensing electrodes are centered on the limb at the same level as the anterior electrodes.
Upon arriving at the testing location, participants sat for 10 minutes with their prosthesis donned to reach a homeostatic state. At the initial visit, the researchers collected participant health history, date of birth, activity information, date of amputation, amputation etiology, height, weight, residual limb length, and mid-limb circumference. The research prosthetist reviewed activity information and indicated an MFCL for each participant. Researchers also recorded characteristics of each prosthesis including EV type, sock use, EV settings, and prosthetic components such as liner type. All aspects of the prosthesis (e.g. socket, sleeve, socks, and liner) were maintained across test sessions. Participants were asked not to consume caffeine or alcohol and to maintain a consistent diet for each test day.
Participants then removed their prosthesis and liner, and researchers applied electrodes to their residual limb before beginning the activity protocol. Each test protocol was broken into three cycles (Figure 2). These cycles consisted of three, half-hour intervals of varying activity compositions. The first two intervals were of low activity (2 minutes of walking, 22 minutes of sitting, and 6 minutes of standing), and the third interval was of high activity (15 minutes of walking and 15 minutes of standing). Participants walked at a comfortable, self-selected walking speed. Twenty-minute seated rests were conducted between each cycle. Data collected during stands were used in analysis rather than walking data so as to maintain a consistent reference posture at each measurement. Participants were asked to stand with equal weight on each leg for several seconds at the start and end of each activity, as per prior studies.16,31 A low sodium meal (i.e. lunch) was provided for the participants after the first cycle of each session, and the same meal was provided for both test sessions.
Figure 2.
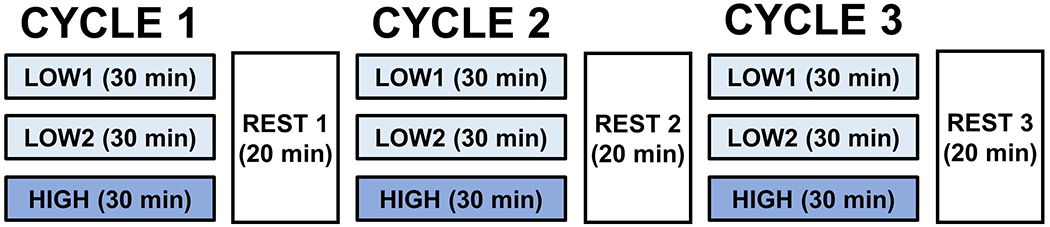
Standardized activity protocol conducted at each session. Each cycle consisted of two low-activity intervals (predominantly sitting, some walking and standing) followed by a high-activity interval (walking and standing). Twenty-minute seated rests were conducted between each cycle.
The fluid volume after the first cycle was considered the reference volume for each participant, consistent with previous efforts that used a similar activity protocol (Figure S1).7 Fluid volume was then expressed as a percent change with respect to that reference. The overall rate of limb fluid volume change was calculated as the percent change from the reference volume to the end of the protocol divided by elapsed time. Using rates of change accounted for slight protocol timing variations between sessions. For each region (i.e. anterior and posterior), the overall rate of fluid volume change for each test condition (i.e. EV and SS) was compared using the Wilcoxon signed-rank test. Non-parametric statistical tests were used because of the small sample size and non-normal distribution of the data (as determined by the Shapiro-Wilk test of normality). A threshold value of 0.05 was used for all comparisons except where a Bonferroni correction for multiple comparisons was applied. Statistical tests were conducted using SPSS (IBM SPSS Statistics, Version 24.0, Armonk, NY, USA).
The rate of fluid volume change during each of the three cycles was determined to assess how the short-term rates of fluid volume change differed between test conditions and throughout the protocol. A least-squares regression was applied to the equal-weight standing points of each cycle, and a Wilcoxon signed-rank test was used to compare rates of fluid volume change during corresponding cycles across test conditions for each limb region. A Friedman test with pairwise comparisons evaluated if the rates of fluid volume change within each condition differed by cycle.
For each limb region, fluid volume change for each activity (i.e. sit, stand, walk) was segmented and summed following the reference volume after Cycle 1 and divided by the time spent conducting each activity. This cumulative fluid volume rate of change for each activity was then compared across test conditions with the Wilcoxon signed-rank test. When the distribution of median differences between test conditions was not symmetrical, the sign test was used to evaluate differences.
RESULTS
Participant Demographics
Twelve individuals with transtibial amputation were tested, including nine males and three females (Table 1). None of the participants reported a history of peripheral vascular disease.
Table 1.
Participant characteristics.
| ID # | Gender | MFCL | Tobacco | Diabetes/CV Issues | Etiology | Age (years) | Since Amputation (years) | Mass (kg) | Height (cm) | BMI | Limb Length (cm) | Mid Limb Circ (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | K3 | Y | N/Y | Infection | 41.7 | 5.5 | 78.0 | 177.8 | 24.7 | 13.5 | 31.1 |
| 2 | M | K4 | N | N/N | Traumatic | 51.7 | 26.7 | 98.9 | 175.3 | 32.2 | 13.2 | 29.5 |
| 3 | F | K3 | Y | Y/N | Traumatic | 40.5 | 13.7 | 78.0 | 160.0 | 30.5 | 16.2 | 26.7 |
| 4 | M | K3 | N | N/N | Traumatic | 40.1 | 8.8 | 131.5 | 188.0 | 37.2 | 19.5 | 32.1 |
| 5 | M | K3 | N | Y/N | Traumatic | 66.6 | 46.9 | 102.1 | 167.6 | 36.3 | 15.2 | 29.4 |
| 6 | M | K3 | N | Y/Y | Infection | 70.9 | 7.0 | 81.6 | 172.7 | 27.4 | 10.5 | 28.9 |
| 7 | M | K3 | N | Y/Y | Infection | 72.5 | 7.6 | 127.5 | 195.6 | 33.3 | 16.5 | 30.7 |
| 8 | F | K3 | N | Y/N | Infection | 52.6 | 4.0 | 77.6 | 167.6 | 27.6 | 16.0 | 27.0 |
| 9 | M | K3 | N | N/N | Infection | 64.8 | 45.0 | 97.1 | 177.8 | 30.7 | 10.0 | 31.3 |
| 10 | M | K4 | N | N/N | Traumatic | 36.8 | 14.8 | 90.0 | 189.2 | 25.1 | 11.0 | 31.6 |
| 11 | M | K3 | N | N/Y | Traumatic | 68.9 | 16.1 | 101.8 | 180.3 | 31.3 | 15.0 | 30.2 |
| 12 | M | K3 | N | N/N | Traumatic | 48.3 | 3.9 | 122.9 | 172.7 | 41.2 | 19.0 | 37.0 |
| Mean | 54.6 | 16.7 | 98.9 | 177.1 | 31.5 | 14.6 | 30.4 | |||||
| Median | 52.1 | 11.3 | 98.0 | 176.5 | 31.0 | 15.1 | 30.4 | |||||
| SD | 13.4 | 15.1 | 19.5 | 10.1 | 5.0 | 3.1 | 2.7 | |||||
| Min | 36.8 | 3.9 | 77.6 | 160.0 | 24.7 | 10.0 | 26.7 | |||||
| Max | 72.5 | 46.9 | 131.5 | 195.6 | 41.2 | 19.5 | 37.0 | |||||
BMI: body mass index; Circ: circumference; CV: cardiovascular; M: male; F: female; Y: yes, N: no; MFCL: Medicare Functional Classification Level; SD: standard deviation
Participant Prosthetic Systems
Socket and suspension characteristics for each participant are listed in Table 2. All participants in this study used the LimbLogic EV system (WillowWood, Mt Sterling, OH, USA). Three participants used a traditional configuration with the pump mounted below the carbon fiber socket with an outer sealing sleeve. One participant used the One System which featured the distally mounted pump with an inner flexible socket and inner sealing sleeve. Eight participants used a custom prosthetic system with the EV pump mounted to a Surlyn™ flexible inner socket sealed with a sealing sleeve. A modified plate with locking pin distal to the pump then secured this inner flexible socket within a laminated carbon fiber frame. Participants used the same configuration of socks and sheaths during each session and did not make changes during either session.
Table 2.
Socket and suspension characteristics. Range indicates how much vacuum may be lost before the pump is activated.
| ID # | Suspension | User Max (inHg) | Range (inHg) | Seal Type | Socks | Liner |
|---|---|---|---|---|---|---|
| 1 | Vacuum/Pin | −20 | 6 | Inner sleeve | 3 sheaths | Ottobock Uneo Unique |
| 2 | Vacuum/Pin | −20 | 4 | Inner sleeve | 1 ply | Ottobock Uneo Unique |
| 3 | Vacuum/Pin | −20 | 4 | Inner sleeve | 2 sheaths | Ottobock Uneo Unique |
| 4 | Vacuum/Pin | −20 | 6 | Inner sleeve | 4 sheaths | Ottobock Uneo Unique |
| 5 | Vacuum/Pin | −20 | 6 | Inner sleeve | 4 sheaths | Ottobock Uneo Unique |
| 6 | Vacuum/Pin | −20 | 6 | Inner sleeve | 1 sheath | Ottobock Uneo Unique |
| 7 | Vacuum | −20 | 6 | Outer sleeve | 1 sheath | Ottobock Uneo Unique |
| 8 | Vacuum/Pin | −20 | 6 | Inner sleeve | 1 sheath | Ottobock Uneo Unique |
| 9 | Vacuum/Pin | −20 | 6 | Inner sleeve | 2 sheaths | Ottobock Uneo Unique |
| 10 | Vacuum | −20 | 5 | Outer sleeve | None | Ossur Iceross Dermo |
| 11 | Vacuum | −20 | 4 | Outer sleeve | None | Ossur Iceross Dermo |
| 12 | Vacuum | −10 | 4 | Inner sleeve | 2 gel socks | WillowWood Alpha Duo |
Protocol Deviations
A pump malfunction required Participant 6 to remove the inner socket from the carbon fiber frame to reset the pump early in the second cycle of the EV condition, shifting residual limb fluid volume. As a result, the first equal weight stand point following the pump reset was selected as the reference fluid volume for this participant. All other analyses involving the second cycle of both the EV and SS session of Participant 6 began at this reference point. Bioimpedance signal noise caused a poor model fit in the posterior channel of Participant 9. To acquire acceptable measurement quality, data processing was modified to include a limited range of current frequencies in the Cole model. The anterior channel was unaffected. Participant 3 and Participant 11 reported consuming caffeine on the day of the first test session. These two participants repeated caffeine consumption for the second test session.
Overall Rate of Limb Fluid Volume Change
Overall rates of fluid volume change were not significantly different (ppost = 0.39, pant = 0.16) between the EV and SS conditions in either limb region (Figure 3A, Table S1). Examining individual results in Figure 3B, eleven participants experienced limb fluid volume benefit (i.e. lower rates of fluid volume loss) in at least one region of the limb when using EV. Seven participants experienced benefit in both anterior and posterior limb regions, and only one experienced higher rates of overall fluid volume loss in both regions with EV.
Figure 3.
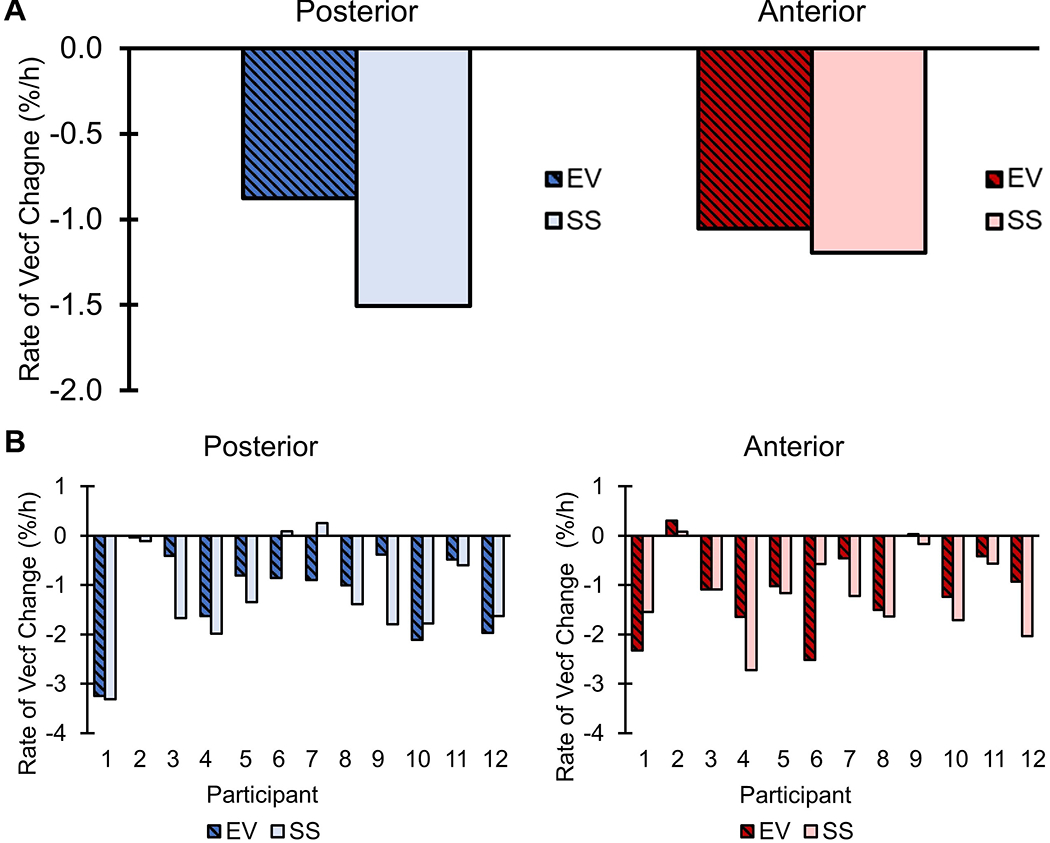
Overall rate of percent fluid volume change for elevated vacuum and suction suspension sockets. (A) Overall median extracellular fluid volume rate of change (%/h) calculated from the reference point for both anterior and posterior limb regions. (B) Overall extracellular fluid volume change (%/h) over the course of each session for each participant. Vecf = extracellular fluid volume. EV = elevated vacuum. SS = suction suspension.
Rate of Limb Fluid Volume Change by Cycle
Posterior fluid volume rates of loss were greater (p = 0.014) in Cycle 2 than Cycle 3 for the EV condition (Figure 4, Table S2). Additionally, in Cycle 3, rates of limb fluid volume loss in the posterior region were significantly greater in the SS condition (p = 0.03) (Table S3).
Figure 4.
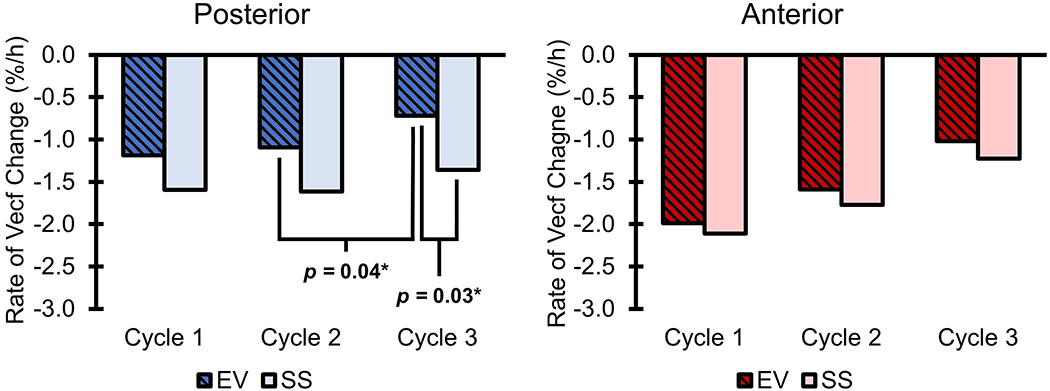
Median rates of percent fluid volume change during each cycle and test condition. Vecf = extracellular fluid volume. *Statistically significant difference.
Rate of Limb Fluid Volume Change by Activity
For both posterior (pwalk = 0.14, pstand = 0.24, psit = 1.00) and anterior (pwalk = 0.77, pstand = 0.24, psit = 0.35) limb regions, no statistical differences existed between rates of limb fluid volume change in EV and SS conditions for each activity type (Figure 5A, Table S4). Trends varied between activity types. Median rates of limb fluid volume change were positive while walking and were negative while sitting and standing. Individual results (Figure 5B) showed that only Participant 2 lost fluid in a region of their limb when walking with EV.
Figure 5.
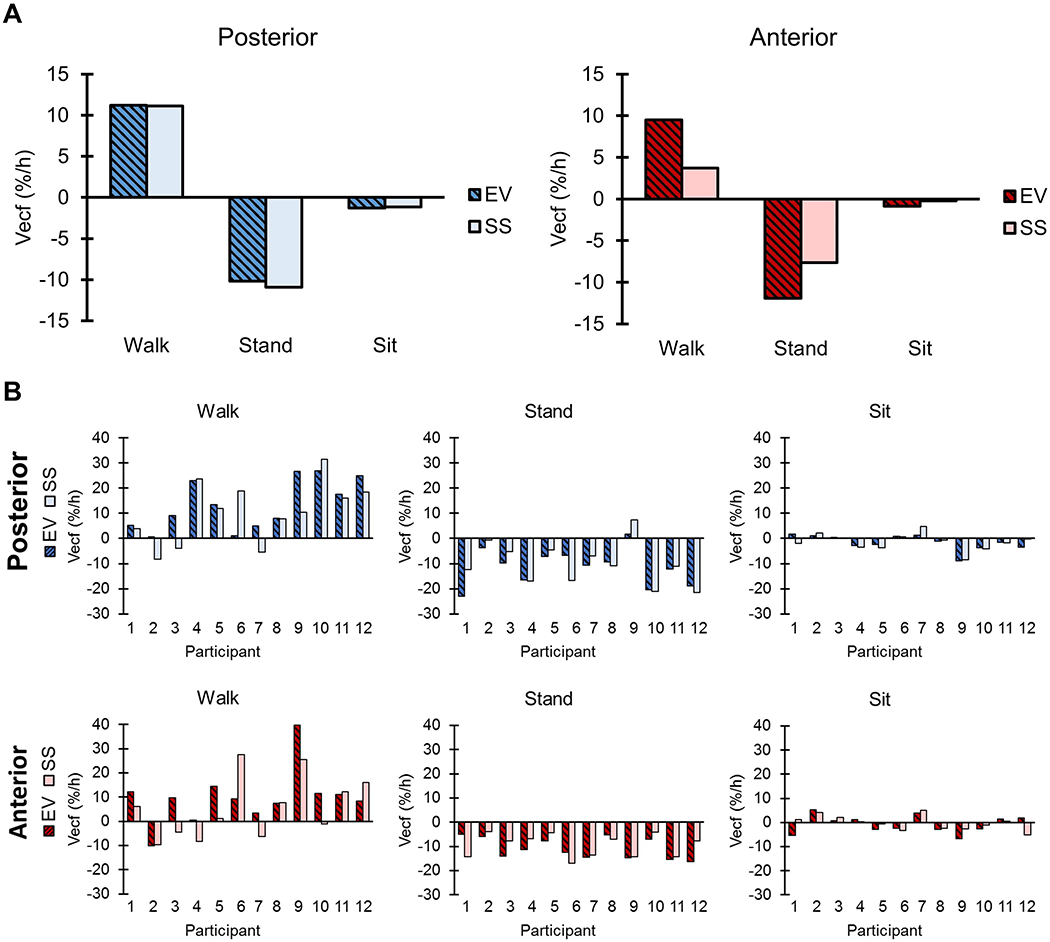
Rate of percent fluid volume change by activity. (A) Median cumulative extracellular fluid volume rate of change by activity. Note that because each activity was normalized to time individually, the activities do not sum to the overall median rate of change. (B) Individual cumulative extracellular fluid volume rate of change by activity. Vecf = extracellular fluid volume.
DISCUSSION
The purpose of this study was to evaluate the effectiveness of EV in managing residual limb fluid volume compared to SS during various activities over multiple hours of a day. Studies have demonstrated the effectiveness of EV to secure the limb within the socket;10–14 however, limitations such as the use of out-of-socket volume measurements and short, single-activity protocols have made interpretation of limb volume studies difficult.11,12,16–18 This study attempted to address these methodological limitations by using an in-socket fluid volume measurement technique (i.e. bioimpedance analysis) and by incorporating activities that are more representative of typical daily activities. This study contributes to the evidence regarding the ability of EV to influence residual limb volume which will allow prosthetists to make more informed clinical decisions regarding volume accommodation strategies to improve daily socket fit.
Previous limb volume studies comparing the use of EV with SS have shown less volume loss with EV.11,17 Exact volume changes experienced by users in prior studies are difficult to compare to the current study due to the differences in measurement techniques (i.e. bioimpedance analysis is limited to measuring extracellular limb fluid volume whereas total limb volume was measured in previous studies). In this study of EV users with transtibial amputation, use of EV significantly reduced the rate of posterior residual limb fluid volume change relative to SS during Cycle 3. This change may be due to the activity accumulation and volume loss prior to Cycle 3. Though the clinical significance of this difference is unclear, this may suggest that EV is more effective as a volume management strategy after activity accumulation. This issue is important because excess daily activity may lead to discomfort for prosthesis users as they are likely reaching their lowest daily limb fluid volume.7 The significant difference likely occurred posteriorly due to the large amount of soft tissue relative to the anterior region. The posterior soft tissue may expand to the wall of the socket more easily, increasing tissue volume and likely lowering the pressure in the interstitial space to encourage volume recovery.16
Despite lower rates of posterior limb fluid volume loss after activity accumulation, walking with EV compared with SS did not significantly improve limb fluid volume recovery in either posterior or anterior limb regions as suggested by prior studies, both those assessing total limb volume and those assessing extracellular fluid volume.11,16,17 Further, no significant differences were found between EV and SS regarding rates of limb fluid volume change by activity type (i.e. sitting, standing, or walking). The length of activity bouts and the preceding activity likely influenced the rate of limb fluid volume change. Additionally, walking conditions such as speed likely varied by each individual and may have affected results. In prior studies, single bouts of an activity such as walking were compared; whereas, bouts of activity in the current study varied throughout the protocol and were summed for analysis. Consistently longer bouts of an activity may be required to observe differences between EV and SS. Rates of limb fluid volume change did vary by activity type. As indicated by previous bioimpedance studies, gaining limb fluid volume while walking is not uncommon and may be due to the increased muscle activity in combination with larger arterial-to-interstitial transport.31
We also note that EV sockets typically require more detail than SS sockets to eliminate loading in unwanted areas. Because EV pulls areas of bony prominence into the socket wall, the prosthetist carefully relieves these areas during design and fitting. SS sockets would not necessarily include these details, but they did in the present study because the same socket was used for both EV and SS configurations. This feature of our study design may have reduced differences between EV and SS results.
Factors such as socket fit, socket components, socket vacuum pressure, individual health, and residual limb tissue content may have affected each user’s limb fluid volume management and suspension with EV. The magnitude of differences between overall residual limb fluid volume change under EV and SS also varied greatly between individuals, suggesting that there may be opportunity to optimize EV to each individual by tuning system parameters to meet individual needs. For the majority of study participants, vacuum systems were set to the highest available vacuum pressure by their prosthetist. This did not permit an evaluation of intermediate vacuum pressure settings. Intermediate vacuum pressures may improve residual limb fluid volume management in cases where EV resulted in higher rates of loss than SS. However, higher vacuum pressures have been shown to reduce limb-socket displacement during ambulation.14 Even if intermediate or low vacuum pressures benefit residual limb fluid volume management, the effects on suspension may be detrimental. Thus, finding the balance between the limb fluid volume management and limb-socket displacement could be valuable to maximize the benefits of EV to patient care.
This study had several limitations including that the order of intervention was not randomized, thus an order effect could not be evaluated. Additionally, results may have been affected by the existing use of EV, the different EV socket designs used in the study, and the lack of accommodation period for SS. Though no participants reported peripheral vascular disease, the presence of comorbidities such as diabetes and other cardiovascular issues may have affected results. Also, the EV socket with the deactivated pump may not have been an ideal representation of SS due to added weight and potentially different than ideal socket designs. Results may have differed had EV been compared to a suspension featuring no negative pressure (i.e. locking pin). Limb fluid volume data used in analysis were collected during standing with equal weight-bearing rather than walking so as to ensure a consistent posture and insensitivity to gait deviations across the 5.5-hour study protocol.16,31 In the future, studies investigating limb fluid volume differences within steps for EV compared with other socket configurations may provide additional insight into how to use EV most effectively for individual prosthesis users.
CONCLUSION
EV reduced posterior limb fluid volume change compared to SS during the final cycle of a 5.5-hour activity protocol after an accumulation of activity. This suggests EV may be more effective than SS in managing daily residual limb fluid volume particularly after an accumulation of activity. Additionally, EV effectiveness appeared to vary by individual and activity. This may indicate that EV can be further optimized for individual users. Additional research into balancing the limb fluid volume and suspension benefits of EV across a range of vacuum pressures could further inform the clinical implementation of EV. Future studies of EV volume changes during the fitting process might reveal other important variables that differentiate EV sockets from other socket designs.
Supplementary Material
Clinical Relevance:
A better understanding of how EV affects residual limb fluid volume will allow prosthetists to make more informed clinical decisions regarding accommodation strategies designed to improve daily socket fit.
Acknowledgments
Funding
The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article. This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Congressionally Directed Medical Research Program under Award No. W81XWH-10-1-1035 and the National Institutes of Health under Award No. R01HD060585. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Defense or the National Institutes of Health.
REFERENCES
- 1.Sanders JE, Fatone S. Residual limb volume change: Systematic review of measurement and management. J Rehabil Res Dev 2011; 48: 949–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Legro MW, Reiber G, del Aguila M, et al. Issues of importance reported by persons with lower limb amputations and prostheses. J Rehabil Res Dev 1999; 36: 155–163. [PubMed] [Google Scholar]
- 3.Sanders JE, Zachariah SG, Jacobsen AK, et al. Changes in interface pressures and shear stresses over time on trans-tibial amputee subjects ambulating with prosthetic limbs: comparison of diurnal and six-month differences. J Biomech 2005; 38: 1566–1573. [DOI] [PubMed] [Google Scholar]
- 4.Hargens AR. Tissue Nutrition and Viability. Springer Science & Business Media, 2012. [Google Scholar]
- 5.Sanders JE, Cagle JC, Harrison DS, et al. How does adding and removing liquid from socket bladders affect residual-limb fluid volume? J Rehabil Res Dev 2013; 50: 845–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders J, Harrison D, Allyn K, et al. How do sock ply changes affect residual-limb fluid volume in people with transtibial amputation? J Rehabil Res Dev 2012; 49(2): 241–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Youngblood RT, Hafner BJ, Allyn KJ, et al. Effects of activity intensity, time, and intermittent doffing on daily limb fluid volume change in people with transtibial amputation. Prosthet Orthot Int 2019; 43: 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanders JE, Hartley TL, Phillips RH, et al. Does temporary socket removal affect residual limb fluid volume of trans-tibial amputees? Prosthet Orthot Int 2016; 40: 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brzostowski JT, Larsen BG, Youngblood RT, et al. Adjustable sockets may improve residual limb fluid volume retention in transtibial prosthesis users. Prosthet Orthot Int 2019; 43: 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Street GM Vacuum suspension and its effects on the limb. Orthopädie Technik 2007; 4: 1–7. [Google Scholar]
- 11.Board WJ, Street GM, Caspers C. A comparison of trans-tibial amputee suction and vacuum socket conditions. Prosthet Orthot Int 2001; 25: 202–209. [DOI] [PubMed] [Google Scholar]
- 12.Klute GK, Berge JS, Biggs W, et al. Vacuum-Assisted Socket Suspension Compared With Pin Suspension for Lower Extremity Amputees: Effect on Fit, Activity, and Limb Volume. Arch Phys Med Rehabil 2011; 92: 1570–1575. [DOI] [PubMed] [Google Scholar]
- 13.Darter BJ, Sinitski K, Wilken JM. Axial bone-socket displacement for persons with a traumatic transtibial amputation: The effect of elevated vacuum suspension at progressive body-weight loads. Prosthet Orthot Int 2016; 40: 552–557. [DOI] [PubMed] [Google Scholar]
- 14.Gerschutz MJ, Hayne ML, Colvin JM, et al. Dynamic Effectiveness Evaluation of Elevated Vacuum Suspension: J Prosthet Orthot 2015; 27: 161–165. [Google Scholar]
- 15.Beil TL, Street GM, Covey SJ. Interface pressures during ambulation using suction and vacuum-assisted prosthetic sockets. J Rehabil Res Dev 2002; 39: 693–700. [PubMed] [Google Scholar]
- 16.Sanders JE, Harrison DS, Myers TR, et al. Effects of elevated vacuum on in-socket residual limb fluid volume: case study results using bioimpedance analysis. J Rehabil Res Dev 2011; 48: 1231–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goswami J, Lynn R, Street G, et al. Walking in a vacuum-assisted socket shifts the stump fluid balance. Prosthet Orthot Int 2003; 27: 107–113. [DOI] [PubMed] [Google Scholar]
- 18.Gerschutz MJ, Denune JA, Colvin JM, et al. Elevated Vacuum Suspension Influence on Lower Limb Amputee’s Residual Limb Volume at Different Vacuum Pressure Settings. J Prosthet Orthot 2010; 22(4): 252–256. [Google Scholar]
- 19.Chino N, Pearson JR, Cockrell JL, et al. Negative pressures during swing phase in below-knee prostheses with rubber sleeve suspension. Arch Phys Med Rehabil 1975; 56: 22–26. [PubMed] [Google Scholar]
- 20.Zachariah SG, Saxena R, Fergason JR, et al. Shape and volume change in the transtibial residuum over the short term: preliminary investigation of six subjects. J Rehabil Res Dev 2004; 41: 683–694. [DOI] [PubMed] [Google Scholar]
- 21.Major MJ, Caldwell R, Fatone S. Evaluation of a Prototype Hybrid Vacuum Pump to Provide Vacuum-Assisted Suspension for Above-Knee Prostheses. J Med Devices 2015; 9: 044504–044504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimnes S, Martinsen OG. Bioimpedance and Bioelectricity Basics. 2nd ed. London, England: Academic Press, 2008. [Google Scholar]
- 23.Zhu F, Sarkar S, Kaitwatcharachai C, et al. Methods and reproducibility of measurement of resistivity in the calf using regional bioimpedance analysis. Blood Purif 2003; 21: 131–136. [DOI] [PubMed] [Google Scholar]
- 24.Salinari S, Bertuzzi A, Mingrone G, et al. Bioimpedance analysis: a useful technique for assessing appendicular lean soft tissue mass and distribution. J Appl Physiol 2003; 94: 1552–1556. [DOI] [PubMed] [Google Scholar]
- 25.Hinrichs P, Cagle JC, Sanders JE. A portable bioimpedance instrument for monitoring residual limb fluid volume in people with transtibial limb loss: A technical note. Med Eng Phys 2019; 68: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders JE, Redd CB, Cagle JC, et al. Preliminary evaluation of a novel bladder-liner for facilitating residual limb fluid volume recovery without doffing. J Rehabil Res Dev 2016; 53: 1107–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders J, Moehring M, Rothlisberger T, et al. A Bioimpedance Analysis Platform for Amputee Residual Limb Assessment. IEEE Trans Biomed Eng 2016; 63: 1760–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Lorenzo A, Andreoli A, Matthie J, et al. Predicting body cell mass with bioimpedance by using theoretical methods: a technological review. J Appl Physiol 1997; 82: 1542–1558. [DOI] [PubMed] [Google Scholar]
- 29.Hanai T, Koizumi N, Gotoh R. Dielectric properties of emulsions. Kolloid-Z Z Für Polym 1962; 184: 143–148. [Google Scholar]
- 30.Fenech M, Jaffrin MY. Extracellular and intracellular volume variations during postural change measured by segmental and wrist-ankle bioimpedance spectroscopy. IEEE Trans Biomed Eng 2004; 51: 166–175. [DOI] [PubMed] [Google Scholar]
- 31.Sanders J, Cagle J, Allyn K, et al. How do walking, standing, and resting influence trans-tibial amputee residual limb fluid volume? J Rehabil Res Dev 2014; 51(2): 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


